Abstract
Purpose
Compared with computed tomography, magnetic resonance (MR) image guidance offers significant advantages for radiation therapy (RT) that may be particularly beneficial for reirradiation (reRT). However, clinical outcomes of MR-guided reRT are not well described in the published literature.
Methods and Materials
We performed a single-institution retrospective safety and efficacy analysis of reRT patients treated on the MRIdian Linac to targets within the abdomen or pelvis using continuous intrafraction MR-based motion management with automatic beam triggering. Fiducial markers were not used.
Results
We evaluated 11 patients who received prior RT to a median of 50 Gy (range, 30-58.8 Gy) in 25 fractions (range, 5-28 fractions). The median interval to reRT was 26.8 months. The most frequently retreated sites were nodal metastases (36.4%) and pancreatic cancer (27.3%). The median reRT dose was 40 Gy (range, 25-54 Gy) in 6 fractions (range, 5-36 fractions); ultrahypofractionation (63.6%) was more common than hyperfractionation (36.4%). Daily on-table adaptive replanning was used for 3 patients (27.3%). With a median of 14 months’ follow-up from reRT completion (range, 6-32 months), the median and 1-year freedom from local progression were 29 months and 88.9%, respectively, and the median and 1-year overall survival were 17.5 months and 70.0%, respectively. One patient (9.1%) experienced acute grade 2 toxic effects; there were no acute or late treatment-related toxic effects of grade 3 or greater.
Conclusions
Magnetic resonance–guided reRT appeared to be feasible and may facilitate safe dose escalation. Additional follow-up is needed to better assess long-term efficacy and late toxic effects. Prospective evaluation of this novel treatment strategy is warranted.
Introduction
Reirradiation (reRT) may provide significant benefit for carefully selected patients with locally recurrent or progressive cancer. However, it must be used with caution because of potentially severe or fatal toxic effects from cumulatively high organ-at-risk (OAR) doses.1,2 As such, reRT is a complex undertaking in which an appropriate balance must be realized between delivering an effectively high target dose while simultaneously achieving appropriately low OAR doses. Prescription doses for ReRT are typically modest out of necessity to prioritize patient safety.3,4 However, safely achieving prescription dose intensification may improve long-term local control (LC) and also overall survival (OS).4, 5, 6
During the past several decades, technological advancements have improved the therapeutic ratio of reRT through improved image guidance, motion management, and more highly conformal delivery techniques.7, 8, 9, 10 The recent advent of magnetic resonance (MR)–guided RT has further contributed to improving outcomes of patients,11 especially those with cancers in high-risk anatomic locations such as the pancreas and central lung.12,13
The unique imaging and on-table adaptive replanning capabilities of MR-guided RT are ideal to minimize OAR dose during reRT and potentially reduce severe toxic effects, although published clinical outcomes of this treatment strategy are limited to case reports.14,15 The purpose of this analysis was to report our institutional MR-guided reRT outcomes.
Methods and Materials
After obtaining institutional review board approval, we performed a single-institution retrospective analysis of patients who received reRT to the abdomen or pelvis using the ViewRay MRIdian Linac (ViewRay Inc, Oakwood Village, OH) between April 2018 and December 2020. Patient, tumor, treatment, toxic effect, and disease progression details were evaluated.
Simulation for reRT on the MRIdian Linac included a planning 0.35 T balanced steady-state free precession sequence (TrueFISP) MR scan acquired over at least 17 to 25 seconds and immediately followed by a planning computed tomography (CT) scan. Simulation was typically done in the supine position for abdominal targets and in the prone position for pelvic targets to facilitate small bowel displacement and sparing. Fiducial markers were not placed because the treated lesions were directly visualized throughout treatment using continuous intrafraction sagittal plane MR imaging at 4 to 8 frames per second. Intravenous or oral contrast were not used because both the target and OARs can be well visualized without contrast on the TrueFISP MR scan, which served as the primary scan for segmentation and treatment planning.
Target volume and OAR contours were delineated on the TrueFISP MR simulation scan and exported to the MRIdian treatment planning system. The simulation CT was exported to the MRIdian treatment planning system and deformably registered to the simulation MR scan for electron density information for dose calculation purposes. For some cases, bulk density assignment to the vertebral bodies as bone, external as water, and any abdominal gas as air was used to account for changes in anatomy between the simulation CT and MR image (MRI).
The gross tumor volume (GTV) was defined as the visible tumor on diagnostic and simulation scans. For most patients (8 of 11), a clinical target volume was not used; for the 3 others, it was defined by a 5- to 10-mm uniform expansion from the GTV. The planning target volume (PTV) was defined by a 3- to 5-mm uniform expansion of the GTV (or clinical target volume if present). No internal target volume was used.
