Abstract
Objectives
This study aims to determine the histopathological patterns and biological characteristics of ameloblastoma.
Methods
This was a cross-sectional retrospective laboratory-based study using 82 formalin-fixed paraffin-embedded tissue blocks from patients diagnosed histologically with ameloblastoma. Information regarding age, sex, anatomical location of the lesion, histopathologic type, and biological behaviour or characteristics of the types of ameloblastoma was obtained from laboratory request forms. Categorical and continuous variables were summarized in percentage and mean ± standard deviation, respectively. The cohort was conducted on Ugandan patients diagnosed between 2016 and 2019.
Results
Most patients (66.3%) were clinically presenting a painless jaw swelling, and a follicular pattern was common (39%) followed by the plexiform pattern (12.2%). All the ameloblastoma cases (100%) were benign, with the majority (76.8%) cases being non-recurrent while the remaining (23.2%) were recurrent, and the plexiform pattern was the commonest recurrent histopathological pattern.
Conclusion
This study reports a relatively significant rate of recurrence in almost a quarter of the study population. The plexiform histopathologic type was the dominant type in recurrence cases. Therefore, this finding provides insightful information to clinicians to ensure close follow-up for patients diagnosed with such a variant to prevent possible relapse of the disease.
Keywords: Ameloblastoma, Biological behaviour, Histopathological patterns
الملخص
أهداف البحث
تهدف هذه الدراسة إلى تحديد الأنماط النسيجية المرضية والخصائص البيولوجية للورم الأرومي المينائي.
طرق البحث
كانت هذه دراسة معملية مستعرضة تضمنت 82 كتلة نسيجية مثبتة بالبارافين مثبتة في الفورمالين من المرضى الذين تم تشخيص إصابتهم نسيجيا بالورم الأرومي المينائي. تم الحصول على المعلومات المتعلقة بالعمر والجنس والموقع التشريحي للآفة والنوع النسيجي المرضي والسلوك البيولوجي أو خصائص أنواع معلومات الورم الأرومي المينائي من استمارات طلب المختبر. تم تلخيص المتغيرات الفئوية والمستمرة باستخدام النسبة المئوية والمتوسط مع الانحراف المعياري على التوالي. تم إجراء الدراسة على مرضى أوغنديين تم تشخيصهم بين عامي 2016 و 2019 في قسم علم الأمراض.
النتائج
غالبية المرضى (66.3٪) تعرضوا سريريا لتورم في الفك غير مؤلم وكان النمط الجريبي الأكثر شيوعا بنسبة 39٪ يليه نمط الضفيرة الذي وجد في 12.2٪. جميع حالات الورم الأرومي المينائي (100٪) في هذه الدراسة كانت حميدة وغالبيتها (76.8٪) كانت حالات غير متكررة بينما 23.2٪ المتبقية كانت متكررة وكان نمط الضفيرة هو النمط النسيجي المرضي الأكثر شيوعا.
الخلاصة
تشير هذه الدراسة إلى معدل تكرار كبير نسبيا لما يقرب من ربع مجتمع الدراسة. أيضا، فإن حالات التكرار سيطر عليها النوع النسيجي الضفيري. لذلك، توفر هذه النتائج معلومات ثرية للأطباء أنه يجب عليهم دائما ضمان المتابعة الدقيقة للمرضى الذين تم تشخيصهم بمثل هذا المتغير لضمان الانتكاس المبكر المحتمل للمرض.
