Abstract
Background
Ovarian cancer is a life-threatening disease with a high mortality rate in women. Our previous work presented that long non-coding RNA (lncRNA) activated by transforming growth factor beta (TGF-β) (lncRNA ATB) played a role of oncogene in ovarian cancer. However, whether exosomal lncRNA ATB from ovarian cancer cells could regulate the tumorigenesis of ovarian cancer remains unclear.
Methods
RT-qPCR assay was performed to evaluate the level of lncRNA ATB in cancer cells (SKOV3 and A2780). In addition, ovarian cancer cells-secreted exosomes were collected with ultracentrifugation. CCK8 assay was performed to detect the viability of ovarian cells and HUVECs. Meanwhile, Western blot was performed to detect the expression of mechanism related protein and tube formation assay was used to observe the angiogenesis of HUVECs. Finally, xenograft mice model was used to verify the role of ovarian cancer cell-derived exosomes in vivo.
Results
Ovarian cancer cells-derived exosomes promoted the viability, angiogenesis and migration of HUVECs; however, knockdown of lncRNA ATB in HUVECs reversed these phenomena. In addition, exosomal lncRNA ATB promoted the tumorigenesis of ovarian cancer via regulating miR-204-3p/TGFβR2 axis. Furthermore, ovarian cancer cells-secreted exosomal lncRNA ATB increased tumor growth in vivo.
Conclusion
Exosomal lncRNA ATB derived from ovarian cancer cells could improve tumor microenvironment via regulating miR-204-3p/TGFβR2 axis. Thus, this study might provide new knowledge for the treatment of ovarian cancer.
Keywords: ovarian cancer, exosome, lncRNA ATB, TGFβR2, tumor microenvironment
Introduction
Ovarian cancer is a malignant tumor disease occurring in the ovaries,1 and this disease can occur at any age.2 For example, germ ovarian cancer is common in women under the age of 20,3 while borderline tumors tend to occur in women between the ages of 30 and 40.4 Overall, the vast majority of ovarian cancer occurs in women over age 50.5 At present, the main treatments for malignant ovarian cancer including surgery, chemotherapy, targeted therapy, endocrine therapy and radiotherapy.6,7 Although multimodality approaches were implemented, 5-year survival rate of patient with ovarian cancer is less than 50%.8 Since the etiology of ovarian cancer is still not clear, and may be related to aging, genetic factors and endocrine disorders,9 the treatment of ovarian cancer remains challenging. Therefore it is very necessary to explore the pathogenesis of ovarian cancer.
Exosomes originally referred to discoidal vesicles with a diameter of 40~100 nm that could be released into extracellular matrix.10 Exosomes carrying a large number of biomolecules including proteins, lipids, nucleic acids (microRNAs (miRNAs), messenger RNAs (mRNAs), long non-coding RNAs (lncRNAs), circular RNAs (circRNAs), DNA, etc.) and could be transferred to other cells or tissues in the circulatory system.11 This ability endow exosomes with a variety of biological functions including information transmission between cells, regulation of tumor growth and migration, etc.12 For example, Feng et al reported that tumor cells could secrete a large number of exosomes; these exosomes could improve the distant tumor microenvironment for tumor metastasis by regulating intercellular communication between ovarian cancer cells and cancer-associated fibroblasts, as well as other cells.13,14 In other words, exosomes from tumor cells improve the local and distant microenvironment to a large extent.13
Tumor microenvironment refers to the surrounding microenvironment in which tumor cells exist.15 The tumor microenvironment consists of surrounding blood vessels, non-tumor cells (tumor-associated macrophages, human umbilical vein endothelial cells (HUVECs), extracellular matrix and so on.16 In addition, it has been reported that tumor microenvironment could directly affect the proliferation and migration ability of cancer cells.17 In our previous research, knockdown of lncRNA activated by transforming growth factor beta (TGF-β) (lncRNA ATB) could notably inhibit the proliferation and viability via inducing the apoptosis of ovarian cancer cells. Moreover, we found lncRNA ATB promote the tumorigenesis of ovarian cancer cells in vitro and in vivo by regulating miR-204-3p/Nidogen 1 axis.18 In the current study, we aimed to explore the relationship between exosomal lncRNA ATB and tumor microenvironment in ovarian cancer.
Materials and Methods
Cell Culture
Human umbilical vein endothelial cells and ovarian cancer cell lines SKOV3 and A2780 were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured in RPMI-1640 supplemented with 2 mM Glutamine (Sigma Aldrich, St. Louis, MO, USA) and 10% FBS at 37°C with 5% CO2. ITD-1 was purchased from MedChemExpress (Monmouth Junction, NJ, USA).
Cell Transfection
Previously we showed that lncRNA ATB was highly expressed in SKOV3 and A2780 cells,18 vector-control (ctrl), lncRNA ATB small interfering RNA (siRNA) 1 and lncRNA ATB siRNA 2 were transfected into SKOV3 and A2780 cells using Lipofectamine® 2000 (Thermo Fisher Scientific, Waltham, MA, USA). Vector-ctrl sequences: GAGGTTGAATAAGAAGAACCTCACA; lncRNA ATB siRNA 1 sequences: CCACAGCTGGTAATGTGCATAGTCT; and lncRNA ATB siRNA 2 sequences: CAGGTACTGTGAGTGCTCACCTGAT. All these siRNAs were obtained from RiboBio (Guangzhou, China).
