Abstract
Toads of the genus Atelopus are chemically defended by a unique combination of endogenously synthesized cardiotoxins (bufadienolides) and neurotoxins which may be sequestered (guanidinium alkaloids). Investigation into Atelopus small-molecule chemical defenses has been primarily concerned with identifying and characterizing various forms of these toxins while largely overlooking their ecological roles and evolutionary implications. In addition to describing the extent of knowledge about Atelopus toxin structures, pharmacology, and biological sources, we review the detection, identification, and quantification methods used in studies of Atelopus toxins to date and conclude that many known toxin profiles are unlikely to be comprehensive because of methodological and sampling limitations. Patterns in existing data suggest that both environmental (toxin availability) and genetic (capacity to synthesize or sequester toxins) factors influence toxin profiles. From an ecological and evolutionary perspective, we summarize the possible selective pressures acting on Atelopus toxicity and toxin profiles, including predation, intraspecies communication, disease, and reproductive status. Ultimately, we intend to provide a basis for future ecological, evolutionary, and biochemical research on Atelopus.
Keywords: Atelopus toxins, Tetrodotoxin, Bufadienolides, Chemical defense, Bacterial symbiosis, Methodological bias
Graphical abstract
Highlights
-
•
We review the quantity and diversity of toxins in Atelopus toads.
-
•
Sampling and methodological biases likely affect known toxin quantity and diversity.
-
•
Atelopus extinctions threaten the loss of undescribed toxins.
-
•
Few data exist on the ecology and evolution of Atelopus chemical defenses.
-
•
Atelopus is a promising study system for toxin sequestration and synthesis.
1. Introduction
Harlequin toads (Anura: Bufonidae: Atelopus) are small, diurnal, and poisonous amphibians native to South and Central America (Lötters et al., 2011). Many species are brightly colored on all or part of their bodies (Fig. 3c; Lötters et al., 2011), and these colors may act as aposematic signals to warn potential predators of their toxicity (Rößler et al., 2019). Harlequin toads are smooth skinned and lack the large parotoid glands commonly observed in other bufonids. Instead, Atelopus granular glands are small and evenly distributed across their bodies (Mcdiarmid, 1971). Concentrated within the granular glands and skin epithelium (Mebs et al., 2018a) are two classes of toxic chemicals: bufadienolides and guanidinium alkaloids (Daly et al., 1997). With the possible exception of Clinotarsus curtripes (see Section 4.1.1; Gosavi et al., 2014), the cooccurrence of these toxins is unique to Atelopus, and extensive research has focused on describing the chemicals found in Atelopus skin – uncovering several toxins found nowhere else in the natural world (Yotsu-Yamashita et al., 2004; Yotsu et al., 1990b). However, toxin assessment of Atelopus species has been geographically and taxonomically biased, and most species have not been evaluated. Furthermore, the ecology and evolution of Atelopus chemical defenses have received little investigation.
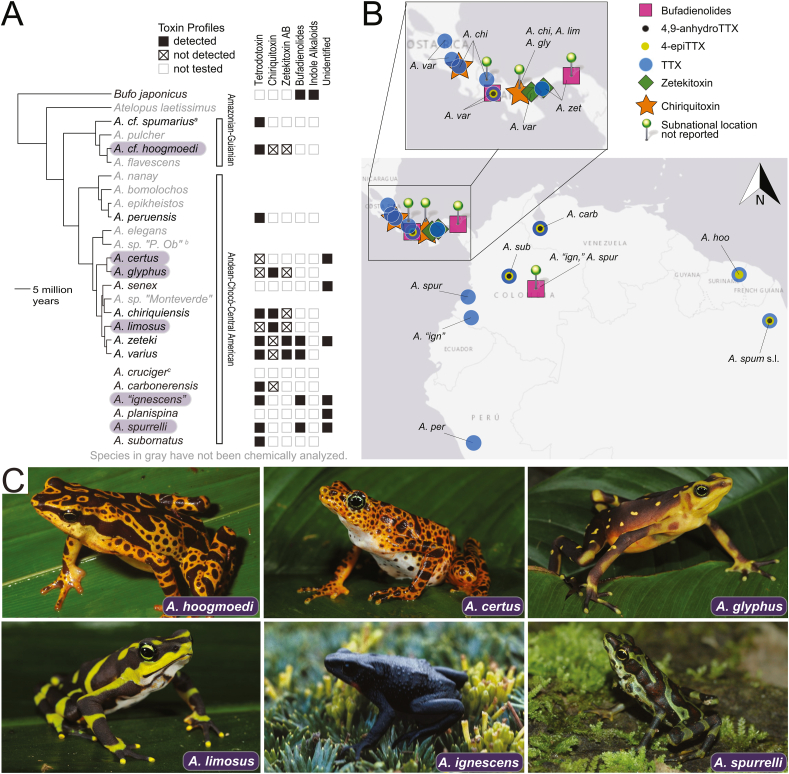
Fig. 3.
A) The phylogenetic distribution of toxic non-proteinaceous chemicals in skin, granular gland, and egg extracts of Atelopus. Bars to the right of the chronogram correspond to clades described by Lötters et al. (2011) and supported by Ramírez et al. (2020). Species listed below the chronogram were not included in the original phylogenetic analysis (Ramírez et al., 2020), and have been placed in the Andean-Chocó-Central American clade based on Lötters et al. (2011) and/or geographic range (Amphibiaweb, 2021). Species names highlighted in purple have corresponding images in Fig. 3c. a Whereas A. cf. spumarius samples from Ecuador were used in the estimation of the chronogram (Ramírez et al., 2020), the associated toxin profile data is derived from A. spumarius sensu lato collected in Colombia (Table S2; Daly et al., 1994). b “P. Ob” is an abbreviation of “Puerto Obaldia-Capurgana.” cA. cruciger are nontoxic (Mebs and Schmidt, 1989). References: Atelopus: See Table S1. Bufo japonicus: (Erspamer et al., 1964, Inoue et al., 2020). B) Geographic distribution of Atelopus toxins. Samples for which subnational data weren't reported (green pin) are mapped only when they are the sole sample containing a particular toxin collected from a given species in that country. The locations of these points were selected for ease of visualization. See Supplementary Table S2 for coordinate data. C) Selected images of Atelopus species which have been subjected to chemical analysis. Photo credits: A. hoogmoedi by Pedro L. V. Peloso via calphotos.berkeley.edu (© 2010, with permission); A. certus, A. glyphus, and A. limosus by Brian Freiermuth via calphotos.berkeley.edu (© 2013, with permission); A. ignescens by Luis A. Coloma via bioweb.bio (CC BY-NC-ND 4.0), A. spurrelli by RD Tarvin (2014, Termales, Chocó, Colombia). For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.
Amphibians have experienced severe and widespread declines in recent decades (Stuart et al., 2004). Atelopus have suffered a particularly drastic decline; a major survey in 2005 found that, of species with sufficient population trend data (52 of 113 known species), 81% were in decline and 56% were possibly extinct. Chytridiomycosis, a disease caused by the fungal pathogen Batrachochytrium dendrobatidis, is implicated in many of the declines (La Marca et al., 2005; Lampo et al., 2017), and habitat loss and degradation are likely also important drivers (Gómez-Hoyos et al., 2020; Jorge et al., 2020b; Santa-Cruz et al., 2017). Recently, several Atelopus species thought to be extinct or locally extirpated have been rediscovered (Barrio Amorós et al., 2020; Enciso-Calle et al., 2017; Escobedo-Galván et al., 2013; Tapia et al., 2017); however, these rediscovered populations are still at risk of extinction due to habitat loss, invasive species, low genetic diversity, and chytridiomycosis (Byrne et al., 2020; González-Maya et al., 2018; Kardos et al., 2021). Atelopus extinctions not only risk the loss of irreplaceable biodiversity but also threaten the persistence of toxins that are unique to the genus.
Here we review the available data on Atelopus small-molecule (i.e., non-peptide) chemical defenses and identify geographic and taxonomic gaps in Atelopus toxin sampling. We describe known Atelopus toxin diversity, as well as the chemical features, pharmacology, and sources of individual toxins. Then we collate the methods used to assess Atelopus toxins and detail their capabilities and pitfalls. Finally, while taking into account these methodological biases and gaps in sampling, we review the available data from an ecological and evolutionary perspective. We aim to provide a foundation for future research programs on the chemical defenses of this highly threatened genus of Neotropical toads.
2. Methods
2.1. Literature review
We examined peer-reviewed literature published prior to November 2021 describing the composition and toxicity of Atelopus chemical defenses as well as auxiliary literature that describes the pharmacology of relevant toxins, toxin detection and quantification methods, and Atelopus ecology, morphology, taxonomy, and evolution. Articles were found using keyword searches through the UC Berkeley Library, Google Scholar, and Google Search with phrases such as “Atelopus toxic,” “Atelopus peptides,” “atelopidtoxin,” “chiriquitoxin,” “zetekitoxin,” “Atelopus bufadienolides,” etc. An exhaustive search was performed specifically for literature detailing the detection, quantification, and identification of Atelopus small-molecule toxins; in total, seventeen peer-reviewed papers were identified that met one or more of these criteria (see Supplementary Table S1 for a complete list). Becker et al. (2011) claims to have detected zetekitoxins in Atelopus zeteki via HPLC, a method which would require a zetekitoxin AB standard for positive identification (Table 1). Given the extraordinary rarity of purified zetekitoxin AB (Yotsu-Yamashita et al., 2004), it seems highly unlikely Becker et al. (2011) had access to sufficient quantities for use as an HPLC standard. Thus, we exclude this paper from our analyses. Additionally, we reviewed a PhD thesis (Brown, 1972) describing toxicity assessments of several Atelopus species, as well as the isolation and detection of guanidinium alkaloids in A. zeteki. Some of the data presented therein appears to have been published elsewhere (Brown et al., 1977; Fuhrman et al., 1969), but we include the unpublished data from Brown (1972) in our analyses. Two additional papers were identified that detailed the presence or absence of skin peptides produced by Atelopus that may or may not be used in defense (Ellison et al., 2014; Woodhams et al., 2006). Owing to a lack of information on Atelopus skin peptide diversity and function, we focus our review on guanidinium alkaloids and cardiac glycosides.
Table 1.
Detection and quantification methods utilized in studies of Atelopus toxins.
| Method | Capabilities | Limitations | Relevant Atelopus Studies |
|---|---|---|---|
| BIOASSAYS | |||
| Mouse Bioassay (MBA) |
|
|
(Brown, 1972; Brown et al., 1977; Daly et al., 1994; Fuhrman et al., 1969, 1976; Kim et al., 1975; Mebs et al., 1995; Mebs and Schmidt, 1989; Pavelka et al., 1977; Shindelman et al., 1969; Yotsu-Yamashita et al., 1992, 2004; Yotsu-Yamashita and Tateki, 2010; Yotsu et al., 1990b) |
| Binding Inhibition Assays |
|
|
(Daly et al., 1994, 1997; Flier et al., 1980) |
| IMMUNOLOGICAL | |||
| Immunohistochemistry (IH) |
|
Mebs et al. (2018a) | |
| PHYSICOCHEMICAL | |||
| Nuclear Magnetic Resonance (NMR) |
|
|
(Fuhrman et al., 1976; Kim et al., 1975; Pavelka et al., 1977; Shindelman et al., 1969; Yotsu-Yamashita et al., 2004; Yotsu-Yamashita and Tateki, 2010; Yotsu et al., 1990b) |
| Thin Layer Chromatography (TLC) |
|
|
(Brown, 1972; Brown et al., 1977; Daly et al., 1994; Flier et al., 1980; Kim et al., 1975; Mebs and Schmidt, 1989; Shindelman et al., 1969; Yotsu-Yamashita et al., 2004; Yotsu et al., 1990b) |
| High Pressure Liquid Chromatography (HPLC) |
|
|
Flier et al. (1980) |
| Liquid Chromatography with Fluorescence Detection (LC-FLD) |
|
|
(Daly et al., 1994; Mebs et al., 1995; Yotsu-Yamashita et al., 1992; Yotsu-Yamashita and Tateki, 2010) |
| Gas Chromatography with Mass Spectrometry (GC-MS) |
|
|
(Daly et al., 1984; Mebs and Schmidt, 1989) |
| Electrospray Ionization with Mass Spectrometry (ESI-MS) |
|
(Mebs et al., 1995; Yotsu-Yamashita and Tateki, 2010) | |
| High Resolution Hydrophilic Interaction Liquid Chromatography/Mass Spectrometry (HR-HILIC-LC/MS) |
|
Mebs et al. (2018a) | |
2.2. Geographic and phylogenetic mapping of Atelopus toxin profiles
Sixteen of the eighteen Atelopus toxin assessment papers (including Brown, 1972) compiled during literature review described sampling location. In a few papers, only country-level sampling locations were provided or the species identification was dubious, so we excluded some of these samples from our combined assessments (see Table S2 for details). When GPS coordinates were not provided, we obtained coordinates using the geocoding service provided by Google Maps (https://developers-dot-devsite-v2-prod.appspot.com/maps/documentation/utils/geocoder). If the specific location name provided in a paper was not available, coordinates were determined by inputting larger geographic regions known to contain the locations of interest. See Supplementary Table S2 for a complete inventory of sampling locations, location names, and coordinates. Maps were generated through the ArcGIS Online application, Map Viewer Classic (Esri, Redlands, CA, USA), and edited using Adobe Illustrator (Adobe Inc., 2021).
To visualize the phylogenetic distribution of Atelopus toxins (Fig. 3a), we obtained a chronogram of Atelopus species from Ramírez et al. (2020) and pruned it to include a single tip per species in R v3.6.1 (R Core Team, 2019) using packages phytools v0.7.70 (Revell, 2012) and ape v5.5 (Paradis and Schliep, 2019).
3. Taxonomic and geographic gaps in Atelopus toxin assessments
The literature review yielded toxicity and small-molecule toxin composition data for sixteen Atelopus species (Fig. 3a, Table S1), approximately 15% of the recognized diversity of the genus (Amphibiaweb, 2021). The amount of research dedicated to each of the sixteen species screened for toxins or toxicity varies: nine have been investigated in a single study, and four species have been investigated in four or more studies (Table S1). Some species identifications in older papers make interpretation of the data difficult. In one case, Brown (1972) measured the toxicities of two Atelopus populations identified as A. varius ambulatorius and A. cruciger. Based on reported collection location, and the known distribution of these species, these individuals were likely misidentified and may represent other species. Furthermore, the identification of populations classified as A. spumarius (collected in Amapá, Brazil; Daly et al., 1994; Mebs et al., 1995) and A. ignescens (collected in Colombia and Ecuador; Brown, 1972; Daly et al., 1994; Flier et al., 1980) is ambiguous based on the collection locations. We designate these populations as A. spumarius sensu lato and A. “ignescens,” following Lötters et al. (2002) and Quilindo et al. (2005), respectively. Similarly, the population of toads designated A. oxyrhynchus by Mebs and Schmidt (1989) and Yotsu-Yamashita et al. (1992) has since been identified as A. carbonerensis, which is likely extinct (see Table S2 for a more complete discussion of taxonomy; Lötters et al., 2019).
The extent of toxin research on Atelopus is geographically biased, with Central American Atelopus receiving the most focus. Of the nine described Central American harlequin toads (Ramírez et al., 2020; Veselý and Batista, 2021), seven (A. certus, A. glyphus, A. limosus, A. zeteki, A. chiriquiensis, A. varius, A. senex) have been tested for toxins and six (A. senex excluded) have had their toxins chemically analyzed (Table S1). However, the majority of Atelopus species are found outside of Central America and therefore large geographic and taxonomic gaps in sampling exist (Fig. 3b). Amazonian and Central Andean species have received particularly little investigation. Although Ecuador is a center of Atelopus diversity (25 described species, of which 17 are endemic; Tapia et al., 2017), populations from only two Atelopus species (A. planispina and A. “ignescens”) in Ecuador have been assessed (Table S2). We note that the inconsistent toxin sampling of Atelopus limits the generalizability of conclusions drawn in this review.
4. Atelopus toxins – chemical structures, pharmacology, and sources
Two chemically and pharmacologically distinct toxin classes have been detected in Atelopus tissues: guanidinium alkaloids, which are neurotoxins that may be sequestered from exogenous sources (i.e. symbiotic bacteria; Magarlamov et al., 2017), and bufadienolides, which are cardiac glycosides that are endogenously synthesized (Chiadao and Osuch, 1969; Garraffo and Gros, 1986; Porto and Gros, 1971). Atelopus do not appear to possess lipophilic alkaloids (Daly et al., 1984), and have not been assessed for indole alkaloids, a class of compounds commonly detected in amphibian skin and found in particularly large quantities in other bufonids (Rodríguez et al., 2017; Roseghini et al., 1976, 1988, 1989). In this section we review the modes of action, relative strengths, and possible sources of guanidinium and bufadienolide toxins detected in Atelopus, paying special attention to the five guanidinium alkaloids with described structures: tetrodotoxin, 4-epitetrodotoxin, 4,9-anhydrotetrodotoxin, chiriquitoxin, and zetekitoxin AB. We also review toxins which have been detected but whose properties and structures are relatively unknown.
4.1. Guanidinium alkaloids
Guanidinium alkaloids are low molecular weight neurotoxins that target voltage-gated sodium channels (VGSCs). The eponymous positively charged guanidinium moiety (Fig. 1) interacts with the extracellular facing end of the sodium ion channel, while the rest of the molecule effectively seals off the pore (Narahashi, 2008). With the flow of sodium ions occluded, nerves lose the ability to produce action potentials and thus can no longer send signals (Narahashi et al., 1964). Guanidinium alkaloid poisoning is characterized by tingling, ataxia, paralysis, and death by respiratory failure or bradycardia (Durán-Riveroll and Cembella, 2017; How et al., 2003).
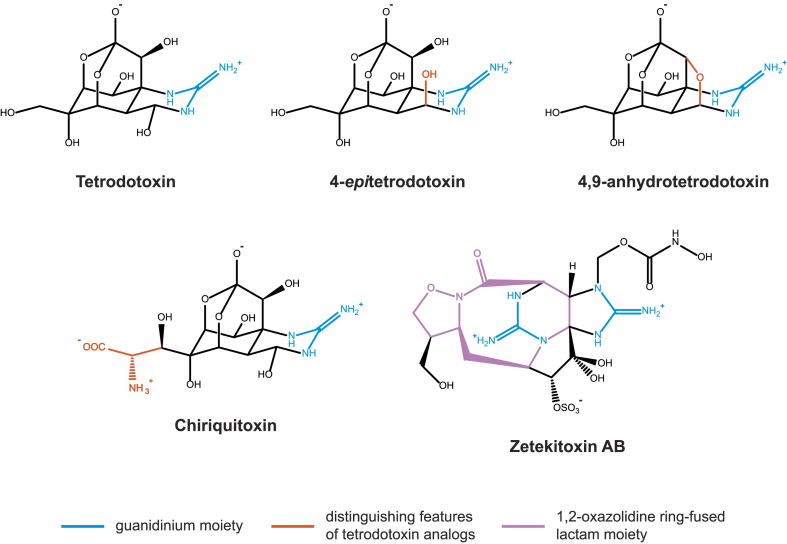
Fig. 1.
