Abstract
Excessive interleukin (IL)-6 production is a driver for malignancy and drug resistance in colorectal cancer (CRC). Our study investigated a seven-week post-treatment with the anti-inflammatory drug, Diacerein (Diac), alone or in combination with 5-fluorouracil (5-FU), using a 1,2-dimethylhydrazine (DMH) rat model of CRC. Diac alone and 5-FU+Diac reduced serum levels of carcino-embryonic antigen (CEA), while all regimens decreased serum levels of colon cancer-specific antigen (CCSA), a more specific CRC biomarker. Additionally, Diac, 5-FU and their combination suppressed colonic content/gene expression of IL-6, its downstream oncogene, Kirsten rat sarcoma viral oncogene homolog (K-Ras), and consequently Notch intracellular domain and nuclear factor-kappa B (NF-κB) p65. In turn, NF-κB downstream factors, viz., matrix metalloproteinase-9 (MMP-9), vascular endothelial growth factor (VEGF), c-Myc, and B-cell lymphoma-2 (Bcl-2) were also downregulated, while E-cadherin was elevated. Additionally, the drugs reduced the immunoreactivity of CD31 to prove their anti-angiogenic effect, while the TUNEL assay confirmed the apoptotic effect. The apoptotic effect was confirmed by transferase dUTP nick-end labeling assay. Moreover, these drugs inhibited colon content of p-Akt, β-catenin, and cyclin D1 immunoreactivity. The drugs also activated the tumor suppressor glycogen synthase kinase 3- β (GSK3-β) and upregulated the expression of the Nur77 gene, which represents the second arm of IL-6 signaling. However, only 5-FU upregulated miR-200a, another K-Ras downstream factor. The in-vitro cytotoxic and migration/invasion assays verified the molecular trajectories. Accordingly, we evaluated the antineoplastic effect of Diac alone and its possible chemosensitization effect when added to 5-FU. This combination may target critical oncogenic pathways, including the IL-6/K-Ras/Notch/NF-κB p65 axis, p-Akt/GSK3-β/β-catenin/cyclin D-1 hub, and Nur77.
Abbreviations: VEGF, vascular endothelial growth factor; Akt, protein kinase B; ANOVA, analysis of variance; Bcl-2, B-cell lymphoma-2; BSA, bovine serum albumin; CCSA-2, colon cancer specific antigen; CD 31, cluster of differentiation 31; CDK, cyclin dependent kinase; CEA, carcino-embryonic antigen; CRC, colorectal cancer; Ct, cycle threshold; DAB, 3, 3'- Diaminobenzidine; Diac, diacerein; Dll-1, Delta-like 1; DMH, di-methylhydrazine; ELISA, Enzyme-Linked immunosorbent Assay; EMT, epithelial mesenchymal transition; 5-FU, 5-flurouracil; gp130, glycoprotein 130; GSK3-β, glycogen synthase kinase 3- β; H&E, hematoxylin & eosin; Hes-1, hairy enhance of split-1; HRP, horse-radish peroxidase
Keywords: Colorectal cancer, Diacerein, Notch, 5-fluorouracil, IL-6, β-catenin
1. Introduction
Colorectal cancer (CRC) is a common malignancy with high prevalence and low survival (Metwally et al., 2018). CRC is a heterogeneous disease with many factors playing key roles in its pathogenesis. A chief signaling axis involved in CRC is the Notch pathway, which is initiated by binding of Notch ligand, as Delta-like (Dll)-1, Dll-3, Dll-4, Jagged-1, and Jagged-2, to its receptor in two adjacent cells. This binding is followed by proteolytic cleavage of the receptor by γ-secretase and other proteases to liberate the active Notch intracellular domain (NICD). The latter is transported to the nucleus, where it binds to multiple transcription cofactors causing expression of Notch target genes, including hairy enhance of split-1family (Hes-1) and related family (Hey-1), nuclear factor-kappa B (NF-κB), cyclin D1, and c-Myc (Miele and Osborne, 1999, Miele, 2006, Miele et al., 2006, Miele et al., 2006, Miele, 2006). Thus, the Notch pathway includes several processes involved in oncogenesis, such as cell proliferation and growth, metastasis, anti-apoptosis (Sun et al., 2007, Maniati et al., 2011, Wang et al., 2015), and angiogenesis (Zeng et al., 2005).
Other signaling processes, such as the Wnt/β-catenin pathway, are critical players in the pathogenesis of CRC. When the Wnt pathway is downregulated, the β-catenin molecule is captured in a multiprotein complex that includes glycogen synthase kinase 3-β (GSK3-β) that phosphorylates β-catenin and marks it for proteasomal degradation. However, binding of Wnt to its receptor results in the suppression of GSK3-β and the appearance of non-phospholyrated β-catenin. The latter is translocated into the nucleus, where it binds to transcription factors T-cell factor and lymphoid enhancing factor (TCF/LEF) to express target genes, such as cyclin D1(Nusse and Clevers, 2017), c-Myc, vascular endothelial growth factor (VEGF), Jagged 1, and matrix metalloproteinase-7 (MMP-7) (Bertrand et al., 2012). Therefore, Wnt/β-catenin signaling promotes various oncogenic processes, such as cell proliferation, cell survival, angiogenesis (Li et al., 2014), and metastasis (Ahn et al., 2017, Zhang et al., 2017).
Chemotherapeutic agents have been used widely for many years to treat CRC. Positive outcomes in treating or reducing the size of tumors are achieved, but serious side effects emerge either upon their use or years after the end of treatment (He et al., 2003, Miyamoto et al., 2013). Acquired drug resistance, including environmental and genetic factors, is among these side effects and is a critical hindrance to successful cancer treatment. Several molecules play a role in the emergence of chemoresistance, such as interleukin-6 (IL-6) (Wang, Y et al., 2010), Kirsten rat sarcoma viral oncogene (K-Ras) (Jiang et al., 2004, McCubrey et al., 2006), Notch pathway (Huang et al., 2015, Li et al., 2018a), NF-κB molecule (Kuang et al., 2017, Yang et al., 2020) and its downstream proteins B-cell lymphoma-2 (Bcl-2), c-Myc, cyclin D1, MMP-9 (Laios et al., 2013, Xia et al., 2017, Elbadawy et al., 2019, Fu et al., 2019), phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) signaling (Liu et al., 2017, Li et al., 2020), GSK 3- β/β-catenin axis and GSK3-β/Bcl-2 cue (Zheng et al., 2010, Blondy et al., 2020). Novel highly selective drugs are needed to overcome chemoresistance in neoplastic cells and avoid major side effects (Singer, 2010, Broekman et al., 2011). A novel smart strategy is known as “drug repositioning”; defined as utilizing old drugs for new applications, thus, shortening the time needed for developing new drugs (Ashburn and Thor, 2004, Pantziarka et al., 2014).
Diacerein/diacerhein (Diac) is an anti-inflammatory and analgesic anthraquinone approved for the treatment of osteoarthritis (Lohberger et al.,2019). Diac reduces the release and production of pro-inflammatory cytokines, viz: IL-6 (Bharti et al., 2016) and IL-1β (Gadotti et al., 2012, Fouad et al., 2020). The latter is amply reviewed for its role in the progression of CRC (Fan et al., 2020, Baker et al., 2019) via inducing several tumor-related processes (Shimizu et al., 1996, Nakao et al., 2005, Chattopadhyay et al., 2021). Additionally, crosstalk between the two cytokines is reported, where IL-1β is an upstream stimulator of IL-6 (Parikh et al., 2000, Nagasaki et al., 2014). A synergistic interaction was encountered between the two ILs in a human colon cancer cell line (Street et al., 2003) and patients with CRC (Maihöfner et al., 2003).
The role of IL6 cannot be ruled out at this time. The cytokine may trigger the proliferation of malignant cells (Lee et al., 2003), drug resistance (Wang, Y et al., 2010), angiogenesis (Wei et al., 2003) and metastasis (Chang et al., 2013). Recent in-vitro studies showed an antineoplastic effect of Diac against breast cancer cells mediated by blocking the binding of IL-6 to its receptor (IL-6R). This inhibition led to downregulation in several cancer-related pathways, such as (PI3K/Akt) and rat sarcoma/rapidly accelerated fibrosarcoma/mitogen-activated protein kinase (RAS/RAF/ MAPK) (Bharti et al., 2016, Bharti et al., 2017). Additionally, Diac was reported to be a metabolic precursor of Rhein, an anthraquinone compound that is extracted from Rhubarb leaves. A previous study showed that Rhein enhances the effect of chemotherapeutic agents and reduces adverse effects and resistance (Chai et al., 2010). Chemopreventative potential against various kinds of cancer viz., pulmonary, cervical, hepatic, as well as CRC is also reported (Henamayee et al., 2020). Further, this metabolite displays antiproliferative, antiangiogenic and proapoptotic potential (Yang et al., 2019, Zhuang et al., 2019, Wang et al., 2020).
Accordingly, we evaluated possible antineoplastic effects of Diac alone compared to 5-fluorouracil (5-FU), a standard antitumor drug in CRC, using a 1,2-dimethylhydrazine (DMH) rat model. We also studied the possible add-on effect of Diac to enhance the chemosenstivity to 5-FU via targeting different oncogenic/resistance-inducing pathways.
2. Material and methods
2.1. Chemicals
Diac was a gift from Eva Pharma Company (Cairo, Egypt), and 1, 2- DMH and 5-FU (Utoral® ampoule; 250 mg/5 ml) were purchased from Sigma-Aldrich (MO, USA) and EIMC United Pharmaceuticals (Cairo, Egypt), respectively.
2.2. Animals
Adult male Wistar rats (200–250 g) were supplied by the Modern Veterinary Office for Laboratory Animals (Cairo, Egypt) and maintained on standard diet pellets purchased from El-Fagr Co. (Alexandria, Egypt) and water ad libitum. Rats were acclimated for two weeks before starting the experiment and were lodged in polypropylene cages at 25 ± 3 °C and kept on a 12 h light/dark cycle with 60%–70% humidity. The investigation complied with ARRIVE guidelines from the National Centre for the Replacement, Refinement, and Reduction of Animals in Research (NC3Rs) (Kilkenny et al., 2010) and was conducted in agreement with the EU Directive, 2010/63/EU for animal experiments (Directive, 2010). All procedures were approved by the Research Ethics Committee at the Faculty of Pharmacy, Cairo University (Cairo, Egypt; PT: 2148).
2.3. Induction of CRC
DMH was freshly dissolved in saline and injected subcutaneously at a dose of 20 mg/kg once weekly for 15 weeks (Muthu et al., 2013, Umesalma et al., 2014). The induction of CRC was verified by the presence of aberrant crypt foci in four colon tissues stained with hematoxylin & eosin (H&E) 24 h after the last DMH dose [pilot experiment; data not shown].
2.4. Experimental design and sampling
Fifty adult male rats were divided evenly among five groups. Rats in group 1 were injected with saline to serve as control animals. Rats in the remaining four groups received DMH for 15 weeks. These animals were either left untreated as positive controls or treated with 5-FU (50 mg/kg; i.p.) administered once weekly for 7 weeks (Tao et al., 2015), Diac (50 mg/kg; p.o, daily for 7 weeks (Gadotti et al., 2012, Refaie and El-Hussieny, 2017); or a combination of both treatments (Fig. 1).
Fig. 1.
Schematic illustration of the time plan. The CRC inducer DMH (20 mg/kg; S.C) was administered for 15 weeks. Treatment started on week 16 and lasted for 7 weeks, during which Diac (50 mg/kg; p.o) was administered daily, whereas 5-FU (50 mg/kg; i.p) was injected once per week. The same regimens were used in the combination group. Euthenization was carried out at the end of week 22. CONT: control group; CRC: colorectal cancer; Diac: Diacerein; DMH: 1, 2-dimethylhydrazine; 5-FU: 5-fluorouracil.
At the end of the experimental period and 24 h after the last administration of Diac/5-FU, rats were fasted overnight, anesthetized with a thiopental overdose (200 mg/kg; i.p) and blood samples were collected by cardiac puncture. Sera were separated and stored at −80 °C until analysis. Animals were then sacrificed by cervical dislocation and, after laparotomy, colon tissues were immediately resected, weighed, and lengths measured from the ascending colon to anus. Each specimen was thoroughly inspected for gross abnormalities. Colon specimens were cut longitudinally into two parts along the medial axis; the 1st part was fixed in 10% neutral buffered formaldehyde solution for 1 day for histopathological (n = 10 per group) and immunohistochemical (n = 3 per group) assessment, while the other part was further divided into three segments to be used for the estimation of biochemical parameters using Enzyme-Linked immunosorbent assay (ELISA), Western blotting, and polymerase chain reaction (PCR).
2.5. ELISA
2.5.1. Evaluation of serum CCSA-2, CEA, and MMP-9 levels
Carcinoembryonic antigen (CEA), colon cancer-specific antigen (CCSA-2), and MMP-9 were estimated using the corresponding rat MyBioSource ELISA kits (CA, USA) following the manufacturer’s instructions.
2.5.2. Measurement of NF-κB, IL-6, VEGF, E-cadherin, GSK3-β, Bcl-2 and p-Akt
The following ELISA kits and their sources (in parentheses) were used to assess the colon contents of NF-κB (Novus Biologicals, CO, USA; Cat # NBP2- 29661), Bcl-2 (Novus Biologicals; Cat# NBP2-69947), E-cadherin (PicoKine ELISA Kit; Boster Biological Technology, CA, USA; Cat# EK1434), VEGF (MyBioSource; Cat # MBS724516), IL-6 (Biovision, CA, USA; Cat # K4145-100), GSK3-β (Aviva Systems Biology, CA, USA; Cat # OKEH03023) and pS473-Akt (DRG, NJ, USA; Cat # EIA-3997) following the manufacturers, protocols.
2.6. Quantitative real-time PCR (qRT-PCR)
Frozen colonic tissue segment (30 mg) was for the detection of K-Ras, Nur77 and c-Myc gene expression. RNA was isolated using the GeneJET RNA purification kit (Thermo Fisher Scientific, MA, USA), and the miRNeasy extraction kit (Qiagen, CA, USA) was used to extract the total miRNA. RNA yield was determined spectrophotometrically at 260/280 nm using the nano drop instrument (Thermo Fisher Scientific). K-Ras, Nur77, and c-Myc expression was assessed with qRT-PCR using a QuantiTect reverse transcription kit (Qiagen, MD, USA) and miR-200a assessed using Applied Biosystems (Foster City, USA). DNA denaturation was performed at 95 °C for 15 s, annealing at 60 °C for 30 s and further extension occurred at 72 °C for 30 s. This cycle was repeated 40 times to support the detection of amplified DNA. Primer sequences are provided in Table 1. The housekeeping gene for K-Ras, Nur77, and c-Myc was β-actin, and miR-200a was standardized to RNU6B. Relative quantitation (RQ) of target genes was calculated using cycle threshold (Ct) values as: RQ = 2− ΔΔCt, where ΔΔCt = [(Ct target gene in Sample)-(Ct ref gene in Sample)]-[(Ct target gene in Control)-(Ct ref gene in Control)] (Sahdev et al., 2017).
Table 1.