All patients were treated with continuous intrafraction MR imaging. The treatment delivery system was set so that if more than 5% of the tracking structure, which included the GTV, extended beyond a 3-mm boundary, the beam would be automatically held until the tracking structure returned inside the boundary, at which time treatment delivery would resume (Fig 1). Patients with abdominal targets subject to potentially significant respiratory motion were treated in breath hold, whereas patients with pelvic targets were usually treated with free-breathing beam gating because they were not subject to significant respiratory motion. On-table adaptive replanning was performed for selected patients, especially when gross disease was located within 5 mm of gastrointestinal OARs on simulation scans, to ensure that OAR dose constraints were not exceeded either owing to interfraction anatomic changes or otherwise to compensate for changes in tumor anatomy and position.
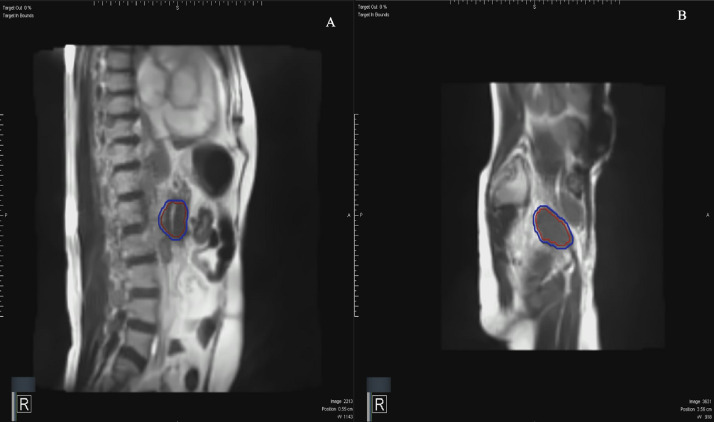
Fig. 1.
Intrafraction motion management in the sagittal plane acquired using magnetic resonance imaging on the MRIdian Linac (ViewRay Inc, Oakwood Village, OH) at 8 frames per second with the tracking structure encompassing gross disease in the red region of interest for (A) pancreas and (B) pelvic lymph node reirradiation. Automated beam gating occurred when greater than 5% of the deformed red region of interest moved outside the static 3-mm expanded blue tracking boundary region of interest.
Because different dose fractionation reRT schedules were used, the biologically equivalent schedule in 2-Gy fractions (EQD2) was calculated for all patients using the following formula: EQD2 = D × (d + α/β) / (2.0 + α/β), where D was the total dose, d was the dose per fraction, and α/β was equal to 3 Gy for OARs and 10 Gy for the tumor. There was variability in OAR dose constraints used across different radiation oncologists, given the lack of defined constraints for reRT in the literature. A priority, however, for all physicians was to limit the volume of tissue outside of the PTV that received a dose greater than the prescription dose. Cumulative dose constraints across the original and reRT plans for gastrointestinal luminal organs were not used for plan optimization, although they were reviewed for awareness and routinely exceeded 90 to 100 Gy EQD23. Dose constraints for both ultrahypofractionated and hyperfractionated plans typically included the duodenum, stomach, small bowel, large bowel, and rectum, each V30 to V35 ≤ 0.5 cm3 and V33 to V38 ≤ 0.03 cm3 on the reRT plan; bladder, V30 to V35 ≤ 0.5 cm3 on the reRT plan; spinal cord, cumulative V50 to V60 ≤ 0.03 cm3 EQD23; kidneys, cumulative mean ≤ 18 Gy EQD23; and liver, cumulative mean ≤ 30 Gy EQD23.16
Statistical analysis was performed using SAS, version 9.4 (SAS Institute, Cary, North Carolina). Clinical outcomes were assessed using the Kaplan-Meier method from the date of reRT start. Freedom from local progression (FFLP) was defined as the time to local progression and was assessed using Response Evaluation Criteria in Solid Tumors, version 1.1. Progression free survival (PFS) was the time to the first occurrence of local progression, distant progression, or death. Overall survival (OS) was calculated as the time to death or otherwise last follow-up. Toxic effects were assessed per the Common Terminology Criteria for Adverse Events, version 5.0. Acute toxic effects were defined as being present during or within 3 months from the completion of reRT. All toxic effects were assessed by a physician at least once weekly during RT and at each follow-up visit, usually performed at 3-month intervals after completion of reRT.
Results
A total of 11 patients were evaluated (Table 1). The median age was 62 years (range, 34-88 years), and the majority of patients (90.9%) had an Eastern Cooperative Oncology Group performance status of 0 to 1. Most had a primary malignancy of the rectum (36.4%), pancreas or bile duct (36.4%), or cervix (18.2%). Eight patients (72.7%) experienced local progression after definitive surgery and either neoadjuvant or adjuvant RT, and the others experienced progression after definitive chemoradiation therapy. The median total prescription dose for the prior RT course was 50 Gy (range, 30-58.8 Gy) in 25 fractions (range, 5-28 fractions). For most patients (63.6%), chemotherapy was given before reRT after a diagnosis of local progression, whereas surgical resection was also performed for 2 patients (18.2%).
