Abstract
BRAF V600E and TERT promoter mutations, particularly their genetic duet, are well known to be associated with poor clinical outcomes of papillary thyroid cancer (PTC). Loss of radioactive iodine (RAI) avidity in recurrent PTC is a major cause of treatment failure and hence poor clinical outcomes. This study investigated the role of mutation patterns in loss of RAI avidity in recurrent PTC. Methods: This was a retrospective study of the relationship between loss of RAI avidity in structural recurrent PTC and the genotype patterns of BRAF V600E and TERT promoter mutations in 164 patients (104 women and 60 men) with a median age of 50 y (interquartile range, 35–62 y). Results: The overall prevalence of RAI avidity loss in recurrent PTC was 62.8% (103/164). When the cohort was divided into mutation and wild-type groups, the RAI avidity loss was 80.4% versus 33.9% (P < 0.001) in BRAF V600E versus wild-type BRAF patients, with an adjusted odds ratio of 7.11 (95% confidence interval [CI], 3.24–16.27), and 89.4% versus 52.1% (P < 0.001) in TERT mutation versus wild-type patients, with an adjusted odds ratio of 6.89 (95% CI, 2.28–25.66). When the cohort was divided into 4 genotypes, the RAI avidity loss was 70.3% (45/64), 55.6% (5/9), and 97.4% (37/38) in patients with BRAF V600E alone, TERT mutation alone, and the genetic duet of coexisting BRAF and TERT mutations, versus 30.2% (16/53) in patients with neither mutation (P < 0.001, = 0.251, and < 0.001, respectively). These corresponded to odds ratios of 5.39 (95% CI, 2.31–13.13), 2.84 (95% CI, 0.53–16.32), and 81.04 (95% CI, 11.67–3559.83), respectively. The synergy index was 13.28 (95% CI, 1.54–114.46; P = 0.019) between BRAF V600E and TERT mutation in cooperatively affecting RAI avidity. A similar genotype-associated expression pattern was observed for thyroid iodide-handling genes. Conclusion: BRAF V600E alone and, particularly, coexisting BRAF V600E and TERT promoter mutations are strongly associated with loss of RAI avidity and impairment of the iodide-metabolizing machinery in recurrent PTC, showing a robust predictive value for failure of RAI treatment of PTC.
Keywords: BRAF V600E, TERT promoter mutation, radioiodine therapy, thyroid cancer, recurrence
Papillary thyroid cancer (PTC), comprising about 85%–90% of all thyroid cancers, is a common endocrine malignancy (1–3) that is histologically classified into several variants, among which conventional PTC accounts for most (4–6). With the current standard treatments, PTC generally has an excellent clinical prognosis (7). Conventional radioactive iodine (RAI) therapy using 131I contributes to this excellent performance of clinical treatment of PTC by reducing disease recurrence. The treatment takes the advantage of the unique ability of thyroid cells to take up iodide through iodide-metabolizing molecules specifically expressed in thyroid cells. These molecules include sodium-iodide symporter (NIS), which transports iodide into the thyroid cell from the bloodstream; thyroid peroxidase (TPO), which oxidizes iodide into iodine; and thyroglobulin (TG), which incorporates iodine into its tyrosine residuals to produce thyroid hormone (4). These genes are regulated by specific transcriptional factors, such as PAX8 (8,9). Normal function of this iodide-metabolizing machinery is critical for thyroid hormone biosynthesis in normal thyroid physiology and for RAI uptake and trapping in cancer cells in RTC RAI treatment, which is upregulated by thyroid-stimulating hormone (TSH) receptor (TSHR).