الكلمات المفتاحية: الورم الأرومي المينائي, سلوك بيولوجي, أنماط نسيجية
Introduction
Odontogenic tumours (OTs) originate from epithelial or ectomesenchymal tissues, which are part of the tooth-forming apparatus. Ameloblastomas are a form OTs that are relatively rare and comprise about 11% of OTs and 1% of all the jaw tumors.1 The existing literature has reported on the variation in the incidence of ameloblastoma. Ochsenius et al. reported the prevalence of ameloblastomas at 20.4%.2 Two other studies reported a higher incidence of ameloblastoma than the combined incidence of all other OTs, constituting 3% of the radiolucent jaw lesions.1,3 A study conducted in Nigeria reported ameloblastoma as the commonest type of OT, comprising 75.5% of all OTs included in the study.29 A similar study in sub-Saharan Africa (SSA) reported ameloblastoma prevalence at 80%.4 In Uganda, the prevalence of ameloblastoma in comparison with other OTs, was reported at 73%.5 However, these findings from African countries seem to contradict studies from Europe30 and America31 where odontomas have been reported to be the most prevalent OTs.31
Clinically, ameloblastoma is a benign tumour and is usually painless. However, it may become painful when it transforms to other forms of neoplasia or it grows by compressing adjacent nerves.6,7 Commonly, ameloblastomas involve the mandible, however, in a few cases, they also develop in the maxilla.8 The molar region (ramus) of the mandible is by far the commonest site followed by the anterior (symphysis menti).9, 10, 11 Clinically, the vast majority of patients with ameloblastoma present a painless jaw mass, and some patients may also show displaced teeth, mobile teeth, and ulceration.10,11
In 2017, the World Health Organization (WHO) updated a classification of ameloblastomas.12 In their study, Cadavid et al. (2019) reported WHO's updated classification of ameloblastomas11 into three groups: conventional, peripheric, and unicystic. The conventional type consists of six histological forms: plexiform, follicular, acanthomatous, desmoplastic, granular, and basal cell type. The unicystic type is subdivided into mural, luminal, and intraluminal. Conventional ameloblastoma is the most clinically significant OT, which is often locally aggressive and has a significant impact that may lead to patient's morbidity and mortality.13 Studies show that a follicular pattern is the most widespread followed by the plexiform pattern.9,10,14 In addition, follicular type has the highest rate of recurrence and up to 30% has been documented compared to other variants such as unicystic ameloblastoma, which has a low recurrence rate.15 In one of the studies done at Mayo clinic in the United States, it was found that, ameloblastomas, which have a very aggressive behaviour, grow by pushing into the jawbone and cause swelling and pain. Such aggressiveness is more pronounced in the maxilla than in the mandible.16
Biologically, ameloblastomas have also been found to transform into malignant entities (i.e. ameloblastic carcinoma and metastatic ameloblastoma) at a prevalence rate of only 2%.3 Metastatic ameloblastoma typically demonstrates well-differentiated benign histology, similar to the conventional type of ameloblastoma at the primary site, but additional foci of the benign histology are identified in location(s) remote from the primary and are considered to be a metastasis.17
This study aims to study the histopathological patterns and biological behaviour or characteristics of ameloblastoma in a cohort of Ugandan patients diagnosed between 2016 and 2019 at the department of pathology of Makerere University College of Health Science (MakCHS).
Materials and Methods
Study design and setting
This was a cross-sectional descriptive laboratory-based study. The study was conducted at the department of pathology of Makerere University College of Health Science (MakCHS). The department is located at Upper Mulago Hill in Mulago National Referral Hospital (MNRH). Specifically, the hospital facilitates teaching, research, offering diagnostic biopsy and autopsy services for the entire country. The department receives and processes an average of eight thousand tissue biopsies per year.
Patients’ specimens
The study was conducted on patients’ tissue blocks, previously diagnosed as ameloblastoma during the period from January 2016 to December 2019. These were the preserved formalin-fixed paraffin-embedded (FFPE) tissue blocks of ameloblastoma at the department of pathology (MakCHS) during the study period. New cases that were received during the study period were also included. The FFPE tissue blocks from patients with histological diagnosis of ameloblastoma were retrieved from archives during the study period, and laboratory request forms with relevant information including age, sex, tribe, geographical location, oral anatomical site, and summary of clinical history were included. Histologically confirmed cases of ameloblastoma with no relevant data provided on the biodata form, missing blocks, or blocks with missing/insufficient tissue, and tissues with diagnostic variation were excluded.
Sampling procedure and staining with haematoxylin and eosin
Laboratory requisition forms were used to select cases. Thereafter, we assigned each case a unique identification number for ensuring patients' anonymity. The FFPE tissue blocks were sectioned serially at a thickness of 4.0 microns and stained with haematoxylin and eosin stains (H and E) for re-confirming the previous diagnosis, and the histopathologic characterization of the tumours was performed by two independent and experienced pathologists who were blinded of patients’ previous diagnosis and clinical information. In case of disagreement between the two pathologists, a third pathologist was invited as a tie breaker for reaching confirmatory diagnosis.
Data analysis
Data were analysed using IBM SPSS version 23.0. Continuous and categorical variables were summarized in terms of mean ± standard deviation (SD) and proportions, respectively.
