Abstract
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the virus that is responsible for the current coronavirus disease pandemic and the vaccines currently developed are administered to prevent this infection. CoronaVac is a vaccine produced by the inactivated virus method. Ocular side effects such as anterior uveitis, optic neuritis, vision loss, episcleritis, allergic reaction and paracentral acute middle maculopathy have been reported after receiving CoronaVac vaccine. We assume that with this study, we can identify potential changes in posterior segment structures and posterior segment vascular density of people who received CoronaVac vaccine with optical coherence tomography angiography (OCTA) device.
Material method
Forty healthcare professionals who applied to the Health Sciences University Antalya Training and Research Hospital Ophthalmology Clinic for routine eye control were included in the study. The subjects who do not have any systemic condition and would be administered CoronaVac vaccine were chosen to assess. OCTA images of the patients before and within 1 week after vaccination were captured, then retinal and optic disc vascular values, foveal avascular zone (FAZ), choriocapillary blood flow (CBF), subfoveal choroidal thickness (SCT) and retinal thickness were analyzed and compared.
Results
Two of the 40 patients had burning and stinging in the eye (5%), two of the 40 patients had redness (5%) and itching (5%) in the eye. 36 patients did not have any ocular symptoms.No statistically significant difference was found in the retinal and optic disc vascular density values, FAZ, CBF, SCT and retinal thickness values of the patients before and after vaccination.
Conclusion
This is among the first studies in the literature to evaluate the changes in retinal and optic disc vascular values in people who received CoronaVac vaccine. In this study, we observed that CoronaVac vaccine did not effect retinal and optic disc vascular density significantly.
Keywords: CoronaVac, Coronavirus, COVID-19, Optical coherence tomography angiography, Sinovac
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the virus which is responsible for the current coronavirus disease pandemic and it is extremely contagious. Being an enveloped virus, coronavirus belongs to the RNA virus family. The disease caused by this virus is named COVID-19 (where “CO” stands for corona, “VI” for virus, “D” for disease, and “19″ indicates the year in which it emerged). COVID-19, which was first detected in Wuhan, China, spread all over the world in a short time and turned into a pandemic [1,2].
COVID-19 is a disease that primarily affects the respiratory tract. Its general findings are fever, cough, fatigue, and sore throat [3]. There are also ocular findings in this infection. Eye watering, itching, foreign body sensation, blurred vision, limitation of eye movements, conjunctivitis and Guillain–Barré syndrome are some of the ocular findings of COVID-19 disease [4,5].
CoronaVac is a vaccine developed by Sinovac company. It is a dead vaccine inactivated with formaldehyde and using aluminum as an adjuvant. The technique of producing a vaccine from inactivated virus, which has been used for a long time, is an experienced and reliable method. In studies, side effects such as redness and itching at the injection site, fever, headache, muscle pain, fatigue, cough, diarrhea, nausea and vomiting, shortness of breath, joint pain, lymphadenopathy and anaphylactic reaction were reported in the patients after CoronaVac vaccination [6].
Optical coherence tomography angiography (OCTA) is a non-invasive imaging modality that has been used recently for imaging retinal and choroidal vascular blood flow. Optical coherence tomography angiography can measure both the superficial and deep vessel density in the macular capillary plexus by detecting the motion contrast in the blood flow [7]. In previous studies, retinal and choroidal vascular density parameters in COVID-19 patients were compared with the control group using OCTA, and statistically significant results were obtained [8,9]. The aim of this study was to use the OCTA device to compare the retina, choroid, and optic disc vascular density parameters of CoronaVac vaccine recipients before and after vaccination.
We assume that with this research, we can observe the changes that may occur in the posterior segment structures and posterior segment vascular density of the people who received CoronaVac vaccine through the OCTA device.
