Abstract
Background
Pharmacovigilance enhances post-market drug safety. However, analytical reports of a pattern of adverse drug reactions (ADRs) experienced by patients in Saudi Arabia are demanded.
Objective
To describe patterns of ADRs submitted to the Saudi Central National Pharmacovigilance and Drug Safety Center (NPC), Saudi Food and Drug Administration (SFDA), from its inception in 2015 until the end of 2017 to understand the pattern of ADR reporting in Saudi Arabia.
Methods
In this retrospective study, data from cases reported to the NPC were used to determine ADRs and identify the most common associated drug classes based on anatomical therapeutic chemical (ATC) classification system.
Result
A total of 17,730 ADR cases were reported during study period. An annual increase in ADRs was clearly evident. Approximately 54% of the total ADRs reported were serious. Most commonly reported ATC drug classes were anti-infective agents for systemic use (22.27%), antineoplastic and immunomodulating agents (21.49%), alimentary tract and metabolism (15.48 %), cardiovascular system (11.11%) and nervous system (10.23%). Vancomycin (2.7%), ceftiraxone (1.8%), fingolimod (1.4%) and paracetamol (1.4%) were the most common drugs associated with serious ADRs.
Conclusion
This study provide valuable insights in hypothesis generation for future studies on drug-event interactions and amplification studies. The NPC educational programs and awareness campaigns to promote systematic reporting of ADRs among healthcare professionals and general public should be continued.
Keyword: Pharmacovigilance, Adverse drug reactions, SFDA, Saudi Arabia
1. Introduction
Pharmacovigilance enhances post-market drug safety through evaluation, detection, prevention, and understanding the adverse effects of medication or any other medication-related problem (World Health Organization, 2006). Adverse Drug Reactions (ADRs) reporting is the foundation of pharmacovigilance and identifies unwanted, undesirable effect of a drug that occurs during normal clinical use (Saudi Food and Drug Authority, 2015). ADRs are common causes of mortality and morbidity worldwide (World Health Organization, 2002; World Health Organization, 2012) and represent a fundamental economic burden on any given health system (World Health Organization, 2012; Mazzitello et al., 2013). ADRs that occur in medical practice cannot always be predicted by premarket data owing to intrinsic limitations of clinical trials such as inadequate number of patients and limited follow-up time. Therefore, post-marketing surveillance is a necessary tool for early detection of severe and unexpected ADRs (World Health Organization, 2006).
In Saudi Arabia, the SFDA established pharmacovigilance activities to oversee the risk–benefit balance of all registered products during their active marketing cycle in in Saudi Arabia (Alharf et al., 2018). The SFDA monitors and assesses national ADRs spontaneous reports from both healthcare professionals and the general public (Alharf et al., 2018). This includes post-marketing surveillance of drugs and medical products, ADR signal detection, receiving notifications related to drug safety and quality, follow up on safety updates and make appropriate recommendations regarding the safety of the circulating pharmaceutical preparations through the decisions of the Pharmacovigilance Advisory Committee. In 2015, SFDA established the second Saudi Pharmacovigilance Guidelines on Good Pharmacovigilance Practices (GVP), which became functional nationwide (Alharf et al., 2018). These guidelines describe the roles and responsibilities of all relevant stakeholders, including marketing authorization holders (MAHs), healthcare professionals, and other international regulatory agencies.
It is noteworthy that legislations and the infrastructure of pharmacovigilance systems also vary among WHO member countries (World Health Organization, 2002; World Health Organization, 2006). It has been evident from previous literature that the information obtained from diverse geographic areas may not always be suitable or appropriate to specific communities (Lobo et al., 2013; Morimoto et al., 2011; NMPA, 2019. China’s Annual Report), (Ozcan, et al., 2016; Sonawane et al., 2018). In order to support detect regional ADR signals and take necessary actions to reduce the risks associated with drug-related events, countries need to regularly manage, analyse, and monitor pharmacovigilance databases. Thereby efforts to enhance ADR reporting, and support systems to evaluate the effectiveness of pharmacovigilance activities and regulation could be optimized. Therefore, in the present study, we analysed NPC data to understand ADR reporting pattern in Saudi Arabia by determining the proportion and characteristics of ADRs reported, and provide clues to future studies on drug related adverse reactions.