Guanidinium alkaloids detected in Atelopus. Purified quantities of Zetekitoxin C have been insufficient to estimate chemical structure (Brown et al., 1977).
Although guanidinium alkaloids have been detected in many marine animals (Chau et al., 2011), their occurrence in terrestrial taxa is limited to five amphibian families: Salamandridae, Dendrobatidae (Colostethus), Brachycephalidae, Rhacophoridae (Polypedates), and Bufonidae (Atelopus; Daly et al., 1994; Kim et al., 1975; Lüddecke et al., 2018; Pires et al., 2005; Tanu et al., 2001). Tetrodotoxin has also been reported in a single species of the salamander family Ambystomatidae (Yotsu et al., 1990a), however that finding has since been called into question (Hanifin, 2010). Lastly, tetrodotoxin has been suggested to cooccur with bufadienolides in Clinotarsus cultripes (Gosavi et al., 2014), a ranid, and chiriquitoxin has been suggested to occur in Hypsiboas crepitans, a hylid (Lamadrid-Feris et al., 2015); however, these findings are based on preliminary data that have not been verified by more sensitive techniques. Five guanidinium alkaloids have been detected and structurally identified in Atelopus: tetrodotoxin, 4,9-anhydrotetrodotoxin, 4-epitetrodotoxin, chiriquitoxin, and zetekitoxin AB (Fig. 1). While not structurally identified, zetekitoxin C has been detected in Atelopus zeteki and is likely also a guanidinium alkaloid (Brown et al., 1977).
4.1.1. Tetrodotoxin
Tetrodotoxin (TTX) has a complex structure, of which the most functionally important portion is its single guanidinium group (Woodward, 1964). The strength of TTX binding is dependent on the characteristics of a given voltage gated sodium channel. In mammals, for instance, VGSC subtypes NaV1.5, NaV1.8 and NaV1.9 are considered TTX-resistant (Thottumkara et al., 2014; Tsukamoto et al., 2017). Multiple vertebrate taxa (including some pufferfish, newts, and snakes) have evolved TTX resistance in NaV proteins 1.4 and/or 1.7, which is thought to minimize or prevent TTX poisoning (Feldman et al., 2012; Hanifin and Gilly, 2015; McGlothlin et al., 2016; Venkatesh et al., 2005). Tetrodotoxin-sensitive calcium channels have been identified in canine heart tissue (Hegyi et al., 2012, 2013).
Source. While a bacterial origin of TTX is well-supported for marine taxa (Campbell et al., 2009, Chau et al., 2011, Li et al., 2020, Magarlamov et al., 2017, Wu et al., 2005), the source of TTX in amphibians remains unresolved (see Hanifin, 2010; Stokes et al., 2014; Lukowski and Narayan, 2019). Although a complete review of all evidence regarding the source of TTX defenses in amphibians is outside the scope of this text, we evaluate existing data from Atelopus considering recent research in Taricha newts. Specifically, we propose that the absence of TTX in captive-born A. varius and newts (Daly et al., 1997; Kudo et al., 2015, 2017) and the detection of TTX-producing bacteria in newts (Vaelli et al., 2020) are suggestive of an exogenous, bacterial origin of TTX defenses in amphibians.
Although adult, captive-born Atelopus and newts lack TTX (Daly et al., 1997; Mebs and Yotsu-Yamashita, 2021; Kudo et al., 2015, 2017), wild-caught Atelopus and newts retain (Mebs et al., 1995, 2018a; Yotsu-Yamashita et al., 1992) or accumulate TTX in captivity (but see Yotsu-Yamashita et al., 2012; Hanifin et al., 2002). Together these data suggest that captive conditions do not necessarily prevent individuals from being toxic, yet without exposure to a natural environment, individuals appear incapable of initiating toxicity. Along these lines, wild-caught newts forced to secrete gland contents are able to replenish their TTX defenses over time (Cardall et al., 2004). The maintenance and regeneration of TTX in captive-held, wild amphibians has been interpreted as evidence for endogenous production (Cardall et al., 2004; Mailho-Fontana et al., 2019). However, we propose that similar patterns of TTX upkeep and accumulation might be expected in a system where symbiotic bacteria obtained from a natural environment produce toxins for the captive amphibians.
The detection of TTX-producing bacteria is complicated by the unknown genetic basis of TTX synthesis (Lukowski and Narayan, 2019). While one early study was unable to detect bacterial DNA in TTX-rich tissues of the salamandrid Taricha granulosa (Lehman et al., 2004), multiple strains of TTX-producing bacteria have recently been cultured from the skin of the same species (Vaelli et al., 2020). These findings bolster the possibility that Atelopus similarly hosts bacteria capable of guanidinium alkaloid biosynthesis. Future research could assess whether wild-caught Atelopus individuals possess TTX-producing bacteria or attempt to inoculate captive toads with isolated strains of TTX-producing bacteria.
It is worth noting that, while a dietary origin for TTX defense is unsupported in newts (Cardall et al., 2004; Gall et al., 2012; Hanifin et al., 2002), we cannot rule it out in Atelopus given the current evidence. Pufferfish, for instance, are chemically defended by TTX and while TTX-producing bacteria have been cultured from their tissues (Campbell et al., 2009, Li et al., 2020, Wu et al., 2005, Yu et al., 2011), pufferfish are also capable of sequestering TTX from their diet (Honda et al., 2005; Itoi et al., 2018; Zhang et al., 2020). TTX has been detected in a terrestrial invertebrate (Stokes et al., 2014), which could serve as a source of TTX in terrestrial food chains.
In summary, the origin of TTX in amphibians generally, and Atelopus specifically, remains unclear, as conclusive evidence for bacterial or endogenous production is lacking. With this caveat in mind and given the overwhelming evidence for a bacterial origin in marine systems along with ambiguous evidence for endogenous production of TTX by any vertebrate, we consider the bacterial origin more likely and discuss its implications in the remainder of this review (see sections 6, 7, 8).
4.1.2. 4,9-Anhydrotetrodotoxin and 4-epitetrodotoxin
4,9-anhydrotetrodotoxin (4,9-anhydroTTX) is a tetrodotoxin analog wherein the two hydroxyl substituents at positions C4 and C9 have been replaced with an ester linkage connecting the carbons (Fig. 1; Deguchi, 1967). 4,9-anhydroTTX is generally a weaker VGSC ligand than TTX, with 40–231 times as much 4,9-anhydroTTX needed to achieve the same inhibition as a given amount of TTX on a human VGSC (Rosker et al., 2007). As a result, 4,9-anhydroTTX is the least toxic TTX analog found in Atelopus: the LD50 (mouse, intravenous injection) is more than a hundred times that of TTX (Deguchi, 1967). Interestingly, 4,9-anhydroTTX is also more selective in its binding targets, strongly inhibiting the human NaV1.6 (Rosker et al., 2007; Teramoto et al., 2012) and NaV1.1 proteins (Denomme et al., 2020). Despite differences in targeting and strength between the toxins, the symptoms of 4,9-anhydroTTX poisoning are similar to those of TTX poisoning (Deguchi, 1967).
4-epitetrodotoxin (4-epiTTX) is a simple epimer of TTX, meaning it has the same chemical formula and differs only by the arrangement of substituents at the C4 position (Fig. 1). This change results in a sevenfold reduction in toxicity (Nakamura and Yasumoto, 1985). There seems to have been less investigation into the pharmacological nature of 4-epiTTX as compared to other TTX analogs.
Source. In aqueous solutions, TTX readily undergoes epimerization and subsequent dehydration to form 4-epiTTX and 4,9-anhydroTTX, respectively (Watanabe et al., 2019). These two analogs have been found in almost all terrestrial taxa that possess TTX (Hanifin, 2010). 4,9-anhydroTTX is the most stable of the three under basic conditions (Goto et al., 1965). Given that frog skin is slightly basic (Civan and Peterson-Yantorno, 1986), it might be expected for all TTX to be ultimately converted to the less toxic 4,9-anhydroTTX in Atelopus. However, this is inconsistent with observations of TTX analog ratios in Atelopus, where TTX is present in larger amounts than 4-epiTTX and 4,9-anhydroTTX (Daly et al., 1994; Mebs et al., 1995, 2018a; Yotsu-Yamashita et al., 1992). Similar data are observed in wild-caught pufferfish, which maintain a relatively constant ratio of the three chemicals across their tissues (Nakamura and Yasumoto, 1985). In lab-raised pufferfish, the fate of TTX is dependent on the route of administration: intramuscularly injected TTX is mostly converted to 4,9-anhydroTTX while dietarily administered TTX remains unmodified as the major component (Kono et al., 2008). Thus, it is important to use biologically relevant administration methods when conducting toxin metabolism and sequestration experiments. Future research could investigate whether TTX-binding proteins, which are known from pufferfish and gastropods (Hwang et al., 2007; Matsui et al., 2000; Matsumoto et al., 2010; Yotsu-Yamashita et al., 2001), can prevent the interconversion of TTX and analogs.
4.1.3. Chiriquitoxin
Chiriquitoxin (CHTX) is a tetrodotoxin analog found exclusively in Atelopus toads. It differs from tetrodotoxin by the replacement of a hydroxyl substituent with a glycine residue at the C11 position (Fig. 1; Yotsu et al., 1990b). CHTX binds with particularly low affinity to human NaV1.7, which may be attributable to the loss of a ligand/channel hydrogen bond which involves the C11 hydroxyl group in TTX (Tsukamoto et al., 2017). Unlike TTX, CHTX can also interfere with the function of potassium voltage gated ion channels (Yang and Kao, 1992). Nevertheless, CHTX is only slightly less toxic than TTX upon injection in mice, and produces similar symptoms (Fuhrman et al., 1976).
Source. Chiriquitoxin is the most structurally complex tetrodotoxin analog found in Atelopus, and, unlike 4,9-anhydroTTX and 4-epiTTX, is not an aqueous equilibrium product of TTX. It has been proposed that CHTX is generated by a reaction between glycine and either tetrodotoxin or an oxidized derivative thereof (Yotsu et al., 1990b). Whether this conversion is performed by the toads themselves or by microorganisms living on their skin is unknown, but there is precedence for amphibians modifying sequestered toxins. Four species of dendrobatid poison frogs (Dendrobates auratus, D. tinctorius, Adelophobates galactonotus, and A. castaneoticus) metabolize an ingested pumiliotoxin, PTX (+)-251D, stereoselectively hydroxylating it to form a more potent derivative, aPTX (+)-267A (Alvarez-Buylla et al., 2020; Daly et al., 2003). However, preliminary investigations have not shown animals to be capable of interconverting TTX analogs (Yotsu-Yamashita et al., 2013). A study of a TTX-bearing newt (Cynops pyrrhogaster) demonstrated that ingested TTX and putative biosynthetic precursors accumulated in body tissues but remained in their original forms (Kudo et al., 2017). In contrast, parotoid-gland-associated bacteria are known to biotransform bufadienolides in the toad Rhinella marina (Kamalakkannan et al., 2017). Nevertheless, no bacteria have been found that can produce CHTX or modify TTX into any analog (Yotsu-Yamashita et al., 2013).
4.1.4. Zetekitoxin AB
Zetekitoxin AB (ZTX AB) is unique among Atelopus guanidinium alkaloids; it is an analog of the paralytic shellfish toxin saxitoxin and contains two guanidinium moieties (Fig. 1). Furthermore, ZTX AB is the only natural chemical known to possess an 1,2-oxazolidine ring-fused lactam moiety (Yotsu-Yamashita et al., 2004). Despite structural differences, ZTX AB is remarkably similar to TTX in potency, with an LD50 (mouse, intraperitoneal) of 11 μg/kg as compared to 10 μg/kg for TTX (Brown et al., 1977; Fuhrman et al., 1976). Symptomatically, ZTX AB poisoning is virtually indistinguishable from TTX poisoning, except that it more commonly induces cardiac arrhythmia (Brown et al., 1977; Fuhrman et al., 1976). Unlike many other saxitoxin analogs but like TTX, ZTX AB causes hypotension (Brown et al., 1977; Durán-Riveroll and Cembella, 2017). Unfortunately, only limited amounts of ZTX AB have been available for pharmacological and biophysical study. As a result, little is known about its binding specificity.
Source. ZTX AB has only ever been detected in Atelopus zeteki and A. varius, and its source remains uninvestigated. Cyanobacteria and dinoflagellates, however, are well established as the source of saxitoxin (Durán-Riveroll and Cembella, 2017), and saxitoxin-producing cyanobacteria are found in freshwater systems (Smith et al., 2011). Given that Atelopus are riparian and possess skin-associated cyanobacteria (Becker et al., 2014), it seems plausible that ZTX AB has a cyanobacterial origin. Unlike TTX, the genetic basis of saxitoxin synthesis is known (Hackett et al., 2013), so metagenomic techniques could be applied to the Atelopus microbiome to test for the presence of bacteria with gene clusters similar to the saxitoxin gene cluster (Lukowski and Narayan, 2019). While A. zeteki from El Valle de Antón, Panama are the most studied sources of ZTX AB (Table S1), the use of metagenomic analyses on the microbiome of this species is complicated by the possible extinction of A. zeteki in the wild, and uncertainty regarding whether captive A. zeteki retain ZTX AB (Lukowski and Narayan, 2019). However, populations of A. varius persist in El Copé, Coclé, Panama as of 2016 (Byrne et al., 2020) and ZTX AB was detected in A. varius collected near El Copé in 1971 (Yotsu-Yamashita et al., 2004). These A. varius populations could be promising subjects for metagenomic research in search of ZTX AB-producing bacteria.
4.1.5. Zetekitoxin C
What was once referred to as atelopidtoxin (Fuhrman et al., 1969; Shindelman et al., 1969) is now known to be a mixture of ZTX AB and zetekitoxin C (ZTX C). ZTX C appears to only have been isolated once, as a minor component of Atelopus zeteki skin alkaloids. It is much less toxic than ZTX AB. Chemically, ZTX C has features in common with guanidinium alkaloids, including solubility in water and basicity (Brown, 1972; Brown et al., 1977). The symptoms produced by its injection in dogs – hypotension, ventricular fibrillation, and death – are also consistent with inhibition of voltage-gated sodium channels (Brown et al., 1977; Durán-Riveroll and Cembella, 2017; Murtha, 1960). Unfortunately, insufficient quantities of ZTX C were purified for structural analysis (Brown et al., 1977).
4.2. Bufadienolides
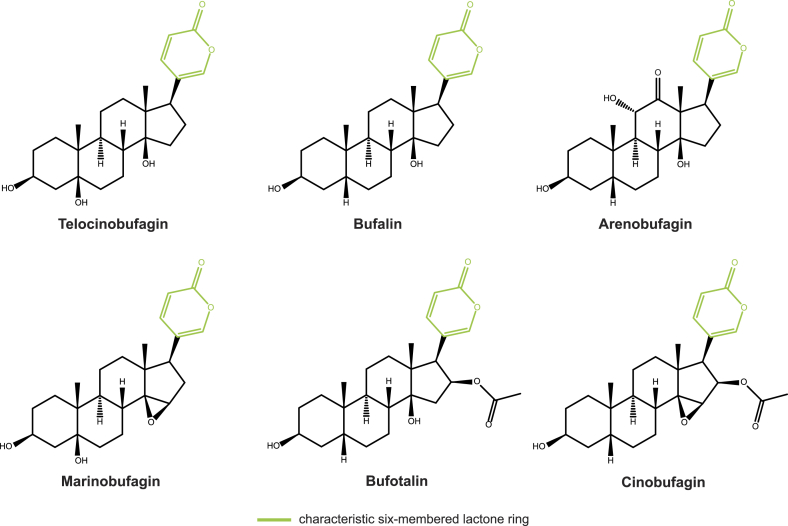
Bufadienolides are cardiac glycosides (CGs), steroidal toxins that bind to and inhibit Na+/K+-ATPases (Fig. 2; Botelho et al., 2019). Na+/K+-ATPase inhibition ultimately causes a buildup of Ca2+ ions within nerve and muscle cells, which increases the contractility of muscle tissues (Blaustein et al., 2009). CG poisoning manifests as hypertension, gastrointestinal distress, abnormal heart rate, and – in high enough doses – death (Roberts et al., 2016). CG inhibition of Na+/K+-ATPase also alters some signaling pathways and is the topic of intense research for potential anticancer therapies (Reddy et al., 2020). Whereas other CGs have a five membered lactone ring attached to the central steroid structure, bufadienolides are characterized by a six membered lactone ring (Fig. 2; Rodríguez et al., 2017).
Fig. 2.
Bufadienolides detected in Atelopus.
Source. All four Atelopus species that have been tested for bufadienolides were found to possess this class of toxins (Daly et al., 1997; Flier et al., 1980). Bufadienolides are endogenously synthesized by toads, likely from cholesterol (Chiadao and Osuch, 1969; Garraffo and Gros, 1986; Porto and Gros, 1971). Interestingly, bufadienolides and other CGs are present at low levels in mammal and amphibian tissues, and likely have a highly conserved role as endogenous hormones (Dmitrieva et al., 2000; Flier et al., 1980; Lenaerts et al., 2018; Schoner and Scheiner-Bobis, 2005). Bufadienolides may also be used for sodium and water regulation in toads. For example, exposure to saline solutions altered the concentration of digitalis-like compounds (likely bufadienolides, see Dufresnes et al., 2019; Rodríguez et al., 2017) in the skin and brain of Bufotes viridis (Lichtstein et al., 1991). Thus, a possible evolutionary pathway for bufadienolide defense in toads is via natural selection on the regulation of endogenous CGs (Flier et al., 1980) coupled with the development of Na+/K+-ATPase target site insensitivity, whereby amino acid substitutions result in a weaker affinity of Na+/K+-ATPase for CGs. Target site insensitivity to CGs has been demonstrated in the α3 Na+/K+-ATPase subunit of bufonid toads – including Atelopus spumarius – and toad-feeding reptiles (Moore et al., 2009; Ujvari et al., 2015) and in a tandem duplicate of the α1 Na+/K+-ATPase in toad-feeding frogs (Leptodactylus; Mohammadi et al., 2021). More than one hundred different bufadienolides have been detected in the skins, eggs, or granular gland secretions of bufonid toads (Rodríguez et al., 2017). The mechanisms underlying the diversity of bufadienolides in toads has been largely uninvestigated, though microbial biotransformation may play a role (Hayes et al., 2009b; Kamalakkannan et al., 2017).
