Abstract
Fecal incontinence has an enormous social and economic impact and may significantly impair quality of life. Even though fecal incontinence is a common complaint in (aging) adults, a structured pathophysiological model of the clinical presentations of fecal incontinence is missing in current literature. The most frequent manifestations of fecal incontinence are passive fecal loss, urge incontinence, or mixed fecal incontinence. At our institution, we treat 400 patients per year with defecation disorders, including a significant number of patients with fecal incontinence. On the basis of this experience, we have tried to create a concept that merges current insight in causes and treatment options in a clinically useful algorithm. By applying the system of anamnesis and physical examination described in this article and expanding it with simple additional anorectal examination, in most patients, one can determine the type of fecal incontinence and choose a targeted therapy.
Keywords: fecal incontinence, proctology, gastroenterology, surgery, sacral neuromodulation, graciloplasty
Introduction
According to the Rome IV criteria, fecal incontinence is defined as the uncontrolled passage of fecal materials that occurred at least two to four times in a four-week period for the past six months [1]. Reported prevalence varies strongly, that is, up to 15% in independently living adults and increasing in (aging) patients living in care facilities [2-4]. Fecal incontinence negatively effects quality of life, but only a small percentage of patients consult a doctor or discuss their complaints with family [4]. Embarrassment may play a part in this.
In their excellent and comprehensive review recently published by this journal, Maeda et al. [5-7] indicated how many aspects should be considered in the analysis and management of fecal incontinence. Fecal incontinence can have many causes, including disorders that cause fecal incontinence in an intact continence mechanism, such as inflammatory bowel disease and overflow diarrhea. If we exclude these secondary causes of fecal incontinence, current literature still lacks a structured pathophysiological model of the clinical presentations of fecal incontinence. In this article, we present a concept that merges current insight in causes and treatment options in a clinically useful algorithm. We have focused on fecal incontinence that has not been provoked by conditions such as inflammatory bowel disease, overflow diarrhea, malignancies, and neurological disorders. However, that does not mean that the therapeutic options cannot be considered in such circumstances.
Part 1: Physiology and Clinical Presentation of Fecal Incontinence
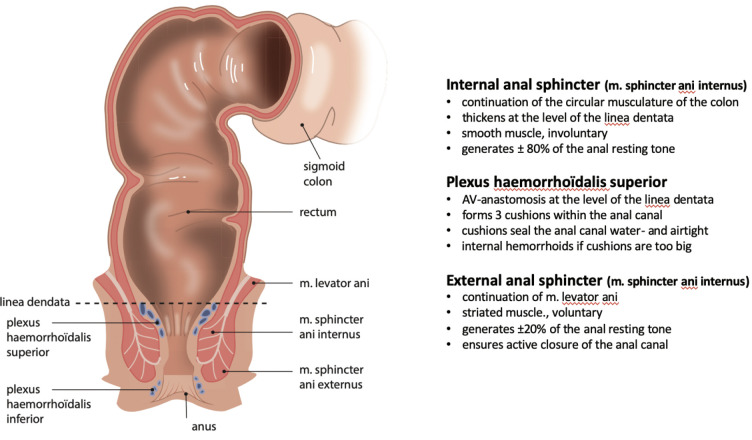
The internal and external anal sphincters, combined with the puborectalis muscle, are the major structures allowing controlled closure and opening of the anal canal. The internal anal sphincter generates approximately 80% of the anal resting tone. The external anal sphincter complements but is mainly responsible for the anal squeezing pressure. The superior hemorrhoidal plexus consists of three hemorrhoidal pads that cause the water- and airtight seal of the anal canal. Figure 1 summarizes the characteristics of the anal sphincters and hemorrhoidal plexus.
Figure 1.
Characteristics of the anal sphincter complex and plexus hemorrhoïdalis superior. Copyright: All illustrations are illustrated on our request, are a reprint, and are previously published in the journal “Aging Academy” [29]. With permission from the illustrator, we reuse these illustrations.
