Abstract
Background:
Psilocybin is a psychedelic drug that has shown lasting positive effects on clinical symptoms and self-reported well-being following a single dose. There has been little research into the long-term effects of psilocybin on brain connectivity in humans.
Aim:
Evaluate changes in resting-state functional connectivity (RSFC) at 1 week and 3 months after one psilocybin dose in 10 healthy psychedelic-naïve volunteers and explore associations between change in RSFC and related measures.
Methods:
Participants received 0.2–0.3 mg/kg psilocybin in a controlled setting. Participants completed resting-state functional magnetic resonance imaging (fMRI) scans at baseline, 1-week and 3-month post-administration and [11C]Cimbi-36 PET scans at baseline and 1 week. We examined changes in within-network, between-network and region-to-region RSFC. We explored associations between changes in RSFC and psilocybin-induced phenomenology as well as changes in psychological measures and neocortex serotonin 2A receptor binding.
Results:
Psilocybin was well tolerated and produced positive changes in well-being. At 1 week only, executive control network (ECN) RSFC was significantly decreased (Cohen’s d = −1.73, pFWE = 0.010). We observed no other significant changes in RSFC at 1 week or 3 months, nor changes in region-to-region RSFC. Exploratory analyses indicated that decreased ECN RSFC at 1 week predicted increased mindfulness at 3 months (r = −0.65).
Conclusions:
These findings in a small cohort indicate that psilocybin affects ECN function within the psychedelic ‘afterglow’ period. Our findings implicate ECN modulation as mediating psilocybin-induced, long-lasting increases in mindfulness. Although our findings implicate a neural pathway mediating lasting psilocybin effects, it is notable that changes in neuroimaging measures at 3 months, when personality changes are observed, remain to be identified.
Keywords: Functional magnetic resonance imaging, resting-state connectivity, psilocybin, psychedelic, executive control network
Introduction
Psilocybin is a prodrug of the psychedelic psilocin (4-hydroxy-N,N-dimethyltryptamine) (Nichols, 2016). Effects include profound alterations in consciousness that last approximately 6 h and are characterised by perceptual alterations and synaesthesia, experiences of non-duality and transcendence and profound changes in affect (Preller and Vollenweider, 2018). Therapeutic effects of psilocybin have been reported following between one and three moderate-to-high doses (0.025–0.42 mg/kg) in brain-related disorders including major depressive disorder (MDD) (Davis et al., 2020), treatment-resistant depression (Carhart-Harris et al., 2018), obsessive-compulsive disorder (Moreno et al., 2006), terminal cancer-associated anxiety (Griffiths et al., 2016), demoralisation (Anderson et al., 2020), as well as smoking (Johnson et al., 2017) and alcohol addiction (Garcia-Romeu et al., 2019). Psilocybin is currently in phase 2b for the treatment of treatment-resistant depression (COMPASS Pathways Ltd., London, UK) and in phase 2a for major depressive disorder (Usona Institute, Madison, WI, USA).
Persistent changes in personality and mood have also been observed in healthy volunteers following a single medium-to-high dose of psilocybin. These include, for example, increases in personality traits openness and extraversion, decreases in neuroticism and increases in mindful awareness (Erritzoe et al., 2018; MacLean et al., 2011; Madsen et al., 2020). These therapeutic and personality effects appear to persist for at least months, and in some cases have been reported to last more than a year (Gasser et al., 2014; Johnson et al., 2017; MacLean et al., 2011).
The medicalisation of psychedelic drugs is expanding rapidly despite a limited understanding of the neurobiology underpinning therapeutic effects. Psychological theories of psychedelic therapy, such as reduced negative affect (Barrett et al., 2020), increased mindfulness (Madsen et al., 2020; Murphy-Beiner and Soar, 2020; Smigielski et al., 2019), increased cognitive flexibility (Murphy-Beiner and Soar, 2020) and reduced experiential avoidance (Zeifman et al., 2020) have been proposed, as well as increased acceptance and processing of traumatic autobiographical memories (Sloshower et al., 2020), but these have no current grounding in neurobiology. Thus, in order to maximise psilocybin’s safety and efficacy as a potential therapeutic, it is important to investigate mechanisms by which psilocybin exerts its effects.
Functional magnetic resonance imaging (fMRI) resting-state functional connectivity (RSFC) measures correlations between blood-oxygen-level-dependent (BOLD) signals in participants instructed to simply let their mind wander (Lee et al., 2013). Despite not being focused on any task, the brain remains organised into networks (Raichle, 2015), the character of which correlates with personality traits (Cai et al., 2020; Hsu et al., 2018) and aligns with known functional and structural topology (Straathof et al., 2019). During the psychedelic experience, psilocybin produces a reduction in the synchronised BOLD activity of the major hubs of the default mode network (DMN) (Carhart-Harris et al., 2012; Mason et al., 2020), increases between-network RSFC (Roseman et al., 2014) and increases global RSFC across the sensory cortex while decreasing the global connectivity in associative regions (Preller et al., 2020). Similarly, lysergic acid diethylamide (LSD) increases RSFC between high-level association cortices, which correlates with subjective reports of ego-dissolution (Tagliazucchi et al., 2016). Although understanding the neurological basis of the acute psychedelic experience is widely informative, the long-term psychological effects of psychedelics may be distinct (Carhart-Harris et al., 2016).
Five studies to date have reported effects on human brain function after the psychoactive effects of a classical psychedelic have subsided: two studies with ayahuasca and three with psilocybin (Barrett et al., 2020; Carhart-Harris et al., 2017; Pasquini et al., 2020; Sampedro et al., 2017; Smigielski et al., 2019). Post-drug brain imaging was performed within 24 h after the psychedelic session in all but one study (Barrett et al., 2020), during which time ‘afterglow’ effects and potential residual drug availability confounds relating effects to lasting changes (Madsen et al., 2019; Majić et al., 2015). By ‘afterglow’, we allude to the experience of ‘elevated and energetic mood with a relative freedom from concerns of the past and from guilt and anxiety’ up to 2 weeks after the experience, as described as early as during the 1960s and the ‘first-wave’ of psychedelic research (Grob et al., 1996; Pahnke, 1969). More recently, transient elevations in mood have been reported (Majić et al., 2015; Murphy-Beiner and Soar, 2020). Barrett et al. (2020) reported an increase in the number of significant RSFC across the brain in 12 healthy individuals from baseline to 1-week and 1-month post-psilocybin, hypothesising that psilocybin may increase emotional and brain plasticity. None of these previous studies evaluated correlations between change in RSFC and change in personality or other psychological traits. Furthermore, none of these studies have explored neuromolecular mechanisms mediating these effects. The psychoactive effects of psilocybin stem from agonism at the serotonin 2A receptor (5-HT2AR) (Vollenweider et al., 1998). Positron emission tomography (PET) with the radiotracer [11C]Cimbi-36 enables the quantification of brain 5-HT2AR levels in humans in vivo, which has been previously associated with aspects of the psychedelic experience (Ettrup et al., 2014, 2016; Finnema et al., 2014; Madsen et al., 2020; Stenbæk et al., 2020). Combining [11C]Cimbi-36 PET with RSFC would provide insight into the neuromolecular mechanisms associated with psychedelic effects on brain connectivity.
In the current study, we evaluated the effect of a single psilocybin dose on RSFC in 10 healthy psychedelic-naïve individuals at 1 week and 3 months after administration, evaluating changes in within- and between-network RSFC. Further, we sought to replicate a previous finding of changes in region-to-region RSFC (Barrett et al., 2020). Lastly, in an exploratory analysis, we assessed correlations between network RSFC change and variables associated with increased well-being. These included personality measures, well-being and mindfulness, which we recently showed were altered 3 months after psilocybin, as well as correlated with change in the neocortex 5-HT2A binding (Madsen et al., 2020). Additionally, the baseline neocortex 5-HT2A binding was related to the temporal character of the psychedelic experience (Stenbæk et al., 2020). Finally, we examined whether the self-reported experience was correlated with long-term changes in brain connectivity.
