Abstract
Background
The most dreaded pandemic grappling world now, the Coronavirus Disease 2019 (COVID-19), chiefly involves the respiratory system; nevertheless, it is a multisystem disorder. Its involvement of the hepatic system is considerable; however, still emerging are its clinical implications and the effects on morbidity and mortality.
Aim
The aim of this study is to report on the various aspects of its hepatic involvement by describing the alterations in tests of liver function and its significance in the disease outcome in a cohort of hospitalized COVID-19 patients at a tertiary center in northern India.
Methods
This is a retrospective cohort study conducted in a tertiary-care hospital in northern India. All confirmed hospitalized COVID-19 cases aged 15 and above from Apr to Oct 2020 with no pre-existing liver disease were included. The primary endpoint was death at 28 days. Statistical analysis included descriptive analysis, sensitivity-specificity, and univariable and multivariable regression analysis as well as survival analysis.
Results
A total of 708 patients with COVID-19 fulfilled the inclusion criteria included 561 (79.2%) males and 147 (20.8%) females. The median age was 49 (IQR = 25) years. Mild and moderate/severe disease were seen in 508 (71.8%) and 200 (28.2) patients, respectively. Serum bilirubin, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) were elevated in 6.92%, 69.91%, and 80.22% of patients, respectively. In univariable logistic regression, AST [odds ratio; OR 1.008 95% CI (1.005–1.012) per 1 IU/L increase] and ALT [OR 1.005 95% CI (1.002–1.007) per 1 IU/L increase] were significantly associated with the odds of moderate to severe disease but only AST was significant after adjustment to age, sex, and comorbidity [adjusted odds ratio; aOR 1.007 95% CI (1.003–1.011) per 1 IU/L increase]. Serum albumin was negatively associated with the odds of moderate to severe disease and remained significant in the adjusted model [aOR 0.217 95%CI (0.149–0.316) per 1 g/dL increase].
Ninety-six patients succumbed to illness [case fatality rate; CFR 13.6%). In adjusted Cox Proportional-Hazards Model for mortality, AST [adjusted hazard ratio; aHR 1.002 95% CI (1.000–1.003) per 1 IU/L increase] and serum albumin [aHR 0.396 95% CI (0.285–0.549) per 1 g/dL increase] showed significant association with mortality.
Conclusion
Liver function abnormalities are common in patients with COVID-19. In particular, AST and serum albumin levels are effective predictors of disease severity and mortality and can be used as markers of fatal disease in the management as well as prognostication of COVID-19.
Keywords: COVID-19, liver functions, severe disease, serum albumin, mortality
Abbreviation: ACG, American College of Gastroenterology; ALC, Absolute Lymphocyte Count; ALP, Alkaline Phosphatase; ALT, Alanine Aminotransferase/Alanine Transaminase; ANC, Absolute Neutrophil Count; AST, Aspartate Aminotransferase/Aspartate Transaminase; AUC, Area Under the Curve; COVID-19, Coronavirus Disease 2019; CRP, C Reactive Protein; GGT, Gamma Glutamyl Transferase; Hb, Hemoglobin; IQR, Interquartile Range; NLR, Neutrophil to Lymphocyte Ratio; OR, Odds Ratio; PLT, Platelet; PT, Prothrombin Time; ROC, Receiver Operating characteristic Curve; RT PCR, Real Time Transcription Polymerase chain reaction; SpO2, Saturation of oxygen by pulse oximetry; TLC, Total Leukocyte Count; ULN, Upper Limit of Normal
Coronavirus Disease 2019 (COVID-19), the pandemic of the 21st century, has created a significant burden on health care resources, infrastructures and the world economy. No country has been spared. COVID-19 primarily manifests as a lower respiratory tract infection and involves other organs or organ systems as well such as the gastrointestinal tract, liver and kidneys along with neurological and hematological manifestations.
An initial cohort study describing the epidemiological and clinical characteristics of 99 cases of COVID-19 pneumonia in Wuhan, China revealed elevated levels of the liver enzyme Alanine Aminotransferase (ALT) in 28% of patients and that of total bilirubin in 18% of patients.1 With no direct cytopathic effects being observed on liver biopsy specimens obtained from fatal cases, the exact mechanism of liver damage in COVID-19 was not clear.2,3 Subsequently, numerous studies have shown varying degrees of liver damage in patients with COVID-19 with a prevalence ranging from 15 to 65%.4, 5, 6, 7, 8, 9, 10 However, the studies reporting on liver damage and abnormality of liver function tests associated with COVID-19 are small and mostly inconclusive in reporting the prognostic significance of these abnormalities. The present study reports on the characteristics of liver function abnormalities and their significance in disease severity and outcomes in a large cohort of 708 patients with COVID-19 hospitalized in a tertiary center in northern India.
Materials and Methods
This retrospective cohort study included 708 real-time reverse transcription-polymerase chain reaction (RT PCR) confirmed patients with COVID-19 who were 15 years or older and were hospitalized in a tertiary hospital in northern India over a 6-month period from April to October 2020. Cases that did not have complete data were excluded. Data collected included demographic data, signs and symptoms, comorbidities, vitals at admission inclusive of pulse rate, blood pressure and oxygen saturation by pulse oximetry (SpO2) and data on the outcome (i.e., discharge or death). History of pre-existing liver disease and alcohol consumption was recorded. Routine laboratory tests at admission were done on the day of admission and were inclusive of complete blood count (CBC) including total leukocyte-count (TLC), hemoglobin (Hb), platelet count (PLT), and liver and renal function tests comprising of serum bilirubin, aspartate transaminase(AST), alanine transaminase (ALT), alkaline phosphatase (ALP), gamma glutamyl transferase (GGT), serum total protein, serum albumin and serum creatinine. Viral hepatitis screening was done with hepatitis B surface antigen (HbSAg) and anti-hepatitis C antibody in addition to human immunodeficiency virus (HIV) using rapid test Markers of inflammation such as ferritin and C-reactive protein (CRP) were investigated for, and prothrombin time (PT) was measured, and international normalized ratio (INR) estimated as and when indicated. Those who had abnormal liver functions underwent ultrasound imaging of the abdomen to rule out underlying chronic liver ailments such as hepatic steatosis, biliary obstruction. Markers for hepatitis A and Hepatitis E were done if indicated.
