Abstract
During human infection, Aspergillus fumigatus secretes a 18-kDa protein that can be detected as an immunodominant antigen in the urine of infected patients. Recently, this protein was shown to be mitogillin, a ribotoxin that cleaves a single phosphodiester bond of the 29S rRNA of eukaryotic ribosomes. We proved the immunogenic capacity of mitogillin in a rabbit animal model, indicating its usefulness as an antigen for serological diagnosis of invasive aspergillosis. The mitogillin gene from A. fumigatus was transferred from plasmid pMIT+ to expression vector pQE30 and expressed in Escherichia coli as a fusion protein. Purified recombinant mitogillin was recognized by serum immunoglobulin G (IgG) of polyclonal rabbit sera that were obtained by immunization with purified native mitogillin. Consequently, we developed an enzyme-linked immunosorbent assay for detection of IgG, IgM, and IgA antibodies to recombinant mitogillin. In serum samples of patients suffering from aspergilloma (AO; n = 32), invasive pulmonary aspergillosis (IPA; n = 42), or invasive disseminated aspergillosis (IDA; n = 40), a good correlation of production of IgG antibody against mitogillin and clinical disease was observed (for patients with AO, 100% [32 of 32] were positive; for patients with IPA, 64% [31 of 42] were positive; for patients with IDA, 60% [24 of 40] were positive). In contrast, positive titers for serum IgG and IgM antibodies against mitogillin were found in only 1.3% of the serum samples of healthy volunteers and positive titers for IgA antibody were found in only 1.0% of the serum samples of healthy volunteers (n = 307; specificity = 95.4%). These results indicate that recombinant mitogillin expressed in E. coli can be used for improvement of the serodiagnosis of A. fumigatus-associated diseases.
Aspergillus fumigatus is the causative agent of a variety of diseases in humans, such as allergic bronchopulmonary aspergillosis (ABPA), asthma, sinusitis, aspergilloma (AO), invasive pulmonary aspergillosis (IPA), and invasive disseminated aspergillosis (IDA). Deleterious invasive aspergillosis (IA) is an increasing problem in the immunocompromised host, and the rate of lethality of IA is estimated to be as high as 95% (8, 27).
An efficient antifungal therapy for IA depends on an early diagnosis, but this is limited due to the absence of specific clinical symptoms at the early stage of disease. Specific diagnostic tools that lead to an early and sufficient therapy could substantially improve the clinical outcome (1, 4, 6), but conventional serological tests face some major obstacles. Most immunoassays for detection of circulating antibodies are based on crude extracts of A. fumigatus. These extracts contain complex and undefined mixtures of polysaccharide and protein components and are derived from various sources such as conidial, mycelial, cytoplasmic, metabolic, or cell wall fractions of the fungus (13–15, 28). Consequently, there is little knowledge about the role of the humoral immune response, the cross-reactivities of different epitopes in these crude antigenic preparations, the diagnostic value of class-specific antibody detection, and antibody kinetics in human aspergillosis (7).
The utilization of well-characterized immunodominant fungal antigens could substantially improve the serodiagnosis in Aspergillus-related diseases. Galactomannan (GM), the immunodominant polysaccharide cell wall antigen of Aspergillus spp., has been studied extensively in the past (9, 23, 24, 33), and recently, monoclonal antibody EB-A2 has successfully been used in a sensitive direct double-sandwich enzyme-linked immunosorbent assay (ELISA) to detect circulating GM during IA (9, 29, 30, 33). A drawback of this method, however, is the high frequency of false-positive results (34, 35, 37), which may be due to cross-reactivity with other fungi or other unidentified serum components (35, 36).
Few data are available about A. fumigatus protein antigens that are preferentially produced in vivo. Mitogillin is a small basic protein of approximately 18 kDa with cytotoxic activity released by A. fumigatus (16, 17, 31). Together with the related toxins A. fumigatus allergen I (AspfI), restrictocin from Aspergillus restrictus (20), and α-sarcin from Aspergillus giganteus (26), mitogillin is a member of a family of conserved RNases that cleave a single phosphodiester bond of the 29S rRNA of eukaryotic ribosomes (11, 16, 17).
An indication that mitogillin is produced in vivo during infection was provided by Lamy et al. (21), who detected mitogillin within kidney cells of mice infected with A. fumigatus in regions of necrosis surrounding fungal colonies. Furthermore, Arruda et al. (3) demonstrated that 85% of the patients with immunoglobulin E (IgE) antibodies to A. fumigatus also had IgE antibodies to AspfI, which they defined as a major allergen of the fungus. Interestingly, mitogillin was found to be one of the major A. fumigatus antigens detectable in human urine (12, 21, 23, 25), and among the water-soluble ethanol-precipitated proteins (WSEPs) from broth cultures of A. fumigatus, mitogillin was recognized by pooled sera of aspergilloma patients (25), suggesting that the ribotoxin can successfully be used as a specific diagnostic marker for A. fumigatus-associated diseases.
To study the diagnostic potential of mitogillin in human aspergillosis, we expressed mitogillin from A. fumigatus in Escherichia coli and purified the recombinant protein to homogeneity. After protein analysis, the recombinant mitogillin was used for specific IgG, IgM, and IgA antibody detection in patients suffering from different forms of aspergillosis.
MATERIALS AND METHODS
Fungal strains and plasmids.
A. fumigatus strain M2045 was isolated from the tracheal aspirate of a patient suffering from cystic fibrosis. A. fumigatus strain M5299 was obtained from the bronchoalveolar lavage fluid of an AIDS patient suffering from disseminated IA. Strain M5299 was deposited at the Centraalbureau voor Schimmelcultures (CBS) culture collection (CBS 109032). The isolates were maintained on Sabouraud dextrose agar slants. Plasmid pMIT+ carrying the gene encoding mitogillin (17) was a gift from Jacqui Shea (Royal Postgraduate Medical School, Hammersmith Hospital, London, United Kingdom).
Culture and WSEPs.
Culture, fungal antigen extraction, and the isolation of WSEPs from the culture filtrate were performed as described by Latgé et al. (25). Briefly, conidia of A. fumigatus strains M2045 and M5299 were inoculated in 50 ml of Sabouraud liquid medium (2% [wt/vol] glucose, 1% [wt/vol] Mycopeptone). The cultures were incubated for 4 to 5 days at 25°C and 100 rpm. The shaken liquid cultures were transferred to 1 liter of Sabouraud medium and cultured at 25°C and 600 rpm. Maximal fungal growth was observed after 48 to 72 h. The culture filtrates from the strains were precipitated with 4 volumes of ethanol overnight at 4°C. The precipitate was washed two times with ethanol and resuspended in water (approximately 20 mg [dry weight] per ml). The undissolved precipitate was removed by centrifugation, and the WSEPs were aliquoted and stored at −80°C for further analysis.
Construction of the mitogillin expression vector.
The coding region without the leader sequence of the mitogillin gene was amplified by PCR from plasmid pMIT+ with primer RES1-f (5′-AGGGAGCTCATGGCGACCTGGACATGCATC-3′), which contains a SacI restriction site, and primer RES1-r (5′-AACTGCAGCTAATGAGAACACAGTCTCAAGT-3′), which contains a PstI restriction site. PCR was performed in a volume of 50 μl (500 ng of plasmid pMIT+ DNA; 20 mM [each] dATP, dCTP, dGTP, and dTTP; 25 pmol of each primer; 2.5 U of Taq DNA polymerase [Amersham, Braunschweig, Germany]; 5 μl of 10× Taq buffer [Amersham]; 3 μl of MgCl2 [25 mM]; 38.5 μl of sterile water). Amplification was performed in an Omni-Gene temperature cycler (Hybaid; MWG-Biotech, Ebersberg, Germany). For a hot start, the PCR reagent mixture was preheated to 94°C before DNA was added. Twenty-five cycles were carried out, with each cycle consisting of (i) denaturation for 1 min at 94°C, (ii) annealing for 1 min at 59°C, and (iii) extension for 90 s at 72°C. Final extension was performed for 10 min at 72°C after the last cycle.
Plasmid pQE30 (Qiagen, Hilden, Germany) was cut with SacI and PstI and treated with calf intestinal alkaline phosphatase (New England Biolabs, Frankfurt, Germany). The PCR product was digested with SacI and PstI, purified, and integrated in frame into the polylinker region of linearized pQE30 to generate pQEMW1.
Recombinant expression and purification of mitogillin.
