Abstract
We assessed the genetic diversity of 26 Neisseria lactamica strains from epidemiologically related sources, i.e., groups of kindergartens and primary schools in three Bavarian towns, by the partial sequencing of the argF, rho, recA, and 16S ribosomal genes. We found a total of 17 genotypes, of which 12 were found only in one strain. The genotypes comprised 5 alleles of the argF gene, 9 of rho, 8 of recA, and 10 of the 16S ribosomal DNA. Sequence analysis by determination of homoplasy ratios and split decomposition analysis revealed abundant recombination within N. lactamica.
Neisseria lactamica is a commensal bacterium colonizing the human nasopharynx. The most prominent biochemical feature of the species is the production of acid from lactose, which distinguishes N. lactamica from all other Neisseria spp. described until now (14). Gram-negative cocci utilizing lactose were described in 1933 (17). The novel species N. lactamica was proposed by Hollis et al. in 1969 (14). 16S ribosomal DNA sequence analysis (28), chromosomal DNA-DNA hybridiziation studies (13), and representational difference analysis (6) clearly support the separation of the two species N. lactamica, which is apathogenic, and Neisseria meningitidis, which is a facultative pathogen. Reports on infections due to N. lactamica seem to have only anecdotal character (8). N. lactamica most frequently colonizes the nasopharynges of young children (3, 5, 12, 20, 25). With respect to age groups, there is an inverse relationship between colonization by N. lactamica and by N. meningitidis (3, 5, 12, 24, 25). It was suggested that nasopharyngeal colonization with N. lactamica protects the host from colonization with pathogenic strains of N. meningitidis by the induction of immune protection (7, 12). This suggestion is supported by the observation of cross-reactive antigens in the two species (18, 19, 31, 37).
Neisseria species are naturally competent, and inter- and intraspecific horizontal gene transfer has been described (4, 9, 10, 21, 22, 26, 28, 35, 36). Because of frequent recombination among Neisseria spp. (11), the phylogeny of Neisseria can be described by interconnected networks rather than by phylogenetic trees (15, 28). Although there is evidence that horizontal gene transfer occurs in N. lactamica (28), the population structure of N. lactamica has not been defined yet by multilocus enzyme electrophoresis or multilocus sequence typing (23). The recent report on recombination in Neisseria spp. (28) included only six N. lactamica strains obtained from sources in Sweden, the United Kingdom, and Canada (2). Therefore, it is unclear to what extent epidemiologically related and unrelated strains of N. lactamica differ genetically. In the present study, we analyzed the genetic variability of 26 N. lactamica strains isolated from epidemiologically defined kindergarten or school groups in three neighboring towns in Bavaria, Germany. For this purpose we determined genotypes (GT) by sequencing four genes (28) which are not under immune selection according to the philosophy of multilocus sequence typing used for N. meningitidis (23). Furthermore, we tested recombination by homoplasy ratios and split decomposition analysis (16, 27).
MATERIALS AND METHODS
Isolation and identification of N. lactamica strains.
N. lactamica strains were collected within the context of the Bavarian meningococcal carriage study performed November 1999 through March 2000. The isolates were obtained from Bavarian children aged 3 to 11 years living in the towns of Munich, Augsburg, and Ingolstadt. This study was approved by the ethical committee of the Medical Faculty of the University of Würzburg. Pharyngeal swabs were taken from the back of the throat with cotton swabs and plated immediately on Martin-Lewis agar containing trimethoprim lactate, vancomycin, colistin, amphotericin B, and IsoVitaleX (kind gift from Becton Dickinson, Heidelberg, Germany). After overnight growth at 37°C in a 5% CO2 atmosphere, colonies were screened with oxidase reagent (Heipha, Heidelberg, Germany). Oxidase-positive Neisseria colonies were tested for β-galactosidase and γ-glutamyltransferase (GGT) activity. β-Galactosidase activity was tested using o-nitrophenyl-β-d-galactopyranoside (ONPG) as the substrate. Bacteria were resuspended to a final optical density at 600 nm of 10 in an ONPG-containing solution (2 mM ONPG [AppliChem, Darmstadt, Germany], 113 μM sodium dodecyl sulfate, 124 mM NaCl, 16 mM K-phosphate, pH 7). ONPG-positive strains were yellow after incubation at 37°C for 1 h. GGT activity was tested by incubation of bacteria for 8 h at 37°C in 1 mM l-glutamic acid–γ-3-carboxy-4-nitroanilide–NH4 salt (Sigma-Aldrich, Schnelldorf, Germany). For both assays, N. lactamica reference strain DSM 4691 (German type culture collection, Braunschweig, Germany) and N. meningitidis strain MC58 (ET-5 complex, serogroup B; kindly provided by E. R. Moxon, Oxford, United Kingdom) were used as positive and negative controls, respectively. The diagnosis of N. lactamica for oxidase-positive, ONPG-positive, and GGT-negative strains was confirmed by use of API NH (BioMérieux, Marcy-1'Etoile, France).
