Abstract
The therapeutic potential for messenger RNA (mRNA) in infectious diseases and cancer was first realized almost three decades ago, but only in 2018 did the first lipid nanoparticle-based small interfering RNA (siRNA) therapy reach the market with the United States Food and Drug Administration (FDA) approval of patisiran (Onpattro™) for hereditary ATTR amyloidosis. This was largely made possible by major advances in the formulation technology for stabilized lipid-based nanoparticles (LNPs). Design of the cationic ionizable lipids, which are a key component of the LNP formulations, with an acid dissociation constant (pKa) close to the early endosomal pH, would not only ensure effective encapsulation of mRNA into the stabilized lipoplexes within the LNPs, but also its subsequent endosomal release into the cytoplasm after endocytosis. Unlike other gene therapy modalities, which require nuclear delivery, the site of action for exogenous mRNA vaccines is the cytosol where they get translated into antigenic proteins and thereby elicit an immune response. LNPs also protect the mRNA against enzymatic degradation by the omnipresent ribonucleases (RNases). Cationic nano emulsion (CNE) is also explored as an alternative and relatively thermostable mRNA vaccine delivery vehicle. In this review, we have summarized the various delivery strategies explored for mRNA vaccines, including naked mRNA injection; ex vivo loading of dendritic cells; CNE; cationic peptides; cationic polymers and finally the clinically successful COVID-19 LNP vaccines (Pfizer/BioNTech and Moderna vaccines)—their components, design principles, formulation parameter optimization and stabilization challenges. Despite the clinical success of LNP-mRNA vaccine formulations, there is a specific need to enhance their storage stability above 0 °C for these lifesaving vaccines to reach the developing world.
Key Points
| Messenger RNA (mRNA) has immense therapeutic potential in infectious diseases and cancer immunotherapy. |
| Clinically successful lipid nanoparticles (LNPs) are smart nano-sized lipid-based carrier systems for mRNA delivery into the cytosol. |
| Engineering cationic ionizable lipids of LNPs with a suitable acid dissociation constant (pKa) for enhanced complexation and subsequent endosomal release is being actively researched. |
| There is a specific need to enhance the storage stability of LNP-mRNA vaccines to above 0 °C to bring these lifesaving vaccines to the developing world. |
Introduction
Messenger RNA (mRNA)-based vaccines have profound applications in infectious diseases and cancer immunotherapy [1]. The aim of vaccination is to stimulate acquired-, adaptive-immunity against the pathogen and thereby protect the human body from disease upon subsequent exposure to the same pathogen. Vaccine-mediated acquired immunity is elicited in the body by both humoral responses, i.e., by production of antigen-specific antibodies, which act as a first line of defense in eliminating the incoming pathogens, and the more persistent cell-mediated immune response by the lymphocytes (memory B-cells and T-cells) against the antigens present in the pathogen [2].
To understand how mRNA works inside the cell, one needs to comprehend the central dogma of molecular biology. Inside the normal living cells, the DNA is transcribed to RNA in the nucleus, which is translated into functional proteins through ribosomes in the cytoplasm. Hence, RNA plays a central role in the flow of genetic information from DNA to functional proteins. Therefore, mRNA-encoding pathogenic antigens (exogenous mRNA) can act as a potential therapeutic vaccine candidate, which would be translated in the normal cells after delivery. However, exogenous mRNAs, upon administration, become further degraded by ribonucleases (RNases) that are present ubiquitously all over the body [3]. Further, mRNA needs to enter the cytosol in full length for effective translation. Hence this requires a meticulous design of nano-particulate drug delivery system to deliver the intact exogenous mRNA cargo to its site of action, i.e., cytoplasm, where they express the antigenic proteins and thus elicit immune response [4–6].
No mRNA vaccines were approved until 2020. The COVID-19 (SARS-CoV-2 virus) pandemic brought mRNA vaccines to the limelight, with US Food and Drug Administration (FDA) providing emergency use approval/authorization for two mRNA-based vaccine candidates, i.e., Pfizer–BioNTech (approved on 11th December 2020) [7] and Moderna (approved on 18th December 2020) [8] vaccines. Since 11 December 2020, the Pfizer-BioNTech COVID-19 vaccine has been available under EUA in individuals aged ≥ 16 years, and the authorization was expanded on 10 May 2021 to include those aged 12–15 years [9]. Recently, on 23 August 2021, Pfizer-BioNTech COVID-19 vaccine received full approval from US FDA and is marketed as ComirnatyTM. Moderna has also completed its submission to the FDA for full approval of its COVID-19 vaccine for ages ≥ 18 years.
Both approved COVID-19 mRNA vaccines used a lipid-based nanoparticle (LNP) delivery system and are administered by intra-muscular injection into the deltoid muscle. The route of administration of mRNA vaccines determines its fate and efficacy; for example, the intradermal injection provides direct access of vaccine to antigen presenting cells (APCs) such as dendritic cells and macrophages, which can internalize them; whereas intra-muscular injection needs infiltration of APCs from large surrounding blood vessels to the injection site or alternatively, mRNA nanoparticles may be drained to the nearest lymph nodes by lymphatic drainage where they may transfect into the APCs present in the lymph nodes [10–13]. Hence, the intra-muscular route is the most widely used administration route for adjuvanted vaccines [14].