The expression plasmid pQEMW1 was transformed by electroporation into E. coli host strain M15 containing a repressor plasmid (pREP4). Successful transformation was confirmed by PCR analysis of the colonies with primers RES1-f and RES1-r. Twenty milliliters of Luria-Bertani (LB) broth containing 100 μg of ampicillin per ml and 25 μg of kanamycin per ml was inoculated with a single colony of the transformed strain (37°C, vigorous shaking, overnight). For large-scale expression, 1 liter of LB broth (with 100 μg of ampicillin per ml and 25 μg of kanamycin per ml) was inoculated 1:50 with the overnight culture. Growth of the bacteria was continued at 37°C with vigorous shaking until the A600 reached 0.7 to 0.9. The culture was induced with 2 mM isopropyl-β-d-thiogalactopyranoside and continued to grow at 37°C for another 5 h. Bacterial cells were harvested by centrifugation at 4,000 × g for 10 min. To avoid protein degradation, purification of the recombinant mitogillin was done immediately after growth, induction, and cell lysis. Protein purification was performed with an Ni2+ HiTrap chelating affinity column and the GradiFrac system (Pharmacia, Freiburg, Germany) according to the instructions of the manufacturer. The human immunoglobulin preparations Pentaglobin and Intraglobin F (Biotest Pharma GmbH, Dreieich, Germany) were used in Western blot analysis to detect contaminating antigens from E. coli in the recombinant antigen preparation. Protein refolding was done by dialysis by the protocol for the Qiaexpressionist kit (Qiagen). The protein concentration was determined by the bicinchoninic acid protein assay (Pierce, Rockford, Ill.).
Infection and immunization procedures.
Four New Zealand White rabbits (weight, 3.0 to 3.5 kg) were used. Two rabbits received 108 viable conidia via subcutaneous injection. The first was infected with strain M2045 and the second was infected with strain M5299. The procedure was repeated every second week five times before the animals were killed. Two additional rabbits were used for immunization with native or recombinant mitogillin. For these immunization procedures Antibody-Multiplier normal and Antibody-Multiplier special adjuvants (Linaris, Bettingen, Germany) were used according to the suggestions of the manufacturer. Briefly, a polyclonal rabbit antiserum against native A. fumigatus mitogillin was raised by immunizing a third animal with the protein that was eluted from the gels on which sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of WSEPs from the culture filtrate of strain M2045 was performed. A polyclonal rabbit antiserum against recombinant mitogillin was raised by immunizing a fourth animal with the purified recombinant mitogillin. In total, 3.0 mg of protein was given to each animal. Serum samples were obtained from each animal before the immunization and at 2, 4, 6, 8, 10, and 12 weeks after the first immunization or infection procedure.
Western blotting and ELISA. (i) Antigenic characterization of recombinant mitogillin.
After SDS-PAGE under reducing conditions, recombinant mitogillin and the WSEPs of A. fumigatus strain M2045 were transferred onto nitrocellulose (cellulose nitrate; pore size; 0.45 μm; Schleicher & Schuell, Dassel, Germany) and used as antigens in Western blot analysis. The blots were probed with polyclonal rabbit sera derived either from rabbits infected with A. fumigatus M2045 or M5299 or from rabbits immunized with either recombinant or native mitogillin. In addition, Western blots were carried out with polyclonal antiserum obtained by immunizing mice with putative degradation products of the recombinant protein.
(ii) Recombinant mitogillin ELISA.
A recombinant ELISA was developed to detect specific IgG, IgM, and IgA antibodies to mitogillin from A. fumigatus. A total of 1 μg of the recombinant protein per ml was diluted in coating buffer (0.015 M Na2CO3, 0.035 M NaHCO3 [pH 8.4]), and 100 μl of the antigen solution was coated (4°C, overnight) onto each well of ELISA microtiter plates (high binding, U shape; Greiner, Frickenhausen, Germany) before 150 μl of ELISA blocking reagent (Boehringer, Mannheim, Germany) was added for 30 min. Subsequently, 150 μl of diluted serum (1:100 in phosphate-buffered saline–0.05% [vol/vol] Tween 20) was added to each well for 2 h at 37°C. Thereafter, 50 μl of conjugates was added to each well, and the plates were incubated for 1 h at 37°C. The conjugates were diluted in phosphate-buffered saline–0.05% (vol/vol) Tween 20 as follows: anti-human IgG-horseradish peroxidase (HRP) conjugate (DAKO, Hamburg, Germany), 1:6,000; anti-human IgM-HRP conjugate (DAKO), 1:2,000; anti-human IgA-HRP conjugate (DAKO), 1:4,000; anti-rabbit IgG-peroxidase conjugate (Dianova, Hamburg, Germany), 1:4,000. Five washings with washing buffer (0.35 M NaCl, 0.05 M Tris-HCl [pH 7.4], 0.1% [vol/vol] Tween 20) was performed between each step of the ELISA. Substrate was prepared with 14 mg of 1,2-phenylenediamine dihydrochloride (DAKO), 12 ml of H2O, and 5 μl of 30% (vol/vol) H2O2. One hundred microliters of the substrate was added to each well, and the plate was incubated for 15 min. The reaction was stopped with 100 μl of 0.5 N H2SO4, and the optical density (OD) was measured at a wavelength of 491 nm (reference filter, 620 nm). The OD values were adjusted for any background detected for assays in wells that had not been coated with mitogillin. All serum samples were tested in duplicate.
Reproducibility of the assay.
To ensure the reproducibility of the recombinant mitogillin assay, we have tested (i) control serum samples 1 (negative), 2 (borderline), and 3 (positive) on 10 runs on different days and (ii) we have compared the index values of control serum samples 4 to 8 (samples 4 and 5 were negative, sample 6 was borderline, and samples 7 and 8 were positive) with four different affinity chromatography preparations of purified recombinant mitogillin. All tests were done in duplicate.
Statistical analysis: cutoff values.
The mean values of the ODs for specific IgG, IgM, and IgA antibodies against mitogillin were determined for the serum samples of 307 blood donors. The cutoff value for the mitogillin IgG, IgM, or IgA ELISA was defined as the mean value for 307 donor serum samples plus 3 standard deviations. A test result smaller than the cutoff value but larger than the mean value for the blood donor sera plus 2 times the standard deviation was considered borderline. The results of the mitogillin ELISAs are given as index values, which were calculated as the ratio of a given OD compared to the cutoff value. An index value of ≥1 indicates a positive test result.
A. fumigatus antigen detection.
A. fumigatus GM antigen detection in serum samples was done with the Platelia kit and the Pastorex kit (Sanofi Diagnostics Pasteur, Marnes la Coquette, France) according to the instructions of the manufacturer.
Detection of A. fumigatus antibodies to crude antigens.
Anti-A. fumigatus antibody detection was performed by two conventional assays based on crude Aspergillus extracts. Anti-A. fumigatus complement-fixing antibodies were tested with a commercially available preparation of A. fumigatus metabolic antigen suitable for complement fixation (CF; Immuno-Mycologics, Norman, Okla.), according to the instructions of the manufacturer. Counterimmunoelectrophoresis (IE) was done with a commercially available A. fumigatus antigen for the detection of immunoprecipitating antibodies (HAL GmbH, Düsseldorf, Germany).
Serum samples and patients.
For the determination of the cutoff values and the specificity of the recombinant mitogillin ELISA we examined serum samples of 307 healthy blood donors (mean age, 40.0 years; age range, 19 to 65 years) for specific IgG, IgM, and IgA antibodies against mitogillin. In addition, we tested serum samples of a patient who was suffering from mucormycosis, of a patient who was suffering from histoplasmosis of the lung and who had antibodies against Histoplasma capsulatum, and of a patient who had candidemia and antibodies against Candida albicans. Furthermore, we screened four serum samples of two healthy immunocompetent individuals who showed positive antibody titers in a commercially available anti-A. fumigatus IgG antibody detection kit (HALISA; HAL Allergie GmbH).
To test the sensitivity of the ELISA, we examined 117 serum samples of 36 patients suffering from different forms of A. fumigatus-associated diseases. All serum samples were tested for IgG, IgM, and IgA antibodies against mitogillin. In addition, the samples were screened for antibodies against Aspergillus with two conventional test systems (CF, IE) based on crude metabolic antigen. Finally, the results of antibody detection were matched with the results of A. fumigatus GM antigen detection (Platelia, Pastorex). When tested retrospectively, serum samples were stored at −20°C. Briefly, we tested 32 serum samples from 13 episodes of AO, 42 serum samples from 9 episodes of IPA, 40 serum samples from 11 episodes of IDA, and 3 serum samples of 3 patients who were diagnosed with a type III allergy to A. fumigatus. All patients suffered from proven cases of A. fumigatus-associated diseases. A. fumigatus was isolated from lung tissue of all IPA patients and from the lungs and various organs of the group of patients suffering from IDA (see Table 1). In the study we included serially obtained serum samples of patients with culturally proven and lethal invasive disease. For example, patient 22 was a 39-year-old male who developed IPA within a transplanted lung. Follow-up serum samples were examined 4, 5, 12, and 19 days after transplantation. Patient 28 was an 18-year-old male with hepatitis of unknown origin and panmyelophthisis who suffered from disseminated IA. Sera were obtained 3 months before the onset of respiratory symptoms, at the onset of pneumonia, and at 10, 29, 37, 39, and 43 days after the onset of symptoms.