2. Material method
2.1. Patient selection
Forty healthcare professionals who applied to the Health Sciences University Antalya Training and Research Hospital Ophthalmology Clinic for routine eye control were included in the study. The subjects who do not have any systemic condition and would be administered the first dose of CoronaVac vaccine were chosen to assess. Forty eyes of these 40 cases were included in the study. Vaccination dates of the patients were between 01.01.2021 and 01.03.2021. None of the participants had COVID-19 before.
Exclusion criterias; those with any eye pathology (eg: glaucoma, uveitis, diabetic retinopathy, amblyopia, epiretinal membrane), myopia greater than −6 diopters, those with axial length higher than 26 mm, those who had eye surgery and those with systemic condition (eg: hypertension, diabetes mellitus, systemic lupus erythematosus, vascular diseases).
Demographic characteristics of the patients and thecontrol group (age, gender, medications, systemic disease, etc.) and examination findings were available in their file records. Complete ophthalmological examination and OCTA images were performed before and within 1 week after the CoronaVac vaccination. The findings of the fundus examinations of the patients were evaluated by slit-lampbiomicroscopy. The OCTA images of the patients were interpreted. “Spectral-domain OCTA” (AngioVue; Optovue, Inc, Fremont, CA) device was used in the present study.
2.2. OCTA parameters evaluation
The OCTA measurements of the patients were performed in 6 × 6 mm HD angio retina and 4.5 × 4.5 mm angio optic disc scales. Each OCTA scan underwent automatic scan quality [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], values ≥8 were accepted. OCTA images with a Signal Strength Index (SSI) less than 80, and residual motion artifacts were excluded from the analysis.
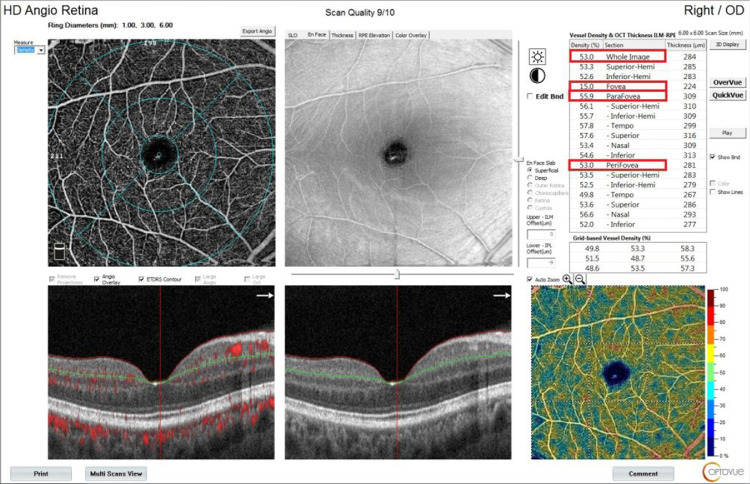
Foveal vascular density: The vascular density in the circle with a diameter of 1 mm in the center of the fovea was taken as a percentage. Parafoveal vascular density: The vascular density in the ring between 1 mm and 3 mm was taken as a percentage. Perifoveal vascular density: The vascular density in the remaining ring between 3 mm and 6 mm was taken as a percentage. Whole image vascular density: The vascular density in a circle with a diameter of 6 mm from the center of the fovea was taken as a percentage (Fig. 1 ).
Fig. 1.
Vascular density values of a vaccinated patient in OCTA imaging.
These areas gave the cross-sectional density measurement of the superficial capillary area in automated mode. Automatically calculated by the software, the ratio of the vascular image (white areas) in these areas to the whole area gives the density as a percentage.
Foveal avascular zone (FAZ): FAZ area was calculated by the device automatically and taken as mm2. Choriocapillaris blood flow (CBF): In the choriocapillaris layer, the value of blood flow in the circular section within a radius of 1 mm and an area of 3.142 mm2 was calculated as mm2 automatically by the device.
