Abstract
Our purpose was to define a clinically useful lower limit of injected dose for 68Ga-prostate-specific membrane antigen (PSMA)-11 PET/CT imaging of prostate cancer. Methods: 68Ga-PSMA-11 PET/CT was performed on 11 patients. PET was acquired in list mode and reconstructed using a 3-min full acquisition, a 2-min acquisition, and a 1-min acquisition to generate images obtained with three thirds (standard dose), two thirds (low dose), and one third (very low dose) of the injected dose, respectively. Overall image quality (5-point scale) was assessed, and the detectability of PSMA-positive lesions was determined by 3 readers and compared with the reference standard. Results: Image quality declined with decreasing dose (mean score of 4.1 ± 0.4 for the standard dose, 3.4 ± 0.7 for the low dose, and 1.9 ± 0.4 for the very low dose; all P < 0.05). Readers 1, 2, and 3 correctly identified the lesions (n = 21) at a rate of 100%, 100%, and 95% with the standard dose; 95%, 81%, and 90% with the low dose; and 71%, 76%, and 59% with the very low dose, respectively. Conclusion: 68Ga-PSMA-11 dose reduction is not feasible without a negative impact on image quality and lesion detectability.
Keywords: image dose, dose reduction, PSMA, prostate cancer
PET/CT using 68Ga-prostate-specific membrane antigen (PSMA)-11 has been adopted rapidly worldwide, given its high sensitivity and specificity for localization of prostate cancer. Thus far, imaging of biochemically recurrent prostate cancer is the most commonly accepted clinical indication for PSMA-ligand PET/CT, as multiple mainly retrospective studies indicate superior detection efficacy compared with conventional imaging and choline-based PET, even at low PSA levels (1–4). For primary high-risk prostate cancer, there is growing evidence that PSMA-ligand PET/CT reliably helps in detecting lymph node and bone metastases (5). Further, emerging data imply that intraprostatic tumor localization, therapy stratification, and therapy monitoring of advanced disease in specific clinical situations might become future clinical indications (6–8).
Despite widely increased use of 68Ga-PSMA-11, the optimal injected dose has not been determined yet and is still under debate. The current joint procedure guideline of the European Association of Nuclear Medicine (EANM) and the Society of Nuclear Medicine and Molecular Imaging (SNMMI) recommends an injected dose of approximately 1.8–2.2 MBq (0.049–0.060 mCi) per kilogram of body weight (BW) (9). However, this recommendation is based on the injected dose in initial publications followed by the rapid adoption of this approach worldwide and not on systematic analyses. 68Ga-PSMA-11 PET availability is limited by 68Ge/68Ga generator capacity and PET scan time. In an attempt to balance availability and quality, different tracer doses for 68Ga-PSMA-11 PET imaging should be investigated to define the lower limit of tracer dose guaranteeing an adequate image quality at a constant scan time.
Therefore, the purpose of this retrospective analysis was to evaluate the relationship between administered tracer dose, image quality, and lesion detection. We approached this evaluation by undersampling of PET list-mode data from standard-dose 68Ga-PSMA-11 PET examinations.
MATERIALS AND METHODS
Patient Population and Data Acquisition/Reconstruction
Eleven consecutive patients (mean ± SD, 69 ± 7 y old; range, 59–81 y) undergoing 68Ga-PSMA-11 PET for primary (n = 2) or biochemically recurrent (n = 9) prostate cancer between August and September 2016 were included in this retrospective analysis. Patient characteristics and injected dose are summarized in Table 1. The retrospective patient selection for this prospective analysis was approved by the Ethics Committee of the Technical University Munich (permit 5665/13).
TABLE 1.