The puborectalis muscle: the often-forgotten pivot of the continence mechanism
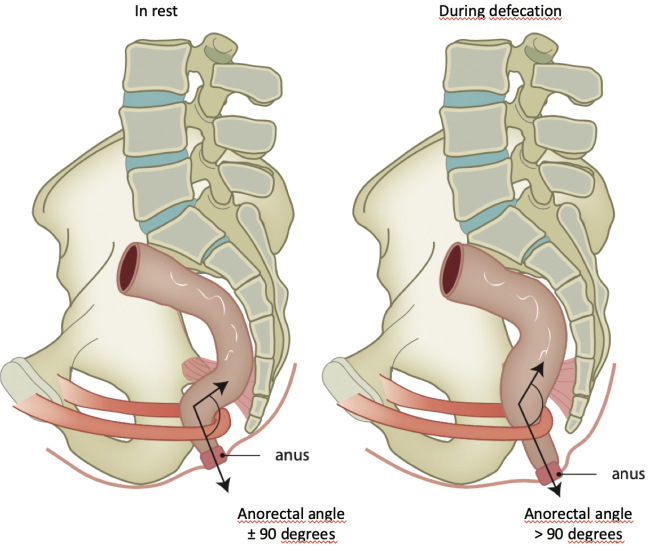
The puborectalis muscle, a striated muscle, is just as important if not more important to continence as the anal sphincter complex is. As an anatomical part of the levator ani muscle, the puborectalis muscle is largely autonomous. With its origin and insertion at the pubic bone, the puborectalis muscle loops around the anorectal junction and, in rest, creates an anorectal angle of 80° to 90° due to partial contraction. During straining, the contraction increases, causes shortening of the puborectalis muscle, and creates a sharper anorectal angle. This narrows the intestinal lumen at the anorectal junction (Figure 2). The sharpening of the anorectal angle forms the first barrier between the rectum and anal canal. A second barrier in continence is formed by the pressure of the anal sphincter complex combined with the hemorrhoidal pads.
Figure 2.
The anatomical position of the puborectalis muscle and the anorectal angle with varying degrees of contraction. Copyright: All illustrations are illustrated on our request, are a reprint, and are previously published in the journal “Aging Academy” [29]. With permission from the illustrator, we reuse these illustrations.
Both anal resting tone and squeeze pressure, as well as the functioning of the puborectalis muscle, can easily be determined by digital rectal examination. The patient is positioned in a left lateral position, and the palmar side of the examiner's digit is placed on the anorectal junction. If the patient squeezes, the digit is pushed ventrally if the puborectalis muscle is functioning. Additionally, during short sudden increase in intra-abdominal pressure such as coughing or sneezing, the puborectalis muscle reflexively contracts, as with squeezing. Combined with the caudally directed increased intra-abdominal pressure that further sharpens the anorectal angle, the barrier between the rectum and anal canal increases. During a Valsalva maneuver (prolonged straining) during evacuation, the puborectalis muscle relaxes. The lumen at the anorectal junction becomes less squeezed, and the anorectal angle becomes obtuse (Figure 2). As a result of the rectal contraction and the increased intra-abdominal pressure, the rectal content can now be expelled unimpeded. If one paradoxically contracts the puborectalis muscle during defecation, one has an expulsion problem. This is called spastic pelvic floor syndrome.
If the external sphincter is damaged, for example after childbirth, one can often be reasonably continent if the puborectalis muscle functions normally. However, the puborectalis muscle regresses after menopause. Some patients then develop fecal incontinence a few years after menopause. A connection with traumatic childbirth is often not made. If the puborectalis muscle is also damaged during childbirth and malfunctioning since, fecal incontinence is often immediately present after birth.
The different clinical manifestations of fecal incontinence
The first step in the analysis of fecal incontinence is to distinguish between spontaneous or passive fecal loss and urge incontinence. Explain the differences to the patients, and let them choose which complaint is most prominent. A combination of complaints can occur, but placement of the most prominent complaints in either category is important. Fecal stress incontinence, like in urinary incontinence, almost never occurs because the short and sudden increase in intra-abdominal pressure results in a puborectalis muscle contraction that squeezes the anorectal junction and causes the sharpening of the anorectal angle. However, a sudden increase in intra-abdominal pressure can lead to a loss of rectal contents in case of a very low anal resting tone and open anus in combination with a malfunctioning puborectalis muscle.
Spontaneous or passive fecal loss
Without having any urge, there appears to be solid feces in the underpants [8-10]. This fundamentally differs from soiling, which is merely fluid loss from the anal canal. The spontaneous fecal loss often occurs after normal defecation earlier that day or while exercising. Most patients also complain regarding the need of large amounts of toilet paper to clean the anus after defecation. Sometimes a combination with urge incontinence occurs.
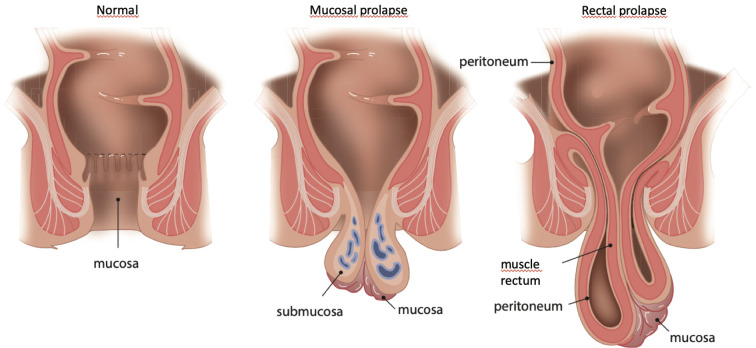
Rectal examination is often normal in patients with spontaneous fecal loss. The anal resting tone and squeeze pressure are normal, assuming that there is no simultaneous urge component. During a Valsalva maneuver, one can see the rectal mucosa descending, resulting in a mucosal prolapse especially on the perineal side. The mucosal prolapse can be partially or completely circumferential. When the muscle layers of the rectum participate, there is rectal prolapse (Figure 3). If there is doubt regarding the existence of a mucosal prolapse during consulting hour, the patient can be asked to make an “anal selfie” at home during complaints.