PCR and sequence analysis.
Oligonucleotides were purchased from ARK Scientific (Darmstadt, Germany). PCR was performed on a thermal cycler obtained from Biometra (Göttingen, Germany). Thermostable DNA polymerase (AmpliTaq) was purchased from Perkin-Elmer (Weiterstadt, Germany). As templates, heat-inactivated bacterial suspensions (optical density at 600 nm of 0.1) in phosphate-buffered saline were used. For genotyping according to the principles of multilocus sequence typing (23), internal fragments of three housekeeping genes, i.e., recA (positions 308 to 639 [GenBank accession no. U57905]), argF (positions 358 to 634 [X64871]), rho (positions 67 to 417 [AJ223910]), and of the 16S ribosomal DNA (positions 52 to 509 [J01859; Escherichia coli ribosomal DNA]), as well as a part of the por gene (positions 379 to 750 [X65533] [9, 33]), were amplified by PCR using the primers shown in Table 1. In the present report the term GT is used throughout, because it was not the aim of the study to set up a generally applicable sequence typing scheme for N. lactamica. recA, argF, rho, and 16S ribosomal DNA had been used in a recent publication studying recombination in the genus Neisseria (28). Sufficient sequence information was available from this publication to be used for the design of conserved primers (Table 1). The PCR conditions for argF (primers DA3 and DA4), rho (primers DA17 and DA18), and por (primers DA14 and DA9) were initial denaturation at 94°C for 10 min, followed by 36 cycles of annealing (56°C for 1 min), extension (72°C for 1 min), and denaturation (94°C for 1 min) and then 1 cycle of annealing (56°C for 1 min) and a final extension (72°C for 10 min). The PCR conditions for the amplification of the recA gene (primers DA1 and DA2) and the 16S ribosomal gene (primers 27f and 907r) were the same, except for the annealing temperatures, which were 62°C for the recA gene and 53°C for the 16S ribosomal DNA. For sequence analysis, PCR products were purified using the QIAquick PCR purification kit or the QIAquick gel extraction kit (Qiagen, Hilden, Germany). Automated DNA sequencing was performed on an Applied Biosystems (Foster City, Calif.) model 377 using the dye terminator cycle method with AmpliTaq. PCR primers were used for sequencing, except for the 16S ribosomal DNA, which was sequenced using primers 27f and 519r. All sequences were determined on both strands. Nucleotide sequence data were analyzed with the Lasergene sequence analysis software (DNASTAR, Madison, Wis.). Each distinct allele of a gene was assigned a number, and GT were defined according to the combination of allele numbers of the four housekeeping genes. The 16S ribosomal gene was further analyzed with the RIDOM database hosted by the server of the Institute of Hygiene and Microbiology (University of Würzburg; http://www.ridom.hygiene.uni-wuerzburg.de/index.html) (D. Harmsen, J. Rothganger, C. Singer, J. Albert, and M. Frosch, Letter, Lancet 353:291, 1999). The homplasy test (27) was performed using the program HOMOPLASY (30). The sequence alignments were converted to NEXUS files using SFE, version 1.0.3 (K. Jolley; http://mlst.zoo.ox.ac.uk/links/SFE103.zip), and split decomposition was analyzed with SPLITSTREE, version 3.1 (16). The tree produced by the unweighted pair group method with arithmetic averages (UPGMA) shown in Fig. 3 was drawn with START (K. Jolley; http://mlst.zoo.ox.ac.uk/links/START.zip).
TABLE 1.
Oligonucleotides used for PCR and sequencing of the argF, recA, rho, por, and 16S ribosomal gene of N. lactamica strains
| Oligonucleotide | Sequence (5′–3′) | Target | Position (accession no.)a |
|---|---|---|---|
| DA1 | GCCAGAAAAACGGCGGCG | recA | 279–296 (U57905) |
| DA2 | TGACTTTGACGCGGGTTTCG | recA | 670–651 (U57905) |
| DA3 | GACGCGCGTTACAACATGG | argF | 329–347 (X64871) |
| DA4 | GCAGGCAGTGCATGAATTTG | argF | 665–646 (X64871) |
| DA9 | CGGTAGCGGCAACTTCGG | por | 793–776 (X65533) |
| DA14 | TGAACAGCATCCTGAAAAGCA | por | 367–347 (X65533) |
| DA17 | TCCGGCACACTCGAAATCC | rho | 37–55 (AJ223910) |
| DA18 | TGCAGCATCACGGTTTTACC | rho | 449–430 (AJ223910) |
| 27f | AGAGTTTGATCMTGGCTCAG | 16S ribosomal DNA | 8–27 (J01859) |
| 907r | CCGTCAATTCMTTTRAGTTT | 16S ribosomal DNA | 926–907 (J01859) |
| 519r | GWATTACCGCGGCKGCTG | 16S ribosomal DNA | 536–519 (J01859) |
Numbering according to accession number.