Materials and methods
Participants
Detailed information about participants and protocol are described in a previous study (Madsen et al., 2020) and one more study, which included these and other participants (Stenbæk et al., 2020). The study was approved by the Danish Medicines Agency (EudraCT ID: 2016-004000-61, amendments: 2017014166, 2017082837, 2018023295) and by the ethics committee for the capital region of Copenhagen (journal ID: H-16028698, with amendments). The study was preregistered at ClinicalTrials.gov (identifier: NCT03289949). Six male and four female participants took part in this study (mean ± SD age = 28.3 ± 3.4 years).
Participants were recruited from a list of individuals who expressed interest in participating in a psilocybin brain scanning study. After obtaining the informed consent, participants underwent screening for somatic illness, including a medical examination, an electrocardiogram (ECG), blood screening for somatic disease, and screening for psychiatric disorders using Mini International Neuropsychiatric Interview, Danish translation version 6.0.0 (Sheehan et al., 1998). Exclusion criteria were: (1) present or previous primary psychiatric disease (DSM axis 1 or WHO ICD-10 diagnostic classifications) or in first-degree relatives; (2) present or previous neurological condition/disease, significant somatic condition/disease; (3) intake of drugs suspected to influence test results; (4) non-fluent Danish language skills; (5) vision or hearing impairment; (6) previous or present learning disability; (7) pregnancy; (8) breastfeeding; (9) magnetic resonance imaging (MRI) contraindications; (10) alcohol or drug abuse; (11) allergy to test drugs; (12) significant exposure to radiation within the past year (e.g. medical imaging investigations); (13) intake of QT-prolonging medication or ECG results indicative of heart disease, (14) blood donation less than 3 months before project participation; (15) bodyweight less than 50 kg; and (16) low plasma ferritin levels (<12 µg/L).
Experimental procedures
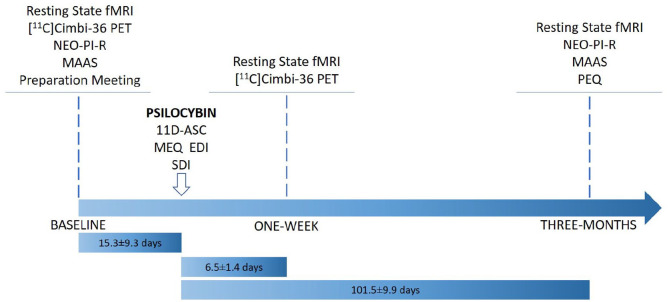
Figure 1 provides an overview of the study design. Prior to inclusion, participants were informed about the study, including safety precautions and potential effects and side effects of psilocybin. Before the psilocybin session, all participants met at least one of the two staff members present on the psilocybin intervention day. A urine test was used to screen for common drugs of abuse (Rapid Response, BTNX Inc., Markham, ON, Canada) on baseline imaging days. At baseline, participants filled out questionnaires including the NEO Personality Inventory-Revised (NEO PI-R) (Costa and McCrae, 2008; Skovdahl-Hansen et al., 2004) and the mindfulness attention and awareness scale (MAAS) (Brown and Ryan, 2003; Jensen et al., 2016) and completed an magnetic resonance imaging (MRI) scan session.
Figure 1.
Flowchart describing study design. Note that not all data collected at a single time point was collected on a single day. Time lengths (mean ± SD) describe time between MRI scan sessions and psilocybin session.
EDI: ego-dissolution inventory; MAAS: mindful attention awareness scale; MEQ: mystical experience questionnaire; MRI: magnetic resonance imaging; NEO-PI-R: revised NEO personality inventory; PEQ: persisting effects questionnaire; SDI: subjective drug intensity.
On a separate day, open-label psilocybin sessions were conducted including two supporting psychologists familiar with effects of psilocybin, safety precautions and interpersonal support methods (Johnson et al., 2008). Psilocybin was administered in the morning; a number of 3 mg capsules were taken with a glass of water to approximate dose (dose: 0.2 mg/kg (n = 4) and 0.3 mg/kg (n = 6)), considered ‘medium’ and ‘high’ doses, respectively (Hasler et al., 2004). Participants listened to a standardised music playlist, adapted from one kindly provided by Prof. Roland Griffiths, Johns Hopkins Medicine. Music was played using a stereo system. Subjective drug intensity (SDI) was measured every 20 min using a 0–10 Likert scale (question: ‘How intense is your experience right now?’ 0=‘Not at all’, 10=‘Very much’). Measurements were obtained from the time of drug administration to the end of the session. Participants responded orally and the supporting psychologists noted their responses. At the end of psilocybin session days, participants completed questionnaires aimed to quantify aspects of the psychedelic experience, including the 11-dimensional altered states of consciousness (11D-ASC) questionnaire (Studerus et al., 2010), the revised mystical experience questionnaire (MEQ30) (Barrett et al., 2015), and the ego-dissolution inventory (EDI) (Nour et al., 2016) (median [range]: 6.4 [5.9–7.4] h after psilocybin intake). One week and 3 months after psilocybin administration, participants returned for MRI scan sessions identical to the baseline scan session. At 3 months, participants filled out questionnaires including the NEO PI-R, MAAS and persisting effects questionnaire (PEQ) (Griffiths et al., 2006, 2011), which measures psychological changes (both positive and negative) that are subjectively perceived to be due to the psilocybin experience. Number of days between psilocybin sessions and follow-up questionnaires: mean (SD) [range] = 97.8 (11.9) [79–120 days]).
Positron emission tomography
The PET data used in this analysis are the same as those reported previously (Madsen et al., 2020; Stenbæk et al., 2020). For a more in-depth description of the PET methods, please refer Madsen et al. (2020). [11C]Cimbi-36 is an agonist radioligand selective for serotonin (5-HT) 2A (5-HT2AR) and 2C receptors (Ettrup et al., 2014). Participants completed 120-min scans on a high-resolution research tomograph (HRRT) PET-scanner (CTI/Siemens, Knoxville, TN, USA) at baseline and 1 week following psilocybin administration. Regional time-activity curves were extracted using Pvelab (Svarer et al., 2005) from a neocortex and cerebellum region for estimation of non-displaceable binding potential (BPND) using the simplified reference tissue model (Ettrup et al., 2016; Innis et al., 2007). Neocortex [11C]Cimbi-36 binding predominantly reflects 5-HT2AR binding (Finnema et al., 2014). The neocortex and cerebellum regions of interest (ROIs) were defined a priori in Pvelab (Svarer et al., 2005). The neocortex ROI comprises occipital, orbitofrontal and parietal cortex as well as pre/post central, middle/inferior frontal, middle/inferior temporal, superior frontal and superior temporal gyri. A composite neocortex ROI was used because the signal is very highly correlated between these regions (Spies et al., 2020). Subcortical [11C]Cimbi-36 binding was not considered because the signal is low and noisy. The cerebellum ROI encompasses only the grey matter of the cerebellum.
Magnetic resonance imaging scan parameters
Participants completed three identical MRI scan sessions: baseline, 1-week, and 3-month post-psilocybin. The MRI data were acquired on a 3T Prisma scanner (Siemens, Erlangen, Germany) using a 64-channel head/neck coil. A high-resolution 3D T1-weighted structural image was acquired: inversion time = 900 ms, echo time = 2.58 ms, repetition time = 1900 ms, flip angle = 9°, in-plane matrix = 256 × 256, in-plane resolution = 0.9 × 0.9 mm, 224 slices, and a slice thickness of 0.9 mm, no gap. Ten minutes of resting-state BOLD fMRI data was acquired: repetition time = 2000 ms, echo time = 30 ms, flip angle = 90°, 32 axial slices with a slice thickness of 3 mm, 0.75 mm gap, in-plane resolution: 3.6 × 3.6 mm, iPAT acceleration factor = 2. A gradient-echo field map of the same spatial dimensions was acquired to resolve spatial distortions due to inhomogeneities in the magnetic field (repetition time = 400 ms, echo times = 4.92 and 7.38 ms). Prior to the resting-state scan sessions, participants were instructed to close their eyes, let their mind wander freely and to not to fall asleep. Resting-state scan sessions were acquired after structural image acquisition (~15–20 min after scanning onset) and prior to task-related fMRI measures not described here.