Table 1.
Patient, tumor, prior therapy, and reirradiation characteristics
| Characteristic | Patients, no. (%)* (N = 11) |
|---|---|
| Age, median (range), y | 62 (34-88) |
| Sex | |
| Male | 9 (81.8) |
| Female | 2 (18.2) |
| ECOG performance status before reRT | |
| 0 | 6 (54.5) |
| 1 | 4 (36.4) |
| 2 | 1 (9.1) |
| Primary tumor site | |
| Rectum | 4 (36.4) |
| Pancreas or bile duct | 4 (36.4) |
| Cervix | 2 (18.2) |
| Lung | 1 (9.1) |
| Histology | |
| Adenocarcinoma | 8 (72.7) |
| Squamous cell carcinoma | 2 (18.2) |
| Non-small cell carcinoma | 1 (9.1) |
| Initial RT dose and fractionation | |
| Total dose, median (Gy) | 50 (30-58.8) |
| Number of fractions, median | 25 (5-28) |
| EQD210 | 56 (40-60) |
| EQD23 | 56 (43.2-100) |
| Definitive surgery after initial RT | 8 (72.7) |
| Low anterior resection | 4 (50) |
| Pancreaticoduodenectomy | 2 (25) |
| Hysterectomy | 1 (12.5) |
| Total pelvic exenteration | 1 (12.5) |
| Interval from initial RT end to reRT start, median (range), mo | 26.8 (7.6-59.0) |
| Reirradiation site | |
| Lymph node | 4 (36.4) |
| Pancreas | 3 (27.3) |
| Presacral | 2 (18.2) |
| Rectum | 1 (9.1) |
| Superior mesenteric artery | 1 (9.1) |
| Reirradiation target volumes, median (range), cm3 | |
| GTV | 24.3 (7.6-142.21) |
| PTV | 38.8 (26.3-179) |
| Reirradiation dose and fractionation, median (range) | |
| Total dose, Gy | 40 (25-54) |
| Fractions, No. | 6 (5-36) |
| EQD210 | 44.7 (31.3-83.3) |
| EQD23 | 56.1 (34.3-83.3) |
| Reirradiation fractionation | |
| Ultrahypofractionation | 7 (63.6) |
| Hyperfractionation | 4 (36.4) |
| Concurrent chemotherapy during reirradiation | |
| Xeloda | 4 (36.4) |
| None | 7 (63.6) |
| Tumor resection after reRT | 0 |
Abbreviations: ECOG = Eastern Cooperative Oncology Group; EQD2 = equivalent dose in 2-Gy fractions; GTV = gross tumor volume; RT = radiation therapy; reRT = reirradiation; PTV = planning target volume.
Data are presented as the number and percentage of patients unless otherwise indicated.
The prior RT and reRT delivered to each patient is summarized in Table 2. The median interval from the completion of prior RT to the start of reRT was 26.8 months (range, 7.6-59.0 months). Most commonly, reRT was delivered to abdominal or pelvic nodal metastasis (36.4%), the pancreas (27.3%), or a presacral recurrence (18.2%). The median GTV and PTV volumes were 24.3 cm3 (range, 7.6-142.21 cm3) and 38.8 cm3 (range, 26.3-190.2), respectively.
Table 2.
