Abstract
Background
Understanding the transmissibility and pathogenicity of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is crucial for control policies, but evidence remains limited.
Methods
We presented a systematic and meta-analytic summary concerning the transmissibility and pathogenicity of COVID-19.
Results
A total of 105 studies were identified, with 35042 infected cases and 897912 close contacts. 48.6% (51/105) of studies on secondary transmissions were from China. We estimated a total SIR of 7.8% (95% confidence interval [CI], 6.8%−8.8%), SAR of 6.6% (95% CI, 5.7%−7.5%), and symptomatic infection ratio of 86.9% (95%CI, 83.9%−89.9%) with a disease series interval of 5.84 (95%CI, 4.92–6.94) days. Household contacts had a higher risk of both symptomatic and asymptomatic infection, and transmission was driven between index cases and second-generation cases, with little transmission occurring in second-to-later-generation cases (SIR, 12.4% vs. 3.6%). The symptomatic infection ratio was not significantly different in terms of infection time, generation, type of contact, and index cases.
Conclusions
Our results suggest a higher risk of infection among household contacts. Transmissibility decreased with generations during the intervention. Pathogenicity of SARS-CoV-2 varied among territories, but didn’t change over time. Strict isolation and medical observation measures should be implemented.
Keywords: COVID-19, SARS-CoV-2, Incidence, Virulence, Asymptomatic infections, Meta-analysis
Introduction
An outbreak of novel pneumonia, called coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in December 2019 and spread rapidly worldwide [1]. As of 27 April 2021, the confirmed cases of COVID-19 have surpassed 100 million in over 120 countries and territories, which resulted in more severe conditions than the severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome outbreaks [2]. The transmissibility of the virus and the disease series interval (SI, also known as generation time) are the key elements that affect the ability to contain an outbreak [3]. A series of existing publications estimated the SI, basic reproductive number (R0), and other parameters associated with transmission dynamics based on investigation data or parameters of similar diseases such as SARS, but most have used mathematical models or statistical models combined with agent-based models [4], [5], [6], [7], [8], [9], [10].
Cluster studies are especially useful for transmissibility assessment because the data obtained from contact tracing can be used to calculate transmissibility by analysing exposure history at the individual level. Although a large number of publications have reported COVID-19 clusters, most publications reported only cluster infections and confirmed the existence of human-to-human transmission through close contacts. There is a lack of analysis related to the general transmissibility of COVID-19 and its influencing factors. In addition, the existing literature had conflicting definitions and report criteria, which increased the difficulty of the estimation. To the best of our knowledge, there is no systematic summary or final conclusion on whether variations in the SARS-CoV-2 affect its pathogenicity.
Thus, we conducted a systematic and meta-analytic summary concerning the transmissibility and pathogenicity of COVID-19 reflected by the secondary infection rate (SIR), secondary attack rate (SAR), and symptomatic infection ratio, with the aim of providing constructive suggestions for the prevention and management of COVID-19.
Material and methods
Literature search
We reviewed both English and Chinese publications extracted from English databases including Web of Science and PubMed, and Chinese databases, including China National Knowledge Infrastructure, WANFANG Database, and the VIP Database for Chinese Technical Periodicals. Both English and Chinese databases were searched for relevant articles with the following terms: (COVID-19 OR 2019-nCoV OR SARS-CoV-2 OR novel coronavirus) AND (attack rate OR secondary attack rate OR super spread OR cluster). The included papers were published on or before 17 August 2020. All eligible studies and their references were retrieved and reviewed.
Inclusion and exclusion criteria
Inclusion criteria
We included all relevant scientific studies aimed at studying the SIR of COVID-19. The publication must have reported both three indexes among close contacts, or at least two of the number of close contacts, the number of infected cases among close contacts, and SIR.
Exclusion criteria
-
(1)
Studies not relevant to COVID-19 or COVID-19 studies not associated with SIR
-
(2)
Data not collected first hand (e.g. data collected from media)
-
(3)
Missing original data
-
(4)
Duplicate studies
-
(5)
Theoretical models, reviews, and publications in languages other than English or Chinese
Processing of overlapping data
Overlapping data refers to different studies that share partial or whole samples. The judgement of overlapping data was conducted as follows:
-
a.
Determination of whether the samples were from the same region. If yes,
-
b.
Determination of whether the samples were from the same clusters. If yes,
-
c.
Determination of whether the report period was the same.
The details are presented in Supplementary Fig. 1. Studies without overlapping data were included, while studies with completely overlapping data were excluded from the analysis. When data partially overlapped, studies with the largest sample size were included. Taking integrity into consideration, we first included all relevant studies and executed the above steps during specific analysis.
Definitions
Infected case
Cases were defined as patients with positive laboratory test results, including symptomatic and asymptomatic cases.
Index cases
Cases that met the infected case definition and were the first to be detected and reported in cases of cluster infection.
Secondary infected cases
Cases infected by index cases and met the definition of infected cases. In this review, we refer to all cases ≥ 2 generations away from the index case as secondary cases. According to the generation of cases, we categorised secondary cases as second-generation cases and ≥third-generation cases in the subgroup analysis.
SIR
This is the probability of a close contact becoming infected over the duration of latent infection in a patient. The denominator of the SIR is the total number of close contacts, and the numerator is the number of close contacts who became infected.
SAR: This is the probability of a close contact becoming infected and symptomatic over the duration of latent infection in the case patient. The denominator of the SAR is the total number of close contacts, and the numerator is the number of close contacts who became infected and symptomatic.
Asymptomatic secondary rate
This is the probability of a close contact becoming infected but having no symptoms over the duration of latent infection in the case patient. The denominator is the total number of close contacts, and the numerator is the number of close contacts who became infected but asymptomatic.