There are often patients with recurrent PTC that is surgically inoperable and has lost RAI avidity because of the silencing of thyroid iodide-metabolizing genes (7), for which predictive molecular markers may be helpful if identified. BRAF V600E is a prominent oncogene in PTC and has an established prognostic value for poor prognosis of this cancer (4,10). TERT promoter mutation, existing in 2 main forms—chr5 1,295,228 C>T (C228T) and chr5 1,295,250 C>T (C250T)—is another major oncogene in PTC that is also associated with poor clinical outcomes (11). The genetic duet of coexisting BRAF V600E and TERT promoter mutations is particularly robustly associated with poor clinical outcomes of PTC (12,13). In the present study, we investigated the role and predictive value of BRAF V600E and TERT promoter mutations in primary PTC for loss of RAI avidity in subsequent recurrent disease.
MATERIALS AND METHODS
Study Subjects
We previously established a large cohort of 1,051 patients with PTC to assess the relationship between genetic variants and the clinical prognosis of PTC (13). In this cohort, structural recurrence of PTC, defined as recurrent tumor confirmed radiographically, cytologically, or pathologically, occurred in 167 patients. Among these 167 patients, 164, including 104 women and 60 men with a median age of 50 y (interquartile range, 35–62 y), had clinical radiology reports available on the whole-body RAI imaging scan to evaluate recurrence of PTC. The present study focused on these 164 patients. Loss of RAI avidity was defined as no RAI uptake in one or more lesions of recurrent PTC on whole-body RAI scans, which included either diagnostic 123I body scans or 131I posttreatment scans. Patients were prepared for RAI body scanning with either thyroid hormone withdrawal or recombinant human TSH stimulation with an achieved TSH level of more than 30 mIU/L. All patients underwent total or near-total thyroidectomy as the initial treatment. Therapeutic neck lymph node dissection and 131I ablation therapy after total thyroidectomy for treatment of the initial disease were pursued as clinically indicated following standard treatment criteria as previously described (12,13). The study was approved by our institutional review board, and informed patient consent was obtained when required. In some patients, PTC specimens were prospectively obtained with written patient consent; in others, clinicopathologic information was retrospectively obtained with institutional review board–approved waiver of patient consent.
Mutation Analysis
Genomic DNA was isolated from primary PTC samples using standard phenol-chloroform extraction and ethanol precipitation procedures. The regions harboring the spots for BRAF V600E and TERT promoter mutations in the BRAF and TERT genes were amplified by respective polymerase chain reactions, followed by Sanger sequencing to detect the BRAF and TERT promoter mutation status as described previously (12,13).
Analysis of Relationship Between Mutations and Expression of Thyroid Iodide-Handling Genes
The BRAF V600E and TERT promoter mutation status and the normalized RNA-sequencing data were acquired from the Cancer Genome Atlas data portal (14). The messenger RNA expression level of 5 thyroid iodide-handing genes, including NIS, TSHR, TPO, TG, and PAX8, was calculated by the log-transformation of the RNA counts.
Statistical Analysis
Continuous data were summarized as medians and interquartile ranges and compared using the Wilcoxon–Mann–Whitney test. Categoric variables were summarized as frequencies and percentages and compared using the χ2 test. Logistic regression models were used to assess the effects of mutations on the risk of RAI avidity loss, with the adjustment for patient age and sex. The interactions between BRAF and TERT mutations in affecting the risk of RAI avidity loss were tested using the synergy index with 95% confidence intervals (CIs) (15). Statistical analyses were performed using Stata/SE, version 12 (Stata Corp). All P values were 2-sided, and P values of less than 0.05 were considered statistically significant.
RESULTS
Relationship Between BRAF V600E, TERT Promoter Mutation, and Loss of RAI Avidity in Recurrent PTC
The overall prevalence of loss of RAI avidity in recurrent PTC was 62.8% (103/164). When the entire cohort of 164 patients was divided into BRAF V600E–positive and –negative groups (Table 1), loss of RAI avidity more commonly occurred in BRAF V600E–positive patients than in BRAF V600E–negative patients (80.4% vs. 33.9%, P < 0.001), with an adjusted odds ratio of 7.11 (95% CI, 3.24–16.27) for BRAF V600E–associated risk for loss of RAI avidity. When the entire cohort of 164 patients was divided into TERT mutation–positive and –negative groups, loss of RAI avidity more commonly occurred in TERT mutation–positive patients than in mutation-negative patients (89.4% vs. 52.1%, P < 0.001), with an adjusted odds ratio of 6.89 (95% CI, 2.28–25.66) for TERT mutation–associated risk for loss of RAI avidity. Similar observations were made when only conventional PTC was analyzed (Table 1).
