Abstract
Purpose
To report a case of Purtscher-like retinopathy treated with systemic steroids in a young woman with chronic kidney disease.
Observations
An 18-year-old female with a stage 3b chronic kidney disease presented with bilateral, sudden vision loss during an influenza-like syndrome. Best corrected visual acuity (BCVA) was 20/32 bilaterally and fundoscopic examination revealed cotton-wool spots, Purtscher flecken and intraretinal haemorrhages. Flourescein angiography showed areas of retinal ischemia with vascular leakage and optical coherence tomography showed cystoid macular oedema. The patient completed a short-course treatment with high-dose oral steroids. After 1 week, BCVA was 20/20 bilaterally. After 1 month, fundoscopy and imaging evaluation revealed complete resolution of the retinal injury. This favorable outcome remained stable throughout the 1-year follow-up.
Conclusions
AND IMPORTANCE: Purtscher-like retinopathy is a rare, sight-threatening retinal disorder. We described a case of retinal injury presumably related to chronic kidney disease and possibly triggered by an influenza-like syndrome, with a favorable visual recovery.
Keywords: Chronic kidney disease, Influenza-like syndrome, Purtscher-like retinopathy, Retinal microvasculopathy, Systemic steroids
HIGHLIGHTS
-
•
Purtscher-like retinopathy is a rare, sight-threatening retinal disorder.
-
•
There is a possible association to chronic kidney disease.
-
•
It frequently leads to sudden but reversible visual loss.
-
•
Fundoscopy reveals posterior pole cotton-wool spots, haemorrhages and Purtscher flecken.
1. Introduction
Purtscher's retinopathy is a rare retinal disorder. In 1910, the Austrian ophthalmologist Otmar Purtscher was the first to report the occurrence of several areas of retinal whitening, haemorrhage and oedema in a patient complaining of sudden blindness after suffering a severe head trauma.1 Later, a similar retinal pattern, that Purtscher fully defined as angiopathia retinae traumatica in 1912, was found in association with numerous types of trauma.2 Over the past 50 years, the same clinical pattern has been attributed to several systemic non-traumatic conditions; namely acute pancreatitis, fat embolism syndrome and autoimmune and connective tissue disorders.3, 4, 5 The term “Purtscher-like” is the correct designation to describe this retinopathy occurring in non-traumatic situations.
The estimated incidence of Purtscher's and Purtscher-like retinopathies (PuR) is 0.24 cases/million annually and its diagnosis is mainly clinical in the presence of an attributable cause.6 The presentation usually includes sudden vision loss which may occur up to 48 hours following the beginning of the precipitating condition.3 Fundoscopy often reveals multiple cotton-wool spots and retinal haemorrhages restricted to the posterior pole (83–92% of cases) and the pathognomonic Purtscher flecken (50% of cases). Bilateral involvement occurs in 60% of cases.3,4
Currently, the exact pathogenesis remains unclear. PuR acts the same way as an occlusive microvasculopathy with all features suggesting microembolic occlusion of the retinal precapillary arterioles.3,4,7
Apart from the treatment of the underlying etiology, no therapeutic guidelines exist for PuR. A conservative observation-only approach may be a reasonable option with many patients experiencing spontaneous visual acuity and retinal recovery during the first 1–3 months following onset. Isolated cases of successful treatment using high-dose steroids were reported but evidence is scarce to support such treatment routinely.5,8
PuR is a rare, underreported and underdiagnosed condition. The current understanding of pathogenesis and treatment is limited and its low incidence restricts the possibility of clinical trials. Therefore, reporting cases of PuR is needed. From our knowledge, only two authors addressed the uncommon occurrence of PuR in the context of chronic kidney disease (CKD).9,10 This report describes a clinical case of Purtscher-like retinopathy with complete visual recovery in a young woman with stable CKD.
2. Case report
An 18-year-old female presented to our emergency department with sudden, bilateral and painless vision loss with onset 7 days prior. The visual impairment started during an influenza-like syndrome that occurred in the previous 2 weeks. No history of ocular or extra-ocular trauma was recorded nor previous ophthalmological disorders.
This young woman was born with a complex anorectal congenital disorder – cloacal malformation - submitted to a posterior sagittal anorectovaginourethroplasty as a newborn. During childhood, she was followed-up due to several organic disorders such as neurogenic bladder, enterovesical fistula, chronic diarrhoea and severe metabolic acidosis. On account of recurrent urinary tract infections, she developed CKD with a stable stage 3b at the admission - basal creatinine level of 1,6 mg/dL, glomerular filtration rate of 36 ml/min/1,73 m.2 She also presented congenital heart defects, namely an interatrial communication and a patent arterial duct, both submitted to surgical closure during childhood.
