Highlights
-
•
Optimization of ultrasound-assisted extraction of makiang seed was evaluated.
-
•
Variable parameters including temperature, time and amplitude were analyzed.
-
•
Ultrasound had a significant effect on bioactive compound of makiang seed.
-
•
Makiang seed extract showed antioxidant and antimicrobial activities.
-
•
Makiang seed extract enhanced microbial stability of orange juice during storage.
Keywords: Ultrasound-assisted extraction, Response surface methodology, Makiang seed, Bioactive compound, Antimicrobial activity, Orange juice
Abstract
An ultrasound-assisted extraction (UAE) was optimized for the extraction of bioactive compound (total phenolic compound and total flavonoid content) with antioxidant activity (DPPH and FRAP assays) using response surface methodology based on Box-Behnken design (BBD). The effect of extraction temperature (X1: 30–70 °C), extraction time (X2: 25–45 min) and amplitude (X3: 30–50%) were determined. In addition, antimicrobial activity and application of optimized makiang seed extract (MSE) were also evaluated. Results showed that the optimum condition of UAE were X1: 51.82 °C, X2: 31.87 min and X3: 40.51%. It was also found that gallic acid was the major phenolic compound of optimized MSE and its minimum inhibitiory concentration (MIC) and minimum bactericidal concentration (MBC) was between 1.56 - 6.25 and 25–100 mg/mL respectively. The addition of MSE could enhance the stability of orange juice and shelf life extension was also obtained. This research finding suggests the beneficial opportunities for ultrasound-assisted extraction for the production of bioactive compound from makiang seed with antioxidant activity leading to an application in medicinal and functional food industry.
1. Introduction
Over the years, it is widely known that health benefit and nutrition are interrelated and consumer’s trend for choosing foods has been focusing on raw material enriched with bioactive compounds which pose a positive effect on human health [31]. In this regard, plant materials have been captured with extended attention because they contain large amounts of bioactive compounds [13]. In general, most of bioactive compounds are located in the cell and required to release to the extracting solvent through the extraction process. Conventional extraction method including maceration, distillation and soxhlet extraction are common extraction methods used to extract bioactive compounds from plant materials; however, these methods provide disadvantages such as low extraction rate, large amount of extracting solvent, high energy consumption and long-time process [30]. Therefore, green extraction has become a topic of interest and has been proposed by many researchers [14], [11]. Green extraction is defined as an extraction method that presents reducing extracting solvent, lowering energy consumption and decreasing process time with an increase in extraction yield. Driven by the green extraction conceptual objective, green extraction methods have been developed as a result of industrial challenges to be ecological, environmental and economical impacts [48].
Ultrasound-assisted extraction is one of green extraction method because it meets all requirements of green extraction method. Ultrasound or ultrasonic wave has been defined as the frequency higher than 20 kHz, which is the threshold for human auditory detection usually between 20 and 10,000 kHz [41]. The source of ultrasound is commonly from vibrating probe which cause vibration of the surrounding solution which initiate energy transfer to adjacent molecules. The application of ultrasound in extraction has been reported mainly in the range of 20–1,000 kHz which cavitation bubbles are produced and collapse are occurred leading to an extreme shear force. This shear force contributes to disruptive effect on cell wall or cell membrane of matrices by cavitation consequently a liberation of bioactive compounds [32]. Nowadays, ultrasound-assisted extraction has gained extensive interest for the extraction of valuable bioactive compound from various plant materials [49]
Cleistocalyx nervosum var. paniala (C. nervosum), also called makiang, is commonly found in Southeast Asia country such as Laos, Vietnam and Thailand; especially in the northern part of Thailand [44]. It is indigenous plant belonging to the Myrtaceae family and its ripe fruit, with a reddish to purple color, is typically used to process beverage and marmalade due to its unique taste and flavour [39]. Makiang fruit are rich in phenolic compound and anthocyanins with relatively high activity against oxidative damage and antimicrobial activity has been documented [43]. Makiang seeds are generally regarded as waste from fruit processing. However, they accumulate high amount of health benefit compounds; thus, they are considered as a promising source of bioactive compounds with antimicrobial activity and can be applied in pharmaceutical and food industries [21].
Food industry has been facing difficulty to enhance process efficiency without an increasing investment and resource utilization. Investigation a best condition for the system or “optimization” of food process is the key element to resolve this dilemma [6]. In the past, optimization process was usually carried out by studying the effect of one-parameter variations on an output while other parameters are set at a constant value; however, interactive effect between specific parameters has not been taken into account and it has raised the absence of interpretation of entire effect of all factors on a response. Furthermore, this technique needs more experiments required to perform and leads to an increasing cost and time [8]. Therefore, multivariate statistic methods have been conducted to optimize the process parameter in food application. Response surface methodology (RSM) is the most popular technique for multivariate statistic methods. RSM is a combination of statistical and mathematical system based on the compatibility of data to polynomial model and this should unveil the behavior of all data with the goal of establishment of mathematic model for predictions [20].
To date, no studies have been performed regarding to the extraction of bioactive compounds from makiang seed by UAE using RSM to determine optimum extraction condition with independent variables including temperature, time and amplitude. Antioxidant properties including total phenolic compound, total flavonoid content and antioxidant activity by DPPH and FRAP assays were determined to investigate the effect of different parameters on UAE efficacy. In addition, antimicrobial activity against spoilage and pathogenic microorganisms of optimized makiang seed extract (MSE) and its application in orange juice were also investigated.
2. Materials and methods
2.1. Sample preparation
Makiang (Cleistocalyx nervosum var. paniala) fruits with uniform size were collected from Lampang province in Northern part of Thailand and seed was separated and was rinsed by clean running water before blanching at 80 °C for 1 min. Blanched seed was dried by tray dryer (Thermotec 2000, Contherm™, Sweden) at 45 °C for 48 h and then grounded by laboratory miller to obtain seed power by using a 60-mesh sieve. Makiang seed powder was kept in aluminium foil laminated bag at 4 °C until being analyzed.
2.2. Ultrasound-assisted extraction (UAE) experiment
UAE experiments were performed with ultrasound processor (UP400S Ultrasonic processor, Hielsher, Germany) with a 400 W power, 0.7 s cycle, 24 kHz and a titanium probe (H22D, 22 mm). In each experiment run, 40 g of makiang seed powder was mixed with 200 mL ethanol solvent (70% v/v). After extraction, sample was cooled down with ice bath for 5 min and then centrifuged at 565 g for 15 min. Supernatant was filtered through Whatman filter paper No. 1 and solvent was removed at 40 °C by rotary evaporator (Buchi Rotavapor R-200, USA). Refined extract of making seed was obtained and placed in amber glass bottle at 4 °C for further experiment. Refined makiang seed extract was analyzed for its antioxidant properties (total phenolic compound, total flavonoid content antioxidant activity by DPPH and FRAP assays)
2.3. Experimental design and model validation
Extraction factors including, extraction temperature, extraction time and amplitude could significantly influence on antioxidant properties of Makiang seed extract. Therefore, response surface methodology (RSM) with Box-Behnken design (BBD was applied to obtain optimal condition and identify correlation between extraction variables. The coded values of extraction parameters and the experimental run from BBD were detailed in Table 1 and Table 2, respectively. The experiment was carried out according to optimal condition to obtain experimental data compared with the model’s predicted values. According to two-decimal places from RSM, the rounding was done to obtain practical numbers (rounding down for value < 0.50 and rounding up for value ≥ 0.50). To determine model validity, %Difference was calculated as
Table 1.
Coded and actual values for Box–Behnken design (BBD).
| Independent variables | Code symbols | Level |
||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Temperature (°C) | X1 | 30 | 50 | 70 |
| Time (min) | X2 | 25 | 35 | 45 |
| Amplitude (%) | X3 | 30 | 40 | 50 |
Table 2.
The experimental run from Box-Behnken design (BBD).
| Run | Extraction conditions |
||
|---|---|---|---|
| X1-Temperature (°C) | X2- Time (min) | X3- Amplitude (%) | |
| 1 | 30 | 25 | 40 |
| 2 | 70 | 25 | 40 |
| 3 | 30 | 45 | 40 |
| 4 | 70 | 45 | 40 |
| 5 | 30 | 35 | 30 |
| 6 | 70 | 35 | 30 |
| 7 | 30 | 35 | 50 |
| 8 | 70 | 35 | 50 |
| 9 | 50 | 25 | 30 |
| 10 | 50 | 45 | 30 |
| 11 | 50 | 25 | 50 |
| 12 | 50 | 45 | 50 |
| 13 | 50 | 35 | 40 |
| 14 | 50 | 35 | 40 |
| 15 | 50 | 35 | 40 |
| 16 | 50 | 35 | 40 |
| 17 | 50 | 35 | 40 |
2.4. Determination of phenolic compound profile of makiang seed extract from optimized condition
High performance liquid chromatography (HPLC) was employed to analyze and quantify phenolic compound of MSE from optimized condition (UAE) and maceration extraction (optimized condition from UAE without ultrasound treatment). Briefly, making seed extract was filtered through 0.45-µm filter paper prior to injection. The chromatographic separation was carried out on a reverse phase uBondapak C18 (300 mm × 4.9 mm, Prostar, Varian, Darmstadt, Germany) and thermostated at 25 °C. The mobile phase was 100% H2O (pH 3) and 100% methanol with a flow rate of 1.0 mL/min and injection volume of 50 μL. UV array detection was performed at 254 nm. Gallic acid, quercetin, caffeic acid and ferulic acid were used as standard and results were expressed as mg/100 g dry wt.
