Abstract
Objective
To provide updated information on the evaluation and management of adverse reactions to vaccines.
Data Sources
PubMed (MEDLINE) search since publication of a practice parameter in 2012.
Study Selections
Original articles and guidelines on adverse reactions to vaccines, including vaccines against severe acute respiratory syndrome coronavirus 2 or coronavirus disease 2019 (COVID-19).
Results
Current guidelines conclude that patients with egg allergy are not at increased risk for reaction to egg-based influenza vaccines. Except for gelatin, most patients with allergy to vaccine constituents tolerate vaccines containing them. Most patients who have immediate reactions after receiving COVID-19 vaccines go on to receive a subsequent dose uneventfully.
Conclusion
The risk of reactions to vaccination should be weighed against the risk of having a vaccine-preventable disease if the vaccine is withheld. There is no need to ask about egg allergy before the administration of influenza vaccines, including on screening forms. In most cases, an allergy to a vaccine constituent is not a contraindication to the vaccine containing it. Patients who have had possible anaphylactic reactions to vaccines should be evaluated by an allergist rather than simply being labeled allergic, because most can go on to receive subsequent doses. Most immediate reactions to COVID-19 vaccines are not allergic, and care should be taken to not label such reactions as anaphylactic. The role, if any, of polyethylene glycol in these reactions has yet to be revealed.
Introduction
A practice parameter on adverse reactions to vaccines was published in 20091 and updated in 2012.2 Although most of the information contained in the 2012 document remains current, additional progress has been made in our understanding of, and approach to, adverse events following immunization, and lessons learned based on these new data will be presented in this update.
Key Messages.
-
•
Weigh the risk of vaccination against the risk of not vaccinating.
-
•
It is not necessary to ask about egg allergy before the administration of influenza vaccines.
-
•
An allergy to a vaccine constituent is different than an allergic reaction to a vaccine.
-
•
Most immediate reactions to coronavirus disease 2019 vaccines are not allergic and should not be labeled as “anaphylactic.”
Alt-text: Unlabelled box
Weigh the Risk of Vaccination Against the Risk of Not Vaccinating
The circumstances in which allergists are asked to provide recommendations relative to vaccine allergy generally fall into 2 categories; patients who may be allergic to a vaccine constituent and are concerned about receiving a vaccine containing that constituent or patients who have had an apparent allergic reaction after receiving a vaccine and are concerned about receiving additional doses of that or other vaccines. Our goal should be for patients to receive recommended vaccines if possible, given their enormous benefit in disease prevention.3 , 4 We must provide advice based on available evidence regarding the likelihood of a vaccine reaction in a patient allergic to a constituent or the likelihood of a reaction to a subsequent vaccine dose in a patient who may have had an allergic reaction to a previous dose. In almost all circumstances, the data are very reassuring that the vaccines will be well-tolerated.5 , 6 Withholding vaccination in patients where there is concern about a reaction to the vaccine may seem prudent or conservative; however, not vaccinating also carries real risk. Whatever risk is posed by receiving a vaccine must be weighed against the risk of not receiving the vaccine and remaining susceptible to a vaccine-preventable disease. We can help patients with their decision about whether to receive a vaccine or not by carefully listening to and addressing their individual concerns about the risks of vaccination. Patients receive a great deal of misinformation about vaccine risks from many sources. In many cases, studies have not revealed any increased risk (eg, autism or infertility), and we do not believe it is possible (biologically plausible) that the vaccine could cause such harm. We must however avoid telling patients that vaccines are completely safe, but rather acknowledge that serious reactions can occur but are exceedingly rare, often in the range of 1 in 1 million, and that the same rare adverse events from vaccination often occur at a higher rate from the disease itself, which can be prevented by vaccination.