Symptomatic infection ratio
This is the number of symptomatic infections per secondary infection. The denominator is the total number of secondary cases, and the numerator is the number of secondary cases with symptoms.
Close contacts
Varied according to the study.
Super spreading event (SSE)
This refers to large clusters of infection in which index cases infected> 10 cases.
SI
This refers to the time interval between onset time in the index case versus the secondary cases.
Quality evaluation and data abstraction
All selected studies were independently assessed by two researchers. If they were uncertain about the quality of the study, the study was reviewed by a third researcher. We assessed the quality of each study based on a modified version of the Newcastle-Ottawa scale [11], [12] (Supplementary Material). We assessed each study’s selection process, comparability, and outcome for a maximum of 11 points. Studies were ranked high if they had a score greater than 66.6%, moderate if they had a score greater than 33.3% and less than or equal to 66.6%, and low if they had a score of less than or equal to 33.3%.
The following data were extracted: first author, location, cluster period, definition of infected cases and close contacts, generation, sex, age, occupation of index and secondary cases, type of contact, number of infected cases, total sample size of close contacts, and SI.
Statistical analyses
Meta-analyses were performed to yield a point estimate and 95% confidence interval (CI) for SIR and SI. I 2 was used to describe the heterogeneity between studies. An I 2>50% indicated significant heterogeneity, in which case a random-effects model was used. Otherwise, a fixed-effects model was used. A subgroup analysis was also conducted to explore heterogeneity. Subgroups were selected based on the characteristics of the index cases and secondary cases. The false discovery rate method was used to adjust the p value when multiple comparisons were conducted within the characteristics of the cases. All tests of significance were at α = 0.05. All analyses were performed in R 3.6.2, using the metafor package [13].
Results
Search results and study characteristics
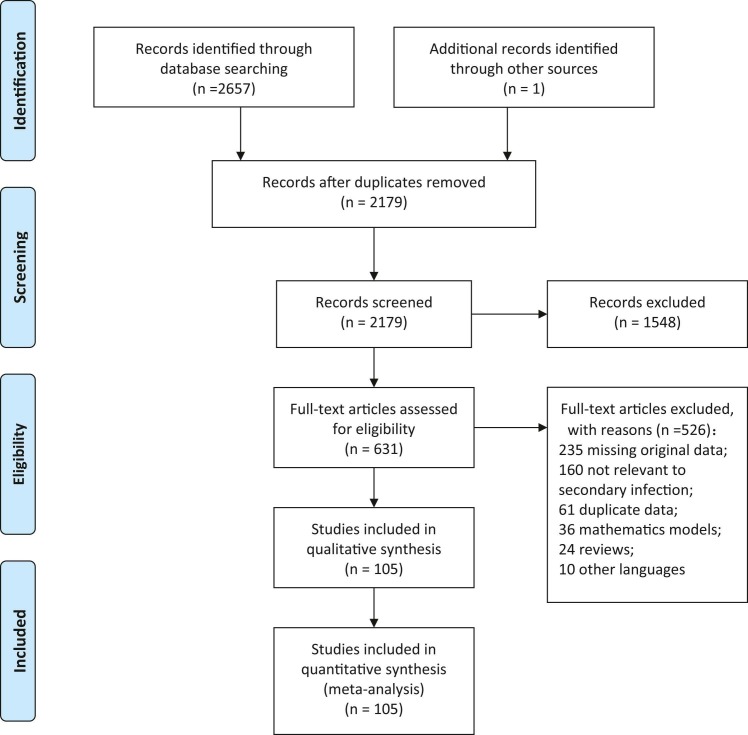
Our search strategy yielded a total of 2657 citations, and one record was identified from the reference list in the included studies. After removal of duplicates and application of the inclusion and exclusion criteria, 105 studies were included in the systematic review and meta-analysis ( Fig. 1, Supplementary Table 1). Of these, 50 were in English and 55 were in Chinese. Among these, 28 studies were of high quality, 66 studies were of moderate quality, and 11 were of low quality (Supplementary Table 1). The average score was 6.13 ± 1.97.
Fig. 1.
Flow diagram of included studies and the selection process.
The majority of studies on secondary transmissions were from China (n = 51), 11 studies were from other countries in Asia except China, seven were from Europe, and three were from North America. A total of 35042 infected cases were identified among 897912 close contacts. An I 2 of 97.15% indicated high heterogeneity among the included studies.
COVID-19 SIR and influencing factors
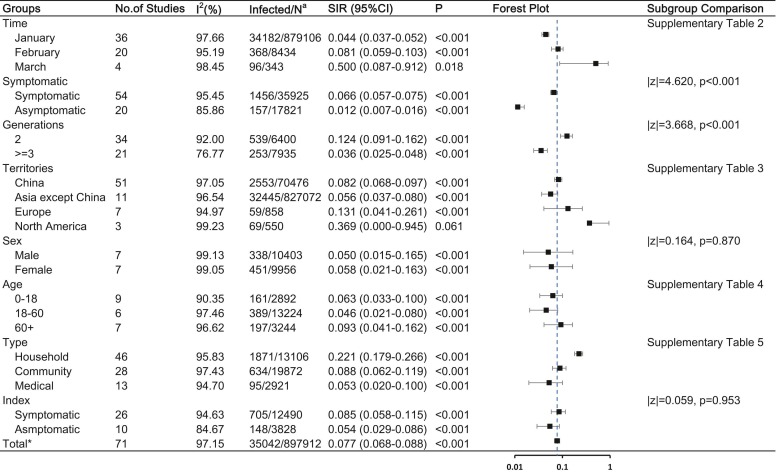
The estimated overall SIR of COVID-19 was 7.8% (95% CI, 6.8–8.8%) with significant heterogeneity (I 2 = 97.15%). Fig. 2 and Supplementary Tables 2–5 summarises the estimated SIRs from the included studies.
