Dear editor,
Since 2019, the COVID-19 epidemic has raged worldwide, with children and adolescents accounting for a quarter of the world's population being greatly threatened. The safety and effectiveness of the COVID-19 vaccine for children have been examined. At present, clinical trials of COVID-19 vaccines for children are gradually increasing. The most common ones are attenuated and inactivated vaccines.The efficacy and safety of many randomized clinical trials (RCTs) and observational trials of the COVID-19 vaccine in children have been published through a systematic search of common databases between 2019, and November 8, 2021.
We read with great interest the report in this Journal by Chappell et al. who found that SARS-CoV-2 infections have occurred in immunocompromised children and young people with no increased risk of severe disease.1 Even without an increased risk of contracting COVID-19 in immunocompromised children and young adults. However, a meta-analysis of the safety and efficacy of COVID-19 vaccines in children and/or adolescents is still warranted. An extensive literature search was performed in PubMed, Web of Science, EMBASE, Elsevier ScienceDirect, and Cochrane Library to find all compliant articles published from January 1, 2020, to November 8, 2021. The following keywords were used on the search strategy: “COVID-19″, “2019-nCoV”, “SARS-CoV-2″, “2019 novel coronavirus”, “coronavirus disease 2019″, “severe acute respiratory syndrome coronavirus 2″, “children”, “child”, “adolescent”, and “teenager”. The reference lists, cited in the included studies and reviews, were eligible as exploratory targets to identify extensive articles. The inclusion criteria included1: adult COVID-19 children/adolescents confirmed by reverse transcriptase-polymerase chain reaction (rt-PCR)2; peer-reviewed original articles in English3; individual study populations being at least fifteen cases4; the key available data tabulated data or effect (95% confidence interval (CI)), must be clearly stated. Case reports, repeated articles, review papers, and preprints were eliminated.
Single-group rates and corresponding 95% CIs were used to assess the association between children/adolescents and the COVID-19 vaccine in a whole random-effects meta-analysis model. The model includes effectiveness rates, adverse effects rates, and injection site pain rates in the COVID-19 group. The I2 statistic was used to quantify the heterogeneity of the effects among the included studies. A sensitivity analysis was perform to determine the robustness of the results. The "META" package of the R software (version 4.1.1) was applied. A significant association was not recognized until the two-tailed P < 0.05.
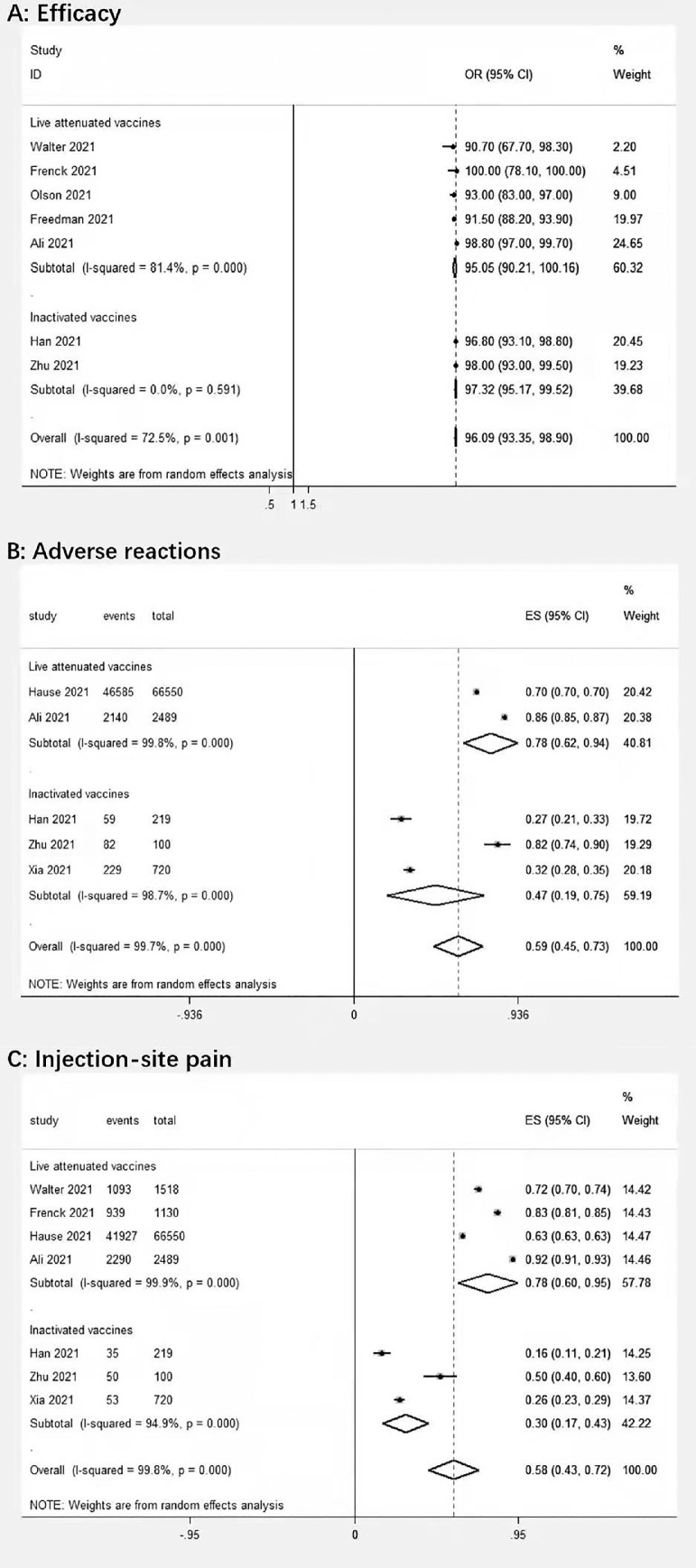
