Abstract
Zoonotic viruses continually pose a pandemic threat. Infection of humans with viruses for which we typically have little or no prior immunity can result in epidemics with high morbidity and mortality. These epidemics can have public health and economic impact and can exacerbate civil unrest or political instability. Changes in human behavior in the past few decades—increased global travel, farming intensification, the exotic animal trade, and the impact of global warming on animal migratory patterns, habitats, and ecosystems—contribute to the increased frequency of cross-species transmission events. Investing in the pre-clinical advancement of vaccine candidates against diverse emerging viral threats is crucial for pandemic preparedness. Replication-defective adenoviral (Ad) vectors have demonstrated their utility as an outbreak-responsive vaccine platform during the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic. Ad vectors are easy to engineer; are amenable to rapid, inexpensive manufacturing; are relatively safe and immunogenic in humans; and, importantly, do not require specialized cold-chain storage, making them an ideal platform for equitable global distribution or stockpiling. In this review, we discuss the progress in applying Ad-based vaccines against emerging viruses and summarize their global safety profile, as reflected by their widespread geographic use during the SARS-CoV-2 pandemic.
Keywords: adenovirus, vaccine, pandemic, emerging, virus, vector, outbreak, pathogen
Graphical abstract
In this special issue on emerging infectious diseases, Coughlan, Kremer, and Shayakhmetov review the history, safety, and real-world efficacy of adenovirus (Ad)-based vaccines. They also address how the development of Ad-based vaccines against other emerging viruses underpinned their use in response to the SARS-CoV-2 pandemic.
Introduction
The ongoing threat posed by emerging viruses has been highlighted following the introduction of a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), into the human population in late 2019. In addition to coronaviruses, several viruses of concern have been identified by the World Health Organization (WHO) in their “blueprint list of priority diseases” and by the National Institute of Allergy and Infectious Diseases (NIAID) as priority pathogens for biodefense research (Table 1). Many priority pathogens are transmitted by mosquitoes or ticks (i.e., Zika virus), by exposure to infected bats (i.e., Nipah virus), or upon inhalation or ingestion of the urine or feces of infected rodents (i.e., Lassa virus). Viruses with a zoonotic reservoir represent an unpredictable threat for spill-over into the human population. Many of these viruses can cause severe illness with high mortality or fatality, and to date, most lack licensed and approved vaccines or effective pharmaceutical countermeasures. To mitigate the future risks posed by emerging viruses, pandemic preparedness is of the utmost importance. In combination with surveillance programs to establish an accurate geographical distribution of endemic risk areas and investment in diagnostics to determine the true seroprevalence and incidence in humans, advancing the early-stage pre-clinical and translational development of a broad repertoire of candidate vaccines will be crucial.
Table 1.
WHO and NIAID priority viral diseases for research and development
| Disease | Causative agent | Classification (Order, Family) | Zoonotic reservoir/host | Mode of transmission | Case fatality rate in humans | Geographic distribution | PMID |
|---|---|---|---|---|---|---|---|
| Ebola virus disease (EVD) Marburg virus disease (MVD) |
Ebola virus (EBOV) Marburg virus (MARV) |
Mononegavirales Filoviridae Mononegavirales Filoviridae |
Fruit bats, family Pteropodidae Fruit bats, family Rousettus |
Exposure to infected animals or humans, body fluids. Hospital or burial ceremonies are high risk. | 25%–90% 24%–90% |
Central and West Africa Central Africa |
29083948 26325242 33309082 |
| Lassa fever | Lassa virus (LASV) | Bunyavirales Arenaviridae | Mastomys rats | Exposure to rat urine, feces, or fluids | 1% or 50%–70% in hospitalized patients. High rates of fetal mortality in third trimester of pregnancy. | West Africa | 31479990 |
| Crimean-Congo hemorrhagic fever (CCHF) | Crimean-Congo hemorrhagic fever virus (CCHFV) | Bunyavirales Nairoviridae | Viremic livestock, Hyalomma ticks | Tick bites, exposure to body fluids of infected livestock or humans (including nosocomial). | 4%–40% up to 80% in clinically infected patients |
Africa, The Balkans, Middle East, Asia |
29083948 28687403 |
| Hantavirus fever renal syndrome (HFRS) Hantavirus pulmonary syndrome (HPS) |
Sin Nombre virus (SNV) and Andes virus (ANDV) | Bunyavirales Hantaviridae | Deer mice, Peromyscus maniculatus | Exposure to rodents and their droppings or body fluids or following inhalation of aerosolized material from rodent urine or feces | 0.1%–15% for HFRS 40%–50% for HPS |
North/South America (SNV, ANDV) Asia, Europe |
19403663 16375712 16375712 |
| Rift Valley fever (RVF) | Rift Valley fever virus (RVFV) | Bunyavirales Phenuiviridae | Ruminants, mosquitoes, Aedes species | Exposure to infected animals or by mosquito bites during high-density circulation | Up to 35% | Africa and Arabian Peninsula | 29083948 |
| Zika fever | Zika virus (ZIKV) | Amarillovirales Flaviviridae | Mosquitoes, Aedes species | Bite from infected mosquito, vertical transmission, or through sexual contact | Rare, but fetal loss following vertical transmission is 14%, with congenital Zika syndrome in ∼21%. | Africa, Asia, Micronesia, Americas |
29083948 31597021 |
Coronaviruses, including SARS-CoV-1, SARS-CoV-2, and MERS-CoV, have been omitted from this table due to the large amount of published data on these viruses. Emerging viruses from the Paramyxoviridae (i.e., Nipah virus) have also been omitted due to space constraints. Table updated from Ewer et al.1
The target product profile (TPP) for disease-specific vaccines prioritizes characteristics relevant to the nature of the pathogen and the type of risk it poses. Different attributes may be desirable for vaccines aimed at non-emergency prophylactic use versus vaccines designed for use in an emergency outbreak scenario. Preferred characteristics include platforms capable of rapid induction of durable protective immunity, those with an established safety and immunogenicity profile in relevant risk groups, the potential to elicit breadth of protection against variants or viral lineages, and vaccines compatible with thermostability and prolonged shelf life. Replication-defective adenoviral (Ad) vaccines have been a prominent platform in the response to the SARS-CoV-2 pandemic, with vaccines based on three Ad types: HAdV-C5 (Ad5); HAdV-D26 (Ad26); and chimpanzee Y25 (ChAdOx1), receiving emergency use authorization (EUA) across the United States, EU, South America, Asia, and Africa and full approval in Russia. In this review, we will outline the benefits, risks, and potential future of Ad-based vaccines against emerging viruses, summarize data from pre-clinical and clinical trials using Ad vaccines for “priority diseases,” and discuss the safety of Ad vaccines, following their extensive global use during the SARS-CoV-2 pandemic.
Background on vaccines
Vaccines: Mechanism of action
Most of us know that vaccines protect us from diseases because they teach our immune system to recognize the pathogen and induce an immune response, which prevents us from being infected by a virus. Vaccines prevent the death of >15 million individuals every year. Due to a successful global vaccination program, smallpox was eradicated in 1980 and is no longer the scourge it was for greater than a millennium. Following the introduction of the measles, mumps, and rubella (MMR) vaccine, deaths attributed to these viral infections declined by 96% since 1980 (when global deaths from measles were in the millions).2,3 Unfortunately, the development of long-lasting, effective vaccines against many viral pathogens can be challenging. Factors that contribute to this include high mutation rates and antigenic diversity, poor immunogenicity of conserved epitopes, technical challenges in engineering structurally authentic immunogens, and a lack of correlates of protection. Therefore, efforts to better understand how different vaccine platforms work, what phenotype of immune response they elicit, and precisely how that response contributes to protection will enable a systematic approach to iterative vaccine design.
Initially, vaccines stimulate the recruitment of effector cells to the site of injection, following an innate immune response. Effective vaccines engage professional antigen-presenting cells (APCs) to present exogenous antigens to naive T cells to initiate B cell and T cell responses (Figure 1). In most cases, we produce T cells that lyse infected cells and neutralizing antibodies (NAbs) that can block viral entry. However, the role of non-neutralizing antibodies (Abs) in mediating protection has become apparent in recent years, and this class of Ab can also contribute to viral clearance. For example, Abs can agglutinate viral particles (vps), which make these large aggregates an easy target for immune cells to phagocytose the complex via Fc receptors (FcRs) and degrade the virus. Alternatively, Abs can also bind to viral glycoproteins expressed on the surface of infected cells and target those cells for destruction via Fc-mediated antibody-dependent cellular cytotoxicity (ADCC)4, 5, 6, 7 or phagocytosis (ADCP).8 Abs can also activate the complement pathway, which opsonizes and promotes the phagocytosis of viruses and/or damages the envelope (phospholipid bilayer) present on some viruses.
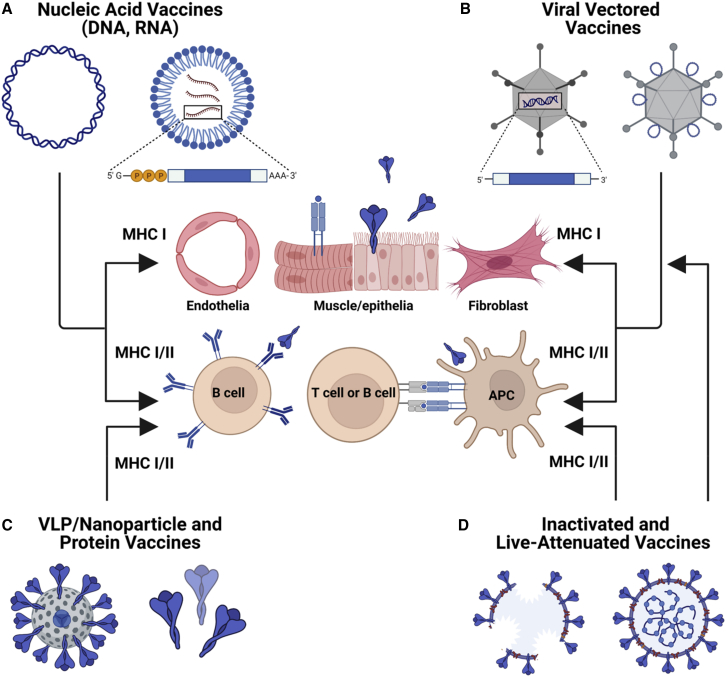
Figure 1.
Vaccine platforms for outbreak pathogens
Schematic diagram showing the range of different platforms that can be used for vaccine development. (A) Nucleic-acid-based vaccines (i.e., DNA or mRNA) encode the vaccine antigen target sequence, allowing for transgene expression in vivo. These vaccines facilitate both MHC class I antigen presentation from cells at the site of injection and MHC class II antigen presentation by APCs. (B) Similarly, viral-vectored vaccines (i.e., Ads) can also encode the transgene antigen sequence or display peptide antigen on the capsid exterior. These vectors allow for in vivo expression and antigen processing via MHC class I and class II. (C) Virus-like-particles (VLPs) or protein-based vaccines are processed in a similar manner to inactivated platforms. (D) Inactivated vaccine platforms are largely scavenged by APCs, resulting in MHC class II presentation, although cross-presentation in dendritic cells (DCs) can facilitate MHC class I presentation. As live attenuated vaccines can infect respiratory epithelia, they can also present antigen via MHC class I. Figure created with BioRender.com.
In non-professional APCs, antigen presentation is thought to be limited to major histocompatibility complex class II (MHC class II) presentation and preferentially induces a Th2-skewed response. However, in professional APCs (e.g., dendritic cells [DCs], Langerhans cells, and macrophages), a phenomenon called cross-presentation occurs, where proteins that are taken up from the extracellular environment can be loaded onto MHC class I molecules to promote a Th1 response. There are several pathways toward cross-presentation in APCs, including cytosolic and vacuolar. Following uptake of exogenous antigen by macropinocytosis or phagocytosis, antigen escape from the early endosome, or fusion of the endosome with the endoplasmic reticulum (ER) and subsequent degradation of antigen by the proteasome, facilitates peptide loading onto recycled cell surface MHC class I molecules. It is worth noting that cross-presentation can operate independently of the proteasome and the transporter associated with antigen presentation. In the vacuolar pathway, peptide antigen can be loaded onto MHC class I in the endosome and lysosome. Of note, it has been shown that CD8+ T cell responses elicited by Ad-based vaccines rely on cross-presentation by subpopulations of DCs.9 It is considered that cooperation between APCs and non-lymphoid cells likely contributes to the kinetics, maintenance, and phenotype of antigen-specific immune responses elicited by Ad-based vaccines.10,11 Collectively, the ability to engage multiple coordinated pathways presumably contributes to their capacity to elicit both cellular and humoral immune responses simultaneously.
Vaccines: Platform selection
Understanding what profile of immune response is desirable in mediating protection for a given disease target is an important consideration when selecting an optimal vaccine platform (Figure 1). In the context of vaccine development for emerging viruses, additional considerations may be required, such as the potential need for manufacturing under high containment (i.e., BSL-3) and associated cost and biosafety implications, suitability for stockpiling, cost-per-dose, and stability under cold-chain free conditions.
Whole viruses: Live attenuated and inactivated vaccines
More than 500 years ago, people in Africa and Asia were using live, unattenuated smallpox to inoculate or “variolate” some members of the community.12 This approach consisted of lancing a ripe pustule of an infected individual and then inserting the lance subcutaneously into a second, healthy individual. While not without risk, this approach must have saved millions of lives. Europeans caught up in the 18th century and added their twist—which was based on the observation that milkmaids who were infected with cowpox appeared to be protected from the ravages of smallpox. This observation translated into the use of cowpox (Vaccinia) as a vaccine against smallpox (Variola), tirelessly promoted by Edward Jenner.13
The advantage of using whole-virus vaccines is the breadth of antigenic targets, as this type of platform can deliver all the proteins in the capsid and possibly internal proteins (which are often highly conserved). Licensed vaccines against influenza virus include live attenuated influenza vaccines (LAIVs) and inactivated influenza vaccines (IIVs): the latter including inactivated whole virion vaccines (WIVs) and split-virion or sub-virion IIV formulations.14 The MMR vaccine is also based on attenuated measles, mumps, and rubella viruses. A live attenuated vaccine is also used to prevent poliomyelitis, which is caused by poliovirus serotypes 1, 2, and 3. The polio vaccine has reduced disease burden by 99%. Unfortunately, use of live polioviruses can cause “vaccine-derived poliovirus” spread and can be problematic if poliovirus is endemic in regions where vaccination rates are low. Another live virus vaccine used for ∼60 years is based on human Ad type 4 and 7 (HAdV-4 and -7) to protect against respiratory illness caused by the same viruses. This oral vaccine has been used almost exclusively in 18- to 30-year-old military recruits since the 1970s.15, 16, 17 The safety profile of HAdV-4 and -7 has been acceptable, and it has saved many lives.18, 19, 20
There are two principal methods for virus inactivation—heat and chemical crosslinking. The rabies vaccine is a whole virus, inactivated with beta-propiolactone (BPL) (as is CoronaVac, BBIBP-CorV, and Covaxin). In many cases, the crude preparation can retain residual viral nucleic acid, which can facilitate stimulation of innate immune signaling (acting as an adjuvant).14,21 When considering the development of live attenuated or inactivated vaccines against viral pathogens that have high fatality, the need to grow virus in high containment makes large-scale manufacture impractical.22 Furthermore, there are additional risks if inactivation procedures are ineffective or sub-optimal or if attenuated strains revert to wild type. These concerns have prompted the development of alternative, next-generation vaccine platforms for emerging viral pathogens.23
Subunit-based vaccines
Protein-based vaccines are a simple, safe, and scalable platform. Before designing a subunit vaccine, one must know enough about the virus to identify which part of the capsid will be the most effective in mediating protection. Considerations include whether the protein is involved in receptor engagement and whether Abs against it will induce virus neutralization. Ideally, the vaccine will also induce a T cell response to allow infected cells to be lysed. Typically, these platforms include a viral protein that was expressed in cells and purified to near homogeneity before being incorporated into a vaccine formulation. Protein production can be in plant, bacteria, yeast, insect, animal, or human cells. An example of a US Food and Drug Administration (FDA)-approved subunit vaccine is the hepatitis B virus vaccine, where the hepatitis B surface antigen (HBsAg) is produced in yeast.24 Each production platform has its strengths and drawbacks with respect to post-translational modification of the protein (in particular glycosylation), production potential, upscaling, upstream and downstream processing, and risk of contaminants. A challenge with protein-based vaccines is often their inability to induce an innate immune response, which can have a negative impact on downstream immunogenicity. A formulation step typically stabilizes the protein and incorporates an adjuvant.
Virus-like particles (VLPs) and self-assembling nanoparticles
VLPs are self-forming structures typically composed of a subset of capsid proteins. Due to their size, symmetry, and particulate composition, VLPs are readily taken up by APCs, allowing for receptor-mediated uptake, clustering and activation of pattern-recognition receptors (PRRs),25 and presentation of particles to lymphocytes so that the immune system will mount an antigen-specific immune response. VLPs can be made from numerous viruses26,27 and can be engineered to contain or present sequences from other viruses.28 In some cases, VLPs can be engineered to package nucleic acids,29 peptides from other pathogens,30 or molecular adjuvants and immunostimulatory molecules on their surfaces.31 VLPs can be produced in cells derived from bacteria, yeast, insect and mammals, and in cell-free expression systems and organisms such as silkworm pupae and various plants.
Like subunit vaccines, VLPs benefit from cross-presentation pathways that allow induction of CD8+ T-cell-mediated cytotoxic immune responses and have been shown to utilize the MHC class I receptor recycling pathway of cross-presentation.32 Several VLP vaccines are commercially available, including Gardasil, a multivalent human papillomavirus (HPV) vaccine.33 Although VLPs represent a promising platform for future development, VLPs derived from bacterial and insect cells can be contaminated with endotoxin or baculovirus, they lack mammalian glycosylation, and there are occasionally issues with formulation stability, precipitation and aggregation, or scale-up to meet global demand.
Another innovative vaccine platform is the use of naturally occurring, self-assembling nanoparticles or computationally designed vaccine scaffolds.34,35 Similar to VLPs, these particles allow for a structurally ordered display of antigen, which, along with their small size, allows them to mimic viruses. Vaccines based on conjugation to bacterial ferritin, which self-assembles into stable nanoparticles allowing for surface presentation of viral glycoprotein ectodomains, have been developed as vaccines against numerous viruses.36, 37, 38, 39 Another bacterial scaffold that has been employed in the development of self-assembling, nanoparticle-based vaccines is lumazine synthase (LS).40,41
Nucleic-acid-based vaccines: DNA and mRNA
A primary difference within nucleic-acid-based vaccines, as compared with inactivated, protein-, or nanoparticle-based platforms, is that the antigen is produced from the cell that takes up the vaccine following immunization. Transgene expression of the target antigen from mRNA likely persists for a few days,42 while DNA vector-based vaccines may provide more sustained antigen presentation.10,43 Antigens encoded by nucleic-acid vaccines can also be targeted to the cell surface to allow more efficient detection by the developing immune response (Figure 2). DNA-based vaccines (plasmids), which have been explored for greater than 3 decades, are rapidly designed, easily produced, scalable, and thermostable. Clever approaches have also allowed the production of plasmids void of antibiotic resistance genes.44 DNA-based vaccines also preferentially induce a Th1-biased immune response. Avoiding degradation prior to reaching the nucleus can be a limiting factor for DNA-based vaccines. However, to date, these vaccines have been encouraging in pre-clinical studies, and significant success in human clinical trials may not be far off.45, 46, 47 Innate responses to DNA and RNA include PRRs that detect uncapped mRNA (TLR7) and unmethylated CpG (TLR9) and those that detect antimicrobial peptides (AMPs) and coagulation factors linked to viral capsid (TLR4).48, 49, 50
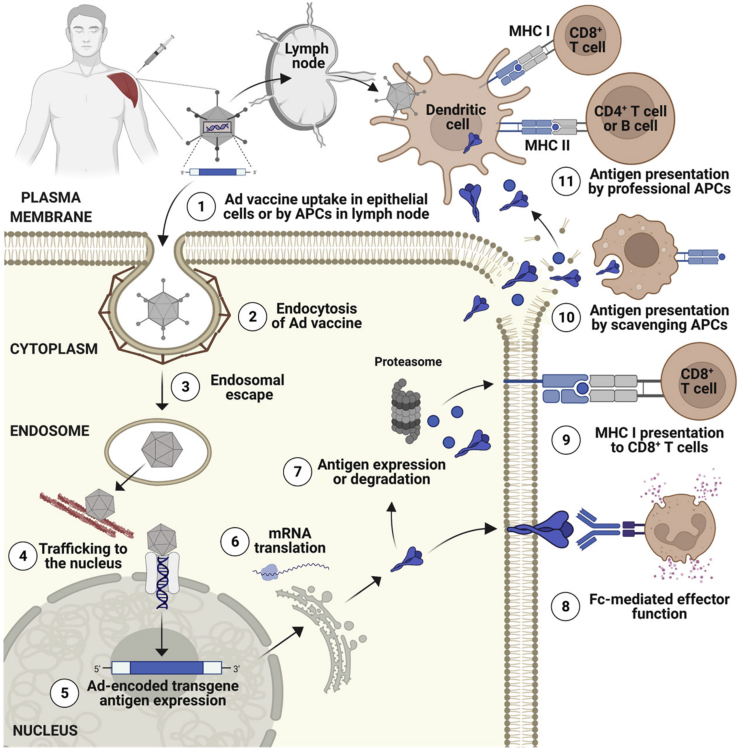
Figure 2.
Schematic diagram outlining the antigen-presentation mechanisms used by Ad-based vaccines
(1) Ad-vaccine is taken up by muscle cells or antigen-presenting cells (APCs) at the site of injection or following trafficking to draining lymph nodes (dLNs). (2) In parenchymal cells (i.e., muscle), uptake can be mediated by endocytosis. (3) Ad vaccine escapes from the endosome. (4) Partially disassembled Ad capsids traffic to the nucleus using the microtubule network. (5) Once in the nucleus, the encoded vaccine transgene antigen is transcribed. (6) mRNA corresponding to the encoded transgene antigen is exported to the cytoplasm and is translated into protein. (7) Antigen is expressed, and some antigen is degraded by the proteasome. (8) Depending on the antigen design, glycoproteins that normally traffic to the plasma membrane will follow this path and can potentially be recognized by Abs, including those capable of Fc-mediated effector function. (9) Degraded peptide antigen can be loaded onto MHC class I for direct presentation to CD8+ T cells. (10) Secreted antigens can be released into the extracellular space or apoptosis of transgene-expressing cells can also facilitate antigen release. Extracellular (exogenous) antigen can be scavenged by macrophages or other APCs at the site of injection. (11) Antigen fragments arriving in the dLN are phagocytosed by professional APCs and peptides processed and presented to T cells via appropriate MHC molecules. Figure created with BioRender.com.