Resting-state fMRI pre-processing
Resting-state fMRI data were pre-processed using SPM12 (Penny et al., 2007). This process included slice-timing correction, realignment and unwarping, co-registration of the high-resolution T1 structural image to the fMRI data, segmentation of the high-resolution T1 structural image, applying warping parameters estimated for the high-resolution T1 into MNI space to fMRI data and smoothing with an 8-mm full width at half maximum (FWHM) Gaussian filter. Additional denoising of time-series data was performed using CONN (version 17.c) (Whitfield-Gabrieli and Nieto-Castanon, 2012). Time series were filtered using a bandpass filter from 0.008 to 0.09 Hz. Additionally, we performed an estimation of physiological noise sources using anatomical component correction (aCompCor): regressing out the time series (and first derivative) of the first five principal components from a decomposition of the time series from white-matter and cerebrospinal fluid voxels, separately. Additionally, we regressed the time series for the six motion parameters (and first derivatives) (Behzadi et al., 2007; Whitfield-Gabrieli and Nieto-Castanon, 2012). Individual outlier volumes were identified and censored using the Artifact Detection Tools (ART) (global variance threshold = 4 and composite motion threshold = 2) (http://web.mit.edu/swg/software.htm). Mean denoised time series were extracted from ROIs for further analysis. We calculated the between-region correlation across the entire time series. The Pearson’s rho correlation estimates were transformed using Fisher’s r-to-z transform (i.e. r-to-z = 0.5 × (ln((1+r)/(1−r))), where r is the Pearson’s rho and ln represent taking the natural logarithm). These r-to-z values were included in the statistical analyses related to the connectivity strength.
Brain atlases
Regions of interest and networks were defined using an a priori defined atlas (Raichle, 2011). This atlas defines 36 regions belonging to seven canonical resting-state networks: network (DMN), dorsal attention network (DAN), executive control network (ECN), salience network (SN), sensorimotor network (SMN), visual network (VN) and auditory network (AN). Montreal Neurological Institute (MNI) coordinates for each network can be found in Raichle (2011) and in Supplemental Table S1. Within-network connectivity was defined as the mean connectivity between each unique pair of ROIs comprising a given network. Henceforth ECN integration and disintegration refer to increased and decreased mean within-network connectivity, respectively. Between-network connectivity was defined as the mean connectivity between all ROIs from two networks, where each pair of ROIs contained a region from each network. To draw comparisons with a similar previous study, we also evaluated a 268-region atlas (https://www.nitrc.org/frs/?group_id=51) described previously (Barrett et al., 2020; Shen et al., 2013).
Statistical analysis
All statistical analyses were calculated in R (v4.0.2) (R Studio Team, 2020). Plots were constructed using the ggplot2 package (Wickham, 2016).
Paired t-tests were performed to investigate if there were any significant differences in ART censored volumes between time points (1 week vs. 3 months). Effects of time (1 week vs. baseline or 3 months vs. baseline) were compared separately using paired t-tests to determine the effect of time on within- and between-network connectivity and related estimates. The p-values across the 28 within- and between-network comparisons at each time point were adjusted using the Bonferroni–Holm method, which controls the family-wise type-I error rate (Holm, 1979). Unadjusted p-values are denoted punc, whereas adjusted p-values are denoted pFWE. Where an effect of psilocybin on connectivity exceeded our statistical significance threshold (pFWE < 0.05), exploratory post hoc analyses were performed. We report the Cohen’s d value for each post hoc effect evaluated. Due to limited statistical power stemming from a small sample, we do not draw inference on statistical significance for post hoc analyses, but instead report standardised effect sizes and 95% confidence intervals.
Correlations
The post hoc Pearson’s product–moment correlations were performed between change in ECN connectivity and change in MAAS, change in neocortex 5-HT2AR (i.e. [11C]Cimbi-36 BPND) (Madsen et al., 2020) as well as measures of the acute psychedelic experience (SDI, EDI, MEQ and 11-D-ASC) and change in personality (NEO-PIR). Change in ECN RSFC was also compared with the PEQ using a linear latent variable model capturing shared covariance in individual behavioural change measures using the lava package (v. 1.6.8 in R (Holst and Budtz-Jørgensen, 2013)).
Barrett replication analysis
We attempted to replicate a previously described analysis framework applied to the Shen268 atlas (Barrett et al., 2020). As described, we applied a one-sample t-test to all ROI-to-ROI connectivity estimates and retained only those edges with a statistically significant non-zero mean connectivity after the Bonferroni correction for 35,778 edges tested at each time point (i.e. punc < 1.4 × 10−6). Paired t-tests evaluating change from baseline at 1 week or 3 months were performed for each edge surviving correction. Suprathreshold edges were identified as either increases or decreases in connectivity following psilocybin.
In our view, the type-I error for the test of interest (i.e. effect of psilocybin) is inflated by the initial ‘edge filtering’ step considering each time point separately and further inflated by not adjusting for the family of tests of interest (695 reported in Barrett et al., 2020). Accordingly, we report paired t-tests evaluating changes from baseline to 1 week or 3 months, adjusting p-values using the Bonferroni–Holm method for the set of edges tested.
The R Notebook containing code used in the production of this manuscript is available at https://github.com/Pneumaethylamine/LastingPsilocybinRSFC
Results
Population
Acute psychedelic effects were well tolerated in all participants, and no serious adverse events occurred. Based on self-report SDI scores throughout the sessions, the psychedelic experiences were characterised by three distinct phases, the onset, peak plateau and descent (Stenbæk et al., 2020). As previously reported, participants in this study self-reported changes in personality, including increased trait openness and mindfulness (Madsen et al., 2020). Self-reported increases in positive attributes from the PEQ (including spirituality) were 25.9% ± 21.5% (mean ± SD), whereas increased negative attributes reported were 1.4% ± 1.7%. Time between baseline and psilocybin intervention was 15.3 ± 9.3 days, intervention and 1 week rescan was 6.5 ± 1.4 days and intervention and 3 month rescan was 101.5 ± 9.9 days (mean ± SD).
Lasting psilocybin effects on network connectivity
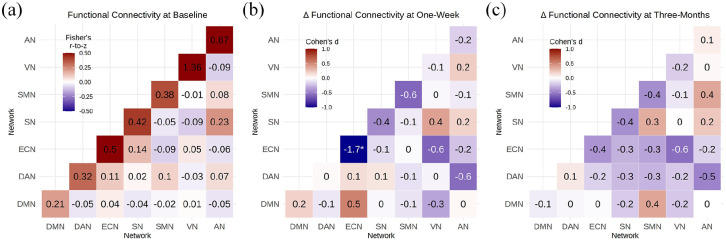
Within- and between-network RSFC structure was as expected, for example, high within-network connectivity and relatively lower between-network connectivity (Figure 2(a)). Mean composite motion and censored volumes were low (3.5 ± 4.0 volumes; mean ± SD) and not statistically significantly different between scan times (punc > 0.05).
Figure 2.
Psilocybin effects on within- and between-network resting-state connectivity: (a) mean within- and between-network functional connectivity, cell values and colour scale represent mean r-to-z values across participants, (b) change in connectivity from baseline to 1-week rescan, (c) change in connectivity from baseline to 3 month rescan. Cell values and colour scale in (b) and (c) represent effect size (Cohen’s d).
* denotes change that is statistically significant after adjustment across 28 tests (i.e. pFWE < 0.05).