TABLE 1.
Specific IgG, IgM, and IgA antibodies against mitogillin in patients with A. fumigatus-associated diseases
| Patient no. | Serum sample no.a | Age (yr) | Sexb | Clinical manifestation and treatment | Underlying disease and treatmentc | Antibodies to A. fumigatus
|
Presence of Aspergillus antigen, GM antigeng | Source of A. fumigatush | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Recombinant mitogillind
|
CFe | IEf | ||||||||||
| IgG | IgM | IgA | ||||||||||
| 1 | 1 (0) | 62 | M | AO | + (2.55) | − (0.69) | + (1.71) | − | + (6) | + (E), + (L) | Sp, BAL | |
| 2 (7) | + (2.56) | − (0.61) | (+) (0.93) | − | + (7) | + (E) | ||||||
| 3 (33) | + (2.81) | − (0.66) | (+) (0.82) | − | + (5) | + (E) | ||||||
| 4 (64) | + (2.61) | (+) (0.82) | (+) (0.94) | − | + (4) | + (E) | ||||||
| 5 (70) | Surgery | + (2.88) | (+) (0.96) | + (1.36) | − | + (4) | + (E) | Lu | ||||
| 2 | 6 (0) | 67 | M | AO | Previous pulmonary tuberculosis | + (2.60) | + (1.20) | (+) 0.80 | − | + (5) | + (E) | Sp, BS |
| 7 (139) | + (2.93) | − (0.55) | − (0.41) | − | + (4) | + (E) | ||||||
| 3 | 8 (0) | 84 | M | AO | Previous pulmonary tuberculosis | + (2.37) | − (0.06) | + (1.32) | + (1:10) | + (2) | NDi | |
| 9 (131) | + (2.48) | − (0.06) | + (1.20) | − | + (2) | + (L) | ||||||
| 10 (234) | Surgery | + (2.49) | − (0.07) | + (1.56) | − | + (3) | ND | Lu | ||||
| 4 | 11 (0) | 49 | M | AO | Previous pulmonary tuberculosis, surgery | + (1.19) | − (0.57) | − (0.41) | − | + (4) | − (E) | Sp |
| 12 (30) | (+) (0.82) | − (0.46) | − (0.39) | − | + (3) | (+) (E) | ||||||
| 13 (434) | + (2.02) | + (1.38) | − (0.58) | − | + (2) | − (E) | ||||||
| 14 (537) | + (1.40) | − (0.48) | − (0.47) | − | + (2) | + (E) | Lu | |||||
| 5 | 15 (0) | 51 | M | AO | Previous pulmonary tuberculosis | + (1.02) | (+) (0.75) | − (0.35) | − | + (2) | − (E) | Sp |
| 16 (77) | + (1.75) | + (1.01) | (+) (0.86) | − | + (2) | − (E) | Lu | |||||
| 6 | 17 (0) | 52 | M | AO (bilateral) | Previous pulmonary tuberculosis | + (2.41) | (+) (0.88) | − (0.46) | + (1:10) | + (2) | − (E) | Sp |
| 18 (160) | + (1.89) | (+) (0.93) | − (0.53) | I | + (4) | − (E) | ||||||
| 19 (583) | + (2.04) | + (1.02) | − (0.50) | + (1:5) | + (3) | − (E) | ||||||
| 20 (722) | + (2.15) | (+) (0.97) | (+) (0.78) | + (1:5) | + (3) | − (E) | ||||||
| 21 (2,437) | Hemoptysis | + (2.49) | + (1.58) | − (0.48) | − | + (5) | + (E) | BAL, Sp | ||||
| 22 (2,473) | + (2.2) | + (1.23) | − (0.35) | − | + (7) | + (E) | ||||||
| 7 | 23 (0) | 63 | M | AO, surgery | Previous pulmonary tuberculosis | + (1.72) | − (0.26) | − (0.32) | − | + (3) | + (E) | Lu |
| 24 (949) | IPA | + (1.74) | − (0.32) | − (0.37) | − | + (2) | − (E) | Pl | ||||
| 8 | 25 | 58 | M | AO | Wegener granulomatosis | + (3.10) | − (0.30) | − (0.60) | I | + (5) | + (E) | BAL, P1 |
| 9 | 26 | 64 | M | AO | Chronic obstructive bronchitis | + (2.60) | + (1.30) | (+) (0.90) | + (1:80) | + (5) | − (E) | Sp, TA, BAL |
| 10 | 27 | 53 | M | AO surgery | Previous pulmonary tuberculosis | + (1.59) | (+) (0.76) | − (0.27) | − | + (2) | − (E) | Lu |
| 11 | 28 (0) | 60 | W | AO | Previous pulmonary tuberculosis | + (2.67) | − (0.40) | − (0.66) | 0 | + (2) | − (E) | Sp |
| 29 (39) | Surgery | + (2.85) | − (0.43) | (+) (0.76) | 0 | + (3) | + (E) | Lu | ||||
| 12 | 30 (0) | 70 | M | AO | Previous pulmonary tuberculosis | + (2.84) | + (2.36) | − (0.64) | I | + (2) | − (E) | Sp, BS |
| 31 (973) | + (2.60) | + (1.40) | − (0.46) | I | + (4) | − (E) | ||||||
| 13 | 32 | 64 | M | AO | Previous pulmonary tuberculosis, hepatitits diabetes mellitus | + (1.07) | (+) (0.98) | (+) (0.86) | + (1:10) | + (3) | + (E) | Lu |
| 14 | 33 (0) | 38 | M | IPA | AML | − (0.37) | − (0.69) | − (0.13) | + | − | − (L) | |
| 34 (16) | (+) (0.79) | − (0.45) | − (0.01) | − | − | − (L) | ||||||
| 35 (23) | + (1.07) | − (0.64) | − (0.01) | − | − | − (L) | ||||||
| 36 (29) | + (1.20) | (+) (0.90) | − (0.07) | − | − | − (L) | ||||||
| 37 (30) | + (1.68) | (+) (0.80) | − (0.04) | − | − | − (L) | ||||||
| 38 (37) | + (1.10) | − (0.40) | − (0.01) | − | − | − (L) | ||||||
| 39 (44) | Death day 53j | (+) (0.79) | − (0.47) | − (0.01) | − | − | − (L) | Lu | ||||
| 15 | 40 (0) | 76 | F | IPA | Neoplasia of the orbita | − (0.23) | − (0.01) | − (0.17) | − | − | ND | |
| 41 (6) | − (0.19) | − (0.12) | − (0.04) | − | − | − (L) | ||||||
| 42 (10) | − (0.51) | − (0.23) | − (0.38) | − | − | − (L) | ||||||
| 43 (16) | (+) (0.96) | − (0.53) | − (0.56) | − | − | − (L) | ||||||
| 44 (20) | Death day 22 | + (1.22) | − (0.57) | (+) (0.83) | − | − | − (L) | Lu | ||||
| 16 | 45 (0) | 18 | F | IPA | Neoplasia of the lung | − (0.28) | − (0.02) | − (0.01) | − | − | − (L) | |
| 46 (17) | − (0.31) | − (0.10) | − (0.06) | − | − | − (L) | ||||||
| 47 (28) | + (1.34) | − (0.35) | − (0.21) | − | − | − (L) | ||||||
| 48 (37) | Death day 37 | + (1.15) | − (0.35) | − (0.17) | − | − | − (L) | Lu | ||||
| 17 | 49 (0) | 39 | M | IPA | AML, BMT | − (0.57) | − (0.31) | − (0.01) | − | − | − (E); − (L) | |
| 50 (5) | + (1.34) | − (0.15) | − (0.38) | − | − | − (E); − (L) | ||||||
| 51 (11) | + (1.36) | − (0.01) | − (0.12) | − | − | + (E); − (L) | ||||||
| 52 (13) | − (0.56) | − (0.09) | − (0.04) | − | − | + (E); − (L) | ||||||
| 53 (14) | − (0.61) | − (0.22) | − (0.07) | − | − | + (E); − (L) | ||||||
| 54 (20) | + (1.13) | − (0.23) | − (0.01) | − | − | + (E); − (L) | ||||||
| 55 (24) | Death day 26 | (+) (0.91) | − (0.16) | − (0.15) | − | − | + (E); + (L) | Lu | ||||