Summary of patients treated with reirradiation on the ViewRay MRIdian Linac
| Patient number | Age | Primary cancer | Prior RT dose, median, Gy/fx | Interval to reRT,mo | ReRT location | ReRT PTV, cm3 | ReRT total dose/ fx | ReRT schedule | ReRT EQD210, median, Gy | ReRT EQD23, median, Gy | On-table adaptive replanning | Concurrent chemotherapy | Local progression | Grade ≥3 toxic effects |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 88 | Pancreas | 30/5 | 12.8 | Pancreas | 31.31 | 25/5 | QD | 31.3 | 40 | No | No | No | No |
| 2 | 58 | Rectum | 50.4/28 | 30.1 | Presacral | 160.71 | 54/36 | bid | 51.8 | 48.6 | No | Xeloda | 21.9 mo after reRT | No |
| 3 | 62 | Rectum | 45/25 | 26.8 | LN | 33.24 | 40.8/34 | bid | 38.1 | 34.3 | No | Xeloda | 32.3 mo after reRT | No |
| 4 | 60 | Lung | 40/5 | 7.6 | LN | 43.4 | 40/5 | QD | 60 | 88 | Yes | No | No | No |
| 5 | 70 | Pancreas | 59.4/33 | 11.9 | Pancreas | 190.2 | 50/5 | QD | 83.3 | 130 | Yes | No | No | No |
| 6 | 34 | Rectum | 58.8/28 | 59.0 | Presacral | 38.84 | 42/28 | bid | 40.3 | 37.8 | No | Xeloda | 8 mo after reRT | No |
| 7 | 42 | Cervix | 59.4/33 | 31.4 | LN | 28.34 | 33/6 | QD | 42.6 | 56.1 | Yes | No | No | No |
| 8 | 62 | Cervix | 59.4/33 | 10.7 | LN | 26.26 | 32.5/5 | QOD | 44.7 | 61.8 | No | No | No | No |
| 9 | 76 | Pancreas | 50.4/28 | 13.7 | Pancreas | 37.84 | 40/5 | QD | 60 | 88 | No | No | No | No |
| 10 | 80 | Rectum | 50.0/25 | 43.4 | Rectum | 143.45 | 42/28 | bid | 40.3 | 37.8 | No | Xeloda | No | No |
| 11 | 78 | Bile duct | 56/28 | 32.4 | SMA | 53.25 | 40/6 | QOD | 55.6 | 77.3 | No | No | No | No |
Abbreviations: bid = twice daily; EQD2 = equivalent dose in 2-Gy fractions; fx = fraction; LN = lymph node; PTV = planning target volume; QD = daily; QOD = every other day; reRT = reirradiation; RT = radiation therapy.
The median total prescription reRT dose for all patients was 40 Gy (range, 25-54 Gy) in 6 fractions (range, 5-36 fractions). The corresponding median reRT prescription EQD210 and EDQ23 were 44.7 Gy (range, 31.3-83.3 Gy) and 56.1 Gy (range, 34.3-130 Gy), respectively. Ultrahypofractionation (5 or 6 fractions) was used to retreat nearly two-thirds (63.6%) of patients, predominantly to the nodal metastasis or the pancreas. The median prescription dose for those who received ultrahypofractionation was 40 Gy (range, 25-50 Gy) in 5 fractions (range, 5-6 fractions) delivered daily (5 patients) or every other day (2 patients). The corresponding median reRT prescription EQD210 and EDQ23 were 55.6 Gy (range, 31.3-83.3 Gy) and 77.3 Gy (range, 40-130 Gy), respectively. All other patients (36.4%) were treated with hyperfractionation to a median total prescription dose of 42 Gy (range, 30-54 Gy) in 28 fractions (range, 25-36 fractions) twice daily. The corresponding median reRT prescription EQD210 and EDQ23 were 40.3 Gy (range, 38.1-51.8 Gy) and 37.8 Gy (range, 34.3-48.6 Gy), respectively.
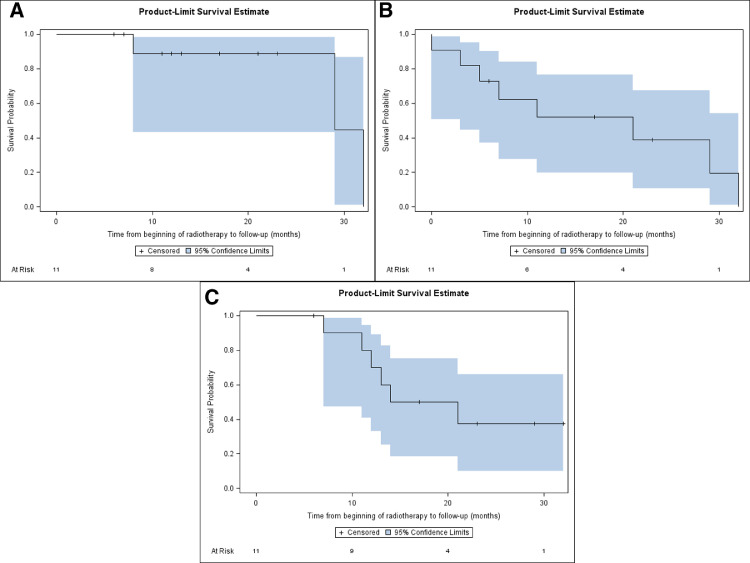
The median follow-up from reRT completion was 14 months (range, 6-32 months). The median and 1-year FFLP for all patients was 29 months and 88.9%, respectively (Fig 2A). The median and 1-year PFS were 21 months and 52.0%, respectively (Fig 2B). Six patients (54.5%) were dead at the time of last follow-up, all owing to distant progression and none because of local progression or reRT. The median and 1-year OS were 17.5 months and 70.0%, respectively (Fig 2C). The only 3 patients during the study period who experienced local progression after reRT had rectal cancer; all were retreated using hyperfractionation to a median of 42 Gy (range, 40.8-54 Gy) in 34 fractions (range, 28-36 fractions) twice daily and experienced local progression at a median of 21.9 months (range, 8.1-32.3 months) after reRT on the MRIdian Linac.
