Abstract
Purpose
Four cases of ibrutinib-related uveitis are presented, which are to the best of our knowledge the first in the literature. Possible mechanisms of ibrutinib-mediated uveitis are explored.
Observations
Case 1 is a 60-year-old female who had been stable on 1 year of ibrutinib for chronic lymphocytic leukaemia. She was diagnosed with ibrutinib-related uveitis, which responded well to topical steroids. Case 2 is a 63-year-old male diagnosed with uveitis after 2 years of ibrutinib treatment for chronic lymphocytic leukaemia. He responded well to topical and oral steroids; however, he continued to have uveitis relapses after weaning steroids. Case 3 is a 69-year-old male diagnosed with uveitis after 18 months of ibrutinib treatment. He was trialed on topical and intravenous steroids, and restarted ibrutinib without worsening of symptoms. Case 4 is a 66-year-old female who developed uveitis after being stable on ibrutinib for 3 years. She responded well to topical steroids.
Conclusions and Importance
Inflammatory complications of tyrosine kinase inhibitors are well described. While ibrutinib, and other kinase inhibitors, are generally well-tolerated, there are increasing reports of ocular toxicities, including uveitis. It is recommended to monitor patients for potential ocular adverse effects and facilitate rapid ophthalmologic assessment.
Keywords: Uveitis, Ibrutinib, Ocular toxicity, Oral chemotherapy, Kinase inhibitors
1. Introduction
The use of oral chemotherapeutic agents, and in particular small molecule kinase inhibitors, has increased in recent years. As of August 2019, 43 small molecule tyrosine kinase inhibitors were approved by the FDA.1 The literature surrounding adverse events is growing constantly. Ocular adverse events may be a predictable side effect of the mechanism of action of the drug, such as the eyelash and corneal epithelial changes seen with epidermal growth factor receptor inhibitors. They may be class specific but unrelated to the intended mechanism of action of the drug such as the retinal pigment epithelial abnormalities and retinal pigment epithelial (RPE) detachment seen with mitogen-activated extracellular signal-regulated kinase (MEK) inhibitors. We have summarized the important known ocular toxicities of small molecule tyrosine kinase inhibitors in Table 1.2 Ocular adverse reactions, while increasingly reported, may still be underestimated.3 We report four cases of ibrutinib-related uveitis.
Table 1.
| Name | Known Target | Indications | Ocular toxicity |
|---|---|---|---|
| Anaplastic lymphoma kinase (ALK) | |||
| Alectinib | ALK and RET | ALK + NSCLC | Nil |
| Brigatinib | ALK, ROS1, IGF-1R, Flt3, EGFR | ALK + NSCLC after crizotinib | Nil |
| Ceritinib | ALK, IGF-1R, InsR, ROS1 | ALK + NSCLC as first-line treatment or after crizotinib resistance | Nonspecitic vision disorder (vision impairment, blurred vision, photopsia, accommodation disorder) |
| Crizotinib | ALK, c-Met (HGFR), ROS1, MST1R | ALK+, ROS1+ NSCLC | Nonspecific vision disorder (diplopia, photopsia, photophobia, blurred vision, visual field defect, floaters) |
| Entrectinib | TRKA/B/C, ROS1, ALK | ROS1+ NSCLC; solid tumors with NTRK fusion proteins | Nil |
| Lorlatinib | ALK | ALK + NSCLC | Nil |
| Bosutinib | BCR-ABL, Src, Lyn, Hck | CML | Nil |
| Dasatinib | BCR-ABL, EGFR, Src, Lck, Yes, Fyn, Kit, EphA2, PDGFRβ | Ph + chronic ML and ALL | Optic neuropathy VKH like illness Dry eye/conjunctivitis Blurred vision Periorbital oedema |
| Imatinib | BCR-ABL, Kit, PDGFR | Ph + CML or ALL, CEL, DFSP, HES, GIST, MDS/MDP | VKH-like illness Glaucoma Neovascularisation, retinal haemorrhage, macular oedema Optic neuropathy/papilloedema Periorbital/eyelid oedema Keratitis/conjunctivitis/blepharitis Scleral/conjunctival haemorrhage Cataract |
| Nilotinib | BCR-ABL, PDGFR, DDR1 | Ph + CLL | Iridocyclitis Periorbital oedema Dry eye Conjunctivitis/blepharitis Conjunctival haemorrhage/hyperaemia Papilloedema |
