Abstract
The psychological trauma associated with events resulting in traumatic brain injury (TBI) is an important and frequently overlooked factor that may impede brain recovery and worsen mental health following a TBI. Indeed, individuals with comorbid post-traumatic stress disorder (PTSD) and TBI have significantly poorer clinical outcomes than individuals with a sole diagnosis. Emotion dysregulation is a common factor leading to poor cognitive and affective outcomes following TBI. Here we synthesize how acute post-injury molecular processes stemming either from physical or emotional trauma may adversely impact circuitry subserving emotion regulation, and ultimately yield long-term systems-level functional and structural changes that are common to TBI and PTSD. In the immediate aftermath of traumatic injury, glucocorticoids stimulate excess glutamatergic activity, particularly in prefrontal cortex-subcortical circuitry implicated in emotion regulation. In human neuroimaging work, assessing this same circuitry well after the acute injury, TBI and PTSD show similar impacts on prefrontal and subcortical connectivity and activation. These neural profiles indicate that emotion regulation may be a useful target for treatment, including for early intervention to prevent the adverse sequelae of TBI. Ultimately, the success of future TBI and PTSD early interventions depends on the fields’ ability to address both the physical and emotional impact of physical injury.
Keywords: traumatic brain injury, posttraumatic stress disorder, postconcussion syndrome, neuroimaging, HPA axis, glutamate
The Mental Health Consequences of Traumatic Injuries
Each year, more than 50 million people worldwide experience a traumatic brain injury (TBI;1,2). There is significant overlap in post-injury outcomes that result from the physical damage of TBI (i.e., white matter degradation, neuronal loss, neuroinflammation, etc.), the emotional response to the trauma, and the distress related to both physical and psychological symptoms of the TBI and injury event. This combination of physical and emotional trauma increases risk for both acute concussion and stress symptoms as well as the development of persistent postconcussion syndrome (PCS). Both physical and emotional trauma are associated with multiple chronic psychiatric conditions including posttraumatic stress disorder (PTSD), major depressive disorder (MDD), general anxiety disorder, and substance use disorder (3-6). Regardless of previous psychiatric history, there is a markedly increased risk of psychiatric disorders after TBI (7), though previous psychiatric history has been shown to exacerbate psychological symptoms after physical trauma (8-10). One possibility for this heightened risk of psychiatric conditions, and associated cognitive, behavioral and affective outcomes, is the presence of acute and chronic emotion dysregulation resulting from physical and/or emotional trauma following traumatic injury.
PTSD is of particular interest after injury, as rates of PTSD are high, ranging from 8 to 40% depending upon mechanism of injury (3). However, prevalence of comorbid PTSD and TBI is more difficult to estimate due to numerous factors including, but not limited to, different sample types (civilian vs. military) and sizes, differences in mechanism of injury, and methodological differences in obtaining PTSD and TBI history. Still, a recent 2020 meta-analysis that combined military and civilian samples reported 27% of those with TBI also met criteria for PTSD, compared to only 11% without TBI who met criteria for PTSD (11). Furthermore, the relative risk for PTSD following TBI for civilians and military samples was 1.2 and 4.8, respectively (11). A 2014 review further noted prevalence of comorbid PTSD and TBI varied according to severity of TBI, with estimates ranging from 3 to 30% for civilian samples and 12 to 89% in military samples where rates increased with severity of TBI (13). Post concussive syndrome (PCS) can occur in addition to PTSD, with an estimated half of mild TBI cases also presenting with PCS symptoms (14). Up to 25% of individuals with TBI show persistent PCS symptoms after three months post-injury (i.e., chronic PCS) (15). Importantly, these prevalence estimates, regardless of sample or TBI severity, are significantly higher than prevalence of PTSD in the general population (~9%) (11) suggesting a shared pathophysiology leading to common symptoms in PTSD and TBI.
Critically, posttraumatic stress symptoms in the acute post-injury window, or symptoms of Acute Stress Disorder (ASD), have been found to be important markers of risk for not only non-remitting PTSD (16), but also, chronic PCS. For those who experience a traumatic injury, ASD is more likely to occur alongside an mTBI diagnosis. (17). The presence of ASD following TBI also predicts greater severity of chronic PCS (8,10). Moreover, the relationship between PTSD and PCS symptoms becomes stronger as time since injury passes (10). The similarity in rates of PCS and PTSD after TBI is not surprising given the overlap in emotion-related symptoms, particularly hyperarousal (16,18).These findings suggest that acute injury- and stress-driven outcomes contribute to the emotion dysregulation that confers risk for chronic PTSD and PCS following TBI. In fact, this suggests there are common neurobiological pathways underlying the regulation of emotions impacted by both the physical and/or psychological aspects of traumatic injury.
While the physical damage from TBI leads to an established array of symptoms (e.g., dizziness, confusion, memory impairments, irritability, fatigue, difficulty concentrating, etc.), the emotional reactivity to a traumatic event, whether ASD or general anxiety, can mimic many of the same symptoms (e.g., difficulty breathing, headache, stomach pain, nausea; 19,20). Therefore, it is difficult to disentangle the specific effects of the physical trauma of the head injury from the psychological consequences of the injury event (19). However, the combination of PTSD and TBI appear more impactful, or more deleterious when considering outcome, than the effects of PTSD or TBI alone. Still, it remains unclear whether coexisting PTSD and TBI (this comorbidity is subsequently referred to as PTSD + TBI) produces an additive or multiplicative effect of dysregulation on shared emotion regulation neurocircuitry. Unpacking the neural effects and concurrent behavior of PTSD + TBI is of great clinical importance. Studies on clinical outcomes suggest that a comorbid diagnosis of PTSD + TBI worsens outcomes significantly more than the outcomes associated with a single diagnosis (21). For example, veterans diagnosed with PTSD + TBI are at an elevated risk of suicidality compared to veterans with PTSD only (22,23). The impact of PTSD + TBI is also evident when examining the rates of PCS; those with PTSD have greater PCS symptoms after experiencing an mTBI than those without PTSD (24).
In service of understanding co-occurring and interactive post-TBI outcomes, we review evidence that the shared aspects of persistent cognitive and affective effects in PTSD and TBI arise from molecular changes within emotion regulation circuits. We focus on both injured military personnel and civilians, particularly for non-sports-related traumatic injury. In both of these populations, the prevalence of PTSD and severity of symptoms suggests the long-term structural and functional neural effect of PTSD + TBI is greater than the effect of either of these diagnoses alone. Indeed, similarities in symptoms of TBI and PTSD may be the result of overlapping disruption at the neural level of emotion regulation networks (13). Finally, we highlight the need to incorporate assessment of emotion dysregulation alongside evaluation of TBI-specific symptoms.