As with plasmid-based vaccines, RNA vaccines have been explored for 3 decades too. The breakthrough that made mRNA viable as a vaccine platform is the modification of their bases, which prevents excessive immunostimulation, allowing evasion of PRR recognition, preventing premature degradation, and therefore enabling increased and sustained transgene antigen expression.51 In addition, advances in formulation chemistry facilitated the encapsulation of modified mRNA in lipid nanoparticles (LNPs), which display biocompatibility and can accommodate a large mRNA payload,51 with the capacity to encode more than one antigen for a multi-valent vaccine.52 Following uptake in target cells at the site of injection, mRNA-based vaccines do not have to enter the nucleus. Therefore, a significant trafficking hurdle is avoided, and mRNA can be very rapidly translated into the targeted protein or antigen in the cytoplasm. The subject of applying mRNA vaccines against infectious diseases is beyond the scope of this review and is covered in detail in comprehensive reviews elsewhere.23,51
Pseudotyped, replication-defective, and replication-competent viral vectors
The concept of using viral vectors to deliver gene expression cassettes encoding targeted antigens is also greater than 3 decades old. Of note, some of the first HAdV type 2 and 5 vectors were “vaccines.”53,54 Advantages of viral-vector-based vaccines include the ability of viruses to be taken up efficiently by cells and the potential to engineer replication-defective vectors and capitalize on their inherent immunostimulatory properties (i.e., their symmetry can act as a PAMP). The immunostimulatory effects facilitate activation of innate immunity that can enhance the response to the target antigen.
Among the most studied viral vectors for vaccine applications are Ads,55, 56, 57, 58 poxvirus: modified vaccinia Ankara (MVA),56,59 adeno-associated virus (AAV),60 rhabdovirus:61 vesicular stomatitis virus (VSV),62,63 paramyxovirus: Newcastle disease virus (NDV),64,65 human parainfluenza virus,66 and Sendai virus.67 An expression cassette encoding one or more antigens can be incorporated into the genome of the vector (i.e., Ad, MVA, and AAV), which is then expressed in cells that take up the vaccine (Figure 2). Typically, vectors are injected intramuscularly (i.m.). Advantages include robust and inexpensive production, high safety profiles, and a tendency to generate Th1-skewed or balanced Th1 and Th2 responses. Drawbacks include pre-existing immunity against the vector, which can reduce vaccine efficacy and may preferentially amplify a pre-existing response (versus generate a robust de novo response against the encoded transgene[s]). Alternatively, viral vectors can be pseudotyped and genetically engineered to display heterologous glycoprotein antigens (i.e., VSV and NDV), vectors can be (re-)targeted to specific cell types via modification of the receptor-binding domains, or (sero)types with a preferential tropism (e.g., APCs) can be selected. Moreover, some vectors can be engineered for a single replication cycle to boost efficacy68 or capsid proteins can be modified to include antigenic epitopes from a target pathogen.69,70 The latter approach allows antigen to be processed and presented by MHC class II during vaccine uptake, and depending on the platform, simultaneous production of genome-encoded antigen can allow for MHC class I presentation.
Specifically, replication-defective Ad vaccines have several characteristics that enhance their potential as an adaptable plug-and-play platform technology well suited to pandemic preparedness initiatives.1 They have a stable double-stranded DNA (dsDNA) genome that can be engineered to encode one or more vaccine antigens;59,71, 72, 73 their broad geographic use during the SARS-CoV-2 pandemic highlights their suitability for rapid manufacturing to meet global demand; they are safe and immunogenic in healthy adults,55,57,74, 75, 76, 77 infants as young as 1 week of age,78,79 the elderly,55 and immunocompromised;80, 81, 82 and they are compatible with thermostabilization and lyophilization procedures,83, 84, 85 allowing them to be stockpiled or distributed without the need for specialized ultra-cold storage. Finally, they are substantially cheaper than mRNA platforms, potentially allowing for a more equitable global vaccine distribution. These factors are all important considerations when developing vaccines against outbreak pathogens, which may be geographically endemic in low- and middle-income countries (LMICs).
Targets for outbreak pathogen vaccine development
Beyond vaccines for SARS-CoV-2, which are outlined in detail in a recent review,86 some of the most advanced Ad-based platforms have been developed against Ebola virus (EBOV), a member of the family Filoviridae. Vaccines based on Ad5, Ad26, and chimpanzee vector ChAd3 have undergone clinical evaluation as standalone or heterologous prime:boost regimens with MVA. The Ad26.ZEBOV + MVA-BN-Filo regimen received regulatory approval on July 1, 2020 by the European Medicines Agency (EMA). To date, Ad-based vaccines for Ebola virus have been shown to be safe and immunogenic in children,87,88 healthy adults,56,89, 90, 91, 92 and HIV-infected individuals (Table 2).81,82 Phase I clinical trials have also been initiated to evaluate Ad vaccines against members of the Flaviviridae (i.e., Zika virus),58 Togaviridae (i.e., Chikungunya virus),93 or Orthomyxoviridae (i.e., H5N1 avian influenza)75,94 families (Table 3). Viral hemorrhagic fevers, including viruses from the Arenaviridae, Nairoviridae, Hantaviridae, and Phenuiviridae (all order Bunyavirales), in addition to emerging viruses from the Paramyxoviridae family (i.e., Nipah virus), are also important targets for vaccine development due to their potential for high mortality, their zoonotic life cycle (resulting in unpredictable outbreaks), and the lack of licensed prophylactic countermeasures. The antigen targets for vaccines against several outbreak pathogens are highlighted in Figures 3A–3D.
Table 2.
Adenoviral vaccine clinical trials for Ebola
| Disease target (antigen) | Vector (source) | Group (phase) | Dose/route | Regimen (type) and interval (time) | T cell response | Antibody response (Post-Ad immunization versus pre-immunization or placebo) | Clinical Trials.gov | PMID (year) |
|---|---|---|---|---|---|---|---|---|
| Ebola ZEBOV GP (Zaire ebolavirus glycoprotein) |
Ad26 (Johnson & Johnson) |
Healthy adults ≥18–50 >50–70 (phase 2) |
5 × 1010 vps (i.m.) | +Boost MVA/28 days MVA/56 days MVA/84 days (1 × 108 IUs) |
IFN-γ ELISpot and flow cytometry but only measured post-MVA boost | GMC (95% CI), EU/mL 332 versus <40 (D29 versus D1) 361 versus <40 (D57 versus D1) 242 versus <40 (D85 versus D1) |
NCT02564523 | 34714820 (2021) |
| HIV+ adults ≥18–50 (phase 2) | +Boost MVA/28 or 56 days (1 × 108 IUs) |
GMC (95% CI), EU/mL 368 versus <40 (D29 versus D1) 291 versus <40 (D57 versus D1) |
||||||
| Ebola ZEBOV GP (Zaire ebolavirus glycoprotein) |
Ad26 (Johnson & Johnson) |
Healthy adults ≥18 years (phase 1, 2) | 5 × 1010 vps (i.m.) | +Boost MVA/56 days (1 × 108 IUs) |
Not reported in this study | GMC (95% CI), EU/mL 236 versus 69 (D57 versus D1) |
NCT02509494 | 34529963 (2021) |
| MVA/56 days (1 × 108 IUs) +Ad26.ZEBOV at 2 years |
GMC (95% CI), EU/mL 269 versus 60 (D57 versus D1) 30,411 versus 279 (D741 versus D720) |
|||||||
| Ebola ZEBOV GP (Zaire ebolavirus glycoprotein) |
Ad26 (Johnson & Johnson) |
Healthy children 1–17 years (phase 2) |
5 × 1010 vps (i.m.) | +Boost MVA/56 days (1 × 108 IUs) |
Not reported in this study | GMC (95% CI), EU/mL D57 versus D1 12–17 years: 314 versus ∼40 4–11 years: 390 versus ∼40 1–3 years: 693 versus ∼40 |
NCT02509494 | 34529962 (2021) |
| Ebola ZEBOV GP (Zaire ebolavirus glycoprotein) |
Ad26 (Johnson & Johnson) |
Healthy adults ≥18–50 years (phase 1) | 5 × 1010 vps (i.m.) | +Boost MVA/28 days MVA/56 days |
IFN-γ ELISpot SFUs/106 (FC of median) G1: 4.1 G2: 2.3 |
GMC (95% CI), EU/mL G1: 532.9 versus 18.3 (D29 versus D1) G2: 581.1 versus 22.0 (D29 versus D1) 854.3 versus 22.0 (D57 versus D1) |
NCT02313077 | 27092831 (2016) |
| Ebola EBO-Z GP (Zaire ebolavirus glycoprotein) |
ChAd3 (GSK) |
Healthy children 1–17 years (phase 2) |
1 × 1011 vps (i.m.) | No boost, single-shot regimen | Geo mean FC (95% CI) CD4+ D30 versus D0 13–17 years: 2.1 6–12 years: 2.3 1–5 years: 2.6 |
GMC (95% CI), EU/mL D30 versus D0 13–17 years: 1,564 versus 30 6–12 years: 1,395 versus 23 1–5 years: 2,406 versus 22 |
NCT02548078 | 32199492 (2020) |
| CD8+ D30 versus D0 13–17 years: 1.7 6–12 years: 2.0 1–5 years: 2.4 |
D365 versus D0 13–17 years: 716 versus 30 6–12 years: 752 versus 23 1–5 years: 1,424 versus 22 |
|||||||
| Ebola BIVALENT EBO GP (Zaire and Sudan ebolavirus glycoprotein) |
ChAd3 (GSK) |
Healthy adults ≥18–50 (phase 1) | G1: 2.0 × 1010 vps G2: 2.0 × 1011 vps (i.m.) |
Single dose | Flow cytometry CD4+ Zaire D28 versus D0 G1: ∼0.1% versus <0.05% G2: ∼0.2% versus <0.05% CD8+ Zaire D14 versus D0 G1: ∼0.01% versus <0.1% G2: ∼0.4% versus <0.1% |
GMT (95% CI), EC90 Zaire GP: D28 G1: 331 versus baseline G2: 2,037 versus baseline |
NCT02231866 | 25426834 (2017) |
| CD4+ Sudan D28 versus D0 G1: ∼0.1% versus <0.01% G2: ∼0.2% versus <0.01% CD8+ Sudan D14 versus D0 G1: ∼0.01% versus <0.01% G2: ∼0.2% versus <0.01% |
Sudan GP: D28 G1: 279 versus baseline G2: 936 versus baseline |
|||||||
| Ebola EBO-Z GP (Zaire ebolavirus glycoprotein) |
ChAd3 (GSK) |
Healthy adults ≥18–50 (phase 1) | 1.0 × 1010 vps 2.5 × 1010 vps 5.0 × 1010 vps (i.m.) |
+Boost MVA/3–10 weeks (1.5 × 108 PFUs 3 × 108 PFUs) |
IFN-γ ELISpot SFU/106 D14 versus D0 633 versus <50 Flow cytometry D14 versus D0 CD4+: 0.2% versus 0.13% CD8+: 0.004% versus ? |
GMT (95% CI) D28 versus rVSV-ZEBOV ChAd3 prime: 752.4 rVSV-ZEBOV: 920.7 |
NCT02240875 | 25629663 (2016) |
| Ebola (GP) | Ad5 (CanSino Biologic) | Healthy adults ≥18–60 years (phase 1) | 4 × 1010 vps 1.6 × 1011 vps (i.m.) |
Single dose or homologous prime:boost | IFN-γ ELISpot SFU/106 | GMT (95% CI), ELISA EC90 Prime D28 versus D0: reported in PMID: 25817373 Prime:boost D196 versus D168: Low dose: 6,110 versus 197.9 High dose: 11,825 versus 575.5 |
NCT02326194 NCT02533791 |
28017642 (2017) |
| Ebola (GP) | Ad5 (CanSino Biologic) | Healthy adults ≥18–60 years (phase 1) | 4 × 1010 vps 1.6 × 1011 vps (i.m.) |
Single dose | IFN-γ ELISpot SFU/106 (median, D14) Low dose: 465 High dose: 765 Flow ICS CD4+/CD8+ IFN-γ, TNF, IL-2 increased |
GMT (95% CI), ELISA EC90 Prime D28 versus placebo: Low dose: 682.7 versus 5 High dose: 1,305.7 versus 5 |
NCT02326194 | 25817373 (2015) |
FC, fold change; GMC, geometric mean concentration; GMT, geometric mean titer; ICS, intracellular cytokine staining; IL, interleukin; IUs, infectious units; SFUs, spot forming units; vps, viral particles.
Table 3.
Adenoviral vaccine clinical trials for emerging viruses
| Disease target (antigen) | Vector (source) | Group (phase) | Dose/route | Regimen (type) and interval (time) | T cell response (IFN-γ ELISpot. SFU/106 PBMCs) | Antibody response (Post-Ad immunization versus pre-immunization or placebo) | Clinical Trials.gov ID | PMID (Year) |
|---|---|---|---|---|---|---|---|---|
| Zika (M + Env) | Ad26 (Johnson & Johnson) | Healthy adults ≥18–50 years (phase 1) | G1: 5 × 1010 vps G2: 1.0 × 1011 vps (i.m.) |
Single dose | D15 versus D1 (Env) G1: ∼600 versus ∼83 G1: 250 versus ∼83 |
GMT (95% CI), MN50 G1: ∼40 versus <10 (D57 versus D1) G2: 103.4 versus <10 (D57 versus D1) |
NCT03356561 | 33587687 |
| Homologous prime:boost | D71 versus D1 (Env) G1: ∼1,100 versus ∼83 G1: 400 versus ∼83 |
GMT (95% CI), MN50 G1: 1,065.6 versus <10 (D71 versus D1) G2: 956.6 versus <10 (D71 versus D1) |
||||||
| Chikungunya (Capsid, E3, E2, 6k, E1) | ChAdOx1 (University of Oxford) | Healthy adults ≥18–50 years (phase 1) | G1: 5 × 109 vps G2: 2.5 × 1010 vps G3: 5 × 1010 vps (i.m.) |
Single dose | D14 versus D0 D14: 1,031 versus 180.1 D28: 541.1 versus 180.1 D56: 398.2 versus 180.1 D182: 352.8 versus 180.1 All groups combined |
GMT (95% CI), PRNT50 G1: ∼32–256 versus <6 (D28 versus D0) G2: ∼64–384 versus <6 (D28 versus D0) G3: ∼64–384 versus <6 (D28 versus D0) Against ×4 CHIKV lineages |
NCT03590392 | 34330906 |
| GMT (95% CI), ELISA units G1: 80.99 versus 4.74 (D182 versus D1) G2: 205.9 versus 4.72 (D182 versus D1) G3: 169.7 versus 3.00 (D182 versus D1) Against E2 protein | ||||||||
| Avian influenza (H5 HA) | Replicating Ad4 (PaxVax) | Healthy adults ≥18–40 years (phase 1) | G1: 1 × 1010 vps (oral, enteric) G2: 1 × 103 vps–1 × 108 vps (tonsillar) G3: 1 × 103vps–1 × 108 vps (i.n.) |
Single dose | Flow cytometry increases in IFN-γ+ CD69+ CD4+/CD8+ in tonsillar and i.n. groups |
Pseudovirus IC50 (median) G1: ∼170 versus ∼35 (W8 versus W0) G2: ∼800 versus ∼35 (W8 versus W0) G3: ∼320 versus ∼35 (W8 versus W0) |
NCT01806909 NCT01443936 |
33529172 |
| Avian influenza (H5 HA) | Replicating Ad4 (PaxVax) | Healthy adults ≥18–49 years (phase 1) | G1: 1 × 1010 vps (oral, enteric) G2: 1 × 103 vps–1 × 108 vps (tonsillar) G3: 1 × 103vps–1 × 108 vps (i.n.) |
Single dose | Not reported in this study | Pseudovirus IC50 (median) G1: ∼210 versus ∼? (W8 versus W0) G2: ∼836 versus ∼? (W8 versus W0) G3: ∼352 versus ∼? (W8 versus W0) |
NCT01443936 | 31004012 |
?, values not provided; HA, hemagglutinin; IC50, half-maximal inhibitory concentration; MN50, microneutralization titer-50; PRNT50, plaque reduction neutralization test-50.
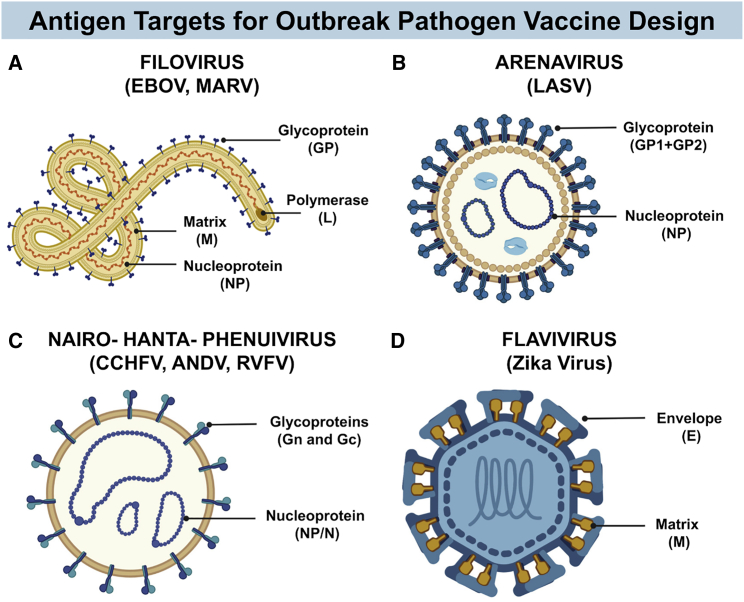
Figure 3.
Schematic diagrams of the structure of several emerging viruses identified as priority pathogens by the WHO
(A) A general structure of the Filoviridae family, highlighting antigen targets that have been employed in vaccine design. (B) Structure of the Arenaviridae family, showing antigen targets for vaccine development. (C) A schematic structure for viruses from the families Nairoviridae, Hantaviridae, or Phenuiviridae, order Bunyavirales, again showing vaccine target antigens. (D) Diagram showing the general structure of Zika virus, a member of the Flaviviridae family, and major targets for vaccine design. Figure created with BioRender.com.
Filoviridae; Marburg virus (MARV) and EBOV
MARV was identified as the causative agent for Marburg virus disease (MVD) following an outbreak in Germany in 1967, and the EBOV was first identified in 1976 in the Democratic Republic of Congo (formerly Zaire). Both viruses are members of the family Filoviridae, in the order Mononegavirales, and possess a viral envelope with a single-stranded, negative-sense RNA genome (Figure 3A). Infection with MARV or EBOV can result in severe viral hemorrhagic fever with fatality rates of 25%–90%.95,96 Survivors of EBOV infection can suffer with long-term sequelae.97,98 Fruit bats of the Pteropodidae or Rousettus families are believed to be natural hosts,99 and initial infection with EBOV or MARV may be through exposure to animals, with epidemics arising following subsequent human-human transmission through direct contact or exposure to infectious body fluids. Filoviruses represent a serious threat due to their high case fatality; the potential for unpredictable, rapidly expanding epidemics; and the risk for bioterrorism (Table 1). The Ebola virus outbreak in 2013–2016 in Africa was responsible for 11,000 deaths.95 This epidemic prompted widespread collaborative efforts to develop vaccines, which led to the regulatory approval of two vaccine regimens: one based on a pseudotyped VSV bearing the EBOV glycoprotein (GP) and a second based on a heterologous prime:boost regimen with an Ad26 prime and boost with MVA, encoding one or more filovirus GPs. Additional Ad-based platforms, chimpanzee Ad vector ChAd3 and Ad5, have also undergone extensive pre-clinical and clinical testing as candidate vaccines against EBOV (Table 2).
Correlates of protection for EBOV have not been conclusively defined.100 Ab responses directed toward the viral GP have been associated with protection in animal models.101 However, a role for GP-specific CD8+ T cells in mediating protection has also been demonstrated in non-human primates (NHPs),102 where passive transfer of high titer sera did not confer protection, but selective depletion of CD8+ T cells abrogated protection in 80% of animals.103 Pioneering studies in the early 2000s tested Ad5-based vaccines encoding the EBOV GP or nucleoprotein (NP) at doses of 1 × 1010 vps in mice or 2 × 1012 vps in NHPs in a single-shot versus homologous prime:boost regimen administered i.m.101,104 As reported for Ad-based vaccines,105 induction of antigen-specific immune responses was rapid (<3 weeks). However, Ab responses to GP were not boosted by the second homologous Ad5-GP immunization, likely due to anti-vector immunity. When a single-shot regimen containing an equal mixture of Ad5-GP/Ad5-NP was tested in NHPs, it conferred complete protection from infection within 1 month of immunization,104 even with a high challenge dose. Building upon these promising findings, the Nabel laboratory subsequently evaluated strategies to modify the encoded GP antigen to eliminate its inherent cytopathic effects while maintaining protective efficacy or used approaches to improve GP-specific immune responses by enhancing transgene expression by codon optimization.106,107
Considering the more advanced pre-clinical state of Ad-based vaccines against Ebola virus relative to other outbreak pathogens, a broader range of studies exist. These include testing mucosal delivery, the use of diverse human and non-human Ad vector platforms, heterologous prime:boost regimens, or the construction of multi-valent vaccine candidates. Ad5 vaccines encoding GP from Zaire ebolavirus, administered intranasally (i.n.) to several animal species (i.e., mice, guinea pigs, and NHPs), have been shown to provide complete protection from lethal challenge, comparable to i.m. immunization,108, 109, 110, 111 and can bypass pre-existing immunity to the Ad5 vector carrier.108,110 Alternative strategies to overcome pre-existing immunity to common Ad serotypes include the use of Ad vectors derived from rare or non-human Ads.10,14,112 Yang and colleagues reported the construction of two chimpanzee Ad vectors, AdC7 and AdC68, expressing the Ebola virus GP from the 2014 outbreak. A single i.m. immunization with each vector at 2 × 1010 vps elicited GP-specific Ab responses, although only AdC68 elicited detectable antigen-specific interferon (IFN)-γ enzyme-linked immune absorbent spot (ELISpot) responses.113 This observation again emphasizes that distinct Ad platforms elicit a range of immunological phenotypes,10 and vaccines will thereby require customization for specific disease targets. When tested in a heterologous prime:boost regimen, AdC7-AdC68 was found to be optimal, inducing GP-binding Abs, pseudovirus NAbs, and GP-specific T cells. ChAd3- and ChAd63-based vaccines have also been tested in NHPs, with the ChAd3 platform being identified as superior in eliciting protection from lethal challenge, a factor associated with a higher magnitude of both cellular and humoral immune responses.102 However, protective immunity waned 10 months post-immunization with a single shot of ChAd3, which may limit its use beyond emergency, reactive-use applications. However, this effect could be overcome by use of a heterologous MVA boost at week 8, which facilitated the maintenance of a high frequency of tumor necrosis factor (TNF) and IFN-γ+ co-producing CD8+ T cells at the 10-month challenge time point, which were associated with increased protection. The ChAd3 platform, as well as its use in a heterologous prime:boost regimen with MVA,56 has now been evaluated in human clinical trials and has been found to be safe and immunogenic in children88 and healthy adults89,92 (Table 2).