Primer sequences.
| Gene symbol | Primer sequence from 5′- 3′ | Gene bank accession number |
|---|---|---|
| K-Ras | F: AAAATGACTGAATATAAACTTGTGG R: CTCTATTGTTGGATCATATTCGTC |
XM014544259 |
| c-Myc | F: AACAGGAACTATGACCTCG R: AGCAGCTCGAATTTCTTC |
NM_012603.2 |
| Nur77 | F:AGGGGGAGACTATTCCATGCC R:CCAGGCCTGAGCAGAAGATGAGC |
NM_024388.2 |
| β-Actin | F: ATGGATGACGATATCGCTGC R: CTTCTGACCCATACCCACCA |
NM_031144.3 |
| miR-200a | F: CTGGGCCTCTGTCCCCATCT R:GATGTTCAAAGGTGACCCA |
NR_031916.1 (Wu et al., 2018) |
| RNU6B | F: AACGCTTCACGATTTGCGT' R: CTCGCTTCGGCAGCACA |
XR_003710259.1 |
2.7. Western blotting analysis
Protein expression of NICD of Notch 1 receptor in colon tissue was measured using western blotting. The last colon segment was homogenized in radioimmunoprecipitation assay (RIPA) lysis buffer with protease and phosphatase inhibitors for protein extraction and protein concentration/sample was measured using Bradford Protein Assay kits (Bio Basic, Ontario, Canada; Cat# SK3041). Proteins (20 μg) were loaded on 12% sodium dodecyl sulfate polyacrylamide electrophoresis gels (Bio-Rad; Cat#161-0181). After separation, proteins were blotted onto polyvinylidene fluoride membranes. Blocking of the membranes used Tris-buffered saline with tween-20 (TBS-T) and 3% bovine serum albumin (BSA) at room temperature for 1 h. The 1ry antibodies, namely, mouse monoclonal NICD of Notch1 receptor (Santa Cruz Biotechnology, TX, USA; Cat#sc-373891) was diluted in TBS-T and incubated overnight at 4 °C following the manufacturer’s instructions. The following day, membranes were rinsed 3–5 times for 5 min with TBS-T and incubated with horse-radish peroxidase (HRP) conjugated 2ry antibody (Novus Biologicals; Cat#NB7160) for 1 h at room temperature. Washing was then repeated. Chemiluminescent Clarity™ ECL Western blotting substrate (Bio-Rad; Cat#170-5060) was used to visualize protein antibody complexes. Image analysis software was used to read band intensity against β-actin (housekeeping protein) by protein normalization on the Chemidoc™ MP imager (Clinx Scientific Instruments, Shanghai, China).
2.8. Estimation of apoptosis using transferase dUTP nick-end labeling (TUNEL) assay DNA fragmentation
The terminal deoxynucleotidyl TUNEL assay was used for detection of DNA fragmentation, a hallmark of apoptosis. This assay used frozen colon sections and an Apo-BrdU In Situ DNA Fragmentation Assay Kit (BioVision; Cat# K401-60) following the manufacturer’s instruction. The number of apoptotic cells exhibiting green fluorescence (TUNEL positive) is counted; and the numbers are expressed as percentage of total cells in the sample (Sharma and Agarwal, 2011). All images were captured using a camera-based fluorescence microscope (Flexacam C1, Leica Microsystems Inc, IL, USA) (200x magnification & 50 µm scale bar). Image analysis was performed using Image–Pro plus version 6.0 (Media Cybernetics Inc., MD, USA).
2.9. Histopathological and immunohistochemical assessments
Fixed colon tissues were embedded in paraffin at 56 °C in a hot air oven for 1 day and 4-µm tissue sections were divided into two sets. The first set was transferred to glass slides, deparaffinized, and stained with H&E for examination with a light microscope (100x magnification and 100 µm scale bar). A scoring system was adopted from Ahmed et al. (2017b) with modification. Grading from (0–4) was used to evaluate morphological alterations detected in colon sections (dysplastic activity in the mucosal layer with hyperchromachia, inflammatory reactions, and edema); 0: Nil (0%–19%); +1: Mild (20%–39%); +2: Moderate (40%–59%), +3: marked (60%–79%), and + 4: Severe (80%–100%).
The 2nd set was used for immunohistochemistry protein expression of β-catenin, cyclin D1 and CD31. The sections were prepared for antigen retrieval by adding 10 mM sodium citrate (pH = 6) and microwaving for 15 min. Subsequently, tissues were blocked in 3% hydrogen peroxide-methanol for 15 min at room temperature and then washed with distilled deionized water and phosphate buffered saline (PBS). Thermo Fisher Scientific 1ry monoclonal antibodies of β-catenin (Cat#13-8400), cyclin D1 (Cat#MA5-16356) and CD31 (Cat# MA5-16337) were diluted in 3% BSA-PBS (at a dilution of 1:100 for β-catenin & cyclin D1 or 1:50 for CD31), and then incubated with the slides at 4 °C for overnight in a humidity chamber. Thereafter, slides were incubated with HRP conjugated 2ry antibody (Agilent Dako, CA, USA; Cat#K8000) for 30 min followed by colorimetric detection using a 3, 3′- diaminobenzidine (DAB) kit (Thermo Fisher Scientific; Cat# 34002). The last step before investigating the slides under a microscope is counterstaining the slides with H stain and dehydration with ethanol and xylene. Histopathological and immunohistological examinations were performed by a pathologist who was blinded to the origin of samples. All images were captured using a digital microscope camera (Tucsen® ISH1000; Fuzhou, China) and Olympus® CX21 microscope (Tokyo, Japan). Image analysis for immunoreactivity was performed using image J software (MA, USA). The mean of stained area percentage was determined for each group (400 x magnification) under the light microscope.
2.10. In-vitro study
2.10.1. Cytotoxicity assay
The tumor HCT116 cells (American Type Culture Collection; Manassas, USA) were maintained at the National Cancer Institute (Cairo, Egypt) by serial sub-culturing. These cells were cultured in a 200 µl fresh medium (RPMI) and left for 24 hr then treated with 5-FU or Diac at different concentration gradients of 31.25, 62.5, 125, 250, 500 μg/ml and 12.5, 25, 50, 100, 200 μg/ml, respectively. Following, the plates were incubated for 72 hr and according to the method of Skehan et al. (1990), sulphorhodamine-B dye was added and incubated at room temperature for half an hour. The optical density (O.D.) of each well was measured spectrophotometrically at 570 nm with an ELISA microplate reader (Sunrise Tecan reader, Germany). The mean background absorbance was automatically subtracted and the mean value of each drug concentration was calculated as follows.
The IC50 values of 5-FU and Diac were calculated using prism version 8 (Skehan et al., 1990). In order to obtain the IC50 value of the combination group, cytotoxicity assay for the combination group was performed using the IC50 of Diac with different concentrations of 5-FU (31.25, 62.5, 125, 250, 500 μg/ml).
2.10.2. Migration and invasion assay
The wound closure assay was used to evaluate the effect of the tested drugs on migration. In this test, a graze is made on a cell monolayer and the IC50 values of 5-FU, Diac and their combination (obtained from the cytotoxicity assays) were added. The tissue culture plates were then placed in an incubator set at room temperature and 5% CO2 for 72 hr. Afterwards, the media was removed from the wells and the wells were treated with 70% ethanol and crystal violet for cell fixation and staining, respectively as described previously by Justus et al. (2014). The migration distance, which is the distance from one side of the wound to the other, was quantified by taking snapshot pictures with Leica L2 inverted microscope (Leica microsystems, Wetzlar, Germany). Pictures were captured at 20 x magnification and 1 mm scale bar.
3. Statistical analysis
Data are presented as means ± standard deviation and statistical analysis was carried out using unpaired Student t-tests or one-way analysis of variance followed by Tukey’s post-hoc test to compare between two or more means, respectively. For non-parametric data, values were analyzed using Kruskal–Wallis test followed by Dunn’s test; Mann–Whitney tests were used to analyze significant differences between two groups. Data analysis and drawings of figures used GraphPad Prism® (GraphPad software, CA, USA) version 6. A probability level of < 0.05 was used as the criterion for significance.
4. Results
4.1. Diac and/or 5-FU ameliorate CRC
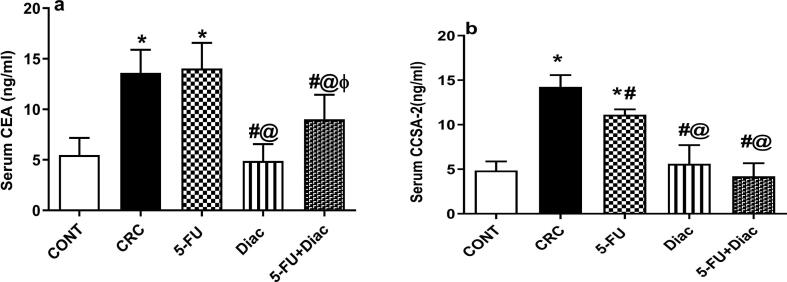
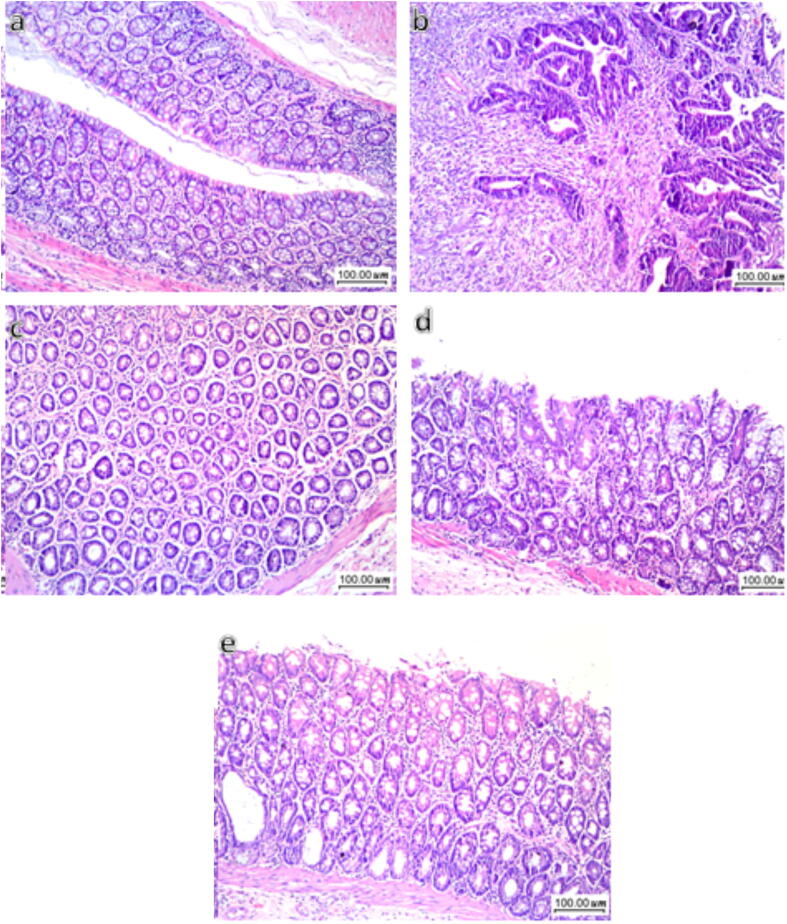
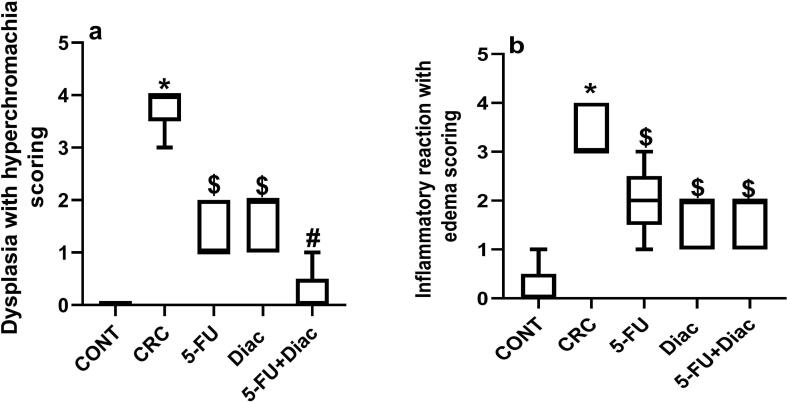
Serum levels of (a) CEA and (b) CCSA-2 in CRC rats exhibited 2.5- and 2.9-fold increases, respectively compared to sera from negative control animals (Fig. 2). Treatment with Diac alone, but not 5-FU, decreased CEA, an effect reflected in combined treatment. Diac alone caused 64% inhibition, but the combination regimen leveled it off only by 34%. Conversely, all treatment regimens reversed changes in the more reliable diagnostic CRC biomarker, CCSA-2. This protein decreased after 5-FU treatment. Diac alone and with 5-FU returned levels back to the normal range. These findings were reflected in the histopathology results (Fig. 3). Control rats (a) exhibited normal colon mucosa formed of regularly arranged tubular glands and crypts and lined by columnar epithelial cells. The latter is characterized by luminal cytoplasmic mucus secretion (goblet cells) and flattened/small rounded basal uniform nuclei. The lamina propria between glands show loose connective tissue without inflammatory infiltration. In the CRC group (b) shows multiple scattered aberrant crypt foci with columnar epithelial cells, loss of goblet cells, and nuclear enlargement with prominent nucleoli, moderate nuclear pleomorphism, hyperchromachia, and coarse chromatin with many mitotic figures leading to an increase in the nucleo-cytoplasmic ratio. Moreover, some glands are fused and form cribriform patterns with invasion beyond the muscularis mucosa and surrounding desmoplasia, indicating frank carcinoma formation. Additionally, surrounding lamina propria is infiltrated by inflammatory cells, mainly lymphocytes and plasma cells. On the contrary, treatment with Diac, 5-FU, or their combination markedly improve dysplastic changes, where sections of (c) 5-FU, (d) Diac and (e) their combination induced improvement in altered morphology; most colonic glands are lined by columnar epithelial cells with preserved goblet cells and regular nuclei. Nonetheless, few scattered glands showed a decrease in goblet cell count and slight enlargement of nuclei with hyperchromachia. Scoring of histopathological changes detected in colon sections for different treatments are provided in Fig. 4.
Fig. 2.
Effect of Diac and/or 5-FU for 7 weeks on serum level of (a) CEA and (b) CCSA-2 in DMH-induced CRC in rats. Data are expressed as means ± SD (n = 6 animals/group). Statistical analysis was carried out using one-way ANOVA followed by Tukey’s Multiple Comparison test; P < 0.05. As compared with (*) CONT, (#) CRC, (@) 5-FU and (ϕ) Diac-treated groups. Treatments started on week 16 after DMH administration and lasted for 7 weeks, where Diac was gavaged daily and 5-FU was injected once weekly. ANOVA: analysis of variance; CCSA-2: colon cancer specific antigen; CEA: carcino-embryonic antigen; CONT: control; CRC: colorectal cancer; Diac: Diacerein; 5-FU: 5-fluorouracil.
Fig. 3.
Effect of Diac and/or 5-FU on CRC-induced morphological alterations. Compared to section of (a) CONT group showing normal architecture, the (b) CRC section depicts dysplasia in the epithelial cells, indicated by a decreased count of goblet cells, nuclear enlargement, pleomorphism, hyperchromachia and rise in mitotic activity. Some glands formed cribriform pattern and invade beyond muscularis mucosa and surrounded by desmoplasia and the lamina propria between glands is infiltrated by inflammatory cells. However, sections of (c) 5-FU, (d) Diac, and (e) 5-FU+Diac show improvement in the dysplastic changes, mostly with goblet cell restoration and epithelial cells with regular nuclei; only very few scattered glands reveal reduced goblet cells count, slight nuclear enlargement and hyperchromachia.
Fig. 4.
