Abstract
Advances in cancer therapy have resulted in more cancer therapy-related cardiac dysfunction (CTRCD), which is the main cause of death in older female survivors of breast cancer. Traditionally, guideline-recommended medications for heart failure, such as beta-blockers and angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (ACEIs/ARBs), are commonly used to prevent or attenuate CTRCD. However, sometimes their effectiveness is not satisfactory. Recently, the drug combination of sacubitril plus valsartan has been proven to be more beneficial for heart failure with reduced ejection fraction in the long term compared with an ACEI/ARB alone. However, there is a lack of evidence of the efficacy and safety of this drug combination in CTRCD. We report a case of worsening CTRCD, despite treatment with traditional medications, in which the patient improved after changing perindopril to sacubitril/valsartan. The patient’s heart function greatly improved after changing this ACEI to sacubitril/valsartan. Changing an ACEI/ARB to sacubitril/valsartan in patients with worsening chemotherapy-induced heart failure is appropriate. Further studies with a high level of evidence are required to assess the efficacy and safety of sacubitril/valsartan for CTRCD.
Keywords: Cancer therapy-related cardiac dysfunction, sacubitril/valsartan, perindopril, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, heart failure, breast cancer
Introduction
Breast cancer is the most commonly diagnosed cancer worldwide.1,2 Advances in early detection and cancer therapy have resulted in not only improved survival, but also more cancer therapy-related cardiac dysfunction (CTRCD) owing to antineoplastic drugs, such as anthracyclines and trastuzumab. CTRD is the main cause of death in older female survivors of breast cancer.3,4
Traditional medications, such as beta-blockers and angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (ACEIs/ARBs), are commonly used to prevent or attenuate chemotherapy-related left ventricular pathological remodeling.5–9 Bosch et al. found that, in 90 patients who received chemotherapy, patients in the treatment group with enalapril and carvedilol had a lower incidence of the combined end point of death or heart failure (HF) compared with those in the control group. 9 A systematic review 7 also reported that, with the help of prophylactic agents including statins, beta-blockers, and angiotensin antagonists, there were 83 cardiac events in the prophylaxis arm compared with 304 in the control arm (relative risk = 0.31, 95% confidence interval: 0.25–0.39). Recently, the drug combination of sacubitril plus valsartan has been shown to reduce all-cause mortality, as well as cardiovascular mortality and hospitalization for HF, compared with a proven dose of the ACE inhibitor enalapril. The benefits of sacubitril/valsartan are rapid and consistent in the left ventricular ejection fraction (LVEF).10–12 Unfortunately, there is only limited evidence of the performance of this drug combination in patients with cancer and HF with reduced ejection fraction.13,14 We report here a patient with breast cancer in whom CTRCD developed, despite combination therapy with an ACEI and beta-blockers. The patient eventually recovered after initiating sacubitril/valsartan.
Case report
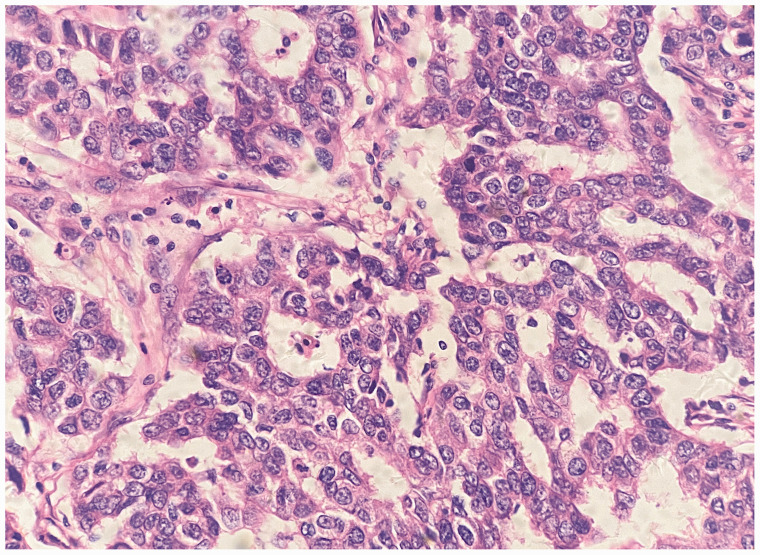