4.3. Unidentified toxins
Toxin diversity in Atelopus is incompletely characterized, and toxins whose identities are unknown have been detected in multiple species. For various reasons, including small quantities and methodological limitations, investigation into these chemicals has been insufficient to clarify their structures, pharmacology, and/or chemical characteristics (see Section 5.2 for a discussion of the methods used to identify Atelopus toxins).
Several unidentified toxins that mirror guanidinium alkaloids in effect or chemistry have been detected in Atelopus. The only toxin found in A. certus is water soluble and likely positively charged, both of which are features of guanidinium alkaloids. While this unknown chemical was determined to not be TTX, too little was purified for further analysis (Yotsu-Yamashita and Tateki, 2010). In competitive binding assays, A. spurrelli skin extracts inhibit saxitoxin binding, a characteristic of guanidinium alkaloids. Given that TTX is a minor component of A. spurrelli skin extracts, one or more unidentified TTX-like toxins are believed responsible for A. spurrelli toxicity (Daly et al., 1994). Similarly, TTX is a trace component in A. “ignescens,” and the tetrodotoxin-like chemicals which account for the remaining toxicity of A. “ignescens” skin extracts to mice are uncharacterized (Daly et al., 1994). Finally, aqueous A. senex skin extracts injected into mice caused the same symptoms as known guanidinium alkaloids (Brown, 1972). While A. senex skin extracts likely contain guanidinium alkaloids, the individual identities of these toxins have not been determined.
There are unidentified Atelopus toxins which either differ substantially from guanidinium alkaloids or whose properties are almost completely unknown. Aqueous skin extracts of A. planispina injected in mice cause symptoms that differ from those of guanidinium alkaloid poisoning, specifically cessation of respiration before cardiac arrest (Fuhrman et al., 1969). The unidentified toxin is unlikely a bufadienolide because bufadienolides are weakly soluble in water (Flier et al., 1980) and do not cause the symptoms observed with A. planispina toxins (Roberts et al., 2016). Thus, A. planispina represents a likely source of novel Atelopus toxins, which warrants further research. Secondly, an unidentified major toxin has been detected in a single specimen of A. zeteki and has received no further investigation (Yotsu-Yamashita and Tateki, 2010). However, the method used on that specimen was incapable of detecting ZTX AB, the most common major toxin found in A. zeteki (Table S2; Yotsu-Yamashita and Tateki, 2010), so it is plausible that the chemical was ZTX AB.
5. Atelopus toxin extraction, quantification, and identification methods
In this section we give a general overview of the methods used to isolate, quantify, and identify toxins in Atelopus. We do not attempt to describe every step, but rather focus on those which impact the accuracy and completeness of the toxin assessment. Furthermore, we describe how these methods have changed over time, and the consequences of those changes. We also note methods which may prove useful in future Atelopus toxin studies.
5.1. Extraction and purification
In most studies, extractions are performed on isolated skin or eggs, though whole-body extractions are also possible (Mebs et al., 2018a; Yotsu-Yamashita et al., 1992). Usually, tissues are broken into small pieces and suspended in a solvent with properties most amenable to the toxin type of interest. If the tissues are homogenized, subsequent dialysis is performed to separate soluble chemicals from the slurry (Fuhrman et al., 1969; Pavelka et al., 1977; Shindelman et al., 1969). A variety of extract cleaning methods can be used, many of which involve some form of filtration via chromatography (Daly et al., 1994; Mebs and Schmidt, 1989; Shindelman et al., 1969). Final toxin separation and purification may be performed through chromatography or free-flowing electrophoresis (Brown et al., 1977; Yotsu-Yamashita et al., 2004).
A study published in 1977 found that higher levels of guanidinium alkaloids were extracted from A. chiriquiensis eggs and skin when 3% acetic acid was used as opposed to water, with the effect most pronounced in egg extractions (Pavelka et al., 1977). Following acid extraction, the toxins exhibited enhanced solubility in water. The authors suggest guanidinium alkaloids in Atelopus may exist to some extent in an insoluble bound form, from which the toxins are released following hydrolysis with acid (Pavelka et al., 1977). Several species of TTX-possessing pufferfish (Matsui et al., 2000; Matsumoto et al., 2010; Yotsu-Yamashita et al., 2001) and gastropods (Hwang et al., 2007) are known to possess TTX-binding proteins, thus another possibility is the acidic denaturation of a guanidinium alkaloid-binding protein. Previous studies (before 1977) used distilled water for the initial extraction, and thus may have reported lower toxin levels than were present in the toads tested. With a few exceptions (Daly et al., 1994, 1997; Mebs et al., 1995), subsequent studies on Atelopus toxins followed Pavelka et al. (1977) and performed acidic extractions.
While bufadienolides have a variety of structures and physical properties (Rodríguez et al., 2017), they tend to be poorly soluble in water (Flier et al., 1980; Li et al., 2009; Zhang et al., 2008). Furthermore, bufadienolides degrade over 24-h timescales in highly acidic or basic solutions (Li et al., 2015). Thus, aqueous and/or acidic extractions of Atelopus tissues may largely exclude bufadienolides and mouse bioassays of such extractions likely do not account of the contribution of bufadienolides to Atelopus toxicity. Bufadienolide-specific toad extractions commonly employ methanol as a solvent (Barnhart et al., 2017; Daly et al., 1997; Flier et al., 1980; Inoue et al., 2020; Petroselli et al., 2018).
Considering the severity of Atelopus declines (La Marca et al., 2005), nonfatal extraction methods may be critical for future research on toxins in wild Atelopus populations. One method involves collecting small skin punches from animals in the field, and has been utilized to measure TTX levels in salamanders but has not been benchmarked yet for accuracy against whole-body extractions (Bucciarelli et al., 2014; Hanifin et al., 2002). Completely noninvasive methods involve the collection of granular gland secretion via manual or electrical stimulation of amphibian skin (Conceição et al., 2007; Rozek et al., 1998). Although these methods have not been thoroughly tested on amphibians that possess guanidinium alkaloids, we suspect that they would be fruitful. The sampling of museum specimens for toxins represents another avenue for Atelopus research and could enable the assessment of species that have gone extinct. In a couple of studies, analyses were performed on the storage alcohol of Atelopus museum specimens (Mebs et al., 1995, 2018a). However, toxins in museum specimens may degrade over time and specimens stored in formalin are not suitable for toxin analyses (Mebs et al., 1995).
5.2. Toxin identification
After extraction and purification, a variety of methods can be applied to determine guanidinium alkaloid identity (Table 1). Thin layer chromatography (TLC) and nuclear magnetic resonance (NMR) were among the earliest and most used of these in Atelopus studies. TLC separates chemicals and allows for assessment of purity. Spots on a TLC plate can be sprayed with the Weber reagent, an aqueous solution of sodium nitroprusside, potassium ferricyanide, and sodium hydroxide (Weber, 1928) that turns red in the presence of fifty or more mouse units of guanidinium alkaloids (approximately equivalent to 11 μg of TTX; Brown et al., 1977). Alternatively, the spotted TLC plate can be sprayed with an alkaline solution and heated (Mebs and Schmidt, 1989). This converts guanidinium alkaloids into 2-aminoquinazoline derivatives that fluoresce under UV light (Nakamura and Yasumoto, 1985). 1H NMR and C-13 NMR spectra give information on the electronic environments, neighboring atoms, and quantities of carbon and hydrogen atoms in a molecule, respectively (Klein, 2017). In addition to serving as a method of detection, NMR has been critical in determining the chemical structures of Atelopus guanidinium alkaloids (Yotsu-Yamashita et al., 2004; Yotsu et al., 1990b).
The next technology that became widely used in Atelopus toxin studies was developed by Yotsu et al. (1989). This liquid chromatography-fluorescence detection system (LC-FLD) takes advantage of the fluorescence of 2-aminoquinazoline derivatives (generated by heating guanidinium alkaloids in alkaline solutions) to identify guanidinium alkaloids that have been separated by liquid chromatography, and was the first method used to detect 4-epiTTX and 4,9-anhydroTTX in Atelopus extracts (Yotsu-Yamashita et al., 1992; Yotsu et al., 1989). LC-FLD utilizes reverse phase chromatography, which is incapable of separating all TTX analogs (Bane et al., 2014). Additionally, because TTX analogs exhibit a wide range of fluorescent intensities, analogs may not all be detectable under the same set of conditions (Shoji et al., 2001). For instance, detection of 11-deoxytetrodotoxin, a common analog in newts and brachycephalid toads (Hanifin, 2010), requires higher post column reaction temperatures than does the detection of TTX (Yotsu et al., 1989). Studies of Atelopus toxins which utilized LC-FLD either employed post-column reaction temperatures below those needed for 11-deoxytetrodotoxin detection (Yotsu-Yamashita et al., 1992) or neglect to specify the post-column reaction temperature (Daly et al., 1994; Mebs et al., 1995; Yotsu-Yamashita and Tateki, 2010). All Atelopus LC-FLD studies utilized a C18 chromatography column but varied in mobile phase composition (heptafluorobutyric acid or trifluoroacetic acid) and concentration of NaOH in the post-column reaction (ranging from 2.8N to 4N; Daly et al., 1994; Mebs et al., 1995; Yotsu-Yamashita et al., 1992, Yotsu-Yamashita and Tateki, 2010). LC-FLD cannot detect low levels of CHTX, and it is incapable of detecting ZTX AB (Yotsu-Yamashita et al., 1992; Yotsu-Yamashita and Tateki, 2010). Four Atelopus species have had their guanidinium alkaloids studied exclusively through LC-FLD: A. “ignescens,” A. spumarius sensu lato, A. spurrelli, and A. subornatus (Daly et al., 1994; Mebs et al., 1995). Thus, these species could have undetected CHTX, ZTX AB, or 11-deoxytetrodotoxin.
Most recently, methods that incorporate electrospray ionization-mass spectrometry (ESI-MS) have emerged as promising guanidinium alkaloid assays in Atelopus studies (Mebs et al., 1995, 2018a; Yotsu-Yamashita and Tateki, 2010). Whereas quantification by LC-FLD requires calibration with standards of each TTX analog being measured, quantification of TTX analogs can be performed by ESI-MS with the exclusive use of a TTX standard (Chen et al., 2011, Nakagawa et al., 2006, Shoji et al., 2001). The simplest use of ESI-MS is to directly screen toad extracts for ions indicative of toxin presence, such as the MH+ ion of TTX (320 m/z; Mebs et al., 1995). However, the presence of other chemicals in the extract/matrix can reduce sensitivity (Bane et al., 2014), thus chromatography can be used prior to ESI-MS for chemical separation (Yotsu-Yamashita and Tateki, 2010). Mebs et al. (2018a) is the only study of Atelopus toxins to employ high resolution hydrophilic interaction liquid chromatography (HR-HILIC), a highly sensitive method which achieves better separation of analogs than reverse phase chromatography (Knutsen et al., 2017). Subsequent analysis by ESI-MS detected only TTX and 4-epiTTX in an A. hoogmoedi extract (Mebs et al., 2018a). As HR-HILIC-LC/MS can detect nearly every major analog of TTX found in nature (Yotsu-Yamashita et al., 2013), the exclusive detection of TTX and 4-epiTTX suggests that A. hoogmoedi lacks other TTX analogs including 6-epitetrodotoxin and 11-deoxytetrodotoxin, which are frequently detected in newts (Hanifin, 2010). This is consistent with the apparent absence of 6-epitetrodotoxin and tetrodonic acid in A. carbonerensis (Mebs et al., 1995; Yotsu-Yamashita et al., 1992). Application of HR-HILIC-LC/MS to other Atelopus species could determine whether 6-epitetrodotoxin, 11-deoxytetrodotoxin, and tetrodonic acid are universally absent in the genus.
HR-HILIC-LC/MS forms the basis of a mass spectrometry-guided screening method developed by Kudo et al. (2014, 2020): it enables the detection of unknown compounds for subsequent structural analysis. The pattern of unique TTX analogs and other chemicals detected in newts via this method has led to the determination of a putative TTX biosynthetic pathway (Kudo et al., 2014, Kudo et al., 2016, Kudo et al., 2020, Kudo et al., 2021, Kudo and Yotsu-Yamashita, 2019). Use of this screening method on Atelopus could help determine the extent of convergence in the biosynthetic pathway(s) of amphibian associated TTX. Furthermore, the search for genes in amphibians and bacteria which encode for inferred biosynthetic enzymes could give powerful insight into the ultimate source of TTX (Kudo et al., 2014).
The identification of individual Atelopus bufadienolides has only been attempted once (Flier et al., 1980). After verification of bufadienolide presence, Flier et al. (1980) performed HPLC on A. “ignescens” skin extractions. By comparing the elution order with those of bufadienolide standards, the preliminary identities of six Atelopus bufadienolides were determined. A variety of methods are now available which make it possible to identify many bufadienolides precisely and sensitively (Zhan et al., 2020).
5.3. Quantification of toxicity and pharmacological activity
Most of the methods described in Section 5.2 can quantify individual chemicals, subject to their detection and identification limitations (Table 1). In contrast, the methods described below can be used to determine the combined toxicity or pharmacological activity of multiple toxins.
The mouse bioassay (MBA) is the most frequently used method of toxicity quantification in Atelopus toxin studies. MBAs may also be used throughout the toxin extraction and purification process to identify toxic fractions (Shindelman et al., 1969; Yotsu-Yamashita and Tateki, 2010; Yotsu et al., 1990b). MBAs involve injecting varying doses of toxin intraperitoneally into mice and measuring survival times (Brown et al., 1977). The symptoms observed during MBAs may suggest the presence or absence of different toxins (Fuhrman et al., 1969). Toxin quantities determined by MBAs are reported in mouse units (MUs). The standard definition of one MU is that it is enough toxin to kill a 20g male mouse in 30 min by intraperitoneal injection and is equivalent to 0.22 μg of tetrodotoxin (Kawabata, 1978; Yasumoto, 1991). However, Atelopus toxin studies published prior to 1988 use conflicting definitions of MUs based on post-injection survival times of 20 min (Brown et al., 1977; Pavelka et al., 1977), 30 min (Mebs and Schmidt, 1989) and 1 h (Kim et al., 1975; Shindelman et al., 1969). Other studies do not specify the survival time used in their MU definition (Fuhrman et al., 1969, 1976). Lastly, female mice and mice of different strains were sometimes used for MBAs (Pavelka et al., 1977). Post 1989, all Atelopus studies that use MBAs for toxin quantification apply the standard MU definition or use the standard MU to TTX equivalent conversion outlined in Yasumoto (1991). The variability in MU definition between Atelopus studies may complicate the comparability of the toxicities they report.
Binding assays can be used to determine the quantity of chemicals with specific pharmacological activities. While other variants of toxin binding assays exist (Stokes et al., 2012), those applied in studies of Atelopus toxins thus far measure the binding inhibition of radioactively labeled reference chemicals in homogenized brain tissue or red blood cells by toad skin extracts. Binding inhibition assays for guanidinium alkaloids and bufadienolides use [3H]Saxitoxin and [3H]Ouabain as reference chemicals, respectively (Daly et al., 1994, 1997; Flier et al., 1980). Modern binding assays can be an order of magnitude more sensitive than the mouse bioassay in detecting guanidinium alkaloids (Kawabata, 1978; Stokes et al., 2012), and have fewer ethical considerations (Stern et al., 2018; Taylor et al., 2011; Wilder-Kofie et al., 2011).
6. Geographic and phylogenetic distribution of Atelopus toxins
Atelopus are distributed throughout much of the Andes from Bolivia to Venezuela, continuing into Central America, with the most northern species found in Costa Rica. A disjunct group of species occupies the eastern Amazonian Basin and the Guiana Shield (Lötters et al., 2011). Atelopus are found at elevations ranging from 0 to 4800m (La Marca et al., 2005) and occupy a variety of habitats, including Chocó-Darién moist forests (Veselý and Batista, 2021), treeless high-altitude páramo (Rueda Solano et al., 2016), and lowland Amazonian rainforest (Jorge et al., 2020a). Harlequin toads live in riparian areas, with males often staying close to streams and females ranging further into the surrounding areas (Mcdiarmid, 1971).
Atelopus toxins similarly exhibit geographic and phylogenetic patterns, with tetrodotoxin found in the majority of species and toxin diversity concentrated in Central American toads (Fig. 3a). It is important to note that the distribution patterns derived from existing research may not reflect the complete distributions of those toxins due to sampling biases (Section 3) and methodological limitations (Section 5.2). For instance, four South American Atelopus species have been analyzed exclusively using a method that cannot detect low levels of CHTX or any amount of ZTX AB (Daly et al., 1994; Mebs et al., 1995, 2018a; Yotsu-Yamashita et al., 2013; Yotsu-Yamashita and Tateki, 2010). In contrast to Central American Atelopus, only a minority of South American species have been chemically analyzed. Thus, there is a possibility that some South American Atelopus could possess CHTX or ZTX AB, or other chemicals that are yet to be discovered. In this section we describe the geographic and phylogenetic distribution of each Atelopus toxin, given the available data, and note any patterns possibly indicative of genetic or environmental factors influencing toxin composition.
6.1. Guanidinium alkaloids
Sequestered toxin profiles are constrained by the availability of toxins (an environmental factor; Mebs et al., 2018b; Yoshida et al., 2020) and the capacity of the organism to sequester those chemicals (a genetic factor; Davison et al., 2021). Atelopus may sequester guanidinium alkaloids from bacteria, and, if so, it is unclear whether such bacteria are horizontally or vertically transmitted. In the case of horizontal transmission, Atelopus toxin profiles would be constrained by the geographic distributions of guanidinium-alkaloid synthesizing bacteria, whereas vertical transmission would ensure the availability of particular guanidinium alkaloids, regardless of any biogeographic patterns in microbial diversity. In both scenarios, Atelopus guanidinium alkaloid profiles would also be shaped by the genetic capacity to sequester specific toxins, establish symbioses with toxin-producing bacteria, or, in the possible case of CHTX, to modify sequestered chemicals (see Section 4.1.3). If, instead, Atelopus guanidinium alkaloids are endogenously synthesized, toxin profiles should reflect genetically controlled synthetic abilities and, possibly, plasticity in defensive responses to environmental cues (e.g., predation attempts, reproductive status, etc). Thus it is likely that both genetic and environmental factors shape Atelopus guanidinium toxin profiles.
