Abstract
Purpose:
This guideline reviews the evidence and provides recommendations for the indications and appropriate techniques of radiation therapy (RT) in the treatment of nonmetastatic cervical cancer.
Methods:
The American Society for Radiation Oncology convened a task force to address 5 key questions focused on the use of RT in definitive and postoperative management of cervical cancer. These questions included the indications for postoperative and definitive RT, the use of chemotherapy in sequence or concurrent with RT, the use of intensity modulated radiation therapy (IMRT), and the indications and techniques of brachytherapy. Recommendations were based on a systematic literature review and created using a predefined consensus-building methodology and system for grading evidence quality and recommendation strength.
Results:
The guideline recommends postoperative RT for those with intermediate risk factors, and chemoradiation for those with high-risk factors. In the definitive setting, chemoradiation is recommended for stages IB3-IVA, and RT or chemoradiation is conditionally recommended for stages IA1-IB2 if medically inoperable. IMRT is recommended for postoperative RT and conditionally recommended for definitive RT, for the purposes of reducing acute and late toxicity. Brachytherapy is strongly recommended for all women receiving definitive RT, and several recommendations are made for target dose and fractionation, the use of intraoperative imaging, volume-based planning, and recommendations for doses limits for organs at risk.
Conclusions:
There is strong evidence supporting the use of RT with or without chemotherapy in both definitive and postoperative settings. Brachytherapy is an essential part of definitive management and volumetric planning is recommended. IMRT may be used for the reduction of acute and late toxicity. The use of radiation remains an essential component for women with cervical cancer to achieve cure.
Introduction
Despite improvements in screening and prevention, cervical cancer remains a significant cause of morbidity and mortality. In the last 2 decades there have been notable advances in surgical procedures, external radiation therapy (RT), brachytherapy techniques, and chemotherapy.
Methods
Task Force Composition
The task force consisted of a multidisciplinary team of radiation oncologists; a gynecologic oncologist, medical oncologist, radiation oncology resident, and medical physicist; and a patient representative. This guideline was developed in collaboration with the American Brachytherapy Society, American Society of Clinical Oncology and the Society of Gynecologic Oncology, who provided representatives and peer reviewers.
Document Review and Approval
The guideline was reviewed by 20 official peer reviewers and revised accordingly. The modified guideline was posted on the ASTRO website for public comment in November 2019. The final guideline was approved by the ASTRO Board of Directors and endorsed by the American Brachytherapy Society, Canadian Association of Radiation Oncology, European Society for Radiotherapy and Oncology, Royal Australian and New Zealand College of Radiologists, and the Society of Gynecologic Oncology.
Evidence Review
A systematic search of human subject studies retrieved from the database Ovid MEDLINE was conducted. The inclusion criteria required research to involve adult women (age ≥18 years), who had received a diagnosis of cervical cancer, published in English, from January 1993 through October 2018, and RT delivered with curative intent. The literature review excluded studies with ≤50 participants; those focused on diagnostic methods; preclinical studies, health economics and cost analyses, comments and editorials; those focused on metastatic disease or recurrent disease, or otherwise not relevant to the scope of the guideline. Because different qualities of evidence were available for each KQ, inclusion criteria were further refined as follows: KQ1 was limited to meta-analyses and randomized controlled trials (RCTs); KQ2 to meta-analyses, RCTs, and prospective nonrandomized trials; and KQs 3, 4, and 5 to meta-analyses, RCTs, prospective nonrandomized trials, and retrospective studies (N≥100). For subquestions with limited data, retrospective study results and expert opinion were relied on to support recommendations as reflected in the low-to-moderate quality of evidence cited in these cases.
All supplementary materials, including the full-text guideline and evidence tables (which summarize the data used to formulate recommendations), are available at https://doi.org/10.1016/j.prro.2020.04.002. The full-text guideline also includes Figure 1 which is the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram showing the number of articles screened, excluded and included in the evidence review; and Appendix 1 (peer reviewer’s disclosure information); Appendix 2 (list of abbreviations) and Appendix 3 (literature search strategy).
Figure 1.
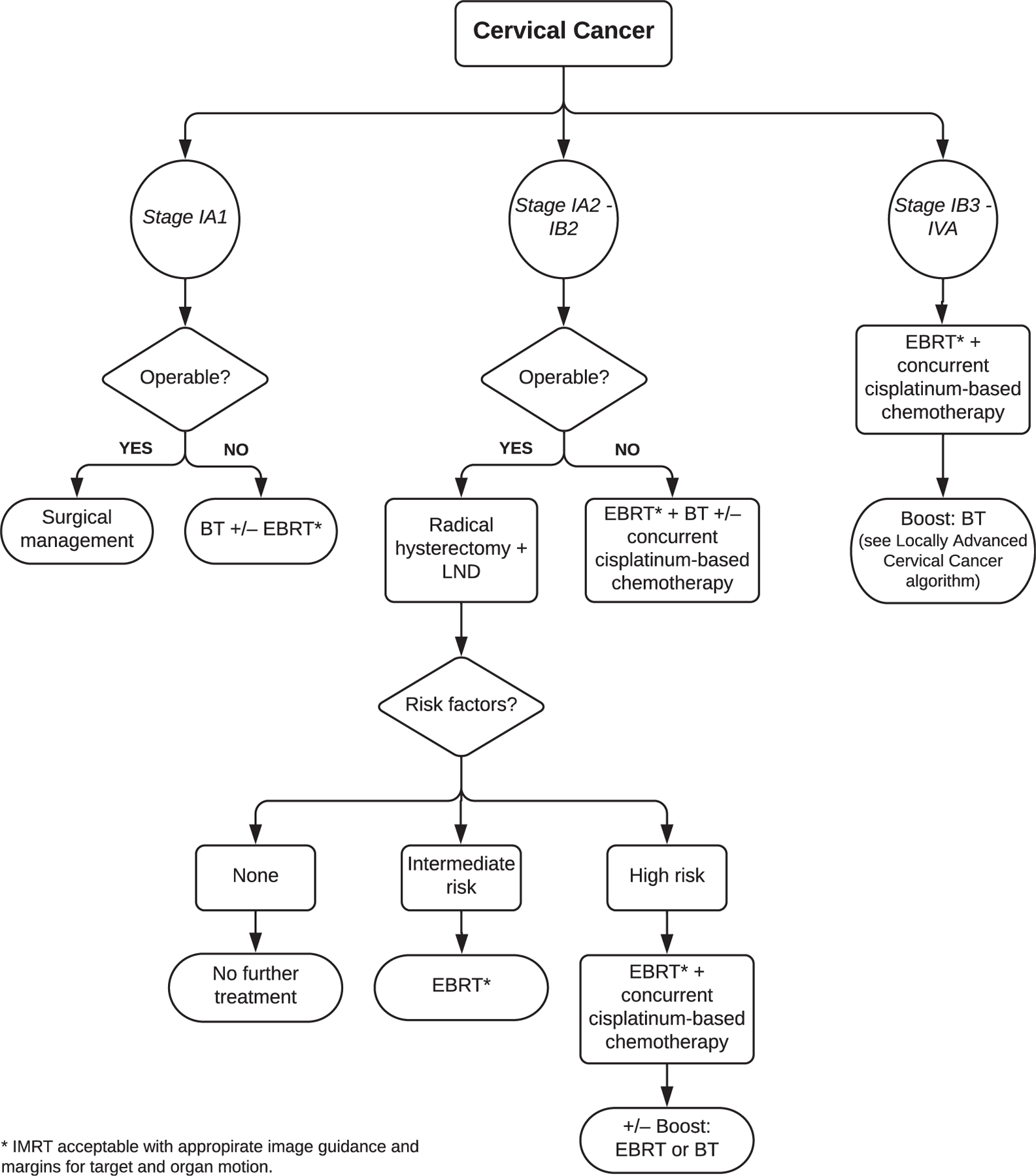
Cervical cancer algorithm. Abbreviations: BT = brachytherapy; EBRT = external beam radiation therapy; IMRT = intensity modulated radiation therapy; LND = lymph node dissection; RT = radiation therapy.
Scope of the Guideline
This guideline covers only the subjects specified in the 5 KQs (see Table 2 in the full-text guideline for KQs and outcomes of interest). The scope is limited to curative management of invasive carcinomas of the uterine cervix, which include squamous cell carcinomas and adenocarcinomas, and does not include rare histologies, noninvasive disease or palliative treatment. It focuses on management of cervical cancer with RT and its indications, techniques, and outcomes. It additionally covers other therapies that modify the efficacy of RT when used concurrently or in sequence (eg, chemotherapy or surgery).
High-risk surgicopathologic findings
The evidence is strong that adjuvant concurrent cisplatin-based chemoradiation improves overall survival and progression-free survival for patients with cervical cancer who have high-risk pathologic features after radical hysterectomy (eg, positive margins or positive lymph nodes or extension into the parametrial tissue)1; the benefit of chemoradiation compared with RT alone is similar to the benefit observed for locally advanced patients with cervical cancer who undergo definitive chemoradiation compared with RT alone.8 For cases meeting these high-risk criteria, whole pelvic RT can be delivered to a total dose of 4500 to 5040 cGy, in 180 cGy fractions, with concurrent weekly cisplatin (40 mg/m2).