Clearance from the ethical committee was obtained for the study.
Inclusion Criteria
-
1.
Confirmed cases of SARS COVID-19 using RTPCR from posterior pharyngeal and nasal swabs
-
2.
Patients aged 15 years and above
Exclusion Criteria
-
1.
Age less than 14 years
-
2.
All suspected cases or cases with a positive rapid antigen test where COVID-19 RT PCR was negative
-
3.
Associated confounding infectious diseases such as dengue, malaria, UTI at admission.
-
4.
Patients with known HIV infection or those who were newly diagnosed during screening
-
5.
Patients with a known history of liver disease such as cirrhosis of the liver, hepatitis B, hepatitis C, alcoholic liver disease and acute viral hepatitis.
-
6.
Those with a history of significant alcohol abuse defined as those who consume more than two drinks per day for male and more than one drink per day for female
-
7.
All those on hepatotoxic drugs
-
8.
Patients admitted with shock
-
9.
Patient with heart failure
-
10.
Pregnant or lactating females
Primary Endpoint
In-hospital all-cause mortality at 28 days.
Definitions
A confirmed case of COVID-19 was defined as a positive result by the RT-PCR assay of nasal and pharyngeal swab specimens.11 Based on national guidelines, the patients were categorized into mild, moderate, and severe disease using clinical parameters. Mild disease included patients with uncomplicated upper respiratory tract symptoms such as fever, sore throat, cough, malaise, and headache without evidence of breathlessness or hypoxia. Patients with an oxygen-saturation of 90–94% or respiratory rate more than or equal to 24 breaths per minute were categorized as moderate disease, and those who had an oxygen saturation of less than 90% or a respiratory rate of more than 30 breaths per minute with clinical signs of pneumonia constituted severe disease.12 As per American College of Gastroenterology (ACG) guidelines, ALT and AST levels up to 33 IU/L for men and up to 25 IU/L for women were considered normal.13
Statistical Analysis
Demographic, clinical parameters and outcomes were initially analyzed using descriptive statistics. Continuous variables were presented as the median and interquartile range (IQR) and were compared by Mann–Whitney U test. Categorical variables were presented as percentages and frequency distribution and compared by Chi-square tests. For continuous variables, receiver operating characteristic (ROC) curves were plotted for sensitivity-specificity analysis. Multiple logistic regression analysis for disease severity at admission was used to determine the predictive effect of deranged liver function tests with odds ratio (OR) and 95% confidence intervals (CI) being reported. Survival analysis was done using Cox-proportional hazards regression. Univariable models were adopted to evaluate independent risk factors pertaining to mortality. Multivariable analysis using backward elimination was done adjusting for potential confounding effects of other parameters or factors, the results being reported as hazards ratio (HR) and 95% CI. A P-value less than 0.05 was considered to be statistically significant at 95% CI. For data handling and analysis, MS Excel 2016 (Microsoft Corporation) and SPSS 23 for Windows (IBM SPSS Statistics for Windows Version 23.0 Armonk NY: IBM Corp) were used, respectively.
Results
Demographic Characteristics, Comorbidities and Laboratory Investigations at Admission
A total of 708 patients fulfilled the inclusion criteria. The median age of patients was 49 years, interquartile range (IQR) of 25 years with 561 (81.9%) males and 147 (19.4%) females. The median duration of admission was 10 (IQR 8) days. Comorbid conditions were present in 316 patients (44.6%). Among those patients with pre-existing comorbidities, 130 (18.4%) patients had diabetes mellitus and 126 (17.8%) patients had primary hypertension. Mild and moderate/severe disease was observed in 508 (71.8%) and 200 (28.2) patients, respectively. The demographic profile and baseline investigations at admissions are shown in Table 1.
Table 1.
Demographic Profile of Patients with COVID-19.
| Characteristics | N = 708 | |
|---|---|---|
| Age (Years) | Mean | SD |
| 50 | 17 | |
| Median | IQR | |
| 49 | 25 | |
| Sex | N | % |
| Male | 561 | 79.2 |
| Female | 147 | 20.8 |
| Duration of admission (Days) | Mean | SD |
| 11 | 7 | |
| Median | IQR | |
| 11 | 8 | |
| Comorbidities | N | % |
| Any comorbidity | 316 | 44.6 |
| DM | 130 | 18.4 |
| Pr HTN | 126 | 17.8 |
| CAD | 54 | 7.6 |
| Malignancy | 36 | 5.1 |
| COPD | 19 | 2.7 |
| CKD | 13 | 1.8 |
| CVA | 6 | 0.8 |
| Others | 131 | 18.5% |
| Disease severity | N | % |
| Mild | 508 | 71.8% |
| Moderate/Severe/critical | 200 | 28.2% |
| Mortality | N | % |
| Died | 96 | 13.6% |
| Survived | 612 | 86.4% |
| Base Line Laboratory investigations | Median | IQR |
| Hb (g/dL) | 13.4 | 2.81 |
| TLC (/μL) | 5890 | 3615 |
| ANC (/μL) | 2976 | 3255.4 |
| ALC (/μL) | 1127 | 1314 |
| NLR | 2.58 | 3.67 |
| Platelets (/μL) | 1,63,500 | 81,000 |
| Total Bili (mg/dL) | 0.40 | 0.30 |
| AST (IU/L) | 41.00 | 35.8 |
| ALT (IU/L) | 50.00 | 45.8 |
| ALP (IU/L) | 116.7 | 124.8 |
| GGT (IU/L) | 46.43 | 20.4 |
| Total Protein (g/dL) | 7.06 | 0.73 |
| Serum Albumin (g/dL) | 3.6 | 0.8 |
| INR | 1.06 | 0.21 |
| BUN (mg/dL) | 25.00 | 15.0 |
| Serum Creatinine (mg/dL) | 1.05 | 0.41 |
| LDH IU/L | 395.7 | 211 |
| Serum Ferritin (ng/ml) | 265 | 425 |
| CPK IU/L | 116 | 128.3 |
| CRP (mg/dL) | 19.50 | 66 |
| D dimer (ng/ml) | 293 | 313 |
| Blood glucose (mg/dL) | 91 | 60 |
ALC, Absolute Lymphocyte Count; ALP, Alkaline Phosphatase; ALT, Alanine Transaminase; ANC, Absolute Neutrophil Count; Bili, Bilirubin; BUN, Blood Urea Nitrogen; CAD, Coronary Artery Disease; AST, Aspartate Transaminase; CKD, Chronic Kidney Disease; COPD, Chronic Obstructive Pulmonary Disease; CPK, Creatinine Phosphokinase; CRP, C-Reactive Protein; CVA, Cerebrovascular Accident; DM, Diabetes Mellitus; GGT, Gamma Glutamyl Transaminase; Hb, Hemoglobin; INR, International Normalized Ratio; IQR, Interquartile Range; LDH, Lactate Dehydrogenase; NLR, Neutrophil-Lymphocyte Ratio; Pr HTN, Primary Hypertension; TLC, Total Leukocyte-Count; SD, Standard Deviation.