The WHO TPP preferred criteria for Ebola virus vaccines considers platforms that confer greater than 80% efficacy in preventing disease, are suitable for use in all age groups, and can rapidly elicit immunity as well as platforms capable of targeting multiple filovirus species with a single vaccine. With these criteria in mind, Ad-based vaccines are suitable. Both Ad26 and ChAd3 platforms have been safely tested in clinical trials in children87,88 and adults.56,90, 91, 92 Species D Ad26 and species B Ad35 vaccines encoding GPs of diverse filoviruses have been shown to elicit cross-reactive B and T cell responses,114 suggesting that combining GP antigens in a single vaccine could elicit broad protection. In support of this, a bivalent formulation for ChAd3 in which both the GP from EBOV and the Sudan strain (SUDV) were encoded102 did not negatively affect protection of macaques from challenge with a lethal dose of EBOV. This vaccine candidate subsequently advanced to phase I clinical testing (Table 2).92 More recently, a study by Sebastian and colleagues described the construction of ChAdOx1 encoding the GP from Zaire ebolavirus, Sudan ebolavirus, as well as Marburg virus and tested its immunogenicity in mice and efficacy following a single-shot regimen in guinea pigs.115 GP-specific Ab responses were elicited against each distinct virus, and guinea pigs were completely protected from lethal challenge with EBOV (although protection against other strains was not confirmed). Recently, innovative technologies in computational antigen design facilitated encoding of conserved T cell epitopes, or “pan-filovirus epitopes,” from NP, matrix (M), and polymerase (L) in ChAdOx1 or MVA vectors (Figure 3A).116 In this approach, the GP was not included as an antigen and no filovirus-specific NAbs were induced. Despite this, a heterologous Ad prime:MVA boost conferred complete protection from challenge with EBOV and MARV in mice, demonstrating the breadth of protection that can be elicited toward highly conserved T cell epitopes.
Arenaviridae: Lassa virus (LASV)
LASV is an enveloped single-stranded, bisegmented, ambisense RNA virus that is a member of the order Bunyavirales (Figure 3B). The virus was first identified in Nigeria in 1969 as the causative agent of an acute viral hemorrhagic fever. Infection is caused by exposure to the urine or feces of infected Mastomys rats, and LASV infects 100,000–500,000 people annually.95 Infection can be asymptomatic-mild in endemic areas, and as such, the true incidence is unclear. However, high mortality can be observed in hospitalized patients (15%–70%) and during the third trimester of pregnancy, where fetal loss is common and mortality can reach 90% (Table 1).117 Long-term health effects in survivors are common, including chronic neurological complications and hearing loss. As a result of its high case fatality rate, documented reports of human-to-human transmission, the potential to cause nosocomial infections, and a history of imported cases in countries outside of West Africa, the development of an effective vaccine suitable for use in high-risk populations is a priority for global health security.
A correlate of protection for Lassa fever has not been conclusively identified. A role for cellular immunity in protection has been inferred from pre-clinical models and human survivors, where the development of NAbs has been found to be delayed or weak.95 In contrast, T cell activation has been associated with control of infection in NHPs.118 Ad-based vaccines are well established to elicit potent cellular immune responses, suggesting they may be a suitable platform for protecting against Lassa fever. To date, vaccines based on Ad5 and chimpanzee Ad vector ChAdOx1 have been tested pre-clinically. Maruyama and colleagues constructed two replication-defective, Ad5-based vaccines against Lassa virus encoding the viral NP or precursor GP complex (GPC) (GP1 + GP2)—the surface GP of arenaviruses and a potential target for Abs.119 Using a homologous prime:boost regimen, the authors sequentially immunized Hartley guinea pigs i.m. with 1 × 1010 infectious units (IUs) of Ad5-NP followed by Ad5-GPC 16 days later and challenged animals with 8 × 104 plaque-forming units (PFUs) of Lassa strain LF2384 at D40 post-immunization. Serum Abs capable of binding both NP and GP were detected in vaccinated animals, but NAbs prior to challenge were low (plaque reduction neutralization test-50 [PRNT50]: 1:10) and were only observed in three out of eight animals. Despite this, all Ad-immunized animals completely survived the challenge and LASV was not detected in the brain, lung, liver, spleen, kidney, or serum, whereas animals immunized with a control Ad succumbed to disease. The authors hypothesized that non-neutralizing anti-NP or anti-GPC Abs capable of engaging Fc-mediated effector functions might contribute to protection, as this mechanism was proposed as a novel correlate of protection in another study.6 However, the latter role was not formally investigated in the Maruyama study, nor were antigen-specific T cell responses.
A more recent study described the construction of a ChAdOx1 vaccine against Lassa fever.120 Again, the LASV GPC antigen was selected and immunogenicity was evaluated in a single-shot or homologous prime:boost regimen in mice, followed by efficacy testing in Hartley guinea pigs using 1 × 105 median tissue culture infectious dose (TCID50) of a guinea-pig-adapted Josiah strain LASV challenge virus. Mice were immunized i.m. with 1 × 108 IUs, and when a boost was administered, the same dose was used with a 28-day interval (D28). T cell responses to both the encoded lineage IV GPC (Josiah strain), as well as cross-reactive responses toward three heterologous strains from lineages I–III, were measured by ELISpot and flow cytometry, with predominantly CD8+>CD4+ responses detected. Similarly, immunization with ChAdOx1-GPC resulted in breadth of reactivity against lineage I–III GPs by ELISA. Interestingly, no benefit of homologous boosting was observed in mice, with comparable levels of T cells or Abs following the single-shot or prime:boost regimen. In contrast, an increase in GP-specific Abs was detected in guinea pigs that received the homologous prime:boost. In support of prior evidence that suggested that NAbs are not required for protection against Lassa fever, the ChAdOx-GPC vaccine did not elicit NAb responses, but guinea pigs were 100% protected from clinical disease. Although complete sterilizing protection was not achieved, only very low levels of LASV RNA were detected in the tissues of vaccinated animals.
The WHO TTP for a vaccine against Lassa virus prioritizes non-emergency preventative use, which could be used in endemic regions and would be suitable for use in healthcare workers and pregnant people. Ideally, this vaccine should provide >90% efficacy in preventing infection or disease, be a single-shot vaccine, elicit breadth against four Lassa virus lineages (I–IV), and confer long-lasting, durable protection. An additional consideration is the possibility to co-administer this vaccine with other vaccines licensed for the same age and population groups without any negative impact on immunogenicity or safety, particularly in the context of co-infection with malaria, Ebola, or HIV.119 The general properties of Ad vaccines fulfill many of these criteria: there are reports of a single-shot immunization in animal models conferring protection121 and Ad-based vaccines can elicit immune responses with substantial breadth121 (with evidence of prolonged somatic hypermutation),122 they can stimulate long-lived immunity in animals123 and humans,124,125 and they have already been safely co-administered with routine Expanded Program on Immunization (EPI) vaccines without affecting their immunogenicity.79 The Coalition for Epidemic Preparedness Innovations (CEPI) (https://cepi.net) is currently supporting the development of a ChAdOx1-based vaccine against Lassa fever in partnership with University of Oxford and Janssen Vaccines & Prevention.126 It is likely that this vaccine will enter phase I clinical trials in the near future.
Nairoviridae: Crimean-Congo hemorrhagic fever virus (CCHFV)
Crimean-Congo hemorrhagic fever (CCHF) is an acute viral infection caused by CCHFV and transmitted by Ixodid ticks, primarily of the Hyalomma genus. The virus belongs to the order Bunyavirales and is enveloped with a tri-segmented, negative-sense RNA viral genome (Figure 3C). There is growing concern regarding increasing reports of imported cases, expanding endemic regions, and broadening geographic distribution of the tick vector due to climate change, habitat disruption, or bird migration.127,128 The pathogen has a wide host range, and humans can become exposed through tick bites or by exposure to body fluids from viremic livestock or humans. Outbreaks in hospital settings have also been reported.129 The high mortality (4%–40%) and a lack of licensed vaccine or treatment highlights the urgency for vaccine development (Table 1). However, to date, this has been hampered by limited availability of immunocompetent models to fully evaluate vaccine efficacy and a lack of information regarding correlates of protection. Furthermore, differences in the ability of distinct vaccine platforms delivering CCHFV antigens to confer protection have been reported,130, 131, 132 suggesting that a specific phenotype of immunity may be preferential (i.e., particular immunoglobulin G [IgG] subclass or phenotype of antigen-specific T cell) or that an effective design approach should consider targeting multiple antigens simultaneously.
Pre-clinical studies with diverse vaccine platforms have indicated that the surface glycoproteins (Gn and Gc) or the nucleocapsid (N) are attractive targets for CCHFV vaccine design. In particular, N is highly conserved between CCHFV strains133 and it is reported to be immunogenic during infection, and vaccines encoding N developed against other members of the order Bunyavirales have been protective.132,134 As such, Zivcec and colleagues evaluated the immunogenicity and efficacy of an Ad5-based vaccine encoding N in IFNAR−/− mice, administered as a single-shot or homologous prime:boost regimen. Mice were immunized i.m. with 1.25 × 107 IUs Ad5-N and boosted on D28 with 1 × 108 IUs of the same construct administered i.n., in an effort to bypass anti-vector immunity. Four weeks later, mice were challenged with 1,000 lethal dose 50 (LD50) CCHFV administered subcutaneously. Although anti-N IgG responses were detected in immunized animals, the single-shot regimen only provided partial protection from lethal challenge (33%), and the prime:boost regimen resulted in 78% survival. However, viremia was substantially reduced, and viral load and infectious titer in the liver and spleen were decreased in the prime:boost regimen. Considering that these experiments were performed in an immunocompromised IFN-signaling-deficient IFNAR−/− mouse model, the partial protection observed in this study supports the rationale for inclusion of N in a vaccine candidate for CCHFV. Recently, structural insights into the Gc trimer135 (the only known target for NAbs)136 or the secreted glycoprotein GP38137 have highlighted their potential as vaccine targets that could be incorporated into Ad vaccines.
Hantaviridae: Sin Nombre virus (SNV) and Andes virus (ANDV)
The Hantaviridae family, order Bunyavirales, contains a number of viruses that cause diseases manifesting in vascular leakage: hantavirus fever with renal syndrome (HFRS) and hantavirus pulmonary syndrome (HPS). The virus is enveloped with a tripartite, negative-sense RNA genome (Figure 3C). Transmitted primarily by Peromyscus maniculatus (deer mice), infection occurs following exposure to infected rodents or by inhalation of aerosolized infectious material from rodent urine, droppings, or body fluids. Two strains, Sin Nombre virus (SNV) and Andes virus (ANDV), cause the most severe disease. Lethality ranges from 0.1%–15% for HFRS and up to 40%–50% for HPS (Table 1).138,139 No licensed vaccine currently exists. The long incubation period, along with reports of human-to-human transmission during outbreaks of ANDV, are of increasing concern.140 The precise correlates of protection from infection have not been conclusively identified. As for LASV and CCHFV, the surface GP precursor GPC, which is co-translationally cleaved into the Gn and Gc envelope proteins, is considered to be an important target for protective immunity. However, the multifunctional nucleoprotein (NP) can also elicit cellular and humoral immune responses and, as such, may represent an additional antigen target.
With this in mind, Maeda and Safronetz et al. engineered Ad5-based vaccines encoding SNV or ANDV N antigen.134,141 Ad encoding SNV N elicited high levels of antigen-specific IFN-γ-producing T cells, which were superior to plasmid DNA or MVA vaccines encoding the same antigen.141 In addition to N, Safronetz and colleagues also constructed Ads encoding -Gn or -Gc glycoproteins as separate Ad vaccines. Using mice to evaluate immunogenicity and a relevant Syrian hamster animal model to model HPS in humans,134 the authors demonstrated that, when administered intraperitoneally (i.p.) as a single vaccine (1 × 108 PFUs), all vectors elicited detectable cellular and humoral immune responses that were capable of protecting hamsters from clinical disease. Interestingly, the authors determined that Ad5-N could completely protect animals from challenge despite a lack of NAbs. In fact, NAbs were not readily detected for any vaccine regimen, yet animals were completely protected from mortality. There was an association with increased control of ANDV replication following challenge in hamsters when immunized with Ad5-Gn > Ad5-Gc. When Ad5-Gn and Ad5-Gc were co-administered, ANDV RNA was undetectable in challenged animals.
Phenuiviridae: Rift Valley fever virus (RVFV)
First identified in the 1930s, RVFV is an arthropod-borne viral zoonosis that can be transmitted by multiple mosquito species. It has largely affected sub-Saharan Africa to date but is expanding geographically, with outbreaks spreading to the Arabian Peninsula and Madagascar. A member of Phenuiviridae family, order Bunyavirales, its structure is similar to CCHFV: it possesses an envelope and contains a tripartite, ambisense, negative-sense RNA viral genome (Figure 3C). RVFV infection predominantly affects ruminants with high rates of mortality, and it is responsible for mass spontaneous abortions and neonatal mortality, with substantial economic impact. The finding that RVFV can infect placental tissue142 has raised concerns that infection may also be associated with risk of miscarriage in human pregnancy.143 Infection of humans can be as a result of contact with infected animals or through mosquito bites during high density circulation in animals.144 Clinical symptoms are wide ranging but can be severe, resulting in hemorrhagic fever with mortality rates of up to 35% in hospitalized patients (Table 1).144 It is important to note that this virus also has biosecurity implications due to the fact that it can be lethal in aerosolized form, and thus, it represents a threat for bioterrorism.144 There are no licensed vaccines for human use, and veterinary vaccines have been associated with some safety issues (i.e., fetal malformations and stillbirths) and are deemed unsafe for use in humans.145 As such, a one-health approach for safe and effective vaccine development would be a worthy consideration.
NAbs are considered to be crucial for conferring sterilizing protection, in particular, NAbs directed toward the viral glycoproteins.146,147 NAbs display a predominance in responses to Gn > Gc in recovered humans.146 T cell responses to the viral glycoproteins have also been detected in RVFV-recovered patients. In 2009, Holman and colleagues evaluated an Ad5-based vaccine encoding Gc and Gc genes from RVFV in CD1 mice.144 Animals were immunized i.p. with 1 × 108 PFUs, and some animals were administered with a homologous boost with the same dose at week 10 (W10). Serum Ab titers (ELISA) against Gn/Gc were detected 2 weeks post-immunization, and immunized mice were 100% protected from lethal challenge with 100 PFU ZH501 strain of RVFV 11 weeks post-immunization. Boosting of Gn- and Gc-specific Ab responses was detected upon homologous boosting with Ad5-GnGc, with Ab titers sustained out to week 26 post-immunization and complete protection from challenge at week 27. In the context of prior immunity to Ad5, the authors demonstrated that pre-existing immunity had a negative impact on Ab titers and survival when a low-dose Ad5-GnGc was used (106 PFUs), although this could be largely overcome by increasing the dose of vaccine used to immunize (108 PFUs).
Subsequent pre-clinical studies have evaluated ChAdOx1 as a vaccine against RVFV. A head-to-head comparison of immunogenicity and efficacy was described for i.m. immunization with 1 × 108 IUs of ChAdOx1 or Ad5 encoding Gn and Gc.148 In addition, the effect of co-administration with commercial adjuvants AddaVax and Matrix-M was evaluated. As previously observed, humoral immune responses elicited by Ad5 were superior to ChAdOx1,112,149 with higher NAb titers detected in Ad5-GnGc-immunized mice. However, adjuvants enhanced NAb responses elicited by ChAdOx1-GnGc, but not Ad5-GnGc. In contrast, AddaVax (but not Matrix-M) enhanced CD8+ IFN-γ+ and TNF responses in Ad5-GnGc-immunized mice but had no effect on the cytokine profile elicited by ChAdOx1-GnGc. These differences highlight that distinct, underlying mechanisms likely contribute to the induction of humoral or cellular immunity induced by these Ad platforms.10 Despite differences, both platforms conferred 100% protection from challenge with 1 × 103 PFUs of RVFV strain 56/74 administered i.p.
Considering that a one-health approach is an appealing strategy for a vaccine against RVFV, studies have shown that the ChAdOx1-GnGc vaccine can elicit protective immunity in sheep, goats, and cattle150 and, importantly, in pregnant sheep and goats.145 A single-shot i.m. immunization with 1 × 109 IUs elicited NAb responses in all three species and conferred 100% protection from challenge with no detectable viremia. In pregnant ruminants immunized in the first trimester, the vaccine was shown to elicit robust NAb titers, provide protection against viremia, and prevent fetal loss, although the latter was incomplete in goats, with 2 out of 23 fetuses found to be autolyzed (1/5 and 1/3 in two does with multi-fetal pregnancies). Interestingly, NAb titers were higher in goats with fetal loss as compared with sheep where no fetal loss was observed, suggesting that species-specific differences in mechanisms of in utero infection or the phenotype of protective immunity may play a role in vaccine efficacy.
The TPP for vaccines against RVFV include three options: (1) a human vaccine for reactive, emergency use to be deployed during outbreaks and in regions in close proximity to outbreaks that is safe for use during pregnancy; (2) a vaccine that could confer longer term protection for individuals with high risk of infection due to their occupation (i.e., slaughterhouse workers, veterinarians, and farmers); and (3) a vaccine suitable for use in ruminants that could prevent transmission between animals and humans. The latter TPP should be affordable, suitable for use in pregnant animals, independent of cold-chain storage requirements, and compatible with differentiating infected from vaccinated animals (DIVA) principles. In terms of a vaccine for humans, the optimal criteria include at least 90% efficacy in preventing disease, rapid onset of immunity (2 weeks), the ability to confer protection against all RVFV lineages for at least 1 year following a single-dose regimen, and suitability for co-administration with other relevant vaccines. Considering the demonstrated immunogenicity and efficacy of Ad vaccines in diverse animal species, in addition to their now extensively documented use in humans, Ad-based platforms would be well suited to future vaccine development for RVFV and other “one-health” vaccine applications.
Flaviviridae: Zika virus (ZIKV)
ZIKV was discovered in Africa in 1947 and subsequently in Asia in 1966.151 Major outbreaks occurred in the Pacific between 2007 and 2015, with a substantial outbreak in the Americas in 2016, resulting in spread to over 70 countries and its declaration as a public health emergency of international concern by the WHO. Transmitted by infected mosquitoes of the Aedes species, the majority of cases are asymptomatic, but the virus can cause a spectrum of fetal and birth defects collectively known as congenital Zika syndrome, and infection has been associated with Guillain-Barré syndrome (GBS). ZIKV is an enveloped, positive-sense RNA virus in the family Flaviviridae and order Amarillovirales (Figure 3D). The family includes other viruses that can cause hemorrhagic fever or encephalitis, such as Dengue virus (DENV), West Nile virus (WNV), or Japanese encephalitis virus (JEV). In 2016, a dramatic increase in cases of microcephaly and other congenital or neurological disorders was associated with infection with ZIKV during pregnancy in Brazil. As the arthropod vector, Aedes mosquitoes, has a broad geographic distribution, there is concern that ZIKV could spread to the northern hemisphere (Table 1). As such, the development of an effective vaccine that is safe for use in individuals of child-bearing age or in pregnant people is a public-health priority.
Antigens that have been evaluated as vaccine targets for ZIKV include the pre-membrane (prM) or envelope (E) proteins that are exposed on the surface of the virion (Figure 2D). In late 2016, Abbink and colleagues described the construction of a species G simian Ad vaccine platform, RhAd52,152 encoding ZIKV prM-Env.153 A single-shot regimen was tested in rhesus monkeys following i.m. immunization with 1 × 1011 vps. The Ad vaccine rapidly induced ZIKV Env-specific NAbs 2 weeks post-immunization with broad epitope recognition, along with Env-specific T cell responses. Importantly, 100% protection from complete protection against subcutaneous (s.c.) challenge with 103 PFUs of ZIKV-BR was observed. Subsequently, an Ad26-based vaccine, a species D Ad vector encoding membrane (M) and Env was evaluated in mice and NHP. In both species, Env binding and NAbs were detected and a single-shot low dose of Ad26 (4 × 107 vps) was capable of providing complete protection from challenge in mice. In NHP, a comparable human dose of 1 × 1011 vps elicited robust protective immune responses and conferred complete protection from viremia. The latter vaccine, Ad26.ZIKV.001, has recently been evaluated in phase I clinical trials (NCT03356561), where it was tested in a single-shot or homologous prime:boost regimen (Table 3). Eight weeks following the prime immunization, homologous boosting of both NAbs and IFN-γ+ ELISpot responses were detected.58
Several other approaches have included vaccines based on Ad5 and ChAdOx1, in which the encoded antigen formulation was modified to identify the optimal transgene cassette. A study by Kim and colleagues encoded the extracellular portion of E, in which the transmembrane domain (TM) was removed and replaced with a heterologous trimerization domain from T4 bacteriophage, fibritin foldon.105 This modified antigen was encoded within Ad5 and used to immunize mice s.c. with 1 × 1011 vps, with a homologous vector boost administered via i.n. or intradermal (i.d.) route on D14 post-prime. As described for other Ad platforms,104,112 rapid induction of ZIKV-specific Abs was detected 2 weeks post-immunization, and NAbs were high 1 month following the boost immunization. Interestingly, this study evaluated protection from disease in ZIKV-challenged pups born to immunized mice. Complete survival was observed in pups from immunized mice versus 12.5% survival pups from PBS-immunized mothers. Furthermore, in the Ad-immunized groups, pups displayed only mild to no symptoms of neurological disease (i.e., hindlimb paralysis), whereas all pups of PBS-immunized dams had neurological disease symptoms.
Although prior studies with DNA-based vaccines identified the optimal prM-Env cassette for use in RhAd52-prM-Env (which retains the TM domain of Env), a separate study determined that prM-EnvΔTM was the optimal antigen configuration when used in ChAdOx1.154 ChAdOx1-based vaccines, with various iterations of the prM-Env cassette, were tested i.m. at a dose of 1 × 108 IUs in mice. ChAdOx1-prM-EnvΔTM elicited NAbs that were maintained for 16 weeks following a single shot, and immunization conferred 100% protection from challenge. In a more recent study, the authors evaluated the same ChAdOx1 vaccine platform, encoding the envelope protein domain III (EDIII) as a sole antigen, on the basis that this domain has previously been reported to be an effective immunogen for other flaviviruses.155 However, despite inducing anti-ENV Abs, NAbs were not elicited and the vaccine candidate failed to control viremia or completely protect against challenge in two mouse models,156 suggesting that EDIII is not an optimal vaccine target for protection against ZIKV.
