Abstract
Background
The objective of this study was to determine the seroprevalence of SARS-CoV-2 antibodies among Healthcare Workers (HCWs).
Methods
We carried out a cross-sectional study among 3644 HCWs at King Saud Medical City (KSMC) during the last two weeks of December 2020. A Google form survey was used to collect data on demographics, underlying health conditions, job duties, infection control competencies, COVID-19 exposure history, symptoms, and confirmed infections.
Findings
26.5% demonstrated seropositivity to SARS-CoV-2 antibodies, 10-fold higher than the national seroprevalence (2.36) conducted in May 2020. Seropositivity was significantly higher among non-Saudi HCWs and participants who lived outside the hospital dormitory p < 0.0001 and 0.01, respectively). Seropositivity was significantly higher among HCWs who worked on clinical areas of high exposure level, and those who spent longer duration working with patients with COVID-19; p = 0.002 and 0.005, respectively).
Conclusion
SARS-CoV-2 infections among HCWs can go unrecognized, which magnifies the importance of complying with universal masking and social distancing directives. Detecting SARS-CoV-2 antibodies in HCWs can help healthcare leaders in considering staff allocations and assignments accordingly.
Keywords: COVID-19, SARS-CoV-2 antibodies, Healthcare workers, Infection Control measures, Saudi Arabia
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) presents with a wide clinical spectrum. In most cases, patients are asymptomatic or have a mild infection, but a small proportion presents with severe acute respiratory syndrome [1]. As of 21 January, 2022, there have been 340 543 962 confirmed cases of coronavirus disease 2019 (COVID-19) and 5 570 163 related deaths worldwide, of which 548,571 confirmed cases and 8793 deaths were reported in Saudi Arabia (KSA) [2].
The main diagnostic test for detecting SARS-CoV-2 infection is the reverse transcription polymerase chain reaction (RT-PCR) [3], [4]. Serologic tests for detecting SARS-CoV-2 antibodies are important for understanding the extent and prevalence of COVID-19 infections and determining the proportion of the population showing an immune response to SARS-CoV-2 [5]. SARS-CoV-2 antibody tests are known to be accurate for detecting prior SARS-CoV-2 infection if performed> 14 days after symptom onset, but they have very low sensitivity in the first week since symptom onset [6].
Many anti-SARS-CoV-2 chemiluminescent microparticle immunoassay (CMIA) IgG have been introduced, however, validation data to verify assay sensitivity and specificity is not sufficient. Early studies demonstrated high sensitivity and specificity of both Abbott and Euroimmun (EI) IgG assays [7].
Healthcare workers (HCWs) constitute a high-risk group for SARS-CoV-2 infection. A recent meta-analysis of 11 studies found that 10.1% of all patients with COVID-19 were SARS-CoV-2-positive HCWs [8].
Few studies conducted in KSA investigating the SARS-CoV-2- antibodies among HCWs and these percentage of positivity varied among the studies. One study conducted in a tertiary care hospital in Riyadh four months earlier to our study reported that the percentage of SARS-CoV-2- antibodies positivity among HCWs is (3.2%) [9].
Knowing the seroprevalence of SARS-CoV-2 antibodies among HCWs is important for understanding the extent of the spread of COVID-19 among HCWs and assessing the success of infection mitigation interventions in the community and in healthcare settings.
The primary objective of our study was to determine the seroprevalence of SARS-CoV-2 antibodies among HCWs at King Saud Medical City (KSMC); the secondary objective was to determine the factors associated with this seroprevalence.
Participants and methods
Study type: We conducted a cross-sectional descriptive study.
Study setting and duration: This study took place at KSMC which is one of the main Ministry of Health (MoH) institutions in the central region of KSA. Being a quaternary care center, it has been among the governmental facilities dedicated to the care of COVID-19 patients – especially critical cases- in the capital city, Riyadh. Our data has been collected in the last two weeks of December 2020 as part of the Saudi Ministry of Health’s wide-reaching COVID-19 serology testing program among random populations across 20 health regions.
Survey tool: A Google form survey was designed by the investigators and modified based on validation of responses from a pilot sample and distributed to all HCWs at KSMC to collect data on demographics, underlying health conditions, job duties, infection control competencies, COVID-19 exposure history, symptoms, and confirmed infections. The form was designed to automatically identify and remove duplicate responses using national identification number as subject’s identifier.
Subjects’ enrollment criteria: Participation in the survey was voluntary, and any HCW either a KSMC staff or an employee of a contracted company who serves at KSMC was eligible for the study. HCWs who experienced any symptoms suggestive of COVID-19 at the time of enrollment were excluded from the study.