FIG. 3.
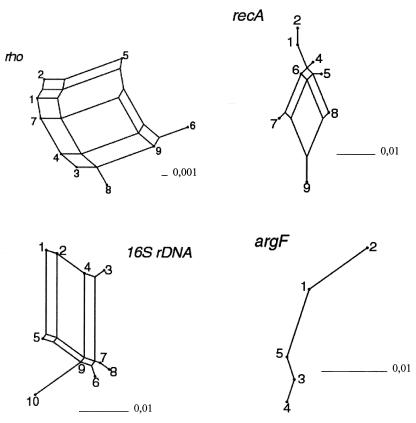
Split graphs for the N. lactamica sequences obtained in this study. Split decomposition was analyzed with SPLITSTREE, version 3.1 (16). The fit values were 100, 100, 88, and 83% for argF, 16S ribosomal DNA, recA, and rho, respectively.
RESULTS
Identification of N. lactamica strains.
During the course of a meningococcal carrier study in the south German federal state of Bavaria (inhabitants: 12,155,000; area: 70,548 km2), 287 GGT-negative, ONPG-positive Neisseria isolates collected from Martin-Lewis agar plates were identified as N. lactamica by biochemical testing using a commercial kit. For the analysis of the genetic diversity of N. lactamica within defined social groups, we selected 40 strains, which were isolated from kindergarten and school groups located in the neighboring towns of Munich, Ingolstadt, and Augsburg. To further confirm the identification as N. lactamica for these 40 strains, we analyzed a partial 16S ribosomal DNA sequence using the RIDOM database (Harmsen et al., letter, 1999). More than 97% identity to the RIDOM database sequence of the N. lactamica type strain, DSM 4691, was defined as a prerequisite for inclusion of a strain in the study. Partial 16S ribosomal DNA sequence analysis showed that the 16S ribosomal genes (positions 52 through 509) of 10 of 40 strains were more related to Neisseria cinerea than N. lactamica (96.8 to 99.1% identity to the N. cinerea 16S ribosomal gene). The 16S ribosomal genes of four further strains showed less than 97% identity to the 16S ribosomal gene of the N. lactamica type strain included in the RIDOM database. Therefore, 26 strains were finally used for genotyping (Table 2). These strains were collected within a period of 1 month (21 February through 23 March 2000).
TABLE 2.
GT of N. lactamica strains isolated in three Bavarian towns
| Isolate no. | Town | Institutiona | Group | Age (yr) | Allele no. for:
|
GT | |||
|---|---|---|---|---|---|---|---|---|---|
| recA | argF | rho | 16S rDNA | ||||||
| 181 | Augsburg | KG 1 | 6 | 5 | 1 | 1 | 1 | 1 | 1 |
| 175 | Augsburg | KG 1 | 4 | 4 | 1 | 1 | 1 | 1 | 1 |
| 176 | Augsburg | KG 1 | 4 | 4 | 1 | 1 | 1 | 1 | 1 |
| 177 | Augsburg | KG 1 | 4 | 5 | 1 | 1 | 1 | 1 | 1 |
| 149 | Augsburg | PS 1 | 1d | 7 | 1 | 3 | 1 | 1 | 2 |
| 163 | Augsburg | PS 1 | 4b | 10 | 1 | 3 | 1 | 1 | 2 |
| 147 | Augsburg | PS 1 | 1d | 7 | 1 | 3 | 1 | 1 | 2 |
| 205 | Ingolstadt | KG 2 | T | 3 | 9 | 3 | 1 | 1 | 3 |
| 208 | Ingolstadt | KG 3 | M | 4 | 6 | 3 | 1 | 1 | 4 |
| 207 | Ingolstadt | KG 2 | T | 4 | 5 | 3 | 8 | 1 | 5 |
| 206 | Ingolstadt | KG 2 | T | 4 | 5 | 3 | 8 | 1 | 5 |
| 155 | Augsburg | PS 1 | 3a | 9 | 6 | 1 | 5 | 2 | 6 |
| 154 | Augsburg | PS 1 | 3a | 8 | 8 | 2 | 4 | 3 | 7 |
| 162 | Augsburg | PS 1 | 4b | 10 | 8 | 2 | 4 | 3 | 7 |
| 185 | Augsburg | KG 1 | 7 | 3 | 8 | 2 | 4 | 3 | 7 |
| 178 | Augsburg | KG 1 | 5 | 4 | 6 | 1 | 9 | 4 | 8 |
| 179 | Augsburg | KG 1 | 5 | 4 | 1 | 5 | 8 | 5 | 9 |
| 265 | Munich | KG 4 | 1 | 4 | 5 | 1 | 8 | 5 | 10 |
| 271 | Munich | KG 4 | 1 | 5 | 4 | 1 | 8 | 6 | 11 |
| 159 | Augsburg | PS 1 | 4a | 11 | 9 | 1 | 2 | 10 | 12 |
| 267 | Munich | KG 4 | 1 | 5 | 9 | 4 | 7 | 6 | 13 |
| 270 | Munich | KG 4 | 1 | 3 | 2 | 1 | 6 | 7 | 14 |
| 156 | Augsburg | PS 1 | 3a | 8 | 1 | 1 | 6 | 8 | 15 |
| 210 | Ingolstadt | KG 3 | M | 3 | 9 | 1 | 2 | 9 | 16 |
| 151 | Augsburg | PS 1 | 1c | 6 | 9 | 1 | 2 | 9 | 16 |
| 183 | Augsburg | KG 1 | 6 | 5 | 7 | 5 | 3 | 9 | 17 |
KG, kindergarten; PS, primary school.