MiR-204-3p agomir and miR-204-3p agomir control (GenePharma, Shanghai, China) were transfected into HUVECs with Lipofectamine® 2000 as well.
Reverse Transcription-Quantitative PCR (RT-qPCR)
Total RNAs were separated from SKOV3, A2780 and HUVEC cells with TRIZOL reagent (Invitrogen, Carlsbad, CA, USA). In addition, cDNA was synthesized using an EntiLink™ 1st Strand cDNA Synthesis Kit (ELK Biotechnology, Wuhan, China). Next, RT-qPCR was performed using with a StepOne™ Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA, USA). β-actin was worked as internal control for lncRNA ATB. The following primers were used: β-actin, Forward: 5ʹ-GTCCACCGCAAATGCTTCTA-3ʹ, reverse: 5ʹ-TGCTGTCACCTTCACCGTTC-3ʹ; lncRNA ATB, forward: 5ʹ-GAGGCTGGTTGACATGCCTT-3ʹ, reverse: 5ʹ-GAGCATCTCTGGGTGCTGGT-3ʹ. Data was analyzed by the 2−ΔΔCt method.
Cell Counting Kit-8 (CCK8) Assay
HUVECs were seeded to 96 well plates (5×103 cells per well) and treated with exosomes that were derived from SKOV3 or A2780 cells (SKOV3 Exo or A2780 Exo) for 24 h. Then, cells were cultured in RPMI-1640 medium and treated with 10 μL CCK8 reagent at 37°C for 2 h. Next, a microplate reader (Bio-Rad, Hercules, CA, USA) was used to measure the optical density at 450 nm. CCK8 solution was provided by Dojindo (Kumamoto, Japan).
Exosomes Isolation, Characterization, Labeling and Uptake
SKOV3 and A2780 cells were cultured in RPMI-1640 medium for 48 h. Then, exosomes were separated from SKOV3 or A2780 cells by ultracentrifugation. The details are as follows: RPMI-1640 medium was enriched and centrifuged at 650 g for 15 min. Next, to continue collecting exosomes from medium, the sample was centrifuged at 155,00 g for 20 min at 4°C. Then a 0.22 μm filter membrane (Millipore, Billerica, MA, USA) was used to filter the sample, followed by centrifuged at 120,000 g for 60 min at 4°C. After washing with 100 μL PBS, the exosomes were enriched. The concentrations of exosomes used in the current study were 50 μg/mL in vitro and 10 mg/kg in vivo, respectively.
Transmission electron microscopy (TEM) was performed to observe the morphology of exosomes. Firstly, 2% paraformaldehyde was used to fix exosomes. Then, phosphotungstic acid (2%) was used to stain exosomes for 2 min. Finally, a TEM (TEM, Hitachi HT7700, Tokyo, Japan) was applied to observe the results of staining. Nanoparticle tracking analysis (NTA) instrument (ZetaView, Particle Metrix, Meerbusch, Germany) was performed to measure the size of exosomes.
Regarding as exosomes uptake, PKH26 labeled-exosomes were cultured with HUVECs for 0, 1, 2, 4 h. Nystatin or heparin was used to inhibit exosomes uptake. Cytoskeleton was stained using fluorescent phalloidin dye (Thermo Fisher Scientific). Finally, a fluorescence microscope (Olympus CX23, Tokyo, Japan) was used to observe the results of staining.
Western Blot Assay
HUVECs were seeded to 6 well plates (2×105 cells per well) and treated with ovarian cancer-derived exosomes with or without lncRNA siRNA2. Then, HUVECs were collected in RIPA lysis buffer (ASPEN Biotechnology CO., LTD, Wuhan, China) and was lysed in the culture plate for 5 min. Next, the protein concentrations were quantified by The BCA protein assay kit (Beyotime, Shanghai, China). Proteins samples (30 μg/lane) were separated by 10% SDS–PAGE, followed by transferred onto PVDF membranes (Millipore). After that, the membranes were incubated with primary antibodies of CD81 (1:1000, Abcam, Cambridge, MA, USA, cat. no. ab109201), TSG101 (1:1000, Abcam, cat. no. ab125011), p-Smad2 (1:1000, Abcam, cat. no. ab280888), Smad2 (1:1000, Abcam, cat. no. ab33875), E-cadherin (1:1000, Abcam, cat. no. ab231303), N-cadherin (1:1000, Abcam, cat. no. ab76011) and β-actin (1:1000, Abcam, cat. no. ab8226) at 4°C overnight. On the following day, the membrane was washed with TBST for three times and incubated with corresponding secondary antibodies for 1 h at room temperature. Finally, an enhanced chemiluminescence (ECL) reagent (Millipore) was used to visualize the bands.
Tube Formation Assay
Matrigel-coated 24-well transwell (8 μm pore size, Corning, New York, NY, USA) was used to perform tube formation assay. HUVECs were cultured into the matrigel-coated well at a density of 1×105 cells at 37°C with 5% CO2. Then, the cells were treated with ovarian cancer cells-derived exosomes with or without lncRNA siRNA2 for 24 h. Finally, a microscope was used to observe the results of tube formation.
