Abstract
Objectives
Risk of hospital-acquired COVID-19 (HA-COVID-19) infection is increased by cohorting infected and non-infected patients together in assessment areas, whist awaiting laboratory PCR results. Molecular point-of-care tests (mPOCT) reduce time to results and improve patient flow but the impact on HA-COVID-19 is unknown.
Methods
In this pre and post implementation study patients were evaluated across two time periods: March 1st to August 13th 2020, prior to the introduction of mPOCT in medical admissions areas, and 14th August 2020 to 1st April 2021, after mPOCT introduction. The primary outcome was proportion of HA-COVID-19 infection among all COVID-19 positive patients. Secondary outcome measures included time to SARS-CoV-2 results, length of time spent in the medical assessment area and comparison of local, regional and national proportions of HA-COVID-19.
Results
1988 patients were admitted through the acute medicine admission cohorting area and tested for SARS-CoV-2 prior to introducing mPOCT and 4640 afterwards. Median (IQR) time to SARS-CoV-2 result was 6.5 (2.1–17.9) hours prior to introducing mPOCT and 1.0 (0.8–1.3) hours afterwards (p < 0.0001). Median (IQR) duration in the assessment cohort area was 12.0 (4.8–20.6) hours prior to introduction of POCT and 3.2 (2.0–5.6) hours afterwards (p < 0.0001). The proportion of hospital-acquired COVID-19 cases was 108 (16.5%) of 654 prior to introducing mPOCT compared with 168 (9.4%) of 1782 afterwards, (HR 0.55, 95%CI 0.43–0.70; p < 0.0001). In the period following the introduction of mPOCT up to 1st April 2021 the median proportion of HA-COVID-19 was 13.6% (95%CI 8.2–18.9%) locally, compared with 43.8% (95%CI 37.8–49.9%) for all acute NHS trusts regionally and 30.9% (95%CI 28.4–33.5%) for all NHS trusts nationally.
Conclusions
Routine mPOCT for SARS-CoV-2 was associated with reduced time to results, time spent in admission cohort areas, and hospital-acquired COVID-19, compared to laboratory PCR.
Keywords: Point-of-care testing, Hospital acquired infection, COVID-19, SARS-CoV-2
Introduction
Timely recognition and management of COVID-19, caused by infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is critical in preventing onward transmission to other patients in hospital.1 NHS data suggests that by May 2021 over 32,000 hospital-acquired COVID-19 cases had occurred with nearly 9000 associated deaths.2 A recent nationwide study has estimated that during the first wave of the pandemic 11.3% of patients with COVID-19 in UK hospitals had acquired their infection in hospital.3 In addition, genome sequencing-based studies from the UK suggest that the vast majority of hospital-acquired COVID-19 (HA-COVID-19) originates from patient-to-patient transmission.4
Although multiple factors are implicated in hospital transmission of SARS-CoV-2, delays in SARS-CoV-2 test results have been recognised as being among the leading causes.1 , 5 The risk of patient-to-patient transmission is increased when single room capacity is exceeded by the number of suspected cases with an unconfirmed COVID-19 status as this leads to the practice of cohorting of acute hospital admissions in assessment wards whilst awaiting SARS-CoV-2 results, inadvertently leading to co-location of infected and non-infected patients in shared bay areas. Reducing the amount of time that patients spend in assessment cohort areas is therefore key to reducing both patient-to-patient transmission. Rapid downstream flow of SARS-CoV-2 positive patients to designated COVID-19 wards allows optimal use of facilities providing patient isolation, adequate ventilation, and clinical care from designated staff with appropriate personal protective equipment.1
Centralized laboratory SARS-CoV-2 polymerase chain reaction (PCR) testing is associated with long delays in returning results, representing the rate-limiting step in effective patient flow through the hospital.6 Molecular point-of-care testing (mPOCT) has been shown to significantly reduce the time from admission to test results for SARS-CoV-2 and to reduce the length of time spent in assessment cohort areas, however its effect on HA-COVID-19 is unknown.6
Methods
Study design and patients
We performed a single center, pre and post implementation study in a tertiary hospital in the UK. We analyzed the records of all medical admissions tested for SARS-CoV-2 with laboratory PCR or mPOCT, and all positive cases across specialities, between 1st March 2020 and 1st April 2021 at University Hospital Southampton Foundation Trust (UHSFT), a large acute teaching hospital in the South of England serving a population of 1.9 million.7
Following local R&D governance review formal application for ethical approval was deemed unnecessary, as only routinely collected, pseudo-anonymised data was used, this study was prospectively approved by senior trust governance. Ref No: SEV/0320.
Point of care testing for SARS-CoV-2
Routine molecular point-of-care testing for SARS-CoV-2 and other respiratory viruses was introduced on the 13th August 2020 for patients admitted under the department of medicine via the acute medical admission pathway. Following device validation and a period of staff training all patients admitted to the Acute Medical Unit (AMU) had a nose and throat swab taken at arrival and tested using the BioFire (Salt Lake City, USA) FilmArray Respiratory PCR Panel 2.1 plus which includes targets for SARS-CoV-2 and 17 other respiratory viruses and atypical bacteria (for full details of the panel targets see supplementary material). The FilmArray SARS-CoV-2 Respiratory Panel 2.1 plus SARS-CoV-2 assay contains gene targets for the S gene and M gene. Patient swabs were collected directly into guanidine thiocyanate containing media tubes (Medical Wire molecular medium) to inactivate viruses and then tested on the FilmArray Torch platform located within a dedicated testing hub within the AMU. Nursing staff wearing appropriate personal protective equipment performed the testing and the run time of the test was around 45 min. The FilmArray systems were integrated with the hospitals Laboratory Information Management System (LIMS) and the electronic patient records so that results were available to clinical and infection control teams as soon as the run was completed.