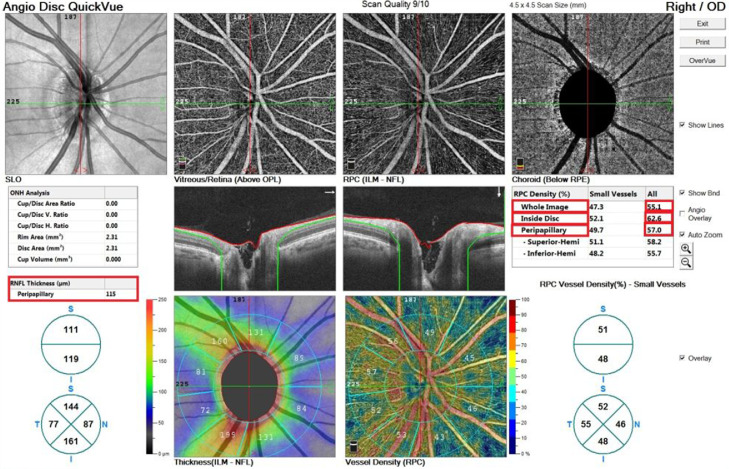
Optic disc vascular density: The vascular density in the 4.5 mm diameter circle centerd on the optic disc was taken as percentage. Optic disc all vessels whole image vascular density, optic disc all vessels inside vascular density and peripapillary all vessels vascular density parameters are examined in this scale. Peripapillary retinal nerve fiber layer thickness was automatically measured and recorded by the device from a 3.4 mm scanning circle centerd on the optic disc (Fig. 2 ).
Fig. 2.
Optic disc vascular density values of a vaccinated patient in OCTA imaging.
Retinal thickness: Retinal thickness between the internal limiting membrane and retinal pigment epithelium (RPE) in the foveal, parafoveal and perifoveal regions was automatically measured and recorded by the device.
Choroidal thickness: In the “Enhanced HD line” mode, assessments were made from the subfoveal area by 2 different observers. The average of the measurements of 2 observers was calculated. RPE and sclerachoroidal junction were accepted as the subfoveal choroidal thickness limit.
2.3. Statistical analysis
The analysis of the data was made using the SPSS 24.0 package program. Descriptive statistics are given as percentage for categorical variables, mean, standard deviation and median, minimum value and maximum value for numerical variables. The conformity of the numerical variables to the normal distribution was evaluated with the Shapiro-Wilk test. Variables are given using mean and standard deviation. Old and new measurements were compared using the Wilcoxon test. The results were evaluated within the 95% confidence interval and p < 0.05 values were considered statistically significant.
3. Results
The mean age of 40 people participating in the study was calculated as 33.05 ± 8.61. Twenty of them were female and the other twenty were male and their mean age is 32.30 ± 8.65 and 33.80 ± 8.97, respectively. Demographic characteristics of the patients are given in Table 1 .
Table 1.
Demographic characteristics of the patients participating in the study.
| Patient | Number |
|---|---|
| Age | 33.05 ± 8.61 |
| Female/Male | 20/20 |
| Female mean age | 32.30 ± 8.65 |
| Male mean age | 33.80 ± 8.97 |
| Doctors | 20 |
| Nurses | 8 |
| Medical secretarias | 6 |
| Health officers | 6 |
| Total number of patients | 40 |
Two of the 40 patients had burning and stinging in the eye (5%), and two of the 40 patients had redness (5%) and itching (5%) in the eye. 36 patients did not have any ocular symptoms. The eye symptoms seen in the patients are given in the table below (Table 2 ).
Table 2.
Eye symptoms seen in patients.
| Symptom | Number of patients | Percent |
|---|---|---|
| Burning and stinging | 2 | 5 |
| Redness | 2 | 5 |
| Itching | 2 | 5 |
| Epiphora | 0 | 0 |
| Eye pain | 0 | 0 |
| Defect of vision | 0 | 0 |
| Conjunctivits | 0 | 0 |
| Neuroophthalmologic symptom | 0 | 0 |
| Asymptomatic patient | 36 | 90 |
The OCTA values of the patients before and after vaccination are given in the Table 3, Table 4, Table 5 .