2. Methods
The current study analysed ADR cases reported to the NPC database between 1 January 2015 and 31 December 2017, using a descriptive cross-sectional study design. The ADR reports in this study were selected by applying the following inclusion and exclusion criteria:
2.1. Inclusion criteria
-
•
All ADRs reported to NPC database from 1 January 2015 to 31 December 2017 were included.
2.2. Exclusion criteria
-
•
ADR reports comprising of adverse events that occurred prior to 2015.
-
•
ADR Reports lacking data on ADR onset.
-
•
Duplicate reports.
The ADRs were reported through paper-based forms, online submission, and other communication channels (e.g. telephone, fax and verbal) to NPC at SFDA. The data in NPC ADRs form include mandatory (drug name and ADR event) and optional (patient characteristics and seriousness category) information. The pharmacists at NPC coding ADR report data using common database Empirica Trace®. The ADRs were classified according to the Medical Dictionary for Regulatory Activities (MedDRA) Alharf et al., 2018). In addition, ADRs were classified into following categories based on seriousness by the person filling the ADR form: life-threatening, fatal, requiring hospitalization or prolongation of hospitalization, resulting in significant disability or incapacity in the reporter’s opinion, regardless of a congenital anomaly (Saudi Food and Drug Authority, 2019). In this study, ADRs from aforementioned categories were collectively considered as serious ADRs. If multiple serious adverse events were listed in a single report, all the evets within the report were considered serious and coded appropriately by NPC coder. However, if the report is unclear regarding ADR seriousness, the coder contacted the reporter for clarification and recorded the seriousness appropriately (Saudi Food and Drug Authority, 2019). Medicines reported in the ADRs were classified according to the Anatomical Therapeutic Chemical (ATC) Classification system into 14 main drug classes, which include: “alimentary tract and metabolism; blpood and blood-forming organs; cardiovascular; dermatological; genito-urinary system and sex hormones; systemic hormonal preparations, excluding sex hormones and insulin; anti-infective agents for systemic use; antineoplastic and immunomodulating agents; anti-parasitic products, insecticides and repellents; musculoskeletal system; nervous system; respiratory system; sensory organs; and various” (WHO Collaborating Centre for Drug Statistics Methodology, 2019).
2.3. Statistical analysis
Descriptive statistics were performed based on ATC drug classes reported as counts and percentages. ADRs seriousness occurring during study period was calculated as frequency (%). The proportion of serious ADRs reported for individual drugs within an ATC therapeutic class were computed in relation to all serious ADRs reported in each ATC class. The analysis focussed on most commonly reported ATC classess, including commonly reported drugs within each therapeutic class. In addition, the proportion of serious ADRs reported among different age groups and gender were computed. Statistical analysis was performed using IBM SPSS (version 25; IBM SPSS, Chicago, IL, USA).
3. Results
Among 17,736 ADR reports submitted to NPC during study period, 17,730 eligible spontaneous and solicited reports for all the marketed drugs were included in current study.
3.1. Adverse Drug Reaction (ADR) Reporting Proportion
The number of ADR reports submitted, annually, increased gradually over the study period (Table 1). It is noteworthy that majority of ADRs reported (53.6%) were found to be serious.
Table 1.
Distribution of adverse drug reports received during the study period, 2015 to 2017.
| Total ADR (n = 17,730) |
Serious (n = 9506) (53.6%) |
Not Serious (n = 7960) (44.9%) |
Missing information on seriousness (n = 264) (1.5%) |
|
|---|---|---|---|---|
| Year | ||||
| 2015 | 3517 | 2117 (60.2%) | 1247 (35.5%) | 153 (4.4%) |
| 2016 | 5943 | 3118 (52.5%) | 2804 (47.2%) | 21 (0.4%) |
| 2017 | 8270 | 4271 (51.6%) | 3909 (47.3%) | 90 (1.1%) |
| Gender | ||||
| Female | 7969 | 4114(51.6%) | 3819(47.9%) | 36 (0.5%) |
| Male | 7872 | 4247(53.9%) | 3571(45.4%) | 54 (0.7%) |
| Missing | 1889 | 1145(60.6%) | 570(30.2%) | 174(9.2%) |
| Age group | ||||
| (0–17) | 2485 | 1518(61.1%) | 952(38.3%) | 15(0.6%) |
| (18–45) | 4911 | 2580(52.5%) | 2318(47.2%) | 13(0.3%) |
| (46–65) | 4426 | 2355(53.2%) | 2051(46.3%) | 20(0.5%) |
| (66–79) | 1721 | 879(51.1%) | 842(48.9%) | 0.00 |
| 80≤ | 313 | 176(56.2%) | 137(43.8%) | 0.00 |
| Missing | 3874 | 1998(51.6%) | 1660 (42.8%) | 216(5.6%) |
3.2. ADRs by Gender
Overall, the proportion of ADR reports received were higher among women (44.9%) compared to men (44.4%). However, the proportion of total serious ADR reports received was higher among men (44.7%) compared to women (43.3%). Majority of ADR reports received among men and women were of serious nature (Table 1). Approximately 11% of the reported ADRs had no information on gender.