Figure 3.
Difference between mucosal prolapse and rectal prolapse. Copyright: All illustrations are illustrated on our request, are a reprint, and are previously published in the journal “Aging Academy” [29]. With permission from the illustrator, we reuse these illustrations.
Urge incontinence
Patients with urge incontinence experience an urge to defecate but are unable to control it before they reach a toilet. Typically, there is no gradual building of the increase of urge. The first feeling of an urge is immediately very intense, and the time between the first urge and loss of stool is often short. Most urge incontinence patients have a history of vaginal delivery with or without rupture. The use of forceps during delivery increases the risk of developing urge incontinence. Additionally, radiotherapy on the prostate or anorectal region and anal surgical procedures can cause urge incontinence.
Physical examination often shows a normal anal resting tone and a clearly reduced squeezing force. Sometimes a sphincter defect is palpable (usually in the perineum in women).
Magnetic Resonance Imaging (MRI) or endo-anal ultrasound (2D or 3D) can visualize lesions on the internal and external sphincters. Anal manometry can confirm anal pressures found with physical examination. With the use of an inflated rectal balloon, rectal sensibility can be measured. This is a combined outcome of the balloon volumes of the first sensation, desire to defecate, and maximum tolerable volume. In most patients with urge incontinence, reduced sensibility appears to be present. The (difficult) determination of the pudendal nerve latency time does not appear to be helpful [11].
Part 2: Treatment of Fecal Incontinence
Spontaneous or passive fecal loss
In case of passive fecal loss, treatment is aimed at preventing the descent of the distal rectal mucosa. Rubber band ligation is usually sufficient [12,13]. The anal resting tone is largely generated by the internal sphincter and ensures the retention of solid feces. The hemorrhoidal pads are important for the water- and airtight seal. Damage to the hemorrhoidal pads can lead to fecal leakage, and therefore, it is important that the rubber bands are placed just proximal to the superior hemorrhoidal plexus. In case of the enlargement of the hemorrhoidal pads, we speak of internal hemorrhoids. The treatment for internal hemorrhoids is similar to the treatment for rectal mucosal prolapse: by tightening the mucosa immediately proximal to the hemorrhoids by rubber band ligation, the hemorrhoids are pushed back into the wall. If the mucosal prolapse is too large for rubber band ligation, one can do a mucopexy or PPH-stapling. Schwandner et al. [14] performed transanal surgery (either stapled or Delorme mucosectomy) for rectal mucosal prolapse associated with fecal incontinence and obstructed defecation. Functional outcome was good in 90% of the patients with fecal incontinence but in only 28% of the patients with obstructed defecation. A rectopexy is indicated for rectal prolapse. If the effect of (multiple sessions of) rubber band ligation is insufficient and a mucosal prolapse still exists, surgical intervention might be necessary. If there is no prolapse or an invasive surgery to correct the prolapse failed, one can try a balloon enema (see urge incontinence) and incontinence panty liners. Thickening of feces can be counterproductive: often having to push harder and, as a result, more descending mucosa.
Urge incontinence
Dietary measures, medical treatment to thicken the stool, and pelvic floor physiotherapy are often effective in mild cases of urge incontinence [6,15]. If these treatment options are not effective, sacral neuromodulation (SNM) or retrograde colonic irrigation can follow. In case of retrograde colonic irrigation, approximately 1 L of lukewarm water is introduced in the colon via a rectal cannula by the patient sitting on the toilet. After the insertion, against the natural direction of feces, a defecation reflex sets in. In fact, the patient is still incontinent, but the colon is now empty, and fecal loss can no longer occur. The administration of a balloon enema is less stressful and less effective because of the small volume. In this case, 400 to 500 mL of liquid is injected via a rectal cannula. The frequencies of these irrigations vary per patient and are practice based. A specialized incontinence nurse can instruct the patient for these irrigations. Sphincter repair is only applied if a clear lesion is seen on endo-anal ultrasound. In our institution, however, a clear lesion is absent in more than half of our patients with urge incontinence. The damage appears to be mainly neurological in nature. In addition, the long-term results of sphincter repair are disappointing [16,17].