It is not Necessary to Ask About Egg Allergy Before the Administration of Influenza Vaccines
An addendum to the 2012 practice parameter on adverse reactions to vaccines was devoted to the issue of administering influenza vaccines to recipients with egg allergy.2 Most influenza vaccines are grown in eggs and contain a residual amount of egg protein measured as ovalbumin content.7 Out of concern that this small amount of ovalbumin would trigger anaphylactic reactions in recipients with egg allergy, for many years, egg allergy was considered a contraindication to receiving influenza vaccination. Nevertheless, a large number of studies have specifically evaluated the administration of egg-based influenza vaccines, both the injectable inactivated influenza vaccine and the intranasal live-attenuated influenza vaccine, to large numbers of patients with egg allergy, including those with severe reactions to the ingestion of egg, and revealed that the recipients have no increased risk of allergic or other adverse reactions.8 , 9 This is almost certainly due to a threshold affect, where the amount of ovalbumin contained in the vaccines (<1 µg per dose) is simply not sufficient to provoke a reaction even in the recipients with most severe egg allergy. The overwhelming majority of patients with egg allergy are children because the allergy is almost always outgrown, that is, egg allergy in adulthood is rare.10 Since 2016, recommendations from the American Academy of Pediatrics have stated that no special precautions are required regarding the administration of egg-based influenza vaccines to children with egg allergy,11 and their guidance regarding the 2021 to 2022 influenza season states unequivocally that such precautions “constitute an unnecessary barrier to immunization” and they further state that “it is not necessary to inquire about an egg allergy before the administration of any influenza vaccine, including on screening forms.”12
The only other vaccine containing egg protein is yellow fever vaccine. Although yellow fever vaccine may contain a somewhat higher quantity of ovalbumin,13 , 14 as with influenza vaccine, the amount of ovalbumin may not be sufficient to provoke a reaction even in patients with egg allergy. The package insert for yellow fever vaccine contains a protocol for the evaluation of recipients with egg allergy.15 It is recommended that patients with egg allergy undergo prick skin testing with the vaccine full-strength, and if the result is negative, undergo intradermal testing with the vaccine diluted 1:100. For patients with negative vaccine skin test results, it is recommended that the vaccine be administered in a single dose under observation. For patients with positive vaccine skin test results, it is recommended that the vaccine be administered in graded doses under observation (Table 1 ). Most patients with egg allergy have negative yellow fever vaccine skin test results and receive the vaccine in a single dose uneventfully.16 , 17 Although studies have validated the safety of administering the vaccine in graded doses to those recipients with egg allergy with positive vaccine skin test results,16 , 17 yellow fever vaccine has also been administered to children with egg allergy with positive yellow fever vaccine skin test results as a single dose without reaction.18 Thus, consideration can be given to administering yellow fever vaccine to recipients with egg allergy as a single dose without prior vaccine skin testing, but with an observation period afterward. If additional studies support the safety of this approach, as with influenza vaccine, it may not be necessary to inquire about egg allergy before the administration of yellow fever vaccine.
Table 1.
Administration of a Vaccine in Graded Dosesa
| Dose/dilution |
|---|
| 0.05 mL of 1:10 dilution |
| 10% of full-dose full-strength |
| 20% of full-dose full-strength |
| 30% of full-dose full-strength |
| 40% of full-dose full-strength |
At 15-minute intervals by the usual route for the vaccine (subcutaneous or intramuscular) prepared to treat systemic allergic reaction.