Results
Demographic characteristics of ameloblastoma patients
For a period of four years (2016–2019), a total of 129 ameloblastoma tumours, comprising 15.7% (n = 129) of all OTs were recorded. About 36.4% (n = 47) ameloblastoma cases were excluded from the study due to missing FFPE tissue blocks, previous wrong diagnosis, spoilt FFPE tissue blocks by insects, and missing clinical files. Figure 1 presents the case selection process in the study.
Figure 1.
Flow chart indicating selection of cases.
A total of 82 patients with ameloblastoma were included in the study. The mean ± SD age of the patients was 31.3 ± 14.098 years (range: 9–72 years). The mean ± SD age of the male and female patients was 31.1 ± 16.747 and 31.5 ± 12.713 years, respectively. There were 59.8% (n = 49) and 40.2% (n = 33) males and females, respectively. The male to female ratio of patients was 1.5:1. Most patients (35.4%) (n = 29) were in age group of 18–27 years. Notably, 42.6% (n = 35) patients were from the central region and 20.7% (n = 17) resided in Uganda's eastern region (Table 1).
Table 1.
Sociodemographic characteristics of patients (N = 82).
| Variable | Frequency (n) | Percentage (%) |
|---|---|---|
| Age (years) | ||
| ≤17 | 12 | 14.6 |
| 18–27 | 29 | 35.4 |
| 28–37 | 13 | 15.9 |
| 38–47 | 14 | 17.1 |
| >47 | 14 | 17.1 |
| Sex | ||
| Male | 49 | 59.8 |
| Female | 33 | 40.2 |
| Region of residence | ||
| Western | 4 | 4.9 |
| Mideastern | 4 | 4.9 |
| Southwestern | 16 | 18.3 |
| Central | 34 | 42.6 |
| Eastern | 17 | 20.7 |
| Northern | 4 | 4.9 |
| Missing | 3 | 3.7 |
Clinical characteristics of patients and anatomical location of tumour
Table 2 presents the clinical characteristics of patients included in the study. Nearly 66.3% (n = 69) patients were presenting a painless swelling involving the lower jaw (mandible). The second common presenting clinical feature was mobile teeth in 17.3% (n = 18) patients. Patients presenting mobile and displaced teeth constituted only 1.0% (n = 1) of the entire study sample.
Table 2.
Clinical presentation of ameloblastoma patients in this study (N = 104).
| Clinical features | Frequency (n) | Percentage (%) |
|---|---|---|
| Painless mass | 69 | 66.3 |
| Teeth mobility | 18 | 17.3 |
| Ulcerative mass | 5 | 4.8 |
| Displaced teeth | 2 | 1.9 |
| Painful mass | 9 | 8.7 |
| Mobile and displaced teeth | 1 | 1.0 |
Regarding involvement of different anatomical sites of the oral cavity, more than three-quarters, that is, 78% (n = 64) patients had mandibular involvement and only 1.2% (n = 1) patients had a tumour involving both the mandible and maxilla (Table 3). Observably, in the case of frequency of involvement of the mandible bone with a tumour, the ramus was involved in 32.9% (n = 27) patients followed by the angle of the mandible, which comprised 26.8% (n = 22).
Table 3.
Oral anatomical site involvement and mandibular involvement ameloblastoma among the study patients (N = 82).
| Variable | Frequency (n) | Percentage (%) |
|---|---|---|
| Oral anatomical site involvement | ||
| Mandible | 64 | 78.0 |
| Maxilla | 7 | 8.5 |
| Mandible and maxilla | 1 | 1.2 |
| Palate | 2 | 2.4 |
| Missing | 8 | 9.8 |
| Side of the mandible bone involvement | ||
| Angle | 22 | 26.8 |
| Ramus | 27 | 32.9 |
| Anterior | 18 | 22.0 |
| Missing | 15 | 18.9 |
Histopathological patterns of ameloblastomas
Table 4 shows the histopathological patterns of ameloblastoma. A follicular pattern was the most predominant histopathological pattern 39.0% (n = 32) followed by plexiform pattern, accounted for 31.7% (n = 26). Considering the distribution of histopathological patterns according to patients’ sex, in the present study (Figure 2), follicular pattern was the most common type 28.0% (n = 23) among males whereas plexiform pattern was dominant among females comprising 18.3% (n = 15). Desmoplastic pattern was equal, 6.1% (n = 5) for both males and females. Other histopathological patterns were as shown in Figure 2. Table 5 presents the distribution of different histopathological patterns according to biological behaviour. Most of the non-recurrent cases 35.4% (n = 29) were follicular pattern followed by plexiform pattern which comprised 20.7% (n = 17). In the recurrent cases, there was a relatively higher number of plexiform patterns 11.0% (n = 9), unlike other histopathological patterns. Figure 3(a)–(d) show the histopathological patterns of plexiform, follicular, desmoplastic, and papilliferous types, respectively.
