Abstract
Background
Safe hospital discharge on parenteral antibiotic therapy is challenging for people who inject drugs (PWID) admitted with serious bacterial infections (SBI). We describe a Comprehensive Care of Drug Addiction and Infection (CCDAI) program involving a partnership between Intermountain Healthcare hospitals and a detoxification facility (DF) to provide simultaneous drug recovery assistance and parenteral antibiotic therapy (DRA-OPAT).
Methods
The CCDAI program was evaluated using a pre-/poststudy design. We compared outcomes in PWID hospitalized with SBI during a 1-year postimplementation period (2018) with similar patients from a historical control period (2017), identified by propensity modeling and manual review.
Results
Eighty-seven patients were candidates for the CCDAI program in the implementation period. Thirty-five participants (40.2%) enrolled in DRA-OPAT and discharged to the DF; 16 (45.7%) completed the full outpatient parenteral antibiotic therapy (OPAT) duration. Fifty-one patients with similar characteristics were identified as a preimplementation control group. Median length of stay (LOS) was reduced from 22.9 days (interquartile interval [IQI], 9.8–42.7) to 10.6 days (IQI, 6–17.4) after program implementation (P < .0001). Total median cost decreased from $39 220.90 (IQI, $23 300.71–$82 506.66) preimplementation to $27 592.39 (IQI, $18 509.45–$48 369.11) postimplementation (P < .0001). Ninety-day readmission rates were similar (23.5% vs 24.1%; P = .8). At 1-year follow-up, all-cause mortality was 7.1% in the preimplementation group versus 1.2% postimplementation (P = .06).
Conclusions
Partnerships between hospitals and community resources hold promise for providing resource-efficient OPAT and drug recovery assistance. We observed significant reductions in LOS and cost without increases in readmission rates; 1-year mortality may have been improved. Further study is needed to optimize benefits of the program.
Keywords: antimicrobial stewardship, OPAT, PWID, substance abuse
We describe a successful treatment model where patients who inject drugs discharge to a community partner for parenteral antibiotics and drug recovery assistance. Hospital length of stay and costs were significantly decreased without increased readmission rates and mortality.
The opioid epidemic has caused a significant increase in hospitalizations for treatment of serious bacterial infections (SBI) in people who inject drugs (PWID) [1–3]. These infections often require prolonged treatment with parenteral antibiotic therapy via a peripherally inserted central catheter (PICC). Outpatient parenteral antibiotic therapy (OPAT) is a safe and cost-effective method to treat patients outside of the inpatient setting. Treatment is usually received at home via home healthcare (HH) or at an infusion center. Historically, PWID have not been considered safe candidates for OPAT due to concerns of line manipulation for illicit drug use, noncompliance, complicated social situations [4], and higher risk of failure [5]. As a result of these concerns, it has become common practice to require PWID with serious infections to remain hospitalized to receive standard-of-care durations of intravenous (IV) antibiotic treatment [4]. These prolonged hospitalizations, sometimes up to 6 weeks, can cause significant financial burden on the hospital and inefficient use of acute care hospital resources [1]. In many instances, the focus during the hospitalization is treatment of the infection, and as a result the underlying substance abuse disorder is not being adequately addressed.
Patients who use drugs and admitted to the hospital for invasive Staphylococcus aureus infection were found to have lower antibiotic completion rates and increased readmission rates due to recurrent or persistent infection [6]. Collaboration between hospital systems and community partners focused on substance use disorder (SUD) may provide PWID with SBI requiring parenteral antibiotics upon discharge a safe environment where they can receive OPAT while having the opportunity to engage in addiction-related treatment.
This study aimed to evaluate a new program that provided Comprehensive Care of Drug Addiction and Infection (CCDAI) to hospitalized PWID with SBI and facilitated transition to a nonprofit residential detox facility (DF) where they received supervised self-administered OPAT and drug recovery assistance.
METHODS
Inpatient Comprehensive Care of Drug Addiction and Infection Team and Patients
From January 2018 to December 2018, a multidisciplinary CCDAI team made up of an infectious diseases (ID) physician, hospitalist, psychiatrist, case management, ID pharmacist, home healthcare nurse, as well as a liaison from the DF met weekly to review patients with SBI and concomitant SUD hospitalized at 4 Salt Lake County Intermountain Healthcare hospitals. Hospitalists, case managers, ID physicians, and pharmacists referred patients to the multidisciplinary team based on clinical concern for SUD and a current diagnosis of SBI likely requiring treatment with prolonged IV antibiotics. These patients were evaluated by members of the CCDAI team during their inpatient admission. Psychiatry consultation and medications for opioid use disorder (MOUD) were provided to patients when appropriate. Amenable patients requiring treatment with outpatient IV antibiotics who met eligibility criteria were enrolled in the drug recovery assistance and parenteral antibiotic therapy (DRA-OPAT) part of the program and were transitioned to the DF. Enrollment eligibility criteria included the following: mandatory inpatient ID consultation, medically stable for discharge, stable mental illness, independent with activities of daily living (ADLs), physical and cognitive ability to self-infuse IV antibiotics, tapering regimen if opioids were needed for pain, and an IV antibiotic regimen that can infuse in <20 minutes or delivered in a 24-hour continuous infusion. Hospitalized patients who met eligibility criteria for DRA-OPAT but declined enrollment in the program were discharged from the hospital on an oral antibiotic salvage regimen determined by the treatment team if disposition to a skilled nursing facility (SNF) was not feasible.