Pre and post implementation time periods
Patients were analyzed over two time periods: from March 1st, 2020 to August 13th, 2020, prior to introducing routine use of mPOCT in the AMU, when medical patients were tested for SARS-CoV-2 using laboratory testing within the on-site PHE microbiology laboratory, and August 14th 2020 to April 1st 2021, after the introduction of routine mPOCT in the AMU. For both periods, patients admitted outside of the acute medical admissions pathway were tested with laboratory PCR.
Outcomes
The primary outcome measure was the proportion of hospital-acquired COVID-19 infection among all COVID-19 positive patients. HA-COVID-19 infection was defined in two ways, firstly as detection of SARS-CoV-2 RNA at any time point after 48 h of admission and secondly where patients had previously tested negative as detection of SARS-CoV-2 RNA at any time point after 7 days of admission, in keeping with the NHS England definition of probable hospital-acquired infection.8
Secondary outcome measures included time to results (defined as time from SARS-CoV-2 test request to time result was available to the clinical teams, in hours) and length of time spent in the AMU assessment area (in hours), the proportion of HA-COVID-19 cases in patients tested with mPOCT and laboratory PCR, the proportions of HA-COVID-19 at UHSFT, across the southern region and nationally (calculated from routinely collected data from acute NHS trusts).
Patients and pathways
For the primary outcome, analysis of proportion of HA-COVID-19 infection was undertaken in all hospitalized COVID-19 patients at UHSFT. This included all patients over the study period admitted under any hospital speciality, testing positive for SARS-CoV-2. Patients who were admitted directly to the intensive care unit or not admitted to a downstream hospital ward were excluded.
For the secondary outcomes, analyses of time to results and time spent in the AMU assessment area were undertaken in all patients admitted under the department of medicine via the acute medical admission pathway and tested for SARS-CoV-2 with laboratory PCR or mPOCT.
Data collection and preparation
Baseline characteristic data was collected for all patients including age, gender, ethnicity, comorbidities and body mass index (BMI). Binary variables were derived for comorbidities from the casemix database using the appropriate codes for: previous myocardial infarction, congestive cardiac failure, peripheral vascular disease, previous stroke or TIA, dementia, COPD, connective tissue disease, peptic ulcer disease, liver disease, diabetes, hemiplegia, chronic kidney disease, cancer (solid/lymphoma/metastatic) and HIV/AIDS. These were used to calculate the Charlson Comorbidity Index (CCI) for each patient.
Data was retrieved to flag use of mPOCT at admission and those patients on a non-medical admission pathway as defined by specialty destination ward code to exclude this as a potential confounder.
Data were extracted from structured and/or unstructured components of the electronic health record (EHR) at our institution. All data was handled securely on-site using python 3.7 and associated packages. Further details regarding data processing including pseudonymization are presented in the supplementary materials – Supplement A.
Statistical analysis
Analysis was done using Prism version 9.2 (GraphPad Software Inc; La Jolla, California), and Python version 3.7 + packages. All continuous parameters were summarised using either mean or median and interquartile range (IQR) as appropriate. Proportions and confidence intervals were used for categorical data. The Mann-Whitney U test was used to compare medians and mean differences and corresponding CIs were calculated with the Hodges-Lehmann estimate. Groups were compared using chi-square tests or Fishers exact test for equality of proportions, as appropriate based on group size.
Multivariate model
We evaluated time from hospital admission to hospital-acquired infection amongst the COVID-19 positive patients accounting for competing risks and right-censored data (i.e. patients still in hospital at the time of censoring) using the Nelson-Aelen and Kaplan-Meier estimators, respectively. We used adjusted and unadjusted cox proportional hazards regression to assess predictors of risk for hospital-acquired COVID-19 infection and their impact compared to our primary covariate – mPOCT. Variables with a p value below 0.05 in adjusted regression were considered significant. Variables such as BMI with a greater than 20% rate of missingness were excluded from regression analysis. The proportional hazards assumptions were evaluated using Schoenfeld's residuals.9 The Ljung-Box and Box-Pierce tests were used to prove that the residuals were not autocorrelated.
Role of funding source
The funders of the study had no role in the study conception, design, conduct, data analysis, or manuscript preparation. The corresponding author had full access to all data and the final responsibility to submit for publication.
Results
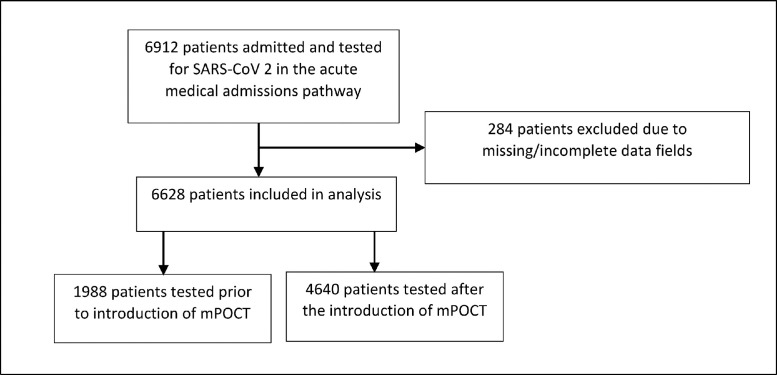
We identified 6944 patients admitted and tested for SARS-CoV-2 through the acute medical admissions pathway with laboratory PCR or mPOCT during the study period from 1st March 2020 to 1st April 2021. Full data on time to results was available for 6628 patients: 1988 prior to introduction of routine mPOCT (1st March 2020 to 13th August 2020) and 4640 after the introduction of routine mPOCT (14th August 2020 to 1 April 2021), shown in Fig. 1 .
