Summary
Background
Low socioeconomic position may affect initiation of sodium-glucose cotransporter-2 inhibitors (SGLT-2i) and glucacon-like-peptide-1 receptor agonists (GLP-1RA) among patients with type 2 diabetes (T2D). We examined the association between socioeconomic position and initiation of SGLT-2i or GLP-1RA in patients with T2D at time of first intensification of antidiabetic treatment.
Methods
Through nationwide registers, we identified all Danish patients on metformin who initiated second-line add-on therapy between December 10, 2012, and December 31, 2020. For each time period (2012-2014, 2015-2017, and 2018-2020), we used multivariable multinomial logistic regression to associate disposable income, as proxy for socioeconomic position, with the probability of initiating a specific second-line treatment at time of first intensification. We reported probabilities standardised to the distribution of demographics and comorbidities of patients included in the last period (2018-2020).
Findings
We included 48915 patients (median age 62 years; 61·7% men). In each time period, high-income patients were more often men and had less comorbidities as compared with low income-patients. In each time period, the standardised probability of initiating a SGLT-2i or a GLP-1RA was significantly higher in the highest income group compared with the lowest: 11·4% vs. 9·5% (probability ratio [PR] 1·21, 95 % confidence interval [CI] 1·01-1·44) in 2012-2014; 22·6% vs. 19.6% (PR 1·15, CI 1·05-1·27) in 2015-2017; and 65·8% vs. 54·8% (PR 1·20, CI 1·16-1·24) in 2018-2020. The differences by income were consistent across multiple subgroups.
Interpretation
Despite a universal healthcare system, low socioeconomic position was consistently associated with a lower probability of initiating a SGLT-2i or a GLP-1RA. These disparities may widen the future socioeconomic gap in cardiovascular outcomes.
Funding
The work was funded by unrestricted grants from ‘Region Sjaelland Den Sundhedsvidenskabelige Forskningsfond’ and ‘Murermester Lauritz Peter Christensen og hustru Kirsten Sigrid Christensens Fond’.
Keywords: Type 2 diabetes, Cardiovascular disease, SGLT-2 inhibitors, GLP-1 receptor agonists, Income, Education, Socioeconomic status, And socioeconomic position
Research in context.
Evidence before this study
Between 2015 and 2017, sodium-glucose cotransporter-2 inhibitors (SGLT-2i) and glucagon-like-peptide-1 receptor agonists were shown to reduce cardiovascular events in patients with type 2 diabetes and cardiovascular disease. Thus, from 2018, international guidelines recommended a broader use of SGLT-2i or GLP-1RA. The literature suggest that low socioeconomic position may negatively affect initiation of new drugs but also that this association may vary between drugs and target populations. We searched PubMed for relevant publications up to November 18, 2021 using the following search terms: ("socioeconomic factors"[Mesh] or "social class" [Mesh] or "socioeconomic" [tiab] or "social" [tiab] or "income" [tiab] or "education*" [tiab] or "depriv*" [tiab]) and ("Sodium-Glucose Transporter 2 Inhibitors"[Mesh] or "sodium-glucose cotransporter-2" [tiab] or "sglt*" [tiab] or "Glucagon-Like Peptide 1"[Mesh] or "glp1*" [tiab] or "glp-1*" [tiab] or "glucagon-like-peptide-1" [tiab]) and ("diabet*" [tiab] or "Diabetes Mellitus, Type 2"[Mesh]). A few studies have investigated socioeconomic differences in initiation SGLT-2i or GLP-1RA in the period parallel to the publication of the cardiovascular outcome trials (2015-2017). Thus, one Australian study and one multinational study found lower likelihood of initiation of SGLT-2i and GLP-1RA in patients with low area-based socioeconomic position or low educational level. Further, one British study found lower use of SGLT-2i in patients from the most deprived areas. In contrast, another English study had opposite findings, with a higher use of GLP-1RA among the most deprived patients and no disparities in use of SGLT-2i. Yet, this study had a high risk of bias, as they did not account for differential needs of intensification. After international guidelines were updated in 2018, the evidence is even more scarce. Two American studies revealed lower likelihood of initiation of these novel drugs among low income patients. However, to our knowledge, after the guideline recommendations changed in 2018, no large studies have investigated the relationship between socioeconomic position and initiation of SGLT-2i or GLP-1RA in countries with a strong universal healthcare system as found in Scandinavia.
Added value of this study
In this Danish nationwide study, we examined the association between socioeconomic position and initiation of either a SGLT-2i or a GLP-1RA at time of first intensification of antidiabetic treatment among metformin-treated patients with type 2 diabetes. In each time-period (2012-2014, 2015-2017, and 2018-2020), we found pronounced socioeconomic differences in initiation of a SGLT-2i or a GLP-1 RA, with significantly lower probabilities of initiating these drugs among patients with low socioeconomic position. This gap was independent of age, sex, ethnicity, clinically established cardiovascular disease, and specialist care. Moreover, disparities were even present in the subgroup of patients with cardiovascular disease, after guidelines recommended SGLT-2i or GLP-1RA to these patients.
Implications of all the available evidence
The observed socioeconomic disparities in initiation with SGLT-2i and GLP-1RA may widen the future socioeconomic gap in cardiovascular outcomes among patients with type 2 diabetes. The differences in treatment were evident even in a Danish system of universal healthcare coverage and limited costs of medication. These findings underscore the importance of considering socioeconomic disparities in dissemination strategies of novel evidence-based drugs.
Alt-text: Unlabelled box
Introduction
Socioeconomic disparities in risk of major adverse cardiovascular events have been shown among patients with type 2 diabetes (T2D).1, 2, 3 In part, inequalities in diabetes care, including treatment with evidence based cardiovascular medication, may contribute to disparities in cardiovascular outcomes.4 Until recently, cardiovascular preventive medication mainly relied on lipid-lowering and blood pressure-lowering drugs, whereas the effect of glucose-lowering drugs were controversial.5 However, from 2015 to 2017, large cardiovascular outcome trials showed substantial cardiovascular protective effects of sodium-glucose cotransporter-2 inhibitors (SGLT-2i) and glucagon-like-peptide-1 receptor agonists (GLP-1RA) in patients with T2D and cardiovascular disease.6, 7, 8, 9 Thus, Danish and international guidelines were revised in 2018.5,10 While metformin remained the recommended drug at first-line, the recommendations of second-line add-on therapy was altered from an individualized choice to initiation of a SGLT-2i or a GLP-1RA in patients with clinically established cardiovascular disease.5,10
A few studies, conducted in the period concurrent with the large outcome trials, suggest that patients with low socioeconomic position, examined by area scores or educational level, are less likely to receive treatment with SGLT-2i or GLP-1RA.11, 12, 13 Yet, after the guidelines recommendations changed in 2018, the evidence is even more limited.14,15 Disparities in adoption of these new evidence-based drugs may adversely affect cardiovascular outcomes in patients with low socioeconomic position. Therefore, the purpose of this study is to examine potential socioeconomic differences in initiation of a SGLT-2i or a GLP-1RA as second-line add-on drug in a contemporary nationwide cohort of metformin-treated patients with T2D. We hypothesized that patients with low socioeconomic were less likely to initiate a SGLT-2i or a GLP-1RA at time of first intensification compared with patients with high socioeconomic position, even after the update of guidelines.