A 55-year-old female non-smoker without any medical history was diagnosed with estrogen receptor/progesterone receptor-negative, HER2-positive ductal carcinoma in the left breast (T2N0M0 stage IIA) (Figure 1). An initial cardiovascular evaluation showed an enlarged left ventricle (left ventricular internal diameter at end-diastole [LVIDD]: 59 mm), with a preserved LVEF (54%) and a normal N-terminal pro-B-type natriuretic peptide (NT-proBNP) concentration, troponin concentration, electrocardiogram, and coronary angiography. A radical mastectomy was performed followed by AC (epirubicin and cyclophosphamide) chemotherapy and cardioprotective therapy with perindopril 2 mg daily, metoprolol 25 mg twice daily, and spironolactone 20 mg daily. This treatment was decided after consultation of a cardio-oncology specialist because of the patient’s left ventricular dilation and the high risk of developing CTRCD. 15 The guideline-recommended medical therapies were gradually titrated with perindopril 8 mg daily, metoprolol 50 mg twice daily, and spironolactone 40 mg twice daily. She received four cycles of chemotherapy with epirubicin 130 mg and cyclophosphamide 900 mg in the following 2 months. A three-dimensional echocardiogram 1.5 months after initiating chemotherapy showed an LVIDD of 59 mm and an LVEF of 52%. During the following 6 months, four-cycle chemotherapy with paclitaxel and trastuzumab followed by four-cycle chemotherapy with only trastuzumab 360 mg were conducted. She was asymptomatic and the LVEF remained at 51% at a 6-month follow-up visit.
Figure 1.
Histopathology shows ductal carcinoma in the left breast (hematoxylin and eosin staining).
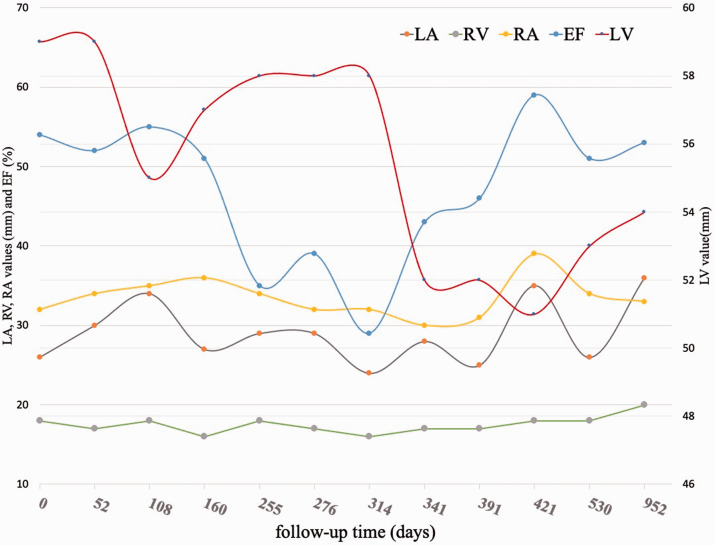
Unfortunately, the patient began complaining of worsening dyspnea and fatigue (New York Heart Association class II) 6.5 months after the initiation of chemotherapy just before the fifth dose of trastuzumab. The NT-proBNP concentration was elevated at 2012 ng/L, a transthoracic echocardiogram showed an LVIDD of 58 mm, and the LVEF was greatly reduced (35%). At this time, because of her worsening symptoms, elevated NT-pro BNP concentration, and considerable decrease in the LVEF, which increased the concern of worsening HF, chemotherapy with trastuzumab was discontinued. Furosemide and hydrochlorothiazide were administered for volume management during hospitalization. Perindopril was then switched to sacubitril/valsartan upon discharge, which was titrated to 100 mg twice daily 2 weeks after their initiation. Within 4 weeks of initiating sacubitril/valsartan, the patient reported that her dyspnea and fatigue had mostly resolved without considerable hypotension, renal dysfunction, or hyperkalemia, and there was no need for hospitalization. The LVIDD and LVEF had also gradually improved with good medication tolerance (Figure 2). A repeat echocardiogram 4 months after switching the medication showed an improvement in left ventricular function (LVEF: 46%) and LVIDD (52 mm). After full evaluation by a cardio-oncology team, she started chemotherapy again at approximately 4 months after initiating sacubitril/valsartan and successfully finished her last six cycles of chemotherapy with only trastuzumab in another 3.5 months. The normalization of left ventricular function (LVEF: 59%) was detected by an echocardiogram and the NT-proBNP concentration returned to normal 20 days after restarting chemotherapy (Figure 2). To date, the patient has been stabilized on sacubitril/valsartan 100 mg twice daily, metoprolol 50 mg twice daily, and spironolactone 40 mg daily.