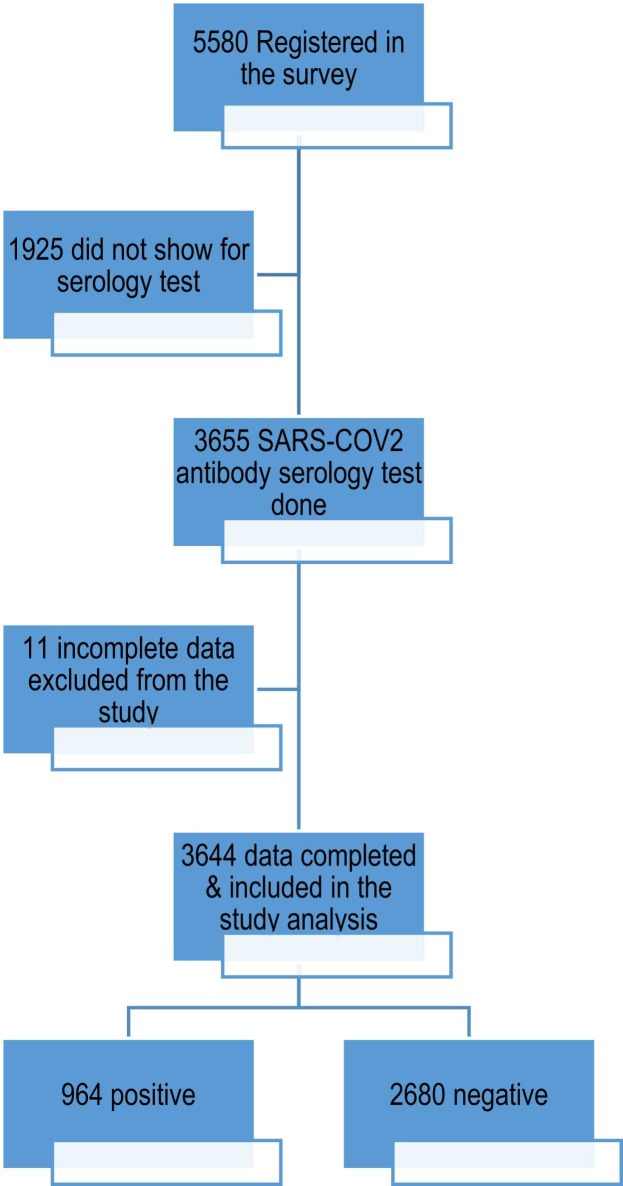
Participants’ responses: A total of 5580 individuals were registered for SARS-CoV-2 antibody serology tests, 3655 of them had their blood sample collected and test results reported. Among those, 3644 were ultimately included in this study, with 11 excluded due to incomplete data.
Laboratory Investigation: A 5-mL blood sample was collected from each participant after obtaining their verbal consent and was sent to the National Health lab within 48 hr at 4–8 °C. A chemiluminescent microparticle immunoassay (CMIA) was used for serum analysis to detect the presence of IgG raised against the nucleocapsid protein of SARS-CoV-2 (Abbott Architect SARSCoV-2 IgG kit, Abbott, USA) [10]. All participants were registered by the study investigators on the national tracing portal, and the test results were retrieved from the same system.
Statistical analysis: IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp was used for data analysis. Statistical significance was set at p < 0.05 for all tests. Summary statistics for the categorical variables were presented as numbers and percentages, whereas those for the continuous variables were presented as mean and standard deviation.
Missing data were kept as missing, and percent was calculated based on the available denominator. Further, chi-squared test and independent t-test were used to assess the association between seropositivity and the various categorical and continuous variables, respectively.
Ethical consideration: The study was conducted in compliance with the KSMC Institutional Research Board regulations, maintaining data confidentiality. Each study participant was anonymously coded by a unique serial number, and all identifiers (names and IDs) were removed from the analysis dataset and from any proposed publication.
Results
A total of 3644 subjects - who appropriately completed the survey and their blood test result for SARS-CoV-2 antibodies was available- had been included in this study ( Fig. 1). SARS-CoV-2 antibodies were detected in 26.5% of the participants (seropositive). The mean ages of the seropositive and seronegative groups were 37.25 ± 8.51 and 36.93 ± 8.81 years, respectively (p = 0.32). There was no statistically significant difference in age and sex between the two study groups. Most participants were Saudi—27.8% of the seropositive participants and 34.6% of the seronegative participants were Saudi. The percentage of seropositive participants was significantly higher than that of seronegative participants among the participants who lived outside the hospital dormitory (67.4% vs. 62.7%, respectively; p = 0.01) ( Table 1).
Fig. 1.
Subjects enrollment:
Table 1.
