Abstract
Brugada syndrome (BrS) presents with a characteristic electrocardiogram (ECG) and is associated with sudden cardiac death. Until now, prolongation of QTc interval and its association with Torsade de Pointe and possible fatal arrhythmia have been the focus of routine baseline ECGs before prescribing psychotropic medication. A semi-systematic literature review was conducted using PubMed. The terms ‘Brugada’, ‘Brugada Syndrome’ AND ‘psychotropic’ ‘antipsychotic’ ‘antidepressant’ ‘mood stabilisers’ ‘clozapine’ ‘Tricyclic Antidepressants’ ‘Lithium’ were searched. From a search that delivered over 200 articles, 82 articles were included. Those that included details around causative medication, doses of medication and where clear timeline on drug cause were included. Where clarification was needed, the manufacturer of the medication was contacted directly. Psychotropic medication can be associated with BrS, Brugada phenocopy or unmasking of BrS, in overdose or in normal doses. Our results include a table summarising a number of psychotropic overdoses that led to BrS unmasking. Routine screening for BrS in patients before prescribing psychotropic medication is a natural extension of the baseline ECG currently routinely done to rule out QTc prolongation. Psychiatrists need to invest in ensuring better skills in interpreting ECGs and work closer with cardiologists in interpreting ECGs.
Keywords: Brugada, Brugada syndrome sudden death, ECG changes, psychotropic medication
Introduction
Brugada syndrome (BrS) is an inherited disease that is presented with a characteristic electrocardiogram (ECG) and can be associated with fatal complications and premature sudden unexpected cardiac death. 1 Having been introduced as a new clinical entity by Pedro and Josep Brugada 2 in 1992, the syndrome is considered responsible for nearly 20% of all sudden cardiac deaths (SCDs) in patients with structurally normal hearts. It is important to realise that any given patient with BrS can have marked day-to-day ECG changes making diagnosis challenging. In a large series of patients with Brugada who had repeated ECGs over the years, only every third ECG showed type 1 and every other third ECG was normal. 3 Some psychotropic medications can unmask BrS or precipitate Brugada-like ECG changes. Brugada pattern ECG has also been found to be more common in those with schizophrenia spectrum disorders. 4
Method
A semi-systematic literature review was conducted using PubMed. The terms ‘Brugada’, ‘Brugada Syndrome’ AND ‘psychotropic’ ‘antipsychotic’ ‘antidepressant’ ‘mood stabilisers’ ‘clozapine’ ‘Tricyclic Antidepressants’ ‘Lithium’ were searched. From a search that delivered over 200 articles, 82 articles were included. Those that included details around causative medication, doses of medication and where clear timeline on drug cause were included. Further articles were identified from the articles reviewed. Where clarification was needed, the manufacturer of the medication was contacted directly.
Definition of Brugada-type ECG
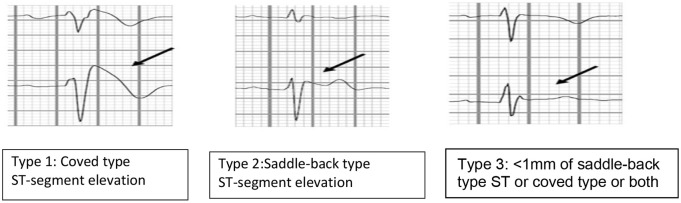
Brugada-type ECG is characterised by several different patterns, of which only type 1 is diagnostic. 5
Type 1 is a prominent cove-shaped ST-T segment with a J-wave amplitude or ST elevation ⩾2 mm (0.2 mV) at its peak followed by a negative T wave, with little or no isoelectric separation. This is the only diagnostic pattern for BrS. 1
Type 2 is a high take-off ST segment with a J-wave amplitude ⩾2 mm with gradually decreasing ST-segment elevation (remaining ⩾1 mm above baseline, followed by a positive or biphasic T wave that gives rise to saddle-back configuration). Type 3 is defined if right precordial ST-segment elevation is <1 mm of a saddle-back type, coved type, or both. Type 2 and type 3 patterns are suggestive of BrS but are not diagnostic in themselves (Figures 1 and 2).
Figure 1.
Three types of Brugada ECG pattern.
ECG, electrocardiogram.
Figure 2.
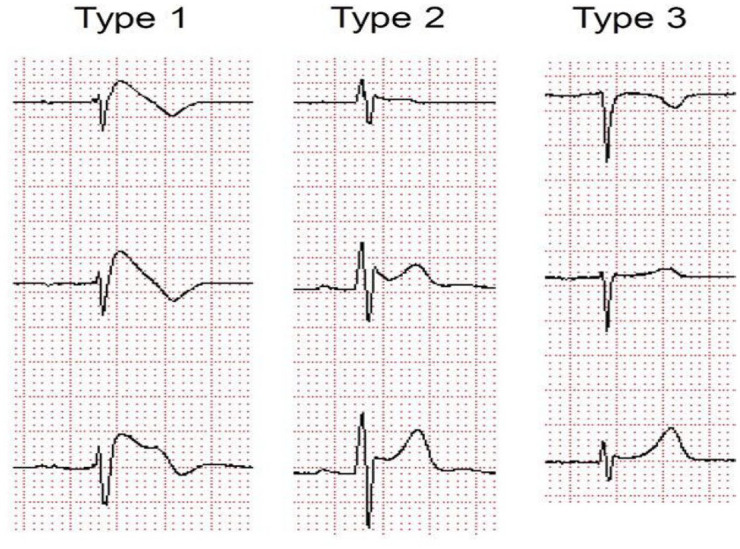
Brugada-type ECG shift.
ECG, electrocardiogram.
Image source: http://circep.aha.journals.org/content/5/3/606/F3.large.jpg.