A total of 9 articles involving 264,674 patients were identified, including 7 RCTs and 2 observational studies. Table 1 describes the detailed characteristics of the effectiveness and safety studies.2, 3, 4, 5, 6, 7, 8, 9, 10 Seven studies have shown that the overall effectiveness of the COVID-19 vaccine is 96.09% (95% confidence interval [CI]:93.35–98.90, p < 0.01) (Fig. 1 A), of the attenuated vaccine is 95.05% (95% [CI]:90.21–100.16, p < 0.01) (Fig. 1A), and the inactivated vaccine 97.32% (95% [CI];95.17–99.52, p > 0.01) (Fig.1A). Safety is important for children. In 5 studies, we found that the adverse reaction was 0.59 (95% [CI]; 0.45–0.73, p < 0.01) (Fig.1B). The adverse reaction of the attenuated vaccine was 0.78 (95% [CI]: 0.62–0.94, p < 0.01) (Fig.1B), the adverse reaction of the inactivated vaccine was 0.47 (95% [CI]: 0.19–0.75, p < 0.01) (Fig.1B) The adverse reactions of inactivated vaccines are significantly less than that of attenuated vaccines. We also counted the pain at the injection site. In 7 studies, the pain at the injection site was 0.58 (95% [CI]: 0.43–0.72, p < 0.01) (Fig.1C), the injection pain of the attenuated vaccine was 0.78 (95% [CI]:0.60–0.95, p < 0.01) (Fig. 1C), the injection pain of the inactivated vaccine was 0.30(95% [CI]: 0.17–0.43, p < 0.01)(Fig. 1C), the injection of inactivated vaccine is less painful and more friendly to children and adolescents.
Table 1.
The basic information of the included literature.
| Study | Population | Study type | Country | Intervention | All person | controls | Vaccine efficacy(95%CI) | Injection-site pain | Adverse reactions |
|---|---|---|---|---|---|---|---|---|---|
| Walter et al.2 | 5–11years | Randomized controlled trial | United States | BNT162b2 mRNA | 2268 | 750 | 90.7% (95% CI, 67.7–98.3) | 1093 | / |
| Frenck et al.3 | 12–15years | Randomized controlled trial | United States | BNT162b2 mRNA | 2260 | 1129 | 100% (95% CI, 78.1 to 100) | 939 | / |
| Olson et al.4 | 12–18years | Randomized controlled trial | United States | Pfizer-BioNTech mRNA.2doses | 464 | 285 | 93% (95% CI = 83–97%) | / | / |
| Hause et al.5 | 12–17years | observational study | United States | BNT162b2 mRNA | 66,550 | / | / | 41,927 | 46,585 |
| Freedman et al.6 | 12–15years | observational study | Israel | BNT162b2 mRNA | 187,707 | / | 91.5% (95% CI 88.2–93.9% | / | / |
| Ali et al.7 | 12–17years | Randomized controlled trial | United States | mRNA-1273 | 3732 | 1243 | 98.8(95%CI= 97.0 to 99.7) | 2290 | 2140 |
| Han et al.8 | 3–17 years | Randomized controlled trial | China | CoronaVac | 333 | 114 | 96•8% [95%CI= 93•1–98•8] | 35 | 59 |
| Zhu et al.9 | 6–17years | Randomized controlled trial | China | Recombinant Adenovirus Type-5–Vectored Coronavirus | 150 | 50 | 98.0% (95%CI= 93.0–99.5) | 50 | 82 |
| Xia et al.10 | 3–17years | Randomized controlled trial | China | BBIBP-CorV | 810 | 90 | 100% | 53 | 229 |
Fig. 1.
Forest plot of Vaccine efficacy (95%CI), njection-site pain, and adverse reactions.