| Ponatinib | BCR-ABL, BCR-ABL T315I, VEGFR, PDGFR, FGFR, EphR, Src family kinases, Kit, RET, Tie2, Flt3 | Ph + CML or ALL | Nil |
| Epidermal growth factor receptor (EGFR) | |||
| Afatinib | EGFR, ErbB2, ErbB4 | NSCLC | Conjunctivitis Keratitis |
| Dacomitinib | EGFR/ErbB2/ErbB4 | EGFR- mutated NSCLC | Nil |
| Erlotinib | EGFR | SCLC and PaC | Ocular toxoplasmosis Trichomegaly and eyelid disease Corneal erosion and ocular surface disease (conjunctivitis/keratitis) Corneal perforation |
| Gefitinib | EGFR | NCLC | Trichomegaly and eyelid disease Corneal erosion and ocular surface disease (conjunctivitis) Corneal perforation Ocular ischaemia/haemorrhage Uveitis |
| Lapatinib | EGFR, ErbB2 | BC | Nil |
| Neratinib | ErbB2/HER2 | HER2+ breast cancer | Nil |
| Osimertinib | EGFR T970 M | NSCLC | Nil |
| Vandetanib | EGFRs, VEGFRs, RET, Brk, Tie2, EphRs, Src family kinases | MTC | Verticillata Conjunctivitis Corneal structural change Glaucoma Cataract |
| FMS-like tyrosine kinase 3 (FLT3) | |||
| Gilteritinib | FLT3 | AMLwith FLT3 mutation5 | Nil |
| Midostaurin | FLT3 | ALL Flt3 mutation+ | Nil |
| Erdafitinib | FGFR1/2/3/4 | Urothelial carcinoma | Dry eye Blurred vision RPE detachment Retinal detachment |
| Ruxolitinib | JAK1 and 2 | MF and PV | Nil |
| Larotrectinib | NTRK | Solid tumors with NTRK gene fusion proteins | Nil |
| Axitinib | VEGFR1/2/3, PDGFRβ | RCC | Retinal artery/vein occlusion |
| Carbozantinib | RET, Met, VEGFR1/2/3, Kit, TrkB, Flt3, Axl, Tie2, ROS1 | Metastatic MTC, advanced RCC and HCC | Nil |
| Lenvatinib | VEGFRs, FGFRs, PDGFR, Kit, RET | DTC | Nil |
| Pazopanib | VEGFR1/2/3, PDGFRα/β, FGFR1/3, Kit, Lck, Fms, Itk | RCC, STS | Ocular surface Lid changes Retinal detachment |
| Regorafenib | VEGFR1/2/3, BCR-ABL, BRAF, BRAF(V600E), Kit, PDGFRα/β, RET, FGFR1/2, Tie2, Eph2A | CRC, GIST | Nil |
| Sorafenib | B/C-Raf, BRAF (V600E), Kit, Flt3, RET, VEGFR1/2/3, PDGFRβ | RCC, DTC and HCC | Ocular surface Lid changes Retinal detachment |
| Sunitinib | PDGFRα/β, VEGFR1/2/3, Kit, Flt3, CSF-1R, RET | RCC, GIST, PNET | Ocular surface Lid changes (periorbital oedema) Retinal detachment |
| Dabrafenib | BRAF | Melanoma and NSCLC with BRAF mutations | Photosensitivity VKH like illness RPE changes and subretinal fluid Uveitis Retinal vein occlusion |
| Encorafenib | BRAFV600E/K | BRAFV600E/K mutant melanoma with binimetinib | Nil |
| Vemurafenib | A/B/C-Raf, BRAF (V600E), SRMS, ACK1, MAP4K5, FGR | Melanoma with BRAFV600E mutation and ECD | Uveitis Conjunctivitis and dry eye CMORPE changes and RPE detachment Retinal vein occlusion Central serous retinopathy |
| TK inhibitors | |||
| Acalabrutinib | Bruton tyrosine kinase | MCL | Nil |
| Ibrutinib | Bruton tyrosine kinase | MCL, CLL, WM, graph vs host disease. | Visual disturbance Cataract Dry eye Subconjunctival haemorrhage Branch retinal artery occlusion Cystoid macular oedema |
| MEK inhibitors | |||
| Binimetinib | MEK1/2 | BRAF V600E/K melanoma with encorafenib | Chorioretinopathy Photopsia/visual impairment Retinal detachment Macular oedema Retinopathy Visual impairment |
| Cobimetinib | MEK1/2 | Melanoma with BRAF V600E/K mutations with vemurafenib | Uveitis CMORPE changes and RPE detachment |
| Trametinib | MEK1/2 | Melanoma (2013) and NSCLC (2017) with BRAF mutations | VKH-like illness RPE changes and subretinal fluid Uveitis Retinal vein occlusion Central serous retinopathy Periorbital oedema Dry eye |
| Abemaciclib | CDK4/6 | HR+, HER– BC | Nil |
| Palbociclib | CDK4/6 | ER+ and HER2– BC | Nil |
| Ribociclib | CDK4/6 | HR + -EGFR– metastatic BC | Nil |
2. Findings
2.1. Case 1
A 60-year-old female diagnosed with chronic lymphocytic leukaemia 9 years prior was stable on 1 year of ibrutinib 420mg daily. She was otherwise well. She presented to the ophthalmologist with a bilateral increase in floaters.