The WHO TPP for a priority vaccine against ZIKV is one that could be used predominantly in an outbreak response, with the main objective being the prevention of pre-natal ZIKV infection and in prevention of clinical illness, namely congenital malformations or complications in pregnancy. The ideal vaccine would be expected to prevent virologically confirmed disease in >80% of the population in a single-dose formulation using a non-replicating platform and should be capable of neutralizing both the Asian and African ZIKV lineages. Additional considerations include suitability for co-administration with other appropriate licensed vaccines (i.e., the WHO EPI program), manufacturing processes in place for rapid scale up, affordability, and shelf-life stability, allowing cold-chain free distribution. Although NAbs are considered to be an important correlate of protection, there is growing appreciation that CD8+ T cells might also contribute to protection.157,158 Ad-based vaccines are known to elicit potent CD8+ responses10,159,160 in addition to Ab and, as previously stated, can exhibit breadth of reactivity. Furthermore, the platform fulfills requirements for co-administration with EPI vaccines79 and the capacity for rapid scale up to meet demand during outbreak scenarios.
Evidence for the safety of adenoviral vaccines
The urgent need for rapid-response vaccines to curtail the global spread of SARS-CoV-2 put unprecedented pressure on the pharmaceutical industry to develop and test vaccine candidates that would provide protection against disease severity and death while simultaneously demonstrating safety in vaccine recipients. Building upon existing blueprints from Ad vaccines to combat HIV,76,161 EBOV,87,91,162,163 influenza virus,75,94,122 respiratory syncytial virus (RSV),164,165 and Middle Eastern respiratory syndrome coronavirus (MERS-CoV),166 which had already been evaluated clinically, vaccines based on Ad5, Ad26, and ChAdOx1 were constructed, manufactured, and rapidly advanced to safety and efficacy studies in early 2020. Several reviews describing the immunogenicity and efficacy of Ad-based vaccines against SARS-CoV-2 have been published23,86 and will not be covered in detail in this review. However, as global-scale evaluation of Ad vaccines has provided a wealth of information regarding the clinical safety profile of distinct Ad-based vectors, we will summarize the latter findings, as they will inform the design of next-generation Ad vaccine platforms for emerging infectious diseases.
Safety data for chimpanzee Ad vector ChAdOx1
The rationale for use of ChAdOx1 as a vaccine against SARS-CoV-2 was based on its low seroprevalence in humans,167 its prior evaluation in phase I clinical trials as a vaccine for other viral pathogens,55,59,93 and promising findings in animal models for a ChAdOx1-based vaccine against a related coronavirus, MERS.168, 169, 170 Initial findings from interim analyses of phase I/II and later phase III clinical trials for Ad-vectored SARS-CoV-2 vaccines reported good tolerability and a lack of serious adverse events (SAEs) related to vaccine administration. Most importantly, these vaccines also provided near-complete protection from death, with significant reductions in the severity of disease and the need for hospitalization. The FDA recommends a toxicity grading scale for measuring adverse events in healthy adults (https://www.fda.gov/media/73679/download), ranging from mild (grade 1), to moderate (grade 2), to severe (grade 3), or potentially life-threatening (grade 4). A preliminary report of the data collected from a single-blind, randomized, controlled phase I/II clinical trial of the AZD1222 SARS-CoV-2 vaccine, based on ChAdOx1 (manufactured by AstraZeneca) showed that both single- and two-dose (28-day interval) vaccine regimens administered i.m. at a dose of 5 × 1010 vps were well tolerated,77 with a profile of adverse reactions similar to prior reports for Ad vaccines (ClinicalTrials.Gov ID: NCT04324606). In this relatively small study, 534 participants were administered with the ChAdOx1 nCoV-19 vaccine, and 533 participants were administered with the meningococcal conjugate vaccine, MenACWY vaccine.77 Solicited local and systemic adverse reactions were recorded at day 3, 7, 14, 28, and 56 post-vaccination, with a follow-up evaluation for safety and efficacy on days 184 and 364. Among solicited local adverse responses recorded during the first 7 days post-vaccination, the most common were mild tenderness, which was reported by 83% of participants, and pain at the injection site (reported by 67% of participants). Mild-to-moderate fatigue (70% of participants), headache (68%), malaise (61%), and muscle ache (60%), followed by chills and feeling feverish, were among the most common systemic adverse reactions reported within 7 days of ChAdOx1 vaccine administration.77 In a two-dose regimen, where prime vaccine administration was followed by a boost 28 days later, mild-to-moderate pain and tenderness remained the most common local adverse reaction, while headache, feeling feverish, chills, malaise, and muscle pain were reported as the most common systemic adverse reactions, similar to participants who received only a single dose of the vaccine. In a two-dose cohort, it was noticed that the reactogenicity profile (or the severity of adverse reactions) after administration of a booster dose of the vaccine was less severe, as compared with the severity of adverse reactions after the prime dose administration.
In a recent report of data collected from the ongoing pivotal double-blind, placebo-controlled phase III study, the safety and efficacy of AZD1222 was evaluated in 21,587 participants who received AZD1222 in a prime:boost regimen and in 10,792 participants who received placebo (NCT04516746).171 Unsolicited adverse events (AEs) were recorded for a duration of 28 days after each dose of vaccine or placebo, while solicited local and systemic AEs were monitored for 7 days post-administration of vaccine or placebo. This study evaluated the safety and efficacy of a vaccine dose of 5 × 1010 vps following i.m. administration, with a 4-week interval between prime and boost immunizations. Participants were also stratified by age into those who were 18–65 years old and those who were over 65 years of age. The majority of participants in this study had comorbidities that are known to increase coronavirus disease 2019 (COVID-19) disease severity, including a history of obesity, type 1 and type 2 diabetes, high blood pressure, and history of smoking, among others. Similar to findings from earlier trials, the majority of solicited local AEs were mild to moderate in intensity, with tenderness (68.7%) and pain at the injection site (58.3%). Upon analysis of systemic AEs, in addition to mild and moderate fatigue, muscle pain, and headache observed in earlier clinical trials with AZD1222, in this larger trial, severe fatigue, muscle pain, headache, and malaise were observed in a subset of participants aged 18–65 years after the first vaccine dose.171 However, the majority of local and systemic AEs were self-limiting and resolved within 1 to 2 days after the onset.
The analysis of vaccine reactogenicity in this larger cohort of participants revealed a spectrum of rare unsolicited AEs, which were observed within 28 days after vaccine administration. Out of 21,587 participants who received at least one dose of the vaccine, 225 participants reported AEs of grade 3 or higher, 1,288 participants (6%) experienced medically attended AEs, and AEs of special interest observed in 58 participants were judged to be related to trial intervention. However, it is important to note that grade 3 AEs, medically attended AEs, and AEs of special interest were also observed in participants receiving the placebo at similar frequencies. Furthermore, the absolute majority of various types of medically attended AEs were experienced only by a single patient and were observed in both the vaccine and placebo arms, making formal association of each particular type of AE with vaccine reactogenicity impossible. Vaccine reactogenicity was stronger after the first administration, compared with a subsequent boost dose, and was less severe in participants over 65 years of age, compared with vaccines from the 18–65 years old group. Overall, this and other clinical trials that analyzed the safety and efficacy of AZD1222 vaccine concluded that the vaccine was safe and effective at preventing symptomatic and severe COVID-19.172,173
Safety data for species D Ad26 vectors
Similar to ChAdOx1, the rationale for use of Ad26 as a vaccine platform for SARS-CoV-2 was based on its low seroprevalence in humans,152,174 its established use in clinical trials,76,157,161,164,165 and its prior approval by the EMA as a component in a vaccine against EBOV.82,87,90,91 The safety and efficacy of a single-dose COVID-19 vaccine Ad26.COV2.S, based on rare human adenovirus Ad26 (developed and distributed by Janssen and Johnson & Johnson), was evaluated in a series of randomized, placebo-controlled clinical trials. Initial studies analyzed two different doses of the vaccine as well as one- or two-dose regimens, where vaccines were administered 56 days apart (NCT04436276).175 In the report of the interim results of a phase I/IIa trial of Ad26.COV2.S vaccine, the reactogenicity of vaccine doses of 5 × 1010 vps (low dose, 323 participants) and 1 × 1011 vps (high dose, 319 participants) was compared with placebo (163 participants). In this trial, participants were also stratified by age into those 18–55 years old and those who were 65 years and older. Similar to findings reported upon analysis of reactogenicity for the AZD1222 vaccine, administration of the Ad26.COV2.S vaccine triggered transient, self-limiting AEs, with the majority recorded as grade 1 and 2 in severity.175 Pain at the injection site was the most frequently reported local AE after Ad26.COV2.S administration, whereas fatigue, headache, myalgia, and nausea were the most frequent systemic AEs reported by the participants in both the low- and the high-dose groups. In this study, participants of 18–55 years of age reported fever as a frequent solicited AE. In this age group, 15% of participants in the low-vaccine-dose group and 39% of participants in the high-dose group reported grade 1 and 2 fevers. Grade 3 fever was reported by 5% and 9% of participants after receiving low and high vaccine doses, respectively. In a group of 65 years and older, grade 3 fever was not observed in participants who received low dose of the vaccine and was observed in 1% of participants who received high vaccine dose. After the second dose of the vaccine, no grade 3 fever was observed in any of the groups and there was no participant discontinuation due to an AE.175
In a subsequent randomized, double-blind, placebo-controlled phase III clinical trial of the Ad26.COV2.S COVID-19 vaccine administered at a single dose of 5 × 1010 vps, the safety and efficacy was evaluated in 19,630 participants who received the vaccine and in 19,691 participants who received placebo (NCT04505722).57 In this study, participants were also stratified by age into two groups: 18–59 years old and 60 years and older. Similar to earlier studies, in this trial, solicited local and systemic AEs were recorded for 7 days and unsolicited AEs were observed and recorded for 28 days after vaccine administration. While unsolicited AEs were monitored in all participants, solicited AEs were monitored in subpopulations that included 3,356 participants who received the vaccine and 3,380 participants who received placebo. In the vaccine group, pain at the injection site was reported by 48.6% of participants and was the most commonly observed local solicited AE. Consistently with earlier observations, headache (reported by 38.9% of participants), fatigue (38.2%), myalgia (33.2%), and nausea (14.2%) were the most common systemic solicited AEs in participants who received the vaccine. Twenty out of 21,895 participants who received the vaccine and 11 out of 21,888 participants who received placebo reported unsolicited AEs of grade 3 or higher, which were considered to be related to the intervention.57 Although an imbalance in the number of unsolicited AEs between vaccine and placebo groups was noted, the majority of these events were only observed in a single patient, making definitive conclusion regarding association of the event with vaccine administration impossible. Similar to findings for the AZD1222 vaccine, the reactogenicity of a single dose of the Ad26.COV2.S vaccine was less pronounced in participants aged 60 years and older, as compared with a cohort of participants 18–59 years old. In summary, a single dose of the Ad26.COV2.S vaccine was found to be well tolerated and safe in humans.
Safety of species C Ad5 vectors
Vaccines to combat COVID-19 based on the common human adenovirus serotype, Ad5, were developed (CanSino Biologics) and evaluated for safety and efficacy in China (NCT04313127).176 In a phase I dose-escalation, open-label, non-randomized clinical trial, reactogenicity was evaluated in three cohorts of participants who received 5 × 1010 vps (low-dose cohort, 36 participants), 1 × 1011 vps (middle-dose cohort, 36 participants), and 1.5 × 1011 vps (high-dose cohort, 36 participants).176 Although the frequency of all reported AEs was similar in all cohorts, grade 3 AEs were more common in participants who received the highest vaccine dose. While pain at the injection site was the most frequent local AE, observed in 47% of participants in the low-dose, 56% of participants in the middle-dose, and 58% of participants in the high-dose cohorts, fever, followed by headache, fatigue, and muscle pain, was the most frequently recorded systemic AEs. Specifically, fever was observed in 42% of participants who received the low, 42% who received the middle, and 56% of participants who received the high vaccine dose. In this trial, grade 3 fever was observed in 14% of participants who received the high dose of the vaccine, while 6% of participants who received low and middle vaccine doses reported grade 3 fever. Overall, the reactogenicity of the Ad5-based vaccine was judged to be dose dependent, and subsequent phase II and III clinical trials were initiated with only low and middle doses of the vaccine.
In the subsequent randomized, double-blind, placebo-controlled phase II clinical trial, the Ad5-based COVID-19 vaccine was administered at a dose of 5 × 1010 vps (129 participants) or 1 × 1011 vps (253 participants), and vaccine reactogenicity was compared with placebo (126 participants). In this larger trial, the dose-dependent increase in the severity of AEs was documented and determined to be highly statistically significant (NCT04341389).176 Specifically, while the majority of reported adverse reactions were mild to moderate in severity, grade 3 adverse reactions were noted in 9% of participants who received vaccine dose of 1 × 1011 vps, which was significantly higher than in participants who received 5 × 1010 vps of the vaccine (1% of participants; p = 0.0011) or placebo (0% participants; p = 0.0004). Similar to findings in the phase I trial described above (NCT04313127),177 fever was the most frequently reported grade 3 adverse reaction, while fatigue was reported as the most frequent systemic adverse reaction: observed in 42% of participants who received 1 × 1011 vps vaccine dose and in 34% of participants who received 5 × 1010 vps dose of the vaccine. In this study, it was noted that high levels of pre-existing anti-Ad5 immunity, older age, and male sex were associated with a significantly lower frequency of fever after vaccination. All grade 3 reactions resolved within 72–96 h without medical intervention. No differences in the occurrence of unsolicited AEs between vaccine and placebo groups were noted during the 14-day observation period post-immunization with either vaccine or placebo. No SAEs were observed within 28 days of vaccination in this trial.176 Data from an ongoing placebo-controlled phase III clinical trial of this Ad5-based COVID-19 vaccine in 20,000 participants who will receive a single dose of vaccine or placebo are due for reporting in early 2022 (NCT04526990).
Safety of heterologous prime:boost with Ad26 and Ad5 vectors
A heterologous Ad vaccine regimen for COVID-19, Gam-COVID-Vac (Sputnik V), was developed, tested, and approved for use in Russia.178 Gam-COVID-Vac consists of a replication-deficient Ad26 vector, which is used for the prime, and an Ad5-based vector used for the boost. Gam-COVID-Vac is administered at a dose of 1 × 1011 vps for each vector component, and the prime and boost vaccine doses are administered 21 days apart. The largest datasets on the safety and efficacy of this vaccine were reported upon interim analysis of findings from a randomized, double-blind, placebo-controlled phase III clinical trial. In this study, 16,427 participants received one dose of the vaccine and were monitored for AEs and SAEs, with the nature and frequency of AEs and SAEs compared with 5,435 participants who received placebo (NCT04530396).178 From 16,427 participants who received the prime dose of the vaccine, 14,964 participants subsequently received a second, boost vaccine dose. AEs were recorded during observational visits on days 28, 42, and 180 post-immunization. The most frequent systemic AEs reported by participants were flu-like illness, headache, and asthenia. Most of the reported AEs were grade 1 and 2 in severity. Grade 3 AEs were observed in 0.38% of the participants. Severe AEs were observed in 0.274% of participants who received the vaccine and in 0.423% of participants who received placebo. None of the severe AEs were considered to be associated with vaccination in this trial.178
Detailed information on the reactogenicity of Gam-COVID-Vac was recently reported by Babamahmoodi and colleagues.179 The authors analyzed side effects and immunogenicity following administration of the Gam-COVID-Vac vaccine in 13,435 healthcare workers in Iran.179 This observational study reported solicited AE data collected from vaccinated participants during the first 8 days post-administration of each of the vaccine doses, in which 3,236 out of 13,435 participants reported AEs post-vaccination. Pain at the injection site was the most frequently reported local AE, reported by 58.2% of respondents after receiving the first dose of the vaccine and by 54.1% of respondents after receiving the second vaccine dose. For vaccinees who reported systemic AEs, the most frequently reported were fatigue (reported by 54.2% after the first and by 44% after the second vaccine doses), body pain (48.6% after the first and 38% after the second vaccine dose), weakness (46.6% after the first and 38.1% after the second vaccine doses), headache (38.1% after the first and 30.8% after the second vaccine dose), and fever (36.5% after the first and 23.8% after the second vaccine dose). The majority of these self-reported AEs subsided within 3 days after the onset, less than 10% of the events lasted 3–7 days, and about 3% of events lasted more than 7 days following either the first or the second vaccine doses.179 Overall, these data are in line with findings from an observational study by Pagotto et al.,180 who reported AEs observed within 72 h of administration of Gam-COVID-Vac vaccine to 707 healthcare workers in Argentina. Out of 683 participants who responded to an AE questionnaire, 57% reported pain at the injection site. Among systemic AEs observed after the first dose of the vaccine, 40% reported fever, 33% reported headache, and 20% reported muscle pain, all of which were mild in severity. However, in this group of vaccinees, muscle pain was reported by 27% of responders as moderate, 10% as severe, and 1% as grade 4. However, because this observational study was small and did not include a placebo group, a definitive conclusion on the association of these severe AEs with vaccine administration could not be drawn.
Safety concerns and considerations for future use of Ad-based vaccines: Vaccine-induced immune thrombotic thrombocytopenia
Although Ad-based vaccines displayed good tolerability and safety in clinical trials that included tens of thousands of participants, administering Ad26.COV2.S and ChAdOx1 (but not Ad5) vaccines to millions of people revealed some extremely rare serious AEs that were suspected to be causally associated with vaccination. On April 9, 2021, two reports published in the New England Journal of Medicine documented cases of unusual thrombosis and thrombocytopenia in recipients of the ChAdOx1 nCov-19 vaccine in Norway181 and in Germany and Austria.182 The report from Norway reported cerebral venous thrombosis (CVT) with thrombocytopenia, observed in 5 out of 132,686 recipients of the ChAdOx1 nCov-19 vaccine. For patients who developed this complication, the time from vaccine administration to hospital admission ranged from 7 to 10 days. All patients had high levels of anti-platelet factor 4 (PF4) Abs in the blood.181 Similarly, high levels of anti-PF4 Abs were found in the blood of 11 patients who developed thrombosis and thrombocytopenia after vaccination with the ChAdOx1 nCov-19 vaccine in Germany and Austria.182 The symptom onset for the latter group of patients ranged from 5 to 13 days after receiving the first dose of the vaccine. Based on the unusual clinical presentation and suspected association with vaccine administration, the syndrome was named vaccine-induced immune thrombotic thrombocytopenia (VITT)181 or thrombosis with thrombocytopenia syndrome (TTS) (Centers for Disease Control [CDC], USA).
Disseminated intravascular coagulation (DIC) was earlier observed after administration of an Ad5 vector during a gene therapy clinical trial that led to the death of a patient in 1999.183 Therefore, it was immediately debated whether the same mechanisms that triggered DIC in that gene therapy trial were responsible for triggering VITT following administration of Ad-based vaccines. Despite both DIC and VITT being thrombotic events, similarities in the mechanisms that trigger these two SAEs are highly unlikely. First, DIC in the gene therapy clinical trial was observed within 36 h after intravenous (i.v.) administration of Ad vector directly into the bloodstream, whereas VITT occurs 5–13 days after i.m. administration of Ad-based vaccines. Second, the dose of vector that triggered DIC was over 500-fold higher, as compared with doses of Ad vectors used for vaccination. Importantly, i.v. administration of Ad vectors in cancer patients at doses 60-fold higher than used in vaccination has not triggered DIC.184 Third, i.v. administration of extremely high doses of Ad vectors, in addition to DIC, triggers cytokine-storm syndrome, involving the systemic activation of innate immune defense mechanisms. In contrast, the onset of clinical presentation with VITT 5–13 days after vaccination suggests activation of adaptive immunity,185 where vaccine administration could trigger a recall response (symptom onset 5–7 days post-vaccination) or a primary PF4-targeted Ab response (symptom onset 7–13 days post-vaccination) upon activation of PF4-specific autoreactive B cells.
While the exact mechanism triggering VITT is currently unknown, should an autoimmune nature be definitively implicated, one might speculate that the incidence of VITT could vary greatly among vaccine recipients in different parts of the world, where genetic and environmental factors that underlie the development of many autoimmune diseases differ dramatically from those present in Western Europe and the United States. The incidence of VITT after ChAdOx1 AZD1222 vaccination is estimated to be ∼10 per million for individuals aged >50, ∼20 per million for individuals <50 years,186 or ∼24.9 per million, as reported in Norway and Denmark.187 These rates are lower following immunization with Ad26.COV2.S, which is estimated at ∼1.7 per million.188,189 However, it is critical to note that infection with SARS-COV-2 that results in symptomatic COVID-19 triggers CVT at an estimated rate of ∼42.8 per million.190 Once again, this provides a rationale for continuing vaccination to limit COVID-19-associated morbidity and mortality.190,191 In-depth observational studies and intervention strategies are currently being developed to promptly diagnose and effectively mitigate the severity of VITT following immunization, as well as to optimize vaccination options for populations most at risk for developing VITT.192
Safety concerns and considerations for future use of Ad-based vaccines: Guillain-Barré syndrome
Among other selected AEs that were reported after Ad-based COVID-19 vaccine administration through the vaccine adverse event reporting system (VAERS), the US CDC lists GBS. GBS is a rare, immune-mediated disorder that can present following non-viral or viral infection193 (including SARS-CoV-2), in which the immune system attacks neurons, leading to acute inflammatory demyelinating polyneuropathy, muscle weakness, and, in rare cases, paralysis. The majority of GBS patients fully recover. GBS events reported to VAERS occurred within 2 weeks of immunization. As of October 27, 2021, the US CDC reported that 244 suspected GBS events were observed after 15.5 million administrations of Ad26.COV2.S vaccine, corresponding to incidence rate of 15.7 cases per million. However, this incidence rate falls within the 8.1–19.1 per million person years range of GBS incidence in the general population, which was determined based on population-based studies from North America and Europe.193 In an observational study and systematic review of GBS reports after vaccination, Shao et al.194 identified 39 cases of GBS that occurred within 2 weeks of vaccination. Out of these 39 confirmed cases of GBS, 25 were reported after vaccination with the ChAdOx1 AZD1222 vaccine, 12 were reported after vaccination with BNT162b2 (Pfizer-BioNTech vaccine), 1 was reported after vaccination with Ad26.COV2.S vaccine, and 1 was reported after administration of the CoronaVac vaccine (inactivated vaccine).194 Due to the very low incidence and the observational nature of studies that reported GBS events after vaccination, definitive conclusions regarding the association of GBS events with vaccine administration cannot be drawn. Because the majority of GBS cases respond well to available pharmaceutical interventions and recover, the benefits of vaccination far outweigh the risk of GBS.