3.3. ADRs by age
Majority of ADR reports received among individual age groups were serious (Table 1). In addition, adults aged 18–45 years (27.1%) and 46–65 years (24.8%) contributed to highest proportion of serious ADR reports received. About 22% of ADR reports received lacked information on age.
3.4. ADRs by therapeutic Groups
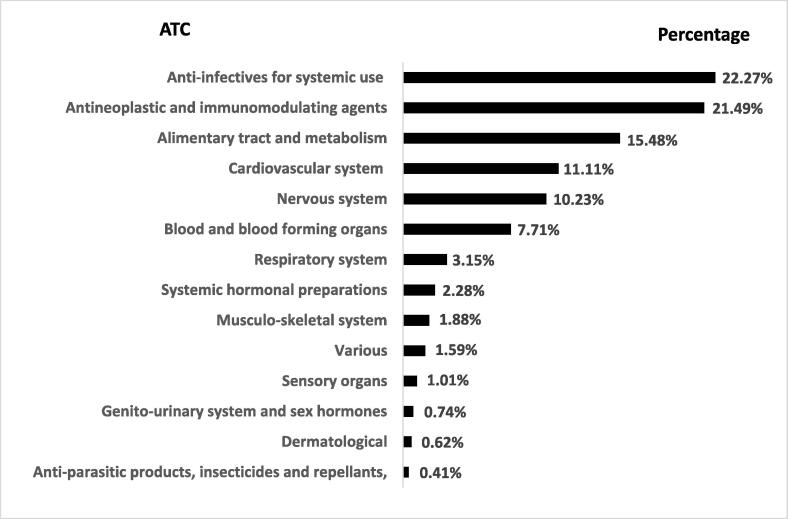
During the study period, 1,274 therapeutic items were reported in 17,730 reports. Most commonly reported ATC classes were anti-infectives for systemic use (22.27 %), followed by antineoplastic and immunomodulating agents (21.49%), alimentary tract and metabolism (16.48%), cardiovascular system (11.11%), and nervous system (10.23%) (Fig. 1). These ATC classes approximately covered 81% of all reported ADRs, and 78.6% of serious ADRs reported during the study period.
Fig. 1.
Adverse Drug Reaction (ADR) reporting proportion by therapeutic class of drugs.
3.5. ADRs by individual Drugs
The individual drugs that were frequently reported for ADRs, including serious ADRs, within each of the commonly reported ATC classes were provided in (Table 2). Interestingly, the drugs most frequently reported for serious ADRs among anti-infective class were vancomycin and ceftriaxone (Table 2). Fingolimod and methotrexate were the most frequent drugs reported with serious ADRs in immunomodulators class (Table 2). Also, the most frequent drugs reported for serious ADRs in alimentary tract and metabolism class were prednisolone and dexamethasone reports (Table 2). Drugs used in cardiovascular diseases, including furosemide, and atorvastatin were frequently reported to be associated with serious ADRs (Table 2). Finally, the most frequently reported drugs with serious ADRs in the nervous system class were paracetamol and morphine (Table 2).
Table 2.