Figure 2.
Echocardiography shows an improvement in cardiac function.
LA, left atrium; RV, right ventricle; EF, ejection fraction; LV, left ventricle.
Discussion
The incidence of left ventricular dysfunction associated with epirubicin, cyclophosphamide, and trastuzumab is 0.9% to 11.4%, 7% 28%, and 1.7% to 20.1%, respectively. 16 The median time between the last dose of anthracycline and the development of cardiotoxicity is approximately 3.5 months in 98% of cases within the first year of follow-up. 17 Therefore, current recommendations are that baseline cardiac function should be assessed before chemotherapy with potential cardiotoxicity. If systolic dysfunction or significant valvular heart disease is found, cardioprotection should be considered. 16 Similar to any other type of cardiomyopathy leading to HF with reduced ejection fraction, early addition of ACEIs/ARBs and beta-blockers in patients with chemotherapy-related cardiomyopathy leads to preservation or improvement of left ventricular function.5–8,18 However, only approximately 11% of patients have a full recovery from CTRCD. 17 Some patients still have worsening HF, despite appropriate pharmacological treatment with ACEIs/ARBs and beta-blockers, which suggests that current chemotherapy should be discontinued, as in our patient.
Based on current evidence, sacubitril/valsartan is recommended for patients with HF with reduced ejection fraction. 19 Unfortunately, evidence on the use of sacubitril/valsartan from previous randomized, clinical trials for the management of worsening HF has not been independently validated in a cancer population. Only a small group of patients with CTRCD were enrolled in two cohort studies.13,14 Martín-Garcia et al. 13 found that sacubitril/valsartan was well tolerated and improved echocardiographic function (LVEF: 33% before vs. 42% after sacubitril/valsartan) and structural parameters, NT-proBNP concentrations (1552 pg/mL before vs. 776 pg/mL after sacubitril/valsartan), and the symptomatic status (New York Heart Association class: 2.2 ± 0.6 before vs. 1.6 ± 0.6 after sacubitril/valsartan) in patients with CTRCD. Gregorietti et al. 14 observed decreased NT-proBNP concentrations, an improved LVEF (26.7 % before vs. 32.3 % after sacubitril/valsartan), and an improved LVIDD (67.5 vs. 60 mm) in 28 patients who developed worsening cardiotoxicity and were treated with sacubitril/valsartan. However, besides these two observational studies, there are currently no data on the use of sacubitril/valsartan, except for some case series.20,21 Sheppard et al. 21 reported that two patients with typical progression of HF symptoms due to anthracycline chemotherapy, despite management with evidence-based HF therapies, significantly improved after treatment with sacubitril/valsartan. Sacubitril/valsartan was also reported to be safe and feasible when administered as the first-line therapy instead of ACEIs. 22
Regarding the mechanisms of the benefits of sacubitril/valsartan in treating chemotherapy-induced HF, some pre-clinical/in vitro studies have reported their association with additional alleviation of dynamin-related protein 1-mediated mitochondrial dysfunction. 23 Boutagy et al. 24 reported that sacubitril/valsartan offered greater protection against left ventricular remodeling and dysfunction by decreasing activation of matrix metalloproteinases in a rodent model of progressive doxorubicin-induced cardiotoxicity compared with standard ARB therapy.