We performed a comprehensive ophthalmologic examination, a spectral-domain optical coherence tomography (SD-OCT) (Spectralis®, Heidelberg Engineering, Heidelberg, Germany), a fluorescein angiography (FA) (TRC-50DX®, Topcon Medical Systems, Inc., Tokyo, Japan) and a complete blood test.
Best corrected visual acuity (BCVA) was 20/32 on US equivalent scale for both eyes. The confrontation visual field examination, pupillary light reflexes and ocular motility tests showed no abnormal findings. Anterior segment slit-lamp examination was unremarkable bilaterally and intraocular pressure measured by applanation tonometry was 18 mmHg. Dilated fundoscopic examination revealed several areas of ill-defined retinal whitening located superficially over the vessels corresponding to cotton-wool spots; multiple, discrete polygonal areas of superficial intraretinal whitening with a clear zone on both sides of retinal vessels consistent with pathognomonic Purtscher flecken; minimal flame-shaped and dot-and-blot intraretinal haemorrhages; and scarce areas of arteriolar narrowing (Fig. 1, Fig. 2). All retinal findings were symmetrical between both eyes. Changes were restricted to the posterior pole retina, scattered through the macular region and nasally to the optic disc, with unremarkable peripheral retina. The vitreous body was regular and no optic disc oedema was observed.
Fig. 1.
Colour fundus photographs of both eyes at presentation showing several ill-defined cotton-wool spots (asterisks); multiple, discrete areas of retinal whitening compatible with Purtscher flecken (white arrows); minimal flame-shaped and dot-and-blot intraretinal haemorrhages (black arrows); and scarce areas of arteriolar narrowing. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 2.
Red-free fundus photographs of both eyes at presentation displaying multiple areas of retinal whitening (asterisks) and intraretinal haemorrhages (white arrows).
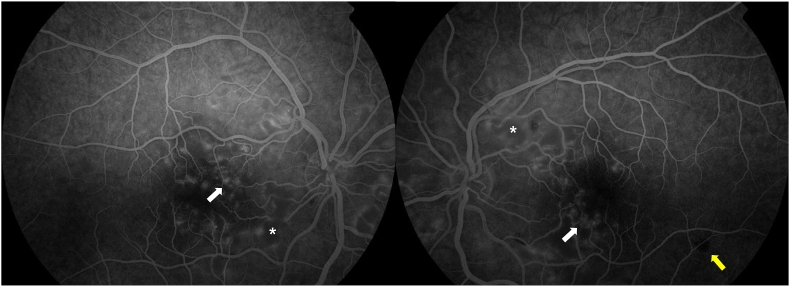
At presentation, FA displayed areas of choroidal hypofluorescence, concealed by the retinal whitening areas or haemorrhages; peripapillary retinal ischemia; late parafoveal leakage; and regular peripheral perfusion (Fig. 3). Optic nerve leakage was not observed. SD-OCT revealed hyperreflective inner retinal layers suggestive of nerve fiber layer ischemia, cystoid macular oedema (central macular thickness of 569 μm), and a subfoveal neurosensorial detachment with a focal disruption of the ellipsoid band (Fig. 4, upper image). Imaging findings were identical between both eyes.
Fig. 3.
Flourescein angiography of both eyes at presentation showing areas of peripapillary retinal ischemia (asterisks), choroidal hypofluorescence (yellow arrow) and late parafoveal leakage (white arrows) with normal peripheral perfusion. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 4.
Spectral-domain optical coherence tomography of the left eye at presentation (top) showing hyperreflective inner retinal layers with classic Purtscher flecken (black arrow), cystoid macular oedema and a subfoveal neurosensorial detachment with a focal disruption of the ellipsoid band. After 1 month (bottom) showing a complete resolution of macular oedema with recovery of foveal depression and preserved photoreceptor's layer.
On admission, the patient was hemodynamically stable with normal blood pressure. Laboratory data failed to indicate an acute systemic disease: a leukocyte count of 12,400/mm3, haemoglobin of 12.0 g/dL and platelet count of 463,000/mm3; blood urea was 97 mg/dL and creatinine level was 1.8 mg/dL (glomerular filtration rate of 31 ml/min/1,73 m2); C-reactive protein was normal with sedimentation rate of 35 mm/h. Infectious diseases screening and autoimmune markers were both negative.
