Abstract
Background
Self-medication with antibiotics is being practiced worldwide with high prevalence, mostly in developing countries. Several factors induce the practice of self-medication, such as irrational and uncontrolled dispensing of medicinal substances, difficulty accessing health-care systems, and cost of diagnosis. Thus, this study assessed the prevalence of self-medication with antibiotics, and its associated factors among the community of Bule-Hora town, South West Ethiopia.
Methods
A community-based cross-sectional study design was used. All households residing in Bule Hora town were used as source population and households in the selected kebeles were included by using a systematic random sampling method. Eight hundred twenty-six study participants were selected for the study. Pre-tested structured questionnaires had been used to collect the required data. Then the collected data were checked for completeness and analyzed by using SPSS version 20. Odds ratios with 95% C.I. were used to measure the association between independent variables and outcome and variables with p-value <0.05 had been considered statistically significant.
Results
Prevalence of self-medication with antibiotics in the past 12 months prior to the data collection was found to be 38.9% [95% CI (1.56, 1.64)]. Being male (AOR = 1.53; 95% CI: 0.489, 0.869) with p value of 0.004, no health insurance scheme (AOR = 2.16; 95% CI: 0.274, 0.779) and availability of some drugs in shop (AOR = 12.98; 95% CI: 0.017, 0.353) with p value of 0.001 were found to be significantly associated with self-medication of antibiotics.
Conclusion
The study revealed that more than one-third of the respondents practiced self-medication. Availability and irrational dispensing of some drugs in the shops were significantly associated with self-medication practice. Therefore, it is important to educate society on the appropriate use of drugs and discourage the use of prescription drugs without medication order.
Keywords: antibiotics, prevalence, self-medication, Bule-Hora
Background
Self-medication (SM) is a practice of taking medicinal agents for self-diagnosed diseases without prior visits or consultation of health-care practitioners.1 Currently, SM is being practiced worldwide with high prevalence. Such excessive use of SM is considered as one of the major health and socio-economic challenges, particularly in developing countries.2 In these countries, although, the type, extent, and reasons for SM practice vary from place to place, the most common factors which persuade the practice of SM are irrational and uncontrolled dispensing of medicinal substances, difficulty to access health-care systems, and the costs of laboratory diagnosis as well as medical consultations.2–4
In developing countries, both over-the-counter and prescription-only medicinal products are commonly consumed for SM, without any control.5–8 People are using them inappropriately; specially prescription-only drugs such as antibiotics.9,10 This predisposes people to the risk of developing resistance as reported by WHO.11
Nowadays, the use of antibiotics without prescription and professional advice is becoming common in the world leads to many consequences including the global emergence of multidrug-resistant pathogens, concealing severe and potentially deadly diseases, the danger of the wrong diagnosis, selection an inappropriate dosage, drug interactions, side effect, and adverse drug reactions.11,12 Treatment failures with antibiotics will also bring about prolonged contagion, which further increases the communicable disease burden in the community and thus expose the entire population to the danger of acquiring a resistant strain of bacteria and pathogens like Multi-Drug Resistant TB which are becoming global problems.13,14
Furthermore, the practice of SM with antimicrobial agents has been linked to a delayed diagnosis of several disorders and ultimately affects the final results of the treatments.15 These might result in extra burden and increased health-care spending in an already underprivileged population.16,17 Despite its effectiveness in primary health care if appropriately utilized, SM with antibiotics has resulted in inappropriate drug use such as taking either high or low doses, lesser or longer periods of taking the medicines. These irrational uses of drugs are complicating the patient’s condition.18,19
The high prevalence in the practice of SM requires urgent attention, as medicines comprise different chemicals which have both beneficial and harmful effects on human health.20 These harmful effects include; dangerous drug interactions when different interacting drugs have been combined, severe adverse drug reactions such as anaphylactic shock, inflammatory bowel syndrome which involves the gastrointestinal system organs, damage to the kidneys, drug intoxication to the liver.
So, comprehensive information is needed to solve this by understanding the burden and associated factors related to SM. Thus, the objective of this study was to determine the prevalence of SM practice and associated factors among Households in BuleHora town, West Guji Zone.