Methods
Setting
In Denmark, most patients with T2D are managed in primary care, with specialist care mainly restricted to patients with complications or patients not reaching treatment targets. From 2018, additional 25000 patients with T2D were obliged to move from outpatient clinics to primary care after the enforcement of a new Danish national primary care contract.16 The Danish healthcare system is mainly tax-funded and offers free access to medical care and automatic threshold-based reimbursements for prescribed drugs according to the patient's total medicine expenses during a one-year period from the time of first redeemed prescription. Patients fully pay for the first 135 Euros, while expenditures exceeding this amount trigger reimbursements in steps of 50%, 75%, 85%, and 100% with a maximum annual expenditure of 574 Euros (December 2020).17
Data sources
We conducted a nationwide observational study by combining individual-level data from five different Danish administrative registers: 1) the Danish Civil Registration System with information on demographics, migration, vital status, and ethnicity;18 2) the Danish National Patient Register with information on all hospital admissions since 1977 and outpatient visits since 1995, coded according to the International Classification of Diseases (ICD-8 until 1993 and ICD-10 since 1994), and procedures since 1996;19 3) the Danish National Prescription Register with information on all redeemed prescriptions since 1995, coded according to the Anatomical Therapeutic Chemical (ATC) system;20 4) the Danish Income Statistics Register with information on income;21 and 5) the Danish Student Register with information on educational level.22
Study population
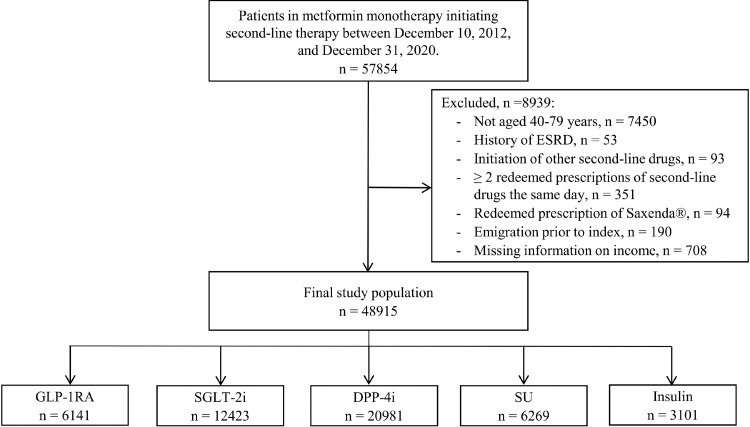
We identified all Danish residents treated with metformin-monotherapy (ATC-code A10AB02), who initiated second-line add-on antidiabetic treatment between December 10, 2012 and December 31, 2020. The study start date (December 10th 2012) was chosen to correspond to the date that SGLT-2i became available for prescription in Denmark (Supplementary table 1). We defined the index date to be the date of initiation of second-line add-on therapy, and defined second-line add-on therapy as: 1) a filled prescription of a second-line drug (see Outcomes and definitions); 2) no prior use of any other glucose-lowering drug besides metformin; 3) at least two prescriptions for metformin, with the latest within 180 days prior to start of second-line therapy; and 4) duration of metformin-monotherapy of ≥ 180 days.
We included patients aged 40 to 79 years at start of second-line treatment. We excluded patients who were prescribed two or more classes of second-line drugs on the same day and all patients initiating the brand-named liraglutide, Saxenda®, as it is only approved for chronic weight management in Denmark. Further, we excluded patients with prescriptions for thiazolidinediones, alpha-glucosidase inhibitors, and meglitinides, since they are rarely prescribed in Denmark. Moreover, alpha-glucosidase inhibitors were withdrawn from the Danish market in 2016.10 Further exclusion criteria are shown in Figure 1.
Figure 1.
Study population flow diagram.
DPP-4i, dipeptidyl peptidase-4 inhibitors; ESRD, end-stage renal disease; GLP-1RA, glucagon-like-peptide-1 receptor agonists; SGLT-2i, sodium-glucose cotransporter-2 inhibitors; SU, sulfonylureas.
Baseline medication and comorbidities
We defined baseline medications as claimed prescriptions in the 180 days prior to initiation of second-line therapy (Supplementary table 3 for ATC-codes). We defined baseline comorbidities from discharge diagnoses codes in the prior 10 years, or by use of condition-specific medications (Supplementary table 2).
Socioeconomic position
We defined socioeconomic position from equivalised disposable income, as income and wealth are suggested as the most proper indicators of socioeconomic position for patients in the active professional life and during the first years of retirement.23 This was measured from the total household income divided by the weighted number of household members according to the Organization for Economic Co-operation and Development (OECD) modified scale (first adult counted as 1, further adults 0.5, and household members younger than 14 years as 0.3).24 We corrected income for inflation to year 2015. To minimise the influence of yearly variations, we used the five-year mean income up to the year prior to the date of initiation of second-line treatment (Supplementary table 2). Within each time period (2012-2014, 2015-2017, and 2018-2020) and age-group (40-64 and 65-79 years), we assigned patients one of three groups of income: lowest quartile (low), two middle quartiles (middle), and the highest quartile (high). We used quartiles to single out the 25% most and 25% least deprived patients at time of first intensification. Further, when investigating subgroups according to sex and ethnicity, quartiles of income were specific according to these variables. In sensitivity analyses, we investigated the highest attained educational level as exposure instead of income. Educational level were divided into three groups in relation to the International Classification of Education (ISCED): 1) basic education (ISCED level 0-2); 2) high school and vocational education (ISCED level 3); and 3) higher education comprising short-term higher education, bachelor's/masters/doctoral degree or equivalent (ISCED level 5 or higher).25
Outcomes and definitions
The primary outcome was defined as prescription of either a SGLT-2i or a GLP-1RA as second-line add-on treatment for type 2 diabetes (identified through ATC codes, see supplementary table 3). The secondary outcomes were initiation of dipeptidyl peptidase-4 inhibitors (DPP-4i), sulfonylurea (SU), insulin, and GLP-1RAs and SGLT-2i as individual outcomes.
In order to account for temporal differences in prescription patterns and in level of evidence due to publication of landmark trials and guideline modifications, we considered three different time periods: 1) from year 2012 to 2014 (prior to publication of the cardiovascular outcome trials); 2) from year 2015 to 2017 (publication of the cardiovascular outcome trials); and 3) from year 2018 to 2020 (update of guidelines). For SGLT-2i and GLP-1RA specifically in the time period 2018-2020, we performed subgroup analyses according to sex, age-group (40-64, 65-79 years), cardiovascular disease status, receiving specialist care, and ethnicity. We chose only to look at this time period, as this period represent the updated guideline recommendations, as well as the most contemporary data on SLGT-2i/GLP-1RA use.
We defined cardiovascular disease as ischemic heart disease, stroke, peripheral vascular disease, or heart failure registered at any time prior to index date (Supplementary table 2). Specialist care for diabetes was defined as out-patient hospital contacts with departments of endocrinology or general internal medicine with diabetes as primary diagnoses (ICD-10 codes E10-E14, O24 (excluding gestational diabetes O24.4) or H36.0).26 The contacts had to be ongoing or finished within 90 days prior to initiation of second-line therapy.
Statistical analyses
We summarized categorical variables using counts and percentages and continuous variables using medians with interquartile ranges (IQR), according to income group. We also stratified baseline characteristics further according to time periods.