Drug Recovery Assistance and Community Partnership
The nonprofit residential DF located in Salt Lake City, Utah provides the community with 83 male beds and a separate 30-bed women’s facility for detoxification and withdrawal management services for up to 14-day stays. The hospital system subsidized the DF with a fixed contractual rate to provide 5 beds at each facility in a private dorm room separate from the detox beds, for patients enrolled in the DRA-OPAT program. The patients received 3 meals daily and could remain at the facility for the duration of their OPAT. Case management was provided to the patients during their stay at the DF and assisted with transitioning patients to residential or outpatient treatment programs upon completion of OPAT. Patients were encouraged to attend 12-Step meetings, Seeking Safety, and peer support groups. The DF partnered with a community nonprofit opioid treatment program that provided clinical assessment and treatment plans that included MOUD in addition to behavior counseling. Random urine drug screens were performed by the DF if drug use was suspected. The HH pharmacy provided IV antibiotics, and the patients were taught by HH nurses to self-administer their IV antibiotics. The HH nurses made weekly and as-needed visits to the patients during their stay at the DF. Physical therapy and wound care were provided by HH if needed. Patients infused their antibiotic in the medication room while being monitored by nonmedical facility staff. The DF provided transportation to medical appointments, and all patients received follow-up care by an ID physician. Patients who enrolled in the DRA-OPAT but either elected to leave the program before completing their prescribed course of IV antibiotics or were dismissed from the DF as a result of a positive random urine drug screen or nonadherence to the facility behavior contract were transitioned to oral antibiotic therapy. The PICC line was removed by a HH nurse, or the patient was transported to the hospital emergency department for PICC removal. Completion of therapy was defined as receiving equal to or greater than the days of OPAT prescribed by the inpatient ID consultation team.
Statistical Analysis
We used a pre-/poststudy design to evaluate the effect of implementation of the CCDAI program. During the postimplementation period (January–December 2018), all PWID hospitalized for SBI requiring IV antibiotics who were referred to the CCDAI team for evaluation and were recorded in a registry (RedCap, Nashville, TN). We will refer to this group of inpatients as program “Candidates” (effectively an intention-to-treat [ITT] population). From this group, patients who were actually discharged to the community partner DF and enrolled in the DRA-OPAT part of the program will be referred to as “Participants” (effectively a per-protocol, or rather per-program [PER-PROGRAM], cohort).
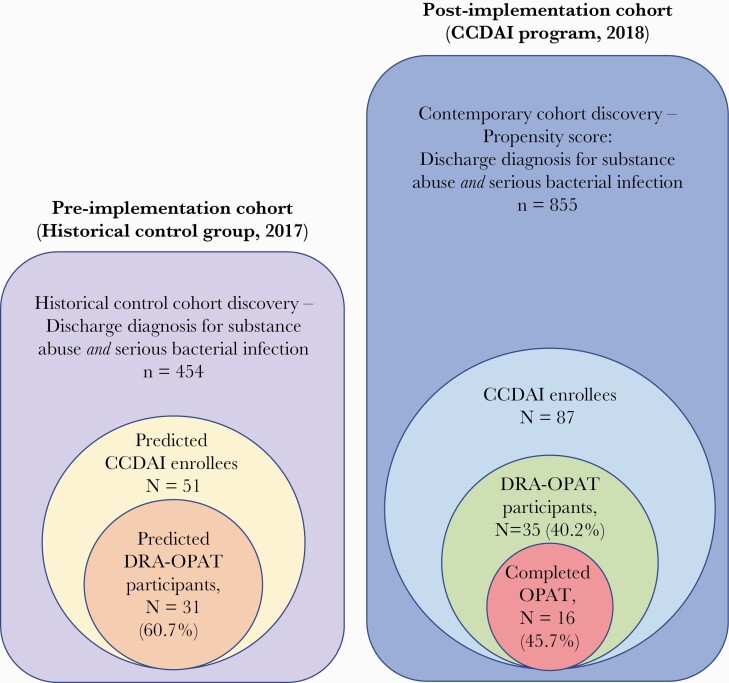
To identify a historical control with comparable clinical characteristics, we used a hybrid approach consisting of initial propensity-score cohort discovery and subsequent manual review. The rationale for this mixed approach was that although there are many billing codes for substance abuse, there is no billing code or other electronic signature that accurately identifies patients who “inject” drugs. Hence, this key data limitation to propensity matching was identified a priori and addressed by manual review. To develop the propensity score, we first identified all discharge diagnosis codes (International Classification of Diseases, 10th Revision) associated with (1) substance abuse and (2) SBI (see Supplemental Data). We then queried all admissions to study hospitals during the program implementation period to identify patients with (1) at least 1 code identifying drug abuse and (2) 1 code identifying SBI (see Figure 1). We confirmed that all program candidates from the ITT population were found within this larger pool. We then excluded patients who would not have been evaluable for the program, including the following: patients who died in the hospital because they were never stable enough to consider disposition; patients discharged to hospice; those discharged to behavioral health units; and those who left against medical advice before an ID consult note was entered, with the rationale that these patients would not have been in the hospital long enough to have been evaluated. We also excluded patients readmitted after a recently diagnosed SBI.