TABLE 1.
Relationship Between BRAF V600E or TERT Promoter Mutations and Loss of RAI Avidity in Recurrent PTC
| Loss of RAI |
Odds ratio |
|||
| Tumor type and mutation status | n | P | Unadjusted | Adjusted* |
| All PTC | ||||
| BRAF V600E | ||||
| Negative | 21/62 (33.9) | 1.00 | 1.00 | |
| Positive | 82/102 (80.4) | <0.001 | 7.88 (3.69–17.51) | 7.11 (3.24–16.27) |
| TERT mutation | ||||
| Negative | 61/117 (52.1) | 1.00 | 1.00 | |
| Positive | 42/47 (89.4) | <0.001 | 7.63 (2.75–26.46) | 6.89 (2.28–25.66) |
| CPTC | ||||
| BRAF V600E | ||||
| Negative | 10/39 (25.6) | 1.00 | 1.00 | |
| Positive | 71/87 (81.6) | <0.001 | 12.52 (4.82–35.27) | 10.78 (4.03–31.38) |
| TERT mutation | ||||
| Negative | 47/91 (51.6) | 1.00 | 1.00 | |
| Positive | 34/35 (97.1) | <0.001 | 31.23 (4.81–1,322.43) | 31.37 (4.39–1,402.18) |
Adjusted for patient age and sex.
CPTC = conventional PTC.
TERT promoter mutations here included, collectively, TERT C228T and TERT C250T. Data in parentheses are percentages or 95% CIs.
Effects of BRAF V600E Alone, TERT Promoter Mutation Alone, or the Genetic Duet of Their Coexistence on Loss of RAI Avidity in Recurrent PTC
When the 164 patients were divided into 4 groups (Table 2), loss of RAI avidity was found in 70.3% (45/64), 55.6% (5/9), and 97.4% (37/38) of patients with BRAF mutation alone, TERT mutation alone, and the genetic duet of coexisting BRAF and TERT mutations, versus 30.2% (16/53) of patients with neither mutation (P < 0.001, 0.251, and < 0.001, respectively). These corresponded to odds ratios of 5.39 (95% CI, 2.31–13.13), 2.84 (95% CI, 0.53–16.32), and 81.04 (95% CI, 11.67–3559.83), respectively, which remained similar after adjustment for patient age and sex (Table 2). These analyses showed that BRAF V600E alone had a significant effect on loss of RAI avidity whereas TERT promoter mutation alone had no significant effect and the genetic duet of the coexisting mutations had a robust effect. Similar observations were made when only conventional PTC was analyzed (Table 2). When only TERT C228T (excluding TERT C250T) in relation to BRAF V600E was analyzed, similar genetic effects on RAI avidity loss in recurrent PTC were found (Table 3).
TABLE 2.
Relationship Between BRAF V600E Alone, TERT Promoter Mutation Alone, or Their Coexistence and Loss of Radioiodine Avidity in Recurrent PTC
| Loss of RAI |
Odds ratio |
|||
| Tumor type and mutation status | n | P | Unadjusted | Adjusted* |
| All PTC | ||||
| No mutation | 16/53 (30.2) | Reference | 1.00 | 1.00 |
| BRAF V600E alone | 45/64 (70.3) | <0.001 | 5.39 (2.31–13.13) | 4.92 (2.07–12.20) |
| TERT mutation alone | 5/9 (55.6) | 0.251 | 2.84 (0.53–16.32) | 2.10 (0.28–16.51) |
| BRAF + TERT mutations | 37/38 (97.4) | <0.001 | 81.04 (11.67–3,559.83) | 103.68 (10.77–5,771.67) |
| CPTC | ||||
| No mutation | 8/36 (22.2) | Reference | 1.00 | 1.00 |
| BRAF V600E alone | 39/55 (70.9) | <0.001 | 8.30 (2.93–25.95) | 7.33 (2.54–23.34) |
| TERT mutation alone | 2/3 (66.7) | 0.156 | 6.57 (0.31–427.22) | 20.44 (0.22–2,768.33) |
| BRAF + TERT mutations | 32/32 (100.00) | <0.001 | 136.12 (20.82–+∞) | 179.58 (20.97–+∞) |
Adjusted for patient age and sex.