Fig. 1.
Patient flow through the study - patients admitted and tested for SARS-CoV-2 through the acute medical admissions pathway.
Median (IQR) age in the pre mPOCT period was 75 years (58–85) and was 74 (54–85) years in the post mPOCT period. 1023 (51.5%) of 1988 patients were male in the pre-mPOCT period compared with 2527 (54.5%) of 4640 in the post mPOCT period. Overall, a higher proportion of patients had co-morbidities in the pre-mPOCT group compared to the post mPOCT group. Baseline characteristics for the patients admitted through the acute medical admission pathway are shown in Table 1 .
Table 1.
Baseline patient characteristics for medical admissions pre and post introduction of molecular point-of-care testing (mPOCT).
| Pre mPOCTgroupn = 1988 | Post mPOCTgroupn = 4640 | Difference(95%CI) | p valuea | |
|---|---|---|---|---|
| Age, years | 75.2 [57.5–85.3] | 73.9 [54.0–84.8] | 1.4 (1.1–1.6) | 0.0019 |
| Male Sex | 1023 (51.5%) | 2527 (54.5%) | 3% (2.2 – 3.8) | 0.0265 |
| BMIb | 26.0[22.28–30.06] | 26.1[22.49–30.27] | 0.2 (0.1–0.3) | 0.2799 |
| BAMEc | 87 (4.6%) | 220 (5.1%) | 0.5% (0.2–0.8) | 0.4611 |
| Asthma | 280 (14.1%) | 478 (10.3%) | 3.8% (3.1–4.4) | < 0.0001 |
| COPD | 332 (16.7%) | 477 (10.3%) | 6.4% (5.7–7.2) | < 0.0001 |
| CKD | 15 (0.8%) | 27 (0.6%) | 0.2% (0.0–0.4) | 0.5204 |
| Diabetes | 198 (10.0%) | 340 (7.3%) | 2.6% (2.1–3.2) | 0.0004 |
| Dementia | 48 (2.4%) | 61 (1.3%) | 1.1% (0.8–1.5) | 0.0018 |
| Hypertension | 861 (43.3%) | 1354 (29.2%) | 14.1% (13.3 15.0) | < 0.0001 |
| IHD | 272 (13.7%) | 417 (9.0%) | 4.7% (4.0–5.4) | < 0.0001 |
| CCF | 170 (8.6%) | 211 (4.6%) | 4.0% (3.4–4.6) | < 0.0001 |
| Cirrhosis | 101 (5.1%) | 123 (2.7%) | 2.4% (1.9–2.9) | < 0.0001 |
| CCI | 3.7 (1.7–5.7) | 3.1 (1.2–5.0) | 0.54 (0.5–0.6) | < 0.0001 |
All data are presented as n (%), median [interquartile range] or mean (SD). CI, confidence interval. BMI, body mass index. BAME, black and minority ethic. COPD, chronic obstructive airways disease. CKD, chronic kidney disease. IHD, ischaemic heart disease. CCF, congestive cardiac failure. CCI, Charlson comorbidity index.
Mann Whitney U Test, Chi squared or Fisher's Exact Test.
Assessed in 1561 and 1946 patients in the pre and post implementation groups, respectively.
Assessed in 1902 and 4357 patients in the pre and post implementation groups, respectively.
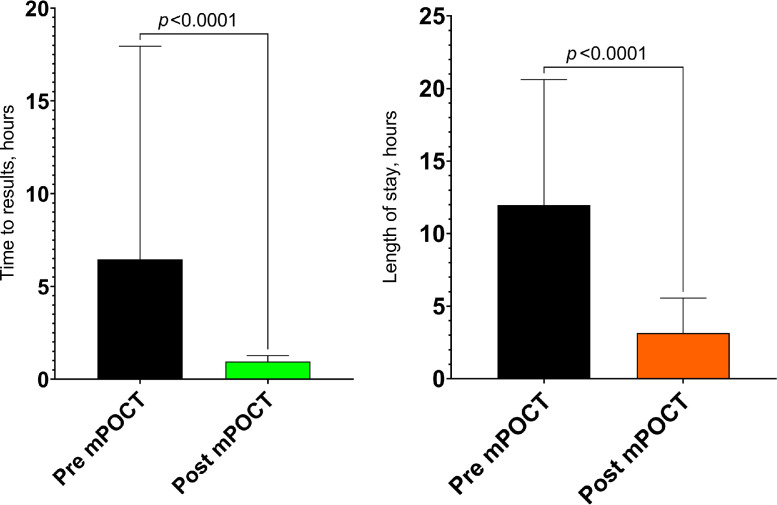
The median (IQR) time from admission to SARS-CoV-2 result was 6.5 (2.1–17.9) hours prior to introducing mPOCT and 1.0 (0.8–1.3) hours afterwards (difference of 5.5 h, 95%CI 5.2 to 5.8; p < 0.0001), shown in Fig. 2 a. Median (IQR) length of stay in the assessment cohort area was 12.0 (4.8 to 20.6) hours prior to the introduction of POCT and 3.2 (2.0–5.6) hours afterwards (difference of 8.8 h, 95%CI 8.5 to 9.1; p < 0.0001), shown in Fig. 2b. This is equivalent to 367 COVID-19 assessment area bed-days saved for every 1000 patient cohort area journeys. Given that there were 9878 patient journeys through the cohorting area after the introduction of mPOCT this is equivalent to 3625 bed days saved in the assessment cohort area in total.