Table 4.
Histopathological patterns of ameloblastoma among the study patients (N = 82).
| Histopathological pattern | Frequency (n) | Percentage (%) |
|---|---|---|
| Plexiform | 26 | 31.7 |
| Desmoplastic | 10 | 12.2 |
| Follicular | 32 | 39.0 |
| Papilliferous | 8 | 9.8 |
| Acanthomatous | 5 | 6.1 |
| Basal cell type | 1 | 1.2 |
Figure 2.
Distribution of histopathological patterns of ameloblastoma according to patients' sex (N = 82).
Table 5.
Histopathological patterns according to the tumour's biological behaviour (N = 82).
| Histopathological pattern | Ameloblastoma biological behaviour |
Total: n (%) | |
|---|---|---|---|
| Non-recurrent: n (%) | Recurrent: n (%) | ||
| Follicular | 29 (35.4) | 3 (3.7) | 32 (39.0) |
| Plexiform | 17 (20.7) | 9 (11.0) | 26 (31.7) |
| Desmoplastic | 4 (4.9) | 6 (7.3) | 10 (12.2) |
| Papilliferous | 7 (8.5) | 1 (1.2) | 8 (9.8) |
| Acanthomatous | 5 (6.1) | 0 (0.0) | 5 (6.1) |
| Basal cell | 1 (1.2) | 0 (0.0) | 1 (1.2) |
Figure 3.
(a) Plexiform pattern of ameloblastoma (H and E stains, ×100). (b) Follicular pattern of ameloblastoma (H and E stains, ×200). (c) Desmoplastic pattern of ameloblastoma (H and E stains, ×100). (d) Papilliferous pattern of ameloblastoma (H and E stains, ×100).
Biological behaviour of ameloblastoma
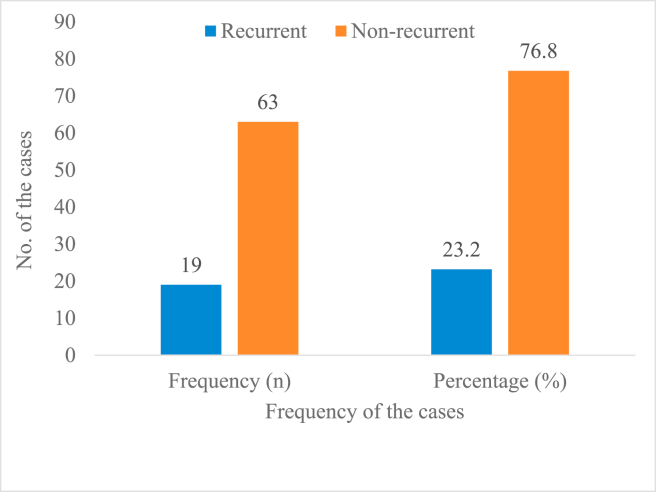
Figure 4 shows the classification of ameloblastoma according to their biological behaviour or characteristics. The vast majority 76.8% (n = 63) were non-recurrent and the remaining 23.2% (n = 19) cases were recurrent. None of the cases included in this study had malignant transformation biological behaviour (neither ameloblastic carcinoma nor malignant ameloblastoma). Based on patients’ age, a significant number of recurrent 13.4% (n = 11) and non-recurrent cases 46.3% (n = 38) were found among those aged between 18 and 44 years. None of the recurrent cases were found in patients below 18 years (Figure 5). Moreover, over half the recurrent cases 52.6% (n = 10) were found among males compared to 47.4% (n = 9) for females (Figure 6).
Figure 4.
Biological behaviour of ameloblastoma among patients (N = 82).
Figure 5.
Biological behaviour of ameloblastoma by patients' age group (N = 82).
Figure 6.
Distribution of cases for biological behaviour according to sex (N = 82).
Regarding duration of recurrence, mean age for the period of recurrence among patients was 50.21 ± 3.527 months with range of 8–156 months. Of the 19 cases that had recurrence, over half 52.6% (n = 10) developed recurrence after a period of more than 37 months. One patient (5.3%) developed recurrence after 13 years and another one (5.3%) had recurrence within 8 months following treatment (Table 6).
Table 6.