2.5. Antimicrobial activity experiment
Microbial cultures including Staphylococcus aureus ATCC 25923, Lactobacillus plantarum TISTR 2081, Salmonella Typhimurium ATCC 13311, Escherichia coli ATCC 25922 and Saccharomyces cerevisiae TISTR 5191 were obtained from Thailand Institute of Scientific and Technological Research, TISTR (Pathum thani, Thailand). Inoculum of each microorganisms was prepared by inoculating with 10 mL sterile growth medium (Muller Hinton Broth and Potato Dextrose Broth for bacterial and yeast cultures, respectively). The test tubes were incubated for 18–24 h at 35 °C and 48 h at 30 °C for bacteria and yeast, respectively. The initial concentration of test microorganisms was approximately 106 CFU/mL.
MSE with optimized condition from previous experiment was used for an analysis of antimicrobial activity by minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) which were conducted by the methods of [36], [37]. The MIC of samples was determined by two-fold serial dilution method. In brief, 200 mg/mL of makiang seed extract sample was added into a test tube containing 1 mL of liquid culture medium and serially diluted separately to achieve 100, 50, 25, 12.50, 6.25, 3.125, 1.56, and 0.78 mg/mL, respectively. The test tubes were incubated for 18–24 h at 35 °C and 48 h at 30 °C for bacteria and yeast, respectively. Controls (a test tube containing 1 mL of extract sample and 1 mL of culture as a negative control and a test tube containing 1 mL of culture medium and 1 mL of culture as a positive control) were used with the test microorganisms. The MIC value was considered as the lowest concentration of the test extract inhibiting the growth of the test microorganisms (no visible growth).
For MBC determination, all the test tubes from the MIC without turbidity were streaked plated on a medium suitable for each culture as follows; Baird–Parker agar for S. aureus, MacConkey agar for E coli, Potato Dextrose agar for S. cerevisiae, Salmonella Shigella agar for S. Typhimurium, Lactobacillus MRS agar for L. plantarum. Then, plates were incubated for 18–28 h at 35 °C for S. aureus, L. plantarum, S. Typhimurium, and E. coli, while S. cerevisiae plates were incubated for 48 h at 30 °C. The concentration was considered MBC if no visible growth on the culture medium was observed.
2.6. Application of optimized makiang seed extract (MSE) in orange juice
Orange fruit (Citrus reticulata Blanco) was purchased from local market in Bangkok (Thailand). After being washed with 200 ppm sodium hypochlorite and peeled, orange fruit was processed using a juice extractor (Philips HR1863, Netherland). Freshly-squeezed orange juice was immediately used after juice extraction. The MSE (1.56 and 3.125 mg/mL of orange juice) was added to orange juice and compared with pasteurized orange juice (80 °C 15 sec) and freshly-squeezed orange juice (control) during storage for 30 days at 4 °C. The physiochemical properties including total phenolic compound, total flavonoid content, antioxidant activity (DPPH and FRAP assay), pH, total soluble solid, color values, total plate count, yeast and mold count and lactic acid bacteria were analyzed every 3 days. The pH meter was used to determine the pH level (Mettler Toledo, S220). Total soluble solid was measured in the range of 0–30 °Brix with a digital hand refractometer (Hanna Instrument, 96801). The color values were obtained by a chroma meter (Minolta, Model CR-300 series, Japan) and CIELAB system; where, L* represents the brightness (0 and 100 represent black and white, respectively), a* represents the red (+a*) or green (-a*) and b* represents the yellow (+b*) and blue. The color difference (ΔE*) was also calculated as follows:
where, subscript “1” as the default color value of the sample and subscript “2” as the color value measured at each time.
2.7. Determination of total phenolic compound
Total phenolic compound was determined by Folin Ciocalteu method previously described by Waterhouse [47] with some modifications. Briefly, 20 µL of sample was mixed with 1.58 mL of distilled waster. 100 µL of Folin Ciocalteu reagent was then added to the mixture. After 5 min incubation, 300 µL of sodium carbonate was added to the mixture and stand for 2 h at room temperature. The absorbance of mixture was measured at 765 nm using spectrophotometer (Thermo Spectronic, Genesys 10 uv, USA). Total phenolic compound was expressed as mg gallic acid equivalent (GAE) / 100 g dry weight (mg GAE/100 g dry wt) or mg GAE/ 100 mL.
2.8. Determination of total flavonoid content
Total flavonoid content was analyzed by aluminium chloride colorimetric method [33]. 0.5 mL of sample was mixed with 2 mL of distilled water, 0.15 mL of 5% (w/v) sodium nitrite and 0.15 mL of 10% (w/v) aluminium chloride. After incubation at ambient temperature for 5 min, mixture was added with 2.2 mL of distilled water and absorbance was measured at 510 nm. The results were expressed as mg quercetin equivalent (QE) / 100 g dry weight (mg QE/100 g dry wt.) or mg QE/100 mL.
2.9. Antioxidant activity by ferric reducing antioxidant power (FRAP) assay
Antioxidant activity by FRAP assay was determined by the method described by Benzie and Strain [9] with slight modification. 50 mL of sample was mixed with 950 µL of warmed (37 °C) FRAP solution. After incubation at ambient temperature for 4 min, absorbance was measured at 593 nm. The reaction was followed up to 30 min and the difference between the final absorbance and blank absorbance was calculated for each sample. Antioxidant activity by FRAP assay was expressed as mM trolox / 100 g dry weight (mM trolox/100 g dry wt.) or mM trolox / 100 mL.
2.10. Antioxidant activity by DPPH assay
The antioxidant activity by DPPH assay was performed according to the previous method described by Brand-Williams and others [10] with modifications. 20 µL of sample or DPPH solution was mixed with 950 µL of DPPH solution and mixture was left in dark and ambient temperature for 15 min. Absorbance was measured at 515 nm and methanol was used as reference sample. Adifference was calculated as a change in absorbance between absorbance of DPPH solution (Aintial) and absorbance of sample (Afinal) as follows;
The results were calculated based on standard curve prepared with different trolox solution (82–5000 µM) and was expressed as mM trolox/100 g dry weight (mM trolox/100 g dry wt.) or mM trolox/100 mL.
2.11. Microbial properties of orange juice [4]
Total plate count was analyzed using pour plate technique by placing a sample of orange juice diluted with 0.85% w/v saline at an appropriate level and a 1 mL pipetted into sterile plate. The plate count agar melted for 15 min at 121 °C and left until the temperature dropped to 45 °C was poured over the sample. Then, plates were incubated for 48 h at 35 °C. The number of colonies formed within the range of 25–250 colonies was counted and reported as colony-forming units/mL (CFU/mL). Yeast and mold count were analyzed using spread plate technique and plates were incubated for 48 h at 30 °C and the number of colonies were reported as CFU/mL. Lactic bacteria were counted by pour plate method by Lactobacillus MRS agar and plates were then incubated in an anaerobic jar for 48 h at 35 °C. The count was reported as CFU/mL.
The shelf life of the treatments was also determined based on the number of total bacteria and yeast and mold as well as physical appearance during the storage.
2.12. Statistical analysis
All experiments were performed in three replicates and data were analyzed by one-way analysis of variance (one-way ANOVA) using SPSS version 20.0 statistic software. Mean values were separated using Duncan’s multiple range test and significant differences were considered at p ≤ 0.05. Data are presented as mean ± standard deviation (SD). The Minitab 16 Statistical Expert software (Stat-Ease, Inc., U.S.A) was used to establish the regression model, composing Box-Behnken design and predicting the optimal conditions for antioxidant properties from UAE experiment.
3. Results and discussion
3.1. Optimization of ultrasound-assisted extraction (UAE) of makiang seed
Several experimental variables including ethanol concentration, amplitude, temperature and time play an essential role on effectiveness of UAE [23]. In this study, ethanol concentration at 70% was set as a fixed factor to determine optimization condition of antioxidant properties from UAE because this condition provided the highest values of antioxidant properties including total phenolic compound, total flavonoid content and antioxidant activity by DPPH and FRAP assays according to preliminary study (data not shown). This is also conformed with previous study that reported 70% ethanol concentration was the appropriate condition to obtain highest total phenolic content, total flavonoid content and antioxidant activity [54]. Therefore, 3 independent variables were established in response surface methodology namely temperature time and amplitude. In addition, the extraction yield of all UAE treatments ranged from 14.19–17.94% (data not shown).
Response surface methodology with Box–Behnken experimental design was performed to determine optimum extraction condition for total phenolic compound, total flavonoid content and antioxidant activity by DPPH and FRAP assays (Table 3). It showed that total phenolic compound, total flavonoid content and antioxidant activity by DPPH and FRAP assays of MSE ranged from 345.33 ± 14.89–585.56 ± 3.67 mg GAE/100 g dry wt., 9.88 ± 0.747–18.59 ± 1.62 mg QE/100 g dry wt., 965.14 ± 47.24–1771.04 ± 13.54 mM trolox/100 g dry wt. and 1106.81 ± 12.50–2233.19 ± 11.27 mM trolox/100 g dry wt., respectively. Table 4 shows analysis of variance (ANOVA) for determination of optimization model fit and it was found that significant (p ≤ 0.05) model F-value with non-significant lack of fit for all responses was observed. In addition, the coefficient of determination (R2) showed a good fit with the experimental data for all responses with R2 higher than 0.9600. The regression coefficients values of the experimental variables are shown in Table 5.
Table 3.