There are many potential benefits of these novel mRNA vaccines over the DNA-based viral vector vaccines and the more traditional live attenuated whole virion vaccines [15, 16]. First, they are safe, that is they are non-infectious, unlike live attenuated vaccine, and non-integrating, unlike DNA-based viral vector vaccines. Hence, there is no risk of infection or insertional mutagenesis. Second, they are highly efficacious and many of them are available as stable and highly translatable versions. Third, mRNAs can be rapidly and inexpensively synthesized with high yield through in vitro transcription reaction and hence are easily scalable to kilogram level quantity [17, 18]. Finally, unlike DNA vaccines, which require transfection of the viral vector delivery system into the nucleus, the mRNA vaccines need to be delivered only to the cytoplasm, which can be achieved through non-viral particulate carrier systems. Hence mRNA, unlike DNA, is devoid of fear from integration into the host genome and it can be efficiently delivered in vivo into the cytoplasm through formulation with various carrier molecules. Despite mRNA’s appealing features as a vaccine candidate, its in vivo delivery remains challenging [6, 14, 19, 20].
The mRNA vaccine field has been rapidly emerging over recent years and, in this review, we highlight the potential of mRNA therapies, currently available technologies for the delivery of mRNA and the role of lipids in mRNA delivery platforms.
mRNA Vaccine Delivery Modalities
Injection of Naked mRNA
Messenger RNA can be delivered without any carrier molecules, namely through naked mRNA injection. Key advantages of this approach are the ease of preparation, storage, and cost effectiveness. In a proper storage buffer like 10% trehalose, the freeze-dried naked mRNA remains stable for 10 months at 4 °C [21]. Before administration, the naked mRNA needs simple reconstitution and dilution in appropriate buffer such as Ringers solution or Lactated Ringers solution [22]. Injection of naked mRNA is susceptible to RNase degradation and, as they do not cross the lipid bilayer, their intracellular delivery is always debatable.
The feasibility to deliver the naked mRNA in vivo was first established in 1990s through intramuscular injection in mice [23]. Several studies [24, 25] hypothesized macropinocytosis, which are active in macrophages and immature dendritic cells, as the mechanism for the intracellular delivery of naked mRNA [26, 27].
Ex Vivo Loading of Dendritic Cells
As dendritic cells are the key players in initiating an antigen-specific immune response, it follows logically to utilize them for the delivery of mRNA vaccine candidates. Dendritic cells internalize the pathogens, proteolytically process and present the antigens to CD8+ and CD4+ T-cells through the major histocompatibility complexes (MHC) class I and MHC class II. Hence this approach utilizes ex vivo loading of dendritic cells with mRNA vaccine candidate (encoding pathogenic antigens), followed by re-infusion of the transfected dendritic cells into autologous recipient to elicit immune response. The ex vivo loading of dendritic cells can be achieved through mild electroporation [28] or via lipid-derived carriers [29]. Electroporation being the most frequently used approach due to its high mRNA delivery efficiency [30].
Most ex vivo loaded dendritic cells exhibit cell-mediated immunity and hence this approach is predominantly utilized for cancer immunotherapy [31]. Further, transfection of autologous dendritic cells and characterization of dendritic cells phenotype post-transfection is a cumbersome process and not feasible for rapid commercial vaccine production as required in the case of a pandemic.
Cationic Nano-emulsion (CNE)
CNE are oil-in-water emulsion-based delivery systems. For example, MF59 and AS03 are two oil-in-water emulsion adjuvants extensively studied for influenza vaccine development during 2009 H1N1 influenza pandemic. MF59 [32, 33] is composed of squalene droplets (4.3%) stabilized with small amount of surfactants like Tween® 80 (0.5%) and SPAN® 85 (0.5%), whereas AS03 [34] is composed of α-tocopherol, squalene and Tween® 80, in an oil-in-water emulsion. Squalene, chemically a triterpene, is a known vaccine adjuvant. Squalene is naturally occurring in humans as precursor molecule in steroid synthesis and as part of the sebum, making it safe, biocompatible, and biodegradable. MF59 has been extensively studied in clinical trials and found to be safe and well tolerated in children and adults, including the elderly [33].
A key component to encapsulate the negatively charged mRNA in these CNEs is a cationic lipid—1,2-dioleoyl-3-trimethylammonium propane (DOTAP), that has been used in the oil phase to complex the mRNA. Therefore, this is also named as cationic lipid nano-emulsion. These CNEs are usually 90 to 130 nm in diameter. They not only aid in the intracellular delivery of the mRNA, but also protect them against degradation from RNases [35].
Most recently, Gennova Biopharmaceuticals Ltd, in collaboration with HDT Biotech Corporation, Seattle, USA, developed a lipid inorganic nanoparticle (LION® technology) for the delivery SARS-CoV2 vaccine candidate HGCO10 (self-amplifying RNA), which is undergoing clinical trials. Gennova claims LION® is a highly stable cationic lipid (DOTAP)-squalene emulsion similar to CNEs that has 15 nm superparamagnetic iron oxide (Fe3O4) nanoparticles (SPIO) (which enables therapeutic and imaging functionalities) embedded in the hydrophobic oil phase. This formulation is shown to have colloidal stability for at least 3 months when stored between 4° and 25 °C [36, 37]. These stability results are promising as cold chain storage and distribution of mRNA vaccines are extremely difficult in tropical and third-world countries, delaying the approval of these life-saving mRNA vaccines in some of these countries, such as India [38]. The showcased colloidal stability is by far better than other emergency-use authorized mRNA vaccines for SARS-CoV2 such as Moderna (shelf-life of up to 6 months at −20 °C; up to 30 days at 2–8 °C and up to 12 h at room temperature) and Pfizer-BioNTech (shelf-life of up to 6 months at −80 to –60 °C; up to 5 days at 2–8 °C and up to 2 h at room temperature). The role of SPIO nanoparticles on the stability enhancement of the mRNA vaccine needs to be explored further.
Cationic Peptides and Polymers
The cationic peptide, protamine has also been shown to stabilize the mRNA against degradation from serum RNases [39] and protect mRNA rabies vaccine from harsh storage conditions [40], which is especially useful for tropical countries. Cationic peptides contain many lysine and arginine residues, which are positively charged and can complex with the negatively charged mRNA [41, 42].