Safety concerns and considerations for future use of Ad-based vaccines: HIV acquisition risk
With the global use of Ad-vectored COVID-19 vaccines, concerns were raised regarding a risk of potential increase in HIV acquisition rates in vaccinated populations, in regions where HIV infections are prevalent.195 This concern is based on the results of two historical, randomized, double-blind, placebo-controlled clinical trials in the Americas, Caribbean, and Australia (STEP trial)196 and in South Africa (Phambili trial).197 These clinical studies were designed to test the efficacy of a preventive HIV-1 vaccine based on the human Ad5 vector in cohorts at high risk for HIV exposure. Early results from these trials, as well as extended follow-up, demonstrated an enhanced risk of HIV acquisition in vaccine recipients, with uncircumcised men with pre-existing Ad5-specific immunity found to be at highest risk.198, 199, 200 Extended follow-up analyses of participants in the STEP trial have shown that the risk of enhanced HIV acquisition wanes 18 months post-vaccination,199 and in the Phambili trial, the risk of enhanced HIV acquisition was observed in men (hazard ratio [HR] = 2.75; 95% confidence interval [CI] 1.49, 5.06; p = 0.001), but not in women (HR = 1.12; 95% CI 0.73, 1.72; p = 0.62).201 Although several potential mechanisms have been proposed,202, 203, 204 the exact factors responsible for the trend toward increased HIV acquisition after vaccination with Ad5-based vectored vaccines remain unknown. It is also unknown whether the same mechanisms that led to enhanced HIV acquisition after vaccination with vectors based on Ad5 are also activated after administration of vaccine vectors based on alternate types, specifically Ad26 or ChAdOx1 (Y25). As of the time of submission of this review for publication, no evidence was found that administration of Ad26.COV2.S or ChAdOx1 COVID-19 vaccines leads to enhanced HIV acquisition. In further support of this, follow-on studies in participants from the STEP trial, in addition to other clinical trials, determined that seropositivity to Ad5 and a range of other Ad types did not present an increased risk for HIV acquisition.205,206 Nevertheless, particular attention to changes in HIV infection in at-risk populations receiving Ad-vectored vaccines or in geographical regions with high HIV prevalence is certainly warranted.96,195
Vaccine efficacy and real-world evidence for protection from severe COVID-19 infection and death
A hierarchy in immunogenicity has been observed when comparing two-dose mRNA vaccines (mRNA-1273 and BNT162b2) versus single-dose Ad26.COV2.S, with mRNA-1273 > BNT162b2 > Ad26.COV2.S.207 In agreement with this, Ad-based vaccination regimens have been shown to elicit lower NAb titers (when measured 4 weeks after full vaccination) than mRNA-based vaccines, with reduced NAb titers against variants of concern (VOCs) also observed for Ad platforms versus mRNA.208 Overall efficacy in preventing symptomatic infection for Ad-based vaccines has also been reported to be lower than for mRNA vaccines (60%–70% versus >90%).171,208, 209, 210, 211 However, importantly, protection from severe-critical disease, hospitalization, or death is high for all vaccine platforms. In a phase III efficacy study with a single dose of Ad26.COV2.S, protection against severe-critical COVID-19 disease >28 days post-immunization was 85.4%57 and efficacy against severe disease (i.e., emergency department visits) was reported to be ∼94% for ChAdOx1 AZD1222171 and 91.6% for Sputnik V.178 Furthermore, real-world protection data are now emerging. A non-randomized study of US insurance claim data reported vaccine effectiveness (VE) of 81% against COVID-19 hospitalization following a single shot of Ad26.CoV2.S.212 Another study comparatively evaluated the VE of Moderna, Pfizer-BioNTech (both two-dose regimens), and Janssen Ad26.COV2.S (one-dose regimen) vaccines against COVID-19 hospitalizations at 21 US hospitals from March to August 2021 (immunocompromised patients were excluded).213 VE was highest for Moderna (93%) followed by Pfizer-BioNTech (88%), with the single-shot Janssen vaccine having 71% protection against hospitalization.213
More recently, the Janssen Ad26.COV2.S vaccine has been tested in a homologous boost regimen. Press releases for the ENSEMBLE 2 study, in which a boost of Ad26.COV2.S was administered at a 2-month interval, have suggested VE of 100% against COVID-19 hospitalization. Very recently, results from the Sisonke 2 phase 3b study were reported, indicating that a booster shot of Ad26.COV2.S administered 6–9 months following initial immunization of South African healthcare workers has a VE >84% against hospital admission when the Omicron variant was dominant.214 Although overall, the VE of Ad26.CoV2.S has been reported to be lower than mRNA vaccines,207 there is evidence of waning protection from infection or hospitalization for mRNA vaccines, whereas VE for Ad26.COV2.S appears to be durable.215 Data are still emerging regarding efficacy and VE against new VOCs. Nonetheless, the robust protection from severe disease for each vaccine, despite differences in immunological potency, suggests a need to better understand the qualitative differences between the immune response elicited by Ad and mRNA platforms in the future and how those parameters translate into correlates of protection.
Lessons learned from the SARS-CoV-2 pandemic
Through the course of the pandemic, several priority areas for pandemic preparedness have become apparent. First, it is clear that we need to (1) optimize and develop vaccines capable of conferring broad, protective immunity that could address the emergence of variants. In the context of Ad-based vaccines, this could be achieved by applying strategies used in “universal” vaccine development, such as focusing on highly conserved viral antigens or epitopes and domains, incorporating molecular or genetic adjuvants, or engineering multi-valent vectors that encode more than one vaccine antigen to increase breadth of protection. Related to this is (2) the crucial importance of antigen selection and the potential for use of stabilized immunogens—optimized through structure-guided approaches—to elicit humoral immune responses against antigenically authentic viral proteins. This was highlighted by reports of a 2P stabilization modification in the spike (S) of SARS-CoV-2, which locked it into a pre-fusion structure and enhanced expression.216,217 This approach was used by both mRNA platforms, in addition to Johnson & Johnson's Ad26.COV2.S vaccine217 but was not used in the ChAdOx1 AZD1222 platform. Although it is difficult to evaluate how differences in antigen design could contribute to differences in efficacy when comparing between two distinct Ad platforms, these questions can be considered for the design of next-generation vaccines against viruses that represent a future emerging pandemic threat.
In addition to these points, questions also arose that were related to (3) increasing our understanding of the precise, step-by-step mechanism of action of vaccines, i.e., can we design optimized vaccines that maximize prevention of infection as well as vaccines that prevent disease? Further to this is the need to better understand how homologous or heterologous Ad prime:boost regimens work (and indeed heterologous Ad + mRNA or + protein)218, 219, 220, 221, 222 in terms of the phenotype of immune response they elicit, how varying intervals between prime:boost affects the downstream immunogenicity or durability of immunity, and how pre-existing immunity (i.e., to Ad vectors) affects subsequent homologous boosting. In addition, there is growing interest in (4) advancing research to develop mucosal vaccines in the future. Ads have an established pre-clinical112,223,224 and clinical track record for mucosal administration74,94,122,225, 226, 227, 228 and may therefore represent useful vaccines for i.n. immunization and protection against a broad range of respiratory pathogens.229 With their low cost, scalability, and suitability for thermostabilization or storage independent of specialized cold-chain, Ad-based vaccines represent an ideal platform for equitable vaccine distribution. Efforts to build capacity in local vaccine manufacturing within LMICs will undoubtedly help to overcome supply issues and will be vital for future pandemic preparedness.
Future directions
A broad range of approaches can be taken to enhance the safety, immunogenicity, and ultimately the efficacy of Ad-based vaccines. A recent study reported direct binding of PF4 to the hexon of the ChAdOx1, Ad26, and Ad5 capsids,230 which may have implications for the induction of Abs directed toward PF4 and the development of VITT in a small number of individuals following immunization. Reassuringly, in the past, it has been possible to successfully modify the hexon of Ad vectors to eliminate the binding of various molecules.231 As such, targeted genetic engineering of the hexon could be used to construct Ads that do not interact with PF4 (or other molecules), thereby potentially enhancing their clinical safety profile. Alternatively, the evaluation of a wider range of Ad vectors could identify platforms that inherently lack off-target interactions with PF4 and other factors and possess biological characteristics optimal for vaccine applications (i.e., robust immunogenicity, high titer growth, genetic stability, etc.).10 Another means of indirectly improving the safety profile of Ad-based vaccines includes strategies to maximize the immunogenicity of the encoded transgene antigen, facilitating the use of reduced vector doses. Such approaches could include using built-in molecular and genetic adjuvants,112 re-targeting the tropism of Ad vectors to specific APCs (including defined DC subsets), or incorporating peptide antigens into the capsid,69,70 in addition to encoding them as a transgene. Furthermore, there is now interest in better understanding how mix-and-match heterologous immunization regimens using Ads and mRNA- or nanoparticle-based vaccine platforms could help maximize VE.218, 219, 220, 221, 222,232 Data emerging on heterologous immunization regimens using Ad-based platforms as a prime and mRNA or nanoparticle boost have been promising, showing increases in the magnitude and breadth of humoral and cellular immune responses.220,222
Summary
In summary, safety evaluations of COVID-19 vaccines based on different Ad types in tens of thousands of clinical trial participants, and in billions of vaccinees globally, now provide clear evidence that these vector-based vaccines are well tolerated. Ad-based vaccines have been a crucial public health intervention during this pandemic233 and have emerged as one of the front-running vaccine platforms due to their cost relative to mRNA platforms ($3–$10 per dose as compared with $19.50–$37, respectively).234 This is reflected in their widespread global distribution: with use of AZD1222 in 168–182 countries235 and use of Ad26.COV2.S in 71 countries worldwide (source: Covid19trackvaccines website/NYT COVID Tracker). In fact, vaccine coverage in Africa, Asia, Russia, and South America has been dominated by Ad-based vaccines (i.e., AstraZeneca, Johnson & Johnson, CanSino, Gamaleya-Sputnik V, and Covishield). Based on the collective safety profile and demonstrated high efficacy at preventing severe disease and death, the risk-benefit analysis strongly points to the exceptional public health benefit of global introduction of Ad-based COVID-19 vaccines and supports their future investment as vaccines for new emerging viral diseases with pandemic potential.
Acknowledgments
This was supported in part by funding from the US National Institutes of Health AI146529 and AI157606 (to L.C.) and AI107960 (to D.M.S.), David C. Lowance Endowment Fund, and Children’s Healthcare of Atlanta Research Trust to D.M.S. E.J.K. is an INSERM fellow.
Declaration of interests
L.C. declares no competing interests. E.J.K. is a member of the EMA vaccine advisory panel and was president of the user supervisory panel of TransVac II. D.M.S. is a paid consultant of Merck and a founder, officer, and shareholder of AdCure Bio, which develops adenovirus technologies for therapeutic use.
Contributor Information
Lynda Coughlan, Email: lcoughlan@som.umaryland.edu.
Eric J. Kremer, Email: eric.kremer@igmm.cnrs.fr.
Dmitry M. Shayakhmetov, Email: dmitryshay@emory.edu.
References
- 1.Ewer K., Sebastian S., Spencer A.J., Gilbert S., Hill A.V.S., Lambe T. Chimpanzee adenoviral vectors as vaccines for outbreak pathogens. Hum. Vaccin. Immunother. 2017;13:3020–3032. doi: 10.1080/21645515.2017.1383575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodson J.L., Seward J.F. Measles 50 years after use of measles vaccine. Infect. Dis. Clin. North Am. 2015;29:725–743. doi: 10.1016/j.idc.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Krishnamoorthy Y., Sakthivel M., Eliyas S.K., Surendran G., Sarveswaran G. Worldwide trend in measles incidence from 1980 to 2016: a pooled analysis of evidence from 194 WHO member states. J. Postgrad. Med. 2019;65:160–163. doi: 10.4103/jpgm.JPGM_508_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DiLillo D.J., Tan G.S., Palese P., Ravetch J.V. Broadly neutralizing hemagglutinin stalk-specific antibodies require FcgammaR interactions for protection against influenza virus in vivo. Nat. Med. 2014;20:143–151. doi: 10.1038/nm.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DiLillo D.J., Palese P., Wilson P.C., Ravetch J.V. Broadly neutralizing anti-influenza antibodies require Fc receptor engagement for in vivo protection. J. Clin. Invest. 2016;126:605–610. doi: 10.1172/JCI84428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abreu-Mota T., Hagen K.R., Cooper K., Jahrling P.B., Tan G., Wirblich C., Johnson R.F., Schnell M.J. Non-neutralizing antibodies elicited by recombinant Lassa-Rabies vaccine are critical for protection against Lassa fever. Nat. Commun. 2018;9:4223. doi: 10.1038/s41467-018-06741-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henry Dunand C.J., Leon P.E., Huang M., Choi A., Chromikova V., Ho I.Y., Tan G.S., Cruz J., Hirsh A., Zheng N.Y., et al. Both neutralizing and non-neutralizing human H7N9 influenza vaccine-induced monoclonal antibodies confer protection. Cell Host Microbe. 2016;19:800–813. doi: 10.1016/j.chom.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He W., Chen C.J., Mullarkey C.E., Hamilton J.R., Wong C.K., Leon P.E., Uccellini M.B., Chromikova V., Henry C., Hoffman K.W., et al. Alveolar macrophages are critical for broadly-reactive antibody-mediated protection against influenza A virus in mice. Nat. Commun. 2017;8:846. doi: 10.1038/s41467-017-00928-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quinn K.M., Zak D.E., Costa A., Yamamoto A., Kastenmuller K., Hill B.J., Lynn G.M., Darrah P.A., Lindsay R.W., Wang L., et al. Antigen expression determines adenoviral vaccine potency independent of IFN and STING signaling. J. Clin. Invest. 2015;125:1129–1146. doi: 10.1172/JCI78280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coughlan L. Factors which contribute to the immunogenicity of non-replicating adenoviral vectored vaccines. Front. Immunol. 2020;11:909. doi: 10.3389/fimmu.2020.00909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bassett J.D., Yang T.C., Bernard D., Millar J.B., Swift S.L., McGray A.J., VanSeggelen H., Boudreau J.E., Finn J.D., Parsons R., et al. CD8+ T-cell expansion and maintenance after recombinant adenovirus immunization rely upon cooperation between hematopoietic and nonhematopoietic antigen-presenting cells. Blood. 2011;117:1146–1155. doi: 10.1182/blood-2010-03-272336. [DOI] [PubMed] [Google Scholar]
- 12.Gross C.P., Sepkowitz K.A. The myth of the medical breakthrough: smallpox, vaccination, and Jenner reconsidered. Int. J. Infect. Dis. 1998;3:54–60. doi: 10.1016/s1201-9712(98)90096-0. [DOI] [PubMed] [Google Scholar]
- 13.Riedel S. Edward Jenner and the history of smallpox and vaccination. Proc. (Bayl. Univ. Med. Cent.) 2005;18:21–25. doi: 10.1080/08998280.2005.11928028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kerstetter L.J., Buckley S., Bliss C.M., Coughlan L. Adenoviral vectors as vaccines for emerging avian influenza viruses. Front. Immunol. 2020;11:607333. doi: 10.3389/fimmu.2020.607333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitchen L.W., Vaughn D.W. Role of U.S. military research programs in the development of U.S.-licensed vaccines for naturally occurring infectious diseases. Vaccine. 2007;25:7017–7030. doi: 10.1016/j.vaccine.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 16.Hoke C.H., Jr., Snyder C.E., Jr. History of the restoration of adenovirus type 4 and type 7 vaccine, live oral (adenovirus vaccine) in the context of the department of defense acquisition system. Vaccine. 2013;31:1623–1632. doi: 10.1016/j.vaccine.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 17.Top F.H., Jr. Control of adenovirus acute respiratory disease in U.S. army trainees. Yale J. Biol. Med. 1975;48:185–195. [PMC free article] [PubMed] [Google Scholar]
- 18.Radin J.M., Hawksworth A.W., Blair P.J., Faix D.J., Raman R., Russell K.L., Gray G.C. Dramatic decline of respiratory illness among US military recruits after the renewed use of adenovirus vaccines. Clin. Infect. Dis. 2014;59:962–968. doi: 10.1093/cid/ciu507. [DOI] [PubMed] [Google Scholar]
- 19.McNeil M.M., Paradowska-Stankiewicz I., Miller E.R., Marquez P.L., Seshadri S., Collins L.C., Jr., Cano M.V. Adverse events following adenovirus type 4 and type 7 vaccine, live, oral in the vaccine adverse event reporting system (VAERS), United States, October 2011-July 2018. Vaccine. 2019;37:6760–6767. doi: 10.1016/j.vaccine.2019.08.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuschner R.A., Russell K.L., Abuja M., Bauer K.M., Faix D.J., Hait H., Henrick J., Jacobs M., Liss A., Lynch J.A., et al. A phase 3, randomized, double-blind, placebo-controlled study of the safety and efficacy of the live, oral adenovirus type 4 and type 7 vaccine, in U.S. military recruits. Vaccine. 2013;31:2963–2971. doi: 10.1016/j.vaccine.2013.04.035. [DOI] [PubMed] [Google Scholar]
- 21.Shingai M., Nomura N., Sekiya T., Ohno M., Fujikura D., Handabile C., Omori R., Ohara Y., Nishimura T., Endo M., et al. Potent priming by inactivated whole influenza virus particle vaccines is linked to viral RNA uptake into antigen presenting cells. Vaccine. 2021;39:3940–3951. doi: 10.1016/j.vaccine.2021.05.065. [DOI] [PubMed] [Google Scholar]
- 22.Subbarao K., Joseph T. Scientific barriers to developing vaccines against avian influenza viruses. Nat. Rev. Immunol. 2007;7:267–278. doi: 10.1038/nri2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gebre M.S., Brito L.A., Tostanoski L.H., Edwards D.K., Carfi A., Barouch D.H. Novel approaches for vaccine development. Cell. 2021;184:1589–1603. doi: 10.1016/j.cell.2021.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyanohara A., Toh-e A., Nozaki C., Hamada F., Ohtomo N., Matsubara K. Expression of hepatitis B surface antigen gene in yeast. Proc. Natl. Acad. Sci. U S A. 1983;80:1–5. doi: 10.1073/pnas.80.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bachmann M.F., Jennings G.T. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010;10:787–796. doi: 10.1038/nri2868. [DOI] [PubMed] [Google Scholar]
- 26.Klausberger M., Wilde M., Palmberger D., Hai R., Albrecht R.A., Margine I., Hirsh A., Garcia-Sastre A., Grabherr R., Krammer F. One-shot vaccination with an insect cell-derived low-dose influenza A H7 virus-like particle preparation protects mice against H7N9 challenge. Vaccine. 2014;32:355–362. doi: 10.1016/j.vaccine.2013.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song J.M., Hossain J., Yoo D.G., Lipatov A.S., Davis C.T., Quan F.S., Chen L.M., Hogan R.J., Donis R.O., Compans R.W., et al. Protective immunity against H5N1 influenza virus by a single dose vaccination with virus-like particles. Virology. 2010;405:165–175. doi: 10.1016/j.virol.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pattinson D.J., Apte S.H., Wibowo N., Rivera-Hernandez T., Groves P.L., Middelberg A.P.J., Doolan D.L. Chimeric virus-like particles and capsomeres induce similar CD8(+) T cell responses but differ in capacity to induce CD4(+) T cell responses and antibody responses. Front. Immunol. 2020;11:564627. doi: 10.3389/fimmu.2020.564627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klimek L., Willers J., Hammann-Haenni A., Pfaar O., Stocker H., Mueller P., Renner W.A., Bachmann M.F. Assessment of clinical efficacy of CYT003-QbG10 in patients with allergic rhinoconjunctivitis: a phase IIb study. Clin. Exp. Allergy. 2011;41:1305–1312. doi: 10.1111/j.1365-2222.2011.03783.x. [DOI] [PubMed] [Google Scholar]
- 30.Sedlik C., Saron M., Sarraseca J., Casal I., Leclerc C. Recombinant parvovirus-like particles as an antigen carrier: a novel nonreplicative exogenous antigen to elicit protective antiviral cytotoxic T cells. Proc. Natl. Acad. Sci. U S A. 1997;94:7503–7508. doi: 10.1073/pnas.94.14.7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim K.H., Kwon Y.M., Lee Y.T., Hwang H.S., Kim M.C., Ko E.J., Wang B.Z., Quan F.S., Kang S.M. Virus-like particles presenting flagellin exhibit unique adjuvant effects on eliciting T helper type 1 humoral and cellular immune responses to poor immunogenic influenza virus M2e protein vaccine. Virology. 2018;524:172–181. doi: 10.1016/j.virol.2018.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Win S.J., Ward V.K., Dunbar P.R., Young S.L., Baird M.A. Cross-presentation of epitopes on virus-like particles via the MHC I receptor recycling pathway. Immunol. Cell Biol. 2011;89:681–688. doi: 10.1038/icb.2010.161. [DOI] [PubMed] [Google Scholar]
