Abstract
Psychological trauma is highly prevalent among psychiatric disorders, however, the relationship between trauma, neurobiology and psychopathology is not yet fully understood. The cerebellum has been recognized as a crucial structure for cognition and emotion, however, it has been relatively ignored in the literature of psychological trauma, as it is not considered as part of the traditional fear neuro-circuitry. The aim of this review is to investigate how psychological trauma affects the cerebellum and to make conclusive remarks on whether the cerebellum forms part of the trauma-affected brain circuitry. A total of 267 unique records were screened and 39 studies were included in the review. Structural cerebellar alterations and aberrant cerebellar activity and connectivity in trauma-exposed individuals were consistently reported across studies. Early-onset of adverse experiences was associated with cerebellar alterations in trauma-exposed individuals. Several studies reported alterations in connectivity between the cerebellum and nodes of large-brain networks, which are implicated in several psychiatric disorders, including the default mode network, the salience network and the central executive network. Also, trauma-exposed individuals showed altered resting state and task based cerebellar connectivity with cortical and subcortical structures that are involved in emotion and fear regulation. Our preferred interpretation of the results is through the lens of the Universal Cerebellar Transform, the hypothesis that the cerebellum, given its homogeneous cytoarchitecture, performs a common computation for motor, cognitive and emotional functions. Therefore, trauma-induced alterations in this computation might set the ground for a variety of psychiatric symptoms.
Highlights
-
•
Structural and functional cerebellar alterations have been associated with exposure to early adverse experiences.
-
•
Altered cerebellar connectivity with nodes of macroscale brain networks, including the salience network, the default mode network and the central executive network, was associated with trauma-related psychiatric symptoms.
1. Introduction
Psychological trauma and post-traumatic stress disorder (PTSD) are highly prevalent among individuals with psychiatric disorders (Grubaugh et al., 2011). Exposure to trauma correlates with substance use, psychotic disorders and mood disorders, and is associated with adverse clinical functioning and increased healthcare burden (Grubaugh et al., 2011). Given that trauma exposure, even in the absence of a PTSD diagnosis, is associated with increased risk of suicidality and hopelessness, an impairing subthreshold PTSD syndrome has been suggested as a chronic condition that contributes to psychopathology (Brancu et al., 2016; Grubaugh et al., 2005). Exposure to adverse experiences, especially early in life, accounts for 54% of the population attributable risk (PAR) for depression (Dube et al., 2003b), 67% of the PAR for suicide attempts (Dube et al., 2003b) and 64% of the PAR for substance use disorders (Dube et al., 2003a). In other words, trauma-exposure is an important risk factor for mental health problems, however, the exact relationship between trauma, neurobiology and psychopathology is not yet fully understood.
The cerebellum has been recently recognized as a crucial structure for cognition (Sokolov et al., 2017), emotion (Adamaszek et al., 2017) and reward (Hull, 2020a; Sendhilnathan et al., 2020; Wagner et al., 2017), beyond its well-established role in sensorimotor learning (Shadmehr et al., 2010). Interestingly, a recent review showed that childhood maltreatment is associated with aberrant cerebellar connectivity with the amygdala and the hippocampus (Teicher et al., 2016), two regions that are traditionally linked to PTSD and fear-related disorders (Shin et al., 2006). Similarly, three model-free whole-brain meta-analyses have shown cerebellar alterations in individuals with PTSD (Hayes et al., 2012; Koch et al., 2016; Wang et al., 2016) and recent studies have suggested that the cerebellum participates in fear prediction and learning (Ernst et al., 2019; Lange et al., 2015a), as well as in the formation and storage of fear memories (Frontera et al., 2020). In addition, several studies have shown that individuals with PTSD show enhanced acquisition and resistance to extinction in eyeblink conditioning, a classical conditioning task that is associated with cerebellar function (Allen et al., 2019). Considering the extensive cerebellar connections with fear-related structures, such as the amygdala, hippocampus, hypothalamus, periaqueductal gray (PAG), raphe nuclei and prefrontal cortex (PFC)(Moreno-Rius, 2019), and the abundancy of stress-modulating endocannabinoid, glucocorticoid, and monoaminergic receptors within the cerebellum (Moreno-Rius, 2019), there is a growing interest in investigating its exact role in aversive and fear-related processes, as the latter are considered fundamental for the development of several psychiatric disorders (Wise and Dolan, 2020).
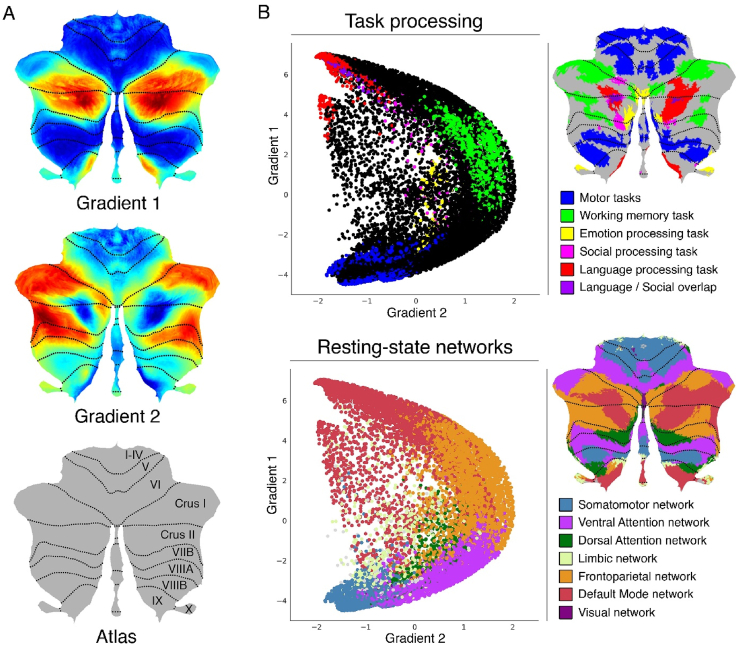
The cerebellum consists of a central region, the vermis, and two cerebellar hemispheres that are divided in 10 lobules (I-X), following an anteroposterior axis. Lobules I–V comprise the anterior lobe, lobules VI-IX the posterior lobe, and lobule X the flocculonodular lobe (Brodal, 2004). All cerebellar efferent and afferent signals pass through the cerebellar peduncles-the structures that connect the cerebellum with the brainstem. Cerebro-cerebellar communication is achieved through parallel loops of connectivity: regions of the cerebral cortex project to the same cerebellar areas from which they receive input (Middleton and Strick, 2001; Strick et al., 2009). Cerebellar activation patterns have been shown to be task-dependent, with a double motor representation in lobules I-VI(first representation) and lobule VIII(second representation) and a triple non-motor representation including lobules VI and Crus I (first representation), Crus II and VIIB (second representation) and lobules IX/X (third representation) (Buckner et al., 2011; Guell et al., 2018a; Schmahmann et al., 2019). More generally, functional topography in the cerebellum follows a gradual organization that progresses away from unimodal to transmodal streams of information processing (Guell et al., 2018c). Non-motor activation in the cerebellum includes activation during working memory (bilateral lobule VI, right lobule VIIB, right Crus I, left Crus II and lobule VIIB), emotional (left VI, left Crus I, bilateral Crus II vermis, bilateral X), social (bilateral VI, Crus I, Crus II, and language (right VI, Crus II, bilateral Crus I, bilateral IX) processing tasks (Guell et al., 2018c). These task-dependent activations were replicated by another study, which also showed that activation in Crus I/II was dominated by autobiographical recall (King et al., 2019). (Fig. 2)
Fig. 2.
Cerebellum gradients and relationship with discrete task activity maps (from Guell et al., 2018a) and resting-state maps (from Buckner et al., 2011). Gradient 1 extended from language task/DMN to motor regions. Gradient 2 isolated working memory/frontoparietal network areas. (A) Cerebellum flatmap atlas and gradients 1 and 2. (B) A scatterplot of the first two gradients. Each dot corresponds to a cerebellar voxel, position of each dot along x and y axis corresponds to position along Gradient 1 and Gradient 2 for that cerebellar voxel, and color of the dot corresponds to task activity (top) or resting-state network (bottom) associated with that particular voxel. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
A crucial function of the cerebellum is error-prediction, which is thought to be used for the update of internal forward models for the refinement of movements (Sokolov et al., 2017). More specifically, sensorimotor control is thought to occur on the following manner: When a movement is generated in the motor cortex, the cerebellum receives an efferent copy of the motor command and generates a representation of the expected sensory outcome of this motor command (Sokolov et al., 2017). This representation is then compared to the actual outcome and, if there is a mismatch between the two, a sensory-prediction error is generated and used to refine subsequent movements (Sokolov et al., 2017). Interestingly, the predictive function of the cerebellum has been suggested to extend beyond supervised error-based learning. More specifically, it has been shown that in some occasions, cerebellar learning meets the criteria described by models of reinforcement learning, highlighting its role in reward-driven behaviors (Hull, 2020b).
Given the relatively homogeneous cytoarchitecture of the cerebellum, its predictive function is thought to be extended to the cognitive and emotional domains and it is hypothesized that it relies on the same computation, the Universal Cerebellar Transform (UCT)(Schmahmann et al., 2019). According to this hypothesis, the heterogeneity of cerebellar functions relies upon its extensive connections with several brain regions, including multiple cerebral cortical regions, as well as the basal ganglia, amygdala, hippocampus, hypothalamus, PAG and nucleus accumbens, among others (Moreno-Rius, 2019; Schmahmann et al., 2019). Alternatively, according to the “multiple functionality” hypothesis, the same cerebellar circuit might be able to realize different computations and different cerebellar functions might depend on different computations carried out within the cerebellum (Diedrichsen et al., 2019). Both hypotheses are compatible with the idea that stress-induced alterations in the cerebellum might affect motor, cognitive and emotional domains and be involved in several types of psychopathology.
Cerebellar alterations have been found in a variety of psychiatric disorders, including psychotic disorders (Brady et al., 2019), mood disorders (Lupo et al., 2019) and addiction (Miquel et al., 2016) and emerged as the most important predictive feature of general psychopathology in adolescents (Moberget et al., 2019). Recent research suggests a substantial overlap in neural signatures of common mental disorders, pointing towards a shared risk for psychopathology (Vanes and Dolan, 2021). For this reason, during the past years, there has been a progressive shift towards a dimensional approach of psychiatric disorders, across diagnostic boundaries (Insel et al., 2010). Interestingly, structural alterations in the cerebellum have been associated with a general liability for common mental disorders (Romer et al., 2018), although the exact mechanism of cerebellar involvement in psychiatric disorders is yet to be discovered.
Taken together, trauma-exposure is a common risk factor among different psychiatric disorders, however, the interaction between trauma, neurobiology and mental health is not yet fully understood. Although there is extensive evidence that the cerebellum is involved in trauma and trauma-related psychopathology, it has been relatively ignored in literature, given that it is not considered part of the traditional fear/aversion neuro-circuitry. Clarifying the way trauma-exposure affects cerebellar function is crucial for a better understanding of trauma-related psychopathology. To this end, the aim of this systematic review is to explore the relationship between trauma-exposure and cerebellar structure and function and to summarize the evidence that supports the inclusion of the cerebellum as part of the trauma-affected circuitry in future studies.
2. Methods
Data for this systematic review were collected according to the PRISMA guidelines (Moher et al., 2009) and have been registered under the number CRD42021253687 at PROSPERO International prospective register of systematic reviews.
2.1. Search strategy
We performed electronic searches using PubMed (1998- March 2021), Science Direct (1997- March 2021) and Scopus (1989-March 2021) databases. A combination of the following keywords was used: ("PTSD" OR "post-traumatic stress disorder" OR "psychological trauma" OR "posttraumatic stress disorder" OR "moral injury" OR "trauma-exposure" OR "maltreatment") AND ("cerebellum" OR "cerebellar"). All studies that were published in English up to March 2021 were included. In addition, the bibliography of included studies was revised in order to add studies that might have been relevant but did not appear in the searches.