An Allergy to a Vaccine Constituent is Different From an Allergic Reaction to a Vaccine
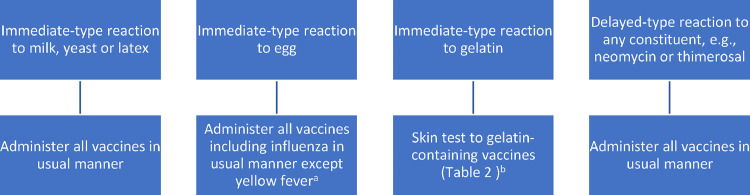
This journey regarding egg allergy and influenza vaccination from contraindication to nonissue has provided some important lessons. Although it was not illogical to be concerned that vaccine excipients might cause reactions in recipients with allergy to those excipients, making egg allergy a contraindication to influenza vaccination without evidence turned out to be inappropriate. There were undoubtedly many patients who did not receive an annual influenza vaccination for this reason and went on to have the consequences of influenza disease, including death. It is essential that concerns about possible allergic reactions to vaccine constituents not be raised as barriers to vaccination without evidence that allergy to the constituent actually causes reactions in vaccine recipients. There are exceedingly rare reports of patients who have had allergic reactions to vaccines due to excipients, such as neomycin, thimerosal, latex, milk, and yeast. However, most of these allergies are quite rare and the overwhelming majority of patients with allergy to any of these substances tolerate vaccines containing them uneventfully, again likely due to an insufficient amount of the allergen being present in the vaccine to provoke a reaction.2 Thus, vaccine or excipient skin testing before the administration of vaccines containing these excipients in patients with reported allergic reactions to them is not required, and the vaccines can be administered in the usual manner (Fig 1 ). However, in patients who have already had an immediate allergic reaction to a vaccine containing one of these excipients, skin testing or serum-specific IgE testing for the constituent may be warranted.
Figure 1.
Recommended approach to patients with previous reactions to vaccine constituents (for recommended approach to patients with previous reactions to vaccine administration, see Fig 2). aFor yellow fever vaccine, skin test with vaccine prick full strength and if negative intradermal diluted 1:100. If positive skin test result, administer in graded doses (Table 1) or consider administration as a single dose without previous vaccine skin testing under observation. bFor gelatin-containing vaccines, skin test with vaccine prick full strength and if negative intradermal diluted 1:100. If positive skin test result, administer in graded doses (Table 1).
The only exception to this general rule that almost all patients tolerate vaccines that contain substances to which they are allergic is gelatin. A number of vaccines contain milligram quantities of gelatin (Table 2 ), and most of the anaphylactic reactions reported to these vaccines have been determined to be due to IgE directed against the gelatin they contain.19, 20, 21, 22, 23 In some circumstances, gelatin-free vaccine alternatives are available (Table 2). Allergy to gelatin is quite rare, but patients describing possible immediate-type allergic reactions to the ingestion of gelatin, or to the receipt of gelatin-containing vaccines, should be evaluated before receiving such vaccines (Fig 1). Nevertheless, once again, even a confirmed gelatin allergy does not constitute a contraindication to receiving gelatin-containing vaccines. Rather, patients with gelatin allergy should undergo skin testing with required gelatin-containing vaccines, and those with negative skin test results can receive the vaccine in a single dose under observation, whereas those with positive skin test results could receive the vaccine in graded doses under observation (Table 1). Gelatin also contains alpha-gal and patients with alpha-gal syndrome may also be at risk for reactions to gelatin-containing vaccines.24 , 25
Table 2.
Gelatin-Containing Vaccines and Possible Alternatives
| Vaccine | Gelatin content (per stated dosage) | Gelatin-free alternatives |
|---|---|---|
| Influenza (FluMist, MedImmune) | 2 mg per 0.2 mL | IIV |
| Measles, mumps, rubella (MMRII, Merck) | 14.5 mg per 0.5 mL | PRIORIX,a GlaxoSmithKline |
| Measles, mumps, rubella, varicella (ProQuad, Merck) | 11 mg per 0.5 mL | PRIORIXa + VARILRIX,a GlaxoSmithKline |
| Rabies (RabAvert, Novartis) | 12 mg per 1.0 mL | Imovax Rabies, Sanofi Pasteur |
| Typhoid Vaccine Live Oral Ty21a (VIVOTIF, Crucell) | capsule | Typhim Vi, Sanofi Pasteur |
| Varicella (VARIVAX, Merck) | 12.5 mg per 0.5 mL | VARILRIX,a GlaxoSmithKline |
| Yellow fever (YF-VAX, Sanofi Pasteur) | 7.5 mg per 0.5 mL | STAMARIL,a Sanofi Pasteur |
| Zoster (ZOSTAVAX, Merck) | 15.58 mg per 0.65 mL | SHINGRIX, GlaxoSmithKline |
Abbreviation: IIV, inactivated influenza vaccine.