Patient Characteristics
| Patient no. | Age (y) | BW (kg) | Dose (MBq) | PSA (ng/mL) | Primary staging | Restaging | Primary therapy | Further prostate-specific therapy |
| 1 | 64 | 67 | 120 | 0.7 | x | RPE | No | |
| 2 | 67 | 73 | 132 | 8.5 | x | EBRT | ADT until 5 mo before PET/CT | |
| 3 | 77 | 68 | 127 | 28 | x | NA | No | |
| 4 | 59 | 73 | 124 | 0.3 | x | RPE | No | |
| 5 | 76 | 70 | 143 | 14.7 | EBRT | ADT until 2 mo before PET/CT | ||
| 6 | 63 | 74 | 160 | 96.7 | x | NA | No | |
| 7 | 75 | 72 | 128 | 4 | x | RPE | Under ADT | |
| 8 | 81 | 81 | 167 | 0.3 | x | RPE | No | |
| 9 | 60 | 85 | 177 | 0.3 | x | RPE | RTx and ADT until 3 y before PET/CT | |
| 10 | 69 | 81 | 156 | 1.5 | x | RPE | No | |
| 11 | 71 | 100 | 192 | 0.2 | x | RPE | No |
PSA = prostate-specific antigen level; RPE = radical prostatectomy; EBRT = external-beam radiation therapy; ADT = androgen deprivation therapy; NA = not applicable; RTx = radiation therapy.
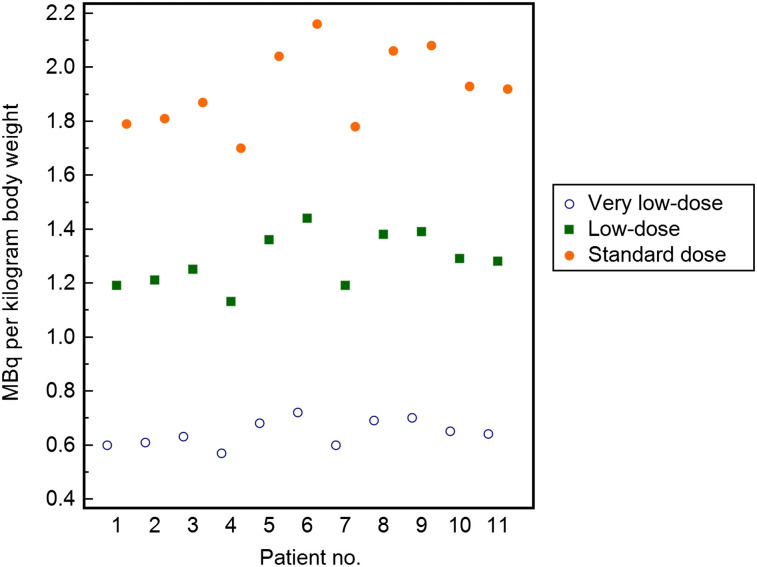
Images were obtained after injection of 68Ga-PSMA-11, which was synthesized as described previously (10). The 68Ga-PSMA-ligand complex solution was injected via an intravenous bolus (mean, 147.8 ± 24.1 MBq; range, 120–192 MBq). The PET acquisition was started at a mean of 55.7 ± 5.7 min after tracer injection (range, 49–66 min). All patients underwent 68Ga-PSMA-11 PET/CT on a Biograph mCT scanner (4-ring TrueV model, 21.6-cm axial field of view; Siemens Medical Solutions) including a diagnostic CT scan. All PET scans were acquired in list mode, including time-of-flight capability, with an acquisition time of 3 min per bed position. The 3-min full acquisition, a 2-min acquisition, and a 1-min acquisition were used to simulate three thirds, two thirds, and one third of the injected dose, referenced as standard dose, low dose, and very low dose. Then, emission data were corrected for randoms, dead time, scatter, and attenuation and were reconstructed iteratively by an ordered-subsets expectation maximization algorithm (4 iterations, 8 subsets), followed by a postreconstruction smoothing gaussian filter (5 mm in full width at half maximum). Standard-dose images were based on injection of 1.9 ± 0.1 (range, 1.7–2.2) MBq of 68Ga-PSMA-11 per kilogram of BW. Undersampling of PET data for the low dose and very low dose equaled a simulated mean dose of 1.3 ± 0.1 (range, 1.1–1.4) MBq/kg of BW and 0.6 ± 0.0 (range, 0.6–0.7) MBq/kg of BW, respectively (Fig. 1).