2.2. Selection criteria
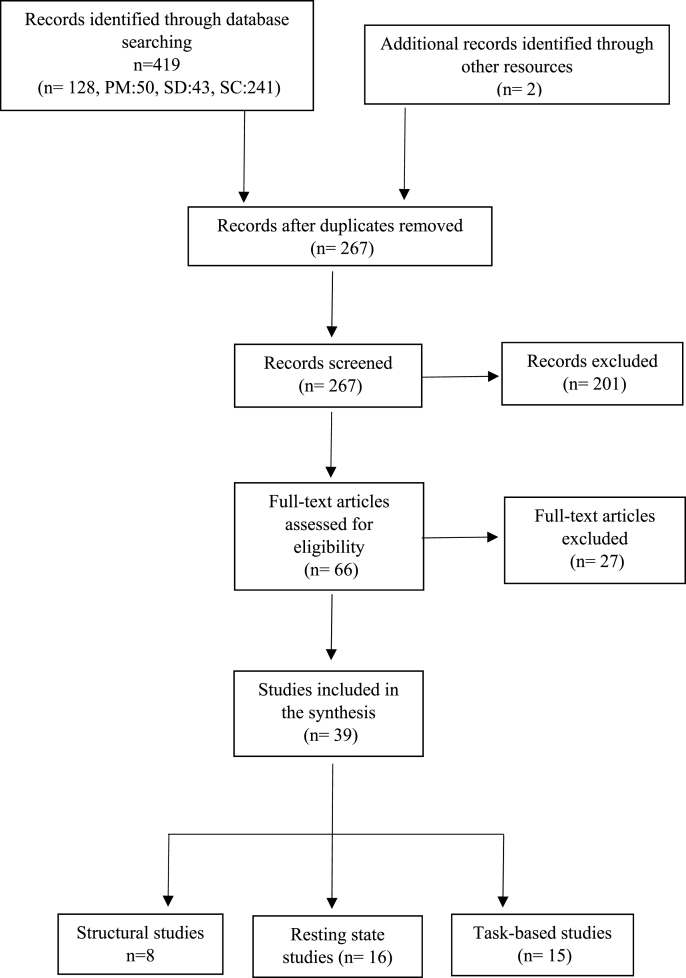
An initial screening of all studies that appeared in the search was carried out. We obtained a total of 267 papers (Fig. 1). Studies that met all of the following criteria were included in the analysis: 1) neuroimaging studies that included the cerebellum in the analysis, 2) studies that investigated effects of trauma exposure in individuals 18 ≥ years old, with or without a PTSD diagnosis, 3) studies that included a comparison group (either a healthy control group or comparing trauma-exposed individuals with PTSD versus trauma-exposed individuals without PTSD). Longitudinal studies with a with-in subject design were also included. Studies that met at least one of the following criteria were excluded from the analysis: 1) animal studies, 2) studies with participants with traumatic brain injury, 3) studies that included participants with severe psychopathology like schizophrenia or bipolar disorder. A total of 39 studies met our inclusion criteria and were included in the review.
Fig. 1.
Flow diagram of included studies. PM: PubMed, SC: Scopus, SD: Science Direct.
2.3. Data extraction
Three reviewers extracted data independently and decisions of inclusion or exclusion were made based on consensus. The following data were extracted from studies that passed the initial screening: authors, year of publication, imaging type and design, type of task in task-based studies, sociodemographic data (sample size, gender and age), trauma characteristics (type of trauma, time window since trauma exposure), psychiatric comorbidities and substance use. For structural and functional imaging data the measures of interest were global and regional volume and global and regional activity.
3. Results
3.1. Structural neuroimaging studies
We identified eight studies that examined structural brain abnormalities in the cerebellum of trauma-exposed individuals with or without PTSD and healthy controls and met our selection criteria (Table 1). For the MRI structural acquisition, 4 of the studies were acquired with 1.5T magnetic resonance (MR) machines, while the other 4 were acquired with 3T MR machines and most of the studies were acquired with isometric voxel sizes of 1 mm. Seven of the studies used structural magnetic resonance imaging (MRI) and one used [11C]NOP-1A positron emission tomography (PET). In total, five studies reported alterations in cerebellar volume or gray matter density (GMD) in individuals with PTSD in comparison to controls (Baldaçara et al., 2011; Holmes et al., 2018; Sui et al., 2010; Sussman et al., 2016; Zhang et al., 2016a) and one study identified differences between groups in nociceptin receptor density in the cerebellum (Narendran et al., 2019). Two studies described no significant between-group differences in the cerebellum (Fennema-Notestine et al., 2003; Levitt et al., 2006).
Table 1.
Structural Neuroimaging studies. PTSD: Post-traumatic stress disorder, TE: Trauma-exposed, HC: Healthy controls, M/F: Male/Female, SD: standard deviation, MRI: Magnetic Resonance Imaging, ND: Not described, MDD: Major depressive disorder, GMD: Gray matter density, AUD: Alcohol Use Disorder, CAPS: Clinician administered PTSD scale, GMV: Gray matter volume, V<SUB>T</SUB>: [11C]NOP-1A total distribution volume.
| Study | Method | PTSD(M/F) | TE (M/F) | HC (M/F) |
Age Mean(SD) |
Trauma type | Trauma wind. Months(SD) | Substance Use | Psych. Com. | Results |
|---|---|---|---|---|---|---|---|---|---|---|
| Fennema-Notestine et al. (2003) | MRI | 11(0/11) | 11(0/11) | 17(0/17) | 33.5(10.3)/35.4(9.6)/35.3(12.5) | Intimate partner violence | ND | No more than 2 years | Several | No differences |
| Levitt et al. (2006) | MRI | 24(24/0) | 24(24/0) | 48(48/0) | 52.5(3.5)/51.7(2.3)/51.8(2.3) | Combat trauma | ND | Yes | MDD | No differences in cerebellar vermis (monozygotic twins sample) |
| Sui et al. (2010) | MRI | 13(0/ 13) |
– | 13(0/ 13) |
24.46(5.77)/26(3.99) | Rape | 54.31(59.79) | ND | ND | PTSD > HC GMD in the left cerebellum |
| Baldaçara et al. (2011) | MRI | 42(15/27) | 42(10/32) | – | 34.9(ND)/38.3(ND) | Several | ND | 1/42 AUD | 34/42 MDD, several | PTSD < HC left cerebellar hemisphere and vermis. Negative correl. with CAPS scores |
| Sussman et al.(2016) | MRI | 23(23/0) | 24(24/0) | – | 37(7)/33(4) | Combat | ND | ND | 16/23 MDD, 4/23 anxiety |
PTSD > TE relative volume of cerebellar lobules VIIB, VIIIA and VIIIB (relative to total cerebellar volume) |
| Zhang et al., 2016a, Zhang et al., 2016b | MRI | 17(5/ 12) |
20(9/ 11) |
20(8/ 12) |
44.41(8.44)/40.35(9.43)/42.52( 7.89) |
Earthquake | 24 | ND | ND | Altered GMV in the right cerebellum in PTSD and TE |
| Holmes et al. (2018) | MRI | 21(10/11) | – | 18(12/6) | 36(8)/35(13) | Several | 213.6(120) | No | 16/21 MDD | PTSD < HC right cerebellar Crus |
| Narendran et al. (2019) | [11C]NOP-1A PET | 9(0/9) | 9(0/9) | 18 (0/18) |
22(2)/22(2) | Rape | 36(22) | No | No | VT in the cerebellum correlate with PTSD symptom severity |
More specifically, Baldaçara et al. showed that individuals with PTSD showed a significantly reduced brain volume and reduced cerebellar left hemisphere and vermal volume in comparison to trauma-exposed healthy controls. In addition, a negative correlation was observed between vermal volume and severity of PTSD symptomatology, as well as with depressive/anxiety symptoms and early traumatic life events (Baldaçara et al., 2011). Individuals with PTSD reported significantly more early traumatic events compared to resilient controls (Baldaçara et al., 2011). Similarly, decreased right cerebellar Crus volume was reported by Holmes et al. in a sample of individuals with PTSD, in comparison to healthy controls (Holmes et al., 2018). Moreover, in order to find inter-regional connections on a group basis, the aforementioned cerebellum region was used as seed to perform an anatomical covariance analysis, where the PTSD group showed significantly lower covariance between the cerebellum and the superior temporal sulcus/middle temporal gyrus in comparison to the control group (Holmes et al., 2018).
Sui at al. performed a Voxel-Based Morphometry (VBM) between female rape survivors with PTSD and healthy controls and found that the PTSD group showed increased left cerebellar GMD in comparison to healthy controls, specifically in the vermal lobules VI, VIII, IX (Sui et al., 2010). Similarly, in another study, combat exposed male soldiers with PTSD showed increased cerebellar lobules VIIB, VIIIA and VIIIB relative to total cerebellar volume, while no similar enlargement was found in combat exposed healthy soldiers. Interestingly, individuals with PTSD also showed a decreased cortical thickness in comparison to controls as well as a lower IQ score (Sussman et al., 2016). Zhang et al. applied a multimodal approach using both gray matter volume and amplitude low-frequency fluctuations (ALFF) from the resting state data to train a Support Vector Machine (SVM) classifier that was capable to distinguish between trauma-exposed individuals and healthy controls. To avoid overfitting during the SVM training, they selected the most discriminative features and found that the cerebellum gray matter volume was among the most relevant features reaching accuracies of 90% (Zhang et al., 2016b). On a different note, Naredran et al. revealed that, in a sample of survivors of sexual violence with or without PTSD, nociceptin receptor density in the midbrain and the cerebellum correlated with PTSD symptom severity (Narendran et al., 2019). Finally, Femenna-Notestine et al. did not find differences between survivors of intimate partner violence (IPV) with or without PTSD and matched controls in any manually-segmented cerebellar measures (Fennema-Notestine et al., 2003). Similarly, Levitt et al. in a 1.5T dataset of monozygotic twin subjects, found no significant differences in cerebellar vermis volume between combat exposed twins vs healthy twins, while there was a significant correlation in vermis volumes between twins (Levitt et al., 2006).
Taken together, five of the eight studies identified in the search observed structural differences on the cerebellum of trauma-exposed individuals with or without PTSD in comparison to healthy controls and one study identified differences in relative cerebellar volumes between trauma exposed individuals with PTSD compared to trauma-exposed individuals without PTSD. These differences include changes in gray matter volume, as well as in nociceptin receptor density in several anatomical areas of the cerebellum. For the MRI acquisitions, all the studies acquired with 3T MR machines obtained significant differences in structural measures for the cerebellum while the 1.5T acquisitions found structural differences in 2 of the 4 selected works.
3.2. Functional neuroimaging studies
3.2.1. Resting state
We included sixteen studies that examined resting state functional magnetic resonance imaging (fMRI) acquisitions, single photon emission computed tomography (SPECT) and fluorodeoxy-glucose positron emission tomography (FDG-PET) in trauma-exposed individuals with and/or without PTSD and/or healthy controls (Table 2). All studies were acquired using 3T field and voxel sizes ranged between 2 mm and 4.75 mm. Fourteen studies identified altered functional connectivity (FC) of the cerebellum in trauma-exposed individuals with or without PTSD. Two studies reported that FC between the cerebellum and the extended amygdala after acute trauma predicted later development of PTSD symptoms.
Table 2.