Such a type of self-adjuvanted RNActive® delivery technology [43] of CureVac uses protamine to complex mRNA and thereby developed vaccines for rabies [44] and for influenza A [45]. Although it stabilizes the mRNA, the intra-cellular delivery of the complex is questionable, and mRNA complexed with protamine are translated poorly in clinical trials [46].
Cationic polymers such as polyethylenimine (PEI), polyamidoamine (PAMAM) dendrimer, condensed and delivered negatively charged RNA molecules and hence have been explored as vaccine delivery candidates over the past few years. Even though these cationic polymers show in vivo efficacy, their potential in mRNA delivery is hampered by cationic polymer’s toxicity and high poly dispersity index (PDI) of the complex [47].
Lipid Nanoparticles (LNPs) for mRNA Delivery
Lipid nanoparticles are smart nano-sized lipid-based carriers for mRNA delivery into the cytosol. Apart from protecting the mRNA against RNAase during systemic circulation, these particulate nanocarriers can efficiently deliver mRNA intracellularly by fusing with the lipid bilayer of the early endosomes and thereby deliver the mRNA into the cytosol [48].
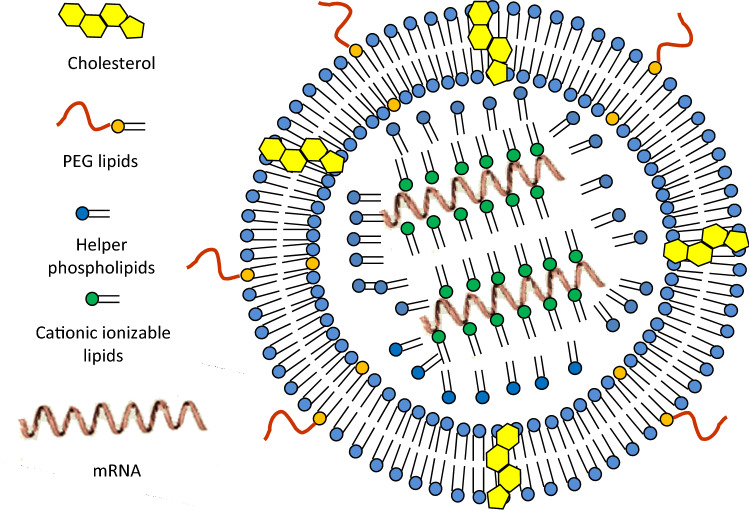
The components of LNPs (Fig. 1) are:
Cationic ionizable lipids are required to form complexation with mRNA (the lipoplex stabilization within LNPs) and further, the complexes are delivered into cytosol by fusing with the endosomal cell membrane (endosomal release once internalized).
- Helper lipids, which are also called structural lipids, include:
- Polyethylene glycol (PEG) lipids usually help to shield any residual charge on the surface of the lipoplexes within the LNPs, making them long systemic circulating nanoparticles. Examples include PEG lipid and PEG cholesterol;
- phospholipids, that support the lipid bilayer structure;
- cholesterol that stabilizes the structure of LNP.
Fig. 1.
Schematic representation of components of lipid nanoparticles (LNPs). PEG polyethylene glycol
Cationic Ionizable Lipids
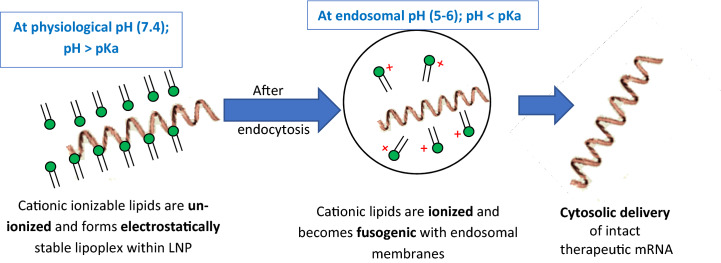
As nucleic acids such as mRNAs are negatively charged, the cationic lipids are a very important ingredient for nucleic acid complexation and delivery. Cationic ionizable lipids are required for complexation and encapsulation of mRNA through electrostatic interactions. These lipids are also essential for the efficient release of mRNA into the cytoplasm. Engineering cationic ionizable lipids with suitable acid dissociation constant (pKa) for enhanced complexation and subsequent endosomal release is a subject of active research [49, 50]. At a pH below the pKa of the ionizable amino head group, these cationic lipids are protonated, and hence acquire a positive charge, but remain un-ionized (no charge) at a pH above the pKa of the amino head group. These cationic ionizable groups play a central role in delivery of mRNA from LNPs by remaining neutral (uncharged; which reduces its systemic toxicity) in the systemic circulation, at a pH of 7.4 (pH > pKa of ionizable amino head group) but become protonated at early endosomal pH (pH ~ 6.5) to facilitate endosomal membrane fusion and subsequent cytosolic release (Fig. 2) [51]. Therefore, ideally these ionizable cationic lipids will have a pKa in the range of 6–7. Some of the commonly used cationic ionizable lipids in the US FDA-approved products are listed in Table 1.
Fig. 2.
Hypothetical cytosolic release of therapeutic mRNAs from lipoplex within lipid nanoparticles (LNPs). pKa the acid dissociation constant of cationic ionizable lipids
Table 1.