Pathophysiology
BrS was initially considered to have an autosomal dominant inheritance pattern with incomplete penetrance and primarily associated with mutations in the SCN5A gene, which encodes for the pore-forming alpha subunit of the cardiac Na+ channels. Over 300 mutations in SCN5A have been identified thus far, accounting for up to 30% of BrS probands. In recent years, however, over a dozen further pathogenic variant genes have been associated with BrS, for example, by altering the normal function of Na+, K+, Ca2+ and hyperpolarization-activated cyclic nucleotide-gated (HCN) channels. These additional gene mutations only account for around 10% of probands however. 6 Both depolarization and repolarization abnormalities have been described in BrS. 7
Although genetic mutations appear to be equally distributed between sexes, the clinical phenotype of BrS is almost 10 times more prevalent in men than in women. 8
Furthermore, women with BrS tend to present with more benign clinical characteristics and less spontaneous type 1 ECG pattern and are more likely to be asymptomatic than men. 9 Although the drivers behind this sex-related distinction remain to be identified, there is evidence suggestive of still unknown, but there is evidence to suggest a hormonal basis. Interestingly, reversion of the phenotype has been noted in two patients with prostate cancer after surgical castration suggesting a possible role for androgens. 1
Recent evidence demonstrating normalisation of the pathognomonic ECG pattern and elimination of the arrhythmic disposition in most patients following radiofrequency ablation of the right ventricular (RV) outflow tract epicardium is suggestive of the possibility that structural abnormalities, likely at a microanatomical scale, also play some role in the pathophysiology of BrS. As such, BrS and arrhythmogenic cardiomyopathy have been proposed to be part of the same disease spectrum. 10
Brugada ECG pattern is more common in those diagnosed with schizophrenia spectrum disorder, conditions which are also associated with ion channel abnormalities. A recent case control study of 338 patients recently diagnosed with a schizophrenia spectrum disorder and 844 healthy controls identified Brugada pattern ECG in 33 patients (8.5%) and 13 healthy controls (1.5%), with an adjusted odds ratio of 3.5 (p < 0.0001). 4 An earlier study found that as many as 4% of patients with schizophrenia had type 1 Brugada ECG compared with an estimated prevalence of 0.05% in the general population. 11
Brugada incidence and prognosis
The worldwide incidence and risk of death in those with a Brugada ECG pattern remains unclear despite a developing understanding of this disorder since its initial description in 1992. 12 Since the first descriptions reported high mortality, the reported incidence of SCD has been declining and there is a clearer understanding that some patients accumulate the highest risk for developing malignant ventricular arrhythmias while others follow a benign course with a long-life expectancy. 13 That being said, BrS has a significant mortality attached and accounts for 12% of all SCDs and around 20% of SCDs in patients with structurally normal hearts. 2 Symptomatic presentation with previous aborted cardiac arrest or sustained ventricular arrhythmia carries the highest risk, with a reported annual rate of repeated arrhythmic event of around 8%. In such cases, secondary prevention with implantable cardioverter-defibrillator (ICD) implantation is recommended. 10
Two meta-analyses reviews have attempted to give a world prevalence; the first gives a prevalence of Brugada ECG pattern as 0.4% with different prevalence according to region – highest in Asia (0.9%) and North America (0.2%) – and according to sex – male (0.9%) and female (0.1%). 12
In another meta-analysis, the worldwide pooled prevalence was predicted as 0.5 per 1000 [95% confidence interval (CI) = 0.3–0.7]. The prevalence in South East Asia was 3.7 in 1000 (95% CI = 0.7–6.7), while the prevalence was much lower in North Africa (0 in 1000). 14
A study of population >55 years old, otherwise healthy Han Chinese population (n = 5214) in Taiwan, found the overall prevalence of Brugada ECG changes to be 3.32%. 15 In a large hospital-based Taiwanese population setting where ECGs were examined as part of routine care (non-cardiology reasons for admission), just under 75,000 ECGs were examined from 20,562 patients and 26 (0.13%) were confirmed to have Brugada-type ECGs. 16 When these 26 patients were followed up, neither SCD nor hospitalised death differed between these patients and those without Brugada-type ECG. 16 Asymptomatic patients with Brugada-type ECGs were not rare in this cohort of hospitalised patients; however, their risk of SCD was not increased. 16 This differed to smaller sample size studies in hospitalised patients where participants had either Brugada-type ECGs or a family history of SCD. 17 By comparison, a Danish general population study of the prevalence and relevance of Brugada-type ECG found a prevalence of 7 in 10,000, and none of the participants presented with type 1 BrS. 18
In a study consisting of 3021 young and middle-aged participants in the Finnish population, no type 1 Brugada ECG shift was found in any participants and 18 participants had type 2 or 3 Brugada shift ECG. 19 No mortality or life-threatening ventricular arrhythmias occurred in the populations studied, and the authors concluded that type 2 or 3 Brugada ECG pattern is a normal variant rather than a specific predictor of life-threatening ventricular arrythmias especially in those without a family history of SCD. 19 In more recent studies where the long-term prognosis of 1029 patients diagnosed with BrS using patients recruited from 11 tertiary centres in four European countries (France, Italy, the Netherlands and Germany), the authors concluded that the cardiac event rate per year was 7.7% in patients with aborted SCD, 1.9% in patients with syncope and 0.5% in asymptomatic patients. 20 In this study, the presence of symptoms and a spontaneous type 1 ECG are the only independent predictors of arrhythmic events. 20 Conversely, sex, family history of SCD, inducibility of ventricular tachyarrhythmias and genetic factors had no predictive value. 20
Screening for BrS involves the following:
A baseline ECG to identify Brugada-type ECG shifts: the identification of which may require expert interpretation of ECG. However, do note the variation associated with ECG presentations previously described. 14
A challenge test: in working-age adults, this can be done using ajmaline 1 mg/kg over 5 min IV, flecainide 2 mg/kg over 10 min IV or 400 mg orally, procainamide 10 mg/kg over 10 min IV or pilsicainide 1 mg/kg over 10 min IV. Following administration, the patient’s ECG is closely monitored to determine the Brugada pattern.21,22
Genetic testing will reveal whether there is a mutation in the sodium ion channel gene SCN5A and hence whether or not there is a familial predisposition to BrS. However, an SCN5A mutation and family history do not have any impact on prognosis, so they are not used for risk stratification, and mutations are only identified in approximately 25% of people with the BrS.23,24
Fever-induced Brugada
Fever-induced Brugada was studied and ECG recordings from 402 febrile and 909 afebrile patients of a similar age were compared. 25 The authors concluded that type 1 Brugada pattern was 20 times (n = 8 in febrile versus n = 1 in afebrile) more prevalent among febrile patients (2% versus 0.1%; p = 0.0001). 25 None of the febrile-induced BrS patients were of South East Asian origin and had an electrolyte imbalance, bradycardia, prolonged PR interval or a family history of SCD. 25 Two of the febrile-induced BrS patients were taking medication associated with BrS: clothiapine and carbamazepine; their ECGs normalised, however, once the fever resolved.