Duration for tumour recurrence among ameloblastoma patients (N = 19).
| Duration for recurrence (months) | Frequency (n) | Percentage (%) |
|---|---|---|
| 8–17 | 2 | 2.4 |
| 18–27 | 4 | 4.9 |
| 28–37 | 3 | 3.7 |
| >37 | 10 | 12.2 |
Treatment modalities and surgical complications
The majority 74.4% (n = 61) of cases were treated by conservative surgery, and the remaining 25.6% (n = 21) were treated by radical surgery. Patients that had recurrence, only 2.4% (n = 2) were treated by radical surgery compared to 20.7% (n = 17) who underwent conservative surgery. Among patients who developed recurrence, 73.7% (n = 14) patients required a second surgery, and 26.3% (n = 5) patients required a third surgery. Regarding surgical complications, 15.9% (n = 13) patients developed wound infections and 4.9% (n = 4) had plate exposure (Table 7).
Table 7.
Treatment modalities and surgical complications among the study patients (N = 82).
| Treatment modality | Frequency n (%) | Recurrent n (%) | Non-recurrent n (%) |
|---|---|---|---|
| Conservative surgery | |||
| Enucleation | 20 (24.4) | 11 (13.4) | 9 (11.0) |
| Marginal resection | 24 (29.3) | 3 (3.7) | 21 (25.6) |
| Partial mandibulectomy | 17 (20.7) | 3 (3.7) | 17 (20.7) |
| Radical surgery | |||
| Maxillectomy | 6 (7.3) | 2 (2.4) | 11 (13.4) |
| Total mandibulectomy | 15 (18.3) | 0 (0.0) | 8 (9.6) |
Discussion
This study describes the histopathological patterns and biological characteristics of ameloblastoma in a cohort of Ugandan patients.
Regarding the histopathological patterns of ameloblastomas presented in this study, follicular type was the most common, accounting for 39% followed by the plexiform pattern at 31.7%. This is similar to the findings of Simon et al.’s study conducted in Tanzania, which found that the follicular type was the most common histopathological pattern, accounting for 51.6%, followed by the plexiform pattern at 23.6%.14 Another study in Nigeria reported that follicular pattern was the most common type (64.9%), while other patterns such as plexiform, desmoplastic, acanthomatous, and basal cell accounted for 13%, 5.2%, 3.9%, and 2.6%, respectively.18
Similar findings were also reported by Gardner et al. in which the follicular pattern was 33.9% and the plexiform pattern was 30.2%.10 However, a study done by Saghravanian et al. in Iran reported findings contrary to the present study and to several other related prior studies in which the plexiform pattern was the most common histopathological pattern at 46.4% followed by follicular (26.8%) and acanthomatous (7.1%).19 Another related review article of Cadavid et al. found the plexiform pattern to be the predominant histopathological pattern (40%), followed by follicular pattern (36%), and other histopathological patterns (24%).11
Ameloblastoma is a locally invasive and highly aggressive tumour with a strong propensity for recurrence and metastasis.20 In this study, the recurrence rate was 23.2%. The recurrence rate reported by Gardner et al.10 was 29.5%, which is slightly higher than the recurrence rate in the current study. Yang et al. reported a recurrence rate of 9.8%, including 890 cases of ameloblastoma and 72 cases had recurrence.21 This is lower than the recurrence rate of 23.2% reported in this study. Another study reported that a surgical margin of less than 2 cm, treatment by curettage or enucleation, and the solid/multicystic feature of the tumour increased the recurrence potential from 55% to 90%.21,22 Ameloblastoma has been reported to occur more frequently from the second to fourth decade of life.23 This is similar to our observation in this study, where over half (59.8%) the cases were found in the same age group, that is, between 18 and 44 years.
In our study, non-recurrent ameloblastomas comprised the vast majority (76.8%) of cases. This observation is a common finding even in previous studies done in both developed and developing countries. For example, studies conducted in Iran and Kenya reported that 96.6% and 84.8% of ameloblastoma cases were non-recurrent, respectively.19,24 From these findings, it can be understood that ameloblastoma is generally a non-recurrent tumour. However, it is not very clear why ameloblastoma becomes aggressive. A few prior studies identify several factors most likely to be responsible for the progression and locally invasive changes, including location in the maxilla, being solid/multicystic in nature, and apoptotic changes in the peripheral basal layer of the tumour. Another molecular aspect for ameloblastoma to progress is the presence of matrix metalloproteinases, which are responsible for matrix degradation during tumour growth, invasion, and induction of angiogenesis.25
Recurrence, which is a biological behaviour of ameloblastoma apart from malignancy transformation, has been reported to occur in young adults to the fifth decade of their life, according to the study conducted in China that involved 87 recurrent cases of ameloblastoma.21 This is consistent with the present study's finding that recurrence was found to occur in young adults up to almost the fifth decade of life (between 18 and 44 years) by 57.9% (n = 11) and was seen to decrease after this age to 47.3%. There was no recurrence below 18 years, although the onset of ameloblastoma was seen to occur in children less than 12 years of age.