Genotyping of the argF, recA, and rho genes.
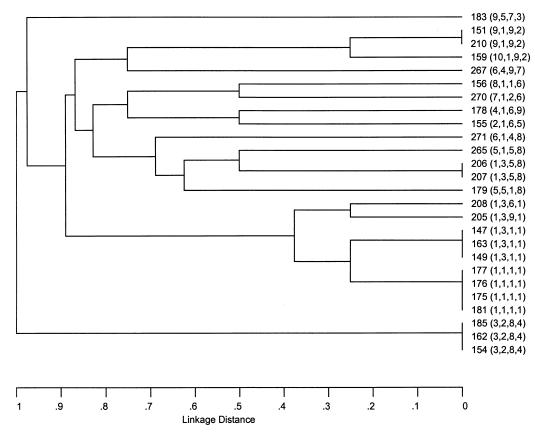
To assess the genetic variability of epidemiologically related N. lactamica strains, we sequenced internal fragments of the argF, recA, and rho genes in addition to the 16S ribosomal gene (28). The analysis of the 16S ribosomal gene indicated a high degree of variability, with 10 alleles among 26 strains (Fig. 1). Sequencing an internal part of argF yielded five alleles. One out of eight polymorphic sites in argF had a nonsynonymous mutation leading to a conservative amino acid substitution (valine to isoleucine). There were nine unique alleles of rho with only synonymous polymorphic sites. We found eight alleles of the recA gene. A total of 24 mutations resulted in two changes of the deduced amino acid sequence; again valine and isoleucine were exchanged. Taken together, the data showed that a high degree of genetic variability could be shown by the use of four genes (argF, recA, rho, and 16S ribosomal DNA). Therefore, we decided to define GT on the basis of these four genes. Table 2 demonstrates the GT in relation to social groups. The linkage distances of the GT are demonstrated by UPGMA analysis in Fig. 2. The branching of the tree shows the high level of discrimination achieved by genotyping. Twelve GT were unique in the 26 strains analyzed. Five of 17 GT, (i.e., GT1, GT2, GT5, GT7, and GT16) occured more than once. Of those, GT1 was restricted to kindergarten 1 in Augsburg, i.e., groups 4 (three isolates) and 6 (one isolate). GT2 occurred in Augsburg primary school 1, i.e., groups 1d (two isolates) and 4b (one isolate). GT5 was restricted to kindergarten 2 in Ingolstadt, and GT7 and GT16 occurred in different institutions. GT16 was the only GT which was found in two different towns.
FIG. 1.
Polymorphic sites (boldface letters) within the argF, recA, rho, and 16S ribosomal genes of N. lactamica. The positions of the polymorphic sites are shown above the nucleotide sequences in vertical format. The numbers were defined according to the sequenced fragments of the genes and do not reflect the position relative to the start codon. Amino acid changes resulting from nonsynonymous mutations are indicated below the nucleotide sequences by single-letter code.
FIG. 2.
Linkage distances of 26 N. lactamica strains analyzed in this study as determined by UPGMA analysis. The strain numbers are on the right, and the allele numbers (parentheses) are given in the following order: 16S ribosomal DNA, argF, recA, rho.
In order to distinguish strains with the same GT, an internal segment of the porin gene (9, 33) was additionally sequenced in 23 of the 26 strains. The segment covered the coding sequence of amino acids 123 through 246 of the N. lactamica porin (GenBank accession no. X65533) (33). This fragment included the sequences encoding surface-exposed, variable loops IV and V of the N. lactamica porin, as defined by Derrick et al. (9) and partially contained loops III and VI. Since the porin is under immune selection, the sequences could be predicted to be more variable than those of the housekeeping genes of our original genotyping system. Accordingly, nonsynonymous mutations (n = 11) were found exclusively in these surface-exposed loops. We found a total of 11 alleles with respect to the DNA sequence of the porin gene fragment. We wondered whether strains with the same GT harbored identical porin gene sequences. All four strains with GT1 carried porin allele 1. Both GT5 strains carried porin allele 3, both GT16 strains carried allele 8, and all three GT7 strains carried allele 9. One of three strains with GT2, which comprised alleles 4 and 7, could be distinguished from the remaining two strains by porin gene sequencing. Taken together, porin sequencing distinguished on a clonal level only one of five N. lactamica GT carried by more than one strain. This finding suggests that a sufficient degree of discrimination was already achieved by sequencing argF, rho, recA, and 16S ribosomal DNA, and the porin gene was thus not included in the genotyping system.