Our patient had an enlarged LVIDD during the initial cardiovascular evaluation and eventually developed HF while being treated with perindopril, metoprolol, and spironolactone at approximately 8 months after initiating the first dose of chemotherapy. Although sacubitril/valsartan is not currently recommended in patients with cancer, the decision to switch from perindopril to sacubitril/valsartan was reached for compassionate use. This switch in treatment soon improved her HF symptoms and normalized her LVEF, even after starting trastuzumab again. Therefore, our experience suggests that comprehensive cardiovascular evaluation before chemotherapy and regular monitoring during chemotherapy are important for physicians to suspect and detect CTRCD early. Switching ACEIs/ARBs to sacubitril/valsartan is a reasonable choice in patients with worsening chemotherapy-induced HF, which might not only normalize heart function, but also save time withholding chemotherapy while waiting for heart function to return to normal again.
Conclusion
The improvement of survival in patients with breast cancer and the appearance of cardiotoxicity of chemotherapies have raised new and important challenges for oncologists and cardiologists. Sacubitril/valsartan is safe and beneficial for improving left ventricular function in patients with worsening CTRCD, despite optimal pharmacological treatment with AECIs/ARBs, beta-blockers, and mineralocorticoid receptor antagonists. Further studies with a high level of evidence are required to assess the efficacy and safety of concomitant sacubitril/valsartan for preventing CTRCD. A randomized, placebo-controlled, multicenter trial has been designed to assess if sacubitril/valsartan administered concomitantly with early breast cancer treatment regimens prevents cardiac dysfunction. 25 More studies are likely to be conducted in the future to determine the potential benefits of sacubitril/valsartan for preventing and treating CTRCD.
The reporting of this study conforms to the CARE guidelines. 26
Acknowledgement
The authors would like to thank our patient for allowing her case to be published.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Ethics statement: The Ethics Committee of Yongchuan Hospital of Chongqing Medical University approved this retrospective study (No. 20190603). Written informed consent was obtained from the patient for her anonymized information to be published in this article.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Zijun Chen https://orcid.org/0000-0001-9442-4936
References
- 1.World Health Organization International Agency for Research on Cancer. The Global Cancer Observatory. 2018 statistics. Available from: http: //gco.iarc.fr/today/data/factsheets/populations/900-world-fact-sheets.pdf (accessed 17 June 2021).
- 2.Sung H, Ferlay J, Siegel R, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021; 71: 209–249. DOI: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Patnaik J, Byers T, DiGuiseppi C, et al. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res 2011; 13: R64. DOI: 10.1186/bcr2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicolazzi M, Carnicelli A, Fuorlo M, et al. Anthracycline and trastuzumab-induced cardiotoxicity in breast cancer. Eur Rev Med Pharmacol Sci 2018; 22: 2175–2185. DOI: 10.26355/eurrev_201804_14752. [DOI] [PubMed] [Google Scholar]
- 5.Cardinale D, Colombo A, Sandri M, et al. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation 2006; 114: 2474–2481. DOI: 10.1161/circulationaha.114.013777. [DOI] [PubMed] [Google Scholar]
- 6.Seicean S, Seicean A, Alan N, et al. Cardioprotective effect of β-adrenoceptor blockade in patients with breast cancer undergoing chemotherapy: follow-up study of heart failure . Circ Heart Fail 2013; 6: 420–426. DOI: 10.1161/circheartfailure.112.000055. [DOI] [PubMed] [Google Scholar]
- 7.Kalam K andMarwick T.. Role of cardioprotective therapy for prevention of cardiotoxicity with chemotherapy: a systematic review and meta-analysis. Eur J Cancer 2013; 49: 2900–2909. DOI: 10.1161/circheartfailure.112.000055. [DOI] [PubMed] [Google Scholar]
- 8.Kheiri B, Abdalla A, Osman M, et al. Meta-Analysis of Carvedilol for the Prevention of Anthracycline-Induced Cardiotoxicity. Am J Cardiol 2018; 122: 1959–1964. DOI: 10.1016/j.amjcard.2018.08.039. [DOI] [PubMed] [Google Scholar]