Post-Trauma Emotion Dysregulation: A Common Framework
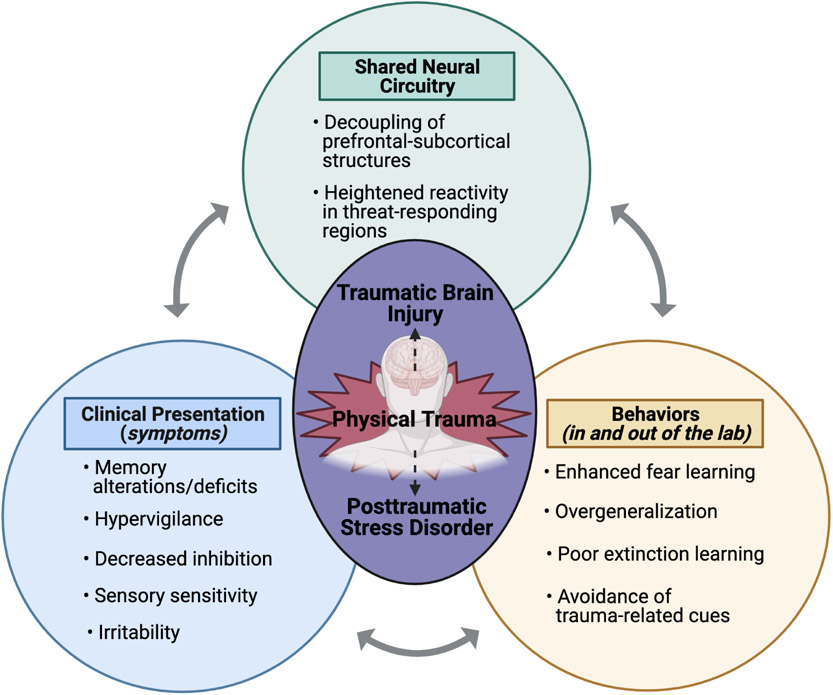
Though reactions to traumatic injury vary across individuals, emotional responses can range from confusion, sadness, and anxiety, to dissociation, depression, and blunted affect (20). One framework shared by research on TBI and trauma-related mental health outcomes involves the role of emotion regulation and the aberrations in brain regions critical for emotion regulation (Figure 1). Emotion regulation broadly refers to complex processes underlying how an individual experiences and expresses emotions (25,26). There is significant overlap in the clinical presentation, behaviors, and neural consequences of PTSD and TBI – many of which can be conceptualized as aspects involving or resulting from emotional dysregulation. Dysregulation may represent poor “bottom-up” processing of emotionally-relevant information (e.g., problems with detecting emotions) or maladaptive “top-down” control of emotions (e.g., difficulty engaging an appropriate coping strategy; 25,26).
Figure 1.
An overview of emotion dysregulation in PTSD and TBI: shared clinical presentations, behaviors, and neural circuitry. Figure created with BioRender.com.
Multiple aspects of emotion dysregulation have been linked with chronic poor outcomes of both TBI and non-TBI trauma. Indeed, two of the defining symptom clusters of PTSD involve emotion dysregulation (i.e., re-experiencing/intrusive thoughts and memories, cognitive and emotional avoidance of trauma reminders) (30). Similarly, in addition to physical and physiological symptoms, TBI is often accompanied by changes in mood and cognition (i.e., increased irritability and decreased inhibition), both of which are associated with regulation of emotion (13). Shared behavioral presentations of PTSD and TBI include enhanced fear learning, fear generalization, and avoidance of trauma-related reminders (27-29). We posit that these behavioral profiles are driven by changes in emotion regulation circuitry (reviewed below).
The neural bases of emotion and emotion regulation have been well-established and involve both cortical and subcortical brain regions (for review see: 32-35). Briefly, motivational features of an emotional stimulus engage primarily subcortical regions, including the amygdala, hippocampus, striatum, periaqueductal grey (PAG), as well as the ventromedial prefrontal cortex (PFC) (32,34). Implicit and explicit (i.e., with or without conscious effort) emotion regulation involves complex bidirectional relationships among PFC-subcortical circuitry (26,33). For instance, threat detection and monitoring, which is heightened in PTSD (35), involves deficient cortical modulation of subcortical structures (i.e., inhibited anterior cingulate cortex (ACC) and reduced PFC modulation of amygdala, striatum, and PAG) (26,33). Subsequent adaptive conscious reappraisal of threat is achieved through increased modulation of ACC and PFC over limbic regions (26,31,32). Collectively, subcortical structures subserving emotional response and PFC regulation of response form the core circuitry for emotion regulation. Molecular changes resulting from physical and/or emotional trauma directly impact emotion regulation circuitry giving rise to the shared emotion dysregulation features of PTSD and TBI.
Acute Stress-Related Molecular Changes Impact Emotion Regulation Circuitry
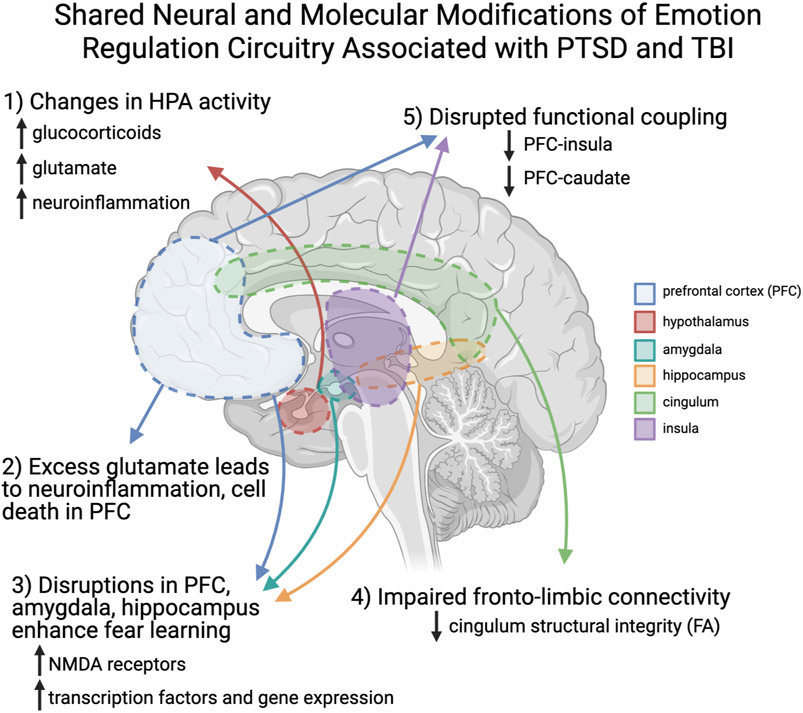
Studies of the acute effects of both non-injury trauma and TBI suggest that a cascade of molecular changes in stress and emotion regulation circuitry (reviewed in Figure 2) may have long-term adverse consequences for this circuitry, increasing risk for PTSD and PCS (36). In this regard, work on the role of neurotransmitters and hormones released through the hypothalamic-pituitary-adrenal (HPA) axis in traumatic injuries has been informative. Briefly, the HPA axis regulates responses to stress by altering levels of neuroendocrine and neural signaling (for review see 39). Poor trauma outcomes are associated with dysregulation of homeostatic HPA functioning (37). TBI causes an initial surge in glucocorticoids (38,39), followed by continued HPA dysregulation that interferences with adaptive responding to stress (40-42). Over time this abnormal stress responding is bidirectionally linked to neuroinflammation, and worse psychiatric and neurocognitive outcomes after TBI (43).