The diagnosis of PuR was assumed based on clinical findings and supported by imaging evaluation. Owing to macular oedema at presentation, we decided to initiate a high-dose systemic steroid - 60 mg of oral prednisolone - for three consecutive days, followed by a slow reduction of 20 mg/day every three days.
One week after the admission, BCVA was 20/20 in both eyes. Fundoscopy revealed a progressive reduction in size and number of cotton-wool spots and Purtscher flecken. SD-OCT displayed a considerable improvement of intraretinal and subretinal fluid.
At 1-month, BCVA was 20/16 bilaterally. Fundoscopy showed resolution of retinal whitening and haemorrhages without any other acute lesions. SD-OCT revealed a complete resolution of macular oedema and neurosensorial detachment with recovery of foveal depression; discrete thinning and disorganization of the inner retinal layers but preserved photoreceptor's layer (Fig. 4, below image). A stable ophthalmological evolution was seen at 6-month and 12-month follow-up visits with no complaints of visual impairment.
3. Discussion
PuR is a rare, sight-threatening condition with no diagnostic and therapeutic recommendations available. The retinal injury classically presents with sudden vision loss, varying from subclinical to severe visual impairment. Visual field defects were also described in up to 90% of cases with peripheral field usually preserved.3,4 However, many authors believe that PuR may frequently be asymptomatic, reason why its real incidence is uncertain. In a systematic review, Miguel et al. established the following diagnostic criteria: presence of Purtscher flecken; presence of flame-shaped or dot-and-blot retinal haemorrhages; presence of cotton-wool spots; reasonable etiology; compatible complementary investigation. Diagnosis of PuR was assumed when at least three of these criteria were met with retinal findings restricted to the posterior pole and absence of direct ocular trauma.4 Our report describes the clinical case of an 18-year-old woman with sudden vision loss and previous history of stage 3b CKD that matches all the aforementioned diagnostic criteria.
Diagnosis of PuR is mainly based in clinical features, however imaging evaluation at presentation supports its assumption. FA may show areas of retinal ischemia, early choroidal hypofluorescence, slower filling of vessels, late leakage, peripapillary staining and precapillary occlusion. Optical coherence tomography may reveal significant retinal oedema.4 All of these features were present in our patient's evaluation, supporting the diagnosis.
It is likely that no single mechanism links all the causes associated with PuR. Clinical appearance suggests an embolic occlusion of the precapillary arterioles by intermediate size emboli as the most accepted pathophysiological mechanism.3 Potential emboli include air, fat emboli, leukocyte aggregates, platelets and fibrin. In a recent study using OCT-angiography, Gil et al. identified multiple areas of capillary non-perfusion in both the superficial and deep retinal plexuses of same distribution as the peripapillary cotton-wool spots, that persisted 5 months later despite complete clearing of fundoscopic findings.7 Moreover, this model respects the anatomical distribution of retinal lesions in PuR. The posterior pole retina is more susceptible to microvascular injury because it is supplied by capillaries with fewer anastomoses when compared to the peripheral retina.10 Activated complement is a common feature for almost all the disorders associated with PuR. It may directly lead to retinal arteriolar occlusion by leukocyte and platelet aggregates or indirectly trigger endothelial injury and clotting cascade initiation. Laboratory studies revealed that complement activation may induce formation of intermediate sized leukocyte aggregates, large enough to cause temporary arteriolar obstruction and classical PuR-related retinal injury.3
Arora et al. (1991) was the first to describe PuR as a cause of sudden, reversible vision loss in a CKD patient under hemodialysis.10 Complement activation with leukocyte aggregation during the exposure of plasma to hemodialysis membranes has been well documented. Later, Stoumbos et al. showed an association of severe PuR with severe CKD and moderate-to-severe anaemia in three young female with late-stage renal injury. Two of these three patients received renal transplants and experienced chronic immune mediated responses of allograft rejection; one of which had been receiving long-term hemodialysis; the other was under immunosuppressive therapy but was not receiving dialysis. The other patient had a familial glomerulonephritis with a recent deterioration of her chronic renal failure, severe leukocytosis, severe anemia and thrombocytosis. Visual loss was severe with only one eye showing a noteworthy recovery. The common feature in these three cases was the end-stage CKD with the severe immune system dysregulation; the authors hypothesized a possible complement activation with leukoembolization of retinal arterioles. Indeed, the majority of cases of PuR associated with CKD previously described in literature occurred in patients with severe chronic renal failure and hemodialysis status or in subjects that underwent renal transplants and experienced chronic allograft rejection. In his paper, Stoumbos et al. also supported that the uremic state and anaemia could make retinal tissue more prone to injury leading to a severe ischemic damage.9