Resting state functional neuroimaging studies. PTSD: Post-traumatic stress disorder, TE: Trauma-exposed, HC: Healthy controls, M/F: Male/Female, SD: standard deviation, fMRI: functional Magnetic Resonance Imaging, ND: Not described, SPECT: Single-photon emission computed tomography, FDG-PET: fluorodeoxyglucose (FDG)-positron emission tomography, ALFF: amplitude of low frequency fluctuation,MDD: Major depressive, *: PTSD + Dissociation, FC: Functional connectivity, BNST: Bed nucleus of the stria terminalis, , PAG: Periaqueductal Gray, ICD: Intrinsic connectivity distribution, dlPFC: dorsolateral prefrontal cortex, mPFC: medial prefrontal cortex, FFG: Fusiform Gyrus, dACC: dorsal anterior cingulate cortex, SN: Salience network (consisting of bilateral posterior insula, supramarginal gyrus, precuneus and cerebellar lobules V,VI).
| Study | Method | PTSD(M/F) | TE (M/F) | HC (M/F) |
Age Mean(SD) |
Trauma type | Trauma window Months(SD) | Substance Use Dis. | Psych. Com. | Results |
|---|---|---|---|---|---|---|---|---|---|---|
| Bonne et al. (2003) | SPECT | 11(4/7) | 17(8/9) | 11(5/6) | 34(9)/35(8)/ 33(12 |
Motor vehicle accident | 6.9(0.38) | No | No | PTSD > HC and PTSD > TE cerebral perfusion in bilateral cerebellum. Correlation with depressive symptoms. |
| Molina et al. (2010) | FDG-PET | 16(16/ 0) |
6(6/0) | – | 39.6(0.19)/39.5(0.34) | Combat | 240 | ND | No | PTSD > TE activity in the left cerebellum |
| Yin et al. (2011) | fMRI | 54(ND) | 72(ND) | – | <60 | Earthquake | 9–15 | ND | No | PTSD < TE ALFF in posterior cerebellum |
| Bing et al. (2013) | fMRI | 20(13/ 7) |
– | 20(14/ 6) |
32.92(8.48)/31.53(7.43) | Motor vehicle accident | 7.2(1.6) | ND | 3/20 MDD | PTSD > HC ALFF in the right cerebellum |
| Nicholson et al. (2015) | fMRI | 36(10/26) | 13(2/ 11)* |
40(11/ 29) |
37(12.9)/37(12.7)/32.3(11.4) | ND | ND | Not 6 months prior | 2/36 and 4/13 depression/anxiety disorders | PTSD + DS > PTSD-DS FC between the amygdala and the left culmen of the cerebellum |
| Insana et al. (2016) | FDG-PET | 30/41 | 41(32/ 9)1 |
– | 29.31(6.09) | Combat and childhood maltreatment | ND | Not 3 months prior | No current | Childhood maltreatment correlated with activation in bilateral vermis and negative associated with the left central lobule and right posterior cerebellum |
| Zhang et al., 2016a, Zhang et al., 2016b | fMRI | 17(5/ 12) |
20(9/ 11) |
20(8/ 12) |
44.41(8.44)/40.35(9.43)/42.52( 7.89) |
Earthquake | 24 | ND | ND | Altered ALFF in bilateral cerebellum in TE vs HC |
| Rabellino et al. (2018a) | fMRI | 70(25/ 45) |
41(8/ 33)* |
50(16/ 34) |
37.83(11.7)/41.12(13.34)/35.2(11.6) | ND | ND | Not 6 months prior | Several | Pos. Correlation between dissociation and FC in the BNST and the left lobules VI-VII |
| Thome et al. (2017) | fMRI | 57(39/ 18) |
– | 41(15/ 26) |
33.98(11.57)/37.11(12.80) | ND | ND | In remission | Several | PTSD > HC FC between PAG and the right cerebellar vermis |
| Holmes et al. (2018) | fMRI | 21(10/ 11) |
– | 18(12/ 6) |
36(8)/35(13) | Several | 213.6(120) | No | 16/21 MDD | PTSD < HC ICD in the right and left dlPFC, left mPFC, and the right cerebellar Crus. Negative correlation with PTSD symptoms. PTSD > HC connectivity between the cerebellum and the right supramarginal gyrus. |
| Rabellino et al., 2018a, Rabellino et al., 2018b | fMRI | 65(25/ 40) |
37(8/ 29)* |
47(15/ 32) |
37.58(11.75)/40.38(13.69)/33.81(11.8) | ND | ND | Not 6 months prior | Several | PTSD > HC between left IV-V + right FFG + hippocampus and between right IV-V+ right posterior insula and planum polare. PTSD < HC FC between left Crus I + frontal gyrus. |
| Chen et al. (2019) | fMRI | 27(7/ 20) |
33(7/ 26) |
30(7/ 23) |
48.4(10.3)/48.5(7.5)/49.9(6.1) | Typhoon | 5–7 | No | Depression and anxiety | PTSD + TE < HC FC between dACC and left cerebellum |
| Belleau et al. (2020) | fMRI | – | 54(19/ 35) |
– | 33.22(11.55) | Several | 2 weeks and 6 months post-trauma | Yes | Several | Negative FC between right amygdala and right cerebellum predicted later development of PTSD symptoms |
| Nicholson et al. (2020) | fMRI | 81(35/ 46) |
49(11/ 38)* |
56(20/ 36) |
39(11.79)/40(13.52)/34(11.98) | ND | ND | In remission | Several | PTSD > HC FC between posterior SN and posterior insula and cuneus/precuneus PTSD < HC FC between SN and right dlPFC and right supramarginal gyrus in PTSD |
| Park et al. (2020) | fMRI | – | 31 (31/0) | 26 (26/0) |
49.8(4.7)/65.3(7.8) | Firefighters | ND | No | No | TE > HC FC between left superior parietal gyrus and right lobules IV, V, VIII, left parahippocampal gyrus, and FFG and between left inferior temporal gyrus and the right lobules VI and IX. (all uncorrected) |
| Suo et al. (2020) | fMRI | – | – | 122(36/86) | – | Earthquake | 10–15 months | No | No | FC between occipital lobe -cerebellum and limbic cortical structures– cerebellum predicted PTSD symptoms |
More specifically, Bonne et al. in a SPECT study showed that the PTSD group exhibited increased cerebral perfusion in large confluent regions of the cerebellum bilaterally, in comparison to both trauma-exposed individuals and healthy controls (Bonne et al., 2003). In addition, PTSD and depressive symptoms correlated with cerebellar perfusion (Bonne et al., 2003). Similarly, Molina et al., in an FDG-PET study, observed increased cerebellar glucose absorption in a group of war veterans with PTSD compared to combat-exposed healthy controls (Molina et al., 2010). On the same line, Insana et al. using FDG-PET data registered to an MR acquisition, showed that childhood maltreatment was positively associated with activation in the cerebellar vermis and negatively associated with activation in the left central lobule and the right posterior cerebellum in a sample of war veterans (Insana et al., 2016). Bing et al. reported increased ALFF values in the right cerebellum of individuals that had motor vehicle accidents and subsequent PTSD in comparison to healthy controls (Bing et al., 2013). The ALFF of the blood oxygen level dependent (BOLD) signal is associated with field potential activity in local brain regions and it measures intrinsic brain responses in resting state aquisitions (Logothetis et al., 2001). As mentioned in the structural section, Zhang et al. trained an SVM using structural and resting state fMRI data. In this paper, they showed that bilateral normalized ALFF measurement for the cerebellum was one of the main discriminative features for the classification of trauma-exposed individuals versus healthy controls (Zhang et al., 2016b).
On the same line, another study showed that subjects with PTSD exhibited greater FC between the periaqueductal gray (PAG) and the right cerebellar vermis, among other regions, in comparison to healthy controls (Thome et al., 2017). Rabellino et al., performed a seed-based FC analysis using the subparcellation of the cerebellum as a seed region of interest (ROI). In comparison with the ALFF, seed-based FC approaches focus on long-distance connectivity correlations. In this study, they found increased FC between the left cerebellar lobules IV-V and the right fusiform gyrus (FFG) and the hippocampus, as well as between the right cerebellar lobules IV-V and the right posterior insula and the planum polare in individuals with PTSD compared to healthy controls (Rabellino et al., 2018b). In addition, there was a positive correlation between state reliving symptoms in the PTSD sample and the FC between bilateral lobules IV-V and bilateral visual cortex, cuneus, lingual and FFG (Rabellino et al., 2018b). Park et al. in a pilot study, using the left superior parietal sulcus as a seed, showed that trauma-exposed firefighters without PTSD showed increased FC in the right cerebellar lobule VIII, left parahippocampal gyrus, right FFG, and right cerebellar lobules IV –V, in comparison to non-exposed healthy controls (uncorrected results)(Park et al., 2020). Seed ROIs were automatically obtained after performing a VBM between groups, and, using the left inferior temporal gyrus as seed it was shown that trauma-exposed firefighters exhibited increased FC in the right cerebellar lobules VI and IX (uncorrected results)(Park et al., 2020).
Nicholson et al., similar to what we have seen in Zhang et al. (2016b), trained a classifier using structural and functional MRI features capable to distinguish among PTSD, its dissociative subtype (PTSD + DS) and healthy controls according to their intrinsic connectivity networks obtained from an Independent Component Analysis (ICA). They showed that the cerebellum Crus I was one of the regions with the highest discriminative weights when classifying groups according to intrinsic connectivity in the Central Executive Network (CEN)(Nicholson et al., 2020). These results show convergent evidence with another study, using a seed-based approach, where they showed that individuals with PTSD + DS show increased amygdala-cerebellum FC, in comparison to individuals in the PTSD group. Interestingly, a positive correlation was observed, by another study, between dissociation and FC in the bed nucleus of the stria terminalis and the left cerebellar lobules VI-VII in PTSD + DS (Rabellino et al., 2018a).
On the other hand, Yin et al. compared earthquake survivors with and without PTSD and showed that the PTSD group exhibited decreased ALFF values in the cerebellum, among other areas, in comparison to the trauma exposed control group (Yin et al., 2011). Similarly, Holmes et al. reported that individuals with PTSD showed lower whole-brain connectivity in the right cerebellar Crus, dorsolateral prefrontal cortex (dlPFC) and medial prefrontal cortex as a result of a seed-based analysis (Holmes et al., 2018). In addition, a significant negative correlation was found between intrinsic connectivity distribution in the cerebellar vermis and PTSD symptomatology (Holmes et al., 2018). On the same line, a seed-based inter-regional FC analysis was conducted in Chen et al., between trauma-exposed individuals with or without PTSD that showed decreased dorsal anterior cingulate cortex (dACC) FC with the right hippocampus and the left cerebellum relative to healthy controls (Chen et al., 2019). In addition, decreased FC was found between left cerebellar Crus I and the frontal gyrus using the first ROI as seed, as well as a negative correlation between symptom severity and FC between cerebellar lobules IV-V and the postcentral gyrus, parietal operculum and supramarginal gyrus. Moreover, using the right Crus I region as a seed, a negative correlation was found between childhood trauma and connectivity with the right visual cortex, middle and superior occipital gyrus and the bilateral cuneus and precuneus within the PTSD sample (Rabellino et al., 2018b).
Finally, two studies showed that FC of the cerebellum after trauma predicted later development of PTSD: More specifically, Belleau et al. reported that more negative amygdala-cerebellum FC predicted greater PTSD symptom severity (Belleau et al., 2020). Likewise, Suo et al. showed that connections of the occipital lobe with the cerebellum and of limbic structures of the cerebral cortex with the cerebellum were primary predictors of PTSD symptom severity (Suo et al., 2020).
3.2.2. Task based fMRI
We identified fifteen studies that explored task-based fMRI cerebellar activation. We classified the tasks into three primary domains: trauma or threat-related tasks, standardized cognitive and emotional tasks and pain-induction paradigms (Table 3). Results are reported with respect to these domains. Most of the fMRI studies were acquired using 3T MR and voxel sizes ranged between 2 mm and 5 mm for the largest axis in anisometric voxel sizes.
Table 3.