Figure 1.
Cohort discovery and pre- and postimplementation patient cohorts. CCDAI, Comprehensive Care of Drug Addiction and Infection; DRA-OPAT, drug recovery assistance and parenteral antibiotic therapy.
Once this larger cohort of PWID with SBI was identified from the postimplementation period, we then developed a propensity score model. This was done by developing a logistic regression model with all actual program candidates as the dependent variable. An optimal model was fitted after exploring all available covariates for their ability to discriminate program candidates from patients who were not. Validation of model discrimination was confirmed via the area under the receiver operator characteristic curve (AUROC) and Hosmer-Lemeshow goodness-of-fit tests.
Once an adequate propensity model was developed, we then turned our attention to identifying a historical control. First, we followed identical methodology as described above to search for all patients, during a 1-year preimplementation period (January–December 2017), who had both (1) a discharge diagnosis for substance abuse and (2) discharge diagnose(es) for SBI. We then applied the propensity score to this cohort from the preimplementation period to stratify patients based on the likelihood that they would have been program candidates had the program existed. We selected a predicted probability cutoff that captured all of the known program candidates in the postimplementation group and applied this to the preimplementation group to identify patients at or above that predicted probability.
Study investigators then manually reviewed the electronic health record (EHR) for these cases to confirm that they had documentation of “injection” drug abuse. This final cohort became the preimplementation group cohort of likely program candidates. Balance of characteristics between the pre- and postimplementation intention-to-treat groups was confirmed using χ2, Fisher’s exact, or Mann-Whitney U tests, as appropriate (see Table 1). These tests were 2-tailed with statistical significance at P < .05; we adjusted patient characteristics and outcomes analyses for a 5% false discovery rate (FDR) using the Benjamini-Hochberg method [7].
Table 1.
Comparison of CCDAI Candidates by Pre- and Postimplementation Period
| Cohort | Preimplementation (2017) | Postimplementation (2018) | P Value |
|---|---|---|---|
| Total, N | 51 | 87 | |
| Age, years (median, IQI) | 39 (35–47) | 39 (30–51) | .55 |
| Male, N (%) | 29 (56.9) | 53 (60.9) | .72 |
| Race, non-white | 6 (9.8) | 12 (13.8) | .6 |
| Cocaine use | 2 (3.9) | 9 (10.3) | .21 |
| Opioid use | 40 (78.4) | 65 (74.7) | .62 |
| Polysubstance use | 38 (74.5) | 55 (63.2) | .17 |
| Stimulant use | 26 (51.0) | 50 (57.5) | .46 |
| Medication for opioid use disorder | 31 (60.8) | 50 (57.5) | .7 |
| Homeless | 24 (47.1) | 30 (34.5) | .14 |
| HIV | 1 (2.0) | 2 (2.3) | 1.0 |
| Hepatitis C | 22 (43.1) | 26 (29.9) | .12 |
| Charlson comorbidity index | 15.5 (6.0–43.5) | 20 (12–36) | .56 |
| Cerebrovascular disease | 5 (9.8) | 8 (9.2) | .91 |
| Congestive heart failure | 16 (31.4) | 13 (14.9) | .02a |
| Diabetes mellitus | 5 (9.8) | 11 (12.6) | .62 |
| Hepatic disease | 21 (41.2) | 35 (40.2) | .91 |
| Chronic pulmonary disease | 27 (52.9) | 29 (33.3) | .02a |
| Kidney disease | 8 (15.7) | 8 (9.2) | .25 |
| Endocarditis | 20 (39.2) | 31 (35.6) | .67 |
| Osteoarticular infection | 16 (31.4) | 27 (31.0) | .97 |
| Skin/soft tissue infection | 26 (51.0) | 47 (54.0) | .73 |
| Vertebral Abscess/discitis/osteomyelitis | 7 (13.7) | 24 (27.6) | .06 |
| Staphylococcus aureus bacteremia | 24 (47.1) | 38 (43.7) | .7 |
| Bone or joint debridement | 7 (13.7) | 19 (21.8) | .24 |
| Cardiac valve replacement | 4 (7.8) | 3 (3.4) | .26 |
| Soft tissue debridement | 16 (31.4) | 30 (34.5) | .71 |
| Spinal debridement | 8 (15.7) | 11 (12.6) | .6 |
| Infectious disease consult | 48 (94.1) | 79 (90.8) | .49 |
| Completed prescribed treatment course | 34 (66.7) | 16 (18.4) | <.0001 |
| 30-day readmission | 4 (7.8) | 11 (12.6) | .09 |
| 90-day readmission | 12 (23.5) | 21 (24.1) | .8 |
| Mortality, 30-day all-cause | 1 (2.0) | 1 (1.1) | .7 |
| Mortality, 1-year | 4 (7.8) | 1 (1.1) | .06 |
| Length of stay, days (median, IQI) | 22.9 (9.8–42.7) | 10.6 (6.0–17.4) | <.0001 |
| Cost, total inpatient ($US, median [IQI]) | 39 220.90 (23 300.71–82 506.66) | 27 592.39 (18 509.45–48 369.11) | .007 |
Abbreviations: CCDAI, Comprehensive Care of Drug Addiction and Infection; HIV, human immunodeficiency virus; IQI, 25%–75% interquartile interval.