Liver Function Tests at Admission
The median (IQR) levels of total bilirubin, AST, ALT, ALP, GGT, total protein, and serum albumin are presented in Table 2.
Table 2.
Liver Function Tests.
| Liver function tests N = 708 | ||
|---|---|---|
| Bili (mg/dl) | n | Percentage (%) |
| <1 | 659 | 93.07 |
| Abnormal > ULN | 49 | 6.92 |
| 1–2 | 35 | 4.94 |
| 2–3 | 3 | 0.42 |
| >3 | 11 | 1.55 |
| AST (IU/L) | n | Percentage (%) |
| ≤ULN IU/L | 213 | 30.08 |
| Abnormal >ULN | 495 | 69.91 |
| >ULN-≤2 ULN | 301 | 42.51 |
| >2ULN-≥3ULN | 101 | 14.26 |
| >3ULN-≤5ULN | 58 | 8.19 |
| >5ULN-≤10ULN | 18 | 2.54 |
| >10 ULN | 17 | 2.40 |
| ALT (IU/L) | n | Percentage (%) |
| ≤ULN IU/L | 140 | 19.77 |
| Abnormal >ULN | 568 | 80.22 |
| >ULN-≤2 ULN | 304 | 42.93 |
| >2ULN-≥3ULN | 127 | 17.93 |
| >3ULN-≤5ULN | 86 | 12.14 |
| >5ULN-≤10ULN | 42 | 5.93 |
| >10 ULN | 9 | 1.25 |
| Serum Albumin | n | Percentage |
| ≥3.5 g/dL | 501 | 70.76 |
| 2.6–3.4 g/dL | 53 | 7.48 |
| 1.6–2.4 | 151 | 21.32 |
| ≤1.5 | 3 | 0.42 |
AST, Aspartate Transaminase; ALT, Alanine Transaminase; Bili, Bilirubin; ULN, Upper Limit of Normal.
Total bilirubin
Total bilirubin was elevated in 49 patients (6.92%). It was between 1–2 mg/dL in 35 (4.94%) patients, 2–3 mg/dL in 3 (0.42%) patients and more than 3 mg/dL in 11 patients (1.55%).
AST
The AST levels were elevated in 495 (69.91%) patients. Most patients had AST levels between 1–2 times ULN (upper limit of normal), which was observed in 301 patients (42.51%). AST levels were between 2–3 times ULN in 101 (14.26%) patients, 3–5 times ULN in 58 (8.19%) patients, 5–10 times ULN in 18 (2.54%) patients, and more than 10 times ULN in 17 (2.4%) patients.
ALT
The ALT levels were elevated in 568 (80.22%) patients. Among these ALT levels were between 1–2 times ULN were observed in 304 patients (42.93%), 2–3 times ULN in 127 (17.93%) patients, 3–5 times ULN in 86 (12.14%) patients, 5–10 ULN in 42 (5.93%) patients and more than 10 ULN in 9 (1.25%) patients.
ALP and GGT
The ALP and GGT levels were normal in most of the patients and hence not included in the analysis.
Serum albumin
Serum albumin was normal (>3.5 g/dL) in 501 (70.76%) patients. It was between 2.6–3.4 g/dL in 53 (7.48%) patients, 1.6–2.5 g/dL in 151 (21.3%) patients, and less than 1.5 g/dL in 3 (0.42%) patients.
PT and INR
PT and INR were normal in most of the patients.
Liver Function as Predictors of Disease Severity
Among 708 patients, 200 (28.2%) had moderate to severe disease during admission. Significant differences existed in the levels of liver function tests, including serum bilirubin, AST, ALT, and serum albumin, with levels of these markers of liver injury being higher in patients with moderate to severe disease (Table 3). The results of the univariable and multivariable logistic regression for predictors of disease severity are shown in Table 4. In the univariable logistic regression, both AST [OR 1.008 95% CI (1.005–1.012) per 1 IU/L increase] and ALT [OR 1.005 95% CI(1.002–1.007) per 1 IU/L increase] were significantly associated with greater odds of moderate to severe disease at presentation, but only AST remained significantly associated with the odds of moderate to severe disease at presentation after adjustment for age, sex, and comorbidity status [adjusted odds ratio; aOR 1.007 95% CI (1.003–1.011) per 1 IU/L increase). Its association is mild, yet significant. Serum albumin was negatively associated with the odds moderate to severe disease [OR 0.145 95% CI (0.103–0.204) per 1 g/dL increase] and was significant even after adjustment for sex, age, and comorbidity status [aOR 0.217 95% CI (0.149–0.316) per 1 g/dL increase] (Table 4).
Table 3.