Available only in some countries outside the United States.
Most Immediate Reactions to Coronavirus Disease 2019 Vaccines are not Allergic and Should not be Labeled as “Anaphylactic”
Vaccines against severe acute respiratory syndrome coronavirus 2 and the coronavirus disease 2019 (COVID-19) it causes were introduced in late 2020. Although no anaphylactic reactions were reported in the clinical trials leading to their approval, shortly after their use in clinical practice, a number of reactions characterized as anaphylaxis were reported, particularly to the messenger RNA (mRNA) vaccines.26 Immunoglobulin (Ig)E-mediated allergic reactions require previous exposure for sensitization and almost all such reactions are because of protein allergens. Thus, the presumably allergic reactions being reported to the mRNA COVID-19 vaccines were unexpected given that no one had previously been exposed to the vaccines and that they do not contain protein. Although some of the reactions described could meet various criteria for anaphylaxis, most of these reactions have subsequently proved to not be allergic in nature, and most patients have gone on to receive second doses uneventfully.27, 28, 29
The demonstration that most reactions to the mRNA COVID-19 vaccines characterized and treated as anaphylaxis turned out not to be anaphylactic reactions also provides an important lesson. It is clear that many patients have immediate adverse events after immunization that involve both symptoms and signs, which are in fact reactions to vaccination, but are not allergic reactions to the vaccine administered. Many such reactions can be characterized as immunization stress-related responses (ISSRs).30 An ISSR may include stress-induced flushing, tachycardia, palpitations and shortness of breath, a vasovagal reaction leading to lightheadedness or syncope, hyperventilation causing tingling sensations, or dissociative neurologic symptoms, such as weakness, abnormal movements, or speech difficulties. Although it is important for vaccine providers to recognize anaphylaxis and treat it promptly, it is also important to realize that ISRRs occur and should be differentiated from anaphylaxis. In ISRRs, symptoms (subjective) often predominate, without signs (objective) to support them. For example, patients may complain of pruritus or tingling without visible skin changes, tongue or throat swelling with a normal oropharyngeal examination, shortness of breath without wheezing or stridor, or lightheadedness with a normal or even elevated blood pressure. Patients who have had these immediate, but not anaphylactic reactions to a COVID-19 vaccine can receive subsequent doses in the usual manner, but be observed for 30 minutes afterward (Fig 2 ). Many vaccine reactions characterized as anaphylaxis do not actually meet current diagnostic criteria for the diagnosis of anaphylaxis, and many that do meet the criteria may not actually be anaphylaxis.31 , 32 The Brighton Collaboration case definition for anaphylaxis as an adverse event following immunization33 is currently undergoing revision to avoid having cases involving only symptoms or nonserious signs classified as anaphylaxis.
Figure 2.
Recommended approach to patients with previous reactions to vaccine administration.