Recombination in N. lactamica.
The four genes sequenced in this study were analyzed for recombination by two different methods: split decomposition analysis (1) and the homoplasy test (27). Split decomposition has been used extensively to analyze the population structures of both bacteria and viruses. Because this method does not make the a priori assumption that the sequences have a tree-like structure, conflicting phylogenetic signals in the data such as evidence of recombination can be visualized, leading to the generation of an interconnected network rather than a tree. Split graphs for the N. lactamica sequences are shown in Fig. 3 Except for argF, split graphs of all genes showed a complex network, consistent with recombination. This result could be confirmed by calculation of homplasy ratios for recA and rho. Homoplasy ratios indicate the frequency of recombination. A freely recombining organism, in which all polymorphic sites are in linkage equilibrium, theoretically exhibits a homoplasy ratio of 1.0; in a nonrecombining, clonal organism, the expected homoplasy ratio is zero. Helicobacter pylori, for example, exhibits the highest homoplasy ratio known, with a mean value of 0.85 calculated for three genes; the value was only 0.34 for meningococci (reviewed in references 29 and 30) (Table 3). The homoplasy ratios (Table 3) for N. lactamica calculated in this study were 0.69 and 0.54 for recA and rho, respectively. Our findings therefore show that in N. lactamica recombination occurs frequently. Homoplasy ratios were higher than those previously reported for meningococci, which may indicate either that recombination occurs more frequently in N. lactamica or that recombinant genotypes are purified less efficiently from the population. KA and Ks values, which represent the frequencies of synonymous and nonsynonymous changes per synonymous or nonsynonymous site, respectively, were rather low for all genes analyzed in this study (Table 3) compared to the values published for meningococci and H. pylori (30). However, this might be explained by the rather small number of alleles tested in this study.
TABLE 3.
Mutation and recombination in N. lactamica
| Organism | Gene or no. of genes | No. of:
|
Mean % (range)
|
Homoplasy ratioa | Reference or source | ||
|---|---|---|---|---|---|---|---|
| bp | Alleles | KA | KS | ||||
| N. lactamica | recA | 432 | 8 | 0.3 | 8.9 | 0.64 ± 0.04 | This study |
| N. lactamica | rho | 351 | 9 | 0.0 | 7.5 | 0.54 ± 0.05 | This study |
| N. lactamica | argF | 276 | 5 | 0.29 | 5.4 | N.D.b | This study |
| N. lactamica | 16S ribosomal DNA | 459 | 10 | 1.84 | N.D. | This study | |
| H. pylori | 3 | 0.7 (0.3–2.5) | 16.8 (14.1–21.4) | 0.85 | 30 | ||
| N. meningitidis | 11 | 0.7 (0.2–7.8) | 13.4 (5.9–26.8) | 0.34 | 30 | ||
Freely recombining organisms, where all polymorphic sites are in linkage equilibrium, theoretically exhibit a homoplasy ratio of 1.0, whereas completely clonal organisms would give rise to a homoplasy ratio of 0.0 (27).
N.D., not done. The homoplasy test (27) could not be applied to argF and the 16S ribosomal DNA gene because at least six alleles are required to perform the test and because the HOMOPLASY program only works with protein-encoding genes.
DISCUSSION
There have been numerous reports illustrating that commensal Neisseria spp. are involved in horizontal gene transfer (4, 9, 10, 21, 22, 26, 28, 35, 36). For example, horizontal transfer of penicillin resistance genes has been described (4, 22). Meningococci rapidly and frequently incorporated tbp alleles from commensals (21). These findings support the definition of a global gene pool of Neisseria spp. (M. C. Maiden, B. Malorny, and M. Achtman, Mol. Microbiol. 21:1297–1298, 1996). N. lactamica is a commensal species of considerable epidemiological importance, because there is evidence suggesting that colonization with N. lactamica protects children from meningococcal infection (7). The population structure of this species has not been analyzed yet. The recent reports on recombination in N. lactamica suggest that the genetic variability of N. lactamica is driven by recombination (28). However, that study used only six N. lactamica strains obtained from sources in Sweden, the United Kingdom, and Canada (2).
