Abstract
Epidermal melanocyte loss in vitiligo, triggered by stresses ranging from trauma to emotional stress, chemical exposure or metabolite imbalance, to the unknown, can stimulate oxidative stress in pigment cells, which secrete damage-associated molecular patterns that then initiate innate immune responses. Antigen presentation to melanocytes leads to stimulation of autoreactive T-cell responses, with further targeting of pigment cells. Studies show a pathogenic basis for cellular stress, innate immune responses and adaptive immunity in vitiligo. Improved understanding of the aetiological mechanisms in vitiligo has already resulted in successful use of the Jak inhibitors in vitiligo. In this review, we outline the current understanding of the pathological mechanisms in vitiligo and locate loci to which therapeutic attack might be directed.
Keywords: antibodies, autoimmunity, cytokines, cytotoxic T cells, Th1/Th2 cells
Introduction
Melanocyte damage and destruction is the underlying pathological event in vitiligo, a skin disease characterized by depigmented patches. Vitiligo has a worldwide prevalence of about one percent, and can be classified into non-segmental, segmental, mixed, and unclassifiable/undetermined types [1, 2]. Differentiating subtypes may be important as they might have different aetiologies. Segmental vitiligo often maps to a blaschkoid or dermatomal distribution [3]. Vitiligo can affect any gender, race, or geographic region [4].
Although non-life threatening, vitiligo can have a serious psychological impact on sufferers [5]. Vitiligo patients commonly experience feelings of stress, fear of disease exacerbation, embarrassment, negative self-image, and self-consciousness [6]. Moreover, patients with vitiligo often experience depression, anxiety, and discrimination and stigmatization from others, resulting in low self-esteem and social isolation [7].
In some countries, vitiligo is still confused with leprosy and patients are stigmatized [8]. Vitiligo can also have a negative impact on the marital prospects of those affected [9–11]. Sufferers with decreased quality of life at treatment initiation face a lower response rate to a given therapy [12]. Development of specific psychological intervention and quality of life measures may benefit treatment outcome and enhance self-esteem [13].
A variety of factors may trigger vitiligo. These include emotional stress, physical trauma, and chemical exposure to imbalances in endogenous neural factors, metabolites, cytokines, or hormones. All of these can stimulate autoimmune responses, in individuals with the appropriate genetic susceptibilities, that ultimately target melanocytes [2]. The melanocytes in vitiligo are highly vulnerable to damage and apoptosis under the action of triggering factors [14]. The treatment of vitiligo, including topical steroids, calcitonin-inhibitors, phototherapies, and surgical procedure, in the past has frequently failed to achieve satisfactory repigmentation, but recently, the Jak inhibitors have shown promise [15].
Histopathological features of vitiligo and melanocyte degeneration
The affected skin in vitiligo demonstrates melanin loss and absence of or reduced numbers of melanocytes in the epidermis [16]. This decrease varies according to the disease stage [13]. Melanocytes as revealed by an appropriate monoclonal antibody technique or the Fontana Masson stain, remain present, though they disappear from affected skin of vitiligo as the disease progress [17]. Immunohistochemical studies of vitiligo lesions show the absence of melanocytes from the basal layer, but they may exist in decreased numbers and can demonstrate degenerative changes [18].
Even though it is clear that depigmentation in vitiligo results from melanocyte loss from the skin, it remains unclear whether this occurs through a degenerative or autoimmune process. Defective in vitro growth of melanocytes derived from individuals with vitiligo [19, 20] and increased susceptibility of vitiligo melanocytes to exogenous stimuli suggests that degeneration may precede melanocyte loss [21].
Melanocytes from vitiligo subjects were shown to poorly proliferate compared to healthy normal melanocytes [19] and also show inadequate antioxidant activity with high levels of superoxide dismutase and low levels of catalase [22]. Under normal circumstances, superoxide dismutase cata-lyses the first step of dismutation by converting the superoxide anion into oxygen and hydrogen peroxide and then the catalase enzyme transforms hydrogen peroxide into water and oxygen, protecting cells from reactive oxygen species (ROS). In fact, melanocytes synthesize high ROS levels as by-product of melanogenesis. Therefore, compensatory media supplements such as growth factors or catalase are required to culture melanocytes derived from vitiligo patients [20, 23]. Also, increased expression of hydrogen peroxide and elevated oxidative by-products within vitiligo patient skin has been reported [22, 24, 25].
In addition, melanocytes from vitiligo patients have been shown to be more sensitive to in vitro oxidative therapies such as cumene hydroperoxide and ultraviolet B irradiation [26, 27]. However, exogenous treatment with catalase in the form of pseudocatalase, which was proposed to cure vitiligo by regulating reactive oxygen species (ROS), was ineffective in treating vitiligo lesions [28]. Thus, dysregulated redox balance in vitiligo might be a consequence, but not a cause, of vitiligo.
Melanocytes from vitiligo patients show morphological and physiological abnor-malities. Those in peri-lesional borders are seen to be enlarged with longer dendritic ends than normal melanocytes [29]. However, rapid regimentation of the skin following engrafting of human vitiligo lesional skin on nude mouse was achieved, indicating that the intrinsic melanocyte defect was not the only cause of melanocyte destruction in vitiligo [30]. Histochemical and immunohistochemical examination shows infiltration of a large number of T lymphocytes at the edge of vitiligo lesions with complete microscopic loss of melanin in lesional skin [31]. Therefore, it is clear that vitiligo melanocytes are abnormal compared to healthy melanocytes.
Responses to stress in vitiligo
Melanocytes in the epidermis are regularly exposed to various environmental stressors e.g. ultraviolet (UV) radiation, pollution, microorganisms, and oxidizing chemicals, all of which can stimulate ROS production [32]. ROS consist of a number of oxygen-based free radicals such as superoxide and hydrogen peroxide, formed during multiple physiological and pathological processes [33]. Such free radicals are constantly scavenged by antioxidants such as superoxide dismutase, catalase, vitamin C, and vitamin E. As mentioned, in vitiligo patients, high levels of superoxide dismutase and low levels of catalase have been observed in the skin [34].
Hydrogen peroxide created from superoxide anion can easily cross melanocyte membranes causing cellular damage [33]. Even though melanin present in the skin protects melanocytes as well as adjacent keratinocytes through its ability to absorb UV radiation, its synthesis likewise results in higher amount of intracellular ROS, causing to be melanocytes more vulnerable to oxidative stress [35, 36].
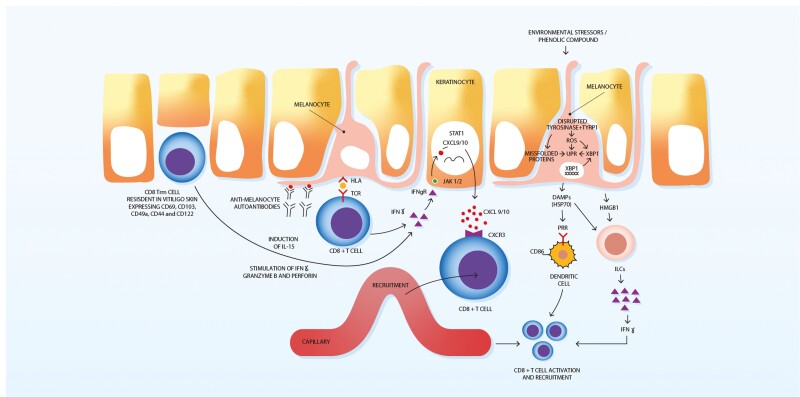
In addition, a decrease in the stability of tyrosinase-related protein-1 (TYRP1), which is required for melanin synthesis, has been observed in vitiligo melanocytes, allowing accumulation of melanin intermediates [37] (Fig. 1). The build-up of intermediate products increases the risk of protein misfolding, hence activating the unfolded protein response (Fig. 1). This in turn induces the restoration of endoplasmic reticulum homeostasis through the halting of protein translation, inducing misfolded protein degradation and promoting the synthesis of chaperons to facilitate protein folding, sustained activation of which leads to apoptosis [2]. Disturbance of UPR can contribute to the development of auto-immune diseases through formation of antigens during misfolded protein degradation, secretion of neo-antigens by apoptotic cells or disruption of immune tolerance [38]. Engagement of UPR in vitiligo pathogenesis is proposed by genetic studies, which revealed that polymorphisms in the gene-encoding X-box-binding protein 1 (XBP1) are correlate with an elevated risk of developing vitiligo [39]. XBP1 is a transcription factor that modulates various downstream UPR targets [39]. Studies showed that exposure of melanocytes to phenolic compounds, known as triggers of vitiligo, activate XBP1, which in turn activates the UPR and increases the expression of cytokines IL6 and IL8 [40] (Fig. 1). Increased levels of IL6 and IL8 were indeed found in the skin and serum of vitiligo patients, indicating sustained UPR activation [40, 41]
Fig. 1.
Melanocytes are regularly exposed to environmental insults such as phenolic compounds can stimulate a state of melanocyte stress through interacting with tyrosinase and TYRP1, leading to production of DAMPs. DAMPs can then stimulate nearby dendritic cells through PRRs. Activated dendritic cells locally synthesize cytokines, inducing CD8+ T cell activation and recruitment to the skin. HMGB1 can activate ILCs with subsequent release of IFN-γ. In the progressive phase of vitiligo, melanocyte-reactive CD8+T cells produce interferon-gamma on encountering melanocyte antigens. These induce keratinocytes to secrete CXCL 9 and CXCL 10, resulting in additional recruitment of lymphocytes to the site through the CXCR3 receptor. CD8+ T cells, resident in vitiligo skin, are involved through the induction of IL-15, which results in the stimulation of IFN-γ, granzyme B and perforin. DAMPs; pathogens or damage-associated molecular patterns, TYRP1; tyrosinase-related protein-1, PRRs; pattern recognition receptors, HMGB1; high-mobility group protein B1, ILCs; innate lymphoid cells, IFN-γ; interferon (IFN)-γ, CXCR3; C-X-C chemokine receptor type 3, CXCL 9/10; C-X-C motif chemokine ligand 9/10, IL-15; interleukin-15.
Intrinsic defects may also render vitiligo melanocytes vulnerable to oxidative stress. Observed anomalies include a dilated endoplasmic reticulum, mitochondrial dysfunction, and an abnormal melanosome structure, all of which suggests that these pigment cells are less capable of dealing with such stressors than those from healthy individuals [32].
ROS-mediated stress has been directly linked with generation of neoantigenic epitopes within beta islet cells [42], and likewise melanocyte stress may generate neoantigens. Elevated ROS in melanocytes of vitiligo subjects has been correlated with peroxidation, and thus it is likely that ROS generates melanocyte neoantigens via protein carbonylation and oxidation [43].
Cellular stress has been found to develop in healthy melanocytes exposed to phenolic derivatives such as 4-tertiary butylphenol and monobenzyl ether of hydroquinone (Fig. 1) [40]. Once melanocytes become stressed, they promote the secretion of inducible heat shock protein 70 (iHSP70), which has been detected in vitiligo melanocytes and seen to correlate with active disease [44] (Fig. 1).
Pathogens or damage-associated molecular patterns (DAMP), which can evoke inflammation via pattern recognition receptors (PRRs) including Toll-like receptors and nucleotide oligomerization domain (NOD)-like receptors (NLRs). Indeed, NLRP1 has been linked with vitiligo in a linkage study [45]. Innate immunity is activated by the release of DAMPs from stressed cells. DAMPs are likely to be constantly released from stressed melanocytes resulting in skin inflammation in patients with vitiligo [46]. In agreement with this, uninvolved skin of vitiligo patients shows increased numbers of lymphocytes in comparison with healthy controls [47].
Innate immunity
Innate immunity is based on the ability of PRRs to detect pathogen-associated molecular patterns found in pathogens or DAMPs released by damaged cells [48]. ROS and iHSP70 produced by stressed melanocytes serve as DAMPs in vitiligo, and PRRs initiate the innate response [46] (Fig. 1). Innate immune cells such as natural killer (NK) cells, macrophage, and dendritic cells show aberrant activation in vitiligo skin and granzyme-B (GZMB)-expressing activated NK cells have been found [47, 49].
Vitiligo skin shows an increase in NK cells activating receptors (CD16+CD56+ and CD3+CD16+CD56+), an upregulation in CLEC2B, an activating ligand of NK cells, and a decrease in the inhibitory receptors (CD16+CD158a+). Vitiligo skin also demonstrates increased numbers of dendritic cells, which can destroy melanocytes when activated by iHSP70 [49]. While chemicals can trigger vitiligo by inducing melanocyte stress, adding HSP70i alone aggravates vitiligo in a mouse model, probably via activation of dendritic cells in the skin [50]. In addition, increased iHSP70 expression in the skin of vitiligo patients causes melanocyte loss [50]. Mice lacking expression of iHSP70 will not develop experimental depigmentation, suggesting a role for iHSP70 in vitiligo [49]. In addition, mutant HSP70i delivery, which interferes with the signalling pathway of endogenous HSP70i, could inhibit depigmentation in mouse and swine models of vitiligo by interfering with dendritic cell activation [49, 51]. Thus, DAMPs, in particular HSP70i, can directly initiate vitiligo in animal models by activating dendritic cells. iHSP70 has been found to have potent adjuvant and chaperone properties [52].
Under conditions of oxidative stress, genetically compromised melanocytes secrete melanosomal peptides-chaperoned iHSP70 that can activate dendritic cells and release the inflammatory signal that initiates the immune response in vitiligo [49]. Secreted iHSP70 from stressed vitiligo melanocytes has been reported to induce dendritic cells to elevate the expression of coactivation markers CD80 and CD86, stimulating immune responses to melanocytes [53, 54] (Fig. 1). Therefore, it is likely that melanocyte stress can contribute to the instigation of autoimmunity through both neoantigen generation and the activation of innate immunity [43]. iHSP70 is thus a link between oxidative stress, as a trigger, and the onset of the autoimmune reaction in vitiligo. Other DAMPs such as calreticulin and high-mobility group protein B1 (HMGB1) have been proven to take a part in the pathogenesis of vitiligo. Some studies found that calreticulin levels were higher in patients with vitiligo than in healthy controls, and its levels were positively correlated with vitiligo lesional area [55]. Calreticulin was shown to induce the secretion of IL-6 and tumour necrosis factor-α (TNF-α), suggesting that calreticulin may stimulate the immune response, thus affecting melanocytes via cellular-mediated immunity [55].