Total phenolic compound, total flavonoid content, antioxidant activity by DPPH and FRAP assay of makiang seed extract (MSE) under different conditions of ultrasound-assisted extraction based on a Box–Behnken design (BBD) for response surface analysis.
| Run | Extraction conditions |
Analytical results |
|||||
|---|---|---|---|---|---|---|---|
| X1-Temperature (°C) | X2- Time (min) | X3- Amplitude (%) | Total phenolic compound (mg GAE/100 g dry wt.) | Total flavonoid content (mg QE/100 g dry wt.) | Antioxidant Activity by DPPH Assay (mM trolox/100 g dry wt.) | Antioxidant Activity by FRAP Assay (mM trolox/100 g dry wt.) | |
| 1 | 30 | 25 | 40 | 438.67 ± 7.05 | 16.63 ± 3.36 | 1551.25 ± 14.47 | 2162.01 ± 14.14 |
| 2 | 70 | 25 | 40 | 469.56 ± 15.41 | 16.33 ± 2.05 | 1479.03 ± 14.44 | 2233.19 ± 11.27 |
| 3 | 30 | 45 | 40 | 404.44 ± 12.69 | 15.07 ± 0.81 | 1261.15 ± 66.39 | 1791.88 ± 44.72 |
| 4 | 70 | 45 | 40 | 510.00 ± 19.00 | 12.22 ± 0.50 | 1448.47 ± 31.82 | 1701.25 ± 71.08 |
| 5 | 30 | 35 | 30 | 570.44 ± 15.00 | 17.56 ± 0.06 | 1247.08 ± 9.55 | 1892.92 ± 71.01 |
| 6 | 70 | 35 | 30 | 345.33 ± 14.89 | 15.77 ± 0.19 | 1270.00 ± 22.53 | 1902.71 ± 11.27 |
| 7 | 30 | 35 | 50 | 354.67 ± 15.93 | 17.33 ± 0.62 | 1385.63 ± 9.26 | 1844.31 ± 40.13 |
| 8 | 70 | 35 | 50 | 502.67 ± 7.86 | 15.57 ± 0.06 | 1413.40 ± 13.43 | 1930.42 ± 44.72 |
| 9 | 50 | 25 | 30 | 402.11 ± 13.00 | 14.66 ± 0.06 | 1672.43 ± 9.39 | 2233.19 ± 61.70 |
| 10 | 50 | 45 | 30 | 452.67 ± 15.14 | 9.88 ± 0.747 | 965.14 ± 47.24 | 1106.81 ± 12.50 |
| 11 | 50 | 25 | 50 | 554.00 ± 1.33 | 12.37 ± 0.06 | 1271.04 ± 19.79 | 1691.88 ± 18.25 |
| 12 | 50 | 45 | 50 | 473.78 ± 15.78 | 12.17 ± 0.87 | 1625.56 ± 6.70 | 1820.56 ± 117.92 |
| 13 | 50 | 35 | 40 | 585.56 ± 3.67 | 17.62 ± 1.62 | 1771.04 ± 13.54 | 2095.35 ± 39.60 |
| 14 | 50 | 35 | 40 | 532.89 ± 14.67 | 18.59 ± 1.62 | 1741.88 ± 13.05 | 2078.68 ± 70.68 |
| 15 | 50 | 35 | 40 | 505.56 ± 10.84 | 18.33 ± 0.62 | 1761.32 ± 10.43 | 2104.38 ± 71.41 |
| 16 | 50 | 35 | 40 | 551.56 ± 14.91 | 17.95 ± 2.12 | 1758.89 ± 14.89 | 2149.86 ± 69.66 |
| 17 | 50 | 35 | 40 | 562.67 ± 8.08 | 17.48 ± 0.81 | 1738.75 ± 15.42 | 2128.68 ± 101.50 |
Table 4.
Analysis of variance for determination of optimization model fit.
| Parameter | Total phenolic compound | Total flavonoid content | Antioxidant activity by DPPH assay | Antioxidant activity by FRAP assay |
|---|---|---|---|---|
| R2 | 0.9610 | 0.9907 | 0.9984 | 0.9916 |
| Adjusted R2 | 0.9108 | 0.9788 | 0.9963 | 0.9808 |
| Lack of fit (F-value) | 0.09 | 0.16 | 1.27 | 3.04 |
| F value | 19.16 | 83.22 | 482.69 | 92.03 |
| P value | 0.000 | 0.000 | 0.000 | 0.000 |
Table 5.
Regression coefficient of the predicted second order polynomial models (BBD) for antioxidant properties.
| Regression coefficient | Total phenolic compound | Total flavonoid content | Antioxidant activity by DPPH assay | Antioxidant activity by FRAP assay |
|---|---|---|---|---|
| Intercept | ||||
| X0 | 574.6* | 17.994* | 1754.38* | 2111.4* |
| Linear | ||||
| X1 | 35.40* | −0.837* | 20.72* | 12.1 |
| X2 | −4.78 | −1.331* | −84.18* | −237.5* |
| X3 | 44.15* | −0.054 | 67.62* | 21.4 |
| Quadratic | ||||
| X12 | −0.91* | 0.00678* | −1.8696* | 0.176 |
| X22 | −0.043 | −0.03610* | −1.3244* | −1.569* |
| X32 | −0.727* | −0.02115* | −2.3839* | −2.414* |
| Cross product | ||||
| X1X2 | 0.15 | −0.00637* | 0.6489* | −0.405 |
| X1X3 | 0.336* | 0.0008 | 0.0121 | 0.141 |
| X2X3 | −0.327* | 0.01145* | 2.6545* | 3.138* |
*Level of significance p ≤ 0.05.
To evaluate the effects of independent variables on the antioxidant properties (total phenolic compound, total flavonoid and antioxidant activity by DPPH and FRAP assays), 3-dimensional response surface plots of the model were graphed for determination of the optimal values to obtain the optimum antioxidant properties of MSE and were used to observe the effect of temperature, time and amplitude on total phenolic compound, total flavonoid and antioxidant activity by DPPH and FRAP assays (Fig. 1, Fig. 2, Fig. 3, Fig. 4, respectively). The response surface plots were formed by fixing two independent variables and changing the remaining one for each response.
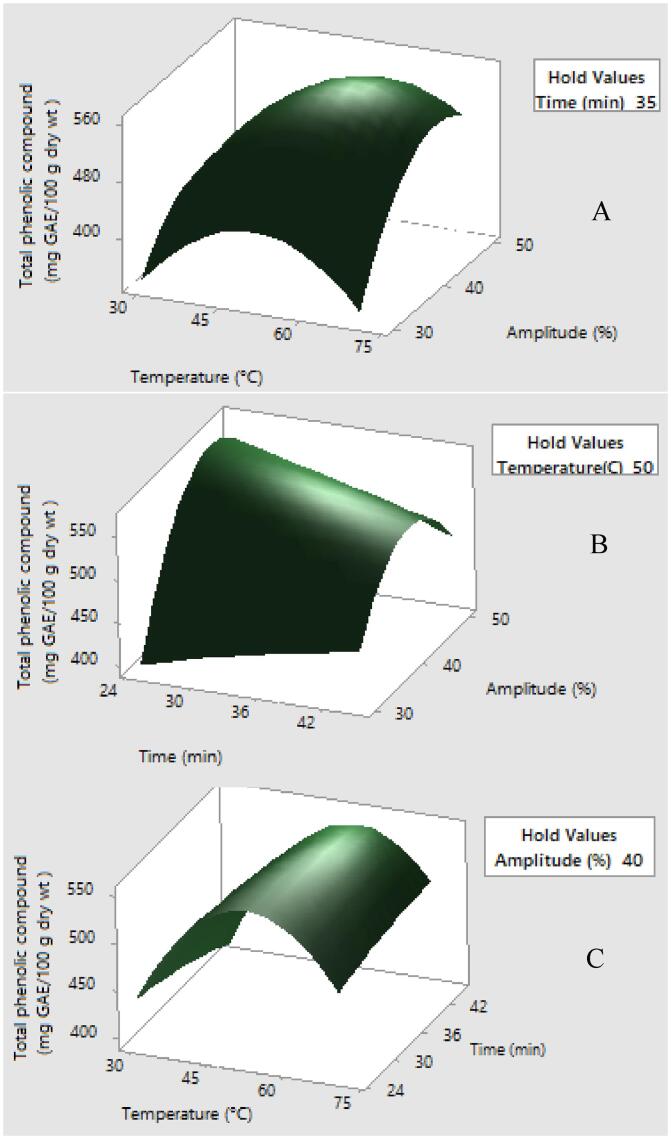
Fig. 1.
Response surface plots showing the effects of variables on the total phenolic compound (mg GAE/100 g dry wt.) (a) time was constant at 35 min (b) temperature was constant at 50 °C and (c) amplitude was constant at 40%
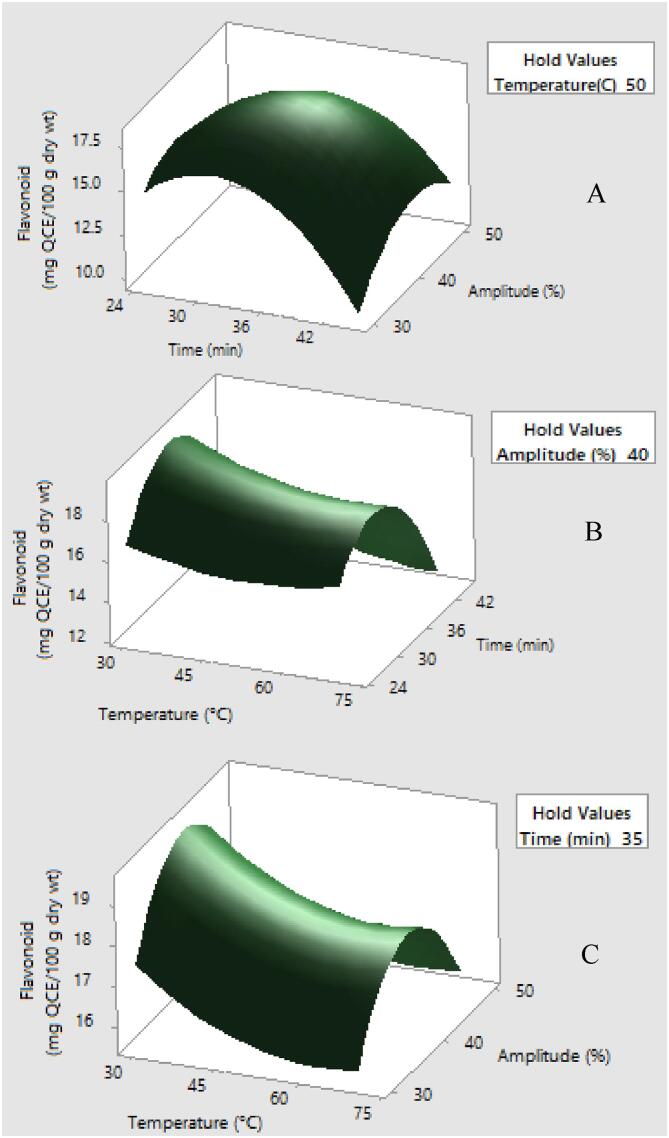
Fig. 2.