On presentation, her best corrected visual acuity (BCVA) was 6/6 bilaterally, with intraocular pressures (IOP) of 20 mmHg in the right and 18 mmHg in the left. Her eye examination demonstrated bilateral fine keratic precipitates, with 2+ anterior chamber cells and 2+ vitreous cells on the right and 3+ anterior chamber cells, 1+ vitreous cells and snowballs on the left. There was no evidence of disc or macular oedema. Initial workup, chemistry panel and full blood examination were normal. No infectious cause was identified on blood tests, and she was negative for HLAB-27. Ibrutinib was presumed to be the cause of this uveitis.
She continued on her regular dose of ibrutinib, and was concurrently commenced on topical steroids (prednisolone acetate 1% one drop bilaterally four times a day). After 8 weeks, her anterior chambers became quiet bilaterally. As she had ongoing vitreous inflammation bilaterally, topical steroids were continued.
5 months after the commencement of topical steroids, her intra-ocular pressures increased to 22 mmHg on the right and 20 mmHg on the left and she was subsequently weaned off the topical steroids. 2 weeks later, she re-developed floaters in her right eye. Her BCVA decreased to 6/7.5 on the right and 6/12 on the left, with intraocular pressures improving to 13 mmHg and 15 mmHg respectively. Her eye examination demonstrated quiet anterior chambers, 1+ vitreous cells, inferior snowballs and cystoid macular oedema, without glaucomatous optic neuropathy (Fig. 1A and B). She had a left-sided epiretinal membrane. She was recommenced on low-dose topical steroids bilaterally, with combined beta-blocker and alpha agonist eye drops (brimonidine 0.2%/timolol 0.5% one drop bilaterally twice a day).
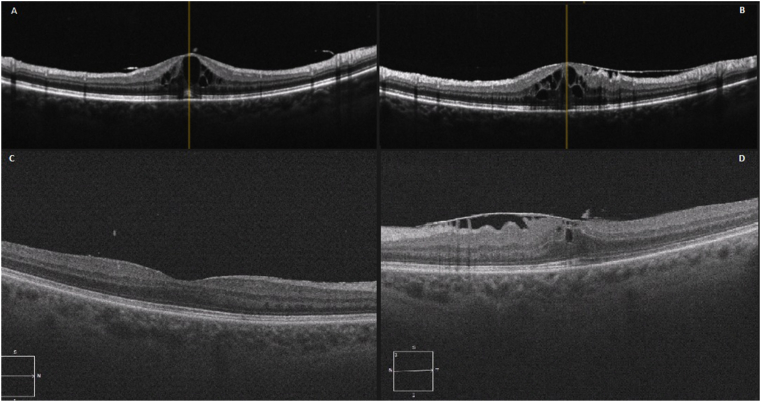
Fig. 1.
Case 1 – OCTsThe OCTs after weaning steroids (5 months post commencement) demonstrates a relapse with significant cystoid macular oedema on the right (A). The left eye OCT shows cystoid macular oedema with an epiretinal membrane (B). 5 months after re-commencing topical steroids, OCTs demonstrate resolved cystoid macular oedema on the right (C) and left (D).
She remained stable on ibrutinib with topical steroids. 10 months after her initial presentation, her BCVA was 6/6 on the right and 6/12 on the left. She had no further inflammation bilaterally, and cystoid macular oedema had resolved (Fig. 1C and D). Her topical steroids were ceased, and her vision remained stable.
2.2. Case 2
A 63-year-old man diagnosed with chronic lymphocytic leukaemia without central nervous system (CNS) involvement had been stable on ibrutinib 420mg daily for 2 years. He was otherwise well. He presented to the ophthalmologist with a floater in his left eye.