| 18 | 56 (0) | 63 | M | IPA | AML | + (1.30) | + (1.17) | − (0.53) | I | − | + (E); | |
| 57 (9) | Death day 9 | + (1.12) | (+) (0.75) | − (0.55) | − | − | (+) (E) | Lu | ||||
| 19 | 58 (0) | 69 | M | IPA | Glomerulonephritis, cortisone therapy | − (0.53) | − (0.01) | − (0.14) | − | − | − (E); − (L) | |
| 59 (11) | − (0.66) | − (0.01) | − (0.01) | − | − | − (E); − (L) | ||||||
| 60 (15) | (+) (0.94) | − (0.09) | − (0.04) | − | − | − (E); − (L) | ||||||
| 61 (20) | Death day 25 | (+) (0.81) | − (0.16) | − (0.12) | − | − | + (E); − (L) | Lu | ||||
| 20 | 62 (0) | 33 | M | IPA | ALL | − (0.57) | − (0.19) | − (0.16) | I | − | − (E); − (L) | |
| 63 (8) | − (0.73) | − (0.41) | − (0.41) | I | − | + (E); − (L) | ||||||
| 64 (10) | (+) (0.90) | − (0.21) | − (0.08) | I | − | + (E); − (L) | ||||||
| 65 (11) | (+) (0.97) | − (0.16) | − (0.06) | − | − | + (E); − (L) | ||||||
| 66 (13) | + (1.10) | − (0.07) | − (0.17) | I | − | − (E); − (L) | ||||||
| 67 (15) | + (1.20) | − (0.12) | − (0.01) | I | − | + (E); − (L) | ||||||
| 68 (18) | Death day 19 | (+) (0.98) | − (0.07) | − (0.12) | I | − | + (E); − (L) | Lu | ||||
| 21 | 69 (0) | 46 | F | IPA | ALL, BMT | − (0.55) | − (0.19) | − (0.01) | − | − | + (E); − (L) | |
| 70 (18) | Death day 25 | + (1.05) | − (0.37) | − (0.29) | − | − | − (E) | Lu | ||||
| 22 | 71 (0) | 40 | M | IPA | Lung Tpx | − (0.71) | − (0.30) | − (0.23) | + (1:5) | − | − (E) | |
| 72 (1) | (+) (0.97) | − (0.34) | − (0.26) | I | − | − (E) | BAL | |||||
| 73 (8) | + (1.80) | − (0.32) | − (0.33) | + (1:5) | + (2) | − (E) | ||||||
| 74 (15) | Death day 44 | + (2.31) | − (0.28) | − (0.44) | I | + (2) | − (E) | Lu | ||||
| 23 | 75 (0) | 54 | M | IDA | AML, BMT | − (0.51) | − (0.41) | − (0.44) | − | − | + (L) | |
| 76 (6) | − (0.51) | − (0.21) | − (0.10) | − | − | + (L) | ||||||
| 77 (7) | (+) (0.78) | − (0.24) | − (0.31) | − | − | − (L) | ||||||
| 78 (14) | Death day 16 | − (0.51) | − (0.28) | − (0.01) | − | − | − (L) | Lu, Coe | ||||
| 24 | 79 | 56 | F | IDA | CML, BMT | + (1.01) | − (0.03) | − (0.32) | − | − | − (L) | Lu, Br |
| 25 | 80 (0) | 37 | M | IDA | AIDS | − (0.65) | − (0.30) | + (3.05) | + (1:5) | − | + (E) | |
| 81 (7) | Death day 9 | − (0.65) | − (0.20) | + (2.45) | + (1:5) | − | + (E) | Lu, Br, BAL, BA | ||||
| 26 | 82 (0) | 42 | M | IDA | AIDS | (+) (0.80) | − (0.25) | + (1.75) | I | − | + (E) | |
| 83 (9) | Death day 12 | (+) (0.76) | − (0.50) | + (1.90) | − | − | + (E) | Lu, Br | ||||
| 27 | 84 | 34 | M | IDA | AIDS | − (0.66) | (+) (0.81) | (+) (0.92) | + (1:5) | − | − (E) | Lu, Br |
| 28 | 85 (−32) | 18 | M | IDA | Subacute liver failure panmyelophthisis | − (0.47) | − (0.13) | − (0.25) | − | − | − (E) | |
| 86 (0) | − (0.56) | − (0.01) | − (0.08) | − | − | − (E) | ||||||
| 87 (10) | (+) (0.89) | − (0.18) | − (0.13) | − | − | − (E) | ||||||
| 88 (29) | + (2.04) | + (1.08) | − (0.63) | + (1:5) | − | − (E) | ||||||
| 89 (37) | − (0.44) | − (0.39) | − (0.08) | + (1:5) | − | − (E) | ||||||
| 90 (39) | − (0.54) | − (0.31) | − (0.16) | − | − | − (E) | ||||||
| 91 (43) | Death day 59 | − (0.54) | − (0.24) | − (0.15) | − | − | − (E) | Lu, Br, | ||||
| 29 | 92 (0) | 66 | M | IDA | Dermatomyositis | − (0.58) | − (0.01) | − (0.09) | − | − | + (L) | |
| 93 (1) | (+) (0.95) | − (0.18) | − (0.24) | − | − | + (L) | ||||||
| 94 (3) | (+) (0.76) | − (0.40) | − (0.26) | − | − | + (L) | ||||||
| 95 (7) | − (0.69) | − (0.07) | − (0.21) | − | − | + (L) | ||||||
| 96 (9) | (+) (0.75) | − (0.01) | − (0.12) | − | − | + (L) | ||||||
| 97 (11) | (+) (0.95) | − (0.11) | − (0.13) | − | − | + (L) | ||||||
| 98 (12) | + (1.36) | − (0.22) | − (0.29) | − | − | + (L) | ||||||
| 99 (13) | Death day 14 | + (2.75) | − (0.39) | + (1.14) | − | − | + (L) | Lu, Sp, He, Th, Ki | ||||
| 30 | 100 (0) | 57 | M | IDA | Liver Tpx (cirrhosis) | + (1.25) | − (0.57) | − (0.50) | − | − | − (L) | |
| 101 (7) | + (1.10) | − (0.33) | (+) 0.76 | − | − | − (L) | ||||||
| 102 (9) | + (1.71) | − (0.36) | + (1.34) | − | − | − (L) | ||||||
| 103 (10) | Death day 16 | + (1.23) | − (0.19) | (+) (0.98) | − | − | − (L) | Lu, Sp, He, Th, Li, Ki | ||||
| 31 | 104 (0) | 37 | M | IDA | CML | − (0.62) | − (0.19) | − (0.32) | I | − | − (L) | |
| BMT | ||||||||||||
| 105 (9) | (+) (0.88) | − (0.40) | − (0.32) | − | − | − (L) | ||||||
| 106 (16) | (+) (0.82) | − (0.02) | − (0.22) | − | − | − (L) | ||||||
| 107 (23) | + (1.05) | − (0.07) | − (0.03) | − | − | − (E) | ||||||
| 108 (30) | Death day 46 | (+) (0.87) | − (0.14) | − (0.22) | − | − | − (E) | Lu, He | ||||
| 32 | 109 (0) | 71 | M | IDA | Infarction, bypass, aorta valve | (+) (0.88) | − (0.25) | − (0.55) | − | − | + (E) | |
| 110 (4) | (+) (0.80) | − (0.22) | − (0.40) | − | − | + (E) | ||||||
| 111 (7) | Death day 7 | − (0.67) | − (0.37) | (+) (0.81) | − | − | + (E) | Lu, He, Th, Li, Ki | ||||
| 33 | 112 (0) | 70 | M | IDA | (+) (0.78) | − (0.10) | − (0.26) | + (1:5) | − | − (L) | ||
| 113 (18) | − (0.44) | − (0.09) | − (0.06) | + (1:5) | − | − (L) | ||||||
| 114 (39) | Death day 42 | (+) (0.80) | − (0.08) | − (0.54) | − | − | − (L) | Br, Lu | ||||
| 34 | 115 | ND | ND | Type III allergy | Farmer's lung | + (2.40) | − (0.05) | − (0.35) | ND | ND | ND | − |
| 35 | 116 | ND | ND | Type III allergy | Lung fibrosis | + (1.18) | − (0.7) | − (0.12) | ND | ND | ND | − |
| 36 | 117 | ND | ND | Type III allergy | Lung fibrosis | + (1.54) | − (0.19) | (+) (0.88) | ND | ND | ND | − |
The day of serum sampling in respect to the day of drawing of first serum sample from each patient is given in parentheses.