Task-based functional neuroimaging. PTSD: Post-traumatic stress disorder, TE: Trauma-exposed, HC: Healthy controls, M/F: Male/Female, SD: standard deviation, fMRI: functional Magnetic Resonance Imaging, ND: Not described, CAPS: Clinician administered PTSD scale, CTQ: Childhood Trauma Questionnaire, BNST: Bed nucleus of the stria terminalis.
| Study | Method | Task | PTSD (M/F) |
TE (M/F) | HC (M/F) |
Age M(SD) |
Trauma type | Trauma wind Months(SD) | Subs. Use Dis. | Psych. Com. | Results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Strigo et al. (2010) | fMRI | Event-related experimental pain paradigm (brief temperature stimul.) | 31(0/31) | – | 20(0/20) | 35.9(8.5)/35.2(12.7) | Intimate partner violence | 30 days < event<5 years | Not within 30 days | Yes | PTSD > HC left cerebellum and PTSD < HC right cerebellum during temperature stimuli. |
| Diener et al. (2012) | fMRI | Painful mechanical stimulation before and after cognitive stressful task | 14(5/9) | 14(6/8) | – | 41(10.39) | 95.5(134.76)/ 90.57(94.8) |
ND | No | PTSD > TE pain threshold post-stress. Negative correl. with cerebellar activity | |
| Vidotto et al. (2014) | fMRI | Disgusting vs neutral pictures | 10(2/8) | – | 25(11/14) | 35.2(14.1)/27.5(9.3) | Earthquake | ND | ND | ND | PTSD > HC in the cerebellum during neutral images. |
| Ke et al.(2015) | fMRI | Trauma-related vs neutral pictures | 12(12/0) | 14(14/0) | – | 37.7(3.8) | Coal mining accident |
24(ND) | No | No | PTSD > TE in vermis. Pos. correl. with CAPS total scores. |
| Ke et al.(2015) | fMRI | Trauma-related vs neutral pictures | 13(13/0) Baseline and follow-up |
– | – | 36.7(4.5) | Coal mining accident |
2 and 24 months after |
No | No | Neg. correlation between cerebellum and reduction of PTSD symptoms |
| Rabellino et al. (2016) | fMRI | Subliminal and supraliminal threat-related vs neutral stimuli | 26(11/15) | – | 20(10/10) | 38.8(12.2)/32.5(11.6) | Childhood maltreat. | ND | Not in the past 6 months | Several, mainly depression | PTSD > HC in the right cerebellar lobule VI in the subliminal threat condition. Neg. Correl. avoidance symptoms-left lobule IV-V |
| Elman et al.(2018) | fMRI | Pleasant vs aversive vs neutral images + pain inducing heat | 12(5/7) | – | 12(6/6) | 38.9(11.9)/39.6(10.2) | ND | ND | Not recent | Only depression and anxiety | PTSD < HC in cerebellar Crus II and lobule VII and VII in rewarding vs neutral condition. PTSD > HC Crus I, Crus II, lobules IV,V, VI, VIII, and IX during pain inducing heat |
| Hall et al. (2018) | fMRI | Involuntary memory task | 21(6/15) | 21(7/14) | – | 21.48(3.34)/22.1(4.75) | Several | ND | ND | ND | Timing differences between the two groups in the cerebellum (among other regions) |
| Naegeli et al. (2018) | fMRI | 15 white noise bursts | 28(4/24) | – | 26(8/18) | 38.5(13.35)/40.35 (11.22) |
Several | 187(168) | No | Yes, mainly depression | PTSD > HC in cerebellar lobule VI during sound bursts |
| Quidé et al. (2018) | fMRI | n-back working memory, emotional go/no-go/mental imagery | 27(0/27) | – | 20(0/20) | 27.22(8.8)/ 28.3(10.07) |
Sexual assault | Within 4 weeks | No | Depression and anxiety | Neg. Correlation bilateral lobule VI,VIII,IX, Crus I and vermis VIII-IX for the happy vs neutral no-go and CAPS scores. Pos. correl. between left lobules VIII-IX, Crus I-II and right VII, vermis VIII-IX, Crus I and CAPS scores in positive vs neutral cond. Mental imagery task |
| Douglas et al. (2019) | fMRI | Trauma-related vs neutral scripts with or without hydrocortisone | 13(6/5) | 11(6/5) | – | 56(10.3)/53.9(9.9) | Earthquake | 46(7.9)/47.1 (10.3) |
No | No severe | No difference between PTSD vs HC. ↓in the left cerebellum in the trauma condition after hydrocortisone |
| Metz et al. (2019) | fMRI | Autobiographical memory task(AMT) with or without hydrocortisone |
19(0/19) | – | 37(0/37) | 31.68(9.04)/ 28.76(7.23) |
Childhood maltreat. | ND | Yes | Yes | No difference between groups in the task. Higher CTQ scores associated with ↑activity in the cerebellum in AMT in the hydrocortisone + condition |
| Terpou et al. (2019) | fMRI | Subliminal and supraliminal threat-related vs neutral stimuli | 26(11/15) | – | 20(10/10) | 38.8(12.2)/32.5(11.6) | Childhood maltreat. | ND | Not in the past 6 months | Several, mainly depression | HC > PTSD in right cerebellar lobule V in the subliminal threat condition. Neg. Correl. lobule V and depersonalization symptoms |
| Awasthi et al. (2020) | fMRI | Positive vs negative vs neutral words | 29(4/25) | – | 23(12/11) | 34.6(9.2)/28.7(7.6) | Sexual assault | 295 (range 12–504) | No | No | PTSD > HC FC between the BNST and cerebellar regions |
| Belleau et al. (2020) | fMRI | Script-driven trauma recall task | – | 54(19/ 35) |
– | 33.22(11.55) | Several | 2 weeks and 6 months post-trauma | Yes | Several | No relationship between FC of the cerebellum during task and later development of PTSD |
3.2.2.1. Trauma-related tasks
We identified nine studies that explored brain responses during presentation of trauma-related versus control stimuli in trauma-exposed individuals with or without PTSD and healthy controls. Awasthi et al. investigated FC between the bed nucleus of the stria terminalis (BNST) and other brain regions in individuals with PTSD and healthy controls during an emotional word paradigm with trauma related vs negative, neutral, and positive random distractors. Findings included increased task based FC between the BNST and cerebellar lobules IX-X, and other limbic and subcortical areas in PTSD patients, while healthy controls showed increased connectivity between the BNST and neocortical areas (Awasthi et al., 2020).
On the same line, Rabellino et al. aimed to investigate the neural correlates of the innate alarm circuit in individuals with PTSD compared to healthy controls, by presenting blocks of subliminal and supraliminal trauma-related or neutral stimuli (facial expressions and words) to participants undergoing fMRI. They found that PTSD patients showed higher activation in the right cerebellar lobule VI and in the right posterior cingulate cortex during subliminal processing of trauma-related words in comparison to healthy controls. In addition, avoidance symptoms in the PTSD group showed a negative correlation with the left cerebellar lobules IV-V, during subliminal trauma-related processing (Rabellino et al., 2016). Terpou et al. replicated this finding in a similar study, in which a negative correlation emerged between Depersonalization/Derealization scores in individuals with PTSD and activation in the right cerebellar lobule V during presentation of subliminal threat. However, in Terpou's study, individuals with PTSD exhibited decreased activation in cerebellar lobule V during subliminal threat presentation compared to controls (Terpou et al., 2019).
Ke et al. compared brain activity of witnesses of a coal mining accident who developed PTSD to those who did not develop PTSD during exposure to trauma-related and neutral pictures. Although no differences were found between groups in the trauma-related vs neutral condition, PTSD patients showed increased BOLD signal variability in the vermis compared to controls in both conditions and the latter correlated with symptom severity (Ke et al., 2015). In a subsequent study, the authors followed up 13 individuals of the PTSD group and found that brain response in the vermis and the left posterior cingulate/precuneus at baseline predicted PTSD symptoms at follow-up. Also, they showed that changes in the activation of the cerebellum were negatively correlated with reduction of the re-experiencing and hyperarousal symptoms (Ke et al., 2016).
Given that there is consensus in scientific literature that the Hypothalamic-Pituitary-Adrenal (HPA) axis is involved in the pathophysiology of PTSD (Dunlop and Wong, 2019), Douglas et al. examined the effect of acute hydrocortisone administration on brain activation in individuals with or without earthquake-related PTSD during traumatic versus neutral blocks in a double blind placebo controlled study. Although no differences were found between groups in the traumatic vs neutral condition, there was reduced regional blood flow in the left cerebellum (among other regions) after hydrocortisone administration during trauma-script presentation. However, these results did not survive correction for multiple comparisons (Douglas et al., 2019). Finally, although Belleau et al. found that amygdala-cerebellum resting state FC predicted later development of PTSD; as previously mentioned, they found no relationship between cerebellar activation during presentation of subject-specific trauma-related scripts and subsequent PTSD symptoms (Belleau et al., 2020).
3.2.2.2. Cognitive and emotional tasks
Three studies investigated neural correlates of standardized cognitive and emotional tasks in trauma-exposed individuals with and/or without PTSD and/or healthy controls. Vidotto et al. compared brain activation of earthquake survivors with PTSD to healthy controls during presentation of disgusting versus scrambled images and found that the PTSD group showed higher activation in the lateral cerebellum in the scrambled condition (Vidotto et al., 2014). Hall et al. found no differences in brain activation between trauma-exposed individuals with PTSD compared to trauma-exposed individuals without PTSD during an involuntary memory task, however, significant timing differences were found between groups. More specifically, the PTSD group exhibited delayed activity in the cerebellum in comparison to trauma-exposed controls, as part of a general delayed activity in the memory network during autobiographical memory recall in PTSD (Hall et al., 2018). Quidé et al. explored changes in cognition, emotional processing and brain activation in women in the early stages after sexual assault using a n-back working memory task, an emotional go/no-go task and a mental imagery task. No between group differences were identified in the n-back and the mental imagery task, however, in the emotional go/no-go task there was a negative correlation between symptom severity and bilateral activation in cerebellar lobules VI, Crus I VIII, IX, I, and vermal VIII-IX when positive content interfered with response inhibition and a positive correlation between symptom severity and cerebellar activation in lobules VIII, vermal VIII-IX and Crus I for the positive versus rest contrast of the mental imagery task (Quidé et al., 2018). Finally, another study that explored the neural correlates of autobiographical memory after hydrocortisone administration in a sample of PTSD patients versus healthy controls showed that although there were no differences between groups in neither of the two conditions, there was a significant positive correlation between childhood trauma exposure and hydrocortisone-induced cerebellar activation (Metz et al., 2019).
3.2.2.3. Stress-induced analgesia paradigms
We identified three studies that explored neural correlates of stress induced analgesia in trauma-exposed individuals with or without PTSD and/or healthy controls. Stress-induced analgesia is a documented pain-suppression response that occurs after aversive stimulation (Butler and Finn, 2009) and it has been shown to be altered in individuals with PTSD (Nishith et al., 2002). Strigo et al. used an event-related pain paradigm of thermal stimulation and showed that women with IPV-related PTSD showed attenuation of subjective pain intensity and differential brain activation compared to healthy controls, including increased activation in the left cerebellum and decreased activation in the right cerebellum, without specifying exact cerebellar regions (Strigo et al., 2010). Diener et al. investigated whether enhanced analgesia occurs in response to cognitive stressors in individuals with PTSD and found that the latter show significantly more analgesia compared to trauma-exposed individuals without PTSD, and that this decreased pain threshold correlates with decreased activity in the cerebellum, among other regions (Diener et al., 2012). Finally, Elman et al. presented pleasant, aversive and neutral images along with pain-inducing heat while individuals with PTSD and healthy controls were undergoing fMRI scans. Deactivation in several clusters including cerebellar Crus II, lobule VII and VIII was found in the PTSD group during presentation of rewarding versus neutral stimuli, while activation in several clusters including the cerebellum was observed when negative versus neutral images were presented in the PTSD group. Similarly, individuals with PTSD showed increased cerebellar activation in the, Crus I, Crus II and lobules IV, V, VI, VIII and IX compared to controls during pain-inducing heat (Elman et al., 2018).
4. Discussion
The aim of this systematic review was to examine the role of the cerebellum in trauma and trauma-related psychopathology. Despite the large heterogeneity among the reviewed studies, there were some consistent findings that allow us to draw several conclusions. Trauma exposure with or without PTSD has been associated with structural and functional alterations in the cerebellum and correlated with depression, anxiety and dissociation symptoms. This suggests that the cerebellum might be one of the key regions that are affected by adverse experiences, setting the ground for the development of several psychiatric symptoms.