FIGURE 1.
Graph illustrating applied standard dose and simulated low and very low doses in all patients in MBq/kg of BW.
Image Quality and Lesion Detectability
All 3 PET datasets (standard dose, low dose, and very low dose) were analyzed by 3 independent board-certified nuclear medicine physicians in 3 different reading sessions at an interval of at least 4 wk. For each reading session, the PET datasets were analyzed in a different and predetermined order. For image quality, the PET datasets were rated on a 5-point scale (1, very poor/nondiagnostic; 2, poor; 3, moderate; 4, good; and 5, excellent) with scores 4 and 5 predefined as appropriate for adequate image analysis. Further, the detectability of all lesions was rated on a 3-point scale (0, not detected; 1, weak image contrast; 2, moderate image contrast; and 3, high image contrast) and grouped into 4 categories: local recurrence or primary prostate cancer, lymph node metastases, bone metastases, and other visceral metastases (e.g., lung or liver). Lesions not detected by a reader in a specific dataset were assigned 0. In patients with up to 5 lesions, all lesions were noted by the readers, whereas in patients with more than 5 lesions, up to 5 lesions identified by 1 reader were included in the analysis. We defined that a reduction in dose should result in detection of more than 90% of lesions present in the standard-dose images. The standard of reference was determined by 1 additional reader on standard-dose PET images considering all clinical and imaging data available for the patient.
Statistical Analysis
Statistical analysis was performed using the MedCalc software package, version 12.3.0.0, for Microsoft Windows and IBM SPSS Statistics, version 25, for Microsoft Windows. To summarize information obtained from the 3 readers, mean values were calculated. Mean values with SD, as well as the minimum and maximum of mean ratings, are presented for image quality and lesion contrast scores. Assessments of images generated under different doses were compared using Friedman tests in a first step. Because a significant association between administered dose and image quality or lesion contrast was observed for all performed tests, Wilcoxon signed-rank tests were conducted for pairwise group comparisons. Assessments of images generated under different doses were compared using Wilcoxon signed-rank tests. To analyze the diagnostic performance of all 3 PET datasets (standard dose, low dose, and very low dose), the detection rate was calculated for each reader on the basis of the total number of suspected lesions determined by the standard of reference. All statistical tests were performed 2-sided at a significance level of 5%.
RESULTS
Image Quality
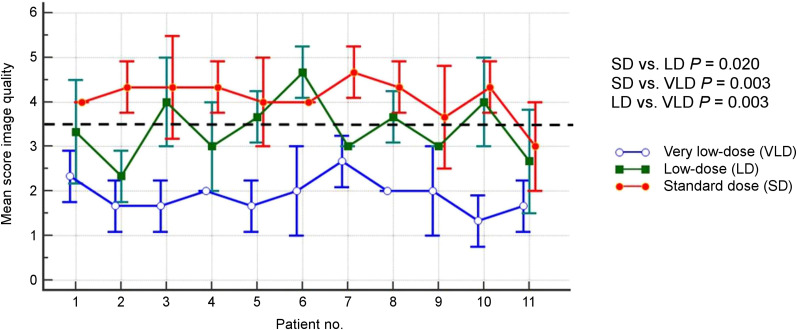
Subjective image quality declined with decreasing simulated tracer dose (mean score of the 3 readers at standard dose, 4.1 ± 0.4 [range, 3.0–4.7]; mean score at the low dose, 3.4 ± 0.7 [range 2.3–4.7]; mean score at the very low dose, 1.9 ± 0.4 [range, 1.3–2.7], respectively; Fig. 2). Mean scores for image quality differed significantly between the 3 dose groups (standard dose vs. low dose, P = 0.020; standard dose vs. very low dose, P = 0.003; low dose vs. very low dose, P = 0.003).