Fig. 2.
Kaplan-Meier plots. (A) Freedom from local progression. (B) Progression-free survival. C, Overall survival from the start of reirradiation.
Treatment was well tolerated with no observed or reported acute or late toxic effects of grade 3 or greater. One patient with pancreas cancer who was heavily pretreated with chemotherapy and then definitive chemoradiation experienced acute grade 2 fatigue after reRT. All other patients experienced no toxic effects greater than grade 1.
Discussion
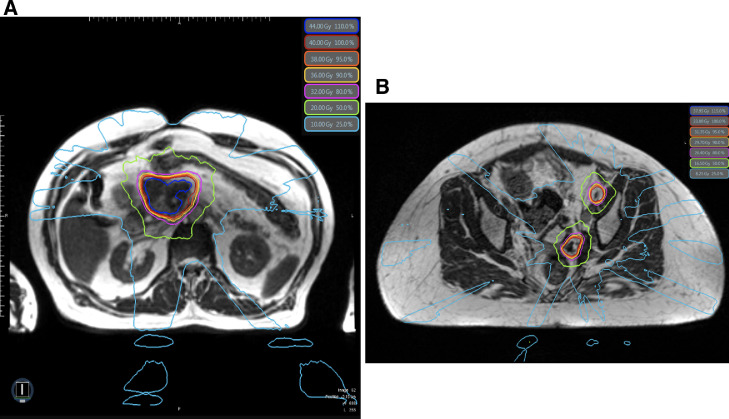
Magnetic resonance–guided RT represents a fundamental paradigm shift in how RT is fundamentally delivered by providing superior soft-tissue visualization compared with CT, continuous intrafraction imaging, advanced motion management capabilities, and on-table adaptive replanning.11 This novel technology can achieve excellent OAR sparing by reducing uncertainty margins, avoiding internal target volumes, and accounting for interfraction changes in anatomy through on-table adaptive replanning that is completed within only a few minutes (Fig 3). Although the dosimetric advantages of MR-guided RT may improve the therapeutic ratio of reRT, the only published clinical outcomes to our knowledge are case reports. Investigators from the University of California, Los Angeles, suggested the feasibility of pelvic reRT on an MR-Cobalt device in a patient with recurrent rectal cancer who was prescribed 35 Gy in 5 fractions, although follow-up was very short.14 We previously reported no significant toxic effects in a patient with pancreas cancer reirradiated to 50 Gy in 5 fractions with on-table adaptive replanning on the MRIdian Linac after previously receiving 59.4 Gy in 33 fractions with concurrent chemotherapy.15
Fig. 3.
(A) Isodose lines from a pancreas reirradiation plan prescribed to 40 Gy in 5 fractions. (B) Isodose lines from a pelvic lymph node reirradiation plan prescribed to 33 Gy in 6 fractions. Daily on-table adaptive replanning was indicated in nearly all fractions for both patients to ensure that organ-at-risk dose constraints were met.
To our knowledge, we report the first case series of abdominopelvic reRT delivered on an MR-Linac. With a median follow-up of 14 months from reRT completion, our early experience has been encouraging compared with that of other published reRT studies (Table 3). In this study, ReRT was tolerated surprisingly well, with only 1 patient experiencing toxic effects of grade 2 and none experiencing toxic effects of grade 3 or greater. Our 1-year FFLP of nearly 90% is notable, especially because no patient had surgery after reRT.6
Table 3.