In addition, studies have revealed that HMGB1 expression was also increased in both blood and lesional samples from vitiligo patients [56]. Moreover, serum level of HMGB1 was elevated in patients with active progressive vitiligo, whereas no change in HMGB1 serum level was shown in patients with slowly progressive vitiligo [57]. HMGB1 is generally located in the cell nucleus and involved in the regulation of gene transcription through interaction with chromatin structures of DNA [58]. Cui et al. (2019) reported abnormal localization of HMGB1 in the cytoplasm of remaining melanocytes in perilesional areas of vitiligo skin. Additionally, in vitro studies have shown that HMGB1 could be released from melanocytes into the extracellular space due to oxidative stress, and then stimulate inflammation via integrating with PRRs [57] (Fig. 1). HMGB1 could induce the expression of inflammatory factors, such as CXCL16, from keratinocytes, which could stimulate the maturation of dendritic cells and the infiltration of cutaneous CD8+ cells [57].
Activated, dendritic cells locally synthesize cytokines, inducing T-cell activation and recruitment to the skin and, in local lymph nodes, the recruitment of cytotoxic T cells, thus bridging the innate with the adaptive immunity [59]. Therefore, delivery of mutant HSP70i may offer a potential treatment for vitiligo by altering innate immunity. The connection in vitiligo between cellular stress and cell-based immunity was illustrated when melanocytes, stressed by exposure to 4-tertiary butyl phenol, were noted to facilitate activation of dendritic cells thus rendering the latter melanocytotoxic in vitro [53].
Other studies have demonstrated that stressed melanocytes can activate melanocyte-specific CD8+ T cells, resulting in an autoimmune response and consequent pigment cell destruction [60]. In addition, perilesional skin of patients with active vitiligo showed infiltrates of plasmacytoid dendritic cells (pDCs), which are associated with a local IFN-α response [61]. Recently, increased presence of innate lymphoid cells (ILCs) have been demonstrated in the skin and blood of vitiligo patients [62] (Fig. 1). Interestingly, ILCs from vitiligo patients synthesized higher amount of IFN-γ when exposed to oxidative stress or stimulated by HSP70 and HMGB1, compared to healthy individuals [62]. ILCs are initial source of IFN-γ, a signature vitiligo cytokine, which is involved in T-cell-mediated destruction of pigment cells [62].
Recently, perilesional keratinocytes from vitiligo skin, under oxidative stress in vitro, have been shown to exhibit increased expressions of NLR family pyrin domain containing 3 (NLRP3) and downstream cytokine IL-1β, an inflammasome regulator that may modulate an innate immune attack on melanocytes [63]. NLRP3 is a cytoplasmic NLR and is an essential constituent of the inflammasome in the innate immunity. The NLRP3 inflammasome is activated by a variety of extracellular inflammatory stimuli, such as bacteria, viruses, and yeasts, in a nuclear factor kB (NF-kB)-mediated signalling manner [64]. In addition, the NLRP3 inflammasome can be activated in response of a wide range of endogenous molecules such as lysosomal destabilization, oxidized mitochondrial DNA, extracellular ATP, potassium efflux and intracellular calcium levels [64]. The priming step induces NLRP3 expression and activates licensing receptors. Importantly, the activation of NLRP3 inflammasome also can be controlled by tyrosine kinase and JNK or Syk kinases, via the recruitment of caspase-1 and regulation of apoptosis-associated speck-like protein containing a CARD (ASC) oligomerization, respectively [64]. In addition, the NLRP3 inflammasome can be activated through transient receptor potential cation channel subfamily M member 2 (TRPM2)-induced intracellular and mitochondrial calcium influx in H2O2-treated keratinocytes [63]. In vitiligo, the NLRP3 inflammasome can be activated by monobenzone, and H2O2. van den Boorn et al. showed that monobenzone induces a melanocyte-specific immune response characterized by the activation of NK cells and the infiltration of macrophages. Indeed, NK cell recruitment to the ear, during treatment with monobenzone, was significantly suppressed in Nlrp3-deficient mice, indicating a key role for the NLRP3 inflammasome in monobenzone-induced inflammation in melanocytes [65]. This suggests that the NLRP3 inflammasome and its downstream inflammatory cytokines could be promising targets for vitiligo therapy. Recently, it has been shown that the activation of the NLRP3 inflammasome is required to stimulate innate immunity in keratinocytes [63]. In addition, NLRP3 inflammasome deactivation diminishes recruitment of CD8+ T cells and suppresses cytokine production in T cells derived from patients with vitiligo [63].
Once activated, the NLRP3 inflammasome mediates caspase-1 cleavage which promotes synthesis of IL-β [66, 67]. Subsequently, the function of CD8+ and CD4+ T cell is strengthened through the IL1-β/IL-1R signalling pathway [63]. IL1-β has been observed to elevate expression of CXCR6 and CXCR3 in CD8+ T cells from vitiligo patients. Similarly, IL1-β has been found to increase synthesis of IL17A/F in CD4+ T cells and interferon (IFN)-γ in both CD8+ and CD4+ T cells [63]. IL1-β in stressed keratinocytes has been also noted to stimulate expression of CXCL10 and CXCL16, ligands of CXCR3 and CXCR6 through NF-kB pathway, which promote migration of cytotoxic T cells into vitiligo areas [63].
Adaptive immunity
Infiltrating T cells and their role in vitiligo
Following proinflammatory signalling in the skin, melanocyte antigens can be processed and presented by dendritic cells in the draining lymph node, resulting in the production of melanocyte-specific cytotoxic T lymphocytes and the generation of melanocyte-specific antibodies by B lymphocytes [68]. Histological studies of vitiligo lesions have shown infiltration of lymphocytes at the border of depigmented lesions. These infiltrates consist mainly of CD8 + T cells, preferentially located in the dermo-epidermal borders neighbouring the melanocytes [69, 70]. CD8+ T cells have been implicated in the destruction of melanocytes in vitiligo.
Melanocytotoxic CD8+ T cells that express the skin homing marker, cutaneous lymphocyte-association antigen, infiltrate the lesional borders of vitiligo skin, and their number correlates with disease extension and severity [71–73].
Furthermore, self-reactive CD8+ T cells in infiltrating perilesional areas of vitiligo recognize the antigens Melan-A/MART, tyrosinase (TYR), and melanocyte-specific protein, all of which are involved in the melanogenic pathway [74]. Vitiligo patients have elevated numbers of CD8+ T cells in their peripheral blood compared to healthy individuals [72].
Moreover, the peri-lesional skin in vitiligo is highly infiltrated by melanocyte-specific CD8+ T cells [70] – cells which are able to kill melanocytes in vitro [70, 75, 76]. CD8+ T cells isolated from the peri-lesional skin of vitiligo patients, have been demonstrated to infiltrate explants of autologous healthy pigmented vitiligo skin and eliminate melanocytes in a manner similar to that observed in the clinical pathology of the disease [76]. Importantly, isolated CD4+ T cells were incapable of inducing melanocyte apoptosis in autologous skin explants [76].
In vitro culture of CD8+ and CD4+ T cells derived from peri-lesional vitiligo skin secreted high levels IFN-γ, a pro-inflammatory cytokine required for melanocyte-specific autoreactive CD8+ T-cell recruitment [77]. These findings provide robust evidence that CD8+ T cells are essential and sufficient for the destruction of melanocytes in vitiligo.
IFN-γ signalling pathway acts as a key driver of CD8+ T-cell recruitment and function in vitiligo
The mechanism by which CD8+ T cells trigger melanocytes apoptosis requires their secretion of proinflammatory cytokines IFN-γ from CD8+ T cells [76]. Analysis of gene expression in human vitiligo lesional skin demonstrated increased expression of the interferon-response gene [78, 79], as well as genes induced by IFN-γ and these include C-X-C chemokine receptor type 3 (CXCR3), a T-cell chemokine receptor, and its ligands: CXCL9, CXCL10, and CXCL11 [79]. In agreement with this finding, skin biopsies from vitiligo patient lesions and from a mouse model of vitiligo also show prominent lymphocyte infiltrates that are primarily CXCR3+ [61, 77, 79, 80]. In addition, melanocyte-specific CD8+ T cells isolated from the skin and blood of human vitiligo subjects predominantly express CXCR3 receptor, and CXCL9 is a specific skin biomarker of active vitiligo [73]. Studies in mouse models of vitiligo have established a critical role for this pathway in the pathogenesis of the disease, since IFN-γ, CXCR3, and CXCL10 are all essential for its development [79, 81]. The neutralization of IFN-γ with antibody treatment or the lack of CXCR3 expression on T cells prevents the migration of autoreactive T cells into the skin, which therefore do not cause depigmentation [79, 81]. Studies employing chemokine reporter mice have shown that CXCL9 and CXCL10 are mostly produced by keratinocytes, and functional studies have demonstrated that keratinocytes are primarily responsible for the recruitment of autoreactive T cells [82]. CXCL9 seems predominantly responsible for the bulk recruitment of T cells to the skin in a mouse model of vitiligo, since, when it is absent, the number of melanocyte-autoreactive T cells within lesional vitiligo skin is decreased tenfold [79]. However, in spite of reduced a number of T cells, vitiligo severity remains unchanged, indicating that the over-recruitment of T cells occurs during the disease process. In contrast, when CXCL10 is absent in mice with established widespread depig-mentation, the incidence and severity of the depigmentation are decreased, even though bulk recruitment of T cells is unchanged [79]. Interestingly, in the Cxcl10−/− mice, the quantity of T cells shown in the epidermis compared to the dermis in the skin is reduced, signifying that CXCL10 is responsible for T-cell localization within the skin and their effector function [79]. Thus, T cells produces IFN-γ, which stimulates the production of CXCL9 and CXCL10 from keratinocytes to recruit more T cells and induce the progression of vitiligo [43]. As well as a role in initiation and progression, the IFN-γ-chemokine pathway is also needed for the maintenance of established vitiligo lesions, as knocking out CXCR3 or blocking the action of CXCL10 in mouse models of vitiligo prevents and reverses depigmentation in the disease [79] (Fig. 1). Indeed, ruxolitinib, a janus kinase (JAK)-1,2 inhibitor, which interferes with IFN-γ signalling pathway through preferential inhibition of JAK1 and JAK2, shows promise in vitiligo. A phase 2 trial of ruxolitinib cream was associated with significant repigmentation of vitiligo up to 52 weeks of treatment, and was well tolerated [83].
Regulatory T cells are suppressors of autoreactive effectors and inhibit depigmentation
Regulatory T (Treg) cells are key factors in the maintenance of appropriate peripheral tolerance, via suppression of self-reactive effector T cells, and so maintain immune homeostasis. Treg cells are a subgroup of CD4+ T cells that primarily represent a phenotype of CD4+, CD25+ and forkhead box P3 (Foxp3), and play a key role in preventing autoimmunity. Patients who suffer from immuno-dysregulation, polyend-ocrinopathy, enteropathy, X-linked syndrome, lack Treg cells because of a mutation in the FOXP3 gene and, as a consequence, suffer from other autoimmune diseases, including vitiligo [84, 85]. Likewise, scurfy mutant mouse strain with defective FOXP3 lack Treg cells and shows widespread autoimmunity, underlining a critical role for Treg cells in the maintenance of tolerance to self-antigens [86]. In a mouse model of vitiligo repigmentation was accompanied by an elevated infiltration of Tregs into the skin, possibly resulting in the prevention of immune responses against melanocytes [87]. In addition, some authors have reported an increase in vitiligo severity when Treg cells were depleted using either CD4 or CD25 antibodies [88, 89]. One study showed an increase in cutaneous Treg cell infiltration and a decrease in depigmentation when the expression of CCL22 was induced in the skin [90]. Another report revealed that adoptive transfer of exogenous Treg cells to vitiligo-prone mice at 3 weeks of age led to elevated number of cutaneous Treg cells and prevented vitiligo [89]. When vitiligo-prone mice were injected with PD-L1P-Fc, Treg cell accumulation in the skin was enhanced and depigmentation was reversed [91]. These findings support the theory that the number of Treg cells in the skin is critical for reducing depigmentation driven by T effectors and therefore helping to control vitiligo progression. Interestingly, recent studies used GD3-reactive chimeric antigen receptor (CAR) Tregs to treat vitiligo in a TCR transgenic mouse model of spontaneous vitiligo [92]. These CAR Tregs demonstrated elevated IL-10 production in response to antigen, suppressed T-cell cytotoxicity towards melanocytes, and they significantly delayed depigmentation compared to untransduced Tregs, thus providing local immune tolerance in perilesional skin of vitiligo [92]. These results were also associated with a greater number of Tregs and melanocytes in CAR Treg treated mice as compared to mice treated with untransduced Tregs, indicating an important role for antigen-specific Tregs in controlling progressive depigmentation [92]. Several research groups have reported disruption of Treg cells function, but there is no consensus as to where the disruption exactly lies: is it in the density of Treg cells, suppressive effects, or in the immigration ability to the skin. A study has reported that peripheral Treg cells isolated from vitiligo patients have shown a decreased ability to inhibit CD8+ T-cell proliferation and activation in vitro [93]. However, another study showed normal Treg cell activity in vitiligo patients but a decreased number in the skin, suggesting that reduced cutaneous localization of Treg cells to the skin, rather than diminished function of Treg cells, contributes to the pathogenesis of vitiligo [94].
Immunohistochemical studies have revealed no significant reduction in Treg cell number in areas of vitiligo [95, 96], whereas another report showed a significant decrease in these cells [97]. It is therefore not clear how the function of Treg is disrupted in vitiligo patients, but effector T-cell phenotype in human vitiligo also indicates the presence of defective Treg T cells. Analysis of the phenotype of peripheral blood mononuclear cells indicates that in vitiligo patients [98]. Further studies are required to reliably determine how a deficiency of Treg cells contributes to the development of vitiligo, and to examine the potential for improving Treg cell function as a treatment.