Intermediate-risk surgicopathologic findings
The Gynecologic Oncology Group (GOG) conducted an RCT (GOG 92) of 277 patients with cervical cancer (including both squamous cell and adenocarcinomas) treated by radical hysterectomy and intermediate-risk Sedlis criteria who were randomized to no further treatment versus adjuvant pelvic RT.9 Adjuvant radiation was associated with a 47% reduction in recurrence (a 12.6% absolute reduction) with acceptable morbidity and a 6% versus 2% grade 3 or 4 adverse event rate.5 A 2012 meta-analysis, which included data from GOG 92, further supports the benefit of adjuvant RT for those with intermediate-risk factors, with a significantly lower risk of disease progression at 5 years.6 For cases meeting these intermediate-risk criteria, whole pelvic RT can be delivered to a total dose of 4500 to 5040 cGy, in 180 cGy per fraction or 4000 to 4400 cGy in 200 cGy per fraction.5
Occult cervical cancer after total hysterectomy
For women who are found to have an occult invasive cervical cancer after total hysterectomy (either for benign disease or uterine cancer), further treatment is needed for stages greater than or equal to IA2 because a radical hysterectomy with lymph node dissection is required for curative surgery in these cases.10 Options would be additional surgery (a parametrectomy, upper vaginectomy, and lymph node dissection) or RT. In practice, if additional surgery is expected to be technically difficult and/or potentially morbid, RT or chemoradiation may be offered as an alternative, particularly if RT is already indicated from surgicopathologic findings. Although prospective evidence is lacking, pelvic RT to 4500 to 5040 cGy, followed by a boost to the sites at high risk of additional occult disease (either with vaginal brachytherapy or external beam radiation therapy (EBRT) depending on location) is a reasonable approach. Concurrent chemotherapy may also be considered depending on factors described earlier in this section.
Integration of chemotherapy with radiation
Multiple RCTs demonstrate an approximately 10% survival benefit at 5 years for radiation with concurrent platinum-based chemotherapy compared with RT alone for women with stage IB3-IVA cervical cancer.8 If treatment of the extended field is indicated, concurrent chemotherapy with cisplatin is administered with appropriate symptom management, consideration of intensity modulated radiation therapy (IMRT; refer to KQ3) to spare bowel; close monitoring of laboratory tests with special attention to assess neutropenia, anemia, and thrombocytopenia; and a potential need to stop chemotherapy before the completion of 5 cycles.22,23
For definitive RT, whole pelvic RT or extended field RT can be delivered to a total dose of 4500 to 5040 cGy, in 180 cGy fractions, with concurrent weekly cisplatin (40 mg/m2). Additional nodal boosts may be included (described in KQ3). This is followed by brachytherapy (described in KQ4 and KQ5), with a goal to limit the total treatment time to ≤7 to 8 weeks.
Hysterectomy after radiation
In the era of combined chemoradiation and image guided brachytherapy (IGBT), pelvic control is very high even for women with bulky stage IB3-IIB cervical cancer. Therefore adjuvant hysterectomy after radiation is not routinely recommended, particularly when IGBT is available. When a lower dose of brachytherapy is given and IGBT is not available, hysterectomy may be considered, especially in the presence of cervix-confined residual disease.
Despite high rates of local control, a small percentage of cancers do not respond well to chemoradiation and have evidence of residual disease after treatment. Time should be allowed for delayed response, with consideration of positron emission tomography imaging approximately 3 months after treatment completion.24 However, if recurrence and/or persistence of disease is confirmed by biopsy as early as 8 to 12 weeks after therapy, there may be a role for salvage hysterectomy or exenteration, if feasible, to improve local control and survival, at the risk of significant morbidity.25
In the treatment of postoperative and definitive cervical cancer, dosimetric studies of IMRT have demonstrated decreased volumes of the bladder, rectum, bowel, and bone marrow receiving clinically significant doses of RT.26,29,31,33 Single and multi-institution series of postoperative RT have demonstrated a favorable toxicity profile with the use of IMRT.32,39 RTOG 1203 (TIME-C) is the only published phase III RCT of 3-dimensional (3-D) RT versus IMRT in the postoperative treatment of patients with early-stage endometrial or cervical cancer.27 This study demonstrated significantly improved acute patient-reported gastrointestinal (primary endpoint) and urinary outcomes, thus supporting the use of IMRT, when available, in these populations.27
Similarly, retrospective comparisons of 2-dimensional (2-D) and 3-D RT to IMRT found decreased acute and chronic toxicities with use of IMRT during the pelvic/para-aortic phase of definitive RT.29,30,36,38 Three prospective randomized trials and one meta-analysis collectively demonstrate decreased acute and late gastrointestinal and urinary toxicities with IMRT compared with 3-D RT.31,33,34,38 IMRT for irradiation of the para-aortic nodal chain is also likely to decrease risk of toxicities compared with 2-D/3-D RT while allowing dose escalation to intact positive nodes, especially for patients receiving concurrent chemotherapy.22,23,40–45 There are, however, no data that IMRT improves disease-specific survival or overall survival over 2-D/3-D techniques. Utmost care must be taken to account for target motion both at time of simulation and throughout treatment with the use of image guidance (Figure 2). Refer to the full-text guideline for a more in-depth discussion of these issues.
Figure 2.
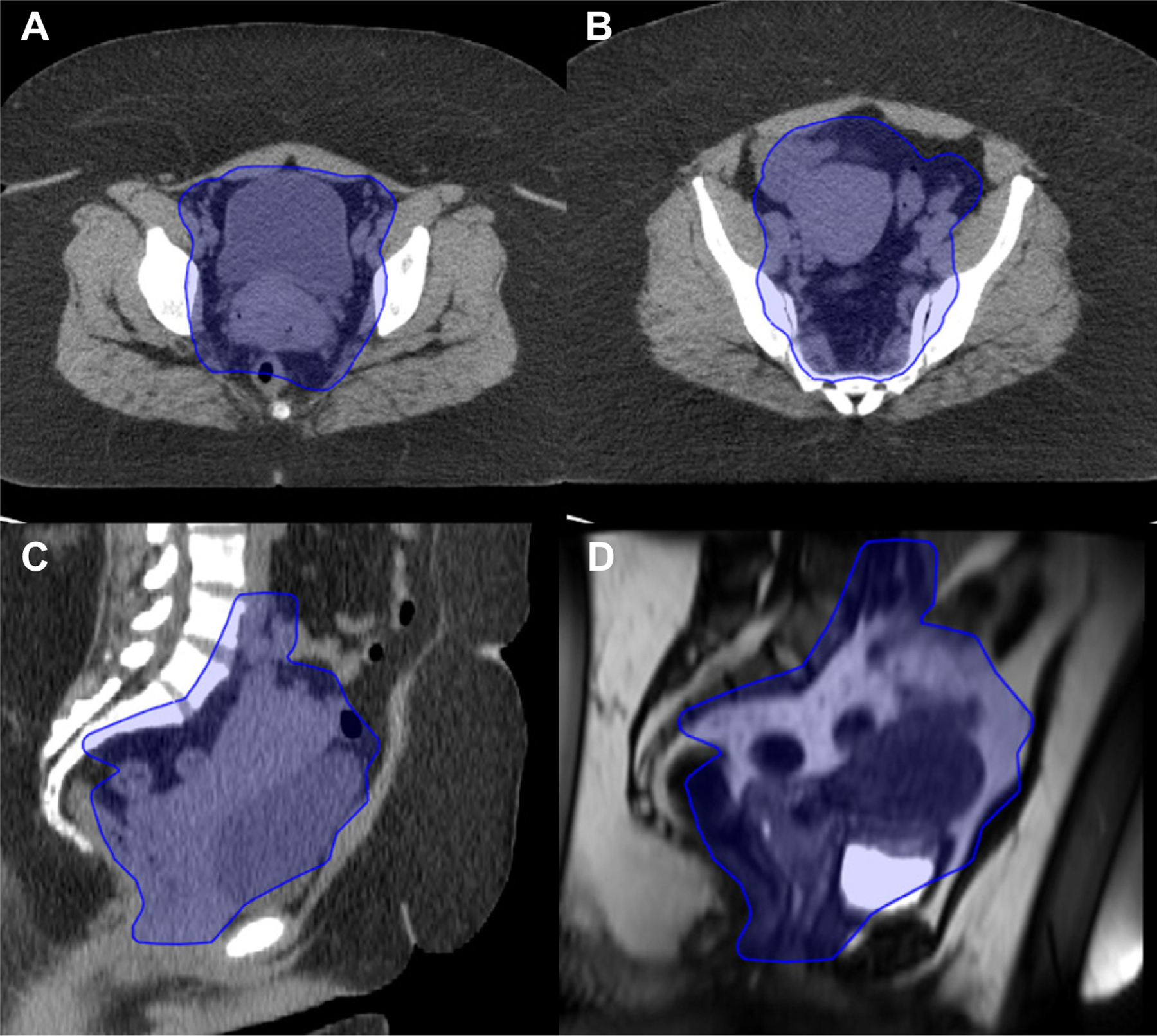
Example of IMRT PTV definition for intact cervical cancer. A and B axial CT images; C sagittal CT images; and D sagtital MRI images, showing uterine motion; refer to the full-text guideline for a detailed description of PTV definitions. Abbreviations: CT = computed tomography; IMRT = intensity modulated radiation therapy; MRI = magnetic resonance imaging; PTV = planning target volume.