Methods
Study Design and Study Area
A community-based cross-sectional study was conducted in Bule-Hora town. The town is located 467 km south of Addis Ababa, Ethiopia, from September to October 2019. Bule-Hora town is the capital of West Guji Zone; Bule-Hora town has an altitude of 1716 meters above sea level and latitude and longitude of 5°35ʹN, 38°15ʹE/5.583°N, 38.250°E/5.583; 38.250. It has three kebeles and a total surface area of 132,703.19 hectares. According to the city population Centre, it has a total population of 27,820 in the last census year, 2007 E.C. Bule-Hora town has one General hospital, one health center, and three health posts, 17 private clinics within different organizations, and 13 drug stores.
Population
In the current study, all households residing in Bule-Hora town were used as a source population. During the above investigation period, there were a total of 11,788 households according to the information obtained from the city administration of Bule-Hora. In this study systematically selected household’s family members whose age is above 18-years, who are not seriously ill, and who can give response to the study queries were included in the study population.
Sample Size Determination
The sample size was determined by using single population proportion by considering the following assumptions: prevalence of SM practice 24.4% (0.244) of the study, conducted in Silte zone, Southern Ethiopia,21 95% confidence interval, Z statistic value of 1.96, and margin of error 3% (0.03); non-response rate which is 5% was added to the calculated sample considered. The number of households to be involved in the study was determined to be 787. The total sample size (n) was then determined to be 826 which is the sum of the calculated value (787 households) and the 5% non-response rate (39 households).
Sampling Procedure
The three kebeles along with their respective households’ numbers were considered as a frame to select sample households. The lists were obtained from respective kebele administration offices. The proportional probability to size sampling method was used to assign proportional sample size for each study kebeles. Then, systematic sampling technique was used after determining the interval by dividing the number of all households in Bule-Hora town by the final sample size then, every Kth who are voluntarily participate was interviewed according to their sequence of house numbers. Whenever two or more eligible respondents were found in the same household, only one of them was selected randomly and included in the study.
Data Collection Procedure
A face-to-face interview with an eligible respondent based on structured pre-tested questionnaires had been done in selected households that were at home during data collection. The adopted questionnaire was used to accommodate all required data. The questionnaires were converted to Amharic and Afan Oromo, then back to the English language by a language expert to ensure consistency. The questionnaire was prepared in five sections: Section A contained the sociodemographic characteristics of respondents; Section B contained questions on self-medication with antibiotics; Section C contained questions on the source of commonly used drugs for self-medication; Section D contained questions on reasons for self-medication and section E contained questions on factors that influence the practice of self-medication. Ten health-care providers, who had previous practice on data gathering were selected and assigned as an interviewer. Three senior pharmacists were assigned as supervisors. Two-day training has been offered by the principal investigator to the data collectors and the supervisors to familiarize themselves with the questionnaires and on data collection techniques. Then the survey form was pre-tested on 5% of the sample size out of the study population before data collection. After informed consent was obtained from the participants, background information and possible factors were collected in a form of a questionnaire. Data on socio-demographic and socioeconomic details (age, gender, educational status, ethnicity, religion, and revenue), the practice of SM with antibiotics and causes of the use of SM, attitude towards SM practices were collected.
Data Quality Control
The process of identification and elimination of any data anomalies has been done through the following measures to ensure that the data to be comprehensive, dependable, correct, repeatable, and consistent using the same methods. Training for data collectors and supervisors was given to qualify them to get the basic skills needed for data gathering and management. A pre-tested data collection tool had been used to get the required information. Necessary adjustments were made before data collection and necessary support was provided to supervisors on a random basis. Brief encounters were held to deal with issues and give clarity on issues that could hinder the gathering of good data with the supervisor found to have problems. An English version questionnaire was translated into Amharic and Afan Oromo languages to make the questions clear and easily understandable for respondent(s).
Data Management and Analysis
Each questionnaire was filled and checked for exhaustiveness before data entry. Then, data were entered and cleaned with EPI-data version 4.3 to minimize errors after coding and will be transported to SPSS software version 20 for analysis. Descriptive statistics namely, frequencies, percentage, cross-tabulation were carried out. Odds ratios with 95% CI were used to measure the association and statistical significance of socio-demographic and other independent variables related to self-medication practices related to antibiotics.
Ethical Considerations
An approval letter to conduct research was obtained from Bule-Hora University, ethical review board via submission of the study protocol before the study commences. The official letters were submitted to the three kebele administrations. Data was gathered after getting permission. Additionally, each respondent was informed about the objective of the survey. The data collectors have discussed the issue of confidentiality and asked for consent before starting data collection and respondents were enlightened that they have full right to decline or discontinue participating in the research. Then, verbal informed consent was obtained from study participants. In this study, verbal informed consent was used after approved by the ethics review committee and the study was conducted in accordance with the Declaration of Helsinki.