Baseline Characteristics, and Comorbidities of 708 RTPCR Confirmed Patients Hospitalized with COVID-19.
| Predictors | Predictor variables | Mild disease (n = 508) | Mod-severe (n = 200) | P value | Died (n = 96) | Survived (n = 612) | P value |
|---|---|---|---|---|---|---|---|
| Age (Years) | Median (±IQR) | 43 (22) | 62.5 (22) | <0.001 | 66 (20.8) | 46 (23) | <0.001 |
| Gender | Male (No %) | 414 (81.5) | 147 (73.5) | 0.023 | 70 (73.0%) | 491 (80.2%) | 0.105 |
| Female (No %) | 94 (18.5) | 53 (26.5) | 26 (27.0%) | 121 (19.8%) | |||
| Severity | Mild disease (SpO2 ≥94%) | – | – | 9 (9.38%) | 499 (81.5%) | <0.001 | |
| Moderate to severe disease (SpO2 <94%) | – | – | 87 (90.6%) | 113 (18.5%) | |||
| Comorbidities | At least one comorbidity (No %) | 180 (35.4) | 136 (68) | <0.001 | 78 (81.3%) | 238 (38.9%) | <0.001 |
| DM (No %) | 68 (13.4) | 62 (31) | <0.001 | 34 (35.4%) | 96 (15.7%) | <0.001 | |
| Pr HTN (No %) | 65 (12.8) | 61 (30.5) | <0.001 | 31 (32.3%) | 95 (15.5%) | <0.001 | |
| CAD (No %) | 27 (5.3) | 27 (13.5) | <0.001 | 15 (15.6%) | 39 (6.4%) | <0.003 | |
| COPD (No %) | 6 (1.2) | 13 (6.5) | <0.001 | 5 (5.2%) | 14 (2.3%) | 0.162 | |
| CKD (No %) | 8 (1.6) | 5 (2.5) | <0.001 | 2 (2.1%) | 11 (1.8%) | 0.693 | |
| Malignancy (No %) | 24 (4.7) | 12 (6) | <0.001 | 12 (12.5%) | 24 (3.9%) | 0.001 | |
| CVA (No %) | 3 (0.6) | 3 (1.5) | <0.001 | 3 (3.1%) | 3 (0.49%) | 0.036 | |
| Vital parameters | SpO2% (Median ± IQR) | 98 (1) | 88 (8) | <0.001 | 84 (12) | 98 (2) | <0.001 |
| Laboratory indices | Sr Bilirubin, mg/dL (Median ± IQR) | 0.4 (0.3) | 0.5 (0.4) | <0.006 | 0.60 (0.5) | 0.40 (0.30) | 0.006 |
| AST, U/L (Median ± IQR) | 38 (27) | 60 (51.0) | <0.001 | 67 (64.8) | 39.00 (31.00) | 0.080 | |
| AST > ULN (No%) | 321 (63.2) | 172 (88) | <0.001 | 87 (90.6) | 408 (6.7) | <0.001 | |
| ALT, U/L (Median ± IQR) | 49 (41.0) | 59 (66.0) | <0.001 | 57.5 (67.00) | 50.0 (42.0) | <0.001 | |
| ALT > ULN (No %) | 396 (78) | 172 (86) | <0.016 | 81 (84.38) | 487 (79.57) | 0.335 | |
| Serum Albumin g/dL (Median ± IQR) | 3.8 (0.8) | 3.2 (0.9) | <0.001 | 2.85 (1.1) | 3.7 (0.7) | <0.001 | |
| TLC, cells/mcL (Median ± IQR) | 5480 (2450.0) | 8220 (6885.0) | <0.001 | 9740.00 (6576.3) | 5650 (2830) | <0.001 | |
| ALC, cells/mcL (Median ± IQR) | 1548.95 (927.9) | 910 (640.75) | <0.001 | 732 (800.6) | 1196 (1290.9) | <0.001 | |
| ANC, cells/mcL (Median ± IQR) | 3104 (1969.6) | 6441 (6770.9) | <0.001 | 7286 (7117.0) | 2774 (2859.0) | <0.001 | |
| NLR (Median ± IQR) | 2.00 (1.7) | 7.08 (8.23) | <0.001 | 10.00 (16.92) | 2.29 (2.23) | <0.001 | |
| Hb, g/dL (Median ± IQR) | 13.8 (2.63) | 12.3 (3.05) | <0.001 | 11.90 (3.26) | 13.67 (2.7) | 0.001 | |
| Platelet, cells/micL (Median ± IQR) | 162,000 (76,750) | 168,000 (121,000) | 0.693 | 1,66,000 (80,000) | 1,50,000 (124,000) | 0.693 | |
| Ferritin, ng/dL (Median ± IQR) | 224.0 (306.0) | 876.0 (734.0) | <0.001 | 934.0 (768.0) | 223.0 (345.0) | <0.001 | |
| CPK IU/L (Median ± IQR)) | 97 (89.5) | 119.5 (166.8) | 0.578 | 177 (301.3) | 114 (108.5) | 0.578 | |
| D dimer ng/mL (Median ± IQR | 262.0 (151.0) | 447.0 (648.0) | <0.001 | 264.0 (214.0) | 741.0 (703.0) | <0.001 | |
| CRP,mg/L (Median ± IQR) | 5.75 (17.0) | 76.75 (43.0) | <0.001 | 87.75 (63.00) | 11.0 (42.00) | <0.001 | |
| BUN (Median ± IQR) | 23.0 (10.0) | 36.0 (37.0) | <0.001 | 53.5 (64.0) | 24.0 (12.0) | <0.001 | |
| Serum Creatinine, mg/dL (Median ± IQR) | 1.02 (0.34) | 1.15 (0.75) | <0.001 | 1.48 (1.34) | 1.01 (0.36) | <0.001 | |
| Blood glucose mg/dL (Median ± IQR) | 95.5 (49.3) | 119.5 (112.8) | <0.001 | 157 (140.3) | 87 (52.5) | <0.001 |
ALC, Absolute Lymphocyte Count; ALP, Alkaline Phosphatase; ALT, Alanine Transaminase; ANC, Absolute Neutrophil Count; AST, Aspartate Transaminase; Bili, Bilirubin; BUN, Blood Urea Nitrogen; CAD, Coronary Artery Disease; CKD, Chronic Kidney Disease; COPD, Chronic Obstructive Pulmonary Disease; CPK, Creatinine Phosphokinase; CRP, C-Reactive Protein; CVA, Cerebrovascular Accident; DM, Diabetes Mellitus; GGT, Gamma Glutamyl Transaminase; Hb, Hemoglobin; INR, International Normalized Ratio; IQR, Interquartile Range; LDH, Lactate Dehydrogenase; NLR, Neutrophil-Lymphocyte Ratio; Pr HTN, Primary Hypertension; TLC, Total Leukocyte-Count.