The scoring of the histopathological changes detected in colon sections of the different studied groups. Panel (a) represents dysplastic activity in mucosal layer associated with hyperchromachia, while panel (b) depicts inflammatory reaction in mucosal layer with edema. Data are presented as boxes and whiskers and expressed as median (min-max) (n = 5/group). Statistical analysis was carried out using Kruskal-Wallis followed by Dunn’s test; Mann-Whitney test was used to analyze significance between 2 groups; P < 0.05. As compared with (*) CONT, (#) CRC treated groups. ($) represents significant versus CRC group using Mann-Whitney test. Treatments started on week 16 after DMH administration and lasted for 7 weeks, where Diac was gavaged daily and 5-FU was injected once weekly. CONT: control; CRC: colorectal cancer; Diac: Diacerein; 5-FU: 5-fluorouracil.
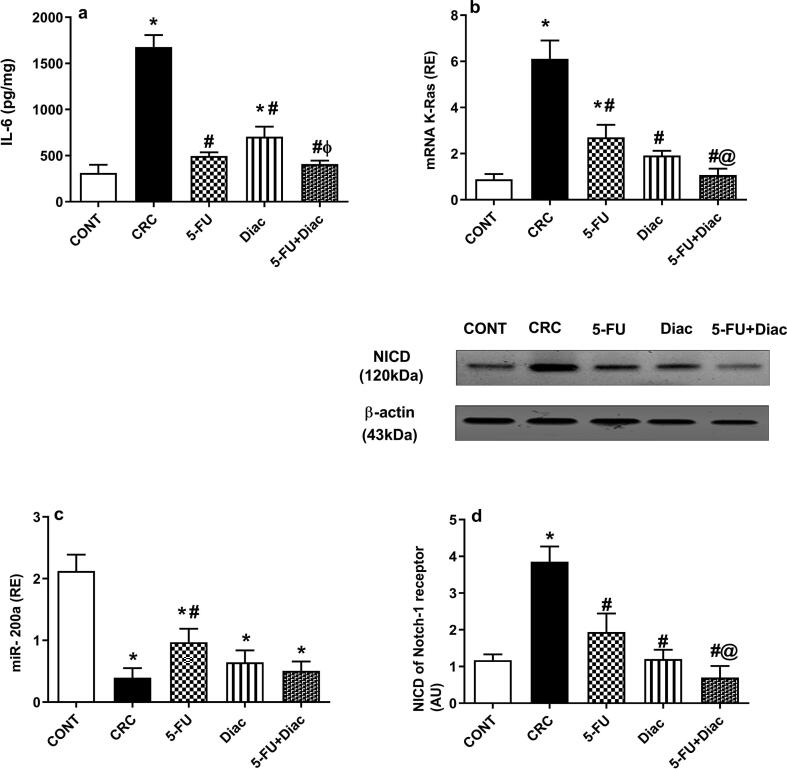
4.2. Diac and/or 5-FU modulation of the IL-6/K-Ras/ NICD pathway and miR-200a in CRC
The colon content of (a) IL-6 was notably enhanced 5.4-fold, an effect associated with sharp upregulation (7 fold) of (b) K-Ras gene expression, but with marked downregulation of (c) miR-200a (Fig. 5), CRC bolstered protein expression of (d) NICD 3.3-fold compared to normal controls. However, treatments variously altered the impact of CRC; Diac and 5-FU abated assessment parameters, only 5-FU succeeded in upregulating gene expression of miR-200a. Notably, the combination regimen showed a better inhibition compared to either drug alone.
Fig. 5.
Effect of Diac and/or 5-FU for 7 weeks on colonic content of (a) IL-6, gene/protein expression of (b) K-Ras, (c) miR-200a, and (d) NICD in DMH-induced CRC rats. Data are expressed as means ± SD (n = 6 animals/group). Statistical analysis was carried out using one-way ANOVA followed by Tukey’s Multiple Comparison test; P < 0.05. As compared with (*) CONT, (#) CRC, (@) 5-FU and (ϕ) Diac-treated groups. Treatments started on week 16 after DMH administration and lasted for 7 weeks, where Diac was gavaged daily and 5-FU was injected once weekly. ANOVA: analysis of variance; CONT: control; CRC: colorectal cancer; Diac: Diacerein; 5-FU: 5-fluorouracil; IL-6: interleukin-6; K-Ras: Kirsten rat sarcoma viral oncogene; NICD: Notch intracellular domain.
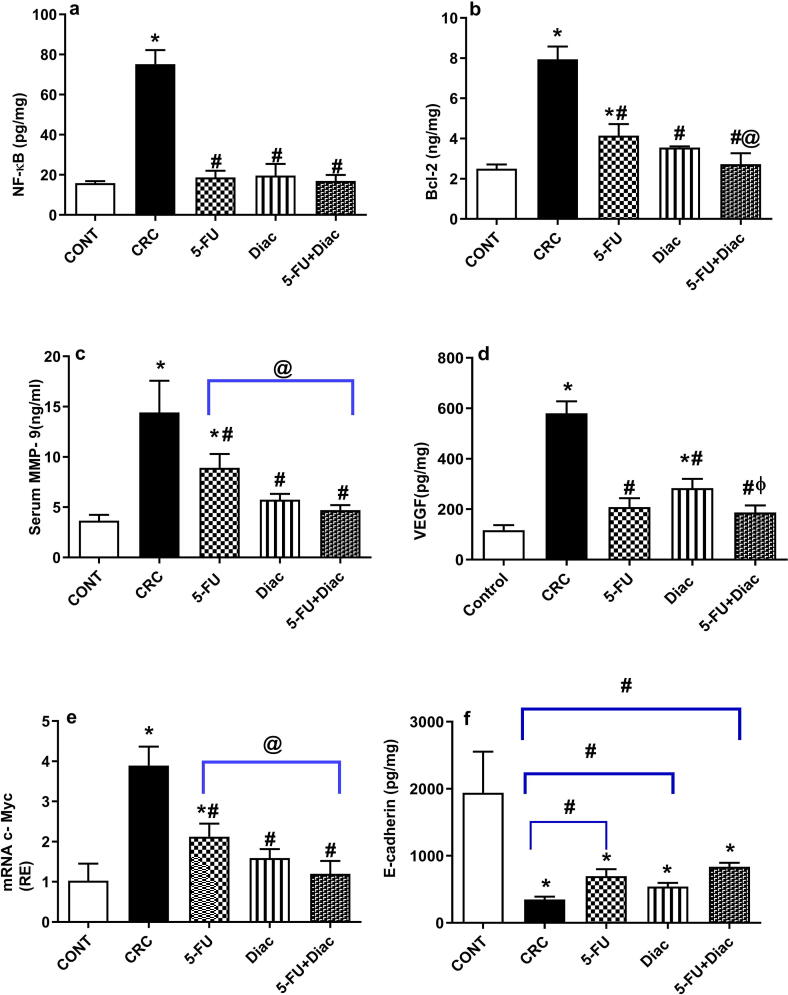
4.3. Diac and/or 5-FU deactivate the NF-κB cue to reduce survival, angiogenesis, invasion, proliferation, and metastasis in CRC
DMH caused a 4.7-fold increase in the colon content of (a) NF-κB; however, treatment with 5-FU, Diac, and 5-FU+Diac normalized NF-κB compared to CRC rats (Fig. 6). Upregulated transcription factors enhanced the content of downstream molecules, namely, (b) Bcl-2, (c) MMP-9, and (d) VEGF, as well as (e) the gene expression of c-Myc. Normalized NF-κB induced downregulation in colon content of (f) the adherens junction protein, E-cadherin. However, all treatments returned NF-κB to baseline, an effect reflected differently for assessed oncogenic markers, where Diac alone or when combined with 5-FU showed a better inhibitory effect relative to that of 5-FU alone, except for VEGF. Nevertheless, these treatments tended to increase E-cadherin to a significant level when analyzed using the Student’s t test.
Fig. 6.
Effect of Diac and/or 5-FU for 7 weeks on colonic/serum contents of (a) NF-κB, (b) Bcl-2, (c) MMP-9, (d) VEGF, and (f) E-cadherin, as well as gene expression of (e) c-Myc in DMH-induced CRC rats. Data are expressed as means ± SD (n = 6 animals/ group). Statistical analysis was carried out using one-way ANOVA followed by Tukey’s Multiple Comparison test; P < 0.05. As compared with (*) CONT, (#) CRC, (@) 5-FU and (ϕ) Diac-treated groups. Symbols on the inverted brackets represent significance using Student’s t test. Treatments started on week 16 after DMH administration and lasted for 7 weeks, where Diac was gavaged daily and 5-FU was injected once weekly. ANOVA: analysis of variance; Bcl-2: B-cell lymphoma-2; CONT: control; CRC: colorectal cancer; Diac: Diacerein; 5-FU: 5-fluorouracil; MMP-9: matrix metalloproteinase-9; NF-κB: nuclear factor-kappa B; VEGF: vascular endothelial growth factor.
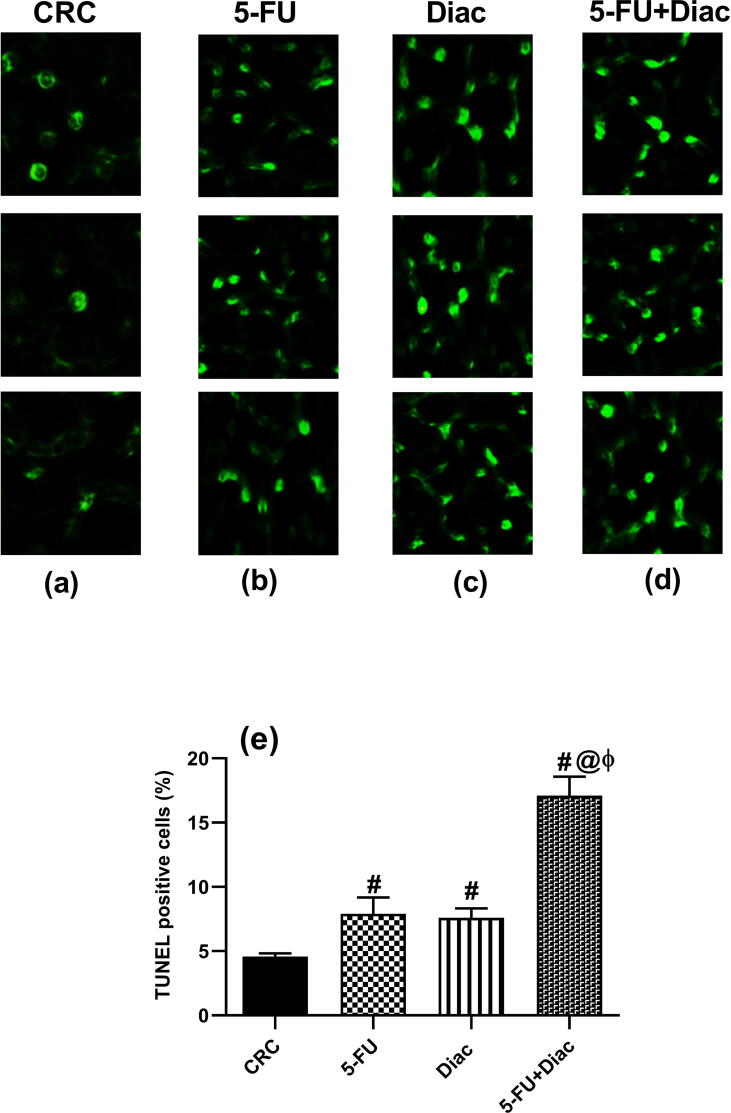
4.4. Diac and/or 5-FU increases apoptotic cell death
TUNEL assays were used to document the apoptotic effect of tested drugs (Fig. 7) Panel (a) shows negative TUNEL cells in the CRC sections, whereas panels (b-d) show the effects in treated animals, which displayed upregulated apoptotic cells indicated as green fluorescence. These results are summarized in panel (e).
Fig. 7.
Effect of Diac and/or 5-FU for 7 weeks on the count of TUNEL positive cells (apoptotic cells) in DMH-induced CRC rats. Section of (a) CRC depicts negative TUNEL cells, while those of (b) 5-FU, (c) Diac and (d) 5-FU+Diac treated groups reveal positive TUNEL cells. These data are summarized in panel (e), where the number of TUNEL positive cells is expressed as percentage of total count of sample. Values are expressed as means of TUNEL positive cells percentage ± SD (n = 6 animals/group) and analyzed using one-way ANOVA followed by Tukey’s Multiple Comparison test; P < 0.05. As compared with (#) CRC, (@) 5-FU and (ϕ) Diac treated groups. Treatments started on week 16 after DMH administration and lasted for 7 weeks, where Diac was gavaged daily and 5-FU was injected once weekly. ANOVA: analysis of variance; CRC: colorectal cancer; Diac: Diacerein; 5-FU: 5-fluorouracil. TUNEL: Terminal deoxynucleotidyl transferase dUTP nick-end labeling.
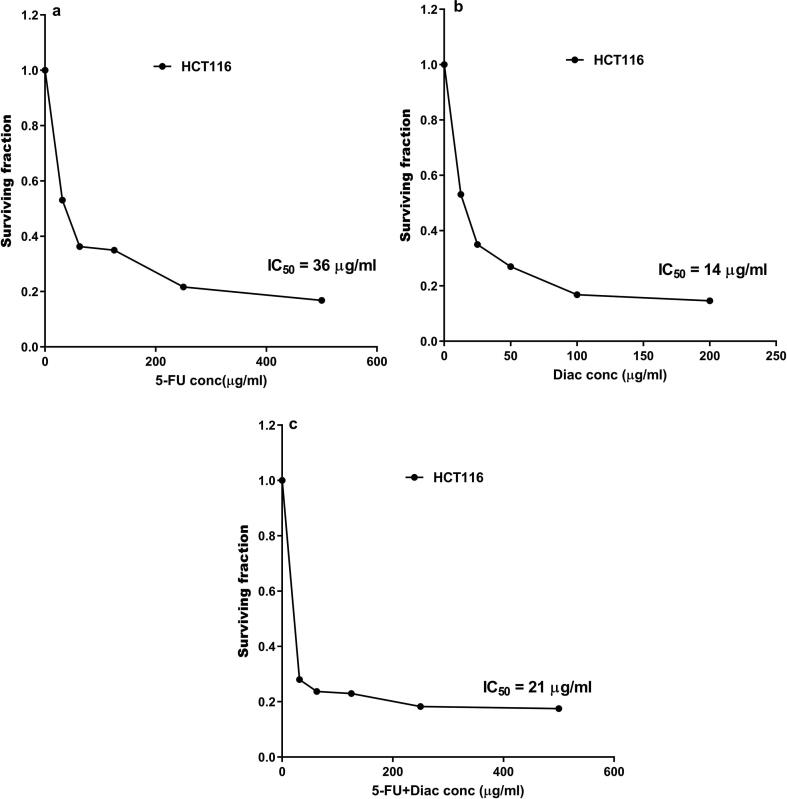
4.5. Diac improved the IC50 of 5-FU
The cytotoxic effect of Diac and/or 5-FU are depicted in Fig. 8, where Diac at a concentration of 14 μg/ml inhibited the growth of 50% of the cells, whereas the IC50 of 5-FU was 36 μg/ml. The effect of Diac IC50 lessened that of 5-FU to reach 21 μg/ml instead of 36 μg/ml.
Fig. 8.
Cell surviving fractions were determined at varying concentrations of (a) 5-FU, (b) Diac and (c) 5-FU+Diac using HCT116 cells after incubation for 72 h. Diac: Diacerein; 5-FU: 5-fluorouracil; HCT116: human colorectal carcinoma cell line.