Figure 2.
An Overview of the Shared Neural and Molecular Modifications Associated with PTSD and TBI. Figure created with BioRender.com.
The HPA axis also plays an important role in stimulating glutamate release following stress, which, if excessive, can adversely impact PFC-subcortical circuitry. Glucocorticoid activity following acute non-injury stress increases release of glutamate (44), especially in the PFC and hippocampus (36,45,46). Heightened glucocorticoid secretion (38,47) and excess glutamatergic activity are also evident acutely following TBI (48-50). Thus, similar glucocorticoid HPA-initiated glutamate release is evident immediately following both non-injury emotional stress and TBI. Moreover, administration of glucocorticoid receptor antagonists blocks this increased glutamate release and reduces anxiety and depressive behaviors (45,51). In addition, CB1 receptor gating of glutamate, a mechanism reducing its release, has also been shown to blunt these adverse effects on PFC (52). Left unchecked, excess glutamate results in excitatory toxicity, including dendritic atrophy (53) and cell death (54), as well as inflammation (55,56) in the PFC and hippocampus. Together, these findings suggest that the acute post-trauma stress response following TBI activates this glucocorticoid-glutamate response which contributes to prolonged distress and neurocognitive problems via its impact on circuitry shared by PTSD and PCS symptoms (57).
Preclinical models of TBI offer an opportunity to better explore how TBI impacts specific emotion regulation behaviors subserved by PFC-subcortical circuitry. The numerous molecular and cellular changes resulting from TBI (induced using different experimental methods; models reviewed in 59) occur in expected subcortical structures, including the amygdala and hippocampus (59). Previous work using Pavlovian conditioning with rodents suggests TBI broadly enhances fear learning, as evidenced by enhanced fear acquisition, greater generalization to fear-related stimuli, and impaired fear extinction (27,28). Consistent with the stress-induced dysregulation of glutamatergic activity detailed above, disruption of glutamate receptors also disrupts fear learning and memory (60). Importantly, these findings translate well to human work indicating TBI is associated with abnormal and heightened fear learning (61). Underlying enhanced fear learning are modifications to specific sub-regions of the amygdala and hippocampus. For example, TBI induces up-regulation of the ionotropic NMDA glutamate receptors in sub-regions of the amygdala which supports long-term potentiation, a mechanism by which fear learning and memories can be enhanced and strengthened (28). Additionally, upregulation of transcriptional factors (62) and gene expression in the canonical “fear-network”, including the amygdala (63), PFC, and hippocampus have been identified following TBI.
As reviewed here there are common acute stress-induced molecular changes that occur as a result of physical injury and emotional trauma. These modifications appear to disrupt emotion regulation circuitry and alter fear learning and memory processes common to both PTSD and TBI. Although the bi-directional pathways in which TBI may contribute to PTSD + TBI comorbidity has not been fully elucidated, recent work suggests modifications in sensory systems may create vulnerability to PTSD (64). Indeed, sensitivity to auditory cues and associated increased activation between sensory brain regions and the amygdala was significantly related to greater PTSD-like behavior in rats (64). How higher-order sensory systems may be impacted by TBI and PTSD and the mechanisms by which these systems influence symptoms is a promising direction for future research.
Long-term Overlapping Neural Consequences of Traumatic Injuries Impact Emotion Regulation Circuitry
The chronic symptoms following TBI likely rest on shared impact of acute and persistent stress-induced molecular changes on emotion regulation neurocircuitry. Of note, there is a significant gap in this literature as there are few structural and functional MRI studies evaluating both TBI and psychological symptoms (i.e., PCS and/or PTSD) in either civilian or military samples. While a substantial body of work has examined resting-state fMRI aberrations in TBI along the severity spectrum (65-69), these studies often do not account for possible psychological symptoms that accompany the TBI (e.g., 74). Similarly, studies in the PTSD literature often neglect to account for TBI (69-71) though a few have examined samples with only mTBI (e.g., 71,72) or a “history of head injury” (e.g., 73). Exclusion of participants with moderate to severe TBI during recruitment is common in the PTSD literature as TBI is expected to “contaminate” fMRI signal and analysis of PTSD-related neural processes (e.g., 74). Despite the sizeable gap in this work, a comprehensive review of the few studies that have examined the overlap in the effects of PTSD + TBI, using various neuroimaging techniques, has been done previously (76). The commonalities in PTSD and TBI are apparent in brain structural morphology and functional connectivity (76-78). Here we emphasize how the overlap in regions which underlie emotion regulation may relate to specific affective PTSD and TBI symptoms. Understanding the underlying neurocircuitry of symptoms for those with PTSD + TBI can guide clinical decisions related to treatment, particularly in the acute aftermath of traumatic injury (79).
Diffusion tensor imaging (DTI) has revealed how integrity of white matter tracts in the brain are impacted by TBI and PTSD. The majority of studies using DTI have examined PTSD and TBI separately though ultimately the findings highlight similarities (76). In both military and trauma-exposed civilian samples with mild to severe TBI, abnormalities in fronto-limbic circuits, including cingulum fiber bundles, which connect the cingulate cortex to the hippocampus, have been reported (65,80,81). Though psychological symptoms were not considered in these samples, white matter pathology (decreased fractional anisotropy, an index of structural integrity derived from DTI) increased with severity of TBI (65,81). Similarly, there is a direct relationship between decreased fractional anisotropy (FA) of the cingulum bundles and PTSD symptom severity, as well as chronicity (80,82-84). Similar white matter abnormalities have been described in veterans with comorbid MDD, PTSD, and mTBI, with more significant decreases of FA in individuals with all three diagnoses (85). Indeed, in veteran samples, widespread decreases in cerebral white matter FA, particularly in the cingulum, are pronounced in those with both mTBI and PTSD compared to those with only mTBI or PTSD (86,87). Furthermore, in a veteran sample, the number of regions with reduced white matter FA mediated the relationship between mTBI and PCS symptoms (88). The cingulum bundle has been implicated in attention modulation of emotion and memory (80). Therefore, reduced integrity of this fronto-limbic pathway may lead to aberrant emotion regulation and memory functions (i.e., hyperarousal, intrusive memories, overgeneralization of fear to trauma-related stimuli), affective symptoms common to both PTSD and TBI (80,89). Collectively, these findings demonstrate white matter integrity, specifically in fronto-limbic circuitry that supports emotion regulation, is not only altered by TBI alone as a result of physical injury, but also impacted by and affects the development of psychological symptoms following injury. Still, more longitudinal research is needed to truly determine how white matter integrity reduction, most notably in fronto-limbic pathways, corresponds to the interaction between PTSD and TBI.