We analysed all metformin-monotherapy patients who intensified treatment in any of the three different time periods (2012-2014, 2015-2017, and 2018-2020) according to income group at the date of intensification. For each calendar time period separately, we used multinomial logistic regression to associate income group with the probability of initiating the specific second-line treatments (SGLT-2i, GLP-1RA, DPP-4i, SU, and insulin) at time of first intensification. The model was adjusted for additive effects of age (restricted cubic splines with three knots), sex, ethnicity, cohabitation status, region of residence (one of five administrative regions in Denmark), duration of T2D, specialist care, diagnoses of ischemic heart disease, stroke, heart failure, renal disease, hypertension, and treatment with loop diuretics, statins, and antithrombotics. We obtained the standardised probabilities of initiating the different second-line treatments in each time period as follows. As our reference population we used all patients that started second-line treatment in the period 2018-2020. We applied the multinomial logistic models to predict the treatment probabilities based on the observed characteristics of each patient in the reference population by also (artificially) setting the income group to a given value, i.e., first to "low", then to "middle", and then to "high”. For all income groups in each time period, we reported the average of the predicted probabilities across the reference population. For SGLT-2i and GLP-1RA, we also reported marginal effects as probability ratios between the standardised probabilities in different income groups. 95% confidence intervals (CI) were obtained based on 1000 bootstrap samples. In sensitivity analyses, we examined educational level instead of income.
For the time period 2018-2020, we performed subgroup analyses according to sex, age-group (40-64, 65-79 years), cardiovascular disease status, and receiving specialist care and reported results for SGLT-2i and GLP-1RA (as a combined outcome and as individual outcomes). We repeated these subgroup analyses after excluding initiators of insulin from the reference group. The statistical significance threshold was set at 5%. All analyses were performed in R, version 4.0.3.27
Ethics
Approval for use of data for this study was granted by The Knowledge Centre on Data Protection Compliance – The Capital Region of Denmark (approval number: P-2019-191). Register-based studies do not need ethical approval in Denmark.
Role of the funding source
The work was funded by unrestricted grants from ‘Region Sjaelland Den Sundhedsvidenskabelige Forskningsfond’ and ‘Murermester Lauritz Peter Christensen og hustru Kirsten Sigrid Christensens Fond’ who had no influence on the analyses or manuscript.
Results
Study population and baseline characteristics
The final study population consisted of 48915 patients (Figure 1). Baseline characteristics differed according to income (Table). Compared with low-income patients, high-income patients were more likely to be men. Moreover, they were slightly older, more educated, less likely to live alone, had a lower prevalence of cardiovascular disease, and were more frequently treated with guideline recommended cardiovascular preventative medication (statins and renin-angiotensin system inhibitors). Further, no income differences in specialist care were observed. Similar patterns were observed for each specific time period (Supplementary table 4-6). Yet, expectedly due to the new Danish national primary care contract, the overall proportion of patients receiving specialist care decreased from 13·0 % in 2012-2014 to 5·6% in 2018-2020.
Table.
Baseline characteristics of the 48915 patients with type 2 diabetes according to income group.
| Variable | Total (n=48915) | Low income (n=12231) | Middle income (n=24455) | High income (n=12229) |
|---|---|---|---|---|
| Age, (years) | 62 [54, 69] | 61 [52, 71] | 62 [53, 69] | 62 [56, 68] |
| Male sex, (%) | 30175 (61·7) | 6829 (55·8) | 14821 (60·6) | 8525 (69·7) |
| Income, (Euros) | 29602 [23061, 39064] | 20223 [17806, 21799] | 29603 [26031, 33752] | 47124 [42520, 55170] |
| Educational level, (%) | ||||
| Basic education | 17329 (35·4) | 6063 (49·6) | 9011 (36·8) | 2255 (18·4) |
| High school or vocational | 21746 (44·4) | 4362 (35·7) | 11572 (47·3) | 5812 (47·5) |
| Higher education | 8517 (17·4) | 1130 (9·2) | 3341 (13·7) | 4046 (33·1) |
| Unknown | 1323 (2·7) | 676 (5·5) | 531 (2·1) | 116 (0·9) |
| Ethnicity (%) | ||||
| Native Danish | 42454 (86·8) | 8568 (70·1) | 22185 (90·7) | 11701 (95·7) |
| Immigrant | 6330 (12·9) | 3624 (29·6) | 2199 (9·0) | 507 (4·1) |
| Descendant | 131 (0·3) | 39 (0·3) | 71 (0·3) | 21 (0·2) |
| Living alone (%) | 17676 (36·1) | 6890 (56·3) | 8636 (35·3) | 2150 (17·6) |
| Region of residence (%) | ||||
| Capital Region of Denmark | 12859 (26·3) | 3457 (28·3) | 5609 (22·9) | 3793 (31·0) |
| Region Zealand | 8367 (17·1) | 1936 (15·8) | 4223 (17·3) | 2208 (18·1) |
| Region of Southern Denmark | 10582 (21·6) | 2749 (22·5) | 5612 (22·9) | 2221 (18·2) |
| Central Denmark Region | 11685 (23·9) | 2705 (22·1) | 6107 (25·0) | 2873 (23·5) |
| North Denmark Region | 5422 (11·1) | 1384 (11·3) | 2904 (11·9) | 1134 (9·3) |
| Specialist care (%) | 4392 (9·0) | 1135 (9·3) | 2160 (8·8) | 1097 (9·0) |
| T2D duration (years) | 4 [2, 6] | 4 [2, 6] | 4 [2, 6] | 4 [2, 6] |
| Comorbidities (%) | ||||
| Ischemic heart disease | 8720 (17·8) | 2398 (19·6) | 4379 (17·9) | 1943 (15·9) |
| Peripheral artery disease | 1789 (3·7) | 513 (4·2) | 946 (3·9) | 330 (2·7) |
| Stroke | 2967 (6·1) | 829 (6·8) | 1502 (6·1) | 636 (5·2) |
| Heart failure | 3014 (6·2) | 865 (7·1) | 1562 (6·4) | 587 (4·8) |
| Atrial fibrillation | 3787 (7·7) | 968 (7·9) | 1935 (7·9) | 884 (7·2) |
| Hypertension | 28259 (57·8) | 6898 (56·4) | 14300 (58·5) | 7061 (57·7) |
| COPD/Asthma | 6844 (14·0) | 2110 (17·3) | 3501 (14·3) | 1233 (10·1) |
| Chronic Kidney Disease | 1975 (4·0) | 545 (4·5) | 1023 (4·2) | 407 (3·3) |
| Cancer | 4735 (9·7) | 1106 (9·0) | 2405 (9·8) | 1224 (10·0) |
| Depression | 7403 (15·1) | 2519 (20·6) | 3812 (15·6) | 1072 (8·8) |
| Bipolar/Psychotic disorders | 2328 (4·8) | 952 (7·8) | 1194 (4·9) | 182 (1·5) |
| Pharmacotherapy (%) | ||||
| SGLT-2i | 12423 (25·4) | 2799 (22·9) | 6276 (25·7) | 3348 (27·4) |
| GLP-1RA | 6141 (12·6) | 1296 (10·6) | 3116 (12·7) | 1729 (14·1) |
| DPP-4i | 20981 (42·9) | 5228 (42·7) | 10425 (42·6) | 5328 (43·6) |
| Sulfonylurea | 6269 (12·8) | 2001 (16·4) | 3040 (12·4) | 1228 (10·0) |
| Insulin | 3101 (6·3) | 907 (7·4) | 1598 (6·5) | 596 (4·9) |
| Statins | 34853 (71·3) | 8520 (69·7) | 17526 (71·7) | 8807 (72·0) |
| RASi | 30934 (63·2) | 7268 (59·4) | 15595 (63·8) | 8071 (66·0) |
| Antithrombotics | 13564 (27·7) | 3514 (28·7) | 6774 (27·7) | 3276 (26·8) |
| Anticoagulants | 4172 (8·5) | 1077 (8·8) | 2107 (8·6) | 988 (8·1) |
| Beta blockers | 12831 (26·2) | 3323 (27·2) | 6567 (26·9) | 2941 (24·0) |
| Loop diuretics | 5723 (11·7) | 1720 (14·1) | 3081 (12·6) | 922 (7·5) |
| Thiazide | 6997 (14·3) | 1621 (13·3) | 3633 (14·9) | 1743 (14·3) |
| Ca channel blockers | 13836 (28·3) | 3282 (26·8) | 7015 (28·7) | 3539 (28·9) |
Data are presented as median values (interquartile ranges [IQR]) for continuous variables and as numbers (percentages) for categorical variables. COPD, chronic obstructive pulmonary disease; DPP-4i, dipeptidyl peptidase-4 inhibitors; GLP-1RA, glucagon-like peptide-1 receptor agonists; RAS, renin-angiotensin system; SGLT-2i, sodium glucose co-transporter 2 inhibitors.