In this example case of stage IIB cervical cancer, a final PTV for 45 Gy can be seen in the blue shaded contour. The PTV includes the primary CTV of the cervix and uterus, proximal vagina, paracervical tissue, parametrial tissue including uterosacral ligaments, and pelvic nodal basins with additional margins for daily setup variation and internal target motion. The PA nodes are not included in this case because of the absence of any concerning nodes in the pelvis or PA chain on PET imaging; thus the superior border is set at the level of the aortic bifurcation (approximately L4–5) and inferiorly into the vagina, to 4 cm distal to extent of disease. At the level of the acetabulum (A), note the anterior extension of the PTV well into the bladder as a result of significant variation in uterine position. Also note posterior extension of the PTV in the rectum to allow for coverage of the uterosacral ligaments and motion of the cervix and the presacral lymph nodes; coverage of the mesorectum may be required in some cases with rectal invasion or posterior uterosacral ligament involvement. At the level of S3 (B), note the extension of the PTV posteriorly to allow for coverage of the uterosacral ligaments. Midsagittal CT (C) and MRI (D) obtained on the same day show significant motion of the uterus with partial bladder emptying. The PTV encompasses this entire excursion of the uterine body (may be several centimeters), with additional margin for daily setup. The use of regular image guidance at the time of treatment is necessary to ensure all targets remain within the PTV, and replanning may be necessary if the PTV margin is found to be too small. This is provided as an example of a large PTV rather than a recommended volume for all cases; reference to the appropriate contouring atlases is indicated for each individual considered for IMRT.
IMRT may also be used to boost selective sites of nodal involvement. The dose required is dependent on the size of the grossly involved node. Generally, between 5500 to 6500 cGy is delivered to involved nodes based on size, location, contribution from brachytherapy, and dose per fraction.46
Brachytherapy is an integral component of definitive treatment for patients with locally advanced cervical cancer. Results from national databases have consistently found improved outcomes using brachytherapy.47,48 In multiple large national retrospective data sets, the use of brachytherapy in women with cervical cancer declined between 2003 to 2011, whereas use of IMRT or SBRT instead increased during this period.47,48 The use of brachytherapy has been consistently associated with improved survival compared with IMRT or SBRT as a boost. The omission of brachytherapy has a stronger negative effect on survival than the exclusion of chemotherapy.48 Other smaller retrospective studies show similar results with improved survival in patients treated with brachytherapy compared with nonbrachytherapy cohorts.49–51 Therefore neither SBRT nor IMRT are a suitable substitute for brachytherapy and should only be considered for those ineligible because of complex medical factors. Referral to tertiary centers for brachytherapy is necessary if the originating facility has a limited capacity to support a patient with complex comorbidities. Previous 2-D prospective cohort studies found high control rates and acceptable toxicities, though these have improved further with 3-D IGBT techniques.8,11–14,17,18,53–65 Prospective and retrospective cohort data of 3-Debased planning for brachytherapy indicates high rates of cervical control and decreased toxicity, so it is emerging as standard practice in many centers.56,58,60,62,63,66
Brachytherapy may be considered in the postoperative setting in the presence of a positive vaginal mucosal margin. For positive margins beyond the vaginal mucosa surface (ie, parametrial, paravaginal) or positive macroscopic margins, an advanced brachytherapy technique (eg, an intracavitary multichannel cylinder) or interstitial needles may be required to adequately deliver conformal doses to the areas at risk. For regions at risk not amenable to brachytherapy, a targeted external beam boost may be considered.
Definitions for volume-based targets were established by the GEC-ESTRO (Groupe Européen de Curiethérapie–European Society for Radiotherapy & Oncology),85 including the gross tumor volume, high-risk clinical target volume, and intermediate-risk clinical target volume (Table 8). Validation of these target concepts comes from multiple retrospective and prospective series. One of the largest of these studies, retroEMBRACE, found that women treated with IGBT had improved local control, reduced toxicity, and an altered pattern of relapse relative to 2-D brachytherapy.56,62,64,66,72,86 Aside from improved local control rates, there is also prospective, though nonrandomized, data indicating significantly reduced grade 3 to 4 toxicities in 3-D versus 2-D planned patients treated with chemoradiation for locally advanced disease (2.6% versus 22.7%, P < .002).63 Taken together these studies support improved outcomes and reduced toxicities when using an image-based brachytherapy approach.
Table 8.
Target volume definitions for image guided brachytherapy81
| Volume | Components | Dose goals |
|---|---|---|
| GTV | Gross tumor at the time of brachytherapy, determined by imaging or examination | At a minimum, dose should be ≥8000 cGy |
| HR-CTV | GTV, the entire cervix, and regions of indeterminate T2-weighted MRI signal (ie, gray zones) | D90 ≥8000 cGy, with consideration of escalation for advanced disease or poor response to initial therapy |
| IR-CTV | HR-CTV with an asymmetrical expansion,* not extending into OARs, and including sites of initial disease involvement | Optional: D90 ≥6000 cGy, with consideration of escalation for advanced disease |
Abbreviations: GTV = gross tumor volume; HR-CTV = high-risk target volume; IR-CTV = intermediate-risk target volume; MRI = magnetic resonance imaging; OARs = organs at risk.
The IR-CTV expansion is 0.5–1.0 cm globally with an additional 0.5 cm superiorly into the uterus, inferiorly into the vagina, and laterally in bilateral paracervical tissues.
Standard tandem and ovoid/ring/mold applicators may not always adequately cover the residual extent of disease after EBRT or allow for optimal sparing of the surrounding organs at risk (OARs). Supplemental interstitial needles in addition to standard tandem and ovoid or tandem and ring applicators may help optimize dose distributions by allowing higher doses to targets, while still meeting normal OAR constraints.87
Intraoperative imaging to evaluate the applicator placement should be performed. Real-time guidance with either transabdominal or transrectal ultrasound is easy to obtain and can reduce the risk of uterine perforation.67
Magnetic resonance imaging (MRI) and computed tomography (CT) are standard for brachytherapy treatment planning. MRI, however, provides superior soft tissue definition, making it easier to visualize the cervix and residual disease compared with CT imaging. Comparisons of MRI versus CT-based planning reveal similar OAR doses, but CT may overestimate the tumor width compared with MRI, particularly in advanced disease.69,88
The combined prescription dose calculation to an equivalent dose of 2 Gy with an α-to-β ratio of 10 (EQD210) of EBRT and brachytherapy should be ≥8000 to 8500 cGy, with doses ≥8500 cGy for tumors with poor response to EBRT or adenocarcinoma histology or for stage III disease at presentation. Suggested brachytherapy doses in combination with EBRT are listed in Table 9 in the full-text guideline.
For cervical cancer brachytherapy, OARs include the bladder, rectum, sigmoid/bowel, and vagina. The dose volume–effect relationships for predicting late rectal morbidity indicate a threshold rectal D2cc be kept to ≤ 6500 cGy.58 In regard to high-grade toxicity, the fistula risk was 12.5% at 3 years for patients who received a D2cc dose ≥7500 cGy compared with 0 to 2.7% for patients receiving lower doses. Single-institution data suggest limiting the bladder D2cc to ≤8000 cGy.82 The EMBRACE study also shows that vaginal stenosis is correlated with the dose to the rectovaginal point (20% at 6500 cGy, 27% at 7500 cGy, and 34% at 8500 cGy) and proposes that this point be kept to ≤6500 cGy.59,60 Although OAR sparing is expected to improve quality of life for many women, control of the cervical tumor continues to be of primary importance. In situations in which OAR constraints cannot be met despite best efforts, tumor coverage may be prioritized after careful discussion with the patient.
Conclusions
Radiation is an integral part of the management of locally advanced disease, either as an adjuvant treatment after surgery in the presence of risk factors or as a primary curative treatment, used in combination with chemotherapy and a brachytherapy boost to the primary site. IMRT and IGBT are effective at reducing normal tissue toxicity and allow for dose escalation to residual disease in the central pelvis (in the case of brachytherapy) or positive nodes (in the case of IMRT). All these factors have resulted in safer and more effective treatment for women with this disease.
Supplementary Material
Figure 3.