FIGURE 2.
Mean image quality and standard dose for all 3 readers of PET datasets obtained with standard dose, low dose, and very low dose. Dashed line indicates cutoff for adequate image quality.
Lesion Detectability
In total, 21 lesions suggestive of prostate cancer (12 lymph node, 5 local recurrence, 2 primary prostate cancer, and 2 bone) were identified in 9 patients according to the standard of reference. For reader 1, the mean lesion detectability scores were 2.7 ± 0.6, 2.5 ± 0.9, and 1.2 ± 1.1 at the standard dose, low dose, and very low dose, respectively. Lesion detectability significantly differed between the standard dose and the very low dose (P < 0.001) and between the low dose and the very low dose (P < 0.001) but not between the standard dose and the low dose (P = 0.157).
For reader 2, the mean detectability scores were 2.4 ± 0.8, 2.0 ± 1.1, and 1.4 ± 1.1 for the standard dose, low dose, and very low dose, respectively, with a statistically significant difference in observed lesion detectability between the standard dose and the low dose (P = 0.021), the standard dose and the very low dose (P = 0.001), and the low dose and the very low dose (P = 0.046). For reader 3, the mean detectability scores were 2.5 ± 0.8, 2.1 ± 0.9, and 1.4 ± 1.2 for the standard dose, low dose, and very low dose, respectively, with lesion detectability being statistically different only between the standard dose and the very low dose (P = 0.001), not between other doses (standard vs. low dose, P = 0.131; low dose vs. very low dose, P = 0.052). Details on lesion detectability are presented in Table 2, Supplemental Table 1, and Supplemental Fig. 1; supplemental materials are available at http://jnm.snmjournals.org). A representative example is given in Figure 3.
TABLE 2.
Mean Lesion Detectability on PET Datasets Obtained with Standard Dose, Low Dose, and Very Low Dose According to Standard of Reference
| Mean detectability |
|||||
| Patient no. | Lesions (n) | Localization | Standard dose | Low dose | Very low dose |
| 1 | 1 | LN | 3 | 2 | 1 |
| 2 | 2 | LR | 3 | 3 | 2 |
| 3 | 3 | Prostate | 3 | 3 | 2.67 |
| 4 | Bone | 2 | 2.33 | 0.33 | |
| 4 | 5 | LR | 0.67 | 0 | 0 |
| 5 | 6 | LN | 3 | 2.67 | 1.67 |
| 7 | LN | 3 | 2.67 | 1.67 | |
| 8 | LN | 2.67 | 2.67 | 1.67 | |
| 9 | LN | 3 | 2.33 | 1 | |
| 10 | LN | 2 | 1.67 | 1.33 | |
| 6 | 11 | LN | 3 | 2.33 | 2.33 |
| 12 | LN | 2 | 1.33 | 1 | |
| 13 | Prostate | 2 | 2.67 | 0.67 | |
| 14 | LN | 2 | 1.67 | 0 | |
| 15 | LN | 2 | 1.67 | 0 | |
| 7 | 16 | LN | 3 | 3 | 3 |
| 17 | LN | 2.67 | 2.33 | 0.67 | |
| 18 | LR | 3 | 3 | 3 | |
| 8 | 19 | LR | 3 | 1 | 3 |
| 20 | Bone | 1.67 | 1.67 | 0.33 | |
| 10 | 21 | LN | 3 | 3 | 1 |
LN = lymph node; LR = local recurrence.