Income inequalities in initiation of SGLT-2i or GLP-1RA
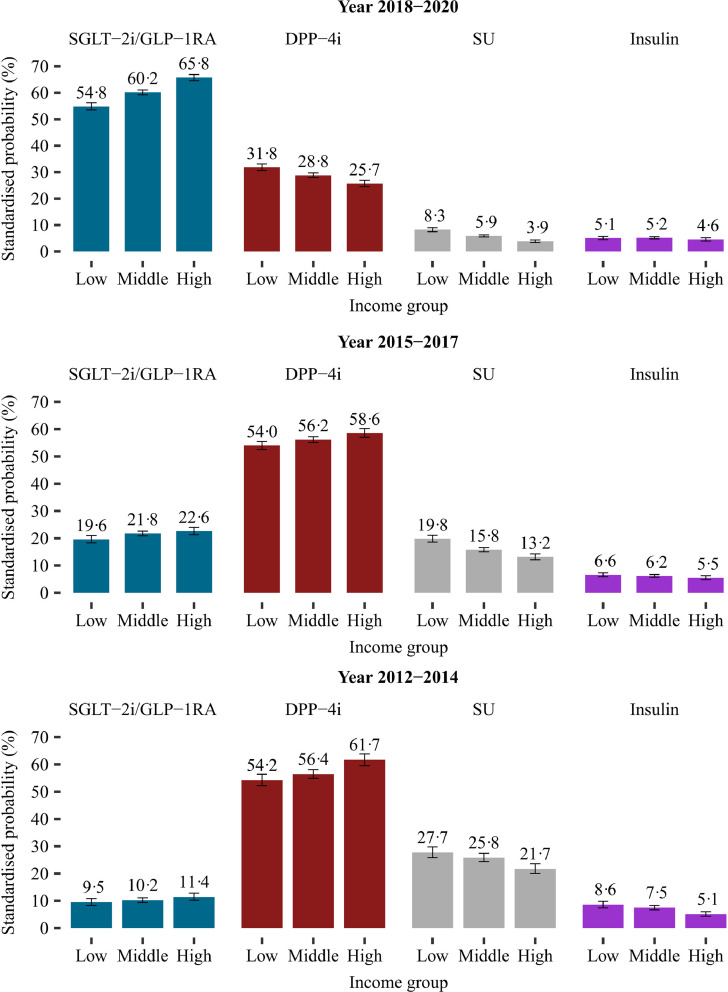
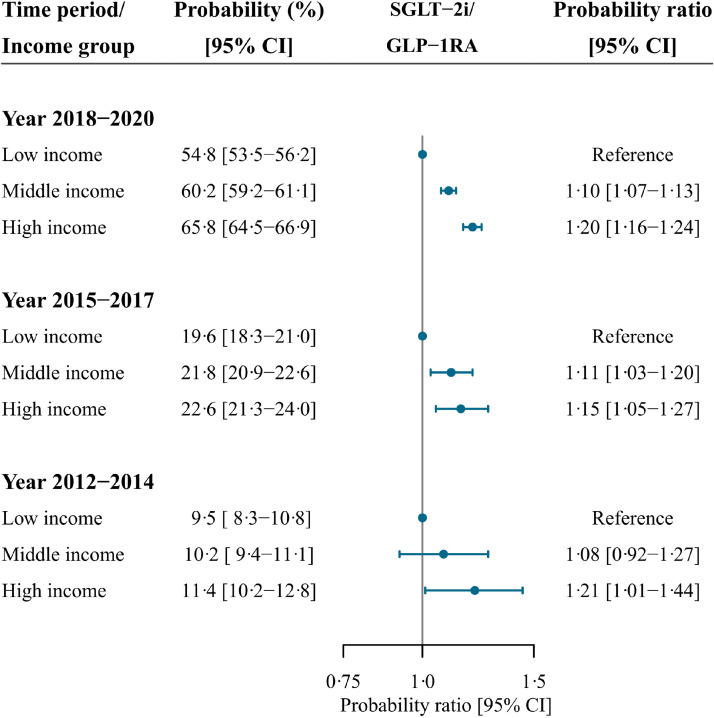
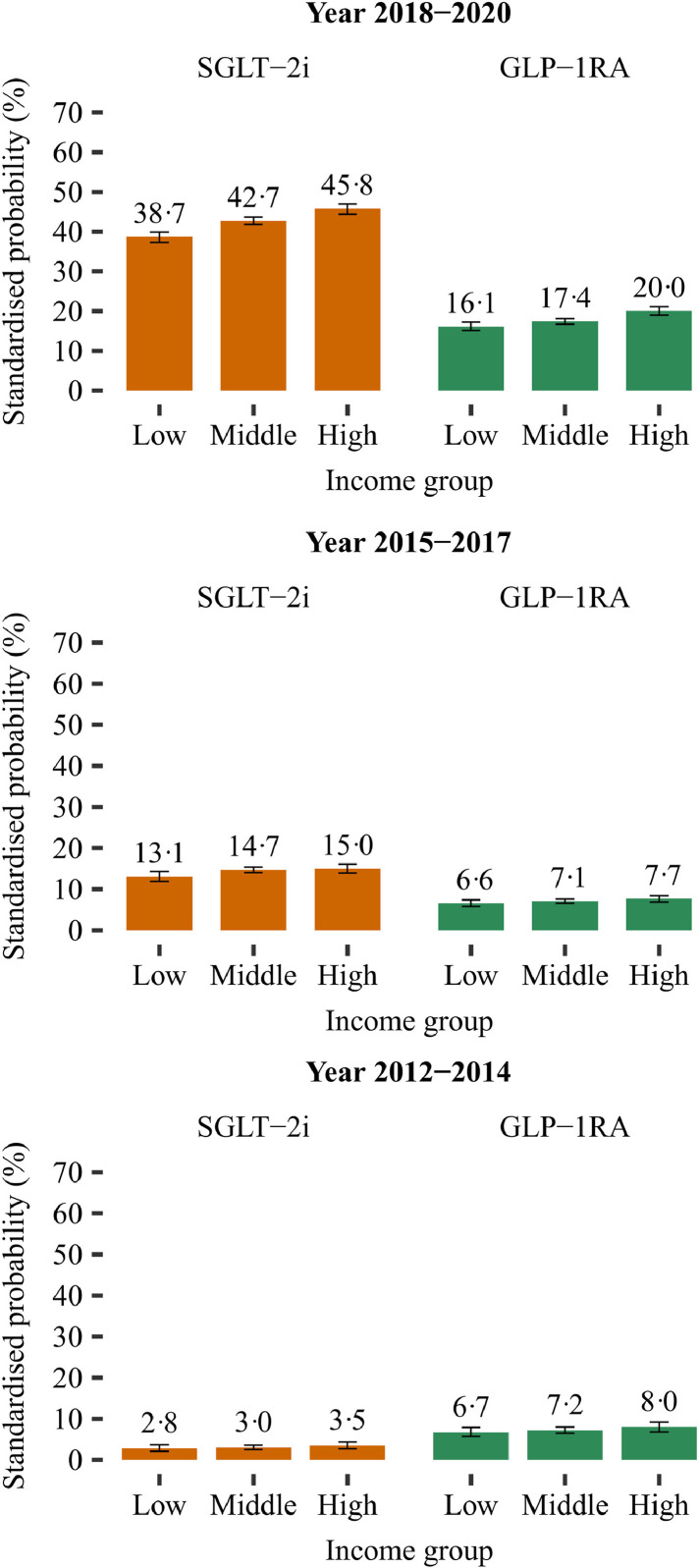
In each time period, the standardised probability of initiating SGLT-2i or GLP-1RA, at time of first intensification, were significantly higher in the highest income group compared with the lowest income group (Figures 2 and 4): probability ratio (PR), 1·21 (CI 1·01-1·44) in 2012-2014; PR, 1·15 (CI 1·05-1·27) in 2015-2017; and PR, 1·20 (CI 1·16-1·24) in 2018-2020. Income inequalities were also present when examining SGLT-2i and GLP-1RA as individual drug classes (Figure 3 and Supplementary figure 1).