Dissemination and Utilization of Results
The results of the study were presented to the Bule-Hora University and to those who need this result and consumed for community health education. A manuscript write-up was undertaken and sent for peer review on international reputed journals.
Result
Socio-Demographic Characteristics
Out of the 826 respondents who participated in this study, 57.9% were males. Majorities (40.6%) of respondents were in the age group of 18–24 years and 57.3% of them were married. As shown in Table 1, most of the respondents (43.8%) had college/university level of education, and 28.6% (95% CI (1.56, 1.69)) of them were classified as middle class according to wealth index analysis.
Table 1.
Socio-Demographics of Study Participants
| Variables | Frequency | Percentage |
|---|---|---|
| Gender | ||
| Male | 478 | 57.9 |
| Female | 348 | 42.1 |
| Age | ||
| ˂24 | 335 | 40.6 |
| 25–34 | 234 | 28.3 |
| 35–44 | 172 | 20.8 |
| ˃45 | 85 | 10.3 |
| Marital Status | ||
| Single | 321 | 38.9 |
| Married | 473 | 57.3 |
| Divorced | 15 | 1.8 |
| Widowed | 11 | 1.3 |
| Separated | 6 | 0.7 |
| Educational level | ||
| Illiterate | 110 | 13.3 |
| Primary | 137 | 16.6 |
| Secondary | 217 | 26.3 |
| College/University | 362 | 43.8 |
| Occupation | ||
| Unemployed | 396 | 47.9 |
| Employed | 203 | 24.6 |
| Business Person | 197 | 23.8 |
| Others | 30 | 3.6 |
| Religion | ||
| Christian | 542 | 65.6 |
| Muslim | 228 | 27.6 |
| Atheist | 31 | 3.8 |
| Others | 25 | 3.0 |
Prevalence of Self-Medication with Antibiotics
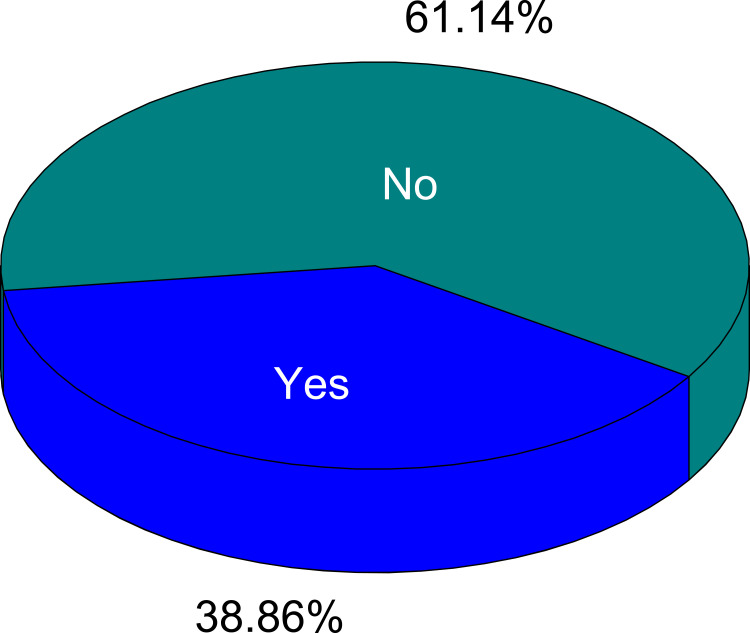
The prevalence of SM with antibiotics in the last 12 months before the data gathering was found to be 38.9% [95% CI (1.56, 1.64)]. The median of self-medication practice was 2 (IQR = 2), the maximum was eight times and most of the respondents exercised once or twice during the twelve months (Figure 1).
Figure 1.
Frequency of self-medication with antibiotics.