Functional magnetic resonance imaging (fMRI) is particularly useful in evaluating how symptoms of TBI and psychiatric disorders may independently and mutually alter functional connectivity at rest (i.e., when the participant is not engaged in a task) and during affective tasks. Civilians with mTBI showed decreased resting-state functional connectivity between the insula and PFC, though neither PCS nor post-traumatic stress symptoms were evaluated (90), even though this decreased connectivity is evident in PTSD. In a veteran sample with mTBI, decoupling of insula-PFC and caudate-PFC connectivity at rest was related to greater posttraumatic intrusion symptoms and more errors processing threatening versus safe stimuli (91). This aligns with the emotion dysregulation framework: the anterior insula contributes to interoceptive awareness, suggesting the observed deficits in PFC inhibition permitted greater internal attention to intrusive thoughts (92). Notably, this reduced connectivity is also linked to impaired cognitive function (e.g., deficits in orientation and abstract thinking) suggesting insula-PFC circuity may underly both TBI and PTSD symptoms (90).
Previous theoretical reviews have implicated the hippocampus, orbitofrontal cortex, and dorsolateral prefrontal cortex (dlPFC) as potential regions where TBI and PTSD may overlap (93,94). Simmons and Matthews (95), conducted a meta-analysis to identify regions which are disrupted in both PTSD and TBI, thereby providing data-driven regions-of-interests for future investigations. Both the caudate, a component of the striatum, and the ACC were identified. The caudate is a region important for associative learning processes (95) and the ACC is implicated in the appraisal of emotional stimuli (26). Together these regions coordinate emotional responses to stimuli and may underlie generalization of responses to trauma and non-trauma related cues. The dlPFC/middle frontal gyrus, a region important for executing goal-directed behavior such as regulating emotional responses (26), also appears vulnerable to both mTBI and PTSD, however, these results have mixed directions, with articles noting both hyper and hypo-activation (95).
Ultimately the structural and functional MRI work indicates the circuitry common to PTSD and TBI involves regions supporting emotion regulation, and that this circuitry is impacted following physical and emotional traumatic injury.
Clinical Implications and Future Directions
The neural consequences of acute injury- and stress-driven changes in the brain from molecular- to systems-level neurocircuitry contribute to the sequelae of emotional dysregulation that confers risk of PCS and ASD, and ultimately risk for lasting symptoms of PTSD and MDD (10). While the impact of TBI on physical and psychological health outcomes has been studied for two decades (96), less clear is how and when to intervene to prevent the negative sequalae of PTSD + TBI. This cascade of neural changes highlights the importance of better understanding the timing and type of interventions used to treat, or ideally prevent, PTSD + TBI and PCS.
Decision making in and timing of clinical care during the “golden-hour” after traumatic injury is critical. In addition to standard medical care, consultation from a clinician trained in trauma-informed care could also improve short- and long-term outcomes (20). Given the substantial overlap in neurocircuitry of PTSD + TBI and affected emotion regulation, it is near impossible to disentangle which condition is driving symptoms. Akin to most bodily injury, the physical damage of TBI takes time to heal even with early medical care. However, early attention to post-traumatic stress symptoms has been shown to improve chronic psychological outcomes (79,97). Therefore, especially in the acute phase, reducing emotional and psychological response to trauma could “free up” bodily resources for healing physical trauma of TBI or improve response to clinical treatments (98,99). However, it is unclear how trauma intensive early treatments are impacted by the symptoms of TBI, a worthy area for future discovery to development PTSD + TBI specific interventions.
Improvement of chronic outcomes can be facilitated by on-going engagement in both physiological/physical and emotional/mental health treatments for the first six months to a year following traumatic injury. For example, psychological intervention after concussion reduces depressive symptoms and risk of PCS (100-102). Cognitive behavioral therapies (CBT) are the gold standard for treating PTSD, and have also been shown to be effective in treating those with long-term PTSD + TBI. Cognitive processing therapy (103) and prolonged exposure therapy (two types of CBT for PTSD; 104), reduce symptoms of PTSD, depression, and PCS. The utility of CBT for not only PTSD but also TBI symptoms may be due in part to CBT leading to functional improvements in brain regions responsible for emotion regulation (105) that are impacted, as outlined above. Moreover, evidence suggests CBT is an effective treatment for ASD + TBI symptoms in the early aftermath of traumatic injury (106), supporting the use of CBT as a secondary prevention technique. Beyond CBT, other common therapeutic techniques (i.e., mindfulness) similarly target emotion regulation processes (20). These promising studies suggests CBT and other therapies known to directly impact emotion regulation circuitry and processes, are effective treatments for TBI symptoms. Early provision of interventions targeting emotion dysregulation may disrupt the cascade of stress-induced molecular changes following TBI, sparing long-term impacts on emotion regulation circuitry and improving TBI and PTSD symptom trajectories. Certainly, additional work is needed to assess the specificity of these early interventions on emotion regulation circuitry as a mediator of PTSD + TBI outcomes.
Improvements in clinical and medical care have significantly reduced mortality rates of TBI (2), however long-term outcomes associated with the emotional and psychological distress of the event leading to injury have been infrequently addressed in the treatment of TBI. Lack of consensus and implementation of best practices in clinical treatment of TBI (e.g., different timing of interventions) may explain variability in chronic outcomes (2). However, administration of psychological interventions to treat trauma-related symptoms should be more readily applied within current TBI treatment protocols. As it remains unclear whether physical damage perpetuates psychological symptoms or vice versa, the medical and psychological clinical care for TBI patients should be weighted equally and a more holistic approach to treatment should be taken.
Emotion dysregulation plays a key role in the shared outcomes of PTSD and TBI as evidenced by the overlap in symptom presentation (i.e., hypervigilance, memory deficits, sensory sensitivity, irritability, etc.), behavior (i.e., avoidance, overgeneralization of fear), and shared neural circuitry. Ultimately, the field needs additional well-controlled studies, specifically framed through the lens of emotion regulation, to determine best practice for prevention and intervention to improve long term quality of life for patients with PTSD + TBI.