CPTC = conventional PTC.
TERT promoter mutations here included, collectively, TERT C228T and TERT C250T. Data in parentheses are percentages or 95% CIs.
TABLE 3.
Relationship Between BRAF V600E Alone, TERT C228T Alone, or Their Coexistence and Loss of RAI Avidity in Recurrent PTC
| Loss of RAI |
Odds ratio |
|||
| Tumor type and mutation status | n | P | Unadjusted | Adjusted* |
| All PTC | 103/164 (62.8) | |||
| No mutation | 16/53 (30.2) | Reference | 1.00 | 1.00 |
| BRAF V600E alone | 49/68 (72.1) | <0.001 | 5.96 (2.71–13.14) | 5.63 (2.49–12.72) |
| TERT C228T alone | 5/9 (55.6) | 0.137 | 2.89 (0.69–12.20) | 2.18 (0.39–12.25) |
| BRAF + TERT mutations | 33/34 (97.1) | <0.001 | 76.31 (9.59–607.18) | 127.69 (11.45–1,423.89) |
| CPTC | 81/126 (64.3) | |||
| No mutation | 8/36 (22.2) | Reference | 1.00 | 1.00 |
| BRAF V600E alone | 43/59 (72.9) | <0.001 | 9.41 (3.55–24.89) | 8.83 (3.23–24.09) |
| TERT C228T alone | 2/3 (66.7) | 0.090 | 7.00 (0.56–87.50) | 28.89 (0.77–1,079.55) |
| BRAF + TERT mutations | 28/28 (100.00) | <0.001 | 118.95 (18.10–+∞) | 162.36 (18.63–+∞) |
Adjusted for patient age and sex.
CPTC = conventional PTC.
TERT promoter mutations here included, collectively, TERT C228T and TERT C250T. Data in parentheses are percentages or 95% CIs.
The risk of RAI avidity loss associated with coexisting BRAF and TERT mutations was dramatically higher than the sum of the effects of the 2 mutations individually, suggesting a synergistic interaction between the 2 mutations. Indeed, synergism analysis revealed a robust synergy index of 13.28 (95% CI, 1.54–114.46; P = 0.019) between BRAF V600E and TERT promoter mutations (C228T + C250T) and a similarly robust synergy index of 10.99 (95% CI, 1.28–94.06; P = 0.029) between BRAF V600E and TERT C228T (Table 4).
TABLE 4.
Synergy Test of Interactions Between BRAF V600E and TERT Promoter Mutations in Their Effect on Loss of RAI Avidity in Recurrent PTC
| Risk of loss of radioiodine avidity |
|||
| TERT mutation | PTC type | Synergy index | P |
| C228T and C250T | All PTC | 13.28 (1.54–114.46) | 0.019 |
| CPTC | – | – | |
| C228T only | All PTC | 10.99 (1.28–94.06) | 0.029 |
| CPTC | – | – | |
Synergy index for risk of loss of radioiodine avidity could not be calculated for conventional PTC (CPTC) because loss of radioiodine avidity occurred in all of cases harboring genetic duet of coexisting BRAF and TERT mutations. Data in parentheses are 95% CIs.