Transwell Assay
HUVECs migration ability was detected by transwell assay. HUVECs were added to the upper chamber supplemented with 100 µL serum-free RPMI-1640 medium. Meanwhile, 600 μL complete RPMI-1640 medium was added to the lower chamber. HUVECs migrated from the upper chamber to the lower chamber after 24 h of incubation. Then, 4% paraformaldehyde was used to fix HUVECs located in the lower chamber. Next, 0.1% crystal violet solution was used to stain the HUVECs for 10 min. Finally, a microscope was used to observe the results of staining.
Wound Healing Assay
HUVECs were seeded to 6 well plates (5×105/cell). After a monolayer of cells had covered the surface of the medium, parallel scratches were made in each hole with 200 μL pipette tips. Next, the floating cells were washed off with PBS and images were taken with a microscope at 0 and 24 h.
Dual-Luciferase Reporter Assay
The pGL6-miR‐based luciferase reporter plasmids containing wild‐type (WT) TGFβR2 3ʹUTR or mutated‐type (MT) TGFβR2 3ʹUTR at the putative binding sites of miR-204-3p were purchased from by Sangon Biotech (Shanghai, China). The luciferase reporter plasmids together with miR-204-3p agomir NC or miR-204-3p agomir (10 nM) were transfected into HUVECs using with Lipofectamine® 2000 for 48 h. Then, the Dual Luciferase Reporter Assay System (Beyotime) was performed to detect luciferase activity.
In vivo Model of Animals
A total of 18 BALB/nude mice (6 weeks old) were obtained from Vital River (Beijing, China). These mice were housed in a specific-pathogen-free (SPF) animal facility at 24 ± 2°C constant temperature with 60% humidity. SKOV3 cells (5×106; 100 μL) were injected into mouse subcutaneously. Tumor size (mm3) was measured weekly according to the equation: (length×width^2)/2. When the tumor volume research about 200 mm3, SKOV3 Exo or A2780 Exo (10 mg/kg) were injected into mice via the tail vein twice weekly. At the end of study, animals were sacrificed and the tumors were collected and pictured. Subsequently, the weight of each tumor was measured. All animal procedures were approved by Taizhou People’s Hospital. The National Institute of Health Guide for the Care and Use of Laboratory Animals was strictly followed.
Immunohistonchemistry (IHC) Staining
IHC staining was performed to detect the relative level of CD31 and p-Smad2. Firstly, 4% paraformaldehyde was used to fix tumors tissues for 10 min. Next, primary antibodies (anti-CD31 (1:1000, Abcam, cat. no. ab182981); p-Smad2 (1:1000, Abcam, cat. no. ab280888)) were incubated with the samples. Then, corresponding secondary antibodies (Goat anti-rabbit IgG antibody (1:1000, Abcam, cat. no. ab150077)) were incubated with the samples. Finally, a microscope was used to observe the results of staining.
Statistical Analysis
The data were analyzed via GraphPad Prism software (version 7.0, La Jolla, CA, USA). All of the data are presented as the mean ± standard deviation (SD). The differences between multiple means were analyzed by One-way analysis of variance (ANOVA), followed by Tukey’s tests. A statistically difference was indicated as *P < 0.05.
Results
Knockdown of lncRNA ATB Inhibits the Viability of Ovarian Cancer Cells
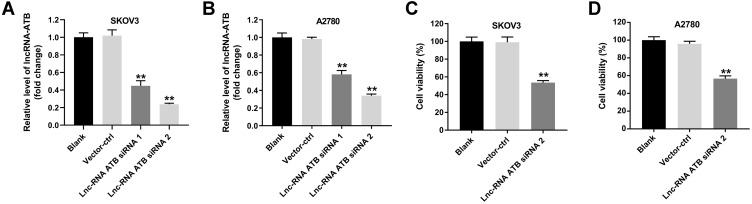
In our previous research, the results showed that lncRNA ATB was highly expressed in ovarian cancer cells.18 To verify the efficiency of lncRNA ATB siRNAs, RT-qPCR assay was performed to evaluate the level of lncRNA ATB in SKOV3 or A2780 cells. As revealed in Figure 1A and B, lncRNA ATB siRNA 1 and lncRNA ATB siRNA 2 obviously downregulated the level of lncRNA ATB in ovarian cancer cells, compared with the control group. Since siRNA 2 exhibited better inhibitory effect on lncRNA ATB expression, siRNA 2 was selected of use in the following experiment. In addition, lncRNA ATB siRNA 2 significantly inhibited the viability of ovarian cancer cells (Figure 1C and D). All in all, lncRNA ATB knockdown notably inhibited the viability of ovarian cancer cells.
Figure 1.
Knockdown of lncRNA ATB inhibits the viability of ovarian cancer cells. (A and B) Vector-control (ctrl), lncRNA ATB siRNA 1 and lncRNA ATB siRNA 2 (20 nM) were transfected into SKOV3 and A2780 cells using Lipofectamine® 2000 for 24 h. RT-qPCR assay was performed to evaluate the level of lncRNA ATB in SKOV3 or A2780 cells. (C and D) CCK8 assay was performed to detect the viability of ovarian cancer cells. **P < 0.01 compared with blank group; n = 3.