M, male; F, female.
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; BMT, bone morrow transplantation; CML, chronic myeloid leukemia; Tpx, transplantation.
+, an index value above the 3-standard deviation cutoff was considered positive; (+), an index value above the 2-standard-deviation cutoff but not above the 3-standard-deviation cutoff was considered borderline. Index values are given in parentheses.
Titers in serum are given in parentheses; I, inhibitory.
The number of precipitins is given in parentheses.
(L), GM antigen detection with Pastorex Aspergillus; +, agglutination positive, −, agglutination negative. (E), GM antigen detection with Platelia Aspergillus; +, an index value of ≥1.5 was calculated as positive; (+), an index value of ≥1 but <1.5 was interpreted as borderline; −, an index value of <1 was considered negative.
BA, bronchial aspirate; BAL, bronchoalveolar lavage; Br, brain, Coe, cecum; He, heart; Ki, kidney; Li, liver; Lu, lung tissue; Pl, pleura; Sp, sputum; TA, tracheal aspirate; Th, thyroid gland.
ND, not determined.
Day of death in respect to the day of drawing of first serum sample.
In the group of patients with AO, A. fumigatus was grown from the lung tissue of eight patients. For the remaining five patients the diagnosis was confirmed by typical clinical and radiological signs in patients at risk for AO combined with repetitive culture of A. fumigatus in a specimen such as pleural effusion fluid or bronchoalveolar lavage fluid and more than two major precipitins by IE.
RESULTS
Generation of recombinant mitogillin and antigen characterization.
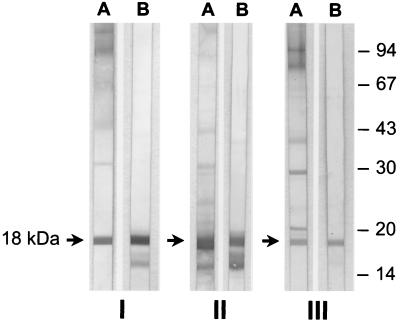
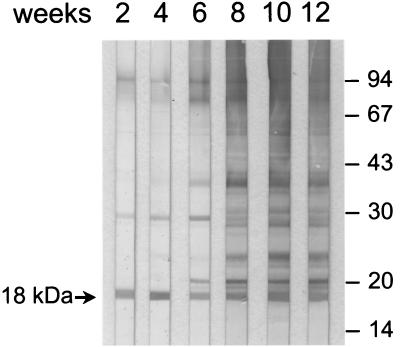
The identity of the PCR product that was cloned into expression vector pQE30 with mitogillin (17) was proved by sequencing. Following affinity chromatography the recombinant mitogillin was analyzed by SDS-PAGE; its molecular mass was slightly larger than that of the native mitogillin due to the 6× HIS affinity tag (data not shown). In Western blot experiments, no contamination with E. coli proteins was detected in the recombinant protein preparation with human Pentaglobin and Intraglobin F. These polyclonal human immunoglobulin preparations reacted well with multiple proteins from E. coli M15, which was used for expression of recombinant mitogillin (data not shown). In Western blots, serum IgG from polyclonal rabbit serum samples against native or recombinant mitogillin as well as IgG from polyclonal rabbit serum samples that were raised by infecting animals with A. fumigatus M2045 or M5299 reacted with recombinant mitogillin. Likewise, serum IgG of polyclonal rabbit serum raised against recombinant mitogillin reacted with native mitogillin of the WSEPs from A. fumigatus strain M2045. In addition, the natural mitogillin of the WSEPs was recognized by IgG from polyclonal rabbit sera generated against native mitogillin and those polyclonal sera raised by infecting the animals with conidia of A. fumigatus clinical strains M2045 and M5299 (Fig. 1). Using follow-up serum samples, we observed that mitogillin is an immunodominant A. fumigatus antigen that elicits an early antibody response compared to the times of the responses elicited by the other A. fumigatus antigens of the WSEPs when rabbits were infected with viable A. fumigatus conidia (Fig. 2). In the Western blots we observed a potential degradation product of mitogillin that reacted with serum IgG of polyclonal rabbit serum samples raised against native and recombinant mitogillin (Fig. 1, lanes B of parts I and II). To test this hypothesis, mice were immunized with the purified degradation product. In Western blots mitogillin was well recognized by these polyclonal mouse serum samples (data not shown).
FIG. 1.
Western blots with WSEPs of A. fumigatus strain M2045 (lanes A) and recombinant mitogillin (lanes B) as antigens. The blots were probed with serum samples of rabbits that were immunized with native mitogillin (I) and recombinant mitogillin (II) and of an animal that was infected with A. fumigatus M2045 (III). Molecular mass markers (in kilodaltons) are shown in the right margin. Every combination results in the reaction of the polyclonal antibody with mitogillin (arrows).
FIG. 2.
Kinetics of antibody production to WSEP antigens of A. fumigatus. The Western blots were probed with consecutively obtained polyclonal serum samples of a rabbit that was infected with viable A. fumigatus conidia of strain M2045. Molecular mass markers (in kilodaltons) are shown in the right margin. Antibodies to mitogillin appear early after infection (arrow).
Control sera.
The reproducibility of the anti-mitogillin antibody assay was shown by testing control serum samples 1 to 3 (negative, borderline, and positive, respectively) in 10 runs on different days. The standard deviations within the runs were 0.10 for control serum sample 1 (mean index value, 0.51), 0.12 for control serum sample 2 (mean index value, 0.92), and 0.14 for control serum sample 3 (mean index value, 2.17). When four different preparations of recombinant mitogillin were compared, the standard deviations for control serum samples 4 to 8 were as follows: 0.03 for control serum sample 4 (mean index value, 0.33), 0.08 for control serum sample 5 (mean index value, 0.57), 0.06 for control serum sample 6 (mean index value, 0.76), 0.07 for control serum sample 7 (mean index value, 1.22), and 0.12 for control serum sample 8 (mean index value, 1.76).
Serum samples of 4 of 307 (1.3%) healthy blood donors had a positive IgG index value to recombinant mitogillin. Ten of the 307 blood donor serum samples (3.3%) showed borderline IgG index values, resulting in a specificity of 95.4%. In four blood donor serum samples (1.3%; n = 307) we found positive IgM index values. Fourteen (4.6%; n = 307) serum samples were borderline for IgM. In three (1.0%; n = 307) blood donor serum samples, positive IgA index values to recombinant mitogillin were found, and four (1.3%; n = 307) serum samples showed borderline IgA index values. No significant increase in specific titers of antibody against mitogillin could be observed when the index values for blood donor sera were matched with the age of the donors (data not shown). In the sera of two A. fumigatus-exposed healthy individuals we detected positive IgG levels using a commercial A. fumigatus antibody ELISA based on crude antigens (ActiTips A. fumigatus and EnzyDex; HAL Allergie GmbH). However, no specific IgG, IgM, or IgA antibodies against mitogillin were found in these serum samples. Likewise, no positive anti-mitogillin antibody index values were found in the serum samples of patients suffering from mucormycosis, histoplasmosis, or candidosis.
Patients with A. fumigatus-associated diseases.
Table 1 presents the results of anti-A. fumigatus antibody and Aspergillus antigen detection in serum samples of patients suffering from AO, IPA, IDA, or type III allergy to A. fumigatus. In all serum samples (n = 32) obtained from 13 episodes of AO, specific IgG antibodies against mitogillin were detected (sensitivity = 100%). In nine episodes (17 serum samples) we detected specific IgM against mitogillin, and in eight episodes (14 serum samples) specific IgA antibodies were present. Precipitating anti-A. fumigatus antibodies were found in all serum samples tested in the AO group (n = 32) by IE. Complement-fixing antibodies were detected in four episodes (six serum samples). GM was detected in nine episodes (16 serum samples) by the Platelia sandwich ELISA. GM antigen was not detected in any of the serum samples obtained from patients 5, 9, 10, and 12 (all tested by the Platelia sandwich ELISA).