4.6. Diac and/or 5-FU reduces invasion and metastasis
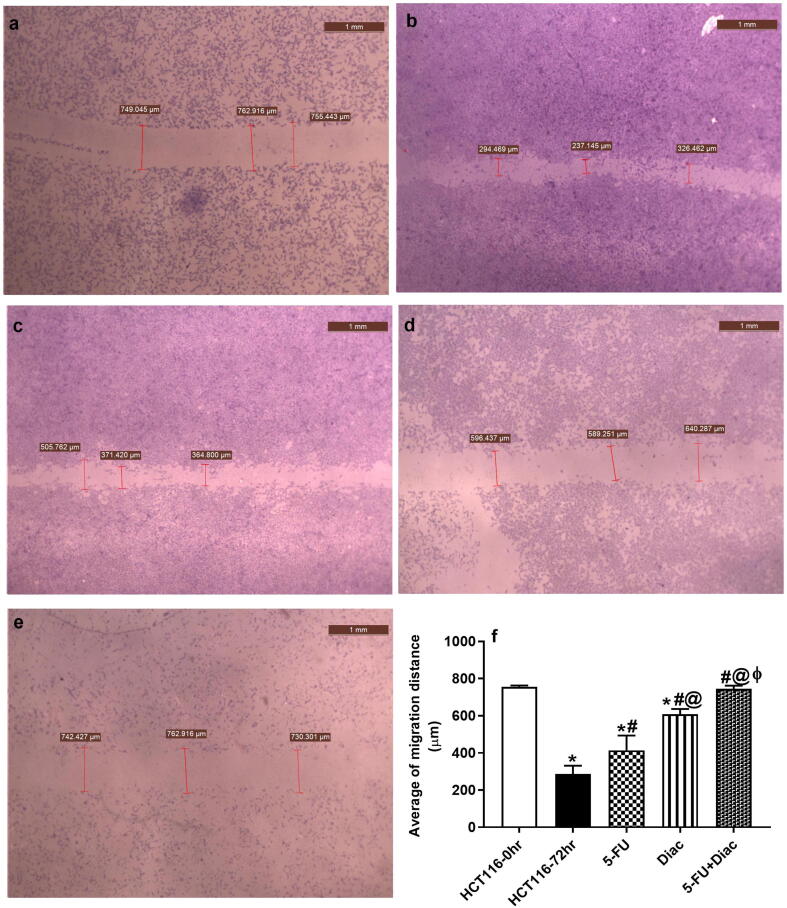
To further verify the anti-metastatic potential of Diac and to investigate the benefits of the add-on therapy on the anti-metastatic effect of 5-FU, an in-vitro cell migration and invasion assay was performed using the wound closure method. As shown in Fig. 9 (a-f), Diac decreased cell migration more than 5-FU did, as represented by increasing the wound width of HCT116 cells. Interestingly, the addition of Diac to 5-FU showed a significant reduction in cell migration verified by the wider wound space compared to the individual drugs to almost reach normal values.
Fig. 9.
Representative wound closure photos of HCT116 cells after 72 h. (a) untreated HCT116 cells at 0 h, (b) untreated HCT116 cells at 72 h, (c) treated with IC50 of 5-FU (d) treated with IC50 of Diac (E) treated with IC50 of 5-FU+Diac. The wound width was measured in μm. Pictures were captured at 20× magnification and 1 mm scale bar. Panel (f) represents the average of the migration distance, where data are illustrated as means ± SD and analyzed using one-way ANOVA followed by Tukey’s Multiple Comparison test; P < 0.05. As compared with (*) HCT116-0hr, (#)HCT116-72 hr, (@) 5-FU and (ϕ) Diac-treated groups. ANOVA: analysis of variance; Diac: Diacerein; 5-FU: 5-fluorouracil; HCT116:human colorectal carcinoma cell line.
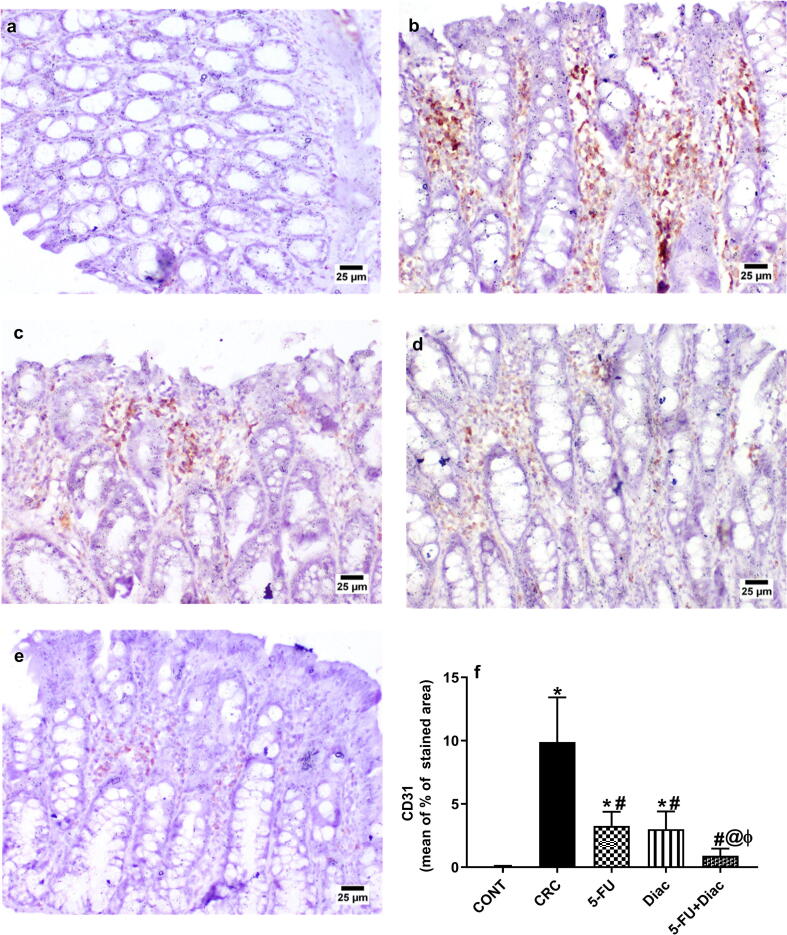
4.7. Diac and/or 5-FU reduces angiogenesis
The immunoreactivity of CD31 was assessed to further confirm the anti-angiogenic effect of the tested drugs (Fig. 10 a-f). Section of the (a) control group panel shows normal colonic epithelium with normal CD31 immunoreactivity, whereas that of (b) CRC reveals excessive CD31 protein expression in the colon mucosa and in the newly formed blood vessels in submucosa. Meanwhile, treatment with (c) 5-FU and (d) Diac depict a moderate CD31 immunoreactivity. Finally, the combination group (e) succeeded to reduce the expression of CD31 to resemble that of the control group. These findings were briefed in panel (f).
Fig. 10.
Effect of Diac and/or 5-FU for 7 weeks on the colonic CD31 immuno-histochemical staining in DMH-induced CRC rats. Section of (a) CONT shows normal colonic epithelium with normal expression of CD31, whereas section of (b) CRC reveals excessive CD31 immunoreactivity in colon mucosa and in newly formed blood vessels in the submucosa. Sections of (c) 5-FU and (d) Diac treated groups show moderate CD31 immunoreactivity, while that of (e) 5-FU+Diac shows subtle CD31immunoreactivity. Panel (f) represents % of stained area, where data are illustrated as means ± SD (n = 6 animals/group) and analyzed using one-way ANOVA followed by Tukey’s Multiple Comparison test; P < 0.05. As compared with (*) CONT, (#) CRC, (@) 5-FU and (ϕ) Diac-treated groups. Treatments started on week 16 after DMH administration and lasted for 7 weeks, where Diac was gavaged daily and 5-FU was injected once weekly. ANOVA: analysis of variance; CD31: cluster of differentiation 31; CONT: control; CRC: colorectal cancer; Diac: Diacerein; 5-FU: 5-fluorouracil.
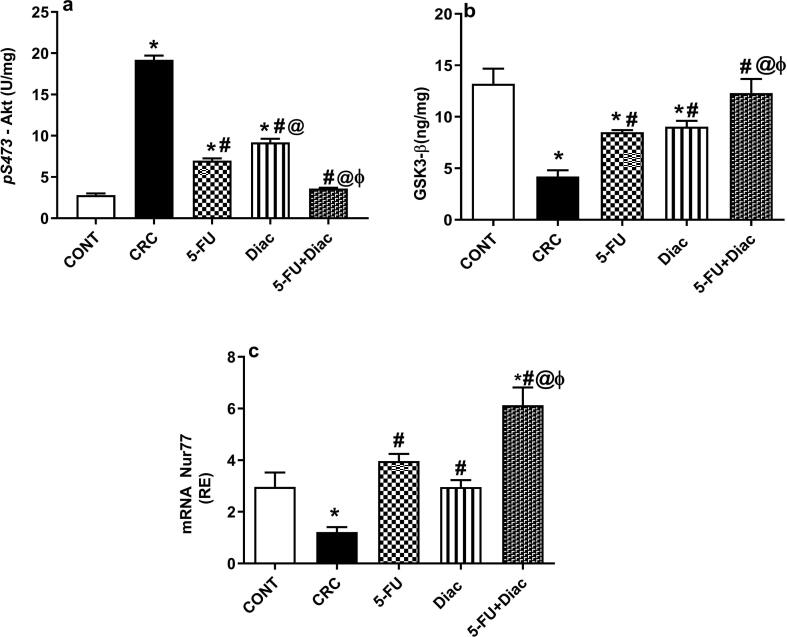
4.8. Diac and/or 5-FU inactivate Akt to activate downstream molecules, Nur77 and GSK3-β, in CRC
The second pathway triggered by the upstream molecule, IL-6; upregulated activation/phosphorylation of (a) Akt in the CRC group about 7-fold, when compared to control rats. Activated protein kinase was associated with inactivation of tumor protector (b) GSK3-β, as well as the orphan nuclear receptor (c) Nur77. These effects were significantly reversed by the administration of either drug alone, while the best effect was seen in rats treated with the drug combination, which normalized both p-Akt and GSK3-β and upregulated Nur77 above controls (Fig. 11).
Fig. 11.
Effect of Diac and/or 5-FU for 7 weeks on protein content of (a) p-Akt, (b) GSK3-β and gene expression of (c) Nur77 in DMH-induced CRC rats. Data are expressed as means ± SD (n = 6 animals/group). Statistical analysis was carried out using one-way ANOVA followed by Tukey’s Multiple Comparison test; P < 0.05. As compared with (*) CONT, (#) CRC, (@) 5-FU and (ϕ) Diac-treated groups. Treatments started on week 16 after DMH administration and lasted for 7 weeks, where Diac was gavaged daily and 5-FU was injected once weekly. ANOVA: analysis of variance; CONT: control; CRC: colorectal cancer; Diac: Diacerein; 5-FU: 5-fluorouracil; GSK3-β: glycogen synthase kinase 3-β; p-Akt: phosphorylated protein kinase B.
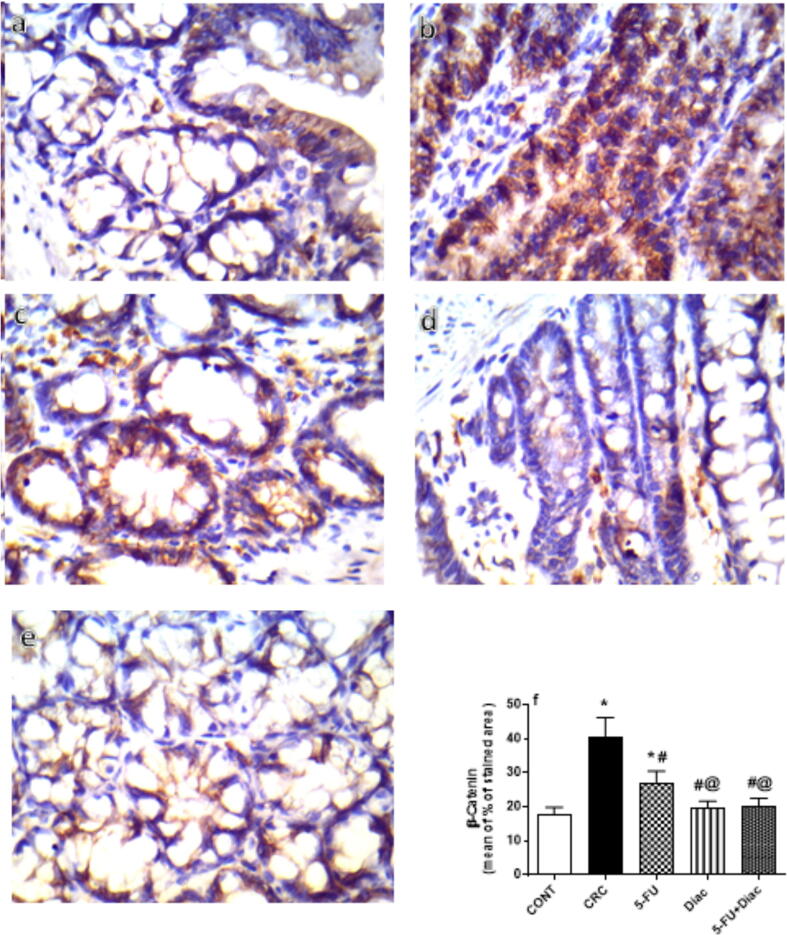
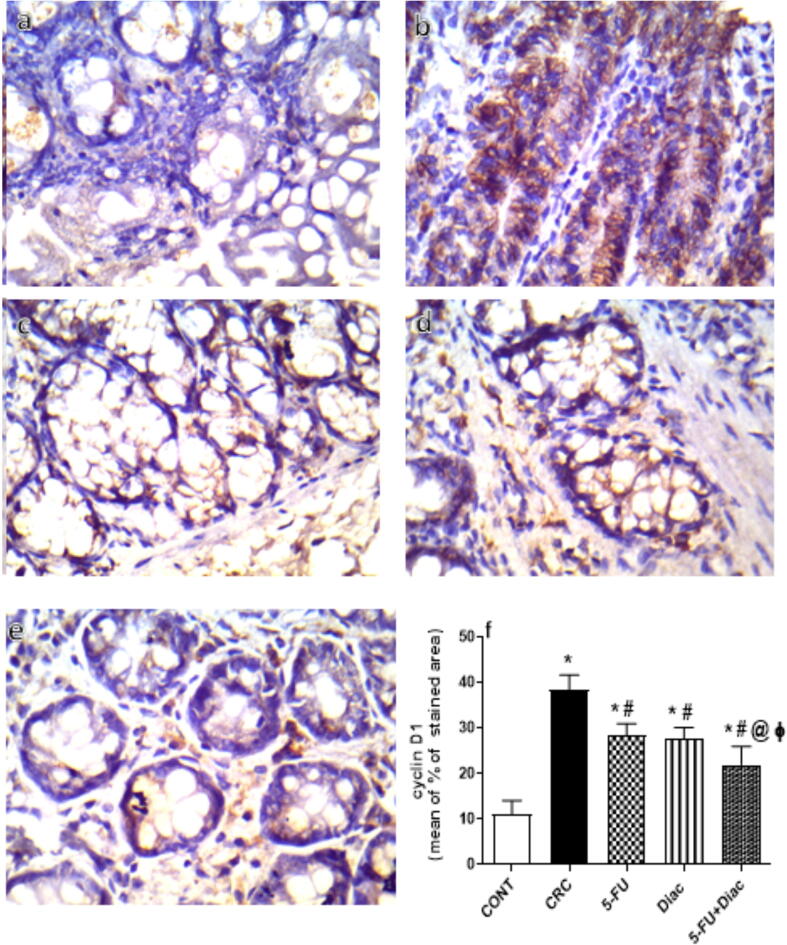
4.9. Diac and/or 5-FU inhibited immunoreactivity of β–catenin and cyclin D1 in CRC
DMH-induced CRC noticeably enhanced the immunoreactivity of GSK3-β-target molecule β–catenin (Fig. 12 a-f), and cyclin D1 (Fig. 13 a-f). Treatment with 5-FU, Diac, and 5-FU+Diac ameliorated CRC effects and inhibited β–catenin and cyclin D1 immunoreactivity. Notably, the effects of both Diac alone and 5-FU+Diac were superior to 5-FU single regimen on β–catenin; for cyclin D1, the effect of the combination regimen was superior to either drug alone.