Immune-dysregulation of T helper cells in vitiligo
The numbers of circulating Th1 cells are increased in vitiligo compared to controls [99]. A mouse model of vitiligo shows a predominantly Th1-mediated pattern with a dominant role of CXCL10 [80]. Recently, decreased frequencies of circulating Th1/Tc1, Th17/Tc17, and Th1/Th17-Tc1/Tc17 cells were shown in patients with vitiligo, suggesting possible migration of these cells into the skin [100]. Indeed, Th1/Th17 and Tc1/Tc17 cells have been touted as potential targets for therapy [101]. Importantly, one study has demonstrated that the production of IL-17 by T cells from vitiligo perilesional skin remains similar to the amount secreted by T cells from healthy skin, indicating that the Th17 pathway does not play an important role in melanocyte destruction [77]. In addition, more recent studies, evaluating the use of the anti-IL-17A biological agent secukinumab for the treatment of vitiligo, have confirmed that IL-17 or Th17 cells had no direct pathogenic role in vitiligo [102, 103].
Resident memory T cells and their function in vitiligo
The loss of pigmentation in therapeutically repigmented skin is common in patients with vitiligo, and is often seen in the first year following cessation of treatment [104]. One study showed that vitiligo patients treated with narrow band-ultraviolet B (NB-UVB) experienced such loss within 2 years of stopping treatment [105]. Notably, relapse occurs in the previously repigmented areas, suggesting that autoimmune memory plays a role in the skin that is not cleared by current treatments [43]. Resident memory T (Trm) cells are a subset of long-lived T cells that persist within most nonlymphoid tissues after inflammation or infection driven by T cells [106, 107].
Trm cells of the skin are known for their long-term residence in the skin. They patrol their surroundings, the epidermis and papillary dermis, and rapidly respond when they encounter their cognate antigen [108]. They are defined by the cell surface expression of CD69, CD103, CD49a, and CD44 [109]. CD103 is implicated in binding E-cadherin expressed on epidermal cells, thereby promoting Trm cell retention [110]. CD69 is required for the development of Trm cells in non-lymphoid tissues, including the skin [111]. Moreover, CD69 has been demonstrated to inhibit the function of sphingosine 1-phosphate receptor 1 (S1PR1) [111]. S1PR1 is required for lymphocyte exit from peripheral tissues, hence its suppression maintains Trm cell residence in the skin [112]. Upregulated expression of CD103 and CD96 on Tm cells is crucial for their development and survival as their deletion on virus-specific T cells caused a significant decrease in the quantity of Trm cell in the skin following a virus infection [113]. Different subsets of Trm cells were identified in vitiligo skin. One study found that stable and active vitiligo perilesional skin was highly infiltrated with a population of CD8 Trm cells that express both CD69 and CD103, compared with control normal human skin [77].
CD8 Trm cells that remain in stable vitiligo could be a vital mediator for disease flares [77]. In addition, CXCR3 was also reported to define a subset of Trm cells in vitiligo [77]. Vitiligo skin was shown to be enriched with CD8 Trm cells expressing CXCR3. Interestingly, high frequencies of melanocyte-specific CD8 cells were demonstrated within CXCR3+ subsets [77].
A study showed that lesional skin from vitiligo patients contained an accumulation of a subset of CD8+ Trm cells characterized by CD49a+ [114]. Activated CD8+ CD49a+ Trm cells were found to be involved in the stimulation of IFN-γ, granzyme B and perforin due to the induction IL-15, and thus promoting a high cytotoxic response [114] (Fig. 1). One study found that human and mouse Trm cells in vitiligo expressed high level of CD122, a subunit of IL-15 receptor, in the blood and lesional skin, and that lesional keratinocytes upregulated CD215 expression [115]. Interestingly, IL-15 is secreted in different peripheral tissues via myeloid and stroma cells and it is most frequently trans-presented to T cells on the surface of myeloid and stromal cells that express CD215, which is required to display the cytokine on the cell surface to induce activation of T cells [116].
Importantly, melanocyte-specific T cells from mouse and human vitiligo express significantly higher levels of CD122 than endogenous memory T cells, indicating that autoreactive T cells are more reliant on this cytokine than are non-autoreactive T cells [115]. Treatment with anti-CD122 antibody reversed disease in mouse vitiligo, inhibited Trm secretion of IFN-γ and depleted Trm cell from skin lesions, while leaving endogenous memory T cells unaffected [115]. Both systemic and local short-term treatment, through injection with anti-CD122 antibody, provided durable repigmentation [115]. These findings are in agreement with there being an essential role for IL-15 in Trm cell maintenance in vitiligo and we propose that targeting CD122 could be highly effective treatment strategy for patients with vitiligo [43].
Very recently, NKG2D was found to define a subset of effector memory T cells with proinflammatory functions in vitiligo [117]. NKG2D+ CD8+ effector memory T cells were characterized by an activated phenotype and have a great ability to secrete high amount of both IFN-γ and TNF-α [117]. The NKG2D ligands MICA-MICB were shown to be expressed by dendritic cells that infiltrated vitiligo skin upon inflammation and could signify a cell subset capable of promoting activation of NKG2D+ CD8+ effector memory T cells in the skin [117]. Interaction of NKG2D with its corresponding ligands MICA-MICB potentiated the synthesis of IFN-γ and TNF-α by CD8+ effector memory T cells [117]. These results infer that NKG2D may play an important role in vitiligo progression, and it could be an interesting target to treat vitiligo [117].
As Trm cells are able to live in tissues for long periods and rapidly evoke immunity reactions against viruses, they were considered strong candidates for stimulating relapse in vitiligo [43]. Trm cell function has been the subject of debate. In some models, these cells sufficiently control viral titres during re-infection with no need for recirculating cells [118, 119]. In other contexts, they have been seen to mainly synthesize cytokines for effector T cell recruitment from the circulation. However, CD8+ Trm cells are shown to have low cytotoxic potential.
In normal human skin, CD8+ Trm cells demonstrated low levels of granzyme B and perforin [120]. Moreover, the effect of CD8+ Trm cells isolated from normal human skin on lysing allogenic target cells were significantly poorer than circulating effector memory T cells [121]. Trm cells mediate the immune response to melanoma via inhibiting tumour outgrowth instead of tumour elimination, indicating that Trm cells lack cytotoxic ability [122]. Based on their low cytotoxic activity and efficient ability of producing cytokines, Trm cells may be involved in recruitment of effector cells from circulation [43]. In vitiligo mouse model, it was found that blockade of T-cell migration to the skin or depletion of recirculating memory T cells reversed vitiligo, although the number of Trm cells was not affected [123]. It was therefore concluded that Trm cells cannot independently maintain vitiligo in the absence of recruitment of further T cells [123]. In this vitiligo mouse model, autoreactive melanocyte-specific CD8+ Trm cells were shown to synthesize IFN-γ and CXCL10, probably employing the IFN-γ-chemokine pathway to maintain vitiligo lesions [123]. Thus, Trm cells are likely to mediate the long-term maintenance and relapse of the disease via cytokine-mediated recruitment of circulating T cells [43].
Notably, patients with stable vitiligo are considered to have a continuing immune response, as evidenced by the existence of Trm cells in perilesional skin [77, 115]. These may be involved during a flare-up, as, by analogy, in psoriasis [124].
Humoral immunity in vitiligo
Besides cellular immunity, humoral (antibody-mediated) immunity adds supportive evidence for a pathological role of autoimmunity in vitiligo. Different antibodies to melanocytes have been identified at significantly elevated levels in the sera of vitiligo patients as compared to healthy controls [125–128], and their levels are directly associated with the extent and activity of vitiligo [126, 129, 130], being present in 93% of patients with wider depigmentation (5–10% skin area involved) and in only 50% of patients with minimal pigment loss (<2% skin area involved) [131]. These anti-melanocyte antibodies belong to the immunoglobulin G (IgG) class [128], including subclasses IgG1, IgG2, and IgG3 [132], though IgA anti-melanocyte antibodies have also been detected [133].
Immunoprecipitation studies using mela-nocyte protein extracts have shown that antibodies in vitiligo patients are most frequently directed to antigens with molecular weights of 35, 40–45, 75, 90, and 150 kDa, these being found on the cell surface [134]. Some of these proteins, including those of 40–45, 75, and 150 kDa, appear to be common tissue antigens, whereas the 35 and 90 kDa proteins are preferentially expressed on melanocytes [135]. Other researchers have identified vitiligo antibody targets of 45, 65, 70, 88, and 110 kDa, specifically expressed in melanocytes [136]. Various melanocyte-associated autoantigens have been reported. Antibodies against tyrosinase, a melanocyte-specific protein, have been extensively detected [137–139], as have antibodies against other proteins of the melanogenic pathway such as L-dopachrome tautomerase, TYRP1, and PMEL, albeit at a low prevalence [140–142]. Considering this autoantibody response, rituximab, a monoclonal directed against CD20 protein expressed on the B cell surface, has shown promise in a clinical trial [143]. A variety of circulating organ-specific antibodies, for example, against gastric parietal cells, pancreatic islet cells, thyroid and adrenal glands, are common in vitiligo patients’ sera, though these are not recognized as major melanocyte antigens [144].
Phage display technology has identified other targets such as melanin-concentrating hormone receptor 1, tyrosine hydroxylase, heat-shock protein 90, osteopontin, ubiquitin-conjugating enzyme, translation-initiation factor 2, GTP-binding protein Rab38, γ-enolase, α-enolase, and lamin A [145–147], as well as four novel autoantigens glycoprotein non-metastatic melanoma protein b, melanocortin 1 receptor, OCA2-encoded P protein and GTP-binding protein Rab27A (unpublished data). Vitiligo-associated antibodies are capable of melanocytotoxicity in vitro and in vivo by complement-mediated cytotoxicity and by antibody-dependent cellular cytotoxicity [148, 149]. Melanin-concentrating hormone receptor 1 blocks the function of the receptor, though it is not known if this affects melanocyte function [150]. Melanocyte expressed of HLA-DR and intercellular adhesion molecule-1 (ICAM-1) induced by anti-melanocyte antibodies may make melanocytes a target for cytotoxic T cells [151]. Presently, it is unclear whether pigment cells are a primary or secondary target of the humoral immunity in vitiligo. Autoantibodies might arise from a genetic susceptibility to immune dysregulation at the T or B lymphocyte level, with lack of tolerance to pigment cell antigens, or from an immune response against melanocytes damaged by other mechanisms, such as T-cell destruction [152].
Repigmentation observed in vitiligo patients receiving immunosuppressive treatments supports the notion of an immune-mediated process in vitiligo [13]. Tacrolimus, a reagent that suppresses T cells by blocking cytokine gene-activating cofactor calcineurin, works in vitiligo [153, 154], as do topical corticosteroids, which suppress T lymphocyte activity and B-cell antibody responses [155]. Phototherapy reduces the number of Langerhans cells in the skin and downregulates expression of vitiligo-associated melanocyte antigens [156].
Pathogenic mechanisms of autoimmunity in vitiligo
The ability of vitiligo-associated antibodies to destroy melanocytes has been demonstrated in vitro by both complement-mediated cytotoxicity and antibody-dependent cellular cytotoxicity [148, 150, 157].
In vivo, the administration of IgG from vitiligo patients into human skin grafted onto nude mice has been shown to induce melanocyte destruction [149]. In a reconstructed epidermis model, sera from 9 out of 13 (69%) vitiligo patients induced the detachment of melanocytes, although this was not related to disease extent nor activity [158]. Vitiligo patient antibodies against MCHR1 were demonstrated to block the function of the receptor [150]. However, it is not known if or how this activity could affect the melanocyte function [150]. Moreover, IgG anti-melanocyte antibodies are able to induce the expression of HLA-DR and ICAM-1 on melanocytes and the release of IL-8 from melanocytes [151]. By enhancing the antigen-presenting activity of melanocytes in this way, they become targets for cytotoxic T cells [159]. Interestingly, the normally intracellular melanocyte antigens TYRP1 and PMEL can be expressed on the cell surface and so can be accessible by antibodies [160, 161]. In addition, pigment cell antibodies in vitiligo might be secondary to destruction of the melanocyte from another immune cause such as T cells, but that once triggered the antibodies are themselves destructive to melanocytes.
While the potential for cytotoxic CD8+ T cells to eliminate melanocytes in both vitiligo and the immunotherapy of malignant melanoma is clear, the exact mechanism that these cells use is not fully understood. Several cytotoxic effector proteins such as perforin, granzyme, Fas ligand, and cytokines can be used to destroy target cells [162]. It is believed that cytotoxic T cells mainly use perforin and granzyne as fast-acting method of destroying cancer cells or virus-infected cells, whereas Fas ligand-driven killing mechanism may act as slower-acting alternative process [163].
In fact, several intracellular signalling pathways promote cytotoxic T-cell killing via perforin, granzyme, and Fas ligand, and therefore it is currently unclear how these pathways are selectively used by T cells and how they communicate [162]. T-cell-mediated killing mechanism in autoimmune vitiligo may differ from that of cancer or viral-infected cells. Therefore, how melanocytes in vitiligo are eliminated is not yet clear. This requires further studies in order to reveal the exact mechanism involved in details. Genes implicated in antigen presentation confer a significant risk for the development of vitiligo [164]. One hypothesis suggests that modified proteins called neo-antigen can be extremely immunogenic, stimulating an immune response against them, since thymic epithelial cells responsible for T-cell education do not synthesize such proteins [165]. This leads to the formation of highly self-reactive T cells with high-affinity receptors that target neo-antigens [165]. Malignant melanoma is immunogenic owing to somatic DNA mutation that result in neo-antigen generation [166]. However, self-reactive T cells are unlikely to target mutated proteins in vitiligo, since untransformed melanocytes do not have the ability to mutate their DNA [43]. Several biochemical processes have been involved in neo-antigen formation as well such processes as deamidation, carbanylation, citrullination, oxidation, and alternative mRNA splicing [42]. These activities are implicated in generating neo-antigens in untransformed beta islet cells [42].