Figure 2.
Standardised probability of initiating different classes of glucose-lowering drugs as second-line add-on therapy at time of first intensification according to income group and time period.
Standardised with the respect to the distribution of age, sex, ethnicity, cohabitation status, duration of type 2 diabetes, specialist care, region of residence, ischemic heart disease, peripheral artery disease, stroke, heart failure, renal disease, hypertension, loop diuretics, statins, and antithrombotics of all included patients in the last time period (2018-2020). Three different time periods are considered according to the cardiovascular outcome trials and the update of guidelines: (i) from year 2012 to 2014 (prior to publication of the cardiovascular outcome trials); (ii) from year 2015 to 2017 (publication of the cardiovascular outcome trials); (iii) from year 2018 to 2020 (update of guidelines).
DPP-4i, dipeptidyl peptidase-4 inhibitors; ESRD, end-stage renal disease; GLP-1RA, glucagon-like-peptide-1 receptor agonists; SGLT-2i, sodium-glucose cotransporter-2 inhibitors; SU, sulfonylureas.
Figure 4.
Forest plot depicting standardised probability ratios of initiating either a SGLT-2i or a GLP-1 RA at time of first intensification according to income group and time period.
Standardised with the respect to the distribution of age, sex, ethnicity, cohabitation status, duration of type 2 diabetes, specialist care, region of residence, ischemic heart disease, peripheral artery disease, stroke, heart failure, renal disease, hypertension, loop diuretics, statins, and antithrombotics of all included patients in the last time period (2018-2020).
CI, confidence interval; GLP-1RA, glucagon-like-peptide-1 receptor agonist; SGLT-2i, sodium-glucose cotransporter-2 inhibitor.
Figure 3.
Standardised probability of initiating a SGLT-2i or a GLP-1 RA, as individual drug classes, according to income group and time period.
Standardised with the respect to the distribution of age, sex, ethnicity, cohabitation status, duration of type 2 diabetes, specialist care, region of residence, ischemic heart disease, peripheral artery disease, stroke, heart failure, renal disease, hypertension, loop diuretics, statins, and antithrombotics of all included patients in the last time period (2018-2020). Three different time periods are considered according to the cardiovascular outcome trials and the update of guidelines: (i) from year 2012 to 2014 (prior to publication of the cardiovascular outcome trials); (ii) from year 2015 to 2017 (publication of the cardiovascular outcome trials); (iii) from year 2018 to 2020 (update of guidelines). GLP-1 RA, glucagon-like-peptide-1 receptor agonists; SGLT-2i, sodium-glucose cotransporter-2 inhibitors.
In general, the standardised probability of initiating either a SGLT-2i or a GLP-1RA, at time of first intensification, increased over time (Figure 2). For all income groups in 2018-2020, SGLT-2i were the most prescribed class of second-line glucose-lowering treatment followed by DPP-4is and GLP-1 RAs. Until 2018, the probability of initiating DPP-4i increased with increasing income (Figure 2). In 2018-2020, income showed an inverse association with DDP-4i, with the lowest probability among the highest income group (Figure 2).
Subgroup analyses of patients initiating SGLT-2i or GLP-1RA between 2018 and 2020
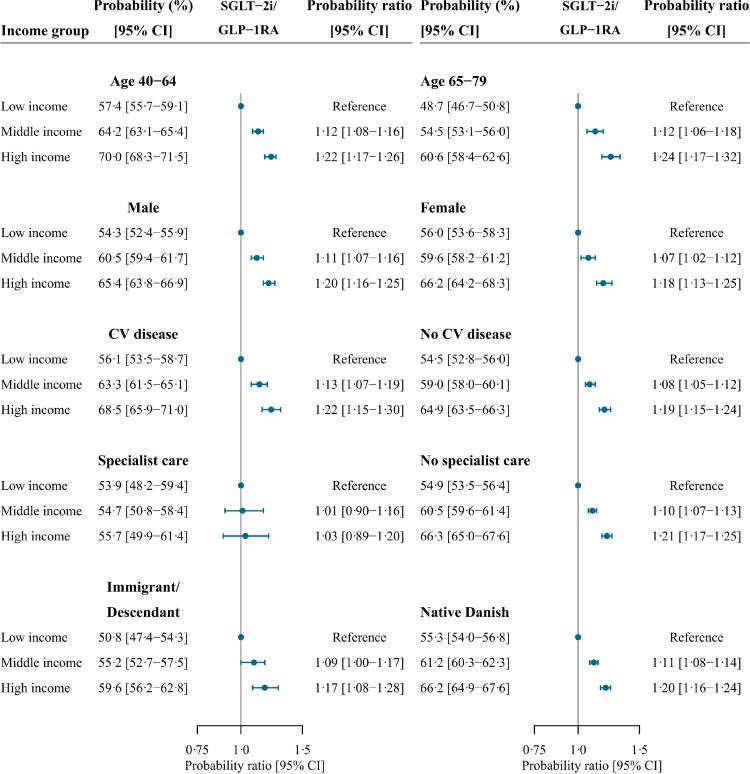
For the time period 2018-2020, income inequalities were consistent in subgroups of age, sex, and ethnicity, with a higher probability of initiating a SGLT-2i or a GLP-1RA with higher income group (Figure 5). In each income group, the estimates were slightly higher for younger patients (40-64 years) compared with older patients (65-79 years) and higher for Danish natives compared with immigrants and descendants. The presence of cardiovascular disease did not modify the socioeconomic disparities in initiation of a SGLT-2i or a GLP-1RA (Figure 5). Among patients receiving specialist care, no significant association with income was observed (Figure 5). Across income groups, we observed slightly lower estimates of initiation of a SGLT-2i or a GLP-1RA in the specialist care group than in the none-specialist care group. Yet, when excluding insulin from the reference group, the preferred drug of choice in patients with very poor glycaemic control, we observed higher estimates across income groups among patients receiving specialist care (Supplementary figure 2). Moreover, among these patients, we observed borderline significant socioeconomic disparities in initiation of a SGLT-2i or a GLP-1RA.