Cationic ionizable lipids used in approved formulations
| Brand name | Cationic ionizable lipid | pKa of the ionizable head group |
|---|---|---|
| OnpattroTM | (6Z,9Z,28Z,31Z)-Heptatriaconta-6,9,28,31 tetraen-19-yl-4-(dimethylamino) butanoate (DLin-MC3-DMA) | 6.44 |
| ComirnatyTM | ((4-Hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis(2-hexyldecanoate), ALC-0315 | 6.09 |
| Spikevax™ | (Heptadecan-9-yl 8-((2-hydroxyethyl) (6-oxo-6-(undecyloxy) hexyl) amino) octanoate}; SM102 | 6.75 |
Previous efforts to transfect mRNA with cationic DOTAP (quaternary amino head group), a pH insensitive cationic lipid that remains protonated and positively charged throughout the physiological pH range, along with fusogenic DOPE, was hindered by its systemic toxicity.
Polyethylene Glycol (PEG) Lipids
Polyethylene glycol lipids provide colloidal stability and prevent protein (opsonins) binding to the nanoparticle, thereby reducing the clearance of the nanoparticles from the systemic circulation by the reticulo-endothelial system (RES) and thus, achieve longer systemic circulation [52]. It is also important to consider the lower fusogenicity of the PEG lipids that might hinder endosomal release of mRNA, the so-called PEG-dilemma. Cleavable PEGylation can be a useful strategy for efficient intracellular delivery of mRNA [53]. PEG lipids may also enhance the storage stability of the LNPs by preventing their physical aggregation in solution, which would otherwise increase the LNPs particle size that could further lead to premature release of the encapsulated mRNA.
Phospholipids
Phospholipids are usually neutral like DSPC and DPPC, which provide bilayer structural stability to the LNPs. They also play a role in fusogenicity and biodistribution (reduced toxicity) of the LNPs [52]. DPPC is a natural lung surfactant and the preferred lipid for pulmonary dosage forms. That is the reason it has been historically used in many pulmonary drug delivery systems (DDS) including surfaxin, arikace, etc. The release profile of the encapsulated mRNA can be altered by combining DSPC, another saturated phospholipid with significantly higher lipid transition temperature (Tm) of 55 °C, with DPPC that has a Tm of 41 °C [54].
Cholesterol
Cholesterol is a neutral lipid that enhances the bilayer stability by improving its rigidity and preventing leakage of the therapeutic ingredient. Additionally, it is believed to play a role in the membrane fusion for LNPs and gene transfer when used in optimum concentration [55].
Formulation of LNPs
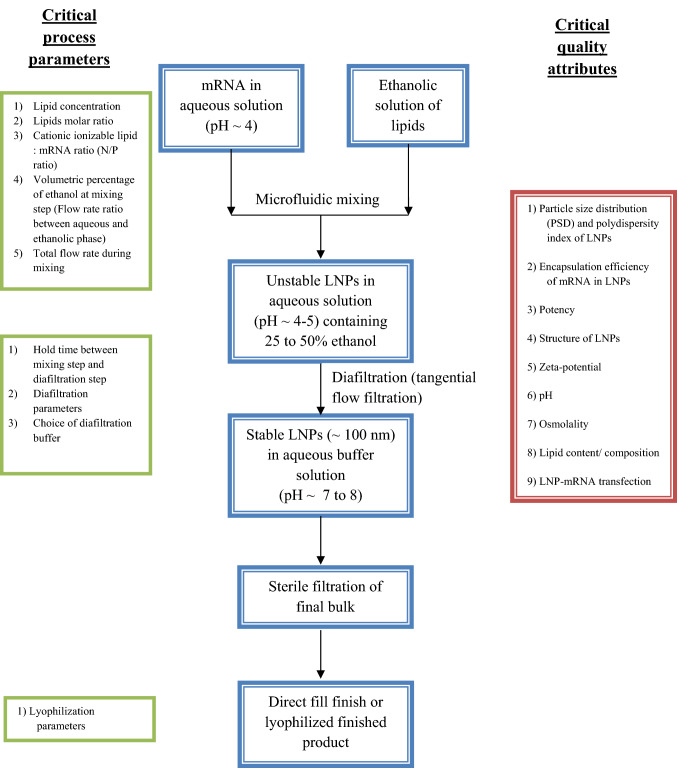
mRNA is prepared in low pH aqueous buffer, pH ~ 4.0 and is micro-fluidically mixed with an ethanolic solution of hydrophobic lipids to form low polydispersity unstable LNPs that contain 25–50% ethanol and a low pH. This pre-bulk of unstable LNPs needs to be dialyzed or buffer exchanged and/or concentrated immediately using tangential flow filtration (TFF) to get the final bulk [48, 56, 57]. This final bulk is sterile filtered using a 0.2 μm sterile grade filter and filled into aseptic containers. The finished drug product could be in either a lyophilized format or in a direct fill-finish format (Fig. 3).
Fig. 3.
Manufacturing process flow of lipid nanoparticles (LNPs) with its associated critical process parameters and critical quality attributes
The older method of ethanol injection and thin film hydration are replaced by microfluidic mixing devices as they produce homogenous narrow particle size distribution with higher encapsulation efficiency. The initial mixing of aqueous and ethanolic phase produces a pre-bulk of pH 5.5, protonating the ionizable lipid (pH < pKa), which allows mRNA binding and encapsulation followed by gradual increase of the bulk pH to neutral by TFF wherein cationic ionizable lipid becomes gradually uncharged and more hydrophobic, thereby driving vesicles to fuse and cause the further sequestration of the ionizable lipid with mRNA into the interior of the solid lipid nanoparticles. The PEG-lipid content stops the fusion process by providing the LNP with a hydrophilic exterior, determining its thermodynamically stable size, and the bilayer forming neutral phospholipids like DSPC is present just underneath this PEG-lipid layer [55]. The various formulation parameters that need optimization are lipid concentration; lipids molar ratio; cationic ionizable lipid:mRNA ratio (N/P ratio) to effect maximum encapsulation of mRNA within LNPs [57]. In a recent study by Moderna, it was shown that the volumetric mixing ratio of aqueous and ethanol streams, as well as total mixing flow rate during the microfluidic mixing step, significantly affects the particle size of the formed LNPs [58, 59]. Different formulation techniques provide different encapsulation efficiencies and different particle size with variable poly dispersity index. Careful choice of formulation parameters would provide stable LNPs that meet the quality target product profile of the finished drug product. Unravelling the structure of LNP-mRNA vaccine formulations would lead to its rational design towards enhanced mRNA delivery [60]. The morphology of LNPs is not like a traditional liposome, characterized by a lipid bilayer surrounding an aqueous core. Indeed, they possess an electron-dense core (as seen in CRYO-TEM), where the cationic/ionizable lipids are organized into inverted micelles around the encapsulated mRNA molecules [56, 61, 62]. LNPs are the carrier of choice for mRNA delivery and have been successfully used in various clinical formulations including COVID-19 mRNA vaccines [7, 8].
