To the Editor:
Primary lateral sclerosis (PLS) is a neurodegenerative disorder characterized by slowly progressive symmetrical upper motor neuron (UMN) signs. Neuropathological features of PLS include degeneration of the primary motor cortex and TDP43-immunoreactive neuronal inclusions accompanied by corticospinal tract degeneration, sparing lower motor neurons in the brainstem and spinal cord. Motor neuron disorders may present as atypical parkinsonism, such as progressive supranuclear palsy and corticobasal syndrome (CBS) (1, 2). Less is known about PLS, but asymmetrical PLS can be difficult to correctly diagnose (3). We report an autopsy case of a patient with PLS who had progressive asymmetrical UMN signs and dystonia that led to antemortem clinical diagnosis of possible CBS.
This patient was a 65-year-old, right-handed Caucasian woman with a 2-year history of difficulty using her left extremities that were noticed shortly after a fall. She subsequently developed difficulty walking, for which she required a walker. She had no significant past medical history or family history of neurological or psychiatric disorders. Neurological examination revealed UMN signs and dystonic movements in the left lower extremity, as well as gait disturbance, with left leg circumduction, due to spasticity. A Babinski sign was elicited on the left. Lower motor neuron signs (atrophy and fasciculations) were absent in all extremities and in the tongue. Parkinsonism, dementia, sensory deficits, ataxia, and autonomic dysfunction were absent. Needle electromyography and MRI scans of the brain, cervical, and thoracic spine were unremarkable. Her initial diagnosis was hemidystonia following traumatic brain injury. Her gait disturbance worsened rapidly, and she required a wheelchair within 9 months of symptomatic onset. Neurological examination at 64 years of age revealed UMN signs and dystonic posturing of the left upper and right lower extremity. Her cognitive function was grossly intact. She was diagnosed with possible CBS. Levodopa treatment provided no benefit. She developed swallowing and speaking difficulties at the end of life.
The autopsy was performed after informed consent from her caregiver who had legal power of attorney to grant permission for an autopsy. The formalin-fixed left hemibrain weighed 540 g, and the calculated whole brain weight was 1080 g. There was no focal atrophy on macroscopic examination (Fig. 1A, B). On hematoxylin and eosin-stained sections, severe neuronal loss and gliosis were evident in the motor cortex (Fig. 1C). There was no hippocampal sclerosis. The putamen had slight gliosis. The forebrain (Fig. 1S) and substantia nigra (Fig. 1T) had no neuronal loss. There was no significant neuronal loss in motor neuron nuclei of the brainstem (Fig. 1L) or spinal cord. Bunina bodies were not observed. The motor cortex and the corticospinal tract in the midbrain (Fig. 1F, I) and medulla (Fig. 1M, L) had degeneration, with infiltration of CD68-immunoreactive macrophages (Fig. 1D, G, K). Immunohistochemistry for phospho-tau (CP13; mouse monoclonal; from the late Dr. Peter Davies, Feinstein Institute, North Shore Hospital, NY) revealed no tau lesions of corticobasal degeneration. Thioflavin-S (Sigma-Aldrich, St. Louis, MO) fluorescent microscopy showed a few senile plaques in the entorhinal cortex (5–10/10× field), in the inferior parietal cortex (0–3/10× field), and midfrontal cortex (0–1/10× field), as well as a few neurofibrillary tangles in the CA1 sector of hippocampus (0–1/10× field) and the subiculum (0–1/10× field).These findings were consistent with Braak II and Thal 2. Immunohistochemistry for p-TDP43 (S409/410; mouse monoclonal; Cosmo Bio, Tokyo, Japan) revealed neuronal cytoplasmic inclusions (NCI), sparse dystrophic neurites, and glial cytoplasmic inclusions (GCIs) in the neocortex (Fig. 1O), including the motor cortex (Fig. 1E). TDP-43 pathology was present in all cortical layers consistent with FTLD-TDP type B (4). The pathologic diagnosis of FTLD-PLS was made. In addition, there was also moderate Lewy-related pathology (i.e. Lewy bodies and Lewy neurites) in temporal (Fig. 1Q) and entorhinal cortices. The brainstem and basal forebrain (Fig. 1R) had sparse Lewy-related pathology. These findings are consistent with cerebral type Lewy body disease (LBD) (5). The semiquantitative scores of neuronal loss, gliosis, p-TDP43, and Lewy-related pathology are shown in the Table.
FIGURE 1.