Relationship Between Expression Level of Iodide-Handling Thyroid Genes and Genotypes of BRAF V600E and TERT Promoter Mutations in PTC
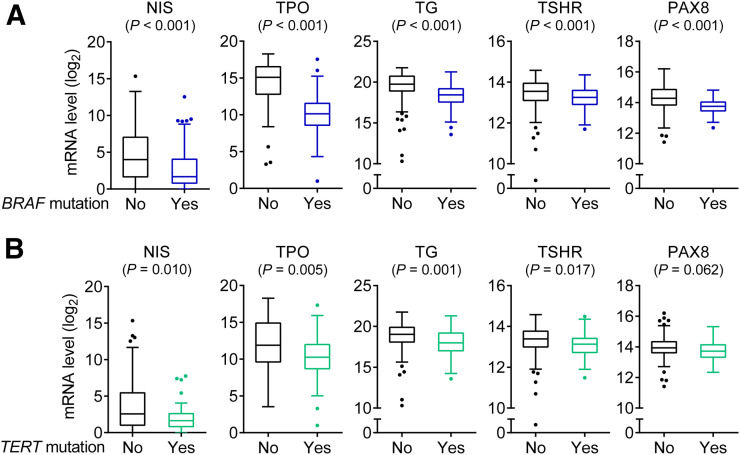
To explore a molecular background for loss of RAI avidity associated with BRAF V600E and TERT mutations in PTC, we used the PTC data in the Cancer Genome Atlas database to analyze the relationship between the genotypes of BRAF V600E and TERT promoter mutations and the expression of classic iodide-handling genes, including NIS, TSHR, TPO, TG, and PAX8, in 386 PTC samples that had information available for the analysis. When the entire cohort was divided into 2 groups of BRAF V600E–positive and –negative cases, the expression of these thyroid iodide-metabolizing genes was significantly lower in the mutation-positive group than the mutation-negative group (Fig. 1A). When the entire cohort was similarly divided into 2 groups of TERT mutation–positive and –negative cases, the expression of the thyroid iodide-metabolizing genes was similarly lower in the mutation-positive group than the mutation-negative group (Fig. 1B).
FIGURE 1.
Box plots of messenger RNA (mRNA) expression of thyroid iodide-handing genes in PTC. (A) Comparison of gene expression levels between BRAF V600E–negative and –positive groups by dividing entire cohort into 2 genotype groups. (B) Comparison of gene expression levels between TERT promoter mutation–negative and –positive groups by dividing entire cohort into 2 genotype groups. Central horizontal lines represent medians, and box boundaries represent interquartile ranges. Sample sizes in BRAF mutation–negative and –positive groups were 160 and 226, respectively. Sample sizes in TERT mutation–negative and –positive groups were 347 and 39, respectively. TERT promoter mutations here included, collectively, TERT C228T and TERT C250T. P values were calculated using 2-sided Wilcoxon–Mann–Whitney test.
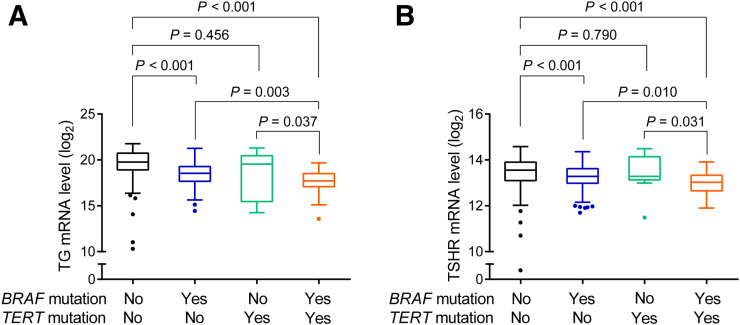
We also examined the relationship between thyroid gene expression and each mutation alone or the genetic duet of coexisting mutations by dividing the cohort into 4 genotype groups. As exemplified by the thyroid TG gene (Fig. 2A) and TSHR gene (Fig. 2B) compared with the group harboring neither mutation, BRAF V600E alone, but not TERT mutation alone, was associated with a significantly lower gene expression level. The gene expression level in the group with the genetic duet of BRAF V600E and TERT mutation was even lower than that in the group harboring neither mutation and, in fact, was the lowest among the 4 genotype groups; it was also significantly lower than the gene expression level in the groups harboring either mutation alone. Similar trends for the relationship between mutations and gene expression were found with NIS, TPO, and PAX8 (Supplemental Fig. 1; supplemental materials are available at http://jnm.snmjournals.org). These patterns of the effects of the 2 gene mutations on thyroid gene expression mirrored those of the effects of the mutations on RAI avidity loss in recurrent PTC.