Response surface plots showing the effects of variables on total flavonoid content (mg QE/100 g dry wt.) (a) temperature was constant at 50 °C (b) amplitude was constant at 40% and (c) time was constant at 35 min.
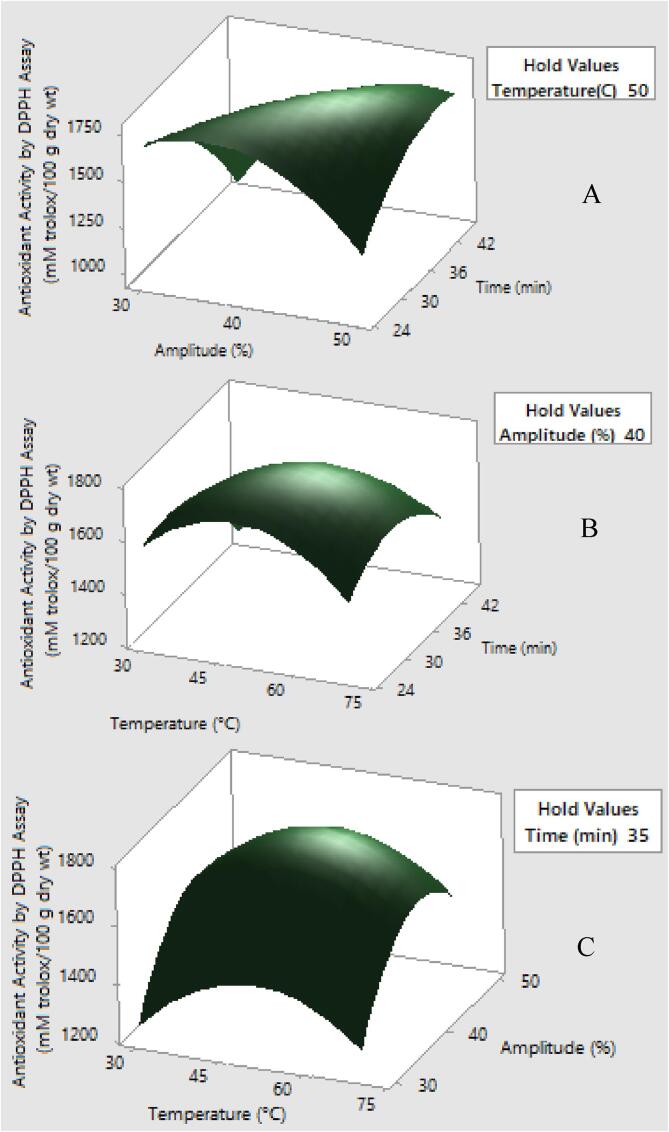
Fig. 3.
Response surface plots showing the effects of variables on antioxidant activity by DPPH assay (mM trolox/100 g dry wt.) (a) temperature was constant at 50 °C (b) amplitude was constant at 40% and (c) time was constant at 35 min.
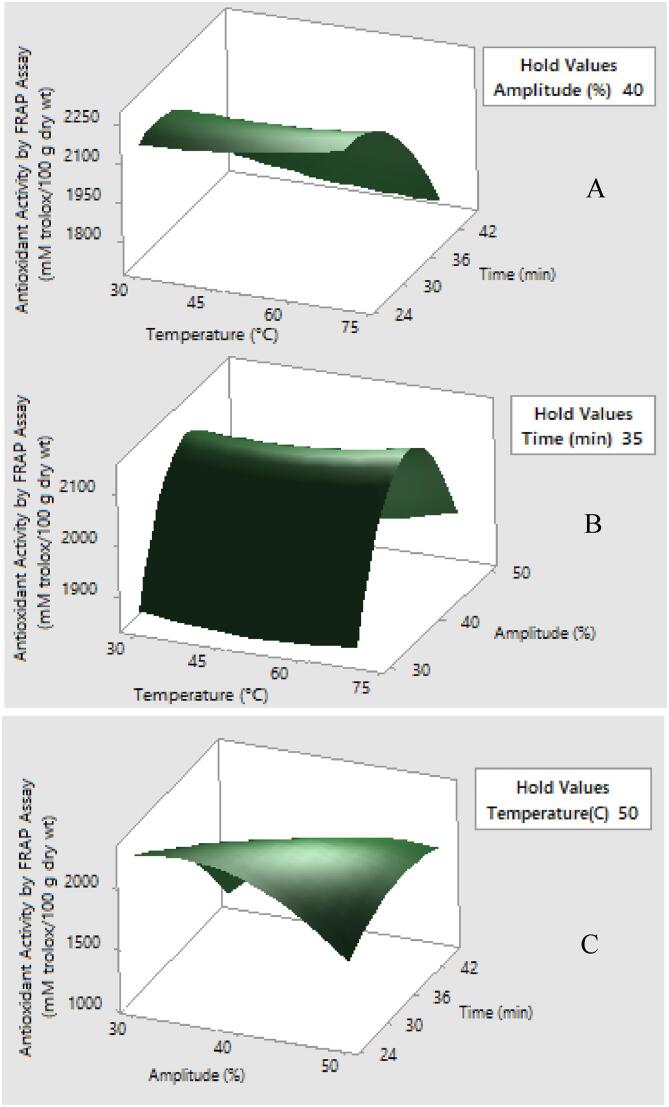
Fig. 4.
Response surface plots showing the effects of variables on antioxidant activity by FRAP assay (mM trolox/100 g dry wt.) (a) amplitude was constant at 40% (b) time was constant at 35 min and (c) temperature was constant at 50 °C.
Effect of extraction parameters on total phenolic compound is depicted in response surface plots as shown in Fig. 1. A temperature increase had a positive effect on yield of total phenolic compound and this result is in accordance with previous study on optimization of phenolic compounds extraction [40]. In addition, this result was also in good agreement with previous study that reported the increased yield of phenolic compounds was observed by heating the extracting solvent using ethanol solvent [19]. The amplitude term was the most positively significant term in the model equation for total phenolic compound whereas quadratic term of temperature (X12) had the greatest negative impact. In addition, extraction variables of total phenolic compound were well correlated with quadratic regression model due to a high regression coefficient (R2 = 0.9610). The findings obtained from our study are in good agreement with previous report from [3] who observed an increased yield of total phenolic compound from lyophilized fig fruit resulted from a rise in extraction temperature. The extraction yield of total phenolic compound could be expressed by the following polynomial equation:
The performance of UAE primarily depends on cavitation phenomenon in the media promoting compression and expansion of solvent and micro-jet formation of solvent is activated. This initiates the formation of micropores in matrix cell expediting the passage of solvent to target compound located in intracellular component [45].
An increase in amplitude promoted the yields of total phenolic compound, total flavonoid content and antioxidant activity by DPPH and FRAP assays. However, above a specific level of amplitude can decrease extraction efficiency due to the small bubble formation is initiated and this consequent a reduced ultrasound energy transmission through solvent [5]. Time is also an important factor that has a direct impact on phenolic compound and flavonoids extracted from makiang seed. Therefore, higher extraction time could lead to a greater yield of extracted compounds; however, excessive extraction time can negatively impact on extracted compound as its degradation [17].
The linear effect of temperature, time, amplitude as well as quadratic effects of temperature (X12), time (X22) and amplitude (X32) and interaction of temperature × time (X1X2) and time × amplitude (X2X3) demonstrated significant effects on total flavonoid content (Table 5). Total flavonoid content was mainly dependent on X2 followed by X3 and X1 according to their regression coefficient. The relationship between extraction yield of total flavonoid content and variable are illustrated as the following second order polynomial equation.
The lack of fit value (F) of 0.16 showed non significance and the model was fit with good prediction (R2 = 0.9907 and adjusted R2 = 0.9788).
The ultrasound-assisted extraction possessed a significant impact on extraction of total phenolic compound and total flavonoid content. When extraction temperature increased to certain value, the extracted amount was enhanced. This result may be to an increase in temperature lead to a greater mass transfer resulting in higher extraction yield, but excessive temperature may cause a damage on bioactive compounds [14]. In addition, several studies have revealed that total phenolic compound and total flavonoid content tend to increase as an increasing amplitude in UAE of plant materials; however, excessive exposure to ultrasound can subsequently cause a degradation of biologically active compounds, Therefore, a suitable amplitude in UAE is critical to achieve high level of bioactive compound with regard to quality and quantity [46].
Different methods for antioxidant activity determination have been well-documented and the most commonly selected methods are DPPH assay (activity to scavenge DPPH radicals or ability to donate H atom) and FRAP assay (activity to reduce metal ions or ability to donate H atom) [28]. In this present study, the influence of the UAE variables on the antioxidant activity of MSE was evaluated by DPPH and FRAP assays. In overall, variations among the antioxidant activity of MSE were significantly (p ≤ 0.05) dependent on the UAE conditions.
Antioxidant activity by DPPH assay under ultrasound-assisted extraction was not significantly affected by quadratic effect of temperature and amplitude (X1X3). Remaining factors showed significant effect on antioxidant activity by DPPH assay (p ≤ 0.05). In addition, linear term of temperature and amplitude contributed to positive impact on antioxidant activity by DPPH assay. The polynomial equation for extraction yield of antioxidant activity by DPPH assay was described as follows;
The ANOVA results (Table 5) exhibit that the polynomial model is greatly significant (p ≤ 0.05) for antioxidant activity by FRAP assays which was mainly influenced by amplitude (X3). The quadratic effect of time (X22) and amplitude (X32) as well as the interaction between time and amplitude (X2X3) revealed a significant effect on antioxidant activity by FRAP assays.