No longer statistically significant after adjusting for 5% false discovery rate using Benjamini-Hochberg method.
From within this preimplementation group of inpatients who likely would have been program candidates (historical ITT cohort), we sought to identify the patients who would have enrolled in the outpatient part of the program and actually been DRA-OPAT participants (per-program group) at the community partner program facility, had it existed. We recognized a priori that propensity matching would not be possible because the many factors that lead to a patient being approved by the evaluation committee and actually matriculating into the program are varied, largely psychosocial, and not available in the EHR.
To address this, we assigned the actual sitting members of the program evaluation committee to independently review the EHR record, including clinical characteristics and social work narrative notes, and adjudicate whether the patient was likely to have been discharged to participate in the program or not. All cases were independently reviewed by 2 different committee members, and the interrater agreement (kappa) was calculated. An independent committee member served as a blinded tiebreaker for cases in which the 2 reviewers differed in their evaluation. In this way, we identified a preimplementation per-program control group consisting of likely DRA-OPAT program participants. Similarly, we compared clinical and demographic characteristics between the pre- and postimplementation groups using appropriate statistical tests as described above (see Table 2). These tests were also 2-tailed with statistical significance at P < .05 and adjusted for a 5% FDR.
Table 2.
Comparison of DRA-OPAT Participants by Pre- and Postimplementation Period
| Cohort | Preimplementation (2017) | Postimplementation (2018) | P Value |
|---|---|---|---|
| Total, N | 31 | 35 | |
| Age, years (median, IQI) | 38 (35–45) | 39 (29–49) | .94 |
| Male, N (%) | 16 (51.6) | 19 (54.3) | .83 |
| Race, non-white | 26 (83.9) | 29 (82.9) | .92 |
| Cocaine use | 1 (3.2) | 2 (5.7) | .63 |
| Opioid use | 26 (83.9) | 28 (80.0) | .68 |
| Polysubstance use | 26 (83.9) | 23 (65.7) | .06 |
| Stimulant use | 17 (54.8) | 23 (65.7) | .37 |
| Medication for opioid use disorder | 21 (67.7) | 22 (62.9) | .68 |
| Homeless | 13 (41.9) | 16 (45.7) | .94 |
| HIV | 1 (3.2) | 0 (0) | .47 |
| Hepatitis C | 16 (51.6) | 10 (28.6) | .03a |
| Charlson comorbidity index | 20 (7.5–51) | 23 (14.5) | .73 |
| Cerebrovascular disease | 4 (12.9) | 3 (8.6) | .57 |
| Congestive heart failure | 9 (29.0) | 4 (11.4) | .07 |
| Diabetes mellitus | 3 (9.7) | 7 (20.0) | .31 |
| Hepatic disease | 14 (45.2) | 13 (37.1) | .37 |
| Chronic pulmonary disease | 16 (51.6) | 11 (31.4) | .16 |
| Kidney disease | 5 (16.1) | 4 (11.4) | .72 |
| Endocarditis | 16 (51.6) | 14 (40.0) | .34 |
| Osteoarticular infection | 8 (25.8) | 15 (42.9) | .22 |
| Skin/soft tissue infection | 14 (45.2) | 20 (57.1) | .33 |
| Vertebral Abscess/discitis/osteomyelitis | 5 (16.1) | 10 (28.6) | .23 |
| Staphylococcus aureus bacteremia | 16 (51.6) | 17 (48.6) | .81 |
| Bone or joint debridementa | 3 (9.7) | 13 (37.1) | .02a |
| Cardiac valve replacement | 2 (6.5) | 0 (0) | .13 |
| Soft tissue debridement | 8 (25.8) | 14 (40.0) | .32 |
| Spinal debridement | 4 (12.9) | 3 (8.6) | .57 |
| Infectious disease consult | 28 (90.3) | 35 (100) | .1 |
| Completed prescribed treatment course | 23 (74.2) | 16 (45.7) | .01 |
| 30-day readmission | 2 (6.5) | 4 (11.4) | .68 |
| 90-day readmission | 6 (19.4) | 9 (25.7) | .57 |
| Mortality, 30-day all-cause | 0 (0) | 0 (0) | -- |
| Mortality, 1-year | 2 (6.5) | 0 (0) | .13 |
| Length of stay, days (median, IQI) | 35.1 (16–746.8) | 12.1 (8.9–17.9) | <.0001 |
| Cost, total inpatient ($US, median [IQI]) | 68 748.20 (34 485.35–112 712.08) | 33 231.88 (24 170.49–46 670.35) | <.0001 |
Abbreviations: DRA-OPAT, drug recovery assistance and parenteral antibiotic therapy; HIV, human immunodeficiency virus; IQI, 25%–75% interquartile interval.