Most commonly reported active substances with serious ADRs among top five ATC classes identified in this study.
| ATC class | Total ADR | Serious ADRs |
|---|---|---|
| Anti-infective for systemic use | (n = 3945) | (n = 2142) |
| Vancomycin | 457 | 258(12.1%) |
| Ceftriaxone | 301 | 173(8.1%) |
| Ciprofloxacin | 179 | 101(4.7%) |
| Meropenem | 118 | 82(3.8%) |
| Piperacillin/Tazobactam | 248 | 152(7.1%) |
| Ombitasvir/Paritaprevir/Ritonavir | 267 | 77(3.5%) |
| Moxifloxacin | 107 | 75(3.5%) |
| Amphotericin B | 80 | 57(2.6%) |
| Ribavirin | 141 | 45(2.1%) |
| Antineoplastic and Immunomodulating Agents | (n = 3810) | (n = 2046) |
| Fingolimod | 205 | 132(6.4%) |
| Methotrexate | 212 | 107(5.2%) |
| Vincristine | 107 | 96(4.6%) |
| Rituximab | 120 | 89(4.3%) |
| Doxorubicin | 77 | 67(3.2%) |
| Cyclophosphamid | 76 | 63(3.0%) |
| Adalimumab | 186 | 60(2.9%) |
| Oxaliplatin | 71 | 51(2.4%) |
| Pegaspargase | 60 | 49(2.3%) |
| Mycophenolate | 51 | 49(2.3%) |
| Tacrolimus | 51 | 47(2.2%) |
| Alimentary tract and metabolism | (n = 2744) | (n = 1358) |
| Prednisolone | 165 | 117(8.6%) |
| Dexamethasone | 138 | 82(6.0%) |
| Metformin Hydrochloride | 202 | 80(5.8%) |
| Omeprazole | 123 | 76(5.5%) |
| Vitamin D | 126 | 52(3.8%) |
| Pantoprazole | 87 | 44(3.2%) |
| Prednisone | 47 | 43(3.1%) |
| Sitagliptin | 94 | 41(3.0%) |
| Hydrocortisone | 86 | 39(2.8%) |
| Ranitidine | 76 | 38(2.7%) |
| Cardiovascular system | (n = 1969) | (n = 1051) |
| Furosemide | 192 | 111(10.6%) |
| Atorvastatin | 240 | 102(9.7%) |
| Alprostadil | 70 | 70(6.6%) |
| Amlodipine | 80 | 42(3.9%) |
| Lisinopril | 80 | 42(3.9%) |
| Simvastatin | 81 | 41(3.9%) |
| Sacubitril/Valsartan | 75 | 34(3.2%) |
| Irbesartan | 50 | 26(2.4%) |
| Nervous system | (n = 1813) | (n = 872) |
| Paracetamol | 238 | 132(15.1%) |
| Morphine | 158 | 85(9.7%) |
| Phenytoin | 83 | 62(7.1%) |
| Carbamazepine | 55 | 32(3.6%) |
| Tramadol | 34 | 29(3.3%) |
| Acetaminophen | 35 | 27(3.0%) |
| Acetylsalicylicacid | 79 | 23(2.6%) |
| Fentanyl | 69 | 23(2.6%) |
| Pethidine | 44 | 18(2.0%) |
| Escitalopram | 27 | 15(1.7%) |
3.6. ADRs according to Seriousness:
A total of 9,506 ADRs (53.6% of all ADRs) were reported as serious events. Drugs with high proportion of serious ADR reports, and their respective ADR terms among the reported ATC classes are listed in Table 3. The most frequent drug with serious ADRs was vancomycin anti-infective agent; this drug was associated with some serious adverse events including rash, itching, redness, red man syndrome and acute kidney injury, either individually or in combination. Incidentally, another anti-infective agent, ceftriaxone was associated various adverse events, including itching, rash, redness, shortness of breath, and acute kidney injury. The third most commonly reported drug with serious ADRs was fingolimod which was associated with conditions such as, bradycardia, relapse, cognitive impairment, fatigue, leukopenia. Interestingly, paracetamol was fourth in the list that was linked to elevated liver enzyme, rash, renal failure, diarrhea, or hypotension.
Table 3.