In literature, complement activation was also described for more moderate levels of CKD, in patients not undergoing dialysis.3,9 Components of the immune system mediate many acute forms of renal disease and play a central role in the progression of CKD, including those initiated by nonimmunological mechanisms.11 Compelling clinical and experimental evidence has strongly implicated complement activation as a pivotal pathogenic mediator of progressive renal fibrosis and loss of function.12 Complement proteins can be filtered when glomerular permeability is impaired and then activated within the tubular compartment, contributing to the chronic, non-resolving inflammatory environment in which CKD progresses.13 In a recent study, Jalal et al. have found that the alternative pathway of complement is activated in the plasma of stage III/IV CKD patients with a noteworthy significant increase of plasma complement activation fragments when compared with healthy subjects. Furthermore, patients with moderate CKD also showed increased plasma levels of factor D - the activating enzyme of the alternative pathway.14
Different from the cases reported by Stoumbos et al., neither late-stage renal injury (only stable stage 3 non-immune mediated CKD) nor moderate-to-severe anemia or leukocytosis (a leukocyte count of 12,400/mm3 and hemoglobin level of 12.0 g/dL) were observed in the current case. It is well-stablished that CKD patients are relatively immunocompromised and more susceptible to local and systemic infections that may trigger complement and immunologic activity.15 Since there was no history of decompensation of the previously stable CKD, we hypothesized that the abnormal systemic complement activation may have been exacerbated in the context of the influenza-like syndrome experienced by our patient - 2 weeks prior to the onset of symptoms. This mechanism could lead to leukocyte aggregation and microembolic occlusion of the retinal precapillary arterioles – the same pathomechanism advocated for microvascular occlusive disorders reported with hemodialysis, allograft rejection and immune-related renal disorders. Usually, PuR is observed within some hours to some days after the onset of the systemic disorder. Unfortunately, we could not test the patient for complement and leukocyte aggregates since the visual loss was reported several days after the event.
Current knowledge supports the idea that PuR is a self-limited disorder. With an observation-only approach, the majority of patients recover totally or partially its visual function within few weeks from the onset, with retinal findings showing a slower recovery. However, successful treatment with high-dose steroids has been reported in isolated cases. Visual improvement is attributed to steroids’ ability to stabilize damaged neuronal membranes and microvascular channels, hastening nerve fiber recovery, and to prevent granulocyte aggregation secondary to complement activation. Despite the apparent benefit, further evidence is needed to corroborate the role of steroids in changing the natural history of PuR and support an advantage over observation.4,5
Poor visual improvement may be associated with optic disk swelling, choroidal hypoperfusion, outer retinal damage, macular oedema, pseudo-cherry-red spot and prior episodes of PuR.3 Indeed, acute-stage macular oedema is the foremost reason for decreased visual acuity at presentation. The duration and severity of this acute retinal changes may play a determinant role in late-stage structural recovery. A high rate of late-stage macular atrophy can be found particularly in cases with severe macular oedema, leading to poor visual prognosis. A therapeutic strategy mainly addressed to promptly resolve the acute retinal oedema may prevent atrophic processes of the retinal layers seen in cases of PuR with poor prognosis.8 This approach is according to the current understanding of PuR. Favorable outcomes after a conservative observation-only approach are likely to occur in the majority of cases.8 Nonetheless, some patients will show restricted visual improvement, mainly those with severe visual impairment at presentation associated with macular oedema. In our patient, visual impairment was mild-to-moderate at presentation but OCT showed moderate-to-severe macular oedema. This finding prompted us to take a therapeutic approach with high-dose oral prednisolone. After 1 week, the patient revealed a complete visual recovery with significant reabsorption of intraretinal fluid.
4. Conclusions
This case report offers insight about the possible association of PuR with CKD. We described a case of sudden but reversible visual loss with macular oedema at presentation. Our patient completed a short-course treatment with high-dose oral steroids. After 1 year, no visual impairment was reported and structural evaluation revealed no retinal sequelae.
Ethical approval and patient consent
Appropriate written informed consent was obtained from the patient for images and clinical information reported in this article. Ethical approval was not required at our institution to publish an anonymous case report. Data sharing is not applicable to this article as no datasets were generated during the current report.