The model for antioxidant activity by FRAP assay is represented in following equation;
The main effect of ultrasound-assisted extraction on antioxidant activity by DPPH and FRAP assays is the increased permeability of cell wall leading to a greater extraction of phenolic compounds and flavonoids [24].
Chemical structures and number of functional groups of phenolic compounds including (1) number and position of alcohol groups on the aromatic ring; (2) molecular size; (3) flexibility/bulkiness; and (4) water solubility are considerably involved in antioxidant activity [2]. Besides, the ability of aromatic hydroxylated rings to donate hydrogen atoms accounted for antioxidant activity of phenolic acids [1]. Relationship between antioxidant activity and total phenolic compound has been well documented and it has been reported that secondary metabolites especially phenolic compounds are key active compound responsible for antioxidant activity [22].
3.2. Optimal UAE condition and its validation
After running several numerical optimizations, predicted optimized conditions were temperature 51.82 °C, time 31.87 min and amplitude 40.51. However, makiang seed was practically extracted under these following conditions; temperature 52 °C, time 32 min and amplitude 41% after rounding to experimentally verify model validity. Then, the yield of total phenolic compound, total flavonoid content and antioxidant activity by DPPH and FRAP assays were determined to verify the reliability of the predicted optimum condition. The predicted values and experimental values of all responses obtained from optimal extraction condition are shown in Table 6. According to the validation results, it revealed that slightly variation or %Difference between the experimental response and predicted values from optimum condition confirmed the validity of the proposed optimum UAE condition. Therefore, predicted model can be applied to optimize the extraction conditions for total phenolic compound, total flavonoid content and antioxidant activity by DPPH and FRAP assays.
Table 6.
Experimental values (data) of the validation of predicted values
| Parameters | Experimental values | Predicted values | % Difference | Unit |
|---|---|---|---|---|
| Total phenolic compound | 518.00 ± 15.87 | 548.08 | 5.49 | mg GAE/100 g dry wt. |
| Total flavonoid content | 18.62 ± 0.92 | 18.00 | 3.44 | mg QE/100 g dry wt. |
| Antioxidant activity by DPPH assay | 1731.46 ± 14.77 | 1754.62 | 1.28 | mM trolox/100 g dry wt. |
| Antioxidant activity by FRAP assay | 2122.08 ± 12.63 | 2111.55 | 0.50 | mM trolox/100 g dry wt. |
3.3. Phenolic compound profile of optimized makiang seed extract
The phenolic compound profile obtained from maceration extraction (ME) was used to compare with ultrasound-assisted extraction (UAE) in this study. All phenolic compounds of MSE from UAE were significantly higher than those from ME (p ≤ 0.05) as shown in Table 7. In the other word, concentration of the phenolic compound identified in the MSE were enhanced by UAE and the most abundant phenolic compound in MSE was characterized as gallic acid (2033.34 ± 30.73 and 1479.96 ± 107.62 mg/100 g dry wt. for UAE and ME, respectively).
Table 7.
Phenolic compound profile of makiang seed from maceration extraction (ME) and ultrasound-assisted extraction (UAE)
| Phenolic compound | UAE | ME | Unit |
|---|---|---|---|
| Gallic acid | 2033.34 ± 30.73a | 1479.96 ± 107.62b | mg/100 g dry wt. |
| Ferulic acid | 1908.53 ± 232.52a | 380.75 ± 33.32b | mg/100 g dry wt. |
| Caffeic acid | 156.29 ± 17.77 | nd | mg/100 g dry wt. |
| Quercetin | 241.97 ± 43.65a | 161.52 ± 31.30b | mg/100 g dry wt. |
a, b: Different superscript letters on the same low show the significant difference between the mean values (p ≤ 0.05).
nd: not detected.
Gallic acid is one of the most important phenolic compound in plant material due to its antioxidant activity. It has been documented that the antioxidant activity of gallic acid is stronger than BHT, BHA and alpha-tocopherol while the long-term use has not shown any adverse effect [27]. Furthermore, gallic acid is heat stable compound that is not decomposed at temperatures up to 100 °C; therefore, thermal degradation should be disregarded and can be used instead of some synthetic antioxidants in food industry [38]. Ferulic acid is also widely known as antioxidant and it is abundant in fruit and vegetable including seed and leaf; therefore, by product from plant can be used as a rich source of ferulic acid [12]. In addition, the caffeic acid commonly found in plant and propolis has been reported that it provides excellent free radical scavenging ability and can be commercially used to protect polyunsaturated oils [51]. Besides, quercetin, a member of flavonoids, is considered as one of the most promising natural antioxidant. It is naturally found in various foods such as fruit, vegetable, tea and wine. Importantly, quercetin has been claimed to provide beneficial health effect including lowering a risk of cancer and cardiovascular disease [9].
3.4. Antimicrobial activity by minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)
Results showed that MSE was able to inhibit the growth of all types of tested microorganisms, namely Staphylococcus aureus, Escherichia coli, Saccharomyces cerevisiae, Lactobacillus plantarum and Salmonella Typhimurium. MIC values were in the range of 1.56–6.25 mg/mL and it exhibited that S. aureus and L. plantarum were the most sensitive microorganisms to MSE according to the lowest MIC values (Table 8). Our results were consistent with the study of Leelapornpisit, Khansuwan, Kittipongpattana, and Rojanakul [29] which showed that the MSE was effective against Propionibacterium acenes and S. aureus. The MBC values showed the best activity against S. aureus, L. plantarum and S. cerevisiae (25 mg/mL), followed by S. Typhimurium (50 mg/mL) and E. coli (100 mg/mL), respectively (Table 8). Due to the differences in microbial structures, phenolic compounds with different antimicrobial activity can penetrate differently, which can affect the MIC and MBC values of each species. It was found that MSE had the best inhibitory effect to the growth of S. aureus, L. plantarum, and S. cerevisiae followed by S. Typhimurium and E. coli, respectively, since S. aureus and L. plantarum were Gram positive bacteria which cell wall structure is less complex than Gram-negative bacteria (S. Typhimurium and E. coli were more resistant to the MSE). Consistently, a study demonstrated that the leaf extract from Limnophila rugosa showed the higher antibacterial activity against S. aureus than E. coli. Gram-negative bacteria have an outer membrane and periplasmic space which are not found in Gram-positive bacteria, and act as barrier to penetration of substances [42].
Table 8.
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of makiang seed extract from optimized ultrasound-assisted extraction.
| Microorganisms | Staphylococcus aureus | Lactobacillus plantarum | Saccharomyces cerevisiae | Salmonella Typhimurium | Escherichia coli |
|---|---|---|---|---|---|
| MIC (mg/mL) | 1.56 | 1.56 | 1.56 | 3.125 | 6.25 |
| MBC (mg/mL) | 25 | 25 | 25 | 50 | 100 |
3.5. Physiochemical properties of orange juice samples added with makiang seed extract during storage at 4 °C
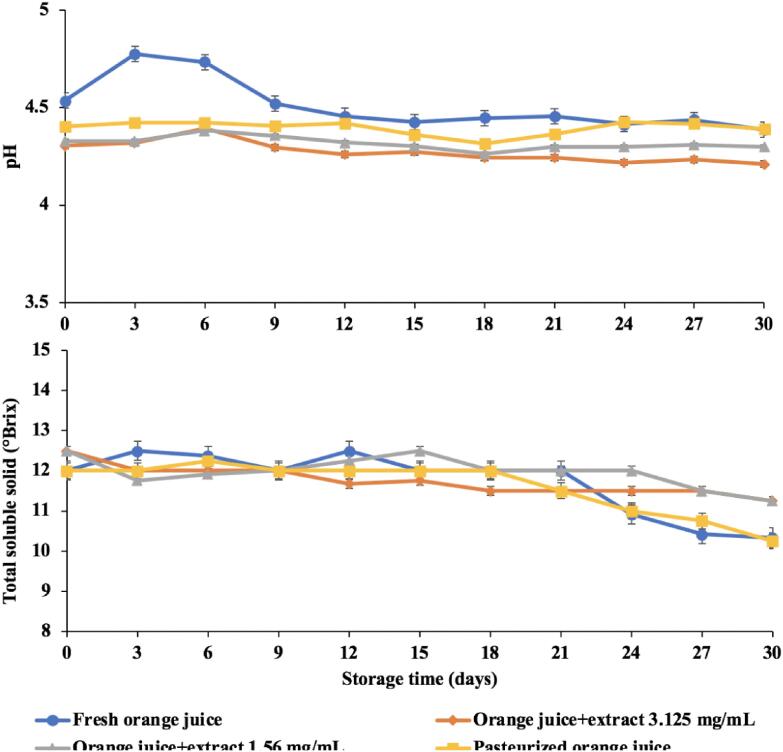
The pH value and total soluble solid decreased as storage increased (Fig. 5). The pH and total soluble solid were in the range of 4.21–4.77 and 10.25–12.50 °Brix, respectively. These results were consistent with Ibrahim [26] examining the changes in pH during storage of pineapple, papaya and watermelon juices for 4 weeks at 4 °C and room temperature. Their results showed that pH values of all fruit juices tended to decrease as the storage increased. They attributed their findings to the growth of microorganisms. Both extract added samples (1.56 and 3.125 mg/mL) resulted in a higher total soluble solid than freshly squeezed orange juice (control) and pasteurized samples at the end of storage (11.25 and 11.25 VS 10.33 and 10.25 °Brix, respectively). The decrease in total soluble solid during the storage was a result of microorganisms using sugar in orange juice to convert into energy and use for growth which resulted in the decreased total soluble solid. Moreover, [25] showed that total soluble solid content in orange juice decreased after 4 weeks at 7 °C. They related their results to the growth of microorganisms.