Apart from Pfizer/BioNTech and Moderna, CureVac and Arcturus (LUNAR® technology) are developing their mRNA COVID-19 vaccines based on LNP technology.
Opportunities to Enhance the Shelf-life and Storage Conditions of mRNA Vaccines
Even though mRNA vaccines have become a reality because of the LNP formulation technology, they are unavailable in many tropical and third-world countries due to the requirement for sub-zero storage and shipment temperatures. There is still a huge scope for improvement in LNP formulation technology to enhance the shelf-life and storage conditions of LNP-mRNA vaccines.
Stability Enhancing Excipients
To enhance the RNA stability, all the excipients intended to be used should be free from RNases. Apart from the basic components of LNPs, other components such as buffers, antioxidants, non-reducing free radical scavengers (e.g., ethanol) and metal chelators can be used to improve the stability of the LNPs. The extent to which each of these excipients enhance the stability of LNPs at different storage conditions (below 0 °C or above 0 °C) needs to be evaluated. For below 0 °C storage condition, buffer selection is crucial, as freezing a solution buffered with sodium phosphate (as used in Pfizer BioNTech COVID-19 vaccine) may cause a drift in bulk pH by up to 3.5 units, which might be detrimental to mRNA’s stability, whereas the buffer component used in Moderna’s COVID-19 vaccine may have a more stabilizing effect as TRIS buffer is a known hydroxyl free-radical scavenger [63]. Further, the osmolyte used in the Pfizer/BioNtech vaccine, sodium chloride, has a eutectic temperature of −21 °C [64]. Aggregation and loss of efficacy were observed by freeze–thaw cycles in these formulations, which were prevented by the addition of lyoprotectants, such as sucrose and trehalose [65]. Both the clinically available LNP-mRNA vaccines have sucrose as cryo/lyo-protectant [66].
According to Schoenmaker et al. [64], pH optimization of the final formulation is also crucial for mRNA vaccine stability, as hydrolysis rate of mRNA is dependent on pH; mRNA being more stable in a weakly basic environment. pH of the finished product also determines the LNPs stability. Both Pfizer/BioNTech and Moderna’s COVID-19 vaccines maintain the finished drug product pH between 7 and 8.
Lyophilization of the LNPs
As mRNA hydrolysis is the determining factor for mRNA-LNP stability [64], it flows logically to freeze-dry or lyophilize the LNP formulation to increase their stability.
According to Philip Dormitzer, head of viral vaccines research at Pfizer, the company has ongoing thermostability studies and is also working on a lyophilized formulation [67].
Currently, there is little published literature on lyophilization techniques to enhance the stability of the LNP formulations. Apart from preserving the integrity of LNPs through the lyophilization process, the lyophilized formulation should retain the structure of LNPs and encapsulation efficiency of mRNA in the LNPs after reconstitution, which is highly challenging [65]. Since lyophilization is a costly and time-consuming, high-energy process, other drying approaches like spray drying/supercritical drying approaches should also be explored [38, 64, 68].
Conclusion
SARS-CoV-2 mRNA vaccines have taken the center stage during this pandemic with the emergency use approval of Pfizer/BioNTech and Moderna vaccines that are based on LNP technology. The ionizable cationic lipids are the key components of the LNPs that need careful design to optimize their complexation and endosomal release properties and have received due attention from the scientific community. Even though LNP-based mRNA vaccines are a reality today in developed countries, much research is required to understand the LNP’s structure, biodistribution, toxicity, etc., and enhancement of its storage stability will unlock the full potential and reach of this transformative treatment modality to the entire world. Lastly, in the context of COVID-19 or any such future pandemics, the most important consideration is the speed at which we can bring the vaccine to the market. In the case of mRNA therapeutics, the development timelines are fast, because once the pathogen is genetically sequenced, it will enable us to generate a rapid response to the infectious disease outbreaks like SARS (severe acute respiratory syndrome), MERS (Middle East respiratory syndrome) or COVID-19.
Declarations
Funding
No funding was received for the publication of this review.
Conflicts of interest/competing interests
Sivakumar Ramachandran, Soumya Ranjan Satapathy and Tathagata Dutta declare no conflicts of interest/competing interests.
Ethics approval
Not applicable
Consent to participate
Not applicable
Consent for publication
Not applicable
Availability of data and material
Not applicable
Code availability
Not applicable
Authors’ contributions
SR and SRS contributed equally to conceptualizing and drafting the manuscript; TD critically reviewed the manuscript. All authors read and approved the final version of the manuscript and agree to be accountable for the work.