ECN within-network connectivity was statistically significantly decreased at 1 week (punc = 0.00039, pFWE = 0.010, Cohen’s d = −1.73; Figures 2(b) and 3). Nine of 10 participants showed numerically reduced ECN RSFC at 1 week. Examination of individual ECN edges showed that nine of 10 edges had decreased connectivity across the 10 participants. At 3 months, ECN RSFC remained numerically decreased as compared to baseline, but this effect was not statistically significant (punc = 0.23, pFWE = 1, Cohen’s d = −0.4). No other within- or between-network connectivity estimates were statistically significantly altered at 1 week or 3 months (Supplemental Table S2). Additional network connectivity effects with a |Cohen’s d| > 0.5 include at 1 week, SMN–SMN, ECN–VN and DAN–AN connectivity decreased (Cohen’s d = −0.6), whereas DMN–ECN connectivity increased (Cohen’s d = 0.5). At 3 months, ECN–VN and DAN–AN connectivity remained decreased (Cohen’s d = −0.6 and −0.5, respectively). A Cohen’s d magnitude >0.5 represents a ‘medium’ effect size (Ferguson, 2009).
Figure 3.
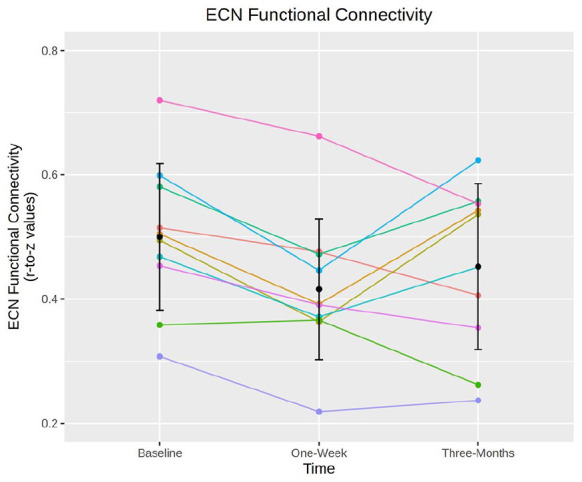
Executive control network (ECN) connectivity by participant. Spaghetti plot showing individual changes in mean ECN connectivity scores (y-axis) and time point (x-axis). Error bars represent mean ± standard deviation. Colours represent individual participants. ECN connectivity is significantly decreased at 1 week but not at 3 months.
Replication of previous study
Later, we attempted to replicate previously reported findings from a similar study (Barrett et al., 2020). Of the 35,778 edges defined by the Shen268 atlas, 405 showed evidence for significant connectivity based on the strategy described by Barrett and colleagues, who reported 695 suprathreshold edges. RSFC was altered in 25 edges at 1 week (19 increased and six decreased) and 18 edges at 3 months (12 increased and six decreased) at a statistical threshold of punc < 0.05. Two of these edges were altered in the same direction at both time points (one increased and one decreased). Although we observed fewer total edges showing evidence for significant connectivity, we observed a similar proportion of edges showing a time effect (i.e. 25/405 and 18/405 are approximately similar to 48/695 and 29/695, respectively). None of the 25 nor 18 edges remained statistically significant after controlling the type-I error for the 405 tests using the Bonferroni–Holm method.
Exploratory associations with ECN functional connectivity
Lastly, we explored the association between change in ECN RSFC at 1 week and self-report measures of the psychedelic experience acquired immediately after the experience, change in neocortex 5-HT2AR (at 1 week), change in personality (at 3 months) and self-reported persisting effects of the psychedelic experience (3 months). A summary of correlations can be found in Supplemental Table S3.
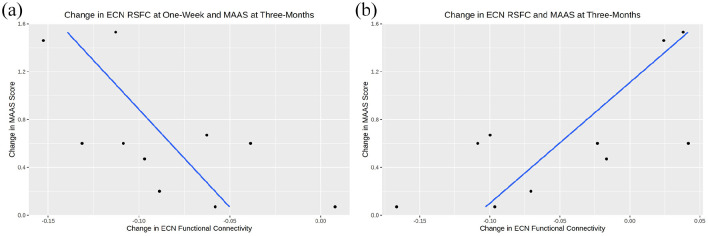
The most promising associations were observed between changes in ECN functional connectivity at 1 week and 3 months with self-reported change in MAAS at 3 months (r [95% CI] = −0.65 [−0.91, −0.04]) and (r [95% CI] = 0.71 [0.15, 0.93]) respectively, that is, the greater the decrease in ECN connectivity at 1 week, the greater the increase in MAAS score at 3 months (Figure 4). A smaller ECN connectivity change from baseline to 3 months was associated with a greater increase in MAAS score.
Figure 4.
Correlations between executive control network (ECN) connectivity and mindful attention awareness scale (MAAS). Scatter plots with linear regressions between MAAS score (y-axis) and (a) change in ECN connectivity at 1 week, and (b) change in ECN connectivity at 3 months. Blue lines represent lines of best fit and black dots denote observed data.
Neocortex 5-HT2AR
Change in neocortex 5-HT2AR binding measured with [11C]Cimbi-36 BPND at 1 week was not correlated with change in ECN connectivity at 1 week (r [95% CI] = 0.52 [−0.15, 0.87]). However, change in neocortex 5-HT2AR at 1 week correlated more strongly with the ECN RSFC change at 3 months (r [95% CI] = −0.67 [−0.91, −0.06]). In other words, greater disintegration of the ECN correlated with more neocortex 5-HT2AR.
Persisting effects questionnaire
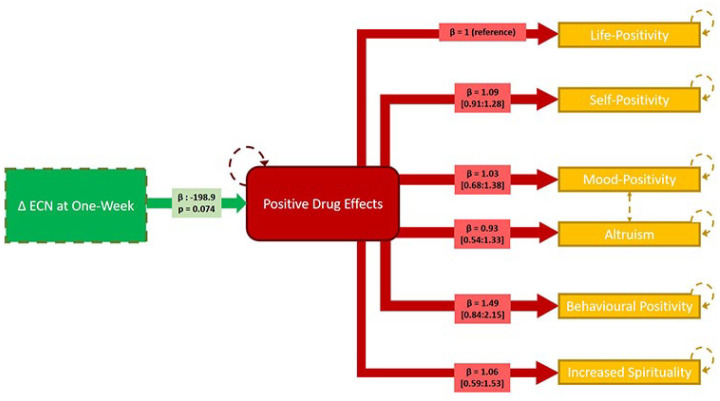
The positive subscales of the PEQ loaded strongly onto a single latent construct, indicating high shared correlation (p < 10−6). Change in ECN RSFC at 1 week was negatively associated with the underlying latent variable (−19.9 [−41.7, 1.93], units: change in Life Positivity PEQ per 0.1-unit change in ECN RSFC; Figure 5). Individual estimates are reported in Figure 5.
Figure 5.
Linear latent variable model linking change in executive control network (ECN) connectivity at 1 week and persisting effects questionnaire (PEQ) responses at 3 months. The green box denotes observed change in ECN connectivity at 1 week. The red box denotes the latent variable (‘positive drug change’). The yellow boxes denote observed PEQ scores. Hatched orange lines between ‘mood-positivity’ and ‘altruism’ indicate additional shared covariance. Hatched lines denote model components estimated with error. The loading parameter, β, reflecting the correlation of the score with the latent variable for each model path is noted in respective boxes (95% confidence intervals indicated for estimates between latent variable and PEQ subscale scores). Significance of the estimated effect of the ECN change on the latent variable is also noted.
Discussion
Our results show that psilocybin, when administered to healthy volunteers in a controlled environment, statistically significantly decreases ECN RSFC at 1 week, but not at 3 months. We observed correlations between ECN RSFC changes and changes in MAAS, neocortex 5-HT2AR and positive aspects of the PEQ, implicating alterations in ECN connectivity as a potential mechanism underlying the clinical and behavioural effects of psilocybin. No other network connectivity estimates were statistically significantly affected at 1 week or 3 months. Our study is small, but nevertheless implicates a candidate brain system underlying lasting psilocybin effects that can be examined in future studies in healthy and patient populations.