Fig. 5.
pH and total soluble solid of orange juice sample during the storage at 4 °C.
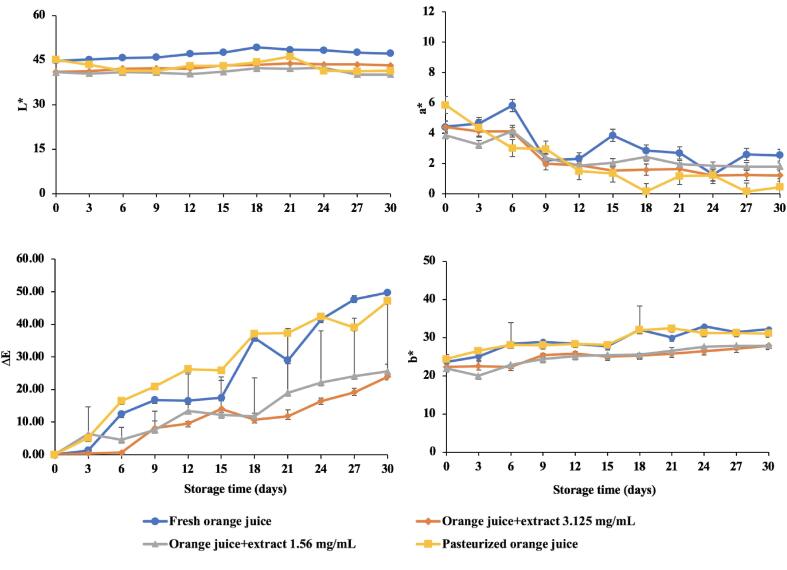
Color is a visible physical property of food and plays an important role in consumer satisfaction [50]. The initial L* and a* values for freshly squeezed orange juice (control), extract added (1.56 and 3.125 mg/mL) and pasteurized orange juice were 47.18, 40.15, 43.82, 45.07 and 4.42, 3.87, 4.40 and 5.86, respectively (Fig. 6). The a* values of all samples tended to decrease as the storage increased . Conversely, the b* and ΔE* values of the samples tended to increase as the storage period increased. It should be noted that both extract added (1.56 and 3.125 mg/mL) samples had lower b* and ΔE* values than control and pasteurized samples. As mentioned earlier, L* value is the brightness of sample (if L* is a large value, the sample is very bright.), a* value is green and red (−a* indicates green and +a* indicates red color), b* value representing yellow and blue (−b* representing blue and +b* representing yellow), and ΔE* value represents the color difference between the pre-storage and after storage of orange juice samples (if the ΔE* value was high, the sample had a very different color from the initial value). As a result, the orange juice samples in our study showed a decrease in brightness and the color were more red than green. Additionally, an increasing b* value indicated the orange juice samples were more yellow than blue. The increased ΔE* value during the storage could be due to the changes in carotenoid content, the main pigment of orange juice, the oxidation of total phenolic compound as well as the non-enzymatic browning reaction resulting from ascorbic acid decomposition [50]. Choi, Kim, and Lee [15] reported an increase in the ΔE* value which was associated with a decrease in the carotenoid contents after 7 weeks at 4.5 °C. The greater difference in ΔE* value of extract added (1.56 and 3.125 mg/mL) samples as compared with that of freshly squeezed orange juice (control) and pasteurized orange juice samples could also be due to the color of the extract which affected the color of the orange juice.
Fig. 6.
Color values (L*, a*, b*) and color difference (ΔE) of orange juice samples during the storage at 4 °C.
3.6. Antioxidant and antioxidant activity (DPPH and FRAP assays) of orange juice samples added with makiang seed extract during storage at 4 °C
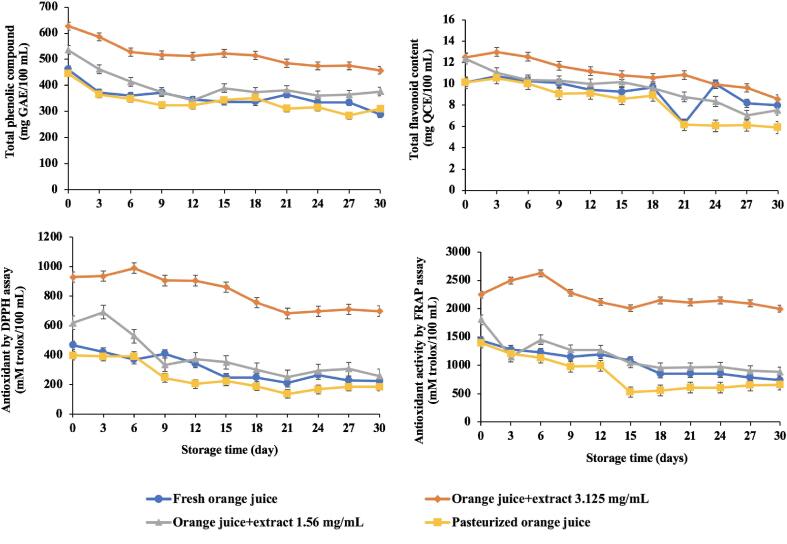
The initial total phenolic compound of control, extract added (1.56 and 3.125 mg/mL) and pasteurized orange juices were; 461.67, 535.50, 627.50 and 446.00 (mg GAE/100 mL), respectively which decreased during the storage (Fig. 7). The lower total phenolic compound and total flavonoid content in pasteurized samples was because the heat from the pasteurization process could damage and decompose phenolic compounds [49]. The reduction in total phenolic compound at the 30 days of storage comparing with the 1st day was 37.50, 29.69, 27.00, and 30.49 (%) for control, extract added (1.56 and 3.125 mg/mL) and pasteurized samples, respectively. This was consistent with a study by Maragò, et al. [34] who reported decreased total phenolic compound during the storage of apple and pomegranate juices at −20 °C, 4 °C and room temperature after 2 weeks. Furthermore, up to 50% reduction of total phenolic compound was observed after storing roselle-mango, okra-papaya and okra-guava juices at 4 °C and 28 °C for 180 days [35] The initial total flavonoid content of control, extract added (1.56 and 3.125 mg/mL) and pasteurized orange juice samples were 10.14, 12.3, 12.50 and 10.12 (mg QE/100 mL), respectively, which decreased during the storage (Fig. 7). The flavonoid contents among the samples showed almost the same trend as total phenolic compound. The control, extract added (1.56 and 3.125 mg/mL) and pasteurized samples in the last storage day (30 day) showed respectively a reduction of 21.40, 40.65, 31.36 and 41.69% compared with the initial day. The flavonoid degradation during storage was consistent with [16] who examined the total flavonoid content of orange juices from various citrus species (Palazzelli, Minneola, Salustiana and Shamouti) and red grape juice. According to that study, the flavonoid contents significantly decreased after 15 days of storage at 4 °C.
Fig. 7.
Antioxidant property (total phenolic compound, total flavonoid content and antioxidant activity by DPPH and FRAP assays) of orange juice samples during the storage at 4 °C.
The initial values of antioxidant activity by DPPH and FRAP assays for control, extract added (1.56 and 3.125 mg/mL) and pasteurized samples were 468.42, 618.83, 929.46, 398.00 (mM trolox/100 mL) and 1439.46, 1809.88, 2252.58 and 1399.04 (mM trolox/100 mL), respectively, which moderately decreased during the storage (Fig. 7). Not surprisingly, extract added samples (3.125 mg/mL and 1.56 mg/mL) had higher antioxidant activity (DPPH and FRAP assay) than control and pasteurized orange juice, respectively which could be due to the high content of phenolic compounds as well as flavonoid content in makiang seed extract (MSE).
3.7. Microbiological properties of orange juice samples added with makiang seed extract (MSE) during storage at 4 °C
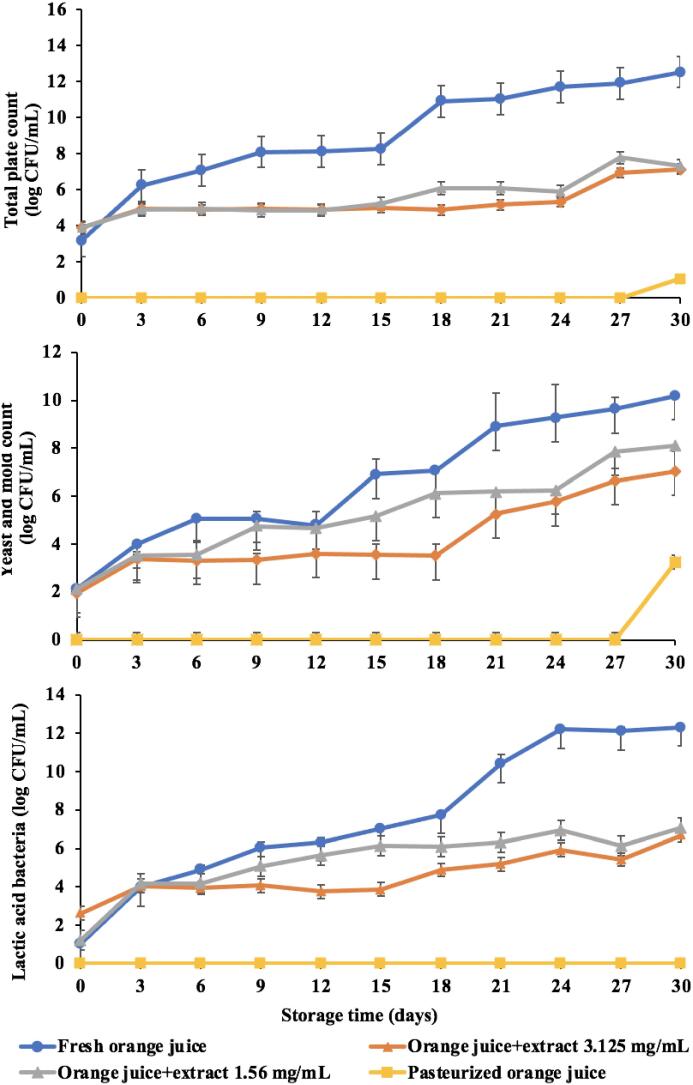
The total microbial count, yeast and mold count and lactic acid bacteria tended to increase as the storage period increased (Fig. 8). The total microbial count (total plate count) in beverages must not exceed 1.0 × 105 CFU/mL [52]. The initial microbial counts of control, extract added (1.56 and 3.125 mg/mL) and pasteurized orange juices were 1.44 × 103, 7.80 × 103, 7.50 × 103 and <10 CFU/mL, respectively and all samples except pasteurization had more than 1.0 × 105 CFU/mL after 3, 15, and 21 days of storage, respectively. Since orange juice contains a relatively low pH (3.0–4.0), it limits the type and number of microorganisms that can survive. At the same time, orange juice had a relatively high content of total soluble solid. Therefore, yeast is often the main cause of the deterioration of orange juice. Yeast and mold found in orange juice must not exceed 5.0x103 CFU/mL [55]. The initial yeast and mold content of control, extract added (1.56 and 3.125 mg/mL) and pasteurized orange juice samples were 1.32 × 102, 1.35 × 102, 8.60 × 10 and <10 CFU/mL, respectively which recorded greater than standard on days 3, 9, and 21, respectively. However, the pasteurization showed the onset of yeast growth only at day 30. Because orange juice has a high sugar content, it is frequently spoiled by bacteria creating lactic acid normally found in orange juice and they are generally approximately 1.0 × 104 CFU/mL [18], and must not exceed 1.0 × 105 CFU/mL to be within the acceptable limits. The initial lactic acid bacteria content of the control, extract added (1.56 and 3.125 mg/mL) and pasteurized orange juice samples were 1.08 × 10, 1.66 × 10, 4.2 × 102 and <1 CFU/mL, respectively and the population exceeded the limit on days 9, 12 and 21, respectively.