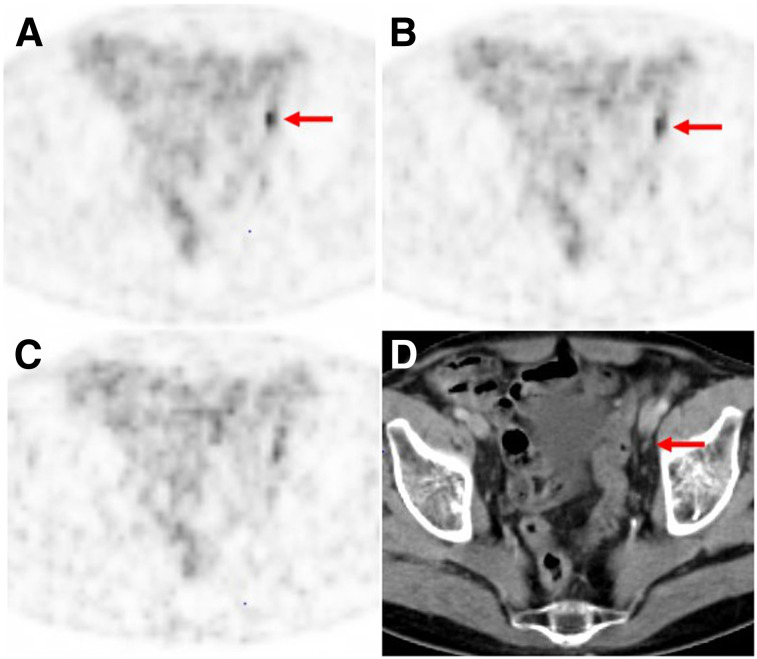
FIGURE 3.
68Ga-PSMA-11 PET/CT images of 75-y-old patient (patient 7) with biochemical recurrence after radical prostatectomy (PSA value, 4 ng/mL at time of imaging). (A and B) Lymph node metastasis (arrows) was classified correctly by all 3 readers on PET performed with standard dose (A) and low dose (B), although mean lesion detectability decreased from 2.67 for standard dose to 2.33 for low dose. (C) On PET performed with very low dose, 2 readers missed this lesion because of increased image noise (mean lesion detectability, 0.67). (D) Corresponding CT image shows small, morphologically unobtrusive lymph node (arrow).
Reader 1 correctly identified 21 lesions (100%) at the standard dose, 20 lesions (95%) at the low dose, and 15 lesions (71%) at the very low dose; reader 2 correctly identified 21 (100%), 17 (81%), and 16 (76%) lesions, respectively; and reader 3 correctly identified 20 (95%), 19 (90%), and 14 (67%) lesions, respectively. No false-positive lesions due to image noise were observed at the very low dose for any reader.
DISCUSSION
The clinical use of 68Ga-PSMA-11 PET has substantially increased since its introduction in 2012, but the lower injected-dose limit that will still allow adequate image quality and lesion detection has not been investigated yet. Both the limited amount of 68Ga from a 68Ge/68Ga generator and the principle of reducing exposure to ionizing radiation as far as possible at equivalent image quality have triggered the present investigation. The results of our study indicate that at constant scan parameters, reduction of the 68Ga-PSMA-11 injected dose below the range recommended in the EANM/SNMMI procedure guideline (1.8–2.2 MBq/kg of BW) is not feasible without having a substantial negative impact on image quality and lesion detectability. Only PET images reconstructed with the full dataset from the 3-min list-mode acquisition representing the total injected dose with a mean of 1.9 ± 0.1 MBq/kg of BW exhibited a score defined as appropriate for reliable image analysis (mean score image quality for the standard dose: 4.1 ± 0.4, vs. 3.4 ± 0.7 and 1.9 ± 0.4 for the low dose and the very low dose, respectively). In addition, lesions were identified correctly at a rate of at least 95% only at the standard dose; the rate was 81%–95% at the low dose and 67%–76% at the very low dose. The only potential scenario for which one could envision the use of the low-dose regimen would be the assessment of a patient with a high tumor burden when the general response to a treatment (progressive disease vs. partial response) is the main indication. This could be a compromise in situations with low generator yield.