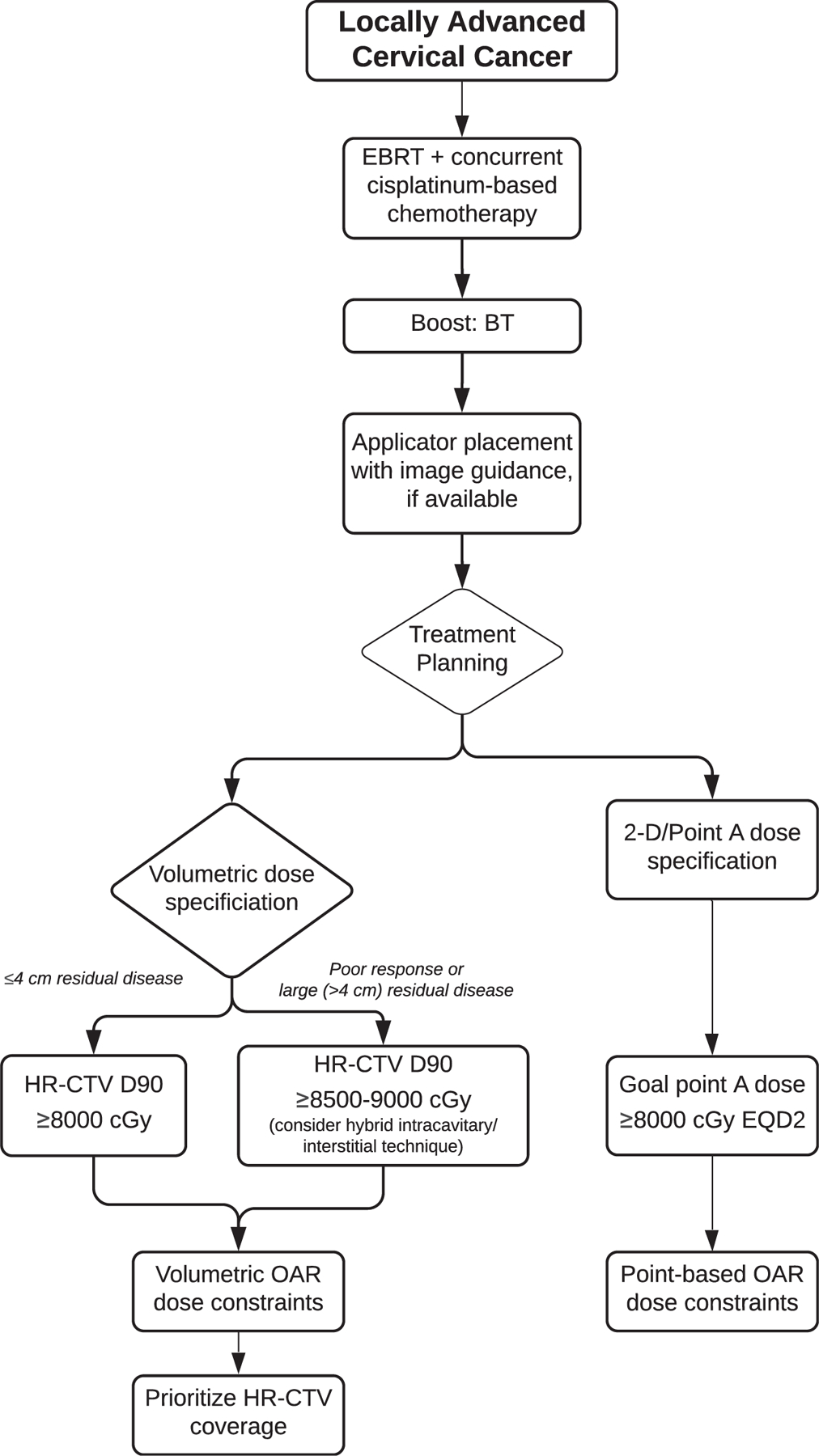
Locally advanced cervical cancer algorithm. Abbreviations: 2-D = 2-dimensional; BT = brachytherapy; EBRT = external beam radiation therapy; EQD2 = equivalent dose at 2 Gy per fraction; HR-CTV = high-risk clinical target volume; OAR = organ at risk.
Table 1.
ASTRO recommendation grading classification system
ASTRO’s recommendations are based on evaluation of multiple factors including the QoE, individual study quality, and panel consensus, all of which inform the strength of recommendation.
| Strength of Recommendation | Definition | Overall QoE Grade | Recommendation Wording |
|---|---|---|---|
| Strong | ● Benefits clearly outweigh risks and burden, or risks and burden clearly outweigh benefits. ● All or almost all informed people would make the recommended choice. |
Any (usually high, moderate, or expert opinion) | “Recommend/Should” |
| Conditional | ● Benefits are finely balanced with risks and burden or appreciable uncertainty exists about the magnitude of benefits and risks. | Any (usually moderate, low, or expert opinion) | “Conditionally Recommend” |
| ● Most informed people would choose the recommended course of action, but a substantial number would not. | |||
| ● Ashared decision-making approach regarding patient values and preferences is particularly important. | |||
| Overall QoE Grade | Type/Quality of Study | Evidence Interpretation | |
|
| |||
| High | ● 2 or more well-conducted and highly generalizable RCTs or meta-analyses of such trials. | The true effect is very likely to lie close to the estimate of the effect based on the body of evidence. | |
| Moderate | ● 1 well-conducted and highly generalizable RCT or a meta-analysis of such trials OR | The true effect is likely to be close to the estimate of the effect based on the body of evidence, but it is possible that it is substantially different. | |
| ● 2 or more RCTs with some weaknesses of procedure or generalizability OR | |||
| ● 2 ormore strong observational studies with consistent findings. | |||
| Low | ● 1 RCT with some weaknesses of procedure or generalizability OR | The true effect may be substantially different from the estimate of the effect. There is a risk that future research may significantly alter the estimate of the effect size or the interpretation of the results. | |
| ● 1 or more RCTs with serious deficiencies of procedure or generalizability or extremely small sample sizes OR | |||
| ● 2 or more observational studies with inconsistent findings, small sample sizes, or other problems that potentially confound interpretation of data. | |||
| Expert Opinion* | ● Consensus of the panel based on clinical judgment and experience, due to absence of evidence or limitations in evidence. | Strong consensus (≥90%) of the panel guides the recommendation despite insufficient evidence to discern the true magnitude and direction of the net effect. Further research may better inform the topic. | |
Abbreviations: ASTRO = American Society for Radiation Oncology; QoE = quality of evidence; RCTs = randomized controlled trials.
A lower quality of evidence, including expert opinion, does not imply that the recommendation is conditional. Many important clinical questions addressed in guidelines do not lend themselves to clinical trials, but there still may be consensus that the benefits of a treatment or diagnostic test clearly outweigh its risks and burden.
Table 2.
Recommendations for postoperative RT with or without systemic therapy
| KQ1 Recommendations | Strength of Recommendation | Quality of Evidence (Refs) |
|---|---|---|
| 1. For women undergoing surgery for cervical cancer who have high surgicopathologic risk factors, adjuvant EBRT and concurrent platinum-based chemotherapy are recommended. Implementation remark: High-risk factors include positive margin(s) or positive lymph node(s) or extension into the parametrial tissue. |
Strong | High 1−4 |
| 2. For women with cervical cancer and intermediate-risk factors, adjuvant EBRT is recommended to decrease locoregional recurrence. Implementation remark: Intermediate-risk factors include*: ● LVSI plus deep one-third cervical stromal invasion with any tumor size ● LVSI plus middle one-third stromal invasion and tumor size ≥2 cm ● LVSI plus superficial one-third stromal invasion and tumor size ≥5 cm ● No LVSI but deep or middle one-third stromal invasion plus tumor size ≥4 cm |
Strong | High 5−7 |
Abbreviations: EBRT = external beam radiation therapy; LVSI = lymphovascular space involvement; RT Z radiation therapy.
The original Gynecologic Oncology Group (GOG) 92 protocol estimated tumor size based on palpation; however, estimation based on pathologic or magnetic resonance imaging findings are an acceptable substitute.
Table 3.
Recommendations for definitive RT with and without systemic therapy and hysterectomy after RT
| KQ2 Recommendations | Strength of Recommendation | Quality of Evidence (Refs) |
|---|---|---|
| 1. For women with FIGO stage IB3-IVA* squamous cell or adenocarcinoma of the cervix, RT with concurrent platinum-based chemotherapy is recommended for definitive treatment. Implementation remark: Recommended dose for cisplatin is 40 mg/m2 weekly for 5–6 cycles. |
Strong | High 8,11−18 |
| 2. For women with FIGO stage IB3-IVA cervical cancer, a planned adjuvant hysterectomy after RT or chemoradiation is not recommended.† | Strong | High 13,19−21 |
| 3. In women with FIGO stage IA1-IB2 that are deemed medically inoperable, RT with or without chemotherapy is conditionally recommended. | Conditional | Expert Opinion |
Abbreviation: FIGO = International Federation of Gynecology and Obstetrics; RT = radiation therapy.
Stage IIA1 cancers may be managed with radical hysterectomy in well-selected (eg, nonbulky, with limited vaginal involvement) cases.
In the setting of biopsy-proven gross residual disease after point-A–based dose specification for brachytherapy, surgery may be an option.
Table 4.
Recommendations for IMRT
| KQ3 Recommendations | Strength of Recommendation | Quality of Evidence (Refs) |
|---|---|---|
| 1. In women with cervical cancer treated with postoperative RT with or without chemotherapy, IMRT is recommended to decrease acute and chronic toxicity. | Strong | Moderate (acute) 26,27 Low (chronic) 26,28 |
| 2. In women with cervical cancer treated with definitive RT with or without chemotherapy, IMRT is conditionally recommended to decrease acute and chronic toxicity. | Conditional | Moderate (acute) 29−34 Moderate (chronic) 29,31,35−38 |
Abbreviations: IMRT = intensity modulated radiation therapy; RT = radiation therapy.
Table 5.
Recommendations for brachytherapy
| KQ4 Recommendations | Strength of Recommendation | Quality of Evidence (Refs) |
|---|---|---|
| 1. For women receiving definitiveRT for intact cervical cancer, brachytherapy is recommended. | Strong | Moderate 47–51 |
| 2. For women with cervical cancer receiving postoperative whole pelvis radiation, a brachytherapy boost is conditionally recommended in the presence of positive margin(s). Implementation remark: The brachytherapy technique selected is based on the location and volume of the positive margin(s). |
Conditional | Low 52 |
Abbreviation: RT = radiation therapy.
Table 6.