Select reirradiation studies
| Authors | Publication year | N | Primary cancer | Prior RT dose, median, Gy/fx, | Interval to reRT, median, mo | reRT total dose/fx, median | reRT EQD210, median, Gy median | reRT EQD23, median, Gy | Surgery after reRT | Follow-up, median, mo | Local control | Overall survival | Acute or late grade ≥3 toxic effects |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Valentini et al2 | 2006 | 59 | Rectum | 50.4/28 | 27 | 40.8/34 | 38.1 | 34.3 | 50.8% | 36 | 1-y 76.3% | 1 y (87.5%) | 5.1%/1.7% |
| Tao et al6 | 2017 | 102 | Rectum | 50.4/28 | 30 | 39/26 | 37.4 | 35.1 | 45% | 28 | 3-y 40% | 3 y (39%) | NR/34% |
| Koroulakis et al10 | 2020 | 28 | Rectum | 54/30 | 48.5 | 44.4/NR | NA | NA | 21.4% | 28.6 | 1-y 66.3% | 1 y (81.8%) | 10.7%/21.4% |
| Lominska et al24 | 2012 | 28 | Pancreas | 50.4/28 | NR | 22.5/3 | 32.8 | 47.3 | 0% | 5.9 | 1-y 85.7% | Median 5.9 mo | 0%/7.1% |
| Dagoglu et al25 | 2016 | 30 | Pancreas | 50.4/28 | 18 | 25/5 | 31.3 | 40 | 0% | 11 | 1-y 78% | 1 y (50%) | 10%/7% |
| Koong et al26 | 2017 | 23 | Pancreas | 50.4/28 | 13 | 25/5 | 31.3 | 40 | 0% | 28 | 1-y 81% | Median 8.5 mo | 8.7%/0% |
| Hunt et al4 | 2018 | 24 | Various | 45/25 | 27.9 | 39/26 | 37.4 | 35.1 | 0% | 16.8 | 1-y 38% | 1 y (50%) | 16.7%/NR |
| Abusaris et al18 | 2012 | 33 | Various | NR | NR | 32/4 | 45.3 | 64 | 0% | 15 | 1-y 64% | 1 y (52%) | 0%/0% |
| Current study | 2021 | 11 | Various | 50/25 | 26.8 | 40/6 | 44.7 | 56.1 | 0% | 14 | 1-y 88.9% | 1 y (70%) | 0%/0% |
Abbreviations: EQD2 = equivalent dose in 2-Gy fractions; fx = fraction; NA = not available; NR = not reported; reRT = reirradiation; RT = radiation therapy.
Historically, reRT has been prescribed to 30 to 40 Gy EQD210 to minimize the risk of severe toxic effects, although these modest doses are not associated with durable LC, especially in patients who do not proceed to surgery.4 Owing to the exceptional OAR sparing uniquely achieved with an MR-Linac, we used a dose escalation strategy for most patients; nearly all were prescribed ≥40 Gy EDQ210, and nearly half were prescribed ≥50 Gy EDQ210. Our use of higher prescription doses was motivated by data suggesting that this can improve long-term treatment efficacy.4,5,17,18 Koom and colleagues reported significantly higher LC among patients with locally recurrent rectal cancer who were prescribed >50 Gy EQD210 and who did not have subsequent surgery.5 Chung and colleagues showed that a subset of patients with recurrent rectal cancer who were prescribed >50 Gy EQD210 had superior LC, PFS, and OS.17 Both studies used generous PTV margins up to 3 cm, which may have contributed to a high incidence (approximately 40%) of late toxic effects of grade 3 or greater. In contrast, Abusaris and colleagues reported no severe toxic effects using stereotactic body radiation therapy (SBRT) reRT with only 2- to 3-mm PTV margins; >60 Gy EQD210 was associated with significantly higher 1-year LC versus ≤60 Gy EQD210 (100% vs 53%; P = .04).18
Most of the published data establishing the feasibility of reRT for abdominopelvic cancers includes patients with locally recurrent rectal cancer, and as shown in a recently published systematic review,19 this has predominantly been delivered using hyperfractionation. In 2006, Valentini and colleagues published the results of a multicenter phase 2 study of 59 patients with recurrent rectal cancer treated with 3-dimensional conformal RT to 40.8 Gy in 1.2-Gy fractions twice daily with a PTV of up to 4 cm, resulting in margins that included minimal toxic effects of grade 3 or greater and encouraging long-term LC and OS.2 Tao and colleagues from the MD Anderson Cancer Center retrospectively evaluated 102 patients with rectal cancer who preoperatively received a median of 50.4 Gy in 28 fractions, and after a median of 30 months, received reRT to a median of 39 Gy in 1.5-Gy fractions twice daily.6 Nearly all were treated with 3-dimensional conformal RT and generous PTV margins up to 2.5 cm. The incidence of toxic effects of grade 3 or greater was thought to be acceptable (34%), and patients who were able to undergo subsequent surgery had especially promising LC and OS. In addition to locally recurrent rectal cancer, reRT is also feasible for anal canal, gynecologic, prostate, and other abdominopelvic cancers.7,20, 21, 22
Whereas radiobiologic principles indicate that hyperfractionation using a lower dose per fraction should be preferred for reRT, in recent years, there has been greater interest in delivering ultrahypofractionated reRT with SBRT that features steep dose gradients and tight margins.23, 24, 25, 26, 27 Abusaris and colleagues reported no toxic effects of grade 3 or greater in 33 patients, most with rectal or cervical cancer, who were reirradiated using SBRT with 2- to 3-mm PTV margins.18 A multi-institution retrospective study reported favorable outcomes in patients with pancreas cancer reirradiated with SBRT to a median of 25 Gy in 5 fractions; most of the patients experienced symptom palliation, 1-year FFLP was 62%, and there were no toxic effects of grade 3 or greater.27 A retrospective study from the United Kingdom of 30 patients with recurrent rectal cancer reirradiated to 30 Gy in 5 fractions reported no toxic effects of grade 3 or greater, improved quality of life after reRT, and 1-year LC of 84.9%.28 Thus, it is reasonable to consider SBRT reRT to the abdomen and pelvis, which we have routinely adopted at our institution, especially when delivered using the enhanced abilities of an MR-Linac.