Executive control network connectivity
A single psilocybin administration decreased ECN RSFC; nine out of 10 participants showed decreased ECN RSFC and nine out of 10 ECN edges showed decreased RSFC across all participants. This distributed effect is consistent with this representing a network-wide effect not driven by a specific participant or edge. Executive functions include, for example, cognitive flexibility, goal setting, attentional control and information processing (Niendam et al., 2012). This is consistent with qualitative reports in addiction patients who report persistent changes in attentional control and goal setting following psilocybin intervention (Nielson et al., 2018; Noorani et al., 2018). Though little research has been performed on ECN RSFC and its association with trait changes in executive functions, one study reported lower FC within the ECN in long-term tai-chi practitioners relative to controls. These practitioners also showed increased trait mindfulness and performed better on emotion regulation tasks (Liu et al., 2018), aligning with our finding that decreased ECN connectivity predicted increased trait mindfulness. Change in ECN connectivity was not associated with any measures of the acute experience (Supplemental Table S3), suggesting it might not simply be mediated by brain psilocin concentration (Madsen et al., 2019).
Psilocybin has been shown in small open-label trials to be efficacious in the treatment of depression (Cohen’s d = −2.1 and −2.5 at 5-week post-administration) (Carhart-Harris et al., 2018; Davis et al., 2020), a disorder, which is partly characterised by deficits in executive functions (Snyder, 2013). Our finding that psilocybin decreased ECN RSFC is consistent with a recent study reporting that unmedicated, first-time MDD patients demonstrated hyper-connectivity between the left dorsolateral prefrontal cortex (PFC) and frontal and parietal regions, nodes which commonly constitute cognitive control networks (Shen et al., 2015). However, another study reported that MDD is characterised by reduced frontoparietal control system connectivity (Kaiser et al., 2015). Although it is intriguing that in healthy individuals we observe an effect of psilocybin on a resting-state network that displays pathological connectivity in depressed patients, future studies are necessary to more clearly establish this network’s relation to treatment-induced changes in measures of personality and well-being. Additionally, it remains to be established whether psilocybin-induced changes in connectivity would produce the well-being states described by such connectivity signatures in healthy, untreated individuals.
The observed effect on ECN RSFC may also align with psilocybin’s potential effects on obsessive-compulsive disorder (OCD) (Moreno et al., 2006) and addiction (Bogenschutz et al., 2015; Johnson et al., 2017), disorders broadly characterised by aberrant control of behaviour. The OCD patients display greater connectivity in ECN regions, including dorsolateral PFC (Chen et al., 2016), suggesting that reducing ECN connectivity could be therapeutically beneficial. Although individuals with addiction disorder show neuropsychological impairment in brain regions associated with cognitive control (Goldstein et al., 2004), RSFC investigations of control networks in addiction have so far utilised alternative network definitions, and thus are not directly comparable to these findings (Sutherland et al., 2012).
As we only see a significant change in ECN connectivity at 1 week and not at 3 months, this change may underlie the ‘afterglow’ effect. Despite the lack of long-term effects, the association between the 1 week effect and long-term measures of well-being suggests a role for this period in mediating long-term effects on well-being, the neural correlates of which were not detected in this study. Detailed quantitative characterisation of the ‘afterglow’ phenomenon could enable optimisation around this potentially clinically important phase of psychedelic psychotherapy by, for example, informing best practice surrounding post-session integration.
Additional effects on connectivity
Besides ECN RSFC at 1 week, all other standardised effect sizes for psilocybin induced change in RSFC (within- and between-network) were small to medium (i.e. |Cohen’s d| < 0.5). This suggests that future studies evaluating similar effects of psilocybin on RSFC would require sample sizes >60 to be adequately statistically powered (i.e. (1−β) > 0.8). Thus, our findings argue against large-scale changes in network connectivity structure insofar as we have quantified them here. This is notable considering participants report substantive changes in personality and well-being lasting for at least 1 year (Erritzoe et al., 2018; MacLean et al., 2011; Madsen et al., 2020) that are likely not entirely explained by ECN changes.
Our results suggest a small effect of psilocybin on DMN RSFC (Cohen’s d = 0.19). This is noteworthy because DMN disintegration has been reported when scanning participants during the psychedelic experience (Carhart-Harris et al., 2012; Preller et al., 2020) and may be implicated in the therapeutic effects of psilocybin as DMN connectivity is elevated in a range of conditions and is reduced following administration of psilocybin to experienced meditators (Smigielski et al., 2019; Whitfield-Gabrieli and Ford, 2012). Although we do not see a persistent effect on DMN connectivity, acute disruption of the DMN may still be therapeutically relevant (Carhart-Harris and Friston, 2019; Mason et al., 2020). Intake of both Salvinorin-A, a 5-HT2AR-independent hallucinogen, and 3,4-methylenedioxymethamphetamine (MDMA), which has a subjective effect profile distinct from serotonergic psychedelics (Doss et al., 2020; Müller et al., 2020; Roseman et al., 2014), leads to a reduction in DMN connectivity. Reduced DMN connectivity is not associated with ego-dissolution in response to psilocybin (Lebedev et al., 2015). This muted effect on DMN RSFC after the psychedelic session is consistent with a study that scanned MDD individuals 1 day after the psychedelic experience (Carhart-Harris et al., 2017) and healthy volunteers 1 week and 1 month after psilocybin (Barrett et al., 2020). Thus, convergent evidence indicates that persistent alterations in DMN RSFC are unlikely to be a critical mechanism underlying lasting psilocybin effects.
Associations with executive control network change
Through exploratory analyses, we observed three notable associations with change in ECN RSFC. Firstly, a greater decrease in ECN RSFC at 1 week and a lesser decrease in ECN RSFC at 3 months both correlated with increased mindfulness score at 3 months. This could reflect that ECN disintegration followed by reintegration produces lasting increases in mindful awareness, a trait that is associated with reduced stress and better mood (Brown and Ryan, 2003). Secondly, building on our previous finding that change in 5-HT2AR correlated negatively with change in mindfulness (Madsen et al., 2020), we observed that a greater decrease in ECN RSFC 1 week and a lesser decrease in ECN RSFC at 3 months was associated with decreased 5-HT2AR at 1 week. Although speculative, our findings suggest that individual change in neocortex 5-HT2AR following psilocybin administration may effect a change in mindfulness that is mediated by changes in ECN connectivity. Thirdly, decrease in ECN at 1 week was positively associated with a latent construct of positive persisting effects, reflecting positive items from the PEQ, at 3 months. This effect was particularly pronounced regarding ‘behavioural positivity’, aligning with qualitative reports from patients in clinical trials (Nielson et al., 2018; Noorani et al., 2018). Although exploratory, these observations provide a framework for linking brain and behavioural changes effected by psilocybin administration in future studies in healthy and clinical cohorts. Although we see changes in personality-trait openness, neuroticism and conscientiousness in this sample (Madsen et al., 2020), these changes were not correlated with change in ECN RSFC (Supplemental Table S3).
Barrett replication
Replication is critical for identifying reliable brain markers of psilocybin effects. Here, we sought to replicate recently reported findings from a study very similar to ours (Barrett et al., 2020). We replicated the scale of region-to-region RSFC estimates that were affected by psilocybin. However, none of these effects remained statistically significant when controlling for multiple comparisons. Ours and the previous study have small sample sizes, which exacerbate the statistical power limitations of an exploratory region-to-region RSFC analysis strategy. Without substantively larger samples, this analysis framework would likely benefit from a hypothesis-driven evaluation of specific region pairs. Notably, the Shen268 atlas does not describe an ECN, and thus these results cannot be compared with our ECN finding using the Raichle atlas. This heterogeneity highlights a case for co-ordination of spatial parcellation within this corner of neuroimaging.