Table 3.
Comparison results of superficial and deep capillary plexus vascular density values over time.
| Group | Mean | Min. | Max. | P | |
|---|---|---|---|---|---|
| SCP wholeimage vascular | Before vac. | 51,11 ± 0,47 | 47,50 | 54,80 | 0,225 |
| density value (%) | After vac. | 50,42 ± 0,57 | 46,70 | 55,30 | |
| SCP foveal vascular | Before vac. | 19,91 ± 1.46 | 7,90 | 32,30 | 0,478 |
| density value (%) | After vac. | 19,53 ± 1.47 | 9,50 | 32,10 | |
| SCP parafovea | Before vac. | 53,64 ± 0,61 | 48,30 | 59,20 | 0,153 |
| vasculardensity value (%) | After vac. | 52,41 ± 0,84 | 45,30 | 58,50 | |
| SCP perifovea | Before vac. | 51,48 ± 0,49 | 46,80 | 55,40 | 0,232 |
| vascular density value (%) | After vac. | 50,84 ± 0,55 | 47,60 | 55,40 | |
| DCP wholeimage vascular | Before vac. | 55,13 ± 1.12 | 48,00 | 63,70 | 0,296 |
| density value (%) | After vac. | 54,09 ± 0,86 | 46,30 | 60,90 | |
| DCP foveal vascular | Before vac. | 38,34 ± 1.74 | 26,60 | 51,30 | 0,695 |
| density value (%) | After vac. | 38,02 ± 1.64 | 26,10 | 51,70 | |
| DCP parafovea | Before vac. | 58,11 ± 0,76 | 51,70 | 63,00 | 0,225 |
| vascular density value (%) | After vac. | 57,37 ± 0,60 | 52,60 | 63,10 | |
| DCP perifovea | Before vac. | 56,83 ± 1.14 | 49,80 | 65,70 | 0,198 |
| vascular density value (%) | After vac. | 55,54 ± 0,95 | 46,20 | 63,30 |
S.D: Standarddeviation Min: Minimum Max: Maximum SCP: Superficial capillary plexus DCP: Deep capillary plexus Vac: Vaccination.
Table 4.
Comparison results of foveal avascular zone, choriocapillary blood flow, subfoveal choroidal thickness and total retinal thickness over time.
| Group | Mean | Min. | Max. | P | |
|---|---|---|---|---|---|
| Foveal avascular zone (mm2) | Before vac. | 0,27 ± 0.02 | 0,08 | 0,42 | 0,191 |
| After vac. | 0,28 ± 0.02 | 0,10 | 0,42 | ||
| Choriocapillaris blood flow (mm2) | Before vac. | 2,10 ± 0.02 | 1,90 | 2,25 | 0,502 |
| After vac. | 2,11 ± 0.02 | 1,94 | 2,31 | ||
| Subfovealchoroid thickness (μm) | Before vac. | 318 ± 10,83 | 207 | 411 | 0,08 |
| After vac. | 344 ± 8.46 | 298 | 426 | ||
| Total retina fovea thickness (μm) | Before vac. | 246,95 ± ± 4,79 | 214 | 282 | 0,241 |
| After vac. | 246,16 ± 4,57 | 213 | 284 | ||
| Total retina parafovea thickness (μm) | Before vac. | 316 ± 3.89 | 287 | 351 | 0,354 |
| After vac. | 314,37 ± 3.50 | 292 | 341 | ||
| Total retina perifovea thickness (μm) | Before vac. | 290,42 ± 2.41 | 276 | 317 | 0,968 |
| After vac. | 290,79 ± 2.14 | 279 | 312 |
S.D.: Standarddeviation Min: Minimum Max: Maximum Vac: Vaccination.
Table 5.