Ovarian Cancer Cells-Derived Exosomes are Transferred into HUVECs
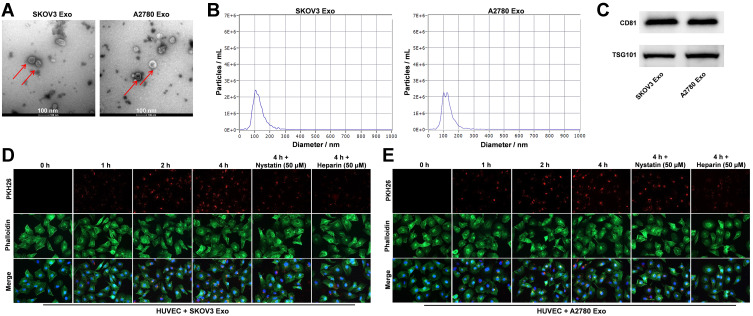
Evidence has shown that tumor cells-secreted exosomes could influence the tumor microenvironment.13 In order to explore the relationship between exosomal lncRNA ATB and tumor microenvironment, ovarian cancer cells-derived exosomes were collected in the current study. Exosome characterization was performed with TEM, NTA and Western blotting assays. As indicated in Figure 2A and B, ovarian cancer cells-derived exosomes were small vesicles approximately 30–150 nm in diameter with a typical lipid bilayer structure. Western blot data indicated that the levels of CD81 and TSG101 (exosome markers) were highly expressed in SKOV3 Exo and in A2780 Exo (Figure 2C). These results revealed that ovarian cancer cells-derived exosomes were successfully collected. Additionally, it has been reported that exosomes secreted from cancer cells could be transported to HUVECs.19 Therefore, we next investigate whether exosomes secreted from ovarian cancer cells could be transported to HUVECs. As showed in Figure 2D and E, PKH26 labeled exosomes were absorbed by HUVECs in a time dependent manner, and these phenomena were notably reversed in the presence of nystatin or heparin. To sum up, ovarian cancer cells-secreted exosomes were collected and transferred into HUVECs.
Figure 2.
Ovarian cancer cells-derived exosomes are transferred into HUVECs. (A and B) Exosomes were isolated from SKOV3 or A2780 cells and exosome characterization was performed with TEM, NTA. (C) Western blot was performed to detect the levels of CD81 and TSG101 in SKOV3 Exo or in A2780 Exo. (D and E) Exosomes uptake by HUVECs was detected using PKH26 dye. Red color: exosome; green color: HUVECs. N = 3.
Exosomal lncRNA ATB Derived from Ovarian Cancer Cells Promotes the Viability and Angiogenesis of HUVECs
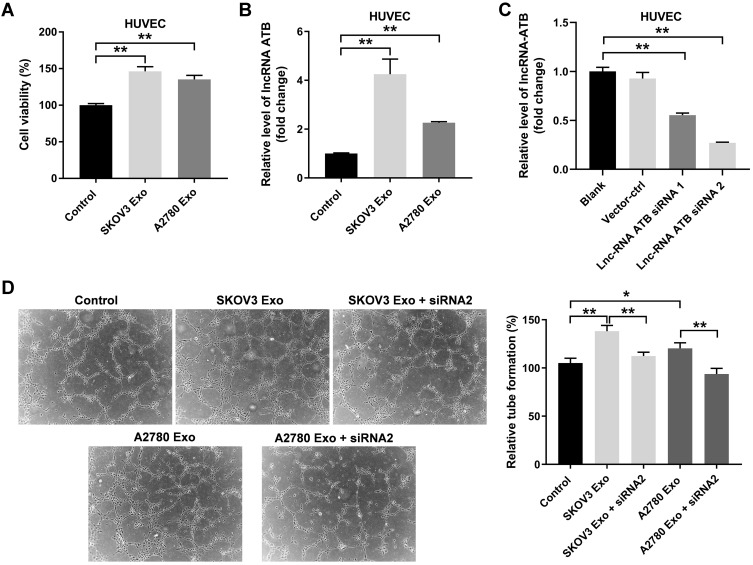
Next, to investigate the effect of ovarian cancer cells-secreted exosomes on HUVECs, CCK8 assay was performed. As indicated in Figure 3A, the viability of HUVECs was significantly enhanced by SKOV3 Exo or A2780 Exo. Meanwhile, SKOV3 Exo or A2780 Exo exposure significantly increased the expressions of lncRNA ATB in HUVECs (Figure 3B). Expectedly, lncRNA ATB siRNA2 remarkably decreased the level of lncRNA ATB in HUVECs that were treated with SKOV3 Exo or A2780 Exo (Figure 3C). In addition, SKOV3 Exo or A2780 Exo significantly promoted the tube formation of HUVECs; however, these phenomena were reversed in the presence of lncRNA ATB siRNA2 (Figure 3D). All these data suggested that exosomal lncRNA ATB derived from ovarian cancer cells could promote the viability and angiogenesis of HUVECs.
Figure 3.
Exosomal lncRNA ATB derived from ovarian cancer cells promotes the viability and angiogenesis of HUVECs. HUVECs were treated with ovarian cancer cells-derived exosomes with or without lncRNA ATB siRNA for 24 h. (A) CCK8 assay was performed to detect the viability of HUVECs. (B and C) RT-qPCR assay was performed to evaluate the level of lncRNA ATB in HUVECs. (D) A microscope was used to observe the result of tube formation. *P < 0.05, **P < 0.01; n = 3.