It is well known that variations in injected dose may be caused by patient logistics and the short half-life of 68Ga. Further, in our experience, variable elution activities caused by the age of the 68Ge/68Ga generator, the frequency and time of the first and last elutions, and variations in radiochemical product yield still remain a challenge. Therefore, a low output of the 68Ge/68Ga radionuclide generator can cause major problems in institutions with high patient throughput. However, to ensure a high diagnostic quality of the 68Ga-PSMA-11 PET/CT examination, it is not recommended to reduce the injected dose below the EANM/SNMMI-recommended range. Our data indicated that adaptation of patient throughput to generator output (e.g., reduction of number of patients examined) should be considered. Therefore, logistics and technical workflow should be optimized to ensure the minimal possible delay between radiotracer production and injection.
Our study evaluated image quality and lesion detectability at different simulated tracer doses while keeping image acquisition time constant. In theory, reduction of the 68Ga-PSMA-11 dose is possible by extending the PET acquisition time above 2–4 min per bed position as recommended in the EANM/SNMMI procedure guideline (9). Most recently, Lütje et al. evaluated the role of variations in PET acquisition time in patients with local and metastatic prostate cancer undergoing 68Ga-PSMA-11 PET/MRI (11). In hybrid simultaneous PET/MRI, PET acquisition times can easily be extended without relevant changes in the total examination time, as MRI of a specific region usually takes longer than 2–4 min. Use of PET/MRI thus may potentially allow for a reduction in administered tracer dose and, subsequently, a reduction in patient dose. In their study (11), PET image quality increased with rising PET acquisition times, plateauing at acquisition times of at least 4 min. Lesion-based analysis revealed the same trend for tumor lesions of the prostate and lymph node metastases. Shorter image acquisitions were accompanied by increased image noise as an estimate of objective image quality. However, no information on possible dose reduction by extending PET acquisition times was included, as this study aimed only to optimize the PET imaging protocols of 68Ga-PSMA-11 PET/MRI examinations. For 68Ga-PSMA-11 PET/CT, the role of variations in PET acquisition time has not been evaluated yet. However, this approach seems not economically feasible in institutions with high patient throughput and was not the aim of our study.
Our study had several limitations. First, the number of patients analyzed was limited (n = 11), and our patient population was rather inhomogeneous. However, we intended to reflect a routine clinical patient collective including both recurrent and primary prostate cancer at different metastatic stages. Nevertheless, our results are preliminary and have to be confirmed in larger studies (e.g., a controlled prospective trial). Second, imaging was performed using only a single PET scanner model, the Biograph mCT with TrueV. As this model is one of the more sensitive PET scanners available, it is likely that dose reduction on other, less sensitive, scanners will result in even more significant image quality degradation. In addition, histopathology as a standard of reference was not available for most patients. Nevertheless, we primarily aimed for intrapatient comparison between different simulated doses rather than analysis of the performance of 68Ga-PSMA-11 PET as validated by histopathology. Further, we have not investigated whether a higher dose might result in an even higher number of lesions detected than was possible with the standard dose regimen recommended by the EANM/SNMMI guideline.
CONCLUSION
The results of our study indicate that reduction of the 68Ga-PSMA-11 dose below the range recommended in the EANM/SNMMI procedure guideline is not feasible without a substantial negative impact on image quality and lesion detectability. Therefore, we recommend the use of 1.8–2.2 MBq of 68Ga-PSMA-11 per kilogram of BW to maintain high diagnostic quality.
DISCLOSURE
Matthias Eiber received funding from the SFB 824 (DFG Sonderforschungsbereich 824, project B11) from the Deutsche Forschungsgemeinschaft, Bonn, Germany. Siemens Medical Solutions (Erlangen, Germany) supported the application of Biograph mCT as part of an academic collaboration. Wolfgang Fendler received a scholarship from the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG, grant 807122). Johannes Czernin is the recipient of a Challenge Award from the Prostate Cancer Foundation. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is reduction of 68Ga-PSMA-11 possible without a negative impact on image quality and lesion detectability?