Fig. 2.
COVID-19 SIR and the influencing factors Abbreviations: SIR, secondary infected rate; CI, confidence interval Note: a: Number of infected cases / total number of close contacts. * : Having removed studies with overlap data.
The SIR did not significantly differ according to sex, age, and region. However, SIR differed in terms of symptoms and generation of secondary cases, as well as infection time and type of contact. SIR in March (50.0%, 95% CI [8.7%−91.2%]) was higher than that in January (4.4%, 95% CI [3.7%−5.2%]; p = 0.026) and February (8.1%, 95% CI [5.9%−10.3%]; p = 0.037). Second-generation cases had an SIR of 12.4% (95% CI, 9.1–16.2%), which was higher than that of ≥third-generation cases (SIR, 3.6%, 95% CI [2.5–4.8%]; p < 0.001).
Based on the included studies, we defined three types of contact. Household contact refers to contact with families or family members. Community contact refers to contact in work, study, travel, daily communication, or other social activities. Medical contact refers to contact in a medical setting. Household contact was more frequent (n = 46) and had a higher SIR (22.1, 95% CI [17.9–26.6%]) than community contact (SIR, 8.8%, 95% CI [6.2%−11.9%]; p = 0.006) and medical contact (SIR, 5.3%, 95% CI [2.0–10.0%]; p < 0.001).
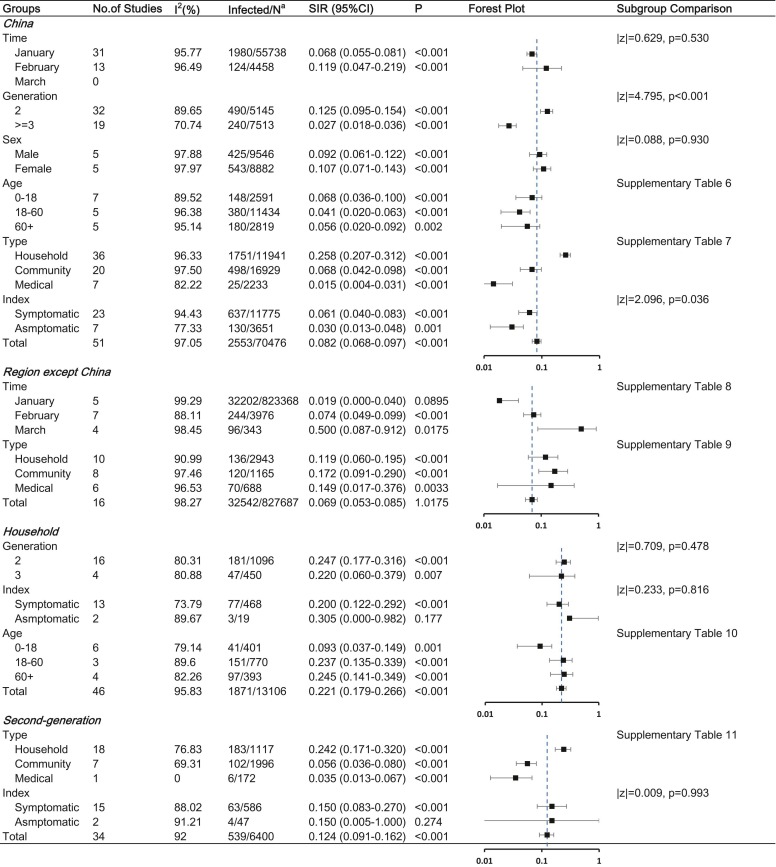
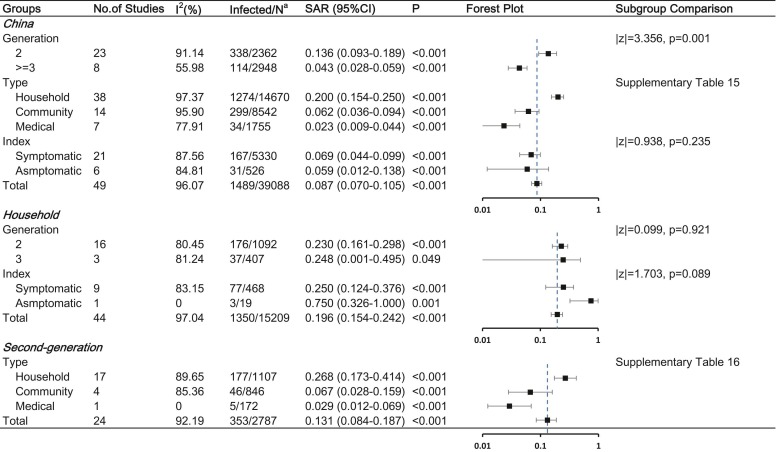
To better explore the factors influencing COVID-19 SIR, we carried out a stratified analysis. In China, symptomatic index cases resulted in more secondary cases than asymptomatic index cases (SIR, 6.1% [95% CI, 4.0%−8.3%] vs. 3.0% [95% CI, 1.3%−4.8%], p = 0.036). The SIR was highest in the household, followed by the community, and least in medical contact. As of the search date, China has had no cluster cases since March. However, regions except China showed no significant difference among the three types of contact and had the largest SIR of 50.0% (95% CI, 8.7–91.2%) in March. As for second-generation cases, household contact (SIR, 24.2%, 95% CI [17.1–32.0%]) resulted in more second-generation cases than community (SIR, 5.6%, 95% CI [3.6–8.0%]; p < 0.001) or medical contact (SIR, 3.5%, 95% CI [1.3–6.7%]; p = 0.010). The details are shown in Fig. 3 and Supplementary Table 6–11.