Perceived Complaints About Self-Medication with Antibiotics
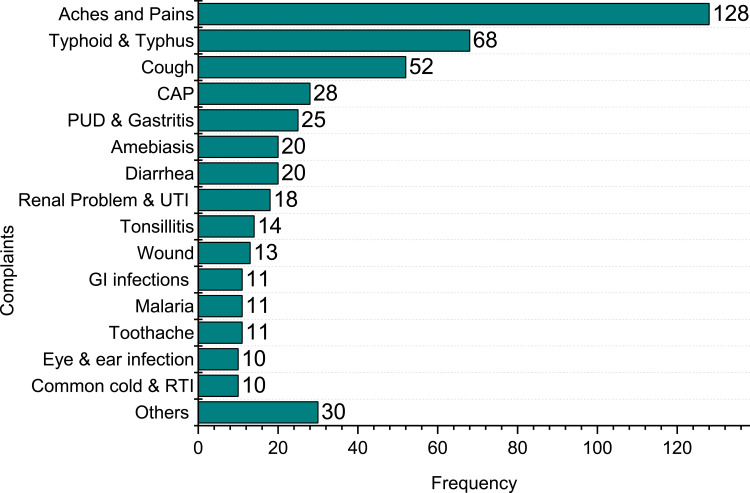
SM with antibiotics was practiced for aches and pains (15.5%), typhoid and typhus (8.1%), cough (6.3%), community-acquired pneumonia (CAP) (3.4%), diarrhea (4.3%), amebiasis (2.5%), tonsillitis (1.7%), wound (1.5) etc. (Figure 2).
Figure 2.
Frequency of chief complaints for which antibiotic was used for self-medication. Others include anaemia, arthritis, asthma, cellulitis, nasal congestion, skin infection.
Reason for Self-Medication with Antibiotics
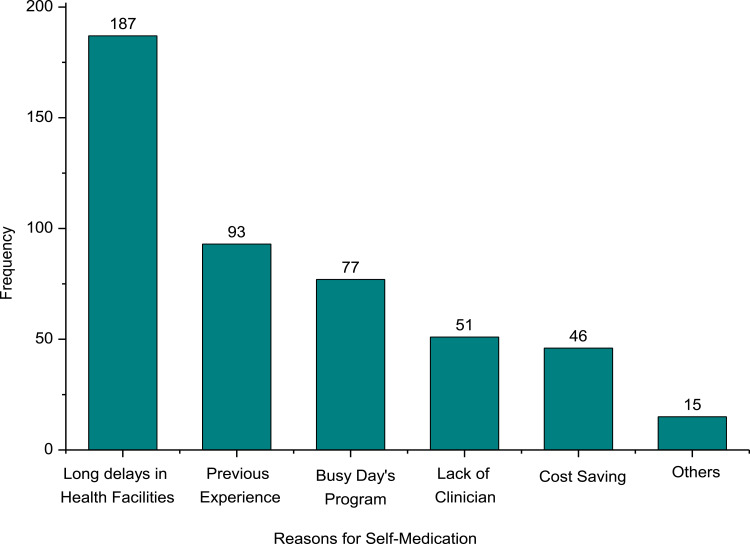
The main reasons for SM with antibiotics were prolonged waiting to get service in health institutions (39.9%), medical treatment of the previous similar symptoms (19.8%), and lack of time to visit health institutions (16.4%) (Figure 3).
Figure 3.
Reasons for self-medication with antibiotic.
Most Frequently Used Antibiotics and Their Sources
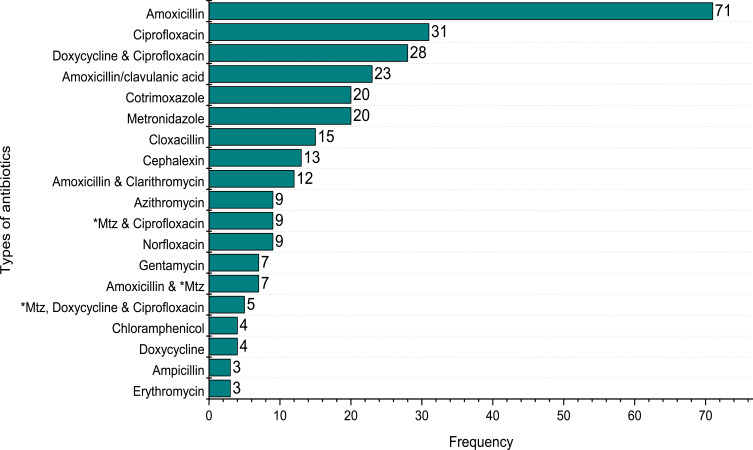
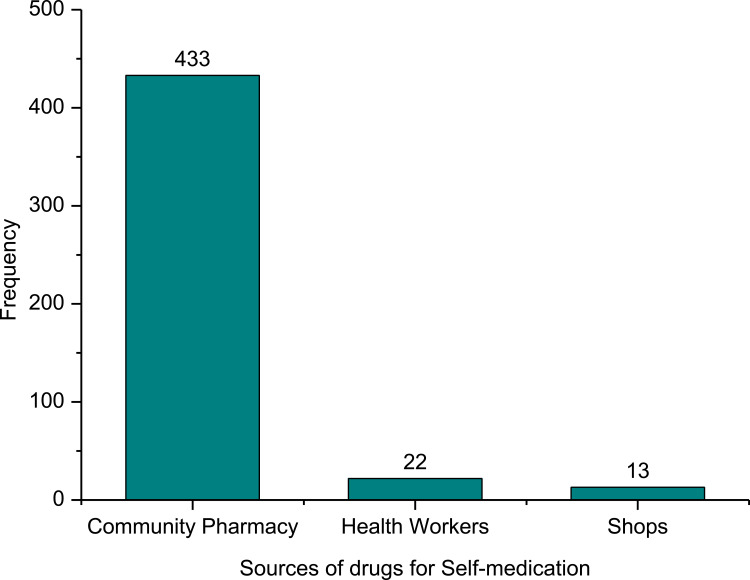
Of those respondents who self-medicated, 22.1% used amoxicillin at least once in the 12 months. It was followed by ciprofloxacin (9.7%), doxycycline and ciprofloxacin (8.7%), co-trimoxazole (6.2%), metronidazole (6.2%), etc (Figure 4). Antimicrobials used for SM were procured mainly from community pharmacies (dispensaries) (92.3%), health workers (4.7%), friends, and/or relatives (2.8%) (Figure 5). Study respondents obtained information for the practice of the antibiotics among others were medicine dispensers (70.8%), previous doctor’s prescription (25.6%), friends (3.6%).