This study has some limitations, including its retrospective nature, the small number of patients, heterogeneous dose fractionation schedules, and various tumor types and histologies. Although the median follow-up was relatively short, it was similar to that of other published reRT studies (Table 3) and long enough to meaningfully assess acute toxic effects and early treatment efficacy.
In conclusion, our early experience suggests that dose-escalated reRT to the abdomen and pelvis using an MR-Linac is feasible. Based on these encouraging outcomes, we are planning to conduct a prospective trial for dose-escalated MR-guided reRT.
Footnotes
Sources of support: This work had no specific funding.
Disclosures: Dr Chuong reports receiving grants, personal fees, and nonfinancial support from ViewRay, grants from AstraZeneca and Novocure, and personal fees from Sirtex, Accuray, and Advanced Accelerator Applications outside the submitted work. Dr Kotecha reports receiving personal fees and nonfinancial support from Elekta, grants from Novocure, Blue Earth Diagnostics, Medtronic, AstraZeneca, and Exelixis, and personal fees from Accuray and ViewRay outside the submitted work. Dr Alvarez reports receiving grants from Sirtex outside the submitted work. Dr Mehta reports receiving nonfinancial support from Sapience, Karyopharm, and Mevion and other from Oncoceutics outside the submitted work. Dr Gutierrez reports receiving personal fees and nonfinancial support from ViewRay outside the submitted work. Dr Mittauer reports receiving personal fees and nonfinancial support from ViewRay and other from MR Guidance, LLC, outside the submitted work. All other authors have no disclosures to declare.
The research data related to this study are available upon request.
References
- 1.Haque W, Crane CH, Krishnan S, et al. Reirradiation to the abdomen for gastrointestinal malignancies. Radiat Oncol. 2009;4:55. doi: 10.1186/1748-717X-4-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valentini V, Morganti AG, Gambacorta MA, et al. Preoperative hyperfractionated chemoradiation for locally recurrent rectal cancer in patients previously irradiated to the pelvis: A multicentric phase II study. Int J Radiat Oncol Biol Phys. 2006;64:1129–1139. doi: 10.1016/j.ijrobp.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 3.Glimelius B. Recurrent rectal cancer. The pre-irradiated primary tumour: Can more radiotherapy be given? Colorectal Dis. 2003;5:501–503. doi: 10.1046/j.1463-1318.2003.00501.x. [DOI] [PubMed] [Google Scholar]
- 4.Hunt A, Das P, Minsky BD, et al. Hyperfractionated abdominal reirradiation for gastrointestinal malignancies. Radiat Oncol. 2018;13:143. doi: 10.1186/s13014-018-1084-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koom WS, Choi Y, Shim SJ, et al. Reirradiation to the pelvis for recurrent rectal cancer. J Surg Oncol. 2012;105:637–642. doi: 10.1002/jso.23023. [DOI] [PubMed] [Google Scholar]
- 6.Tao R, Tsai CJ, Jensen G, et al. Hyperfractionated accelerated reirradiation for rectal cancer: An analysis of outcomes and toxicity. Radiother Oncol. 2017;122:146–151. doi: 10.1016/j.radonc.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 7.Barsky AR, Reddy VK, Plastaras JP, et al. Proton beam re-irradiation for gastrointestinal malignancies: A systematic review. J Gastrointest Oncol. 2020;11:187–202. doi: 10.21037/jgo.2019.09.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mesko S, Wang H, Tung S, et al. Estimating PTV margins in head and neck stereotactic ablative radiation therapy (SABR) through target site analysis of positioning and intrafractional accuracy. Int J Radiat Oncol Biol Phys. 2020;106:185–193. doi: 10.1016/j.ijrobp.2019.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mantel F, Flentje M, Guckenberger M. Stereotactic body radiation therapy in the re-irradiation situation—A review. Radiat Oncol. 2013;8:7. doi: 10.1186/1748-717X-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koroulakis A, Molitoris J, Kaiser A, et al. Reirradiation for rectal cancer using pencil beam scanning proton therapy: A single institutional experience. Adv Radiat Oncol. 2021;6 doi: 10.1016/j.adro.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall WA, Paulson ES, van der Heide UA, et al. The transformation of radiation oncology using real-time magnetic resonance guidance: A review. Eur J Cancer. 2019;122:42–52. doi: 10.1016/j.ejca.2019.