Acknowledgements
Christine Larson was supported by NIH grants R01 MH124076, U24 DA041147, and R01 MH106574. Terri deRoon-Cassini was supported by R56 MH116656-01A1. E.K.W. was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Numbers 2UL1TR001436 and 2TL1TR001437.
Footnotes
Disclosures
None of the authors have biomedical financial interests or potential conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dewan MC, Rattani A, Gupta S, Baticulon RE, Hung Y-C, Punchak M, et al. (2018): Estimating the global incidence of traumatic brain injury. J Neurosurg 1–18. [DOI] [PubMed] [Google Scholar]
- 2.Maas AIR, Menon DK, Adelson PD, Andelic N, Bell MJ, Belli A, et al. (2017): Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol 16: 987–1048. [DOI] [PubMed] [Google Scholar]
- 3.Bryant RA, O’Donnell ML, Creamer M, McFarlane AC, Clark CR, Silove D (2010): The Psychiatric Sequelae of Traumatic Injury. Am J Psychiatry 167: 312–320. [DOI] [PubMed] [Google Scholar]
- 4.Silver JM, Kramer R, Greenwald S, Weissman M (2001): The association between head injuries and psychiatric disorders: findings from the New Haven NIMH Epidemiologic Catchment Area Study. Brain Inj 15: 935–945. [DOI] [PubMed] [Google Scholar]
- 5.Stocchetti N, Zanier ER (2016): Chronic impact of traumatic brain injury on outcome and quality of life: a narrative review. Crit Care 20: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Praag DLG, Cnossen MC, Polinder S, Wilson L, Maas AIR (2019): Post-Traumatic Stress Disorder after Civilian Traumatic Brain Injury: A Systematic Review and Meta-Analysis of Prevalence Rates. J Neurotrauma 36: 3220–3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashman TA, Spielman LA, Hibbard MR, Silver JM, Chandna T, Gordon WA (2004): Psychiatric challenges in the first 6 years after traumatic brain injury: cross-sequential analyses of axis I disorders11No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit upon the authors(s) or upon any organization with which the author(s) is/are associated. Arch Phys Med Rehabil 85: 36–42. [DOI] [PubMed] [Google Scholar]
- 8.Broshek DK, De Marco AP, Freeman JR (2015): A review of post-concussion syndrome and psychological factors associated with concussion. Brain Inj 29: 228–237. [DOI] [PubMed] [Google Scholar]
- 9.Leddy JJ, Baker JG, Wilier B (2016): Active Rehabilitation of Concussion and Post-concussion Syndrome. Phys Med Rehabil Clin N Am 27: 437–454. [DOI] [PubMed] [Google Scholar]
- 10.Meares S, Shores EA, Taylor AJ, Batchelor J, Bryant RA, Baguley IJ, et al. (2011): The prospective course of postconcussion syndrome: the role of mild traumatic brain injury. Neuropsychology 25: 454–465. [DOI] [PubMed] [Google Scholar]
- 11.Loignon A, Ouellet M-C, Belleville G (2020): A Systematic Review and Meta-analysis on PTSD Following TBI Among Military/Veteran and Civilian Populations. J Head Trauma Rehabil 35: E21–E35. [DOI] [PubMed] [Google Scholar]
- 12.Alway Y, McKay A, Gould KR, Johnston L, Ponsford J (2016): Factors associated with posttraumatic stress disorder following moderate to severe traumatic brain injury: a prospective study: Research Article: Predictors of PTSD Following TBI. Depress Anxiety 33: 19–26. [DOI] [PubMed] [Google Scholar]
- 13.Bahraini NH, Breshears RE, Hernández TD, Schneider AL, Forster JE, Brenner LA (2014): Traumatic Brain Injury and Posttraumatic Stress Disorder. Psychiatr Clin North Am 37: 55–75. [DOI] [PubMed] [Google Scholar]
- 14.King NS (2003): Post-concussion syndrome: clarity amid the controversy? Br J Psychiatry 183: 276–278. [DOI] [PubMed] [Google Scholar]
- 15.Whittaker R, Kemp S, House A (2007): Illness perceptions and outcome in mild head injury: a longitudinal study. J Neurol Neurosurg Psychiatry 78: 644–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bryant RA (2011): Acute stress disorder as a predictor of posttraumatic stress disorder: a systematic review. J Clin Psychiatry 72: 233–239. [DOI] [PubMed] [Google Scholar]
- 17.Broomhall LGJ, Clark CR, McFarlane AC, O’Donnell M, Bryant R, Creamer M, Silove D (2009): Early Stage Assessment and Course of Acute Stress Disorder After Mild Traumatic Brain Injury. J Nerv Ment Dis 197: 178–181. [DOI] [PubMed] [Google Scholar]
- 18.Qureshi KL, Upthegrove R, Toman E, Sawlani V, Davies DJ, Belli A (2019): Post-traumatic stress disorder in UK civilians with traumatic brain injury: an observational study of TBI clinic attendees to estimate PTSD prevalence and its relationship with radiological markers of brain injury severity. BMJ Open 9: e021675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore EL, Terryberry-Spohr L, Hope DA (2006): Mild traumatic brain injury and anxiety sequelae: A review of the literature. Brain Inj 20: 117–132. [DOI] [PubMed] [Google Scholar]
- 20.Substance Abuse and Mental Health Services Administration (2014): Understanding the Impact of Trauma. Trauma-Informed Care in Behavioral Health Services. Rockville, MD: HHS Publication No. (SMA) 13–4801. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK207201/pdf/Bookshelf_NBK207201.pdf [PubMed] [Google Scholar]
- 21.Marshall RD, Olfson M, Hellman F, Blanco C, Guardino M, Struening EL (2001): Comorbidity, Impairment, and Suicidality in Subthreshold PTSD. Am J Psychiatry 158: 1467–1473. [DOI] [PubMed] [Google Scholar]
- 22.Blakey SM, Wagner HR, Naylor J, Brancu M, Lane I, Sallee M, et al. (2018): Chronic Pain, TBI, and PTSD in Military Veterans: A Link to Suicidal Ideation and Violent Impulses? J Pain 19: 797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finley EP, Bollinger M, Noël PH, Amuan ME, Copeland LA, Pugh JA, et al. (2015): A national cohort study of the association between the polytrauma clinical triad and suicide-related behavior among US Veterans who served in Iraq and Afghanistan. Am J Public Health 105: 380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aase DM, Babione JM, Proescher E, Greenstein JE, DiGangi JA, Schroth C, et al. (2018): Impact of PTSD on post-concussive symptoms, neuropsychological functioning, and pain in post-9/11 veterans with mild traumatic brain injury. Psychiatry Res 268: 460–466. [DOI] [PubMed] [Google Scholar]
- 25.Mennin DS, Heimberg RG, Turk CL, Fresco DM (2002): Applying an emotion regulation framework to integrative approaches to generalized anxiety disorder. Clin Psychol Sci Pract 9: 85–90. [Google Scholar]