Our present study was aimed at elucidating the genetic variability of N. lactamica strains from epidemiologically linked individuals. We used a genotyping system based on a set of four genes (28) and showed that this system has a satisfying degree of discrimination between strains. Seventeen GT could be defined. Sequencing the porin gene did not add significant information to the typing scheme. The epidemiological analysis of the GT suggested clonal spread within social groups of children, but it was very unusual to find identical isolates in two epidemiologically unrelated hosts. This conclusion is best illustrated by the fact that only one of 17 GT was found in two different towns. Analysis of homoplasy ratios of the recA and rho alleles as well as split decomposition analysis of 16S ribosomal RNA, recA, and rho showed frequent recombination in N. lactamica, and the observed homoplasy ratios were higher than those previously reported for meningococci. While the comparison of recombination frequencies in different species by means of homoplasy ratios has to be interpreted with some caution, our data nevertheless suggest that frequent recombination diversifies N. lactamica and that efficient purification mechanisms (e.g., sequential bottlenecks) eliminating new variants from the population are lacking. Clonal spread seemed to occur if close contact between carriers existed. It is impossible to explain the epidemiological basis for the maintenance of a high level of genetic variability in N. lactamica due to a lack of epidemiological and experimental data. A variety of issues, therefore, will have to be addressed. The mean duration of carriage of N. lactamica in an individual host is unknown, and it is unclear how effectively N. lactamica is transmitted between hosts. Future experiments should, furthermore, analyze the accumulation of genetic variability within a host during carriage and assess the velocity of microevolution during spread of a clone in a community.
It was recently shown that the 16S ribosomal gene of N. lactamica is subject to recombination (28). Consistent with this finding, we could detect 10 alleles of the 16S ribosomal gene in 26 strains, with evidence for extensive recombination. Furthermore, our initial process of strain selection for this study revealed that a considerable number of GGT-negative isolates utilizing lactose carried 16S ribosomal sequences related to N. cinerea. Although recombination within the rrn operon has also been demonstrated for other bacterial species (32, 34), our findings illustrate the difficulties of species identification by 16S ribosomal DNA sequencing in frequently recombining bacteria. We suggest that molecular diagnosis of such species should include sequencing complete genes of both the 16S and the 23S ribosomal DNA. Furthermore, the ribosomal DNA sequences have to be examined for mosaic structures evolved by recombination.
In conclusion, our study illustrated genetic diversity in N. lactamica, which is driven by recombination. The knowledge of the population structure of N. lactamica should help to define those clones and strains of N. lactamica which induce natural immunity against meningcoccal disease and those which do not. Such investigations will be of importance for the development of live vaccines against meningococcal disease based on apathogenic species such as N. lactamica.
ACKNOWLEDGMENTS
We thank Gabi Heinze for expert technical assistance and Silke Getzlaff, Frank Hessler, and Carmen Sorger for help with strain collection. The government of Bavaria (Bayerisches Staatsministerium für Arbeit und Sozialordnung, Familie, Frauen und Gesundheit) is gratefully acknowledged for logistic support during the collection of strains within the context of the Bavarian meningococcal carrier study. We are grateful to Becton Dickinson and BioMerieux for their support to the Bavarian meningococcal carrier study by providing Martin-Lewis agar plates and the API NH identification system, respectively.
REFERENCES
- 1.Bandelt H J, Dress A W. Split decomposition: a new and useful approach to phylogenetic analysis of distance data. Mol Phylogenet Evol. 1992;1:242–252. doi: 10.1016/1055-7903(92)90021-8. [DOI] [PubMed] [Google Scholar]
- 2.Barrett S J, Sneath P H. A numerical phenotypic taxonomic study of the genus Neisseria. Microbiology. 1994;140:2867–2891. doi: 10.1099/00221287-140-10-2867. [DOI] [PubMed] [Google Scholar]
- 3.Blakebrough I S, Greenwood B M, Whittle H C, Bradley A K, Gilles H M. The epidemiology of infections due to Neisseria meningitidis and Neisseria lactamica in a northern Nigerian community. J Infect Dis. 1982;146:626–637. doi: 10.1093/infdis/146.5.626. [DOI] [PubMed] [Google Scholar]