Fig. 12.
Effect of Diac and/or 5-FU for 7 weeks on the colonic β-catenin immuno-histochemical staining in DMH-induced CRC rats. Section of (a) CONT shows normal colonic epithelium of β-catenin expressed in the membrane and cytoplasm, whereas section of (b) CRC reveals excessive β-catenin immunoreactivity. Section of (c) 5-FU treated group shows moderate β-catenin immunoreactivity, while those of (d) Diac and (e) 5-FU+Diac show normal β-catenin immunoreactivity. Panel (f) represents % of stained area, where data are illustrated as means ± SD (n = 6 animals/group) and analyzed using one-way ANOVA followed by Tukey’s Multiple Comparison test; P < 0.05. As compared with (*) CONT, (#) CRC and (@) 5-FU treated groups. Treatments started on week 16 after DMH administration and lasted for 7 weeks, where Diac was gavaged daily and 5-FU was injected once weekly. ANOVA: analysis of variance; CONT: control; CRC: colorectal cancer; Diac: Diacerein; 5-FU: 5-fluorouracil.
Fig. 13.
Effect of Diac and/or 5-FU for 7 weeks on the colonic cyclin D1 immuno-histochemical staining in DMH-induced CRC rats. Panel (a) of CONT group reveals normal colonic epithelium of cyclin D1expressed in the membrane and cytoplasm, while excessive cyclin D1 immunoreactivity is detected in panel (b) of CRC group. Sections from (c) 5-FU and (d) Diac treated groups display moderate cyclin D1 immunoreactivity, effect that is more prominent in the combined (e) 5-FU+Diac regimen. Panel (f) represents % of stained area, where data are illustrated as means ± SD (n = 6 animals/group) and analyzed using one-way ANOVA followed by Tukey’s Multiple Comparison test; P < 0.05. As compared with (*) CONT, (#) CRC and (@) 5-FU treated groups. Treatments started on week 16 after DMH administration and lasted for 7 weeks, where Diac was gavaged daily and 5-FU was injected once weekly. ANOVA: analysis of variance; CONT: control; CRC: colorectal cancer; Diac: Diacerein; 5-FU: 5-fluorouracil.
5. Discussion
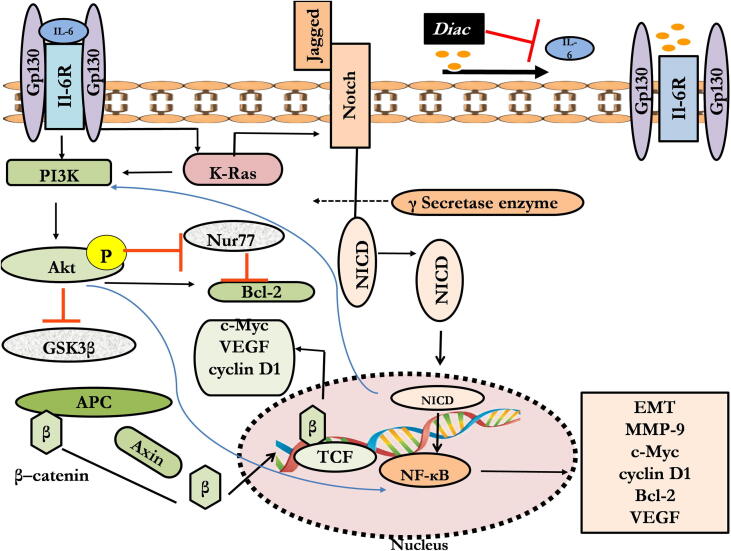
The present research shows for the first time in-vivo antineoplastic properties of Diac alone compared with a standard antitumor drug, 5-FU, in a rat model of CRC. Diac and 5-FU ameliorated tumor progression via inhibition of angiogenesis, proliferation, and metastasis, while promoting apoptosis. These effects were further documented by an increase in morphological indices. Such effects can be a consequence of downregulation of IL-6 and its downstream factors; viz., K-Ras/NICD/NF-κB p65/Bcl-2, MMP-9, and Akt/GSK3-β/β–catenin, long with common target molecules VEGF, c-Myc, and cyclin D1. Additionally, 5-FU alone upregulated gene expression of miR-200a, while the two drugs, as well as their combination, succeeded in upregulating gene expression of Nur77 (Fig. 14).
Fig. 14.
Schematic presentation of diacerin antineoplastic effect. Diac: Diacerein; IL-6: interleukin-6; K-Ras: Kirsten rat sarcoma viral oncogene; NICD: Notch intracellular domain; MMP-9: matrix metalloproteinase-9; NF-κB: nuclear factor-kappa B; VEGF: vascular endothelial growth factor; GSK3-β: glycogen synthase kinase 3-β; p-Akt: phosphorylated protein kinase B.
The antitumor effect of Diac, the first focus of our work, showed effects on several oncogenic targets. Diac and/5-FU significantly reduced serum levels of the diagnostic tumor marker, CCSA, with reference to CRC rats. Meanwhile, serum levels of CEA dropped in rats treated with Diac alone or in combination with 5-FU. Animals treated with 5-FU alone showed no statistically significant changes compared to CRC controls. Inconsistent results were reported, CEA is reported as overexpressed after exposure of colon cancer cells to 5-FU (Aquino et al., 2000, Prete et al., 2008, Tao et al., 2015), but is also reported to decrease 5-FU levels of this marker (Lee et al., 2016, Ahmed et al., 2017a). This discrepancy highlights the low specificity and sensitivity CEA serum test as a diagnostic and prognostic marker for CRC patients. Conversely, CCSA-2 shows more consistent results and is more sensitive (Xue et al., 2014).
Notably, inflammation is an intricate part of CRC tumorigenesis. Tumor cells secrete a variety of inflammatory molecules, including cytokines, in their microenvironment that drive tumor progression (Setrerrahmane and Xu, 2017). Cytokine IL-6 induces cancer proliferation(Lee et al., 2003), survival (Liu et al., 2010), resistance to chemotherapy (Wang, Y et al., 2010), epithelial mesenchymal transition (EMT) (Sullivan et al., 2009), angiogenesis (Wei et al., 2003), invasion, and metastasis (Chang et al., 2013). IL-6 binding to its receptor activates glycoprotein 130 (gp130) to promote several oncogenic pathways, such as RAS/MAPK and PI3K/Akt(Hideshima et al., 2001, Cavarretta et al., 2007). Accordingly, our study highlights the possible impact of Diac alone and with 5-FU on the IL-6 downstream factors.
K-Ras is part of the RAS/MAPK pathway that acts as a switch for signal transmission, including the Notch gene. Notably, K-Ras is synergistic with the Notch pathway (De La O et al., 2008, De La O and Murtaugh, 2009 and enhances the expression of Notch -1 via p38MAPK (Wang et al., 2008). This pathway is the first hinge for Diac-mediated effects. Diac and/or 5-FU inhibit IL-6, K-Ras, γ- secretase, and NICD of the Notch-1 receptor. Additionally, miR-200a likely cross talks with K-Ras/Notch axis. K-Ras downregulates miR-200a and the latter abates the Notch pathway (Wang, Z et al., 2010a), however, this process was confirmed for 5-FU exposure only. miR-200a was upregulated by 5-FU, but not by Diac, a finding that suggests that K-Ras is not the sole regulator of miR-200a.
Following the release of NICD from the Notch receptor, it is translocated to activate transcription factor NF-κB (Harbuzariu et al., 2018) and its target genes. These genes have a critical role in tumorigenesis. These genes are hallmarks of cancer progression, including cell growth, proliferation, anti-apoptosis, angiogenesis, EMT, and inflammation (Aggarwal, 2004, Karin, 2006, Naugler and Karin, 2008, Zhao et al., 2015). Both Diac and 5-FU inhibited protein expression of NICD and colon content of NF-κB. This activity caused a decrease in target molecules, namely c-Myc (La Rosa et al., 1994, Rayet and Gelinas, 1999), cyclin D1(Cusack et al., 2000), Bcl-2 (Karin et al., 2002), and MMP-9 (Rangaswami et al., 2004), as well as VEGF (Ping et al., 2015). These results shed light on the possible anticancer effects of Diac. Previous studies reported a reduction in Notch-1 signaling and NF-κB activity (Wang et al., 2006) and, hence, tumorigenesis (Zeng et al., 2005, Maniati et al., 2011, Wang et al., 2015).
The epithelial marker E-cadherin is a critical marker for preventing EMT and hindering cellular movement, invasion, cell survival and metastasis (Mittal, 2018). Tested drugs in our work upregulated E-cadherin to improve cell-cell adherence and impair cellular metastasis. These findings concur with earlier studies, where Diac upregulates E-cadherin in-vitro study using a breast cell line (Bharti et al., 2018). Again, the inhibitory effect of Diac on NF-κB expression is one possible reason for increased E-cadherin. The expression of E-cadherin is regulated by various signaling molecules, including NF-κB (De Craene and Berx, 2013, Lamouille et al., 2014). However, to the best of our knowledge, the effects of 5-FU on E-cadherin has not been previously reported.
Another molecule that fosters metastasis is the oncogenic marker, MMP-9. This factor is also a downstream molecule of NF-κB (Ping et al., 2015) and is considered as a target gene of Notch (Wang et al., 2008). This marker plays a vital role in cell migration, invasion, and metastasis through hydrolysis of extracellular matrix collagen and mediation of extracellular matrix reshaping (Huang, 2018). Serum levels of MMP-9 in our experiment were markedly diminished in rats treated with Diac, 5-FU or their combination., a finding consistent with a previous study on breast cancer cells (Bharti et al., 2018) and that might partly explain its anti-metastatic effect.
Additionally, the two drugs and their combination leveled off the colon content of VEGF, another target molecule of NF-κB (Wang et al., 2006, Ping et al., 2015). VEGF exhibits a pleiotropic role in tumor progression, including metastasis (Qiu et al., 2016) and angiogenesis (Goel and Mercurio, 2013). VEGF is a critical factor in the release of MMP-2 and MMP-9 from tumor cells. This release leads to the degradation of collagen allowing subsequent metastases (Munaut et al., 2003). Moreover, this angiogenic factor is released from tumor cells to endothelial cells. It binds to its receptor and initiates mitosis and consequently angiogenesis (Marti, 2003). Moreover, initiation of angiogenesis can be linked to Notch signaling that enhances the expression of Jagged 1 that binds to the endothelial Notch receptor to trigger angiogenesis(Li and Harris, 2005). This process supports tumor growth, migration, invasion, and metastases of the tumor cells. Hence, inhibiting this marker may help further explain their anti-metastatic and anti-angiogenesis properties. Moreover, the anti-angiogenic potential of our tested drugs was reinforced by immunohistochemistry analysis of CD31 and their anti-metastatic potential was supported by in-vitro functional metastasis assay.
Additionally, cyclin D1 and c-Myc are other downstream targets of NF-κB and mediate important processes in cancer cell proliferation and the progression through the cancer cell cycle (Baldin et al., 1993, Dang, 2012). Cyclin D1 forms a complex with cyclin-dependent kinase enzymes (CDKs that leads to the transition of the cell cycle from G1 phase to S phase (Dong et al., 2018). Further, c-Myc stimulates cyclins and CDKs, and downregulates levels or blocks the activity of cell cycle inhibitors, such as of p15, p21, and p27(García-Gutiérrez et al., 2019). The current study first documented the ability of Diac to counteract these molecules to further support anti-proliferative and antitumor potential. Further, Diac, similar to 5-FU, enhanced cell death by downregulating the anti-apoptotic marker Bcl-2 as reported in a breast cancer cell line (Bharti et al., 2016). Diac thus acted via turning off IL-6/gp130/Janus kinase 2 (JAK2)/signal transduction and activator of transcription 3 (STAT3) pathways, another cascade to the activated IL-6. The cytotoxic assay further confirmed the ability of the tested drugs to inhibit the growth of the CRC cells.
Our study highlights the modulatory effect of Diac on another IL-6-associated carcinogenic pathway; viz., the Akt/GSK3-β/β-catenin cascade. Treatment with Diac, 5-FU, or their combination discernibly lowered protein content of p-Akt when compared to CRC rats. This effect is consistent with the previous work of Bharti et al. (2016). IL-6-mediated phosphorylation of gp130 was reported to activate PI3K with the subsequent phosphorylation/activation of Akt (Hideshima et al., 2001). In turn, GSK3-β is phosphorylated/inactivated (Dey et al., 2015) to stabilize and translocate β-catenin to the nucleus (Silva-García et al., 2019). These findings support the current results recorded for Diac and/or 5-FU treated rats that showed an increase in the non-phosphorylated/active form of GSK3- β, along with a decrease in its upstream factor, p-Akt, and downstream β-catenin. The latter molecule, as well as NF-κB p65 represent crosstalk points of the two IL-6 cascades; viz., the IL-6/K-Ras/NICD/NF-κB p65 and IL-6/Akt/GSK3-β/β-catenin signaling pathways. This concept can be confirmed by the ability of β-catenin to enhance the transcription of its target genes; viz., VEGF, c-Myc and cyclin D1(Agarwal et al., 2005, Sanjari et al., 2020); molecules that are also the target of their upstream regulator NF-κB, as discussed above.
Additionally, previous studies indicate that activation of Akt enhances anti-apoptotic marker Bcl-2 expression (Dey et al., 2015). Hence, the current apoptotic potential of Diac could be mediated by inhibition of Bcl-2, which represents a meeting point of the two pathways. Bcl-2 is a downstream molecule of both NF-κB and p-Akt.
Another explanation for the Diac-mediated inhibition of Bcl-2 is upregulated Nur77 (NR4A1 or TR3), an orphan nuclear receptor family that plays a crucial role in colon cancer. The nuclear export of Nur77 initiates apoptosis through targeting mitochondria. This factor binds to anti-apoptotic Bcl-2 forming a pro-apoptotic complex(Cho et al., 2008, Hu et al., 2019). The mRNA level of Nur77 in our study was appreciably amplified by Diac and/or 5-FU thus enhancing apoptotic processes.
The PI3K/Akt pathway is reported to increase NF-κB, as p-Akt induces IκB kinase (IKK) to activate the p65 subunit of NF-κB(Agarwal et al., 2005). Further, GSK3 represses RAS via phosphorylation at its C-terminus leading to its degradation (Ahn et al., 2017). The RAS pathway induces the PI3K/Akt axis (Lee and Gilliland, 2008). Additionally, activation of the Notch pathway induces PI3K/Akt and vice versa (Gutierrez and Look, 2007), an interaction confirmed when blocking of Notch-1 decreased phosphorylation of Akt and promoted its inactivation (Wang, Z et al., 2010b). Moreover, the impact of Nur77 cannot be ruled out. Cytosolic Nur77 suppresses β-catenin via enhancing its proteasomal degradation through activating GSK3-β (Sun et al., 2012). Moreover, a previous study reported that p-Akt inhibits Nur77 expression through phosphorylation at serine 350 (Pekarsky et al., 2001). Accordingly, the crosstalk between assessed pathways can explain the present effects of Diac and 5-FU.