Most commonly reported drugs and their respective serious adverse events during 2015 – 2017 in Saudi Arabia.
| Suspected drug |
Total Serious ADR (n = 9506) |
Serious ADR terms |
|---|---|---|
| Vancomycin | 258 (2.7%) | Rash, Itching, Redness, Red Man Syndrome, Acute Kidney Injury |
| Ceftriaxone | 173 (1.8%) | Itching, Rash, Redness, Shortness of Breath, Acute Kidney Injury |
| Fingolimod | 132 (1.4%) | Bradycardia, Relapse, Cognitive Impairment, Fatigue, Leukopenia |
| Paracetamol | 132 (1.4%) | Elevated Liver Enzyme, Rash, Renal Failure, Diarrhea, Hypotension |
| Prednisolone | 117 (1.2%) | Hyperglycaemia, Acute Graft Rejection, Bacterial Endocarditis, Depression, Emotional Instability |
| Furosemide | 111 (1.2%) | Hypokalaemia, Metabolic Alkalosis, Rash, Decrease Haemoglobin, Diarrhea |
| Methotrexate | 107 (1.1%) | Chemotherapy Induced Arthritis, Bacterial Endocarditis, Cholestasis, Depression, Emotional Instability |
| Atorvastatin | 102 (1.1%) | Muscle Pain, Fatigue, Elevated Liver Enzyme, Bone Pain, Increase Bilirubin |
| Morphine | 85 (0.9%) | Itching, Redness, Hypotension, Rash, Hives |
| Dexamethasone | 82 (0.9%) | Typhlitis, Thrombosis, Sepsis, Chemotherapy Induced Arthritis, Hyperglycaemia |
4. Discussion
The current study identified a steady increase in ADRs reported to NPC since its inception in 2015. Interestingly, drugs from five main classes contributed to most reported serious ADRs. This study identifies potential contribution of vancomycin, fingolimod, and paracetamol to serious ADRs in Saudi Arabia.
Our analysis showed that ADRs increased remarkably over three years since the inception of NPC. Most remarkable increases were observed in 2017. This could be due to increased awareness of ADRs and reporting channels, and increased access to sources of information. Similarly, in a recent review by (Alharf et al., 2018) the investigators reviewed the Saudi vigilance program and identified a significant increase in ADRs reporting over the years (Alharf et al., 2018). The observed rise in the reporting could also be attributed to several measures implemented by the NPC since 2014, including improved communication with private and public healthcare providers, increased oversight on pharmaceutical companies, and robust awareness campaigns conducted in public places, such as shopping malls and mass media broadcasts. Further, the implementation of new pharmacovigilance guidelines issued at the end of 2015 may have enhanced ADRs reporting (Alharf et al., 2018). In addition, ADR reports, including serious reports received were prevalent among adults in our study; these findings were consistent with current evidence (Almubark et al., 2020; Suggett, et al., 2016). Furthermore, the prevalence of ADRs reported was higher among women compared to men in our study. Although these results were consistent with reported association between female gender and ADRs (Suggett, et al., 2016; Almubark et al., 2020), it is interesting to note that serious ADRs reported in our study were predominant among men. Therefore, the possible role of gender related perceptions and attitudes towards ADRs, and/ or awareness in reporting the events should be explored in future studies.
Interestingly, in the present study 53.6% of the reports involved one type or other serious ADRs. Although the total number of serious ADRs reported has been increasing annually, the percentage of serious ADRs reported each year is in decline, reflecting increased awareness on ADR reporting strategy and/or a surge in an actual number of non-serious adverse drug events. In addition, high prevalence of serious ADR reports received in Saudi Arabia could be attributed to likely bias in reporting serious events by healthcare professionals and other stakeholders (AlShammari et al., 2018; Aldryhim et al., 2019; Matsuda et al., 2015). The positive bias in reporting serious events was evident in a cross-sectional analysis of ADR reporting attitudes among health care professionals in Saudi Arabia; about 64% of health care professional surveyed were more inclined to reporting serious adverse events (AlShammari et al., 2018). A retrospective analysis of ADR reports in USA from 2006 to 2014 illustrated a similarly steady increase in number of serious ADR reports (Sonawane et al., 2018). However, serious ADR reports varied from 10.3% in China (NMPA, 2019. China’s Annual Report) to 33% in Japan (Morimoto et al., 2011). These findings indicate a possible role of geographic and cultural factors on ADR profile.