TTX. Tetrodotoxin is the most widespread Atelopus toxin, having been detected in ten of the sixteen chemically assessed species (Fig. 3a). It is usually a major toxin component and has been found in Atelopus toads from across the entire geographic range of the genus (Fig. 3b) and within both major clades (Fig. 3a). Atelopus is the only taxon in Bufonidae known to possess guanidinium alkaloids (Rodríguez et al., 2017); toxin assessments of species within the sister taxon of Atelopus (Oreophrynella; Kok et al., 2018) and other “atelopodid” bufonids (Melanophryniscus and Dendrophryniscus; Graybeal, 1997) have failed to detect guanidinium alkaloids (Daly et al., 1994; Mebs et al., 1995). Thus, the phylogenetic distribution of TTX within Bufonidae suggests a single origin of TTX defense in the common ancestor of Atelopus with possible secondary losses in some species, i.e., A. glyphus, A. limosus, and A. cruciger (Fig. 3a). If TTX is sequestered from bacteria in Atelopus, the absence of TTX could be reflective of differences in microbiome composition rather than the loss of sequestration ability. However, better sampling of the Amazonian-Guianan Atelopus clade and of Bufonidae more generally is needed before a definitive model of the origin and evolution of TTX defense in Atelopus can be proposed.
4,9-anhydroTTX and 4-epiTTX frequently cooccur with TTX, which is expected given the aqueous equilibrium between the three chemicals. Interestingly, 4-epiTTX has once been detected in the absence of 4,9-anhydroTTX in a single male specimen of A. hoogmoedi from Suriname (Fig. 3b; Mebs et al., 2018a).
CHTX. Chiriquitoxin was discovered in the now-extinct Atelopus chiriquiensis in 1975 and was thought to be unique to that species until its detection in A. limosus and A. glyphus more than three decades later (Kim et al., 1975; Yotsu-Yamashita and Tateki, 2010). CHTX is a major component in all three species (Table S1), and appears to be restricted to Central America, having only been found in Costa Rican and Panamanian Atelopus (Fig. 3b). The three species with CHTX form a polyphyletic group. A. certus and A. senex possess guanidinium alkaloid-like toxins (Brown, 1972; Fuhrman et al., 1969), and their phylogenetic placement (Fig. 3a) and Central American ranges (Kahn et al., 2005; Veselý and Batista, 2021) suggest they would be promising targets for CHTX testing. However, A. senex is extinct (IUCN, 2021). TTX was also a major component in A. chiriquiensis, consistently detected alongside CHTX (Table S1). Although TTX is the likely metabolic precursor of CHTX (Yotsu et al., 1990b), TTX is not known to be present in A. glyphus or A. limosus (Yotsu-Yamashita and Tateki, 2010), indicating that TTX, if present in these species, could be completely converted to CHTX.
ZTX AB. Zetekitoxin AB (ZTX AB) has only been found in two of the seven assessed Central American Atelopus: A. varius and A. zeteki (Fig. 3a). These sister species are closely related (Fig. 3a; Lötters et al., 2011; Ramírez et al., 2020) and a recent whole-genome analysis does not support the species boundary between them (Byrne et al., 2020), suggesting that A. varius and A. zeteki are the same species. A. varius and A. zeteki exhibit intraspecific variation in the presence and absence of TTX and ZTX AB. The exclusive presence of ZTX AB in A. varius and A. zeteki suggests that ZTX AB sequestration or synthesis is under some degree of genetic control. This is corroborated by the occurrence of Colostethus panamensis, a dendrobatid poison frog, in El Valle de Antón. C. panamensis occupies the same habitat as A. zeteki but possesses only TTX, not ZTX AB (Daly et al., 1994). Nevertheless, the paucity of data makes it difficult to draw any conclusion on the broader phylogenetic or geographic distribution of this toxin.
6.2. Bufadienolides
Bufadienolides have been detected in all Atelopus which have been tested with methods that are sensitive to these substances: A. “ignescens,” A. spurrelli, A. varius, and A. zeteki (Fig. 3a; Daly et al., 1997; Flier et al., 1980). Thus, while cardiac glycosides appear geographically restricted to Andean and Central American harlequin toads (Fig. 3b), this is likely an artifact of incomplete sampling. Identification of Atelopus bufadienolides has been attempted only once, revealing the likely presence of major components telocinobufagin and bufotalin, and minor components including marinobufagin, cinobufagin, bufalin, and arenobufagin, as well as two unidentified bufadienolides (Fig. 2; Flier et al., 1980). In other bufonids, bufadienolide profiles are highly variable between populations, species, and life history stages. Factors implicated in this variation include population structuring, environmental factors like climate and habitat quality, and microbial toxin biotransformation (Bókony et al., 2016, 2019; Cao et al., 2019; Hayes et al., 2009a, 2009b; Inoue et al., 2020; Kamalakkannan et al., 2017). Consequently, there is likely undiscovered bufadienolide diversity and variation within Atelopus.
7. Atelopus chemical defense characteristics: ecological and evolutionary perspectives
The ecological roles of Atelopus toxins have not been investigated. However, the localization of toxins within granular glands that can be emptied in response to threatening stimuli and the possibly aposematic colorations of many Atelopus species (see Fig. 3c and Rößler et al., 2019) suggest that Atelopus toxins may serve as an antipredator defense (Mebs et al., 2018a). There are few known Atelopus predators. Erythrolamprus epinephalus is a colubrid snake that has been observed eating A. varius and A. zeteki in the wild (Greene, 1997; Lindquist et al., 2007) and has consumed A. elegans and A. zeteki while in captivity to no ill effect (Myers et al., 1978). However, Myers et al. (1978) do not specify whether these toads were wild-caught or captive-raised. The genetic basis of guanidinium alkaloid resistance in Erythrolamprus snakes may include amino acid substitutions in the skeletal muscle VGSC, NaV1.4 (Feldman et al., 2012; Ramírez-Castañeda, 2017). Interestingly, E. epinephalus is also resistant to the effects of dendrobatid lipophilic alkaloids (Myers et al., 1978). In 2019, a fish (Hoplerythrinus unitaeniatus) and aquatic insect (Abedus spp.) were observed preying on A. hoogmoedi and A. varius, respectively (González-Maya et al., 2019; Lima et al., 2019). The predators appeared to suffer no ill effects, which suggests multiple predator species may possess resistance to Atelopus toxins. It remains to be seen whether Atelopus toxins prevent attacks or consumption by predators that lack resistance to bufadienolides and/or guanidinium alkaloids.
Atelopus toxins may have functions that extend beyond antipredator defense, as seen in other amphibian systems. Toxins can serve as intraspecific cues: Taricha larvae can detect tetrodotoxin and use this cue to avoid cannibalism by toxic adults (Zimmer et al., 2006). Rhinella marina tadpoles cannibalize eggs and are attracted to the bufadienolides found within them (Crossland et al., 2012). In another toad species, Bufo bufo, tadpoles produce greater concentrations of bufadienolides when living in ponds with high tadpole densities, suggesting bufadienolides could also act as a control mechanism for competition (Bókony et al., 2016). Defensive bufadienolides may also act as regulators of sodium and water levels in toads and could have evolved from endogenous chemicals (see Section 4.2). Lastly, bufadienolides and guanidinium alkaloids may also play a role in the immune system or as antimicrobial defenses. Two bufadienolides found in A. “ignescens,” marinobufagin and telocinobufagin, have antimicrobial activities (Cunha-Filho et al., 2005; Flier et al., 1980). Along with arenobufagin, another known Atelopus bufadienolide (Flier et al., 1980), telocinobufagin inhibits in vitro growth of the pathogen, Batrachochytrium dendrobatidis (Bd; Barnhart et al., 2017). In Taricha, TTX levels are negatively associated with total parasite richness and likelihood of Bd infection, but not with nematode infection load (Johnson et al., 2018). Similarly, although TTX exposure reduces survivorship of trematodes in the lab (Calhoun et al., 2017), there is no relationship between TTX levels and infection by nematode, trematode, and cestode endoparasites in Notophthalmus viridescens (Mebs et al., 2020). The dynamic between Atelopus toxins and Bd is important considering the role of Bd in Atelopus declines (La Marca et al., 2005), though a direct mechanism is not immediately obvious given the absence of Na+/K + -ATPases and VGSCs in fungi (Johnson et al., 2018; Rodríguez-Navarro and Benito, 2010). In summary, complex selective pressures relating to predation, communication, physiology, and immune function may be acting on Atelopus chemical defenses, underscoring the necessity of further research into Atelopus chemical ecology. In this section, we propose explanations for patterns in Atelopus toxin profiles and overall toxicity and suggestions for further research.
7.1. Atelopus toxin profiles
While many individual Atelopus toxins have been detected and characterized both chemically and pharmacologically, the adaptive importance of the specific compositions of Atelopus toxin cocktails remains uninvestigated. From an antipredator perspective, for instance, it is unclear whether possessing ZTX AB or CHTX rather than TTX as a major toxin component would affect harlequin toad fitness. It is possible that the unique binding patterns of different guanidinium alkaloids (see Section 4.1) result in functional differences relevant to warding off specific predators. Alternatively, considering the similar toxicities of TTX, CHTX, and ZTX to mice, the identity of the major alkaloid component in each Atelopus species may be of no adaptive significance. Future studies that involve exposing potential Atelopus predators to different guanidinium alkaloids could determine whether major toxin component identity influences the effectiveness of Atelopus chemical defenses.
The implications of simultaneously maintaining guanidinium alkaloids and cardiac glycosides, two chemically and pharmacologically distinct toxin classes, are worth consideration. Having diverse toxin types can enable organisms to be defended against multiple natural enemies, as demonstrated in chemically defended plants (Lindroth and Hwang, 1996). Furthermore, toxins can synergize to magnify each other's effects (Nelson and Kursar, 1999; Raaymakers et al., 2017). The respective targets of bufadienolides and guanidinium alkaloids, VGSC and Na+/K+-ATPase proteins, both influence sodium ion concentrations and may thus interact physiologically. In astrocytes, a type of glial cell, inhibition of VGSCs results in lower Na+/K+-ATPase activity. VGSCs may maintain Na+ ion concentrations at levels necessary for Na+/K+-ATPase function (Sontheimer et al., 1994). The interaction between these membrane proteins could have consequences for the function of Atelopus toxin cocktails. Interestingly, tetrodotoxin reduces the toxicity of cardiac glycosides (CGs) when injected directly into the brain of cats but potentiates CG toxicity when given intravenously (Peres-Gomes and Ribeiro, 1979). The difference in effect between TTX administered to the brain and TTX administered intravenously is probably a consequence of the impermeability of the blood-brain barrier to TTX (Zimmer, 2010). Tissue-specific expression of uncharacterized receptors for toxin-binding proteins could also result in delivery-dependent differences in poisoning symptoms. In dogs, intravenous TTX increases survival times and reduces cardiac arrhythmia following cardiac glycoside poisoning (Bernstein, 1969). The prevalence of system- and delivery-dependent results highlight the need to investigate the interactions of bufadienolide and guanidinium alkaloids in predator systems that are biologically relevant to Atelopus.
If Atelopus sequester guanidinium alkaloids, bufadienolide biosynthesis could provide some level of chemical defense when said sequestration is disrupted. Captive-raised Atelopus that lack guanidinium alkaloids retain bufadienolides (Daly et al., 1997). Melanophryniscus is another genus of bufonid toads where autogenous toxin production may compensate for variability in toxin sequestration (Cei et al., 1968; Hantak et al., 2013). Although Melanophryniscus that are fed an alkaloid-free diet gradually lose lipophilic alkaloids from their skins (Mebs et al., 2018b) Melanophryniscus may be able to upregulate the synthesis of indole alkaloids when lipophilic alkaloids are low (Jeckel et al., 2015). Similarly, the myobatrachid, Pseudophryne semimarmorata, synthesizes more indole alkaloids when raised in captivity without access to dietary lipophilic alkaloids (Smith et al., 2002). In contrast to Melanophryniscus and Pseudophryne, bufadienolide quantities are similar in A. varius with and without guanidinium alkaloids, suggesting that bufadienolide production is not upregulated in response to low TTX levels (Daly et al., 1997). More investigation is needed to clarify the functional role and regulation of autogenous toxins in Atelopus.
7.2. Variation in toxicity between and within species
A common characteristic of chemically defended organisms is variation in toxicity, from the individual to the species level and in response to temporal, environmental, and physiological changes (Speed et al., 2012). Atelopus is no different. Individual harlequin toads range from completely nontoxic to toxic enough to kill thousands of mice (Fig. 4 inset). The causes of this variation are unknown and presumably depend on the source of a toxin and the ability of the toad to bioaccumulate the toxin or host its producers; however, some patterns do emerge which parallel those observed in better-studied systems. It is important to note that the toxicity values reported for Atelopus are primarily reflective of the guanidinium alkaloids present in their skin because the acidic aqueous extraction methods commonly used prior to toxin quantification likely exclude some or all bufadienolides from the resulting toxic fractions (see Section 5.1). When methods sensitive to bufadienolides were used, bufadienolide quantities in Atelopus were found to be large enough to contribute to the overall toxicity of the toads (Daly et al., 1997; Flier et al., 1980). Studies published prior to 1989 employed alternative and conflicting mouse unit definitions, which impede the comparability of reported Atelopus toxicities (see Section 5.3 for an expanded discussion).
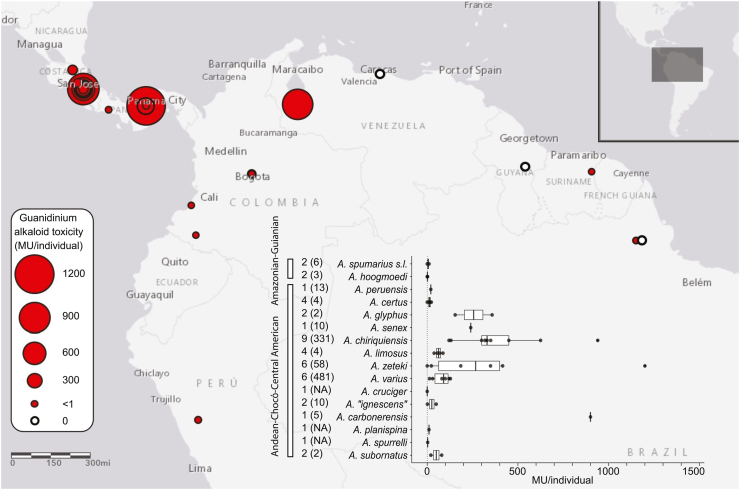
Fig. 4.
Geographic (N = 892 individuals) and species-level (inset) variation in adult Atelopus toxicity. Toxicity values primarily reflect quantity of guanidinium alkaloids (see section 5.1). Numbers left of species names detail the total number of toxicity assessments and number of specimens assessed (in parentheses) for each species. One mouse unit (MU) is sufficient to kill a single average-weight mouse in 30 min upon injection (Yasumoto, 1991). When toxicity values were given in TTX equivalents or when TTX quantity alone was given, conversion to MUs used the conversion factor 1 MU = 0.22 μg TTX (Yasumoto, 1991). See Supplementary Table S2 for coordinates, toxicity values, species names, sources, and details on unreported sample size data. Toxicity data summarized and graphed using ggplot2 v3.3.3 (Wickham, 2016), dplyr v1.0.6 (Wickham et al., 2021), and cowplot v1.1.1 (Wilke, 2020).
The most toxic harlequin toads (presently known) are found in Central America (e.g. up to 1200 MU/toad for A. zeteki and 948 MU/toad for A. chiriquiensis; Kim et al., 1975; Pavelka et al., 1977) and the montane cloud forests of the state of Mérida, Venezuela (A. carbonerensis, up to 1000 MU/toad; Dole and Durant, 1974; Yotsu-Yamashita et al., 1992). Atelopus from the Guiana Shield and the Andes south of Venezuela generally have low toxin levels (Fig. 4). However, while a minimum of 889 Central American harlequin toads have been assessed for toxicity, at least 39 from all other geographic regions have been tested (Fig. 4 inset, see Table S2 for details on reported sample sizes). The lack of standardization in older Atelopus toxicity studies also prevents confirmation of these patterns. Future research on Atelopus toxicity could prioritize the sampling of toads from the Andes and Guiana Shield.
Atelopus toxicity may be influenced by selection pressure from predators. In Taricha, a genus of newts that possesses as many as 60,000 MU of TTX per individual, high toxin levels are thought to be driven by a coevolutionary relationship with TTX-resistant predatory garter snakes (Thamnophis) (Hague et al., 2020, Hanifin et al., 2002, Williams et al., 2003). The intensity of reciprocal selection between these species varies geographically, resulting in populations with drastically different toxicities and toxin resistances (Hague et al., 2020). A similar situation is possible between Atelopus and one or more predator species (such as Erythrolamprus epinephalus). Future studies could investigate covariance in Atelopus predator toxin resistance and Atelopus toxicity across the sympatric ranges of both taxa to see if a coevolutionary arms race is taking place.
Some of the observed intrapopulation variation in Atelopus toxicity (Daly et al., 1994; Kim et al., 1975; Mebs et al., 1995; Pavelka et al., 1977) might be attributable to the experiences of sampled individuals. While also found in the skin epithelium and the liver, Atelopus toxins are primarily localized in the granular glands, which are distributed across the body and can eject their contents when a toad feels threatened (Mebs et al., 2018a; Toledo and Jared, 1995). A toad that was recently attacked may have temporarily diminished its skin-associated stores of alkaloids and steroids. Over longer time periods, encounters with predators could lead to higher toxicity in Atelopus individuals. Predator cue exposure and simulated predator attacks induce increased toxicity in some amphibian species that possess guanidinium alkaloids or bufadienolides (Benard and Fordyce, 2003; Bucciarelli et al., 2017), but not in others (Brossman et al., 2014; Üveges et al., 2017). The plasticity of Atelopus chemical defenses needs investigation and could provide insight into the ecological significance of their toxins.
Reproductive cycles and development may also play a role in toxicity variation. Gravid Atelopus chiriquiensis females have lower skin-associated toxin levels than males but are comparably poisonous when the toxicities of their eggs are accounted for (Pavelka et al., 1977). Thus, Atelopus may provision their eggs with toxins, possibly as a defensive measure, and this process likely involves the diversion of skin toxins into the reproductive system (Pavelka et al., 1977; Yotsu-Yamashita and Tateki, 2010). More generally, toxicity may vary with Atelopus age and/or metamorphic stage, as seen in other bufadienolide-defended toads (Hayes et al., 2009a; Üveges et al., 2017) and tetrodotoxin-defended newts (Gall et al., 2011; Tsuruda et al., 2002) and octopi (Williams, 2008; Williams et al., 2011). In other toxin-sequestering frog species, body size has been positively correlated with granular gland capacity (Saporito et al., 2010) and overall toxin quantity (Jeckel et al., 2015). Atelopus toxin provisioning and ontogenetic changes in toxicity could be investigated for mechanisms involved in toxin transport and accumulation.