Participant demographics.
| Characteristics | SARS-CoV-2 serology results |
P | |
|---|---|---|---|
| Positive n = 964 (26.5%) |
Negative n = 2680 (73.5%) |
||
| Median age (IQR), years | 35.0 (11.0) | 34.0 (10.0) | 0.32 |
| Mean age ± SD, years | 37.25 ± 8.51 | 36.93 ± 8.81 | |
| Sex | 0.44 | ||
| Male | 195 (20.2) | 574 (21.4) | |
| Female | 769 (79.8) | 2106 (78.6) | |
| Nationality* | < 0.0001 | ||
| Saudi | 268 (27.8) | 926 (34.6) | |
| Non-Saudi Arab | 64 (6.6) | 185 (6.9) | |
| Filipino | 254 (26.4) | 782 (29.2) | |
| Indian | 349 (36.2) | 691 (25.8) | |
| Other Asian | 24 (2.5) | 70 (2.6) | |
| Western (+other African, as there were only 3 participants) | 4 (0.4) | 20 (0.7) | |
| Living condition* | 0.01 | ||
| In the hospital dormitory | 311 (32.6) | 991 (37.3) | |
| Outside the hospital dormitory/with family members/alone/shared flat with colleagues | 642 (67.4) | 1665 (62.7) | |
| Body mass index (kg/m2)* | 0.26 | ||
| < 18.5 | 15 (1.7) | 71 (3.0) | |
| 18.5–24.9 | 349 (40.5) | 985 (41.4) | |
| 25–29.9 | 332 (38.6) | 854 (35.9) | |
| 30–39.9 | 152 (17.7) | 427 (17.9) | |
| > 40 | 13 (1.5) | 42 (1.8) | |
| Blood group* | 0.01 | ||
| A | 228 (26.3) | 557 (23.5) | |
| B | 219 (25.3) | 571 (24.1) | |
| AB | 71 (8.2) | 148 (6.2) | |
| O | 349 (40.3) | 1093 (46.1) | |
| Underlying health condition | |||
| Any comorbidity | 250 (26.2) | 680 (25.6) | 0.69 |
| Asthma | 91 (9.4) | 210 (7.8) | 0.12 |
| Hypertension | 58 (6.0) | 132 (4.9) | 0.19 |
| Diabetes mellitus | 54 (5.6) | 167 (6.2) | 0.48 |
| Obesity (self-reported) | 47 (4.9) | 134 (5.0) | 0.88 |
| Pregnancy | 19 (2.0) | 56 (2.1) | 0.82 |
| Thyroid function disorder | 15 (1.6) | 25 (0.9) | 0.11 |
| Chronic neurological impairment | 6 (0.6) | 14 (0.5) | 0.72 |
| Cancer | 6 (0.6) | 15 (0.6) | 0.83 |
| Chronic hematological disorder | 4 (0.4) | 18 (0.7) | 0.38 |
| Chronic kidney disease | 1 (0.1) | 9 (0.3) | 0.24 |
| Chronic lung disease | 1 (0.1) | 11 (0.4) | 0.15 |
| Chronic liver disease | 0 (0.0) | 3 (0.1) | 0.30 |
| Immunodeficiency/organ recipient | 0 (0.0) | 3 (0.1) | 0.30 |
IQR, interquartile range; SD, standard deviation
* : incomplete data, percent was calculated out of available data
In terms of blood groups, there was a statistically significant difference in the percentage of seronegative and seropositive participants with O blood group (46.1% vs. 40.3%, respectively; p = 0.01). There was no significant association between body mass index and seropositivity. Among the seropositive participants, 26.2% had at least one underlying health condition, with asthma being the most frequently reported comorbidity (9.4%), with no significant difference detected between the two study groups regarding any of the reported underlying health condition (Table 1).
Table 2 presents the qualifications and occupational information of the participants. There were no statistically significant differences between the seropositive and seronegative groups in terms of highest educational degree (p = 0.10), although there was a significant difference between the groups in terms of total years of experience. There was a difference between the nurses and the other clinical staff in term of positivity; nurses were more likely to be seropositive than seronegative (71.9% vs. 66.6%). Although not statistically significant, the seropositive participants were slightly more likely to have provided direct patient care (78% vs. 75.2%) than the seronegative participants. The seropositive participants were also more likely to have a high occupational exposure level compared with seronegative participants (40.8% vs. 34.9%, respectively; p = 0.002). An insignificantly larger number of staff members in the seropositive group were reassigned from their original department during the pandemic to work in critical care units and isolation wards (37.5% vs. 34.1%). The mean duration of work with patients with COVID-19 differed significantly between the seropositive and seronegative groups (3.98 ± 3.44 and 3.61 ± 3.46 months, respectively; p = 0.005). Conversely, the difference in regular daily duty hours and work at other institutions between the seropositive and seronegative groups was not statistically significant.
Table 2.