Author contributions
Individual contributions to the paper using the relevant CRedIT roles.
Christophe Pinto: Methodology, Validation, Formal analysis, Conceptualization, Data curation, Writing – Original draft preparation, Writing – Reviewing and Editing.
Tiago Fernandes: Data curation, Investigation, Writing – Reviewing and Editing, Supervision, Project Administration.
Petra Gouveia: Writing – Reviewing and Editing, Supervision, Project Administration.
Keissy Sousa: Writing – Reviewing and Editing, Supervision, Project Administration.
Acknowledgements and disclosures
This report received no specific funding. The authors declare no conflicts of interest. All authors attest that they meet the current ICMJE criteria for Authorship.
References
- 1.Purtscher O. Noch unbekannte befunde nach schadeltrauma. Ber Dtsch Ophthalmol Ges. 1910;36:294–301. [Google Scholar]
- 2.Purtscher O. Angiopathia retinae traumatica. Lymphorrhagien des Augengrundes. Graefes Arch Ophthal. 1912;82:347–371. [Google Scholar]
- 3.Agrawal A., McKibbin M.A. Purtscher's and purtscher-like retinopathies. A Review Surv Ophthalmol. 2006;51(2):129–136. doi: 10.1016/j.survophthal.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Miguel A.I.M., Henriques F., Azevedo L.F.R., Loureiro A.J.R., Maberley D.A.L. Systematic review of Purtscher's and Purtscher-like retinopathies. Eye. 2013;27(1):1–13. doi: 10.1038/eye.2012.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xia D., Chen X., Zhou Q., et al. Efficacy of Purtscher's retinopathy treatments: a systematic review. Curr Eye Res. 2017;42(6):908–917. doi: 10.1080/02713683.2016.1255335. [DOI] [PubMed] [Google Scholar]
- 6.Agrawal A., McKibbin M. Purtscher's retinopathy: epidemiology, clinical features and outcome. Br J Ophthalmol. 2007;91(11):1456–1459. doi: 10.1136/bjo.2007.117408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gil P., Raimundo M., Marques J.P., Póvoa J., Silva R. Optical coherence tomography angiography characterization of acute and late stage Purtscher retinopathy. Eur J Ophthalmol. 2018;28(4):NP1–NP6. doi: 10.1177/1120672118769788. [DOI] [PubMed] [Google Scholar]
- 8.Gil P., Pires J., Costa E., Matos R., Cardoso M.S., Mariano M. Purtscher retinopathy: to treat or not to treat? Eur J Ophthalmol. 2015;25(6):e112–e115. doi: 10.5301/ejo.5000623. [DOI] [PubMed] [Google Scholar]
- 9.Stoumbos V.D., Klein M.L., Goodman S. Purtscher’s-like retinopathy in chronic renal failure. Ophthalmology. 1992;99(12):1833–1839. doi: 10.1016/S0161-6420(92)31716-6. [DOI] [PubMed] [Google Scholar]
- 10.Arora N., Lambrou F.H., Jr., Stewart M.W., Vidrine-Parks L., Sandroni S. Sudden blindness associated with central nervous symptoms in a hemodialysis patient. Nephron. 1991;59(3):490–492. doi: 10.1159/000186615. [DOI] [PubMed] [Google Scholar]
- 11.Tecklenborg J., Clayton D., Siebert S., Coley S.M. The role of the immune system in kidney disease: the immune system in kidney disease. Clin Exp Immunol. 2018;192(2):142–150. doi: 10.1111/cei.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen S.-F., Chen M. In: Liu B.-C., Lan H.-Y., Lv L.-L., editors. vol. 1165. Adv Exp Med Biol. Springer; Singapore: 2019. Complement activation in progression of chronic kidney disease; pp. 423–441. (Renal Fibrosis: Mechanisms and Therapies). [DOI] [PubMed] [Google Scholar]
- 13.Fearn A. Complement activation in progressive renal disease. World J Nephrol. 2015;4(1):31. doi: 10.5527/wjn.v4.i1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jalal D., Renner B., Laskowski J., et al. Endothelial microparticles and systemic complement activation in patients with chronic kidney disease. J Am Heart Assoc. 2018;7(14) doi: 10.1161/JAHA.117.007818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Syed-Ahmed M., Narayanan M. Immune dysfunction and risk of infection in chronic kidney disease. Adv Chron Kidney Dis. 2019;26(1):8–15. doi: 10.1053/j.ackd.2019.01.004. [DOI] [PubMed] [Google Scholar]