References
- 1.Sullenger BA, Nair S. From the RNA world to the clinic. Science. 2016;352:1417–1420. doi: 10.1126/science.aad8709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amanna IJ, Slifka MK. Contributions of humoral and cellular immunity to vaccine-induced protection in humans. Virology. 2011;411:206–215. doi: 10.1016/j.virol.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Houseley J, Tollervey D. The many pathways of RNA degradation. Cell. 2009;136:763–776. doi: 10.1016/j.cell.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 4.Wu Z, Li T. Nanoparticle-mediated cytoplasmic delivery of messenger RNA vaccines: challenges and future. Perspect Pharm Res. 2021;38:473–478. doi: 10.1007/s11095-021-03015-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maugeri M, Nawaz M, Papadimitriou A, Angerfors A, Camponeschi A, Na M, et al. Linkage between endosomal escape of LNP-mRNA and loading into EVs for transport to other cells. Nat Commun. 2019;10:4333. doi: 10.1038/s41467-019-12275-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fortner A, Schumacher D. First COVID-19 vaccines receiving the US FDA and EMA emergency use authorization. Discoveries (Craiova) 2021;9:e122. doi: 10.15190/d.2021.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ols S, Lore K. Imaging the early fate of mRNA vaccines. Nat Biomed Eng. 2019;3:331–332. doi: 10.1038/s41551-019-0399-y. [DOI] [PubMed] [Google Scholar]
- 11.Trevaskis NL, Kaminskas LM, Porter CJ. From sewer to saviour—targeting the lymphatic system to promote drug exposure and activity. Nat Rev Drug Discov. 2015;14:781–803. doi: 10.1038/nrd4608. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura T, Harashima H. Dawn of lipid nanoparticles in lymph node targeting: potential in cancer immunotherapy. Adv Drug Deliv Rev. 2020;167:78–88. doi: 10.1016/j.addr.2020.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura T, Kawai M, Sato Y, Maeki M, Tokeshi M, Harashima H. The effect of size and charge of lipid nanoparticles prepared by microfluidic mixing on their lymph node transitivity and distribution. Mol Pharm. 2020;17:944–953. doi: 10.1021/acs.molpharmaceut.9b01182. [DOI] [PubMed] [Google Scholar]
- 14.Zeng C, Zhang C, Walker PG, Dong Y. Formulation and delivery technologies for mRNA vaccines. Curr Top Microbiol Immunol 2020;1–40. [DOI] [PMC free article] [PubMed]
- 15.Liu MA. A comparison of plasmid DNA and mRNA as vaccine technologies. Vaccines (Basel) 2019;7:37. doi: 10.3390/vaccines7020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Satapathy SR, Sahoo RN, Pattnaik KP, Panigrahi L, Mallick S. Developmental biology of Corona vaccine: lipid nanoparticle mRNA-encapsulation, a safe approach. Minerva Biotechnol Biomol Res. 2021;33:166–173. doi: 10.23736/S2724-542X.21.02742-7. [DOI] [Google Scholar]
- 17.Jackson NAC, Kester KE, Casimiro D, Gurunathan S, DeRosa F. The promise of mRNA vaccines: a biotech and industrial perspective. NPJ Vaccines. 2020;5:11. doi: 10.1038/s41541-020-0159-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuchs AL, Neu A, Sprangers R. A general method for rapid and cost-efficient large-scale production of 5′ capped RNA. RNA. 2016;22:1454–1466. doi: 10.1261/rna.056614.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20:101–124. doi: 10.1038/s41573-020-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao Y, Gao GF. mRNA vaccines: a matter of delivery E. ClinicalMedicine. 2021;32:100746. doi: 10.1016/j.eclinm.2021.100746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones KL, Drane D, Gowans EJ. Long-term storage of DNA-free RNA for use in vaccine studies. Biotechniques. 2007;43:675–681. doi: 10.2144/000112593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Probst J, Weide B, Scheel B, Pichler BJ, Hoerr I, Rammensee HG, et al. Spontaneous cellular uptake of exogenous messenger RNA in vivo is nucleic acid-specific, saturable and ion dependent. Gene Ther. 2007;14:1175–1180. doi: 10.1038/sj.gt.3302964. [DOI] [PubMed] [Google Scholar]
- 23.Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, et al. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 24.Diken M, Kreiter S, Selmi A, Britten CM, Huber C, Tureci O, et al. Selective uptake of naked vaccine RNA by dendritic cells is driven by macropinocytosis and abrogated upon DC maturation. Gene Ther. 2011;18:702–708. doi: 10.1038/gt.2011.17. [DOI] [PubMed] [Google Scholar]
- 25.Selmi A, Vascotto F, Kautz-Neu K, Tureci O, Sahin U, von Stebut E, et al. Uptake of synthetic naked RNA by skin-resident dendritic cells via macropinocytosis allows antigen expression and induction of T-cell responses in mice. Cancer Immunol Immunother. 2016;65:1075–1083. doi: 10.1007/s00262-016-1869-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weide B, Carralot JP, Reese A, Scheel B, Eigentler TK, Hoerr I, et al. Results of the first phase I/II clinical vaccination trial with direct injection of mRNA. J Immunother. 2008;31:180–188. doi: 10.1097/CJI.0b013e31815ce501. [DOI] [PubMed] [Google Scholar]
- 27.Rittig SM, Haentschel M, Weimer KJ, Heine A, Muller MR, Brugger W, et al. Intradermal vaccinations with RNA coding for TAA generate CD8+ and CD4+ immune responses and induce clinical benefit in vaccinated patients. Mol Ther. 2011;19:990–999. doi: 10.1038/mt.2010.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boczkowski D, Nair SK, Snyder D, Gilboa E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J Exp Med. 1996;184:465–472. doi: 10.1084/jem.184.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tateshita N, Miura N, Tanaka H, Masuda T, Ohtsuki S, Tange K, et al. Development of a lipoplex-type mRNA carrier composed of an ionizable lipid with a vitamin E scaffold and the KALA peptide for use as an ex vivo dendritic cell-based cancer vaccine. J Control Release. 2019;310:36–46. doi: 10.1016/j.jconrel.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Van Tendeloo VF, Ponsaerts P, Lardon F, Nijs G, Lenjou M, Van Broeckhoven C, et al. Highly efficient gene delivery by mRNA electroporation in human hematopoietic cells: superiority to lipofection and passive pulsing of mRNA and to electroporation of plasmid cDNA for tumor antigen loading of dendritic cells. Blood. 2001;98:49–56. doi: 10.1182/blood.V98.1.49. [DOI] [PubMed] [Google Scholar]