Most speculatively, unidentified environmental factors may influence the success of microbe-toad symbioses, which are possible sources of guanidinium alkaloids in Atelopus. Some Atelopus species have extremely high site fidelity and individuals may thus be exposed to relatively constant microenvironments throughout their lives (Crump, 1986; Tarvin et al., 2014). Atelopus occupy a variety of habitats, and the possibility of covariance between toxicity and abiotic factors such as temperature and precipitation remains an area of interest.
8. Concluding remarks
A half-century of research into Atelopus chemical defenses has resulted in the discovery of individual chemicals and toxin profiles found in no other biological system. Yet, only a fraction of Atelopus species have been assessed for toxins, and the most characterized species are geographically and phylogenetically clustered. Furthermore, varying standards and detection abilities reduce what conclusions can be drawn from existing data. There is likely undiscovered toxin diversity in the genus, representing chemicals with possible medical or scientific value. Of the known Atelopus toxins, several appear restricted to a few species or populations of harlequin toads. For instance, the only known extant source of ZTX AB is A. varius (Yotsu-Yamashita et al., 2004). ZTX AB cannot be synthesized at this time (Adachi et al., 2014, 2019), and is consequently at risk of disappearing. If efforts by host countries or by collaborations supported by host countries are not made to chemically analyze declining Atelopus species, novel chemicals could be lost before being identified and characterized. Such a situation may have already occurred with the unidentified A. planispina toxin which induced unique poisoning symptoms (Fuhrman et al., 1969). A. planispina was last observed in 1985 and may be extinct (IUCN, 2021).
Many questions remain regarding the evolution of Atelopus chemical defenses. While it is commonly assumed that Atelopus toxins provide protection against predators, the potential ecological and physiological roles of toxins in Atelopus remain unstudied. Few Atelopus predators are known, and investigation into Atelopus predator-prey relationships could bring a clearer understanding of what causes toxicity variation within and between species as well as the adaptive significance of Atelopus toxin cocktails. We suggest that toxins could have additional functions unrelated to antipredator defense, including communication, defense against pathogens, and physiological regulation.
The clade of endemic Central American Atelopus, which diverged from South American Atelopus more than three million years ago (Ramírez et al., 2020), has the highest diversity of guanidinium alkaloids. If not a result of sampling biases, it is unclear what shapes the chemical defense characteristics that are potentially unique to this subclade. Have Central American toads evolved unique sequestration or synthesis mechanisms? Are they forming symbiotic relationships with different guanidinium alkaloid-producing or modifying cyanobacteria? The genetic underpinnings of putative toxin sequestration and bacterial symbioses in Atelopus have not been studied. Furthermore, the case for bacteria as the source of Atelopus guanidinium alkaloids is speculative and requires further investigation. Feeding experiments and bacterial inoculation studies are likely to be fruitful areas of research in the future.
Krogh's principle holds that the answers to biological questions can be most efficiently pursued through the study of organisms with features relevant to those questions (Krebs, 1975; Krogh, 1929). Thus, loss of organismal diversity necessarily impedes research in the life sciences. Harlequin toad chemical defenses represent a promising study system for multiple broad evolutionary and ecological questions – including the interplay between VGSCs and Na+/K+-ATPase in regulating vertebrate physiology, the evolution of toxin sequestration and synthesis, and the regulation of bacteria-amphibian symbioses – however, Atelopus have experienced precipitous declines in recent decades (La Marca et al., 2005). A few species are stable in the wild (Lampo et al., 2017; Lips, 2008), and several conservation efforts (e.g., Centro Jambatu: http://www.anfibiosecuador.ec/, Atelopus Survival Initiative: https://www.atelopus.org/ and see Valencia and Marin da Fonte (2022), Amphibian Rescue & Conservation Project: http://amphibianrescue.org/) are ensuring the ex-situ survival of species at risk of extinction. There is much to be discovered by studying Atelopus and their toxins, highlighting the importance of continued investment in conservation.
Credit author statement
Kannon Pearson: Conceptualization, Investigation, Visualization, Writing – Original Draft, Writing – Review and Editing. Rebecca Tarvin: Supervision, Visualization, Writing – Review and Editing, Software.
Ethical statement
No experiments were performed for this review.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank María José Navarrete-Méndez, Gary Bucciarelli, Tyler Douglas, Erica Bree Rosenblum, and Michelle St. John for their valuable input on the focus and content of this review. We also thank J.P. Ramírez and colleagues for providing a copy of their chronogram and Aurora Alvarez-Buylla for assistance in obtaining literature. We are grateful to the four anonymous reviewers whose commentary improved this work. We acknowledge Brian Freiermuth, John P. Clare, Luis A. Coloma, and Pedro L. V. Peloso for the photographs of Atelopus which were incorporated in the graphical abstract and Fig. 3c. RDT was supported by UC Berkeley start-up funding. We dedicate this paper to all the people past and present working to save Atelopus from extinction.
Handling Editor: Dr. Glenn King
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.toxcx.2022.100092.
Contributor Information
Kannon C. Pearson, Email: kannonpearson@berkeley.edu.
Rebecca D. Tarvin, Email: rdtarvin@berkeley.edu.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Adachi M., Imazu T., Sakakibara R., Satake Y., Isobe M., Nishikawa T. Total synthesis of chiriquitoxin, an analogue of tetrodotoxin isolated from the skin of a dart frog. Chem. Eur J. 2014;20:1247–1251. doi: 10.1002/chem.201304110. [DOI] [PubMed] [Google Scholar]
- Adachi K., Ishizuka H., Odagi M., Nagasawa K. Synthetic approaches to zetekitoxin AB, a potent voltage-gated sodium channel inhibitor. Mar. Drugs. 2019;18:1–19. doi: 10.3390/md18010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A., Payne C.Y., Vidoudez C., Trauger S.A., O'Connell L.A. 2020. Pumiliotoxin Metabolism and Molecular Physiology in a Poison Frog. bioRxiv 2020.11.03.367524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amphibiaweb Amphibiaweb [WWW Document] 2021. https://amphibiaweb.org 8.20.21Univ. California, Berkeley, CA, USA. URL.
- Bane V., Lehane M., Dikshit M., O'Riordan A., Furey A. Tetrodotoxin: chemistry, toxicity, source, distribution and detection. Toxins. 2014 doi: 10.3390/toxins6020693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnhart K., Forman M.E., Umile T.P., Kueneman J., McKenzie V., Salinas I., Minbiole K.P.C., Woodhams D.C. Identification of bufadienolides from the boreal toad, Anaxyrus boreas, active against a fungal pathogen. Microb. Ecol. 2017;74:990–1000. doi: 10.1007/s00248-017-0997-8. [DOI] [PubMed] [Google Scholar]
- Barrio Amorós C.L., Costales M., Vieira J., Osterman E., Kaiser H., Arteaga A. Back from extinction: rediscovery of the harlequin toad Atelopus mindoensis Peters, 1973 in Ecuador. Herpetol. Notes. 2020;13:325–328. [Google Scholar]
- Becker M.H., Harris R.N., Minbiole K.P.C., Schwantes C.R., Rollins-Smith L.A., Reinert L.K., Brucker R.M., Domangue R.J., Gratwicke B. Towards a better understanding of the use of probiotics for preventing chytridiomycosis in Panamanian golden frogs. EcoHealth. 2011;8:501–506. doi: 10.1007/s10393-012-0743-0. [DOI] [PubMed] [Google Scholar]
- Becker M.H., Richards-Zawacki C.L., Gratwicke B., Belden L.K. The effect of captivity on the cutaneous bacterial community of the critically endangered Panamanian golden frog (Atelopus zeteki) Biol. Conserv. 2014;176:199–206. doi: 10.1016/j.biocon.2014.05.029. [DOI] [Google Scholar]
- Benard M.F., Fordyce J.A. 2003. Are Induced Defenses Costly? Consequences of Predator-Induced Defenses in Western Toads, Bufo Boreas. Ecology. [DOI] [Google Scholar]
- Bernstein M.E. Pharmacologic effects of tetrodotoxin: cardiovascular and antiarrhythmic activities. Toxicon. 1969;7:287–302. doi: 10.1016/0041-0101(69)90029-4. [DOI] [PubMed] [Google Scholar]
- Blaustein M.P., Zhang J., Chen L., Song H., Raina H., Kinsey S.P., Izuka M., Iwamoto T., Kotlikoff M.I., Lingrel J.B., Philipson K.D., Wier W.G., Hamlyn J.M. The pump, the exchanger, and endogenous ouabain: signaling mechanisms that link salt retention to hypertension. Hypertension. 2009;53:291–298. doi: 10.1161/HYPERTENSIONAHA.108.119974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bókony V., Móricz Á.M., Tóth Zsófia, Gál Z., Kurali A., Mikó Z., Pásztor K., Szederkényi M., Tóth Zoltán, Ujszegi J., Üveges B., Krüzselyi D., Capon R.J., Hoi H., Hettyey A. Variation in chemical defense among natural populations of common toad, Bufo bufo, tadpoles: the role of environmental factors. J. Chem. Ecol. 2016;42:329–338. doi: 10.1007/s10886-016-0690-2. [DOI] [PubMed] [Google Scholar]
- Bókony V., Üveges B., Verebélyi V., Ujhegyi N., Móricz Á.M. Toads phenotypically adjust their chemical defences to anthropogenic habitat change. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-39587-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botelho A.F.M., Pierezan F., Soto-Blanco B., Melo M.M. A review of cardiac glycosides: structure, toxicokinetics, clinical signs, diagnosis and antineoplastic potential. Toxicon. 2019 doi: 10.1016/j.toxicon.2018.11.429. [DOI] [PubMed] [Google Scholar]
- Brossman K.H., Carlson B.E., Stokes A.N., Langkilde T. Eastern newt (Notophthalmus viridescens) larvae alter morphological but not chemical defenses in response to predator cues. Can. J. Zool. 2014;92:279–283. doi: 10.1139/cjz-2013-0244. [DOI] [Google Scholar]
- Brown G.B. Stanford University; 1972. Atelopidtoxin: Purification and Chemistry. [Google Scholar]
- Brown G.B., Kim Y.H., Küntzel H., Mosher H.S., Fuhrman G.J., Fuhrman F.A. Chemistry and pharmacology of skin toxins from the frog Atelopus zeteki (atelopidtoxin: zetekitoxin) Toxicon. 1977;15:115–128. doi: 10.1016/0041-0101(77)90030-7. https://doi.org/https://doi.org/10.1016/0041-0101(77)90030-7. [DOI] [PubMed] [Google Scholar]
- Bucciarelli G.M., Li A., Kats L.B., Green D.B. Quantifying tetrodotoxin levels in the California newt using a non-destructive sampling method. Toxicon. 2014;80:87–93. doi: 10.1016/j.toxicon.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Bucciarelli G.M., Bradley Shaffer H., Green D.B., Kats L.B. An amphibian chemical defense phenotype is inducible across life history stages. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-08154-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne A.Q., Richards-Zawacki C.L., Voyles J., Bi K., Ibáñez R., Rosenblum E.B. Whole exome sequencing identifies the potential for genetic rescue in iconic and critically endangered Panamanian harlequin frogs. Global Change Biol. 2020;27:50–70. doi: 10.1111/gcb.15405. [DOI] [PubMed] [Google Scholar]
- Calhoun D.M., Bucciarelli G.M., Kats L.B., Zimmer R.K., Johnson P.T.J. Noxious newts and their natural enemies: experimental effects of tetrodotoxin exposure on trematode parasites and aquatic macroinvertebrates. Toxicon. 2017;137:120–127. doi: 10.1016/j.toxicon.2017.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S., Harada R.M., Defelice S.V., Bienfang P.K., Li Q.X. Bacterial production of tetrodotoxin in the pufferfish Arothron hispidus. Nat. Prod. Res. 2009;23:1630–1640. doi: 10.1080/14786410903003780. [DOI] [PubMed] [Google Scholar]
- Cao Y., Cui K., Pan H., Wu J., Wang L. The impact of multiple climatic and geographic factors on the chemical defences of Asian toads (Bufo gargarizans Cantor) Sci. Rep. 2019;9:1–11. doi: 10.1038/s41598-019-52641-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardall B.L., Brodie E.D., Brodie E.D., Hanifin C.T. Secretion and regeneration of tetrodotoxin in the rough-skin newt (Taricha granulosa) Toxicon. 2004;44:933–938. doi: 10.1016/j.toxicon.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Cei J.M., Erspamer V., Roseghini M. Taxonomic and evolutionary significance of biogenic amines and polypeptides in amphibian skin. II. Toads of the genera Bufo and Melanophryniscus. Syst. Zool. 1968;17:232–245. https://doi.org/https://doi.org/10.1093/sysbio/17.3.232. [PubMed] [Google Scholar]
- Chau R., Kalaitzis J.A., Neilan B.A. On the origins and biosynthesis of tetrodotoxin. Aquat. Toxicol. 2011 doi: 10.1016/j.aquatox.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Chen X.W., Liu H.X., Jin Y.B., Li S.F., Bi X., Chung S., Zhang S.S., Jiang Y.Y. Separation, identification and quantification of tetrodotoxin and its analogs by LC-MS without calibration of individual analogs. Toxicon. 2011;57:938–943. doi: 10.1016/j.toxicon.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Chiadao C., Osuch M.V. Biosynthesis of bufadienolides-3β-hydroxycholanates as precursors in Bufo marinus bufadienolides synthesis. Biochem. Pharmacol. 1969;18:1797–1802. doi: 10.1016/0006-2952(69)90273-1. [DOI] [PubMed] [Google Scholar]
- Civan M.M., Peterson-Yantorno K. Intracellular pH regulation in frog skin: a 31P-nuclear magnetic resonance study. Am. J. Physiol. 1986;251 doi: 10.1152/ajprenal.1986.251.5.f831. [DOI] [PubMed] [Google Scholar]
- Conceição K., Miriane Bruni F., Antoniazzi M.M., Jared C., Camargo A.C.M., Lopes-Ferreira M., Pimenta D.C. Major biological effects induced by the skin secretion of the tree frog Phyllomedusa hypochondrialis. Toxicon. 2007;49:1054–1062. doi: 10.1016/j.toxicon.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Crossland M.R., Haramura T., Salim A.A., Capon R.J., Shine R. Exploiting intraspecific competitive mechanisms to control invasive cane toads (Rhinella marina) Proc. R. Soc. B. 2012;279:3436–3442. doi: 10.1098/rspb.2012.0821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump M.L. Homing and site fidelity in a Neotropical frog, Atelopus varius (Bufonidae) Copeia. 1986;1986:438–444. https://doi.org/https://doi.org/10.2307/1445001. [Google Scholar]
- Cunha-Filho G.A., Schwartz C.A., Resck I.S., Murta M.M., Lemos S.S., Castro M.S., Kyaw C., Pires O.R., Leite J.R.S., Bloch C., Schwartz E.F. Antimicrobial activity of the bufadienolides marinobufagin and telocinobufagin isolated as major components from skin secretion of the toad Bufo rubescens. Toxicon. 2005;45:777–782. doi: 10.1016/j.toxicon.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Daly J.W., Highet R.J., Myers C.W. Occurrence of skin alkaloids in non-dendrobatid frogs from Brazil (Bufonidae), Australia (Myobatrachidae) and Madagascar (Mantellinae) Toxicon. 1984;22:905–919. doi: 10.1016/0041-0101(84)90182-X. [DOI] [PubMed] [Google Scholar]