Limitations
As noted previously, our sample size of 10 individuals limits statistical power. Nevertheless, the data reported here provide a firmer foundation for future studies in clinical and healthy cohorts with larger samples. Although psilocybin seems to have positive behavioural and mood effects in healthy individuals, it is not clear how closely our observed effects on brain connectivity would generalise to clinical cohorts. Additional studies in patient groups are needed to delineate the neurobiological basis of therapeutic effects of serotonergic psychedelics including psilocybin. There is variation across resting-state atlases in how cognitive control networks are defined, limiting our ability to draw firm conclusions with previous related studies (Raichle, 2015; Schaefer et al., 2018; Shen et al., 2020). Alternative analytic strategies (e.g. dynamic functional connectivity and entropy analyses) or task-based fMRI may reveal more pronounced effects on brain function and connectivity than those reported here. Future studies integrating fMRI with PET markers may offer deeper insights into the neurobiological mechanisms mediating psilocybin effects on behaviour (Fisher and Hariri, 2012). Although the observed effect on ECN may be related to individual differences in pharmacodynamics (i.e. drug availability and metabolism), we did not measure individual plasma psilocin levels (Madsen et al., 2019).
Conclusion
In conclusion, we report effects of a single psilocybin administration on RSFC networks at 1 week and 3 months in a cohort of 10 individuals. Although a small sample, we identified a statistically significant reduction in ECN RSFC at 1 week but not at 3 months (although numerically decreased). Exploratory correlations with change in ECN at 1 week suggest that it may be associated with change in neocortex 5-HT2AR at 1 week as well as change in mindfulness and persistent positive psychological effects at 3 months. Nevertheless, future studies are necessary to more thoroughly map psilocybin effects on to changes in brain function and connectivity that may mediate its lasting clinical and behavioural effects.
Supplemental Material
Supplemental material, sj-docx-1-jop-10.1177_02698811211026454 for Lasting effects of a single psilocybin dose on resting-state functional connectivity in healthy individuals by Drummond E-Wen McCulloch, Martin Korsbak Madsen, Dea Siggaard Stenbæk, Sara Kristiansen, Brice Ozenne, Peter Steen Jensen, Gitte Moos Knudsen and Patrick MacDonald Fisher in Journal of Psychopharmacology
Acknowledgments
Psilocybin was kindly supplied by National Institute of Mental Health, Klecany, Czech Republic and Forensic Laboratory of Biologically Active Compounds, Department of Chemistry of Natural Compounds, University of Chemistry and Technology Prague, Prague, Czech Republic.
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: DEM’s salary was supported by an unrestricted grant from COMPASS Pathways Ltd., which had no involvement in this manuscript or related data collection. GMK has received honoraria as consultant for Sanos and for Sage Therapeutics. All other authors declare that there are no conflicts of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Innovation Fund Denmark (grant number: 4108-00004B), Independent Research Fund Denmark (grant number: 6110-00518B), and Ester M. og Konrad Kristian Sigurdssons Dyreværnsfond (grant number: 850-22-55166-17-LNG). MKM was supported by the Rigshospitalet Research Council (grant number: R130-A5324). BO was supported by the Lundbeck Foundation (grant number: R231-2016-3236) and Marie-Curie-NEUROMODEL (grant number: 746850). DEM was supported by an unrestricted grant from COMPASS Pathways Ltd.
ORCID iDs: Drummond E-Wen McCulloch  https://orcid.org/0000-0001-6360-5224
https://orcid.org/0000-0001-6360-5224
Dea Siggaard Stenbæk  https://orcid.org/0000-0002-5439-4637
https://orcid.org/0000-0002-5439-4637
Patrick MacDonald Fisher  https://orcid.org/0000-0002-8115-0611
https://orcid.org/0000-0002-8115-0611
Supplemental material: Supplemental material for this article is available online.
References
- Anderson BT, Danforth A, Daroff R, et al. (2020) Psilocybin-assisted group therapy for demoralized older long-term AIDS survivor men: An open-label safety and feasibility pilot study. EClinicalMedicine 27: 100538. DOI: 10.1016/j.eclinm.2020.100538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett FS, Doss MK, Sepeda ND, et al. (2020) Emotions and brain function are altered up to one month after a single high dose of psilocybin. Sci Rep 10: 2214. DOI: 10.1038/s41598-020-59282-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett FS, Johnson MW, Griffiths RR. (2015) Validation of the revised Mystical Experience Questionnaire in experimental sessions with psilocybin. J Psychopharmacol 29: 1182–1190. DOI: 10.1177/0269881115609019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, et al. (2007) A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage 37: 90–101. DOI: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogenschutz MP, Forcehimes AA, Pommy JA, et al. (2015) Psilocybin-assisted treatment for alcohol dependence: A proof-of-concept study. J Psychopharmacol 29: 289–299. DOI: 10.1177/0269881114565144. [DOI] [PubMed] [Google Scholar]
- Brown KW, Ryan RM. (2003) The benefits of being present: Mindfulness and its role in psychological well-being. J Pers Soc Psychol 84: 822–848. DOI: 10.1037/0022-3514.84.4.822. [DOI] [PubMed] [Google Scholar]
- Cai H, Zhu J, Yu Y. (2020) Robust prediction of individual personality from brain functional connectome. Soc Cogn Affect Neurosci 15: 359–369. DOI: 10.1093/scan/nsaa044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Friston KJ. (2019) REBUS and the anarchic brain: Toward a unified model of the brain action of psychedelics. Pharmacol Rev 71: 316–344. DOI: 10.1124/pr.118.017160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Bolstridge M, Day CMJ, et al. (2018) Psilocybin with psychological support for treatment-resistant depression: Six-month follow-up. Psychopharmacology 235: 399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Erritzoe D, Williams T, et al. (2012) Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc Natl Acad Sci U S A 109: 2138–2143. DOI: 10.1073/pnas.1119598109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Kaelen M, Bolstridge M, et al. (2016) The paradoxical psychological effects of lysergic acid diethylamide (LSD). Psychol Med 46: 1379–1390. DOI: 10.1017/S0033291715002901. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, Roseman L, Bolstridge M, et al. (2017) Psilocybin for treatment-resistant depression: FMRI-measured brain mechanisms. Sci Rep 7: 13187. DOI: 10.1038/s41598-017-13282-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Meng X, Hu Q, et al. (2016) Altered resting-state functional organization within the central executive network in obsessive–compulsive disorder. Psychiatry Clin Neurosci 70: 448–456. DOI: 10.1111/pcn.12419. [DOI] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. (2008) The revised NEO personality inventory (NEO-PI-R). In: Boyle GJ, Matthews G, Saklofske DH. (eds) The SAGE Handbook of Personality Theory and Assessment: Volume 2 - Personality Measurement and Testing. London: SAGE, pp.179–198. DOI: 10.4135/9781849200479.n9. [DOI] [Google Scholar]