FIGURE 2.
Box plots of messenger RNA (mRNA) expression of thyroid iodide-handling genes in PTC in different genotype backgrounds. (A) Expression of TG gene. (B) Expression of TSHR gene. For each thyroid gene, entire cohort was divided into 4 genotype groups: no mutation, BRAF V600E alone, TERT promoter mutation alone, and genetic duet of 2 coexisting mutations. Central horizontal lines represent medians, and box boundaries represent interquartile ranges. Sample sizes in no mutation, BRAF V600E, TERT promoter mutation, and genetic duet groups were 149, 198, 11, and 28, respectively. TERT promoter mutations here included, collectively, TERT C228T and TERT C250T. P values were calculated using 2-sided Wilcoxon–Mann–Whitney test.
DISCUSSION
In recent years, BRAF V600E has been introduced as a marker for poor prognosis of PTC, including the risk for loss of RAI avidity in recurrent disease (4,16). In 2005, BRAF V600E was first shown to be associated with loss of RAI avidity in recurrent PTC (10), a phenomenon that has now been widely observed (4). In vitro studies functionally showed a direct link of BRAF V600E to the impairment or even complete silencing of thyroid iodide-metabolizing genes (17), which was subsequently reproduced in in vivo studies (18). BRAF V600E was also shown to be associated with mislocalization of NIS in the cytoplasm of thyroid cancer cells (19). These studies provide a molecular explanation for the association of BRAF V600E with loss of RAI avidity in PTC and support BRAF V600E as an increased risk for loss of RAI avidity in PTC.
TERT promoter mutation has now become recognized as another important oncogene and genetic prognostic marker in thyroid cancer (11). In particular, the initial finding of the coexistence of TERT promoter mutations and BRAF V600E in PTC in 2013 (20), which has been confirmed in many studies (11), stimulated tremendous interest in its potential clinical and biologic relevance. Indeed, the genetic duet of BRAF V600E and TERT promoter mutations has been demonstrated to have a robust synergistic adverse effect on clinical outcomes of PTC, including disease recurrence (12) and patient mortality (13). This robust synergistic oncogenesis-promoting role of the 2 mutations uses a novel molecular mechanism that requires both BRAF V600E and TERT promoter mutations to synergistically promote TERT expression by activating a novel BRAF/MAP kinase/FOS/GABP/TERT pathway system (21). It is thus well established today that BRAF V600E and TERT promoter mutations are the most prominent oncogenic genetic driver events in PTC.
Different from these previous studies, the present study emphasized the role of both BRAF V600E and TERT promoter mutations, particularly the genetic duet of their coexistence, in the RAI avidity status of recurrent PTC. We found that BRAF V600E alone but not TERT mutation alone was significantly associated with loss of RAI avidity in recurrent PTC and that the genetic duet of the 2 coexisting mutations had the most robust effect on loss of RAI avidity in recurrent PTC. We also investigated, for the first time to our knowledge, the role of the 4 genotypes in the silencing of thyroid genes as a molecular mechanism for loss of RAI avidity. We similarly demonstrated that the most robust effect of the genetic duet of BRAF and TERT mutations was on impairment of the expression of thyroid iodide-handling genes. Interestingly, unlike BRAF V600E alone, TERT promoter mutation alone also had no significant effect on thyroid gene expression, mirroring its lack of effect on RAI avidity status. A recent elegant study demonstrated an association of BRAF V600E and TERT promoter mutations with RAI refractoriness in distant metastatic PTC (22). That study also showed that the genetic duet of BRAF V600E and TERT promoter mutations had the most robust effect.