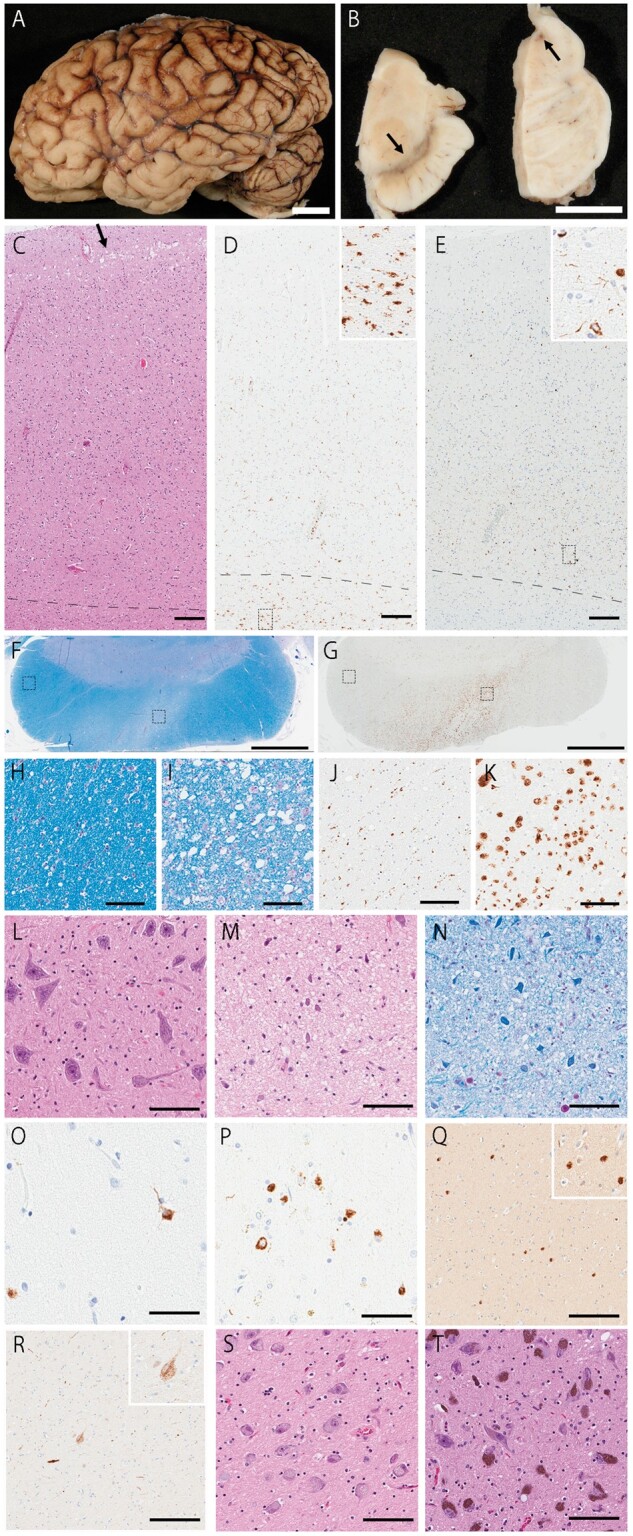
Macroscopic and histopathologic images. (A) The brain shows no focal atrophy. (B) The substantia nigra has normal degree of pigment. (C, D) The motor cortex shows microvacuolation (arrow; C) and many CD68-positive microglia (D). (E) p-TDP43-positive NCIs and GCIs are observed in all layers in the motor cortex. (F–K) The cerebral peduncle shows myelin pallor (I) in the corticospinal tract, while myelination is well preserved in other areas, such as corticopontine fibers (H). (G) Many CD68-positive microglia are observed in the corticospinal tract (K), but minimal in corticopontine fibers (J). (L) The hypoglossal nucleus is well preserved. Microvacuolation (M) and myelin pallor (N) is observed in the corticospinal tract in the medulla. Many p-TDP43-positive NCIs are present in the superior temporal cortex (O) and putamen (P). (Q) Many Lewy bodies are observed in the superior temporal cortex. (R) The basal nucleus of Meynert shows a few α-synuclein-positive cells. (S, T) Neuronal population is well preserved in the basal nucleus of Meynert (S) and substantia nigra (T). C, L, M, S, T: H&E staining; F, H, I, N: LFB staining; D, G, J, K: CD68 immunostaining; E, O, P: pTDP43 immunostaining; Q, R: NACP staining. Scale bars: C–E, Q, R = 200 μm; F, G = 3 mm; H–N, S, T = 100 μm; O, P = 50 μm.