4.1. Structural neuroimaging studies
Structural alterations in the cerebellum after trauma exposure were reported in six of the eight studies identified in the search, however, there was high variability in the types of alterations. Gray matter volume in the cerebellum was among the most discriminative features between trauma exposed individuals and healthy controls (Zhang et al., 2016b) and enlargement of relative cerebellar volume in lobules VII and VIII in contrast to decreased cerebral cortical thickness was reported in individuals with PTSD compared to controls (Sussman et al., 2016). Also, it was shown that individuals with PTSD exhibited increased GMD in the vermis and decreased GMD in the frontal cortex compared to healthy controls (Sui et al., 2010). Interestingly, a recent study reported that GMD is associated with neuroreceptor and neurotransmitter availability (Manninen et al., 2021), suggesting that alterations in GMD found in the cerebellum might be related to stress-induced regional alterations in neurotransmission. This hypothesis is in line with the results of Narendran et al., who showed that nociceptin receptor density in the cerebellum correlated with PTSD symptom severity (Narendran et al., 2019), and with a recent review that suggests that altered endocannabinoid neurotransmission might be a key feature of PTSD (Hill et al., 2018).
Two studies found volumetric reductions in the cerebellar hemispheres, vermis and lobule VII in individuals with PTSD compared to healthy and trauma-exposed controls. Importantly, in the second study, individuals with PTSD scored significantly higher in early adverse experiences compared to resilient controls, suggesting that a reduction in cerebellar volume might occur after early exposure to stressors. This is in line with several studies that found cerebellar reduction in maltreated children and adolescents (De Bellis and Kuchibhatla, 2006) and with a recent study that showed a significant reduction of gray matter volume in cerebellar lobules I-IV, V and VI in individuals that were exposed to early adversity (Van Dam et al., 2014). It has been suggested that cerebellar function might be particularly important during critical periods of development, regulating cortical circuitries related to the cognitive and social domains (Badura et al., 2018). Adverse experiences in different developmental periods might differentially affect subsequent cerebellar and whole-brain development (Badura et al., 2018). Most studies included in this review did not report on potential presence of early adverse experiences/age of potential exposure and differences in the former might partially explain inconsistent findings between studies. This highlights the necessity of consistent reporting of potential early exposure to adversity in future studies.
Finally, two studies found no structural differences between trauma-exposed vs non-exposed individuals (Fennema-Notestine et al., 2003; Levitt et al., 2006). Remarkably, one of those studies was carried out in monozygotic twins, discordant for combat exposure (Levitt et al., 2006). This suggests that cerebellar alterations might predispose individuals to trauma-related psychiatric symptoms and not vice versa. However, future studies are needed to clarify this matter. All in all, six studies reported structural alterations in the cerebellum in trauma-exposed individuals and these alterations might be related to adverse experiences early in life. Future studies should control for early exposure to adversity and report the time window between the realization of the study and trauma exposure.
4.2. Resting-state neuroimaging studies
Regarding effects of trauma-exposure on cerebellar function, sixteen studies explored cerebellar resting state activity in individuals with or without PTSD and/or healthy controls. Activity in bilateral cerebellum was among the most discriminative features that classified trauma-exposed individuals versus healthy controls (Zhang et al., 2016b). Three studies reported increased cerebellar resting state activity in individuals with PTSD compared to both trauma-exposed and healthy controls (Bing et al., 2013; Bonne et al., 2003; Molina et al., 2010), in line with a recent meta-analysis (Wang et al., 2016), and one study reported decreased cerebellar resting state activity in the PTSD group compared to trauma-exposed controls (Yin et al., 2011). Activity in the left central lobule and the right posterior cerebellum was negatively associated with childhood trauma in a sample of war veterans, highlighting again that exposure to childhood aversity might be a crucial factor when interpreting cerebellar alterations in trauma-related psychopathology.
Trauma-exposed individuals showed aberrant connectivity between the cerebellum and several other regions: For example, five studies identified functional alterations in connectivity between the cerebellum and nodes of large-scale brain networks, including the default mode network (DMN), the central executive network (CEN) and the salience network (SN). This is in line with recent results suggesting that cerebellar regions, including Crus I, Crus II, lobules VIIB, IX and X, are extensively connected with attention/executive networks, as well as the DMN(Guell et al., 2018c).
More specifically, individuals with PTSD showed reduced anticorrelation between central and peripheral nodes of the DMN, including the medial prefrontal cortex (PFC) and the cerebellum, and the right supramarginal gyrus (Holmes et al., 2018), a potential node of the frontoparietal CEN (Igelström and Graziano, 2017). The authors suggested that this reduced anticorrelation might reflect a “blurring” between these two normally opposed networks that represents a reduced capacity to switch between the internal and external world in PTSD, which in turn might lead to over-vigilance and generalized fear responses (Holmes et al., 2018). Alterations in the SN were also reported, with another study considering the cerebellar lobules V-VI together with the posterior insula and the precuneus as part of the posterior Salience Network (SN) and reporting that individuals with PTSD showed increased connectivity between the latter and the posterior insula, the cuneus and precuneus, while they exhibited decreased connectivity between the posterior SN and the dlPFC and the supramarginal gyrus (Nicholson et al., 2020). This is in line with a recent study which showed that decreased connectivity between cerebellar lobule VI and Crus I with the precuneus was associated with a decrease in clinical symptoms in a PTSD sample, after a trauma-focused intervention (Verger et al., 2020) and another study that showed that ALFF in the precuneus and the cerebellar Crus I, among other regions, predicted whether PTSD patients would be responsive to paroxetine treatment (Yuan et al., 2018) Also, decreased FC was reported between the cerebellum and the dorsal anterior cingulate cortex (dACC), another major player of the SN(Seeley et al., 2007), in trauma-exposed individuals with and without PTSD versus healthy controls (Chen et al., 2019).
All in all, these results are in line with recent reviews that propose network alterations as a key feature of PTSD, including alterations in the DMN, the CEN and the SN, as well as in the interaction between them (Akiki et al., 2017; Neria, 2021). More specifically, it has been suggested that an hyperactive SN destabilizes the interaction between the DMN and the CEN in PTSD (Akiki et al., 2017), which is in line with the increased posterior SN connectivity and alterations in connectivity of basic CEN and DMN nodes reported in the included studies. Remarkably, aberrant SN connectivity and subsequent alterations in the DMN and CEN have been proposed as a common feature of several psychiatric disorders, including depression, anxiety, schizophrenia and addiction (Menon, 2011; Zhang and Volkow, 2019). Here, SN overactivity might reflect constant monitoring of external and internal information and increased detection of salient/threatening stimuli. Given the well-established predictive function of the cerebellum, its role in trauma-related psychopathology might be related to an increased prediction of threatening outcomes after detection of, otherwise, neutral cues.
Finally, increased connectivity was reported between the right cerebellar vermis and the PAG(Thome et al., 2017) in individuals with PTSD compared to healthy controls. The PAG is involved in the central autonomic network and it has been associated with the initiation of fear-related behaviors (Tovote et al., 2016). Given that a recent study showed that cerebellar projections to the PAG control fear learning and memory (Frontera et al., 2020), the increased connectivity between the two regions might be related to a hyperarousal state that leads to increased fear learning in individuals with PTSD (Maddox et al., 2019).
4.3. Resting state neuroimaging studies and psychiatric symptoms
Interestingly, aberrant cerebellar activity was associated with several psychiatric symptoms. For example, intrinsic connectivity distribution in the cerebellum negatively correlated with all clusters of PTSD symptoms (Holmes et al., 2018). Also, FC between the somatosensory cerebellum and the primary visual cortex and visual processing and integration areas was associated with reliving symptoms, while FC between the somatosensory cerebellum and the primary somatosensory cortex and the supramarginal gyrus was negatively correlated with symptom severity (Rabellino et al., 2018b). These results are in line with a recent paper that suggested the cerebellar sensorimotor system as a crucial player for higher cognitive and emotional functions via the notion of embodied cognition (Guell et al., 2018b).
Remarkably, two studies reported that dissociation symptoms in individuals with PTSD were associated with increased FC between the extended amygdala and the cerebellar vermis (Nicholson et al., 2015; Rabellino et al., 2018a), while another study suggested that reduced FC between the cerebellum and the amygdala after trauma-exposure predicted later development of PTSD symptoms. This is in line with a recent review that suggested that signals from the sensorimotor cerebellum might be sent to the amygdala during aversive conditioning to suppress amygdala-dependent arousal, after the acquisition of the conditioned avoidance response (Lindquist, 2020).
4.4. Task-based neuroimaging studies
We identified fifteen studies that explored task-based fMRI activation. Tasks included presentation of trauma-related versus neutral stimuli, standardized cognitive and emotional tests and stress-induced analgesia tasks; results for each of these tasks are discussed in the sections that follow.
4.5. Trauma-related tasks
Regarding trauma-related tasks, two studies used subliminal presentation of trauma-related cues to investigate fast responses to salient threatening stimuli in childhood maltreatment survivors with PTSD versus healthy controls. Individuals with PTSD exhibited increased activation in the cerebellar lobule VI(Rabellino et al., 2016), posterior cingulate cortex (Rabellino et al., 2016), PAG(Terpou et al., 2019), superior colliculus (Terpou et al., 2019) and reticular formation (Terpou et al., 2019) during subliminal processing of trauma-related cues, while decreased activation was reported in the lobule V (Terpou et al., 2019). Also, in line with aforementioned results, a negative correlation was found between avoidance symptoms and activation in the sensorimotor cerebellum during subliminal threat processing (lobules IV-V)(Terpou et al., 2019), while increased activation in lobule VI was reported during PTSD symptom provocation and during presentation of trauma-related pictures respectively (Ke et al., 2015; Naegeli et al., 2018).
Increased activation in lobule VI during presentation of subliminal trauma-related cues together with regions involved in the innate alarm system suggests that nonmotor regions of the cerebellum might participate in early detection of potentially threatening stimuli. Interestingly, activation in lobule VI has been also associated with recall of traumatic autobiographic memories in a recent meta-analysis, suggesting that the same cerebellar circuitry might be involved in a variety of functions related to PTSD (Thome et al., 2020). On the other hand, the negative correlation between activation in cerebellar lobules IV-V and avoidance symptoms suggests, as mentioned previously, that the sensorimotor cerebellum might also play a role in PTSD, potentially through avoidance-induced regulation of fear-related regions such as the amygdala and the hippocampus, as has been recently suggested (Lindquist, 2020).
Finally, increased connectivity was reported in individuals with PTSD during presentation of trauma-related stimuli between the extended amygdala and the cerebellar lobules IX-X and other limbic and subcortical areas, while healthy controls showed increased connectivity between the extended amygdala and neocortical regulatory regions (Awasthi et al., 2020). This is not surprising given that lobules IX-X have been associated with emotional processing (Adamaszek et al., 2017). Increased connectivity of lobules IX-X with the extended amygdala of individuals with PSTD in contrast to increased cortex-BNST connectivity of healthy controls might represent a decreased cerebral cortical regulation of augmented emotional responses in PTSD.
4.6. Cognitive, emotional and stress-inducing tasks
Only four studies used standardized cognitive and emotional tasks. Results were not consistent due to the fact that each study used different tasks. Generally, no between-group differences were found in task performance, although in some cases trauma-exposed individuals exhibited alterations in task-based cerebellar activation patterns or the latter correlated with symptom severity. Interestingly, one study showed that hydrocortisone administration affected cerebellar activation during an autobiographical memory task and it was associated with childhood trauma exposure. This suggests that early adverse experiences might affect cerebellar structure and function in a glucocorticoid-dependent manner (Metz et al., 2019).
Finally, results were mixed in stress-induced analgesia tasks: trauma-exposed individuals showed both increased and decreased activation in large cerebellar regions during pain-inducing heat, and opposed activation patterns were found in different cerebellar regions during reward and aversive processing respectively (Elman et al., 2018; Strigo et al., 2010). The authors suggested that alterations in both positive and negative valence processing might be a key feature of PTSD-related neuropsychopathology (Elman et al., 2018). Given that the cerebellum is involved in both reward (Hull, 2020a; Wagner et al., 2017) and aversion (Ernst et al., 2019) prediction, it might be one of the key regions that mediates this feature.