Recommendations for brachytherapy technique
| KQ5 Recommendations | Strength of Recommendation | Quality of Evidence (Refs) |
|---|---|---|
| Optimal imaging and technique for the delivery of brachytherapy | ||
| 1. For women receiving brachytherapy for cervical cancer, intra-procedure imaging is recommended if available. |
Strong | Low 67 |
| 2. For women receiving brachytherapy for cervical cancer, MRI or CT-based planning to a volume-based prescription is recommended. |
Strong | Moderate 56,62,63,68−72 |
| 3. For women receiving brachytherapy for cervical cancer, if volume-based planning cannot be performed, then 2-D/point-based planning is rec006Fmmended. |
Strong | Moderate 8,11–13,73 |
| Optimal dose/fractionation schedule for the delivery of brachytherapy | ||
| 4. For women treated with definitive RT for cervical cancer, the total EQD210 of EBRT and brachytherapy should be ≥8000 cGy. (Table 9 in the full-text guideline) |
Strong | Moderate 56,74 |
| 5. For women with cervical cancer receiving volume-based brachytherapy, HR-CTV D90 greater than or equal to prescription dose (≥8000 cGy) is conditionally recommended, with careful consideration of normal tissue constraints. (Table 7) |
||
|
Implementation remark: ● For patients with poor response or large-volume (>4 cm) disease, D90 ≥8500 cGy is reasonable. ● Utilization of a hybrid intracavitary/interstitial technique can help improve the dose distribution when not achieving appropriate target and/or OAR dose constraints with an intracavitary alone approach. |
Conditional | Moderate 57,75−77 |
| Optimal OAR constraints of brachytherapy | ||
| 6. In women treated with brachytherapy for intact cervical cancer, volumetric contouring of the OARs and use of appropriate dose constraints are recommended. | Strong | Moderate 60,63,72,77−79 |
| 7. If volumetric planning is not available for women treated with brachytherapy for intact cervical cancer, 2-D/point-based dose constraints should be applied. | Strong | Moderate 8,11−13 |
Abbreviations: 2-D = 2-dimensional; CT = computed tomography; EBRT = external beam radiation therapy; EQD210 = dose calculation to an equivalent dose of 2 Gy with an α-to-β ratio of 10; HR-CTV = high-risk clinical target volume; MRI = magnetic resonance imaging; OARs = organs at risk; RT = radiation therapy.
Table 7.
Dose constraints
| Organ at risk | Ideal dose constraint (cGy) (EQD23) | Maximum* dose constraint (cGy) (EQD23) | ICRU point (cGy) (EQD23) | References |
|---|---|---|---|---|
| Rectum | <6500 D2cc | <7500 D2cc | <7500 point dose | 58,65,78,80,81 |
| Bladder | <8000 D2cc | <9000 D2cc | <9000 point dose | 78,80−82 |
| Vagina (recto-vaginal point)† | <6500 point dose | <7500 point dose | – | 59,60 |
| Sigmoid‡ | <7000 D2cc | <7500 D2cc | – | 83 |
| Bowel‡ | <7000 D2cc | <7500 D2cc† | – | 83,84 |
Abbreviations: ICRU = International Commission of Radiation Units and Measurements; EQD23 = dose calculation to an equivalent dose of 2 Gy with an α-to-β ratio of 3. D2cc is the minimal dose to the 2 cm3 (2 mL) of the organ at risk receiving the maximal dose.
There will be occasions when exceeding these maximum constraints is necessary to adequately treat the targets of therapy, according to the clinical judgment of the treating physician.
The rectovaginal point is defined 5 mm posterior to the vaginal mucosa from the center of the vaginal sources.
Dose constraints for sigmoid and bowel are based largely on expert opinion because there is minimal evidence of a dose response.
Preamble.
As the leading organization in radiation oncology, the American Society for Radiation Oncology (ASTRO) is dedicated to improving quality of care and patient outcomes. A cornerstone of this goal is the development and dissemination of clinical practice guidelines based on systematic methods to evaluate and classify evidence, combined with a focus on patient-centric care and shared decision making. ASTRO develops and publishes guidelines without commercial support, and members volunteer their time.
Disclosure Policy —
ASTRO has detailed policies and procedures related to disclosure and management of industry relationships to avoid actual, potential, or perceived conflicts of interest. All task force members are required to disclose industry relationships and personal interests from 12 months before initiation of the writing effort. Disclosures go through a rigorous review process with final approval by ASTRO’s Conflict of Interest Review Committee. For the purposes of full transparency, task force members’ comprehensive disclosure information is included in this publication. The complete disclosure policy for formal papers is online.
Selection of Task Force Members —
The Guideline Subcommittee strives to avoid bias by selecting a multidisciplinary group of experts with variation in geographic region, gender, ethnicity, race, practice setting, and areas of expertise. Representatives from organizations and professional societies with related interests and expertise are also invited to serve on the task force.
Methodology —
The task force uses evidence-based methodologies to develop guideline recommendations in accordance with the National Academy of Medicine standards. The evidence identified from key questions (KQs) is assessed using the Population, Intervention, Comparator, Outcome, Timing, Setting (PICOTS) framework. A systematic review of the KQs is completed, which includes creation of evidence tables that summarize the evidence base task force members use to formulate recommendations. Table 1 describes ASTRO’s recommendation grading system.
Consensus Development —
Consensus is evaluated using a modified Delphi approach. Task force members (except for the patient representative) confidentially indicate their level of agreement on each recommendation based on a 5-point Likert scale, from “strongly agree” to “strongly disagree.” A prespecified threshold of ≥75% (≥90% for expert opinion recommendations) of raters who select “strongly agree” or “agree” indicates consensus is achieved. Recommendation(s) that do not meet this threshold are removed or revised. Recommendations edited in response to task force or reviewer comments are resurveyed before submission of the document for approval.
Annual Evaluation and Updates —
Guidelines are evaluated annually beginning 2 years after publication for new potentially practice-changing studies that could result in a guideline update. In addition, the Guideline Subcommittee will commission a replacement or reaffirmation within 5 years of publication.
Full-Text Guideline —
The reader is encouraged to consult the full-text guideline supplement for the supportive text, abbreviations list, and additional information on radiation therapy for cervical cancer because the executive summary contains limited information.
Key Questions and Recommendations.
Key Question 1: Postoperative RT with and without systemic therapy (Table 2)
See evidence tables in supplementary materials for the data supporting the recommendations for KQ1.
Following primary surgery for cervical cancer, when is it appropriate to deliver postoperative RT with or without systemic therapy?
Key Question 2: Definitive RT with and without systemic therapy; hysterectomy after RT (Table 3)
See evidence tables in supplementary materials for the data supporting the recommendations for KQ2 and Figure 1 for a visual representation of the cervical cancer recommendations.
When is it appropriate to deliver definitive RT with and without systemic therapy? When is it appropriate to perform a hysterectomy after RT for cervical cancer?
Key Question 3: Intensity modulated radiation therapy (Table 4)
See evidence tables in supplementary materials for the data supporting the recommendations for KQ3.
For patients receiving definitive or postoperative RT for cervical cancer, when is it appropriate to deliver IMRT?
Key Question 4: Brachytherapy (Table 5)
See evidence tables in supplementary materials for the data supporting the recommendations for KQ4.
For patients receiving definitive or postoperative RT for cervical cancer, when is brachytherapy indicated?
Key Question 5: Brachytherapy technique (Table 6)
See evidence tables in supplementary materials for the data supporting the recommendations for KQ5, and see Figure 3 for a visual representation of the recommendations for locally advanced cervical cancer.
For patients receiving definitive RT for cervical cancer, what is the optimal dose/fractionation schedule, imaging, and technique for the delivery of brachytherapy?
Acknowledgements
We are grateful to Yimin Geng, MSLIS, MS, the University of Texas MD Anderson research medical librarian, for her assistance with creating the search strategy for this guideline. The task force also thanks Elisha Fredman, MD, Sarah Hazell, MD, Blair Murphy, MD, Steven Seyedin, MD, Sarah Stephens, MD, and Michael Stolten, MD, for literature review assistance.
The task force thanks the peer reviewers for their comments and time spent reviewing the guideline. See Appendix 1 in the full-text guideline for their names and disclosures.
Footnotes
Task Force Members’ Disclosure Statements
All task force members’ disclosure statements were rigorously reviewed before being invited and were shared with other task force members throughout the guideline’s development. Those disclosure are published within this report. Where potential conflicts were detected, remedial measures to address them were taken.