Brugada phenocopy
Brugada phenocopy (BrP) is a clinical condition which leads to Brugada-like ECG but is secondary to an underlying pathological condition rather than BrS itself such as hyperkalaemia, hypokalaemia, left ventricular aneurysm, pericarditis or pulmonary embolism. 7 Once the aetiology is resolved, the BrP ECG pattern normalises. 26 Differentiating between BrP and BrS is challenging but important, not least because while the clinical implications of BrP remain uncertain, patients with BrS are at increased risk of SCD and may require an ICD. 27
Medication used to treat Brugada
Management of acute presentation in A&E
Whenever a patient presents with syncope or cardiac arrest, BrS needs to be one of the differential diagnoses and an ECG must be performed.21,22 Alongside supportive management of the patient, sodium bicarbonate IV has demonstrated efficacy when used to promote the recovery of a ‘normal’ ECG pattern.28 –31 Any fever should be reduced immediately; paracetamol can be prescribed for this. 21 Isoproterenol, a non-selective β adrenoceptor agonist, has been found to be effective and is a drug of choice for the acute treatment of Brugada, particularly in the context of electrical storm as further outlined below. 32
Ongoing management
In the longer term, the most important action is to discontinue any medicine that precipitated BrP or unmasked BrS and avoid other medicines with high risk of precipitating it. 21 The website www.brugadadrugs.org includes lists of medicines to be avoided, medicines to be avoided preferentially and medicines that are potentially anti-arrhythmic. Close collaboration between the patient, the patient’s carer(s) and all health professionals involved in the patient’s care is recommended to plan ongoing management of any co-morbid conditions, whether mental ill health or physical ill health. 33 Where BrS is clearly medicine-induced, further intervention may not be needed unless the patient also has other underlying risk factors.21,33 The patient needs to be educated to avoid excessive alcohol intake.
To determine which intervention is necessary to manage the BrS, the patient’s risk will be classified as follows:
Numerous factors have been put forward in relation to risk stratification though syncope and spontaneous type 1 ECG pattern are the only ones that have remained consistent in their predictive role across multiple studies. 34 There remains controversy around the value of electrophysiologic study (EPS) for risk stratification in patients with BrS. 35
The intervention that is most evidence-based in those deemed at higher risk of SCD with BrS, and therefore first-line, is implantation of an ICD.21,22 However, this procedure is not without risks and complications, particularly in younger, more active patients.
If a patient suffers an electrical storm, which is defined as three or more episodes of ventricular tachycardia/ventricular fibrillation (VT/VF) in 24 h, this is treated with outward potassium current blockers, for example, (hydro)quinidine, and beta-sympathomimetics, for example, isoproterenol. 21 Anything that may have precipitated this, including fever or hypokalemia, must be treated concurrently. In the case of a patient with a spontaneous type 1 Brugada ECG who is asymptomatic, (hydro)quinidine can be considered.
Psychotropic medications associated with Brugada
As already described, a significant number of factors can influence the electrocardiographic and arrhythmic manifestations of BrS. With regard to psychotropic medication, the principal mode of action is blockade of ventricular sodium and calcium channels. 36 This differs from the most common mechanism underlying QT prolongation in psychotropic drugs, namely blockade of potassium channels in the myocardium. 37
In some of the cases reviewed in this article, a particular psychotropic medicine unmasked BrS while in others may have precipitated BrP.28 –31,38 –40 For example, intoxication with drugs that inhibit cardiac sodium channels, such as tricyclic antidepressants, can trigger BrP in otherwise normal individuals. 31 Differentiating between the two is challenging but important in informing the patient’s future care plan.
Although women are considered to be at greater risk of arrhythmia associated with prolonged QT interval, interestingly, in BrS, men are at higher risk of both developing the clinical phenotype for the disorder and also of suffering cardiac events following exposure to psychotropic medication that can unmask the disorder. 41
Although there is little in the literature on the topic, age is potentially a further complicating factor in relation to the presentation of Brugada-type ECG, in the context of psychotropic drug use and indeed more generally. For example, there is an increased likelihood of experiencing comorbidity as we grow older, including cardiac comorbidity. There is also likely to be additional medication burden that could include drugs that impact sodium and calcium channels.
Tricyclic antidepressants
The cardiovascular adverse effects of tricyclic antidepressants, both in therapeutic doses and in overdose, are well documented. This section will review case reports that specifically mention the BrS.28 –31,38 –40 Please see Table 2 for further details of the cases.
Table 2.
Summary of Brugada case studies involving tricyclic antidepressants. .