Fig. 2.
a. Median (IQR) time from admission to results, hours. b. Median (IQR) length of stay in assessment area, hours.
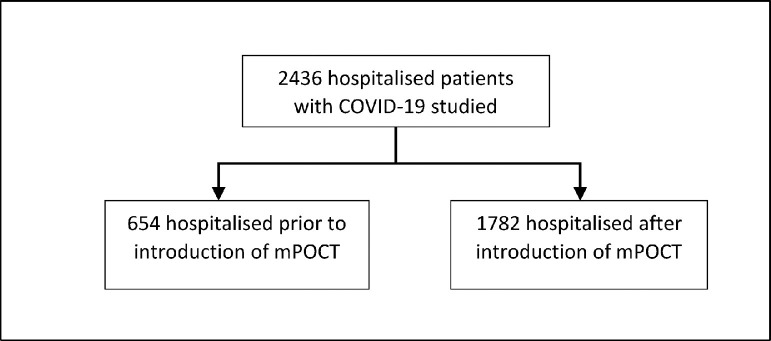
2436 individuals were admitted with COVID-19 or were diagnosed whilst in hospital, during the study period; 654 prior to the introduction of routine mPOCT and 1782 afterwards, shown in Fig. 3 . Baseline characteristics for both groups are shown in Table 2 .
Fig. 3.
Patient flow through the study - patients testing positive for COVID-19.
Table 2.
Baseline patient characteristics for hospitalized patients with COVID-19, pre and post introduction of molecular point-of-care testing (mPOCT).
| Pre mPOCTgroupn = 654 | Post mPOCTgroupn = 1782 | Difference(95%CI) | p valuea | |
|---|---|---|---|---|
| Age, years | 72.6 [55.9–83.4] | 64.8 [49.7–79.4] | 7.8 (7.3–8.4) | < 0.0001 |
| Male Sex | 280 (42.8%) | 832 (46.7%) | 3.9% (2.4–5.4) | 0.0977 |
| BMIb | 26.9 [23.8–31.1] | 27.9 [23.9–32.9] | 1 (0.8–1.2) | 0.0039 |
| BAMEc | 59 (10.2%) | 156 (10.1%) | 0.1% (0.1–0.1) | 1.0000 |
| Asthma | 115 (17.6%) | 298 (16.7%) | 0.9% (0.3–2.1) | 0.6591 |
| COPD | 123 (18.8%) | 261 (14.6%) | 4.2% (2.8–5.5) | 0.0149 |
| CKD | 123 (18.8%) | 228 (12.8%) | 6.0% (4.6–7.5) | 0.0002 |
| Diabetes | 179 (27.4%) | 407 (22.8%) | 4.5% (3.1–6) | 0.0235 |
| Dementia | 75 (11.5%) | 145 (8.1%) | 3.3% (2.15–4.5) | 0.0138 |
| Hypertension | 256 (39.1%) | 526 (29.5%) | 9.6% (8–11.3) | < 0.0001 |
| IHD | 166 (25.4%) | 400 (22.4%) | 2.9% (1.5–4.3) | 0.1426 |
| CCF | 154 (23.5%) | 322 (18.1%) | 5.5% (4.0–7.0) | 0.0030 |
| Cirrhosis | 41 (6.3%) | 126 (7.1%) | 0.8% (0.1–1.5) | 0.5463 |
| CCI | 5.2 (1.8–8.7) | 4.2 (0.75–7.6) | 1 (0.9–1.1) | < 0.0001 |
All data are presented as n (%), median [interquartile range] or mean (SD). CI, confidence interval. BMI, body mass index. BAME, black and minority ethic. COPD, chronic obstructive airways disease. CKD, chronic kidney disease. IHD, ischaemic heart disease. CCF, congestive cardiac failure. CCI, Charlson comorbidity index.
Mann Whitney U Test, Chi squared or Fisher's Exact Test.
Assessed in 494 and 1255 patients in the pre and post implementation groups, respectively.
Assessed in 581 and 1551 patients in the pre and post implementation groups, respectively.