Another treatment option after failure of SNM and/or retrograde colonic irrigation is an appendicostomy (Malone stoma), through which antegrade colonic irrigation can be effectuated. It is a small stoma of the appendix pulled toward the abdominal wall and can be camouflaged more easily than a colostomy. The same idea of antegrade colon irrigation applies to a CHAIT-catheter, which is placed in the cecum or proximal colon through endoscopy. A colostomy is a last resort treatment, and fortunately, this is rarely necessary.
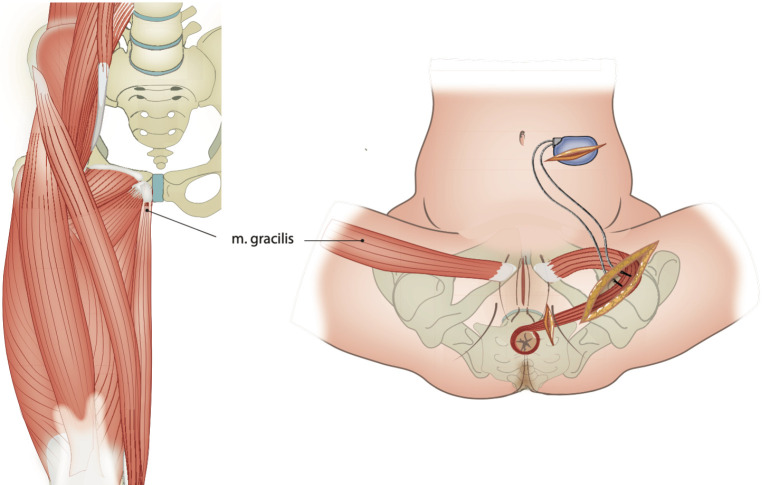
Another rarely used surgical treatment is graciloplasty [18]. In this technique, the gracilis muscle is used to create a neo-sphincter. The muscle is prepared in the thigh, the neurovascular bundle is left intact, and the muscle is wrapped around the anus. An option is to stimulate the gracilis muscle directly with a pacemaker, and this is called dynamic graciloplasty (Figure 4). The pacemaker must be switched off prior to defecation. In our clinic, we only use graciloplasty without a pacemaker, also called nondynamic graciloplasty, and only in case of predominantly passive fecal incontinence combined with an anus that could be moved ventrally to nearly the pubic bone (see later).
Figure 4.
Dynamic graciloplasty as a neo-sphincter. Copyright: All illustrations are illustrated on our request, are a reprint, and are previously published in the journal “Aging Academy” [29]. With permission from the illustrator, we reuse these illustrations.
Sacral neuromodulation
SNM is a successful treatment for urge incontinence [19]. Depending on sensory or motor response, an electrode is placed in foramen S3 or S4. As a trial, the effect is measured by external pacing ambulatory over a period of three weeks. If this proves to be effective (at least 50% less moments of incontinence), a permanent electrode and pacemaker are implanted. With careful selection of patients, 74%-86% has a positive result [19]. Depending on the setting, the pacemaker will last for five to seven years. A new pacemaker can be connected to the electrode in situ. The latest series of pacemakers are rechargeable and, just like the newer leads, MRI resistant. SNM is a comfortable therapy for patients and, compared to irrigation, is less onerous. Therefore, in patients with urge incontinence after failure of conservative treatment options, SNM is our preferred following treatment option.
SNM - mode of action
The effect on the squeeze pressure appears to be absent [19,20]. Most authors today believe that a modulation of rectal or upper anal canal sensation is likely [19]. The cerebral perception of fecal urge at a particular level of rectal filling plays a central role in the continence mechanism. The idea is that the responsible receptors are located in the rectal wall, but the type and location of these receptors are still under investigation. Because the feeling of rectal filling persists after complete rectal excision with a colo-anal anastomosis [21], colon-pouch-anal anastomosis [22], or an ileal pouch-anal anastomosis [23], this cannot be fully explained by the receptors in the rectal wall. There are indications that the pressure in the upper anal canal correlates better with the sense of urge than the pressure in the rectum [24]. During labor, women often experience a feeling of fecal urgency while the rectum is empty. Pressure from the child on the pelvic floor and thus on the upper anal canal (or receptors in the pelvic floor) could theoretically activate the same receptors. Consistent with this is the momentary feeling of urge when the rectal finger pulls the perineum caudally on physical examination. The urge disappears immediately after the pull has stopped. This sensation can be generated several times in a row. An indication that the proximal anal canal has its own role in sensing urgency is the finding that in patients with idiopathic fecal incontinence, SNM lowers the threshold for inducing urge sensation from the proximal anal canal [25].
How can we link these observations together?