- 4.Bowler L D, Zhang Q Y, Riou J Y, Spratt B G. Interspecies recombination between the penA genes of Neisseria meningitidis and commensal Neisseria species during the emergence of penicillin resistance in N. meningitidis: natural events and laboratory simulation. J Bacteriol. 1994;176:333–337. doi: 10.1128/jb.176.2.333-337.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cartwright K A, Stuart J M, Jones D M, Noah N D. The Stonehouse survey: nasopharyngeal carriage of meningococci and Neisseria lactamica. Epidemiol Infect. 1987;99:591–601. doi: 10.1017/s0950268800066449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Claus H, Friedrich A, Frosch M, Vogel U. Differential distribution of two novel restriction-modification systems in clonal lineages of Neisseria meningitidis. J Bacteriol. 2000;182:1296–1303. doi: 10.1128/jb.182.5.1296-1303.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coen P G, Cartwright K, Stuart J. Mathematical modelling of infection and disease due to Neisseria meningitidis and Neisseria lactamica. Int J Epidemiol. 2000;29:180–188. doi: 10.1093/ije/29.1.180. [DOI] [PubMed] [Google Scholar]
- 8.Denning D W, Gill S S. Neisseria lactamica meningitis following skull trauma. Rev Infect Dis. 1991;13:216–218. doi: 10.1093/clinids/13.2.216. [DOI] [PubMed] [Google Scholar]
- 9.Derrick J P, Urwin R, Suker J, Feavers I M, Maiden M C. Structural and evolutionary inference from molecular variation in Neisseria porins. Infect Immun. 1999;67:2406–2413. doi: 10.1128/iai.67.5.2406-2413.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feil E, Zhou J, Smith J M, Spratt B G. A comparison of the nucleotide sequences of the adk and recA genes of pathogenic and commensal Neisseria species: evidence for extensive interspecies recombination within adk. J Mol Evol. 1996;43:631–640. doi: 10.1007/BF02202111. [DOI] [PubMed] [Google Scholar]
- 11.Feil E J, Enright M C, Spratt B G. Estimating the relative contributions of mutation and recombination to clonal diversification: a comparison between Neisseria meningitidis and Streptococcus pneumoniae. Res Microbiol. 2000;151:465–469. doi: 10.1016/s0923-2508(00)00168-6. [DOI] [PubMed] [Google Scholar]
- 12.Gold R, Goldschneider I, Lepow M L, Draper T F, Randolph M. Carriage of Neisseria meningitidis and Neisseria lactamica in infants and children. J Infect Dis. 1978;137:112–121. doi: 10.1093/infdis/137.2.112. [DOI] [PubMed] [Google Scholar]
- 13.Hoke C, Vedros N A. Taxonomy of the Neisseriae: deoxyribonucleic acid base composition, interspecific transformation, and deoxyribonucleic acid hybridization. Int J Syst Bacteriol. 1982;32:57–66. [Google Scholar]
- 14.Hollis D G, Wiggins G L, Weaver R E. Neisseria lactamicus sp. n., a lactose-fermenting species resembling Neisseria meningitidis. Appl Microbiol. 1969;17:71–77. doi: 10.1128/am.17.1.71-77.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmes E C, Urwin R, Maiden M C. The influence of recombination on the population structure and evolution of the human pathogen Neisseria meningitidis. Mol Biol Evol. 1999;16:741–749. doi: 10.1093/oxfordjournals.molbev.a026159. [DOI] [PubMed] [Google Scholar]
- 16.Huson D H. SplitsTree: analyzing and visualizing evolutionary data. Bioinformatics. 1998;14:68–73. doi: 10.1093/bioinformatics/14.1.68. [DOI] [PubMed] [Google Scholar]
- 17.Jessen J. Studien ueber gramnegative Kokken. Zentbl Bakteriol Parasitenkd Infektkrankh. 1993;133:75–88. [Google Scholar]
- 18.Kim J J, Mandrell R E, Griffiss J M. Neisseria lactamica and Neisseria meningitidis share lipooligosaccharide epitopes but lack common capsular and class 1, 2, and 3 protein epitopes. Infect Immun. 1989;57:602–608. doi: 10.1128/iai.57.2.602-608.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kremastinou J, Tzanakaki G, Pagalis A, Theodondou M, Weir D M, Blackwell C C. Detection of IgG and IgM to meningococcal outer membrane proteins in relation to carriage of Neisseria meningitidis or Neisseria lactamica. FEMS Immunol Med Microbiol. 1999;24:73–78. doi: 10.1111/j.1574-695X.1999.tb01267.x. [DOI] [PubMed] [Google Scholar]
- 20.Kremastinou J, Tzanakaki G, Velonakis E, Voyiatzi A, Nickolaou A, Elton R A, Weir D, Blackwell C. Carriage of Neisseria meningitidis and Neisseria lactamica among ethnic Greek school children from Russian immigrant families in Athens. FEMS Immunol Med Microbiol. 1999;23:13–20. doi: 10.1111/j.1574-695X.1999.tb01711.x. [DOI] [PubMed] [Google Scholar]
- 21.Linz B, Schenker M, Zhu P, Achtman M. Frequent interspecific genetic exchange between commensal neisseriae and Neisseria meningitidis. Mol Microbiol. 2000;36:1049–1058. doi: 10.1046/j.1365-2958.2000.01932.x. [DOI] [PubMed] [Google Scholar]