Cytokines imbalance and their role in vitiligo pathogenesis
Cytokine imbalance has been shown in vitiligo skin [167]. Elevated serum levels of soluble IL-2 receptor, which correlates with disease activity in vitiligo patients, have been reported, as have increased synthesis of IL-6, a cytokine that induces ICAM-1 expression on melanocytes which could facilitate leukocyte–melanocyte interaction, in addition to elevated levels of IL-8, a recruiter of neutrophils, T lymphocytes, and basophils [168, 169]. Recently, it was described that T helper (TH) 1 (IL-2, IFN-γ, TNF-β), Th2 (IL-4, IL-5, IL-10, IL-13), and Th17 (IL-17, IL-23)-innate cytokines were elevated in the sera of all 44 vitiligo patients examined, with a higher ratio of Th1/Th2 cytokines [170]. Expression of the pro-inflammatory cytokine TNF-α is significantly high in lesional and peri-lesional vitiligo patient skin, whereas a variety of melanogenic mediators such as endothelin-1, stem cell factor, basic fibroblastic growth factor, and granulocyte monocyte-colony stimulating factor are expressed at lower levels in vitiligo patches [171]. Melanocyte adhesion is disrupted in vitiligo skin through increased levels of MMP-9, produced by keratinocytes in response to IFN-γ and TNF-α, and which disturbs E-cadherin on the pigment cells [172]. In both human vitiligo and in a mouse model, high levels of IFN-γ, the cytokine required for the cutaneous recruitment of melanocyte-specific autoreactive CD8+ T lymphocytes, and of IFN-γ-induced cytokine CXCL10, and its receptor CXCR3, found on autoreactive CD8+ T cells, have been demonstrated [79]. Knocking out CXCR3 or blocking CXCL10 action prevents and reverses depigmentation in vitiligo [173].
Malignant melanoma-associated vitiligo
Malignant melanoma is a type of skin cancer resulted from uncontrolled growth of melanocytes. Although the exact mechanism implicated in the pathogenesis of vitiligo associated with malignant melanoma is still unclear, the immune reactivity against malignant melanoma cells, especially CD8+ T cells, is thought to play a critical role [174].
Malignant melanoma-associated vitiligo may arise from immune response directed against antigens expressed by both melanocytes and malignant cells. Indeed, antibodies reactive to tyrosinase [175], TYRP1, dopachrome tautomerase, and Pmel17 [176] have been found in the sera of some patients with malignant melanoma. These melanocyte-specific antigens were recognized by self-reactive CD8+ T cells [177]. Following immunotherapy for metastatic malignant melanoma, enhanced efficacy is associated with CD8+ T cells. Immunotherapy for malignant melanoma involves blocking T-cell checkpoint inhibitors, which interfere with T-cell tolerance in tissues, and adoptive cell therapy, in which T cells that infiltrate autologous tumour are expanded ex vivo for therapeutic reinjection [43]. Importantly, tumour infiltration with CD8+ T cells is vital in the effectiveness of both strategies [178]. These cells are thought to regulate malignant melanoma via perforin-dependent cytolysis [162].
New-onset vitiligo occurs in about 4% of patients treated with immunotherapy [179]. In patients suffering from melanoma, treatment with the immune checkpoint inhibitors; anti-PD-1 therapy, is correlated with development of vitiligo [180]. One study showed that vitiligo occurs following anti-DP-1 treatment in one of four melanoma patients but not in other cancers, indicating that vitiligo is melanocyte lineage-specific immune adverse event [180]. The same study revealed that development of vitiligo in patients suffering from melanoma is correlated with greater response rates to anti-PD-1 therapy [180].
Notably, vitiligo patches initiated by malignant melanoma immunotherapy are packed with CD8+ T cells that are specific to melanocytes, in a similar manner to the situation in idiopathic vitiligo [181]. More recent study demonstrated that in patients with melanoma, anti-PD-1 treatment resulted in an expansion of melanocyte-specific CD8+ T cells and only 5 of 13 responders developed vitiligo [182]. Thus, CD8+ T cells are crucial to both the eradication of malignant melanoma and the pathogenesis of vitiligo. Therefore, the immune response in malignant melanoma patients which causes melanocyte damage is suggested to be cell-mediated, driven by CD8+ T cells and not humoral. Melanocyte-specific antibodies in malignant melanoma are likely to arise as a secondary immune response after melanocyte destruction via cell-mediated effects. The serum titres of malignant melanoma-related antibodies are low and their levels do not differ in patients with and without malignant melanoma-associated vitiligo. Vitiligo-like lesions in malignant melanoma patients receiving immunotherapy are considered as a good prognostic factor, correlated with a longer survival [183]. Therefore, the relationship between vitiligo and melanoma is suggested to be the result of an immune response to antigens shared by normal melanocytes and melanoma cells. However, it is not clear whether the autoimmune response against normal melanocyte antigens contributes to clearance of melanoma or whether development of vitiligo is merely a correlating adverse event [182]. In addition, there appears to be mutually exclusive relationship between a predisposition to vitiligo and a predisposition to melanoma, which implies a genetic dysregulation of the immune surveillance affecting melanocytic system [184]. Of particular interest is the association found in European-derived whites between vitiligo and SNPs in TYR, the gene encoding tyrosinase [185]. The causal genetic variation of TYR is inversely correlated between vitiligo and malignant melanoma [186], thus suggesting vitiligo may develop from the dysregulation of normal immune surveillance against melanoma [186].
Interestingly, a correlation between the minor TYR allele, 402Q and predisposition to malignant melanoma has been described [187], suggesting that such a variant may be less accessible to the immune defences. Therefore, in melanoma cases with the 402Q variant, these neoplastic melanocytes perhaps evade immune surveillance [185, 186]. On the other hand, the TYR 402R variant possibly presented to the immune defences more effectively than 402Q variant leading to a more efficient immunological responses to neoplastic melanocytes, but equally contributes to susceptibility to vitiligo [185, 186]. Better understanding of immunity against melanocytes in patients with melanoma, who develop vitiligo, may help in the identification of more targets for immunotherapy against both melanoma and vitiligo.
Genetic factors
Genetic involvement in vitiligo is obvious from a simple examination of family histories: 15–20% of vitiligo patients have at least one affected first-grade relative [188]. However, vitiligo does not show a Mendelian mechanism of inheritance, but is polygenic with multiple susceptibility loci [164].
The concordance rate in monozygotic twins is 23%, suggesting involvement of non-genetic factors [189]. Genome-wide association studies have revealed that approximately half of vitiligo susceptibility genes encode immune-regulatory proteins, while the remainder encode melanocyte-specific proteins [164].
Several studies have shown that multiple loci contribute to vitiligo susceptibility (Table 1). An observed association between HLA types and vitiligo, and other autoimmune disorders, can be elucidated by several pathways that ultimately result in disruption in self-antigen recognition. This can lead to autoreactive T-cell development and/or failure to produce effective Tregs population [68]. Genome-wide association studies have also reported a subset of other immune regulatory genes that are key mediators of adaptive immunity, such as CD80 (activation of T cells), cytotoxic T lymphocyte antigen-4; CTLA4 (inhibition of T cells), GZMB (cytotoxicity of T cells), forkhead box protein P3; FOXP3 (development and function of regulatory T cells), lymphoid protein tyrosine phosphatase non-receptor type 22; PTPN22 (down-regulation of T-cell activation), and arginine–glutamic acid dipeptide repeats protein – RERE (regulation of cell apoptosis) [190]. An association of vitiligo with genes that play a role in innate immunity, such as NACHT leucine-rich-repeat protein 1 (NLRP1), interferon-induced helicase C domain 1 (IFIH1) and caspase-7 (CASP7), has also been found in genome-wide association studies [191]. In addition to immune regulatory genes, several genes that are only expressed in melanocytes and involved in melanocyte function have been identified as vitiligo susceptibility loci. These include TYR, PMEL, melanocortin 1 receptor (MC1R), OCA2 [192]. Such genes encode for enzymes or proteins recognized as autoantigens in vitiligo, facilitating an anti-melanocyte autoimmune response [139, 141].
Table 1.
A summary of genes associated with susceptibility to vitiligo
| Type | Gene | Protein | Function | References |
|---|---|---|---|---|
| HLA | HLA–A | HLA class I histocompatibility antigen, A | Peptide antigen presentation to the immune system | [185, 193, 194] |
| HLA-DRB1 and DQA1 | HLA class II histocompatibility antigen, DRB1 and DQA1 | Peptide antigen presentation to the immune system | [185, 195] | |
| HLA class III | HLA class III histocompatibility antigen | Involved in inflammation and cytokine production | [196] | |
| Immune-regulatory | AIRE | Autoimmune regulator | Maintenance of immune tolerance | [197, 198] |
| BACH2 | Transcription regulator protein BACH2 | B cell transcriptional repressor encodes a transcriptional repressor of B cells, which is a key regulator of CD4+ T-cell differentiation that prevents inflammatory disease by controlling the balance between tolerance and immunity | [191] | |
| C1QTNF6 | Complement C1q tumour necrosis factor-related protein 6 | Induces monocyte IL-10 expression | [185] | |
| CASP7 | Caspase-7 | Executor protein of apoptosis and inflammation | [191] | |
| CCR6 | Chemokine-cytokine receptor 6 | Regulates differentiation and function of B and T lymphocytes and dendritic cells | [185, 196] | |
| CD44 | CD44 antigen | Involves in T-cell development | [191] | |
| CD80 | Tlymphocyte activation antigen CD80 | Co-stimulates activation of T cells | [191] | |
| CLNK | Cytokine-dependent hematopoietic cell linker | Positively regulates immune-receptor signalling | [191] | |
| CTLA4 | Cytotoxic Tlymphocyte protein 4 | Inhibition of T cells | [199–201] | |
| CXCR5 | C-X-C chemokine receptor type 5 | Involves in B lymphocyte migration | [192] | |
| FASLG | FAS ligand | Regulate immune apoptosis | [202] | |
| FOXP1 | Forkhead box protein P1 | Regulates B cell development | [203] | |
| FOXP3 | Forkhead box protein P3 | Regulates development and function of regulatory T cells | [85] | |
| GZMB | Granzyme B | Mediates the process of immune-induced cell death with contribution of cytotoxic T lymphocytes and natural killer cells | [185, 204] | |
| IFIH1 | Interferon-induced helicase C domain 1 | Regulates innate immune response | [191] | |
| IKZF4 | Zinc finger protein Eos | Regulates T-cell activation | [191, 192] | |
| IL2RA | Interleukin2 receptor subunit alpha | Regulates regulatory T cells | [185, 192] | |
| NLRP1 | NACHT leucine-rich-repeat protein 1 | Regulates innate immune system | [45, 205–207] | |
| PTPN22 | Lymphoid protein tyrosine phosphatase non-receptor type 22 | Negative regulation of T-cell activation | [185, 208–213] | |
| SH2B3 | SH2B adapter protein 3 | Development of B and T lymphocytes | [191] | |
| SLA | Src-like-adapter | Regulates antigen receptor signalling | [191] | |
| TOB2 | Protein TOB2 | Involves in T-cell tolerance | [191] | |
| TSLP | Thymic stromal lymphopoietin | Cytokine regulator of maturation of skin dendritic cells and T cells | [85] | |
| UBASH3A | Ubiquitin-associated and SH3 domain–containing A protein | Suppresses T-cell receptor signalling | [185] | |
| XBP1 | Xbox-binding protein 1 | Regulator of major histocompatibility complex class II | [39, 85] | |
| Melanocyte function | ASP | Agouti signalling protein | Melanogenesis regulator via MC1R | [214] |
| FOXD3 | Forkhead box D3 | Regulator of melanoblast development | [215] | |
| MC1R | Melanocortin 1 receptor | Regulator of melanogenesis | [191, 214] | |
| OCA2 | OCA2 gene | Melanosomal transporter | [191] | |
| PMEL | Melanocyte-specific protein PMEL | Structural organization of premelanosomes | [192] | |
| TYR | Tyrosinase | Regulator of melanogenesis | [185, 216] | |
| ZMIZ1 | Zinc finger protein MIZ type 1 | Regulates development, function and survival of melanocytes | [196, 217] | |
| Metabolism-related genes | ACE | Angiotensin-converting enzyme | Regulator of inflammation and blood pressure | [218] |
| CAT | Catalase | Protects cells from oxidative stress by breakdown of hydrogen peroxide | [219–221] | |
| EDN-1 | Endothelin-1 | Regulator of melanocyte growth and function | [222] | |
| LPP | Lipoma-preferred partner | Potential coactivator | [185] | |
| RERE | Arginine–glutamic acid dipeptide repeats protein | Regulates cell apoptosis | [185] | |
| RNASET2 | Ribonuclease T2 | Oxidative stress regulator | [196] |
Neuronal theory, linking neuropeptides and the immune response, and the convergence theory
Neuronal elements were originally thought to have a role in vitiligo through catecholamine released from epidermal nerve endings, which might be cytotoxic to melanocytes, or by autonomic dysfunction producing pigment cell destruction [223, 224]. Clinical pointers towards neural involvement included the distribution along blaschkoid lines in segmental vitligo, and the occasional observation of a true dermatomal appearance, e.g. in the trigeminal areas [190, 224].
Ultrastructurally, vitiligo skin shows degeneration of fine cutaneous nerves in 42% of cases, with Schwann cells showing thickened basement membrane in 75% of instances and axonal damage in a half [223, 225]. Changes in neuropeptides, notably neuropeptide Y, are evident at the margins of vitiligo patches [225]. This led to the postulation that neuropeptides, potentially neuropeptide Y, which has effects on the immune system through receptors located on several immunologically active cells including T cells, NK cells, dendritic cells, granulocytes, and macrophages, released from nerve ending release next to melanocytes, could provoke a local immune reaction and melanocyte destruction [226, 227].