Fig. 3.
Stratified analysis of COVID-19 SIR Abbreviations: SIR, secondary infected rate; CI, confidence interval Note: a: Number of infected cases / total number of close contacts.
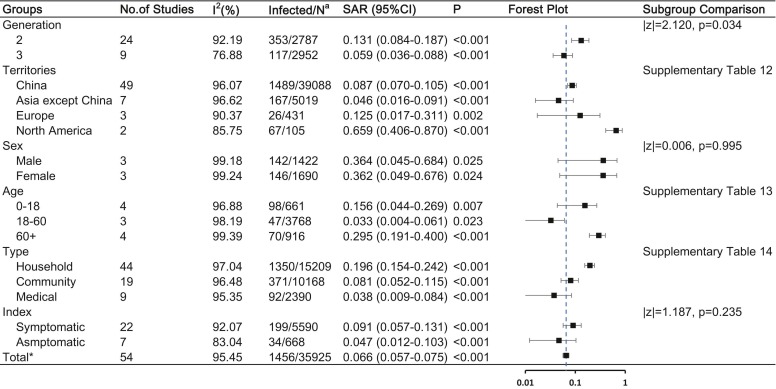
COVID-19 SAR and influencing factors
The estimated overall SAR of COVID-19 was 6.6% (95% CI, 5.7%−7.5%). Fig. 4 and Supplementary Table 12–14 illustrates the result of the subgroup analysis that the generation of secondary cases and type of contact might affect secondary infection. Second-generation cases had an SAR of 13.1% (95% CI, 8.4–18.7%), which was higher than that of ≥third-generation cases (SAR, 5.9%, 95% CI [3.6–8.7%]; p = 0.034). Household contact had a higher SAR (19.6%, 95% CI [15.4–24.2%]) than community contact (SAR, 8.1%, 95% CI [5.2–11.5%]; p = 0.013) and medical contact (SAR, 3.8%, 95% CI [0.9–8.4%]; p < 0.001).
Fig. 4.
COVID-19 SAR and the influencing factors Abbreviations: SAR, secondary attack rate; CI, confidence interval Note: a: Number of infected cases / total number of close contacts. * : Having removed studies with overlap data.
Stratified analysis was also performed ( Fig. 5 and Supplementary Table 15–16). A significant difference between second-generation and ≥third-generation cases was also found in China (SAR, 13.6% [95% CI, 9.3–18.9%] vs. 4.3% [95% CI, 2.8%–−5.9%], p = 0.001). Household contact (SAR, 26.8%, 95% CI [17.3–41.4%]) resulted in more second-generation cases than community (SAR, 6.7%, 95% CI [2.8–15.9%]; p = 0.016) or medical contact (SAR, 2.9%, 95% CI [1.2–6.9%]; p = 0.001).
Fig. 5.
Stratified analysis of COVID-19 SIR Abbreviations: SAR, secondary attack rate; CI, confidence interval Note: a: Number of infected cases / total number of close contacts.
Symptomatic infection ratio of COVID-19
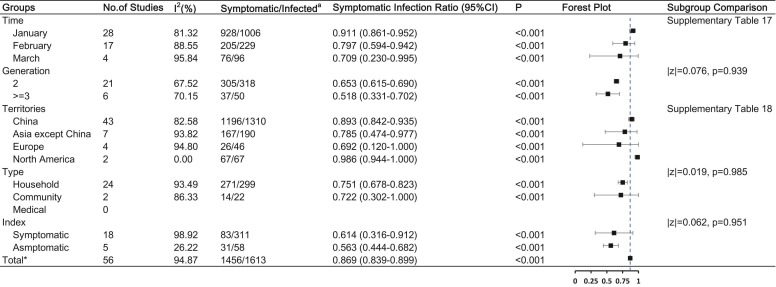
The pooled symptomatic infection ratio of COVID-19 from 56 studies was 86.9% (95% CI, 83.9%−89.9%). The symptomatic infection ratio did not significantly differ according to infection time, generation, type of contact, and index cases ( Fig. 6 and Supplementary Table 17–18). However, a subgroup comparison indicated that the symptomatic infection ratio of COVID-19 in North America (SIR, 98.6%, 95% CI [94.4–100.0%]) was higher than that in China (89.3%, 95% CI [84.2–93.5%]; p = 0.017). The stratified analysis showed that the SIR did not change over time in China (January vs. February, 90.6% vs. 91.3%, p = 0.614).
Fig. 6.
COVID-19 symptomatic infection ratio and the influencing factors Note: a: Number of symptomatic secondary cases / total number of secondary cases. * : Having removed studies with overlap data.
Characteristics of asymptomatic infection
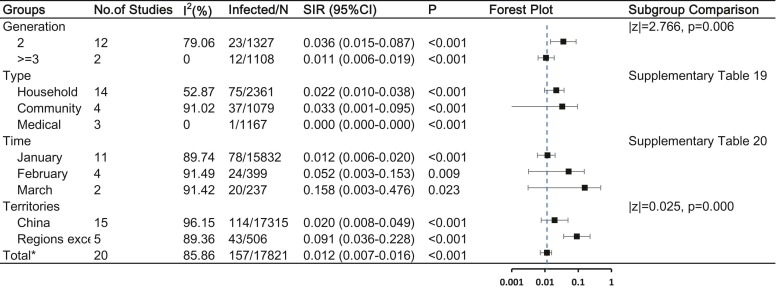
Thirty-three studies reported secondary asymptomatic cases and had an estimated overall asymptomatic secondary rate of 1.2% (95%CI, 0.7–1.6%). Asymptomatic secondary cases occurred more frequently in the second generation and in regions other than China ( Fig. 7 and Supplementary Table 19–20).