No longer statistically significant after adjusting for 5% false discovery rate using Benjamini-Hochberg method.
The prespecified coprimary analyses were to compare pre- and postimplementation length of hospital stay and total cost in the program candidates (ITT) group. For cost data, we used total inpatient costs and the cost to maintain the community partner beds but did not include antibiotic costs. Secondary analyses included (1) total cost and length of stay (LOS) in the participants (per-program) group and (2) 30-day all-cause readmission and 90-day all-cause readmission and 30-day all-cause mortality and 1-year all-cause mortality in both ITT and per-program groups, respectively. Mortality data were obtained from the Utah Population Database (UPDB), which permits ascertainment of that outcome independent of the limitations of the hospital network or medical record. Finally, we planned a post hoc sensitivity analysis using a multiple linear regression for LOS and total cost in the ITT and per-program groups to control for any remaining imbalanced covariates in the pre- and postimplementation cohorts. The study was approved by the Intermountain Healthcare Institutional Review Board. All statistical analyses were performed using SPSS version 25 (IBM Corporation, Armonk, NY).
RESULTS
Cohort Discovery
During cohort discovery, 855 patients were identified with discharge diagnoses for both an SBI and substance abuse during the postimplementation period, and 454 patients were identified during the preimplementation period (see Figure 1). The differences in sample sizes were reflective of a new EHR that was being implemented during the preimplementation period. The variables included in the final logistic regression model developed as a propensity score for identifying the historical cohort are listed in Supplementary Table 1. Discrimination (AUROC.949) and classification (93.2% accuracy) of the model was excellent. Kappa agreement was 0.6 for determining likely historical program participants.
Clinical Characteristics and Outcomes
During the postimplementation period, 87 hospitalized patients were identified as CCDAI program candidates (ITT population). Thirty-five patients (40.2%) were ultimately enrolled and discharged to the DRA-OPAT facility (per-program group) (Figure 1); of these, 16 (45.7%) completed the full OPAT duration at the center. In the preimplementation group, 51 patients were identified as likely to have been candidates had the program existed. Of these, the program evaluation committee determined that 31 (60.8%) would likely have been DRA-OPAT participants. Patient characteristics for the program candidates (ITT) group by pre- and postimplementation periods are displayed in Table 1. Patients in both groups were well balanced in terms of demographics, patterns of drug abuse, type of infection, and surgical intervention (see Table 1). There were more patients with congestive heart failure (CHF) and chronic pulmonary disease in the historical control group and more patients with spinal infections in the postimplementation group; however, these differences were not statistically significant after adjusting for FDR.
Median LOS was reduced from 22.9 days (IQI, 9.8–42.7) to 10.6 days (IQI, 6–17.4) after program implementation (P < .0001) (Table 1). Total cost per patient was also decreased from $39 220.90 (IQI, $23 300.71–$82 506.66) preimplementation to $27 592.39 (IQI, $18 509.45–$48 369.11) postimplementation (P < .0001) (Supplementary Figure 1). More patients completed the duration and route of antibiotic course as originally prescribed in the preimplementation group (66.7% vs 18.4%); this difference largely reflects IV-to-oral transitions to complete therapy in patients who declined participation in the program and those who decided to leave the community-partner facility before the originally anticipated stop date. Thirty-day readmission was numerically more frequent after program implementation (7.8% vs 12.6%, P = .09), but there was no difference in 90-day readmission (23.5% vs 24.1%, P = .8). At 1-year follow-up, all-cause mortality was 7.1% in the preimplementation group versus 1.2% (P = .06). Preplanned sensitivity analyses adjusting for residual imbalanced factors (CHF, chronic pulmonary disease, and spinal infection) confirmed significant reductions in LOS (unstandardized β coefficient −12.7 days; 95% confidence interval [CI], −17.7 to −7.6; P < .0001) and total cost (β coefficient $−19 925.82; 95% CI, −31 194.50 to −8 657.15; P = .001).
Patient characteristics were similar between pre- and postimplementation groups in the per-protocol cohort of DRA-OPAT participants (see Table 2). In this subgroup, median LOS was 35.1 days (IQI, 16.7–46.8) preimplementation versus 12.1 days (IQI, 8.9–17.9) postimplementation (P < .0001) (Table 2). Total inpatient costs were $68 748.20 (IQI, $34 485.35–$112 712.08) preimplementation versus $33 231.88 (IQI, $24 170.49–$46 670.35) postimplementation (P < .0001). In sensitivity analyses, reductions in LOS (unstandardized β coefficient −21.3 days; 95% CI, −28.4 to −14.1; P < .0001) and total cost (β coefficient $−36 285.50; 95% CI, −53 028.29 to −19 542.70; P < .0001) remained significant.
