Abstract
Bone metastases in prostate cancer (PCa) have important prognostic significance, and imaging modalities used for PCa staging should have high sensitivity for detecting such lesions. Prostate-specific membrane antigen (PSMA)–targeted PET radiotracers are promising new agents for imaging PCa. We undertook a head-to-head comparison of PSMA-targeted 2-(3-{1-carboxy-5-[(6-18F-fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (18F-DCFPyL) PET to Na18F PET to determine which modality was more sensitive for the detection of lesions suggestive of bone metastases in a group of patients with metastatic PCa. Methods: Patients with progressive, metastatic PCa were prospectively imaged with both 18F-DCFPyL and Na18F PET/CT, with both scans occurring within 24 h of each other. A consensus 2-reader central review was performed to identify all bone lesions suggestive of sites of PCa involvement on both scans, and maximized SUVs corrected for body weight (SUVmax) and lean body mass (SULmax) were recorded. Soft-tissue lesions were also noted on both scans, and SUVmax, SULmax, and PSMA reporting and data system (RADS) version 1.0 scores were recorded. Data from the 2 scans were compared using a generalized estimating equation. Results: In total, 16 patients meeting all inclusion criteria were enrolled in this study, and 15 of the 16 (93.8%) were imaged with both PET radiotracers. In total, 405 bone lesions suggestive of sites of PCa were identified on at least 1 scan. On 18F-DCFPyL PET/CT, 391 (96.5%) were definitively positive, 4 (1.0%) were equivocally positive, and 10 (2.5%) were negative. On Na18F PET/CT, the corresponding values were 388 (95.8%), 4 (1.0%), and 13 (3.2%). Of the definitively negative lesions on 18F-DCFPyL PET, 8 of 10 (80.0%) were sclerotic and 2 of 10 (20.0%) were infiltrative or marrow-based. Additionally, 12 of 13 (92.3%) of the definitively negative lesions on Na18F PET were infiltrative or marrow-based and 1 of 13 (7.7%) was lytic. Also identified were 78 PSMA-RADS-4, 17 PSMA-RADS-5, and 1 PSMA-RADS-3C soft-tissue lesions. Conclusion: PET/CT imaging using 18F-DCFPyL and Na18F PET had nearly identical sensitivities for the detection of bone lesions in patients with metastatic PCa. As would be expected, PSMA-targeted PET provides more information on soft-tissue disease. There may be little additional value to imaging PCa patients with Na18F after a PSMA-targeted PET scan has already been performed.
Keywords: prostate-specific membrane antigen, PSMA, prostate cancer, bone metastases, 18FNa, PyL, PET/CT
Prostate cancer (PCa) is the most common noncutaneous malignancy in men (1) and frequently metastasizes to bone (2). Despite the considerable importance of accurately identifying patients with bone metastases, anatomic imaging with CT or functional imaging with 99mTc-methylene diphosphonate bone scans have generally shown poor sensitivity for identifying such metastases (3). The relatively low sensitivity of conventional imaging modalities for detecting bone metastases from PCa has prompted an extensive search for tumor-specific PET imaging agents (4). Prostate-specific membrane antigen (PSMA) is a type II transmembrane glycoprotein that is highly expressed by PCa epithelial cells (5–7). Small-molecule ligands that bind to the active site of PSMA and are labeled with radionuclides for PET imaging including 18F (8–10) and 68Ga (11,12) have been extensively investigated as molecular imaging agents for PCa. Our group has focused primarily on 18F-labeled radiotracers because of their favorable imaging characteristics, including a near-ideal 110-min half-life and low positron energy leading to short path-length annihilation and intrinsic high spatial resolution (13,14).
Previously we have shown that the PSMA-targeted PET radiotracer 2-(3-{1-carboxy-5-[(6-18F-fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (18F-DCFPyL) (9) offers a superior lesion detection rate in patients with metastatic PCa as compared with conventional imaging with CT and bone scans (15). Additionally, we have observed a markedly higher bone lesion detection rate with 18F-DCFPyL than with Na18F in a single patient with aggressive castration-resistant metastatic PCa (16). In light of these observations, we sought to further explore the relative detection efficiencies of 18F-DCFPyL PET/CT and Na18F PET/CT for detecting bony metastases in a prospective head-to-head comparative study.