In the present study, adverse reactions were reported for all fourteen classes of drugs. ATC drug classes, including anti-infective agents for systemic use, antineoplastic and immunomodulating agents, alimentary tract and metabolism, cardiovascular system, and nervous system contributed more than 80% of all reported ADRs events. The high prevalence of ADRs observed among these ATC classes could be due to increased drug use. However, the high prevalence of ADRs among the anti-infective class of drugs could be due to the misuse of over the counter antibiotics. This notion was supported by a recent local systemic review that showed a high antibiotic misuse (41% to 92%) in Saudi Arabia, predominantly among pediatric age group (Alnemri et al., 2016). The reasons for this high antibiotic misuse are complicated and possibly related to various contributing factors including cultural factors, behavioural characteristics, socio-economic status and level of education (Ozcan et al., 2016). However, caution should be exercised in interpreting our findings, and should not be viewed as drug-event causation. Incidentally, the high prevalence of ADRs reported in this study could be due to more number of ADRs being reported by volume, or more ADRs occurring due to prevalent use of suspect drugs. Although similar results were observed in previous studies, the top ATC drug classes causing ADRs varied by geographic area or population. (Alnemri et al., 2016; Ozcan et al., 2016; Gharaibeh et al., 1998; Alsbou et al., 2015). For example, Ozcan et al. identified that approximately 64% of all reported drugs causing ADRs belonged to ATC classes - antineoplastic and immunomodulating agents, anti-infectives for systemic use, and nervous system in Turkey (Ozcan et al., 2016). More interestingly, a recent local study by Khan et al., showed that anti-infective and anti-epileptic drugs were the most common classes of medicinal products linked to ADRs in paediatric patients (Khan et al., 2013). In Amman, Jordan, Gharaibeh et al. found that chemotherapeutic drugs were frequently used medications among 3.6% of drug-induced admissions in internal medicine department (Gharaibeh et al, 1998). In another study, Alsbou et al. found that ADRs prevalence rate was 3.2% in a Southern Jordanian teaching hospital; antibiotics and analgesics were the most prevalent classes of drugs that contributed to ADRs (Alsbou et al., 2015). These studies clearly show the variations within the same country which indicate the importance of evaluating ADRs by locality. The top most ATC drug classes reported in our study (e.g. anti-infective and anti-neoplastic) drugs appear to be in agreement with Jordanian studies (Gharaibeh et al., 1998), (Alsbou et al., 2015). The specific drugs that contributed to high proportion of serious ADRs during the study period, and their respective MedDRA terms could aid in hypothesis generation for future studies (Table 3).
It is noteworthy hat the most common drug implicated in serious ADRs in our study was vancomycin, which was associated with adverse events including rash, itching, redness, red man syndrome and acute kidney injury; contributing to 2.7% of all reported serious ADRs. These events might have originated due to high prevalence (35.6%) of methicillin-resistant staphylococcus aureus (MRSA) infection in Saudi Arabia for which vancomycin is the treatment of choice (Al Yousef and Taha, 2016). A similar ADR profile was observed for ceftriaxone, second most commonly reported anti-infective agent, for serious ADRs (Table 3). Interestingly, a study by Lobo et al. demonstrated that risperidone, olanzapine, ceftriaxone, vancomycin, and furosemide were responsible for severe ADRs in Brazil, indicating ADR profile dependence on population being investigated (Lobo, et al., 2013).
In our study, immunomodulating agent, fingolimod was associated with serious bradycardia, disease relapse, cognitive impairment, fatigue and leukopenia. Our findings are in agreement with a systematic review, by Ziemssen et al., that suggested ineffectiveness or adverse events to be primary reasons for fingolimod discontinuation, indicating the need to address this drug related adverse events more closely (Ziemssen et al., 2017).
It is interesting to note that paracetamol, a widely available over-the-counter drug, was associated with 1.4% of the total serious ADRs, including elevated liver enzyme, rash, renal failure, diarrhea, and hypotension. Our results were compatible with findings of another study conducted in a governmental hospital in Saudi Arabia, which found higher ADR proportion (31.6%) for (NSAIDs, Analgesic) indicating a possible regional phenomenon (Alayed et al., 2019). This might also be related to polypharmacy or serious misuse of over-the-counter medications in Saudi Arabia, which should be addressed in future studies.
5. Limitations
Although in our study, some data limitations need to be addressed to facilitate robust analyses and conclusions in future. The missing data and the electronic systems should be evaluated regularly to address inconsistencies in data transcription to enhance and improve the quality of the database. Nonetheless, our findings would provide valuable insights in hypothesis generation for future studies on drug-event interactions.