Of the 35 DRA-OPAT participants, 16 completed the originally recommended duration of therapy at the DF, and 19 did not (Table 3). Numerically stimulant use and polysubstance use were more common among DRA-OPAT participants who did not complete treatment. Two patients absconded with their PICC line, and 2 patients violated the program behavioral contract and had positive urine drugs of abuse screens during their stay. Two patients required readmission due to antibiotic-related adverse events. The median LOS at the DF for those who completed treatment was 24 days (IQI, 19.5–38), compared to 4 days (IQI, 2–9) for those who did not. Factors that correlated with successful completion of therapy included active MOUD (odds ratio [OR], 17.0; 95% CI, 2.1–136; P = .008) and homelessness (OR, 8.1; 95% CI, 1.2–53.7; P = .03). Substance used (opioid vs methamphetamine), race, and length of hospital stay before discharge may also contribute, but we had an inadequate sample size to confirm these observations.
Table 3.
DRA-OPAT Participants by Program Completion
| Cohort | Completed DRA-OPAT | Did Not Complete Program |
|---|---|---|
| N | 16 | 19 |
| Age, years (median, IQI) | 35 (28–53.3) | 40 (34–49) |
| Male, N (%) | 9 (56.3) | 10 (52.6) |
| Race, non-white | 1 (6.3) | 5 (26.3) |
| Cocaine use | 1 (6.3) | 1 (5.3) |
| Opioid use | 15 (93.8) | 13 (68.4) |
| Polysubstance use | 8 (50) | 14 (73.7) |
| Stimulant use | 8 (50) | 15 (78.9) |
| Medication for opioid use disordera | 14 (87.5) | 8 (42.1) |
| Homelessa | 12 (75) | 8 (42.1) |
| Insured | 1 (6.3) | 6 (31.6) |
| Charlson comorbidity index | 32 (12.5) | 20 (12–33) |
| HIV | 0 (0) | 0 (0) |
| Hepatitis C | 4 (25) | 5 (26.3) |
| Cerebrovascular disease | 1 (6.3) | 2 (10.5) |
| Congestive heart failure | 0 (0) | 4 (21.1) |
| Diabetes mellitus | 2 (12.5) | 5 (26.3) |
| Hepatic disease | 5 (31.3) | 7 (36.8) |
| Chronic pulmonary disease | 6 (37.5) | 6 (31.6) |
| Kidney disease | 2 (12.5) | 2 (10.5) |
| Endocarditis | 7 (43.8) | 7 (36.8) |
| Osteoarticular infection | 6 (37.5) | 8 (42.1) |
| Skin/soft tissue infection | 12 (75) | 8 (42.1) |
| Vertebral abscess/discitis/osteomyelitis | 6 (37.5) | 4 (21.1) |
| Staphylococcus aureus bacteremia | 8 (50) | 9 (47.4) |
| Bone or joint debridement | 7 (43.8) | 5 (26.3) |
| Cardiac valve replacement | 0 (0) | 0 (0) |
| Soft tissue debridement | 6 (37.5) | 7 (36.8) |
| Spinal debridement | 2 (12.5) | 1 (5.3) |
| Hospital length of stay, days (median, IQI)a | 13.5 (10.6–18.5) | 9.1 (7.7–13.4) |
| Days at program facilitya | 24 (19.5–38) | 4 (2–9) |
| 30-day readmission | 2 (12.5) | 2 (10.5) |
| 90-day readmission | 5 (31.3) | 4 (21.1) |
| Mortality, 30-day all-cause | 0 (0) | 0 (0) |
| Mortality, 1-year | 0 (0) | 0 (0) |
| Positive toxicology screen during program | 0 (0) | 2 (10.5) |
| PICC line abuse | 0 (0) | 2 (10.5) |
| Adverse event during program treatment | 3 (18.8) | 4 (21.1) |
Abbreviations: DRA-OPAT, drug recovery assistance and parenteral antibiotic therapy; HIV, human immunodeficiency virus; IQI, 25%–75% interquartile interval; PICC, peripherally inserted central venous catheter.
Significant at P < .05.
DISCUSSION
This study describes outcomes data for a new program that used a multidisciplinary approach to inpatient management of drug addiction and infection as well as a unique partnership with a community nonprofit residential DF that provided PWID with concomitant SBI to receive DRA-OPAT. Our results demonstrate significant decrease in hospital LOS and subsequent inpatient costs when these complex patients are no longer kept in the hospital for the duration of their prescribed IV antibiotic course. After initiation of this new program, mortality and readmission rates did not increase despite lower IV antibiotic treatment completion rates. Benefits of shortening the inpatient LOS for this patient population once they become medically stable allows for earlier engagement in outpatient addiction treatment as well as access to outpatient resources focused on psychological, social, and environmental contributors to addiction. These types of resources are not readily available to patients while they remain hospitalized. Prolonged inpatient stays solely for receipt of IV antibiotics are no longer sustainable for many hospitals due to significant expense as well as critical nursing and bed shortages.