MATERIALS AND METHODS
Patient Population
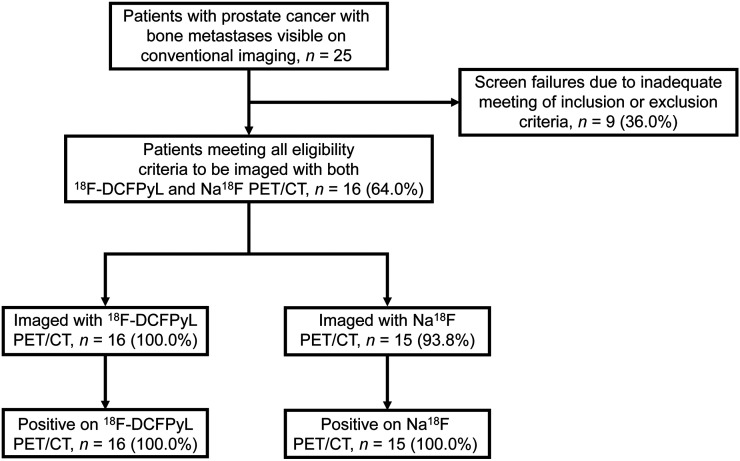
This prospective study (Fig. 1) was approved by the Institutional Review Board of Johns Hopkins Medicine. All participants gave written informed consent and were imaged under a U.S. Food and Drug Administration Investigational New Drug application (121064; ClinicalTrials.gov identifier NCT03497377). Key inclusion criteria were a history of histologic confirmation of PCa; radiologic evidence of new or progressive metastatic PCa on CT, MRI, bone scans, Na18F PET, or 18F-FDG PET; and a rising level of prostate-specific antigen on at least 2 observations taken at least 1 wk apart. Patients could remain on androgen deprivation therapy provided they were on the same regimen before documentation of progressive metastatic disease. Key exclusion criteria were a history of being treated with an investigational drug, biologic, or therapeutic device within 14 d before initial study radiotracer administration; prior radiation or chemotherapy within 2 wk before initial study radiotracer administration; initiation of new therapy for progressive metastatic disease since radiographic documentation of progression; serum creatinine or total bilirubin higher than 3 times the upper limit of normal; or liver transaminases higher than 5 times the upper limit of normal.
FIGURE 1.
Standards for Reporting of Diagnostic Accuracy Studies (STARD) flow diagram.
Imaging Protocol
All patients were imaged with 18F-DCFPyL and Na18F within 24 h. Patients were imaged on either a Biograph mCT 128-slice PET/CT scanner (Siemens Healthineers) or a Discovery RX 64-slice PET/CT scanner (GE Healthcare). For each individual patient, both scans were performed on the same scanner. For the 18F-DCFPyL scans, patients were intravenously injected with no more than 333 MBq (9 mCi) of radiotracer approximately 60 min before image acquisition. For the Na18F scans, patients were intravenously injected with approximately 370 MBq (10 mCi) of radiotracer approximately 60 min before image acquisition. Regardless of the administered radiotracer, the scanners were operated in 3-dimensional emission mode with CT attenuation correction, and images were reconstructed with manufacturer-supplied ordered-subset expectation maximization iterative methods without point-spread function. The field of view was vertex to mid thigh for 18F-DCFPyL and vertex to toes for Na18F.
Image Analysis
All PET/CT scans were collaboratively reviewed by 2 board-certified nuclear medicine specialists with experience in both 18F-DCFPyL and Na18F PET/CT interpretation. A consensus was reached by the reviewers as to the presence of definitive or equivocal lesions on the basis of focal abnormal radiotracer uptake above the background level and outside the expected biodistribution of each radiotracer. The readers evaluated the images from both radiotracers together so as to determine the lesion-level concordance and discordance of the 2 agents. Degenerative change and suspected false-positive lesions were not included in the analysis (Fig. 2; Supplemental Fig. 1 [supplemental materials are available at http://jnm.snmjournals.org]). For each lesion, the maximum SUV was determined for body weight (SUVmax) and lean body mass (SULmax). A spheric volume of interest was also placed over a non–disease-involved site in the right femur such that the maximum area of the sphere on an axial slice matched the axial cross-sectional area of the femur on the same slice. These femur volumes of interest were acquired so that uptake in lesions could be normalized to take into account the intrinsically higher uptake of Na18F in bone. Soft-tissue lesions were also noted on both scans, with SUVmax and SULmax recorded for those lesions appreciated on 18F-DCFPyL PET. All detected lesions were also assigned a PSMA reporting and data system (PSMA-RADS) score (17–20).