Table 4.
Predictors of Moderate to Severe Disease- Multiple Logistic Regression for Adjusted and Unadjusted Odds Ratio.
| Predictors | Predictor variables | CRUDE OR (95% CI) | P Value | ADJUSTED OR (95%CI) | P Value |
|---|---|---|---|---|---|
| General | Age | 1.068 (1.055–1.081) | <0.001 | 1.047 (1.032–1.062) | <0.001 |
| Gender (Male vs Female) | 1.588 (1.080–1.081) | <0.019 | 0.879 (0.548–1.409) | 0.592 | |
| Comorbidity | Any comorbidity | 3.872 (2.733–5.486) | <0.001 | 1.387 (0.880–2.184) | 0.159 |
| DM | 2.907 (1.961–4.310) | <0.001 | – | ||
| Pr HTN | 2.991 (2.009–4.453) | <0.001 | – | ||
| CAD | 2.780 (1.587–4.872) | <0.001 | – | ||
| COPD | 5.816 (2.179–15.525) | <0.001 | – | ||
| CKD | 1.603 (0.518–4.959) | <0.413 | – | ||
| Malignancy | 1.287 (0.631–2.626) | <0.488 | – | ||
| CVA | 2.563 (0.513–12.808) | 0.251 | – | ||
| Liver function tests | Serum Bili | 1.101 (0.978–1.240) | 0.112 | 0.880 (0.739–1.049) | 0.154 |
| Albumin | 0.145 (0.103–0.204) | <0.001 | 0.217 (0.149–0.316) | <0.001 | |
| AST | 1.008 (1.005–1.012) | <0.001 | 1.007 (1.003–1.011) | <0.001 | |
| ALT | 1.005 (1.002–1.007) | <0.001 | 0.999 (0.995–1.003) | 0.682 | |
| ALT > ULN | 1.743 (1.110–2.736) | 0.016 | – | ||
| Other Lab parameters | Hb | 0.734 (0.670–0.804) | <0.001 | ||
| ANC | 1.000 (1.000–1.000) | <0.001 | |||
| ALC | 1.001 (1.000–1.001) | <0.011 | |||
| NLR | 1.212 (1.153–1.273) | <0.001 | |||
| Platelets | 1.00 (1.000–1.000) | 0.04 | |||
| BUN | 1.039 (1.029–1.049) | <0.001 | |||
| Serum Creatinine | 1.276 (1.105–1.474) | 0.001 | |||
| LDH | 1.007 (1.006–1.008) | <0.001 | |||
| CPK | 1.001 (1.000–1.001) | 0.036 | |||
| Blood glucose | 1.006 (1.003–1.009) | <0.001 | |||
| Serum Ferritin | 1.003 (1.002–1.004) | <0.001 | |||
| CRP | 1.042 (1.033–1.051) | <0.001 | |||
| D Dimer | 1.002 (1.001–1.002) | <0.001 |
ALC, Absolute Lymphocyte Count; ALT, Alanine Transaminase; ANC, Absolute Neutrophil Count; AST, Aspartate Transaminase; Bili, Bilirubin; BUN, Blood Urea Nitrogen; CAD, Coronary Artery Disease; CKD, Chronic Kidney Disease; CPK, Creatinine Phosphokinase; COPD, Chronic Obstructive Pulmonary Disease; CRP, C-Reactive Protein; CVA, Cerebrovascular Accident; DM, Diabetes Mellitus; Hb, Hemoglobin; NLR, Neutrophil-Lymphocyte Ratio; LDH, Lactate Dehydrogenase; Pr HTN, Primary Hypertension; ULN, Upper Limit of Normal.
Outcome Predictors
A total of 96 patients succumbed to illness (case fatality rate, CFR 13.6%). Significant differences in the levels of total bilirubin, AST, and ALT were observed between survivors and non-survivors (Table 3). The sensitivity and specificity of an abnormal AST to predict mortality was, respectively, 90.6% and 67%. But its positive predictive value (PPV) was only 17.5%, the negative predictive value (NPV) being 95.73%. Similarly, an abnormal ALT predicted mortality with a sensitivity of 84.4% and a specificity of 89.3%, with PPV of 14.3% and NPV of 89.3%.