Exosomal lncRNA ATB Derived from Ovarian Cancer Cells Promotes the Migration of HUVECs
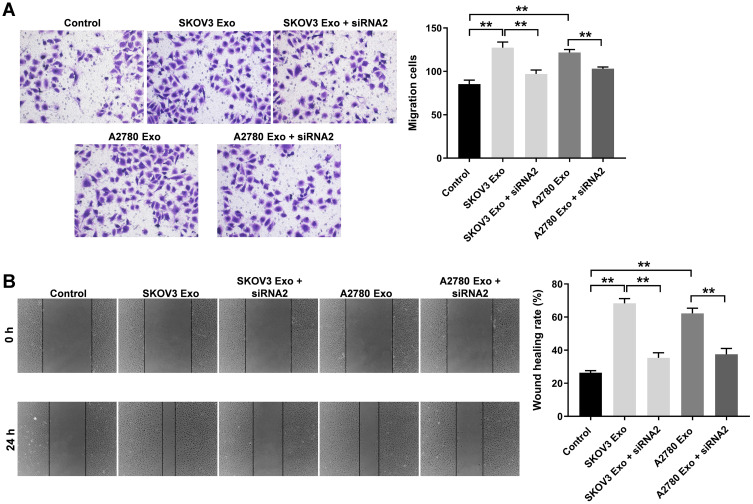
To explore the effect of ovarian cancer cells-secreted exosomes on the migration of HUVECs, transwell migration and wound healing assays were used. The results showed that SKOV3 Exo or A2780 Exo significantly increased the migration ability of HUVECs, compared with the control group. In consistently, these phenomena were notably prevented after transfection of lncRNA ATB siRNA2 (Figure 4A and B). In addition, co-culture of ovarian cancer cells and HUVECs experiment was carried out accordingly. The result indicated the co-culture remarkably promoted the migration and viability of HUVECs, whereas these promotions were completely reversed by lncRNA ATB siRNA2 (Supplementary Figure 1A–C). All these data indicated that exosomal lncRNA ATB derived from ovarian cancer cells promoted the migration of HUVECs.
Figure 4.
Exosomal lncRNA ATB derived from ovarian cancer cells promotes the migration of HUVECs. HUVECs were treated with ovarian cancer cells-derived exosomes with or without lncRNA ATB siRNA for 24 h. (A and B) Transwell and wound healing assays were performed to detect the migration of HUVECs. **P < 0.01; n = 3.
Exosomal lncRNA ATB Derived from Ovarian Cancer Cells Changes the Expression of Epithelial-Mesenchymal Transformation Markers in HUVECs via Regulating miR-204-3p/TGFβR2 Axis
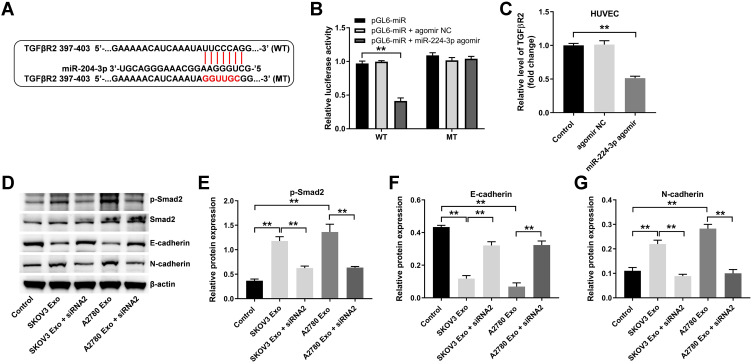
Our previous work presented that miR-204-3p was a direct target of lncRNA ATB.18 To explore the mechanism by which exosomal lncRNA ATB regulated tumor microenvironment, the potential target genes of miR-204-3p was identified by three online bioinformatics databases (TargetScan, miRDB, and miRWalk). There online bioinformatics databases commonly suggested TGFβR2 might be a potential target of miR-204-3p (Figure 5A). In addition, previous research suggested that TGFβR2 plays a crucial role in ovarian cancer.20–22 Therefore, we focused on exploring the relationship between TGFβR2 and miR-204-3p. As indicated in dual luciferase assay, miR-204-3p agomir markedly inhibited the activity of HUVECs harboring WT TGFβR2, but had no effect on cells containing MT TGFβR2 (Figure 5B). Meanwhile, miR-204-3p agomir markedly inhibited the level of TGFβR2 in HUVECs, compared with the control group (Figure 5C).
Figure 5.
Exosomal lncRNA ATB derived from ovarian cancer cells changes the expression of a few EMT markers in HUVECs via regulating miR-204-3p/TGFβR2 axis. (A) Three online bioinformatics databases (Targetscan, miRDB, miRWalk) were used to predict the target genes of miR-204-3p. (B) MiR-204-3p agomir and miR-204-3p agomir NC were transfected into HUVECs with Lipofectamine® 2000. Dual-luciferase reporter assay was used to confirm the binding of miR-204-3p and TGFβR2. (C) RT-qPCR assay was performed to evaluate the level of TGFβR2 in HUVECs. (D–G) Western blot was performed to detect the levels of p-Smad2, Smad2, E-cadherin and N-cadherin in HUVECs. **P < 0.01; n = 3.