The review articles by Pogrel et al. and Gardner et al. reported that recurrence of ameloblastoma is possible at 20 years after the initial surgical removal of the tumour.10,22 This period of recurrence from the initial surgical removal of the tumour is higher than the 13 years of recurrence reported in this study. Other studies found the minimum period of recurrence at 34 months and 104.9 months.21,24 This variation in the time of recurrence, as reported in some previous studies, has been associated with conservative surgery, surgical margins less than 2 cm, and the solid/multicystic nature of the benign tumour.21,22
The recurrence rate varies in the different studies reported previously. In this study, the recurrence rate was higher in the plexiform pattern (11%) followed by the desmoplastic pattern (7.3%). The association between histopathological patterns and recurrence seems to be contradictory. In Regezi et al.’s review article reported that 29.5% of the recurrent cases were of the follicular pattern followed by the plexiform pattern at 16.7%, which is different from the finding in our study.26 However, Anne et al.’s study reported that the plexiform pattern had higher recurrence rate because of higher levels of matrix metalloproteinase (MMP), which is similar to the present study finding.27 Furthermore, Gardner et al. found no recurrence in cases with the desmoplastic pattern, whereas cases with basal cell type pattern had a recurrence rate of 50%.11 This is also in disagreement with the finding of our study in which the desmoplastic pattern was the second most common recurrent histopathological pattern.
The implications of the difference in recurrence rate for the compared studies include the difference in the number of patients treated by conservative surgery, for example, curettage compared to those treated by radical surgery. The rate of recurrence is usually higher in studies with a large number of patients treated by conservative surgery compared to studies in which most of the patients underwent radical surgery. In one study it was reported that the recurrence rate after radical surgery ranges from 13% to 15% compared to the recurrence rate of 90–100% after curettage.28 In addition, the difference in the number of patients with solid/multicystic and peripheric histopathological patterns may also explain the difference in the recurrence rate for the various studies reported in the literature. This is because such pattens have a high recurrence rate compared to unicystic ameloblastomas, which have a low recurrence potential.15
Conclusion
Most of the ameloblastoma tumours included in this study were benign, only a few of them were recurrent and none of them was malignant. The most common histopathological pattern of ameloblastoma in this study was follicular followed by plexiform. For recurrent cases, plexiform was the predominant histopathological pattern in this study followed by the desmoplastic pattern. The duration of recurrence in this study was found to be more than 10 years, following the initial removal of the primary tumour. This indicates that ameloblastoma has a long duration of recurrence and, therefore, there is need for long term monitoring for occurrence of recurrence.
Recommendations
Based on our study findings, we may recommend that patients who are diagnosed with both plexiform and desmoplastic histopathological patterns of ameloblastoma should be closely monitored for follow-up after the initial surgical removal of the primary tumour due to high recurrence potential.
Source of funding
This research receives no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
This study was approved by the institution review board of the School of Biomedical Sciences (SOBS) (SBS-HDREC-655: issued on 23 November 2019) of Makerere college of Health Science (MakCHS), a constituent of Makerere University.
Authors’ contribution
PB: conceptualization, designing, data curation, data analysis, and writing the first draft, JJY: methodology, in-depth literature search, and writing the first draft of the manuscript, GO: supervision, designing, methodology, and writing the first draft of the manuscript, and HW: supervision, designing, methodology, and writing the first draft of the manuscript. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
Acknowledgement
We are grateful to the Uganda Cancer Institute (UCI) for their support.
Footnotes
Peer review under responsibility of Taibah University.