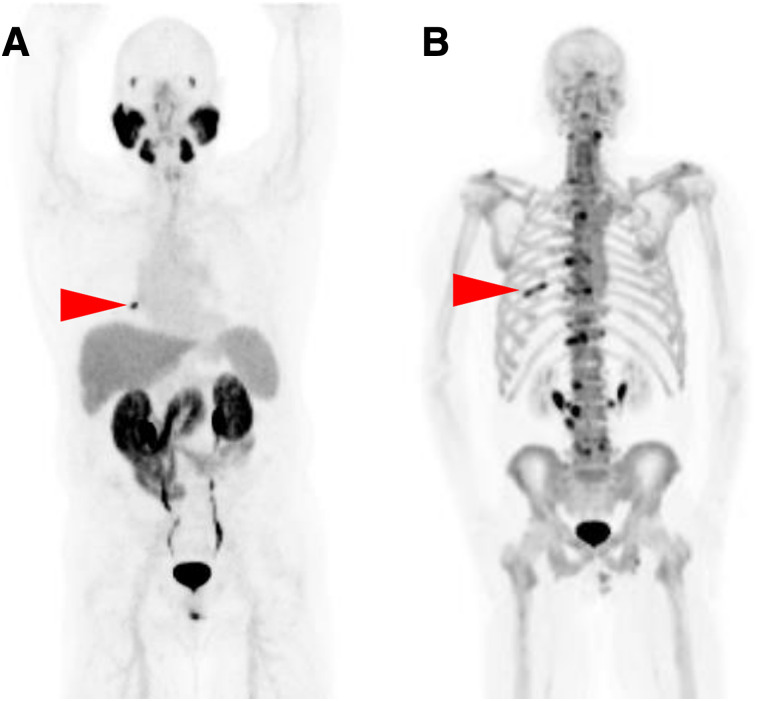
FIGURE 2.
Anterior maximum-intensity projections for both 18F-DCFPyL (A) and Na18F PET/CT (B) demonstrate single bone lesion (arrowheads) suggestive of metastatic disease in right eighth rib. Extensive abnormal uptake in spine on Na18F PET image was attributed to degenerative change by central reviewers.
Statistical Analysis
Because of the mostly descriptive nature of this study, a formal power calculation was not performed. Rather, a sample size of 16 patients was chosen on the basis of the availability of study funding. Descriptive statistics were used for patient demographics and clinical information, the number of lesions identified on each PET modality, and the median and interquartile ranges (IQRs) of the uptake parameters for the bone lesions.
SUVmax and SULmax were normalized by dividing each observation by the mean right femur measurement for that patient, followed by natural log transformation of each normalized value. 18F-DCFPyL and Na18F PET studies were compared for both SUVmax and SULmax (separately) using these log-transformed values in a generalized estimating equation linear regression model, where 18F-DCFPyL SUVmax or SULmax was the independent variable and Na18F SUVmax or SULmax was the dependent variable. When considering lesion detection by either method, dichotomized as definitively positive versus equivocal or definitively negative, a generalized estimating equation logistic regression model was used (21). Two patients had a disproportionately large number of lesions detected (160 and 155 total lesions). In contrast, among the other 14 patients the mean number of lesions was 6.9. Therefore, we repeated the generalized estimating equation linear and logistic regression analyses after excluding those 2 patients.
RESULTS
Between May and November 2016, 16 patients who met all predetermined inclusion criteria were enrolled and imaged in this study, with 1 patient refusing to undergo Na18F PET/CT after having already been imaged with 18F-DCFPyL PET/CT (Fig. 1). An additional 9 screened patients did not meet all inclusion criteria. Supplemental Table 1 lists key demographic and clinical information from the patients in this study. Patients had a mean age of 65.8 y (range, 52–77 y), and 14 of 16 (87.5%) patients were white. At the time of imaging, 14 of 16 (87.5%) patients had been treated, or were being treated, with systemic therapy. The details of the prior or current therapies for the patients are included in Table 1. All patients were in grade group 2 or higher at original diagnosis (6/16 [37.5%] grade group 2–3, 4/16 [25.0%] grade group 4, and 6/16 [37.5%] grade group 5). The median serum prostate-specific antigen level at the time of imaging was 4.4 ng/mL (range, 0.2–224.5 ng/mL), and 10 of 16 (62.5%) patients had undetectable levels of testosterone on current therapy.