- 33.Lowy D.R., Schiller J.T. Prophylactic human papillomavirus vaccines. J. Clin. Invest. 2006;116:1167–1173. doi: 10.1172/JCI28607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ueda G., Antanasijevic A., Fallas J.A., Sheffler W., Copps J., Ellis D., Hutchinson G.B., Moyer A., Yasmeen A., Tsybovsky Y., et al. Tailored design of protein nanoparticle scaffolds for multivalent presentation of viral glycoprotein antigens. Elife. 2020;9 doi: 10.7554/eLife.57659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walls A.C., Miranda M.C., Schafer A., Pham M.N., Greaney A., Arunachalam P.S., Navarro M.J., Tortorici M.A., Rogers K., O'Connor M.A., et al. Elicitation of broadly protective sarbecovirus immunity by receptor-binding domain nanoparticle vaccines. Cell. 2021;184:5432–5447.e16. doi: 10.1016/j.cell.2021.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wuertz K.M., Barkei E.K., Chen W.H., Martinez E.J., Lakhal-Naouar I., Jagodzinski L.L., Paquin-Proulx D., Gromowski G.D., Swafford I., Ganesh A., et al. A SARS-CoV-2 spike ferritin nanoparticle vaccine protects hamsters against Alpha and Beta virus variant challenge. NPJ Vaccines. 2021;6:129. doi: 10.1038/s41541-021-00392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He L., Lin X., Wang Y., Abraham C., Sou C., Ngo T., Zhang Y., Wilson I.A., Zhu J. Single-component, self-assembling, protein nanoparticles presenting the receptor binding domain and stabilized spike as SARS-CoV-2 vaccine candidates. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abf1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Darricarrere N., Qiu Y., Kanekiyo M., Creanga A., Gillespie R.A., Moin S.M., Saleh J., Sancho J., Chou T.H., Zhou Y., et al. Broad neutralization of H1 and H3 viruses by adjuvanted influenza HA stem vaccines in nonhuman primates. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.abe5449. [DOI] [PubMed] [Google Scholar]
- 39.Boyoglu-Barnum S., Ellis D., Gillespie R.A., Hutchinson G.B., Park Y.J., Moin S.M., Acton O.J., Ravichandran R., Murphy M., Pettie D., et al. Quadrivalent influenza nanoparticle vaccines induce broad protection. Nature. 2021;592:623–628. doi: 10.1038/s41586-021-03365-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jardine J., Julien J.P., Menis S., Ota T., Kalyuzhniy O., McGuire A., Sok D., Huang P.S., MacPherson S., Jones M., et al. Rational HIV immunogen design to target specific germline B cell receptors. Science. 2013;340:711–716. doi: 10.1126/science.1234150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu Z., Chokkalingam N., Tello-Ruiz E., Wise M.C., Bah M.A., Walker S., Tursi N.J., Fisher P.D., Schultheis K., Broderick K.E., et al. A DNA-launched nanoparticle vaccine elicits CD8(+) T-cell immunity to promote in vivo tumor control. Cancer Immunol. Res. 2020;8:1354–1364. doi: 10.1158/2326-6066.CIR-20-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pascolo S. Synthetic messenger RNA-based vaccines: from scorn to hype. Viruses. 2021;13 doi: 10.3390/v13020270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Finn J.D., Bassett J., Millar J.B., Grinshtein N., Yang T.C., Parsons R., Evelegh C., Wan Y., Parks R.J., Bramson J.L. Persistence of transgene expression influences CD8+ T-cell expansion and maintenance following immunization with recombinant adenovirus. J. Virol. 2009;83:12027–12036. doi: 10.1128/JVI.00593-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oliveira P.H., Mairhofer J. Marker-free plasmids for biotechnological applications - implications and perspectives. Trends Biotechnol. 2013;31:539–547. doi: 10.1016/j.tibtech.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 45.Modjarrad K., Roberts C.C., Mills K.T., Castellano A.R., Paolino K., Muthumani K., Reuschel E.L., Robb M.L., Racine T., Oh M.D., et al. Safety and immunogenicity of an anti-middle east respiratory syndrome coronavirus DNA vaccine: a phase 1, open-label, single-arm, dose-escalation trial. Lancet Infect. Dis. 2019;19:1013–1022. doi: 10.1016/S1473-3099(19)30266-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trimble C.L., Morrow M.P., Kraynyak K.A., Shen X., Dallas M., Yan J., Edwards L., Parker R.L., Denny L., Giffear M., et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. Lancet. 2015;386:2078–2088. doi: 10.1016/S0140-6736(15)00239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tebas P., Roberts C.C., Muthumani K., Reuschel E.L., Kudchodkar S.B., Zaidi F.I., White S., Khan A.S., Racine T., Choi H., et al. Safety and immunogenicity of an anti-zika virus DNA vaccine. N. Engl. J. Med. 2021;385:e35. doi: 10.1056/NEJMoa1708120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheneau C., Kremer E.J. Adenovirus-extracellular protein interactions and their impact on innate immune responses by human mononuclear phagocytes. Viruses. 2020;12 doi: 10.3390/v12121351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheneau C., Eichholz K., Tran T.H., Tran T.T.P., Paris O., Henriquet C., Bajramovic J.J., Pugniere M., Kremer E.J. Lactoferrin retargets human adenoviruses to TLR4 to induce an abortive NLRP3-associated pyroptotic response in human phagocytes. Front. Immunol. 2021;12:685218. doi: 10.3389/fimmu.2021.685218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Doronin K., Flatt J.W., Di Paolo N.C., Khare R., Kalyuzhniy O., Acchione M., Sumida J.P., Ohto U., Shimizu T., Akashi-Takamura S., et al. Coagulation factor X activates innate immunity to human species C adenovirus. Science. 2012;338:795–798. doi: 10.1126/science.1226625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chaudhary N., Weissman D., Whitehead K.A. mRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nat. Rev. Drug Discov. 2021;20:817–838. doi: 10.1038/s41573-021-00283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Freyn A.W., Ramos da Silva J., Rosado V.C., Bliss C.M., Pine M., Mui B.L., Tam Y.K., Madden T.D., de Souza Ferreira L.C., Weissman D., et al. A multi-targeting, nucleoside-modified mRNA influenza virus vaccine provides broad protection in mice. Mol. Ther. 2020;28:1569–1584. doi: 10.1016/j.ymthe.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Randrianarison-Jewtoukoff V., Perricaudet M. Recombinant adenoviruses as vaccines. Biologicals. 1995;23:145–157. doi: 10.1006/biol.1995.0025. [DOI] [PubMed] [Google Scholar]
- 54.Eloit M., Gilardi-Hebenstreit P., Toma B., Perricaudet M. Construction of a defective adenovirus vector expressing the pseudorabies virus glycoprotein gp50 and its use as a live vaccine. J. Gen. Virol. 1990;71:2425–2431. doi: 10.1099/0022-1317-71-10-2425. [DOI] [PubMed] [Google Scholar]
- 55.Antrobus R.D., Coughlan L., Berthoud T.K., Dicks M.D., Hill A.V., Lambe T., Gilbert S.C. Clinical assessment of a novel recombinant simian adenovirus ChAdOx1 as a vectored vaccine expressing conserved influenza A antigens. Mol. Ther. 2014;22:668–674. doi: 10.1038/mt.2013.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ewer K., Rampling T., Venkatraman N., Bowyer G., Wright D., Lambe T., Imoukhuede E.B., Payne R., Fehling S.K., Strecker T., et al. A monovalent chimpanzee adenovirus Ebola vaccine boosted with MVA. N. Engl. J. Med. 2016;374:1635–1646. doi: 10.1056/NEJMoa1411627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sadoff J., Gray G., Vandebosch A., Cardenas V., Shukarev G., Grinsztejn B., Goepfert P.A., Truyers C., Fennema H., Spiessens B., et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against covid-19. N. Engl. J. Med. 2021;384:2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salisch N.C., Stephenson K.E., Williams K., Cox F., van der Fits L., Heerwegh D., Truyers C., Habets M.N., Kanjilal D.G., Larocca R.A., et al. A double-blind, randomized, placebo-controlled phase 1 study of Ad26.ZIKV.001, an ad26-vectored anti-zika virus vaccine. Ann. Intern. Med. 2021;174:585–594. doi: 10.7326/M20-5306. [DOI] [PubMed] [Google Scholar]
- 59.Coughlan L., Sridhar S., Payne R., Edmans M., Milicic A., Venkatraman N., Lugonja B., Clifton L., Qi C., Folegatti P.M., et al. Heterologous two-dose vaccination with simian adenovirus and poxvirus vectors elicits long-lasting cellular immunity to influenza virus A in healthy adults. EBioMedicine. 2018;29:146–154. doi: 10.1016/j.ebiom.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zabaleta N., Dai W., Bhatt U., Herate C., Maisonnasse P., Chichester J.A., Sanmiguel J., Estelien R., Michalson K.T., Diop C., et al. An AAV-based, room-temperature-stable, single-dose COVID-19 vaccine provides durable immunogenicity and protection in non-human primates. Cell Host Microbe. 2021;29:1437–1453.e8. doi: 10.1016/j.chom.2021.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lundstrom K. Self-replicating RNA viruses for vaccine development against infectious diseases and cancer. Vaccines (Basel) 2021;9 doi: 10.3390/vaccines9101187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Regules J.A., Beigel J.H., Paolino K.M., Voell J., Castellano A.R., Hu Z., Munoz P., Moon J.E., Ruck R.C., Bennett J.W., et al. A recombinant vesicular stomatitis virus Ebola vaccine. N. Engl. J. Med. 2017;376:330–341. doi: 10.1056/NEJMoa1414216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huttner A., Dayer J.A., Yerly S., Combescure C., Auderset F., Desmeules J., Eickmann M., Finckh A., Goncalves A.R., Hooper J.W., et al. The effect of dose on the safety and immunogenicity of the VSV Ebola candidate vaccine: a randomised double-blind, placebo-controlled phase 1/2 trial. Lancet Infect. Dis. 2015;15:1156–1166. doi: 10.1016/S1473-3099(15)00154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sun W., Liu Y., Amanat F., Gonzalez-Dominguez I., McCroskery S., Slamanig S., Coughlan L., Rosado V., Lemus N., Jangra S., et al. A Newcastle disease virus expressing a stabilized spike protein of SARS-CoV-2 induces protective immune responses. Nat. Commun. 2021;12:6197. doi: 10.1038/s41467-021-26499-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tcheou J., Raskin A., Singh G., Kawabata H., Bielak D., Sun W., González-Domínguez I., Sather D.N., García-Sastre A., Palese P., et al. Safety and immunogenicity analysis of a Newcastle disease virus (NDV-HXP-S) expressing the spike protein of SARS-CoV-2 in sprague dawley rats. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.791764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ohtsuka J., Imai M., Fukumura M., Maeda M., Eguchi A., Ono R., Maemura T., Ito M., Yamayoshi S., Kataoka Y., et al. Non-propagative human parainfluenza virus type 2 nasal vaccine robustly protects the upper and lower airways against SARS-CoV-2. iScience. 2021:103379. doi: 10.1016/j.isci.2021.103379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nyombayire J., Anzala O., Gazzard B., Karita E., Bergin P., Hayes P., Kopycinski J., Omosa-Manyonyi G., Jackson A., Bizimana J., et al. First-in-Human evaluation of the safety and immunogenicity of an intranasally administered replication-competent Sendai virus-vectored HIV type 1 gag vaccine: induction of potent T-cell or antibody responses in prime-boost regimens. J. Infect. Dis. 2017;215:95–104. doi: 10.1093/infdis/jiw500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barry M. Single-cycle adenovirus vectors in the current vaccine landscape. Expert Rev. Vaccines. 2018;17:163–173. doi: 10.1080/14760584.2018.1419067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Coughlan L., Uusi-Kerttula H., Ma J., Degg B.P., Parker A.L., Baker A.H. Retargeting adenovirus serotype 48 fiber knob domain by peptide incorporation. Hum. Gene Ther. 2014;25:385–394. doi: 10.1089/hum.2014.016. [DOI] [PubMed] [Google Scholar]
- 70.Vujadinovic M., Vellinga J. Progress in adenoviral capsid-display vaccines. Biomedicines. 2018;6 doi: 10.3390/biomedicines6030081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Asthagiri Arunkumar G., McMahon M., Pavot V., Aramouni M., Ioannou A., Lambe T., Gilbert S., Krammer F. Vaccination with viral vectors expressing NP, M1 and chimeric hemagglutinin induces broad protection against influenza virus challenge in mice. Vaccine. 2019;37:5567–5577. doi: 10.1016/j.vaccine.2019.07.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cicconi P., Jones C., Sarkar E., Silva-Reyes L., Klenerman P., de Lara C., Hutchings C., Moris P., Janssens M., Fissette L.A., et al. First-in-Human randomized study to assess the safety and immunogenicity of an investigational respiratory syncytial virus (RSV) vaccine based on chimpanzee-adenovirus-155 viral vector-expressing RSV fusion, nucleocapsid, and antitermination viral proteins in healthy adults. Clin. Infect. Dis. 2020;70:2073–2081. doi: 10.1093/cid/ciz653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McMahon M., Asthagiri Arunkumar G., Liu W.C., Stadlbauer D., Albrecht R.A., Pavot V., Aramouni M., Lambe T., Gilbert S.C., Krammer F. Vaccination with viral vectors expressing chimeric hemagglutinin, NP and M1 antigens protects ferrets against influenza virus challenge. Front. Immunol. 2019;10:2005. doi: 10.3389/fimmu.2019.02005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Green C.A., Scarselli E., Sande C.J., Thompson A.J., de Lara C.M., Taylor K.S., Haworth K., Del Sorbo M., Angus B., Siani L., et al. Chimpanzee adenovirus- and MVA-vectored respiratory syncytial virus vaccine is safe and immunogenic in adults. Sci. Transl. Med. 2015;7:300ra126. doi: 10.1126/scitranslmed.aac5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gurwith M., Lock M., Taylor E.M., Ishioka G., Alexander J., Mayall T., Ervin J.E., Greenberg R.N., Strout C., Treanor J.J., et al. Safety and immunogenicity of an oral, replicating adenovirus serotype 4 vector vaccine for H5N1 influenza: a randomised, double-blind, placebo-controlled, phase 1 study. Lancet Infect. Dis. 2013;13:238–250. doi: 10.1016/S1473-3099(12)70345-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barouch D.H., Tomaka F.L., Wegmann F., Stieh D.J., Alter G., Robb M.L., Michael N.L., Peter L., Nkolola J.P., Borducchi E.N., et al. Evaluation of a mosaic HIV-1 vaccine in a multicentre, randomised, double-blind, placebo-controlled, phase 1/2a clinical trial (APPROACH) and in rhesus monkeys (NHP 13-19) Lancet. 2018;392:232–243. doi: 10.1016/S0140-6736(18)31364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Folegatti P.M., Ewer K.J., Aley P.K., Angus B., Becker S., Belij-Rammerstorfer S., Bellamy D., Bibi S., Bittaye M., Clutterbuck E.A., et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Afolabi M.O., Tiono A.B., Adetifa U.J., Yaro J.B., Drammeh A., Nebie I., Bliss C., Hodgson S.H., Anagnostou N.A., Sanou G.S., et al. Safety and immunogenicity of Chad63 and MVA ME-TRAP in West African children and infants. Mol. Ther. 2016;24:1470–1477. doi: 10.1038/mt.2016.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mensah V.A., Roetynck S., Kanteh E.K., Bowyer G., Ndaw A., Oko F., Bliss C.M., Jagne Y.J., Cortese R., Nicosia A., et al. Safety and immunogenicity of malaria vectored vaccines given with routine expanded program on immunization vaccines in Gambian infants and neonates: a randomized controlled trial. Front. Immunol. 2017;8:1551. doi: 10.3389/fimmu.2017.01551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Frater J., Ewer K.J., Ogbe A., Pace M., Adele S., Adland E., Alagaratnam J., Aley P.K., Ali M., Ansari M.A., et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in HIV infection: a single-arm substudy of a phase 2/3 clinical trial. Lancet HIV. 2021;8:e474–e485. doi: 10.1016/S2352-3018(21)00103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Churchyard G.J., Snowden M.A., Hokey D., Dheenadhayalan V., McClain J.B., Douoguih M., Pau M.G., Sadoff J., Landry B. The safety and immunogenicity of an adenovirus type 35-vectored TB vaccine in HIV-infected, BCG-vaccinated adults with CD4(+) T cell counts >350 cells/mm(3) Vaccine. 2015;33:1890–1896. doi: 10.1016/j.vaccine.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 82.Barry H., Mutua G., Kibuuka H., Anywaine Z., Sirima S.B., Meda N., Anzala O., Eholie S., Betard C., Richert L., et al. Safety and immunogenicity of 2-dose heterologous Ad26.ZEBOV, MVA-BN-Filo Ebola vaccination in healthy and HIV-infected adults: a randomised, placebo-controlled phase II clinical trial in Africa. PLoS Med. 2021;18:e1003813. doi: 10.1371/journal.pmed.1003813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Afkhami S., LeClair D.A., Haddadi S., Lai R., Toniolo S.P., Ertl H.C., Cranston E.D., Thompson M.R., Xing Z. Spray dried human and chimpanzee adenoviral-vectored vaccines are thermally stable and immunogenic in vivo. Vaccine. 2017;35:2916–2924. doi: 10.1016/j.vaccine.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 84.Alcock R., Cottingham M.G., Rollier C.S., Furze J., De Costa S.D., Hanlon M., Spencer A.J., Honeycutt J.D., Wyllie D.H., Gilbert S.C., et al. Long-term thermostabilization of live poxviral and adenoviral vaccine vectors at supraphysiological temperatures in carbohydrate glass. Sci. Transl. Med. 2010;2:19ra12. doi: 10.1126/scitranslmed.3000490. [DOI] [PubMed] [Google Scholar]
- 85.Berg A., Wright D., Dulal P., Stedman A., Fedosyuk S., Francis M.J., Charleston B., Warimwe G.M., Douglas A.D. Stability of chimpanzee adenovirus vectored vaccines (ChAdOx1 and ChAdOx2) in liquid and lyophilised formulations. Vaccines (Basel) 2021;9:1249. doi: 10.3390/vaccines9111249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mendonca S.A., Lorincz R., Boucher P., Curiel D.T. Adenoviral vector vaccine platforms in the SARS-CoV-2 pandemic. NPJ Vaccines. 2021;6:97. doi: 10.1038/s41541-021-00356-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Afolabi M.O., Ishola D., Manno D., Keshinro B., Bockstal V., Rogers B., Owusu-Kyei K., Serry-Bangura A., Swaray I., Lowe B., et al. Safety and immunogenicity of the two-dose heterologous Ad26.ZEBOV and MVA-BN-Filo Ebola vaccine regimen in children in Sierra Leone: a randomised, double-blind, controlled trial. Lancet Infect. Dis. 2021 doi: 10.1016/S1473-3099(21)00128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tapia M.D., Sow S.O., Mbaye K.D., Thiongane A., Ndiaye B.P., Ndour C.T., Mboup S., Keshinro B., Kinge T.N., Vernet G., et al. Safety, reactogenicity, and immunogenicity of a chimpanzee adenovirus vectored Ebola vaccine in children in Africa: a randomised, observer-blind, placebo-controlled, phase 2 trial. Lancet Infect. Dis. 2020;20:719–730. doi: 10.1016/S1473-3099(20)30019-0. [DOI] [PubMed] [Google Scholar]
- 89.Tapia M.D., Sow S.O., Ndiaye B.P., Mbaye K.D., Thiongane A., Ndour C.T., Mboup S., Ake J.A., Keshinro B., Akintunde G.A., et al. Safety, reactogenicity, and immunogenicity of a chimpanzee adenovirus vectored Ebola vaccine in adults in Africa: a randomised, observer-blind, placebo-controlled, phase 2 trial. Lancet Infect. Dis. 2020;20:707–718. doi: 10.1016/S1473-3099(20)30016-5. [DOI] [PubMed] [Google Scholar]
- 90.Milligan I.D., Gibani M.M., Sewell R., Clutterbuck E.A., Campbell D., Plested E., Nuthall E., Voysey M., Silva-Reyes L., McElrath M.J., et al. Safety and immunogenicity of novel adenovirus type 26- and modified vaccinia ankara-vectored Ebola vaccines: a randomized clinical trial. JAMA. 2016;315:1610–1623. doi: 10.1001/jama.2016.4218. [DOI] [PubMed] [Google Scholar]
- 91.Ishola D., Manno D., Afolabi M.O., Keshinro B., Bockstal V., Rogers B., Owusu-Kyei K., Serry-Bangura A., Swaray I., Lowe B., et al. Safety and long-term immunogenicity of the two-dose heterologous Ad26.ZEBOV and MVA-BN-Filo Ebola vaccine regimen in adults in Sierra Leone: a combined open-label, non-randomised stage 1, and a randomised, double-blind, controlled stage 2 trial. Lancet Infect. Dis. 2021 doi: 10.1016/S1473-3099(21)00125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ledgerwood J.E., DeZure A.D., Stanley D.A., Coates E.E., Novik L., Enama M.E., Berkowitz N.M., Hu Z., Joshi G., Ploquin A., et al. Chimpanzee adenovirus vector Ebola vaccine. N. Engl. J. Med. 2017;376:928–938. doi: 10.1056/NEJMoa1410863. [DOI] [PubMed] [Google Scholar]
- 93.Folegatti P.M., Harrison K., Preciado-Llanes L., Lopez F.R., Bittaye M., Kim Y.C., Flaxman A., Bellamy D., Makinson R., Sheridan J., et al. A single dose of ChAdOx1 Chik vaccine induces neutralizing antibodies against four chikungunya virus lineages in a phase 1 clinical trial. Nat. Commun. 2021;12:4636. doi: 10.1038/s41467-021-24906-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Matsuda K., Migueles S.A., Huang J., Bolkhovitinov L., Stuccio S., Griesman T., Pullano A.A., Kang B.H., Ishida E., Zimmerman M., et al. A replication-competent adenovirus-vectored influenza vaccine induces durable systemic and mucosal immunity. J. Clin. Invest. 2021;131 doi: 10.1172/JCI140794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Prescott J.B., Marzi A., Safronetz D., Robertson S.J., Feldmann H., Best S.M. Immunobiology of Ebola and Lassa virus infections. Nat. Rev. Immunol. 2017;17:195–207. doi: 10.1038/nri.2016.138. [DOI] [PubMed] [Google Scholar]
- 96.Mennechet F.J., Tran T.T., Eichholz K., van de Perre P., Kremer E.J. Ebola virus vaccine: benefit and risks of adenovirus-based vectors. Expert Rev. Vaccines. 2015;14:1471–1478. doi: 10.1586/14760584.2015.1083429. [DOI] [PubMed] [Google Scholar]