| Year | Patient demo | Significant medical history? (Pre-existing Brugada? Y/N) |
Presentation | Observations | Findings | Drug; dose/level; (Overdose? Y/N) | Treatment administered | Response and follow-up |
|---|---|---|---|---|---|---|---|---|
| 2001 | 33-year-old male | Not stated | Needed resuscitation | Not stated | ECG showed sinus rhythm with RBBB, prolonged QTc of 526 ms and ST elevation typical of Brugada syndrome | Nortriptyline; level 476 mcg/ml; (Y) | Not stated | Serial ECGs showed gradual normalisation |
| 2004 | 66-year-old female | Depression, COPD (N) | Comatose; ventilation slightly depressed but did not need mechanical ventilation | BP 80/40 mmHg temp 35.8°C |
ECG changes including ST-segment elevation in the right precordial and inferior leads. Electrolytes, cardiac markers and pH within normal limits; echocardiogram normal |
Imipramine; imipramine level 1460 mcg/L; desipramine level 1170 mcg/L; (Y) | Sodium chloride 0.9% infusion; low-dose noradrenaline; magnesium sulphate 2 × 750 mg; sodium bicarbonate 1.4% 2 L/24 h | Within a few hours regained consciousness and ECG abnormalities disappeared; no family history of syncope or sudden death; flecainide test negative; genetic screening negative |
| 2005 | 48-year-old male | Hypertension, non-cardiac chest pain, negative exercise tolerance test for ischaemia, echocardiogram showed structurally normal heart (not stated) | On admission: normal Next morning: coarse breath sounds at the left base 5 hours later: acutely dyspnoeic with transient loss of consciousness; denied chest pain or palpitations; afebrile |
On admission: HR 72 bpm; BP 132/94 mmHg; RR 16/min; GCS 13/15 Next Morning: HR 90 bpm; BP 100/60 mmHg; O2 sats 70% while on oxygen 4 L/min; RR 20/min; GCS 11/15 5 hours later: HR 105 bpm, BP 125/65 mmHg, GCS 15/15, O2 sat 94% on oxygen at 10 L/min via mask |
On admission: normal electrolytes and glucose; blood gas analysis on room air pH 7.37, PaCO2 6.4 kPa, PaO2 9.0 kPa, lactate 0.9 mmol/L; serum toxicology positive for TCA; no abnormality on chest X-ray; initial ECG showed normal QRS duration 0.12 s Next morning: chest X-ray showed bibasal atelectasis; ECG included isolated ST elevation of <1 mm with concave ST elevation in V2; troponin I <0.3 mcg/L 5 hours later: chest X-ray unchanged; electrolytes, glucose, blood gases, troponin normal; ECG included ST-segment elevation in V1-V2 |
Diazepam, zopiclone, nortriptyline, paroxetine; doses not specified; (Y) | On admission observation only. Next morning CPAP 7.5 cm H2O with FiO2 of 1.0 (sats improved to 97%), IV amoxicillin and clavulanic acid; however declining GCS so intubation and mechanical ventilation; later stabilised so ventilation weaned and extubated; 5 hours later: aspirin 300 mg and cardiology opinion sought – Brugada syndrome diagnosed |
Observed for further 24 h without further event; ECG returned to normal over next 4 days, nortriptyline discontinued with no further events |
| 2005 | 42-year-old male | Depression, asthma, obstructive sleep apnoea; two years before his ECG was normal (Y) |
VF at home; two further episodes of VF in hospital | Not stated | Electrolytes and TSH normal; coronary angiogram normal; gated blood pool SPECT showed left and right ventricular ejection fractions normal; ECG showed Brugada pattern | Desipramine 400 mg nocte (increased from 300 mg nocte 1 month previously), omeprazole 20 mg od, bromocriptine 2.5 mg nocte; (N) | Beta-blocker and ICD | Genetic testing revealed SCN5A polymorphism; desipramine was discontinued and ECG returned to normal |
| 2006 | 44-year-old male | Depression, normal ECG 1 year prior (N) | Denied chest pain, palpitations, syncope, shortness of breath, flushed skin, dry mucus membranes, hypoactive bowel sounds | HR 52 bpm; BP 119/83 mmHg; RR 12/min; temperature 36.0°C; O2 sats 98% on room air; JVP normal; cardiac apical impulse focal and nondisplaced; rest of physical exam normal | Heart auscultation bradycardia, normal rhythm, normal S1 and S2, no murmurs, rubs or gallops, no hepatomegaly; normal U&E’s, bloods and chest X-ray; troponin-I negative; ECG included RBBB and ST-segment elevations in V1-V2 with inverted T waves consistent with Brugada type 1 | Desipramine, clonazepam, trazodone; no doses or levels available; (Y) | IV sodium bicarbonate | Right precordial ST-segment elevations and T-wave inversions normalised after 5 h; sinus bradycardia, first-degree AV block and RBBB persisted; patient remained asymptomatic |
| 2007 | 50-year-old male | Not stated | Cardiopulmonary arrest | Not stated | After initial resuscitation; ECG showed Brugada pattern; no ischaemia | Amitriptyline; 13.6 g, level >1000 ng/ml; (Y) | Sodium bicarbonate 700 mEq | Brugada persisted despite sodium bicarbonate; 5 h after last dose of sodium bicarbonate and 18 h after the event, Brugada pattern resolved |
| 2009 | 56-year-old female | Depression (N) | Found slumped on the floor | Normal vital signs; normal physical examination | Normal chemistries; ECG revealed classic type 1 Brugada pattern | Amitriptyline, temazepam, doses not stated; (Y) | Not stated | ECG done a few days later showed normal ST segments |
| 2009 | 40-year-old male | Depression (Unsure) | First time: syncope with convulsive seizures Second time suddenly fell after standing up Third time syncope while walking outside |
First time: Alert Second time: needed CPR Third time: not specified |
First time: no abnormalities in bloods, neurological findings, brain CT or MRI Second time: 12-lead ECG included saddle-back type ST-segment elevation in lead V2 Third time: 12-lead ECG included slight ST-segment elevation in leads V1 and V2 |
Over previous 3 years nortriptyline, mianserin, sertraline, brotizolam and amantadine; doses not stated; (N) | Not specified | No family history of sudden death First time: none Second time: no neurological deficits observed, admitted briefly but no abnormal findings on brain CT, brain MRI or EEG, ECG abnormal Third time: Pilsicainide provocation test and treadmill exercise test produced coved-type ST-segment changes in leads V1 and V2; head up tilt test negative; echo normal; no stenosis on angiogram; electrophysiologic study could not induce VF; ICD implantation considered and nortriptyline stopped |
| 2010 | 58-year-old male | None (No) | Found lying unconscious in room, responsive only to painful stimuli | HR 106 bpm; BP 126/84 mmHg; RR 18/min; Temp 37.4°C; O2 sats 96% | Creatinine kinase-MR and troponin I normal; ECG showed ST and T-wave abnormality consistent with Brugada | Amitriptyline; 4500 mg; (Y) | Patient intubated; gastric lavage and decontamination with activated charcoal; sodium bicarbonate infusion 400 mmol/L | Mental status improved; ECG changes gradually reverted; weaned off ventilator; genetic testing for Brugada negative |
| 2010 | 57-year-old female | Depression with one suicide attempt (N) | Unconscious, convulsions | HR 70 bpm; BP 87/63 mmHg; GCS 6/15; O2 sats 97%; more extensive physical examination normal | Glucose 143 mg/dL; normal U&E’s, normal bloods and cardiac markers negative; ECG showed Brugada-like pattern, i.e. RBBB and ST elevations in leads V1-V3; CT brain normal | Escitalopram, risperidone, lorazepam, dosulepin; dosulepin level 1190 mcg/L; (Y) | Sedated; mechanically ventilated; activated charcoal 1 g/1 kg repeated every 4 h for 16 h; sodium bicarbonate until pH reached 7.5 (250 mEq total dose) | 2 days later successfully weaned from the ventilator and extubated; ECG pattern normalised; ajmaline test was positive 4 days after the event (type 1 Brugada pattern); repeat ajmaline test 11 days after did not show ECG abnormalities |
| 2019 | 34-year-old female | Depression (no) | Convulsions; cardiac arrest with monomorphic VT | Not stated | Direct cardioversion led to spontaneous circulation; subsequent episodes pulseless VT; ECG showed RBBB pattern with coved ST-segment elevation and inverted T waves in V1 and V2 | Nortriptyline; serum level 1581 ng/ml; (Y) | Direct cardioversion on arrival; subsequent episodes amiodarone infusion; then IV sodium bicarbonate | ECG abnormalities resolved |
AV, atrioventricular; BP, blood pressure; COPD, chronic obstructive pulmonary disease; CPAP, continuous positive airway pressure; CPR, cardiopulmonary resuscitation; CT, computed tomography; ECG, electrocardiogram; EEG, electroencephalogram; GCS, Glasgow Coma Scale; HR, heart rate; ICD, implantable cardioverter-defibrillator; JVP, jugular venous pressure; MRI, magnetic resonance imaging; QRS, is the name of the waves; RBBB, right bundle branch block; RR, respiratory rate; SPECT, Single Photon Emmision Computered Tomography; TCA, tricyclic antidepressant; TSH, thyroid stimulating hormone; VF, ventricular fibrillation; VT, ventricular tachycardia.