Qualification and Occupational Information.
| Characteristics | SARS-CoV-2 serology results |
P | |
|---|---|---|---|
| Positive n = 964 (26.5%) |
Negative n = 2680 (73.5%) |
||
| Highest educational degree* | 0.10 | ||
| None | 19 (2.0) | 40 (1.5) | |
| Bachelor’s degree | 553 (58.0) | 1604 (60.3) | |
| Diploma degree | 236 (24.8) | 575 (21.6) | |
| Residency/Master’s degree | 99 (10.4) | 270 (10.2) | |
| Fellowship/Doctorate degree | 46 (4.8) | 171 (6.4) | |
| Total years of experience * | 0.02 | ||
| < 2 years | 60 (6.3) | 235 (8.8) | |
| 3–5 years | 97 (10.2) | 328 (12.3) | |
| 5–10 years | 337 (35.0) | 880 (33.1) | |
| > 10 years | 462 (48.5) | 1215 (45.7) | |
| Clinical role* | 0.10 | ||
| Nurse | 692 (71.9) | 1783 (66.6) | |
| Physician | 120 (12.5) | 363 (13.6) | |
| Administrative staff | 48 (5.0) | 162 (6.0) | |
| Respiratory therapist | 25 (2.6) | 78 (2.9) | |
| Radiology/X-ray technician | 13 (1.4) | 41 (1.5) | |
| Other technician/specialist | 30 (3.1) | 124 (4.6) | |
| Cleaner | 11 (1.1) | 27 (1.0) | |
| Pharmacist | 6 (0.6) | 37 (1.4) | |
| Anesthesia technician | 3 (0.3) | 7 (0.3) | |
| Laboratory personnel | 4 (0.4) | 13 (0.5) | |
| Physical therapist | 2 (0.2) | 12 (0.4) | |
| Nutritionist/dietician | 1 (0.1) | 12 (0.4) | |
| Engineer | 2 (0.2) | 13 (0.5) | |
| Security staff | 5 (0.5) | 6 (0.20) | |
| Direct patient care, yes | 743 (78.0) | 1998 (75.2) | 0.08 |
| Occupational exposure level * | 0.002 | ||
| High | 389 (40.8) | 928 (34.9) | |
| Medium | 457 (48.0) | 1357 (51.1) | |
| Low | 107 (11.2) | 373 (14.0) | |
| No. of months working with patients with COVID-19 during 2020, median (IQR) | 4.0 (7.0) | 4.0 (7.0) | 0.005 |
| No. of months working with patients with COVID-19 during 2020, mean (SD) | 3.98 ± 3.44 | 3.61 ± 3.46 | |
| Pulled from non-critical care wards, (yes) | 357 (37.5) | 905 (34.1) | 0.06 |
| Regular daily duty hours* | 0.09 | ||
| < 8 | 290 (30.4) | 912 (34.3) | |
| 9–12 |
|
1320 (49.7) | |
| > 12 | 159 (16.7) | 426 (16.0) | |
| Worked at another institution, yes | 195 (20.5) | 476 (17.9) | 0.08 |
IQR, interquartile range; SD, standard deviation
* : incomplete data, percent was calculated out of available data
Table 3 presents the data on infection control competency. The percentage of staff who attended infection control training was significantly higher among seropositive group compared to seronegative group (83.1% vs. 79.8%, respectively; p = 0.03) as the training activity was more focus on clinical units with high levels of occupational exposure.
Table 3.
Infection Control Competency.
| Characteristics | SARS-CoV-2 serology results |
P | |
|---|---|---|---|
| Positive n = 964 (26.5%) |
Negative n = 2680 (73.5%) |
||
| Training received, yes | 792 (83.1) | 2120 (79.8) | 0.03 |
| Modalitya | 0.17 | ||
| None | 173 (18.2) | 525 (19.8) | |
| Virtual training | 504 (52.9) | 1399 (52.7) | |
| Face-to-face training | 102 (10.7) | 225 (8.5) | |
| Both | 173 (18.2) | 504 (19.0) | |
| Proper hand hygiene knowledge, yes | 730 (76.6) | 1948 (73.3) | 0.045 |
| Self-estimated compliance with precautions | |||
| Hand Hygienea | 0.15 | ||
| Always/most of the time | 946 (99.3) | 2616 (98.4) | |
| Occasionally/rarely | 5 (0.5) | 30 (1.1) | |
| Never | 2 (0.2) | 12 (0.5) | |
| Maska | 0.51 | ||
| Always/most of the time | 934 (98.0) | 2596 (97.7) | |
| Occasionally/rarely | 16 (1.7) | 45 (1.7) | |
| Never | 3 (0.3) | 17 (0.6) | |
| Social distancinga | 0.17 | ||
| Always/most of the time | 823 (95.5) | 2064 (93.8) | |
| Occasionally/rarely | 26 (3.0) | 97 (4.4) | |
| Never | 13 (1.5) | 40 (1.8) | |
| N95 mask usea | 0.39 | ||
| Always/most of the time | 612 (82.4) | 1599 (80.0) | |
| Occasionally/rarely | 99 (13.3) | 302 (15.1) | |
| Never | 32 (4.3) | 97 (4.9) | |
| Face shield usea | 0.44 | ||
| Always/most of the time | 641 (86.3) | 1693 (84.7) | |
| Occasionally/rarely | 86 (11.6) | 246 (12.3) | |
| Never | 16 (2.2) | 59 (3.0) | |
| Powered air purifying respirator usea | 0.29 | ||
| Always/most of the time | 469 (63.1) | 1200 (60.1) | |
| Occasionally/rarely | 126 (17.0) | 348 (17.4) | |
| Never | 148 (19.9) | 448 (22.4) | |
| Coverall usea | 0.14 | ||
| Always/most of the time | 602 (81.0) | 1549 (77.6) | |
| Occasionally/rarely | 87 (11.7) | 268 (13.4) | |
| Never | 54 (7.3) | 179 (9.0) | |
: incomplete data, percent was calculated out of available data
Regarding the infection control practices training, seropositivity of the participant was not significantly influenced by the training modality. Almost similar percentage among the two study groups have either attended virtual, or face-to-face training, or both modalities.