Treg cells, seen as central to the mechanism of melanocyte destruction in vitiligo, can be induced by vasoactive intestinal polypeptide, and can alter the Th1/cytotoxic T-cell (Tc)1 balance that is skewed in vitiligo [228]. Hence, the neural and neuro-endocrine systems, neuro-peptides and neurotransmitters, inter-acting with the immune systems, need to be taken into account in the causation of vitiligo [229].
The convergence theory attempts to link together the potential causal mechanisms of vitiligo [230]. It suggests multiple sequential stages in pathogenesis beginning with an elicitation stage perhaps due to mechanical friction and skin trauma, emotional stresses, chemical exposure or imbalances in endogenous neural factors, metabolites, cytokines or hormones [5, 226, 231–234]. Such factors, in an individual whose genetic make-up predisposes to immune activation and melanocyte destruction, it is proposed, result in oxidative stress within melanocytes which subsequently express HSP70 and chaperoned melanocyte antigens, presented by dendritic cells to T cells in regional lymph nodes, resulting in proliferation of melanocyte-specific cytotoxic T cells and melanocyte destruction – the stage of immune activation [60, 152, 189]. Absent or reduced fully-functional skin-infiltrating Tregs contribute to the on-going immune response and disease spread [95]. Antibodies against melanocyte-specific proteins such as tyrosinase, generated in response to melanocyte destruction, damage pigment cells by activating complement or by antibody-dependent cellular cytotoxicity [139, 150].
Conclusion and prospects
Sufficient is now understood about the pathogenesis of vitiligo to permit targeted pharmacological intervention at the appropriate immunological steps. However, the exact modus by which the genetic interacts with the environment, and with the immune system still requires considerable elucidation. What can be said is that both environmental factors and cell-intrinsic defects instigate stress responses in melanocytes, resulting in the synthesis of DAMPs that elicit innate immune cells, which in turn activate adaptive immunity that ultimately targets melanocytes.
A genetic predisposition to autoimmunity may underlie inappropriate immune responses in vitiligo, but immune responses may occur secondarily as a result of melanocyte damage by other factors, as when autoantibodies have been observed directed against intracellular pigment cell proteins – exposure of cryptic epitopes and protein modification occurring during apoptosis [235]. Following processing by dendritic cells, antigenic proteins can be presented to either autoreactive T cells, which have evaded clonal deletion, or to naive T cells, which have not been tolerized against cryptic epitopes [236]. In consequence, antibodies can be secreted following autoreactive B-cell stimulation by activated autoreactive CD4+ T lymphocytes [236], which may then act to further aggravate vitiligo.
However, it is possible that antibodies play no part in the pathogenesis of vitiligo, but might indicate the existence of autoreactive anti-melanocyte T cells capable of destroying melanocytes, a scenario that merits further investigation.
Acknowledgement
S.F. was sponsored by the Ministry of Education in Libya and by the University of Gharyan, Gharyan, Libya.
Glossary
Abbreviations
- UV
Ultraviolet
- TYRP1
tyrosinase-related protein-1
- ROS
reactive oxygen species
- DAMP
damage-associated molecular patterns
- PRRs
pattern recognition receptors
- NOD
nucleotide oligomerization domain
- NLRs
nucleotide oligomerization domain-like receptors
- PAMPs
pathogen-associated molecular patterns
- GZMB
granzyme-B
- NK
natural killer
- HSP
heat shock protein
- IL
interleukin
- TNF-α
tumour necrosis factor-α
- HMGB1
High-mobility group protein B1
- pDCs
plasmacytoid dendritic cells
- ILCs
innate lymphoid cells
- NLRP3
NLR family pyrin domain containing 3
- NF-kB
nuclear factor kB
- ASC
apoptosis-associated speck-like protein containing a CARD
- TRPM2
transient receptor potential cation channel subfamily M member 2
- CXCR3
C-X-C chemokine receptor type 3
- JAK
janus kinase
- Treg
Regulatory T
- Foxp3
forkhead box P3
- CAR
chimeric antigen receptor
- NB-UVB
narrow band-ultraviolet B
- Trm
memory T
- ICAM-1
intercellular adhesion molecule-1
- CTLA4
cytotoxic T lymphocyte antigen-4
- NLRP1
NACHT leucine-rich-repeat protein 1
- IFIH1
interferon-induced helicase C domain 1
- CASP7
caspase-7
- MC1R
melanocortin 1 receptor
- Tc
cytotoxic T cell
- TYRP1
tyrosinase-related protein-1
- PRRs
pattern recognition receptors
- HMGB1
high-mobility group protein B1
- IFN-γ
interferon-γ
Conflict of interest
The authors identify no conflicts of interest. No funding was received for this paper. Ethics approval was not required.
Funding
No funding was made for this paper.
Author contributions
All authors contributed to the manuscript.
Data availability
Not applicable to this paper.
References
- 1. Ezzedine K, Lim HW, Suzuki T, et al.; Vitiligo Global Issue Consensus Conference Panelists . Revised classification/nomenclature of vitiligo and related issues: the Vitiligo Global Issues Consensus Conference. Pigment Cell Melanoma Res 2012, 25, E1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Manga P, Elbuluk N, Orlow SJ.. Recent advances in understanding vitiligo. F1000Research 2016, 5, 2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Al Abadie MS, Gawkrodger DJ.. Integrating neuronal involvement into the immune and genetic paradigm of vitiligo. Clin Exp Dermatol 2021, 46, 646–50. [DOI] [PubMed] [Google Scholar]
- 4. Zhang Y, Cai Y, Shi M, et al.. The prevalence of vitiligo: a meta-analysis. PLoS One 2016, 11, e0163806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kent G, Al’Abadie M.. Psychologic effects of vitiligo: a critical incident analysis. J Am Acad Dermatol 1996, 35, 895–8. [DOI] [PubMed] [Google Scholar]
- 6. Nogueira LS, Zancanaro PC, Azambuja RD.. Vitiligo and emotions. An Bras Dermatol 2009, 84, 41–5. [DOI] [PubMed] [Google Scholar]
- 7. Kostopoulou P, Jouary T, Quintard B, et al.. Objective vs. subjective factors in the psychological impact of vitiligo: the experience from a French referral centre. Br J Dermatol 2009, 161, 128–33. [DOI] [PubMed] [Google Scholar]
- 8. Parsad D, Dogra S, Kanwar AJ.. Quality of life in patients with vitiligo. Health Qual Life Outcomes 2003, 1, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Porter J, Beuf A, Nordlund JJ, Lerner AB.. Personal responses of patients to vitiligo: the importance of the patient-physician interaction. Arch Dermatol 1978, 114, 1384–5. [PubMed] [Google Scholar]
- 10. Sukan M, Maner F.. The problems in sexual functions of vitiligo and chronic urticaria patients. J Sex Marital Ther 2007, 33, 55–64. [DOI] [PubMed] [Google Scholar]
- 11. Alikhan A, Felsten LM, Daly M, Petronic-Rosic V.. Vitiligo: a comprehensive overview Part I. Introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol 2011, 65, 473–91. [DOI] [PubMed] [Google Scholar]
- 12. Parsad D, Pandhi R, Dogra S, Kanwar AJ, Kumar B.. Dermatology Life Quality Index score in vitiligo and its impact on the treatment outcome. Br J Dermatol 2003, 148, 373–4. [DOI] [PubMed] [Google Scholar]
- 13. Ezzedine K, Eleftheriadou V, Whitton M, van Geel N.. Vitiligo. Lancet 2015, 386, 74–84. [DOI] [PubMed] [Google Scholar]
- 14. Boissy RE, Manga P.. On the etiology of contact/occupational vitiligo. Pigment Cell Res 2004, 17, 208–14. [DOI] [PubMed] [Google Scholar]
- 15. Relke N, Gooderham M.. The use of Janus kinase inhibitors in vitiligo: a review of the literature. J Cutan Med Surg 2019, 23, 298–306. [DOI] [PubMed] [Google Scholar]
- 16. Tobin DJ, Swanson NN, Pittelkow MR, Peters EM, Schallreuter KU.. Melanocytes are not absent in lesional skin of long duration vitiligo. J Pathol 2000, 191, 407–16. [DOI] [PubMed] [Google Scholar]
- 17. Ackerman AB, Kerl H, Sánchez J.. A Clinical Atlas of 101 Common Skin Diseases: with Histopathologic Correlation. New York: Ardor Scribendi, 2000. [Google Scholar]
- 18. van den Wijngaard R, Wankowicz-Kalinska A, Le Poole C, Tigges B, Westerhof W, Das P.. Local immune response in skin of generalized vitiligo patients. Destruction of melanocytes is associated with the prominent presence of CLA+ T cells at the perilesional site. Lab Invest 2000, 80, 1299–309. [DOI] [PubMed] [Google Scholar]
- 19. Puri N, Mojamdar M, Ramaiah A.. In vitro growth characteristics of melanocytes obtained from adult normal and vitiligo subjects. J Invest Dermatol 1987, 88, 434–8. [DOI] [PubMed] [Google Scholar]
- 20. Puri N, Mojamdar M, Ramaiah A.. Growth defects of melanocytes in culture from vitiligo subjects are spontaneously corrected in vivo in repigmenting subjects and can be partially corrected by the addition of fibroblast-derived growth factors in vitro. Arch Dermatol Res 1989, 281, 178–84. [DOI] [PubMed] [Google Scholar]
- 21. Dell’Anna ML, Mastrofrancesco A, Sala R, et al.. Antioxidants and narrow band-UVB in the treatment of vitiligo: a double-blind placebo controlled trial. Clin Exp Dermatol 2007, 32, 631–6. [DOI] [PubMed] [Google Scholar]
- 22. Schallreuter KU, Moore J, Wood JM, et al.. In vivo and in vitro evidence for hydrogen peroxide (H2O2) accumulation in the epidermis of patients with vitiligo and its successful removal by a UVB-activated pseudocatalase. J Investig Dermatol Symp Proc 1999, 4, 91–6. [DOI] [PubMed] [Google Scholar]
- 23. Medrano EE, Nordlund JJ.. Successful culture of adult human melanocytes obtained from normal and vitiligo donors. J Invest Dermatol 1990, 95, 441–5. [PubMed] [Google Scholar]
- 24. Dell’Anna ML, Ottaviani M, Albanesi V, et al.. Membrane lipid alterations as a possible basis for melanocyte degeneration in vitiligo. J Invest Dermatol 2007, 127, 1226–33. [DOI] [PubMed] [Google Scholar]
- 25. Shalbaf M, Gibbons NC, Wood JM, et al.. Presence of epidermal allantoin further supports oxidative stress in vitiligo. Exp Dermatol 2008, 17, 761–70. [DOI] [PubMed] [Google Scholar]
- 26. Maresca V, Roccella M, Roccella F, et al.. Increased sensitivity to peroxidative agents as a possible pathogenic factor of melanocyte damage in vitiligo. J Invest Dermatol 1997, 109, 310–3. [DOI] [PubMed] [Google Scholar]
- 27. Jimbow K, Chen H, Park JS, Thomas PD.. Increased sensitivity of melanocytes to oxidative stress and abnormal expression of tyrosinase-related protein in vitiligo. Br J Dermatol 2001, 144, 55–65. [DOI] [PubMed] [Google Scholar]
- 28. Gawkrodger DJ. Pseudocatalase and narrowband ultraviolet B for vitiligo: clearing the picture. Br J Dermatol 2009, 161, 721–2. [DOI] [PubMed] [Google Scholar]
- 29. Spielvogel RL, Kantor GR.. Pigmentary disorders of the skin. In: Elder DE, Elenitsas R, Johansson BL, Murphy GF (eds), Lever’s Histopathology of the Skin. Philadelphia: Lippincott Williams & Wilkins, 2005, 705–13. [Google Scholar]
- 30. Gilhar A, Pillar T, Eidelman S, Etzioni A.. Vitiligo and idiopathic guttate hypomelanosis. Repigmentation of skin following engraftment onto nude mice. Arch Dermatol 1989, 125, 1363–6. [DOI] [PubMed] [Google Scholar]
- 31. Li W, Wang S, Xu AE.. Role of in vivo reflectance confocal microscopy in determining stability in vitiligo: a preliminary study. Indian J Dermatol 2013, 58, 429–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rashighi M, Harris JE.. Vitiligo pathogenesis and emerging treatments. Dermatol Clin 2017, 35, 257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sravani PV, Babu NK, Gopal KV, et al.. Determination of oxidative stress in vitiligo by measuring superoxide dismutase and catalase levels in vitiliginous and non-vitiliginous skin. Indian J Dermatol Venereol Leprol 2009, 75, 268–71. [DOI] [PubMed] [Google Scholar]
- 34. Schallreuter KU, Moore J, Wood JM, et al.. Epidermal H(2)O(2) accumulation alters tetrahydrobiopterin (6BH4) recycling in vitiligo: identification of a general mechanism in regulation of all 6BH4-dependent processes? J Invest Dermatol 2001, 116, 167–74. [DOI] [PubMed] [Google Scholar]
- 35. Xie H, Zhou F, Liu L, et al.. Vitiligo: How do oxidative stress-induced autoantigens trigger autoimmunity? J Dermatol Sci 2016, 81, 3–9. [DOI] [PubMed] [Google Scholar]
- 36. Wang Y, Li S, Li C.. Perspectives of new advances in the pathogenesis of vitiligo: from oxidative stress to autoimmunity. Med Sci Monit 2019, 25, 1017–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ghanem G, Fabrice J.. Tyrosinase related protein 1 (TYRP1/gp75) in human cutaneous melanoma. Mol Oncol 2011, 5, 150–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Todd DJ, Lee AH, Glimcher LH.. The endoplasmic reticulum stress response in immunity and autoimmunity. Nat Rev Immunol 2008, 8, 663–74. [DOI] [PubMed] [Google Scholar]