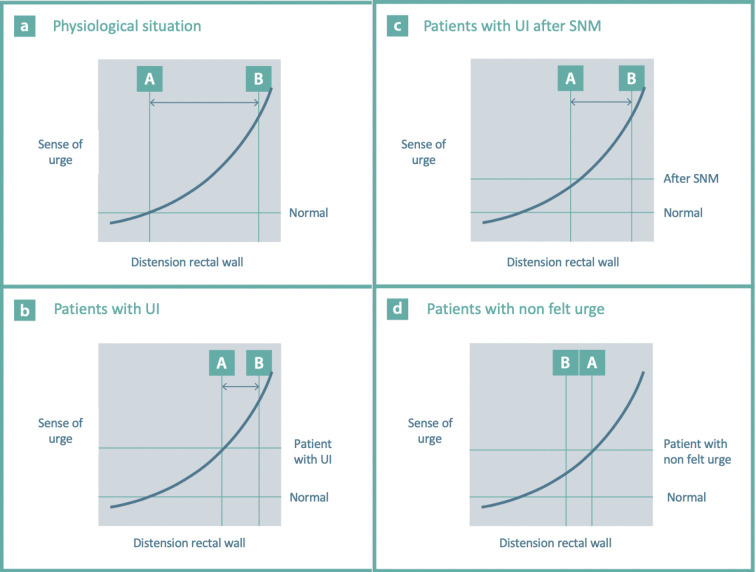
Figure 5 attempts to apply this most held theory of sensibility modulation in schematic form based on the clinical observation after successful sacral neuromodulation. As indicated above, most patients with urge incontinence have anamnestic missing of the first part of their urge curve. The first urge in patients with urge incontinence is felt with larger filling of the rectum and is immediately very strong, and because of the reduced anal squeeze, the period between the first urge and loss of stool is short (see Figure 5B: the time between points A and B is shorter). After a successful therapy with SNM, they appear to have anamnestic, as it were, regained part of that curve: in Figure 5C, the first urge is felt earlier and is less intense than that before SNM. The time that elapses during SNM between the first urge and being unable to hold the stool is now longer, so they have more time to reach the toilet (section AB in Figure 5C). Therefore, SNM does not result in higher squeeze pressure but in more time to reach the toilet on time. The weak external sphincter is thus only a part of a much more extensive, mainly neurological, damage to the pelvic floor, for example due to overstretching of the sacral plexus during delivery. Consistent with this, we see many patients with urge incontinence without apparent anatomical muscle and/or sphincter damage. In addition, there is no difference in success in SNM between the groups with and without muscle sphincter damage nor after a sphincter repair [26].
Figure 5.
A to d concern urge curves. Figure 5a normal situation. Figure 5b: patients with urge incontinence (UI). Figure 5c: patients with UI after sacral neuromodulation (SNM). Figure 5d: patients with nonfelt urge (see later). The first urge is felt at point A. Point B is the point where the external sphincter and the puborectalis muscle are overruled and defecation/loss of stool occurs. The point between A and B is the time during which defecation can be deliberately delayed. Copyright: All illustrations are illustrated on our request, are a reprint, and are previously published in the journal “Aging Academy” [29]. With permission from the illustrator, we reuse these illustrations.
Part 3: Other Forms of Fecal Incontinence
Spontaneous loss without mucosal prolapse
There is a group of patients with spontaneous fecal loss that does not meet the characteristics of the group with spontaneous fecal loss as described in Part 2. This group does not have a mucosal prolapse, and in this group, we find a low anal resting tone and squeeze pressure, as well as a reduced rectal sensation. Some of these patients have a combined picture with urge incontinence. The puborectalis muscle appears to play a major role in this form of spontaneous loss.
To perform its function, the anteriorly directed contraction force of the puborectalis muscle must find a stable structure on its path. If not, all structures are shifted ventrally, and the pinch and kinking mechanism of the puborectalis muscle at the anorectal junction does not function. The stable structure, a counter pressure point, is mainly formed by the transversus perinei superficialis and the inferior diaphragmatis urogenitalis fascia. It is called the perineal body and can clinically be recognized as the perineum (Figure 6). If an already flaccid anus (low resting tone) can easily be opened ventrally, this stable structure appears to be damaged.
Figure 6.