Comparison results of optic disc total vessel vascular density values and retinal nerve fiber layer over time.
| Group | Mean | Min. | Max. | P | |
|---|---|---|---|---|---|
| Optic disc whole image | Before vac. | 56,21 ± 0,44 | 53,10 | 60,80 | 0,513 |
| vascular density value (%) | After vac. | 56,03 ± 0,41 | 51,80 | 59,30 | |
| Optic discinside vascular density value (%) | Before vac. | 60,61 ± 0,76 | 53,2 | 66,50 | 0,601 |
| After vac. | 60,08 ± 0,77 | 52,50 | 64,60 | ||
| Peripapillary vascular density value (%) | Before vac. | 58,34 ± 0,47 | 54 | 62,40 | 0,455 |
| After vac. | 58,14 ± 0,48 | 52,70 | 61,20 | ||
| Peripapilary retinal nerve fiber layer (μm) | Before vac. | 115,53 ± 2.40 | 98 | 136 | 0,529 |
| After vac. | 115,42 ± 2.39 | 97 | 138 |
S.D: Standard deviation Min: Minimum Max: Maximum Vac:Vaccination.
When the tables above are examined, there are comparisons of superficial capillary plexus (SCP), deep capillary plexus (DCP) and optic disc vascular density values, FAZ, CBF, subfoveal choroidal thickness and retinal thickness variables at 2 different time points. The table includes the number of the patients, mean, standard deviation and minimum-maximum values for these variables. No statistically significant difference was found in the comparisons of these data before and after vaccination. However, after the vaccination,the values of superficial capillary plexus, deep capillary plexus and optik disc all vessels vascular densities were lower than the values before vaccination. Neverthless, this difference between the values was not statistically significant.
4. Discussion
COVID-19 is a systemic disease that might affect the entire body. Its general symptoms are fever, cough, fatigue, headache, sore throat, loss of taste, loss of smell, and low back pain [3,10]. In this disease, eye findings also draw attention, sometimes even appearing as the first finding. Eye watering, itching, foreign body sensation, double vision, blurred vision, limitation of eye movements, conjunctivitis, Guillain–Barré syndrome, chemosis, lagophthalmia and ischemic optic neuropathy are the reported ocular findings of COVID-19 infection [5,[11], [12], [13]].
The etiopathogenesis of eye findings seen in COVID-19 is not comprehensively understood. Considering the pathogenesis of eye findings such as eye watering, itching, foreign body sensation and conjunctivitis, it is thought that the ocular surface is used as the entry point to the body and the virus achieves this by using ACE-2 receptors [14]. Peripheral nerve damage, diplopia, and demyelination and inflammation are held responsible for visual impairment [15]. It has been suggested that an autoimmune mechanism against Schwann cells and myelin antigens plays a role in Guillain–Barré syndrome [16].
COVID-19 has caused a worldwide deadly pandemic. It is believed that the vaccination is a breakaway from this pandemic. Vaccines used against coronavirus were produced using different techniques [17]. CoronaVac is a vaccine produced by the inactivated virus method. Inactivated virus vaccines are based on a live virus that has been killed or inactivated and therefore cannot cause clinical disease. Inactivated virus vaccines are usually made by exposing a virulent virus to chemical or physical agents (such as formalin or β-propiolactone) to destroy infectivity while maintaining immunogenicity. Their major disadvantage is that there may not be an adequate antibody response. These live attenuated vaccines are used to protect against mumps, measles, rubella and chickenpox. It is not known for certain whether these vaccines will also need a booster vaccination. In recent studies, it has been suggested that the booster shot is necessary [18].
CoronaVac has been mainly used in China, Indonesia, Brazil and Turkey. This is a dead (chemically inactivated) virus vaccine. It can be distinguished from other vaccines like those on the market from J&J and AstraZeneca which are vector vaccines, where a harmless virus is used to transport the material that will create the important SARS-Cov-2 protein in the cell, and from artificial protein vaccines like the Russian Sputnik V, and the well known mRNA vaccines like those from Moderna and Pfizer-Biontech. CoronaVac 600 SU / 0.5 ml (Sinovac Life Sciences, Beijing, China) is used as a vaccine in Turkey after it has been approved for emergency use. The vaccine is administered intramuscularly in 2 doses with an interval of 28 days [19].