A small number of immediate reactions to COVID-19 vaccines describe reactions that are more convincingly anaphylactic.26 The mechanism of these reactions has yet to be elucidated. As with other vaccines, in patients who have had possible anaphylactic reactions to COVID-19 vaccines, it is appropriate to perform prick skin tests with the vaccine full-strength, and if negative intradermal tests with the vaccine diluted 1:100.2 Negative vaccine skin test results in a patient with such a history implies that the reaction was not mast cell-mediated, or at least not IgE-mediated, and it is recommended that such patients receive the vaccine in a single dose under observation. Positive vaccine skin test results in such patients imply that the vaccine may provoke a reaction, and it is recommended that such patients receive the vaccine in graded doses under observation (Fig 2).34 Another option in this circumstance would be to offer an alternative vaccine (eg, administration of a viral vector vaccine to a patient who reacted to an mRNA vaccine), also administered under observation, although the mRNA vaccines are now generally preferred over the available adenoviral vector vaccine.35 In particularly difficult cases, where there is hesitancy to administer a second dose, consideration can be given to measuring a COVID-19 spike protein antibody titer to evaluate the immune response generated to the dose already administered.36 Nevertheless, it should be noted that a particular titer has not been established as a surrogate for protection and thus the presence of anti–COVID-19 spike protein antibodies does not imply the same level or duration of protection from disease conferred by completing the initial series and appropriate boosters.35
In evaluating possible allergic reactions to the mRNA COVID-19 vaccines, an early candidate allergen was polyethylene glycol (PEG). PEG, although not an ingredient in any non–COVID-19 vaccine, is present in other medications, such as some injectable corticosteroids and oral laxatives, and in some foods and cosmetics.37 , 38 There are very rare reports of patients who have had IgE-mediated reactions to PEG-containing medications.37 , 38 Although not a protein, PEGs, particularly those of higher molecular weights, are large enough molecules to rarely provoke IgE responses. Prior exposure and sensitization to PEG through these other sources could potentially explain how someone might have an allergic reaction after the first dose of a novel vaccine. Nevertheless, the other medications to which reactions have been reported contain much larger amounts of PEG than the vaccines. For example, methylprednisolone acetate injectable suspension contains 29.1 mg PEG 3350 per 1 mL dose and the Pfizer COVID-19 vaccine contains 0.05 mg PEG 2000 per 0.3 mL dose. Furthermore, most patients who have had possible immediate allergic reactions to the COVID-19 mRNA vaccines do not have evidence of IgE antibody to PEG, and some of those who do have gone on to receive second doses uneventfully despite their apparent PEG allergy.29 The PEG in the mRNA COVID-19 vaccines is present in the lipid nanoparticles surrounding the mRNA, and some reports have suggested that these “multivalent” PEGylated nanoparticles may be more likely to cross-link IgE antibody in a way that native PEG may not.39 Another mechanism proposed as a possible cause of apparent mast cell-mediated reactions to the PEG in mRNA COVID-19 vaccines is complement activation-related pseudoallergy (CARPA), where IgG or IgM antibody directed against PEG would activate complement, generating C3a and C5a, which would then lead to mast cell degranulation.40 , 41 A recent report describes 11 patients with suspected allergic reactions to mRNA COVID-19 vaccines evaluated for CARPA.41 None had positive skin test results or serum-specific IgE to PEG, and only 1 had a positive skin test result to the vaccine. Nevertheless, 10 had positive basophil activation test (BAT) results to PEG and all 11 had positive BAT results to the vaccines and measurable serum-specific IgG to PEG. The authors conclude that the reactions are likely because of IgG anti-PEG CARPA but acknowledge that additional studies are needed. Among the factors complicating this interpretation are that some of the reported reactions may not have been mast cell-mediated, it is unknown whether the subjects would have reacted to a second dose, only 3 control subjects were evaluated, and a substantial portion of the general population may have anti-PEG IgG.42 Collectively, these studies argue against PEG skin testing either before administration of or after reactions to mRNA COVID-19 vaccines outside a research setting.43
Conclusion
The 2012 update to the adverse reactions to vaccines practice parameter still serves as useful guidance.2 Evidence published since has allowed us to conclude that we should stop asking about egg allergy before the administration of influenza vaccine, and that, with the exception of gelatin, patients with allergy to vaccine constituents should receive vaccines containing those constituents in the usual manner. Most immediate reactions reported to vaccines against COVID-19 have turned out not to be allergic. Patients with milder reactions to these vaccines should receive subsequent doses in the usual manner, but under observation for 30 minutes. For patients with more severe immediate reactions, consideration can be given to vaccine skin testing and administration in graded doses if positive, or to the administration of an alternative vaccine. PEG skin testing has been found to have no clinical use in the evaluation of such reactions.
Footnotes
Disclosures: The author has no conflicts of interest to report.
Funding: The author has no funding sources to report.