Next, TGFβ signaling pathway associated proteins (p-Smad2 and Smad2) and epithelial-mesenchymal transformation (EMT) associated markers (E-cadherin and N-cadherin) were detected by Western blotting.23,24 As indicated in Figure 5D–G, SKOV3 Exo or A2780 Exo obviously increased the expressions of p-Smad2 and N-cadherin, and decreased the level of E-cadherin in HUVECs, while these changes were markedly reversed by lncRNA ATB siRNA2. Collectively, exosomal lncRNA ATB derived from ovarian cancer cells could change the expression of EMT markers in HUVECs via regulating miR-204-3p/TGFβR2 axis.
Exosomal lncRNA ATB Derived from Ovarian Cancer Cells Promote the Migration of HUVECs via Regulating miR-204-3p/TGFβR2
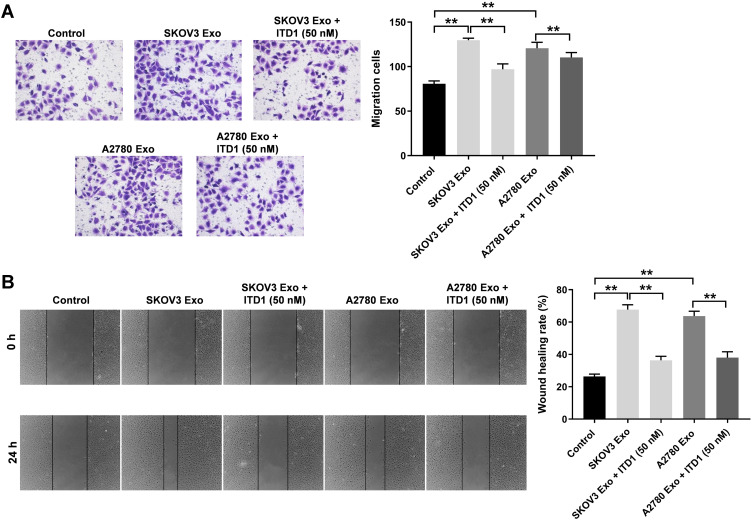
It has been reported that ITD-1 could initiate the degradation of TGF-β receptors, thereby blocking TGF-β signaling.25 In order to further investigate the role of TGFβR2 on cell migration, rescue experiments were performed. As revealed in Figure 6A and B, SKOV3 Exo or A2780 Exo notably promoted the migration of HUVECs, while there effects were revered by ITD-1. These data confirmed that exosomal lncRNA ATB derived from ovarian cancer cells promoted the migration of HUVECs via regulating miR-204-3p/TGFβR2 axis.
Figure 6.
Exosomal lncRNA ATB derived from ovarian cancer cells promote the migration of HUVECs via regulating miR-204-3p/TGFβR2. HUVECs were treated with ovarian cancer cells-derived exosomes with or without 10 μM ITD1 for 24 h. (A and B) Transwell and wound healing assays were performed to detect the migration of HUVECs. **P < 0.01; n = 3.
Exosomal lncRNA ATB Derived from Ovarian Cancer Cells Promote Tumor Growth in vivo
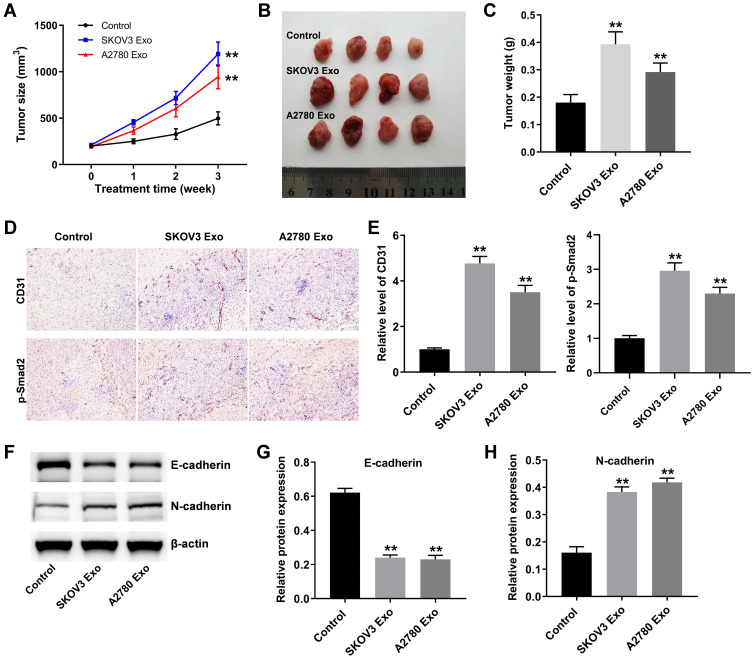
Finally, to investigate the function of ovarian cancer cells-derived exosomes in tumorigenesis, ovarian xenograft mouse model was established. As indicated in Figure 7A–C, SKOV3 Exo or A2780 Exo markedly promoted the tumor volume and tumor weight in mice, compared with the control group. Moreover, IHC staining results showed that ovarian cancer cells-derived exosomes significantly increased the levels of CD31 and p-Smad2 in tumor tissues (Figure 7D and E). Meanwhile, SKOV3 Exo or A2780 Exo obviously decreased the expression of E-cadherin and increased the level of N-cadherin in tumor tissues (Figure 7F–H). These data suggested that exosomal lncRNA ATB derived from ovarian cancer cells could promote tumor growth in vivo.