6. Conclusions
In conclusion, Current NPC educational programs and awareness campaigns should continue promoting the concept of pharmacovigilance to assure the safety of therapeutic products in Saudi market. Further studies are needed to illustrate the underlying mechanism of the prevalent ADRs pertaining to vancomycin, fingolimod and paracetamol and the high proportion of serious spontaneous ADRs reports. Besides, while evidence accumulates, this will instrument the mandate further regulating or revoking dangerous drugs and updating product labels to ensure patient safety and improve public health.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The authors thank NPC for providing permission and approval to use their pharmacovigilance data. We gratefully acknowledge all NPC staff contributing to data collection. All the opinions, thoughts and conclusions expressed in this paper are those of the authors and does not reflect the opinions or policies of the SFDA.
Funding.
This study was not funded.
Ethical approval.
This study received ethics approval from Institutional Review Board office at King Abdullah International Medical Research Centre, Health Affairs, Ministry of National Guard (number SP19.242). The study was conducted following the principles and the current revision of the Declaration of Helsinki, the Good Clinical Practice.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alayed N., Alkhalifah B., Alharbi M., Alwohaibi N., Farooqui M. Adverse Drug Reaction (ADR) as a Cause of Hospitalization at a Government Hospital in Saudi Arabia: A Prospective Observational Study. Curr. Drug Saf. 2019;14(3):192–198. doi: 10.2174/1574886314666190520105330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldryhim A.Y., Alomair A., Alqhtani M., Mahmoud M.A., Alshammari T.M., Pont L.G., Kamal K.M., Aljadhey H., Mekonnen A.B., Alwhaibi M., Balkhi B., Alhawassi T.M. Factors that facilitate reporting of adverse drug reactions by pharmacists in Saudi Arabia. Expert Opinion Drug Saf. 2019;18(8):745–752. doi: 10.1080/14740338.2019.1632287. [DOI] [PubMed] [Google Scholar]
- Alharf A., Alqahtani N., Saeed G., Alshahrani A., Alshahrani M., Aljasser N., Alquwaizani M., Bawazir S. Saudi Vigilance Program: Challenges and lessons learned. Saudi Pharm. J. 2018;26(3):388–395. doi: 10.1016/j.jsps.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alnemri A.R., Almaghrabi R.H., Alonazi N., Alfrayh A.R. Current paediatric research. Curr. Pediatr. Res. 2016;20:169–173. [Google Scholar]
- Alsbou M., Alzubiedi S., Alzobi H., Samhadanah N.A., Alsaraireh Y., Alrawashdeh O., Aqel A., Al-Salem K. Adverse drug reactions experience in a teaching hospital in Jordan. Int. J. Clin. Pharm. 2015;37(6):1188–1193. doi: 10.1007/s11096-015-0185-1. [DOI] [PubMed] [Google Scholar]
- AlShammari T.M., Almoslem M.J. Knowledge, attitudes & practices of healthcare professionals in hospitals towards the reporting of adverse drug reactions in Saudi Arabia: A multi-centre cross sectional study. Saudi Pharm. J.: SPJ: Off. Publication Saudi Pharm. Soc. 2018;26(7):925–931. doi: 10.1016/j.jsps.2018.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almubark R.A., Aljadani R.H., Alqahtani A.S., Alshammari T.M., BinDhim N.F. National Cross-Sectional Study of Community-Based Adverse Drug Reactions in Saudi Arabia. Drugs - real world outcomes. 2020;7(2):161–170. doi: 10.1007/s40801-020-00186-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Yousef S., Taha E. Methicillin-resistant staphylococcus aureus in Saudi Arabia: Genotypes distribution review. Saudi J. Med. Med. Sci. 2016;4(1):2. doi: 10.4103/1658-631X.170880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharaibeh M., Zmeili S., Abu-Rajab A., Daoud Z. Drug-induced admissions to medical wards at Jordan University Hospital. Int. J. Clin. Pharmacol. Ther. 1998;36(9):478–482. [PubMed] [Google Scholar]