In the group of patients suffering from IPA, specific anti-mitogillin IgG antibodies were detected in all nine episodes examined (27 of 42 serum samples were positive; sensitivity = 64%). Anti-mitogillin IgM was detected in two episodes (four serum samples), and IgA was detected in one episode (one serum sample). By IE and CF antibodies against A. fumigatus were detected in only one episode (two serum samples of patient 22). A. fumigatus GM antigen was present in five of the nine episodes. GM antigen was absent from patient 22 (tested by the Platelia sandwich ELISA) and patients 14, 15, and 16 (tested by the Pastorex latex agglutination test). However, specific anti-mitogillin IgG antibodies were present in these four patients. In follow-up serum samples of two patients (patients 17 and 19), antibodies against mitogillin were observed earlier than GM antigen was (tested by the Platelia sandwich ELISA). In the follow-up serum samples of two other patients (patients 20 and 21), GM was detected earlier. In three IPA patients (patients 17, 20, and 21), we observed within one episode single serum samples that were anti-mitogillin antibody positive but GM negative (serum samples 50, 66, and 70) and samples that were antibody negative but GM antigen positive (serum samples 52, 53, 63, and 69). In the IPA group the sensitivity increased to 74% (31 of 42 serum samples were positive) when combined testing of the sera (anti-mitogillin IgG antibodies and GM antigen detection) was performed (Table 2).
TABLE 2.
Sensitivity, specificity, and positive and negative predictive values of anti-mitogillin IgG antibody detection and of combined testing (anti-mitogillin IgG antibody and GM antigen detection) in the patient groups examined
| Patient group | Test type | Sensitivity (%) | Specificity (%) | Predictive value (%)
|
|
|---|---|---|---|---|---|
| Positive | Negative | ||||
| AO | Anti-mitogillin IgG antibodies | 100 | 95 | 0.95 | 1 |
| IPA | 64 | 95 | 0.93 | 0.73 | |
| IDA | 60 | 95 | 0.93 | 0.70 | |
| AO | Combined testing | 100 | 87 | 0.89 | 1 |
| IPA | 74 | 87 | 0.85 | 0.77 | |
| IDA | 78 | 87 | 0.86 | 0.80 | |
In the group of patients with IDA, anti-mitogillin IgG antibodies were present in 9 of 11 episodes examined (24 of 40 serum samples were positive; sensitivity = 60%). Anti-mitogillin IgA was detected in the two IgG-negative episodes (patients 25 and 27). Altogether, six episodes (10 serum samples) were IgA positive, and IgM was found in two episodes (two serum samples). Complement-fixing anti-Aspergillus antibodies were detected in four episodes (seven serum samples). Precipitating antibodies were not present in the 40 serum samples of the IDA group. Five episodes were positive for GM antigen, but six episodes were negative (patients 27, 28, and 31 tested by the Platelia sandwich ELISA). Anti-mitogillin IgG was detected in five of these GM-negative episodes, (patients 24, 28, 30, 31, and 33), and IgA was present in one GM-negative episode (patient 27). In two episodes GM was detected earlier than anti-mitogillin IgG (patients 23 and 29). In consecutively obtained serum samples of patient 23 we observed samples that were GM antigen positive but anti-mitogillin antibody negative (serum samples 75 and 76) and a sample that was positive for anti-mitogillin IgG but negative for GM antigen (serum sample 77). Combined testing (anti-mitogillin IgG antibodies and GM antigen detection) increased the sensitivity to 78% (31 of 40 positive samples) in this group (Table 2).
In the group of patients with type III allergy to A. fumigatus all sera were positive by the IgG mitogillin ELISA, and one serum sample had a borderline IgA level. GM antigen detection was not performed with samples of this group.
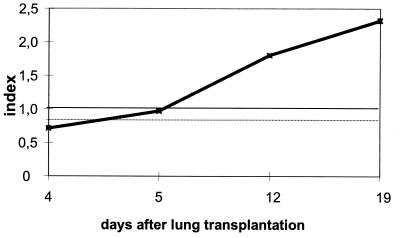
To evaluate the kinetics of antibody production to mitogillin during human A. fumigatus infection, serially obtained serum samples of patients suffering from IA were tested. Patient 22 developed severe respiratory symptoms after a lung transplantation. Antibodies to mitogillin were not present in a serum sample that was obtained 4 days after the transplantation (serum sample 71; Fig. 3). Severe IA localized within the transplanted lung was observed during a bronchoscopy that was done 5 days after surgery. A. fumigatus was repeatedly cultured from bronchoalveolar lavage specimens. The follow-up serum sample that was obtained on the day of the bronchoscopy (serum sample 72) showed a borderline IgG antibody index value against mitogillin. Positive titers for IgG antibody against mitogillin were found in the serum samples of the patient that were drawn on day 12 (serum sample 73) and day 19 (serum sample 74) after the transplantation. Specific IgM and IgA antibodies against mitogillin and A. fumigatus GM antigen were absent from all serum samples of patient 22. The patient died 44 days after the diagnosis of IA.
FIG. 3.
Index values for specific IgG antibodies to mitogillin obtained with serum samples of patient 22 suffering from IPA. The cutoff is indicated by a solid line. Borderline test results are indicated by a dotted line.
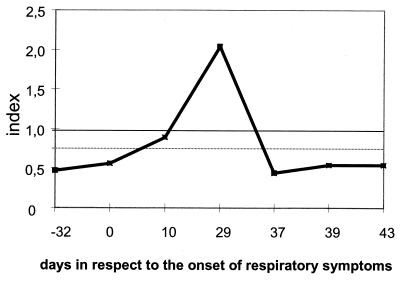
Patient 28 (Fig. 4) suffered from hepatitis of unknown origin with subacute liver failure. Three months after the spontaneous remission of the liver disease, he developed panmyelophthisis which was refractory to any therapy. During pancytopenia, the patient suffered a fatal severe destructive IA in the right upper lobe and the right apical medial lobe of the lung. A. fumigatus was cultured from a lung biopsy specimen obtained with the aid of computed tomography, and hyphae were detected in a histological examination with Grocott-Gomori stain. After death, pathological examination confirmed the mycotic necrotic focus in the right upper and middle lobe and revealed multiple A. fumigatus foci in the remaining lobes of the lung. In addition, embolic lesions with diameters of up to 0.8 cm were found in the parietal brain. Three months before the onset of severe respiratory symptoms, IgG antibodies against mitogillin were not detected in the serum of this patient (serum sample 85). Despite panmyelophthisis, the patient developed a borderline IgG antibody titer to mitogillin 10 days after the onset of the respiratory symptoms (serum sample 87). Highly positive IgG titers and positive IgM titers were found on day 29 (serum sample 88). The IgG and IgM antibody titers dropped on day 37 (serum sample 89). No detectable antibodies against mitogillin were found on day 39 or day 43 (serum samples 90 and 91 respectively). The patient died as a direct consequence of the IDA 22 days after a dramatic decrease in the level of anti-mitogillin antibodies (day 59). None of the serum samples of patient 28 showed detectable amounts of GM antigen.
FIG. 4.
Index values for specific IgG antibodies to mitogillin obtained with serum samples of patient 28 suffering from IDA. The cutoff is indicated by a solid line. Borderline test results are indicated by a dotted line.
DISCUSSION
Our knowledge about the role of the humoral immune response in human aspergillosis is still limited (7). Crude and undefined antigen preparations of A. fumigatus are commonly used for antibody detection, but standardized immunodominant antigens which indicate invasive disease are still not available for A. fumigatus. As a consequence, profound difficulties exist in the interpretation of serological test results (5, 7). We decided to investigate the usefulness of mitogillin for improving the serodiagnosis of aspergillosis. We identified this antigen as an 18-kDa protein in all WSEP preparations of 12 clinical A. fumigatus isolates (M. Weig, data not shown). These data are in accordance with the observations of Latgé et al. (25), who showed that a major immunodominant protein in the WSEPs of different A. fumigatus reference strains had a molecular mass of approximately 18 kDa.
In the present study we show that mitogillin can be used as a standardized marker for the early diagnosis of A. fumigatus-associated diseases. In all 13 episodes of AO (32 of 32 serum samples were positive; sensitivity = 100%) and in 18 of 20 episodes of IA (51 of 82 serum samples were positive; sensitivity = 62.2%), IgG antibodies to mitogillin were detected when repetitive sampling was performed. Two episodes of IA (in two AIDS patients) were anti-mitogillin IgG negative but IgA positive. In comparison, Aspergillus GM antigen was found in only 9 of 13 episodes of AO and in 10 of 20 episodes of IA. In 2 episodes of IA, anti-mitogillin antibodies were detected prior to GM antigen, GM antigen was detected earlier in 4 episodes, and in 10 episodes only anti-mitogillin antibodies were detected but GM antigen was absent. It is important that 8 of these 14 GM antigen-negative but anti-mitogillin antibody-positive episodes were tested by the sensitive Platelia sandwich ELISA. These findings underline the value of repetitive and combined testing for the diagnosis of IA. At this point we cannot draw a definite conclusion on the diagnostic value of serum IgM and IgA antibodies in the stage-specific diagnosis of A. fumigatus-associated diseases. However, the ubiquitous nature of the fungus and the high average daily level of exposure to A. fumigatus conidida might explain why IgG antibodies against mitogillin are superior to IgM antibodies as diagnostic markers in the diagnosis of A. fumigatus-associated diseases.