Figure 5.
Forest plot depicting standardised probability ratios of initiating either a SGLT-2i or a GLP-1RA at time of first intensification according to income group and subgroups between 2018 and 2020.
CI, confidence interval; CV disease, cardiovascular disease defined as ischemic heart disease, peripheral artery disease, stroke, or heart failure; GLP-1RA, glucagon-like-peptide-1 receptor agonist; SGLT-2i, sodium-glucose cotransporter-2 inhibitor.
In separate subgroup analyses, the results of SGLT-2i and GLP-1RA were almost consistent with the results of the combined outcome (Supplementary figures 3 and 5). Yet, among immigrants and descendants, we observed pronounced income differences for GLP-1RA, but no significant differences for SGLT-2i. Moreover, after exclusion of insulin from the reference group, we observed borderline income inequalities only for GLP-1RA among patients receiving specialist care (Supplementary figures 4 and 6).
Educational level
Baseline characteristics differed according to educational level in a similar pattern as according to income (Supplementary table 7). Further, in each time-period the probabilities of initiating SGLT-2i or GLP-1RA were higher in patients with highest educational level compared with lowest educational level: PR 1·17 (CI, 0.98-1·39) in 2012-2014, PR 1·19 (CI, 1·10-1·30) in 2015-2017, and PR 1·08 (CI, 1·04-1·11) in 2018-2020 (Supplementary figures 7-8).
Discussion
This nationwide study is the first large European study investigating socioeconomic differences in treatment with SGLT-2i and GLP-1RA after guidelines have been modified to recommend these drugs as second-line add-on treatment in patients with T2D and established cardiovascular disease. In each time-period, we found pronounced socioeconomic differences in initiation of a SGLT-2i or a GLP-1RA over other second-line add-on drugs at time of first intensification, with significantly lower probabilities of initiating these drugs among patients with low socioeconomic position. This gap was independent of age, sex, ethnicity, prevalent cardiovascular disease, and specialist care. Further, in the subgroup of patients with cardiovascular disease, inequalities were evident even after the update of guidelines. This indicates that there is much room for improvement and that patients with low socioeconomic position may be less likely to be treated with new evidence-based medication. Of note, these socioeconomic differences were found with the Danish system of universal healthcare coverage and limited costs of medication. Socioeconomic disparities in use of evidence-based medication may be even more prominent in other countries with higher levels of social inequalities. Nevertheless, our results support the inverse equity hypothesis, which postulates that new health interventions are initially adopted by the wealthy individuals and thus increase inequalities.28 Thus, in the long-term, these disparities may widen the socioeconomic gap in cardiovascular outcomes in patients with T2D. Importantly, this gap may widen even further, since patients with low socioeconomic position may have benefitted the most from treatment with SGLT-2i or GLP-1RA, as low socioeconomic position has been linked to several (unmeasured) cardiovascular risk factors such as smoking and higher levels of blood pressure and body mass index, especially among women.29,30
A few studies have investigated socioeconomic differences in use of SGLT-2i or GLP-1RA concurrent with the cardiovascular outcome trials11, 12, 13,31 and two large studies after the update of guidelines in 2018.14,15 The results from these studies are mixed. Further, the variations in study design make interpretation and comparison difficult. We strictly investigated prescription patterns at time of first intensification in metformin-treated patients. In this way, we ensured comparability by accounting for differential needs of intensification across socioeconomic position, as patients with low socioeconomic position may develop more advanced diabetes. Moreover, we were able to reflect recent changes in decision making in clinical practice, to reflect guidelines with metformin as first choice without the biased influence of other drugs at first-line, and to account for relevant patient characteristics at time of intensification.
In contrast to our findings, an English study between 2012 and 2016, found that low area-based socioeconomic position was associated with increased prescriptions of GLP-1RA and all other classes of glucose-lowering drugs, aside from SGLT-2i where no differences were observed.31 However, these results were most likely downwardly biased by a higher need for intensification of antidiabetic treatment in the most deprived patients, as they investigated total prescriptions without considerations of stages of intensification. Moreover, due to non-differential misclassification, using area-based measures of socioeconomic position may have biased the true individual-level effect towards the null. Interpretation of the results were further complicated as British guidelines only recommended SGLT-2i at first intensification if SU were contraindicated and did not recommend GLP-1RA in any case at first instensification.32
One Australian study, one multinational study, and one British study presented results consistent with our findings.11, 12, 13 Despite not considering different stages of antidiabetic treatment, the Australian study found lower likelihood of add-on treatment of SGLT-2i (2013-2015) and GLP-1RA (2010-2015) among patients with T2D and low area-based socioeconomic position.11 The British study (2014-2017) reported significant lower odds of initiating SGLT-2i at second-line add-on therapy on top of metformin in patients from the most deprived areas.13 However, this was evident in a British setting where patients initiating SGLT-2i may represent a selected patient group due to local guidelines.32 Between 2014 and 2016, the multinational study reported lower odds of receiving a SGLT-2i or a GLP-1RA relative to a SU at first time of intensification in patients with low educational level.12 These disparities were observed despite an overrepresentation of patients from urban areas in each country, leading to a possible underestimation of disparities. Yet, the results only reflected the very early adoption of these drugs, since the overall use of SGLT-2i and GLP-1RA as second-line treatment were low (1·3% vs. 4·3 %). In comparison, the use of SGLT-2i and GLP-1RA in our study increased over time across all income groups and were much more widespread (overall use of SGLT-2i and GLP-RA in 2018-2020: 42·5% vs. 17·8%).
Despite the fact that all patients were insured by commercial health plans or Medicare Advance, two large American studies, McCoy et al. (2016-2019) and Eberly et al. (2015-2019), found significantly lower likelihood of overall prescriptions for SGLT-2i and/or GLP-1RA among patients with low zip-coded income.14,15 These results were consistent with our findings. Nevertheless, as heterogeneity exist in commercial health plans, the results from an American setting may not be applicable into a Danish setting with universal health care.
We found a relatively high usage of SGLT-2i or GLP-1RA among patients with cardiovascular disease. Yet, in this subgroup, the probabilities were only modestly higher across socioeconomic position compared with patients without cardiovascular disease. Thus, our results indicate that the positive results from clinical trials in patients with cardiovascular disease may have increased knowledge, awareness, and confidence in prescribing these drugs in general, but also suggest a need for increasing focus on prescribing these beneficial drugs in patients with cardiovascular disease across socioeconomic position.
As a key secondary finding, we found that the initial disparities of prescription of DPP-4i were inversed between 2018 and 2020 after the update of guidelines. As DPP-4i, SGLT-2i, and GLP-1RA are all expensive drugs, this suggest that even when expensive medication is decided, patients with high socioeconomic position receive the most beneficial treatment.