Prior published studies have described nurse-administered OPAT to PWID in a respite facility, residential addiction treatment facility, and homelike alterative to a hospital [8–11]. Our program differed from these because the OPAT site did not have any onsite medical staff. The patients that participated in the OPAT-DRA program were responsible for infusing their own antibiotics. The onsite staff observed and documented the antibiotic infusion, but they were not trained to assist. The 30-day readmission rate for patients enrolled in the DRA-OPAT program was 11.4%, which falls within the 6%–25% range of OPAT readmission in the general population [12, 13]. Our study demonstrates that PWID are capable of safely self-administering their OPAT in a supervised setting without onsite medical staff. Residential addiction treatment programs are often unwilling to accept patients requiring OPAT due to their perception that these patients are higher medical acuity requiring more intense medical staffing. Hiring additional medically trained staffed is usually cost-prohibitive for these facilities. Our findings may compel more residential addiction treatment programs to admit PWID requiring OPAT when enrolled in a structured OPAT program and closely followed by an ID specialist.
Recruitment of patients for these programs can be challenging. Englander et al [9] described a similar program that used a medically enhanced residential treatment model for integration of SUD and prolonged IV antibiotics. They had a low recruitment and retention rate and ultimately had to close the program after 6 months. Most patients who declined admission remained in the hospital or were discharged to an SNF. Our program enrolled 35 of 85 (40.2%) of the CCDAI candidates into the DRA-OPAT part of the program. Some patients did not meet eligibility criteria due to inability to perform ADLs or infuse their own IV antibiotics. Others were not amenable to being discharged to the DF due to their prior experience at acute detox or the stigma associated with this facility. The DF had a strict policy prohibiting cell phones, internet access, unsupervised visitation, and leaving the center other than for medical or legal obligations. These restrictions deterred some patients from participating in the program and contributed to early attrition from the DRA-OPAT program. Eligible patients for DRA-OPAT who declined transition to the DF were not given the choice to remain in the hospital and were discharged on oral salvage antibiotic therapy when transition to an SNF was not feasible. This new policy may have contributed to higher recruitment than other programs described in the literature.
Assessment and initiation of MOUD by the hospitalist or psychiatry consult service during the inpatient stay was an integral part of our program. Treatment with MOUD was correlated with successful completion of antibiotic therapy in the DRA-OPAT participants and should be considered essential in any OPAT program for PWID. Our study found amphetamine and polysubstance use were more common in the DRA-OPAT participants who did not complete IV antibiotic treatment, although the impact of specific substance used on completion of therapy could not be determined due to inadequate sample size. Recent data suggest methamphetamine use adversely affects MOUD receipt, retention, and opioid abstinence in patients with opioid use disorder [14].
Most of our DRA-OPAT participants who were treated with MOUD received methadone rather than buprenorphine due to cost and the DF’s established relationship with a community nonprofit opioid treatment program. After completion of DRA-OPAT, arrangements were made for the participants to continue MOUD. The CCDAI candidates who were initiated on MOUD inpatient but declined to participate in DRA-OPAT were also provided the opportunity to continue MOUD outpatient. Although most PWID hospitalized with SBI are not seeking addiction treatment, hospitalization creates an opportunity to engage patients in treatment and facilitate transition to an outpatient treatment program [15, 16]. Inpatient induction and linkage with outpatient buprenorphine compared with inpatient detoxification has been associated with lower illicit drug use at 6 months and increased entrance into outpatient buprenorphine treatment program [17]. We hypothesize treatment with MOUD and/or referral to addiction treatment may have positively influenced the mortality and 90-day readmission rate of this group despite fewer patients completing standard-of-care IV antibiotic treatment for their infections [18, 19].
Limitations
This study was limited by retrospective study design and a relatively small sample size. We acknowledge that although the historical control group was well matched to the contemporary group, unmeasured differences may have been present. We were unable to accurately assess drug-rehabilitation/remission status, continuity of outpatient MOUD, as well as engagement with other addiction treatment programs. This information would be useful for determining ultimate success of the program, as would larger sample size and longer follow-up period. The hospitals that participated in this study and the home health pharmacy and nursing services were within the same healthcare corporation, which allowed for access to a shared EHR, frequent communication, and close coordinated care that may not be generalizable. Our hospital system comprises a large percentage of admissions in the geographic area studied and includes the primary urban hospital as well. However, our data do not capture readmissions to hospitals outside of our system. Mortality data were obtained from the UPDB that is inclusive of all healthcare facilities in Utah.
Finally, we recognize that this is only one of several novel therapeutic options now available to PWID requiring treatment of SBI. Future innovation should focus on enhancing the DRA component and studying other antibiotic options including early oral transition and novel depot glycopeptide formulations.
CONCLUSIONS
In summary, this study demonstrates a successful treatment model that incorporates creative partnerships between hospitals and community resources to provide resource efficient OPAT and DRA. More research needs to be done on methods to improve program retention and identifying factors that influence which patients receive the greatest benefit from treatment in this program model.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
In Memoriam: Dr. James Kimball, MD, a caring and capable psychiatrist whose commitment to serving the underserved, including people who inject drugs, is greatly missed.