- 39. Ren Y, Yang S, Xu S, et al.. Genetic variation of promoter sequence modulates XBP1 expression and genetic risk for vitiligo. PLoS Genet 2009, 5, e1000523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Toosi S, Orlow SJ, Manga P.. Vitiligo-inducing phenols activate the unfolded protein response in melanocytes resulting in upregulation of IL6 and IL8. J Invest Dermatol 2012, 132, 2601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Singh M, Jadeja SD, Vaishnav J, et al.. Investigation of the role of interleukin 6 in vitiligo pathogenesis. Immunol Invest 2020, 1–18. [DOI] [PubMed] [Google Scholar]
- 42. James EA, Pietropaolo M, Mamula MJ.. Immune recognition of β-Cells: Neoepitopes as key players in the loss of tolerance. Diabetes 2018, 67, 1035–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Frisoli ML, Essien K, Harris JE.. Vitiligo: Mechanisms of pathogenesis and treatment. Annu Rev Immunol 2020, 38, 621–48. [DOI] [PubMed] [Google Scholar]
- 44. Doss RW, El-Rifaie AA, Abdel-Wahab AM, Gohary YM, Rashed LA.. Heat Shock Protein-70 expression in vitiligo and its relation to the disease activity. Indian J Dermatol 2016, 61, 408–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Levandowski CB, Mailloux CM, Ferrara TM, et al.. NLRP1 haplotypes associated with vitiligo and autoimmunity increase interleukin-1β processing via the NLRP1 inflammasome. Proc Natl Acad Sci USA 2013, 110, 2952–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Richmond JM, Frisoli ML, Harris JE.. Innate immune mechanisms in vitiligo: danger from within. Curr Opin Immunol 2013, 25, 676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yu R, Broady R, Huang Y, et al.. Transcriptome analysis reveals markers of aberrantly activated innate immunity in vitiligo lesional and non-lesional skin. PLoS One 2012, 7, e51040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sun L, Liu W, Zhang LJ.. The role of toll-like receptors in skin host defense, psoriasis, and atopic dermatitis. J Immunol Res 2019, 2019, 1824624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mosenson JA, Zloza A, Nieland JD, et al.. Mutant HSP70 reverses autoimmune depigmentation in vitiligo. Sci Transl Med 2013, 5, 174ra28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Denman CJ, McCracken J, Hariharan V, et al.. HSP70i accelerates depigmentation in a mouse model of autoimmune vitiligo. J Invest Dermatol 2008, 128, 2041–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Henning SW, Fernandez MF, Mahon JP, et al.. HSP70iQ435A-encoding DNA repigments vitiligo lesions in sinclair swine. J Invest Dermatol 2018, 138, 2531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang H, Wang W, Li Q, Huang W.. Fusion protein of ATPase domain of Hsc70 with TRP2 acting as a tumor vaccine against B16 melanoma. Immunol Lett 2006, 105, 167–73. [DOI] [PubMed] [Google Scholar]
- 53. Kroll TM, Bommiasamy H, Boissy RE, et al.. 4-Tertiary butyl phenol exposure sensitizes human melanocytes to dendritic cell-mediated killing: relevance to vitiligo. J Invest Dermatol 2005, 124, 798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mosenson JA, Flood K, Klarquist J, et al.. Preferential secretion of inducible HSP70 by vitiligo melanocytes under stress. Pigment Cell Melanoma Res 2014, 27, 209–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang Y, Liu L, Jin L, et al.. Oxidative stress-induced calreticulin expression and translocation: new insights into the destruction of melanocytes. J Invest Dermatol 2014, 134, 183–91. [DOI] [PubMed] [Google Scholar]
- 56. Kim JY, Lee EJ, Seo J, Oh SH.. Impact of high-mobility group box 1 on melanocytic survival and its involvement in the pathogenesis of vitiligo. Br J Dermatol 2017, 176, 1558–68. [DOI] [PubMed] [Google Scholar]
- 57. Cui T, Zhang W, Li S, et al.. Oxidative Stress-Induced HMGB1 release from melanocytes: a paracrine mechanism underlying the cutaneous inflammation in vitiligo. J Invest Dermatol 2019, 139, 2174–84.e4. [DOI] [PubMed] [Google Scholar]
- 58. Andersson U, Yang H, Harris H.. High-mobility group box 1 protein (HMGB1) operates as an alarmin outside as well as inside cells. Semin Immunol 2018, 38, 40–8. [DOI] [PubMed] [Google Scholar]
- 59. Rodrigues M, Ezzedine K, Hamzavi I, Pandya AG, Harris JE; Vitiligo Working Group . New discoveries in the pathogenesis and classification of vitiligo. J Am Acad Dermatol 2017, 77, 1–13. [DOI] [PubMed] [Google Scholar]
- 60. van den Boorn JG, Picavet DI, van Swieten PF, et al.. Skin-depigmenting agent monobenzone induces potent T-cell autoimmunity toward pigmented cells by tyrosinase haptenation and melanosome autophagy. J Invest Dermatol 2011, 131, 1240–51. [DOI] [PubMed] [Google Scholar]
- 61. Bertolotti A, Boniface K, Vergier B, et al.. Type I interferon signature in the initiation of the immune response in vitiligo. Pigment Cell Melanoma Res 2014, 27, 398–407. [DOI] [PubMed] [Google Scholar]
- 62. Tulic MK, Cavazza E, Cheli Y, et al.. Innate lymphocyte-induced CXCR3B-mediated melanocyte apoptosis is a potential initiator of T-cell autoreactivity in vitiligo. Nat Commun 2019, 10, 2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Li S, Kang P, Zhang W, et al.. Activated NLR family pyrin domain containing 3 (NLRP3) inflammasome in keratinocytes promotes cutaneous T-cell response in patients with vitiligo. J Allergy Clin Immunol 2020, 145, 632–45. [DOI] [PubMed] [Google Scholar]
- 64. Tang L, Zhou F.. Inflammasomes in common immune-related skin diseases. Front Immunol 2020, 11, 882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. van den Boorn JG, Jakobs C, Hagen C, et al.. Inflammasome-dependent induction of adaptive NK cell memory. Immunity 2016, 44, 1406–21. [DOI] [PubMed] [Google Scholar]
- 66. Thornberry NA, Bull HG, Calaycay JR, et al.. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature 1992, 356, 768–74. [DOI] [PubMed] [Google Scholar]
- 67. Gu Y, Kuida K, Tsutsui H, et al.. Activation of interferon-gamma inducing factor mediated by interleukin-1beta converting enzyme. Science 1997, 275, 206–9. [DOI] [PubMed] [Google Scholar]
- 68. Speeckaert R, van Geel N.. Vitiligo: An update on pathophysiology and treatment options. Am J Clin Dermatol 2017, 18, 733–44. [DOI] [PubMed] [Google Scholar]
- 69. Badri AM, Todd PM, Garioch JJ, Gudgeon JE, Stewart DG, Goudie RB.. An immunohistological study of cutaneous lymphocytes in vitiligo. J Pathol 1993, 170, 149–55. [DOI] [PubMed] [Google Scholar]
- 70. Wańkowicz-Kalińska A, van den Wijngaard RM, Tigges BJ, et al.. Immunopolarization of CD4+ and CD8+ T cells to Type-1-like is associated with melanocyte loss in human vitiligo. Lab Invest 2003, 83, 683–95. [DOI] [PubMed] [Google Scholar]
- 71. Le Poole IC, van den Wijngaard RM, Westerhof W, Das PK.. Presence of T cells and macrophages in inflammatory vitiligo skin parallels melanocyte disappearance. Am J Pathol 1996, 148, 1219–28. [PMC free article] [PubMed] [Google Scholar]
- 72. Ogg GS, Rod Dunbar P, Romero P, Chen JL, Cerundolo V.. High frequency of skin-homing melanocyte-specific cytotoxic T lymphocytes in autoimmune vitiligo. J Exp Med 1998, 188, 1203–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Strassner JP, Rashighi M, Ahmed Refat M, Richmond JM, Harris JE.. Suction blistering the lesional skin of vitiligo patients reveals useful biomarkers of disease activity. J Am Acad Dermatol 2017, 76, 847–855.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. van Geel NA, Mollet IG, De Schepper S, et al.. First histopathological and immunophenotypic analysis of early dynamic events in a patient with segmental vitiligo associated with halo nevi. Pigment Cell Melanoma Res 2010, 23, 375–84. [DOI] [PubMed] [Google Scholar]
- 75. Palermo B, Campanelli R, Garbelli S, et al.. Specific cytotoxic T lymphocyte responses against Melan-A/MART1, tyrosinase and gp100 in vitiligo by the use of major histocompatibility complex/peptide tetramers: the role of cellular immunity in the etiopathogenesis of vitiligo. J Invest Dermatol 2001, 117, 326–32. [DOI] [PubMed] [Google Scholar]
- 76. van den Boorn JG, Konijnenberg D, Dellemijn TA, et al.. Autoimmune destruction of skin melanocytes by perilesional T cells from vitiligo patients. J Invest Dermatol 2009, 129, 2220–32. [DOI] [PubMed] [Google Scholar]
- 77. Boniface K, Jacquemin C, Darrigade AS, et al.. Vitiligo skin is imprinted with resident memory CD8 T cells expressing CXCR3. J Invest Dermatol 2018, 138, 355–64. [DOI] [PubMed] [Google Scholar]
- 78. Grimes PE, Morris R, Avaniss-Aghajani E, Soriano T, Meraz M, Metzger A.. Topical tacrolimus therapy for vitiligo: therapeutic responses and skin messenger RNA expression of proinflammatory cytokines. J Am Acad Dermatol 2004, 51, 52–61. [DOI] [PubMed] [Google Scholar]
- 79. Rashighi M, Agarwal P, Richmond JM, et al.. CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci Transl Med 2014, 6, 223ra23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wang XX, Wang QQ, Wu JQ, et al.. Increased expression of CXCR3 and its ligands in patients with vitiligo and CXCL10 as a potential clinical marker for vitiligo. Br J Dermatol 2016, 174, 1318–26. [DOI] [PubMed] [Google Scholar]
- 81. Harris JE, Harris TH, Weninger W, Wherry EJ, Hunter CA, Turka LA.. A mouse model of vitiligo with focused epidermal depigmentation requires IFN-γ for autoreactive CD8+ T-cell accumulation in the skin. J Invest Dermatol 2012, 132, 1869–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Richmond JM, Bangari DS, Essien KI, et al.. Keratinocyte-derived chemokines orchestrate T-cell positioning in the epidermis during vitiligo and may serve as biomarkers of disease. J Invest Dermatol 2017, 137, 350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Rosmarin D, Pandya AG, Lebwohl M, et al.. Ruxolitinib cream for treatment of vitiligo: a randomised, controlled, phase 2 trial. Lancet 2020, 396, 110–20. [DOI] [PubMed] [Google Scholar]
- 84. Moraes-Vasconcelos D, Costa-Carvalho BT, Torgerson TR, Ochs HD.. Primary immune deficiency disorders presenting as autoimmune diseases: IPEX and APECED. J Clin Immunol 2008, 28 Suppl 1, S11–9. [DOI] [PubMed] [Google Scholar]
- 85. Birlea SA, Jin Y, Bennett DC, et al.. Comprehensive association analysis of candidate genes for generalized vitiligo supports XBP1, FOXP3, and TSLP. J Invest Dermatol 2011, 131, 371–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lahl K, Loddenkemper C, Drouin C, et al.. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J Exp Med 2007, 204, 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Eby JM, Kang HK, Klarquist J, et al.. Immune responses in a mouse model of vitiligo with spontaneous epidermal de- and repigmentation. Pigment Cell Melanoma Res 2014, 27, 1075–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gregg RK, Nichols L, Chen Y, Lu B, Engelhard VH.. Mechanisms of spatial and temporal development of autoimmune vitiligo in tyrosinase-specific TCR transgenic mice. J Immunol 2010, 184, 1909–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Chatterjee S, Eby JM, Al-Khami AA, et al.. A quantitative increase in regulatory T cells controls development of vitiligo. J Invest Dermatol 2014, 134, 1285–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Eby JM, Kang HK, Tully ST, et al.. CCL22 to activate treg migration and suppress depigmentation in vitiligo. J Invest Dermatol 2015, 135, 1574–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Miao X, Xu R, Fan B, et al.. PD-L1 reverses depigmentation in Pmel-1 vitiligo mice by increasing the abundance of Tregs in the skin. Sci Rep 2018, 8, 1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Mukhatayev Z, Dellacecca ER, Cosgrove C, et al.. Antigen specificity enhances disease control by tregs in vitiligo. Front Immunol 2020, 11, 581433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lili Y, Yi W, Ji Y, Yue S, Weimin S, Ming L.. Global activation of CD8+ cytotoxic T lymphocytes correlates with an impairment in regulatory T cells in patients with generalized vitiligo. PLoS One 2012, 7, e37513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Klarquist J, Denman CJ, Hernandez C, et al.. Reduced skin homing by functional Treg in vitiligo. Pigment Cell Melanoma Res 2010, 23, 276–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ben Ahmed M, Zaraa I, Rekik R, et al.. Functional defects of peripheral regulatory T lymphocytes in patients with progressive vitiligo. Pigment Cell Melanoma Res 2012, 25, 99–109. [DOI] [PubMed] [Google Scholar]
- 96. Terras S, Gambichler T, Moritz RK, Altmeyer P, Lambert J.. Immunohistochemical analysis of FOXP3+ regulatory T cells in healthy human skin and autoimmune dermatoses. Int J Dermatol 2014, 53, 294–9. [DOI] [PubMed] [Google Scholar]