Since guidelines are based on a shared decision-making approach with considerations of patient preferences and differential costs of therapy, it is essential to investigate disparities in adoption of new guideline recommended medication after showing beneficial effects.5,10 Thus, the literature suggests that physicians try to balance efficacy and costs. Yet, physicians seem to be less reluctant to prescribe more costly, but also more effective drugs.33 Nevertheless, costs had less influence in a Danish universal healthcare setting with patient reimbursements as compared with other countries. Socioeconomic differences in health literacy may also in part explain the observed disparities in treatment, as patients with limited health literacy may be less prone to search for new beneficial treatment regimes.34 Another important explanation may be differences between general practitioners (GP), since the vast majority of patients were treated in primary care. These differences in drug choices between GPs may be due to differential options for participations in research, conferences, and symposia and for interactions with pharmaceutical companies.33 Of note, we did not observe disparities in treatment among patients receiving specialist care, suggesting a higher degree of standardisation of treatment across socioeconomic position among specialists. However, these results are likely to be influenced by referral bias, as referred patients are more likely to have very poor glycaemic control, in which insulin would be the first-choice add-on drug. Thus, after excluding patients initiating insulin, although less pronounced as among the non-specialist group, we found borderline significant disparities of SGLT-2i/GLP-1RA among specialists. In separate sub-group analyses among patients receiving specialist care, we found borderline significant disparities in initiation of GLP-1RA but not in initiation of SGLT-2i. This pattern was even more pronounced among immigrants and descendants. This suggests that when it is decided to initiate one of the two new beneficial evidence based drugs, low-income patients, especially among immigrants and descendants and patients with specialist care, are less likely than high-income patients to initiate GLP-1RA. This may be explained by slightly higher costs, slightly larger anti-obesity effects, and by greater administration barriers of GLP-1 RA (subcutaneous injections) as compared with SGLT-2i. In addition, across socioeconomic position, we found consistently lower probabilities of initiating SGLT-2i or GLP-1RA among immigrants and descendants compared with Danish natives. This was mainly driven by differences in GLP-1RA. In addition to differential levels of health literacy, drug prices, and differences in GPs, cultural factors as well as language and physician-patient communication barriers may in particular have contributed to these ethnic disparities.33, 34, 35 Hence, the administration barriers of GLP-1RA may be especially challenging among immigrant patients, particularly in lower socioeconomic groups, but also regardless of socioeconomic position.
Strengths and limitations
The major strengths of our study are the completeness of nationwide individual-level data with minimal risk of selection bias. Yet, our findings should be interpreted in the context of several limitations. First, we cannot completely rule out unmeasured confounding, as we did not have data on important clinical variables such as HbA1c and body mass index. However, the design of the study minimized the influence of this as already discussed. Second, in order to reliably define second-line add-on treatment, patients had to survive as well as not initiate second-line add-on therapy within the first 6 months after initiation of metformin. This selection of patients favoured patients with higher socioeconomic position and may have resulted in more conservative estimates. Third, due to lack of information on HbA1c and the rapid temporal changes in initiation patterns in combination with differential rates of survival and differential needs of intensification, we were not able to reliably determine disparities in receiving effective treatment in time when needed. Thus, we did not investigate the probability of receiving second-line add-on therapy during a certain follow-up period. Yet, we reported clear socioeconomic differences in initiation of new evidence-based drugs among patients with a medical evaluated need of intensification, with a minimal risk of bias. At the same time, we were able to report recent changes in decision making in clinical practice. Fourth, we did not investigate patient adherence after dispensing of prescriptions. However, all patients had already showed initial adherence of metformin. Lastly, due to the observational design of the study, no causal conclusion can be drawn.
Future perspectives and clinical implications
To reduce inequalities in treatment with these newer and more beneficial antidiabetic drugs, multiple interventions are required. First, to increase patient awareness and health literacy, patient-centred interventions, including diabetes self-management education and support, are especially needed in the most deprived patients. Second, focused interventions towards GPs with large number of patients with low socioeconomic position are needed. This may include resources and personnel education. Future studies may answer whether societal costs of such initiatives and increased drug expenses will outbalance the associated health benefits. Reducing inequalities in treatment with SGLT-2i or GLP-1RA may reduce disparities in cardio-renal outcomes in both primary and secondary prevention patients. Thus, influenced by recent cardio-renal clinical trials from 2018 to 2019,36, 37, 38 new updated recommendations from 2019 advocate initiation of SGLT-2i or GLP-1RA in patients with either established cardiovascular disease, renal disease, or at high cardio-renal risk, independently of background therapy, glycaemic control, or individualised treatment goals.39,40 These changes were not incorporated in the minor revisions of Danish guidelines from October 2019, which primarily included initiation of a SGLT-2i in preference to a GLP-1RA in patients with renal disease or heart failure at time of intensification.41 As Danish guidelines may be modified in accordance with the international guidelines, future studies may answer whether socioeconomic differences in usage of these drugs will exist in this future setting.
Conclusions
In patients with T2D, low socioeconomic position was consistently associated with a lower probability of initiating treatment with a SGLT-2i or a GLP-1RA at time of first intensification, even after guidelines recommended these drugs to patients with established cardiovascular disease. These differences in initiation of new evidence based cardiovascular medications may contribute to future socioeconomic disparities in cardiovascular outcomes. Future efforts to reduce disparities in adoption of new evidence based drugs are needed.
Declaration of interests
ACF has received grants from ‘Region Sjaelland Den Sundhedsvidenskabelige Forskningsfond’ and from ‘Murermester Lauritz Peter Christensen og hustru Kirsten Sigrid Christensens Fond’ for the conduct of this study. EF has received grants from Novo Nordisk, unrelated to the submitted work. LK has received speaker honorarium from Novo Nordisk, AstraZeneca, Novartis, and Boehringer Ingelheim, unrelated to the submitted work. CTP has received grants from Bayer and Novo Nordisk, unrelated to the submitted work. GHG has Novo Nordisk stocks. MS has received speaker honorarium from Novo Nordisk, AstraZeneca, Boehringer Ingelheim, and Novartis, unrelated to the submitted work. NEB has received grants from Novo Nordisk, unrelated to the submitted work. ACR has received speaker honorarium from Novartis, unrelated to the submitted work. All other authors have no declaration of interests.
Acknowledgments
Contributors
ACF, MS, TAG, NEB, and ACR contributed to the conception or design of the work. ACF, ACR, and TAG managed the data and the statistical analyses. ACF drafted the manuscript. All authors critically reviewed the results, revised the manuscript, gave final approval and agree to be accountable for all aspects of the work ensuring integrity and accuracy.
Data sharing
Data are available at the highly protected servers held by Statistics Denmark, the central authority on Danish statistics. Data are anonymised, in accordance with Danish law, and all analyses need to be performed at the servers of Statistics Denmark. Only Danish research environments are granted authorisation from Statistics Denmark. However, foreign researchers, can get access through an affiliation to a Danish authorised research environment.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanepe.2022.100308.
Appendix. Supplementary materials
References
- 1.Jackson CA, Jones NR, Walker JJ, et al. Area-based socioeconomic status, type 2 diabetes and cardiovascular mortality in Scotland. Diabetologia. 2012;55(11):2938–2945. doi: 10.1007/s00125-012-2667-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rawshani A, Svensson AM, Zethelius B, Eliasson B, Rosengren A, Gudbjörnsdottir S. Association Between Socioeconomic Status and Mortality, Cardiovascular Disease, and Cancer in Patients With Type 2 Diabetes. JAMA internal medicine. 2016;176(8):1146–1154. doi: 10.1001/jamainternmed.2016.2940. [DOI] [PubMed] [Google Scholar]
- 3.Falkentoft AC, Zareini B, Andersen J, et al. Socioeconomic position and first-time major cardiovascular event in patients with type 2 diabetes: a Danish nationwide cohort study. European journal of preventive cardiology. 2021 doi: 10.1093/eurjpc/zwab065. [DOI] [PubMed] [Google Scholar]
- 4.Brown AF, Ettner SL, Piette J, et al. Socioeconomic position and health among persons with diabetes mellitus: a conceptual framework and review of the literature. Epidemiologic reviews. 2004;26:63–77. doi: 10.1093/epirev/mxh002. [DOI] [PubMed] [Google Scholar]
- 5.Davies MJ, D'Alessio DA, Fradkin J, et al. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes care. 2018;41(12):2669–2701. doi: 10.2337/dci18-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. The New England journal of medicine. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 7.Marso SP, Bain SC, Consoli A, et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. The New England journal of medicine. 2016;375(19):1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 8.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. The New England journal of medicine. 2016;375(4):311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. The New England journal of medicine. 2017;377(7):644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 10.Danish Society for General Practice and Danish Endocrine Society: Pharmacological treatment of type 2 diabetes - aims and algorithms 2018. 2018. https://vejledninger.dsam.dk/media/files/4/guidelines-2018-final.pdf. Accessed 26 November 2021.