Diac is known to act by inhibiting IL-6, which enhances 5-FU resistance (Li, S et al., 2018), but the inhibition of this cytokine did not differ among the three treatment regimens. Thus, we searched for other mechanisms to explain the improved chemosensitivity to 5-FU seen after combined treatment – the second goal of our study. Possibly, the combination of Diac to 5-FU augmented the inhibitory effect of 5-FU on the K-Ras oncogene and its downstream molecule, NICD. Jiang et al. (2004) showed that K-Ras enhances chemoresistance to 5-FU mediated apoptosis. Further, Li D.D. et al. (2018) reported that inhibition of Notch signaling chemosensitizer CRC cells to 5-FU. These results support our findings. Additionally, the inhibitory effect of the combination of drugs on MMP-9, a downstream molecule of Notch (Ranganathan et al., 2011), superseded that of 5-FU alone. Notably, inhibition of MMP-9 shares a pivotal role in the response of cancer cells to chemotherapy (Laios et al., 2013).
Another Notch downstream molecule is transcription factor, NF-κB, that was reduced by the three treatment regimens in a comparable fashion. Despite the role of NF-κB in the development of 5-FU resistance (Nakanishi and Toi, 2005, Yang et al., 2020), the combination of drugs produced no greater effect than 5-FU alone. Adding Diac to 5-FU enhanced the suppressive effect of 5-FU on NF-κB downstream oncogenic proteins. These molecules, with known involvement in chemoresistance, are c-Myc (Kugimiya et al., 2015, Wang et al., 2016) and cyclin D1(Xia et al., 2017). Hence, other upstream molecules in the GSK3-β/β-catenin pathway (Bertrand et al., 2012, Nusse and Clevers, 2017) are documented for involvement in 5-FU chemoresistance (Zheng et al., 2010, Blondy et al., 2020).
This concept is supported here by the further suppression of c-Myc and cyclin D1 after combined treatment compared to 5-FU monotherapy. Ample evidence supports the chemoresistance potential of the GSK3-β/β-catenin axis (Zheng et al., 2010, Blondy et al., 2020). Suppression of GSK3-β stimulates thymidylate synthase, the ultimate enzyme in 5-FU metabolism, and enhances β-catenin and Bcl-2 to promote CRC cell survival. Moreover, the anti-apoptotic marker Bcl-2 impedes the intrinsic apoptotic pathway (Nie et al., 2012, Wu et al., 2015), where it hampers the release of cytochrome c from mitochondria and consequently inhibits the activation of caspase 3 (Yang et al., 1997). Thus, the ability of combined treatment to upregulate GSK3-β and inhibit β-catenin and Bcl-2, as well as their downstream molecules, c-Myc and cyclin D1, indicate that the effects of 5-FU alone can support the synergistic effect of Diac in combination therapy.
Conversely, Nur77 abolishes the anti-apoptotic effect induced by Bcl-2. After mitochondrial translocation, Nur77 interacts with Bcl-2 and reverses its effect (Moll et al., 2006, Zhang, X.k., 2007), thus increasing chemosensitivity (Chen et al., 2019). Similarly, our results show that combination treatment upregulated Nur77 gene expression over that mediated by 5-FU monotherapy, a finding that supports the beneficial effect of add-on therapy with Diac. The latter drug may counteract chemoresistance by modulating the Nur77/Bcl-2 axis.
Our results also showed that K-Ras can organize another oncogenic pathway implicated in chemoresistance, namely, PI3K/p-Akt (Peyssonnaux et al., 2000, Li et al., 2020). The latter hub is reported to enhance resistance to 5-FU in the breast cancer cells (McCubrey et al., 2006) and CRC cells (Liu et al., 2017, Li et al., 2020). The addition of Diac to 5-FU in our study enhanced the inhibitory effect of 5-FU on the K-Ras/p-Akt pathway to offer another possible mechanism for the improved effect of 5-FU.
Overall, one can postulate that the combination of Diac with 5-FU may overcome the chemoresistance of CRC to 5-FU via targeting various interacting pathways. These pathways include K-Ras/Notch/MMP-9, GSK-3β/β-catenin/Bcl-2/c-Myc & cyclin D-1, Nur77/Bcl-2 and K-Ras/PI3K/Akt.
6. Conclusion
Our work highlights the antineoplastic potential of Diac to improve CRC treatment via inhibiting IL-6 and its downstream oncogenic cascade; viz., K-Ras/ NICD/NF-κB and Akt/GSK3-β/β-catenin. These pathways intermingle to curb known tumor markers, VEGF, c-Myc, cyclin D1, Bcl-2, and MMP-9. The possible role of E-cadherin and Nur77 is also highlighted. Moreover, the present findings demonstrate a beneficial use of Diac as an add-on therapy to increase chemosensitivity to 5-FU and consequently decrease 5-FU dose and side effects. Clinical use will require further studies.
7. Limitations of the study
Target molecules were assessed by single techniques and the CRC metastasis should be documented by examining other organs.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
Authors wish to thank pathologist/Mohammed K. Kherbetway at Faculty of Medicine in Suez Canal University for providing help in histopathological/immunohistochemical examination.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Peer review under responsibility of King Saud University.
References
- Agarwal A., Das K., Lerner N., Sathe S., Cicek M., Casey G., Sizemore N. The AKT/IκB kinase pathway promotes angiogenic/metastatic gene expression in colorectal cancer by activating nuclear factor-κB and β-catenin. Oncogene. 2005;24(6):1021–1031. doi: 10.1038/sj.onc.1208296. [DOI] [PubMed] [Google Scholar]
- Aggarwal B.B. Nuclear factor-κB: the enemy within. CancerCell. 2004;6(3):203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Ahmed H.H., El-Abhar H.S., Hassanin E.A.K., Abdelkader N.F., Shalaby M.B. Punica granatum suppresses colon cancer through downregulation of Wnt/β-Catenin in rat model. Rev. Bras. Farmacogn. 2017;27(5):627–635. doi: 10.1016/j.bjp.2017.05.010. [DOI] [Google Scholar]
- Ahmed H.H., El-Abhar H.S., Hassanin E.A., Abdelkader N.F., Shalaby M.B. Ginkgo biloba L. leaf extract offers multiple mechanisms in bridling N-methylnitrosourea–mediated experimental colorectal cancer. Biomed. Pharmacother. 2017;95:387–393. doi: 10.1016/j.biopha.2017.08.103. [DOI] [PubMed] [Google Scholar]
- Ahn S.Y., Yang J.H., Kim N.H., Lee K., Cha Y.H., Yun J.S., Kang H.E., Lee Y., Choi J., Kim H.S., Yook J.I. Anti-helminthic niclosamide inhibits Ras-driven oncogenic transformation via activation of GSK-3. Oncotarget. 2017;8(19):31856–31863. doi: 10.18632/oncotarget.16255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquino A., Prete S., Guadagni F., Greiner J., Giuliani A., Orlando L., Masci G., De Santis S., Bonmassar E., Graziani G. Effect of 5-fluorouracil on carcinoembryonic antigen expression and shedding at clonal level in colon cancer cells. Anticancer Res. 2000;20:3475–3484. [PubMed] [Google Scholar]
- Ashburn T.T., Thor K.B. Drug repositioning: identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004;3(8):673–683. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- Baker K.J., Houston A., Brint E. IL-1 family members in cancer; two sides to every story. Front. Immunol. 2019;10:1197. doi: 10.3389/fimmu.2019.01197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldin V., Lukas J., Marcote M.J., Pagano M., Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes & Dev. 1993;7:812–821. doi: 10.1101/gad.7.5.812. https://doi:10.1101/gad.7.5.812 [DOI] [PubMed] [Google Scholar]
- Bertrand F.E., Angus C.W., Partis W.J., Sigounas G. Developmental pathways in colon cancer: crosstalk between WNT, BMP, Hedgehog and Notch. Cell Cycle. 2012;11:4344–4351. doi: 10.4161/cc.22134. https://doi:10.4161/cc.22134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti R., Dey G., Banerjee I., Dey K.K., Parida S., Kumar B.P., Das C.K., Pal I., Mukherjee M., Misra M., Pradhan A.K., Emdad L., Das S.K., Fisher P.B., Mandal M. Somatostatin receptor targeted liposomes with Diacerein inhibit IL-6 for breast cancer therapy. Cancer Lett. 2017;388:292–302. doi: 10.1016/j.canlet.2016.12.021. [DOI] [PubMed] [Google Scholar]
- Bharti R., Dey G., Das A.K., Mandal M. Differential expression of IL-6/IL-6R and MAO-A regulates invasion/angiogenesis in breast cancer. Br. J. Cancer. 2018;118(11):1442–1452. doi: 10.1038/s41416-018-0078-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti R., Dey G., Ojha P.K., Rajput S., Jaganathan S.K., Sen R., Mandal M. Diacerein-mediated inhibition of IL-6/IL-6R signaling induces apoptotic effects on breast cancer. Oncogene. 2016;35(30):3965–3975. doi: 10.1038/onc.2015.466. [DOI] [PubMed] [Google Scholar]
- Blondy S., David V., Verdier M., Mathonnet M., Perraud A., Christou N. 5-Fluorouracil resistance mechanisms in colorectal cancer: from classical pathways to promising processes. Cancer Sci. 2020;111(9):3142–3154. doi: 10.1111/cas.14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekman F., Giovannetti E., Peters G.J. Tyrosine kinase inhibitors: multi-targeted or single-targeted? World. J. Clin. Oncol. 2011;2:80–93. doi: 10.5306/wjco.v2.i2.80. https://doi:10.5306/wjco.v2.i2.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavarretta I.T., Neuwirt H., Untergasser G., Moser P.L., Zaki M.H., Steiner H., Rumpold H., Fuchs D., Hobisch A., Nemeth J.A., Culig Z. The antiapoptotic effect of IL-6 autocrine loop in a cellular model of advanced prostate cancer is mediated by Mcl-1. Oncogene. 2007;26(20):2822–2832. doi: 10.1038/sj.onc.1210097. [DOI] [PubMed] [Google Scholar]
- Chai S., To K.K., Lin G. Circumvention of multi-drug resistance of cancer cells by Chinese herbal medicines. Chin. Med. 2010;5:1–9. doi: 10.1186/1749-8546-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q., Bournazou E., Sansone P., Berishaj M., Gao S.P., Daly L., Wels J., Theilen T., Granitto S., Zhang X., Cotari J., Alpaugh M.L., de Stanchina E., Manova K., Li M., Bonafe M., Ceccarelli C., Taffurelli M., Santini D., Altan-Bonnet G., Kaplan R., Norton L., Nishimoto N., Huszar D., Lyden D., Bromberg J. The IL-6/JAK/Stat3 feed-forward loop drives tumorigenesis and metastasis. Neoplasia. 2013;15(7):848–IN45. doi: 10.1593/neo.13706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay I., Ambati R., Gundamaraju R., Kleinjan A. Exploring the crosstalk between inflammation and epithelial-mesenchymal transition in cancer. Mediators Inflamm. 2021;2021:1–13. doi: 10.1155/2021/9918379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Cao X., Tu X., Alitongbieke G., Xia Z., Li X., Chen Z., Yin M., Xu D., Guo S., Li Z., Chen L., Zhang X., Xu D., Gao M., Liu J., Zeng Z., Zhou H.u., Su Y., Zhang X.-K. BI1071, a Novel Nur77 modulator, induces apoptosis of cancer cells by activating the Nur77-Bcl-2 apoptotic pathway. Mol Cancer Ther. 2019;18(5):886–899. doi: 10.1158/1535-7163.MCT-18-0918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S.D., Lei P., Abdelrahim M., Yoon K., Liu S., Guo J., Papineni S., Chintharlapalli S., Safe S. 1, 1-bis (3′-indolyl)-1-(p-methoxyphenyl) methane activates Nur77-independent proapoptotic responses in colon cancer cells. Mol. Carcinog. 2008;47(4):252–263. doi: 10.1002/mc.20378. [DOI] [PubMed] [Google Scholar]
- Cusack, J.C., Liu, R., Baldwin, A.S., 2000. Inducible chemoresistance to 7-ethyl-10-[4-(1-piperidino)-1-piperidino] carbonyloxycamptothecin (CPT-11) in colorectal cancer cells and a xenograft model is overcome by inhibition of nuclear factor-κB activation. Cancer Res. 60(9), 2323-2330. [PubMed]
- Dang C. MYC on the path to cancer. Cell. 2012;149(1):22–35. doi: 10.1016/j.cell.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Craene B., Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat. Rev. Cancer. 2013;13(2):97–110. doi: 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- De La O J.-P., Emerson L.L., Goodman J.L., Froebe S.C., Illum B.E., Curtis A.B., Murtaugh L.C. Notch and Kras reprogram pancreatic acinar cells to ductal intraepithelial neoplasia. Proc. Natl. Acad. Sci. 2008;105(48):18907–18912. doi: 10.1073/pnas.0810111105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La O, J.P., Murtaugh, L.C., 2009. Notch and Kras in pancreatic cancer: at the crossroads of mutation, differentiation and signaling. Cell Cycle 8, 1860-1864. https://doi.org/10.4161/cc.8.12.8744. [DOI] [PMC free article] [PubMed]
- Dey G., Bharti R., Dhanarajan G., Das S., Dey K.K., Kumar B.P., Sen R., Mandal M. Marine lipopeptide Iturin A inhibits Akt mediated GSK3β and FoxO3a signaling and triggers apoptosis in breast cancer. Sci. Rep. 2015;5:10316. doi: 10.1038/srep10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Directive E. 63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union. 2010;276:33–74. [Google Scholar]
- Dong P., Zhang C., Parker B.-T., You L., Mathey-Prevot B., Liu X. Cyclin D/CDK4/6 activity controls G1 length in mammalian cells. PLoS ONE. 2018;13(1):e0185637. doi: 10.1371/journal.pone.0185637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbadawy M., Usui T., Yamawaki H., Sasaki K. Emerging roles of C-Myc in cancer stem cell-related signaling and resistance to cancer chemotherapy: a potential therapeutic target against colorectal cancer. Int. J. Mol. Sci. 2019;20:2340. doi: 10.3390/ijms20092340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H., Liao X., Zhang J., Zheng H. Macrophages promote cell proliferation in colorectal cancer via IL-1β-mediated downregulation of miR-28-3p. J. Biol. Regul. Homeost. Agents. 2020;34(5) doi: 10.23812/20-210-A. [DOI] [PubMed] [Google Scholar]
- Fouad A.A., Abdel-Aziz A.M., Hamouda A.A. Diacerein downregulates NLRP3/Caspase-1/IL-1β and IL-6/STAT3 pathways of inflammation and apoptosis in a rat model of cadmium testicular toxicity. Biol. Trace Elem. Res. 2020;195(2):499–505. doi: 10.1007/s12011-019-01865-6. [DOI] [PubMed] [Google Scholar]