- 97.Tiffany A., Vetter P., Mattia J., Dayer J.A., Bartsch M., Kasztura M., Sterk E., Tijerino A.M., Kaiser L., Ciglenecki I. Ebola virus disease complications as experienced by survivors in Sierra Leone. Clin. Infect. Dis. 2016;62:1360–1366. doi: 10.1093/cid/ciw158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mattia J.G., Vandy M.J., Chang J.C., Platt D.E., Dierberg K., Bausch D.G., Brooks T., Conteh S., Crozier I., Fowler R.A., et al. Early clinical sequelae of Ebola virus disease in Sierra Leone: a cross-sectional study. Lancet Infect. Dis. 2016;16:331–338. doi: 10.1016/S1473-3099(15)00489-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Leroy E.M., Kumulungui B., Pourrut X., Rouquet P., Hassanin A., Yaba P., Delicat A., Paweska J.T., Gonzalez J.P., Swanepoel R. Fruit bats as reservoirs of Ebola virus. Nature. 2005;438:575–576. doi: 10.1038/438575a. [DOI] [PubMed] [Google Scholar]
- 100.Antonello J., Grant-Klein R.J., Nichols R., Kennedy S.B., Dubey S., Simon J.K. Serostatus cutoff levels and fold increase to define seroresponse to recombinant vesicular stomatitis virus - Zaire Ebola virus envelope glycoprotein vaccine: an evidence-based analysis. Vaccine. 2020;38:4885–4891. doi: 10.1016/j.vaccine.2020.04.061. [DOI] [PubMed] [Google Scholar]
- 101.Sullivan N.J., Sanchez A., Rollin P.E., Yang Z.Y., Nabel G.J. Development of a preventive vaccine for Ebola virus infection in primates. Nature. 2000;408:605–609. doi: 10.1038/35046108. [DOI] [PubMed] [Google Scholar]
- 102.Stanley D.A., Honko A.N., Asiedu C., Trefry J.C., Lau-Kilby A.W., Johnson J.C., Hensley L., Ammendola V., Abbate A., Grazioli F., et al. Chimpanzee adenovirus vaccine generates acute and durable protective immunity against ebolavirus challenge. Nat. Med. 2014;20:1126–1129. doi: 10.1038/nm.3702. [DOI] [PubMed] [Google Scholar]
- 103.Sullivan N.J., Hensley L., Asiedu C., Geisbert T.W., Stanley D., Johnson J., Honko A., Olinger G., Bailey M., Geisbert J.B., et al. CD8+ cellular immunity mediates rAd5 vaccine protection against Ebola virus infection of nonhuman primates. Nat. Med. 2011;17:1128–1131. doi: 10.1038/nm.2447. [DOI] [PubMed] [Google Scholar]
- 104.Sullivan N.J., Geisbert T.W., Geisbert J.B., Xu L., Yang Z.Y., Roederer M., Koup R.A., Jahrling P.B., Nabel G.J. Accelerated vaccination for Ebola virus haemorrhagic fever in non-human primates. Nature. 2003;424:681–684. doi: 10.1038/nature01876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim E., Erdos G., Huang S., Kenniston T., Falo L.D., Jr., Gambotto A. Preventative vaccines for Zika virus outbreak: preliminary evaluation. EBioMedicine. 2016;13:315–320. doi: 10.1016/j.ebiom.2016.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Richardson J.S., Yao M.K., Tran K.N., Croyle M.A., Strong J.E., Feldmann H., Kobinger G.P. Enhanced protection against Ebola virus mediated by an improved adenovirus-based vaccine. PLoS One. 2009;4:e5308. doi: 10.1371/journal.pone.0005308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sullivan N.J., Geisbert T.W., Geisbert J.B., Shedlock D.J., Xu L., Lamoreaux L., Custers J.H., Popernack P.M., Yang Z.Y., Pau M.G., et al. Immune protection of nonhuman primates against Ebola virus with single low-dose adenovirus vectors encoding modified GPs. PLoS Med. 2006;3:e177. doi: 10.1371/journal.pmed.0030177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Richardson J.S., Pillet S., Bello A.J., Kobinger G.P. Airway delivery of an adenovirus-based Ebola virus vaccine bypasses existing immunity to homologous adenovirus in nonhuman primates. J. Virol. 2013;87:3668–3677. doi: 10.1128/JVI.02864-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Patel A., Zhang Y., Croyle M., Tran K., Gray M., Strong J., Feldmann H., Wilson J.M., Kobinger G.P. Mucosal delivery of adenovirus-based vaccine protects against Ebola virus infection in mice. J. Infect. Dis. 2007;196:S413–S420. doi: 10.1086/520603. [DOI] [PubMed] [Google Scholar]
- 110.Choi J.H., Schafer S.C., Zhang L., Kobinger G.P., Juelich T., Freiberg A.N., Croyle M.A. A single sublingual dose of an adenovirus-based vaccine protects against lethal Ebola challenge in mice and Guinea pigs. Mol. Pharm. 2012;9:156–167. doi: 10.1021/mp200392g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wong G., Richardson J.S., Cutts T., Qiu X., Kobinger G.P. Intranasal immunization with an adenovirus vaccine protects Guinea pigs from Ebola virus transmission by infected animals. Antivir. Res. 2015;116:17–19. doi: 10.1016/j.antiviral.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 112.Bliss C.M., Parsons A.J., Nachbagauer R., Hamilton J.R., Cappuccini F., Ulaszewska M., Webber J.P., Clayton A., Hill A.V.S., Coughlan L. Targeting antigen to the surface of EVs improves the in vivo immunogenicity of human and non-human adenoviral vaccines in mice. Mol. Ther. Methods Clin. Dev. 2020;16:108–125. doi: 10.1016/j.omtm.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yang X., Wang X., Song Y., Zhou P., Li D., Zhang C., Jin X., Huang Z., Zhou D. Chimpanzee adenoviral vector prime-boost regimen elicits potent immune responses against Ebola virus in mice and rhesus macaques. Emerg. Microbes Infect. 2019;8:1086–1097. doi: 10.1080/22221751.2019.1644968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zahn R., Gillisen G., Roos A., Koning M., van der Helm E., Spek D., Weijtens M., Grazia Pau M., Radosevic K., Weverling G.J., et al. Ad35 and ad26 vaccine vectors induce potent and cross-reactive antibody and T-cell responses to multiple filovirus species. PLoS One. 2012;7:e44115. doi: 10.1371/journal.pone.0044115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sebastian S., Flaxman A., Cha K.M., Ulaszewska M., Gilbride C., Sharpe H., Wright E., Spencer A.J., Dowall S., Hewson R., et al. A multi-filovirus vaccine candidate: co-expression of Ebola, Sudan, and Marburg antigens in a single vector. Vaccines (Basel) 2020;8 doi: 10.3390/vaccines8020241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rahim M.N., Wee E.G., He S., Audet J., Tierney K., Moyo N., Hannoun Z., Crook A., Baines A., Korber B., et al. Complete protection of the BALB/c and C57BL/6J mice against Ebola and Marburg virus lethal challenges by pan-filovirus T-cell epigraph vaccine. PLoS Pathog. 2019;15:e1007564. doi: 10.1371/journal.ppat.1007564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Price M.E., Fisher-Hoch S.P., Craven R.B., McCormick J.B. A prospective study of maternal and fetal outcome in acute Lassa fever infection during pregnancy. BMJ. 1988;297:584–587. doi: 10.1136/bmj.297.6648.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Baize S., Marianneau P., Loth P., Reynard S., Journeaux A., Chevallier M., Tordo N., Deubel V., Contamin H. Early and strong immune responses are associated with control of viral replication and recovery in Lassa virus-infected cynomolgus monkeys. J. Virol. 2009;83:5890–5903. doi: 10.1128/JVI.01948-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Maruyama J., Mateer E.J., Manning J.T., Sattler R., Seregin A.V., Bukreyeva N., Jones F.R., Balint J.P., Gabitzsch E.S., Huang C., et al. Adenoviral vector-based vaccine is fully protective against lethal Lassa fever challenge in Hartley Guinea pigs. Vaccine. 2019;37:6824–6831. doi: 10.1016/j.vaccine.2019.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fischer R.J., Purushotham J.N., van Doremalen N., Sebastian S., Meade-White K., Cordova K., Letko M., Jeremiah Matson M., Feldmann F., Haddock E., et al. ChAdOx1-vectored Lassa fever vaccine elicits a robust cellular and humoral immune response and protects Guinea pigs against lethal Lassa virus challenge. NPJ Vaccines. 2021;6:32. doi: 10.1038/s41541-021-00291-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bliss C.M., Freyn A.W., Caniels T.G., Leyva-Grado V.H., Nachbagauer R., Sun W., Tan G.S., Gillespie V.L., McMahon M., Krammer F., et al. A single-shot adenoviral vaccine provides hemagglutinin stalk-mediated protection against heterosubtypic influenza challenge in mice. Mol. Ther. 2022 doi: 10.1016/j.ymthe.2022.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Matsuda K., Huang J., Zhou T., Sheng Z., Kang B.H., Ishida E., Griesman T., Stuccio S., Bolkhovitinov L., Wohlbold T.J., et al. Prolonged evolution of the memory B cell response induced by a replicating adenovirus-influenza H5 vaccine. Sci. Immunol. 2019;4 doi: 10.1126/sciimmunol.aau2710. [DOI] [PubMed] [Google Scholar]
- 123.Colloca S., Barnes E., Folgori A., Ammendola V., Capone S., Cirillo A., Siani L., Naddeo M., Grazioli F., Esposito M.L., et al. Vaccine vectors derived from a large collection of simian adenoviruses induce potent cellular immunity across multiple species. Sci. Transl. Med. 2012;4:115ra112. doi: 10.1126/scitranslmed.3002925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Collier A.Y., Yu J., McMahan K., Liu J., Chandrashekar A., Maron J.S., Atyeo C., Martinez D.R., Ansel J.L., Aguayo R., et al. Differential kinetics of immune responses elicited by covid-19 vaccines. N. Engl. J. Med. 2021;385:2010–2012. doi: 10.1056/NEJMc2115596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Barnes E., Folgori A., Capone S., Swadling L., Aston S., Kurioka A., Meyer J., Huddart R., Smith K., Townsend R., et al. Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Sci. Transl. Med. 2012;4:115ra111. doi: 10.1126/scitranslmed.3003155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Purushotham J., Lambe T., Gilbert S.C. Vaccine platforms for the prevention of Lassa fever. Immunol. Lett. 2019;215:1–11. doi: 10.1016/j.imlet.2019.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zivcec M., Safronetz D., Scott D.P., Robertson S., Feldmann H. Nucleocapsid protein-based vaccine provides protection in mice against lethal Crimean-Congo hemorrhagic fever virus challenge. PLoS Negl. Trop. Dis. 2018;12:e0006628. doi: 10.1371/journal.pntd.0006628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dowall S.D., Carroll M.W., Hewson R. Development of vaccines against Crimean-Congo haemorrhagic fever virus. Vaccine. 2017;35:6015–6023. doi: 10.1016/j.vaccine.2017.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bente D.A., Forrester N.L., Watts D.M., McAuley A.J., Whitehouse C.A., Bray M. Crimean-Congo hemorrhagic fever: history, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antivir. Res. 2013;100:159–189. doi: 10.1016/j.antiviral.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 130.Hinkula J., Devignot S., Akerstrom S., Karlberg H., Wattrang E., Bereczky S., Mousavi-Jazi M., Risinger C., Lindegren G., Vernersson C., et al. Immunization with DNA plasmids coding for Crimean-Congo hemorrhagic fever virus capsid and envelope proteins and/or virus-like particles induces protection and survival in challenged mice. J. Virol. 2017;91 doi: 10.1128/JVI.02076-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kortekaas J., Vloet R.P., McAuley A.J., Shen X., Bosch B.J., de Vries L., Moormann R.J., Bente D.A. Crimean-Congo hemorrhagic fever virus subunit vaccines induce high levels of neutralizing antibodies but No protection in STAT1 knockout mice. Vector Borne Zoonotic Dis. 2015;15:759–764. doi: 10.1089/vbz.2015.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dowall S.D., Buttigieg K.R., Findlay-Wilson S.J., Rayner E., Pearson G., Miloszewska A., Graham V.A., Carroll M.W., Hewson R. A Crimean-Congo hemorrhagic fever (CCHF) viral vaccine expressing nucleoprotein is immunogenic but fails to confer protection against lethal disease. Hum. Vaccin. Immunother. 2016;12:519–527. doi: 10.1080/21645515.2015.1078045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ozdarendeli A., Canakoglu N., Berber E., Aydin K., Tonbak S., Ertek M., Buzgan T., Bolat Y., Aktas M., Kalkan A. The complete genome analysis of Crimean-Congo hemorrhagic fever virus isolated in Turkey. Virus Res. 2010;147:288–293. doi: 10.1016/j.virusres.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 134.Hooper J.W., Larsen T., Custer D.M., Schmaljohn C.S. A lethal disease model for hantavirus pulmonary syndrome. Virology. 2001;289:6–14. doi: 10.1006/viro.2001.1133. [DOI] [PubMed] [Google Scholar]
- 135.Mishra A.K., Hellert J., Freitas N., Guardado-Calvo P., Haouz A., Fels J.M., Maurer D.P., Abelson D.M., Bornholdt Z.A., Walker L.M., et al. Structural basis of synergistic neutralization of Crimean-Congo hemorrhagic fever virus by human antibodies. Science. 2021;375:eabl6502. doi: 10.1126/science.abl6502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fels J.M., Maurer D.P., Herbert A.S., Wirchnianski A.S., Vergnolle O., Cross R.W., Abelson D.M., Moyer C.L., Mishra A.K., Aguilan J.T., et al. Protective neutralizing antibodies from human survivors of Crimean-Congo hemorrhagic fever. Cell. 2021;184:3486–3501.e21. doi: 10.1016/j.cell.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mishra A.K., Moyer C.L., Abelson D.M., Deer D.J., El Omari K., Duman R., Lobel L., Lutwama J.J., Dye J.M., Wagner A., et al. Structure and characterization of Crimean-Congo hemorrhagic fever virus GP38. J. Virol. 2020;94 doi: 10.1128/JVI.02005-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Safronetz D., Hegde N.R., Ebihara H., Denton M., Kobinger G.P., St Jeor S., Feldmann H., Johnson D.C. Adenovirus vectors expressing hantavirus proteins protect hamsters against lethal challenge with andes virus. J. Virol. 2009;83:7285–7295. doi: 10.1128/JVI.00373-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li K., Li P.Y., Wu X.A., Zhang L., Liu Z.Y., Yu L., Zhang L., Cheng L.F., Bai W.T., Zhang F.L., et al. Induction of Hantaan virus-specific immune responses in C57BL/6 mice by immunization with a modified recombinant adenovirus containing the chimeric gene, GcS0.7. Int. J. Mol. Med. 2013;32:709–716. doi: 10.3892/ijmm.2013.1421. [DOI] [PubMed] [Google Scholar]
- 140.Martinez V.P., Bellomo C., San Juan J., Pinna D., Forlenza R., Elder M., Padula P.J. Person-to-person transmission of Andes virus. Emerg. Infect. Dis. 2005;11:1848–1853. doi: 10.3201/eid1112.050501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Maeda K., West K., Hayasaka D., Ennis F.A., Terajima M. Recombinant adenovirus vector vaccine induces stronger cytotoxic T-cell responses than recombinant vaccinia virus vector, plasmid DNA, or a combination of these. Viral Immunol. 2005;18:657–667. doi: 10.1089/vim.2005.18.657. [DOI] [PubMed] [Google Scholar]
- 142.McMillen C.M., Arora N., Boyles D.A., Albe J.R., Kujawa M.R., Bonadio J.F., Coyne C.B., Hartman A.L. Rift Valley fever virus induces fetal demise in Sprague-Dawley rats through direct placental infection. Sci. Adv. 2018;4:eaau9812. doi: 10.1126/sciadv.aau9812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.McMillen C.M., Hartman A.L. Rift Valley fever: a threat to pregnant women hiding in plain sight? J. Virol. 2021;95 doi: 10.1128/JVI.01394-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Holman D.H., Penn-Nicholson A., Wang D., Woraratanadharm J., Harr M.K., Luo M., Maher E.M., Holbrook M.R., Dong J.Y. A complex adenovirus-vectored vaccine against Rift Valley fever virus protects mice against lethal infection in the presence of preexisting vector immunity. Clin. Vaccine Immunol. 2009;16:1624–1632. doi: 10.1128/CVI.00182-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Stedman A., Wright D., Wichgers Schreur P.J., Clark M.H.A., Hill A.V.S., Gilbert S.C., Francis M.J., van Keulen L., Kortekaas J., Charleston B., et al. Safety and efficacy of ChAdOx1 RVF vaccine against Rift Valley fever in pregnant sheep and goats. NPJ Vaccines. 2019;4:44. doi: 10.1038/s41541-019-0138-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wright D., Allen E.R., Clark M.H.A., Gitonga J.N., Karanja H.K., Hulswit R.J.G., Taylor I., Biswas S., Marshall J., Mwololo D., et al. Naturally acquired Rift Valley fever virus neutralizing antibodies predominantly target the Gn glycoprotein. iScience. 2020;23:101669. doi: 10.1016/j.isci.2020.101669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Faburay B., LaBeaud A.D., McVey D.S., Wilson W.C., Richt J.A. Current status of Rift Valley fever vaccine development. Vaccines (Basel) 2017;5 doi: 10.3390/vaccines5030029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Warimwe G.M., Lorenzo G., Lopez-Gil E., Reyes-Sandoval A., Cottingham M.G., Spencer A.J., Collins K.A., Dicks M.D., Milicic A., Lall A., et al. Immunogenicity and efficacy of a chimpanzee adenovirus-vectored Rift Valley fever vaccine in mice. Virol. J. 2013;10:349. doi: 10.1186/1743-422X-10-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dicks M.D., Spencer A.J., Coughlan L., Bauza K., Gilbert S.C., Hill A.V., Cottingham M.G. Differential immunogenicity between HAdV-5 and chimpanzee adenovirus vector ChAdOx1 is independent of fiber and penton RGD loop sequences in mice. Sci. Rep. 2015;5:16756. doi: 10.1038/srep16756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Warimwe G.M., Gesharisha J., Carr B.V., Otieno S., Otingah K., Wright D., Charleston B., Okoth E., Elena L.G., Lorenzo G., et al. Chimpanzee adenovirus vaccine provides multispecies protection against Rift Valley fever. Sci. Rep. 2016;6:20617. doi: 10.1038/srep20617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Musso D., Ko A.I., Baud D. Zika virus infection - after the pandemic. N. Engl. J. Med. 2019;381:1444–1457. doi: 10.1056/NEJMra1808246. [DOI] [PubMed] [Google Scholar]
- 152.Abbink P., Maxfield L.F., Ng'ang'a D., Borducchi E.N., Iampietro M.J., Bricault C.A., Teigler J.E., Blackmore S., Parenteau L., Wagh K., et al. Construction and evaluation of novel rhesus monkey adenovirus vaccine vectors. J. Virol. 2015;89:1512–1522. doi: 10.1128/JVI.02950-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Abbink P., Larocca R.A., De La Barrera R.A., Bricault C.A., Moseley E.T., Boyd M., Kirilova M., Li Z., Ng'ang'a D., Nanayakkara O., et al. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science. 2016;353:1129–1132. doi: 10.1126/science.aah6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lopez-Camacho C., Abbink P., Larocca R.A., Dejnirattisai W., Boyd M., Badamchi-Zadeh A., Wallace Z.R., Doig J., Velazquez R.S., Neto R.D.L., et al. Rational Zika vaccine design via the modulation of antigen membrane anchors in chimpanzee adenoviral vectors. Nat. Commun. 2018;9:2441. doi: 10.1038/s41467-018-04859-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Beasley D.W., Barrett A.D. Identification of neutralizing epitopes within structural domain III of the West Nile virus envelope protein. J. Virol. 2002;76:13097–13100. doi: 10.1128/jvi.76.24.13097-13100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lopez-Camacho C., De Lorenzo G., Slon-Campos J.L., Dowall S., Abbink P., Larocca R.A., Kim Y.C., Poggianella M., Graham V., Findlay-Wilson S., et al. Immunogenicity and efficacy of Zika virus envelope domain III in DNA, protein, and ChAdOx1 adenoviral-vectored vaccines. Vaccines (Basel) 2020;8 doi: 10.3390/vaccines8020307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cox F., van der Fits L., Abbink P., Larocca R.A., van Huizen E., Saeland E., Verhagen J., Peterson R., Tolboom J., Kaufmann B., et al. Adenoviral vector type 26 encoding Zika virus (ZIKV) M-Env antigen induces humoral and cellular immune responses and protects mice and nonhuman primates against ZIKV challenge. PLoS One. 2018;13:e0202820. doi: 10.1371/journal.pone.0202820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Elong Ngono A., Vizcarra E.A., Tang W.W., Sheets N., Joo Y., Kim K., Gorman M.J., Diamond M.S., Shresta S. Mapping and role of the CD8(+) T cell response during primary Zika virus infection in mice. Cell Host Microbe. 2017;21:35–46. doi: 10.1016/j.chom.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tatsis N., Fitzgerald J.C., Reyes-Sandoval A., Harris-McCoy K.C., Hensley S.E., Zhou D., Lin S.W., Bian A., Xiang Z.Q., Iparraguirre A., et al. Adenoviral vectors persist in vivo and maintain activated CD8+ T cells: implications for their use as vaccines. Blood. 2007;110:1916–1923. doi: 10.1182/blood-2007-02-062117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Quinn K.M., Da Costa A., Yamamoto A., Berry D., Lindsay R.W., Darrah P.A., Wang L., Cheng C., Kong W.P., Gall J.G., et al. Comparative analysis of the magnitude, quality, phenotype, and protective capacity of simian immunodeficiency virus gag-specific CD8+ T cells following human-, simian-, and chimpanzee-derived recombinant adenoviral vector immunization. J. Immunol. 2013;190:2720–2735. doi: 10.4049/jimmunol.1202861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Baden L.R., Walsh S.R., Seaman M.S., Tucker R.P., Krause K.H., Patel A., Johnson J.A., Kleinjan J., Yanosick K.E., Perry J., et al. First-in-human evaluation of the safety and immunogenicity of a recombinant adenovirus serotype 26 HIV-1 Env vaccine (IPCAVD 001) J. Infect. Dis. 2013;207:240–247. doi: 10.1093/infdis/jis670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Li J.X., Hou L.H., Meng F.Y., Wu S.P., Hu Y.M., Liang Q., Chu K., Zhang Z., Xu J.J., Tang R., et al. Immunity duration of a recombinant adenovirus type-5 vector-based Ebola vaccine and a homologous prime-boost immunisation in healthy adults in China: final report of a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Glob. Health. 2017;5:e324–e334. doi: 10.1016/S2214-109X(16)30367-9. [DOI] [PubMed] [Google Scholar]
- 163.Zhu F.C., Hou L.H., Li J.X., Wu S.P., Liu P., Zhang G.R., Hu Y.M., Meng F.Y., Xu J.J., Tang R., et al. Safety and immunogenicity of a novel recombinant adenovirus type-5 vector-based Ebola vaccine in healthy adults in China: preliminary report of a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet. 2015;385:2272–2279. doi: 10.1016/S0140-6736(15)60553-0. [DOI] [PubMed] [Google Scholar]