References
- 1.Kelso JM, Li JT, Nicklas RA, Blessing-Moore J, Cox L, Lang DM, et al. Adverse reactions to vaccines. Ann Allergy Asthma Immunol. 2009;103(4 Suppl 2):S1–S14. doi: 10.1016/s1081-1206(10)60350-x. [DOI] [PubMed] [Google Scholar]
- 2.Kelso JM, Greenhawt MJ, Li JT, Nicklas RA, Bernstein DI, Blessing-Moore J, et al. Adverse reactions to vaccines practice parameter 2012 update. J Allergy Clin Immunol. 2012;130(1):25–43. doi: 10.1016/j.jaci.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Greenwood B. The contribution of vaccination to global health: past, present and future. Philos Trans R Soc Lond B Biol Sci. 2014;369(1645) doi: 10.1098/rstb.2013.0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta S, Cantor J, Simon KI, Bento AI, Wing C, Whaley CM. Vaccinations against COVID-19 may have averted up to 140,000 deaths in the United States. Health Aff (Millwood) 2021;40(9):1465–1472. doi: 10.1377/hlthaff.2021.00619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li PH, Wagner A, Rutkowski R, Rutkowski K. Vaccine allergy: a decade of experience from 2 large UK allergy centers. Ann Allergy Asthma Immunol. 2017;118(6):729–731. doi: 10.1016/j.anai.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Cheung A, Choo S, Perrett KP. Vaccine allergy? Skin testing and challenge at a tertiary pediatric hospital in Melbourne, Australia. J Allergy Clin Immunol Pract. 2019;7(5):1541–1549. doi: 10.1016/j.jaip.2019.01.025. [DOI] [PubMed] [Google Scholar]
- 7.Grohskopf LA, Alyanak E, Ferdinands JM, Broder KR, Blanton LH, Talbot HK, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices, United States, 2021-22 influenza season. MMWR Recomm Rep. 2021;70(5):1–28. doi: 10.15585/mmwr.rr7005a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelso JM. Administering influenza vaccine to egg-allergic persons. Expert Rev Vaccines. 2014;13(8):1049–1057. doi: 10.1586/14760584.2014.933079. [DOI] [PubMed] [Google Scholar]
- 9.Greenhawt M, Turner PJ, Kelso JM. Administration of influenza vaccines to egg-allergic recipients: a practice parameter update 2017. Ann Allergy Asthma Immunol. 2018;120(1):49–52. doi: 10.1016/j.anai.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 10.Sicherer SH, Sampson HA. Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014;133(2):291–307. doi: 10.1016/j.jaci.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 11.Committee on Infectious Diseases. Recommendations for prevention and control of influenza in children, 2016-2017. Pediatrics. 2016;138(4) doi: 10.1542/peds.2016-2527. [DOI] [PubMed] [Google Scholar]
- 12.Committee on Infectious Diseases. Recommendations for prevention and control of influenza in children, 2021-2022. Pediatrics. 2021;148(4) doi: 10.1542/peds.2021-053744. [DOI] [PubMed] [Google Scholar]
- 13.Smith D, Wong P, Gomez R, White K. Ovalbumin content in the yellow fever vaccine. J Allergy Clin Immunol Pract. 2015;3(5):794–795. doi: 10.1016/j.jaip.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 14.Sharma K, Perrett KP, Wood N. Yellow fever vaccination in EGG-allergic children. Pediatr Infect Dis J. 2020;39(6):e76–e78. doi: 10.1097/INF.0000000000002625. [DOI] [PubMed] [Google Scholar]
- 15.YF-Vax Package Insert Sanofi Pasteur Inc., Swiftwater, PA 18370, USA; 2020.