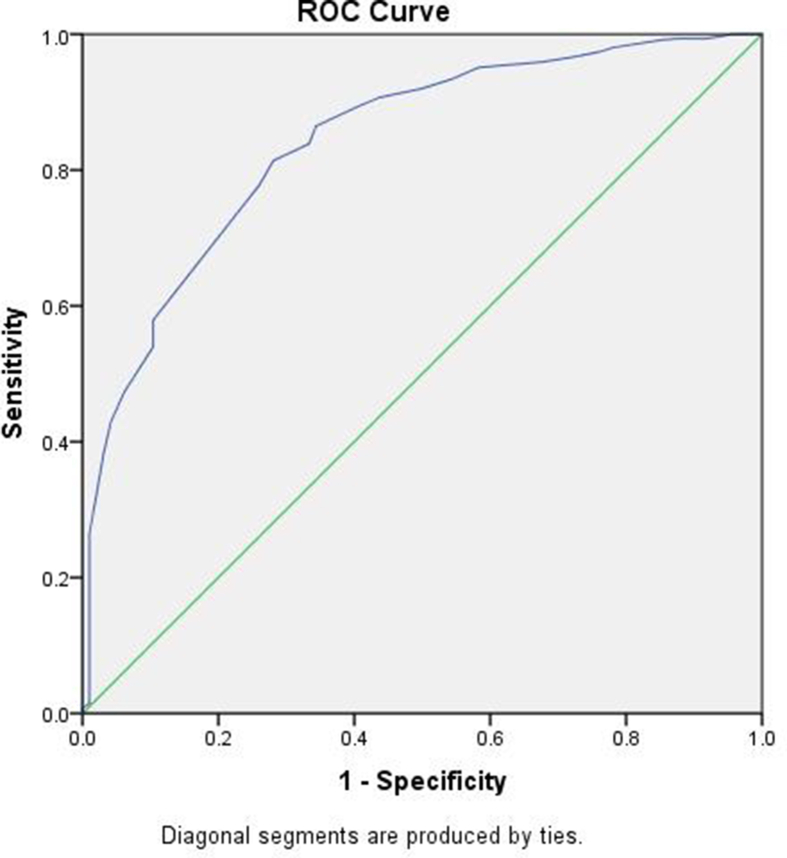
The results of the univariable and multivariable Cox proportional hazard regression are presented in Table 5. Among Liver function tests in univariable Cox regression analysis, serum bilirubin, AST, ALT, and serum albumin were significant predictors of fatal outcome. However, after adjustment for age, sex, comorbidities and SpO2 at admission, only AST was significantly associated with fatality [adjusted hazard ratio; aHR 1.002 95% CI (1.000–1.003) per 1 IU/L increase]. The ROC curve revealed an area under the curve (AUC) of 0.726, indicating moderate prognostic value (Figure 1). Higher serum albumin was a negative predictor of mortality that remained statistically significant in the multivariable analysis [aHR 0.396 95% CI (0.285–0.549) per 1 g/dL increase] (Table 5). The ROC curve revealed an AUC of 0.842 for survival indicating a relatively good prognostic survival value (Figure 2).
Table 5.
Predictors of Mortality-Univariable and Multivariable Cox Proportional Hazard Regression.
| Predictors | Predictor variables | CRUDE HR (95% CI) | P Value | ADJUSTED HR (95%CI) | P Value |
|---|---|---|---|---|---|
| General | Age | 1.055 (1.043–1.067) | <0.001 | 1.020 (1.005–1.035) | 0.007 |
| Gender | 1.660 (1.057–2.606) | <0.028 | 0.718 (0.431–1.197) | 0.204 | |
| Mild Disease vs Severe disease | 27.861 (14.017–55.376) | <0.001 | |||
| Comorbidity | Any comorbidity | 6.374 (3.815–10.651) | <0.001 | 2.516 (1.417–4.466) | 0.002 |
| DM | 2.963 (1.945–4.513) | <0.001 | – | ||
| Pr HTN | 2.178 (1.419–3.345) | <0.001 | – | ||
| CAD | 2.612 (1.504–4.538) | <0.001 | – | ||
| COPD | 2.723 (1.104–6.721) | <0.030 | – | ||
| CKD | 1.147 (0.282–4.657) | <0.848 | – | ||
| Malignancy | 2.882 (1.573–5.283) | <0.001 | – | ||
| CVA | 6.194 (1.952–19.659) | 0.002 | – | ||
| Clinical parameter | SpO2 | 0.917 (0.906–0.928) | <0.001 | 0.945 (0.929–0.962) | <0.001 |
| Liver function tests | Serum Bili | 1.098 (1.044–1.155) | <0.001 | 1.037 (0.966–1.112) | 0.315 |
| Serum Albumin | 0.206 (0.160–0.266) | <0.001 | 0.396 (0.285–0.549) | <0.001 | |
| AST | 1.004 (1.003–1.005) | <0.001 | 1.002 (1.000–1.003) | 0.016 | |
| AST > ULN | 4.679 (2.354–9.298) | <0.001 | – | ||
| ALT | 1.003 (1.002–1.005) | <0.001 | 1.000 (0.998–1.002) | 0.958 | |
| ALT > ULN | 1.335 (0.769–2.318) | 0.304 | – | ||
| Other Lab parameters | Hb | 0.720 (0.663–0.782) | <0.001 | – | |
| ANC | 1.000 (1.000–1.000) | <0.001 | – | ||
| ALC | 1.000 (1.000–1.001) | <0.195 | |||
| NLR | 1.024 (1.017–1.030) | <0.001 | |||
| Platelets | 1.00 (1.000–1.000) | 0.651 | |||
| BUN | 1.021 (1.018–1.024) | <0.001 | |||
| Serum Creatinine | 1.275 (1.018–1.359) | 0.001 | |||
| LDH | 1.002 (1.002–1.002) | <0.001 | |||
| CPK | 1.000 (1.000–1.000) | 0.09 | |||
| Blood glucose | 1.005 (1.004–1.007) | <0.001 | |||
| Serum Ferritin | 1.002 (1.001–1.003) | <0.001 | |||
| CRP | 1.024 (1.017–1.031) | <0.001 | |||
| D Dimer | 1.000 (1.000–1.001) | <0.001 |
ALC, Absolute Lymphocyte Count; ALT, Alanine Transaminase; ANC, Absolute Neutrophil Count; AST, Aspartate Transaminase; Bili, Bilirubin; BUN, Blood Urea Nitrogen; CAD, Coronary Artery Disease; CKD, Chronic Kidney Disease; COPD, Chronic Obstructive Pulmonary disease; CPK, Creatinine Phosphokinase; CRP, C-Reactive Protein; CVA, Cerebrovascular Accident; DM, Diabetes Mellitus; Hb, Hemoglobin; NLR, Neutrophil-Lymphocyte Ratio; LDH, Lactate Dehydrogenase; Pr HTN, Primary Hypertension; SpO2, Oxygen saturation at room air by pulse oximetry; ULN, Upper Limit of Normal.
Figure 1.
ROC for AST and mortality with AUC-0.726. AUC, area under the curve; AST, aspartate aminotransferase; ROC, receiver operating characteristic curve.
Figure 2.
ROC curve for Serum Albumin at admission and Survival AUC = 0.842. AUC, area under the curve; ROC, receiver operating characteristic curve.
Discussion
This is one of the largest studies addressing liver dysfunction in hospitalized patients with COVID-19. This study attempts to systematically evaluate the impact of liver function profile at admission on the severity of COVID-19 at admission and further analyses its prognostic role.