- Fu J., Xu Y., Yang Y., Liu Y., Ma L., Zhang Y. Aspirin suppresses chemoresistance and enhances antitumor activity of 5-Fu in 5-Fu-resistant colorectal cancer by abolishing 5-Fu-induced NF-κB activation. Sci. Rep. 2019;9:1–11. doi: 10.1038/s41598-019-53276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadotti V.M., Martins D.F., Pinto H.F., Oliveira G., Kaster M.P., Quintão N.L., Santos A.R.S. Diacerein decreases visceral pain through inhibition of glutamatergic neurotransmission and cytokine signaling in mice. Pharmacol. Biochem. Behav. 2012;102(4):549–554. doi: 10.1016/j.pbb.2012.06.018. [DOI] [PubMed] [Google Scholar]
- García-Gutiérrez L., Delgado M.D., León J. MYC Oncogene contributions to release of cell cycle brakes. Genes. 2019;10:244. doi: 10.3390/genes10030244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel H.L., Mercurio A.M. VEGF targets the tumour cell. Nat. Rev. Cancer. 2013;13(12):871–882. doi: 10.1038/nrc3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez A., Look A.T. NOTCH and PI3K-AKT pathways intertwined. Cancer Cell. 2007;12(5):411–413. doi: 10.1016/j.ccr.2007.10.027. [DOI] [PubMed] [Google Scholar]
- Harbuzariu A., Oprea-Ilies G.M., Gonzalez-Perez R.R. The role of notch signaling and leptin-notch crosstalk in pancreatic cancer. Medicines. 2018;5:68. doi: 10.3390/medicines5030068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y.C., Chen J.W., Cao J., Pan D.Y., Qiao J.G. Toxicities and therapeutic effect of 5-fluorouracil controlled release implant on tumor-bearing rats. World J. Gastroenterol. 2003;9:1795–1798. doi: 10.3748/wjg.v9.i8.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henamayee S., Banik K., Sailo B.L., Shabnam B., Harsha C., Srilakshmi S., Vgm N., Baek S.H., Ahn K.S., Kunnumakkara A.B. Therapeutic emergence of rhein as a potential anticancer drug: a review of its molecular targets and anticancer properties. Molecules. 2020;25(10):2278. doi: 10.3390/molecules25102278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hideshima T., Nakamura N., Chauhan D., Anderson K.C. Biologic sequelae of interleukin-6 induced PI3-K/Akt signaling in multiple myeloma. Oncogene. 2001;20(42):5991–6000. doi: 10.1038/sj.onc.1204833. [DOI] [PubMed] [Google Scholar]
- Hu Y., French S.W., Chau T., Liu H.-X., Sheng L., Wei F., Stondell J., Garcia J.C., Du Y., Bowlus C.L., Wan Y.-J. RARβ acts as both an upstream regulator and downstream effector of miR-22, which epigenetically regulates NUR77 to induce apoptosis of colon cancer cells. FASEB. 2019;33(2):2314–2326. doi: 10.1096/fj.201801390R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H. Matrix metalloproteinase-9 (MMP-9) as a cancer biomarker and MMP-9 biosensors: recent advances. Sensors. 2018;18:3249. doi: 10.3390/s18103249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, R., Wang, G., Song, Y., Tang, Q., You, Q., Liu, Z., Chen, Y., Zhang, Q., Li, J., Muhammand, S., 2015. Colorectal cancer stem cell and chemoresistant colorectal cancer cell phenotypes and increased sensitivity to Notch pathway inhibitor. Mol. Med. Rep. 12, 2417-2424. https://doi.org/10.3892/mmr.2015.3694. [DOI] [PMC free article] [PubMed]
- Jiang K., Delarue F.L., Sebti S.M. EGFR, ErbB2 and Ras but not Src suppress RhoB expression while ectopic expression of RhoB antagonizes oncogene-mediated transformation. Oncogene. 2004;23(5):1136–1145. doi: 10.1038/sj.onc.1207236. [DOI] [PubMed] [Google Scholar]
- Justus C.R., Leffler N., Ruiz-Echevarria M., Yang L.V. In vitro cell migration and invasion assays. JoVE. 2014;88 doi: 10.3791/51046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M. Nuclear factor-κB in cancer development and progression. Nature. 2006;441(7092):431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- Karin, M., Cao, Y., Greten, F.R., Li, Z.-W., 2002. NF-κB in cancer: from innocent bystander to major culprit. Nat. Rev. Cancer 2(4), 301-310. https://doi.org/10.1038/nrc780. [DOI] [PubMed]
- Kilkenny C., Browne W., Cuthill I.C., Emerson M., Altman D.G. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br. J.pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang C.-M., Fu X., Hua Y.-J., Shuai W.-d., Ye Z.-H., Li Y., Peng Q.-H., Li Y.-Z., Chen S., Qian C.-N., Huang W., Liu R.-y. BST2 confers cisplatin resistance via NF-κ B signaling in nasopharyngeal cancer. Cell Death Dis. 2017;8(6):e2874. doi: 10.1038/cddis.2017.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugimiya N., Nishimoto A., Hosoyama T., Ueno K., Enoki T., Li T.S., Hamano K. The c-MYC-ABCB5 axis plays a pivotal role in 5-fluorouracil resistance in human colon cancer cells. J. Cell. Mol. Med. 2015;19:1569–1581. doi: 10.1111/jcmm.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa, F., Pierce, J.W.,Sonenshein, G.E., 1994. “Differential regulation of the c-myc oncogene promoter by the NF-kappa B rel family of transcription factors. Mol. Cell. Biol.14(2), 1039-1044. https://doi.org/10.1128/mcb. [DOI] [PMC free article] [PubMed]
- Laios A., Mohamed B., Kelly L., Flavin R., Finn S., McEvoy L., Gallagher M., Martin C., Sheils O., Ring M., Davies A., Lawson M., Gleeson N., D'Arcy T., d'Adhemar C., Norris L., Langhe R., Saadeh F., O'Leary J., O'Toole S. Pre-treatment of platinum resistant ovarian cancer cells with an MMP-9/MMP-2 inhibitor prior to cisplatin enhances cytotoxicity as determined by high content screening. Int. J. Mol. Sci. 2013;14(1):2085–2103. doi: 10.3390/ijms14012085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamouille S., Xu J., Derynck R. Molecular mechanisms of epithelial–mesenchymal transition. Nat. Rev. Mol. Cell. Biol. 2014;15(3):178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B.H., Gilliland D.G. K-RasG12D–induced T-cell lymphoblastic lymphoma/leukemias harbor Notch1 mutations and are sensitive to-secretase inhibitors. Blood. 2008;112 doi: 10.1182/blood-2008-03-147587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.J., Beumer J.H., Chu E. Therapeutic drug monitoring of 5-fluorouracil. Cancer Chemother. Pharmacol. 2016;78(3):447–464. doi: 10.1007/s00280-016-3054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.O., Lou W., Hou M., De Miguel F., Gerber L., Gao A.C. Interleukin-6 promotes androgen-independent growth in LNCaP human prostate cancer cells. Clin. Cancer Res. 2003;9:370–376. [PubMed] [Google Scholar]
- Li D.-D., Zhao C.-H., Ding H.-W., Wu Q., Ren T.-S., Wang J., Chen C.-Q., Zhao Q.-C. A novel inhibitor of ADAM 17 sensitizes colorectal cancer cells to 5-Fluorouracil by reversing Notch and epithelial-mesenchymal transition in vitro and in vivo. Cell Prolif. 2018;51(5):e12480. doi: 10.1111/cpr.2018.51.issue-510.1111/cpr.12480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.-L., Harris A.L. Notch signaling from tumor cells: a new mechanism of angiogenesis. Cancer Cell. 2005;8(1):1–3. doi: 10.1016/j.ccr.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Li S., Tian J., Zhang H., Zhou S., Wang X., Zhang L., Yang J., Zhang Z., Ji Z. Down-regulating IL-6/GP130 targets improved the anti-tumor effects of 5-fluorouracil in colon cancer. Apoptosis. 2018;23(5-6):356–374. doi: 10.1007/s10495-018-1460-0. [DOI] [PubMed] [Google Scholar]
- Li T., Si W., Zhu J., Yin L., Zhong C. Emodin reverses 5-Fu resistance in human colorectal cancer via downregulation of PI3K/Akt signaling pathway. Am. J. Transl. Res. 2020;12:1851–1861. [PMC free article] [PubMed] [Google Scholar]
- Li Y., Li P.-K., Roberts M.J., Arend R.C., Samant R.S., Buchsbaum D.J. Multi-targeted therapy of cancer by niclosamide: a new application for an old drug. Cancer Lett. 2014;349(1):8–14. doi: 10.1016/j.canlet.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Liu Y., Zhao L., Pan Y., Shan Y., Li Y., Jia L.i. Upregulation of microRNA-135b and microRNA-182 promotes chemoresistance of colorectal cancer by targeting ST6GALNAC2 via PI3K/AKT pathway. Mol. Carcinog. 2017;56(12):2669–2680. doi: 10.1002/mc.22710. [DOI] [PubMed] [Google Scholar]
- Liu Y., Li P.-K., Li C., Lin J. Inhibition of STAT3 signaling blocks the anti-apoptotic activity of IL-6 in human liver cancer cells. J. Biol. Chem. 2010;285(35):27429–27439. doi: 10.1074/jbc.M110.142752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohberger, B., Kaltenegger, H., Weigl, L., Mann, A., Kullich, W., Stuendl, N., Leithner, A. andSteinecker-Frohnwieser, B., 2019. Mechanical exposure and diacerein treatment modulates integrin-FAK-MAPKs mechanotransduction in human osteoarthritis chondrocytes. Cell. Signal. 56, 23-30. https://doi.org/10.1016/j.cellsig.2018.12.010. [DOI] [PubMed]
- Maihöfner C., Charalambous M.P., Bhambra U., Lightfoot T., Geisslinger G., Gooderham N.J. Expression of cyclooxygenase-2 parallels expression of interleukin-1beta, interleukin-6 and NF-kappaB in human colorectal cancer. Carcinogenesis. 2003;24(4):665–671. doi: 10.1093/carcin/bgg006. [DOI] [PubMed] [Google Scholar]
- Maniati E., Bossard M., Cook N., Candido J.B., Emami-Shahri N., Nedospasov S.A., Balkwill F.R., Tuveson D.A., Hagemann T. Crosstalk between the canonical NF-κB and Notch signaling pathways inhibits Pparγ expression and promotes pancreatic cancer progression in mice. J Clin Invest. 2011;121(12):4685–4699. doi: 10.1172/JCI4579710.1172/JCI45797DS1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti, H.H., 2003. Vascular endothelial growth factor, Molecular and Cellular Biology of Neuroprotection in the CNS. Springer, pp. 375-394.
- McCubrey J.A., Steelman L.S., Abrams S.L., Lee J.T., Chang F., Bertrand F.E., Navolanic P.M., Terrian D.M., Franklin R.A., D’Assoro A.B., Salisbury J.L., Mazzarino M.C., Stivala F., Libra M. Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv. Enzyme Regul. 2006;46(1):249–279. doi: 10.1016/j.advenzreg.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Metwally I.H., Shetiwy M., Elalfy A.F., Abouzid A., Saleh S.S., Hamdy M. Epidemiology and survival of colon cancer among Egyptians: a retrospective study. J. Coloproctol. (Rio J.) 2018;38(01):024–029. doi: 10.1016/j.jcol.2017.09.418. [DOI] [Google Scholar]
- Miele L. Notch signaling. Clin Cancer Res. 2006;12(4):1074–1079. doi: 10.1158/1078-0432.CCR-05-2570. [DOI] [PubMed] [Google Scholar]
- Miele, L., Miao, H., Nickoloff, B., 2006. NOTCH signaling as a novel cancer therapeutic target. Curr. Cancer Drug Targets 6, 313-323. https://doi: 10.2174/156800906777441771. [DOI] [PubMed]
- Miele L., Osborne B. Arbiter of differentiation and death: notch signaling meets apoptosis. J. Cell. Physiol. 1999;181:393–409. doi: 10.1002/(SICI)1097-4652(199912)181:3<393::AID-JCP3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Mittal V. Epithelial mesenchymal transition in tumor metastasis. Annu. Rev. Pathol. 2018;13(1):395–412. doi: 10.1146/annurev-pathol-020117-043854. [DOI] [PubMed] [Google Scholar]
- Miyamoto S., Nakanishi M., Rosenberg D.W. Suppression of colon carcinogenesis by targeting Notch signaling. Carcinogenesis. 2013;34:2415–2423. doi: 10.1093/carcin/bgt191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll U.M., Marchenko N., Zhang X.-k. p 53 and Nur77/TR3–transcription factors that directly target mitochondria for cell death induction. Oncogene. 2006;25(34):4725–4743. doi: 10.1038/sj.onc.1209601. [DOI] [PubMed] [Google Scholar]
- Munaut C., Noël A., Hougrand O., Foidart J.-M., Boniver J., Deprez M. Vascular endothelial growth factor expression correlates with matrix metalloproteinases MT1-MMP, MMP-2 and MMP-9 in human glioblastomas. Int. J. Cancer. 2003;106(6):848–855. doi: 10.1002/ijc.11313. [DOI] [PubMed] [Google Scholar]
- Muthu R., Thangavel P., Selvaraj N., Ramalingam R., Vaiyapuri M. Synergistic and individual effects of umbelliferone with 5-flurouracil on the status of lipid peroxidation and antioxidant defense against 1, 2-dimethylhydrazine induced rat colon carcinogenesis. Biomed. Prev. Nutr. 2013;3(1):74–82. doi: 10.1016/j.bionut.2012.10.011. [DOI] [Google Scholar]
- Nagasaki T., Hara M., Nakanishi H., Takahashi H., Sato M., Takeyama H. Interleukin-6 released by colon cancer-associated fibroblasts is critical for tumour angiogenesis: anti-interleukin-6 receptor antibody suppressed angiogenesis and inhibited tumour–stroma interaction. Br. J. Cancer. 2014;110(2):469–478. doi: 10.1038/bjc.2013.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi C., Toi M. Nuclear factor-κB inhibitors as sensitizers to anticancer drugs. Nat. Rev. Cancer. 2005;5(4):297–309. doi: 10.1038/nrc1588. [DOI] [PubMed] [Google Scholar]
- Nakao S., Kuwano T., Tsutsumi-Miyahara C., Ueda S.-I., Kimura Y.N., Hamano S., Sonoda K.-H., Saijo Y., Nukiwa T., Strieter R.M., Ishibashi T., Kuwano M., Ono M. Infiltration of COX-2–expressing macrophages is a prerequisite for IL-1β–induced neovascularization and tumor growth. J. Clin. Invest. 2005;115(11):2979–2991. doi: 10.1172/JCI23298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naugler W.E., Karin M. NF-κB and cancer—identifying targets and mechanisms. Curr. Opin. Genet. Dev. 2008;18(1):19–26. doi: 10.1016/j.gde.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie J., Liu L., Zheng W., Chen L., Wu X., Xu Y., Du X., Han W. microRNA-365, down-regulated in colon cancer, inhibits cell cycle progression and promotes apoptosis of colon cancer cells by probably targeting Cyclin D1 and Bcl-2. Carcinogenesis. 2012;33:220–225. doi: 10.1093/carcin/bgr245. [DOI] [PubMed] [Google Scholar]