- Davis AK, Barrett FS, May DG, et al. (2020) Effects of psilocybin-assisted therapy on major depressive disorder: A randomized clinical trial. JAMA Psychiatry 78: 481–489. DOI: 10.1001/jamapsychiatry.2020.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doss MK, May DG, Johnson MW, et al. (2020) The acute effects of the atypical dissociative hallucinogen salvinorin A on functional connectivity in the human brain. Sci Rep 10: 16392. DOI: 10.1038/s41598-020-73216-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erritzoe D, Roseman L, Nour MM, et al. (2018) Effects of psilocybin therapy on personality structure. Acta Psychiatr Scand 138: 368–378. DOI: 10.1111/acps.12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettrup A, da Cunha-Bang S, McMahon B, et al. (2014) Serotonin 2A receptor agonist binding in the human brain with [11 C]Cimbi-36. J Cereb Blood Flow Metab 34: 1188–1196. doi: 10.1038/jcbfm.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettrup A, Svarer C, McMahon B, et al. (2016) Serotonin 2A receptor agonist binding in the human brain with [11C]Cimbi-36: Test-retest reproducibility and head-to-head comparison with the antagonist [18F]altanserin. NeuroImage 130: 167–174. DOI: 10.1016/j.neuroimage.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Ferguson CJ. (2009) An effect size primer: A guide for clinicians and researchers. Prof Psychol Res Pr 40: 532–538. DOI: 10.1037/a0015808. [DOI] [Google Scholar]
- Finnema SJ, Stepanov V, Ettrup A, et al. (2014) Characterization of [11C]Cimbi-36 as an agonist PET radioligand for the 5-HT2A and 5-HT2C receptors in the nonhuman primate brain. NeuroImage 84: 342–353. DOI: 10.1016/j.neuroimage.2013.08.035. [DOI] [PubMed] [Google Scholar]
- Fisher PM, Hariri AR. (2012) Linking variability in brain chemistry and circuit function through multimodal human neuroimaging. Genes Brain Behav 11: 633–642. DOI: 10.1111/j.1601-183X.2012.00786.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Romeu A, Davis AK, Erowid F, et al. (2019) Cessation and reduction in alcohol consumption and misuse after psychedelic use. J Psychopharmacol 33: 1088–1101. DOI: 10.1177/0269881119845793. [DOI] [PubMed] [Google Scholar]
- Gasser P, Holstein D, Michel Y, et al. (2014) Safety and efficacy of lysergic acid diethylamide-assisted psychotherapy for anxiety associated with life-threatening diseases. J Nerv Ment Dis 202: 513–520. DOI: 10.1097/NMD.0000000000000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Leskovjan AC, Hoff AL, et al. (2004) Severity of neuropsychological impairment in cocaine and alcohol addiction: Association with metabolism in the prefrontal cortex. Neuropsychologia 42: 1447–1458. DOI: 10.1016/j.neuropsychologia.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW, Carducci MA, et al. (2016) Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J Psychopharmacol 30: 1181–1197. DOI: 10.1177/0269881116675513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW, Richards WA, et al. (2011) Psilocybin occasioned mystical-type experiences: Immediate and persisting dose-related effects. Psychopharmacology 218: 649–665. DOI: 10.1007/s00213-011-2358-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Richards WA, McCann U, et al. (2006) Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology 187: 268–283. DOI: 10.1007/s00213-006-0457-5. [DOI] [PubMed] [Google Scholar]
- Grob CS, McKenna DJ, Callaway JC, et al. (1996) Human psychopharmacology of hoasca, a plant hallucinogen used in ritual context in Brazil. J Nerv Ment Dis 184: 86–94. DOI: 10.1097/00005053-199602000-00004. [DOI] [PubMed] [Google Scholar]
- Hasler F, Grimberg U, Benz MA, et al. (2004) Acute psychological and physiological affects of psilocybin in healthy humans: A double-blind, placebo-controlled dose-effect study. Psychopharmacology 172: 145–156. DOI: 10.1007/s00213-003-1640-6. [DOI] [PubMed] [Google Scholar]
- Holm S. (1979) A simple sequentially rejective multiple test procedure. Scand J Stat 6: 65–70. [Google Scholar]
- Holst KK, Budtz-Jørgensen E. (2013) Linear latent variable models: The lava-package. Comput Stat 28: 1385–1452. DOI: 10.1007/s00180-012-0344-y. [DOI] [Google Scholar]
- Hsu WT, Rosenberg MD, Scheinost D, et al. (2018) Resting-state functional connectivity predicts neuroticism and extraversion in novel individuals. Soc Cogn Affect Neurosci 13: 224–232. DOI: 10.1093/scan/nsy002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, et al. (2007) Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 27: 1533–1539. DOI: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Jensen CG, Niclasen J, Vangkilde SA, et al. (2016) Research on translations of tests: General inattentiveness is a long-term reliable trait independently predictive of psychological health: Danish validation studies of the mindful attention awareness scale. Psychol Assess 28: e70–e87. DOI: 10.1037/pas0000196. [DOI] [PubMed] [Google Scholar]
- Johnson MW, Garcia-Romeu A, Griffiths RR. (2017) Long-term follow-up of psilocybin-facilitated smoking cessation. Am J Drug Alcohol Abuse 43: 55–60. DOI: 10.3109/00952990.2016.1170135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Richards WA, Griffiths RR. (2008) Human hallucinogen research: Guidelines for safety. J Psychopharmacol 22: 603–620. DOI: 10.1177/0269881108093587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser RH, Andrews-Hanna JR, Wager TD, et al. (2015) Large-scale network dysfunction in major depressive disorder: A meta-analysis of resting-state functional connectivity. JAMA Psychiatry 72: 603–611. DOI: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebedev AV, Lövdén M, Rosenthal G, et al. (2015) Finding the self by losing the self: Neural correlates of ego-dissolution under psilocybin. Hum Brain Mapp 36: 137–153. DOI: 10.1002/hbm.22833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MH, Smyser CD, Shimony JS. (2013) Resting-state fMRI: A review of methods and clinical applications. Am J Neuroradiol 34: 1866–1872. DOI: 10.3174/ajnr.A3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Wu Y, Li L, et al. (2018) Functional connectivity within the executive control network mediates the effects of long-term Tai Chi exercise on elders’ emotion regulation. Front Aging Neurosci 10: 315. doi: 10.3389/fnagi.2018.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean KA, Johnson MW, Griffiths RR. (2011) Mystical experiences occasioned by the hallucinogen psilocybin lead to increases in the personality domain of openness. J Psychopharmacol 25: 1453–1461. DOI: 10.1177/0269881111420188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen MK, Fisher PM, Burmester D, et al. (2019) Psychedelic effects of psilocybin correlate with serotonin 2A receptor occupancy and plasma psilocin levels. Neuropsychopharmacology 44: 1328–1334. DOI: 10.1038/s41386-019-0324-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen MK, Fisher PM, Stenbæk DS, et al. (2020) A single psilocybin dose is associated with long-term increased mindfulness, preceded by a proportional change in neocortical 5-HT2A receptor binding. Eur Neuropsychopharmacol 33: 71–80. DOI: 10.1016/j.euroneuro.2020.02.001. [DOI] [PubMed] [Google Scholar]
- Majić T, Schmidt TT, Gallinat J. (2015) Peak experiences and the afterglow phenomenon: When and how do therapeutic effects of hallucinogens depend on psychedelic experiences? J Psychopharmacol 29: 241–253. DOI: 10.1177/0269881114568040. [DOI] [PubMed] [Google Scholar]
- Mason NL, Kuypers KPC, Müller F, et al. (2020) Me, myself, bye: Regional alterations in glutamate and the experience of ego dissolution with psilocybin. Neuropsychopharmacology 45: 2003–2011. DOI: 10.1038/s41386-020-0718-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno FA, Wiegand CB, Keolani Taitano E, et al. (2006) Safety, tolerability, and efficacy of psilocybin in 9 patients with obsessive-compulsive disorder. J Clin Psychiatry 67: 1735–1740. DOI: 10.4088/JCP.v67n1110. [DOI] [PubMed] [Google Scholar]
- Müller F, Holze F, Dolder P, et al. (2020) MDMA-induced changes in within-network connectivity contradict the specificity of these alterations for the effects of serotonergic hallucinogens. Neuropsychopharmacology 46: 545–553. DOI: 10.1038/s41386-020-00906-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Beiner A, Soar K. (2020) Ayahuasca’s “afterglow”: Improved mindfulness and cognitive flexibility in ayahuasca drinkers. Psychopharmacology 237: 1161–1169. DOI: 10.1007/s00213-019-05445-3. [DOI] [PubMed] [Google Scholar]