A higher percentage of HCWs in the seropositive group had proper hand hygiene knowledge compared with those in the seronegative group (76.6% and 73.3%, respectively; p = 0.045). There were no statistically significant differences between the seropositive and seronegative groups in terms of the self-estimation of compliance with hand hygiene, universal masking, social distancing, and use of N95 respirators, face shields, powered air purifying respirators, and coveralls (if indicated).
Table 4 presents the history of COVID-19 exposure, suggestive symptoms, and confirmed COVID-19 infections. Seropositivity rates were significantly lower among the participants who had no history of COVID-19 exposure compared with those who had exposure history (6.1% vs. 13.8%, respectively; p < 0.0001). Percentage of HCWs who previously reported positive for COVID-19 was significantly higher among the seropositive group compared to seronegative group (74.1% vs. 9.8%, respectively; p < 0.0001). In the seropositive group, 78.4% had at least one symptom, compared with 47.6% in the seronegative group (p < 0.0001). The three most frequent symptoms were fever, headache, and cough, with statistically significant differences between the seropositive and seronegative groups (50.8% vs. 12.0%, 48.4% vs. 24.6%, and 43.8% vs. 17.2%, respectively; p < 0.0001).
Table 4.
History of exposure, suggestive symptoms, and confirmed COVID-19 infection.
| Characteristics | SARS-CoV-2 serology results |
P | |
|---|---|---|---|
| Positive n = 964 (26.5%) |
Negative n = 2680 (73.5%) |
||
| Exposure* | |||
| Household | 55 (5.8) | 81 (3.0) | < 0.0001 |
| Public/social | 92 (9.7) | 340 (12.8) | |
| Occupational | 211 (22.1) | 464 (17.5) | |
| More than one source | 537 (56.3) | 1406 (52.9) | |
| None | 58 (6.1) | 366 (13.8) | |
| Previously reported with confirmed COVID-19, yes | 576 (74.1) | 150 (9.8) | < 0.0001 |
| Symptoms | |||
| Any symptoms | 756 (78.4) | 1275 (47.6) | < 0.0001 |
| Fever | 490 (50.8) | 322 (12.0) | < 0.0001 |
| Headache | 467 (48.4) | 659 (24.6) | < 0.0001 |
| Cough | 422 (43.8) | 461 (17.2) | < 0.0001 |
| Body ache/myalgia | 421 (43.7) | 421 (15.7) | < 0.0001 |
| Loss of smell | 352 (36.5) | 66 (2.5) | < 0.0001 |
| Loss of taste | 339 (35.2) | 79 (2.9) | < 0.0001 |
| Sore throat | 319 (33.1) | 448 (16.7) | < 0.0001 |
| Diarrhea | 249 (25.8) | 207 (7.7) | < 0.0001 |
| Fatigue | 236 (24.5) | 264 (9.9) | < 0.0001 |
| Shortness of breath | 232 (24.1) | 151 (5.6) | < 0.0001 |
| Back pain | 219 (22.7) | 344 (12.8) | < 0.0001 |
| Muscle pain | 204 (21.2) | 213 (7.9) | < 0.0001 |
| Runny nose | 181 (18.8) | 302 (11.3) | < 0.0001 |
| Loss of appetite | 172 (17.8) | 65 (2.4) | < 0.0001 |
| Sneezing | 167 (17.3) | 394 (14.7) | 0.053 |
| Bone/joint pain | 136 (14.1) | 135 (5.0) | < 0.0001 |
| Cold | 110 (11.4) | 139 (5.2) | < 0.0001 |
| Leg pain | 108 (11.2) | 156 (5.8) | < 0.0001 |
| Nasal congestion | 108 (11.2) | 139 (5.2) | < 0.0001 |
| Chills | 101 (10.5) | 49 (1.8) | < 0.0001 |
| Nausea | 91 (9.4) | 102 (3.8) | < 0.0001 |
| Chest pain | 87 (9.0) | 68 (2.5) | < 0.0001 |
| Dizziness | 86 (8.9) | 76 (2.8) | < 0.0001 |
| Vomiting | 80 (8.3) | 48 (1.8) | < 0.0001 |
| Abdominal pain | 68 (7.1) | 93 (3.5) | < 0.0001 |
| Eye pain | 65 (6.7) | 68 (2.5) | < 0.0001 |
| Sweating | 63 (6.5) | 48 (1.8) | < 0.0001 |
| Laziness | 63 (6.5) | 60 (2.2) | < 0.0001 |
| Difficulty swallowing | 43 (4.5) | 27 (1.0) | < 0.0001 |
| Tachycardia | 37 (3.8) | 31 (1.2) | < 0.0001 |
| Bitter smell | 27 (2.8) | 12 (0.4) | < 0.0001 |
| Rashes | 10 (1.0) | 19 (0.7) | 0.33 |
| If any symptoms reported, time from symptom onset to serology specimen collection, median (IQR), days | 159.0 (67.0) | 144.0 (143.7) | 0.56 |
| If any symptoms reported, time from symptom onset to serology specimen collection, mean (SD), days | 243.31 ± 1585.35 | 293.19 ± 1539.04 | |
IQR, interquartile range; SD, standard deviation
* : incomplete data, percent was calculated out of available data.