On examination, his BCVA on the right was 6/6 and on the left was 6/7.5, with normal intraocular pressures. His right eye examination showed a mix of large and stellate keratic precipitates in the inferior cornea, with a single posterior synechia (Fig. 2) and 1+ anterior chamber cells. On the left, he had fine keratic precipitates and occasional anterior chamber cells. He had bilateral optic nerve head swelling, worse on the right than the left, associated with small retinal haemorrhages (Fig. 3). He had no evidence of posterior uveitis. An MRI scan showed mildly increased fluid in both optic nerve sheaths, without signs of perineuritis. A lumbar puncture was unremarkable, with a normal opening pressure, glucose and protein and negative flow cytometry, microscopy and culture. A full blood examination and biochemistry panel were normal, with negative ANCA, serum angiotensin converting enzyme (ACE), HLAB27, rheumatoid factor, Treponema pallidum, Bartonella henselae and Lyme disease serology. Ibrutinib was presumed to be the cause for his uveitis.
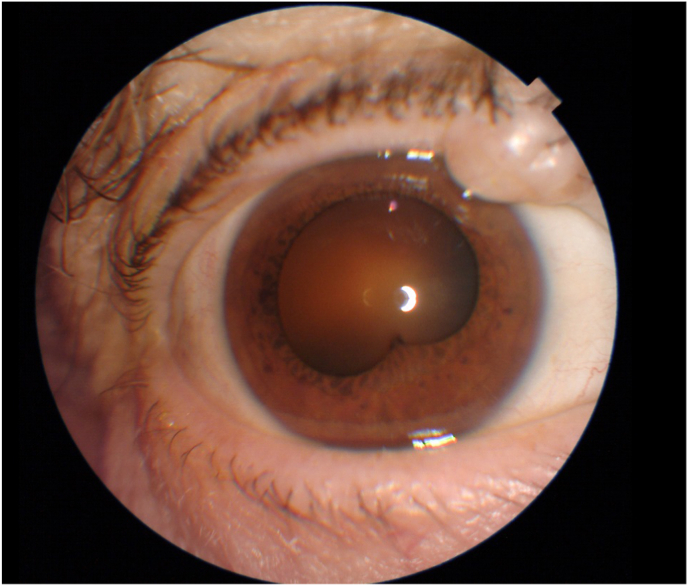
Fig. 2.
Case 2 – Anterior Segment (Initial Presentation) Clinical photograph of the right eye on initial presentation, demonstrating a single posterior synechia and keratic precipitates in the inferior cornea.
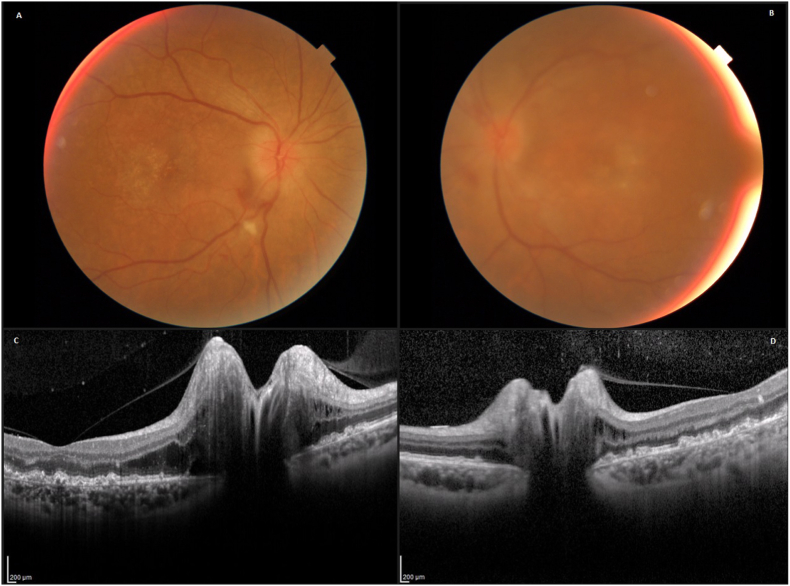
Fig. 3.
Case 2 – Posterior Segment (Initial Presentation) The fundal photograph of the right eye demonstrates global papilloedema with blurred optic nerve edges and elevation, worse on the right (A) than left (B). OCTs demonstrate optic nerve head oedema, worse on the right (C) than left (D).
He remained on ibrutinib but was concurrently commenced on topical steroids (prednisolone acetate 1% one drop bilaterally four times a day). Two weeks later, he started oral steroids (prednisolone 25mg daily) for presumed systemic inflammatory complications of his ibrutinib and ceased topical steroids. A month after commencing steroids, his uveitis fully resolved, with a stable visual acuity. However, he had persistent nerve head swelling, with no change on examination or nerve fibre layer OCT. 8 months after his commencement of steroids, his BCVA improved to 6/6 bilaterally, and his optic nerve swelling decreased bilaterally on oral steroids (Fig. 4). He had no ongoing uveitis, and oral steroids were weaned.