- 31.Benteyn D, Heirman C, Bonehill A, Thielemans K, Breckpot K. mRNA-based dendritic cell vaccines. Expert Rev Vaccines. 2015;14:161–176. doi: 10.1586/14760584.2014.957684. [DOI] [PubMed] [Google Scholar]
- 32.Bogers WM, Oostermeijer H, Mooij P, Koopman G, Verschoor EJ, Davis D, et al. Potent immune responses in rhesus macaques induced by nonviral delivery of a self-amplifying RNA vaccine expressing HIV type 1 envelope with a cationic nanoemulsion. J Infect Dis. 2015;211:947–955. doi: 10.1093/infdis/jiu522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Hagan DT, Ott GS, Nest GV, Rappuoli R, Giudice GD. The history of MF59((R)) adjuvant: a phoenix that arose from the ashes. Expert Rev Vaccines. 2013;12:13–30. doi: 10.1586/erv.12.140. [DOI] [PubMed] [Google Scholar]
- 34.Garcon N, Vaughn DW, Didierlaurent AM. Development and evaluation of AS03, an Adjuvant System containing alpha-tocopherol and squalene in an oil-in-water emulsion. Expert Rev Vaccines. 2012;11:349–366. doi: 10.1586/erv.11.192. [DOI] [PubMed] [Google Scholar]
- 35.Brito LA, Chan M, Shaw CA, Hekele A, Carsillo T, Schaefer M, et al. A cationic nanoemulsion for the delivery of next-generation RNA vaccines. Mol Ther. 2014;22:2118–2129. doi: 10.1038/mt.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erasmus JH, Khandhar AP, O’Connor MA, Walls AC, Hemann EA, Murapa P, et al. An alphavirus-derived replicon RNA vaccine induces SARS-CoV-2 neutralizing antibody and T cell responses in mice and nonhuman primates. Sci Transl Med. 2020;12:eabc9396. doi: 10.1126/scitranslmed.abc9396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Erasmus JH, Khandhar AP, Walls AC, Hemann EA, O’Connor MA, Murapa P, et al. Single-dose replicating RNA vaccine induces neutralizing antibodies against SARS-CoV-2 in nonhuman primates. bioRxiv 2020.
- 38.Crommelin DJA, Anchordoquy TJ, Volkin DB, Jiskoot W, Mastrobattista E. Addressing the cold reality of mRNA vaccine stability. J Pharm Sci. 2021;110:997–1001. doi: 10.1016/j.xphs.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoerr I, Obst R, Rammensee HG, Jung G. In vivo application of RNA leads to induction of specific cytotoxic T lymphocytes and antibodies. Eur J Immunol. 2000;30:1–7. doi: 10.1002/1521-4141(200001)30:1<1::AID-IMMU1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 40.Stitz L, Vogel A, Schnee M, Voss D, Rauch S, Mutzke T, et al. A thermostable messenger RNA based vaccine against rabies. PLoS Negl Trop Dis. 2017;11:e0006108. doi: 10.1371/journal.pntd.0006108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grau M, Walker PR, Derouazi M. Mechanistic insights into the efficacy of cell penetrating peptide-based cancer vaccines. Cell Mol Life Sci. 2018;75:2887–2896. doi: 10.1007/s00018-018-2785-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qiu Y, Man RCH, Liao Q, Kung KLK, Chow MYT, Lam JKW. Effective mRNA pulmonary delivery by dry powder formulation of PEGylated synthetic KL4 peptide. J Control Release. 2019;314:102–115. doi: 10.1016/j.jconrel.2019.10.026. [DOI] [PubMed] [Google Scholar]
- 43.Kallen KJ, Heidenreich R, Schnee M, Petsch B, Schlake T, Thess A, et al. A novel, disruptive vaccination technology: self-adjuvanted RNActive® vaccines. Hum Vaccin Immunother. 2013;9:2263–2276. doi: 10.4161/hv.25181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schnee M, Vogel AB, Voss D, Petsch B, Baumhof P, Kramps T, et al. An mRNA vaccine encoding rabies virus glycoprotein induces protection against lethal infection in mice and correlates of protection in adult and newborn pigs. PLoS Negl Trop Dis. 2016;10:e0004746. doi: 10.1371/journal.pntd.0004746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petsch B, Schnee M, Vogel AB, Lange E, Hoffmann B, Voss D, et al. Protective efficacy of in vitro synthesized, specific mRNA vaccines against influenza A virus infection. Nat Biotechnol. 2012;30:1210–1216. doi: 10.1038/nbt.2436. [DOI] [PubMed] [Google Scholar]
- 46.Weide B, Pascolo S, Scheel B, Derhovanessian E, Pflugfelder A, Eigentler TK, et al. Direct injection of protamine-protected mRNA: results of a phase 1/2 vaccination trial in metastatic melanoma patients. J Immunother. 2009;32:498–507. doi: 10.1097/CJI.0b013e3181a00068. [DOI] [PubMed] [Google Scholar]
- 47.Kowalski PS, Rudra A, Miao L, Anderson DG. Delivering the messenger: advances in technologies for therapeutic mRNA delivery. Mol Ther. 2019;27:710–728. doi: 10.1016/j.ymthe.2019.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hou X, Zaks T, Langer R, Dong Y. Lipid nanoparticles for mRNA delivery. Nat Rev Mater. 2021;6:1078–1094. doi: 10.1038/s41578-021-00358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim M, Jeong M, Hur S, Cho Y, Park J, Jung H, et al. Engineered ionizable lipid nanoparticles for targeted delivery of RNA therapeutics into different types of cells in the liver. Sci Adv. 2021;7:eabf4398. doi: 10.1126/sciadv.abf4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Payne JE, Chivukula P, Karmali P, Tanis SP. Ionizable cationic lipid for RNA delivery US10961188B2 Mar. 30, 2021.