Figure 4.
Types of antibiotics used for self-medication. (*Mtz = Metronidazole).
Figure 5.
Sources of supply of drugs for self-medication with antibiotics at Bule-Hora town.
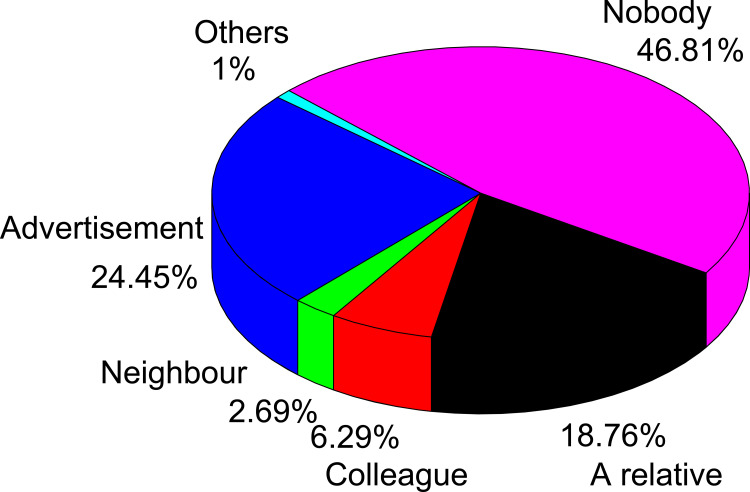
Places of Information for Self-Medication
In this survey, the most common places from which drug information was obtained were self-decision without any information (46.81%), followed by promotion about medicine (24.45%), relatives (18.75%), colleagues (6.29%), and neighbors (2.69%) (Figure 6).
Figure 6.
Sources of information for self-medication with an antibiotic.
Factors Associated with Self-Medication Practices
Being male (AOR = 1.53; 95% CI: 0.489, 0.869) with p value of 0.004, no health insurance scheme (AOR = 2.16; 95% CI: 0.274, 0.779) and availability of some drugs in shop (AOR = 12.98; 95% CI: 0.017, 0.353) with p value of 0.001 were found to be significantly associated with SM of antibiotics.
Discussion
This study aimed to assess the prevalence of SM with antibiotics and associated factors among households at Bule-Hora town, Oromia regional state, Ethiopia. In our survey, the prevalence of self-medication with antibiotics in the past 12 months was 38.86%.