4.7. Limitations and future directions
Taken altogether, trauma-related alterations in several cerebellar regions have been identified in this review. Cerebellar alterations were reported in the majority of structural studies; however, findings were not consistent across them. One factor that might explain the heterogeneity of the results is that most studies did not report on participants’ potential exposure to childhood adversity. Early exposure to adversity alters trajectories of neurodevelopment in several levels, including sensory systems, network architecture as well as circuits involved in threat detection, emotion and reward (Teicher et al., 2016). Childhood adversity was a factor that correlated with cerebellar alterations and psychiatric symptoms in functional studies too. This highlights the necessity of reporting potential exposure to early traumatic experiences in future studies.
Resting state and task-based functional studies suggested involvement of several cerebellar regions in trauma-related processing, including regions traditionally associated with emotional processing (lobules IX-X), as well as regions important for sensorimotor control (IV–V), autobiographical memory, working memory and attention (lobules VI, VII). These regions are consistent with first motor representation and all three nonmotor representations in the cerebellum (Guell et al., 2018c). In addition, given that several studies reported aberrant cerebellar connectivity with nodes of large-scale brain networks, including the DMN, the CEN and the SN in trauma-exposed individuals, and the recently suggested network-based neurobiological model of PTSD (Akiki et al., 2017), future research should further investigate cerebellar involvement in large-scale network alterations. Future studies should report on specific anatomical regions of the cerebellum, as different cerebellar regions might participate in different or even opposing functions. Importantly, a recent meta-analysis showed that all regions identified in this review participate in fear learning (Lange et al., 2015b)
One important limitation of this review is that most of the included studies were cross-sectional and therefore no causality can be inferred between trauma-exposure and cerebellar alterations. That is, preexisting differences in the cerebellum might be a risk factor for developing trauma-related psychopathology. Cerebellar abnormalities that develop after trauma exposure might also be secondary to environmental or psychological phenomena that are associated with, but not caused by, the traumatic event. It is also possible that cerebellar abnormalities associated with trauma exposure are the result of a compensatory reorganization of brain structure and function, rather than a pathological manifestation of psychiatric disease; only studies with an interventional component such as stimulation or modulation experiments that can alter cerebellar structure or function would be able to determine the functional significance (compensatory or pathological) of these abnormalities.
Another methodological limitation is that in our search methods we used the words “cerebellum” or “cerebellar” and this might have led to a confirmation bias. To overcome this possibility we revised the bibliography of the included studies as well as three meta-analyses for potential negative results (Hayes et al., 2012; Koch et al., 2016; Wang et al., 2016). Finally, although inclusion and exclusion criteria were clearly defined and followed, the included studies were highly heterogeneous, limiting the capacity of drawing definitive conclusions in several matters. For example, many studies did not report potential substance use of participants, and individuals with a variety of comorbid psychiatric disorders who were exposed to different types of traumatic events were included in the studies. This highlights the necessity for standardized ways to measure traumatic events across disorders, including the type of traumatic experience and the time window between trauma exposure and the realization of the study. Also, substance use and psychiatric comorbidities should be taken into account and reported.
Finally, fMRI results should be treated cautiously considering the heterogeneity of the acquisition parameters of the selected studies. The complex architecture of the cerebellar gray matter remains below the resolution of the acquisition, especially after the post-processing of the fMRI acquisitions. In this sense, this effect is a more pronounced limitation in the selected functional studies than structural studies as most of the selected structural studies have an isometric resolution of 1 mm and they compare volumes of well-defined structures much larger than the voxel size. However, for the selected functional studies, voxel size ranges from 2 mm to 5 mm and are normalized to the MNI space of 3 mm isometric voxel by interpolations and then applied a Gaussian kernel of up to 8 mm. In addition, most of those studies include voxel-by-voxel comparisons rather than comparing well-defined structures formed by larger sets of voxels. Nevertheless, the solution to this challenge is not trivial since decreasing the voxel size for these acquisitions leads to a reduction in the signal-to-noise ratio (SNR), so we are facing a trade-off between resolution and SNR.
Within the limitations that we have discussed so far, our preferred interpretation of the results presented here is through the lens of the UCT, the hypothesis that the cerebellum, given its homogeneous cytoarchitecture, performs a common computation for a variety of functions (Schmahmann et al., 2019). Our results indicate that structural alterations in the cerebellum are associated with early adversity and that aberrant cerebellar resting and task-based connectivity with several cortical, subcortical and limbic regions might be a common feature of trauma-related psychopathology. The cerebellum has been traditionally associated with sensorimotor learning and cerebellar damage usually leads to dysmetria – a degradation of the precision, efficiency and/or coordination of the movement (Sokolov et al., 2017). According to the UCT theory, non-motor disorders that are related to cerebellar dysfunction present the non-motor equivalent of motor dysmetria: dysmetria of thought (Schmahmann et al., 2019). That is, a dysfunction in the speed, capacity, consistency and/or appropriateness of cognitive and emotional processes. Therefore, the role of the cerebellum in trauma-related psychopathology might be viewed as an “overshoot” or “undershoot” of responses to internal or external cues (Guell et al., 2018b). Future research should determine whether different cerebellar regions are involved in different functions of trauma-related processing or whether involvement of different regions represent different levels of processing, from lower to higher order, according to the functional gradients of the cerebellum (Guell et al., 2018c) that are involved in each domain. Also, trauma-exposure should be investigated across disorders in order to assess commonalities and discordances in cerebellar function between them.
Financial support
The project that gave rise to these results received the support of a fellowship from “La Caixa” Foundation (ID 100010434). The fellowship code is LCF/BQ/DR19/11740014. This work was supported by a grant PI20/00506 from the National R + D + I and funded by the Carlos III Health Institute-Deputy General Assessment and the Fondo Europeo de Desarrollo Regional (FEDER).
Author contribution
CB, LN and LM were responsible for the study concept and design and carried out the data extraction. CB carried out the data analysis and drafted the manuscript. LN, LM, XG and SPD provided critical revision of the manuscript for important intellectual content. All authors critically reviewed content and approved the final version for publication.
Declaration of competing interest
Laia Miquel has received honoraria from Lundbeck and Neuraxpharma, outside the work for this project. Antoni Gual has received honoraria and travel grants from Lundbeck, Janssen, D&A Pharma, and Servier, all outside the work for this project. The other authors declare that they have no competing interests.
Acknowledgments
CERCA Programme/Generalitat de Catalunya.
Contributor Information
C. Blithikioti, Email: blithikioti@clinic.cat.
L. Miquel, Email: miquel@clinic.cat.
Data availability
Data will be made available on request.
References
- Adamaszek M., D ’Agata F., Ferrucci R., Habas C., Keulen S., Kirkby K.C., Leggio M., Marien P., Molinari M., Moulton E., Orsi L., Van Overwalle F., Papadelis C., Priori A., Sacchetti B., Schutter J D., Styliadis C., Verhoeven J. Consensus paper: cerebellum and emotion. Cerebellum. 2017;16:552–576. doi: 10.1007/s12311-016-0815-8. [DOI] [PubMed] [Google Scholar]
- Akiki T.J., Averill C.L., Abdallah C.G. A network-based neurobiological model of PTSD: evidence from structural and functional neuroimaging studies. Curr. Psychiatr. Rep. 2017 doi: 10.1007/s11920-017-0840-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen M.T., Handy J.D., Miller D.P., Servatius R.J. Avoidance learning and classical eyeblink conditioning as model systems to explore a learning diathesis model of PTSD. Neurosci. Biobehav. Rev. 2019 doi: 10.1016/j.neubiorev.2019.03.003. [DOI] [PubMed] [Google Scholar]
- Awasthi S., Pan H., LeDoux J.E., Cloitre M., Altemus M., McEwen B., Silbersweig D., Stern E. The bed nucleus of the stria terminalis and functionally linked neurocircuitry modulate emotion processing and HPA axis dysfunction in posttraumatic stress disorder. NeuroImage Clin. 2020;28 doi: 10.1016/j.nicl.2020.102442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badura A., Verpeut J.L., Metzger J.W., Pereira T.D., Pisano T.J., Deverett B., Bakshinskaya D.E., Wang S.S.H. Normal cognitive and social development require posterior cerebellar activity. Elife. 2018;7 doi: 10.7554/eLife.36401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldaçara L., Jackowski A.P., Schoedl A., Pupo M., Andreoli S.B., Mello M.F., Lacerda A.L.T., Mari J.J., Bressan R.A. Reduced cerebellar left hemisphere and vermal volume in adults with PTSD from a community sample. J. Psychiatr. Res. 2011;45:1627–1633. doi: 10.1016/j.jpsychires.2011.07.013. [DOI] [PubMed] [Google Scholar]
- Belleau E.L., Ehret L.E., Hanson J.L., Brasel K.J., Larson C.L., DeRoon-Cassini T.A. Amygdala functional connectivity in the acute aftermath of trauma prospectively predicts severity of posttraumatic stress symptoms: functional connectivity predicts future PTSD symptoms. Neurobiol. Stress. 2020;12 doi: 10.1016/j.ynstr.2020.100217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing X., Qiu M.G., Ye Z., Zhang J.N., Min L., Han C., Yu Z., Zhang J.J., Jian W., Wei C., Du H.J., Zhang S.X. Alterations in the cortical thickness and the amplitude of low-frequency fluctuation in patients with post-traumatic stress disorder. Brain Res. 2013;1490:225–232. doi: 10.1016/j.brainres.2012.10.048. [DOI] [PubMed] [Google Scholar]
- Bonne O., Gilboa A., Louzoun Y., Brandes D., Yona I., Lester H., Barkai G., Freedman N., Chisin R., Shalev A.Y. Resting regional cerebral perfusion in recent posttraumatic stress disorder. Biol. Psychiatr. 2003;54:1077–1086. doi: 10.1016/S0006-3223(03)00525-0. [DOI] [PubMed] [Google Scholar]
- Brady R.O., Gonsalvez I., Lee I., Öngür D., Seidman L.J., Schmahmann J.D., Eack S.M., Keshavan M.S., Pascual-Leone A., Halko M.A. Cerebellar-prefrontal network connectivity and negative symptoms in schizophrenia. Am. J. Psychiatr. 2019;176:512–520. doi: 10.1176/appi.ajp.2018.18040429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancu M., Mann-Wrobel M., Beckham J.C., Wagner H.R., Elliott A., Robbins A.T., Wong M., Berchuck A.E., Runnals J.J. Subthreshold posttraumatic stress disorder: a meta-analytic review of DSM-IV prevalence and a proposed DSM-5 approach to measurement. Psychol. Trauma Theory, Res. Pract. Policy. 2016;8:222–232. doi: 10.1037/tra0000078. [DOI] [PubMed] [Google Scholar]