In our study we have compared the detection of antibodies to mitogillin by antibody tests based on crude Aspergillus extracts (CF, IE). In an evaluation of eight antibody tests for the diagnosis of IA, Kappe et al. (18) reported that CF (with the metabolic antigen of A. fumigatus) was superior to indirect hemagglutination tests and enzyme immunoassays. However, unsatisfactory sensitivity was observed for all antibody tests based on crude Aspergillus extracts (18). IE is one of the diagnostic procedures most commonly used to detect anti-Aspergillus antibodies in immunocompetent patients suffering from AO and ABPA (for a review, see reference 22). This method is sufficiently insensitive for the elimination of false-positive results that occur in Aspergillus-exposed healthy individuals. The disadvantages of this method are the inability to quantitate the immune response, the lack of standardization due to the use of crude extracts, and the fact that a minor contamination during the production of large quantities of antigen leads to erroneous results. These disadvantages are reflected in the results of our study. As expected, IE was useful in the diagnosis of AO. However, both conventional methods (IE, CF) failed to contribute sufficiently to the serodiagnosis of IA.
In follow-up sera of rabbits that were infected with viable A. fumigatus conidia, we observed that anti-mitogillin antibodies appear early in the course of the infection in comparison with the time of appearance of antibodies against different antigens of the WSEPs of A. fumigatus. To evaluate the kinetics of antibody production in human aspergillosis, we examined follow-up serum samples of patients suffering from fatal IPA and IDA and correlated the test results with the clinical course of disease. The earliest time possible for infection in patient 22 was the day of the lung transplantation, as IPA was restricted to the transplanted lung. Five days after surgery the patient's serum showed borderline levels of IgG against mitogillin, and the IgG levels were highly positive on day 12. In patient 28, IgG antibodies against mitogillin were observed 10 days after the onset of respiratory symptoms. Circulating GM antigens were not detected in any of the serum samples of patients 22 and 28. These results stress the diagnostic value of detection of antibody against mitogillin for the early diagnosis of A. fumigatus-associated diseases. We observed a sharp decrease in the levels of IgG antibody against mitogillin in patient 28 prior to his death. It could be speculated that antibodies to mitogillin play a key role in the control and humoral immune defense of human IA. We are therefore evaluating the value of detection of antibody against mitogillin as a prognostic marker of IA.
False-negative test results and a high frequency of samples with false-positive results presumably due to cross-reactivity with other polysaccharide fungal antigens or other serum components are major drawbacks of solely GM antigen-based serodiagnosis of aspergillosis (34, 35, 37). Mitogillin shares a high degree of amino acid sequence homology only with restrictocin and, to lower extents, with α-sarcin (17) and clavin (D. Parente et al., unpublished data). These proteins are expressed by species of the genus Aspergillus (A. restrictus, A. giganteus, and Aspergillus clavatus) (21). Antigens showing cross-reactivity with mitogillin have been identified in cultures of Aspergillus fischeri, Aspergillus ochraceus, Neosartoria aureola, and Neosartoria stramenia but were absent from other genera of medically important fungi (25).
A. fumigatus is ubiquitous in nature, and Smith et al. (32) calculated that the average number of inhaled conidia is approximately 30 per day. Individuals working in gardens, in animal quarters, or with compost heaps might be burdened with massive numbers of A. fumigatus conidia. High levels of exposure to conidia lead to the production of antibodies against several A. fumigatus antigens in healthy individuals (25). This makes it difficult to discriminate exposure from infection by conventional serological techniques (19) based on crude extracts. However, the array of A. fumigatus antigens expressed depends largely on environmental conditions such as the medium used, the origin of the crude extract, or the type of propagule (i.e., conidium versus mycelium) (25). Ribotoxins of the mitogillin family are scarcely found in resting conidia but are highly expressed during active growth of the fungus (2). For these reasons, we speculated that a mitogillin-based serological test system is highly specific for the diagnosis of Aspergillus-related diseases. Sera from patients who suffered from mucormycosis, candidemia, or histoplasmosis showed no mitogillin-reactive antibodies. In addition, only 4 (1.3%) of the 307 blood donor serum samples examined in our study were positive for IgG antibody against mitogillin, 1.3% were positive for IgM antibody, and 1.0% were positive for IgA antibody by the recombinant mitogillin ELISA. These data obtained for the blood donors confirm the high degree of specificity (95.4%) of the mitogillin-based test system. Similar ratios were identified by Heymann and colleagues (10), who detected AspfI-reactive IgG antibodies in 6% of control patients. We tested serum samples of blood donors with an age range of from 19 to 65 years. Although prolonged exposure to A. fumigatus conidia in older donors is likely, higher levels of antibody against mitogillin were not observed, resulting in a high degree of specificity of the test. The limited number of positive blood donor sera might reflect the prevalence of A. fumigatus allergy in the population. Our data indicate that even high levels of exposure to A. fumigatus conidia do not elicit production of antibody against mitogillin, as serum samples of healthy immunocompetent individuals positive for A. fumigatus antibody with a commercial kit based on crude antigens showed no significant levels of antibody against mitogillin.
The availability of recombinant mitogillin for specific antibody detection provides a new serological tool for the diagnosis of A. fumigatus-associated diseases. Our data indicate a high degree of correlation of production of antibody against mitogillin and the clinical course of disease. Finally, the standardized antigen will be used in further studies to define the role of the humoral immune response in human aspergillosis.
ACKNOWLEDGMENTS
We thank Jacqui Shea for providing plasmid pMIT+ and Klaus Fischer, Stefan Ziesing, and Jean Lumovici for providing serum samples. The excellent technical assistance of Thanh Hang Nguyen and Mechthild Schulze is acknowledged.