Table 1.
Risk stratification and treatment of Brugada Syndrome. 21 .
| Was Brugada symptomatic? | Finding | Class of recommendation | Recommended management |
|---|---|---|---|
| Yes | Aborted sudden cardiac death or documented VT with/without syncope | I | ICD |
| Yes | Spontaneous type 1 ECG with syncope | IIa | ICD |
| Yes | Electrical storm | IIa | Medication in first instance, then ICD if not already present |
| No | VT/VF induced by programmed ventricular stimulation | IIb | ICD |
| No | Spontaneous type 1 ECG | IIb | Medication |
| No | Medicine-induced type 1 ECG: positive family history | III | ICD not indicated |
ECG, electrocardiogram; ICD, implantable cardioverter-defibrillator; VF, ventricular fibrillation; VT, ventricular tachycardia.
Demographically, the cases include both men and women aged between 33 years and 66 years. In some of the cases, there was no evidence of pre-existing BrS.28,30,31 However, with others, it was difficult to tell whether the antidepressant precipitated the ECG pattern or unmasked an existing BrS. 39 A baseline ECG may have clarified this.
It is noteworthy that in two of the cases, the patient presented as collapsed and unconscious.38,39 In two other cases, clinical signs were normal on first presentation, though subsequently deteriorated. 39 In a further four cases, heart rate, temperature, blood pressure and respiratory rate were found to be deranged while all other results were normal.28,30 It was only on performing an ECG that BrS was identified. Therefore, whenever a patient, who is prescribed a tricyclic antidepressant, presents with syncope, an ECG is recommended to screen for BrS whether or not any other cardiovascular signs are present.
Minoura et al. 42 investigated the possible underlying ionic and cellular mechanisms by examining the effects of amitriptyline on the experimental model of a coronary-perfused canine RV wedge preparation. Their results suggested that because of its potent blockade of the fast sodium current with little inhibition of the transient outward potassium channel current, amitriptyline can cause Brugada in patients with a genetic predisposition. In a subsequent study, Chen et al. 43 explored both the acute and long-term effects of some tricyclic antidepressants using neonatal rat ventricular myocytes. The acute effects of amitriptyline on the gating properties of the Nav1.5 cardiac sodium channel resulted in decreased peak current and delayed recovery from activation. In the longer term, there was a significant reduction in the expression of the Nav1.5 protein on the cell membrane, suggesting that amitriptyline caused the protein to be retained within the cell. Furthermore, the interaction between Nav1.5 and the Ankyrin-G or dystrophin antibodies was disrupted. Thus, with both amitriptyline and clomipramine, long-term treatment produces greater decreases in peak sodium current than acute treatment. With nortriptyline, both acute treatment and long-term treatment resulted in similar decreases in peak sodium current. There were no significant changes, either acute or long-term, with desipramine.
Mood stabilisers
Unmasking of BrS has been reported with lithium treatment.43 –45 Similarly, Brugada-type ECG changes have been reported with long-term lithium treatment. 46 In one reported case, lithium was being taken together with lamotrigine and ziprasidone. The patient’s electrolytes were normal, and his lithium level was 0.7 mEq/L. The patient’s ECG normalised when his lithium was withheld. It is thought that lithium exerts this effect via its ability to block sodium-ion channels.
In another case, a 52-year-old woman with depression presented with atypical precordial pain having taken lamotrigine for roughly 5 months. 47 Her ECG suggested type 1 Brugada pattern, although subsequent ECGs were less typical. Examination revealed normal coronary arteries. Her flecainide test was positive. Stopping the lamotrigine led to reversal of the Brugada-pattern ECG.
A 61-year-old man with schizophrenia who was treated with carbamazepine was hospitalised and diagnosed with pneumonia. On the following day, electrocardiography showed Brugada-pattern ST elevation in the right precordial leads and a blood examination revealed that the patient’s carbamazepine concentration was at the upper limit of the standard range. The patient’s ECG normalised after the withdrawal of carbamazepine. 48
Antipsychotics
A 62-year-old man with diabetes, chronic ischemic cerebrovascular disease and bipolar disorder, who was taking pregabalin and quetiapine, presented with dyspnoea and chest discomfort. 49 His temperature on admission was 36.6°C, his pH was 7.2 and his potassium levels were 3.4 mEq/L. His ECG on admission showed Brugada-like pattern. Initially, acute coronary syndrome was suspected. However, coronary angiography showed only mild disease, echocardiography did not reveal abnormalities and troponin levels were normal. Pneumonia was identified via lung opacities and consolidation found by chest radiography. He received positive airways pressure ventilation and antibiotics and recovered after 10 days. The mechanisms by which quetiapine and pregabalin may have exacerbated this presentation are not clear. Possibly, quetiapine metabolites may impair noradrenaline recycling via their affinities for the noradrenaline reuptake transporter, the serotonin receptors and the alpha (1B)-adrenergic receptor. A proposed mechanism for pregabalin is binding the alpha2-delta protein, which is an auxiliary subunit of the voltage-gated calcium channels.