Christina Annunziata (American Society of Clinical Oncology representative): MaxCyte, Medivir, and Precision Biologics (research), Horizon Pharma and Merck (provided drugs for clinical trial), BMC Cancer and Frontiers in Oncology (editor); Sushil Beriwal: American Board of Radiology (board examiner), Brachy Journal and iJROBP (editorial board); Eisai, Institute of Education, and Via Oncology (honoraria), International Journal of Radiation Oncology, Biology, Physics (senior editor); Varian (consultant), XOFT (DSMB); Junzo Chino (vice chair): American Board of Radiology (board examiner); NanoScint (stock); International Journal of Radiation Oncology, Biology, Physics (editorial board); Matthew Harkenrider: ACR (program director and trustee), AstraZeneca (advisory board [ended]), International Journal of Radiation Oncology, Biology, Physics (editorial board); Varian (advisory board [ended]); Christine Holschneider (Society of Gynecologic Oncology representative): NRG-GOG and GOG Foundation (research), National Institutes of Health grants (research—family member), UpToDate (honoraria); Mitchell Kamrava: American Board of Radiology (board examiner), Augmenix (speaker’s bureau), Brachytherapy and International Journal of Radiation Oncology, Biology, Physics (editorial board); Lilie Lin: American Board of Radiology (board examiner); AstraZeneca (research); Jyoti Mayadev: AstraZeneca (consultant), NRG GOG Foundation (member), NRG Oncology Cervical Board (cochair), Varian (advisory board); Marc Morcos: Elekta (travel); Daniel Petereit (American Brachytherapy Society representative and President): American Board of Radiology (board examiner), BMS Foundation (research and salary support), Irving A Hansen Memorial Foundation (patient funding), Ralph Lauren Pink Pony Foundation (board member); Akila Viswanathan (chair): NCI Uterine Task force (cochair), American Board of Radiology (board examiner), Brachytherapy and Gynecologic Oncology Journal (editorial board), Springer textbook (chapter editor); Beth Erickson: American Brachytherapy Society (CME cochair); ASTRO (MOC-CME cochair); Brachytherapy and International Journal of Radiation Oncology, Biology, Physics (editorial board), Elekta (research and travel), Springer textbook (chapter editor). Emma Fields, KathrynJane Fitch (patient representative), Eric Leung, and Chika Nwachukwu reported no disclosures.
Disclaimer and Adherence: American Society for Radiation Oncology (ASTRO) guidelines present scientific, health, and safety information and may reflect scientific or medical opinion. They are available to ASTRO members and the public for educational and informational purposes only. Commercial use of any content in this guideline without the prior written consent of ASTRO is strictly prohibited.
Adherence to this guideline does not ensure successful treatment in every situation. This guideline should not be deemed inclusive of all proper methods of care or exclusive of other methods reasonably directed to obtaining the same results. The physician must make the ultimate judgment regarding therapy considering all circumstances presented by the patient. ASTRO assumes no liability for the information, conclusions, and findings contained in its guidelines. This guideline cannot be assumed to apply to the use of these interventions performed in the context of clinical trials. This guideline is based on information available at the time the task force conducted its research and discussions on this topic. There may be new developments that are not reflected in this guideline and that may, over time, be a basis for ASTRO to revisit and update the guideline.
Supplementary materials
Supplementary material for this article can be found at https://doi.org/10.1016/j.prro.2020.04.002.
References
- 1.Peters WA, Liu PY, Barrett RJ, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol 2000;18:1606–1613. [DOI] [PubMed] [Google Scholar]
- 2.Qin AQ, Liang ZG, Ye JX, et al. Significant efficacy of additional concurrent chemotherapy with radiotherapy for postoperative cervical cancer with risk factors: A systematic review and meta-analysis. Asian Pac J Cancer Prev 2016;17:3945–3951. [PubMed] [Google Scholar]
- 3.Yang J, Yin J, Yan G, Huang D, Wang J. Postoperative chemoradiotherapy versus radiotherapy alone for cervical cancer: A systematic review and meta-analysis. J Obstet Gynaecol 2016;36: 641–648. [DOI] [PubMed] [Google Scholar]
- 4.Falcetta FS, Medeiros LR, Edelweiss MI, Pohlmann PR, Stein AT, Rosa DD. Adjuvant platinum-based chemotherapy for early stage cervical cancer. Cochrane Database Syst Rev 2016;11: CD005342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: A Gynecologic Oncology Group Study. Gynecol Oncol 1999;73:177–183. [DOI] [PubMed] [Google Scholar]
- 6.Rogers L, Siu SS, Luesley D, Bryant A, Dickinson HO. Radiotherapy and chemoradiation after surgery for early cervical cancer. Cochrane Database Syst Rev 2012:CD007583. [DOI] [PMC free article] [PubMed]
- 7.Rotman M, Sedlis A, Piedmonte MR, et al. A phase III randomized trial of postoperative pelvic irradiation in Stage IB cervical carcinoma with poor prognostic features: Follow-up of a gynecologic oncology group study. Int J Radiat Oncol Biol Phys 2006;65:169–176. [DOI] [PubMed] [Google Scholar]
- 8.Eifel PJ, Winter K, Morris M, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: An update of radiation therapy oncology group trial (RTOG) 90–01. J Clin Oncol 2004;22:872–880. [DOI] [PubMed] [Google Scholar]
- 9.Delgado G, Bundy B, Zaino R, Sevin BU, Creasman WT, Major F. Prospective surgical-pathological study of disease-free interval in patients with stage IB squamous cell carcinoma of the cervix: A Gynecologic Oncology Group study. Gynecol Oncol 1990;38:352–357. [DOI] [PubMed] [Google Scholar]
- 10.Park JY, Kim DY, Kim JH, Kim YM, Kim YT, Nam JH. Management of occult invasive cervical cancer found after simple hysterectomy. Ann Oncol 2010;21:994–1000. [DOI] [PubMed] [Google Scholar]
- 11.Whitney CW, Sause W, Bundy BN, et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: A Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol 1999;17:1339–1348. [DOI] [PubMed] [Google Scholar]
- 12.Rose PG, Ali S, Watkins E, et al. Long-term follow-up of a randomized trial comparing concurrent single agent cisplatin, cisplatin-based combination chemotherapy, or hydroxyurea during pelvic irradiation for locally advanced cervical cancer: A Gynecologic Oncology Group Study. J Clin Oncol 2007;25:2804–2810. [DOI] [PubMed] [Google Scholar]
- 13.Keys HM, Bundy BN, Stehman FB, et al. Radiation therapy with and without extrafascial hysterectomy for bulky stage IB cervical carcinoma: A randomized trial of the Gynecologic Oncology Group. Gynecol Oncol 2003;89:343–353. [DOI] [PubMed] [Google Scholar]
- 14.Shrivastava S, Mahantshetty U, Engineer R, et al. Cisplatin chemoradiotherapy vs radiotherapy in FIGO stage IIIB squamous cell carcinoma of the uterine cervix: A randomized clinical trial. JAMA Oncol 2018;4:506–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marita A, Ordeanu C, Rancea A, Nicolae T, Nagy VM. Long-term survival following neoadjuvant chemotherapy and concomitant radiochemotherapy in locally advanced cervical cancer: Results of the Oncology Institute “Prof. Dr. Ion Chiricuta” experience. J Med Life 2018;11:42–50. [PMC free article] [PubMed] [Google Scholar]
- 16.Keys HM, Bundy BN, Stehman FB, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. [Erratum appears in N Engl J Med 1999 Aug 26;341(9):708]. N Engl J Med 1999; 340:1154–1161. [DOI] [PubMed] [Google Scholar]
- 17.Zuliani AC, Esteves SC, Teixeira LC, Teixeira JC, Souza GAD, Sarian LO. Concomitant cisplatin plus radiotherapy and high-dose-rate brachytherapy versus radiotherapy alone for stage IIIB epidermoid cervical cancer: A randomized controlled trial. J Clin Oncol 2014;32:542–547. [DOI] [PubMed] [Google Scholar]
- 18.Mitra D, Ghosh B, Kar A, Basu S, Deb AR, Sur PK. Role of chemoradiotherapy in advanced carcinoma cervix. J Indian Med Assoc 2006;104:432, 434, 436 passim. [PubMed] [Google Scholar]
- 19.Shi D, Liang Z, Zhang C, Zhang H, Liu X. The effect of surgery on the survival status of patients with locally advanced cervical cancer after radiotherapy/chemoradiotherapy: A meta-analysis. BMC Cancer 2018;18:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shim SH, Kim SN, Chae SH, Kim JE, Lee SJ. Impact of adjuvant hysterectomy on prognosis in patients with locally advanced cervical cancer treated with concurrent chemoradiotherapy: A meta-analysis. J Gynecol Oncol 2018;29:e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cetina L, Gonzalez-Enciso A, Cantu D, et al. Brachytherapy versus radical hysterectomy after external beam chemoradiation with gemcitabine plus cisplatin: A randomized, phase III study in IB2-IIB cervical cancer patients. Ann Oncol 2013;24:2043–2047. [DOI] [PubMed] [Google Scholar]