- 22.Lujan R, Zhang Q Y, Saez Nieto J A, Jones D M, Spratt B G. Penicillin-resistant isolates of Neisseria lactamica produce altered forms of penicillin-binding protein 2 that arose by interspecies horizontal gene transfer. Antimicrob Agents Chemother. 1991;35:300–304. doi: 10.1128/aac.35.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maiden M C, Bygraves J A, Feil E, Morelli G, Russell J E, Urwin R, Zhang Q, Zhou J, Zurth K, Caugant D A, Feavers I M, Achtman M, Spratt B G. Multilocus sequence typing: a protable approach to the identification of clones within populations of pathogenic microorganisms. Proc Natl Acad Sci USA. 1998;95:3140–3145. doi: 10.1073/pnas.95.6.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olsen S F, Djurhuus B, Rasmussen K, Joensen H D, Larsen S O, Zoffman H, Lind I. Pharyngeal carriage of Neisseria meningitidis and Neisseria lactamica in households with infants within areas with high and low incidences of meningococcal disease. Epidemiol Infect. 1991;106:445–457. doi: 10.1017/s0950268800067492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saez Nieto J A, Dominguez J R, Monton J L, Cristobal P, Fenoll A, Vazquez J, Casal J, Taracena B. Carriage of Neisseria meningitidis and Neisseria lactamica in a school population during an epidemic period in Spain. J Hyg. 1985;94:279–288. doi: 10.1017/s0022172400061507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saez Nieto J A, Lujan R, Martinez Suarez J V, Berron S, Vazquez J A, Vinas M, Campos J. Neisseria lactamica and Neisseria polysaccharea as possible sources of meningococcal beta-lactam resistance by genetic transformation. Antimicrob Agents Chemother. 1990;34:2269–2272. doi: 10.1128/aac.34.11.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith J M, Smith N H. Detecting recombination from gene trees. Mol Biol Evol. 1998;15:590–599. doi: 10.1093/oxfordjournals.molbev.a025960. [DOI] [PubMed] [Google Scholar]
- 28.Smith N H, Holmes E C, Donovan G M, Carpenter G A, Spratt B G. Networks and groups within the genus Neisseria: analysis of argF, recA, rho, and 16S rRNA sequences from human Neisseria species. Mol Biol Evol. 1999;16:773–783. doi: 10.1093/oxfordjournals.molbev.a026162. [DOI] [PubMed] [Google Scholar]
- 29.Suerbaum S. Genetic variability within Helicobacter pylori. Int J Med Microbiol. 2000;290:175–181. doi: 10.1016/S1438-4221(00)80087-9. [DOI] [PubMed] [Google Scholar]
- 30.Suerbaum S, Smith J M, Bapumia K, Morelli G, Smith N H, Kunstmann E, Dyrek I, Achtman M. Free recombination within Helicobacter pylori. Proc Natl Acad Sci USA. 1998;95:12619–12624. doi: 10.1073/pnas.95.21.12619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Troncoso G, Sanchez S, Moreda M, Criado M T, Ferreiros C M. Antigenic cross-reactivity between outer membrane proteins of Neisseria meningitidis and commensal Neisseria species. FEMS Immunol Med Microbiol. 2000;27:103–109. doi: 10.1111/j.1574-695X.2000.tb01419.x. [DOI] [PubMed] [Google Scholar]
- 32.Ueda K, Seki T, Kudo T, Yoshida T, Kataoka M. Two distinct mechanisms cause heterogeneity of 16S rRNA. J Bacteriol. 1999;181:78–82. doi: 10.1128/jb.181.1.78-82.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ward M J, Lambden P R, Heckels J E. Sequence analysis and relationships between meningococcal class 3 serotype proteins and other porins from pathogenic and non-pathogenic Neisseria species. FEMS Microbiol Lett. 1992;73:283–289. doi: 10.1016/0378-1097(92)90644-4. [DOI] [PubMed] [Google Scholar]
- 34.Yap W H, Zhang Z, Wang Y. Distinct types of rRNA operons exist in the genome of the actinomycete Thermomonospora chromogena and evidence for horizontal transfer of an entire rRNA operon. J Bacteriol. 1999;181:5201–5209. doi: 10.1128/jb.181.17.5201-5209.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou J, Bowler L D, Spratt B G. Interspecies recombination, and phylogenetic distortions within the glutamine synthetase and shikimate dehydrogenase genes of Neisseria meningitidis and commensal Neisseria species. Mol Microbiol. 1997;23:799–812. doi: 10.1046/j.1365-2958.1997.2681633.x. [DOI] [PubMed] [Google Scholar]
- 36.Zhou J, Spratt B G. Sequence diversity within the argF, fbp and recA genes of natural isolates of Neisseria meningitidis: interspecies recombination within the argF gene. Mol Microbiol. 1992;6:2135–2146. doi: 10.1111/j.1365-2958.1992.tb01387.x. [DOI] [PubMed] [Google Scholar]
- 37.Zorgani A A, James V S, Stewart J, Blackwell C C, Elton R A, Weir D M. Serum bactericidal activity in a secondary school population following an outbreak of meningococcal disease: effects of carriage and secretor status. FEMS Immunol Med Microbiol. 1996;14:73–81. doi: 10.1111/j.1574-695X.1996.tb00273.x. [DOI] [PubMed] [Google Scholar]