Fig. 8.
Microbial stability (total plate count, yeast and mold count and lactic acid bacteria) of orange juice samples during the storage at 4 °C.
Total microorganism, yeast and mold and lactic acid bacteria were used together with the changes in physical characteristics of the orange juice as criteria in the determination of shelf life in orange juice. When considering the amount of yeast, which is the main microorganism that causes deterioration in orange juice, together with changes in color, smell and appearance we concluded that control, extract added (1.56 and 3.125 mg/mL) and pasteurized orange juice samples had a shelf life of approximately 3, 9, 21 and 30 days, respectively as preserved at 4 °C. Since freshly squeezed orange juice is unprocessed, we expected the less shelf life as compared with other samples. Our results in terms of shelf life were consistent with [53] who examined the application of apple peel extracts in tomato juice stored for 10 days at 4 °C. Their results proved a longer shelf life in extract added to tomato juice VS control. It was also suggested that plant extracts could be applied in fruit juices to increase the shelf life of juices.
4. Conclusion
In this present study, BBD with RSM was successfully employed to determine optimal experimental parameters of UAE for antioxidant properties (total phenolic compound, total flavonoid content and antioxidant activity by DPPH and FRAP assays) from makiang seed. Three extraction parameters including temperature, time and amplitude were optimized to obtain the highest antioxidant properties. The optimal condition for three parameters was analyzed as temperature 51.82 °C, time 31.87 min and amplitude 40.51%. The results showed that there were no significant differences between predicted and experimental values of antioxidant properties obtained from makiang seed extract (MSE). In addition, HPLC analysis indicated that gallic acid was the most abundant phenolic compound found in optimized MSE. Based on antimicrobial activity against pathogenic and spoilage microorganisms, MSE showed a promising antimicrobial property. The addition of MSE in orange juice significantly preserved antioxidant property and extended the shelf life of orange juice samples during cold storage. Therefore, this study confirmed the application of emerging extraction technology of ultrasound for bioactive compound extraction with antioxidant activity from plant by-products according to industrial demand and sustainable development. Consequently, the application of makiang seed extract may be considered to improve antioxidant property and stability of orange juice during cold storage. In addition, this extract may be recommended as potentially functional food ingredient for health-conscious consumer and up-scaling of this advanced extraction technology needs to be performed for mass production as an industrial operation.
CRediT authorship contribution statement
Thitirat Sirichan: Investigation, Formal analysis, Data curation, Writing - original draft. Isaya Kijpatanasilp: Investigation, Data curation, Writing - original draft. Nicha Asadatorn: Investigation, Formal analysis, Data curation, Writing - original draft. Kitipong Assatarakul: Conceptualization, Data curation, Funding acquisition, Project administration, Supervision, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
The authors would like to acknowledge The 90th Anniversary of Chulalongkorn University Fund (Ratchadaphiseksomphot Endowment Fund), Chulalongkorn University for providing financial support for this study under graduate research grant.
Data availability statements
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Adefegha S.A. Functional foods and nutraceuticals as dietary intervention in chronic diseases; novel perspectives for health promotion and disease prevention. Journal of Dietary Supplements. 2017;15(6):977–1009. doi: 10.1080/19390211.2017.1401573. [DOI] [PubMed] [Google Scholar]
- 2.Agatonovic-Kustrin S., Morton D.W., Ristivojevic P. Probing into the molecular requirements for antioxidant activity in plant phenolic compounds utilizing a combined strategy of PCA and ANN. Comb. Chem. High Throughput Screening. 2017;20(1):25–34. doi: 10.2174/1386207320666170102123146. [DOI] [PubMed] [Google Scholar]
- 3.Ana B.K., Mirela P., Srećko T., Stela J., Ibrahim M., Mate B., Darko V. Effect of extraction conditions on the extractability of phenolic compounds from lyophilised fig fruits (Ficus Carica L.) Polish Journal of Food and Nutrition Sciences. 2011;61(3):195–199. doi: 10.2478/v10222-011-0021-9. [DOI] [Google Scholar]
- 4.AOAC . 17th ed. Association of official Analytical Chemists; Washington, DC: 2005. Methods of analysis. [Google Scholar]
- 5.Ashokkumar M. Applications of ultrasound in food and bioprocessing. Ultrason. Sonochem. 2015;25:17–23. doi: 10.1016/j.ultsonch.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Baş D., Boyacı İ.H. Modeling and optimization I: usability of response surface methodology. Food Engineering. 2007;78(3):836–845. doi: 10.1016/j.jfoodeng.2005.11.024. [DOI] [Google Scholar]
- 7.Benzie I.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 8.Bezerra M.A., Santelli R.E., Oliveira E.P., Villar L.S., Escaleira L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta. 2008;76(5):965–977. doi: 10.1016/j.talanta.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 9.Boots A.W., Haenen G.R.M.M., Bast A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008;583(2–3):325–337. doi: 10.1016/j.ejphar.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Brand-Williams W., Cuvelier M.E., Berset C.J. Use of a free radical method to evaluate antioxidant activity. LWT - Food Science and Technology. 1995;28(1):25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 11.Bubalo M.C., Ćurko N., Tomašević M., Ganić K.K., Redovniković I.R. Green extraction of grape skin phenolics by using deep eutectic solvents. Food Chem. 2016;200:159–166. doi: 10.1016/j.foodchem.2016.01.040. [DOI] [PubMed] [Google Scholar]
- 12.Calabrese E.J., Agathokleous E., Calabrese V. Ferulic acid and hormesis: Biomedical and environmental implications. Mech. Ageing Dev. 2021;198:111544. doi: 10.1016/j.mad.2021.111544. [DOI] [PubMed] [Google Scholar]
- 13.Cartea M.E., Francisco M., Soengas P., Velasco P. Phenolic compounds in Brassica vegetables. Molecules. 2010;16:251–280. doi: 10.3390/molecules16010251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chemat F., Rombaut N., Meullemiestre A., Turk M., Perino S., Fabiano-Tixier A.-S., Abert-Vian M. Review of green food processing techniques. Preservation, transformation, and extraction. Innovative Food Sci. Emerg. Technol. 2017;41:357–377. doi: 10.1016/j.ifset.2017.04.016. [DOI] [Google Scholar]
- 15.Choi M.H., Kim G.H., Lee H.S. Effects of ascorbic acid retention on juice color and pigment stability in blood orange (Citrus sinensis) juice during refrigerated storage. Food Res. Int. 2002;35(8):753–759. doi: 10.1016/S0963-9969(02)00071-6. [DOI] [Google Scholar]
- 16.Del Caro A., Piga A., Vacca V., Agabbio M. Changes of flavonoids, vitamin C and antioxidant capacity in minimally processed citrus segments and juices during storage. Food Chem. 2004;84(1):99–105. doi: 10.1016/S0308-8146(03)00180-8. [DOI] [Google Scholar]
- 17.Deng J., Xu Z., Xiang C., Liu J., Zhou L., Li T., Yang Z., Ding C. Comparative evaluation of maceration and ultrasonic-assisted extraction of phenolic compounds from fresh olives. Ultrason. Sonochem. 2017;37:328–334. doi: 10.1016/j.ultsonch.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 18.Ding W.K., Shah N.P. Survival of free and microencapsulated probiotic bacteria in orange and apple juices. International Food Research Journal. 2008;15(2):219–232. [Google Scholar]
- 19.Ghitescu R.-E., Volf I., Carausu C., Bühlmann A.-M., Gilca I.A., Popa V.I. Optimization of ultrasound-assisted extraction of polyphenols from spruce wood bark. Ultrason. Sonochem. 2015;22:535–541. doi: 10.1016/j.ultsonch.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 20.Ghorbannezhad P., Bay A., Yolmeh M., Yadollahi R., Moghadam J.Y. Optimization of coagulation-flocculation process for medium density fiberboard (MDF) wastewater through response surface methodology. Desalin. Water Treat. 2016;57(56):26916–26931. doi: 10.1080/19443994.2016.1170636. [DOI] [Google Scholar]
- 21.Górnaś P., Rudzińska M. Seeds recovered from industry by-products of nine fruit species with a high potential utility as a source of unconventional oil for biodiesel and cosmetic and pharmaceutical sectors. Ind. Crops Prod. 2016;83:329–338. doi: 10.1016/j.indcrop.2016.01.021. [DOI] [Google Scholar]