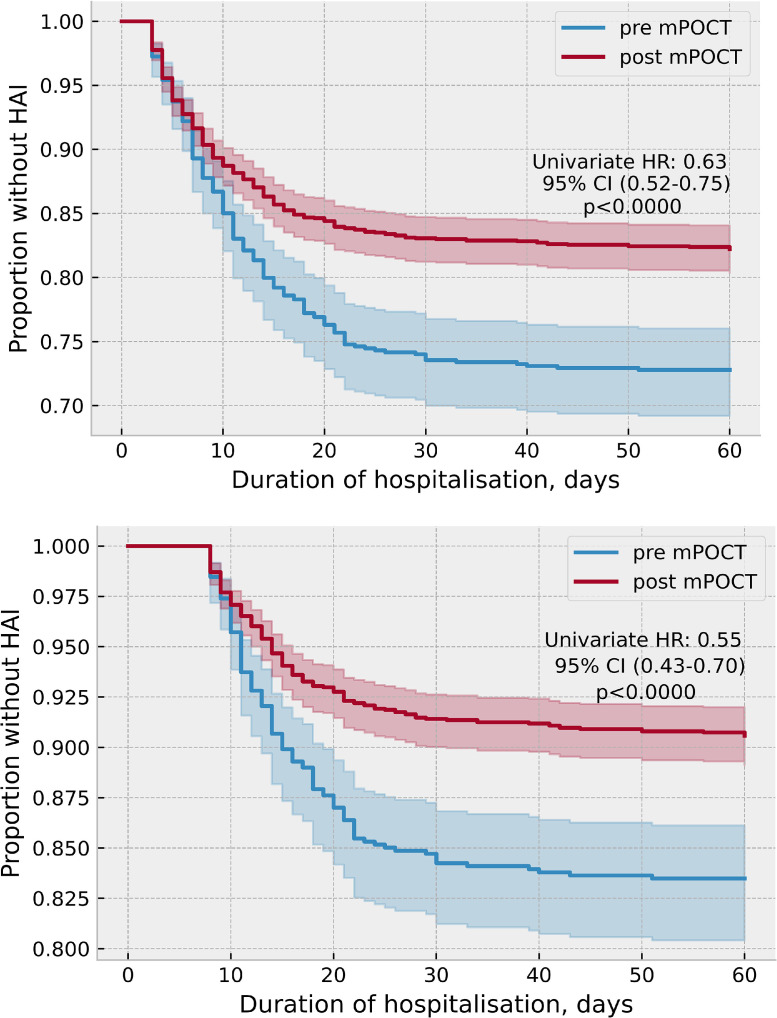
Following the introduction of routine mPOCT, the proportion of HA-COVID-19 fell from 178 (27.2%) of 654 to 317 (17.8%) of 1782 (HR 0.63, 95%CI 0.52–0.75; p < 0.0001), when defined as a SARS-CoV-2 PCR positivity after 48 h of admission, and from 108 (16.5%) of 654 to 168 (9.4%) of 1782 (HR 0.55, 95%CI 0.43–0.70; p < 0.0001), when defined as SARS-CoV-2 PCR positively after 7 days of admission, shown in Fig. 4 a and b.
Fig. 4.
a. Proportion of HA-COVID-19 before and after introduction of mPOCT, when defined as a positive PCR after 48 h of admission. b. Proportion of HA-COVID-19 before and after introduction of mPOCT, when defined as a positive PCR after 7 days of admission.
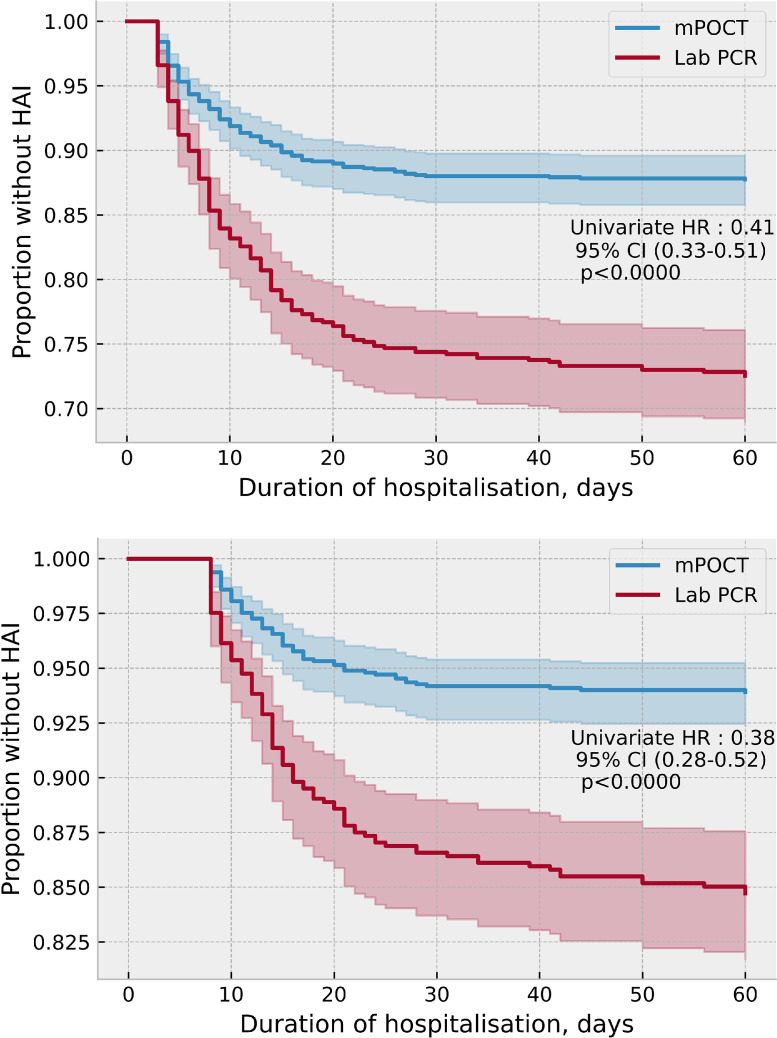
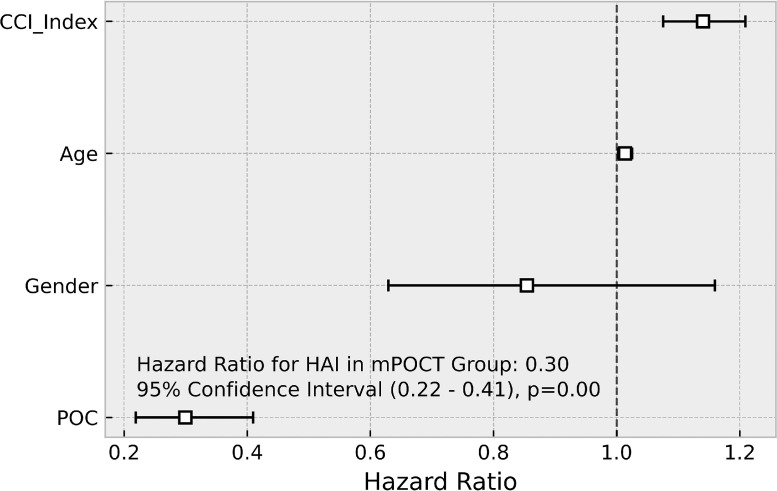
Following the introduction of mPOCT, the proportion of HA-COVID-19 across the hospital was lower in the patients who were tested with mPOCT compared with laboratory testing, both when defined as SARS-CoV-2 PCR positivity after 48 h of admission; 139 (12.3%) of 1133 vs 178 (27.4%) of 649 (HR 0.41, 95% CI 0.33–0.51; p < 0.0001) and after 7 days 69 (6.1%) of 1133 Vs 99 (15.3%) of 649 (HR 0.38, 95%CI 0.28–0.52; p < 0.0001), shown in Fig. 5 a and b. Multivariate time series regression, adjusting for age, gender and CCI demonstrated a similar reduction in HA-COVID-19 infection (HR 0.30, 95% CI 0.22–0.41; p < 0.0001) with comorbidity (CCI) also associated with the risk of HA-COVID-19 (HR 1.13, 95% CI 1.07–1.21; p < 0.0001), shown in Fig. 6 .
Fig. 5.
a. Proportion of HA-COVID-19 when tested with mPOCT or laboratory testing, when defined as a positive PCR after 48 h of admission. b. Proportion of HA-COVID-19 when tested with mPOCT or laboratory testing, when defined as a positive PCR after 7 days of admission.
Fig. 6.
Multivariate model for HA-COVID-19 when tested with mPOCT or laboratory testing.