TABLE 1.
Number of Each Type of CT Morphologic Lesion Detected by 18F-DCFPyL and Na18F PET
| Modality | Lesion morphology on CT | Definitively positive for uptake on PET | Equivocally positive for uptake on PET | Negative for uptake on PET |
| 18F-DCFPyL PET | Sclerotic | 263 | 4 | 8 |
| Infiltrative/marrow-based | 119 | 0 | 2 | |
| Lytic | 9 | 0 | 0 | |
| Na18F PET | Sclerotic | 272 | 2 | 0 |
| Infiltrative/marrow-based | 108 | 2 | 12 | |
| Lytic | 8 | 0 | 1 |
After review by the 2 readers, 405 bone lesions suggestive of sites of PCa involvement were identified on at least one of the modalities. The patient who refused Na18F imaging was excluded from the imaging portion of the analysis. The numbers of positive, equivocal, and negative lesions with each modality for each patient are tabulated in Supplemental Table 1. With regard to CT morphology, 275 (67.9%) of the lesions were sclerotic, 121 (29.9%) were infiltrative or marrow-based, and 9 (2.2%) were lytic. On 18F-DCFPyL PET, 391 (96.5%) were definitively positive, 4 (1.0%) were equivocally positive, and 10 (2.5%) were negative. On Na18F PET, 388 (95.8%) were definitively positive, 4 (1.0%) were equivocally positive, and 13 (3.2%) were negative. Of the definitively negative lesions on 18F-DCFPyL PET, 8 of 10 (80.0%) were sclerotic and 2 of 10 (20.0%) were infiltrative or marrow-based. Conversely, 12 of 13 (92.3%) of the definitively negative lesions on Na18F PET were infiltrative or marrow-based, whereas 1 of 13 (7.7%) was lytic. Additional details on bone lesion detection by the modalities are found in Tables 1 and 2 and in Supplemental Table 2. Relevant examples of discordant findings between the 2 PET scans are shown in Figures 3 and 4. Additional representative images from patients with varying disease burdens are shown in Supplemental Figure 2.
TABLE 2.
Two-by-Two Table Comparing Lesion Detection Between 18F-DCFPyL and Na18F PET
| Na18F |
||
| 18F-DCFPyL | Positive | Negative |
| Positive | 382 | 13 |
| Negative | 10 | NA |
NA = not applicable.
Equivocal lesions were considered positive for this comparison.
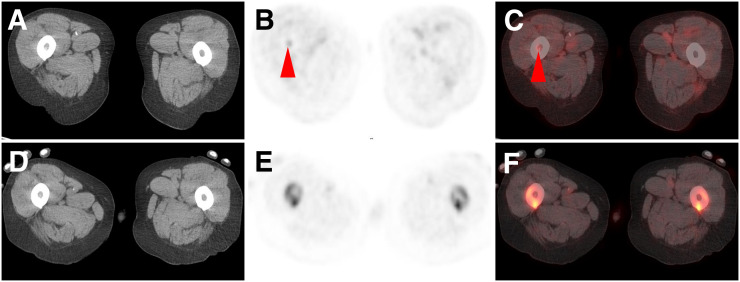
FIGURE 3.
CT (A), 18F-DCFPyL PET (B), 18F-DCFPyL PET/CT (C) and, at same level, CT (D), Na18F PET (E), and Na18F PET/CT (C) axial images of patient with widely metastatic PCa. Both modalities showed numerous lesions, but only 18F-DCFPyL shows uptake (subtle but definitively present and suggestive) in proximal right femur marrow space (arrowheads). This type of lesion constituted most lesions not appreciable with Na18F PET in this study.
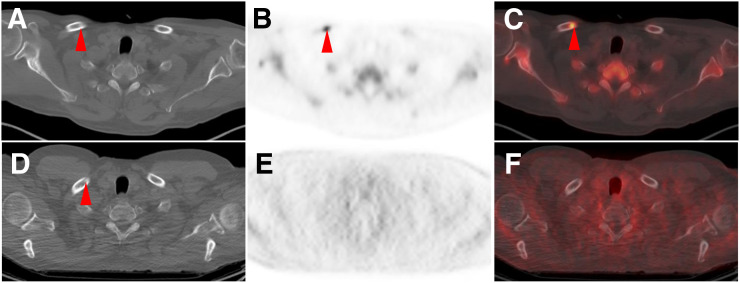
FIGURE 4.