This finding is in line with other studies carried out elsewhere in the world like Thailand (37.37%), Sri Lanka (39%), India (39.4%),27 Cameron (41.9%), and Beirut (42%).28 This result is also similar to studies carried out in different parts of Ethiopia such as Jijiga (37.5%).25 On the other hand, the prevalence of SMP in the current finding was lower than studies done in different parts of Ethiopia such as Gondar town (52.2%),23 and Kolladiba (62.8%)24 and higher than studies carried out in Bahir Dar (12.8%),29 and Worabe (16.9%).22 The variation in the prevalence of SM with antibiotics might be due to the differences in socio-demographic factors such as educational status, socioeconomic status, living status, community mindfulness, and sample sizes. Moreover, according to Mathewos et al 2021, it is not easy to compare the prevalence of SM across different studies due to the varying nature of definitions used, recall period considered, region selected and methodology adopted.30,32
In the current study, the main perceived illnesses that led them to exercise SM with antibiotics were aches/pains followed by typhoid and typhus, and cough/cold. There is a similarity between this study and other studies conducted in various parts of Ethiopia.23,25 The main driving reasons for SM with antibiotics were a prior experience of medical treatment of the same symptoms; long delays in a health facility; easy accessibility of drug retail outlets; and more time-saving. These reasons are consistent with studies conducted in Addis Ababa,33 Mekelle,34 and Mozambique.35
SM with antibiotics is being a usual exercise among the respondents. According to this study, amoxicillin was found to be the most frequently self-medicated antibiotic and followed by ciprofloxacin, doxycycline, and cotrimoxazole. Similar findings were also reported in the previous studies done in the countries like Indonesia, Pakistan, Bangladesh, and Mozambique.28,36,37 The great use of amoxicillin may be due to its inexpensiveness and easy accessibility. It may be a widely known antibiotic to the community compared to other antibiotics. The sources of these antibiotics were found to be the pharmacy retail outlets and health workers.
In this study, it has been revealed that antibiotics are inappropriately used to treat aches and pains as well as cough and the common cold. SM using multiple antibiotics was also observed. Such irrational and irresponsible use of antibiotics for the SM may lead to increased risk of mistreatment, size effect, worsening of infectious disease, development of antibiotic resistance, and limitation of therapeutic choices.26,38,39
Our study indicated that the odds of SM with antibiotics among widowed participants were 5.74 times higher than the odds of married participants. The higher malpractice of SM by widowed participants could be due to peer pressure. The influence of peers on SM was also reported by studies carried out elsewhere in Ethiopia like Gondar Town23 and Meket District.31 The odds of SM with antibiotics among households who were influenced by neighbors were 1.798 times higher than that of the study participants who did not influence by their neighbors. The possible explanation might be due to sharing of their previous practices among them.
In this finding, the odds of SM with antibiotics among study participants who had access to shop were 12.98 times higher compared with the participants who had no access. The possible reason might be due to lack of time to visit health institutions in general and health-care professionals in particular. Therefore, the SM practices may be reduced by improving awareness of the community about the quality of health-care services, teaching the danger of SM with antibiotics and regular supervision and regulation of community pharmacies services.
Conclusion
This study revealed that more than one-third of the study participants practiced SM. According to the study results, amoxicillin was found to be the most frequently used antibiotic for SM. Factors like no insurance scheme, availability of some drugs in a shop, advice by neighbors, and long busy working days were significantly associated with SM practice. Therefore, SM with antibiotics without prescription should be discouraged and hence health-care professionals should counsel the community to refrain from such use and raise awareness of the consequences of inappropriate use of antibiotics. Besides, drug retail outlets should be regulated to decrease the practice. From the foregoing, it is evident that the integrated effort of individuals, communities, health facilities, and the regulatory bodies is crucial to reducing the practice of SM in the community.
Acknowledgment
The authors are thankful to Bule-Hora University for sponsoring this study.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Albarrán KF, Zapata LV. Analysis and quantification of self-medication patterns of customers in community pharmacies in southern Chile. Pharm World Sci. 2008;30:863–868. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. The role of the pharmacist in self-care and self-medication: report of the 4th WHO Consultative Group on the Role of the Pharmacist, The Netherlands; 1998. Available from: https://apps.who.int/iris/handle/10665/65860. Accessed December 13, 2019.