- 26.Fitzgerald JM, DiGangi JA, Phan KL (2018): Functional Neuroanatomy of Emotion and Its Regulation in PTSD: Harv Rev Psychiatry 26: 116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffman AN, Taylor AN (2019): Stress reactivity after tbi, implications for comorbid ptsd. Behav Pharmacol 30: 115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reger ML, Poulos AM, Buen F, Giza CC, Hovda DA, Fanselow MS (2012): Concussive brain injury enhances fear learning and excitatory processes in the amygdala. Biol Psychiatry 71: 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wijenberg MLM, Stapert SZ, Verbunt JA, Ponsford JL, Van Heugten CM (2017): Does the fear avoidance model explain persistent symptoms after traumatic brain injury? Brain Inj 31: 1597–1604. [DOI] [PubMed] [Google Scholar]
- 30.American Psychiatric Association (2013): Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. American Psychiatric Association. 10.1176/appi.books.9780890425596 [DOI] [Google Scholar]
- 31.Dixon ML, Thiruchselvam R, Todd R, Christoff K (2017): Emotion and the prefrontal cortex: An integrative review. Psychol Bull 143: 1033–1081. [DOI] [PubMed] [Google Scholar]
- 32.Etkin A, Büchel C, Gross JJ (2015): The neural bases of emotion regulation. Nat Rev Neurosci 16: 693–700. [DOI] [PubMed] [Google Scholar]
- 33.Martin RE, Ochsner KN (2016): The neuroscience of emotion regulation development: implications for education. Curr Opin Behav Sci 10: 142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rice TR (2016): Commentary: The Neural Bases of Emotion Regulation. Front Psychol 7. 10.3389/fpsyg.2016.00476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lanius RA, Rabellino D, Boyd JE, Harricharan S, Frewen PA, McKinnon MC (2017): The innate alarm system in PTSD: conscious and subconscious processing of threat. Curr Opin Psychol 14: 109–115. [DOI] [PubMed] [Google Scholar]
- 36.McEwen BS, Bowles NP, Gray JD, Hill MN, Hunter RG, Karatsoreos IN, Nasca C (2015):Mechanisms of stress in the brain. Nat Neurosci 18: 1353–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morris MC, Rao U (2013): Psychobiology of PTSD in the Acute Aftermath of Trauma: Integrating Research on Coping, HPA Function and Sympathetic Nervous System Activity. Asian J Psychiatry 6: 3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cernak I, Savic VJ, Lazarov A, Joksimovic M, Markovic S (1999): Neuroendocrine responses following graded traumatic brain injury in male adults. Brain Inj 13: 1005–1015. [DOI] [PubMed] [Google Scholar]
- 39.Zuckerman A, Ram O, Ifergane G, Matar MA, Kaplan Z, Hoffman JR, et al. (2019): Role of Endogenous and Exogenous Corticosterone on Behavioral and Cognitive Responses to Low-Pressure Blast Wave Exposure. J Neurotrauma 36: 380–394. [DOI] [PubMed] [Google Scholar]
- 40.Aimaretti G, Ambrosio MR, Di Somma C, Fusco A, Cannavò S, Gasperi M, et al. (2004): Traumatic brain injury and subarachnoid haemorrhage are conditions at high risk for hypopituitarism: screening study at 3 months after the brain injury. Clin Endocrinol (Oxf) 61: 320–326. [DOI] [PubMed] [Google Scholar]
- 41.Russell AL, Tasker JG, Lucion AB, Fiedler J, Munhoz CD, Wu T-YJ, Deak T (2018): Factors promoting vulnerability to dysregulated stress reactivity and stress-related disease. J Neuroendocrinol 30: e12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor AN, Rahman SU, Sanders NC, Tio DL, Prolo P, Sutton RL (2008): Injury severity differentially affects short- and long-term neuroendocrine outcomes of traumatic brain injury. J Neurotrauma 25: 311–323. [DOI] [PubMed] [Google Scholar]
- 43.Tapp ZM, Godbout JP, Kokiko-Cochran ON (2019): A Tilted Axis: Maladaptive Inflammation and HPA Axis Dysfunction Contribute to Consequences of TBI. Front Neurol 10. 10.3389/fneur.2019.00345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Popoli M, Yan Z, McEwen BS, Sanacora G (2011): The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci 13: 22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Musazzi L, Milanese M, Farisello P, Zappettini S, Tardito D, Barbiero VS, et al. (2010): Acute Stress Increases Depolarization-Evoked Glutamate Release in the Rat Prefrontal/Frontal Cortex: The Dampening Action of Antidepressants ((Bartolomucci A, editor)). PLoS ONE 5: e8566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuen EY, Liu W, Karatsoreos IN, Feng J, McEwen BS, Yan Z (2009): Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc Natl Acad Sci 106:14075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCullers DL, Sullivan PG, Scheff SW, Herman JP (2002): Traumatic brain injury regulates adrenocorticosteroid receptor mRNA levels in rat hippocampus. Brain Res 947: 41–49. [DOI] [PubMed] [Google Scholar]
- 48.Chamoun R, Suki D, Gopinath SP, Goodman JC, Robertson C (2010): Role of extracellular glutamate measured by cerebral microdialysis in severe traumatic brain injury. J Neurosurg 113: 564–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dorsett CR, McGuire JL, DePasquale EAK, Gardner AE, Floyd CL, McCullumsmith RE (2017): Glutamate Neurotransmission in Rodent Models of Traumatic Brain Injury. J Neurotrauma 34: 263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guerriero RM, Giza CC, Rotenberg A (2015): Glutamate and GABA imbalance following traumatic brain injury. Curr Neurol Neurosci Rep 15: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nasca C, Bigio B, Zelli D, Nicoletti F, McEwen BS (2015): Mind the gap: glucocorticoids modulate hippocampal glutamate tone underlying individual differences in stress susceptibility. Mol Psychiatry 20: 755–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zoppi S, Pérez Nievas BG, Madrigal JLM, Manzanares J, Leza JC, García-Bueno B (2011): Regulatory role of cannabinoid receptor 1 in stress-induced excitotoxicity and neuroinflammation. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol 36: 805–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martin KP, Wellman CL (2011): NMDA receptor blockade alters stress-induced dendritic remodeling in medial prefrontal cortex. Cereb Cortex N Y N 1991 21: 2366–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gao J, Wang H, Liu Y, Li Y-Y, Chen C, Liu L-M, et al. (2014): Glutamate and GABA imbalance promotes neuronal apoptosis in hippocampus after stress. Med Sci Monit Int Med J Exp Clin Res 20: 499–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Pablos RM, Villarán RF, Argüelles S, Herrera AJ, Venero JL, Ayala A, et al. (2006): Stress increases vulnerability to inflammation in the rat prefrontal cortex. J Neurosci Off J Soc Neurosci 26: 5709–5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haroon E, Miller AH, Sanacora G (2017): Inflammation, Glutamate, and Glia: A Trio of Trouble in Mood Disorders. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol 42: 193–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O’Neil DA, Nicholas MA, Lajud N, Kline AE, Bondi CO (2018): Preclinical Models of Traumatic Brain Injury: Emerging Role of Glutamate in the Pathophysiology of Depression. Front Pharmacol 9: 579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xiong Y, Mahmood A, Chopp M (2013): Animal models of traumatic brain injury. Nat Rev Neurosci 14:128–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meyer DL, Davies DR, Barr JL, Manzerra P, Forster GL (2012): Mild traumatic brain injury in the rat alters neuronal number in the limbic system and increases conditioned fear and anxiety-like behaviors. Exp Neurol. 235: 574–587. [DOI] [PubMed] [Google Scholar]
- 60.Gillespie CF, Ressler KJ (2005): Emotional Learning and Glutamate: Translational Perspectives. CNS Spectr 10: 831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Glenn DE, Acheson DT, Geyer MA, Nievergelt CM, Baker DG, Risbrough VB, MRS-II Team (2017): Fear learning alterations after traumatic brain injury and their role in development of posttraumatic stress symptoms: GLENN et al. Depress Anxiety 34: 723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tronson NC, Corcoran KA, Jovasevic V, Radulovic J (2012): Fear conditioning and extinction: emotional states encoded by distinct signaling pathways. Trends Neurosci 35: 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blaze J, Choi I, Wang Z, Umali M, Mendelev N, Tschiffely AE, et al. (2020): Blast-Related Mild TBI Alters Anxiety-Like Behavior and Transcriptional Signatures in the Rat Amygdala. Front Behav Neurosci 14. 10.3389/fnbeh.2020.00160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoffman AN, Lam J, Hovda DA, Giza CC, Fanselow MS (2019): Sensory sensitivity as a link between concussive traumatic brain injury and PTSD [no. 1]. Sci Rep 9: 13841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM (2007): White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain J Neurol 130: 2508–2519. [DOI] [PubMed] [Google Scholar]
- 66.Bryer EJ, Medaglia JD, Rostami S, Hillary FG (2013): Neural recruitment after mild traumatic brain injury is task dependent: a meta-analysis. J Int Neuropsychol Soc JINS 19: 751–762. [DOI] [PubMed] [Google Scholar]
- 67.Cook MJ, Gardner AJ, Wojtowicz M, Williams WH, Iverson GL, Stanwell P (2020): Task-related functional magnetic resonance imaging activations in patients with acute and subacute mild traumatic brain injury: A coordinate-based meta-analysis. NeuroImage Clin 25: 102129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O’Neill TJ, Davenport EM, Murugesan G, Montillo A, Maldjian JA (2017): Applications of Resting State Functional MR Imaging to Traumatic Brain Injury. Neuroimaging Clin N Am 27: 685–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eierud C, Craddock RC, Fletcher S, Aulakh M, King-Casas B, Kuehl D, LaConte SM (2014): Neuroimaging after mild traumatic brain injury: Review and meta-analysis. Neuroimage Clin 4: 283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Patel R, Spreng RN, Shin LM, Girard TA (2012): Neurocircuitry models of posttraumatic stress disorder and beyond: A meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev 36: 2130–2142. [DOI] [PubMed] [Google Scholar]
- 71.Ju Y, Ou W, Su J, Averill CL, Liu J, Wang M, et al. (2020): White matter microstructural alterations in posttraumatic stress disorder: An ROI and whole-brain based meta-analysis. J Affect Disord 266: 655–670. [DOI] [PubMed] [Google Scholar]
- 72.Yuan H, Phillips R, Wong CK, Zotev V, Misaki M, Wurfel B, et al. (2018): Tracking resting state connectivity dynamics in veterans with PTSD. Neuroimage Clin 19: 260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koch S, Zuiden M van, Nawijn L, Frijling JL, Veltman DJ, Olff M (2016): Aberrant resting state brain activity in posttraumatic stress disorder: a meta-analysis and systematic review. Depress Anxiety 33: 592–605. [DOI] [PubMed] [Google Scholar]
- 74.Dennis EL, Disner SG, Fani N, Salminen LE, Logue M, Clarke EK, et al. (2019): Altered white matter microstructural organization in posttraumatic stress disorder across 3047 adults: results from the PGC-ENIGMA PTSD consortium. Mol Psychiatry, 10.1038/s41380-019-0631-x [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou Y, Wang Z, Qin L, Wan J, Sun Y, Su S, et al. (2012): Early Altered Resting-State Functional Connectivity Predicts the Severity of Post-Traumatic Stress Disorder Symptoms in Acutely Traumatized Subjects ((Dickey CA, editor)). PLoS ONE 7: e46833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Spadoni AD, Huang M, Simmons AN (2018): Emerging Approaches to Neurocircuits in PTSD and TBI: Imaging the Interplay of Neural and Emotional Trauma. Curr Top Behav Neurosci 38: 163–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bryant RA (2011): Post-traumatic stress disorder vs traumatic brain injury. Dialogues Clin Neurosci 13: 251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kaplan GB, Leite-Morris KA, Wang L, Rumbika KK, Heinrichs SC, Zeng X, et al. (2018): Pathophysiological Bases of Comorbidity: Traumatic Brain Injury and Post-Traumatic Stress Disorder. J Neurotrauma 35: 210–225. [DOI] [PubMed] [Google Scholar]
- 79.Bryant RA (2021): A critical review of mechanisms of adaptation to trauma: Implications for early interventions for posttraumatic stress disorder. Clin Psychol Rev 85: 101981. [DOI] [PubMed] [Google Scholar]