CT (A), Na18F PET (B), Na18F PET/CT (C) and, at same level, CT (D), 18F-DCFPyL PET (E), and 18F-DCFPyL PET/CT (F) axial images of patient with widely metastatic PCa. Both modalities show subtle sclerotic lesion in right clavicle (arrowheads), but only Na18F shows associated intense uptake compatible with site of PCa. Most lesions with Na18F uptake that lacked 18F-DCFPyL uptake were sclerotic.
For those lesions that were definitively or equivocally positive on 18F-DCFPyL, the median SUVmax was 7.4 (IQR, 4.2–12.9) and the median SULmax was 5.5 (IQR, 3.2–9.5). For those lesions that were definitively or equivocally positive on Na18F PET, the median SUVmax was 18.0 (IQR, 11.5–29.3) and the median SULmax was 13.4 (IQR, 8.7–22.6).
In total, 11 of 15 (73.3%) patients had soft-tissue findings suggestive of sites of PCa on 18F-DCFPyL PET/CT. The CT portion of the Na18F PET/CT demonstrated at least 1 suggestive soft-tissue finding in 6 of 15 (40.0%) patients. All suggestive soft-tissue findings on Na18F PET/CT were found to have uptake on 18F-DCFPyL PET/CT, and all patients with such findings had evidence of more widespread soft-tissue involvement on 18F-DCFPyL PET/CT. In total, 78 PSMA-RADS-4 lesions were identified on 18F-DCFPyL PET/CT (i.e., sites of uptake suggestive of PCa involvement without a corresponding CT abnormality), with a range of 0–18 lesions per patient. Correspondingly, 17 PSMA-RADS-5 lesions were identified on 18F-DCFPyL PET/CT with CT abnormalities on Na18F PET/CT, with a range from 0–7 lesions per patient. One additional soft-tissue finding (a thyroid nodule) was categorized as PSMA-RADS-3C (i.e., a finding with uptake that is suggestive of a non-PCa malignancy). In the analysis of SUVs, we observed a statistically significant positive linear association between the natural logarithm of normalized (LnN) 18F-DCFPyL SUVmax and LnN Na18F SUVmax (slope, 0.638; 95% confidence interval [CI], 0.493–0.783; P < 0.0001) among definitively and equivocally positive lesions. There was also a statistically significant positive linear association between LnN 18F-DCFPyL SULmax and LnN Na18F SULmax (slope, 0.636; 95% CI, 0.494–0.779; P < 0.0001). When the 2 patients with the largest number of lesions (160 and 155 lesions) were excluded, the statistically significant positive correlations between the uptake parameters remained: the positive linear association between LnN 18F-DCFPyL SUVmax and LnN Na18F SUVmax demonstrated a slope of 0.877 (95% CI, 0.753–0.9998; P < 0.0001), and the positive linear association between LnN 18F-DCFPyL SULmax and LnN Na18F SULmax demonstrated a slope of 0.765 (95% CI, 0.669–0.861; P < 0.0001).
With regard to lesion detection, 380 of 405 (93.8%) observations were exactly concordant between 18F-DCFPyL and Na18F. When detection was dichotomized as definitively positive versus equivocal or definitively negative, the odds ratio from the generalized estimating equation model was statistically significant (odds ratio, 4.32; 95% CI, 1.04–18.06; P = 0.045). This indicates that a definitively positive lesion on the 18F-DCFPyL PET scan predicts an approximately 4-fold higher likelihood that the lesion would be definitively positive on Na18F. After excluding the 2 patients with a disproportionately large number of lesions, the results were very similar (odds ratio, 4.01; 95% CI, 0.96–16.74; P = 0.057).