Adverse effects seen after vaccination include swelling at the injection site, rash, itching, fever, headache, myalgia, fatigue, cough, diarrhea, nausea and vomiting, shortness of breath, joint pain, fainting, bleeding gums, urticaria, loss of taste, xerostomia, anaphylactic reaction, itchy and swollen lymph nodes [17], [18], [19].
In the study of Naharci and Tasci, an 88-year-old female patient developed blurred vision and delirium one day after the CoronaVac vaccine [20]. In the study of Leber et al., bilateral optic neuritis was found after vaccination in a patient who had CoronaVac vaccine [21].In another study, anterior uveitis was found in 2 eyes of a patient 5 days after receiving the inactivated coronavirus vaccine [22].
In the review by Pichi et al., episcleritis in 1 patient, anterior scleritis in 2 patients, acute macular neuroretinopathy in 2 patients, paracentral acute middle maculopathy in 1 patient, and subretinal fluid in 1 patient after inactivated coronavirus vaccine were reported [23].
Riad et al. in their study on 780 people in Turkey, they investigated the side effects seen within 30 days after vaccination. Out of the 780 included participants, 487 (62.5%) participants reported that they suffered from at least one side effect after receiving the vaccine. 49.3% of the side effects were observed in the first 24 h, and 9% after the first week [24]. Das et al. in his compilation study, he suggested that significant ocular side effects (allergic reactions) occur within the first 7 days after the vaccine [25].
The etiology of the eye side effects of the CoronaVac vaccine is not yet clearly known. It is considered that these side effects may be caused by a component of the vaccine, a hypersensitivity reaction, or autoimmunity. Another suggested possibility is that the spike antigen, human adenovirus or other viral antigens might be responsible for these side effects. [26], [27], [28], [29].
Various OCTA studies have been conducted to explain the etiopathogenesis of ocular findings in COVID-19 infection. Cennoma et al., in their study, compared the OCTA parameters of 40 COVID-19 patients, taken 6 months after recovery, with 40 healthy individuals. Optic disc total vascular density, superficial capillary plexus total vascular density in the group with COVID-19 infection compared to the control group; deep capillary plexus total, parafovea and fovea vascular density values were found to be statistically decreased (p<0.001, p = 0.038, p = 0.029, p = 0.016, p = 0.027, respectively) [8].
Turker et al. compared the OCTA images taken 1 week after recovery of 27 patients who had COVID-19 with healthy individuals. Superficial capillary plexus and deep capillary plexus parafovea vascular density values were found to be statistically decreased in the group with COVID-19 disease compared to the control group (p < 0.05). In addition, in this study, CBF value in the patient group was found to be higher than in the control group (p = 0.042) [9].
Abrisami et al., in their study, compared the OCTA parameters of 30 patients who had COVID-19, taken 2 weeks after recovery, with 23 healthy individuals. Superficial capillary plexus and deep capillary plexus parafovea and fovea vascular density values were found to be statistically decreased in the group with COVID-19 (p < 0.05) [30].
Similar to the studies mentioned above, we compared the vascular density values of the patients before and after the vaccination in order to investigate the vascular etiopathogenesis of the side effects seen in CoronaVac vaccine.
In our study, the OCTA images of the patients were taken before and 1 week after the vaccine. No significant difference was found between vascular density values and retinal thickness values before and after vaccination. This demonstrates that CoronaVac vaccine has no side effects on retinal and optic disc vascular flow and retinal thickness.
Considering the limitations of this study, it can be stated that the number of patients is small. Further studies can be conducted with a higher number of patients. Another issue is that only one type of vaccine was examined in our study. In future studies, adding other vaccine groups and looking at vascular density values will contribute to the literature.