References
- 1.Celur S., Babu K.S. Plexiform ameloblastoma. Int J Clin Pediatr Dent. 2012;5(1):78. doi: 10.5005/jp-journals-10005-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ochsenius G., Ortega A., Godoy L., Peñafiel C., Escobar E. Odontogenic tumors in Chile: a study of 362 cases. J Oral Pathol Med. 2002;31(7):415–420. doi: 10.1034/j.1600-0714.2002.00073.x. [DOI] [PubMed] [Google Scholar]
- 3.Ladeinde A.L., Ajayi O.F., Ogunlewe M.O., Adeyemo W.L., Arotiba G.T., Bamgbose B.O., et al. Odontogenic tumors: a review of 319 cases in a Nigerian teaching hospital. Oral Surgery. Oral Med Oral Pathol Oral Radiol Endodontol. 2005;99(2):191–195. doi: 10.1016/j.tripleo.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 4.Cestaro G., De Monti M., Alkayyali S., Fasolini F., Salmoiraghi F. Umbilical metastasis mimicking symptomatic hernia: report of a case of sister mary Joseph syndrome. Int J Surg Case Rep. 2017;41:105–106. doi: 10.1016/j.ijscr.2017.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamulegeya A., Kalyanyama B.M. Oral maxillofacial neoplasms in an East African population a 10 year retrospective study of 1863 cases using histopathological reports. BMC Oral Health. 2008;8(1):1–11. doi: 10.1186/1472-6831-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kallianpur S., Jadwani S., Misra B., Sudheendra U.S. Ameloblastic carcinoma of the mandible: report of a case and review. J Oral Maxillofac Pathol. 2014;18(5):96–102. doi: 10.4103/0973-029X.141336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pereira F. de AC., Melo L. de A., Gurgel C.A.S., Cangussu M.C.T., Azevedo RA de, Santos JN dos. Clinicopathological and demographic characteristics of ameloblastomas in a population from Bahia, Brazil. Rev Odonto Ciência. 2010;25(3):250–255. [Google Scholar]
- 8.Ebenezer V., Ramalingam B. A cross-sectional survey of prevalence of odontogenic tumours. J Maxillofac Oral Surg. 2010;9(4):369–374. doi: 10.1007/s12663-011-0170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krishnapillai R., Angadi P.V. A clinical, radiographic, and histologic review of 73 cases of ameloblastoma in an Indian population. Quintessence Int. 2010;41(5):e90–100. [PubMed] [Google Scholar]
- 10.Gardner D.G. Critique of the 1995 review by Reichart et al. of the biologic profile of 3677 ameloblastomas. Oral Oncol. 1999;35(4):443–449. doi: 10.1016/s1368-8375(99)00012-3. [DOI] [PubMed] [Google Scholar]
- 11.Cadavid A.M.H., Araujo J.P., Coutinho-Camillo C.M., Bologna S., Junior C.A.L., Lourenço S.V. Ameloblastomas: current aspects of the new WHO classification in an analysis of 136 cases. Surg Exp Pathol. 2019;2(1):4–9. [Google Scholar]
- 12.Laborde A., Nicot R., Wojcik T., Ferri J., Raoul G. Ameloblastoma of the jaws: management and recurrence rate. Eur Ann Otorhinolaryngol Head Neck Dis. 2017;134(1):7–11. doi: 10.1016/j.anorl.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Bachmann A.M., Linfesty R.L. Ameloblastoma, solid/multicystic type. Head Neck Pathol. 2009;3(4):307–309. doi: 10.1007/s12105-009-0144-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simon E.N.M., Merkx M.A.W., Vuhahula E., Ngassapa D., Stoelinga P.J.W. A 4-year prospective study on epidemiology and clinicopathological presentation of odontogenic tumors in Tanzania. Oral Surgery. Oral Med Oral Pathol Oral Radiol Endodontol. 2005;99(5):598–602. doi: 10.1016/j.tripleo.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Barboza C.A.G., Pereira Pinto L., Freitas R.D.A., Costa A.D.L.L., De Souza L.B. Proliferating cell nuclear antigen (PCNA) and p53 protein expression in ameloblastoma and adenomatoid odontogenic tumor. Braz Dent J. 2005;16(1):56–61. doi: 10.1590/s0103-64402005000100010. [DOI] [PubMed] [Google Scholar]
- 16.Sehdev M.K., Huvos A.G., Strong E.W., Gerold F.P., Willis G.W. Ameloblastoma of maxilla and mandible. Cancer. 1974;33(2):324–333. doi: 10.1002/1097-0142(197402)33:2<324::aid-cncr2820330205>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 17.Dhir K., Sciubba J., Tufano R.P. Ameloblastic carcinoma of the maxilla. Oral Oncol. 2003;39(7):736–741. doi: 10.1016/s1368-8375(03)00036-8. [DOI] [PubMed] [Google Scholar]