DISCUSSION
In this prospective study of 16 patients with metastatic PCa, the PSMA-targeted radiotracer 18F-DCFPyL showed sensitivity similar to that of Na18F for detection of suspected bone lesions, and uptake within those lesions correlated strongly between the 2 modalities. Although this overall result differs from our previously reported case report comparing these 2 modalities in a single patient (16), it is in keeping with some recent reports that have investigated similar hypotheses. For example, Zacho et al. recently performed a prospective study on 68 patients with biochemically recurrent PCa and found that Na18F and a 68Ga-labeled PSMA-targeted PET radiotracer had similarly high rates for identifying PCa bone metastases (22). Further, a patient-level analysis by Dyrberg et al. of patients with PCa undergoing imaging with both Na18F and a 68Ga-labeled PSMA-targeted PET radiotracer also found no difference in diagnostic ability between the agents (23).
Other studies have found Na18F to be advantageous for the detection of bone lesions relative to PSMA-targeted radiotracers. Harmon et al. used the PSMA-targeted agent 18F-DCFBC in a prospective comparison to Na18F and found significantly fewer bone lesions in patients with relatively less advanced disease, although findings between the 2 radiotracers were similar in advanced metastatic castration-resistant PCa (24). In that study, the use of 18F-DCFBC, an agent with high blood-pool activity and lower tumor-to-background ratios than most other PSMA-targeted agents being investigated, may have led to limited lesion detectability relative to Na18F (3). Interestingly, another study that compared a 68Ga-labeled PSMA-targeted PET radiotracer to Na18F also found a lower rate of lesion detection with the PSMA-targeted compound (25). For the patients in that study, radionuclide therapy with either 177Lu-PSMA-617 or 223Ra was being planned, and many of the patients had been pretreated with systemic therapy before the PET scans (25). This may have led to a proportion of the lesions in those patients being effectively treated or healed, limiting the detection efficiency of a tumor-specific radiotracer such as a PSMA-targeted agent.
Expanding on that idea, a potential reason for the relatively similar rates of lesion detection in our current study, compared with our previous case report suggesting that a PSMA-targeted agent would detect more lesions (16), may lie in the type of patients being imaged, how aggressive the phenotypes of their disease were, and the degree to which they had been pretreated. Patients with very aggressive disease that is rapidly progressing may have a disproportionate number of infiltrative or marrow-based bone metastases that are better detected on PSMA-targeted PET. Those patients whose disease course is very advanced may have some bone metastases that have been effectively treated, leading to loss of uptake of PSMA-targeted radiotracers despite persistent uptake of Na18F, thus producing a higher lesion detection efficiency for Na18F. It is also possible that a proportion of such heavily treated patients may have had neuroendocrine differentiation, which is known to lead to downregulation of PSMA and decreased uptake of radiotracers targeting this tumor marker (26).
Beyond bone lesion detection, PSMA-targeted radiotracers offer the distinct advantage relative to Na18F of characterizing soft-tissue lesions. This was certainly the case in this cohort. Most of the patients had evidence of soft-tissue PCa involvement, and in every one of those patients the soft-tissue sites were either exclusively appreciable on 18F-DCFPyL PET or more sites of disease were seen with the PSMA-targeted agent. In addition to the PSMA-RADS-4 lesions that were seen only on the 18F-DCFPyL PET scan (such as subcentimeter lymph nodes; and, by definition, PSMA-RADS-4 lymph nodes should not be appreciable with a non–tumor-specific radiotracer given that they are not abnormal on anatomic imaging), a single PSMA-RADS-3C lesion (17,18) that was suspected (although indeterminate) of being a coexisting malignancy was also noted in 1 patient, demonstrating the ability of PSMA-targeted agents to have uptake in non-PCa malignancies (27).
As with any study, there are limitations to the presented work. Although this was a prospective study, it was a single-center experience with a relatively small number of enrolled patients. Further, all but 2 of the patients had received at least 1 line of systemic therapy, making this a relatively heavily pretreated patient population, potentially confounding the analysis of active versus treated lesions on Na18F. We believe the large number of lesions in the lesion-by-lesion analysis ameliorates some of the disadvantage of having relatively few patients. For these reasons, head-to-head studies of PSMA-targeted radiotracers versus Na18F with patients carefully stratified by prior therapy may help clarify the respective roles of these radiotracers in patients with a variety of disease states. Furthermore, given the relatively widespread disease in these patients, there was no specific standard of care for obtaining tissue confirmation of lesions, and that lack of available histology is another limitation of this study.
CONCLUSION
PSMA-targeted 18F-DCFPyL PET has nearly identical sensitivity to Na18F PET for detecting bone lesions suggestive of PCa in patients with known metastatic disease. However, a unique benefit of PSMA-targeted PET is the ability to image soft-tissue sites of metastasis. Together, these findings argue in favor of the routine use of PSMA-targeted PET imaging in place of Na18F PET for this patient population. Expanding these conclusions to other disease states (e.g., biochemical recurrence) will require further study.