The prevalence of comorbidities in our study was 44.6% which was similar to studies from Wuhan, where it was 37.3%,14 and an Indian study which showed 47.11% patients having at least one comorbidity.15
The median AST and ALT levels in our study were 41 (IQR 35.8) IU/L and 50 (IQR 45.8) IU/L, respectively, whereas a study from China involving 1141 patients the median AST and ALT were 65.8 ± 12.7 IU/L and 66.4 ± 13.2 IU/L, respectively,16 which were higher.
AST levels were abnormally high in 69.91% of patients in our study, corroborating with the results of an early Indian study involving 170 patients wherein raised liver enzymes were observed in 58.5%.17 Similarly, in a large study from the US involving 5700 patients, AST levels were reported to be elevated in 58.4% patients.8 In comparison, multiple Chinese cohort studies reported the prevalence of elevated AST levels in patients with COVID-19 to be ranged between 4% and 53%.18 In a systematic review of 6537 patients, elevated AST levels were observed in 53.3% patients.19 A recently published Indian study from southern India involving 445 patients with COVID-19 described abnormalities in AST levels to be present in63.9% patients.20 Our study shows a slightly higher prevalence of elevated AST levels.
Elevated ALT levels were seen in 80.22% of our patients in contrast to Chinese studies where in it ranged between 4% and 33%, whilst a US study reported abnormal ALT levels to be observed in 39% of patients.8,17 A systematic review analyzing the impact of COVID-19 on liver dysfunction placed this prevalence at 36.65%.18 In a recent Indian study, 42.4% of patients had elevated ALT leves.21 In comparison, our study showed a higher prevalence. These differences could possibly be attributed to the cut-offs considered in the respective studies. The cut-off considered in our study was as per the American College of Gastroenterology13 (25 IU/L for women and 33 IU/L for men), whereas most other studies have taken 40 U/L as the cut-off. This could also be due to the increased severity of patients with COVID-19 , as most of our patients were symptomatic and had significant comorbidities. Total bilirubin was elevated in only 6.92% patients, which was similar to a Chinese study20 that reported 4.1% patients with elevated bilirubin levels. A recent systematic review reported the prevalence of patients with elevated bilirubin levels to be 3%.20 An Indian study observed hyperbilirubinemia in 4.2% patients.21
Liver Functions as a Predictor of Severity and Mortality
Our study finds AST and ALT to be significantly associated with the odds of moderate to severe disease in the univariable logistic regression. However, only AST was significant after adjustment for age, sex, and comorbidity status, with AST being associated with 0.7% greater odds per 1 IU/L increase in its levels [aOR 1.007 95% CI (1.003–1.011)]. A large study from the US involving 3381 patients showed severe liver injury defined by >5 times ULN to be associated with severity of COVID-19, need for intubation, renal replacement therapy as well as mortality in 42% patients.22 Another large study from China involving 5771 patients showed that elevated liver enzyme was associated with a significant mortality risk.23 A systematic review and metanalysis of 2115 patients showed the association of elevated liver enzymes with severity of COVID-19 as well as that with mortality, reporting OR of 2.57 in its association with disease severity and OR of 1.66 associated with mortality.24
A large meta-analysis of 20 retrospective studies with 3428 patients with COVID-19 revealed that higher levels of ALT, AST, and bilirubin were associated with a significant increase in the severity of COVID-19 infection.25 Another large study estimated a ninefold risk of severe COVID-19 infection in patients with liver injury.26 The reason for liver enzyme abnormalities have been postulated to be multifactorial. Liver histopathology has shown nonspecific findings with steatosis, mild lobular and/or periportal inflammation, and vascular pathology. The potential contributors possibly stem from immune-mediated inflammatory response, hepatic congestion, systemic hypoxemia, direct infection of hepatocyte, cytokine release, ischemic hepatitis and venous and arterial thrombosis.27
Our study shows a negative association of serum albumin with the odds of moderate to severe disease [OR 0.145 95% CI (0.103–0.204) per 1 g/dL increase], which retained its significance even after adjustment age sex, age, and presence of pre-existing comorbidities [aOR 0.217 95% CI (0.149–0.316)]. Hypoalbuminemia has been described in patients with severe COVID- 19 and may not parallel changes in AST and ALT. In a retrospective cohort of 299 patients, 106 (35.5%) patients had low albumin, with significant differences in the albumin levels between survivors and non-survivors (37.6 g/L vs. 30.5 g/L).28
In our study, in the multivariable Cox proportional hazard regression, only the AST and serum albumin were significant predictors of mortality. A Chinese study showed AST to be a potential diagnostic marker, and its level was significantly higher in non-survivors than survivors, with its ROC curve revealing an AUC of 0.854 indicating its prognostic value.9 Another meta-analysis linked elevated admission levels of these markers to patient mortality.29 Albumin levels have also been reported in other studies to be an independent predictive factor for mortality.23 In a meta-analysis of factors associated with disease outcomes in 1990 patients hospitalized COVID-19, hypoalbuminemia was observed to be associated with severe disease as well as death.30 Serum albumin is a good prognostic marker and reflects not only liver function but also the nutritional status. It is a robust marker for survival with AUC of 0.842 for survival. Additionally, it is also a negative acute phase reactant which correlates with immune markers.29
Strength and limitations
This study provides results from an in-depth analysis of data from a large cohort of patients and involving a considerable number of risk factors on the association of deranged liver function tests on COVID-19 severity and mortality. Routinely available lab tests such as AST and albumin can be used to prognosticate the disease among hospitalized patients which, at the same time, is cost economical as well as time accurate. The AST and ALT as negative predictive value is unique to our study. Limitations of our study include its retrospective nature and absence of pathological or radiological correlation. As the study included patients admitted to the hospital, the data involve a slight under-representation of patients with milder disease.
Abnormalities in liver function, being observed in over 80% of patients hospitalized with COVID-19 is indeed a common phenomenon, however, its association with disease severity and mortality is strong and statistically significant. In particular, elevated AST levels and hypoalbuminemia are effective indicators of disease severity and mortality. Our study advocates the utilization of these readily available laboratory indices in triage, management and prognostication of patients with COVID-19 pneumonia.