- 3.Novignon J, Mussa R, Msonda T, Nonvignon J. The use of non-prescription medicine versus self- assessed health: evidence from Malawi. Int Arch Med. 2011;4(1):38. doi: 10.1186/1755-7682-4-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elseviers M, Wettermark B, Almarsdóttir AB, et al. Drug utilization research methods and applications. Can J Hosp Pharm. 2017;70(4):325. [Google Scholar]
- 5.Sharma A, Madaan A, Nagappa AN. Medication storage and self-medication practice among the youth in Karnataka region, India. Int J Pharm Sci Res. 2012;3(8):2795–2800. [Google Scholar]
- 6.Abay SM, Amelo W. Assessment of self-medication practices among medical, pharmacy, and health science students in Gondar University, Ethiopia. J Young Pharm. 2010;2(3):306–310. doi: 10.4103/0975-1483.66798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shankar PR, Partha P, Shenoy N. Self-medication and non-doctor prescription practices in Pokhara valley, Western Nepal: a questionnaire-based study. BMC Fam Pract. 2002;3:17. doi: 10.1186/1471-2296-3-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kafle KK, Madden JM, Shrestha AD, et al. Can licensed drug sellers contribute to safe motherhood? A survey of the treatment of pregnancy related anemia in Nepal. Soc Sci Med. 2006;42:1577–1588. doi: 10.1016/0277-9536(95)00294-4 [DOI] [PubMed] [Google Scholar]
- 9.Fuentes K, Villa Z. Analysis and quantification of self-medication patterns of customers in community pharmacies in southern Chile. Pharm World Sci. 2008;30:863–868. [DOI] [PubMed] [Google Scholar]
- 10.Balamurugani E, Ganesh K. Prevalence and pattern of self medication use in coastal regions of South India. Br J Med Pract. 2011;4(3):a438. [Google Scholar]
- 11.Skliros E, Merkouris P, Papazafiropoulou A, et al. Self-medication with antibiotics in rural population in Greece: a cross-sectional multicenter study. BMC Fam Pract. 2010;11:58. doi: 10.1186/1471-2296-11-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaghaghi A, Asadi M, Allahverdipou H. Predictors of self medication behavior: a systematic review. Iranian J Public Health. 2014;43(2):136–146. [PMC free article] [PubMed] [Google Scholar]
- 13.Donkor ES, Tetteh-Quarcoo PB, Nartey P, Agyeman IO. Self-medication practices with antibiotics among tertiary level students in Accra, Ghana: a cross-sectional study. Int J Environ Res Public Health. 2012;9(10):3519–3529. doi: 10.3390/ijerph9103519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Awad A, Eltayeb I, Matowe L, Thalib L. Self-medication with antibiotics and antimalarials in the community of Khartoum State, Sudan. J Pharm Pharm Sci. 2005;8(2):326–331. [PubMed] [Google Scholar]
- 15.MOH. National Guidelines for cancer Management in Kenya; 2013. Available from: https://knh.or.ke/wp-content/uploads/2017/08/National-Cancer-Treatment-Guidelines2.pdf. Accessed December 13, 2019.
- 16.Zafar S, Syed R, Waqar S, et al. Self-medication amongst University Students of Karachi: prevalence, knowledge and attitudes. J Med Assoc Stud Corner. 2008;58(4):214–217. [PubMed] [Google Scholar]
- 17.Zafar SN, Syed R, Waqar S, et al. Self-medication amongst university students of Karachi: prevalence, knowledge and attitudes. J Pak Med Assoc. 2008;58(4):214–217. [PubMed] [Google Scholar]
- 18.Nokes K, Prince J, Achieng R. (2000). Children and medicines: self-treatment of common illnesses among Luo schoolchildren in western Kenya. Soc Sci Med. 2000;50(12):1771–1783. doi: 10.1016/S0277-9536(99)00428-1 [DOI] [PubMed] [Google Scholar]
- 19.Ali SE, Ibrahim MI, Palaian S. Medication storage and self-medication behaviour amongst female students in Malaysia. Pharm Pract (Granada). 2010;8(4):226–232. doi: 10.4321/S1886-36552010000400004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tillement JP, Delaveau P. Automédication et sécurité [Self-medication and safety]. Bull Acad Natl Med. 2007;191(8):1517–1526. [PubMed] [Google Scholar]
- 21.Omolase CO, Adeleke OE, Afolabi AO, Afolabi OT. Self-medication amongst general outpatients in a Nigerian Community Hospital. Ann Ib Postgrad Med. 2007;5(2):64–67. doi: 10.4314/aipm.v5i2.64032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wabe NT, Ahmed D, Angamo MT. Self-medication with antibiotics and antimalarials in the community of Silte Zone, South Ethiopia. Taf Prev Med Bull. 2012;11(5):529–536. doi: 10.5455/pmb.1-1314892446 [DOI] [Google Scholar]