Fig. 7.
COVID-19 asymptomatic infection and the influencing factors Abbreviations: SIR, secondary infected rate; CI, confidence interval Note: a: Number of infected cases / total number of close contacts. * : Having removed studies with overlap data.
Characteristics of SSEs
Five studies reported SSEs and had an estimated overall SIR of 40.8% (95% CI, 28.4–53.2%). All SSEs developed from symptomatic index cases. Most secondary cases were symptomatic (SAR, 36.4%; 95% CI, 21.9–51.0%).
COVID-19 SI
We analysed the SI of COVID-19 from 14 reported studies with a total sample size of 973 pairs. The pooled SI was 5.84 ± 1.09 (95% CI, 4.92–6.94) days.
Discussion
With the increasing availability of COVID-19 reports, we reviewed and extracted 105 studies of COVID-19 SIR worldwide. This meta-analysis estimated the COVID-19 SIR, SAR, and symptomatic infection ratio based on existing English and Chinese literature. We estimated a total SIR of 7.8%, SAR of 6.6%, and symptomatic infection ratio of 86.9% with an SI of 5.84 days. The pooled SI was similar to other estimates [14], [15]. Although there are publications summarising the total SIR/SAR [16], [17] or SIR/SAR in specific types [18], [19], [20], [21], our study has the following strengths and innovations. First, we subdivided the definitions of SIR and SAR to better understand the characteristics of COVID-19 transmissibility. Second, a stratified analysis was conducted on the basis of parameter estimation so that we could further explore the source of heterogeneity. Furthermore, we introduced the symptomatic infection ratio to reflect the pathogenicity intensity.
The pooled SIR of COVID-19 was higher in March than in January and February. The stratified analysis indicated that no Chinese clusters were reported since March up until the date we conducted the search, which was probably due to the effective intervention conducted by the Chinese government [22]. This result suggests that public health intervention practices in China can be learned and applied.
The World Health Organisation officially declared COVID-19 a global pandemic on 11 March 2020 and suggested that the focus of the epidemic had shifted to Europe [23]. The stratified analysis confirmed that COVID-19 secondary infection in regions other than China increased from January to March, which was consistent with the COVID-19 condition. We also collected data from John Hopkins University [24], [25] and drew the epidemiological curve (Supplementary Fig. 2), demonstrating that China had a very low incidence since March 2020, while regions besides China were still in the COVID-19 rapid diffusion period, corroborating our viewpoint above. Unfortunately, due to limitations in the dynamic processing of COVID-19 and publication bias, we had no access to reports on cluster infections after March in the search, which may reduce the credibility of the conclusions.
Our study provided further evidence that household contacts had a higher risk of both symptomatic and asymptomatic infections, especially in China, which is consistent with previous studies [17], [21]. Considering the period of peak incidence during the Chinese Spring Festival, the prominence might be due to the tradition of family gatherings and dinners. This suggests that strict isolation and medical observation measures should be implemented for close contacts as soon as possible, and centralised isolation medical observation should be carried out as much as possible to avoid transmission within the family [26].
A previous study proved that the family SAR of the spouses of the family index cases is higher than that of their children, parents, and other family members [27]. However, due to the lack of data, we were unable to explore the associations between family relationships and SAR. Notably, the influence of different contact types was not observed in regions other than China, which might be explained by different lifestyles. For example, religious activities are more popular in regions other than China. An SSE in Buddhist gatherings was also reported in China [28]. There was a total SAR of only 3.8% reported among medical contacts, and 0% in asymptomatic infection. However many regions have reported nosocomial infection [29], [30], [31], [32], [33], [34], and discussions on how to avoid nosocomial infection have been raised and should be paid attention to [35].
It is also worth noting that severe acute respiratory syndrome coronavirus 1 could be transmitted by faecal shedding and air movement of contaminated bio-aerosols, which may have contributed to a 187-person outbreak [36]. Publications have also pointed out that the COVID-19 outbreak may be associated with air conditioning [37], [38]. Given the situation that there is an apparent increase in the infectiousness of SARS-CoV-2 compared to earlier coronaviruses, it would be prudent for hospitals to exercise precautions as part of infection control initiatives, such as covering toilets or using non-flushing commodes, and ensuring robust environmental decontamination protocols [39]. However, the definitions of the different modes were vague and subjective. There was non-negligible information bias during the investigation and data collection. Thus, this conclusion requires further consideration.
The SAR in the second generation was significantly higher than that in the ≥third generation. A possible reason is the early isolation and strict management of close contacts. Contrary to most existing agreements, there was no significant difference in COVID-19 SAR between age groups [16], [40], [41], [42]. A possible reason for this is that due to limitations in the available data, we roughly divided the patients into only three groups. However, previous studies have revealed differences between age groups divided by smaller intervals [43]. On the other hand, a study reported no significant differences in the transmissibility or susceptibility based on the ages of the index cases or contacts [21]. We did not observe a difference in SAR between sexes either, but there have been studies showing that the prevalence of symptomatic COVID-19 was higher in male than in female patients [44].
The symptomatic infection ratio can provide insights into the pathogenicity of SARS-CoV-2. Using meta-analysis, we illustrated that the number of symptomatic cases per secondary case varied among territories, although estimates in Asia, except in China and Europe, were similar on average. North America had a larger symptomatic infection ratio than China, which might be associated with the different virus lineages [45] and types [46], but this still needs further confirmation [47].