- 164.Sadoff J., De Paepe E., DeVincenzo J., Gymnopoulou E., Menten J., Murray B., Bastian A.R., Vandebosch A., Haazen W., Noulin N., et al. Prevention of respiratory syncytial virus infection in healthy adults by a single immunization of Ad26.RSV.preF in a human challenge study. J. Infect. Dis. 2021 doi: 10.1093/infdis/jiab003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Williams K., Bastian A.R., Feldman R.A., Omoruyi E., de Paepe E., Hendriks J., van Zeeburg H., Godeaux O., Langedijk J.P.M., Schuitemaker H., et al. Phase 1 safety and immunogenicity study of a respiratory syncytial virus vaccine with an adenovirus 26 vector encoding prefusion F (Ad26.RSV.preF) in adults aged >/=60 years. J. Infect. Dis. 2020;222:979–988. doi: 10.1093/infdis/jiaa193. [DOI] [PubMed] [Google Scholar]
- 166.Folegatti P.M., Bittaye M., Flaxman A., Lopez F.R., Bellamy D., Kupke A., Mair C., Makinson R., Sheridan J., Rohde C., et al. Safety and immunogenicity of a candidate middle east respiratory syndrome coronavirus viral-vectored vaccine: a dose-escalation, open-label, non-randomised, uncontrolled, phase 1 trial. Lancet Infect. Dis. 2020;20:816–826. doi: 10.1016/S1473-3099(20)30160-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Dicks M.D., Spencer A.J., Edwards N.J., Wadell G., Bojang K., Gilbert S.C., Hill A.V., Cottingham M.G. A novel chimpanzee adenovirus vector with low human seroprevalence: improved systems for vector derivation and comparative immunogenicity. PLoS One. 2012;7:e40385. doi: 10.1371/journal.pone.0040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Alharbi N.K., Padron-Regalado E., Thompson C.P., Kupke A., Wells D., Sloan M.A., Grehan K., Temperton N., Lambe T., Warimwe G., et al. ChAdOx1 and MVA based vaccine candidates against MERS-CoV elicit neutralising antibodies and cellular immune responses in mice. Vaccine. 2017;35:3780–3788. doi: 10.1016/j.vaccine.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Munster V.J., Wells D., Lambe T., Wright D., Fischer R.J., Bushmaker T., Saturday G., van Doremalen N., Gilbert S.C., de Wit E., et al. Protective efficacy of a novel simian adenovirus vaccine against lethal MERS-CoV challenge in a transgenic human DPP4 mouse model. NPJ Vaccines. 2017;2:28. doi: 10.1038/s41541-017-0029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Alharbi N.K., Qasim I., Almasoud A., Aljami H.A., Alenazi M.W., Alhafufi A., Aldibasi O.S., Hashem A.M., Kasem S., Albrahim R., et al. Humoral immunogenicity and efficacy of a single dose of ChAdOx1 MERS vaccine candidate in dromedary camels. Sci. Rep. 2019;9:16292. doi: 10.1038/s41598-019-52730-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Falsey A.R., Sobieszczyk M.E., Hirsch I., Sproule S., Robb M.L., Corey L., Neuzil K.M., Hahn W., Hunt J., Mulligan M.J., et al. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) covid-19 vaccine. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2105290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Al Bahrani S., Albarrak A., Alghamdi O.A., Alghamdi M.A., Hakami F.H., Al Abaadi A.K., Alkhrashi S.A., Alghamdi M.Y., Almershad M.M., Alenazi M.M., et al. Safety and reactogenicity of the ChAdOx1 (AZD1222) COVID-19 vaccine in Saudi Arabia. Int. J. Infect. Dis. 2021;110:359–362. doi: 10.1016/j.ijid.2021.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mennechet F.J.D., Paris O., Ouoba A.R., Salazar Arenas S., Sirima S.B., Takoudjou Dzomo G.R., Diarra A., Traore I.T., Kania D., Eichholz K., et al. A review of 65 years of human adenovirus seroprevalence. Expert Rev. Vaccines. 2019;18:597–613. doi: 10.1080/14760584.2019.1588113. [DOI] [PubMed] [Google Scholar]
- 175.Sadoff J., Le Gars M., Shukarev G., Heerwegh D., Truyers C., de Groot A.M., Stoop J., Tete S., Van Damme W., Leroux-Roels I., et al. Interim results of a phase 1-2a trial of Ad26.COV2.S covid-19 vaccine. N. Engl. J. Med. 2021;384:1824–1835. doi: 10.1056/NEJMoa2034201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhu F.C., Guan X.H., Li Y.H., Huang J.Y., Jiang T., Hou L.H., Li J.X., Yang B.F., Wang L., Wang W.J., et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396:479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhu F.C., Li Y.H., Guan X.H., Hou L.H., Wang W.J., Li J.X., Wu S.P., Wang B.S., Wang Z., Wang L., et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395:1845–1854. doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., Tukhvatulin A.I., Zubkova O.V., Dzharullaeva A.S., Kovyrshina A.V., Lubenets N.L., Grousova D.M., Erokhova A.S., et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Babamahmoodi F., Saeedi M., Alizadeh-Navaei R., Hedayatizadeh-Omran A., Mousavi S.A., Ovaise G., Kordi S., Akbari Z., Azordeh M., Ahangarkani F., et al. Side effects and Immunogenicity following administration of the Sputnik V COVID-19 vaccine in health care workers in Iran. Sci. Rep. 2021;11:21464. doi: 10.1038/s41598-021-00963-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Pagotto V., Ferloni A., Mercedes Soriano M., Diaz M., Braguinsky Golde N., Gonzalez M.I., Asprea V., Staneloni M.I., Zingoni P., Vidal G., et al. Active monitoring of early safety of Sputnik V vaccine in Buenos Aires, Argentina. Medicina (B Aires) 2021;81:408–414. [PubMed] [Google Scholar]
- 181.Schultz N.H., Sorvoll I.H., Michelsen A.E., Munthe L.A., Lund-Johansen F., Ahlen M.T., Wiedmann M., Aamodt A.H., Skattor T.H., Tjonnfjord G.E., et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N. Engl. J. Med. 2021;384:2124–2130. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N. Engl. J. Med. 2021;384:2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Raper S.E., Chirmule N., Lee F.S., Wivel N.A., Bagg A., Gao G.P., Wilson J.M., Batshaw M.L. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol. Genet. Metab. 2003;80:148–158. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 184.Garcia M., Moreno R., Gil-Martin M., Cascallo M., de Olza M.O., Cuadra C., Piulats J.M., Navarro V., Domenech M., Alemany R., et al. A phase 1 trial of oncolytic adenovirus ICOVIR-5 administered intravenously to cutaneous and uveal melanoma patients. Hum. Gene Ther. 2019;30:352–364. doi: 10.1089/hum.2018.107. [DOI] [PubMed] [Google Scholar]
- 185.McGonagle D., De Marco G., Bridgewood C. Mechanisms of immunothrombosis in vaccine-induced thrombotic thrombocytopenia (VITT) compared to natural SARS-CoV-2 infection. J. Autoimmun. 2021;121:102662. doi: 10.1016/j.jaut.2021.102662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Pavord S., Scully M., Hunt B.J., Lester W., Bagot C., Craven B., Rampotas A., Ambler G., Makris M. Clinical features of vaccine-induced immune thrombocytopenia and thrombosis. N. Engl. J. Med. 2021;385:1680–1689. doi: 10.1056/NEJMoa2109908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Pottegard A., Lund L.C., Karlstad O., Dahl J., Andersen M., Hallas J., Lidegaard O., Tapia G., Gulseth H.L., Ruiz P.L., et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population based cohort study. BMJ. 2021;373:n1114. doi: 10.1136/bmj.n1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.See I., Su J.R., Lale A., Woo E.J., Guh A.Y., Shimabukuro T.T., Streiff M.B., Rao A.K., Wheeler A.P., Beavers S.F., et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325:2448–2456. doi: 10.1001/jama.2021.7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Muir K.L., Kallam A., Koepsell S.A., Gundabolu K. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination. N. Engl. J. Med. 2021;384:1964–1965. doi: 10.1056/NEJMc2105869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Taquet M., Husain M., Geddes J.R., Luciano S., Harrison P.J. Cerebral venous thrombosis and portal vein thrombosis: a retrospective cohort study of 537,913 COVID-19 cases. EClinicalMedicine. 2021;39:101061. doi: 10.1016/j.eclinm.2021.101061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Siegler J.E., Klein P., Yaghi S., Vigilante N., Abdalkader M., Coutinho J.M., Abdul Khalek F., Nguyen T.N. Cerebral vein thrombosis with vaccine-induced immune thrombotic thrombocytopenia. Stroke. 2021;52:3045–3053. doi: 10.1161/STROKEAHA.121.035613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Perry R.J., Tamborska A., Singh B., Craven B., Marigold R., Arthur-Farraj P., Yeo J.M., Zhang L., Hassan-Smith G., Jones M., et al. Cerebral venous thrombosis after vaccination against COVID-19 in the UK: a multicentre cohort study. Lancet. 2021;398:1147–1156. doi: 10.1016/S0140-6736(21)01608-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Shahrizaila N., Lehmann H.C., Kuwabara S. Guillain-Barre syndrome. Lancet. 2021;397:1214–1228. doi: 10.1016/S0140-6736(21)00517-1. [DOI] [PubMed] [Google Scholar]
- 194.Shao S.C., Wang C.H., Chang K.C., Hung M.J., Chen H.Y., Liao S.C. Guillain-barre syndrome associated with COVID-19 vaccination. Emerg. Infect. Dis. 2021;27 doi: 10.3201/eid2712.211634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Buchbinder S.P., McElrath M.J., Dieffenbach C., Corey L. Use of adenovirus type-5 vectored vaccines: a cautionary tale. Lancet. 2020;396:e68–e69. doi: 10.1016/S0140-6736(20)32156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Buchbinder S.P., Mehrotra D.V., Duerr A., Fitzgerald D.W., Mogg R., Li D., Gilbert P.B., Lama J.R., Marmor M., Del Rio C., et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Gray G.E., Allen M., Moodie Z., Churchyard G., Bekker L.G., Nchabeleng M., Mlisana K., Metch B., de Bruyn G., Latka M.H., et al. Safety and efficacy of the HVTN 503/Phambili study of a clade-B-based HIV-1 vaccine in South Africa: a double-blind, randomised, placebo-controlled test-of-concept phase 2b study. Lancet Infect. Dis. 2011;11:507–515. doi: 10.1016/S1473-3099(11)70098-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Gray G.E., Moodie Z., Metch B., Gilbert P.B., Bekker L.G., Churchyard G., Nchabeleng M., Mlisana K., Laher F., Roux S., et al. Recombinant adenovirus type 5 HIV gag/pol/nef vaccine in South Africa: unblinded, long-term follow-up of the phase 2b HVTN 503/Phambili study. Lancet Infect. Dis. 2014;14:388–396. doi: 10.1016/S1473-3099(14)70020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Duerr A., Huang Y., Buchbinder S., Coombs R.W., Sanchez J., del Rio C., Casapia M., Santiago S., Gilbert P., Corey L., et al. Extended follow-up confirms early vaccine-enhanced risk of HIV acquisition and demonstrates waning effect over time among participants in a randomized trial of recombinant adenovirus HIV vaccine (step study) J. Infect. Dis. 2012;206:258–266. doi: 10.1093/infdis/jis342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Huang Y., Follmann D., Nason M., Zhang L., Huang Y., Mehrotra D.V., Moodie Z., Metch B., Janes H., Keefer M.C., et al. Effect of rAd5-vector HIV-1 preventive vaccines on HIV-1 acquisition: a participant-level meta-analysis of randomized trials. PLoS One. 2015;10:e0136626. doi: 10.1371/journal.pone.0136626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Moodie Z., Metch B., Bekker L.G., Churchyard G., Nchabeleng M., Mlisana K., Laher F., Roux S., Mngadi K., Innes C., et al. Continued follow-up of Phambili phase 2b randomized HIV-1 vaccine trial participants supports increased HIV-1 acquisition among vaccinated men. PLoS One. 2015;10:e0137666. doi: 10.1371/journal.pone.0137666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Benlahrech A., Harris J., Meiser A., Papagatsias T., Hornig J., Hayes P., Lieber A., Athanasopoulos T., Bachy V., Csomor E., et al. Adenovirus vector vaccination induces expansion of memory CD4 T cells with a mucosal homing phenotype that are readily susceptible to HIV-1. Proc. Natl. Acad. Sci. U S A. 2009;106:19940–19945. doi: 10.1073/pnas.0907898106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Perreau M., Pantaleo G., Kremer E.J. Activation of a dendritic cell-T cell axis by Ad5 immune complexes creates an improved environment for replication of HIV in T cells. J. Exp. Med. 2008;205:2717–2725. doi: 10.1084/jem.20081786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.O'Brien K.L., Liu J., King S.L., Sun Y.H., Schmitz J.E., Lifton M.A., Hutnick N.A., Betts M.R., Dubey S.A., Goudsmit J., et al. Adenovirus-specific immunity after immunization with an Ad5 HIV-1 vaccine candidate in humans. Nat. Med. 2009;15:873–875. doi: 10.1038/nm.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Stephenson K.E., Hural J., Buchbinder S.P., Sinangil F., Barouch D.H. Preexisting adenovirus seropositivity is not associated with increased HIV-1 acquisition in three HIV-1 vaccine efficacy trials. J. Infect. Dis. 2012;205:1806–1810. doi: 10.1093/infdis/jis285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Curlin M.E., Cassis-Ghavami F., Magaret A.S., Spies G.A., Duerr A., Celum C.L., Sanchez J.L., Margolick J.B., Detels R., McElrath M.J., et al. Serological immunity to adenovirus serotype 5 is not associated with risk of HIV infection: a case-control study. AIDS. 2011;25:153–158. doi: 10.1097/QAD.0b013e328342115c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Naranbhai V., Garcia-Beltran W.F., Chang C.C., Mairena C.B., Thierauf J.C., Kirkpatrick G., Onozato M.L., Cheng J., St. Denis K.J., Lam E.C., et al. Comparative immunogenicity and effectiveness of mRNA-1273, BNT162b2 and Ad26.COV2.S COVID-19 vaccines. J. Infect. Dis. 2021 doi: 10.1093/infdis/jiab593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.van Gils M.J., Lavell A.H.A., van der Straten K., Appelman B., Bontjer I., Poniman M., Burger J.A., Oomen M., Bouhuijs J.H., van Vught L.A., et al. Four SARS-CoV-2 vaccines induce quantitatively different antibody responses against SARS-CoV-2 variants. medRxiv. 2022 doi: 10.1101/2021.09.27.21264163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Perez Marc G., Moreira E.D., Zerbini C., et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lopez Bernal J., Andrews N., Gower C., Gallagher E., Simmons R., Thelwall S., Stowe J., Tessier E., Groves N., Dabrera G., et al. Effectiveness of covid-19 vaccines against the B.1.617.2 (delta) variant. N. Engl. J. Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Polinski J.M., Weckstein A.R., Batech M., Kabelac C., Kamath T., Harvey R., Jain S., Rassen J.A., Khan N., Schneeweiss S. Effectiveness of the single-dose Ad26.COV2.S COVID vaccine. medRxiv. 2021 doi: 10.1101/2021.09.10.21263385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Self W.H., Tenforde M.W., Rhoads J.P., Gaglani M., Ginde A.A., Douin D.J., Olson S.M., Talbot H.K., Casey J.D., Mohr N.M., et al. Comparative effectiveness of Moderna, pfizer-BioNTech, and Janssen (Johnson & Johnson) vaccines in preventing COVID-19 hospitalizations among adults without immunocompromising conditions - United States, March-August 2021. Morb. Mortal. Wkly. Rep. 2021;70:1337–1343. doi: 10.15585/mmwr.mm7038e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Gray G.E., Collie S., Garrett N., Goga A., Champion J., Zylstra M., Reddy T., Yende N., Seocharan I., Takalani A., et al. Vaccine effectiveness against hospital admission in South African health care workers who received a homologous booster of Ad26.COV2 during an omicron COVID19 wave: preliminary results of the Sisonke 2 study. medRxiv. 2021 doi: 10.1101/2021.12.28.21268436. [DOI] [Google Scholar]
- 215.Zheutlin A., Ott M., Sun R., Zemlianskaia N.Z., Rubel M., Hayden J., Neri B., Kamath T., Khan N., Schneeweiss S., et al. Durability of protection against COVID-19 breakthrough infections and severe disease by vaccines in the United States. medRxiv. 2022 doi: 10.1101/2022.01.05.22268648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bos R., Rutten L., van der Lubbe J.E.M., Bakkers M.J.G., Hardenberg G., Wegmann F., Zuijdgeest D., de Wilde A.H., Koornneef A., Verwilligen A., et al. Ad26 vector-based COVID-19 vaccine encoding a prefusion-stabilized SARS-CoV-2 spike immunogen induces potent humoral and cellular immune responses. NPJ Vaccines. 2020;5:91. doi: 10.1038/s41541-020-00243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Stuart A.S.V., Shaw R.H., Liu X., Greenland M., Aley P.K., Andrews N.J., Cameron J.C., Charlton S., Clutterbuck E.A., Collins A.M., et al. Immunogenicity, safety, and reactogenicity of heterologous COVID-19 primary vaccination incorporating mRNA, viral-vector, and protein-adjuvant vaccines in the UK (Com-COV2): a single-blind, randomised, phase 2, non-inferiority trial. Lancet. 2022;399:36–49. doi: 10.1016/S0140-6736(21)02718-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Liu X., Shaw R.H., Stuart A.S.V., Greenland M., Aley P.K., Andrews N.J., Cameron J.C., Charlton S., Clutterbuck E.A., Collins A.M., et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet. 2021;398:856–869. doi: 10.1016/S0140-6736(21)01694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Atmar R.L., Lyke K.E., Deming M.E., Jackson L.A., Branche A.R., El Sahly H.M., Rostad C.A., Martin J.M., Johnston C., Rupp R.E., et al. Heterologous SARS-CoV-2 booster vaccinations - preliminary report. medRxiv. 2021 doi: 10.1101/2021.10.10.21264827. [DOI] [Google Scholar]
- 221.Schmidt T., Klemis V., Schub D., Mihm J., Hielscher F., Marx S., Abu-Omar A., Ziegler L., Guckelmus C., Urschel R., et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat. Med. 2021;27:1530–1535. doi: 10.1038/s41591-021-01464-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kim Huat N.K., Er Lim J.M., Gill U.S., de Alwis R., Tan N., Nan Toh J.Z., Abbott J.E., Usai C., Ooi E.E., Hong Low J.G., et al. Differential immunogenicity of homologous versus heterologous boost in Ad26.COV2.S vaccine recipients. medRxiv. 2021 doi: 10.1101/2021.10.14.21264981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Khan A., Sayedahmed E.E., Singh V.K., Mishra A., Dorta-Estremera S., Nookala S., Canaday D.H., Chen M., Wang J., Sastry K.J., et al. A recombinant bovine adenoviral mucosal vaccine expressing mycobacterial antigen-85B generates robust protection against tuberculosis in mice. Cell Rep. Med. 2021;2:100372. doi: 10.1016/j.xcrm.2021.100372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Hassan A.O., Shrihari S., Gorman M.J., Ying B., Yaun D., Raju S., Chen R.E., Dmitriev I.P., Kashentseva E., Adams L.J., et al. An intranasal vaccine durably protects against SARS-CoV-2 variants in mice. Cell Rep. 2021;36:109452. doi: 10.1016/j.celrep.2021.109452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Van Kampen K.R., Shi Z., Gao P., Zhang J., Foster K.W., Chen D.T., Marks D., Elmets C.A., Tang D.C. Safety and immunogenicity of adenovirus-vectored nasal and epicutaneous influenza vaccines in humans. Vaccine. 2005;23:1029–1036. doi: 10.1016/j.vaccine.2004.07.043. [DOI] [PubMed] [Google Scholar]
- 226.Green C.A., Scarselli E., Voysey M., Capone S., Vitelli A., Nicosia A., Cortese R., Thompson A.J., Sande C.S., de Lara C., et al. Safety and immunogenicity of novel respiratory syncytial virus (RSV) vaccines based on the RSV viral proteins F, N and M2-1 encoded by simian adenovirus (PanAd3-RSV) and MVA (MVA-RSV); protocol for an open-label, dose-escalation, single-centre, phase 1 clinical trial in healthy adults. BMJ Open. 2015;5:e008748. doi: 10.1136/bmjopen-2015-008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Green C.A., Sande C.J., Scarselli E., Capone S., Vitelli A., Nicosia A., Silva-Reyes L., Thompson A.J., de Lara C.M., Taylor K.S., et al. Novel genetically-modified chimpanzee adenovirus and MVA-vectored respiratory syncytial virus vaccine safely boosts humoral and cellular immunity in healthy older adults. J. Infect. 2019;78:382–392. doi: 10.1016/j.jinf.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Tasker S., Wight O'Rourke A., Suyundikov A., Jackson Booth P.G., Bart S., Krishnan V., Zhang J., Anderson K.J., Georges B., Roberts M.S. Safety and immunogenicity of a novel intranasal influenza vaccine (NasoVAX): a phase 2 randomized, controlled trial. Vaccines (Basel) 2021;9 doi: 10.3390/vaccines9030224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Telenti A., Arvin A., Corey L., Corti D., Diamond M.S., Garcia-Sastre A., Garry R.F., Holmes E.C., Pang P.S., Virgin H.W. After the pandemic: perspectives on the future trajectory of COVID-19. Nature. 2021;596:495–504. doi: 10.1038/s41586-021-03792-w. [DOI] [PubMed] [Google Scholar]
- 230.Baker A.T., Boyd R.J., Sarkar D., Teijeira-Crespo A., Chan C.K., Bates E., Waraich K., Vant J., Wilson E., Truong C.D., et al. ChAdOx1 interacts with CAR and PF4 with implications for thrombosis with thrombocytopenia syndrome. Sci. Adv. 2021;7:eabl8213. doi: 10.1126/sciadv.abl8213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Alba R., Bradshaw A.C., Coughlan L., Denby L., McDonald R.A., Waddington S.N., Buckley S.M., Greig J.A., Parker A.L., Miller A.M., et al. Biodistribution and retargeting of FX-binding ablated adenovirus serotype 5 vectors. Blood. 2010;116:2656–2664. doi: 10.1182/blood-2009-12-260026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kremer E.J. Pros and cons of adenovirus-based SARS-CoV-2 vaccines. Mol. Ther. 2020;28:2303–2304. doi: 10.1016/j.ymthe.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.The Lancet COVID-19 Commissioners, Task Force Chairs, and Commission Secretariat Operation warp speed: implications for global vaccine security. Lancet Glob. Health. 2021;9:e1017–e1021. doi: 10.1016/S2214-109X(21)00140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.So A.D., Woo J. Reserving coronavirus disease 2019 vaccines for global access: cross sectional analysis. BMJ. 2020;371:m4750. doi: 10.1136/bmj.m4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Flaxman A., Marchevsky N.G., Jenkin D., Aboagye J., Aley P.K., Angus B., Belij-Rammerstorfer S., Bibi S., Bittaye M., Cappuccini F., et al. Reactogenicity and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 in the UK: a substudy of two randomised controlled trials (COV001 and COV002) Lancet. 2021;398:981–990. doi: 10.1016/S0140-6736(21)01699-8. [DOI] [PMC free article] [PubMed] [Google Scholar]