- 80.Bubb EJ, Metzler-Baddeley C, Aggleton JP (2018): The cingulum bundle: Anatomy, function, and dysfunction. Neurosci Biobehav Rev 92: 104–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mac Donald CL, Johnson AM, Cooper D, Nelson EC, Werner NJ, Shimony JS, et al. (2011): Detection of blast-related traumatic brain injury in U.S. military personnel. N Engl J Med 364: 2091–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Averill CL, Averill LA, Wrocklage KM, Scott JC, Akiki TJ, Schweinsburg B, et al. (2018): Altered White Matter Diffusivity of the Cingulum Angular Bundle in Posttraumatic Stress Disorder. Complex Psychiatry 4: 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kennis MPhD, van Rooij SJHPhD, Reijnen AMSc, Geuze EPhD (2017): The predictive value of dorsal cingulate activity and fractional anisotropy on long-term PTSD symptom severity. Depress Anxiety 34: 410–418. [DOI] [PubMed] [Google Scholar]
- 84.Kim SJ, Jeong D-U, Sim ME, Bae SC, Chung A, Kim MJ, et al. (2006): Asymmetrically altered integrity of cingulum bundle in posttraumatic stress disorder. Neuropsychobiology 54: 120–125. [DOI] [PubMed] [Google Scholar]
- 85.Isaac L, Main KL, Soman S, Gotlib IH, Furst AJ, Kinoshita LM, et al. (2015): The impact of depression on Veterans with PTSD and traumatic brain injury: a diffusion tensor imaging study. Biol Psychol 105: 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Davenport ND, Lamberty GJ, Nelson NW, Lim KO, Armstrong MT, Sponheim SR (2016): PTSD confounds detection of compromised cerebral white matter integrity in military veterans reporting a history of mild traumatic brain injury. Brain Inj 30: 1491–1500. [DOI] [PubMed] [Google Scholar]
- 87.Costanzo ME, Chou Y-Y, Leaman S, Pham DL, Keyser D, Nathan DE, et al. (2014): Connecting combat-related mild traumatic brain injury with posttraumatic stress disorder symptoms through brain imaging. Neurosci Lett 577: 11–15. [DOI] [PubMed] [Google Scholar]
- 88.Miller DR, Hayes JP, Lafleche G, Salat DH, Verfaellie M (2016): White matter abnormalities are associated with chronic postconcussion symptoms in blast-related mild traumatic brain injury: White Matter Abnormalities and PCS. Hum Brain Mapp 37: 220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fani N, King TZ, Clendinen C, Hardy RA, Surapaneni S, Blair JR, et al. (2019): Attentional control abnormalities in posttraumatic stress disorder: Functional, behavioral, and structural correlates. J Affect Disord 253: 343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lu L, Li F, Chen H, Wang P, Zhang H, Chen Y-C, Yin X (2020): Functional connectivity dysfunction of insular subdivisions in cognitive impairment after acute mild traumatic brain injury. Brain Imaging Behav 14: 941–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Spielberg JM, McGlinchey RE, Milberg WP, Salat DH (2015): Brain network disturbance related to posttraumatic stress and traumatic brain injury in veterans. Biol Psychiatry 78: 210–216. [DOI] [PubMed] [Google Scholar]
- 92.Jeong H, Chung Y-A, Ma J, Kim J, Hong G, Oh JK, et al. (2019): Diverging roles of the anterior insula in trauma-exposed individuals vulnerable or resilient to posttraumatic stress disorder [no.1]. Sci Rep 9: 15539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stein MB, McAllister TW (2009): Exploring the convergence of posttraumatic stress disorder and mild traumatic brain injury. Am J Psychiatry 166: 768–776. [DOI] [PubMed] [Google Scholar]
- 94.Vasterling JJ, Verfaellie M, Sullivan KD (2009): Mild traumatic brain injury and posttraumatic stress disorder in returning veterans: perspectives from cognitive neuroscience. Clin Psychol Rev 29: 674–684. [DOI] [PubMed] [Google Scholar]
- 95.Simmons AN, Matthews SC (2012): Neural circuitry of PTSD with or without mild traumatic brain injury: a meta-analysis. Neuropharmacology 62: 598–606. [DOI] [PubMed] [Google Scholar]
- 96.Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA (2008): Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N Engl J Med 358: 453–463. [DOI] [PubMed] [Google Scholar]
- 97.Rothbaum BO, Kearns MC, Price M, Malcoun E, Davis M, Ressler KJ, et al. (2012): Early intervention may prevent the development of posttraumatic stress disorder: a randomized pilot civilian study with modified prolonged exposure. Biol Psychiatry 72: 957–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Broadbent E, Kahokehr A, Booth RJ, Thomas J, Windsor JA, Buchanan CM, et al. (2012): A brief relaxation intervention reduces stress and improves surgical wound healing response: A randomised trial. Brain Behav Immun 26: 212–217. [DOI] [PubMed] [Google Scholar]
- 99.Gouin J-P, Kiecolt-Glaser JK (2011): The impact of psychological stress on wound healing: methods and mechanisms. Immunol Allergy Clin North Am 31:81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dwyer B, Katz DI (2018): Postconcussion syndrome. Handbook of Clinical Neurology, vol. 158. Elsevier, pp 163–178. [DOI] [PubMed] [Google Scholar]
- 101.Ghaffar O, McCullagh S, Ouchterlony D, Feinstein A (2006): Randomized treatment trial in mild traumatic brain injury. J Psychosom Res 61: 153–160. [DOI] [PubMed] [Google Scholar]
- 102.Silverberg ND, Hallam BJ, Rose A, Underwood H, Whitfield K, Thornton AE, Whittal ML (2013): Cognitive-behavioral prevention of postconcussion syndrome in at-risk patients: a pilot randomized controlled trial. J Head Trauma Rehabil 28: 313–322. [DOI] [PubMed] [Google Scholar]
- 103.Chard KM, Schumm JA, McIlvain SM, Bailey GW, Parkinson RB (2011): Exploring the efficacy of a residential treatment program incorporating cognitive processing therapy-cognitive for veterans with PTSD and traumatic brain injury. J Trauma Stress 24: 347–351. [DOI] [PubMed] [Google Scholar]
- 104.Wolf GK, Strom TQ, Kehle SM, Eftekhari A (2012): A preliminary examination of prolonged exposure therapy with Iraq and Afghanistan veterans with a diagnosis of posttraumatic stress disorder and mild to moderate traumatic brain injury. J Head Trauma Rehabil 27: 26–32. [DOI] [PubMed] [Google Scholar]
- 105.Helpman L, Marin M-F, Papini S, Zhu X, Sullivan GM, Schneier F, et al. (2016): Neural changes in extinction recall following prolonged exposure treatment for PTSD: A longitudinal fMRI study. Neuroimage Clin 12: 715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bryant RA, Moulds M, Guthrie R, Nixon RDV (2003): Treating acute stress disorder following mild traumatic brain injury. Am J Psychiatry 160: 585–587. [DOI] [PubMed] [Google Scholar]
- 107.Gilbert KS, Kark SM, Gehrman P, Bogdanova Y (2015): Sleep disturbances, TBI and PTSD Implications for treatment and recovery. Clin Psychol Rev 40: 195–212. [DOI] [PMC free article] [PubMed] [Google Scholar]