- 18.Okada H., Yamamoto H., Tilakaratne W.M. Odontogenic tumors in Sri Lanka: analysis of 226 cases. J Oral Maxillofac Surg. 2007;65(5):875–882. doi: 10.1016/j.joms.2006.06.293. [DOI] [PubMed] [Google Scholar]
- 19.Saghravanian N., Salehinejad J., Ghazi N., Shirdel M., Razi M. A 40-year retrospective clinicopathological study of ameloblastoma in Iran. Asian Pac J Cancer Prev APJCP. 2016;17(2):619–623. doi: 10.7314/apjcp.2016.17.2.619. [DOI] [PubMed] [Google Scholar]
- 20.Abdel-Aziz A., Amin M.M. EGFR, CD10 and proliferation marker Ki67 expression in ameloblastoma: possible role in local recurrence. Diagn Pathol. 2012;7(1) doi: 10.1186/1746-1596-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang R., Liu Z., Gokavarapu S., Peng C., Cao W., Ji T. Recurrence and cancerization of ameloblastoma : multivariate analysis of 87 recurrent craniofacial ameloblastoma to assess risk factors associated with early recurrence and secondary ameloblastic carcinoma. Chin J Cancer Res. 2017;29(3):189–195. doi: 10.21147/j.issn.1000-9604.2017.03.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pogrel M.A., Dds D.M.M. Is there a role for enucleation in the management of ameloblastoma? Int J Oral Maxillofac Surg. 2009:807–812. doi: 10.1016/j.ijom.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 23.Aregbesola B., Soyele O., Effiom O., Gbotolorun O., Taiwo O. Odontogenic tumours in Nigeria : a multicentre study of 582 cases and review of the literature. Med Oral, Patol Oral Cirugía Bucal. 2018;23(6):3–8. doi: 10.4317/medoral.22473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adeline V.L., Dimba E.A.O., Wakoli K.A., Njiru A.K., Awange D.O., Onyango J.F., et al. Clinicopathologic features of ameloblastoma in Kenya: a 10-year audit. J Craniofac Surg. 2005:1589–1593. doi: 10.1097/SCS.0b013e31818c0504. [DOI] [PubMed] [Google Scholar]
- 25.Sandra F., Hendarmin L., Kukita T., Nakao Y. Ameloblastoma induces osteoclastogenesis : a possible role of ameloblastoma in expanding in the bone. Oral Oncol. 2005:637–644. doi: 10.1016/j.oraloncology.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 26.Regezi J.A., Kerr D.A. Odontogenic tumors: analysis of 706 cases. J Oral Surg. 1978:771–778. [PubMed] [Google Scholar]
- 27.Anne R., Krisnuhoni E. Matrix metalloproteinase-9 ( MMP-9 ) expression in different subtypes of ameloblastoma. J Maxillofacial and Oral Surg. 2014;13(3):281–285. doi: 10.1007/s12663-013-0538-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chapelle K.A.O.M., Stoelinga P.J.W., Wilde PCM De, Brouns J.J.A., Voorsmit R.A.C.A. Rational approach to diagnosis and treatment of ameloblastomas and odontogenic keratocysts. Br J Oral Maxillofac Surg. 2004;42:381–390. doi: 10.1016/j.bjoms.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Aregbesola B, Soyele O, Effiom O, Gbotolorun O, Taiwo O, Amole I. Odontogenic tumours in Nigeria: a multicentre study of 582 cases and review of the literature. Med Oral Patol Oral Cir Bucal. 2018 Nov 1;23(6):e761–e766. doi: 10.4317/medoral.22473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siriwardena B.S.M.S., Crane H, O’Neill N, Abdelkarim R, Brierley DJ., Franklin CD., et al. Odontogenic tumors and lesions treated in a single specialist oral and maxillofacial pathology unit in the United Kingdom in 1992–2016. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019 Feb;127(2):151–166. doi: 10.1016/j.oooo.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 31.Avelar RL, Antunes AA, Santos Thiago de Santana, Andrade Emanuel Sávio de Souza, Dourado Edwaldo. Odontogenic tumors: clinical and pathology study of 238 cases. Br J Otorhinolaryngol. 2008;74(5):668–673. doi: 10.1016/S1808-8694(15)31375-6. [DOI] [PMC free article] [PubMed] [Google Scholar]