- 23.Jember E, Feleke A, Debie A, Asrade G. Self-medication practices and associated factors among households at Gondar town, Northwest Ethiopia: a cross-sectional study. BMC Res Notes. 2019;12(1):153. doi: 10.1186/s13104-019-4195-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kassa F, Abrha S, Melkam W. Self-medication practice: the case of Kolladiba Town, North West Ethiopia. Int J Pharm Sci Res. 2014;5:670–677. [Google Scholar]
- 25.Amaha MH, Alemu BM, Atomsa GE. Self-medication practice and associated factors among adult community members of Jigjiga town, Eastern Ethiopia. PLoS One. 2019;14(6):e0218772. doi: 10.1371/journal.pone.0218772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ayalew MB. Self-medication practice in Ethiopia: a systematic review. Patient Prefer Adherence. 2017;11:401–413. doi: 10.2147/PPA.S131496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nepal G, Bhatta S. Self-medication with antibiotics in WHO Southeast Asian Region: a systematic review. Cureus. 2018;10(4):e2428. doi: 10.7759/cureus.2428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aslam A, Gajdács M, Zin CS, et al. Evidence of the practice of self-medication with antibiotics among the lay public in low-and middle income countries: a scoping review. Antibiotics. 2020;9:597. doi: 10.3390/antibiotics9090597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mihretie TM Self-Medication Practices with Antibiotics among Urban Dwellers of Bahir Dar Town, North West Ethiopia [master’s thesis]. Addis Ababa: Addis Ababa University; 2014. [Google Scholar]
- 30.Mathewos T, Daka K, Bitew S, Daka D. Self-medication practice and associated factors among adults in Wolaita Soddo town, Southern Ethiopia. Inter J Infection Control. 2021;17(1):20322. doi: 10.3396/ijic.v17.20322 [DOI] [Google Scholar]
- 31.Kassie AD, Bifftu BB, Mekonnen HS. Self-medication practice and associated factors among adult household members in Meket district, Northeast Ethiopia. BMC Pharmacol Toxicol. 2018;19:15. doi: 10.1186/s40360-018-0205-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torres NF, Chibi B, Middleton LE, Solomon VP, Mashamba-Thompson TP. Evidence of factors influencing self-medication with antibiotics in low and middle-income countries: a systematic scoping review. Public Health. 2019;168:92–101. doi: 10.1016/j.puhe.2018.11.018 [DOI] [PubMed] [Google Scholar]
- 33.Tenaw A, Tsige G-M. Self-medication practices in Addis Ababa: a prospective study. Ethiop J Health Sci. 2004;14(1):1–11. [Google Scholar]
- 34.Eticha T, Mesfin K. Self-medication practices in Mekelle, Ethiopia. PLoS One. 2014;9(5):e97464. doi: 10.1371/journal.pone.0097464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Torres NF, Solomon VP, Middleton LE. Patterns of self-medication with antibiotics in Maputo City: a qualitative study. Antimicrob Resist Infect Control. 2019;8:1–12. doi: 10.1186/s13756-019-0618-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Torres NF, Solomon VP, Middleton LE. Identifying the commonly used antibiotics for self-medication in urban Mozambique: a qualitative study. BMJ Open. 2020;10:e041323. doi: 10.1136/bmjopen-2020-041323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torres NF, Solomon VP, Middleton LE. Pharmacists’ practices for non-prescribed antibiotic dispensing in Mozambique. Pharma Pract. 2020;18:1965. doi: 10.18549/PharmPract.2020.3.1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aslam A, Gajdács M, Zin CS, et al. Public awareness and practices towards self-medication with antibiotics among the Malaysian population. A development of questionnaire and pilot-testing. Antibiotics. 2020;9(2):97. doi: 10.3390/antibiotics9020097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zewdie S, Andargie A, Kassahun H. Self-medication practices among Undergraduate University Students in Northeast Ethiopia. Risk Manag Healthc Policy. 2020;13:1375–1381. doi: 10.2147/RMHP.S266329 [DOI] [PMC free article] [PubMed] [Google Scholar]