- Daly J.W., Gusovsky F., Myers C.W., Yotsu-Yamashita M., Yasumoto T. First occurrence of tetrodotoxin in a Dendrobatid frog (Colostethus inguinalis), with further reports for the bufonid genus Atelopus. Toxicon. 1994;32:279–285. doi: 10.1016/0041-0101(94)90081-7. [DOI] [PubMed] [Google Scholar]
- Daly J.W., Padgett W.L., Saunders R.L., Cover J.F. Absence of tetrodotoxins in a captive-raised riparian frog, Atelopus varius. Toxicon. 1997;35:705–709. doi: 10.1016/s0041-0101(96)00165-1. [DOI] [PubMed] [Google Scholar]
- Daly J.W., Garraffo H.M., Spande T.F., Clark V.C., Ma J., Ziffer H., Cover J.F. Evidence for an enantioselective pumiliotoxin 7-hydroxylase in dendrobatid poison frogs of the genus Dendrobates. Proc. Natl. Acad. Sci. U.S.A. 2003;100:11092–11097. doi: 10.1073/pnas.1834430100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison I., Saporito R.A., Schulte L.M., Summers K. Piperidine alkaloids from fire ants are not sequestered by the green and black poison frog (Dendrobates auratus) Chemoecology. 2021 doi: 10.1007/s00049-021-00357-1. [DOI] [Google Scholar]
- Deguchi T. Structure and activity in tetrodotoxin derivatives. Jpn. J. Pharmacol. 1967;17:267–278. doi: 10.1254/jjp.17.267. https://doi.org/https://doi.org/10.1254/jjp.17.267. [DOI] [PubMed] [Google Scholar]
- Denomme N., Lukowski A.L., Hull J.M., Jameson M.B., Bouza A.A., Narayan A.R.H., Isom L.L. The voltage-gated sodium channel inhibitor, 4,9-anhydrotetrodotoxin, blocks human Nav1.1 in addition to Nav1.6. Neurosci. Lett. 2020;724:134853. doi: 10.1016/j.neulet.2020.134853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitrieva R.I., Bagrov A.Y., Lalli E., Sassone-Corsi P., Stocco D.M., Doris P.A. Mammalian bufadienolide is synthesized from cholesterol in the adrenal cortex by a pathway that is independent of cholesterol side-chain cleavage. Hypertension. 2000;36:442–448. doi: 10.1161/01.HYP.36.3.442. [DOI] [PubMed] [Google Scholar]
- Dole J.W., Durant P. Movements and seasonal activity of Atelopus oxyrhynchus (Anura: atelopodidae) in a Venezuelan cloud forest. Copeia. 1974;230 doi: 10.2307/1443028. 1974. [DOI] [Google Scholar]
- Dufresnes C., Mazepa G., Jablonski D., Oliveira R.C., Wenseleers T., Shabanov D.A., Auer M., Ernst R., Koch C., Ramírez-Chaves H.E., Mulder K.P., Simonov E., Tiutenko A., Kryvokhyzha D., Wennekes P.L., Zinenko O.I., Korshunov O.V., Al-Johany A.M., Peregontsev E.A., Masroor R., Betto-Colliard C., Denoël M., Borkin L.J., Skorinov D.V., Pasynkova R.A., Mazanaeva L.F., Rosanov J.M., Dubey S., Litvinchuk S. Fifteen shades of green: the evolution of Bufotes toads revisited. Mol. Phylogenet. Evol. 2019;141 doi: 10.1016/j.ympev.2019.106615. [DOI] [PubMed] [Google Scholar]
- Durán-Riveroll L.M., Cembella A.D. Guanidinium toxins and their interactions with voltage-gated sodium ion channels. Mar. Drugs. 2017;15 doi: 10.3390/md15100303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison A.R., Savage A.E., DiRenzo G.V., Langhammer P., Lips K.R., Zamudio K.R. Fighting a losing battle: vigorous immune response countered by pathogen suppression of host defenses in the chytridiomycosis-susceptible frog Atelopus zeteki. G3 Genes, Genomes, Genet. 2014;4:1275–1289. doi: 10.1534/g3.114.010744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enciso-Calle M.P., Viuche-Lozano A., Anganoy-Criollo M., Bernal M.H. Rediscovery of Atelopus subornatus werner, 1899 (Anura: Bufonidae), with a redescription of the tadpole. Zootaxa. 2017;4344:160–162. doi: 10.11646/zootaxa.4344.1.7. [DOI] [PubMed] [Google Scholar]
- Erspamer V., Bertaccini G., Urakawa N. Biogenic amines and active polypeptides in the skin of ten Japanese amphibian species. Jpn. J. Pharmacol. 1964;14:468–473. doi: 10.1254/jjp.14.468. [DOI] [PubMed] [Google Scholar]
- Escobedo-Galván A.H., Wyatt S.A., González-Maya J.F., Schipper J., Belant J.L., Fischer A., Hoepker A., Cruz-Lizano I., Cardenal J., Castañeda F., Corrales D. Renewing hope: the rediscovery of Atelopus varius in Costa Rica. Amphib. Reptil. 2013;34:573–578. doi: 10.1163/15685381-00002910. [DOI] [Google Scholar]
- Feldman C.R., Brodie E.D., Brodie E.D., Pfrender M.E. Constraint shapes convergence in tetrodotoxin resistant sodium channels of snakes. Proc. Natl. Acad. Sci. Unit. States Am. 2012;109:4556–4561. doi: 10.1073/pnas.1113468109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flier J., Edwards M.W., Daly J.W., Myers C.W. Widespread occurrence in frogs and toads of skin compounds interacting with the ouabain site of Na+,K+-ATPase. Science. 1980 doi: 10.1126/science.6245447. [DOI] [PubMed] [Google Scholar]
- Fuhrman F.A., Fuhrman G.J., Mosher H.S. Toxin from skin of frogs of the genus Atelopus: differentiation from Dendrobatid toxins. Science 84. 1969;165:1376–1377. doi: 10.1126/science.165.3900.1376. [DOI] [PubMed] [Google Scholar]
- Fuhrman F.A., Fuhrman G.J., Kim Y.H., Mosher H.S. Pharmacology and chemistry of chiriquitoxin: a new tetrodotoxin-like substance from the Costa Rican frog Atelopus chiriquiensis. PWPSA (Proc. West. Pharmacol. Soc.) 1976;19:381–384. [PubMed] [Google Scholar]
- Gall B.G., Stokes A.N., French S.S., Schlepphorst E.A., Brodie E.D., Brodie E.D. Tetrodotoxin levels in larval and metamorphosed newts (Taricha granulosa) and palatability to predatory dragonflies. Toxicon. 2011;57:978–983. doi: 10.1016/j.toxicon.2011.03.020. [DOI] [PubMed] [Google Scholar]
- Gall B.G., Stokes A.N., French S.S., Brodie E.D., Brodie E.D. Female newts (Taricha granulosa) produce tetrodotoxin laden eggs after long term captivity. Toxicon. 2012;60:1057–1062. doi: 10.1016/j.toxicon.2012.07.017. [DOI] [PubMed] [Google Scholar]
- Garraffo H.M., Gros E.G. Biosynthesis of bufadienolides in toads. VI. experiments with [1,2-3H]cholesterol, [21-14C]coprostanol, and 5β-[21-14C]pregnanolone in the toad Bufo arenarum. Steroids. 1986;48:251–257. doi: 10.1016/0039-128X(86)90008-5. [DOI] [PubMed] [Google Scholar]
- Gómez-Hoyos D.A., Seisdedos-de-Vergara R., Schipper J., Allard R., González-maya J.F. Potential effect of habitat disturbance on reproduction of the critically endangered harlequin frog Atelopus varius in Las Tablas, Costa Rica. Anim. Biodivers. Conserv. 2020;43:1–7. doi: 10.32800/abc.2020.43.0001. [DOI] [Google Scholar]
- González-Maya J.F., Gómez-Hoyos D.A., Cruz-Lizano I., Schipper J. From hope to alert: demography of a remnant population of the critically endangered Atelopus varius from Costa Rica. Stud. Neotrop. Fauna Environ. 2018;53:194–200. doi: 10.1080/01650521.2018.1460931. [DOI] [Google Scholar]
- González-Maya J.F., Gómez-Hoyos D.A., Seisdedos-de-Vergara R., Cruz-Lizano I., Schipper J. Water-bug (abedus sp.; belostomatidae) predation on the critically endangered Atelopus varius (Bufonidae) at las tablas protected zone, Costa Rica. Acta Biol. Colomb. 2019;24:403–406. doi: 10.15446/abc.v24n2.76924. [DOI] [Google Scholar]
- Gosavi S.M., Gaikwad P.S., Gramapurohit N.P., Kumar A.R. Occurrence of parotoid glands in tadpoles of the tropical frog, Clinotarsus curtipes and their role in predator deterrence. Comp. Biochem. Physiol., A. 2014;170:31–37. doi: 10.1016/j.cbpa.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Goto T., Kishi Y., Takahashi S., Hirata Y. Tetrodotoxin. Tetrahedron. 1965;21 doi: 10.1016/S0040-4020(01)98344-9. 2059–2088. [DOI] [PubMed] [Google Scholar]
- Graybeal A. Phylogenetic relationships of bufonid frogs and tests of alternate macroevolutionary hypotheses characterizing their radiation. Zool. J. Linn. Soc. 1997;119:297–338. doi: 10.1006/zjls.1996.0068. [DOI] [Google Scholar]
- Greene H.W. First. ed. University of California Press; 1997. Snakes: the Evolution of Mystery in Nature. [Google Scholar]
- Hackett J.D., Wisecaver J.H., Brosnahan M.L., Kulis D.M., Anderson D.M., Bhattacharya D., Gerald Plumley F., Erdner D.L. Evolution of saxitoxin synthesis in cyanobacteria and dinoflagellates. Mol. Biol. Evol. 2013;30:70–78. doi: 10.1093/molbev/mss142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hague M.T.J., Stokes A.N., Feldman C.R., Brodie E.D., Brodie E.D. The geographic mosaic of arms race coevolution is closely matched to prey population structure. Evol. Lett. evl3. 2020;184 doi: 10.1002/evl3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanifin C.T. The chemical and evolutionary ecology of tetrodotoxin (TTX) toxicity in terrestrial vertebrates. Mar. Drugs. 2010 doi: 10.3390/md8030577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanifin C.T., Gilly W.F. Evolutionary history of a complex adaptation: tetrodotoxin resistance in salamanders. Evolution. 2015;69:232–244. doi: 10.1111/evo.12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanifin C.T., Brodie E.D., Brodie E.D. Tetrodotoxin levels of the rough-skin newt, Taricha granulosa, increase in long-term captivity. J. Chem. Ecol. 2002;40:1149–1153. doi: 10.1016/s0041-0101(02)00115-0. https://doi.org/https://doi.org/10.1016/S0041-0101(02)00115-0Get. [DOI] [PubMed] [Google Scholar]
- Hantak M.M., Grant T., Reinsch S., Mcginnity D., Loring M., Toyooka N., Saporito R.A. Dietary alkaloid sequestration in a poison frog: an experimental test of alkaloid uptake in Melanophryniscus stelzneri (Bufonidae) J. Chem. Ecol. 2013;39:1400–1406. doi: 10.1007/s10886-013-0361-5. [DOI] [PubMed] [Google Scholar]
- Hayes R.A., Crossland M.R., Hagman M., Capon R.J., Shine R. Ontogenetic variation in the chemical defenses of cane toads (Bufo marinus): toxin profiles and effects on predators. J. Chem. Ecol. 2009;35:391–399. doi: 10.1007/s10886-009-9608-6. [DOI] [PubMed] [Google Scholar]
- Hayes R.A., Piggott A.M., Dalle K., Capon R.J. Microbial biotransformation as a source of chemical diversity in cane toad steroid toxins. Bioorg. Med. Chem. Lett. 2009;19:1790–1792. doi: 10.1016/j.bmcl.2009.01.064. [DOI] [PubMed] [Google Scholar]
- Hegyi B., Bárándi L., Komáromi I., Papp F., Horváth B., Magyar J., Bányász T., Krasznai Z., Szentandrássy N., Nánási P.P. Tetrodotoxin blocks L-type Ca 2+ channels in canine ventricular cardiomyocytes. Pflugers Arch. Eur. J. Physiol. 2012;464:167–174. doi: 10.1007/s00424-012-1114-y. [DOI] [PubMed] [Google Scholar]
- Hegyi B., Komáromi I., Kistamás K., Ruzsnavszky F., Váczi K., Horváth B., Magyar J., Bányász T., Nánási P.P., Szentandrássy N. Tetrodotoxin blockade on canine cardiac L-type Ca2+ channels depends on pH and redox potential. Mar. Drugs. 2013;11:2140–2153. doi: 10.3390/md11062140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda S., Arakawa O., Takatani T., Tachibana K., Yagi M., Tanigawa A., Noguchi T. Toxification of cultured puffer fish Takifugu rubripes by feeding on tetrodotoxin-containing diet. Nippon Suisan Gakkaishi. 2005;71:815–820. doi: 10.2331/suisan.71.815. [DOI] [Google Scholar]
- How C.K., Chern C.H., Huang Y.C., Wang L.M., Lee C.H. Tetrodotoxin poisoning. Am. J. Emerg. Med. 2003;21:51–54. doi: 10.1053/ajem.2003.50008. [DOI] [PubMed] [Google Scholar]
- Hwang P.A., Tsai Y.H., Lin H.P., Hwang D.F. Tetrodotoxin-binding proteins isolated from five species of toxic gastropods. Food Chem. 2007;103:1153–1158. doi: 10.1016/j.foodchem.2006.10.021. [DOI] [Google Scholar]
- Inoue T., Nakata R., Savitsky A., Yoshinaga N., Mori A., Mori N. Variation in bufadienolide composition of parotoid gland secretion from three taxa of Japanese toads. J. Chem. Ecol. 2020;46 doi: 10.1007/s10886-020-01217-y. [DOI] [PubMed] [Google Scholar]
- Itoi S., Ueda H., Yamada R., Takei M., Sato T., Oshikiri S., Wajima Y., Ogata R., Oyama H., Shitto T., Okuhara K., Tsunashima T., Sawayama E., Sugita H. Including planocerid flatworms in the diet effectively toxifies the pufferfish. Takifugu niphobles. Sci. Rep. 2018;8:1–10. doi: 10.1038/s41598-018-30696-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IUCN The IUCN redlist of threatened species version 2021-1 [WWW Document] 2021. https://www.iucnredlist.org 11.18.21.
- Jeckel A.M., Grant T., Saporito R.A. Sequestered and synthesized chemical defenses in the poison frog Melanophryniscus moreirae. J. Chem. Ecol. 2015;41:505–512. doi: 10.1007/s10886-015-0578-6. [DOI] [PubMed] [Google Scholar]
- Johnson P.T.J., Calhoun D.M., Stokes A.N., Susbilla C.B., McDevitt-Galles T., Briggs C.J., Hoverman J.T., Tkach V.V., de Roode J.C. Of poisons and parasites—the defensive role of tetrodotoxin against infections in newts. J. Anim. Ecol. 2018;87:1192–1204. doi: 10.1111/1365-2656.12816. [DOI] [PubMed] [Google Scholar]
- Jorge R.F., Ferrão M., Lima A.P. Out of bound: a new threatened harlequin toad (Bufonidae, Atelopus) from the outer borders of the Guiana shield in Central Amazonia described through integrative taxonomy. Diversity. 2020;12 doi: 10.3390/D12080310. [DOI] [Google Scholar]
- Jorge R.F., Magnusson W.E., Silva D.A. da, Polo É.M., Lima A.P. Urban growth threatens the lowland Amazonian Manaus harlequin frog which represents an evolutionarily significant unit within the genus Atelopus (Amphibia: Anura: Bufonidae) J. Zool. Syst. Evol. Res. 2020;58:1195–1205. doi: 10.1111/jzs.12390. [DOI] [Google Scholar]
- Kahn T.R., Rada M., Santiago S. In: Rana Arlequines. Conservación Internacional. Almonacid J.V.R., Rodríguez-Mahecha J.V., La Marca E., Lötters S., Kahn T.R., Angulo A., editors. 2005. Atelopus senex; p. 108. Bogotá, Colombia. [Google Scholar]
- Kamalakkannan V., Salim A.A., Capon R.J. Microbiome-mediated biotransformation of cane toad bufagenins. J. Nat. Prod. 2017;80 doi: 10.1021/acs.jnatprod.7b00134. 2012–2017. [DOI] [PubMed] [Google Scholar]
- Kardos M., Armstrong E., Fitzpatrick S., Hauser S., Hedrick P., Miller J., Tallmon D.A., Funk W.C. The crucial role of genome-wide genetic variation in conservation. bioRxiv. 2021;118:2021. doi: 10.1073/pnas.2104642118. 07.05.451163. https://doi.org/10.1073/pnas.2104642118/-/DCSupplemental.Published. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata T. vol. II. Japan Food Hygiene Association; Tokyo, Japan: 1978. Assay method for tetrodotoxin; pp. 232–240. (Food Hygiene Examination Manual). [Google Scholar]
- Kim Y.H., Brown G.B., Mosher H.S., Fuhrman F.A. Tetrodotoxin: occurrence in atelopid frogs of Costa Rica. Science. 1975;189(80-):151–152. doi: 10.1126/science.1138374. [DOI] [PubMed] [Google Scholar]
- Klein D. In: Organic Chemistry. Sladjana B., Kalkut J., editors. John Wiley and Sons, Inc.; 2017. Nuclear magnetic resonance spectroscopy; pp. 650–700. [Google Scholar]
- EFSA CONTAM Panel. Knutsen H.K., Alexander J., Barregård L., Bignami M., Brüschweiler B., Ceccatelli S., Cottrill B., Dinovi M., Edler L., Grasl‐Kraupp B., Hogstrand C., Hoogenboom L., Ron ), Nebbia C.S., Oswald I.P., Rose M., Roudot A., Schwerdtle T., Vleminckx C., Vollmer G., Wallace H., Arnich N., Benford D., Botana L., Viviani B., Arcella D., Binaglia M., Horvath Z., Steinkellner H., van Manen M., Petersen A. Risks for public health related to the presence of tetrodotoxin (TTX) and TTX analogues in marine bivalves and gastropods. EFSA J. 2017;15 doi: 10.2903/j.efsa.2017.4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok P.J.R., Ratz S., MacCulloch R.D., Lathrop A., Dezfoulian R., Aubret F., Means D.B. Historical biogeography of the palaeoendemic toad genus Oreophrynella (Amphibia: Bufonidae) sheds a new light on the origin of the Pantepui endemic terrestrial biota. J. Biogeogr. 2018;45:26–36. doi: 10.1111/jbi.13093. [DOI] [Google Scholar]