Cases of Brugada have also been noted with clozapine.11,50 In the detailed, published report, the patient was a 44-year-old man with a long history of schizophrenia. He had risk factors of smoking, a sedentary lifestyle and poor diet. However, he did not have abnormal plasma lipids, hypertension, diabetes, high bodyweight or a family history of cardiac disease. His antipsychotic history included chlorpromazine, zuclopenthixol, olanzapine and risperidone. During a previous trial of clozapine, his ECGs were unremarkable and there were no changes indicative of arrhythmogenic RV cardiomyopathy. Retitration of clozapine was started and his ECG on day 7 was normal. On day 13, he presented with retrosternal chest pain episodes and his ECG was consistent with Brugada type 1 pattern. He was afebrile and his blood tests including serial troponins were normal. Nothing abnormal was found on his echocardiogram or on chest X-ray. Clozapine was stopped and olanzapine was substituted. ECG was performed daily and had normalised 4 days after clozapine was stopped. The flecainide challenge test was positive. The mechanism whereby clozapine was associated with Brugada is not clear. The authors acknowledge the possibility of co-incidence because of the increased ECG monitoring when clozapine is initiated (Table 2).
In conclusion, should we routinely screen for BrS before prescribing psychotropics?
Risk of hospitalisation due to cardiac arrhythmia is increased with the use of antispsychotics. 51 Case control studies suggest that the risk of SCD is increased with most antipsychotics.52 –54
In first episode psychosis, the risk of prolongation of QTc interval is associated with antipsychotic use in the first 2–4 weeks of therapy. 55 While there remains controversy surrounding the relationship between prolongation of QTc and chances of arrhythmia, clear guidance exists for a range of QTc intervals that are likely to be safe and some consensus that QTc intervals longer than 500 ms increase the arrhythmogenic potential and a QTc interval longer than 650 ms is associated with Torsade de Pointes. 56 Despite the uncertainties, QTc interval remains an important measure of arrhythmogenic potential, and in the United Kingdom, it is generally assumed that all antipsychotics are associated with the potential to increase risk of SCD. There is accepted guidance to monitor baseline ECG and yearly thereafter, before prescribing antipsychotics and some antidepressants, in particular to monitor for QTc prolongation. 56
Since its initial description by Pedro and Josep Brugada in 1992, there have been significant developments in the understanding of the pathophysiology of BrS and the associated risk of SCD.1,57 Despite the fact that BrS is responsible for around 20% of SCDs in patients with structurally normal hearts, and there is an established link between certain psychotropic medications and the unmasking of BrS or the precipitation of BrP, there is no equivalent framework in place for routine assessment of Brugada pattern. Given the known risks associated with BrS and psychotropic medication’s potential impact on cardiac ion channels, should we now include BrS ECG pattern screening as well?
There is certainly a gap in our understanding of the implications of asymptomatic Brugada ECG pattern and BrP on mortality; however, as noted above, BrS is the leading cause of SCD in those with apparently structurally normal hearts and there is a developing understanding of the risk factors which are associated with a higher incidence of arrythmia. Given the numerous psychotropic drugs that can unmask BrS or precipitate a Brugada pattern ECG as a result of their effect on ion channels, prescribers should be aware of this disorder and the associated risks. This is particularly the case given the increased prevalence of Brugada pattern ECG among people with schizophrenia.
We would propose that, alongside with the existing recommendations for monitoring QT interval, when prescribing antipsychotic medications and other higher risk psychotropic medications, baseline and annual ECGs should be screened for Brugada pattern as well as QT interval. Consideration should also, of course, be given to other abnormalities present on the ECG.
We would recommend a move to more structured guidance in relation to screening for Brugada pattern ECGs in commonly referenced texts and prescribing guidelines to raise awareness and provide a framework for clinicians to follow. We would further recommend that in addition to a baseline ECG being carried out to identify pre-existing abnormalities, further ECGs should be carried out once steady state of an initiated drug which may unmask BrS or precipitate BrP has been reached and when further dose increments are made.
This does of course raise the question of psychiatrists’ confidence in interpretation of ECGs and the relative lack of awareness of BrS among prescribers in mental health settings. 58 There has long been a call for more training in the interpretation of ECG among psychiatric trainees, non-trainee grades and consultants. 59 This would be of benefit in the general identification of ECG abnormalities which increase the risk of SCD and, with regard to this article, BrS specifically. 60
Abnormalities found on screening ECGs including, for example, ST elevation in leads V1–V3 with a right bundle branch block (RBBB) appearance should prompt the search for cardiology advice to ascertain whether Brugada pattern is likely. Where a medication is known to ‘unmask’ ECG Brugada pattern or Brugada pattern is suspected on an ECG, it is important that a multidisciplinary approach is taken, involving cardiology and mental health services, working together to coordinate more detailed assessment and consider further interventions in relation to both mental health disorder and the potential BrS.
Assessment of the risks and benefits of specific treatments will have to be made with the likely discontinuation of any medicine that precipitated BrP or unmasked BrS. Close collaboration between the patient, the patient’s carer(s) and health professionals involved in the patient’s care is recommended with a view to identifying the least risky pharmacological interventions and considering mitigation strategies or enhanced monitoring, an area which itself is likely to expand considerably in future with the ongoing, rapid development of wearable devices to monitor cardiac rhythm.
Footnotes
Author contribution: Azizah Attard: Conceptualization; Data curation; Methodology; Project administration; Writing – original draft.
Claire Stanniland: Data curation; Formal analysis; Validation; Writing – review & editing.
Stephen Attard: Conceptualization; Validation; Writing – review & editing.
Andrew Iles: Conceptualization; Formal analysis; Writing – review & editing.
Kim Rajappan: Supervision; Validation.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Azizah Attard  https://orcid.org/0000-0001-5590-4515
https://orcid.org/0000-0001-5590-4515
Contributor Information
Azizah Attard, Department of Pharmacy, West London NHS Trust, Southall UB1 3EUN2 PCN, Virtually Healthcare, London.
Claire Stanniland, Department of Pharmacy, West London NHS Trust, Southall, UK.
Stephen Attard, Central and North West London NHS Foundation Trust, London, UK.
Andrew Iles, Surrey and Borders Partnership NHS Foundation Trust, Leatherhead, UK.
Kim Rajappan, Oxford University Hospitals NHS Foundation Trust, Oxford, UK.