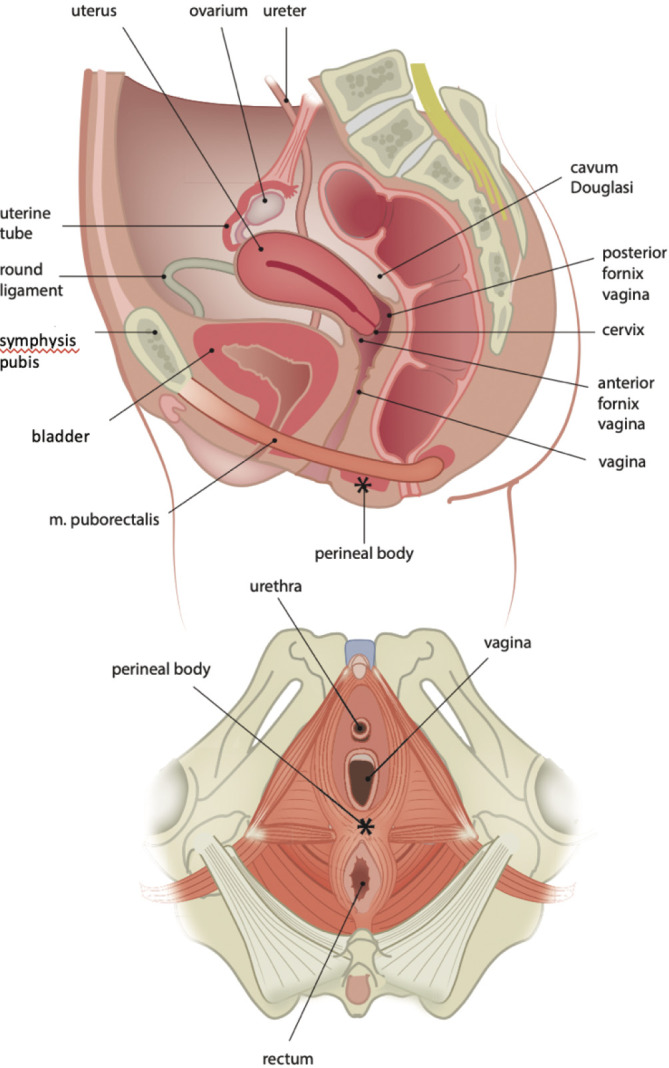
Anatomy of the pelvis and pelvic floor. Copyright: All illustrations are illustrated on our request, are a reprint, and are previously published in the journal “Aging Academy” [29]. With permission from the illustrator, we reuse these illustrations.
In this group of patients, the rectum normally fills with feces. Owing to reduced rectal sensibility in this patient group, this is not felt when the filling is not too large. A slight contraction of the rectum will follow as a normal response to filling. Because there are also a low resting tone, poor squeeze pressure, and insufficient puborectalis muscle function, spontaneous loss occurs: both the first (puborectalis muscle) and second (sphincter complex) lines are overruled before filling/urge is felt at all. The mechanism works the same as that with urge incontinence, except that the impending loss is not felt: spontaneous loss as an unfelt urge (see Figure 5D).
This form of spontaneous loss therefore has a different cause compared with the loss due to the mucosal prolapse. A logical consequence is to restore the counter pressure point of the puborectalis muscle with this unfelt urge. This is only useful if a rectal examination shows that the puborectalis muscle itself still has contraction force. This can be properly assessed during a physical examination, as described in Part 1.
On the basis of the above hypothesis, we performed a retrospective study among our patients who underwent a nondynamic graciloplasty [27]. Thirty-one patients with predominantly passive fecal incontinence and an anus that could be moved ventrally to nearly the pubic bone were selected and were treated with a nondynamic graciloplasty. Some of them also had complaints of urge incontinence, but to a lesser extent. The graciloplasty was used to strengthen the perineal body or ventral side of the anus.
At three months, 71% of the patients were successfully treated for their passive fecal loss. At six months, the success rate increased to 77%, and at 12 months, the results dropped to 58% successfully treated for their passive fecal loss. The decline in success rate was explained by one-third of the patients not having a routine follow-up at one year. After contacting these patients, the success rate increased to 81%. One of the patients developed an urge incontinence afterward, and this was not present preoperatively. Presumably, first line reinforcement of the puborectalis muscle eliminated the passive fecal loss, allowing further filling of the rectum to a level where urge can be felt. Another explanation may be that because of the graciloplasty, the anal resting tone was increased to such an extent that not only the passive loss but also the urge incontinence was cured in some of the patients. Some of the patients whose urge incontinence persisted postoperatively were successfully treated with SNM. In Figure 5D, this presumed mechanism is linked to the possible explanation on which, according to most authors, the effect of SNM is based.
Urge incontinence due to decreased rectal compliance
Urge incontinence can also arise without a dysfunctional continence mechanism. With a rigid rectum due to radiation, a long-standing proctitis, or a status after low anterior resection (reduced capacity) or colo- or ileoanal anastomosis, a rectal motor response on filling can overrule the normal continence mechanism. With anal manometry and rectal sensibility (now hypersensitive) measurement as mentioned above, this type of urge incontinence can be distinguished from the urge incontinence due to a reduced function of the anal sphincter complex and/or puborectalis muscle and reduced rectal sensibility. In case of hypersensitivity of the rectum with a normal continence mechanism, one can try to apply biofeedback to influence awareness of the rectal sensation. Through training with an intrarectal balloon, one can be taught to feel a sensation of urge earlier. At the same time, patients learn to contract the pelvic floor muscles immediately and strongly in response to this early sensation. The purpose of this biofeedback is twofold. First, going to the toilet at an earlier stage prevents fecal incontinence from occurring with further rectal filling. Second, if muscle training succeeds in making the pelvic floor muscles contract more powerfully, the patient may be able to tolerate larger volumes [28].