- 51.Gilleron J, Querbes W, Zeigerer A, Borodovsky A, Marsico G, Schubert U, et al. Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat Biotechnol. 2013;31:638–646. doi: 10.1038/nbt.2612. [DOI] [PubMed] [Google Scholar]
- 52.Cheng X, Lee RJ. The role of helper lipids in lipid nanoparticles (LNPs) designed for oligonucleotide delivery. Adv Drug Deliv Rev. 2016;99:129–137. doi: 10.1016/j.addr.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 53.Fang Y, Xue J, Gao S, Lu A, Yang D, Jiang H, et al. Cleavable PEGylation: a strategy for overcoming the “PEG dilemma” in efficient drug delivery. Drug Deliv. 2017;24:22–32. doi: 10.1080/10717544.2017.1388451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pilkington EH, Suys EJA, Trevaskis NL, Wheatley AK, Zukancic D, Algarni A, et al. From influenza to COVID-19: lipid nanoparticle mRNA vaccines at the frontiers of infectious diseases. Acta Biomater. 2021;131:16–40. doi: 10.1016/j.actbio.2021.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buschmann MD, Carrasco MJ, Alishetty S, Paige M, Alameh MG, Weissman D. Nanomaterial delivery systems for mRNA vaccines. Vaccines (Basel) 2021;9:65–95. doi: 10.3390/vaccines9010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cullis PR, Hope MJ. Lipid nanoparticle systems for enabling gene therapies. Mol Ther. 2017;25:1467–1475. doi: 10.1016/j.ymthe.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Terada T, Kulkarni JA, Huynh A, Chen S, van der Meel R, Tam YYC, et al. Characterization of lipid nanoparticles containing ionizable cationic lipids using design-of-experiments approach. Langmuir ACS J Surf Colloids. 2021;37:1120–1128. doi: 10.1021/acs.langmuir.0c03039. [DOI] [PubMed] [Google Scholar]
- 58.Hassett KJ, Higgins J, Woods A, Levy B, Xia Y, Hsiao CJ, et al. Impact of lipid nanoparticle size on mRNA vaccine immunogenicity. J Control Release. 2021;335:237–246. doi: 10.1016/j.jconrel.2021.05.021. [DOI] [PubMed] [Google Scholar]
- 59.Roces CB, Lou G, Jain N, Abraham S, Thomas A, Halbert GW, et al. Manufacturing considerations for the development of lipid nanoparticles using microfluidics. Pharmaceutics. 2020;12:1095. doi: 10.3390/pharmaceutics12111095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eygeris Y, Patel S, Jozic A, Sahay G. Deconvoluting lipid nanoparticle structure for messenger RNA delivery. Nano Lett. 2020;20:4543–4549. doi: 10.1021/acs.nanolett.0c01386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guevara ML, Persano F, Persano S. Advances in lipid nanoparticles for mRNA-based cancer. Immunotherapy. 2020;8:589959. doi: 10.3389/fchem.2020.589959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Viger-Gravel J, Schantz A, Pinon AC, Rossini AJ, Schantz S, Emsley L. Structure of lipid nanoparticles containing siRNA or mRNA by dynamic nuclear polarization-enhanced NMR spectroscopy. J Phys Chem B. 2018;122:2073–2081. doi: 10.1021/acs.jpcb.7b10795. [DOI] [PubMed] [Google Scholar]
- 63.EMA. Summary of product characteristics (Spikevax; Moderna). 2021 https://www.ema.europa.eu/en/documents/product-information/spikevax-previously-covid-19-vaccine-moderna-epar-product-information_en.pdf. Accessed 13 Dec 21.
- 64.Schoenmaker L, Witzigmann D, Kulkarni JA, Verbeke R, Kersten G, Jiskoot W, et al. mRNA-lipid nanoparticle COVID-19 vaccines: structure and stability. Int J Pharm. 2021;601:120586. doi: 10.1016/j.ijpharm.2021.120586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ball RL, Bajaj P, Whitehead KA. Achieving long-term stability of lipid nanoparticles: examining the effect of pH, temperature, and lyophilization. Int J Nanomed. 2017;12:305–315. doi: 10.2147/IJN.S123062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Uddin MN, Roni MA. Challenges of storage and stability of mRNA-based COVID-19 vaccines. Vaccines (Basel) 2021;9:1033. doi: 10.3390/vaccines9091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dolgin E. How COVID unlocked the power of RNA vaccines. Nature. 2021;589:189–191. doi: 10.1038/d41586-021-00019-w. [DOI] [PubMed] [Google Scholar]
- 68.Crommelin DJA, Volkin DB, Hoogendoorn KH, Lubiniecki AS, Jiskoot W. The science is there: key considerations for stabilizing viral vector-based Covid-19 vaccines. J Pharm Sci. 2021;110:627–634. doi: 10.1016/j.xphs.2020.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]