The analysis of the asymptomatic infection rate also corroborated that China had fewer asymptomatic secondary cases, but reports from the Chinese Centre for Disease Control and Prevention suggested that the majority of cases in China were asymptomatic since the second half of 2020 [48], [49]. Nevertheless, our study showed that both symptomatic infection ratio and asymptomatic infection rate did not change over time, but it should be noted that our samples were not sufficient.
A systematic review estimated that at least one-third of SARS-CoV-2 infections are asymptomatic, based on available data from 17 November 2020 [50]. Asymptomatic infections have two outcomes: the first is referred to as a true asymptomatic infected case, or someone who received positive results on the nucleic acid test or specific IgM antibody detection but did not show symptoms from the beginning to the end of follow-up. The other outcome, however, is the transitional asymptomatic infection in the disease process, which is also referred to as “presymptomatic” [51]. Thus, the increase in asymptomatic infection rate may be due to improvements in detection technology.
There was no significant difference in the symptomatic infection ratio among the generations. A series of reports of SARS-CoV-2 variants have been published, but evidence of pathogenicity characteristics is still lacking [52], [53]. Based on available data, we failed to establish a relationship between sex and symptomatic infection ratio, but a meta-analysis conducted by Mount Sinai Hospital concluded that male patients were more likely to develop severe symptoms than female patients [54].
There are some limitations to this review. First, publication bias might have omitted some meaningful reports. Although this review focused on studies worldwide, the majority of the data were obtained from China. The epidemic occurred in China first, which contributed to the large scale of relevant publications reported from China as the date of search. Also, we failed to extract virus types for the same reason. Retrospectively, to make full use of the information from existing literature, we designed a process for overlapping data, but this did not eliminate the possibility that data could still be partly duplicated among different studies since we could not completely define the exact data source of each study. Additionally, due to the lack of unified normalisation, the variance in the definitions of variables could lead to information bias during investigation and analysis. For example, we could extract only the date of index cases reported by specific studies as the time of infection; thus, the exposure date, onset date, or diagnosis date all could have been recorded. Although these dates were almost similar for the same case, the possibility that the result was affected cannot be eliminated. Moreover, limited by the available studies, we know for certain the number of patients who were asymptomatic only in retrospect, and this may affect the estimates of SAR and symptomatic infection ratio.
Conclusions
Pooled estimates of SIR and SAR of COVID-19 based on currently available data show a higher infection risk among household contacts. Transmissibility decreased with generations during intervention. Pathogenicity of SARS-CoV-2 varied among territories, but didn’t change over time. Strict isolation and medical observation measures should be implemented, and centralised isolation medical observation should be carried out for close contacts as much as possible to avoid transmission within clusters.
Ethics Statement
The authors confirm that the ethical policies of the journal, as noted on the journal’s author guidelines page, have been adhered to. No ethical approval was required as this is a review article with no original research data.
Funding
This work was supported by the Chinese National Natural Fund (81573258); Science Technology Demonstration Project for Emerging Infectious Diseases Control and Prevention (BE2017749); Jiangsu Provincial Six Talent Peak (WSN-002); Southeast University Novel Coronavirus Research (3225002001C1); and Jiangsu Provincial Key Medical Discipline (ZDXKA2016008).
Role of the funding source
The funders of the study had no role in the study design, data collection and analysis, data interpretation, writing of the article or its publishing. The corresponding author had access to all the data in the study and had final responsibility for the decision to submit the article for publication.
Contribution
NYS and HJ designed the study. NYS and JXH conducted the literature search and review. NYS and JA reviewed citations and extracted data. NYS, QW, and TTC analyzed the data. HJ, NYS, and LQY interpreted the results. HJi, CJB, and HJ critically revised the manuscript for important intellectual content.
Data Availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Declaration of interest
We declare no competing interests.
Acknowledgments
We thank all the reviewers for their suggestions.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jiph.2022.01.015.
Appendix A. Supplementary material
Supplementary material.
.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Organization WH. Weekly epidemiological update on COVID-19–27 April 2021. 2020 22 April. Report No.
- 3.Fraser C., Riley S., Anderson R., Ferguson N. Factors that make an infectious disease outbreak controllable. Proc Natl Acad Sci USA. 2004;101:6146–6151. doi: 10.1073/pnas.0307506101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desjardins M.R., Hohl A., Delmelle E.M. Rapid surveillance of COVID-19 in the United States using a prospective space-time scan statistic: detecting and evaluating emerging clusters. Appl Geogr. 2020;118 doi: 10.1016/j.apgeog.2020.102202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ganyani T., Kremer C., Chen D., Torneri A., Faes C., Wallinga J., et al. Estimating the generation interval for coronavirus disease (COVID-19) based on symptom onset data, March 2020. Eurosurveillance. 2020;25(17):12–19. doi: 10.2807/1560-7917.Es.2020.25.17.2000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark A., Jit M., Warren-Gash C., Guthrie B., Wang H.H.X., Mercer S.W., et al. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. Lancet Glob Health. 2020;8(8):e1003–e1017. doi: 10.1016/s2214-109x(20)30264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.James N., Menzies M. Cluster-based dual evolution for multivariate time series: analyzing COVID-19. Chaos. 2020;30(6) doi: 10.1063/5.0013156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jung S.-M., Akhmetzhanov A.R., Hayashi K., Linton N.M., Yang Y., Yuan B., et al. Real-time estimation of the risk of death from novel coronavirus (COVID-19) infection: inference using exported cases. J Clin Med. 2020;9(2) doi: 10.3390/jcm9020523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mizumoto K., Kagaya K., Chowell G. Early epidemiological assessment of the transmission potential and virulence of coronavirus disease 2019 (COVID-19) in Wuhan City, China, January-February, 2020. BMC Med. 2020;18(1) doi: 10.1186/s12916-020-01691-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park S.W., Bolker B.M., Champredon D., Earn D.J.D., Li M., Weitz J.S., et al. Reconciling early-outbreak estimates of the basic reproductive number and its uncertainty: framework and applications to the novel coronavirus (SARS-CoV-2) outbreak. J R Soc Interface. 2020;17(168) doi: 10.1098/rsif.2020.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet]. [cited 2019/6/4]. Available from: 〈http://www.ohri.ca/programs/clinical_epidemiology/nosgen.pdf〉.