- 97. Abdallah M, Lotfi R, Othman W, Galal R.. Assessment of tissue FoxP3+, CD4+ and CD8+ T-cells in active and stable nonsegmental vitiligo. Int J Dermatol 2014, 53, 940–6. [DOI] [PubMed] [Google Scholar]
- 98. Maeda Y, Nishikawa H, Sugiyama D, et al.. Detection of self-reactive CD8+ T cells with an anergic phenotype in healthy individuals. Science 2014, 346, 1536–40. [DOI] [PubMed] [Google Scholar]
- 99. Zhen Y, Yao L, Zhong S, Song Y, Cui Y, Li S.. Enhanced Th1 and Th17 responses in peripheral blood in active non-segmental vitiligo. Arch Dermatol Res 2016, 308, 703–10. [DOI] [PubMed] [Google Scholar]
- 100. Martins C, Darrigade AS, Jacquemin C, et al.. Phenotype and function of circulating memory T cells in human vitiligo. Br J Dermatol 2020, 183, 899–908. [DOI] [PubMed] [Google Scholar]
- 101. Matos TR. Is targeting circulating T blood cells a therapeutic option for vitiligo? Br J Dermatol 2020, 183, 803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Speeckaert R, Mylle S, van Geel N.. IL-17A is not a treatment target in progressive vitiligo. Pigment Cell Melanoma Res 2019, 32, 842–7. [DOI] [PubMed] [Google Scholar]
- 103. Giordano D, Magri F, Persechino F, et al.. Vitiligo with progressive repigmentation during secukinumab treatment in a patient with Psoriatic Arthritis: A case report. Case Rep Dermatol 2021, 13, 209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Cavalié M, Ezzedine K, Fontas E, et al.. Maintenance therapy of adult vitiligo with 0.1% tacrolimus ointment: a randomized, double blind, placebo-controlled study. J Invest Dermatol 2015, 135, 970–4. [DOI] [PubMed] [Google Scholar]
- 105. Sitek JC, Loeb M, Ronnevig JR.. Narrowband UVB therapy for vitiligo: does the repigmentation last? J Eur Acad Dermatol Venereol 2007, 21, 891–6. [DOI] [PubMed] [Google Scholar]
- 106. Mueller SN, Gebhardt T, Carbone FR, Heath WR.. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol 2013, 31, 137–61. [DOI] [PubMed] [Google Scholar]
- 107. Steinbach K, Vincenti I, Merkler D.. Resident-memory T cells in tissue-restricted immune responses: for better or worse? Front Immunol 2018, 9, 2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Dijkgraaf FE, Matos TR, Hoogenboezem M, et al.. Tissue patrol by resident memory CD8+ T cells in human skin. Nat Immunol 2019, 20, 756–64. [DOI] [PubMed] [Google Scholar]
- 109. Topham DJ, Reilly EC.. Tissue-resident memory CD8+ T cells: from phenotype to function. Front Immunol 2018, 9, 515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Frączek A, Owczarczyk-Saczonek A, Placek W.. The role of T(RM) cells in the pathogenesis of vitiligo-a review of the current state-of-the-art. Int J Mol Sci 2020, 21, 552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Mackay LK, Braun A, Macleod BL, et al.. Cutting edge: CD69 interference with sphingosine-1-phosphate receptor function regulates peripheral T cell retention. J Immunol 2015, 194, 2059–63. [DOI] [PubMed] [Google Scholar]
- 112. Skon CN, Lee JY, Anderson KG, Masopust D, Hogquist KA, Jameson SC.. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat Immunol 2013, 14, 1285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Mackay LK, Rahimpour A, Ma JZ, et al.. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat Immunol 2013, 14, 1294–301. [DOI] [PubMed] [Google Scholar]
- 114. Cheuk S, Schlums H, Gallais Sérézal I, et al.. CD49a expression defines tissue-resident CD8+ T cells poised for cytotoxic function in human skin. Immunity 2017, 46, 287–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Richmond JM, Strassner JP, Zapata L, et al.. Antibody blockade of IL-15 signaling has the potential to durably reverse vitiligo. Sci Transl Med 2018, 10, eaam7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Budagian V, Bulanova E, Paus R, Bulfone-Paus S.. IL-15/IL-15 receptor biology: a guided tour through an expanding universe. Cytokine Growth Factor Rev 2006, 17, 259–80. [DOI] [PubMed] [Google Scholar]
- 117. Jacquemin C, Martins C, Lucchese F, et al.. NKG2D Defines a subset of skin effector memory CD8 T Cells with proinflammatory functions in vitiligo. J Invest Dermatol 2020, 140, 1143–1153.e5. [DOI] [PubMed] [Google Scholar]
- 118. Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS.. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature 2012, 483, 227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Mackay LK, Stock AT, Ma JZ, et al.. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc Natl Acad Sci USA 2012, 109, 7037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Seidel JA, Vukmanovic-Stejic M, Muller-Durovic B, et al.. Skin resident memory CD8+ T cells are phenotypically and functionally distinct from circulating populations and lack immediate cytotoxic function. Clin Exp Immunol 2018, 194, 79–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. McMaster SR, Wilson JJ, Wang H, Kohlmeier JE.. Airway-Resident Memory CD8 T Cells provide antigen-specific protection against respiratory virus challenge through rapid IFN-γ production. J Immunol 2015, 195, 203–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Malik BT, Byrne KT, Vella JL, et al.. Resident memory T cells in the skin mediate durable immunity to melanoma. Sci Immunol 2017, 2, eaam6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Richmond JM, Strassner JP, Rashighi M, et al.. Resident memory and recirculating memory T cells cooperate to maintain disease in a mouse model of vitiligo. J Invest Dermatol 2019, 139, 769–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Matos TR, O’Malley JT, Lowry EL, et al.. Clinically resolved psoriatic lesions contain psoriasis-specific IL-17-producing αβ T cell clones. J Clin Invest 2017, 127, 4031–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Naughton GK, Eisinger M, Bystryn JC.. Antibodies to normal human melanocytes in vitiligo. J Exp Med 1983, 158, 246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Naughton GK, Reggiardo D, Bystryn JC.. Correlation between vitiligo antibodies and extent of depigmentation in vitiligo. J Am Acad Dermatol 1986, 15, 978–81. [DOI] [PubMed] [Google Scholar]
- 127. Rocha IM, Oliveira LJ, De Castro LC, et al.. Recognition of melanoma cell antigens with antibodies present in sera from patients with vitiligo. Int J Dermatol 2000, 39, 840–3. [DOI] [PubMed] [Google Scholar]
- 128. Farrokhi S, Hojjat-Farsangi M, Noohpisheh MK, Tahmasbi R, Rezaei N.. Assessment of the immune system in 55 Iranian patients with vitiligo. J Eur Acad Dermatol Venereol 2005, 19, 706–11. [DOI] [PubMed] [Google Scholar]
- 129. Harning R, Cui J, Bystryn JC.. Relation between the incidence and level of pigment cell antibodies and disease activity in vitiligo. J Invest Dermatol 1991, 97, 1078–80. [DOI] [PubMed] [Google Scholar]
- 130. Yu HS, Kao CH, Yu CL.. Coexistence and relationship of antikeratinocyte and antimelanocyte antibodies in patients with non-segmental-type vitiligo. J Invest Dermatol 1993, 100, 823–8. [DOI] [PubMed] [Google Scholar]
- 131. Abu Tahir M, Pramod K, Ansari SH, Ali J.. Current remedies for vitiligo. Autoimmun Rev 2010, 9, 516–20. [DOI] [PubMed] [Google Scholar]
- 132. Xie P, Geoghegan W, Jordon R. Vitiligo autoantibodies. Studies of subclass distribution and complement activation. J Invest Dermatol 1991, 96, 627. [Google Scholar]
- 133. Aronson PJ, Hashimoto K.. Association of IgA anti-melanoma antibodies in the sera of vitiligo patients with active disease. J Invest Dermatol 1987, 88, 475. [Google Scholar]
- 134. Zhu MC, Liu CG, Wang DX, Zhan Z.. Detection of serum anti-melanocyte antibodies and identification of related antigens in patients with vitiligo. Genet Mol Res 2015, 14, 16060–73. [DOI] [PubMed] [Google Scholar]
- 135. Cui J, Arita Y, Bystryn JC.. Characterization of vitiligo antigens. Pigment Cell Res 1995, 8, 53–9. [DOI] [PubMed] [Google Scholar]
- 136. Park YK, Kim NS, Hann SK, Im S.. Identification of autoantibody to melanocytes and characterization of vitiligo antigen in vitiligo patients. J Dermatol Sci 1996, 11, 111–20. [DOI] [PubMed] [Google Scholar]
- 137. Song YH, Connor E, Li Y, Zorovich B, Balducci P, Maclaren N.. The role of tyrosinase in autoimmune vitiligo. Lancet 1994, 344, 1049–52. [DOI] [PubMed] [Google Scholar]
- 138. Baharav E, Merimski O, Shoenfeld Y, et al.. Tyrosinase as an autoantigen in patients with vitiligo. Clin Exp Immunol 1996, 105, 84–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Kemp EH, Gawkrodger DJ, MacNeil S, Watson PF, Weetman AP.. Detection of tyrosinase autoantibodies in patients with vitiligo using 35S-labeled recombinant human tyrosinase in a radioimmunoassay. J Invest Dermatol 1997, 109, 69–73. [DOI] [PubMed] [Google Scholar]
- 140. Kemp EH, Gawkrodger DJ, Watson PF, Weetman AP.. Immunoprecipitation of melanogenic enzyme autoantigens with vitiligo sera: evidence for cross-reactive autoantibodies to tyrosinase and tyrosinase-related protein-2 (TRP-2). Clin Exp Immunol 1997, 109, 495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Kemp EH, Gawkrodger DJ, Watson PF, Weetman AP.. Autoantibodies to human melanocyte-specific protein pmel17 in the sera of vitiligo patients: a sensitive and quantitative radioimmunoassay (RIA). Clin Exp Immunol 1998, 114, 333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Kemp EH, Waterman EA, Gawkrodger DJ, Watson PF, Weetman AP.. Autoantibodies to tyrosinase-related protein-1 detected in the sera of vitiligo patients using a quantitative radiobinding assay. Br J Dermatol 1998, 139, 798–805. [DOI] [PubMed] [Google Scholar]
- 143. Ruiz-Argüelles A, García-Carrasco M, Jimenez-Brito G, et al.. Treatment of vitiligo with a chimeric monoclonal antibody to CD20: a pilot study. Clin Exp Immunol 2013, 174, 229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Mandry RC, Ortíz LJ, Lugo-Somolinos A, Sánchez JL.. Organ-specific autoantibodies in vitiligo patients and their relatives. Int J Dermatol 1996, 35, 18–21. [DOI] [PubMed] [Google Scholar]
- 145. Kemp EH, Waterman EA, Hawes BE, et al.. The melanin-concentrating hormone receptor 1, a novel target of autoantibody responses in vitiligo. J Clin Invest 2002, 109, 923–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Waterman EA, Gawkrodger DJ, Watson PF, Weetman AP, Kemp EH.. Autoantigens in vitiligo identified by the serological selection of a phage-displayed melanocyte cDNA expression library. J Invest Dermatol 2010, 130, 230–40. [DOI] [PubMed] [Google Scholar]
- 147. Faraj S, Gawkrodger D, Weetman A, Kemp EH.. An investigation of lamin A autoantibodies in vitiligo. J Invest Dermatol 2017, 137, S14. [Google Scholar]
- 148. Norris DA, Kissinger RM, Naughton GM, Bystryn JC.. Evidence for immunologic mechanisms in human vitiligo: patients’ sera induce damage to human melanocytes in vitro by complement-mediated damage and antibody-dependent cellular cytotoxicity. J Invest Dermatol 1988, 90, 783–9. [DOI] [PubMed] [Google Scholar]
- 149. Gilhar A, Zelickson B, Ulman Y, Etzioni A.. In vivo destruction of melanocytes by the IgG fraction of serum from patients with vitiligo. J Invest Dermatol 1995, 105, 683–6. [DOI] [PubMed] [Google Scholar]
- 150. Gottumukkala RV, Gavalas NG, Akhtar S, et al.. Function-blocking autoantibodies to the melanin-concentrating hormone receptor in vitiligo patients. Lab Invest 2006, 86, 781–9. [DOI] [PubMed] [Google Scholar]
- 151. Yohn JJ, Morelli JG, Walchak SJ, Rundell KB, Norris DA, Zamora MR.. Cultured human keratinocytes synthesize and secrete endothelin-1. J Invest Dermatol 1993, 100, 23–6. [DOI] [PubMed] [Google Scholar]
- 152. Kemp EH, Waterman EA, Weetman AP.. Autoimmune aspects of vitiligo. Autoimmunity 2001, 34, 65–77. [DOI] [PubMed] [Google Scholar]
- 153. Homey B, Assmann T, Vohr HW, et al.. Topical FK506 suppresses cytokine and costimulatory molecule expression in epidermal and local draining lymph node cells during primary skin immune responses. J Immunol 1998, 160, 5331–40. [PubMed] [Google Scholar]
- 154. Hartmann A, Bröcker EB, Hamm H.. Occlusive treatment enhances efficacy of tacrolimus 0.1% ointment in adult patients with vitiligo: results of a placebo-controlled 12-month prospective study. Acta Derm Venereol 2008, 88, 474–9. [DOI] [PubMed] [Google Scholar]
- 155. Gawkrodger DJ, Ormerod AD, Shaw L, et al.. Vitiligo: concise evidence based guidelines on diagnosis and management. Postgrad Med J 2010, 86, 466–71. [DOI] [PubMed] [Google Scholar]
- 156. Lee CH, Wu SB, Hong CH, Yu HS, Wei YH.. Molecular mechanisms of UV-induced apoptosis and its effects on skin residential cells: the implication in UV-based phototherapy. Int J Mol Sci 2013, 14, 6414–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Fishman P, Azizi E, Shoenfeld Y, et al.. Vitiligo autoantibodies are effective against melanoma. Cancer 1993, 72, 2365–9. [DOI] [PubMed] [Google Scholar]