PERTINENT FINDINGS: In this study, only PET images reconstructed with the full dataset from the 3-min list-mode acquisition representing the total injected dose with a mean of 1.9 ± 0.1 MBq/kg of BW exhibited a score defined as appropriate for reliable image analysis (mean score image quality for the standard dose: 4.1 ± 0.4, vs. 3.4 ± 0.7 and 1.9 ± 0.4 for the low dose and the very low dose, respectively). In addition, lesions were identified correctly at a rate of at least 95% only for the standard dose; the rate was 81%–95% for the low dose and 67%–76% for the very low dose.
IMPLICATIONS FOR PATIENT CARE: Reduction of the 68Ga-PSMA-11 dose below the EANM/SNMMI-recommended range is not feasible without a substantial negative impact on image quality and lesion detectability.
REFERENCES
- 1.Rauscher I, Duwel C, Haller B, et al. Efficacy, predictive factors, and prediction nomograms for 68Ga-labeled prostate-specific membrane antigen-ligand positron-emission tomography/computed tomography in early biochemical recurrent prostate cancer after radical prostatectomy. Eur Urol. 2018;73:656–661. [DOI] [PubMed] [Google Scholar]
- 2.Perera M, Papa N, Roberts M, et al. Gallium-68 prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: updated diagnostic utility, sensitivity, specificity, and distribution of prostate-specific membrane antigen-avid lesions—a systematic review and meta-analysis. Eur Urol. February 14, 2019 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 3.Afshar-Oromieh A, Zechmann CM, Malcher A, et al. Comparison of PET imaging with a 68Ga-labelled PSMA ligand and 18F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2014;41:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rauscher I, Maurer T, Beer AJ, et al. Value of 68Ga-PSMA HBED-CC PET for the assessment of lymph node metastases in prostate cancer patients with biochemical recurrence: comparison with histopathology after salvage lymphadenectomy. J Nucl Med. 2016;57:1713–1719. [DOI] [PubMed] [Google Scholar]
- 5.Maurer T, Gschwend JE, Rauscher I, et al. Diagnostic efficacy of 68gallium-PSMA positron emission tomography compared to conventional imaging for lymph node staging of 130 consecutive patients with intermediate to high risk prostate cancer. J Urol. 2016;195:1436–1443. [DOI] [PubMed] [Google Scholar]
- 6.Schwarzenboeck SM, Rauscher I, Bluemel C, et al. PSMA ligands for PET imaging of prostate cancer. J Nucl Med. 2017;58:1545–1552. [DOI] [PubMed] [Google Scholar]
- 7.Eiber M, Weirich G, Holzapfel K, et al. Simultaneous 68Ga-PSMA HBED-CC PET/MRI improves the localization of primary prostate cancer. Eur Urol. 2016;70:829–836. [DOI] [PubMed] [Google Scholar]
- 8.Seitz AK, Rauscher I, Haller B, et al. Preliminary results on response assessment using 68Ga-HBED-CC-PSMA PET/CT in patients with metastatic prostate cancer undergoing docetaxel chemotherapy. Eur J Nucl Med Mol Imaging. 2018;45:602–612. [DOI] [PubMed] [Google Scholar]
- 9.Fendler WP, Eiber M, Beheshti M, et al. 68Ga-PSMA PET/CT: joint EANM and SNMMI procedure guideline for prostate cancer imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2017;44:1014–1024. [DOI] [PubMed] [Google Scholar]
- 10.Eder M, Neels O, Muller M, et al. Novel preclinical and radiopharmaceutical aspects of [68Ga]Ga-PSMA-HBED-CC: a new PET tracer for imaging of prostate cancer. Pharmaceuticals (Basel). 2014;7:779–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lütje S, Blex S, Gomez B, et al. Optimization of acquisition time of 68Ga-PSMA-ligand PET/MRI in patients with local and metastatic prostate cancer. PLoS One. 2016;11:e0164392. [DOI] [PMC free article] [PubMed] [Google Scholar]