- 11.Morton JI, Ilomӓki J, Magliano DJ, Shaw JE. The association of socioeconomic disadvantage and remoteness with receipt of type 2 diabetes medications in Australia: a nationwide registry study. Diabetologia. 2021;64(2):349–360. doi: 10.1007/s00125-020-05304-3. [DOI] [PubMed] [Google Scholar]
- 12.Nicolucci A, Charbonnel B, Gomes MB, et al. Treatment patterns and associated factors in 14 668 people with type 2 diabetes initiating a second-line therapy: Results from the global DISCOVER study programme. Diabetes, obesity & metabolism. 2019;21(11):2474–2485. doi: 10.1111/dom.13830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilkinson S, Douglas IJ, Williamson E, et al. Factors associated with choice of intensification treatment for type 2 diabetes after metformin monotherapy: a cohort study in UK primary care. Clinical epidemiology. 2018;10:1639–1648. doi: 10.2147/CLEP.S176142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eberly LA, Yang L, Eneanya ND, et al. Association of Race/Ethnicity, Gender, and Socioeconomic Status With Sodium-Glucose Cotransporter 2 Inhibitor Use Among Patients With Diabetes in the US. JAMA network open. 2021;4(4) doi: 10.1001/jamanetworkopen.2021.6139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCoy RG, Van Houten HK, Deng Y, et al. Comparison of Diabetes Medications Used by Adults With Commercial Insurance vs Medicare Advantage, 2016 to 2019. JAMA network open. 2021;4(2) doi: 10.1001/jamanetworkopen.2020.35792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.OVERENSKOMST om almen praksis. PRAKTISERENDE LÆGERS ORGANISATION. 2018. https://www.laeger.dk/sites/default/files/55101_almen_praksis_2018_uden_vejledning.pdf. Accessed 4 November 2021.
- 17.Danish Medicines Agency. Reimbursement thresholds. https://laegemiddelstyrelsen.dk/en/reimbursement/calculate-reimbursement/reimbursement-thresholds/. Accessed 4 November 2021.
- 18.Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. European journal of epidemiology. 2014;29(8):541–549. doi: 10.1007/s10654-014-9930-3. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clinical epidemiology. 2015;7:449–490. doi: 10.2147/CLEP.S91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pottegård A, Schmidt SAJ, Wallach-Kildemoes H, Sørensen HT, Hallas J, Schmidt M. Data Resource Profile: The Danish National Prescription Registry. International journal of epidemiology. 2017;46(3):798. doi: 10.1093/ije/dyw213. -f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baadsgaard M, Quitzau J. Danish registers on personal income and transfer payments. Scandinavian journal of public health. 2011;39(7 Suppl):103–105. doi: 10.1177/1403494811405098. [DOI] [PubMed] [Google Scholar]
- 22.Jensen VM, Rasmussen AW. Danish Education Registers. Scandinavian journal of public health. 2011;39(7 Suppl):91–94. doi: 10.1177/1403494810394715. [DOI] [PubMed] [Google Scholar]
- 23.Galobardes B, Shaw M, Lawlor DA, Lynch JW, Davey Smith G. Indicators of socioeconomic position (part 1) Journal of epidemiology and community health. 2006;60(1):7–12. doi: 10.1136/jech.2004.023531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Organisation for Economic Co-operation and Development. WHAT ARE EQUIVALENCE SCALES? http://www.oecd.org/els/soc/OECD-Note-EquivalenceScales.pdf. Accessed 26 May 2021.
- 25.ISCED 2011. http://uis.unesco.org/sites/default/files/documents/international-standard-classification-of-education-isced-2011-en.pdf. Accessed 13 November 2021.
- 26.Carstensen B, Kristensen JK, Marcussen MM, Borch-Johnsen K. The National Diabetes Register. Scandinavian journal of public health. 2011;39(7 Suppl):58–61. doi: 10.1177/1403494811404278. [DOI] [PubMed] [Google Scholar]
- 27.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2021. R: A language and environment for statistical computing.https://www.R-project.org/ Accessed 25 May 2021. [Google Scholar]
- 28.Victora CG, Vaughan JP, Barros FC, Silva AC, Tomasi E. Explaining trends in inequities: evidence from Brazilian child health studies. Lancet (London, England) 2000;356(9235):1093–1098. doi: 10.1016/S0140-6736(00)02741-0. [DOI] [PubMed] [Google Scholar]
- 29.Ariansen I, Mortensen LH, Graff-Iversen S, Stigum H, Kjøllesdal MK, Næss Ø. The educational gradient in cardiovascular risk factors: impact of shared family factors in 228,346 Norwegian siblings. BMC public health. 2017;17(1):281. doi: 10.1186/s12889-017-4123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newton S, Braithwaite D, Akinyemiju TF. Socio-economic status over the life course and obesity: Systematic review and meta-analysis. PloS one. 2017;12(5) doi: 10.1371/journal.pone.0177151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whyte MB, Hinton W, McGovern A, et al. Disparities in glycaemic control, monitoring, and treatment of type 2 diabetes in England: A retrospective cohort analysis. PLoS medicine. 2019;16(10) doi: 10.1371/journal.pmed.1002942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Institute for Heatlh and Care Excellence (NICE) 2015. Type 2 diabetes in adults: management.https://www.nice.org.uk/guidance/ng28/resources/type-2-diabetes-in-adults-management-pdf-1837338615493 Accessed 13 December 2021. [PubMed] [Google Scholar]
- 33.Lublóy Á. Factors affecting the uptake of new medicines: a systematic literature review. BMC health services research. 2014;14:469. doi: 10.1186/1472-6963-14-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magnani JW, Mujahid MS, Aronow HD, et al. Health Literacy and Cardiovascular Disease: Fundamental Relevance to Primary and Secondary Prevention: A Scientific Statement From the American Heart Association. Circulation. 2018;138(2):e48–e74. doi: 10.1161/CIR.0000000000000579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mikhail N, Wali S, Brown AF. Ethnic Disparities in Diabetes. Endocrinology and metabolism clinics of North America. 2021;50(3):475–490. doi: 10.1016/j.ecl.2021.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet (London, England) 2019;394(10193):121–130. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 37.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. The New England journal of medicine. 2019;380(24):2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 38.Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet (London, England) 2018;392(10157):1519–1529. doi: 10.1016/S0140-6736(18)32261-X. [DOI] [PubMed] [Google Scholar]
- 39.Buse JB, Wexler DJ, Tsapas A, et al. 2019 Update to: Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes care. 2020;43(2):487–493. doi: 10.2337/dci19-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. European heart journal. 2020;41(2):255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 41.Danish Society for General Practice and Danish Endocrine Society: Pharmacological treatment of type 2 diabetes - aims and algorithms 2018. 2019. https://endocrinology.dk/nbv/diabetes-melitus/behandling-og-kontrol-af-type-2-diabetes/. Accessed 6 May 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.