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henke LE, Olsen JR, Contreras JA, et al. Stereotactic MR-guided online adaptive radiation therapy (SMART) for ultracentral thorax malignancies: Results of a phase 1 trial. Adv Radiat Oncol. 2019;4:201–209. doi: 10.1016/j.adro.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chuong MD, Bryant J, Mittauer KE, et al. Ablative 5-fraction stereotactic magnetic resonance-guided radiation therapy with on-table adaptive replanning and elective nodal irradiation for inoperable pancreas cancer. Pract Radiat Oncol. 2021;11:134–147. doi: 10.1016/j.prro.2020.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Levin-Epstein R, Cao M, Lee P, et al. Magnetic resonance-guided inter-fraction monitoring opens doors to delivering safer reirradiation: An illustrative case report and discussion. Cureus. 2018;10:e2479. doi: 10.7759/cureus.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doty D, Chuong MD, Gomez AG, et al. Stereotactic MR-guided online adaptive radiotherapy reirradiation (SMART reRT) for locally recurrent pancreatic adenocarcinoma: A case report [e-pub ahead of print] Med Dosim. 2021 doi: 10.1016/j.meddos.2021.04.006. Accessed August 13, 2021. [DOI] [PubMed] [Google Scholar]
- 16.Kotecha R, Dea N, Detsky JS, et al. Management of recurrent or progressive spinal metastases: Reirradiation techniques and surgical principles. Neurooncol Pract. 2020;7:i45–i53. doi: 10.1093/nop/npaa045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung SY, Koom WS, Keum KC, et al. Treatment outcomes of re-irradiation in locoregionally recurrent rectal cancer and clinical significance of proper patient selection. Front Oncol. 2019;9:529. doi: 10.3389/fonc.2019.00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abusaris H, Hoogeman M, Nuyttens JJ. Re-irradiation: Outcome, cumulative dose and toxicity in patients retreated with stereotactic radiotherapy in the abdominal or pelvic region. Technol Cancer Res Treat. 2012;11:591–597. doi: 10.7785/tcrt.2012.500261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Meij W, Rombouts AJ, Rutten H, et al. Treatment of locally recurrent rectal carcinoma in previously (chemo)irradiated patients: A review. Dis Colon Rectum. 2016;59:148–156. doi: 10.1097/DCR.0000000000000547. [DOI] [PubMed] [Google Scholar]
- 20.Moningi S, Ludmir EB, Polamraju P, et al. Definitive hyperfractionated, accelerated proton reirradiation for patients with pelvic malignancies. Clin Transl Radiat Oncol. 2019;19:59–65. doi: 10.1016/j.ctro.2019.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sadozye AH. Re-irradiation in gynaecological malignancies: A review. Clin Oncol (R Coll Radiol) 2018;30:110–115. doi: 10.1016/j.clon.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 22.Munoz F, Fiorica F, Caravatta L, et al. Outcomes and toxicities of re-irradiation for prostate cancer: A systematic review on behalf of the re-irradiation working group of the Italian Association of Radiotherapy and Clinical Oncology (AIRO) Cancer Treat Rev. 2021;95 doi: 10.1016/j.ctrv.2021.102176. [DOI] [PubMed] [Google Scholar]
- 23.Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol. 1989;62:679–694. doi: 10.1259/0007-1285-62-740-679. [DOI] [PubMed] [Google Scholar]
- 24.Lominska CE, Unger K, Nasr NM, et al. Stereotactic body radiation therapy for reirradiation of localized adenocarcinoma of the pancreas. Radiat Oncol. 2012;7:74. doi: 10.1186/1748-717X-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dagoglu N, Callery M, Moser J, et al. Stereotactic body radiotherapy (SBRT) reirradiation for recurrent pancreas cancer. J Cancer. 2016;7:283–288. doi: 10.7150/jca.13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koong AJ, Toesca DAS, von Eyben R, et al. Reirradiation with stereotactic body radiation therapy after prior conventional fractionation radiation for locally recurrent pancreatic adenocarcinoma. Adv Radiat Oncol. 2017;2:27–36. doi: 10.1016/j.adro.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wild AT, Hiniker SM, Chang DT, et al. Re-irradiation with stereotactic body radiation therapy as a novel treatment option for isolated local recurrence of pancreatic cancer after multimodality therapy: Experience from two institutions. J Gastrointest Oncol. 2013;4:343–351. doi: 10.3978/j.issn.2078-6891.2013.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith T, O'Cathail SM, Silverman S, et al. Stereotactic body radiation therapy reirradiation for locally recurrent rectal cancer: Outcomes and toxicity. Adv Radiat Oncol. 2020;5:1311–1319. doi: 10.1016/j.adro.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]