Discussion
This cross-sectional study investigated the seroprevalence of SARS-CoV-2 antibodies among a convenience sample of HCWs at KSMC, Riyadh, KSA. The study was conducted as part of Saudi Arabia’s wide-reaching COVID-19 serology testing in random populations. The study described and compared the characteristics of HCWs with and without SARS-CoV-2 antibodies.
Among the participants, 26.5% demonstrated seropositivity to SARS-CoV-2 antibodies, a percentage almost 10-fold higher than that reported by Alserehi et al. (2.36%) among HCWs from 85 hospitals in different regions of Saudi Arabia. This wide discrepancy in the reported prevalence could be related to the differences in the study periods and settings [11]. Alserehi et al. conducted their study in the last two weeks of May 2020, just before the peak of COVID-19 cases in Saudi Arabia, whereas our study was conducted in the last two weeks of December 2020, after a large number of hospital admissions of patients with COVID-19 at KSMC (one of the main referral hospitals) and after COVID-19 outbreaks among HCWs in our hospital. The inclusion of control hospitals where no COVID-19 patients had been admitted, the larger number of participants, and other factors could have reduced the percentage of cases in the Alserehi study compared with our study.
Seropositivity was significantly higher among non-Saudi HCWs. This finding matches with our report on the prevalence of COVID-19 among HCWs at our institution where most of the positive cases were non-Saudi [12], [13]. Similarly, in their study of blood donors in Saudi Arabia, Banjar et al. reported that non-citizens were 13.6 times more likely to be positive for SARS-CoV-2 antibodies than citizens. The authors indicated that the initial cases were reported more frequently among non-citizens [14].
On the other hand, we found that the percentage of the expat HCWs who live in residences belonging to the healthcare facility was significantly lower among the seropositive participants, a finding that can be related to the fact that most of the study participants reported a history of occupational exposure rather than household exposure. Expat HCWs’ dormitory is a unique condition and as far as we know, studying the impact of this living condition on COVID-19 infection or antibodies detection seropositivity was not addressed in other studies.
Considering the occupational factors, the percentage of seropositive participants significantly increased with the increasing level of occupational exposure, HCWs working in high and medium occupational exposure level represented 40.8% and 48% respectively of the seropositive group vs 11.2% working in low occupational exposure units. Similarly, another Saudi study showed that HCWs in “case” hospitals where patients with COVID-19 were admitted had higher positivity rates compared with those working in “control” hospitals that did not admit patients with COVID-19 [11]. This relation was also supported by other studies, Korth et al. reported that the level of occupational exposure among HCWs was correlated with higher seroprevalence, particularly among those working in an intermediate-risk unit [15], and another found that 17.14% of HCWs who were in contact with patients with COVID-19 were seropositive despite negative SARS-CoV-2 tests [16]. However, another study showed that seropositivity was similar among HCWs with high and low COVID-19 occupational exposure levels [17].
In this study, we showed that a history of exposure to COVID-19 infected persons - which could be occupational exposure, household exposure, or exposure in public areas - was significantly high among the seropositive group. Exposures to an individual with known coronavirus disease 2019 was also reported by Jacob et al. as a significant risk factor associated with SARS-CoV-2 seropositivity in their study among of 24 749 HCP in 3 US states. They also reported that majority of those exposures was outside the workplace [18].
It was interesting to note that the percentage of HCWs who underwent training on infection control precautions and who had proper hand hygiene knowledge was significantly higher in the seropositive group than in the seronegative group. Prioritizing training efforts toward clinical units with high levels of occupational exposure could explain why the HCWs working in those units have higher competency but are still affected by high exposure. We found no significant difference in the self-reported compliance with hand hygiene and the use of personal protection equipment between the two study groups, which could be related to the reliability of self-estimation of compliance with infection prevention measures.