References
- 1. Argenziano M, Antzelevitch C. Recent advances in the treatment of Brugada Syndrome. Expert Rev Cardiovasc Ther 2018; 16: 387–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Khabra N, Gupta R, Aronow WS, et al. Sudden cardiac death in Brugada Syndrome. Card Rev 2020; 28: 203–207. [DOI] [PubMed] [Google Scholar]
- 3. Richter S, Sarkozy A, Veltmann C, et al. Variability of the diagnostic ECG pattern in an ICD patient population with Brugada Syndrome. J Cardiovasc Electrophysiol 2009; 20: 69–75. [DOI] [PubMed] [Google Scholar]
- 4. Sutterland A, Blom MT, Ladee K, et al. Increased prevalence of ECG suspicious for Brugada Syndrome in recent onset schizophrenia spectrum disorders. Schizophr Res 2019; 210: 59–65. [DOI] [PubMed] [Google Scholar]
- 5. Wilde AM, Antzelevitch C, Borggrefe M, et al. Proposed diagnostic criteria for the Brugada syndrome. Circulation 2002; 106: 2514–2519. [DOI] [PubMed] [Google Scholar]
- 6. Antzelevitch C. J wave syndromes: molecular and cellular mechanisms. J Electrocardiol 2013; 46: 510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li KHC, Lee S, Yin C, et al. Brugada syndrome: a comprehensive review of pathophysiological mechanisms and risk stratification strategies. Int J Cardiol Heart Vasc 2020; 26: 100468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Di Diego JM, Cordeiro JM, Goodrow RJ, et al. Ionic and cellular basis for the predominance of the Brugada syndrome phenotype in males. Circulation 2002; 106: 2004–2011. [DOI] [PubMed] [Google Scholar]
- 9. Benito B, Sarkozy A, Mont L, et al. Gender differences in clinical manifestations of Brugada syndrome. J Am Coll Cardiol 2008; 52: 1567–1573. [DOI] [PubMed] [Google Scholar]
- 10. Snir AD, Raju H. Current controversies and challenges in Brugada syndrome. Eur Cardiol 2019; 14: 169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blom MT, Cohen D, Seldenrijk A, et al. Brugada syndrome ECG is highly prevalent in schizophrenia. Circ Arrhythm Electrophysiol 2014; 7: 384–391. [DOI] [PubMed] [Google Scholar]
- 12. Quan XQ, Li S, Liu R, et al. A meta-analytic review of prevalence for Brugada ECG patterns and the risk for death. Medicine (Baltimore) 2016; 95: e5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iglesias DG, Rubin J, Perez D, et al. Insights for stratification of risk in Brugada syndrome. Eur Cardiol 2019; 14: 45–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vutthikraivit W, Rattanawong P, Putthapiban P, et al. Worldwide prevalence of Brugada syndrome: a systematic review and meta-analysis. Acta Cardiol Sin 2018; 34: 267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Juang JMJ, Chen CY, Chen YH, et al. Prevalence and prognosis of Brugada electrocardiogram patterns in an elderly Han Chinese population: a nation-wide community-based study (HALST cohort). Europace 2015; 17(Suppl. 2): ii54–ii62. [DOI] [PubMed] [Google Scholar]
- 16. Juang JM, Phan WL, Chen PC, et al. Brugada-type electrocardiogram in the Taiwanese population-is it a risk factor for sudden death? J Formos Med Assoc 2011; 110: 230–238. [DOI] [PubMed] [Google Scholar]
- 17. Brugada J, Brugada R, Brugada P. Right bundle-branch block and ST-segment elevation in leads V1 through V3: a marker for sudden death in patients without demonstrable structural heart disease. Circulation 1998; 97: 457–460. [DOI] [PubMed] [Google Scholar]
- 18. Pecini R, Cedergreen P, Theilade S, et al. The prevalence and relevance of the Brugada-type electrocardiogram in the Danish general population: data from the Copenhagen City Heart Study. Europace 2010; 12: 982–986. [DOI] [PubMed] [Google Scholar]
- 19. Junttila MJ, Raatikainen MJP, Karjalainen J, et al. Prevalence and prognosis of subjects with Brugada-type ECG pattern in a young and middle-aged Finnish population. Eur Heart J 2004; 25: 874–878. [DOI] [PubMed] [Google Scholar]
- 20. Probst V, Veltmann C, Eckardt L, et al. Long-term prognosis of patients diagnosed with Brugada syndrome: results from the FINGER Brugada Syndrome Registry. Circulation 2010; 121: 635–643. [DOI] [PubMed] [Google Scholar]
- 21. Steinfurt J, Biermann J, Bode C, et al. The diagnosis, risk stratification, and treatment of Brugada syndrome. Dtsch Arztebl Int 2015; 112: 394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Duffett SA, Roberts JD. Brugada syndrome: evolving insights and emerging treatment strategies. J Innov Card Rhythm Manag 2017; 8: 2613–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hedley PL, Jørgensen P, Schlamowitz S, et al. The genetic basis of Brugada syndrome: a mutation update. Hum Mutat 2009; 30: 1256–1266. [DOI] [PubMed] [Google Scholar]
- 24. Kapplinger JD, Tester DJ, Alders M, et al. An international compendium of mutations in the SCN5A-encoded cardiac sodium channel in patients referred for Brugada syndrome genetic testing. Heart Rhythm 2010; 7: 33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Adler A, Topaz G, Heller K, et al. Fever-induced Brugada pattern: how common is it and what does it mean? Heart Rhythm 2013; 10: 1375–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khmao P, Long IV, Ku N, et al. Brugada phenocopy or congenital Brugada syndrome in a patient with spontaneous pneumopericardium and pericarditis. J Electrocardiol 2019; 55: 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gottschalk BH, Anselm DD, Brugada J, et al. Expert cardiologists cannot distinguish between Brugada phenocopy and Brugada syndrome electrocardiogram patterns. Europace 2016; 18: 1095–1100. [DOI] [PubMed] [Google Scholar]
- 28. Akhtar M, Goldschlager NF. Brugada electrocardiographic pattern due to tricyclic antidepressant overdose. J Electrocardiol 2006; 39: 336–339. [DOI] [PubMed] [Google Scholar]
- 29. Bebarta VS, Waksman JC. Amitriptyline-induced Brugada pattern fails to respond to sodium bicarbonate. Clin Toxicol (Phila) 2007; 45: 186–188. [DOI] [PubMed] [Google Scholar]