- Brodal P. vol. 251. Oxford Uviversity Press; 2004. (The Central Nervous System: Structure and Function). [DOI] [Google Scholar]
- Buckner R.L., Krienen F.M., Castellanos A., Diaz J.C., Thomas Yeo B.T. The organization of the human cerebellum estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106:2322–2345. doi: 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler R.K., Finn D.P. Stress-induced analgesia. Prog. Neurobiol. 2009 doi: 10.1016/j.pneurobio.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Chen H.J., Zhang L., Ke J., Qi R., Xu Q., Zhong Y., Pan M., Li J., Lu G.M., Chen F. Altered resting-state dorsal anterior cingulate cortex functional connectivity in patients with post-traumatic stress disorder. Aust. N. Z. J. Psychiatr. 2019;53:68–79. doi: 10.1177/0004867418812674. [DOI] [PubMed] [Google Scholar]
- De Bellis M.D., Kuchibhatla M. Cerebellar volumes in pediatric maltreatment-related posttraumatic stress disorder. Biol. Psychiatr. 2006;60:697–703. doi: 10.1016/j.biopsych.2006.04.035. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J., King M., Hernandez-Castillo C., Sereno M., Ivry R.B. Universal Transform or multiple functionality? Understanding the contribution of the human cerebellum across task domains. Neuron. 2019 doi: 10.1016/j.neuron.2019.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener S.J., Wessa M., Ridder S., Lang S., Diers M., Steil R., Flor H. Enhanced stress analgesia to a cognitively demanding task in patients with posttraumatic stress disorder. J. Affect. Disord. 2012;136:1247–1251. doi: 10.1016/j.jad.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Douglas K.M., Groves S., Porter R.J., Jordan J., Wilson L., Melzer T.R., Wise R.G., Bisson J.I., Bell C.J. Traumatic imagery following glucocorticoid administration in earthquake-related post-traumatic stress disorder: a preliminary functional magnetic resonance imaging study. Aust. N. Z. J. Psychiatr. 2019;53:1167–1178. doi: 10.1177/0004867419851860. [DOI] [PubMed] [Google Scholar]
- Dube S.R., Felitti V.J., Dong M., Chapman D.P., Giles W.H., Anda R.F. Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: the adverse childhood experiences study. Pediatrics. 2003;111:564–572. doi: 10.1542/peds.111.3.564. [DOI] [PubMed] [Google Scholar]
- Dube S.R., Felitti V.J., Dong M., Giles W.H., Anda R.F. The impact of adverse childhood experiences on health problems: evidence from four birth cohorts dating back to 1900. Prev. Med. 2003;37:268–277. doi: 10.1016/S0091-7435(03)00123-3. [DOI] [PubMed] [Google Scholar]
- Dunlop B.W., Wong A. The hypothalamic-pituitary-adrenal axis in PTSD: pathophysiology and treatment interventions. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2019 doi: 10.1016/j.pnpbp.2018.10.010. [DOI] [PubMed] [Google Scholar]
- Elman I., Upadhyay J., Langleben D.D., Albanese M., Becerra L., Borsook D. Reward and aversion processing in patients with post-traumatic stress disorder: functional neuroimaging with visual and thermal stimuli. Transl. Psychiatry. 2018;8:240. doi: 10.1038/s41398-018-0292-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T.M., Brol A.E., Gratz M., Ritter C., Bingel U., Schlamann M., Maderwald S., Quick H.H., Merz C.J., Timmann D. The cerebellum is involved in processing of predictions and prediction errors in a fear conditioning paradigm. Elife. 2019;8 doi: 10.7554/elife.46831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennema-Notestine C., Stein M.B., Kennedy C.M., Archibald S.L., Jernigan T.L. Erratum: brain morphometry in female victims of intimate partner violence with and without posttraumatic stress disorder (Biological Psychiatry. Biol. Psychiatr. 2003;52(10):1089–1101. doi: 10.1016/S0006-3223(03)00166-5. [DOI] [PubMed] [Google Scholar]
- Frontera J.L., Baba Aissa H., Sala R.W., Mailhes-Hamon C., Georgescu I.A., Léna C., Popa D. Bidirectional control of fear memories by cerebellar neurons projecting to the ventrolateral periaqueductal grey. Nat. Commun. 2020;11 doi: 10.1038/s41467-020-18953-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubaugh A.L., Magruder K.M., Waldrop A.E., Elhai J.D., Knapp R.G., Frueh B.C. Subthreshold PTSD in primary care: prevalence, psychiatric disorders, healthcare use, and functional status. J. Nerv. Ment. Dis. 2005 doi: 10.1097/01.nmd.0000180740.02644.ab. [DOI] [PubMed] [Google Scholar]
- Grubaugh A.L., Zinzow H.M., Paul L., Egede L.E., Frueh B.C. Trauma exposure and posttraumatic stress disorder in adults with severe mental illness: a critical review. Clin. Psychol. Rev. 2011 doi: 10.1016/j.cpr.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guell X., Gabrieli J.D.E., Schmahmann J.D. Triple representation of language, working memory, social and emotion processing in the cerebellum: convergent evidence from task and seed-based resting-state fMRI analyses in a single large cohort. Neuroimage. 2018;172:437–449. doi: 10.1016/j.neuroimage.2018.01.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guell X., Gabrieli J.D.E., Schmahmann J.D. Embodied cognition and the cerebellum: perspectives from the dysmetria of thought and the universal cerebellar Transform theories. Cortex. 2018;100:140–148. doi: 10.1016/j.cortex.2017.07.005. [DOI] [PubMed] [Google Scholar]
- Guell X., Schmahmann J.D., Gabrieli J.D.E., Ghosh S.S. Elife; 2018. Functional Gradients of the Cerebellum: A Fundamental Movement-To-Thought Principle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall S.A., Brodar K.E., LaBar K.S., Berntsen D., Rubin D.C. Neural responses to emotional involuntary memories in posttraumatic stress disorder: differences in timing and activity. NeuroImage Clin. 2018;19:793–804. doi: 10.1016/j.nicl.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J.P., Hayes S.M., Mikedis A.M. Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biol. Mood Anxiety Disord. 2012;2:1. doi: 10.1186/2045-5380-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M.N., Campolongo P., Yehuda R., Patel S. Integrating endocannabinoid signaling and cannabinoids into the biology and treatment of posttraumatic stress disorder. Neuropsychopharmacology. 2018 doi: 10.1038/npp.2017.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes S.E., Scheinost D., DellaGioia N., Davis M.T., Matuskey D., Pietrzak R.H., Hampson M., Krystal J.H., Esterlis I. Cerebellar and prefrontal cortical alterations in PTSD: structural and functional evidence. Chronic Stress. 2018;2 doi: 10.1177/2470547018786390. 247054701878639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull C. Prediction signals in the cerebellum: beyond supervised motor learning. Elife. 2020 doi: 10.7554/eLife.54073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull C. Prediction signals in the cerebellum: beyond supervised motor learning. Elife. 2020 doi: 10.7554/eLife.54073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igelström K.M., Graziano M.S. The inferior parietal lobule and temporoparietal junction: a network perspective. Neuropsychologia. 2017;105:70–83. doi: 10.1016/j.neuropsychologia.2017.01.001. [DOI] [PubMed] [Google Scholar]
- Insana S.P., Banihashemi L., Herringa R.J., Kolko D.J., Germain A. Childhood maltreatment is associated with altered frontolimbic neurobiological activity during wakefulness in adulthood HHS Public Access. Dev. Psychopathol. 2016;28:551–564. doi: 10.1017/S0954579415000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T., Cuthbert B., Garvey M., Heinssen R., Pine D.S., Quinn K., Sanislow C., Wang P. Research Domain Criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatr. 2010 doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Ke J., Zhang L., Qi R., Li W., Hou C., Zhong Y., He Z., Li L., Lu G. A longitudinal fMRI investigation in acute post-traumatic stress disorder (PTSD) Acta Radiol. 2016;57:1387–1395. doi: 10.1177/0284185115585848. [DOI] [PubMed] [Google Scholar]
- Ke J., Zhang L., Qi R., Xu Q., Li W., Hou C., Zhong Y., Zhang Z., He Z., Li L., Lu G. Altered blood oxygen level-dependent signal variability in chronic post-traumatic stress disorder during symptom provocation. Neuropsychiatric Dis. Treat. 2015;11:1805–1815. doi: 10.2147/NDT.S87332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M., Hernandez-Castillo C.R., Poldrack R.A., Ivry R.B., Diedrichsen J. Functional boundaries in the human cerebellum revealed by a multi-domain task battery. Nat. Neurosci. 2019;22:1371–1378. doi: 10.1038/s41593-019-0436-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S.B.J., van Zuiden M., Nawijn L., Frijling J.L., Veltman D.J., Olff M. Aberrant resting-state brain activity in posttraumatic stress disorder: a meta-analysis and systematic review. Depress. Anxiety. 2016 doi: 10.1002/da.22478. [DOI] [PubMed] [Google Scholar]
- Lange I., Kasanova Z., Goossens L., Leibold N., De Zeeuw C.I., van Amelsvoort T., Schruers K. The anatomy of fear learning in the cerebellum: a systematic meta-analysis. Neurosci. Biobehav. Rev. 2015 doi: 10.1016/j.neubiorev.2015.09.019. [DOI] [PubMed] [Google Scholar]
- Lange I., Kasanova Z., Goossens L., Leibold N., De Zeeuw C.I., van Amelsvoort T., Schruers K. The anatomy of fear learning in the cerebellum: a systematic meta-analysis. Neurosci. Biobehav. Rev. 2015 doi: 10.1016/j.neubiorev.2015.09.019. [DOI] [PubMed] [Google Scholar]
- Levitt J.J., Chen Q.C., May F.S., Gilbertson M.W., Shenton M.E., Pitman R.K. Volume of cerebellar vermis in monozygotic twins discordant for combat exposure: lack of relationship to post-traumatic stress disorder. Psychiatry Res. Neuroimag. 2006;148:143–149. doi: 10.1016/j.pscychresns.2006.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist D.H. Emotion in motion: a three-stage model of aversive classical conditioning. Neurosci. Biobehav. Rev. 2020 doi: 10.1016/j.neubiorev.2020.04.025. [DOI] [PubMed] [Google Scholar]
- Logothetis N.K., Pauls J., Augath M., Trinath T., Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Lupo M., Siciliano L., Leggio M. From cerebellar alterations to mood disorders: a systematic review. Neurosci. Biobehav. Rev. 2019 doi: 10.1016/j.neubiorev.2019.06.008. [DOI] [PubMed] [Google Scholar]
- Maddox S.A., Hartmann J., Ross R.A., Ressler K.J. Deconstructing the gestalt: mechanisms of fear, threat, and trauma memory encoding. Neuron. 2019;102:60–74. doi: 10.1016/j.neuron.2019.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manninen S., Karjalainen T., Tuominen L.J., Hietala J., Kaasinen V., Joutsa J., Rinne J., Nummenmaa L. Cerebral grey matter density is associated with neuroreceptor and neurotransporter availability: a combined PET and MRI study. Neuroimage. 2021;235 doi: 10.1016/j.neuroimage.2021.117968. [DOI] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cognit. Sci. 2011 doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Metz S., Fleischer J., Gärnter M., Golde S., Duesenberg M., Roepke S., Wolf O.T., Otte C., Wingenfeld K. Effects of hydrocortisone on autobiographical memory retrieval in patients with posttraumatic stress disorder and borderline personality disorder: the role of childhood trauma. Neuropsychopharmacology. 2019;44:2038–2044. doi: 10.1038/s41386-019-0459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton F.A., Strick P.L. Cerebellar projections to the prefrontal cortex of the primate. J. Neurosci. 2001;21:700–712. doi: 10.1523/JNEUROSCI.21-02-00700.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquel M., Vazquez-Sanroman D., Carbo-Gas M., Gil-Miravet I., Sanchis-Segura C., Carulli D., Manzo J., Coria-Avila G.A. Have we been ignoring the elephant in the room? Seven arguments for considering the cerebellum as part of addiction circuitry. Neurosci. Biobehav. Rev. 2016 doi: 10.1016/j.neubiorev.2015.11.005. [DOI] [PubMed] [Google Scholar]