5. Conclusion
In conclusion, this is among the first studies in the literature to evaluate the changes in retinal and optic disc vascular values in people who received CoronaVac vaccine. In this study, we compared the posterior segment structures and vascular density parameters of people who received CoronaVac vaccine before and after vaccination. Although CoronaVac vaccine has many side effects, we observed that it did not change the retinal and optic disc vascular density values.
Funding
No funding was received for this research.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study. This article does not contain any studies with human participants performed by any of the authors.
CRediT authorship contribution statement
Birumut Gedik: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Yigit Caglar Bozdogan: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. Sibel Yavuz: Validation, Supervision, Software, Project administration, Investigation, Formal analysis, Data curation. Dogan Durmaz: Conceptualization, Data curation, Formal analysis, Project administration, Writing – original draft. Muhammet Kazim Erol: Writing – review & editing, Writing – original draft, Supervision, Project administration, Investigation, Formal analysis, Data curation, Conceptualization.
Declaration of Competing Interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers' bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
References
- 1.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu R., Zhao X., Li J., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gulati A., Pomeranz C., Qamar Z., et al. A comprehensive review of manifestations of novel coronaviruses in the context of deadly COVID-19 global pandemic. Am. J. Med. Sci. 2020;360(1):5–34. doi: 10.1016/j.amjms.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xia J., Tong J., Liu M., et al. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J. Med. Virol. 2020;92(6):589–594. doi: 10.1002/jmv.25725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertoli F., Veritti D., Danese C., et al. Ocular findings in COVID-19 patients: a review of direct manifestations and indirect effects on the eye. J. Ophthalmol. 2020;2020:9. doi: 10.1155/2020/4827304. Article ID 4827304pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Djanas D., Yusirwan Martini RD, et al. Survey data of COVID-19 vaccine side effects among hospital staff in a national referral hospital in Indonesia. Data Br. 2021;36 doi: 10.1016/j.dib.2021.107098. article 107098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yun C., Ahn J., Kim M., et al. Characteristics of retinal vessels in surgically closed macular hole: an optical coherence tomography angiography study. Graefe's Arch. Clin. Exp. Ophthalmol. 2017;255(10):1923–1934. doi: 10.1007/s00417-017-3742-6. [DOI] [PubMed] [Google Scholar]
- 8.Cennamo G., Reibaldi M., Montorio D., et al. Optical coherence tomography angiography features in post-COVID-19 pneumonia patients: a pilot study. Am. J. Ophthalmol. 2021;227:182–190. doi: 10.1016/j.ajo.2021.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turker I.C., Dogan C.U., Guven D., et al. Optical coherence tomography angiography findings in patients with COVID-19. Can. J. Ophthalmol. 2021;56(2):83–87. doi: 10.1016/j.jcjo.2020.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watts C.H., Vallance P., Whitty C.J.M. Coronavirus: global solutions to prevent a pandemic. Nature. 2020;578(7795):363. doi: 10.1038/d41586-020-00457-y. [DOI] [PubMed] [Google Scholar]