REFERENCES
- 1.Aisner J, Wiernik P H, Schimpff S C. Treatment of invasive aspergillosis: relation of early diagnosis and treatment to response. Ann Intern Med. 1977;86:539–543. doi: 10.7326/0003-4819-86-5-539. [DOI] [PubMed] [Google Scholar]
- 2.Arruda L K, Mann B J, Chapman M D. Selective expression of a major allergen and cytotoxin, Asp f I, in Aspergillus fumigatus. Implications for the immunopathogenesis of Aspergillus-related diseases. J Immunol. 1992;149:3354–3359. [PubMed] [Google Scholar]
- 3.Arruda L K, Platts-Mills T A, Fox J W, Chapman M D. Aspergillus fumigatus allergen I, a major IgE-binding protein, is a member of the mitogillin family of cytotoxins. J Exp Med. 1990;172:1529–1532. doi: 10.1084/jem.172.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnes R A, Rogers T R. Response rates to a staged antibiotic regimen in febrile neutropenic patients. J Antimicrob Chemother. 1988;22:759–763. doi: 10.1093/jac/22.5.759. [DOI] [PubMed] [Google Scholar]
- 5.Brouwer J. Detection of antibodies against Aspergillus fumigatus: comparison between double immunodiffusion, ELISA and immunoblot analysis. Int Arch Allergy Appl Immunol. 1988;85:244–249. doi: 10.1159/000234510. [DOI] [PubMed] [Google Scholar]
- 6.Burch P A, Karp J E, Merz W G, Kuhlman J E, Fishman E K. Favorable outcome of invasive aspergillosis in patients with acute leukemia. J Clin Oncol. 1987;5:1985–1993. doi: 10.1200/JCO.1987.5.12.1985. [DOI] [PubMed] [Google Scholar]
- 7.Casadevall A. Antibody immunity and invasive fungal infections. Infect Immun. 1995;63:4211–4218. doi: 10.1128/iai.63.11.4211-4218.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denning D W, Stevens D A. Antifungal and surgical treatment of invasive aspergillosis: review of 2,121 published cases. Rev Infect Dis. 1990;12:1147–1201. doi: 10.1093/clinids/12.6.1147. [DOI] [PubMed] [Google Scholar]
- 9.De Repentigny L, Boushira M, Ste-Marie L, Bosisio G. Detection of galactomannan antigenemia by enzyme immunoassay in experimental invasive aspergillosis. J Clin Microbiol. 1987;25:863–867. doi: 10.1128/jcm.25.5.863-867.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El Dahr J M, Fink R, Selden R, Arruda L K, Platts-Mills T A E, Heymann P W. Development of immune responses to Aspergillus at an early age in children with cystic fibrosis. Am J Respir Crit Care Med. 1994;150:1513–1518. doi: 10.1164/ajrccm.150.6.7952609. [DOI] [PubMed] [Google Scholar]
- 11.Fando J L, Alaba I, Escarmis C, Fernandez-Luna J L, Mendez E, Salinas M. The mode of action of restriction and mitogillin on eukaryotic ribosomes. Inhibition of brain protein synthesis, cleavage and sequence of the ribosomal RNA fragment. Eur J Biochem. 1985;149:29–34. doi: 10.1111/j.1432-1033.1985.tb08888.x. [DOI] [PubMed] [Google Scholar]
- 12.Haynes K A, Latgé J P, Rogers T R. Detection of Aspergillus antigens associated with invasive infection. J Clin Microbiol. 1990;28:2040–2044. doi: 10.1128/jcm.28.9.2040-2044.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hearn V M. Serodiagnosis of aspergillosis. In: Vanden Bossche H, Mackenzie D W R, Cauwenbergh G, editors. Aspergillus and aspergillosis. New York, N.Y: Plenum Press; 1988. pp. 43–71. [Google Scholar]
- 14.Hearn V M, Latgé J P, Prevost M C. Immunolocalization of Aspergillus fumigatus mycelial antigens. J Med Vet Mycol. 1991;29:73–81. [PubMed] [Google Scholar]
- 15.Hearn V M, Wilson E V, Latgé J P, Mackenzie D W R. Immunochemical studies of Aspergillus fumigatus mycelial antigens by polyacrylamide gel electrophoresis and western blotting techniques. J Gen Microbiol. 1990;136:1525–1535. doi: 10.1099/00221287-136-8-1525. [DOI] [PubMed] [Google Scholar]
- 16.Kao R, Davies J. Fungal ribotoxins: a family of naturally engineered targeted toxins? Biochem Cell Biol. 1995;73:1151–1159. doi: 10.1139/o95-124. [DOI] [PubMed] [Google Scholar]
- 17.Kao R, Shea J E, Davies J, Holden D W. Probing the active site of mitogillin, a fungal ribotoxin. Mol Microbiol. 1998;29:1019–1027. doi: 10.1046/j.1365-2958.1998.00987.x. [DOI] [PubMed] [Google Scholar]
- 18.Kappe R, Schulze-Berge A, Sonntag H G. Evaluation of eight antibody tests and one antigen test for the diagnosis of invasive aspergillosis. Mycoses. 1996;39:13–23. doi: 10.1111/j.1439-0507.1996.tb00078.x. [DOI] [PubMed] [Google Scholar]
- 19.Kurup V P, Kumar A, Kenealy W R, Greenberger P A. Aspergillus ribotoxins react with IgE and IgG antibodies of patients with allergic bronchopulmonary aspergillosis. J Lab Clin Med. 1994;123:749–756. [PubMed] [Google Scholar]
- 20.Lamy B, Davies J. Isolation and nucleotide sequence of the Aspergillus restrictus gene coding for the ribonucleolytic toxin restrictocin and its expression in Aspergillus nidulans: the leader sequence protects producing strains from suicide. Nucleic Acids Res. 1991;19:1001–1006. doi: 10.1093/nar/19.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamy B, Moutaouakil M, Latgé J P, Davies J. Secretion of a potential virulence factor, a fungal ribonucleotoxin, during human aspergillosis infections. Mol Microbiol. 1991;5:1811–1815. doi: 10.1111/j.1365-2958.1991.tb01930.x. [DOI] [PubMed] [Google Scholar]
- 22.Latgé J P. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev. 1999;12:310–350. doi: 10.1128/cmr.12.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Latgé J P, Debeaupuis J P, Sarfati J, Diaquin M, Paris S. Cell wall antigens in Aspergillus fumigatus. Arch Med Res. 1993;24:269–274. [PubMed] [Google Scholar]
- 24.Latgé J P, Kobayashi H, Debeaupuis J P, Diaquin M, Sarfati J, Wieruszeski J M, Parra E, Bouchara J P, Fournet B. Chemical and immunological characterization of the extracellular galactomannan of Aspergillus fumigatus. Infect Immun. 1994;62:5424–5433. doi: 10.1128/iai.62.12.5424-5433.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Latgé J P, Moutaouakil M, Debeaupuis J P, Bouchara J P, Haynes K, Prevost M C. The 18-kilodalton antigen secreted by Aspergillus fumigatus. Infect Immun. 1991;59:2586–2594. doi: 10.1128/iai.59.8.2586-2594.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oka T, Natori Y, Tanaka S, Tsurugi K, Endo Y. Complete nucleotide sequence of cDNA for the cytotoxin alpha sarcin. Nucleic Acids Res. 1990;18:1897. doi: 10.1093/nar/18.7.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pannuti C S, Gingrich R D, Pfaller M A, Wenzel R P. Nosocomial pneumonia in adult patients undergoing bone marrow transplantation: a 9-year study. J Clin Oncol. 1991;9:77–84. doi: 10.1200/JCO.1991.9.1.77. [DOI] [PubMed] [Google Scholar]
- 28.Reiss E, Hearn V M, Poulain D, Shepherd M G. Structure and function of the fungal cell wall. J Med Vet Mycol. 1992;30(Suppl. 1):143–156. doi: 10.1080/02681219280000841. [DOI] [PubMed] [Google Scholar]
- 29.Rogers T R, Haynes K A, Barnes R A. Value of antigen detection in predicting invasive pulmonary aspergillosis. Lancet. 1990;336:1210–1213. doi: 10.1016/0140-6736(90)92831-2. [DOI] [PubMed] [Google Scholar]
- 30.Rohrlich P, Sarfati J, Mariani P, Duval M, Carol A, Saint-Martin C, Bingen E, Latgé J P, Vilmer E. Prospective sandwich enzyme-linked immunosorbent assay for serum galactomannan: early predictive value and clinical use in invasive aspergillosis. Pediatr Infect Dis J. 1996;15:232–237. doi: 10.1097/00006454-199603000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Smith J M, Davies J E, Holden D W. Construction and pathogenicity of Aspergillus fumigatus mutants that do not produce the ribotoxin restrictocin. Mol Microbiol. 1993;9:1071–1077. doi: 10.1111/j.1365-2958.1993.tb01236.x. [DOI] [PubMed] [Google Scholar]
- 32.Smith J M, Tang C M, Van Noorden S, Holden D W. Virulence of Aspergillus fumigatus double mutants lacking restriction and an alkaline protease in a low-dose model of invasive pulmonary aspergillosis. Infect Immun. 1994;62:5247–5254. doi: 10.1128/iai.62.12.5247-5254.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ste-Marie L, Senechal S, Boushira M, Garzon S, Strykowski H, Pedneault L, de Repentigny L. Production and characterization of monoclonal antibodies to cell wall antigens of Aspergillus fumigatus. Infect Immun. 1990;58:2105–2114. doi: 10.1128/iai.58.7.2105-2114.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stynen D, Goris A, Sarfati J, Latgé J P. A new sensitive sandwich enzyme-linked immunosorbent assay to detect galactofuran in patients with invasive aspergillosis. J Clin Microbiol. 1995;33:497–500. doi: 10.1128/jcm.33.2.497-500.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sulahian A, Tabouret M, Ribaud P, Sarfati J, Gluckman E, Latgé J P, Derouin F. Comparison of an enzyme immunoassay and latex agglutination test for detection of galactomannan in the diagnosis of invasive aspergillosis. Eur J Clin Microbiol Infect Dis. 1996;15:139–145. doi: 10.1007/BF01591487. [DOI] [PubMed] [Google Scholar]
- 36.Swanink C M A, Meis J F, Rijs A J, Donnelly J P, Verweij P E. Specificity of a sandwich enzyme-linked immunosorbent assay for detecting Aspergillus galactomannan. J Clin Microbiol. 1997;35:257–260. doi: 10.1128/jcm.35.1.257-260.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verweij P E, Stynen D, Rijs A J, De Pauw B E, Hoogkamp-Korstanje J A, Meis J F. Sandwich enzyme-linked immunosorbent assay compared with Pastorex latex agglutination test for diagnosing invasive aspergillosis in immunocompromised patients. J Clin Microbiol. 1995;33:1912–1914. doi: 10.1128/jcm.33.7.1912-1914.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]