In conclusion, our research shows that the current COVID-19 vaccine for children is effective and safe, and is more effective than the adult COVID-19 vaccine. There is a gap between the effectiveness of attenuated and inactivated vaccines, and the effectiveness of inactivated vaccines is the highest. We found that the inactivated vaccine is less painful at the injection site and is more friendly to children and adolescents. More carefully designed further studies based on risk factor adjusted estimates are necessary to confirm our findings.
Funding
This study was supported by Hangzhou Science and Technology Bureau fund (No. 20191203B96;No. 20191203B105;No. 20191231Y039); Youth Fund of Zhejiang Academy of Medical Sciences (No. 2019Y009); Medical and Technology Project of Zhejiang Province (No. 2020362651, No. 2021KY890); Clinical Research Fund of Zhejiang Medical Association (No. 2020ZYC-A13); Hangzhou Health and Family Planning Technology Plan Key Projects (No.2017ZD02); Hangzhou Medical and Health Technology Project (No. 0020290592). Zhejiang Traditional Chinese Medicine Scientific Research Fund Project (No.2022ZB280).
Data sharing statement
All the data and materials mentioned in the manuscript are available.
Ethics approval and consent to participate
This study was approved by the ethics committee of Affiliated Hospital of Hangzhou Normal University. This study was carried out according to the Declaration of Helsinki.
Declaration of Competing Interest
The authors declare no competing interests.
Acknowledgment
The work was supported by the Key medical disciplines of Hangzhou.
References
- 1.Chappell H., Patel R., Driessens C., Tarr A.W., Irving W.L., Tighe P.J., et al. Immunocompromised children and young people are at no increased risk of severe COVID-19. J Infect. 2022;84(1):31–39. doi: 10.1016/j.jinf.2021.11.005. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walter E.B., Talaat K.R., Sabharwal C., Gurtman A., Lockhart S., Paulsen G., et al. Evaluation of the BNT162b2 COVID-19 vaccine in children 5 to 11 years of age. N Engl J Med. 2021 doi: 10.1056/NEJMoa2116298. Nov 9NEJMoa2116298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frenck R.W., Klein N.P., Kitchin N., Gurtman A., Absalon J., Lockhart S., Perez J.L., et al. Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. N Engl J Med. 2021;385(3):239–250. doi: 10.1056/NEJMoa2034577. Jul 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olson S.M., Newhams M.M., Halasa N.B., Price A.M., Boom J.A., Sahni L.C., et al. Effectiveness of Pfizer-BioNTech mRNA vaccination against COVID-19 hospitalization among persons aged 12–18 years — United States,June–September 2021. MMWR Morb Mortal Wkly Rep. 2021;70(42):1483–1488. doi: 10.15585/mmwr.mm7042e1. Oct 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hause A.N., Gee J., Baggs J., Abara W.E., Marquez P., Thompson D., et al. COVID-19 vaccine safety in adolescents aged 12-17 years - United States, December 14, 2020-July 16, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(31):1053–1058. doi: 10.15585/mmwr.mm7031e1. Aug 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freedman A.G., Hershkovitz Y., Kaufman Z., Dichtiar R., Keinan-Boker L., Bromberg M. Effectiveness of BNT162b2 vaccine in adolescents during outbreak of SARS-CoV-2 delta variant infection, Israel, 2021. Emerg Infect Dis. 2021;27(11):2919–2922. doi: 10.3201/eid2711.211886. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ali K., Berman G., Zhou H.H., Deng W.P., Faughnan V., Voges M.C. Evaluation of mRNA-1273 SARS-CoV-2vaccine in adolescents. N Engl J Med. 2021;385(24):2241–2251. doi: 10.1056/NEJMoa2109522. Dec 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han B.H., Song Y.F., Li C.G., Yang W.Q., Ma Q.X., Jiang Z.W., et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy children and adolescents: a double-blind, randomised, controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(12):1645–1653. doi: 10.1016/S1473-3099(20)30831-8. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu F.C., Jin P.F., Zhu T., Wang W.J., Ye H.Y., Pan H.X., et al. Safety and immunogenicity of a recombinant adenovirus type-5-vectored COVID-19 vaccine with a homologous prime-boost regimen in healthy participants aged 6 years and above: a randomised, double-blind, placebo-controlled, phase 2b trial. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab845. Sep 22ciab845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xia S.L., Zhang Y.T., Wang Y.X., Wang H., Yang Y.K., Gao G.F., et al. Safety and immunogenicity of an inactivated COVID-19 vaccine, BBIBP-CorV, in people younger than 18 years: a randomised, double-blind, controlled, phase 1/2 trial. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00462-X. Sep 15S1473-3099(21)00462-X. [DOI] [PMC free article] [PubMed] [Google Scholar]