Conclusion
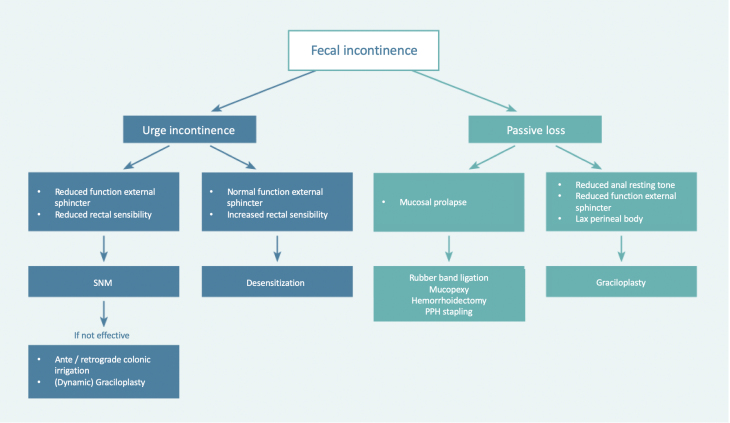
By applying the system of anamnesis and physical examination described in this article and expanding it with simple additional anorectal examination, one can appoint the type of fecal incontinence in most patients and choose a targeted therapy. In Figure 7, this is summarized in an algorithm.
Figure 7.
Clinical algorithm. Copyright: All illustrations are illustrated on our request, are a reprint, and are previously published in the journal “Aging Academy” [29]. With permission from the illustrator, we reuse these illustrations.
Conflicts of Interest
There are no conflicts of interest.
Author Contributions
all authors contributed to the conception and drafting of the manuscript.
Approval by Institutional Review Board (IRB)
not applicable
References
- 1.Simren M, Palsson OS, Whitehead WE. Update on Rome IV criteria for colorectal disorders: Implications for clinical practice. Current Gastroenterology Reports 2017 Apr; 19(4): 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bharucha AE, Dunivan G, Goode PD, et al. Epidemiology, pathophysiology, and classification of fecal incontinence: State of the science summary for the national institute for diabetes and digestive and kidney diseases. American Journal of Gastroenterology 2015 Jan; 110: 127-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng KS, Sivakumaran Y, Nassar N, et al. Fecal incontinence: Community prevalence and associated factors - A systematic review. Diseases of the colon and rectum 2015 Dec; 58(12): 1194-209. [DOI] [PubMed] [Google Scholar]
- 4.Bharucha AE, Zinsmeister AR, Locke GR, et al. Prevalence and burden of fecal incontinence: A population-based study in women. Gastroenterology 2005 Jul; 129(1): 42-9. [DOI] [PubMed] [Google Scholar]
- 5.Maeda K, Yamana T, Takao Y, et al. Japanese Practice Guidelines for Fecal Incontinence Part 1 - Definition, epidemiology, etiology, pathophysiology and causes, risk factors, clinical evaluations, and symptomatic scores and QoL questionnaire for clinical evaluations - English version. Journal of the Anus, Rectum and Colon. 2021 Jan; 5(1): 52-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maeda K, Mimura T, Yoshioka K, et al. Japanese Practice Guidelines for Fecal Incontinence Part 2 - Examination and conservative treatment for fecal incontinence - English version. Journal of the Anus, Rectum and Colon. 2021 Jan; 5(1): 67-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maeda K, Katsuno H, Tsunoda A, et al. Japanese Practice Guidelines for Fecal Incontinence Part 3 - Surgical treatment for fecal incontinence, fecal incontinence in a special conditions - English version. Journal of the Anus, Rectum and Colon. 2021 Jan; 5(1): 84-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rao SS, Bharucha AE, Chiarioni G, et al. Functional anorectal disorders. Gastroenterology 2016 May; 150(6): 1430-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meegdenburg MM van, Meinds RJ, Trzpis M, et al. Subtypes and symptoms of fecal incontinence in the Dutch population: A cross-sectional study. International Journal of Colorectal Disease 2018 Jul; 33(7): 919-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wald A. Diagnosis and management of fecal incontinence. Current Gastroenterology Reports 2018 Mar; 20(3): 9. [DOI] [PubMed] [Google Scholar]
- 11.Diamant NE, Kamm MA, Wald A, et al. AGA technical review on anorectal testing techniques. Gastroenterology 1999 Mar; 116(3): 735-60. [DOI] [PubMed] [Google Scholar]
- 12.Mattana C, Maria G, Pescatori M. Rubber band ligation of hemorrhoids and rectal mucosal prolapse in constipated patients. Diseases of the Colon and Rectum 1989 May; 32(5): 372-5. [DOI] [PubMed] [Google Scholar]