- 12.Fung H.F., Martinez L., Alarid-Escudero F., Salomon J.A., Studdert D.M., Andrews J.R., et al. The household secondary attack rate of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): a rapid review. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3) doi: 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- 14.Rai B., Shukla A., Dwivedi L.K. Estimates of serial interval for COVID-19: a systematic review and meta- analysis. Clin Epidemiol Glob Health. 2021;9:157–161. doi: 10.1016/j.cegh.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. New Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koh W.C., Naing L., Chaw L., Rosledzana M.A., Alikhan M.F., Jamaludin S.A., et al. What do we know about SARS-CoV-2 transmission? A systematic review and meta-analysis of the secondary attack rate and associated risk factors. Plos One. 2020;15(10) doi: 10.1371/journal.pone.0240205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian T., Huo X. Secondary attack rates of COVID-19 in diverse contact settings, a meta-analysis. J Infect Dev Ctries. 2020;14(12):1361. doi: 10.3855/jidc.13256. [DOI] [PubMed] [Google Scholar]
- 18.Fung H.F., Martinez L., Alarid-Escudero F., Salomon J.A., Studdert D.M., Andrews J.R., et al. The household secondary attack rate of SARS-CoV-2: a rapid review. Clin Infect Dis Publ Infect Dis Soc Am. 2020 doi: 10.1093/cid/ciaa1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madewell Z.J., Yang Y., Longini I.M., Jr., Halloran M.E., Dean N.E. Household transmission of SARS-CoV-2 a systematic review and meta-analysis. JAMA Netw Open. 2020;3(12) doi: 10.1001/jamanetworkopen.2020.31756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madewell ZJ, Yang Y., Longini IM, Jr., Halloran ME, Dean NE. Household transmission of SARS-CoV-2: a systematic review and meta-analysis of secondary attack rate. medRxiv: the preprint server for health sciences. 2020. 10.1101/2020.07.29.20164590. [DOI] [PMC free article] [PubMed]
- 21.Thompson H.A., Mousa A., Dighe A., Fu H., Arnedo-Pena A., Barrett P., et al. SARS-CoV-2 setting-specific transmission rates: a systematic review and meta-analysis. Clin Infect Dis: Publ Infect Dis Soc Am. 2021 doi: 10.1093/cid/ciab100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.China NHCotPsRo. COVID-19 Prevention and Control Program (Sixth Edition). 〈http://www.chinacdc.cn/jkzt/crb/zl/szkb_11803/jszl_11815/202003/t20200309_214221.html2020〉.
- 23.WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020 [Internet]. 2020. Available from: 〈https://www.who.int/zh/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020〉.
- 24.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–534. doi: 10.1016/s1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coronavirus Source Data [Internet]. 2020 [cited March17, 2021].
- 26.COVID-19 Epidemiology Group of Emergency response Mechanism CCfDCaP The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Chin J Epidemiol. 2020;41(2):145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [Google Scholar]
- 27.Sun W.W., Ling F., Pan J.R., Cai J., Miao Z.P., Liu S.L., et al. Epidemiological characteristics of 2019 novel coronavirus family clustering in Zhejiang Province. Chin J Prev Med. 2020;54(0) doi: 10.3760/cma.j.cn112150-20200227-00199. E027-E. doi: [DOI] [PubMed] [Google Scholar]
- 28.Ye L.X., Wang H.B., Lu H.C., Chen B.B., Zhu Y.Y., Gu S.H., et al. Investigation of a cluster epidemic of COVID-19 in Ningbo. Zhonghua liu xing Bing xue za zhi = Zhonghua liuxingbingxue zazhi. 2020;41(0) doi: 10.3760/cma.j.cn112338-20200316-00362. [DOI] [PubMed] [Google Scholar]
- 29.Sun H., Lu M., Chen S., Cheng Z., Xiong Y., Wang X. Nosocomial SARS-CoV-2 infection among nurses in Wuhan at a single centre. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ji H., Li L., Huang T., Zhu Y. Nosocomial infections in psychiatric hospitals during the new coronavirus pneumonia outbreak. Eur J Psychiatry. 2020 doi: 10.1016/j.ejpsy.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He Y., Li W., Wang Z., Chen H., Tian L., Liu D. Nosocomial infection among patients with coronavirus disease-2019: a retrospective data analysis of 918 cases from a single center in Wuhan city, China. Infect Control Hosp Epidemiol. 2020:1–6. doi: 10.1017/ice.2020.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vanhems P. Fast nosocomial spread of SARS-CoV2 in a French geriatric unit lyon study group on Covid-19 infection. Infect Control Hosp Epidemiol. 2020:1–4. doi: 10.1017/ice.2020.99. Epub 2020/04/01. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong S.C., Kwong R.T., Wu T.C., Chan J.W.M., Chu M.Y., Lee S.Y., et al. Risk of nosocomial transmission of coronavirus disease 2019: an experience in a general ward setting in Hong Kong. J Hosp Infect. 2020 doi: 10.1016/j.jhin.2020.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Q., Gao Y., Wang X., Liu R., Du P., Wang X., et al. Nosocomial infections among patients with COVID-19, SARS and MERS: a rapid review and meta-analysis. Ann Transl Med. 2020;8(10) doi: 10.21037/atm-20-3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhong Y., Xiao H., Varvares M.A. How to avoid nosocomial spread during tracheostomy for Covid-19 patients. Head Neck. 2020 doi: 10.1002/hed.26167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu I.T., Li Y., Wong T.W., Tam W., Chan A.T., Lee J.H., et al. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N Engl J Med. 2004;350(17):1731–1739. doi: 10.1056/NEJMoa032867. [DOI] [PubMed] [Google Scholar]