- 9.Bosch X, Rovira M, Sitges M, et al. Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: the OVERCOME trial (preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies). J Am Coll Cardiol 2013; 61: 2355–2362. DOI: 10.1016/j.jacc.2013.02.072. [DOI] [PubMed] [Google Scholar]
- 10.McMurray J, Packer M, Desai A, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993–1004. DOI: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 11.Ferrari L andSada S.. Efficacy of angiotensin-neprilysin inhibition versus enalapril in patient with heart failure with a reduced ejection fraction. Intern Emerg Med 2015; 10: 369–371. DOI: 10.1007/s11739-014-1173-5. [DOI] [PubMed] [Google Scholar]
- 12.Desai A, Claggett B, Packer M, et al. Influence of Sacubitril/Valsartan (LCZ696) on 30-Day Readmission After Heart Failure Hospitalization. J Am Coll Cardiol 2016; 68: 241–248. DOI: 10.1016/j.jacc.2016.04.047. [DOI] [PubMed] [Google Scholar]
- 13.Martín-Garcia A, López-Fernández T, Mitroi C, et al. Effectiveness of sacubitril-valsartan in cancer patients with heart failure. ESC Heart Fail 2020; 7: 763–767. DOI: 10.1002/ehf2.12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gregorietti V, Fernandez T, Costa D, et al. Use of Sacubitril/valsartan in patients with cardio toxicity and heart failure due to chemotherapy. Cardiooncology 2020; 6: 24. DOI: 10.1186/s40959-020-00078-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bloom M, Hamo C, Cardinale D, et al. Cancer Therapy-Related Cardiac Dysfunction and Heart Failure: Part 1: Definitions, Pathophysiology, Risk Factors, and Imaging. Circ Heart Fail 2016; 9: e002661. DOI: 10.1161/circheartfailure.115.002661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zamorano J, Lancellotti P, Rodriguez Muñoz D, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur J Heart Fail 2017; 19: 9–42. DOI: 10.1002/ejhf.654. [DOI] [PubMed] [Google Scholar]
- 17.Cardinale D, Colombo A, Bacchiani G, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015; 131: 1981–1988. DOI: 10.1161/circulationaha.114.013777. [DOI] [PubMed] [Google Scholar]
- 18.Finet J. Management of Heart Failure in Cancer Patients and Cancer Survivors. Heart Fail Clin 2017; 13: 253–288. DOI: 10.1016/j.hfc.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 19.McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021; 42: 3599–3726. DOI: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 20.De Vecchis R andPaccone A.. A case series about the favorable effects of sacubitril/valsartan on anthracycline cardiomyopathy. SAGE Open Med Case Rep 2020; 8: 2050313X20952189. DOI: 10.1177/2050313x20952189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheppard C andAnwar M.. The use of sacubitril/valsartan in anthracycline-induced cardiomyopathy: A mini case series. J Oncol Pharm Pract 2019; 25: 1231–1234. DOI: 10.1177/1078155218783238. [DOI] [PubMed] [Google Scholar]
- 22.Dankowski R, Sacharczuk W, Łojko-Dankowska A, et al. Sacubitril/valsartan as first-line therapy in anthracycline-induced cardiotoxicity. Kardiol Pol 2021; 9: 1040–1041. DOI: 10.33963/KP.a2021.0052. [DOI] [PubMed] [Google Scholar]
- 23.Xia Y, Chen Z, Chen A, et al. LCZ696 improves cardiac function via alleviating Drp1-mediated mitochondrial dysfunction in mice with doxorubicin-induced dilated cardiomyopathy. J Mol Cell Cardiol 2017; 108: 138–148. DOI: 10.1016/j.yjmcc.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Boutagy NE, Feher A, Pfau D, et al. Dual Angiotensin Receptor-Neprilysin Inhibition With Sacubitril/Valsartan Attenuates Systolic Dysfunction in Experimental Doxorubicin-Induced Cardiotoxicity. JACC CardioOncol 2020; 2: 774–787. DOI: 10.1016/j.jaccao.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mecinaj A, Gulati G, Heck SL, et al. Rationale and design of the PRevention of cArdiac Dysfunction during Adjuvant breast cancer therapy (PRADA II) trial: a randomized, placebo-controlled, multicenter trial. Cardiooncology 2021; 7: 33. DOI: 10.1186/s40959-021-00115-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. Headache 2013; 53: 1541–1547. DOI: 10.1111/head.12246 [DOI] [PubMed] [Google Scholar]