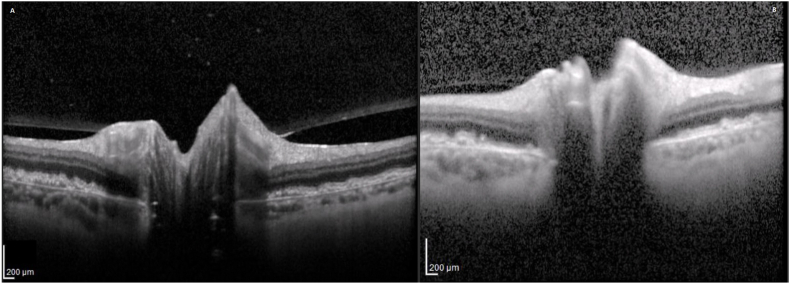
Fig. 4.
Case 2 – OCTs (8 Months of Steroid Therapy) After 8 months of steroid therapy, OCTs demonstrate markedly less optic disc oedema on the right (A) and left (B).
He continued to suffer uveitis relapses with cystoid macular oedema, without nerve head swelling, after weaning steroids while on ibrutinib therapy. These episodes resolved with short doses of steroids (oral or topical), but recurred after weaning.
2.3. Case 3
A 69-year-old male with a known history of chronic lymphocytic leukaemia, without CNS involvement, had been on ibrutinib 420mg a day for the previous 18 months. His other medical history included hypertension, gastro-oesophageal reflux disease, a hemithyroidectomy for a benign thyroid tumour, and shingles affecting his right L1/L2 dermatome 8 weeks prior.
He initially presented with a 2-week history of bilateral paresthesia in his hands and feet. This was followed by a 1-week history of bilateral facial paresthesia, right sided facial weakness, loss of his right inferior visual field and floaters. He had self-ceased ibrutinib 1 week prior to presentation due to his symptoms.
On examination, he had a right facial nerve palsy (House-Brackmann grade III), with normal ear canal and tympanic membrane examinations. There was a right relative afferent pupillary defect, visual acuity was 6/9 bilaterally and intraocular pressures were normal. There were trace cells in both anterior chambers and 2+ cells with 1+ haze in the right vitreous cavity, and trace cells only in the left vitreous cavity. Snowballs were present, more numerous on the right than the left. Fundus exam revealed no cystoid macular oedema, no pallor or swelling of the optic discs and no other signs of posterior uveitis. Automated perimetry (Humphry visual field 24–2) revealed a right inferior field defect. Ishihara plates were 14/15 on the right and 15/15 on the left. The rest of the cranial and peripheral nerve examination was unremarkable.
The patient underwent a vitreous aspirate and intravitreal injection of foscarnet (2.4mg/0.1mL) to cover for a viral cause and was commenced on prednisolone acetate 1% four times a day in both eyes.
Herpetic viral polymerase chain reaction (PCR) was negative on the vitreous aspirate. A full blood examination showed a mild macrocystosis, a mild thrombocytopenia, and evidence of large blastic appearing lymphoma cells. Syphilis serology, anti-myeline oligodendrocyte glycoprotein (MOG) antibody IgM, and anti-neuromyelitis optica (NMO) IgG were negative, and serum ACE levels were within normal limits. His CT brain and temporal bones was unremarkable. His MRI brain and orbits showed normal optic nerves and cavernous sinus and normal facial nerves.
On review 3 days later, his right facial droop had resolved. At two weeks his anterior chambers were quiet and his vitreous cells and haze had resolved on prednisolone acetate 1%. There were some persistent inferior snowballs and he developed pallor of his right optic disc superiorly. His prednisolone acetate drops were subsequently weaned. Although it was deemed unlikely to change his outcome, it was decided by neurology and oncology to trial a 3-day course of intravenous methylprednisolone 1g daily at 1 month post presentation. Due to COVID-19, the patient was subsequently reviewed by telephone as he lived a substantial distance from the hospital. Subjectively, he stated there was no improvement and his symptoms remained stable, at which point his oncologist restarted his ibrutinib without subsequent worsening of his symptoms.
2.4. Case 4
A 66-year-old female with a known history of chronic lymphocytic leukaemia without CNS involvement was on ibrutinib 420mg a day for the past 3 years. Her medical history included bilateral pseudophakia, hypertension and breast cancer (mastectomy and radiotherapy in 2005).