74/1782 (4.2%) of patients in this time period were admitted via a surgical admission pathway. The proportion of HA-COVID-19 was 32 (43.2%) of 74 patients in this patient group (HR 3.82, 95% CI 2.54 – 5.74; p < 0.0001, compared with 136 (8.0%) of 1708 amongst patients admitted via medical and other pathways (HR 0.38, 95% CI 0.27 – 0.53; p < 0.0001), shown in Fig. S1. Schoenfeld residual testing revealed no autocorrelation within the model with the Ljung-box test and Box Peirce tests confirming this (p = 0.989 and 0.999, respectively) suggesting that the variables in our time series analysis are independent of each other. 527 (29.5%) of 1782 patients lacked either a usable height or weight thus BMI could not be used for regression. 226 (12.7%) of 1782 patients had their ethnicity recorded as ‘other’ or ‘unknown’. The addition of this variable had no significant impact on the model, p = 0.111.
Across the entire study period 417 (17.0%) of 2436 COVID-19 patients died whilst in hospital. 68 (24.6%) of 276 patients with HA-COVID-19 died compared with 349 (16.2%) of 2160 with community-acquired infection (RR 1.75 ,95%CI: 1.40–2.19; p < 0.0001). mPOCT was associated with a reduced risk of HA-COVID-19 (RR of 0.34, 95%CI 0.26–0.43; p < 0.0001) with a number needed to test (NNT) of 8.8 (95%CI: 7.2–11.3) to prevent a single HA-COVID-19 infection. This suggests that around 140 HA-COVID-19 episodes were prevented at UHSFT after the introduction of mPOCT, resulting in around 35 fewer deaths.
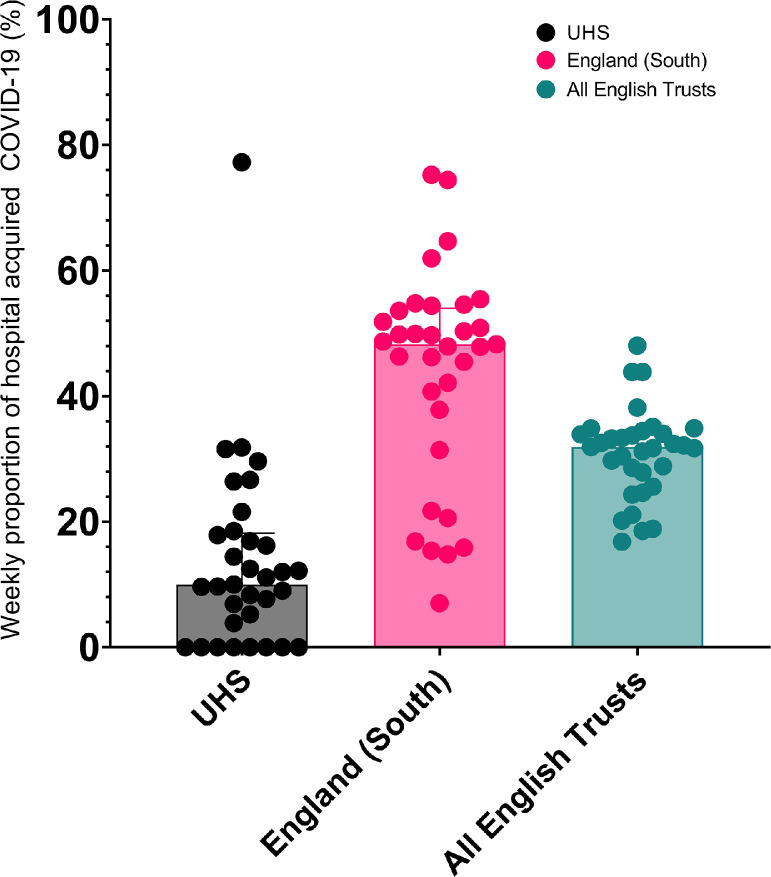
In the period following the introduction of mPOCT up to 1st April 2021 the median proportion of HA-COVID-19, defined as PCR positivity > 7 days after admission to hospital, was 13.6% (95% CI 8.2%–18.9%) at UHSFT compared with 43.8% (95% CI 37.8%−49.9%) for all acute NHS trusts in the South of England and 30.9% (95% CI 28.4%−33.5%) for all NHS trusts nationally, shown in Fig. 7 .