- Nichols DE. (2016) Psychedelics. Pharmacol Rev 68: 264–355. DOI: 10.1124/pr.115.011478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielson EM, May DG, Forcehimes AA, et al. (2018) The psychedelic debriefing in alcohol dependence treatment: Illustrating key change phenomena through qualitative content analysis of clinical sessions. Front Pharmacol 9: 132. DOI: 10.3389/fphar.2018.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niendam TA, Laird AR, Ray KL, et al. (2012) Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci 12: 241–268. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noorani T, Garcia-Romeu A, Swift TC, et al. (2018) Psychedelic therapy for smoking cessation: Qualitative analysis of participant accounts. J Psychopharmacol 32: 756–769. DOI: 10.1177/0269881118780612. [DOI] [PubMed] [Google Scholar]
- Nour MM, Evans L, Nutt D, et al. (2016) Ego-dissolution and psychedelics: Validation of the ego-dissolution inventory (EDI). Front Hum Neurosci 10: 269. DOI: 10.3389/fnhum.2016.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahnke WN. (1969) The psychedelic mystical experience in the human encounter with death. Harv Theol Rev 62: 1–21. DOI: 10.1017/S0017816000027577. [DOI] [Google Scholar]
- Pasquini L, Palhano-Fontes F, Araujo DB. (2020) Subacute effects of the psychedelic ayahuasca on the salience and default mode networks. J Psychopharmacol 34: 623–635. DOI: 10.1177/0269881120909409. [DOI] [PubMed] [Google Scholar]
- Penny W, Friston K, Ashburner J, et al. (2007) Statistical Parametric Mapping: The Analysis of Functional Brain Images, Statistical Parametric Mapping: The Analysis of Functional Brain Images. Academic Press. DOI: 10.1016/B978-0-12-372560-8.X5000-1. [DOI] [Google Scholar]
- Preller KH, Vollenweider FX. (2018) Phenomenology, structure, and dynamic of psychedelic states. Curr Top Behav Neurosci 36: 221–256. DOI: 10.1007/7854_2016_459. [DOI] [PubMed] [Google Scholar]
- Preller KH, Duerler P, Burt JB, et al. (2020) Psilocybin induces time-dependent changes in global functional connectivity. Biol Psychiatry 88: 197–207. DOI: 10.1016/j.biopsych.2019.12.027. [DOI] [PubMed] [Google Scholar]
- R Studio Team (2020) R Studio. Available at: http://www.rstudio.com/
- Raichle ME. (2011) The restless brain. Brain Connect 1: 3–12. doi: 10.1089/brain.2011.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME. (2015) The restless brain: How intrinsic activity organizes brain function. Philos Trans R Soc Lond B Biol Sci 370: 20140172. DOI: 10.1098/rstb.2014.0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseman L, Leech R, Feilding A, et al. (2014) The effects of psilocybin and MDMA on between-network resting state functional connectivity in healthy volunteers. Front Hum Neurosci 8: 204. DOI: 10.3389/fnhum.2014.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampedro F, de la Fuente Revenga M, Valle M, et al. (2017) Assessing the psychedelic “after-glow” in ayahuasca users: Post-acute neurometabolic and functional connectivity changes are associated with enhanced mindfulness capacities. Int J Neuropsychopharmacol 20: 698–711. DOI: 10.1093/ijnp/pyx036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer A, Kong R, Gordon EM, et al. (2018) Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cereb Cortex 28: 3095–3114. DOI: 10.1093/cercor/bhx179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, et al. (1998) The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59: 22–33. [PubMed] [Google Scholar]
- Shen K, Welton T, Lyon M, et al. (2020) Structural core of the executive control network: A high angular resolution diffusion MRI study. Hum Brain Mapp 41: 1226–1236. DOI: 10.1002/hbm.24870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen T, Li C, Wang B, et al. (2015) Increased cognition connectivity network in major depression disorder: A fMRI study. Psychiatry Investig 12: 227–234. DOI: 10.4306/pi.2015.12.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Tokoglu F, Papademetris X, et al. (2013) Groupwise whole-brain parcellation from resting-state fMRI data for network node identification. NeuroImage 82: 403–415. DOI: 10.1016/j.neuroimage.2013.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skovdahl-Hansen H, Mortensen EL, Scioetz H. (2004) Dokumentation for den danske udgave af NEO PI-R og NEO PI-R Kort Version. Copenhagen, Denmark: Dansk Psykologisk Forlag. [Google Scholar]
- Sloshower J, Guss J, Krause R, et al. (2020) Psilocybin-assisted therapy of major depressive disorder using acceptance and commitment therapy as a therapeutic frame. J Contextual Behav Sci 15: 12–19. DOI: 10.1016/j.jcbs.2019.11.002. [DOI] [Google Scholar]
- Smigielski L, Scheidegger M, Kometer M, et al. (2019) Psilocybin-assisted mindfulness training modulates self-consciousness and brain default mode network connectivity with lasting effects. NeuroImage 196: 207–215. DOI: 10.1016/j.neuroimage.2019.04.009. [DOI] [PubMed] [Google Scholar]
- Snyder HR. (2013) Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: A meta-analysis and review. Psychol Bull 139: 81–132. DOI: 10.1037/a0028727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spies M, Nasser A, Ozenne B, et al. (2020) Common HTR2A variants and 5-HTTLPR are not associated with human in vivo serotonin 2A receptor levels. Hum Brain Mapp 41: 4518–4528. DOI: 10.1002/hbm.25138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenbæk DS, Madsen MK, Ozenne B, et al. (2021) Brain serotonin 2A receptor binding predicts subjective temporal and mystical effects of psilocybin in healthy humans. J Psychopharmacol 35: 459–468. DOI: 10.1177/0269881120959609. [DOI] [PubMed] [Google Scholar]
- Straathof M, Rt Sinke M, Dijkhuizen RM, et al. (2019) A systematic review on the quantitative relationship between structural and functional network connectivity strength in mammalian brains. J Cereb Blood Flow Metab 39: 189–209. DOI: 10.1177/0271678X18809547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studerus E, Gamma A, Vollenweider FX. (2010) Psychometric evaluation of the altered states of consciousness rating scale (OAV). PLoS One 5: e12412. DOI: 10.1371/journal.pone.0012412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, McHugh MJ, Pariyadath V, et al. (2012) Resting state functional connectivity in addiction: Lessons learned and a road ahead. NeuroImage 62: 2281–2295. DOI: 10.1016/j.neuroimage.2012.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svarer C, Madsen K, Hasselbalch SG, et al. (2005) MR-based automatic delineation of volumes of interest in human brain PET images using probability maps. NeuroImage 24: 969–979. DOI: 10.1016/j.neuroimage.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Tagliazucchi E, Roseman L, Kaelen M, et al. (2016) Increased global functional connectivity correlates with LSD-induced ego dissolution. Curr Biol 26: 1043–1050. DOI: 10.1016/j.cub.2016.02.010. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Bäbler A, et al. (1998) Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. NeuroReport 9: 3897–3902. DOI: 10.1097/00001756-199812010-00024. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Ford JM. (2012) Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol 8: 49–76. DOI: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A. (2012) Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2: 125–141. DOI: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Wickham H. (2016) ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlag. Available at: https://ggplot2.tidyverse.org/ [Google Scholar]
- Zeifman RJ, Wagner AC, Watts R, et al. (2020) Post-psychedelic reductions in experiential avoidance are associated with decreases in depression severity and suicidal ideation. Front Psychiatry 11: 782. DOI: 10.3389/fpsyt.2020.00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jop-10.1177_02698811211026454 for Lasting effects of a single psilocybin dose on resting-state functional connectivity in healthy individuals by Drummond E-Wen McCulloch, Martin Korsbak Madsen, Dea Siggaard Stenbæk, Sara Kristiansen, Brice Ozenne, Peter Steen Jensen, Gitte Moos Knudsen and Patrick MacDonald Fisher in Journal of Psychopharmacology