- Kono M., Matsui T., Furukawa K., Takase T., Yamamori K., Kaneda H., Aoki D., Jang J.-H., Yotsu-Yamashita M. Examination of transformation among tetrodotoxin and its analogs in the living cultured juvenile puffer fish, kusafugu, Fugu niphobles by intramuscular administration. Toxicon. 2008;52:714–720. doi: 10.1016/j.toxicon.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Krebs H.A. The August Krogh principle: “for many problems there is an animal on which it can be most conveniently studied. J. Exp. Zool. 1975;194:221–226. doi: 10.1002/jez.1401940115. [DOI] [PubMed] [Google Scholar]
- Krogh A. The progress of physiology. Am. J. Physiol. 1929;90:243–251. [Google Scholar]
- Kudo Y., Yotsu-Yamashita M. Isolation and biological Activity of 8-epitetrodotoxin and the structure of a possible biosynthetic shunt product of tetrodotoxin, Cep-226A, from the newt Cynops ensicauda popei. J. Nat. Prod. 2019;82:1656–1663. doi: 10.1021/acs.jnatprod.9b00178. [DOI] [PubMed] [Google Scholar]
- Kudo Y., Yamashita Y., Mebs D., Cho Y., Konoki K., Yasumoto T., Yotsu-Yamashita M. C5-C10 directly bonded tetrodotoxin analogues: possible biosynthetic precursors of tetrodotoxin from newts. Angew. Chem. Int. Ed. 2014;53:14546–14549. doi: 10.1002/anie.201408913. [DOI] [PubMed] [Google Scholar]
- Kudo Y., Chiba C., Konoki K., Cho Y., Yotsu-Yamashita M. Confirmation of the absence of tetrodotoxin and its analogues in the juveniles of the Japanese fire-bellied newt, Cynops pyrrhogaster, captive-reared from eggs in the laboratory using HILIC-LC-MS. Toxicon. 2015;101:101–105. doi: 10.1016/j.toxicon.2015.05.008. [DOI] [PubMed] [Google Scholar]
- Kudo Y., Yasumoto T., Mebs D., Cho Y., Konoki K., Yotsu-Yamashita M. Cyclic guanidine compounds from toxic newts support the hypothesis that tetrodotoxin is derived from a monoterpene. Angew. Chem. Int. Ed. 2016;55:8728–8731. doi: 10.1002/anie.201602971. [DOI] [PubMed] [Google Scholar]
- Kudo Y., Chiba C., Konoki K., Cho Y., Yotsu-Yamashita M. Dietary administration of tetrodotoxin and its putative biosynthetic intermediates to the captive-reared non-toxic Japanese fire-bellied newt, Cynops pyrrhogaster. Toxicon. 2017;137:78–82. doi: 10.1016/j.toxicon.2017.07.016. [DOI] [PubMed] [Google Scholar]
- Kudo Y., Hanifin C.T., Kotaki Y., Yotsu-Yamashita M. Structures of N-hydroxy-type tetrodotoxin analogues and bicyclic guanidinium compounds found in toxic newts. J. Nat. Prod. 2020;83:2706–2717. doi: 10.1021/acs.jnatprod.0c00623. [DOI] [PubMed] [Google Scholar]
- Kudo Y., Hanifin C.T., Yotsu-Yamashita M. Identification of tricyclic guanidino compounds from the tetrodotoxin-bearing newt Taricha granulosa. Org. Lett. 2021;23:3513–3517. doi: 10.1021/acs.orglett.1c00916. [DOI] [PubMed] [Google Scholar]
- La Marca E., Lips K.R., Lötters S., Puschendorf R., Ibáñez R., Rueda-Almonacid J.V., Schulte R., Marty C., Castro F., Manzanilla-Puppo J., Garcia-Pérez J.E., Bolaños F., Chaves G., Pounds J.A., Toral E., Young B.E. Catastrophic population declines and extinctions in neotropical harlequin frogs (Bufonidae: Atelopus) Biotropica. 2005;37:190–201. doi: 10.1111/j.1744-7429.2005.00026.x. [DOI] [Google Scholar]
- Lamadrid-Feris F., Coy-Barrera E.D., Franco O.L., Valencia J.A., Arboleda V.J.W. Identification of compounds from white frog (Anura: hylidae) cutaneous secretions with potential to be used in biotechnological processes. Pharmacologyonline. 2015;2:118–123. [Google Scholar]
- Lampo M., Señaris C., García C.Z. Population dynamics of the critically endangered toad Atelopus cruciger and the fungal disease chytridiomycosis. PLoS One. 2017;12:1–18. doi: 10.1371/journal.pone.0179007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman E.M., Brodie E.D., Brodie E.D., III No evidence for an endosymbiotic bacterial origin of tetrodotoxin in the newt Taricha granulosa. Toxicon. 2004;44:243–249. doi: 10.1016/j.toxicon.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Lenaerts C., Bond L., Tuytten R., Blankert B. Revealing of endogenous Marinobufagin by an ultra-specific and sensitive UHPLC-MS/MS assay in pregnant women. Talanta. 2018;187:193–199. doi: 10.1016/j.talanta.2018.05.020. [DOI] [PubMed] [Google Scholar]
- Li F., Yang R., Weng Y., Tang X. Preparation and evaluation of lyophilized liposome-encapsulated bufadienolides. Drug Dev. Ind. Pharm. 2009;35:1048–1058. doi: 10.1080/03639040902762987. [DOI] [PubMed] [Google Scholar]
- Li W., Lin X., Yang Z., Zhang W., Ren T., Qu F., Wang Y., Zhang N., Tang X. A bufadienolide-loaded submicron emulsion for oral administration: stability, antitumor efficacy and toxicity. Int. J. Pharm. 2015;479:52–62. doi: 10.1016/j.ijpharm.2014.12.054. [DOI] [PubMed] [Google Scholar]
- Li Z., Tian J., Lai Y., Lee C.H., Cai Z., Yu C.F. Puffer fish gut microbiota studies revealed unique bacterial co-occurrence patterns and new insights on tetrodotoxin producers. Mar. Drugs. 2020;18 doi: 10.3390/md18050278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtstein D., Gati I., Babila T., Haver E., Katz U. Effect of salt acclimation on digitalis-like compounds in the toad. Biochem. Biophys. Acta. 1991;1073:65–68. doi: 10.1016/0304-4165(91)90183-H. [DOI] [PubMed] [Google Scholar]
- Lima J.D., Lima J.R.F., Sobrinho A.F. Predation of a harlequin toad, Atelopus hoogmoedi Lescure, 1974 by the gold wolf fish, Hoplerythrinus unitaeniatus (Spix and Agassiz, 1829), in a stream of the Cajari river extractive reserve, Amapá, Brazil. Herpetol. Notes. 2019;12:587–589. [Google Scholar]
- Lindquist E.D., Sapoznick S.A., Rodriguez E.J.G., Johantgen P.B., Criswell J.M. Nocturnal position in the Panamanian golden frog, Atelopus zeteki (Anura, Bufonidae), with notes on fluorescent pigment tracking. Phyllomedusa. 2007;6:37–44. doi: 10.11606/issn.2316-9079.v6i1p37-44. [DOI] [Google Scholar]
- Lindroth R.L., Hwang S.-Y. In: Phytochemical Diversity and Redundancy in Ecological Interactions. Romeo J.T., Saunders J.A., Barbosa P., editors. Springer; 1996. Diversity, redundancy, and multiplicity in chemical defense systems of aspen; pp. 25–56. [DOI] [Google Scholar]
- Lips K.R. Decline of a tropical montane amphibian fauna. Conserv. Biol. 2008;12:106–117. doi: 10.1111/j.1523-1739.1998.96359.x. [DOI] [Google Scholar]
- Lötters S., Haas W., Schick S., Böhme W. On the systematics of the harlequin frogs (Amphibia: Bufonidae: Atelopus) from Amazonia. II: redescription of Atelopus pulcher (Boulenger, 1882) from the eastern Andean versant in Peru. Salamandra. 2002;38:165–184. [Google Scholar]
- Lötters S., Meijden A. Van Der, Coloma L.A., Boistel R., Cloetens P., Ernst R., Lehr E., Veith M. Assessing the molecular phylogeny of a near extinct group of vertebrates: the Neotropical harlequin frogs (Bufonidae; Atelopus) Syst. Biodivers. 2011;9:45–57. doi: 10.1080/14772000.2011.557403. [DOI] [Google Scholar]
- Lötters S., Mebs D., Köhler G., Vargas J., Marca E. La. The voice from the hereafter: vocalisations in three species of Atelopus from the Venezuelan Andes, likely to be extinct. HERPETOZOA. 2019;32:267–275. doi: 10.3897/herpetozoa.32.e39192. [DOI] [Google Scholar]
- Lüddecke T., Schulz S., Steinfartz S., Vences M. A salamander's toxic arsenal: review of skin poison diversity and function in true salamanders, genus Salamandra. Sci. Nat. 2018 doi: 10.1007/s00114-018-1579-4. [DOI] [PubMed] [Google Scholar]
- Lukowski A.L., Narayan A.R.H. Natural voltage-gated sodium channel ligands: biosynthesis and biology. Chembiochem. 2019;20:1231–1241. doi: 10.1002/cbic.201800754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magarlamov T., Melnikova D., Chernyeshev A. Tetrodotoxin-producing bacteria: detection, distribution and migration of the toxin in aquatic systems. Toxins. 2017;9 doi: 10.3390/toxins9050166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailho-Fontana P.L., Jared C., Antoniazzi M.M., Sciani J.M., Pimenta D.C., Stokes A.N., Grant T., Brodie E.D., Brodie E.D. Variations in tetrodotoxin levels in populations of Taricha granulosa are expressed in the morphology of their cutaneous glands. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-54765-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T., Yamamori K., Furukawa K., Kono M. Purification and some properties of a tetrodotoxin binding protein from the blood plasma of kusafugu. Takifugu niphobles. Toxicon. 2000;38:463–468. doi: 10.1016/S0041-0101(99)00166-X. [DOI] [PubMed] [Google Scholar]
- Matsumoto T., Tanuma D., Tsutsumi K., Jeon J.K., Ishizaki S., Nagashima Y. Plasma protein binding of tetrodotoxin in the marine puffer fish Takifugu rubripes. Toxicon. 2010;55:415–420. doi: 10.1016/j.toxicon.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Mcdiarmid R.W. vol. 12. Prof. Publ. Los Angeles Cty. Museum; 1971. (Comparative Morphology and Evolution of Frogs of the Neotropical Genera Atelopus, Dendrophryniscus, Melanophryniscus, and Oreophrynella). [Google Scholar]
- McGlothlin J.W., Kobiela M.E., Feldman C.R., Castoe T.A., Geffeney S.L., Hanifin C.T., Toledo G., Vonk F.J., Richardson M.K., Brodie E.D., Pfrender M.E., Brodie E.D. Historical contingency in a multigene family facilitates adaptive evolution of toxin resistance. Curr. Biol. 2016;26:1616–1621. doi: 10.1016/j.cub.2016.04.056. [DOI] [PubMed] [Google Scholar]
- Mebs D., Schmidt K. Occurrence of tetrodotoxin in the frog Atelopus oxyrhynchus. Toxicon. 1989;27:819–822. doi: 10.1016/0041-0101(89)90049-4. [DOI] [PubMed] [Google Scholar]
- Mebs D., Yotsu-Yamashita M. Acquiring toxicity of a newt, Cynops orientalis. Toxicon. 2021;198:32–35. doi: 10.1016/j.toxicon.2021.04.025. [DOI] [PubMed] [Google Scholar]
- Mebs D., Yotsu-Yamashita M., Yasumoto T., Lötters S., Schlüter A. 1995. Further Report of the Occurrence of Tetrodotoxin in Atelopus Species (Family: Bufonidae), Toxicon. [DOI] [PubMed] [Google Scholar]
- Mebs D., Lorentz M., Yotsu-Yamashita M., Rößler D.C., Ernst R., Lötters S. Geographic range expansion of tetrodotoxin in amphibians – first record in Atelopus hoogmoedi from the Guiana Shield. Toxicon. 2018;150:175–179. doi: 10.1016/j.toxicon.2018.05.011. [DOI] [PubMed] [Google Scholar]
- Mebs D., Pogoda W., Toennes S.W. Loss of skin alkaloids in poison toads, Melanophryniscus klappenbachi (Anura: Bufonidae) when fed alkaloid-free diet. Toxicon. 2018;150:267–269. doi: 10.1016/j.toxicon.2018.06.075. [DOI] [PubMed] [Google Scholar]
- Mebs D., Yotsu-Yamashita M., Hartmann K., Elbert C., Zehner R., Toennes S.W. Revisited - failure of tetrodotoxin to protect red-spotted newts, Notophthalmus viridescens, from endoparasites. Toxicon. 2020;178:77–81. doi: 10.1016/j.toxicon.2020.02.026. [DOI] [PubMed] [Google Scholar]
- Mohammadi S., Yang L., Harpak A., Herrera-Álvarez S., del Pilar Rodríguez-Ordoñez M., Peng J., Zhang K., Storz J.F., Dobler S., Crawford A.J., Andolfatto P. Concerted evolution reveals co-adapted amino acid substitutions in Na+K+-ATPase of frogs that prey on toxic toads. Curr. Biol. 2021;31:2530–2538. doi: 10.1016/j.cub.2021.03.089. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D.J., Halliday D.C.T., Rowell D.M., Robinson A.J., Keogh J.S. Positive Darwinian selection results in resistance to cardioactive toxins in true toads (Anura: Bufonidae) Biol. Lett. 2009;5:513–516. doi: 10.1098/rsbl.2009.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtha E.F. Pharmacological study of poisons from shellfish and puffer fish. Ann. N. Y. Acad. Sci. 1960;90:820–836. doi: 10.1111/j.1749-6632.1960.tb26425.x. [DOI] [PubMed] [Google Scholar]
- Myers C.W., Daly J.W., Malkin B. A dangerously toxic new frog (Phyllobates) used by Embera Indians of Western Colombia, with discussion of blowgun fabrication and dart poisoning. Bull. Am. Mus. Nat. Hist. 1978;161:307–366. [Google Scholar]
- Nakagawa T., Jang J., Yotsu-Yamashita M. Hydrophilic interaction liquid chromatography-electrospray ionization mass spectrometry of tetrodotoxin and its analogs. Anal. Biochem. 2006;352(1):142–144. doi: 10.1016/j.ab.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Yasumoto T. Tetrodotoxin derivatives in puffer fish. Toxicon. 1985;23:271–276. doi: 10.1016/0041-0101(85)90149-7. [DOI] [PubMed] [Google Scholar]
- Narahashi T. Tetrodotoxin - a brief history. Proc. Japan Acad. Ser. B Phys. Biol. Sci. 2008;84:147–154. doi: 10.2183/pjab.84.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T., Moore J.W., Scott W.R. Tetrodotoxin blockage of sodium conductance increase in lobster giant axons. J. Gen. Physiol. 1964;47:965–974. doi: 10.1085/jgp.47.5.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson A.C., Kursar T.A. Interactions among plant defense compounds: a method for analysis. Chemoecology. 1999;9:81–92. doi: 10.1007/s000490050037. [DOI] [Google Scholar]
- Paradis E., Schliep K. Ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics. 2019;35:526–528. doi: 10.1093/bioinformatics/bty633. [DOI] [PubMed] [Google Scholar]
- Pavelka L.A., Kim Y.H., Mosher H.S. Tetrodotoxin and tetrodotoxin-like compounds from the eggs of the Costa Rican frog, Atelopus chiriquiensis. Toxicon. 1977;15:135–139. doi: 10.1016/0041-0101(77)90032-0. https://doi.org/https://doi.org/10.1016/0041-0101(77)90032-0. [DOI] [PubMed] [Google Scholar]
- Peres-Gomes F., Ribeiro J.A. Modification of the cardiotoxic effects of ouabain by acepromazine, tetrodotoxin and magnesium sulphate. Pharmacology. 1979;18:80–90. doi: 10.1159/000137234. [DOI] [PubMed] [Google Scholar]
- Petroselli G., Raices M., Jungblut L.D., Pozzi A.G., Erra-Balsells R. MALDI-MS argininyl bufadienolide esters fingerprint from parotoid gland secretions of Rhinella arenarum: age, gender, and seasonal variation. J. Mass Spectrom. 2018;53:465–475. doi: 10.1002/jms.4082. [DOI] [PubMed] [Google Scholar]
- Pires O.R., Sebben A., Schwartz E.F., Morales R.A.V., Bloch C., Schwartz C.A. Further report of the occurrence of tetrodotoxin and new analogues in the Anuran family Brachycephalidae. Toxicon. 2005;45:73–79. doi: 10.1016/j.toxicon.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Porto A.M., Gros E.G. Biosynthesis of the bufadienolide marinobufagin in toads Bufo paracnemis from cholesterol-20-14C. Experientia. 1971;27:506. doi: 10.1007/BF02147562. [DOI] [PubMed] [Google Scholar]
- Quilindo B.C., Vicente J., Almonacid R. In: Almonacid J.V.R., Rodríguez-Mahecha J.V., La Marca E., Lötters S., Kahn T.R., Angulo A., editors. 2005. Atelopus complejo “ignescens,”. (Rana Arlequines. Conservación Internacional). 135'136. Bogotá, Colombia. [Google Scholar]
- R Core Team R: a language and environment for statistical computing. 2019. https://www.R-project.org/ URL.