DISCLOSURE
We acknowledge funding from CA134675, CA183031, CA184228, EB024495, the Prostate Cancer Foundation Young Investigator Award, and the European Union’s Horizon 2020 research and innovation program under Marie Sklodowska-Curie grant agreement 701983. Martin Pomper is a coinventor on a U.S. patent covering 18F-DCFPyL and as such is entitled to a portion of any licensing fees and royalties generated by this technology. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict-of-interest policies. Steven Rowe is a consultant to Progenics Pharmaceuticals, the licensee of 18F-DCFPyL. Michael Gorin has served as a consultant to Progenics Pharmaceuticals. Steven Rowe, Michael Gorin, Kenneth Pienta, and Martin Pomper receive research funding from Progenics Pharmaceuticals. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: How does the sensitivity of the PSMA-targeted PET radiotracer 18F-DCFPyL compare with Na18F PET for bone lesion detection in patients with metastatic PCa?
PERTINENT FINDINGS: In a prospective head-to-head comparison of 18F-DCFPyL PET versus Na18F PET in 16 patients with metastatic PCa, the 2 agents detected a nearly identical number of bone lesions (395 positive and equivocal lesions on 18F-DCFPyL PET and 392 positive and equivocal lesions on Na18F PET). There was no statistically significant difference in bone lesion detection efficiency between the 2 modalities. 18F-DCFPyL PET does, however, provide additional information regarding soft-tissue findings.
IMPLICATIONS FOR PATIENT CARE: These findings argue in favor of the routine use of PSMA-targeted PET imaging in place of Na18F PET for bone lesion detection in the staging of PCa patients, once PSMA-targeted agents such as 18F-DCFPyL achieve regulatory approval.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Gandaglia G, Karakiewicz PI, Briganti A, et al. Impact of the site of metastases on survival in patients with metastatic prostate cancer. Eur Urol. 2015;68:325–334. [DOI] [PubMed] [Google Scholar]
- 3.Rowe SP, Macura KJ, Ciarallo A, et al. Comparison of prostate-specific membrane antigen-based 18F-DCFBC PET/CT to conventional imaging modalities for detection of hormone-naive and castration-resistant metastatic prostate cancer. J Nucl Med. 2016;57:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li R, Ravizzini GC, Gorin MA, et al. The use of PET/CT in prostate cancer. Prostate Cancer Prostatic Dis. 2018;21:4–21. [DOI] [PubMed] [Google Scholar]
- 5.Ross JS, Sheehan CE, Fisher HA, et al. Correlation of primary tumor prostate-specific membrane antigen expression with disease recurrence in prostate cancer. Clin Cancer Res. 2003;9:6357–6362. [PubMed] [Google Scholar]
- 6.Marchal C, Redondo M, Padilla M, et al. Expression of prostate specific membrane antigen (PSMA) in prostatic adenocarcinoma and prostatic intraepithelial neoplasia. Histol Histopathol. 2004;19:715–718. [DOI] [PubMed] [Google Scholar]
- 7.Perner S, Hofer MD, Kim R, et al. Prostate-specific membrane antigen expression as a predictor of prostate cancer progression. Hum Pathol. 2007;38:696–701. [DOI] [PubMed] [Google Scholar]
- 8.Cho SY, Gage KL, Mease RC, et al. Biodistribution, tumor detection, and radiation dosimetry of 18F-DCFBC, a low-molecular-weight inhibitor of prostate-specific membrane antigen, in patients with metastatic prostate cancer. J Nucl Med. 2012;53:1883–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szabo Z, Mena E, Rowe SP, et al. Initial evaluation of [18F]DCFPyL for prostate-specific membrane antigen (PSMA)-targeted PET imaging of prostate cancer. Mol Imaging Biol. 2015;17:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giesel FL, Hadaschik B, Cardinale J, et al. F-18 labelled PSMA-1007: biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur J Nucl Med Mol Imaging. 2017;44:678–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Afshar-Oromieh A, Malcher A, Eder M, et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imaging. 2013;40:486–495. [DOI] [PubMed] [Google Scholar]