Fig. 7.
Median proportions of HA-COVID-19 for UHSFT, the South of England and all of England.
Discussion
To our knowledge, this real-world study is the first to assess the impact of routine use of mPOCT in an admission pathway, upon HA-COVID-19 infection rates. Consistent with the results of our previous trials, we have demonstrated that routine use of mPOCT in acute hospital admissions significantly reduced the time to SARS-CoV-2 results and the time that patients spent in assessment cohort areas.6 In addition, we have also demonstrated in this study that the introduction of mPOCT in our institution was associated with a large reduction in the rate of HA-COVID-19. Molecular point-of-care testing is likely to reduce HA-COVID-19 by providing a rapid accurate result, allowing patients with SARS-CoV-2 infection to be identified early and transferred to definitive care areas before they are able to transmit infection to other patients in the assessment areas. According to nationally available data10 280,737 patients were admitted to hospital or diagnosed with COVID-19 whilst in hospital in England during our study period. Based on these data 86,832 of these patients are likely to have contracted COVID-19 in hospital. Extrapolating from our data around 52,100 of these infections could have been prevented by nationally deploying mPOCT for acute admissions, potentially resulting in 13,025 fewer COVID-19 related deaths. Although the availability of mPOCT test platforms for SARS-CoV-2 was severely limited during the early part of the pandemic, there are now several widely available test platforms with high levels of accuracy demonstrated though national validation11 that can be deployed in hospitals at the point-of-care or in near-patient settings.
The strengths of the study include its real-world nature. We have performed a pre and post implementation study in a typical acute NHS setting with a large number of patients over a prolonged period of time, suggesting that our results are generalisable to similar UK and international centres. Our study also has a number of potential weaknesses. It was observational, not interventional, and outside the setting of a randomised control interventional trial we are unable to definitively attribute the observed reduction in hospital-acquired COVID-19 infection rates to the introduction of routine mPOCT. There were potential confounding variables within our study as it took place during a period of time with a rapidly changing landscape as the United Kingdom responded to the COVID-19 pandemic. Following the first waves of the pandemic there were a number of changes introduced during the study period in addition to mPOCT that could have influenced HA-COVID-19 rates including staff screening, changes to PPE and infection control practices and staff and community vaccination programmes. We have attempted to control for these by comparing HA-COVID-19 with mPOCT and laboratory testing after the introduction of mPOCT and also by including regional and national data. As most of these interventions were introduced nationally and at the same time, the lack of a fall in HA-COVID-19 either regionally or nationally over the study period suggests that the changes seen at UHSFT were the result of mPOCT rather than other interventions.
At the end of December 2020, the SARS-CoV-2 lineage B.1.1.7 (VOC 202,012/01, ‘alpha variant’) became the dominant SARS-CoV-2 lineage in the UK.12 This may also be a confounding factor as altered strain dynamics may impact upon likelihood of nosocomial transmission. Notably, this variant has been associated with increased transmissibility13 when compared with prior lineages, and therefore is unlikely to be associated with a reduction in hospital-acquired cases. We did not routinely analyze sequencing data from cases within this study, but the local prevalence of the alpha variant was already > 50% by December 2020.
NHSE defines probable hospital-acquired COVID-19 as a positive test for SARS-CoV-2 after 7 days of hospital admission8 to account for the incubation period of SARS-CoV-2, although for other infections hospital-acquired infection is conventionally defined as an infection occurring greater than 48 h after hospital admission14 and therefore, use of NHSE definition may significantly underestimate the true rates of HA-COVID-19 infection. Our results show a similar impact of mPOCT upon HA-COVID-19 infection when defined as infection occurring after either 48 h or after 7 days.
In conclusion, the use of mPOCT as part of the medical admission pathway for COVID-19 significantly reduced the time to results, the time spent on assessment cohort wards and the proportion of HA-COVID-19 infection. Routine use of mPOCT should therefore become the standard of care in hospital admission pathways.
Data sharing
The data analyzed and presented in this study are available from the corresponding author on reasonable request, providing this meets local ethical and research governance criteria.
Funding
This study was funded by University Hospital Southampton NHS Foundation Trust.
CRediT authorship contribution statement
Robert Livingstone: Writing – original draft. Hlaing Lin: Writing – original draft. Nathan J. Brendish: Writing – review & editing. Stephen Poole: Writing – review & editing. Alex R. Tanner: Writing – review & editing. Florina Borca: Data curation, Formal analysis. Trevor Smith: Writing – review & editing. Matthew Stammers: Data curation, Formal analysis, Writing – original draft. Tristan W. Clark: Writing – review & editing, Visualization, Writing – original draft.