We express our gratitude to Drs. Dean Mayer and Michael Coudreaut who were instrumental in the development of this program. We also acknowledge and thank the following: Dr. Katie Carlson, Lorraine Linford, Dr. Bert Lopansri, Keely Morrical, Toni Palmer, Kim Rawlins, Dr. Erin Stahl, Intermountain Healthcare Division of Infectious Diseases, Intermountain Healthcare Hospitalist Service, Intermountain Healthcare Community Health Program, Intermountain Healthcare Medical Specialties Clinical Program, Intermountain Homecare and Hospice, Intermountain Homecare Pharmacy, Project Reality, and Volunteers of America Utah.
Author contributions. S. S. G. and B. J. W. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. S. S. G., B. J. W., and E. S. contributed to study concept and design. N. G., S. S. G., B. J. W., R. A. F., N. T., and E. S. contributed to acquisition, analysis, or interpretation of data. S. S. G. and B. J. W. contributed to drafting the manuscript. All authors contributed to critical review of the manuscript for important intellectual content. B. J. W. contributed to statistical analysis.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Ronan MV, Herzig SJ.. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002-12. Health Aff (Millwood) 2016; 35:832–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schranz AJ, Fleischauer A, Chu VH, Wu LT, Rosen DL.. Trends in drug use-associated infective endocarditis and heart valve surgery, 2007 to 2017: a study of statewide discharge data. Ann Intern Med 2019; 170:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wurcel AG, Anderson JE, Chui KK, et al. Increasing infectious endocarditis admissions among young people who inject drugs. Open Forum Infect Dis 2016; 3:ofw157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rapoport AB, Fischer LS, Santibanez S, Beekmann SE, Polgreen PM, Rowley CF.. Infectious diseases physicians’ perspectives regarding injection drug use and related infections, United States, 2017. Open Forum Infect Dis 2018; 5:ofy132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buehrle DJ, Shields RK, Shah N, Shoff C, Sheridan K.. Risk factors associated with outpatient parenteral antibiotic therapy program failure among intravenous drug users. Open Forum Infect Dis 2017; 4:ofx102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Appa A, Adamo M, Le S, et al. Comparative one-year outcomes of invasive Staphylococcus aureus infections among persons with and without drug use: an observational cohort study. Clin Infect Dis 2021. doi: 10.1093/cid/ciab367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I.. Controlling the false discovery rate in behavior genetics research. Behav Brain Res 2001; 125:279–84. [DOI] [PubMed] [Google Scholar]
- 8. Beieler AM, Dellit TH, Chan JD, et al. Successful implementation of outpatient parenteral antimicrobial therapy at a medical respite facility for homeless patients. J Hosp Med 2016; 11:531–5. [DOI] [PubMed] [Google Scholar]
- 9. Englander H, Wilson T, Collins D, et al. Lessons learned from the implementation of a medically enhanced residential treatment (MERT) model integrating intravenous antibiotics and residential addiction treatment. Subst Abus 2018; 39:225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jafari S, Joe R, Elliot D, Nagji A, Hayden S, Marsh DC.. A community care model of intravenous antibiotic therapy for injection drug users with deep tissue infection for “reduce leaving against medical advice”. Int J Ment Health Addict 2015; 13:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jewell C, Weaver M, Sgroi C, Anderson K, Sayeed Z.. Residential addiction treatment for injection drug users requiring intravenous antibiotics: a cost-reduction strategy. J Addict Med 2013; 7:271–6. [DOI] [PubMed] [Google Scholar]
- 12. Allison GM, Muldoon EG, Kent DM, et al. Prediction model for 30-day hospital readmissions among patients discharged receiving outpatient parenteral antibiotic therapy. Clin Infect Dis 2014; 58:812–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chapman AL, Dixon S, Andrews D, Lillie PJ, Bazaz R, Patchett JD.. Clinical efficacy and cost-effectiveness of outpatient parenteral antibiotic therapy (OPAT): a UK perspective. J Antimicrob Chemother 2009; 64:1316–24. [DOI] [PubMed] [Google Scholar]
- 14. Frost MC, Lampert H, Tsui JI.. The impact of methamphetamine/amphetamine use on receipt and outcomes of medications for opioid use disorder: a systematic review. Addict Sci Clin Pract 2021. doi: 10.1186/s13722-021-00266-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fanucchi LC, Lofwall MR, Nuzzo PA, Walsh SL.. In-hospital illicit drug use, substance use disorders, and acceptance of residential treatment in a prospective pilot needs assessment of hospitalized adults with severe infections from injecting drugs. J Subst Abuse Treat 2018; 92:64–9. [DOI] [PubMed] [Google Scholar]
- 16. Suzuki J, DeVido J, Kalra I, et al. Initiating buprenorphine treatment for hospitalized patients with opioid dependence: a case series. Am J Addict 2015; 24:10–4. [DOI] [PubMed] [Google Scholar]
- 17. Liebschutz JM, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med 2014; 174:1369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kimmel SD, Walley AY, Li Y, et al. Association of treatment with medications for opioid use disorder with mortality after hospitalization for injection drug use-associated infective endocarditis. JAMA Network Open 2020; 3:e2016228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rodger L, Glockler-Lauf SD, Shojaei E, et al. Clinical characteristics and factors associated with mortality in first-episode infective endocarditis among persons who inject drugs. JAMA Network Open 2018; 1:e185220. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.