- Raaymakers C., Verbrugghe E., Hernot S., Hellebuyck T., Betti C., Peleman C., Claeys M., Bert W., Caveliers V., Ballet S., Martel A., Pasmans F., Roelants K. Antimicrobial peptides in frog poisons constitute a molecular toxin delivery system against predators. Nat. Commun. 2017;8 doi: 10.1038/s41467-017-01710-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez J.P., Jaramillo C.A., Lindquist E.D., Crawford A.J., Ibáñez R. Recent and rapid radiation of the highly endangered harlequin frogs (Atelopus) into Central America inferred from mitochondrial DNA sequences. Diversity. 2020;12 doi: 10.3390/D12090360. [DOI] [Google Scholar]
- Ramírez-Castañeda V. University of Los Andes; Bogotá, Colombia: 2017. Origin of Mutations in the NaV Gene Family Associated with Resistance to Neurotoxins in Snakes (Erythrolamprus Sp.) Predators of Poison Dart Frogs (Dendrobatidae) [Google Scholar]
- Reddy D., Kumavath R., Barh D., Azevedo V., Ghosh P. Anticancer and antiviral properties of cardiac glycosides: a review to explore the mechanism of actions. Molecules. 2020;25 doi: 10.3390/molecules25163596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revell L.J. phytools: an R package for phylogenetic comparative biology (and other things) Methods Ecol. Evol. 2012;3:217–223. doi: 10.1111/j.2041-210X.2011.00169.x. [DOI] [Google Scholar]
- Roberts D.M., Gallapatthy G., Dunuwille A., Chan B.S. Pharmacological treatment of cardiac glycoside poisoning. Br. J. Clin. Pharmacol. 2016;81:488–495. doi: 10.1111/bcp.12814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez C., Rollins-Smith L., Ibáñez R., Durant-Archibold A.A., Gutiérrez M. Toxins and pharmacologically active compounds from species of the family Bufonidae (Amphibia, Anura) J. Ethnopharmacol. 2017 doi: 10.1016/j.jep.2016.12.021. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Navarro A., Benito B. Sodium or potassium efflux ATPase. A fungal, bryophyte, and protozoal ATPase. Biochim. Biophys. Acta. 2010;1798:1841–1853. doi: 10.1016/j.bbamem.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Roseghini M., Erspamer V., Endean R. Indole-, imidazole- and phenyl-alkylamines in the skin of one hundred amphibian species from Australia and Papua New Guinea. Comp. Biochem. Physiol. Part C, Comp. 1976;54:31–43. doi: 10.1016/0306-4492(76)90022-8. [DOI] [PubMed] [Google Scholar]
- Roseghini M., Erspamer G.F., Severini C. Biogenic amines and active peptides in the skin of fifty-two African amphibian species other than bufonids. Comp. Biochem. Physiol. 1988;91:281–286. doi: 10.1016/0742-8413(88)90030-8. [DOI] [PubMed] [Google Scholar]
- Roseghini M., Erspamer G.F., Severini C., Simmaco M. Biogenic amines and active peptides in extracts of the skin of thirty-two European amphibian species. Comp. Biochem. Physiol. Part C, Comp. 1989;94:455–460. doi: 10.1016/0742-8413(89)90097-2. [DOI] [PubMed] [Google Scholar]
- Rosker C., Lohberger B., Hofer D., Steinecker B., Quasthoff S., Schreibmayer W. The TTX metabolite 4,9-anhydro-TTX is a highly specific blocker of the Nav1.6 voltage-dependent sodium channel. Am. J. Physiol. Cell Physiol. 2007;293:783–789. doi: 10.1152/ajpcell.00070.2007. [DOI] [PubMed] [Google Scholar]
- Rozek T., Waugh R.J., Steinborner S.T., Bowie J.H., Tyler M.J., Wallace J.C. The maculatin peptides from the skin glands of the tree frog Litoria genimaculata. A comparison of the structures and antibacterial activities of maculatin 1.1 and caerin 1.1. J. Pept. Sci. 1998;4:111–115. doi: 10.1002/(SICI)1099-1387(199804)4:2<111::AID-PSC134>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Rößler D.C., Lötters S., Mappes J., Valkonen J.K., Menin M., Lima A.P., Pröhl H. Sole coloration as an unusual aposematic signal in a Neotropical toad. Sci. Rep. 2019;9:1–11. doi: 10.1038/s41598-019-55021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda Solano L.A., Navas C.A., Carvajalino-Fernández J.M., Amézquita A. Thermal ecology of montane Atelopus (Anura: Bufonidae): a study of intrageneric diversity. J. Therm. Biol. 2016;58:91–98. doi: 10.1016/j.jtherbio.2016.04.007. [DOI] [PubMed] [Google Scholar]
- Santa-Cruz R., Delgado W.L., Medina C.E., Treviño I., Von May R. Distribution and conservation status of the critically endangered harlequin frog Atelopus epikeisthos (Anura: Bufonidae) Salamandra. 2017;53:423–425. [Google Scholar]
- Saporito R.A., Isola M., Maccachero V.C., Condon K., Donnelly M.A. Ontogenetic scaling of poison glands in a dendrobatid poison frog. J. Zool. 2010;282:238–245. doi: 10.1111/j.1469-7998.2010.00732.x. [DOI] [Google Scholar]
- Schoner W., Scheiner-Bobis G. Endogenous cardiac glycosides: hormones using the sodium pump as signal transducer. Semin. Nephrol. 2005;25:343–351. doi: 10.1016/j.semnephrol.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Shindelman J., Mosher H.S., Fuhrman F.A. Atelopidtoxin from the Panamanian frog, Atelopus zeteki. Toxicon. 1969;7:315–319. doi: 10.1016/0041-0101(69)90031-2. [DOI] [PubMed] [Google Scholar]
- Shoji Y., Yotsu-Yamashita M., Miyazawa T., Yasumoto T. Electrospray ionization mass spectrometry of tetrodotoxin and its analogs: liquid chromatography/mass spectrometry, tandem mass spectrometry, and liquid chromatography/tandem mass spectrometry. Anal. Biochem. 2001;290:10–17. doi: 10.1006/abio.2000.4953. [DOI] [PubMed] [Google Scholar]
- Smith B.P., Tyler M.J., Kaneko T., Garraffo H.M., Spande T.F., Daly J.W. Evidence for biosynthesis of pseudophrynamine alkaloids by an Australian myobatrachid frog (Pseudophryne) and for sequestration of dietary pumiliotoxins. J. Nat. Prod. 2002;65:439–447. doi: 10.1021/np010506a. [DOI] [PubMed] [Google Scholar]
- Smith F.M.J., Wood S.A., van Ginkel R., Broady P.A., Gaw S. First report of saxitoxin production by a species of the freshwater benthic cyanobacterium, Scytonema agardh. Toxicon. 2011;57:566–573. doi: 10.1016/j.toxicon.2010.12.020. [DOI] [PubMed] [Google Scholar]
- Smolowitz R., Doucette G. Immunohistochemical localization of saxitoxin in the siphon epithelium of the butter clam, Saxidomus giganteus. Biol. Bull. 1995:229–230. doi: 10.1086/BBLv189n2p229. [DOI] [PubMed] [Google Scholar]
- Sontheimer H., Fernandez-Marques E., Ullrich N., Pappas C.A., Waxman S.G. Astrocyte Na+ channels are required for maintenance of Na+/K+-ATPase activity. J. Neurosci. 1994;14:2464–2475. doi: 10.1523/jneurosci.14-05-02464.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speed M.P., Ruxton G.D., Mappes J., Sherratt T.N. Why are defensive toxins so variable? An evolutionary perspective. Biol. Rev. 2012;87:874–884. doi: 10.1111/j.1469-185X.2012.00228.x. [DOI] [PubMed] [Google Scholar]
- Stern D., von Berg L., Skiba M., Dorner M.B., Dorner B.G. Replacing the mouse bioassay for diagnostics and potency testing of botulinum neurotoxins – progress and challenges. Berl. Münchener Tierärztliche Wochenschr. 2018;131:375–394. doi: 10.2376/0005-9366-17110. [DOI] [Google Scholar]
- Stokes A.N., Williams B.L., French S.S. An improved competitive inhibition enzymatic immunoassay method for tetrodotoxin quantification. Biol. Proced. Online. 2012;14:3. doi: 10.1186/1480-9222-14-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes A.N., Ducey P.K., Neuman-Lee L., Hanifin C.T., French S.S., Pfrender M.E., Brodie E.D., Brodie E.D. Confirmation and distribution of tetrodotoxin for the first time in terrestrial invertebrates: two terrestrial flatworm species (Bipalium adventitium and Bipalium kewense) PLoS One. 2014;9:2–7. doi: 10.1371/journal.pone.0100718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart S.N., Chanson J.S., Cox N.A., Young B.E., Rodrigues A.S.L., Fischman D.L., Waller R.W. Status and trends of amphibian declines and extinctions worldwide. Science 84. 2004;306:1783–1786. doi: 10.1126/science.1103538. [DOI] [PubMed] [Google Scholar]
- Suenaga K., Kotoku S. Detection of tetrodotoxin in autopsy material by gas chromatography. Arch. Toxicol. 1980;44:291–297. doi: 10.1007/BF00278036. [DOI] [PubMed] [Google Scholar]
- Tanu M.B., Mahmud Y., Tsuruda K., Arakawa O., Noguchi T. Occurrence of tetrodotoxin in the skin of a rhacophoridid frog Polypedates sp. from Bangladesh. Toxicon. 2001;39:937–941. doi: 10.1016/S0041-0101(00)00231-2. [DOI] [PubMed] [Google Scholar]
- Tapia E.E., Coloma L.A., Pazmiño-Otamendi G., Peñafiel N. Rediscovery of the nearly extinct longnose harlequin frog Atelopus longirostris (Bufonidae) in Junín, Imbabura, Ecuador. Neotrop. Biodivers. 2017;3:157–167. doi: 10.1080/23766808.2017.1327000. [DOI] [Google Scholar]
- Tarvin R.D., Peña P., Ron S.R. Changes in population size and survival in Atelopus spumarius (Anura: Bufonidae) are not correlated with chytrid prevalence. J. Herpetol. 2014;48:291–297. doi: 10.1670/11-269. [DOI] [Google Scholar]
- Taylor A.D., Vaisocherová H., Deeds J., DeGrasse S., Jiang S. Tetrodotoxin detection by a surface plasmon resonance sensor in pufferfish matrices and urine. J. Sensors. 2011 doi: 10.1155/2011/601704. 2011. [DOI] [Google Scholar]
- Teramoto N., Zhu H.L., Yotsu-Yamashita M., Inai T., Cunnane T.C. Resurgent-like currents in mouse vas deferens myocytes are mediated by NaV1.6 voltage-gated sodium channels. Pflügers Arch. - Eur. J. Physiol. 2012;464:493–502. doi: 10.1007/s00424-012-1153-4. [DOI] [PubMed] [Google Scholar]
- Thottumkara A.P., Parsons W.H., Du Bois J. Saxitoxin. Angew. Chemie - Int. Ed. 2014;53:5760–5784. doi: 10.1002/anie.201308235. [DOI] [PubMed] [Google Scholar]
- Toledo R.C., Jared C. Cutaneous granular glands and amphibian venoms. Comp. Biochem. Physiol. 1995:1–29. https://doi.org/https://doi.org/10.1016/0300-9629(95)98515-I. [Google Scholar]
- Tsukamoto T., Chiba Y., Wakamori M., Yamada T., Tsunogae S., Cho Y., Sakakibara R., Imazu T., Tokoro S., Satake Y., Adachi M., Nishikawa T., Yotsu-Yamashita M., Konoki K. Differential binding of tetrodotoxin and its derivatives to voltage-sensitive sodium channel subtypes (Nav1.1 to Nav1.7) Br. J. Pharmacol. 2017;174:3881–3892. doi: 10.1111/bph.13985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruda K., Arakawa O., Kawatsu K., Hamano Y., Takatani T., Noguchi T. Secretory glands of tetrodotoxin in the skin of the Japanese newt Cynops pyrrhogaster. Toxicon. 2002;40:131–136. doi: 10.1016/S0041-0101(01)00198-2. [DOI] [PubMed] [Google Scholar]
- Ujvari B., Casewell N.R., Sunagar K., Arbuckle K., Wüster W., Lo N., O'Meally D., Beckmann C., King G.F., Deplazes E., Madsen T., Hillis D.M. Widespread convergence in toxin resistance by predictable molecular evolution. Proc. Natl. Acad. Sci. U.S.A. 2015;112:11911–11916. doi: 10.1073/pnas.1511706112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Üveges B., Fera G., Móricz Á.M., Krüzselyi D., Bókony V., Hettyey A. Age- and environment-dependent changes in chemical defences of larval and post-metamorphic toads. BMC Evol. Biol. 2017;17 doi: 10.1186/s12862-017-0956-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaelli P.M., Theis K.R., Williams J.E., O’connell L.A., Foster J.A., Eisthen H.L. The skin microbiome facilitates adaptive tetrodotoxin production in poisonous newts. Elife. 2020;9 doi: 10.7554/eLife.53898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia L., Marin da Fonte L. Collaborative work brings hope for threatened harlequin toads. Oryx. 2022;56(1) doi: 10.1017/S0030605321001319. 12–12. [DOI] [Google Scholar]
- Venkatesh B., Lu S.Q., Dandona N., See S.L., Brenner S., Soong T.W. Genetic basis of tetrodotoxin resistance in pufferfishes. Curr. Biol. 2005;15:2069–2072. doi: 10.1016/j.cub.2005.10.068. [DOI] [PubMed] [Google Scholar]
- Veselý M., Batista A. A new species of Atelopus (Amphibia: Bufonidae) from eastern Panama. Zool. Res. 2021;42:272–279. doi: 10.24272/j.issn.2095-8137.2020.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Fan Y., Yao Z. Isolation of a Lysinibacillus fusiformis strain with tetrodotoxin-producing ability from puffer fish Fugu obscurus and the characterization of this strain. Toxicon. 2010;56:640–643. doi: 10.1016/j.toxicon.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Watanabe R., Tanioka M., Uchida H., Matsushima R., Oikawa H., Matsumiya M., Yotsu-yamashita M., Suzuki T. Quantitation of tetrodotoxin and its analogues with a combination of liquid chromatography−tandem mass spectrometry and quantitative 1 H-NMR spectroscopy. J. Agric. Food Chem. 2019;67:12911–12917. doi: 10.1021/acs.jafc.9b06380. [DOI] [PubMed] [Google Scholar]
- Weber C.J. The determination of the guanidine bases in urine. J. Biol. Chem. 1928;78:465–473. doi: 10.1016/s0021-9258(18)84005-4. [DOI] [Google Scholar]
- Wickham H. second ed. Springer-Verlag; New York: 2016. ggplot2: Elegant Graphics for Data Analysis. [Google Scholar]
- Wickham H., François R., Henry L., Müller K. dplyr: a grammar of data manipulation. 2021. https://CRAN.R-project.org/package=dplyr
- Wilder-Kofie T.D., Lúquez C., Adler M., Dykes J.K., Coleman J.A.D., Maslanka S.E. An alternative in vivo method to refine the mouse bioassay for botulinum toxin detection. Comp. Med. 2011;61:235–242. [PMC free article] [PubMed] [Google Scholar]
- Wilke C.O. cowplot: streamlined plot theme and plot annotations for “ggplot2. 2020. https://CRAN.R-project.org/package=cowplot
- Williams B.L. University of California; Berkeley: 2008. Distribution, Ontogenetic Profile, and Anti-predator Efficacy of Tetrodotoxin in Two Species of Blue-Ringed Octopuses (Hapalochlaena Lunulata and H. Fasciata) [Google Scholar]
- Williams B.L., Brodie E.D., Jr., Brodie E.D., III Coevolution of deadly toxins and predator resistance: self-assessment of resistance by garter snakes leads to behavioral rejection of toxic newt prey. Herpetologica. 2003;59(2):155–163. [Google Scholar]
- Williams B.L., Hanifin C.T., Brodie E.D., Caldwell R.L. Ontogeny of tetrodotoxin levels in blue-ringed octopuses: maternal investment and apparent independent production in offspring of Hapalochlaena lunulata. J. Chem. Ecol. 2011;37:10–17. doi: 10.1007/s10886-010-9901-4. [DOI] [PubMed] [Google Scholar]
- Woodhams D.C., Voyles J., Lips K.R., Carey C., Rollins-Smith L.A. Predicted disease susceptibility in a Panamanian amphibian assemblage based on skin peptide defenses. J. Wildl. Dis. 2006;42:207–218. doi: 10.7589/0090-3558-42.2.207. [DOI] [PubMed] [Google Scholar]
- Woodward R.B. The structure of tetrodotoxin. Pure Appl. Chem. 1964;9:49–74. doi: 10.1351/pac196409010049. [DOI] [Google Scholar]
- Wu Z., Yang Y., Xie L., Xia G., Hu J., Wang S., Zhang R. Toxicity and distribution of tetrodotoxin-producing bacteria in puffer fish Fugu rubripes collected from the Bohai Sea of China. Toxicon. 2005;46:471–476. doi: 10.1016/j.toxicon.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Yang L., Kao C.Y. Actions of chiriquitoxin on frog skeletal muscle fibers and implications for the tetrodotoxin/saxitoxin receptor. J. Gen. Physiol. 1992;100:609–622. doi: 10.1085/jgp.100.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasumoto T. The Manual for the Methods of Food Sanitation Tests. Ministry of Health and Welfare. Bureau of Environmental Health; Japan: 1991. Pufferfish toxin; pp. 296–300. Tokyo. [Google Scholar]
- Yoshida T., Ujiie R., Savitzky A.H., Jono T., Inoue T., Yoshinaga N., Aburaya S., Aoki W., Takeuchi H., Ding L., Chen Q., Cao C., Tsai T.S., de Silva A., Mahaulpatha D., Nguyen T.T., Tang Y., Mori N., Mori A. Dramatic dietary shift maintains sequestered toxins in chemically defended snakes. Proc. Natl. Acad. Sci. U.S.A. 2020;117:5964–5969. doi: 10.1073/pnas.1919065117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotsu M., Endo A., Yasumoto T. An improved tetrodotoxin analyzer. Agric. Biol. Chem. 1989;53:893–895. doi: 10.1080/00021369.1989.10869337. [DOI] [Google Scholar]
- Yotsu M., Iorizzi M., Yasumoto T. Distribution of tetrodotoxin, 6-epitetrodotoxin, and 11-deoxytetrodotoxin in newts. Toxicon. 1990;28:238–241. doi: 10.1016/0041-0101(90)90419-8. [DOI] [PubMed] [Google Scholar]
- Yotsu M., Yasumoto T., Kim Y.H., Naoki H., Kao C.Y. The structure of chiriquitoxin from the Costa Rican frog Atelopus chiriquiensis. Tetrahedron Lett. 1990;31:3187–3190. https://doi.org/https://doi.org/10.1016/S0040-4039(00)94728-2. [Google Scholar]
- Yotsu-Yamashita M., Tateki E. First report on toxins in the Panamanian toads Atelopus limosus, A. glyphus and A. certus. Toxicon. 2010;55:153–156. doi: 10.1016/j.toxicon.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Yotsu-Yamashita M., Mebs D., Yasumoto T. Tetrodotoxin and its analogues in extracts from the toad Atelopus oxyrhynchus (family: Bufonidae) Toxicon. 1992;30 doi: 10.1016/0041-0101(92)90526-b. 1499–1492.https://doi.org/https://doi.org/10.1016/0041-0101(92)90526-B. [DOI] [PubMed] [Google Scholar]
- Yotsu-Yamashita M., Sugimoto A., Terakawa T., Shoji Y., Miyazawa T., Yasumoto T. Purification, characterization, and cDNA cloning of a novel soluble saxitoxin and tetrodotoxin binding protein from plasma of the puffer fish, Fugu pardalis. Eur. J. Biochem. 2001;268:5937–5946. doi: 10.1046/j.0014-2956.2001.02547.x. [DOI] [PubMed] [Google Scholar]
- Yotsu-Yamashita M., Kim Y.H., Dudley S.C., Choudhary G., Pfahnl A., Oshima Y., Daly J.W. The structure of zetekitoxin AB, a saxitoxin analog from the Panamanian golden frog Atelopus zeteki: a potent sodium-channel blocker. Proc. Natl. Acad. Sci. Unit. States Am. 2004;101:4346–4351. doi: 10.1073/pnas.0400368101. https://doi.org/https://doi.org/10.1073/pnas.0400368101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotsu-Yamashita M., Gilhen J., Russell R.W., Krysko K.L., Melaun C., Kurz A., Kauferstein S., Kordis D., Mebs D. Variability of tetrodotoxin and of its analogues in the red-spotted newt, Notophthalmus viridescens (Amphibia: urodela: Salamandridae) Toxicon. 2012;59:257–264. doi: 10.1016/j.toxicon.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Yotsu-Yamashita M., Abe Y., Kudo Y., Ritson-Williams R., Paul V.J., Konoki K., Cho Y., Adachi M., Imazu T., Nishikawa T., Isobe M. First identification of 5,11-dideoxytetrodotoxin in marine animals, and characterization of major fragment ions of tetrodotoxin and its analogs by high resolution ESI-MS/MS. Mar. Drugs. 2013;11:2799–2813. doi: 10.3390/md11082799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Vincent, Yu Peter, Ho Kin-Chung, Lee Fred. Isolation and identification of a new tetrodotoxin-producing bacterial species, Raoultella terrigena, from Hong Kong marine puffer fish Takifugu niphobles. Marine Drugs. 2011;9(11):2384–2396. doi: 10.3390/md9112384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X., Wu Huan, Hong Wu, Wang R., Luo C., Gao B., Chen Z., Li Q. Metabolites from Bufo gargarizans (Cantor, 1842): a review of traditional uses, pharmacological activity, toxicity and quality control. J. Ethnopharmacol. 2020 doi: 10.1016/j.jep.2019.112178. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Tang X., Liu X., Li F., Lin X. Simultaneous determination of three bufadienolides in rat plasma after intravenous administration of bufadienolides extract by ultra performance liquid chromatography electrospray ionization tandem mass spectrometry. Anal. Chim. Acta. 2008;610:224–231. doi: 10.1016/j.aca.2008.01.029. [DOI] [PubMed] [Google Scholar]
- Zhang X., Zong J., Chen S., Li M., Lu Y., Wang R., Xu H. Accumulation and elimination of tetrodotoxin in the pufferfish Takifugu obscurus by dietary administration of the wild toxic gastropod Nassarius semiplicata. Toxins. 2020;12 doi: 10.3390/toxins12050278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer T. Effects of tetrodotoxin on the mammalian cardiovascular system. Mar. Drugs. 2010;8:741–762. doi: 10.3390/md8030741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer R.K., Schar D.W., Ferrer R.P., Krug P.J., Kats L.B., Michel W.C. The scent of danger: tetrodotoxin (TTX) as an olfactory cue of predation risk. Ecol. Monogr. 2006;76:585–600. https://doi.org/https://doi.org/10.1890/0012-9615(2006)076[0585:TSODTT]2.0.CO;2. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.