Our findings on the effects of mutations on RAI avidity and thyroid gene expression in the present study are remarkably consistent with previous findings on the patterns of the effects of these mutations on clinicopathologic outcomes for PTC: BRAF V600E alone had a significant effect whereas TERT mutation alone barely had any effect and the genetic duet of the 2 mutations had a robustly synergistic effect on aggressive pathologic behaviors and recurrence of PTC (12). These previous clinicopathologic findings with respect to the genotypes can now be explained by our present findings of the similar effects of the mutations on thyroid gene silencing and RAI avidity loss, which makes the tumor resistant to RAI treatment, thus resulting in poor clinical outcomes (e.g., increased disease recurrence).
A limitation of this study was our inability to use the criteria of Schlumberger et al. to define “RAI refractoriness” (23) because of incomplete information in the old clinical records of our study subjects. For example, our patients were treated mostly with only one dose of 131I at the first occurrence of recurrent disease, when the RAI body scans were obtained and used for the present study. Thus, the criterion of “accumulated dose of 600 mCi” in the definition of Schlumberger et al. could not be used. We therefore defined “loss of RAI avidity” in the present study as no uptake of RAI in one or more recurrent PTC lesions on RAI body scans. Also, either a diagnostic 123I body scan or a 131I posttreatment body scan was used alone to detect RAI avidity in our analyses, with the former being known to be associated with suboptimal sensitivities. These methodologic limitations may explain the relatively high rate of loss of RAI avidity found in the present study. Nevertheless, as a novel and large study that for the first time addressed the role and potential clinical utility of these unique genetic patterns in the context of the challenges associated with RAI treatment of recurrent thyroid cancer, the findings have important clinical ramifications. Better-controlled future studies are required to definitively establish the clinical utility of BRAF V600E and TERT promoter mutations in predicting the failure of RAI treatment of thyroid cancer.
CONCLUSION
We demonstrated that BRAF V600E and TERT promoter mutations are synergistically associated with loss of RAI avidity in recurrent PTC, mirroring a similar pattern of expression impairment of thyroid iodide-handling genes and corresponding to the previously reported similar pattern of clinical outcomes associated with these genotypes. These results explain the association between BRAF V600E and TERT promoter mutations and RAI treatment failure and poor clinical outcomes for PTC. The unique genetic duet of BRAF V600E and TERT promoter mutations represents a genetic background that may help identify PTC patients for whom there is a potential that RAI treatment of recurrent or persistent disease may fail. This fact emphasizes the importance of disease eradication in the initial treatment of PTC harboring the genetic duet to minimize the risk of persistence of RAI-refractory disease or development of RAI-refractory recurrent disease.
DISCLOSURE
This study was supported partly by U.S. National Institutes of Health grants R01CA215142 and R01CA189224 to Mingzhao Xing. Mingzhao Xing receives royalties as a coholder of a licensed U.S. patent related to BRAF V600E mutation in thyroid cancer. No other potential conflict of interest relevant to this article was reported.
Acknowledgments
The results published here are in part based on data generated by the Cancer Genome Atlas Project (http://cancergenome.nih.gov/).
KEY POINTS
QUESTION: What is the role of BRAF V600E and TERT promoter mutations, particularly their coexisting duet, in loss of RAI avidity in recurrent PTC?
PERTINENT FINDINGS: BRAF V600E alone, but not TERT promoter mutation alone, was significantly associated—and the genetic duet of coexisting 2 mutations were particularly robustly associated—with loss of RAI avidity in recurrent PTC as well as impaired expression of thyroid iodide-metabolizing genes in primary PTC.
IMPLICATIONS FOR PATIENT CARE: Knowledge of the status of BRAF V600E and the genetic duet of coexisting BRAF and TERT mutations may help predict the RAI avidity status of recurrent PTC and correspondingly help guide appropriate treatments.