- 22.Poorvu PD, Sadow CA, Townamchai K, Damato AL, Viswanathan AN. Duodenal and other gastrointestinal toxicity in cervical and endometrial cancer treated with extended-field intensity modulated radiation therapy to paraaortic lymph nodes. Int J Radiat Oncol Biol Phys 2013;85:1262–1268. [DOI] [PubMed] [Google Scholar]
- 23.Osborne EM, Klopp AH, Jhingran A, Meyer LA, Eifel PJ. Impact of treatment year on survival and adverse effects in patients with cervical cancer and paraortic lymph node metastases treated with definitive extended-field radiation therapy. Pract Radiat Oncol 2017;7:e165–e173. [DOI] [PubMed] [Google Scholar]
- 24.Schwarz JK, Siegel BA, Dehdashti F, Grigsby PW. Association of posttherapy positron emission tomography with tumor response and survival in cervical carcinoma. JAMA 2007;298:2289–2295. [DOI] [PubMed] [Google Scholar]
- 25.Azria E, Morice P, Haie-Meder C, et al. Results of hysterectomy in patients with bulky residual disease at the end of chemoradiotherapy for stage IB2/II cervical carcinoma. Ann Surg Oncol 2005;12:332–337. [DOI] [PubMed] [Google Scholar]
- 26.Luo HC, Lin GS, Liao SG, et al. Cervical cancer treated with reduced-volume intensity-modulated radiation therapy base on Sedlis criteria (NCCN VS RTOG). Br J Radiol 2018;91:20170398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klopp AH, Yeung AR, Deshmukh S, et al. Patient-reported toxicity during pelvic intensity-modulated radiation therapy: NRG OncologyeRTOG 1203. J Clin Oncol 2018;36:2538–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shih KK, Hajj C, Kollmeier M, et al. Impact of postoperative intensity-modulated radiation therapy (IMRT) on the rate of bowel obstruction in gynecologic malignancy. Gynecol Oncol 2016;143: 18–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Du XL, Tao J, Sheng XG, et al. Intensity-modulated radiation therapy for advanced cervical cancer: A comparison of dosimetric and clinical outcomes with conventional radiotherapy. Gynecol Oncol 2012;125:151–157. [DOI] [PubMed] [Google Scholar]
- 30.Chang Y, Yang ZY, Li GL, et al. Correlations between radiation dose in bone marrow and hematological toxicity in patients with cervical cancer: A comparison of 3DCRT, IMRT, and RapidARC. Int J Gynecol Cancer 2016;26:770–776. [DOI] [PubMed] [Google Scholar]
- 31.Gandhi AK, Sharma DN, Rath GK, et al. Early clinical outcomes and toxicity of intensity modulated versus conventional pelvic radiation therapy for locally advanced cervix carcinoma: A prospective randomized study. Int J Radiat Oncol Biol Phys 2013;87: 542–548. [DOI] [PubMed] [Google Scholar]
- 32.Mell LK, Sirak I, Wei L, et al. Bone Marrow-sparing intensity modulated radiation therapy with concurrent cisplatin for stage IB-IVA cervical cancer: An international multicenter phase II clinical trial (INTERTECC-2). Int J Radiat Oncol Biol Phys 2017;97:536–545. [DOI] [PubMed] [Google Scholar]
- 33.Naik A, Gurjar OP, Gupta KL, Singh K, Nag P, Bhandari V. Comparison of dosimetric parameters and acute toxicity of intensity-modulated and three-dimensional radiotherapy in patients with cervix carcinoma: A randomized prospective study. Cancer Radiother 2016;20:370–376. [DOI] [PubMed] [Google Scholar]
- 34.Yu C, Zhu W, Ji Y, et al. A comparative study of intensity-modulated radiotherapy and standard radiation field with concurrent chemotherapy for local advanced cervical cancer. Eur J Gynaecol Oncol 2015;36:278–282. [PubMed] [Google Scholar]
- 35.Ioffe YJ, Hillen TJ, Zhou G, et al. Postradiation damage to the pelvic girdle in cervical cancer patients: is intensity-modulated radiation therapy safer than conventional radiation? Int J Gynecol Cancer 2014;24:806–812. [DOI] [PubMed] [Google Scholar]
- 36.Kidd EA, Siegel BA, Dehdashti F, et al. Clinical outcomes of definitive intensity-modulated radiation therapy with fluorodeoxyglucose-positron emission tomography simulation in patients with locally advanced cervical cancer. Int J Radiat Oncol Biol Phys 2010;77:1085–1091. [DOI] [PubMed] [Google Scholar]
- 37.Lin Y, Chen K, Lu Z, et al. Intensity-modulated radiation therapy for definitive treatment of cervical cancer: A meta-analysis. Radiat 2018;13:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin AJ, Kidd E, Dehdashti F, et al. Intensity modulated radiation therapy and image-guided adapted brachytherapy for cervix cancer. Int J Radiat Oncol Biol Phys 2018;103:1088–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X, Shen Y, Zhao Y, et al. Adjuvant intensity-modulated radiotherapy (IMRT) with concurrent paclitaxel and cisplatin in cervical cancer patients with high risk factors: A phase II trial. Eur J Surg Oncol 2015;41:1082–1088. [DOI] [PubMed] [Google Scholar]
- 40.Grigsby PW, Heydon K, Mutch DG, Kim RY, Eifel P. Long-term follow-up of RTOG 92–10: Cervical cancer with positive para-aortic lymph nodes. Int J Radiat Oncol Biol Phys 2001;51:982–987. [DOI] [PubMed] [Google Scholar]
- 41.Small W, Winter K, Levenback C, et al. Extended-field irradiation and intracavitary brachytherapy combined with cisplatin chemotherapy for cervical cancer with positive para-aortic or high common iliac lymph nodes: Results of ARM 1 of RTOG 0116. Int J Radiat Oncol Biol Phys 2007;68:1081–1087. [DOI] [PubMed] [Google Scholar]
- 42.Marnitz S, Martus P, Kohler C, et al. Role of surgical versus clinical staging in chemoradiated FIGO stage IIB-IVA cervical cancer patients: Acute toxicity and treatment quality of the Uterus-11 Multicenter Phase III Intergroup Trial of the German Radiation Oncology Group and the Gynecologic Cancer Group. Int J Radiat Oncol Biol Phys 2016;94:243–253. [DOI] [PubMed] [Google Scholar]
- 43.Xu KM, Rajagopalan MS, Kim H, Beriwal S. Extended field intensity modulated radiation therapy for gynecologic cancers: Is the risk of duodenal toxicity high? Pract Radiat Oncol 2015;5:e291–e297. [DOI] [PubMed] [Google Scholar]
- 44.Wakatsuki M, Kato S, Ohno T, et al. Multi-institutional observational study of prophylactic extended-field concurrent chemoradiotherapy using weekly cisplatin for patients with pelvic node-positive cervical cancer in East and Southeast Asia. Int J Radiat Oncol Biol Phys 2019;105:183–189. [DOI] [PubMed] [Google Scholar]
- 45.Verma J, Sulman EP, Jhingran A, et al. Dosimetric predictors of duodenal toxicity after intensity modulated radiation therapy for treatment of the para-aortic nodes in gynecologic cancer. Int J Radiat Oncol Biol Phys 2014;88:357–362. [DOI] [PubMed] [Google Scholar]
- 46.Vargo JA, Kim H, Choi S, et al. Extended field intensity modulated radiation therapy with concomitant boost for lymph node-positive cervical cancer: Analysis of regional control and recurrence patterns in the positron emission tomography/computed tomography era. Int J Radiat Oncol Biol Phys 2014;90:1091–1098. [DOI] [PubMed] [Google Scholar]
- 47.Han K, Milosevic M, Fyles A, Pintilie M, Viswanathan AN. Trends in the utilization of brachytherapy in cervical cancer in the United States. Int J Radiat Oncol Biol Phys 2013;87:111–119. [DOI] [PubMed] [Google Scholar]
- 48.Gill BS, Lin JF, Krivak TC, et al. National Cancer Data Base analysis of radiation therapy consolidation modality for cervical cancer: The impact of new technological advancements. Int J Radiat Oncol Biol Phys 2014;90:1083–1090. [DOI] [PubMed] [Google Scholar]
- 49.Bandera L, Face BL, Antonioli C, et al. Survival and toxicity of radical radiotherapy (with or without brachytherapy) for FIGO stage I and II cervical cancer: A mono-institutional analysis. Eur J Gynaecol Oncol 2014;35:121–127. [PubMed] [Google Scholar]
- 50.Karlsson J, Dreifaldt AC, Mordhorst LB, Sorbe B. Differences in outcome for cervical cancer patients treated with or without brachytherapy. Brachytherapy 2017;16:133–140. [DOI] [PubMed] [Google Scholar]
- 51.Tran PL, Morice P, Chirpaz E, Lazaro G, Boukerrou M. Impact of management on mortality in patients with invasive cervical cancer in Reunion Island. Eur J Obstet Gynecol Reprod Biol 2017;215:164–170. [DOI] [PubMed] [Google Scholar]
- 52.Li R, Shinde A, Chen YJ, et al. Survival benefit of adjuvant brachytherapy after hysterectomy with positive surgical margins in cervical cancer. Int J Radiat Oncol Biol Phys 2018;102:373–382. [DOI] [PubMed] [Google Scholar]
- 53.Nagy V, Coza O, Ordeanu C, et al. Radiotherapy versus concurrent 5-day cisplatin and radiotherapy in locally advanced cervical carcinoma. Long-term results of a phase III randomized trial. Strahlenther Onkol 2009;185:177–183. [DOI] [PubMed] [Google Scholar]
- 54.Stehman FB, Ali S, Keys HM, et al. Radiation therapy with or without weekly cisplatin for bulky stage 1B cervical carcinoma: Follow-up of a Gynecologic Oncology Group trial. Am J Obstet Gynecol 2007;197:503.e501–503.e506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Viswanathan AN, Moughan J, Small W, et al. The quality of cervical cancer brachytherapy implantation and the impact on local recurrence and disease-free survival in radiation therapy oncology group prospective trials 0116 and 0128. Int J Gynecol Cancer 2012; 22:123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Potter R, Georg P, Dimopoulos JC, et al. Clinical outcome of protocol based image (MRI) guided adaptive brachytherapy combined with 3D conformal radiotherapy with or without chemotherapy in patients with locally advanced cervical cancer. Radiother Oncol 2011;100:116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Potter R, Dimopoulos J, Georg P, et al. Clinical impact of MRI assisted dose volume adaptation and dose escalation in brachytherapy of locally advanced cervix cancer. Radiother Oncol 2007;83: 148–155. [DOI] [PubMed] [Google Scholar]