- 30. Palaniswamy C, Selvaraj DR, Chugh T, et al. Brugada electrocardiographic pattern induced by amitriptyline overdose. Am J Ther 2010; 17: 529–532. [DOI] [PubMed] [Google Scholar]
- 31. Otero D, Lopez P, Calderon Eder C, et al. Brugada pattern: an unusual presentation of tricyclic antidepressant overdose. J Am Coll Cardiol 2019; 73: 2666. [Google Scholar]
- 32. Veerakul G, Nademanee K. Treatment of electrical storms in Brugada syndrome. J Arrhythm 2013; 29: 117–124. [Google Scholar]
- 33. Chen JJ, Sangha RS. Treatment of anxiety and depression in a patient with Brugada syndrome. Case Rep Psychiatry 2014; 2014: 478397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Honarbakhsh S, Providencia R, Lambiase PD. Risk stratification in Brugada syndrome: current status and emerging approaches. Arrhythm Electrophysiol Rev 2018; 7: 79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McDonagh, Metra M, Adamo M, et al. 2921 ESC Guidelines for the diagnosis and treament of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failue of the European Society of Cardiology (ESC) Eith special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heat J 2021; 42: 3599–3726. [Google Scholar]
- 36. Fish JM, Antzelevitch C. Role of sodium and calcium channel block in unmasking the Brugada syndrome. Heart Rhythm 2004; 1: 210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mackin P. Cardiac side effects of psychiatric drugs. Hum Psychopharmacol 2008; 23(Suppl. 1): S3–S14. [DOI] [PubMed] [Google Scholar]
- 38. Chow BJW, Gollob M, Birnie D. Brugada syndrome precipitated by a tricyclic antidepressant. Heart 2005; 91: 651–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ahmed SH, Omar B. Brugada pattern unmasked by tricyclic antidepressant. Clin Cardiol 2009; 32: E3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tashiro N, Sato N, Talib AK, et al. Brugada syndrome case: difficult differentiation between a concealed form and tricyclic antidepressant-induced Brugada sign. Intern Med 2009; 48: 1535–1539. [DOI] [PubMed] [Google Scholar]
- 41. Konigstein M, Rosso R, Topaz G, et al. Drug-induced Brugada syndrome: clinical characteristics and risk factors. Heart Rhythm 2016; 13: 1083–1087. [DOI] [PubMed] [Google Scholar]
- 42. Minoura Y, Di Diego JM, Barajas-Martínez H, et al. Ionic and cellular mechanisms underlying the development of acquired Brugada syndrome in patients treated with antidepressants. J Cardiovasc Electrophysiol 2012; 23: 423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen X, Zhu C, Zhou H, et al. Key role of the membrane trafficking of Nav1.5 channel protein in antidepressant-induced Brugada syndrome. Front Physiol 2018; 9: 1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Darbar D, Yang T, Churchwell K, et al. Unmasking of Brugada syndrome by lithium. Circulation 2005; 112: 1527–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chandra PA, Chandra AB. Brugada syndrome unmasked by lithium. South Med J 2009; 102: 1263–1265. [DOI] [PubMed] [Google Scholar]
- 46. Wright D, Salehian O. Brugada-type electrocardiographic changes induced by long-term lithium use. Circulation 2010; 122: e418–e419. [DOI] [PubMed] [Google Scholar]
- 47. Rodrigues R, Amador P, Rassi L, et al. Brugada pattern in a patient medicated with lamotrigine. Rev Port Cardiol 2013; 32: 807–810. [DOI] [PubMed] [Google Scholar]
- 48. Ota H, Kawamura Y, Sato N, et al. A carbamazepine-induced Brugada-type electrocardiographic pattern in a patient with schizophrenia. Intern Med 2017; 56: 3047–3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Brunetti ND, Ieva R, Correale M, et al. Inferior ST-elevation acute myocardial infarction or an inferior-lead Brugada-like electrocardiogram pattern associated with the use of pregabalin and quetiapine? Am J Ther 2016; 23: e1057–e1059. [DOI] [PubMed] [Google Scholar]
- 50. Sawyer M, Goodison G, Smith L, et al. Brugada pattern associated with clozapine initiation in a man with schizophrenia. Intern Med J 2017; 47: 831–833. [DOI] [PubMed] [Google Scholar]
- 51. Liperoti R, Gambassi G, Lapane KL, et al. Conventional and atypical antipsychotics and the risk of hospitalization for ventricular arrhythmias or cardiac arrest. Arch Intern Med 2005; 165: 696–701. [DOI] [PubMed] [Google Scholar]
- 52. Straus SM, Bleumink GS, Dieleman JP, et al. Antipsychotics and the risk of sudden cardiac death. Arch Intern Med 2004; 164: 1293–1297. [DOI] [PubMed] [Google Scholar]
- 53. Ray WA, Chung CP, Murray KT, et al. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med 2009; 360: 225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Murray-Thomas T, Jones ME, Patel D, et al. Risk of mortality (including sudden cardiac death) and major cardiovascular events in atypical and typical antipsychotic users: a study with the general practice research database. Cardiovasc Psychiatry Neurol 2013; 2013: 247486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhai D, Lang Y, Dong G, et al. QTc interval lengthening in first-episode schizophrenia (FES) patients in the earliest stages of antipsychotic treatment. Schizophr Res 2017; 179: 70–74. [DOI] [PubMed] [Google Scholar]
- 56. Taylor DM, Barnes TRE, Young AH. The Maudsley prescribing guidelines in psychiatry. Oxford: Wiley-Blackwell, 2021. [Google Scholar]
- 57. Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol 1992; 20: 1391–1396. [DOI] [PubMed] [Google Scholar]
- 58. Yadav R, Vidyarthi A. Electrocardiogram interpretation skills in psychiatry trainees. Psychiatrist 2013; 37: 94–97. [Google Scholar]
- 59. Solomons L, Treloar A, Noronha R. Competence of psychiatric clinicians in interpreting electrocardiograms and QT intervals: can they do this? Does it matter? Psychiatric Bull 2008; 32: 291–294. [Google Scholar]
- 60. Holkeri A, Eranti A, Haukilahti MAE, et al. Predicting sudden cardiac death in a general population using an electrocardiographic risk score. Heart 2020; 106: 427–433. [DOI] [PubMed] [Google Scholar]