REFERENCES
- 1.Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. JAMA. 2017;317:1338–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 3.Noone AM, Howlader N, Krapcho M, et al. SEER cancer statistics review (CSR), 1975-2015. SEER website. https://seer.cancer.gov/csr/1975_2015/. Updated September 10, 2018. Accessed August 27, 2019.
- 4.Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer. 2013;13:184–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mao Y, Xing M. Recent incidences and differential trends of thyroid cancer in the USA. Endocr Relat Cancer. 2016;23:313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi X, Liu R, Basolo F, et al. Differential clinicopathological risk and prognosis of major papillary thyroid cancer variants. J Clin Endocrinol Metab. 2016;101:264–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasca di Magliano M, Di Lauro R, Zannini M. Pax8 has a key role in thyroid cell differentiation. Proc Natl Acad Sci USA. 2000;97:13144–13149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Presta I, Arturi F, Ferretti E, et al. Recovery of NIS expression in thyroid cancer cells by overexpression of Pax8 gene. BMC Cancer. 2005;5:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xing M, Westra WH, Tufano RP, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab. 2005;90:6373–6379. [DOI] [PubMed] [Google Scholar]
- 11.Liu R, Xing M. TERT promoter mutations in thyroid cancer. Endocr Relat Cancer. 2016;23:R143–R155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xing M, Liu R, Liu X, et al. BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J Clin Oncol. 2014;32:2718–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu R, Bishop J, Zhu G, Zhang T, Ladenson PW, Xing M. Mortality risk stratification by combining BRAF V600E and TERT promoter mutations in papillary thyroid cancer: genetic duet of BRAF and TERT promoter mutations in thyroid cancer mortality. JAMA Oncol. 2017;3:202–208. [DOI] [PubMed] [Google Scholar]
- 14.Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159:676–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersson T, Alfredsson L, Kallberg H, Zdravkovic S, Ahlbom A. Calculating measures of biological interaction. Eur J Epidemiol. 2005;20:575–579. [DOI] [PubMed] [Google Scholar]
- 16.Xing M, Haugen BR, Schlumberger M. Progress in molecular-based management of differentiated thyroid cancer. Lancet. 2013;381:1058–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu D, Hu S, Hou P, Jiang D, Condouris S, Xing M. Suppression of BRAF/MEK/MAP kinase pathway restores expression of iodide-metabolizing genes in thyroid cells expressing the V600E BRAF mutant. Clin Cancer Res. 2007;13:1341–1349. [DOI] [PubMed] [Google Scholar]
- 18.Chakravarty D, Santos E, Ryder M, et al. Small-molecule MAPK inhibitors restore radioiodine incorporation in mouse thyroid cancers with conditional BRAF activation. J Clin Invest. 2011;121:4700–4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riesco-Eizaguirre G, Gutierrez-Martinez P, Garcia-Cabezas MA, Nistal M, Santisteban P. The oncogene BRAF V600E is associated with a high risk of recurrence and less differentiated papillary thyroid carcinoma due to the impairment of Na+/I− targeting to the membrane. Endocr Relat Cancer. 2006;13:257–269. [DOI] [PubMed] [Google Scholar]
- 20.Liu X, Bishop J, Shan Y, et al. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr Relat Cancer. 2013;20:603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu R, Zhang T, Zhu G, Xing M. Regulation of mutant TERT by BRAF V600E/MAP kinase pathway through FOS/GABP in human cancer. Nat Commun. 2018;9:579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang X, Li J, Li X, et al. TERT promoter mutation predicts radioiodine-refractory character in distant metastatic differentiated thyroid cancer. J Nucl Med. 2017;58:258–265. [DOI] [PubMed] [Google Scholar]
- 23.Schlumberger M, Brose M, Elisei R, et al. Definition and management of radioactive iodine refractory differentiated thyroid cancer. Lancet Diabetes Endocrinol. 2014;2:356–358. [DOI] [PubMed] [Google Scholar]