- 11.Wu P., Duan F., Luo C., et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei province, China. JAMA Ophthalmol. 2020;138(5):575–578. doi: 10.1001/jamaophthalmol.2020.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou L., Xu Z., Castiglione G.M., et al. ACE2 and TMPRSS2 are expressed on the human ocular surface, suggesting susceptibility to SARS-CoV-2 infection. Ocul. Surf. 2020;18(4):537–544. doi: 10.1016/j.jtos.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lusi M.E., Fernandes D.H., Mota C., et al. Eye and brain. A review of neuro-ophthalmological manifestations of human coronavirus infection. Eye Brain. 2020;12:129–137. doi: 10.2147/EB.S268828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mao L., Jin H., Wang M., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dinkin M., Gao V., Kahan J., et al. COVID-19 presenting with ophthalmoparesisfrom cranial nerve palsy. Neurology. 2020;95(5):221–223. doi: 10.1212/WNL.0000000000009700. [DOI] [PubMed] [Google Scholar]
- 16.Gutiérrez-Ortiz C., Méndez A., Rodrigo-Rey S., et al. Miller fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020;95(5):601–605. doi: 10.1212/wnl.0000000000009619. [DOI] [PubMed] [Google Scholar]
- 17.Pormohammad A., Zarei M., Ghorbani S., et al. Efficacy and safety of COVID-19 vaccines: a systematic review and meta-analysis of randomized clinical trials. Vaccines (Basel) 2021;9(5):467. doi: 10.3390/vaccines9050467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bogdanov G., Bogdanocv I., Kazandjieva J., Tsankov N. Cutaneous adverse effects of the available COVID-19 vaccines. Clin. Dermatol. 2021;20:38. doi: 10.1016/j.clindermatol.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Binay U., Karakecili F., Barkay O., et al. Level of SARS-CoV-2 IgG antibodies after two doses CoronaVac vaccine: primarily report. Res. Sq. 2021 doi: 10.21203/rs.3.rs-388073/v1. [DOI] [Google Scholar]
- 20.Naharci M.I., Tasci I. Delirium in a patient with Alzheimer's dementia following COVID-19 vaccination. Psychogeriatrics. 2021 doi: 10.1111/psyg.12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leber H.M., Sant'Ana L., Raio M.C., et al. Acute thyroiditis and bilateral optic neuritis following SARS-CoV-2 vaccination with CoronaVac: a case report. Ocul. Immunol. Inflamm. 2021 doi: 10.1080/09273948.2021.1961815. [DOI] [PubMed] [Google Scholar]
- 22.ElSheikh R.M., Haseeb A., Eleiwa T.H., et al. Acute uveitis following COVID-19 vaccination. Ocul. Immunol. Inflamm. 2021 doi: 10.1080/09273948.2021.1962917. [DOI] [PubMed] [Google Scholar]
- 23.Pichi F., Aljneibi S., Neri P., et al. Association of ocular adverse events with inactivated COVID-19 vaccination in patients in Abu Dhabi. JAMA Ophthalmol. 2021 doi: 10.1001/jamaophthalmol.2021.3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abanoub R., Sagıroglu D., Ustun B., et al. Prevalence and risk factors of CoronaVac side effects: an independent cross-sectional study among healthcare workers in Turkey. J. Clin. Med. 2021;10(12):2629. doi: 10.3390/jcm10122629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Das D., Meghana A., Paul P., Ghosh S. Are we ready for COVID-19 vaccines? – A general side effects overview. J. Curr. Med. Res. Opin. 2021;4(02):830–841. doi: 10.15520/jcmro.v4i02.398. [DOI] [Google Scholar]
- 26.Vojdani A., Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin. Immunol. 2020;217 doi: 10.1016/j.clim.2020.108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riad A., Pokorná A., Attia S., et al. Prevalence of COVID-19 vaccine side effects among healthcare workers in the Czech Republic. J. Clin. Med. 2021;10(7):1428. doi: 10.3390/jcm10071428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jampol L.M., Tauscher R., Schwarz H.P. COVID-19, COVID-19 vaccinations, and subsequent abnormalities in the retina: causation or coincidence? JAMA Ophthalmol. 2021 doi: 10.1001/jamaophthalmol.2021.3483. [DOI] [PubMed] [Google Scholar]
- 29.Chau C.Y.C., Chow L.L.W., Sridhar S., et al. Ophthalmological considerations for COVID-19 vaccination in patients with inflammatory eye diseases and autoimmune disorders. Ophthalmol Ther. 2021;10:201–209. doi: 10.1007/s40123-021-00338-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abrishami M., Emamverdian Z., Shoeibi N., et al. Optical coherence tomography angiography analysis of the retina in patients recovered from COVID-19: a case-control study. Can. J. Ophthalmol. 2021;56(1):24–30. doi: 10.1016/j.jcjo.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]