- 58.Mazeron R, Fokdal LU, Kirchheiner K, et al. Dose-volume effect relationships for late rectal morbidity in patients treated with chemoradiation and MRI-guided adaptive brachytherapy for locally advanced cervical cancer: Results from the prospective multicenter EMBRACE study. Radiother Oncol 2016; 120:412–419. [DOI] [PubMed] [Google Scholar]
- 59.Kirchheiner K, Nout RA, Tanderup K, et al. Manifestation pattern of early-late vaginal morbidity after definitive radiation (chemo)therapy and image-guided adaptive brachytherapy for locally advanced cervical cancer: An analysis from the EMBRACE study. Int J Radiat Oncol Biol Phys 2014;89:88–95. [DOI] [PubMed] [Google Scholar]
- 60.Kirchheiner K, Nout RA, Lindegaard JC, et al. Dose-effect relationship and risk factors for vaginal stenosis after definitive radio(chemo)therapy with image-guided brachytherapy for locally advanced cervical cancer in the EMBRACE study. Radiother Oncol 2016;118:160–166. [DOI] [PubMed] [Google Scholar]
- 61.Kirchheiner K, Nout RA, Czajka-Pepl A, et al. Health related quality of life and patient reported symptoms before and during definitive radio(chemo)therapy using image-guided adaptive brachytherapy for locally advanced cervical cancer and early recovery: A mono-institutional prospective study. Gynecol Oncol 2015;136:415–423. [DOI] [PubMed] [Google Scholar]
- 62.Sturdza A, Potter R, Fokdal LU, et al. Image guided brachytherapy in locally advanced cervical cancer: Improved pelvic control and survival in RetroEMBRACE, a multicenter cohort study. Radiother Oncol 2016;120:428–433. [DOI] [PubMed] [Google Scholar]
- 63.Charra-Brunaud C, Harter V, Delannes M, et al. Impact of 3D image-based PDR brachytherapy on outcome of patients treated for cervix carcinoma in France: Results of the French STIC prospective study. Radiother Oncol 2012;103:305–313. [DOI] [PubMed] [Google Scholar]
- 64.Lindegaard JC, Fokdal LU, Nielsen SK, Juul-Christensen J, Tanderup K. MRI-guided adaptive radiotherapy in locally advanced cervical cancer from a Nordic perspective. Acta Oncol 2013;52: 1510–1519. [DOI] [PubMed] [Google Scholar]
- 65.Jensen NBK, Potter R, Kirchheiner K, et al. Bowel morbidity following radiochemotherapy and image-guided adaptive brachytherapy for cervical cancer: Physician- and patient reported outcome from the EMBRACE study. Radiother Oncol 2018;127:431–439. [DOI] [PubMed] [Google Scholar]
- 66.Viswanathan AN. Cervical carcinoma. In: Halperin EC, ed. Principles and Practice of Radiation Oncology 7th ed. Philadelphia, PA: Wolters Kluwer; 2019:1651–1739. [Google Scholar]
- 67.Sapienza LG, Jhingran A, Kollmeier MA, et al. Decrease in uterine perforations with ultrasound image-guided applicator insertion in intracavitary brachytherapy for cervical cancer: A systematic review and meta-analysis. Gynecol Oncol 2018;14:14. [DOI] [PubMed] [Google Scholar]
- 68.Mahantshetty U, Krishnatry R, Hande V, et al. Magnetic resonance image guided adaptive brachytherapy in locally advanced cervical cancer: An experience from a tertiary cancer center in a low and middle income countries setting. Int J Radiat Oncol Biol Phys 2017;99:608–617. [DOI] [PubMed] [Google Scholar]
- 69.Wang F, Tang Q, Lv G, et al. Comparison of computed tomography and magnetic resonance imaging in cervical cancer brachytherapy: A systematic review. Brachytherapy 2017;16:353–365. [DOI] [PubMed] [Google Scholar]
- 70.Gill BS, Kim H, Houser CJ, et al. MRI-guided high-dose-rate intracavitary brachytherapy for treatment of cervical cancer: The University of Pittsburgh experience. Int J Radiat Oncol Biol Phys 2015;91:540–547. [DOI] [PubMed] [Google Scholar]
- 71.Castelnau-Marchand P, Chargari C, Maroun P, et al. Clinical outcomes of definitive chemoradiation followed by intracavitary pulsed-dose rate image-guided adaptive brachytherapy in locally advanced cervical cancer. Gynecol Oncol 2015;139:288–294. [DOI] [PubMed] [Google Scholar]
- 72.Rijkmans EC, Nout RA, Rutten IH, et al. Improved survival of patients with cervical cancer treated with image-guided brachytherapy compared with conventional brachytherapy. Gynecol Oncol 2014;135:231–238. [DOI] [PubMed] [Google Scholar]
- 73.Mayadev J, Viswanathan A, Liu Y, et al. American Brachytherapy Task Group Report: A pooled analysis of clinical outcomes for high-dose-rate brachytherapy for cervical cancer. Brachytherapy 2017;16:22–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rao BS, Das P, Subramanian BV, et al. A comparative analysis of two different dose fractionation regimens of high dose rate intracavitary brachytherapy in treatment of carcinoma of uterine cervix: A prospective randomized study. J Clin Diagn Res 2017;11:XC06–XC10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tanderup K, Fokdal LU, Sturdza A, et al. Effect of tumor dose, volume and overall treatment time on local control after radiochemotherapy including MRI guided brachytherapy of locally advanced cervical cancer. [Erratum appears in Radiother Oncol. 2017 Apr;123(1):169; PMID: 28237399]. Radiother Oncol 2016;120:441–446. [DOI] [PubMed] [Google Scholar]
- 76.Dimopoulos JC, Lang S, Kirisits C, et al. Dose-volume histogram parameters and local tumor control in magnetic resonance image-guided cervical cancer brachytherapy. Int J Radiat Oncol Biol Phys 2009;75:56–63. [DOI] [PubMed] [Google Scholar]
- 77.Mazeron R, Castelnau-Marchand P, Escande A, et al. Tumor dose-volume response in image-guided adaptive brachytherapy for cervical cancer: A meta-regression analysis. Brachytherapy 2016;15: 537–542. [DOI] [PubMed] [Google Scholar]
- 78.Georg P, Potter R, Georg D, et al. Dose effect relationship for late side effects of the rectum and urinary bladder in magnetic resonance image-guided adaptive cervix cancer brachytherapy. Int J Radiat Oncol Biol Phys 2012;82:653–657. [DOI] [PubMed] [Google Scholar]
- 79.Mazeron R, Gouy S, Chargari C, et al. Post radiation hysterectomy in locally advanced cervical cancer: Outcomes and dosimetric impact. Radiother Oncol 2016;120:460–466. [DOI] [PubMed] [Google Scholar]
- 80.Viswanathan AN, Thomadsen B, American Brachytherapy Society Cervical Cancer Recommendations Committee, American Brachytherapy Society. American Brachytherapy Society consensus guidelines for locally advanced carcinoma of the cervix. Part I: General principles. Brachytherapy 2012;11:33–46. [DOI] [PubMed] [Google Scholar]
- 81.Prescribing, Recording and Reporting Brachytherapy for Cancer of the Cervix. J ICRU 2016;13:NP. [DOI] [PubMed] [Google Scholar]
- 82.Manea E, Escande A, Bockel S, et al. Risk of late urinary complications following image guided adaptive brachytherapy for locally advanced cervical cancer: Refining bladder dose-volume parameters. Int J Radiat Oncol Biol Phys 2018;101:411–420. [DOI] [PubMed] [Google Scholar]
- 83.Potter R, Tanderup K, Kirisits C, et al. The EMBRACE II study: The outcome and prospect of two decades of evolution within the GEC-ESTRO GYN working group and the EMBRACE studies. Clin Transl Radiat Oncol 2018;9:48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Petit C, Dumas I, Chargari C, et al. MRI-guided brachytherapy in locally advanced cervical cancer: Small bowel [Formula: see text] and [Formula: see text] are not predictive of late morbidity. Brachytherapy 2016;15:463–470. [DOI] [PubMed] [Google Scholar]
- 85.Haie-Meder C, Potter R, Limbergen EV, et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (I): Concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother Oncol 2005;74:235–245. [DOI] [PubMed] [Google Scholar]
- 86.Tan LT, Potter R, Sturdza A, et al. Change in patterns of failure after image-guided brachytherapy for cervical cancer: Analysis from the RetroEMBRACE Study. Int J Radiat Oncol Biol Phys 2019;104: 895–902. [DOI] [PubMed] [Google Scholar]
- 87.Fokdal L, Sturdza A, Mazeron R, et al. Image guided adaptive brachytherapy with combined intracavitary and interstitial technique improves the therapeutic ratio in locally advanced cervical cancer: Analysis from the retroEMBRACE study. Radiother Oncol 2016; 120:434–440. [DOI] [PubMed] [Google Scholar]
- 88.Viswanathan AN, Dimopoulos J, Kirisits C, Berger D, Potter R. Computed tomography versus magnetic resonance imaging-based contouring in cervical cancer brachytherapy: Results of a prospective trial and preliminary guidelines for standardized contours. Int J Radiat Oncol Biol Phys 2007;68:491–498. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