She initially presented with a 3-week history of bilateral floaters. On examination, her visual acuity was 6/12 in the right and 6/9 in the left. Intraocular pressures were normal. Both corneas had fine keratic precipitates and 0.5+ cells in the right anterior chamber and 1+ in the left. The anterior vitreous had 1+ cells in each eye without haze. There was evidence of peripheral perivenular sheathing, but no evidence of occlusion or retinitis. There was no cystoid macular oedema.
While continuing the ibrutinib, she underwent a right vitreous tap for viral PCR. She was treated empirically with a right intravitreal injection of foscarnet 2.4mg/0.1mL followed by oral valaciclovir 2g three times daily and topical prednisolone acetate 1% drops hourly in each eye. The herpes multiplex PCR performed on the vitreous tap was negative, at which point valaciclovir was ceased. Syphilis serology and tuberculosis interferon gamma release assay were negative and the serum ACE level was within normal limits. The cells in the anterior chamber and vitreous cavity as well as the peripheral perivenular sheathing resolved with continued topical steroids. The topical steroids were then weaned over the next 2 months at which point the inflammation remained quiet. Her vision improved to 6/5 in the right and 6/4.5 in the left.
3. Discussion
Uveitis, defined as intraocular inflammation, is the fifth leading cause of vision loss in the United States, causing 10–15% of visual impairment in the western world. Around one half of patients with uveitis have no obvious associated infectious or inflammatory disease. Local ocular and systemic drugs are increasingly being identified as a cause of (or at being seen in association with) uveitis.4
Ibrutinib is an oral chemotherapeutic agent used primarily in chronic lymphocytic leukaemia. It is an orally bioavailable Bruton tyrosine kinase (BTK) inhibitor, forming an irreversible bond to BTK, ultimately causing decreased proliferation of and increased apoptosis in malignant B-cells.5 While it is primarily a BTK inhibitor, it does have off-target inhibition of other kinases including interleukin-2-inducible T-cell kinase (ITK), tec protein tyrosine kinase (TEC), BMX and epidermal growth factor receptor (EGFR).6
While ibrutinib is generally well tolerated, it has multiple known adverse reactions. The most common include diarrhoea, upper respiratory tract infections, bleeding, fatigue and cardiac side effects. These reactions are usually mild.5 Notably, it has been associated with peripheral neuropathy.7,8 Ibrutinib also has known ocular adverse effects, including red/dry eyes, subconjunctival haemorrhage, branch retinal artery occlusion and cystoid macular oedema.9,10
To the best of our knowledge, this is the first report of ibrutinib-related uveitis and optic neuropathy cases.
Ocular toxicities are underestimated and under-reported in chemotherapeutic agents, including oral kinase inhibitors (Table 1).3 Every structure of the eye, including eyelids, has been reported to be affected. In the anterior segment, corneal erosions, ulcers, and perforation, subconjunctival haemorrhage, conjunctivitis, abnormal lacrimation and uveitis have been reported. In the posterior segment, vitreous haemorrhage, chorioretinopathy, retinopathy, serous retinal detachment, retinal vascular occlusion, retrobulbar neuritis and optic neuritis have been reported.11, 12, 13 In particular, MEK inhibitors are associated with a specific and unusual ocular toxicity with RPE changes and focal RPE detachments (so called MEK associated retinopathy or MEKAR).14 The MEK pathway is component of the MAPK pathway which itself plays an important role in the maintenance and protection and repair of the human retinal pigment epithelium.15 While there is some off-target activity with the tyrosine kinase inhibitors, MEKAR has not been seen with other (non MEK inhibitor) tyrosine kinase inhibitors. Patients with pre-existing ophthalmic conditions have been noted to be at higher risk of developing ocular adverse effects.11 It has been recommended that these patients have close follow-up, with prompt ophthalmologic evaluation for concerning symptoms.
Uveitis has been reported as an adverse reaction to other kinase inhibitors. In particular, vemurafenib, dabrafenib, and trametinib have been linked with uveitis.16 Uveal inflammation in mitogen-activated protein kinase (MEK) inhibitors, such as trametinib, has been postulated to be linked dysregulation of tight junctions of the endothelial cells in the ciliary body.3,17 However, the mechanisms of other kinase inhibitors in inciting uveitis remain unclear.