- 22.Heim K.E., Tagliaferro A.R., Bobilya D.J. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. The Journal of Nutritional Biochemistry. 2002;13(10):572–584. doi: 10.1016/s0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- 23.Herrera M.C., Luque de Castro M.D. Ultrasound-assisted extraction of phenolic compounds from strawberries prior to liquid chromatographic separation and photodiode array ultraviolet detection. Chromatography A. 2005;1100(1):1–7. doi: 10.1016/j.chroma.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 24.Huang H., Xu Q., Belwal T., Li L., Aalim H., Wu Q., Duam Z., Zhang X., Luo Z. Ultrasonic impact on viscosity and extraction efficiency of polyethylene glycol: a greener approach for anthocyanins recovery from purple sweet potato. Food Chem. 2019;283:59–67. doi: 10.1016/j.foodchem.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 25.Humayun A., Gautam C.K., Madhav M., Sourav S., Ramalingam C. Effect of citric and malic acid on shelf life and sensory characteristics of orange juice. Int. J. Pharm. Pharm. Sci. 2014;6:117–119. [Google Scholar]
- 26.Ibrahim M.A. Effect of different storage condition on pH and vitamin C content in some selected fruit juices (pineapple, pawpaw and watermelon) Int. J. Biochem. Res. Rev. 2016;11(2):1–5. doi: 10.9734/IJBCRR/2016/23462. [DOI] [Google Scholar]
- 27.Kosar M., Bozan B., Temelli F., Baser K.H.C. Antioxidant activity and phenolic composition of sumac (Rhus coriaria L.) extracts. Food Chem. 2007;103(3):952–959. doi: 10.1016/j.foodchem.2006.09.049. [DOI] [Google Scholar]
- 28.Kranl K., Schlesier K., Bitsch R., Hermann H., Rohe M., Bohm V. Comparing antioxidative food additives and secondary plant products-use of different assays. Food Chem. 2005;93(1):171–175. doi: 10.1016/j.foodchem.2004.11.012. [DOI] [Google Scholar]
- 29.Leelapornpisit P., Khansuwan U., Kittipongpattana N., Rojanakul J. In Proceedings of the Research and Development of Functional Food Products Symposium II. 2004. Chemical properties and antioxidant activities of Makiang seed extract for functional food and cosmetic used. [Google Scholar]
- 30.Lianfu Z., Zelong L. Optimization and comparison of ultrasound/microwave assisted extraction (UMAE) and ultrasonic assisted extraction (UAE) of lycopene from tomatoes. Ultrason. Sonochem. 2008;15(5):731–737. doi: 10.1016/j.ultsonch.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Lobo V., Patil A., Phatak A., Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacol. Rev. 2010;4(8):118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luque-García J.L., Luque de Castro M.D. Ultrasound: a powerful tool for leaching. Trends Anal. Chem. 2003;22(1):41–47. doi: 10.1016/S0165-9936(03)00102-X. [DOI] [Google Scholar]
- 33.Maisuthisakul P., Suttajit M., Pongsawatmanit R. Assessment of phenolic content and free radical-scavenging capacity of some Thai indigenous plants. Food Chem. 2007;100(4):1409–1418. doi: 10.1016/j.foodchem.2005.11.032. [DOI] [Google Scholar]
- 34.Maragò E., Iacopini P., Camangi F., Scattino C., Ranieri A., Stefani A., Sebastiani L. Phenolic profile and antioxidant activity in apple juice and pomace: effects of different storage conditions. Fruits. 2015;70(4):213–223. doi: 10.1051/fruits/2015015. [DOI] [Google Scholar]
- 35.Mgaya‐Kilima Beatrice, Remberg Siv Fagertun, Chove Bernard Elias, Wicklund Trude. Influence of storage temperature and time on the physicochemical and bioactive properties of roselle-fruit juice blends in plastic bottle. Food Science & Nutrition. 2014;2(2):181–191. doi: 10.1002/fsn3.2014.2.issue-210.1002/fsn3.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.NCCLS . National Committee for Clinical Laboratory Standards; Wayne, PA (USA): 1998. Performance Standards for Antimicrobial Susceptbility Testing; Eighth Informational Supplement. National Committee for Clinical Laboratory Standards document M100–S9. [Google Scholar]
- 37.NCCLS . National Committee for Clinical Laboratory Standards; Wayne, PA (USA): 1999. Performance Standards for Antimicrobial Susceptbility Testing; Eighth Informational Supplement. National Committee for Clinical Laboratory Standards document M100–S9. [Google Scholar]
- 38.Özcan Musa. Antioxidant activities of rosemary, sage, and sumac extracts and their combinations on stability of natural peanut oil. J. Med. Food. 2003;6(3):267–270. doi: 10.1089/10966200360716698. [DOI] [PubMed] [Google Scholar]
- 39.Patthamakanokporn O., Puwastien P., Nitithamyong A., Sirichakwal P.P. Changes of antioxidant activity and total phenolic compounds during storage of selected fruits. Food Composition and Analysis. 2008;21(3):241–248. doi: 10.1016/j.jfca.2007.10.002. [DOI] [Google Scholar]
- 40.Reungoat V., Gaudin M., Flourat A.L., Isidore E., Mouterde L.M.M., Allais F., Ducatel H., Ioannou I. Optimization of an ethanol/water based sinapine extraction from mustard bran using response surface methodology. Food Bioprod. Process. 2020;122:322–331. doi: 10.1016/j.fbp.2020.06.001. [DOI] [Google Scholar]
- 41.Sanderson B. Applied sonochemistry-the uses of power ultrasound in chemistry and processing. Chem. Eng. Process. 2004;79:207–208. doi: 10.1002/352760054X.ch7. [DOI] [Google Scholar]
- 42.Shan B., Cai Y.-Z., Brooks J.D., Corke H. The in vitro antibacterial activity of dietary spice and medicinal herb extracts. Int. J. Food Microbiol. 2007;117(1):112–119. doi: 10.1016/j.ijfoodmicro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 43.Slemmer J., Weber J. Assessing antioxidant capacity in brain tissue: methodologies and limitations in neuroprotective strategies. Antioxidants. 2014;3(4):636–648. doi: 10.3390/antiox3040636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taya Sirinya, Punvittayagul Charatda, Inboot Wanida, Fukushima Shoji, Wongpoomchai Rawiwan. Cleistocalyx nervosum extract ameliorates chemical-induced oxidative stress in early stages of rat hepatocarcinogenesis. Asian Pac. J. Cancer Prev. 2014;15(6):2825–2830. doi: 10.7314/APJCP.2014.15.6.2825. [DOI] [PubMed] [Google Scholar]
- 45.Vinatoru M. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason. Sonochem. 2001;8(3):303–313. doi: 10.1016/s1350-4177(01)00071-2. [DOI] [PubMed] [Google Scholar]
- 46.Vinatoru M., Mason T.J., Calinescu I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. Trends Anal. Chem. 2017;97:159–178. doi: 10.1016/j.trac.2017.09.002. [DOI] [Google Scholar]
- 47.Waterhouse A.L. Wrolstad Ed. John Wiley and Sons; New York: 2002. Determination of total phenolics (R. E. [Google Scholar]
- 48.Wei Z.-F., Wang X.-Q., Peng X., Wang W., Zhao C.-J., Zu Y.-G., Fu Y.-J. Fast and green extraction and separation of main bioactive flavonoids from Radix Scutellariae. Ind. Crops Prod. 2015;63:175–181. doi: 10.1016/j.indcrop.2014.10.013. [DOI] [Google Scholar]
- 49.Wen C., Zhang J., Zhang H., Dzah C.S., Zandile M., Duan Y., Ma H., Luo X. Advances in ultrasound assisted extraction of bioactive compounds from cash crops-A review. Ultrason. Sonochem. 2018;48:538–549. doi: 10.1016/j.ultsonch.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 50.Wibowo S., Vervoort L., Tomic J., Santiago J.S., Lemmens L., Panozzo A., Grauwet T., Hendrickx M., Van Loey A. Colour and carotenoid changes of pasteurised orange juice during storage. Food Chem. 2015;171:330–340. doi: 10.1016/j.foodchem.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 51.Aladedunye F., et al. Novel caffeic acid amide antioxidants: Synthesis, radical scavenging activity and performance under storage and frying conditions. Food Chem. 2012 doi: 10.1016/j.foodchem.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 52.EMIRATES AUTHORITY FOR STANDARDIZATION & METROLOGY . Microbiological criteria for food stuffs-part 1. EMIRATES AUTHORITY FOR STANDARDIZATION & METROLOGY; 2000. [Google Scholar]
- 53.Massini L., Rico D., Martin-Diana A.B., Barry-Ryan C. Apple peel flavonoids as natural antioxidants for vegetable juice applications. Eur. Food Res.Technol. 2016 doi: 10.1007/s00217-016-2646-8. [DOI] [Google Scholar]
- 54.Sembiring E.N., Elya B., Sauriasari R. Phytochemical Screening, Total Flavonoid and Total Phenolic Content and Antioxidant Activity of Different Parts of Caesalpinia bonduc (L.) Roxb. Pharmacogno. J. 2018 doi: 10.5530/pj.2018.1.22. [DOI] [Google Scholar]
- 55.http://bqsf.dmsc.moph.go.th/bqsfWeb/index.php/sdm_downloads/dmsc-micro/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.