Figure 7.
Exosomal lncRNA ATB derived from ovarian cancer cells promote tumor growth in vivo. (A) Tumor volume was measured weekly. (B and C) In the end of study, the tumors were pictured and weighted. (D and E) IHC staining was performed to evaluate the level of CD31 and p-Smad2 in tumor tissues. (F–H) Western blot was used to detect the levels of E-cadherin and N-cadherin in tumor tissues. **P < 0.01 compared with control group; n = 6.
Discussion
In recent years, exosomes have become the focus of research. Tumor cell-derived exosomes can alters tumor microenvironment and promote tumorigenesis.26 For example, the expression of epithelial ovarian cancer (EOC) serum derived exosome lncRNA aHIF significantly promotes ovarian cancer development.27 We have previously demonstrated that lncRNA-ATB promotes the tumorigenesis of ovarian cancer via binding miR-204-3p.18 Here, the relationship between ovarian cancer cells-derived exosomes and tumor microenvironment was investigated as well. The result showed that SKOV3 Exo or A2780 Exo significantly increased the expression of lncRNA ATB in HUVECs. These results are consistent with previous studies which reported that exosomes from different tumor cells (leukemia, myeloma, breast cancer, osteosarcoma, etc.) could promote the proliferation and metastasis of tumor cells by transmitting genetic information (protein, microRNA, lncRNA, etc.).28 These phenomena indicate that these exosomes can mediate exchange of information and material between cells by carrying a variety of biologically active substances such as lncRNA.
As we know, mesenchymal stem cells (MSCs), macrophages and HUVECs constitute the tumor microenvironment, which affects the migration and growth of tumors.29 The outcomes of current study showed that exosomal lncRNA ATB derived from ovarian cancer cells promoted the viability, angiogenesis and migration of HUVECs; however, knockdown of lncRNA ATB reversed these phenomena. A previous study suggested that exosomal miR-205 isolated from ovarian cancer cells could manage the information transmission between ovarian cancer cells and HUVECs and promote the metastasis of ovarian cancer cells.30 Different from this study, we found lncRNA ATB could be transferred into HUVECs via exosome and alters tumor microenvironment.
It must be admitted that there are some limitations in the current study. For example, the mechanism by which exosomal lncRNA ATB regulates the development of ovarian cancer has not been thoroughly studied. In addition to miR-204-3p, lncRNA ATB alters tumor microenvironment might binding other miRNAs. In addition, the effect of exosomal lncRNA ATB on macrophage polarization remains unclear and more experiments are needed to be performed.
Based on the outcomes of our previous work and current study, lncRNA ATB directly promote the development of ovarian cancer. In addition, exosomal lncRNA ATB derived from ovarian cancer cells could improve tumor microenvironment via regulating miR-204-3p/TGFβR2 axis and thereby promotes tumorigenesis of ovarian cancer.
In conclusion, knockdown of lncRNA ATB not only directly inhibit the tumorigenesis of ovarian cancer, but also inhibit the angiogenesis in the tumor microenvironment. These data may provide new knowledge for the treatment of ovarian cancer.
Funding Statement
There is no funding to report.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding authors on reasonable request.
Ethics Approval and Consent to Participate
All animal procedures were approved by Taizhou People’s Hospital. The National Institute of Health Guide for the Care and Use of Laboratory Animals was strictly followed.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, agreed to the submitted journal, and agreed to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest for this work.
References
- 1.Webb PM, Jordan SJ. Epidemiology of epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol. 2017;41:3–14. doi: 10.1016/j.bpobgyn.2016.08.006 [DOI] [PubMed] [Google Scholar]
- 2.Prat J, D’Angelo E, Espinosa I. Ovarian carcinomas: at least five different diseases with distinct histological features and molecular genetics. Hum Pathol. 2018;80:11–27. doi: 10.1016/j.humpath.2018.06.018 [DOI] [PubMed] [Google Scholar]
- 3.Veneris JT, Mahajan P, Frazier AL. Contemporary management of ovarian germ cell tumors and remaining controversies. Gynecol Oncol. 2020;158(2):467–475. doi: 10.1016/j.ygyno.2020.05.007 [DOI] [PubMed] [Google Scholar]
- 4.Spanheimer PM, Murray MP, Zabor EC, et al. Long-term outcomes after surgical treatment of malignant/borderline phyllodes tumors of the breast. Ann Surg Oncol. 2019;26(7):2136–2143. doi: 10.1245/s10434-019-07210-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roett MA, Evans P. Ovarian cancer: an overview. Am Fam Physician. 2009;80(6):609–616. [PubMed] [Google Scholar]
- 6.Guan LY, Lu Y. New developments in molecular targeted therapy of ovarian cancer. Discov Med. 2018;26(144):219–229. [PubMed] [Google Scholar]