- 13.Mathai V, Seow-Choen F. Anterior rectal mucosal prolapse: An easily treated cause of anorectal symptoms. British Journal of Surgery 1995 Jun; 82(6): 753-4. [DOI] [PubMed] [Google Scholar]
- 14.Schwandner O, Schrinner B. Rectal mucosal prolapse in males: Surgery is effective for fecal incontinence but not for obstructed defecation. Techniques in Coloproctology. 2014 Oct; 18(10): 907-14. [DOI] [PubMed] [Google Scholar]
- 15.Berghmans LCM, Groot JAM, Heeswijk-Faase IC van, et al. Dutch evidence statement for pelvic physical therapy in patients with anal incontinence. International Urogynecology Journal 2015 Apr; 26: 487-96. [DOI] [PubMed] [Google Scholar]
- 16.Rieger NA, Sarre RG, Saccone GT, et al. Postanal repair for faecal incontinence: Long-term follow-up. The Australian and New Zealand Journal of Surgery 1997 Aug; 67(8): 566-70. [DOI] [PubMed] [Google Scholar]
- 17.Setti CP, Kamm MA, Nicholls RJ. Long-term results of postanal repair for neurogenic faecal incontinence. British Journal of Surgery 1994; 81(1): 140-4. [DOI] [PubMed] [Google Scholar]
- 18.Rongen MJ, Uludag O, El Naggar K, et al. Long-term follow-up of dynamic graciloplasty for fecal incontinence. Diseases of Colon and Rectum 2003 Jun; 46(6): 716-21. [DOI] [PubMed] [Google Scholar]
- 19.Janssen PTJ, Komen N, Melenhorst J, et al. Sacral neuromodulation for fecal incontinence: A review of the central mechanisms of action. Journal of Clinical Gastroenterology 2017 Sep; 51(8): 669-76. [DOI] [PubMed] [Google Scholar]
- 20.Sheldon R, Kliff ES, Clarke A, et al. Sacral nerve stimulation reduces corticoanal excitability in patients with faecal incontinence. British Journal of Surgery. 2005 Nov; 92(11): 1423-31. [DOI] [PubMed] [Google Scholar]
- 21.Lane RH, Parks AG. Function of the anal sphincters following colo-anal anastomosis. British Journal of Surgery 1977 Aug; 64: 596-9. [DOI] [PubMed] [Google Scholar]
- 22.Williams N, Seow-Choen F. Physiological and functional outcome following ultra-low anterior resection with colon pouch-anal anastomosis. British Journal of Surgery 1998 Aug; 85: 1029-35. [DOI] [PubMed] [Google Scholar]
- 23.Broens P, Penninckx F. Filling sensations after restorative proctocolectomy. Acta Chirurgica Belgica 2002 Feb; 102(1): 20-3. [DOI] [PubMed] [Google Scholar]
- 24.Broens P, Vanbeckevoort D, Bellon E, et al. Combined radiologic and manometric study of rectal filling sensation. Diseases of the Colon and Rectum 2002 Aug; 45: 1016-22. [DOI] [PubMed] [Google Scholar]
- 25.Haas S, Brock C, Krogh K, et al. Does sacral nerve stimulation improve continence through enhanced sensitivity of the anal canal? A pilot study. Diseases of the Colon and Rectum 2016 Nov; 59(11): 1039-46. [DOI] [PubMed] [Google Scholar]
- 26.Brouwer R, Duthie G. Sacral Nerve neuromodulation is effective treatment for fecal incontinence in the presence of a sphincter defect, pudendal neuropathy, or previous sphincter repair. Diseases of the Colon & Rectum 2010 Mar;53(3): 273-8. [DOI] [PubMed] [Google Scholar]
- 27.Knol ME, Snijders HS, DeRuiter MC, et al. Non-dynamic graciloplasty is an effective treatment for patients with passive fecal incontinence. Techniques in Coloproctology. 2021 Jul; 25(7): 849-55. [DOI] [PubMed] [Google Scholar]
- 28.Bols EMJ, Berghmans BCM, Hendriks EJM, et al. A randomized physiotherapy trial in patients with fecal incontinence: Design of the PhysioFIT-study. BMC Public Health. 2007 Dec; 7: 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knol M, Snijders H, Baeten C, et al. Fecale incontinentie: het belang van een helder ‘denkraam’. Aging Academy. 2021 Mar; 1(2): 48-56. [Google Scholar]