- 37.Lu J., Gu J., Li K., Xu C., Su W., Lai Z., et al. COVID-19 outbreak associated with air conditioning in restaurant, Guangzhou, China, 2020. Emerg Infect Dis. 2020;26(7) doi: 10.3201/eid2607.200764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu W.S., Li Y.G., Wei Z.F., Zhou P.H., Lyu L.K., Zhang G.P., et al. Investigation and analysis on characteristics of a cluster of COVID-19 associated with exposure in a department store in Tianjin. Chin J Epidemiol. 2020;41(4):489–493. doi: 10.3760/cma.j.cn112338-20200221-00139. [DOI] [PubMed] [Google Scholar]
- 39.McDermott C.V., Alicic R.Z., Harden N., Cox E.J., Scanlan J.M. Put a lid on it: Are faecal bio-aerosols a route of transmission for SARS-CoV-2? J Hosp Infect. 2020 doi: 10.1016/j.jhin.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mizumoto K., Omori R., Nishiura H. Age specificity of cases and attack rate of novel coronavirus disease (COVID-19). medRxiv [Preprint] March 9, 2020 [cited 2020 Apr 8].
- 41.Viner R.M., Mytton O.T., Bonell C., Melendez-Torres G.J., Ward J., Hudson L., et al. Susceptibility to SARS-CoV-2 infection among children and adolescents compared with adults a systematic review and meta-analysis. JAMA Pediatr. 2021;175(2):143–156. doi: 10.1001/jamapediatrics.2020.4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu Y., Bloxham C.J., Hulme K.D., Sinclair J.E., Tong Z.W.M., Steele L.E., et al. A meta-analysis on the role of children in SARS-CoV-2 in household transmission clusters. Clin Infect Dis Publ Infect Dis Soc Am. 2020 doi: 10.1093/cid/ciaa1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheongju Coronavirus disease-19: summary of 2,370 contact investigations of the first 30 cases in the Republic of Korea. Osong Public Health Res Perspect. 2020;11(2):81–84. doi: 10.24171/j.phrp.2020.11.2.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abate B.B., Kassie A.M., Kassaw M.W., Aragie T.G., Masresha S.A. Sex difference in coronavirus disease (COVID-19): a systematic review and meta-analysis. BMJ Open. 2020;10(10) doi: 10.1136/bmjopen-2020-040129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu J., Cui J., Qian Z., Wang Y., Zhang H., Duan Y., et al. On the origin and continuing evolution of SARS-CoV-2. Natl Sci Rev. 2020;7(6):1012–1023. doi: 10.1093/nsr/nwaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Forster P., Forster L., Renfrew C., Forster M. Phylogenetic network analysis of SARS-CoV-2 genomes. Proc Natl Acad Sci USA. 2020;117(17):9241–9243. doi: 10.1073/pnas.2004999117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mavian C., Pond S.K., Marini S., Magalis B.R., Vandamme A.M., Dellicour S., et al. Sampling bias and incorrect rooting make phylogenetic network tracing of SARS-COV-2 infections unreliable. Proc Natl Acad Sci USA. 2020;117(23):12522–12523. doi: 10.1073/pnas.2007295117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Real time tracking of COVID-19 [Internet]. 2021.
- 49.The latest situation of Novel Coronavirus pneumonia (April 6th, 2021) [Internet]. 2021.
- 50.Oran D.P., Topol E.J. The proportion of SARS-CoV-2 infections that are asymptomatic: a systematic review. Annu Intern Med. 2021 doi: 10.7326/M20-6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zunyou W. Contribution of asymptomatic and pre-symptomatic cases of COVID-19 in spreading virus and targeted control strategies. Chin J Epidemiol. 2020;41(06):801–805. doi: 10.3760/cma.j.cn.112338-20200406-00517. [DOI] [PubMed] [Google Scholar]
- 52.Volz E., Mishra S., Chand M., Barrett JC, Johnson R., Geidelberg L., et al. Transmission of SARS-CoV-2 Lineage B.1.1.7 in England: Insights from linking epidemiological and genetic data. medRxiv. 2021. 10.1101/2020.12.30.20249034. [DOI]
- 53.Tegally H., Wilkinson E., Giovanetti M., Iranzadeh A., Fonseca V., Giandhari J., et al. Emergence and rapid spread of a new severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) lineage with multiple spike mutations in South Africa. medRxiv. 2020. 10.1101/2020.12.21.20248640. [DOI]
- 54.Ueyama H., Kuno T., Takagi H., Krishnamoorthy P., Vengrenyuk Y., Sharma S.K., et al. Gender difference is associated with severity of coronavirus disease 2019 infection: an insight from a meta-analysis. Crit Care Explor. 2020;2(6) doi: 10.1097/cce.0000000000000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.