Credit authorship contribution statement
Padmaprakash KV: Study concept/Design, Statistical analysis, Drafting and manuscript revision, Final approval of published version. Sandeep Thareja: Conduct of Study. Nishant Raman: Statistical analysis. Sowmya Karantha C: Drafting and manuscript revision. J Muthukrishnan: Drafting and manuscript revision. Vasu Vardhan: Drafting and manuscript revision
Conflicts of interest
The authors have none to declare.
Acknowledgements
None.
Funding
Any grants/equipment/drugs, and/or other support that facilitated the conduct of research/writing of the manuscript.
References
- 1.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu Z., Shi L., Wang Y., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chai X., Hu L., Zhang Y., et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. bioRxiv. 2020 doi: 10.1101/2020.02.03.931766. 2020.02.03.931766. [DOI] [Google Scholar]
- 4.Goyal P., Choi J.J., Pinheiro L.C., et al. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 2020;382:2372–2374. doi: 10.1056/nejmc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elmunzer B.J., Spitzer R.L., Foster L.D., et al. Digestive manifestations in patients hospitalized with coronavirus disease 2019. Clin Gastroenterol Hepatol. 2021;19:1355–1365.e4. doi: 10.1016/j.cgh.2020.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hundt M.A., Deng Y., Ciarleglio M.M., Nathanson M.H., Lim J.K. Abnormal liver tests in COVID-19: a retrospective observational cohort study of 1,827 patients in a major U.S. hospital network. Hepatology. 2020;72:1169–1176. doi: 10.1002/hep.31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Youssef M., H Hussein M., Attia A.S., et al. COVID-19 and liver dysfunction: a systematic review and meta-analysis of retrospective studies. J Med Virol. 2020;92:1825–1833. doi: 10.1002/jmv.26055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richardson S., Hirsch J.S., Narasimhan M., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA, J Am Med Assoc. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan W., Ni Z., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/nejmoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sultan S., Altayar O., Siddique S.M., et al. AGA institute rapid review of the gastrointestinal and liver manifestations of COVID-19, meta-analysis of international data, and recommendations for the consultative management of patients with COVID-19. Gastroenterology. 2020;159:320–334.e27. doi: 10.1053/j.gastro.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Government of India, M of H and FW, Services DG of H (EMR Division) 2020. Clinical management protocol: COVID-19.https://www.mohfw.gov.in/pdf/ClinicalManagementProtocolforCOVID19.pdf [Google Scholar]
- 13.Kwo P.Y., Cohen S.M., Lim J.K. ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol. 2017;112:18–35. doi: 10.1038/ajg.2016.517. [DOI] [PubMed] [Google Scholar]
- 14.Wu C., Chen X., Cai Y., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dosi R., Jain G., Mehta A. Clinical characteristics, Comorbidities,and outcome among 365 patients of coronavirus disease 2019 at a tertiary care centre in Central India. J Assoc Phys India. 2020;68:20–23. [PubMed] [Google Scholar]
- 16.Luo S., Zhang X., Xu H. Don't overlook digestive symptoms in patients with 2019 novel coronavirus disease (COVID-19) Clin Gastroenterol Hepatol. 2020;18:1636. doi: 10.1016/J.CGH.2020.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saini R., Saini N., Ram S., et al. COVID-19 associated variations in liver function parameters: a retrospective study. Postgrad Med J. 2020 doi: 10.1136/POSTGRADMEDJ-2020-138930. [DOI] [PubMed] [Google Scholar]
- 18.Wu Z., Yang D. A meta-analysis of the impact of COVID-19 on liver dysfunction. Eur J Med Res. 2020;25:1–9. doi: 10.1186/S40001-020-00454-X. 2020 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Israfil S.M.H., Sarker M.M.R., Rashid P.T., et al. Clinical characteristics and diagnostic challenges of COVID−19: an update from the global perspective. Front Public Health. 2020;8 doi: 10.3389/FPUBH.2020.567395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao F., Chen L., Li J., et al. Clinical features of COVID-19-related liver functional abnormality. Clin Gastroenterol Hepatol. 2020;18:1561–1566. doi: 10.1016/J.CGH.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saithanyamurthi H., Munirathinam M., Ananthavadivelu M. Prevalence of liver injury in 445 patients with Corona Virus Disease-19-Single-centre experience from southern India. Indian J Gastroenterol. 2021;40:303–308. doi: 10.1007/S12664-021-01147-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phipps M., Barraza L., LaSota E., et al. Acute liver injury in COVID-19: prevalence and association with clinical outcomes in a large U.S. Cohort. Hepatology. 2020;72:807–817. doi: 10.1002/HEP.31404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lei F., Liu Y.M., Zhou F., et al. Longitudinal association between markers of liver injury and mortality in COVID-19 in China. Hepatology. 2020;72:389–398. doi: 10.1002/hep.31301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yadav D., Singh A., Zhang Q., et al. Involvement of liver in COVID-19: systematic review and meta-analysis. Gut. 2021;70:807–809. doi: 10.1136/GUTJNL-2020-322072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cai Q., Huang D., Yu H., et al. COVID-19: abnormal liver function tests. J Hepatol. 2020;73:566–574. doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang J., Cheng A., Kumar R., et al. Hypoalbuminemia predicts the outcome of COVID-19 independent of age and co-morbidity. J Med Virol. 2020;92:2152–2158. doi: 10.1002/JMV.26003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marjot T., Webb G.J., Barritt A.S., et al. COVID-19 and liver disease: mechanistic and clinical perspectives. Nat Rev Gastroenterol Hepatol. 2021;18:348–364. doi: 10.1038/s41575-021-00426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kulkarni A.V., Kumar P., Tevethia H.V., et al. Systematic review with meta-analysis: liver manifestations and outcomes in COVID-19. Aliment Pharmacol Ther. 2020;52:584–599. doi: 10.1111/apt.15916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu W., Tao Z.W., Wang L., et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl). 2020;133:1032–1038. doi: 10.1097/CM9.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]