Declaration of Competing Interest
None
TWC has received speaker fees, honoraria, consultancy fees, travel reimbursement, and equipment and consumables free of charge for the purposes of research outside of this submitted study, from BioFire diagnostics and BioMerieux. He has received speaker fees and discounted equipment and consumables from QIAGEN. He has received consultancy fees from Shionogi, Synairgen research, Randox laboratories and Cidara therapeutics. He has been a member of advisory boards for Roche, Janssen and Cepheid. He is a member of two independent data monitoring committees for trials sponsored by Roche. He has acted as the UK chief investigator for a trial sponsored by Janssen
Acknowledgments
We acknowledge and thank all the patients who kindly participated in this study and all the clinical staff at University Hospital Southampton who cared for them. We also acknowledge the Department of Health and Social Care Antimicrobial Research Capital Funding Award (reference NIHR200638).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2022.01.034.
Appendix. Supplementary materials
References
- 1.HSIB . HSIB; 2021. COVID-19 Transmission in Hospitals: Management of The Risk-A Prospective Safety Investigation.https://www.hsib.org.uk/investigations-and-reports/covid-19-transmission-in-hospitals-management-of-the-risk/ [Internet][citedSep 5]. Available from. [Google Scholar]
- 2.D. Campbell, A. Bawden, Up to 8,700 patients died after catching Covid in English hospitals. May 24, 2021, The Guardian [Internet]. [cited 2021 Sep 5]. Available from: http://www.theguardian.com/world/2021/may/24/up-to-8700-patients-died-after-catching-covid-in-english-hospitals
- 3.Read J.M., Green C.A., Harrison E.M., Docherty A.B., Funk S., Harrison J., et al. Hospital-acquired SARS-CoV-2 infection in the UK's first COVID-19 pandemic wave. Lancet. 2021;398(10305):1037–1038. doi: 10.1016/S0140-6736(21)01786-4. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Illingworth C.J., Hamilton W.L., Warne B., Routledge M., Popay A., Jackson C., et al. Superspreaders drive the largest outbreaks of hospital onset COVID-19 infections. eLife. 2021;10:e67308. doi: 10.7554/eLife.67308. Walczak AM, Ogbunugafor CB, Cobey SE, editors. Aug 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oliver D. Could we do better on hospital-acquired COVID-19 in a future wave? BMJ. 2021;372:n70. doi: 10.1136/bmj.n70. Jan 13. [DOI] [PubMed] [Google Scholar]
- 6.Brendish N.J., Poole S., Naidu V.V., Mansbridge C.T., Norton N.J., Wheeler H., et al. Clinical impact of molecular point-of-care testing for suspected COVID-19 in hospital (COV-19POC): a prospective, interventional, non-randomised, controlled study. Lancet Respir Med. 2020;8(12):1192–1200. doi: 10.1016/S2213-2600(20)30454-9. Dec 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Care Quality Commision, Chief Inspector Praises ‘Impatience to Improve’ At University Hospitals Southampton NHS Foundation Trust. June 15, 2017, Care Quality Commission [Internet]. [cited 2021 Jul 13]. Available from: https://www.cqc.org.uk/news/releases/chief-inspector-praises-%E2%80%98impatience-improve%E2%80%99-university-hospitals-southampton-nhs.
- 8.R. May, S. Powis, P. Issar, P. Philip, Healthcare Associated COVID-19 Infections – Further Actions. June 24, 2020, NHS England and NHS improvement [Internet]. [cited 2021 Sep 5]. Available from: https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/06/Healthcare-associated-COVID-19-infections–further-action-24-June-2020.pdf.
- 9.Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69(1):239–241. [Google Scholar]
- 10.COVID-19 NHS Situation Report, COVID-19 Hospital Activity, Weekly Admissions and Beds up to 6 April 2021. May 13, 2021, NHS England [Internet]. [cited 2021 Aug 20]. Available from: https://www.england.nhs.uk/statistics/statistical-work-areas/covid-19-hospital-activity/.
- 11.UK Health Security Agency, National Technical Validation Process for Manufacturers of SARS-CoV-2 (COVID-19) Tests. June 3, 2020, GOV.UK [Internet]. [cited 2021 Sep 5]. Available from: https://www.gov.uk/government/publications/assessment-and-procurement-of-coronavirus-covid-19-tests/coronavirus-covid-19-serology-and-viral-detection-testing-uk-procurement-overview.
- 12.Office for National Statistics, COVID-19 infection survey: estimates of COVID-19 cases to 23 december for England, regions of England and by cases compatible with the new variant - [Internet]. [cited 2021 Jun 17]. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/adhocs/12716covid19infectionsurvey-estimatesofcovid19casesto23decemberforenglandregionsofenglandandbycases-compatiblewiththenewvariant.
- 13.Volz E., Mishra S., Chand M., Barrett J.C., Johnson R., Geidelberg L., et al. Assessing transmissibility of SARS-CoV-2 lineage B.1.1.7 in England. Nature. 2021;593(7858):266–269. doi: 10.1038/s41586-021-03470-x. May. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization . World Health Organization; 2002. Prevention of Hospital-Acquired Infections: A Practical Guide.https://apps.who.int/iris/handle/10665/67350 [Internet][cited 2021 Nov 13]. Report No.: WHO/CDS/CSR/EPH/2002.12. Available from: [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.