Among the participants with positive test results for SARS-CoV-2 antibodies, approximately 75% previously reported COVID-19 positivity by PCR, and nearly 80% had history of symptoms suggestive for COVID-19. Similarly, other studies strongly linked the SARS-CoV2 seropositivity among HCWs with being previously diagnosed with COVID-19 by PCR (75.6%) and with having history of symptoms suggestive for corona infection [19], [20].
Presence of 25% with no previous positive COVID-19 test results and around 20% who could not recall any symptoms suggestive of COVID-19 in the preceding months among the seropositive group could be possibly because SARS-CoV-2 infection can sometimes be asymptomatic due to the under-reporting of symptoms, or due to symptomatic HCWs who were either not tested or received false-negative PCR results.
Among the participants with negative test results for SARS-CoV-2 antibodies, approximately 10% had confirmed COVID-19, nearly 50% had reported a history of COVID-related symptoms, and 87% recalled exposure to confirmed COVID-19 cases. Numerous factors can contribute to the non-detection of SARS-CoV-2 antibodies in these situations, such as the durability of natural immunity for SARS-CoV-2 infections and the personal interpretation of exposure in terms of distance and duration, which can be overestimated due to the fear and panic of getting infected.
The reporting of a history of symptoms suggestive of COVID-19 was significantly higher in the seropositive group than in the seronegative group, and the most frequently reported symptoms were fever, cough, shortness of breath, and body ache.
Similar to other studies using online data collection tool, our study could not limit the access to the questionnaire by subjects beyond the study population. In addition it lacks the face to face advantage which may affect the reliability of the collected data.
In summary, SARS-CoV-2 infections among HCWs can go unrecognized, which magnifies the importance of complying with universal masking and social distancing directives. A solid surveillance system is also crucial for the early detection and testing of HCWs with acute respiratory illnesses. The risk of occupational exposure should be controlled through the proper use and sustainability of personal protection equipment, and alternative duty schedules should be considered to minimize the exposure period. Training resources and supplies should be prioritized in clinical units with high exposure levels.
Serology tests for SARS-CoV-2 antibodies are of limited value in terms of detecting prior infection, given that they are affected by the timing and sensitivity of the test. Detecting SARS-CoV-2 antibodies in HCWs will identify their immunity status, which can help healthcare leaders in considering staff allocations and assignments accordingly. However, further studies are warranted to determine the persistence of these antibodies and their role in protection and determine whether they are developed as a result of natural immunity following infection or in response to vaccine administration. These studies will help in understanding the effectiveness of various types of vaccines and enable policy makers to decide on the release phases of community and border restrictions.
Acknowledgment
The assistance provided by nursing affairs (Ms. Batla A. Alshammari, Ms. Shorooq ALfarsani, Ms. Lizette Lessing), community health administration (Dr. Youssef Alomran, Dr. Ghassan Watfa) and clinical laboratory (Dr. Fatima Afrazon) was greatly appreciated.
Author’s contribution
All authors contributed equally to the development of the manuscript.
Competing Interest
All coauthors declare no conflict of interest.
Funding
No funding was used for this project.
References
- 1.Shields A., Faustini S.E., Perez-Toledo M., Jossi S., Aldera E., Allen J.D., et al. SARS- CoV-2 seroprevalence and asymptomatic viral carriage in healthcare workers: a cross- sectional study. Thorax. 2020;75:1089–1094. doi: 10.1136/thoraxjnl-2020-215414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.COVID-19 Coronavirus pandemic- Oct31, 2021,〈https://www.worldometers.info/coronavirus/〉.
- 3.Xiang B., Li P., Yang X., Zhong S., Manyande A., Feng M. The impact of novel coronavirus SARS-CoV-2 among healthcare workers in hospitals: an aerial overview. Am J Infect Control. 2020;48:915–917. doi: 10.1016/j.ajic.2020.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deeks J.J., Dinnes J., Takwoingi Y., Davenport C., Spijker R., Taylor-Phillips S. Cochrane COVID-19 diagnostic test accuracy group. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst Rev. 2020;6 doi: 10.1002/14651858.CD013652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galanis P., Vraka I., Fragkou D., Bilali A., Kaitelidou D. Seroprevalence of SARS-CoV-2 antibodies and associated factors in health care workers: a systematic review and meta-analysis. Journal of Hospital Infection. 10.1016/j.jhin.2020.11.008. [DOI] [PMC free article] [PubMed]
- 6.Rudberg AS, Havervall S., Månberg A., et al. SARS-CoV-2 exposure, symptoms and seroprevalence in healthcare workers in Sweden. NATURE COMMUNICATIONS | https://doi.org/10.1038/s41467-020-18848–0. [DOI] [PMC free article] [PubMed]
- 7.Meschi S., Colavita F., Bordi L., Matusali G., Lapa D., Amendola A., et al. Performance evaluation of Abbott ARCHITECT SARS-CoV-2 IgG immunoassay in comparison with indirect immunofluorescence and virus microneutralization test. J Clin Virol. 2020;129 doi: 10.1016/j.jcv.2020.104539. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7336910/# (Aug) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sahu A.K., Amrithanand V.T., Mathew R., Aggarwal P., Nayer J., Bhoi S. COVID-19 in health care workers-A systematic review and meta-analysis. Am J Emerg Med. 2020;38:1727–1731. doi: 10.1016/j.ajem.2020.05.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albaadani A.M., Alsufyani E.A., Mursi M.I., Haris M.H., Kalam K.K., Alsherbeeni N.M., et al. SARS-CoV-2 seroprevalence among healthcare workers from a tertiary care center in Riyadh, Saudi Arabia. Saudi Med J. 2021;42(11):1243–1246. doi: 10.15537/smj.2021.42.11.20210391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abbott Architect SARSCoV-2 IgG kit, Abbott, USA. 〈https://www.fda.gov/media/137383/download〉.