TABLE.
The Distribution and Severity of pTDP, Lewy Pathology, Neuronal Lloss, and Gliosis
| pTDP-43 | NL/Gliosis | Lewy-related Pathology | |
|---|---|---|---|
| Midfrontal cortex | ± | −/− | − |
| Motor cortex | +++ | +++/+++ | − |
| Superior temporal cortex | ± | −/− | +++ |
| Inferior pariental cortex | ± | −/− | + |
| Cingulate | + | −/− | − |
| Hyppocampus | + | −/− | − |
| Parahippocumpal | +++ | −/− | +++ |
| Amygdala | +++ | −/− | − |
| The basal nucleus of Myenert | − | −/− | ++ |
| Putamen | +++ | −/± | − |
| Caudate nucleus | + | −/− | − |
| Thalamus | − | −/− | − |
| Subthalamic nucleus | ± | −/− | − |
| Subtantia nigra | − | −/− | ± |
| Oculomotor nucleus | − | −/− | − |
| Corticospinal tract | /+++ | ||
| Locus ceruleus | − | −/− | ± |
| Pontine base | ± | −/− | − |
| Dorsal motor nucleus of vagal nerve | − | −/− | − |
| The nucleus of ambiguous | − | −/− | − |
| Inferior olive nucleus | − | −/− | − |
| Medullullar tegmentum | ± | −/− | − |
| Hypoglossal nucleus | − | −/− | − |
| Pyramidal tract | /+++ | ||
| Cerebellum | − | −/− | − |
| Anterior horn | − | −/− | − |
The severity of each tau lesion type is shown in 5-point scale: −, none; ±, rare; +, mild; ++, moderate; +++, severe.
Patients with motor neuron disease, such as amyotrophic lateral sclerosis and PLS, are sometimes misdiagnosed as parkinsonian disorders (1–3) because it is challenging to distinguish pyramidal slowness from bradykinesia and spasticity from rigidity (1). Although documentation of her signs supporting a diagnosis of possible CBS was limited, we assume that her asymmetrical spasticity was interpreted as asymmetrical rigidity, a characteristic feature of CBD. In addition, her difficulty with speech might have mimicked oral apraxia.
Interestingly, we found cerebral type LBD, which is characterized by cortical Lewy-related pathology, but minimal involvement of the brainstem and limbic regions and none of amygdala. Patients with neocortical Lewy-related pathology, classified as stage 5 in the Braak stage or as diffuse neocortical type in the McKeith stage, usually also have Lewy-related pathology in the amygdala. Therefore, this rare subtype may have distinct progression patterns of Lewy-related pathology from typical diffuse neocortical LBD. The absence of characteristic features of diffuse LBD, such as dementia, visual hallucinations, and REM sleep behavior disorder, suggests that our case might be considered “preclinical” cerebral type LBD.
A limitation of the present study is that we assessed only the left hemibrain, although the right hemibrain was likely more affected due to her left-predominant symptoms.
In summary, we report a patient with asymmetrical PLS where FTLD-TDP mimicked CBS. An important lesson from this patient is that PLS can be asymmetric. Thus, careful evaluation is needed to distinguish asymmetrical spasticity from rigidity.
FUNDING
Dr. Dickson receives support from the NIH (Award Number: P50-NS072187).
COMPETING INTERESTS
The authors have no duality or conflicts of interest to declare.
REFERENCES
- 1. Norlinah IM, Bhatia KP, Ostergaard K, et al. Primary lateral sclerosis mimicking atypical parkinsonism. Mov Disord 2007;22:2057–62 [DOI] [PubMed] [Google Scholar]
- 2. Coon EA, Whitwell JL, Jack CR, et al. Primary lateral sclerosis as progressive supranuclear palsy: diagnosis by diffusion tensor imaging. Mov Disord 2012;27:903–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tando S, Kasai T, Mizuta I, et al. An autopsy case of corticobasal syndrome due to asymmetric degeneration of the motor cortex and substantia nigra with TDP-43 proteinopathy, associated with Alzheimer's disease pathology. Neuropathology 2021;41:214–25 [DOI] [PubMed] [Google Scholar]
- 4. Mackenzie IR, Neumann M, Baborie A, et al. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol 2011;122:111–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kosaka K, Iseki E, Odawara T, et al. Cerebral type of Lewy body disease. Neuropathology 1996;16:32–5 [Google Scholar]