- Khan L.M., Al-Harthi S.E., Saadah O.I. Adverse drug reactions in hospitalized pediatric patients of Saudi Arabian University Hospital and impact of pharmacovigilance in reporting ADR. Saudi Pharm. J. 2013;21(3):261–266. doi: 10.1016/j.jsps.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo M.G. A. de A., Pinheiro S.M.B., Castro J.G.D., Momenté V.G., Pranchevicius M.C.S. Adverse drug reaction monitoring: Support for pharmacovigilance at a tertiary care hospital in Northern Brazil. BMC Pharmacol. Toxicol. 2013;14(1) doi: 10.1186/2050-6511-14-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzitello C., Esposito S., De Francesco A., Capuano A., Russo E., De Sarro G. Pharmacovigilance in Italy: An overview. J. Pharmacol. Pharmacother. 2013;4(5):20. doi: 10.4103/0976-500X.120942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda S., Aoki K., Kawamata T., Kimotsuki T., Kobayashi T., Kuriki H., Nakayama T., Okugawa S., Sugimura Y., Tomita M., Takahashi Y., Morishita R. Bias in spontaneous reporting of adverse drug reactions in Japan. PLoS ONE. 2015;10(5):e0126413. doi: 10.1371/journal.pone.0126413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Collaborating Centre for Drug Statistics Methodology, 2019. Guidelines for ATC classification and DDD assignment. Available at: https://www.whocc.no/atc_ddd_index_and_guidelines/guidelines/
- Morimoto T., Sakuma M., Matsui K., Kuramoto N., Toshiro J., Murakami J., Fukui T., Saito M., Hiraide A., Bates D.W. Incidence of adverse drug events and medication errors in Japan: The JADE study. J. Gen. Intern. Med. 2011;26(2):148–153. doi: 10.1007/s11606-010-1518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NMPA, 2019. China’s Annual Report for National Adverse Drug Reaction Monitoring (2019) Released [WWW Document]. Natl. Advers. Drug React. Monit. URL http://english.nmpa.gov.cn/2020-04/10/c_500154.htm)
- Ozcan G., Aykac E., Kasap Y., Nemutlu N.T., Sen E., Aydinkarahaliloglu N.D. Adverse Drug Reaction Reporting Pattern in Turkey: Analysis of the National Database in the Context of the First Pharmacovigilance Legislation. Drugs - Real World Outcomes. 2016;3(1):33–43. doi: 10.1007/s40801-015-0054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saudi Food & Drug Authority, 2015. Guideline on Good Pharmacovigilance [WWW Document]. Guidel. Good Pharmacovigil. Pract. URL https://old.sfda.gov.sa/ar/drug/resources/DocLib2/Guideline on Good Pharmacovigilance Practices (GVP).pdf
- Saudi Food & Drug Authority ,2019. [WWW Document]. ADR Report. Forms. URL https://old.sfda.gov.sa/en/drug/about/sector_departments/national_pharmacovigilance_center/pages/reporting_forms.Aspx
- Sonawane K.B., Cheng N., Hansen R.A. Serious Adverse Drug Events Reported to the FDA: Analysis of the FDA Adverse Event Reporting System 2006–2014 Database. J. Manag. Care Spec. Pharm. 2018;24(7):682–690. doi: 10.18553/jmcp.2018.24.7.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suggett E., Marriott J. Risk Factors Associated with the Requirement for Pharmaceutical Intervention in the Hospital Setting: A Systematic Review of the Literature. Drugs - real world outcomes. 2016;3(3):241–263. doi: 10.1007/s40801-016-0083-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO), 2012. SAFETY MONITORING of MEDICINAL PRODUCTS. WHO Publ.https://www.who.int/medicines/areas/quality_safety/safety_efficacy/ConsumerReporting.pdf?ua=1.
- World Health Organization W.H.O. Safety of Medicines. World Heal. Organ. Geneva. 2002;2002(2):20. doi: 10.1021/jp5101347. [DOI] [Google Scholar]
- World Health Organization The Safety of Medicines in Public health programmes: Pharmacovigilance an essential tool. WHO Libr Cat. Data. 2006;61 https://www.who.int/medicines/areas/quality_safety/safety_efficacy/Pharmacovigilance_B.pdf?ua=1 [Google Scholar]
- Ziemssen T., Medin J., Couto C.A.M., Mitchell C.R. Multiple sclerosis in the real world: A systematic review of fingolimod as a case study. Autoimmun. Rev. 2017;16(4):355–376. doi: 10.1016/j.autrev.2017.02.007. [DOI] [PubMed] [Google Scholar]