- 16.Cancado B, Aranda C, Mallozi M, Weckx L, Sole D. Yellow fever vaccine and egg allergy. Lancet Infect Dis. 2019;19(8):812. doi: 10.1016/S1473-3099(19)30355-X. [DOI] [PubMed] [Google Scholar]
- 17.Gerhardt CMB, Castro APBM, Pastorino AC, MdB Dorna, CdJ Nunes-Santos, Aquilante BP, et al. Safety of yellow fever vaccine administration in confirmed egg-allergic patients. Vaccine. 2020;38(42):6539–6544. doi: 10.1016/j.vaccine.2020.08.020. [DOI] [PubMed] [Google Scholar]
- 18.Bédard M-A, Graham F, Paradis L, Samaan K, Bégin P, Des Roches A. Single-dose yellow fever vaccination is well tolerated in egg-allergic children despite positive intradermal test to the vaccine. J Allergy Clin Immunol Pract. 2021;9(11):4170–4172.e1. doi: 10.1016/j.jaip.2021.06.050. [DOI] [PubMed] [Google Scholar]
- 19.Kelso JM, Jones RT, Yunginger JW. Anaphylaxis to measles, mumps, and rubella vaccine mediated by IgE to gelatin. J Allergy Clin Immunol. 1993;91(4):867–872. doi: 10.1016/0091-6749(93)90344-f. [DOI] [PubMed] [Google Scholar]
- 20.Sakaguchi M, Ogura H, Inouye S. IgE antibody to gelatin in children with immediate-type reactions to measles and mumps vaccines. J Allergy Clin Immunol. 1995;96(4):563–565. doi: 10.1016/s0091-6749(95)70304-7. [DOI] [PubMed] [Google Scholar]
- 21.Sakaguchi M, Nakayama T, Inouye S. Food allergy to gelatin in children with systemic immediate-type reactions, including anaphylaxis, to vaccines. J Allergy Clin Immunol. 1996;98(6 Pt 1):1058–1061. doi: 10.1016/s0091-6749(96)80191-6. [DOI] [PubMed] [Google Scholar]
- 22.Sakaguchi M, Yamanaka T, Ikeda K, Sano Y, Fujita H, Miura T, et al. IgE-mediated systemic reactions to gelatin included in the varicella vaccine. J Allergy Clin Immunol. 1997;99(2):263–264. doi: 10.1016/s0091-6749(97)70108-8. [DOI] [PubMed] [Google Scholar]
- 23.Kelso JM. The gelatin story. J Allergy Clin Immunol. 1999;103(2 Pt 1):200–202. doi: 10.1016/s0091-6749(99)70490-2. [DOI] [PubMed] [Google Scholar]
- 24.Mullins RJ, James H, Platts-Mills TAE, Commins S. Relationship between red meat allergy and sensitization to gelatin and galactose-α-1,3-galactose. J Allergy Clin Immunol. 2012;129(5):1334–1342.e1. doi: 10.1016/j.jaci.2012.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stone CA, Jr, Hemler JA, Commins SP, Schuyler AJ, Phillips EJ, Peebles RS, Jr, et al. Anaphylaxis after zoster vaccine: implicating alpha-gal allergy as a possible mechanism. J Allergy Clin Immunol. 2017;139(5):1710–1713.e2. doi: 10.1016/j.jaci.2016.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimabukuro TT, Cole M, Su JR. Reports of anaphylaxis after receipt of mRNA COVID-19 vaccines in the US-December 14, 2020-January 18, 2021. JAMA. 2021;325(11):1101–1102. doi: 10.1001/jama.2021.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelso JM. Misdiagnosis of systemic allergic reactions to mRNA COVID-19 vaccines. Ann Allergy Asthma Immunol. 2021;127(1):133–134. doi: 10.1016/j.anai.2021.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krantz MS, Kwah JH, Stone CA, Jr Phillips EJ, Ortega G, Banerji A, et al. Safety evaluation of the second dose of messenger RNA COVID-19 vaccines in patients with immediate reactions to the first dose. JAMA Intern Med. 2021;181(11):1530–1533. doi: 10.1001/jamainternmed.2021.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolfson AR, Robinson LB, Li L, McMahon AE, Cogan AS, Fu X, et al. First-dose mRNA COVID-19 vaccine allergic reactions: limited role for excipient skin testing. J Allergy Clin Immunol Pract. 2021;9(9):3308–3320.e3. doi: 10.1016/j.jaip.2021.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gold MS, MacDonald NE, McMurtry CM, Balakrishnan MR, Heininger U, Menning L, et al. Immunization stress-related response—redefining immunization anxiety-related reaction as an adverse event following immunization. Vaccine. 2020;38(14):3015–3020. doi: 10.1016/j.vaccine.2020.02.046. [DOI] [PubMed] [Google Scholar]