- Nusse R., Clevers H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169(6):985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- Pantziarka, P., Bouche, G., Meheus, L., Sukhatme, V., Sukhatme, V.P., Vikas, P., 2014. The repurposing drugs in oncology (ReDO) project. ecancermedicalscience 8. https://doi: 10.3332/ecancer.2014.442. [DOI] [PMC free article] [PubMed]
- Parikh A.A., Moon M.R., Pritts T.A., Fischer J.E., Szabo C., Hasselgren P.O., Salzman A.L. IL-1beta induction of NF-kappaB activation in human intestinal epithelial cells is independent of oxyradical signaling. Shock(Augusta, Ga.) 2000;13(1):8–13. doi: 10.1097/00024382-200013010-00002. [DOI] [PubMed] [Google Scholar]
- Pekarsky Y., Hallas C., Palamarchuk A., Koval A., Bullrich F., Hirata Y., Bichi R., Letofsky J., Croce C.M. Akt phosphorylates and regulates the orphan nuclear receptor Nur77. Proc. Natl. Acad. Sci. 2001;98(7):3690–3694. doi: 10.1073/pnas.051003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyssonnaux C., Provot S., Felder-Schmittbuhl M.P., Calothy G., Eychène A. Induction of postmitotic neuroretina cell proliferation by distinct ras downstream signaling pathways. Mol Cell Biol. 2000;20(19):7068–7079. doi: 10.1128/mcb.20.19.7068-7079.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping F.M., Liu G.J., Liu Z.J., Li H.B., Zhai J.W., Li S.X., Liu Y.M., Li B.W., Wei H. Expression of RKIP, E-cadherin and NF-kB p65 in esophageal squamous cell carcinoma and their correlations. Int. J. Clin. Exp. pathol. 2015;8:10164. [PMC free article] [PubMed] [Google Scholar]
- Prete S.P., Turriziani M., Massara M.C., De Rossi A., Correale P., De Vecchis L., Torino F., Bonmassar L., Aquino A. Combined effects of 5-fluorouracil, folinic acid and oxaliplatin on the expression of carcinoembryonic antigen in human colon cancer cells: pharmacological basis to develop an active antitumor immunochemotherapy. J. Exp. Clin. Cancer Res. 2008;27:5. doi: 10.1186/1756-9966-27-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y., Yu H., Shi X., Xu K.e., Tang Q., Liang B.o., Hu S., Bao Y., Xu J., Cai J., Peng W., Cao Q., Yin P. microRNA-497 inhibits invasion and metastasis of colorectal cancer cells by targeting vascular endothelial growth factor-A. Cell Prolif. 2016;49(1):69–78. doi: 10.1111/cpr.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan P., Weaver K.L., Capobianco A.J. Notch signalling in solid tumours: a little bit of everything but not all the time. Nat. Rev. Cancer. 2011;11(5):338–351. doi: 10.1038/nrc3035. [DOI] [PubMed] [Google Scholar]
- Rangaswami, H., Bulbule, A.,Kundu, G.C., 2004. Nuclear factor-inducing kinase plays a crucial role in osteopontin-induced MAPK/IκBα kinase-dependent nuclear factor κB-mediated promatrix metalloproteinase-9 activation. J. Biol. Chem. 279(37), 38921-38935. https://doi.org/10.1074/jbc.M404674200. [DOI] [PubMed]
- Rayet, B.,Gelinas, C., 1999. Aberrant rel/nfkb genes and activity in human cancer. Oncogene 18(49), 6938-6947. https://doi.org/10.1038/sj.onc.1203221. [DOI] [PubMed]
- Refaie M.M., El-Hussieny M. The role of interleukin-1b and its antagonist (diacerein) in estradiol benzoate-induced endometrial hyperplasia and atypia in female rats. Fundam. Clin. Pharmacol. 2017;31(4):438–446. doi: 10.1111/fcp.12285. [DOI] [PubMed] [Google Scholar]
- Sahdev A.K., Raj V., Singh A.K., Rai A., Keshari A.K., De A., Samanta A., Kumar U., Rawat A., Kumar D., Nath S., Prakash A., Saha S. Ameliorative effects of pyrazinoic acid against oxidative and metabolic stress manifested in rats with dimethylhydrazine induced colonic carcinoma. Cancer Biol. Ther. 2017;18(5):304–313. doi: 10.1080/15384047.2017.1310341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjari M., Kordestani Z., Safavi M., Mashrouteh M., FekriSoofiAbadi M., Tafreshi A.G. Enhanced expression of Cyclin D1 and c-myc, a prognostic factor and possible mechanism for recurrence of papillary thyroid carcinoma. Sci. Rep. 2020;10:1–9. doi: 10.1038/s41598-020-61985-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setrerrahmane S., Xu H. Tumor-related interleukins: old validated targets for new anti-cancer drug development. Mol. Cancer. 2017;16:1–17. doi: 10.1186/s12943-017-0721-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R., Agarwal A. In: Sperm Chromatin. Zini A., Agarwal A., editors. Springer New York; New York, NY: 2011. Laboratory evaluation of sperm chromatin: TUNEL assay; pp. 201–215. [DOI] [Google Scholar]
- Shimizu S., Nishikawa Y., Kuroda K., Takagi S., Kozaki K., Hyuga S., Saga S., Matsuyama M. Involvement of transforming growth factor β1 in autocrine enhancement of gelatinase B secretion by murine metastatic colon carcinoma cells. Cancer Res. 1996;56(14):3366–3370. [PubMed] [Google Scholar]
- Skehan P., Storeng R., Scudiero D., Monks A., McMahon J., Vistica D., Warren J.T., Bokesch H., Kenney S., Boyd M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. JNCI. 1990;82(13):1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- Silva-García O., Valdez-Alarcón J.J., Baizabal-Aguirre V.M. Wnt/β-catenin signaling as a molecular target by pathogenic bacteria. Front. Immunol. 2019;10:2135. doi: 10.3389/fimmu.2019.02135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer C.F. Principles and method of action of targeted therapies. Wien. Med. Wochenschr. 2010 doi: 10.1007/s10354-010-0817-y. [DOI] [PubMed] [Google Scholar]
- Street M.E., Miraki-Moud F., Sanderson I.R., Savage M.O., Giovannelli G., Bernasconi S., Camacho-Hubner C. Interleukin-1beta (IL-1beta) and IL-6 modulate insulin-like growth factor-binding protein (IGFBP) secretion in colon cancer epithelial (Caco-2) cells. J. Endocrinol. 2003;179(3):405–415. doi: 10.1677/joe.0.1790405. [DOI] [PubMed] [Google Scholar]
- Sullivan N.J., Sasser A.K., Axel A.E., Vesuna F., Raman V., Ramirez N., Oberyszyn T.M., Hall B.M. Interleukin-6 induces an epithelial–mesenchymal transition phenotype in human breast cancer cells. Oncogene. 2009;28(33):2940–2947. doi: 10.1038/onc.2009.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H., Ghaffari S., Taneja R. bHLH-Orange transcription factors in development and cancer. Transl. oncogenomics. 2007;2:107. doi: 10.4137/tog.s436. https://doi:10.4137/tog.s436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z., Cao X., Jiang M.-M., Qiu Y., Zhou H., Chen L., Qin B., Wu H., Jiang F., Chen J., Liu J., Dai Y., Chen H.-F., Hu Q.-Y., Wu Z., Zeng J.-Z., Yao X.-S., Zhang X.-K. Inhibition of β-catenin signaling by nongenomic action of orphan nuclear receptor Nur77. Oncogene. 2012;31(21):2653–2667. doi: 10.1038/onc.2011.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao L., Yang J.K., Gu Y., Zhou X., Zhao A.G., Zheng J., Zhu Y.J. Weichang’an and 5-fluorouracil suppresses colorectal cancer in a mouse model. World J. Gastroenterol. 2015;21:1125. doi: 10.3748/wjg.v21.i4.1125. https://doi:10.3748/wjg.v21.i4.1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umesalma S., Nagendraprabhu P., Sudhandiran G. Antiproliferative and apoptotic-inducing potential of ellagic acid against 1, 2-dimethyl hydrazine-induced colon tumorigenesis in Wistar rats. Mol. Cell. Biochem. 2014;388(1-2):157–172. doi: 10.1007/s11010-013-1907-0. [DOI] [PubMed] [Google Scholar]
- Wang A., Jiang H., Liu Y., Chen J., Zhou X., Zhao C., Chen X., Lin M. Rhein induces liver cancer cells apoptosis via activating ROS-dependent JNK/Jun/caspase-3 signaling pathway. J. Cancer. 2020;11:500. doi: 10.7150/jca.30381. https://doi:10.7150/jca.30381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Xue M., Chung D.C. c-Myc is regulated by HIF-2α in chronic hypoxia and influences sensitivity to 5-FU in colon cancer. Oncotarget. 2016;7(48):78910–78917. doi: 10.18632/oncotarget.12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.-C., Lin X.-L., Wang H.-Y., Qin Y.-J., Chen L., Li J., Jia J.-S., Shen H.-F., Yang S., Xie R.-Y., Wei F., Gao F., Rong X.-X., Yang J., Zhao W.-T., Zhang T.-T., Shi J.-W., Yao K.-T., Luo W.-R., Sun Y., Xiao D. Hes1 triggers epithelial-mesenchymal transition (EMT)-like cellular marker alterations and promotes invasion and metastasis of nasopharyngeal carcinoma by activating the PTEN/AKT pathway. Oncotarget. 2015;6(34):36713–36730. doi: 10.18632/oncotarget.5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Niu X.L., Qu Y.e., Wu J., Zhu Y.Q., Sun W.J., Li L.Z. Autocrine production of interleukin-6 confers cisplatin and paclitaxel resistance in ovarian cancer cells. Cancer Lett. 2010;295(1):110–123. doi: 10.1016/j.canlet.2010.02.019. [DOI] [PubMed] [Google Scholar]
- Wang Z., Banerjee S., Li Y., Rahman K.W., Zhang Y., Sarkar F.H. Down-regulation of Notch-1 Inhibits Invasion by Inactivation of Nuclear Factor-κB, Vascular Endothelial Growth Factor, and Matrix Metalloproteinase-9 in Pancreatic Cancer Cells. Cancer Res. 2006;66(5):2778–2784. doi: 10.1158/0008-5472.CAN-05-4281. [DOI] [PubMed] [Google Scholar]
- Wang Z., Li Y., Kong D., Ahmad A., Banerjee S., Sarkar F.H. Cross-talk between miRNA and Notch signaling pathways in tumor development and progression. Cancer Lett. 2010;292(2):141–148. doi: 10.1016/j.canlet.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Li Y., Banerjee S., Kong D., Ahmad A., Nogueira V., Hay N., Sarkar F.H. Down-regulation of Notch-1 and Jagged-1 inhibits prostate cancer cell growth, migration and invasion, and induces apoptosis via inactivation of Akt, mTOR, and NF-κB signaling pathways. J.cell. biochem. 2010;109:726–736. doi: 10.1002/jcb.22451. [DOI] [PubMed] [Google Scholar]
- Wang Z., Li Y., Banerjee S., Sarkar F.H. Exploitation of the Notch signaling pathway as a novel target for cancer therapy. Anticancer Res. 2008;28:3621–3630. [PubMed] [Google Scholar]
- Wei L.-H., Kuo M.-L., Chen C.-A., Chou C.-H., Lai K.-B., Lee C.-N., Hsieh C.-Y. Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene. 2003;22(10):1517–1527. doi: 10.1038/sj.onc.1206226. [DOI] [PubMed] [Google Scholar]
- Wu D.-M., Wang S., Wen X., Han X.-R., Wang Y.-J., Shen M., Fan S.-H., Zhuang J., Zhang Z.-F., Shan Q., Li M.-Q., Hu B., Sun C.-H., Lu J., Zheng Y.-L. Inhibition of microRNA-200a Upregulates the Expression of Striatal Dopamine Receptor D2 to Repress Apoptosis of Striatum via the cAMP/PKA Signaling Pathway in Rats with Parkinson’s Disease. Cell. Physiol. Biochem. 2018;51(4):1600–1615. doi: 10.1159/000495649. [DOI] [PubMed] [Google Scholar]
- Wu D.-W., Huang C.-C., Chang S.-W., Chen T.-H., Lee H. Bcl-2 stabilization by paxillin confers 5-fluorouracil resistance in colorectal cancer. Cell Death Differ. 2015;22(5):779–789. doi: 10.1038/cdd.2014.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W., Lo C.M., Poon R.Y., Cheung T.T., Chan A.C., Chen L., Yang S., Tsao G.S., Wang X.Q. Smad inhibitor induces CSC differentiation for effective chemosensitization in cyclin D1-and TGF-β/Smad-regulated liver cancer stem cell-like cells. Oncotarget. 2017;8(24):38811–38824. doi: 10.18632/oncotarget.16402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue G., Wang X., Yang Y., Liu D., Cheng Y., Zhou J., Cao Y., Peng DunFa. Colon cancer-specific antigen-2 may be used as a detecting and prognostic marker in colorectal cancer: a preliminary observation. PLoS ONE. 2014;9(4):e94252. doi: 10.1371/journal.pone.0094252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Liu X., Bhalla K., Kim C.N., Ibrado A.M., Cai J., Peng T.-I., Jones D.P., Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275(5303):1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- Yang L., Lin S., Kang Y., Xiang Y., Xu L., Li J., Dai X., Liang G., Huang X., Zhao C. Rhein sensitizes human pancreatic cancer cells to EGFR inhibitors by inhibiting STAT3 pathway. J. Exp. Clin. Cancer Res. 2019;38:1–13. doi: 10.1186/s13046-018-1015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Ma L., Xu Y., Liu Y., Li W., Cai J., Zhang Y. Enalapril overcomes chemoresistance and potentiates antitumor efficacy of 5-FU in colorectal cancer by suppressing proliferation, angiogenesis, and NF-κB/STAT3-regulated proteins. Cell Death Dis. 2020;11:1–13. doi: 10.1038/s41419-020-2675-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Q., Li S., Chepeha D.B., Giordano T.J., Li J., Zhang H., Polverini P.J., Nor J., Kitajewski J., Wang C.-Y. Crosstalk between tumor and endothelial cells promotes tumor angiogenesis by MAPK activation of Notch signaling. Cancer Cell. 2005;8(1):13–23. doi: 10.1016/j.ccr.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Zhang C., Zhang Z., Zhang S., Wang W., Hu P. Targeting of Wnt/β-catenin by anthelmintic drug pyrvinium enhances sensitivity of ovarian cancer cells to chemotherapy. Med. Sci. Monit. 2017;23:266. doi: 10.12659/MSM.901667. https://doi:10.12659/MSM.901667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X.k., 2007. Targeting nur77 translocation. Expert Opin. Ther. Targets 11, 69-79. https://doi.org/10.1517/14728222.11.1.69. [DOI] [PubMed]
- Zhao Q., Yue J., Zhang C., Gu X., Chen H., Xu L.u. Inactivation of M2 AChR/NF-κB signaling axis reverses epithelial-mesenchymal transition (EMT) and suppresses migration and invasion in non-small cell lung cancer (NSCLC) Oncotarget. 2015;6(30):29335–29346. doi: 10.18632/oncotarget.5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z., Li J., He X., Chen X., Yu B., Ji J., Zhang J., Wang T., Gu Q., Zhu Z. Involvement of RhoGDI2 in the resistance of colon cancer cells to 5-fluorouracil. Hepatogastroenterology. 2010;57:1106. [PubMed] [Google Scholar]
- Zhuang Y., Bai Y., Hu Y., Guo Y., Xu L., Hu W., Yang L., Zhao C., Li X., Zhao H. Rhein sensitizes human colorectal cancer cells to EGFR inhibitors by inhibiting STAT3 pathway. OncoTargets Ther. 2019;12:5281. doi: 10.2147/OTT.S206833. [DOI] [PMC free article] [PubMed] [Google Scholar]