The mechanisms of uveitis in ibrutinib remain unclear. Notably, ibrutinib has been found to inhibit MEK as a downstream effect in some cancer cell lines.18 Furthermore, ibrutinib has been shown to trigger inflammatory processes, including autoimmune phenomena.19,20 These include early autoimmune skin lesions, palindromic rheumatoid arthritis, bullous pemphigoid, autoimmune haemolytic anaemia, autoimmune cytopenia, severe arthritic syndrome and autoimmune myelitis.21,22 These, like the presented cases, have been found to be responsive to steroids.21,23 Autoimmune phenomena have been postulated to be related to ibrutinib's inhibition of interleukin-2-inducible T-cell kinase, thereby causing a Th1 shift and a pro-inflammatory response of Th1 cells.24,25 Th1-mediated immune responses, primarily driven by INF-y, TNF-a and IL-2, have been associated with autoimmune disorders including uveitis.21,26
4. Conclusions
To the best of our knowledge, this is the first report of ibrutinib-related uveitis. Given reports of association between multiple other kinase inhibitors and uveitis, this is an unsurprising relationship. While the mechanism of ibrutinib-related uveitis remains unclear, ibrutinib has been demonstrated to trigger other steroid-responsive inflammatory and autoimmune phenomena.
Patient consent
Consent to publish each case was not obtained. This report does not contain any personal information that could lead to the identification of the patients. Alfred Health Ethics Committee does not require approval for case series of more than one patient.
Acknowledgements and Disclosures.
Funding
No funding or grant support
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Declaration of competing interest
Anthony J Hall has served on advisory boards for Novartis and Bayer and AbbVie. He has received lecture fees from AbbVie and Novartis. His institution has received research support from Novartis.
Zelia K Chiu, Jonathan KS Goh, Cecilia Ling and Ming-Lee Lin have no financial disclosures.
Acknowledgements
None.
References
- 1.Pottier C., Fresnais M., Gilon M., Jérusalem G., Longuespée R., Sounni N.E. Tyrosine kinase inhibitors in cancer: breakthrough and challenges of targeted therapy. Cancers. 2020;12 doi: 10.3390/cancers12030731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hager T., Seitz B. Ocular side effects of biological agents in oncology: what should the clinician be aware of? OncoTargets Ther. 2013;7:69–77. doi: 10.2147/OTT.S54606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niro A., Strippoli S., Alessio G., Sborgia L., Recchimurzo N., Guida M. Ocular toxicity in metastatic melanoma patients treated with mitogen-activated protein kinase kinase inhibitors: a case series. Am J Ophthalmol. 2015;160:959. doi: 10.1016/j.ajo.2015.07.035. 967.e1. [DOI] [PubMed] [Google Scholar]
- 4.Moorthy R.S., Moorthy M.S., Cunningham E.T., Jr. Drug-induced uveitis. Curr Opin Ophthalmol. 2018;29:588–603. doi: 10.1097/ICU.0000000000000530. [DOI] [PubMed] [Google Scholar]
- 5.Paydas S. Management of adverse effects/toxicity of ibrutinib. Crit Rev Oncol Hematol. 2019;136:56–63. doi: 10.1016/j.critrevonc.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Cheng S., Ma J., Guo A., et al. BTK inhibition targets in vivo CLL proliferation through its effects on B-cell receptor signaling activity. Leukemia. 2014;28:649–657. doi: 10.1038/leu.2013.358. [DOI] [PubMed] [Google Scholar]
- 7.Byrd J.C., Brown J.R., O'Brien S., et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371:213–223. doi: 10.1056/NEJMoa1400376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cömert P., Albayrak M., Yıldız A., Şahin O., Öztürk H.B., Reis Aras M. Ibrutinib-induced polyneuropathy: a case report. J Oncol Pharm Pract. 2020;26:1501–1504. doi: 10.1177/1078155220903357. [DOI] [PubMed] [Google Scholar]
- 9.Kunkler A.L., Binkley E.M., Mantopoulos D., et al. Known and novel ocular toxicities of biologics, targeted agents, and traditional chemotherapeutics. Graefes Arch Clin Exp Ophthalmol. 2019;257:1771–1781. doi: 10.1007/s00417-019-04337-8. [DOI] [PubMed] [Google Scholar]
- 10.Saenz-de-Viteri M., Cudrnak T. Bilateral cystoid macular edema in a patient with chronic lymphocytic leukemia treated with ibrutinib. Leuk Lymphoma. 2019;60:842–844. doi: 10.1080/10428194.2018.1508673. [DOI] [PubMed] [Google Scholar]