- 31.Greenhawt M, Abrams EM, Oppenheimer J, Vander Leek TK, Mack DP, Singer AG, et al. The COVID-19 pandemic in 2021: avoiding overdiagnosis of anaphylaxis risk while safely vaccinating the world. J Allergy Clin Immunol Pract. 2021;9(4):1438–1441. doi: 10.1016/j.jaip.2021.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hourihane JO, Byrne AM, Blϋmchen K, Turner PJ, Greenhawt M. Ascertainment bias in anaphylaxis safety data of COVID-19 vaccines. J Allergy Clin Immunol Pract. 2021;9(7):2562–2566. doi: 10.1016/j.jaip.2021.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rϋggeberg JU, Gold MS, Bayas J-M, Blum MD, Bonhoeffer J, Friedlander S, et al. Anaphylaxis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25(31):5675–5684. doi: 10.1016/j.vaccine.2007.02.064. [DOI] [PubMed] [Google Scholar]
- 34.Mustafa SS, Ramsey A, Staicu ML. Administration of a second dose of the moderna COVID-19 vaccine after an immediate hypersensitivity reaction with the first dose: two case reports. Ann Intern Med. 2021;174(8):1177–1178. doi: 10.7326/L21-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Interim clinical considerations for use of COVID-19 vaccines currently authorized in the United States. Available at: https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html. Accessed November 1, 2021.
- 36.Gilbert PB, Montefiori DC, McDermott AB, Fong Y, Benkeser D, Deng W, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 2022;375(6576):43–50. doi: 10.1126/science.abm3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wenande E, Garvey LH. Immediate-type hypersensitivity to polyethylene glycols: a review. Clin Exp Allergy. 2016;46(7):907–922. doi: 10.1111/cea.12760. [DOI] [PubMed] [Google Scholar]
- 38.Stone CA, Jr, Liu Y, Relling MV, Krantz MS, Pratt AL, Abreo A, et al. Immediate hypersensitivity to polyethylene glycols and polysorbates: more common than we have recognized. J Allergy Clin Immunol Pract. 2019;7(5):1533–1540.e8. doi: 10.1016/j.jaip.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Troelnikov A, Perkins G, Yuson C, Ahamdie A, Balouch S, Hurtado PR, et al. Basophil reactivity to BNT162b2 is mediated by PEGylated lipid nanoparticles in patients with PEG allergy. J Allergy Clin Immunol. 2021;148(1):91–95. doi: 10.1016/j.jaci.2021.04.032. [DOI] [PubMed] [Google Scholar]
- 40.Klimek L, Novak N, Cabanillas B, Jutel M, Bousquet J, Akdis CA. Allergenic components of the mRNA-1273 vaccine for COVID-19: possible involvement of polyethylene glycol and IgG-mediated complement activation. Allergy. 2021;76(11):3307–3313. doi: 10.1111/all.14794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Warren CM, Snow TT, Lee AS, Shah MM, Heider A, Blomkalns A, et al. Assessment of allergic and anaphylactic reactions to mRNA COVID-19 vaccines with confirmatory testing in a US Regional Health System. JAMA Netw Open. 2021;4(9) doi: 10.1001/jamanetworkopen.2021.25524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hong L, Wang Z, Wei X, Shi J, Li C. Antibodies against polyethylene glycol in human blood: a literature review. J Pharmacol Toxicol Methods. 2020;102 doi: 10.1016/j.vascn.2020.106678. [DOI] [PubMed] [Google Scholar]
- 43.Greenhawt M, Abrams EM, Shaker M, Chu DK, Khan D, Akin C, et al. The risk of allergic reaction to SARS-CoV-2 vaccines and recommended evaluation and management: a systematic review, meta-analysis, GRADE assessment, and international consensus approach. J Allergy Clin Immunol Pract. 2021;9(10):3546–3567. doi: 10.1016/j.jaip.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]