- 11.Alserehi H.A., Alqunaibet A.M., Al-Tawfiq J.A., Alharbi N.K., Alshukairi A.N., Alanazi K.H., et al. Seroprevalence of SARS-CoV-2 (COVID-19) among healthcare workers in Saudi Arabia: comparing case and control hospitals. Diagn Microbiol Infect Dis. 2020;99 doi: 10.1016/j.diagmicrobio.2020.115273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abohamr S.I., Aldossari M.A., Alaklobi F.A., Amer H.A., Alzarzour S.H., Abdelhamid S.W., et al. Clinical characteristics and in-hospital outcome of medical staff infected with COVID-19 in Saudi Arabia A retrospective single-center study. Saudi Med J. 2020;41(12):1336–1343. doi: 10.15537/smj.2020.12.25514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amer H.A., Abdallah H.A., Alkheledan H.S., Gul N.S., Altayieb J.A., Alsalam M., et al. Characteristics of healthcare workers with COVID-19: a retrospective descriptive study in a quaternary care center in Riyadh. Saudi Arab Ann Med Surg. 2021;72 doi: 10.1016/j.amsu.2021.103069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banjar A., Al-Tawfiq J.A., Alruwaily A., Alserehi H., Al-Qunaibet A., Alaswad R., et al. Seroprevalence of antibodies to SARS-CoV-2 among blood donors in the early months of the pandemic in Saudi Arabia. Int J Infect Dis. 2021;104:452–457. doi: 10.1016/j.ijid.2021.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korth J., Wilde B., Dolff S., Anastasiou O.E., Krawczyk A., Jahn M., et al. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J Clin Virol. 2020;128 doi: 10.1016/j.jcv.2020.104437. 〈https://pubmed.ncbi.nlm.nih.gov/32434708/〉 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y., Tong X., Wang J., Huang W., Yin S., Huang R., et al. High SARS-CoV-2 antibody prevalence among healthcare workers exposed to COVID-19 patients. J Infect [Internet]. 2020 Sep 1 [cited 2020 Oct 23];81(3):420–6. Available from: 〈https://pubmed〉. [DOI] [PMC free article] [PubMed]
- 17.Hunter B.R., Dbeibo L., Weaver C.S., Beeler C., Saysana M., Zimmerman M.K., et al. Seroprevalence of SARS-CoV-2 antibodies among healthcare workers with differing levels of COVID-19 patient exposure. Infect Control Hosp Epidemiol. 2020;41:1441–1442. doi: 10.1017/ice.2020.390. 〈https://pubmed.ncbi.nlm.nih.gov/32741406/〉 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacob J.T., Baker J.M., Fridkin S.K., Lopman B.A., Steinberg J.P., Christenson R.H., et al. Risk factors associated with SARS-CoV-2 seropositivity among US health care personnel. JAMA Netw Open. 2021;4(3) doi: 10.1001/jamanetworkopen.2021.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozdmir A., Cuha M.D., Dizman G.T., Alp A., Metan G., Sener B. SARS-CoV-2 seroprevalence among healthcare workers: retrospective analysis of the data from a univrsity hospital in Turkey. Mikrobiyol Bul. 2021;55(2):223–232. doi: 10.5578/mb.20219908. [DOI] [PubMed] [Google Scholar]
- 20.Daperno M., Guiotto C., Casonato I., Pagana G., Micalizzi S., Azzolina M., et al. Risk factors of SARS-CoV-2 seroprevalence among hospital employees in Italy: a single-centre study. Intern Med J. 2021;51(7):1049–1059. doi: 10.1111/imj.15201. doi: 10.1111/imj.15201.Epub 2021 Apr 26. [DOI] [PMC free article] [PubMed] [Google Scholar]