- Moberget T., Alnæs D., Kaufmann T., Doan N.T., Córdova-Palomera A., Norbom L.B., Rokicki J., van der Meer D., Andreassen O.A., Westlye L.T. Cerebellar gray matter volume is associated with cognitive function and psychopathology in adolescence. Biol. Psychiatr. 2019;86:65–75. doi: 10.1016/j.biopsych.2019.01.019. [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G., Altman D., Antes G., Atkins D., Barbour V., Barrowman N., Berlin J.A., Clark J., Clarke M., Cook D., D'Amico R., Deeks J.J., Devereaux P.J., Dickersin K., Egger M., Ernst E., Gøtzsche P.C., Grimshaw J., Guyatt G., Higgins J., Ioannidis J.P.A., Kleijnen J., Lang T., Magrini N., McNamee D., Moja L., Mulrow C., Napoli M., Oxman A., Pham B., Rennie D., Sampson M., Schulz K.F., Shekelle P.G., Tovey D., Tugwell P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 2009 doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- Molina M.E., Isoardi R., Prado M.N., Bentolila S. Basal cerebral glucose distribution in long-term post-traumatic stress disorder. World J. Biol. Psychiatr. 2010;11:493–501. doi: 10.3109/15622970701472094. [DOI] [PubMed] [Google Scholar]
- Moreno-Rius J. The cerebellum under stress. Front. Neuroendocrinol. 2019 doi: 10.1016/j.yfrne.2019.100774. [DOI] [PubMed] [Google Scholar]
- Naegeli C., Zeffiro T., Piccirelli M., Jaillard A., Weilenmann A., Hassanpour K., Schick M., Rufer M., Orr S.P., Mueller-Pfeiffer C. Locus coeruleus activity mediates hyperresponsiveness in posttraumatic stress disorder. Biol. Psychiatr. 2018;83:254–262. doi: 10.1016/j.biopsych.2017.08.021. [DOI] [PubMed] [Google Scholar]
- Narendran R., Tollefson S., Fasenmyer K., Paris J., Himes M.L., Lopresti B., Ciccocioppo R., Mason N.S. Decreased nociceptin receptors are related to resilience and recovery in college women who have experienced sexual violence: therapeutic implications for posttraumatic stress disorder. Biol. Psychiatr. 2019;85:1056–1064. doi: 10.1016/j.biopsych.2019.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neria Y. Functional neuroimaging in PTSD: from discovery of underlying mechanisms to addressing diagnostic heterogeneity. Am. J. Psychiatr. 2021 doi: 10.1176/appi.ajp.2020.20121727. [DOI] [PubMed] [Google Scholar]
- Nicholson A.A., Densmore M., Frewen P.A., Théberge J., Neufeld R.W., McKinnon M.C., Lanius R.A. The dissociative subtype of posttraumatic stress disorder: unique resting-state functional connectivity of basolateral and centromedial amygdala complexes. Neuropsychopharmacology. 2015;40:2317–2326. doi: 10.1038/npp.2015.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson A.A., Harricharan S., Densmore M., Neufeld R.W.J., Ros T., McKinnon M.C., Frewen P.A., Théberge J., Jetly R., Pedlar D., Lanius R.A. Classifying heterogeneous presentations of PTSD via the default mode, central executive, and salience networks with machine learning. NeuroImage Clin. 2020;27:102262. doi: 10.1016/j.nicl.2020.102262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishith P., Griffin M.G., Poth T.L. Stress-induced analgesia: prediction of posttraumatic stress symptoms in battered versus nonbattered women. Biol. Psychiatr. 2002;51:867–874. doi: 10.1016/S0006-3223(01)01346-4. [DOI] [PubMed] [Google Scholar]
- Park Y.W., Jun S., Noh J., Chung S.J., Han S., Lee P.H., Kim C., Lee S.K. Structural and resting-state brain alterations in trauma-exposed firefighters: preliminary results. J. Korean Soc. Radiol. 2020;81:676–687. doi: 10.3348/JKSR.2020.81.3.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quidé Y., Cléry H., Andersson F., Descriaud C., Saint-Martin P., Barantin L., Gissot V., Carrey Le Bas M.P., Osterreicher S., Dufour-Rainfray D., Brizard B., Ogielska M., El-Hage W. Neurocognitive, emotional and neuroendocrine correlates of exposure to sexual assault in women. J. Psychiatry Neurosci. 2018;43:318–326. doi: 10.1503/jpn.170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabellino D., Densmore M., Frewen P.A., Théberge J., Lanius R.A. The innate alarm circuit in post-traumatic stress disorder: conscious and subconscious processing of fear- and trauma-related cues. Psychiatry Res. Neuroimag. 2016;248:142–150. doi: 10.1016/j.pscychresns.2015.12.005. [DOI] [PubMed] [Google Scholar]
- Rabellino D., Densmore M., Harricharan S., Jean T., McKinnon M.C., Lanius R.A. Resting-state functional connectivity of the bed nucleus of the stria terminalis in post-traumatic stress disorder and its dissociative subtype. Hum. Brain Mapp. 2018;39:1367–1379. doi: 10.1002/hbm.23925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabellino D., Densmore M., Théberge J., McKinnon M.C., Lanius R.A. The cerebellum after trauma: resting-state functional connectivity of the cerebellum in posttraumatic stress disorder and its dissociative subtype. Hum. Brain Mapp. 2018;39:3354–3374. doi: 10.1002/hbm.24081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer A.L., Knodt A.R., Houts R., Brigidi B.D., Moffitt T.E., Caspi A., Hariri A.R. Structural alterations within cerebellar circuitry are associated with general liability for common mental disorders. Mol. Psychiatr. 2018;23:1084–1090. doi: 10.1038/mp.2017.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann J.D., Guell X., Stoodley C.J., Halko M.A. The theory and neuroscience of cerebellar cognition. Annu. Rev. Neurosci. 2019 doi: 10.1146/annurev-neuro-070918-050258. [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendhilnathan N., Ipata A.E., Goldberg M.E. Neural correlates of reinforcement learning in mid-lateral cerebellum. Neuron. 2020;106:188–198. doi: 10.1016/j.neuron.2019.12.032. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R., Smith M. a, Krakauer J.W. Error correction, sensory prediction, and adaptation in motor control. Annu. Rev. Neurosci. 2010 doi: 10.1146/annurev-neuro-060909-153135. [DOI] [PubMed] [Google Scholar]
- Shin L.M., Rauch S.L., Pitman R.K. Annals of the New York Academy of Sciences. Blackwell Publishing Inc.; 2006. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD; pp. 67–79. [DOI] [PubMed] [Google Scholar]
- Sokolov A.A., Miall R.C., Ivry R.B. The cerebellum: adaptive prediction for movement and cognition. Trends Cognit. Sci. 2017 doi: 10.1016/j.tics.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick P.L., Dum R.P., Fiez J.A. Cerebellum and nonmotor function. Annu. Rev. Neurosci. 2009 doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- Strigo I.A., Simmons A.N., Matthews S.C., Grimes E.M., Allard C.B., Reinhardt L.E., Paulus M.P., Stein M.B. Neural correlates of altered pain response in women with posttraumatic stress disorder from intimate partner violence. Biol. Psychiatr. 2010;68:442–450. doi: 10.1016/j.biopsych.2010.03.034. [DOI] [PubMed] [Google Scholar]
- Sui S.G., Zhang Y., Wu M.X., Xu J.M., Duan L., Weng X.C., Shan B.C., Li L.J. Abnormal cerebellum density in victims of rape with post-traumatic stress disorder: voxel-based analysis of magnetic resonance imaging investigation. Asia Pac. Psychiatr. 2010;2:129–135. doi: 10.1111/j.1758-5872.2010.00076.x. [DOI] [Google Scholar]
- Suo X., Lei D., Li W., Yang J., Li L., Sweeney J.A., Gong Q. Individualized prediction of PTSD symptom severity in trauma survivors from whole-brain resting-state functional connectivity. Front. Behav. Neurosci. 2020;14:234. doi: 10.3389/fnbeh.2020.563152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman D., Pang E.W., Jetly R., Dunkley B.T., Taylor M.J. Neuroanatomical features in soldiers with post-traumatic stress disorder. BMC Neurosci. 2016;17:13. doi: 10.1186/s12868-016-0247-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher M.H., Samson J.A., Anderson C.M., Ohashi K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat. Rev. Neurosci. 2016 doi: 10.1038/nrn.2016.111. [DOI] [PubMed] [Google Scholar]
- Terpou B.A., Densmore M., Thome J., Frewen P., McKinnon M.C., Lanius R.A. The innate alarm system and subliminal threat presentation in posttraumatic stress disorder: neuroimaging of the midbrain and cerebellum. Chronic Stress. 2019;3 doi: 10.1177/2470547018821496. 247054701882149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thome J., Densmore M., Frewen P.A., McKinnon M.C., Théberge J., Nicholson A.A., Koenig J., Thayer J.F., Lanius R.A. Desynchronization of autonomic response and central autonomic network connectivity in posttraumatic stress disorder. Hum. Brain Mapp. 2017;38:27–40. doi: 10.1002/hbm.23340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thome J., Terpou B.A., McKinnon M.C., Lanius R.A. The neural correlates of trauma-related autobiographical memory in posttraumatic stress disorder: a meta-analysis. Depress. Anxiety. 2020 doi: 10.1002/da.22977. [DOI] [PubMed] [Google Scholar]
- Tovote P., Esposito M.S., Botta P., Chaudun F., Fadok J.P., Markovic M., Wolff S.B.E., Ramakrishnan C., Fenno L., Deisseroth K., Herry C., Arber S., Lüthi A. Midbrain circuits for defensive behaviour. Nature. 2016;534:206–212. doi: 10.1038/nature17996. [DOI] [PubMed] [Google Scholar]
- Van Dam N.T., Rando K., Potenza M.N., Tuit K., Sinha R. Childhood maltreatment, altered limbic neurobiology, and substance use relapse severity via trauma-specific reductions in limbic gray matter volume. JAMA Psychiatr. 2014;71:917–925. doi: 10.1001/jamapsychiatry.2014.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanes L.D., Dolan R.J. Transdiagnostic neuroimaging markers of psychiatric risk: a narrative review. NeuroImage Clin. 2021 doi: 10.1016/j.nicl.2021.102634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verger A., Rousseau P.F., Malbos E., Chawki M.B., Nicolas F., Lançon C., Khalfa S., Guedj E. Involvement of the cerebellum in EMDR efficiency: a metabolic connectivity PET study in PTSD. Eur. J. Psychotraumatol. 2020;11:1767986. doi: 10.1080/20008198.2020.1767986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidotto G., Catalucci A., Roncone R., Pino M.C., Mazza M. Neural correlates of observation of disgusting images in subjects with first episode psychosis and post-traumatic stress disorder. J. Biol. Regul. Homeost. Agents. 2014;28:639–650. [PubMed] [Google Scholar]
- Wagner M.J., Kim T.H., Savall J., Schnitzer M.J., Luo L. Cerebellar granule cells encode the expectation of reward. Nature. 2017;544:96–100. doi: 10.1038/nature21726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Liu J., Zhang J., Zhan W., Li L., Wu M., Huang H., Zhu H., Kemp G.J., Gong Q. Altered resting-state functional activity in posttraumatic stress disorder: a quantitative meta-analysis. Sci. Rep. 2016;6 doi: 10.1038/srep27131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise T., Dolan R.J. Associations between aversive learning processes and transdiagnostic psychiatric symptoms in a general population sample. Nat. Commun. 2020;11 doi: 10.1038/s41467-020-17977-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y., Li L., Jin C., Hu X., Duan L., Eyler L.T., Gong Q., Song M., Jiang T., Liao M., Zhang Y., Li W. Abnormal baseline brain activity in posttraumatic stress disorder: a resting-state functional magnetic resonance imaging study. Neurosci. Lett. 2011;498:185–189. doi: 10.1016/j.neulet.2011.02.069. [DOI] [PubMed] [Google Scholar]
- Yuan M., Qiu C., Meng Y., Ren Z., Yuan C., Li Y., Gao M., Lui S., Zhu H., Gong Q., Zhang W. Pre-treatment resting-state functional MR imaging predicts the long-term clinical outcome after short-term paroxtine treatment in post-traumatic stress disorder. Front. Psychiatr. 2018;9:532. doi: 10.3389/fpsyt.2018.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Wu Q., Zhu H., He L., Huang H., Zhang J., Zhang W. Multimodal MRI-based classification of trauma survivors with and without post-traumatic stress disorder. Front. Neurosci. 2016;10 doi: 10.3389/fnins.2016.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Wu Q., Zhu H., He L., Huang H., Zhang J., Zhang W. Multimodal MRI-based classification of trauma survivors with and without post-traumatic stress disorder. Front. Neurosci. 2016;10 doi: 10.3389/fnins.2016.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Volkow N.D. Brain default-mode network dysfunction in addiction. Neuroimage. 2019 doi: 10.1016/j.neuroimage.2019.06.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.