- 12.Afshar-Oromieh A, Hetzheim H, Kratochwil C, et al. The theranostic PSMA ligand PSMA-617 in the diagnosis of prostate cancer by PET/CT: biodistribution in humans, radiation dosimetry, and first evaluation of tumor lesions. J Nucl Med. 2015;56:1697–1705. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez-Crespo A. Comparison of gallium-68 and fluorine-18 imaging characteristics in positron emission tomography. Appl Radiat Isot. 2013;76:55–62. [DOI] [PubMed] [Google Scholar]
- 14.Gorin MA, Pomper MG, Rowe SP. PSMA-targeted imaging of prostate cancer: the best is yet to come. BJU Int. 2016;117:715–716. [DOI] [PubMed] [Google Scholar]
- 15.Rowe SP, Macura KJ, Mena E, et al. PSMA-based [18F]DCFPyL PET/CT is superior to conventional imaging for lesion detection in patients with metastatic prostate cancer. Mol Imaging Biol. 2016;18:411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rowe SP, Mana-Ay M, Javadi MS, et al. PSMA-based detection of prostate cancer bone lesions with 18F-DCFPyL PET/CT: a sensitive alternative to (99mTc-MDP bone scan and Na18F PET/CT? Clin Genitourin Cancer. 2016;14:e115–e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rowe SP, Pienta KJ, Pomper MG, Gorin MA. Proposal for a structured reporting system for prostate-specific membrane antigen-targeted PET imaging: PSMA-RADS version 1.0. J Nucl Med. 2018;59:479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rowe SP, Pienta KJ, Pomper MG, Gorin MA. PSMA-RADS version 1.0: a step towards standardizing the interpretation and reporting of PSMA-targeted PET imaging studies. Eur Urol. 2018;73:485–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin Y, Werner RA, Higuchi T, et al. Follow-up of lesions with equivocal radiotracer uptake on PSMA-targeted PET in patients with prostate cancer: predictive values of the PSMA-RADS-3A and PSMA-RADS-3B categories. J Nucl Med. 2019;60:511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Werner RA, Bundschuh RA, Bundschuh L, et al. Interobserver agreement for the standardized reporting system PSMA-RADS 1.0 on 18F-DCFPyL PET/CT imaging. J Nucl Med. 2018;59:1857–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanley JA, Negassa A, Edwardes MD, Forrester JE. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol. 2003;157:364–375. [DOI] [PubMed] [Google Scholar]
- 22.Zacho HD, Nielsen JB, Afshar-Oromieh A, et al. Prospective comparison of 68Ga-PSMA PET/CT, 18F-sodium fluoride PET/CT and diffusion weighted-MRI at for the detection of bone metastases in biochemically recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2018;45:1884–1897. [DOI] [PubMed] [Google Scholar]
- 23.Dyrberg E, Hendel HW, Huynh THV, et al. 68Ga-PSMA-PET/CT in comparison with 18F-fluoride-PET/CT and whole-body MRI for the detection of bone metastases in patients with prostate cancer: a prospective diagnostic accuracy study. Eur Radiol. 2019;29:1221–1230. [DOI] [PubMed] [Google Scholar]
- 24.Harmon SA, Bergvall E, Mena E, et al. A prospective comparison of 18F-sodium fluoride PET/CT and PSMA-targeted 18F-DCFBC PET/CT in metastatic prostate cancer. J Nucl Med. 2018;59:1665–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uprimny C, Svirydenka A, Fritz J, et al. Comparison of [68Ga]Ga-PSMA-11 PET/CT with [18F]NaF PET/CT in the evaluation of bone metastases in metastatic prostate cancer patients prior to radionuclide therapy. Eur J Nucl Med Mol Imaging. 2018;45:1873–1883. [DOI] [PubMed] [Google Scholar]
- 26.Tosoian JJ, Gorin MA, Rowe SP, et al. Correlation of PSMA-targeted 18F-DCFPyL PET/CT findings with immunohistochemical and genomic data in a patient with metastatic neuroendocrine prostate cancer. Clin Genitourin Cancer. 2017;15:e65–e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salas Fragomeni RA, Amir T, Sheikhbahaei S, et al. Imaging of nonprostate cancers using PSMA-targeted radiotracers: rationale, current state of the field, and a call to arms. J Nucl Med. 2018;59:871–877. [DOI] [PubMed] [Google Scholar]