- 7.Paleari L, DeCensi A. Endocrine therapy in ovarian cancer: where do we stand? Curr Opin Obstet Gynecol. 2018;30(1):17–22. doi: 10.1097/GCO.0000000000000423 [DOI] [PubMed] [Google Scholar]
- 8.Zhang T, Zhang L, Li F. Integrative network analysis identifies potential targets and drugs for ovarian cancer. BMC Med Genomics. 2020;13(Suppl 9):132. doi: 10.1186/s12920-020-00773-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doubeni CA, Doubeni AR, Myers AE. Diagnosis and management of ovarian cancer. Am Fam Physician. 2016;93(11):937–944. [PubMed] [Google Scholar]
- 10.Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367:6478. doi: 10.1126/science.aau6977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalluri R. The biology and function of exosomes in cancer. J Clin Invest. 2016;126(4):1208–1215. doi: 10.1172/JCI81135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azmi AS, Bao B, Sarkar FH. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev. 2013;32(3–4):623–642. doi: 10.1007/s10555-013-9441-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L, Yu D. Exosomes in cancer development, metastasis, and immunity. Biochim Biophys Acta Rev Cancer. 2019;1871(2):455–468. doi: 10.1016/j.bbcan.2019.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng W, Dean DC, Hornicek FJ, Shi H, Duan Z. Exosomes promote pre-metastatic niche formation in ovarian cancer. Mol Cancer. 2019;18(1):124. doi: 10.1186/s12943-019-1049-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arneth B. Tumor microenvironment. Medicina. 2019;56(1). doi: 10.3390/medicina56010015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai H, Gong L, Liu J, Zhou Q, Zheng Z. Diosgenin inhibits tumor angiogenesis through regulating GRP78-mediated HIF-1α and VEGF/VEGFR signaling pathways. Die Pharmazie. 2019;74(11):680–684. doi: 10.1691/ph.2019/9526 [DOI] [PubMed] [Google Scholar]
- 17.Zhang D, Ding L, Li Y, et al. Midkine derived from cancer-associated fibroblasts promotes cisplatin-resistance via up-regulation of the expression of lncRNA ANRIL in tumour cells. Sci Rep. 2017;7(1):16231. doi: 10.1038/s41598-017-13431-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan D, Qian H, Guo T, et al. LncRNA-ATB promotes the tumorigenesis of ovarian cancer via targeting miR-204-3p. Onco Targets Ther. 2020;13:573–583. doi: 10.2147/OTT.S230552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen X, Zhang S, Du K, et al. Gastric cancer-secreted exosomal X26nt increases angiogenesis and vascular permeability by targeting VE-cadherin. Cancer Sci. 2021;112(5):1839–1852. doi: 10.1111/cas.14740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu J, Zhang L, Mei Z, et al. Interaction of E3 ubiquitin ligase MARCH7 with long noncoding RNA MALAT1 and autophagy-related protein ATG7 promotes autophagy and invasion in ovarian cancer. Cell Physiol Biochem. 2018;47(2):654–666. doi: 10.1159/000490020 [DOI] [PubMed] [Google Scholar]
- 21.Christie M, Oehler MK. Molecular pathology of epithelial ovarian cancer. J Br Menopause Soc. 2006;12(2):57–63. doi: 10.1258/136218006777525794 [DOI] [PubMed] [Google Scholar]
- 22.Abedini Bakhshmand E, Mohammad Soltani B, Fasihi A, Mowla SJ. Hsa-miR-5582-3P regulatory effect on TGFβ signaling through targeting of TGFβ-R1, TGFβ-R2, SMAD3, and SMAD4 transcripts. J Cell Biochem. 2018;119(12):9921–9930. doi: 10.1002/jcb.27314 [DOI] [PubMed] [Google Scholar]
- 23.Sanada TJ, Sun XQ, Happé C, et al. Altered TGFβ/SMAD signaling in human and rat models of pulmonary hypertension: an old target needs attention. Cells. 2021;10:1. doi: 10.3390/cells10010084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Odero-Marah V, Hawsawi O, Henderson V, Sweeney J. Epithelial-mesenchymal transition (EMT) and prostate cancer. Adv Exp Med Biol. 2018;1095:101–110. [DOI] [PubMed] [Google Scholar]
- 25.Sun Z, Ettensohn CA. TGF-β sensu stricto signaling regulates skeletal morphogenesis in the sea urchin embryo. Dev Biol. 2017;421(2):149–160. doi: 10.1016/j.ydbio.2016.12.007 [DOI] [PubMed] [Google Scholar]
- 26.Baig MS, Roy A, Rajpoot S, et al. Tumor-derived exosomes in the regulation of macrophage polarization. Inflamm Res. 2020;69(5):435–451. doi: 10.1007/s00011-020-01318-0 [DOI] [PubMed] [Google Scholar]
- 27.Tang X, Liu S, Liu Y, et al. Circulating serum exosomal aHIF is a novel prognostic predictor for epithelial ovarian cancer. Onco Targets Ther. 2019;12:7699–7711. doi: 10.2147/OTT.S220533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kok VC, Yu CC. Cancer-derived exosomes: their role in cancer biology and biomarker development. Int J Nanomed. 2020;15:8019–8036. doi: 10.2147/IJN.S272378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang C, Kim HS, Song G, Lim W. The potential role of exosomes derived from ovarian cancer cells for diagnostic and therapeutic approaches. J Cell Physiol. 2019;234(12):21493–21503. doi: 10.1002/jcp.28905 [DOI] [PubMed] [Google Scholar]
- 30.Zhang H, Lu B. microRNAs as biomarkers of ovarian cancer. Expert Rev Anticancer Ther. 2020;20(5):373–385. doi: 10.1080/14737140.2020.1760095 [DOI] [PubMed] [Google Scholar]