- 11.Gharwan H., Groninger H. Kinase inhibitors and monoclonal antibodies in oncology: clinical implications. Nat Rev Clin Oncol. 2016;13:209–227. doi: 10.1038/nrclinonc.2015.213. [DOI] [PubMed] [Google Scholar]
- 12.Huillard O., Bakalian S., Levy C., et al. Ocular adverse events of molecularly targeted agents approved in solid tumours: a systematic review. Eur J Cancer. 2014;50:638–648. doi: 10.1016/j.ejca.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 13.Huang S., Armstrong E.A., Benavente S., Chinnaiyan P., Harari P.M. Combining Anti-EGFR Antibody with Tyrosine Kinase Inhibitor. Vol. 64. 2004. Dual-agent molecular targeting of the epidermal growth factor receptor (EGFR) pp. 5355–5362. [DOI] [PubMed] [Google Scholar]
- 14.Booth A.E.C., Hopkins A.M., Rowland A., Kichenadasse G., Smith J.R., Sorich M.J. Risk factors for MEK-associated retinopathy in patients with advanced melanoma treated with combination BRAF and MEK inhibitor therapy. Ther Adv Med Oncol. 2020;12 doi: 10.1177/1758835920944359. 1758835920944359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Noll R., Leijen S., Neuteboom G.H., Beijnen J.H., Schellens J.H. Effect of inhibition of the FGFR-MAPK signaling pathway on the development of ocular toxicities. Cancer Treat Rev. 2013;39:664–672. doi: 10.1016/j.ctrv.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Fu C., Gombos D.S., Lee J., et al. Ocular toxicities associated with targeted anticancer agents: an analysis of clinical data with management suggestions. Oncotarget. 2017;8:58709–58727. doi: 10.18632/oncotarget.17634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis M.E. Ocular toxicity of tyrosine kinase inhibitors. Oncol Nurs Forum. 2016;43:235–243. doi: 10.1188/16.ONF.235-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rauf F., Festa F., Park J.G., et al. Ibrutinib inhibition of ERBB4 reduces cell growth in a WNT5A-dependent manner. Oncogene. 2018;37:2237–2250. doi: 10.1038/s41388-017-0079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsang M., Parikh S.A. A concise review of autoimmune cytopenias in chronic lymphocytic leukemia. Curr Hematol Malig Rep. 2017;12:29–38. doi: 10.1007/s11899-017-0366-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rogers K.A., Ruppert A.S., Bingman A., et al. Incidence and description of autoimmune cytopenias during treatment with ibrutinib for chronic lymphocytic leukemia. Leukemia. 2016;30:346–350. doi: 10.1038/leu.2015.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wanner D., Bohn J.-P., Rudzki J., Stockhammer G., Steurer M. Autoimmune myelitis in a CLL patient undergoing treatment with ibrutinib. Ann Hematol. 2019;98:205–207. doi: 10.1007/s00277-018-3381-y. [DOI] [PubMed] [Google Scholar]
- 22.Shaikh H., Khattab A., Faisal M.S., et al. Case series of unique adverse events related to the use of ibrutinib in patients with B-cell malignancies—a single institution experience and a review of literature. J Oncol Pharm Pract. 2018;25:1265–1270. doi: 10.1177/1078155218788707. [DOI] [PubMed] [Google Scholar]
- 23.Mulvey J.J., Nuovo G.J., Magro C.M. Cutaneous, purpuric painful nodules upon addition of ibrutinib to RCVP therapy in a CLL patient: a Distinctive reaction pattern reflecting iatrogenic Th2 to Th1 milieu reversal. Am J Dermatopathol. 2016;38:492–498. doi: 10.1097/DAD.0000000000000441. [DOI] [PubMed] [Google Scholar]
- 24.Bunnell S.C., Diehn M., Yaffe M.B., Findell P.R., Cantley L.C., Berg L.J. Biochemical interactions integrating Itk with the T cell receptor-initiated signaling cascade. J Biol Chem. 2000;275:2219–2230. doi: 10.1074/jbc.275.3.2219. [DOI] [PubMed] [Google Scholar]
- 25.Dubovsky J.A., Beckwith K.A., Natarajan G., et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood. 2013;122:2539–2549. doi: 10.1182/blood-2013-06-507947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caspi R.R. Th1 and Th2 responses in pathogenesis and regulation of experimental autoimmune uveoretinitis. Int Rev Immunol. 2002;21:197–208. doi: 10.1080/08830180212063. [DOI] [PubMed] [Google Scholar]
- 27.Stjepanovic N., Velazquez-Martin J.P., Bedard P.L. Ocular toxicities of MEK inhibitors and other targeted therapies. Ann Oncol. 2016;27:998–1005. doi: 10.1093/annonc/mdw100. [DOI] [PubMed] [Google Scholar]