- 158. Cario-André M, Pain C, Gauthier Y, Taïeb A.. The melanocytorrhagic hypothesis of vitiligo tested on pigmented, stressed, reconstructed epidermis. Pigment Cell Res 2007, 20, 385–93. [DOI] [PubMed] [Google Scholar]
- 159. Kemp EH, Waterman EA, Weetman AP.. Immunological pathomechanisms in vitiligo. Expert Rev Mol Med 2001, 3, 1–22. [DOI] [PubMed] [Google Scholar]
- 160. Takechi Y, Hara I, Naftzger C, Xu Y, Houghton AN.. A melanosomal membrane protein is a cell surface target for melanoma therapy. Clin Cancer Res 1996, 2, 1837–42. [PubMed] [Google Scholar]
- 161. Leonhardt RM, Vigneron N, Rahner C, Cresswell P.. Proprotein convertases process Pmel17 during secretion. J Biol Chem 2011, 286, 9321–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Martínez-Lostao L, Anel A, Pardo J.. How do cytotoxic lymphocytes kill cancer cells? Clin Cancer Res 2015, 21, 5047–56. [DOI] [PubMed] [Google Scholar]
- 163. Hassin D, Garber OG, Meiraz A, Schiffenbauer YS, Berke G.. Cytotoxic T lymphocyte perforin and Fas ligand working in concert even when Fas ligand lytic action is still not detectable. Immunology 2011, 133, 190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Spritz RA, Andersen GH.. Genetics of Vitiligo. Dermatol Clin 2017, 35, 245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Bethune MT, Joglekar AV.. Personalized T cell-mediated cancer immunotherapy: progress and challenges. Curr Opin Biotechnol 2017, 48, 142–52. [DOI] [PubMed] [Google Scholar]
- 166. Leisegang M, Kammertoens T, Uckert W, Blankenstein T.. Targeting human melanoma neoantigens by T cell receptor gene therapy. J Clin Invest 2016, 126, 854–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Singh M, Kotnis A, Jadeja SD, Mondal A, Mansuri MS, Begum R.. Cytokines: the yin and yang of vitiligo pathogenesis. Expert Rev Clin Immunol 2019, 15, 177–88. [DOI] [PubMed] [Google Scholar]
- 168. Singh S, Singh U, Pandey SS.. Serum concentration of IL-6, IL-2, TNF-α, and IFNγ in Vitiligo patients. Indian J Dermatol 2012, 57, 12–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Miniati A, Weng Z, Zhang B, et al.. Stimulated human melanocytes express and release interleukin-8, which is inhibited by luteolin: relevance to early vitiligo. Clin Exp Dermatol 2014, 39, 54–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Gholijani N, Yazdani MR, Dastgheib L.. Predominant role of innate pro-inflammatory cytokines in vitiligo disease. Arch Dermatol Res 2020, 312, 123–31. [DOI] [PubMed] [Google Scholar]
- 171. Moretti S, Fabbri P, Baroni G, et al.. Keratinocyte dysfunction in vitiligo epidermis: cytokine microenvironment and correlation to keratinocyte apoptosis. Histol Histopathol 2009, 24, 849–57. [DOI] [PubMed] [Google Scholar]
- 172. Boukhedouni N, Martins C, Darrigade A-S, et al.. Type-1 cytokines regulate MMP-9 production and E-cadherin disruption to promote melanocyte loss in vitiligo. JCI Insight 2020, 5, e133772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Harris JE, Rashighi M, Nguyen N, Jabbari A, et al.. Rapid skin repigmentation on oral ruxolitinib in a patient with coexistent vitiligo and alopecia areata (AA). J Am Acad Dermatol 2016, 74, 370–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. van Elsas A, Hurwitz AA, Allison JP.. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med 1999, 190, 355–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Fishman P, Merimski O, Baharav E, Shoenfeld Y.. Autoantibodies to tyrosinase: the bridge between melanoma and vitiligo. Cancer 1997, 79, 1461–4. [DOI] [PubMed] [Google Scholar]
- 176. Huang SK, Okamoto T, Morton DL, Hoon DS.. Antibody responses to melanoma/melanocyte autoantigens in melanoma patients. J Invest Dermatol 1998, 111, 662–7. [DOI] [PubMed] [Google Scholar]
- 177. Kirkin AF, Dzhandzhugazyan K, Zeuthen J.. Melanoma-associated antigens recognized by cytotoxic T lymphocytes. APMIS 1998, 106, 665–79. [DOI] [PubMed] [Google Scholar]
- 178. Edwards J, Wilmott JS, Madore J, et al.. CD103 + tumor-resident CD8 + T cells are associated with improved survival in immunotherapy-naïve melanoma patients and expand significantly during anti-PD-1 treatment. Clinical Cancer Research 2018, 24, 3036. [DOI] [PubMed] [Google Scholar]
- 179. Teulings HE, Limpens J, Jansen SN, et al.. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol 2015, 33, 773–81. [DOI] [PubMed] [Google Scholar]
- 180. Hua C, Boussemart L, Mateus C, et al.. Association of vitiligo with tumor response in patients with metastatic melanoma treated with Pembrolizumab. JAMA Dermatol 2016, 152, 45–51. [DOI] [PubMed] [Google Scholar]
- 181. Yee C, Thompson JA, Roche P, et al.. Melanocyte destruction after antigen-specific immunotherapy of melanoma: direct evidence of t cell-mediated vitiligo. J Exp Med 2000, 192, 1637–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Lo JA, Kawakubo M, Juneja VR, et al.. Epitope spreading toward wild-type melanocyte-lineage antigens rescues suboptimal immune checkpoint blockade responses. Sci Transl Med 2021, 13, eabd8636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Tatli AM, Besen AA, Kalender ME, et al.. Association of vitiligo and response in patients with metastatic malignant melanoma on temozolomide. Tumori 2015, 101, e67–9. [DOI] [PubMed] [Google Scholar]
- 184. Bergqvist C, Ezzedine K.. Vitiligo: a review. Dermatology 2020, 236, 571–92. [DOI] [PubMed] [Google Scholar]
- 185. Jin Y, Birlea SA, Fain PR, et al.. Variant of TYR and autoimmunity susceptibility loci in generalized vitiligo. N Engl J Med 2010, 362, 1686–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Spritz RA. The genetics of generalized vitiligo: autoimmune pathways and an inverse relationship with malignant melanoma. Genome Med 2010, 2, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Gudbjartsson DF, Sulem P, Stacey SN, et al.. ASIP and TYR pigmentation variants associate with cutaneous melanoma and basal cell carcinoma. Nat Genet 2008, 40, 886–91. [DOI] [PubMed] [Google Scholar]
- 188. Alkhateeb A, Fain PR, Thody A, Bennett DC, Spritz RA.. Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. Pigment Cell Res 2003, 16, 208–14. [DOI] [PubMed] [Google Scholar]
- 189. Picardo M, Dell’Anna ML, Ezzedine K, et al.. Vitiligo. Nat Rev Dis Primers 2015, 1, 15011. [DOI] [PubMed] [Google Scholar]
- 190. Lerner AB. Vitiligo. J Invest Dermatol 1959, 32, 285–310. [PubMed] [Google Scholar]
- 191. Jin Y, Birlea SA, Fain PR, et al.. Genome-wide association analyses identify 13 new susceptibility loci for generalized vitiligo. Nat Genet 2012, 44, 676–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Tang XF, Zhang Z, Hu DY, et al.. Association analyses identify three susceptibility Loci for vitiligo in the Chinese Han population. J Invest Dermatol 2013, 133, 403–10. [DOI] [PubMed] [Google Scholar]
- 193. Liu JB, Li M, Chen H, et al.. Association of vitiligo with HLA-A2: a meta-analysis. J Eur Acad Dermatol Venereol 2007, 21, 205–13. [DOI] [PubMed] [Google Scholar]
- 194. Jin Y, Hayashi M, Fain PR, et al.. Major association of vitiligo with HLA-A∗02:01 in Japanese. Pigment Cell Melanoma Res 2015, 28, 360–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Fain PR, Babu SR, Bennett DC, Spritz RA.. HLA class II haplotype DRB1∗04-DQB1∗0301 contributes to risk of familial generalized vitiligo and early disease onset. Pigment Cell Res 2006, 19, 51–7. [DOI] [PubMed] [Google Scholar]
- 196. Quan C, Ren YQ, Xiang LH, et al.. Genome-wide association study for vitiligo identifies susceptibility loci at 6q27 and the MHC. Nat Genet 2010, 42, 614–8. [DOI] [PubMed] [Google Scholar]
- 197. Tazi-Ahnini R, McDonagh AJ, Wengraf DA, et al.. The autoimmune regulator gene (AIRE) is strongly associated with vitiligo. Br J Dermatol 2008, 159, 591–6. [DOI] [PubMed] [Google Scholar]
- 198. Oftedal BE, Hellesen A, Erichsen MM, et al.. Dominant mutations in the autoimmune regulator AIRE are associated with common organ-specific autoimmune diseases. Immunity 2015, 42, 1185–96. [DOI] [PubMed] [Google Scholar]
- 199. Kemp EH, Ajjan RA, Waterman EA, et al.. Analysis of a microsatellite polymorphism of the cytotoxic T-lymphocyte antigen-4 gene in patients with vitiligo. Br J Dermatol 1999, 140, 73–8. [DOI] [PubMed] [Google Scholar]
- 200. Birlea SA, Laberge GS, Procopciuc LM, Fain PR, Spritz RA.. CTLA4 and generalized vitiligo: two genetic association studies and a meta-analysis of published data. Pigment Cell Melanoma Res 2009, 22, 230–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Pehlivan S, Ozkinay F, Alper S, et al.. Association between IL4 (-590), ACE (I)/(D), CCR5 (Delta32), CTLA4 (+49) and IL1-RN (VNTR in intron 2) gene polymorphisms and vitiligo. Eur J Dermatol 2009, 19, 126–8. [DOI] [PubMed] [Google Scholar]
- 202. Li M, Sun D, Li C, et al.. Functional polymorphisms of the FAS gene associated with risk of vitiligo in Chinese populations: a case-control analysis. J Invest Dermatol 2008, 128, 2820–4. [DOI] [PubMed] [Google Scholar]
- 203. Jin Y, Birlea SA, Fain PR, et al.. Common variants in FOXP1 are associated with generalized vitiligo. Nat Genet 2010, 42, 576–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Ferrara TM, Jin Y, Gowan K, Fain PR, Spritz RA.. Risk of generalized vitiligo is associated with the common 55R-94A-247H variant haplotype of GZMB (encoding granzyme B). J Invest Dermatol 2013, 133, 1677–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Jin Y, Birlea SA, Fain PR, Spritz RA.. Genetic variations in NALP1 are associated with generalized vitiligo in a Romanian population. J Invest Dermatol 2007, 127, 2558–62. [DOI] [PubMed] [Google Scholar]
- 206. Jin Y, Mailloux CM, Gowan K, et al.. NALP1 in vitiligo-associated multiple autoimmune disease. N Engl J Med 2007, 356, 1216–25. [DOI] [PubMed] [Google Scholar]
- 207. Alkhateeb A, Qarqaz F.. Genetic association of NALP1 with generalized vitiligo in Jordanian Arabs. Arch Dermatol Res 2010, 302, 631–4. [DOI] [PubMed] [Google Scholar]
- 208. Cantón I, Akhtar S, Gavalas NG, et al.. A single-nucleotide polymorphism in the gene encoding lymphoid protein tyrosine phosphatase (PTPN22) confers susceptibility to generalised vitiligo. Genes Immun 2005, 6, 584–7. [DOI] [PubMed] [Google Scholar]
- 209. LaBerge GS, Bennett DC, Fain PR, Spritz RA.. PTPN22 is genetically associated with risk of generalized vitiligo, but CTLA4 is not. J Invest Dermatol 2008, 128, 1757–62. [DOI] [PubMed] [Google Scholar]
- 210. Laberge GS, Birlea SA, Fain PR, Spritz RA.. The PTPN22-1858C>T (R620W) functional polymorphism is associated with generalized vitiligo in the Romanian population. Pigment Cell Melanoma Res 2008, 21, 206–8. [DOI] [PubMed] [Google Scholar]
- 211. Laddha NC, Dwivedi M, Shajil EM, Prajapati H, Marfatia YS, Begum R.. Association of PTPN22 1858C/T polymorphism with vitiligo susceptibility in Gujarat population. J Dermatol Sci 2008, 49, 260–2. [DOI] [PubMed] [Google Scholar]
- 212. Song GG, Kim JH, Lee YH.. The CTLA-4 + 49 A/G, CT60 A/G and PTPN22 1858 C/T polymorphisms and susceptibility to vitiligo: a meta-analysis. Mol Biol Rep 2013, 40, 2985–93. [DOI] [PubMed] [Google Scholar]
- 213. Garcia-Melendez ME, Salinas-Santander M, Sanchez-Dominguez C, et al.. Protein tyrosine phosphatase PTPN22 + 1858C/T polymorphism is associated with active vitiligo. Exp Ther Med 2014, 8, 1433–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Na GY, Lee KH, Kim MK, Lee SJ, Kim DW, Kim JC.. Polymorphisms in the melanocortin-1 receptor (MC1R) and agouti signaling protein (ASIP) genes in Korean vitiligo patients. Pigment Cell Res 2003, 16, 383–7. [DOI] [PubMed] [Google Scholar]
- 215. Alkhateeb A, Fain PR, Spritz RA.. Candidate functional promoter variant in the FOXD3 melanoblast developmental regulator gene in autosomal dominant vitiligo. J Invest Dermatol 2005, 125, 388–91. [DOI] [PubMed] [Google Scholar]
- 216. Jin Y, Ferrara T, Gowan K, et al.. Next-generation DNA re-sequencing identifies common variants of TYR and HLA-A that modulate the risk of generalized vitiligo via antigen presentation. J Invest Dermatol 2012, 132, 1730–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Sun Y, Zuo X, Zheng X, et al.. A comprehensive association analysis confirms ZMIZ1 to be a susceptibility gene for vitiligo in Chinese population. J Med Genet 2014, 51, 345–53. [DOI] [PubMed] [Google Scholar]
- 218. Jin SY, Park HH, Li GZ, et al.. Association of angiotensin converting enzyme gene I/D polymorphism of vitiligo in Korean population. Pigment Cell Res 2004, 17, 84–6. [DOI] [PubMed] [Google Scholar]
- 219. Wood JM, Gibbons NC, Chavan B, Schallreuter KU.. Computer simulation of heterogeneous single nucleotide polymorphisms in the catalase gene indicates structural changes in the enzyme active site, NADPH-binding and tetramerization domains: a genetic predisposition for an altered catalase in patients with vitiligo? Exp Dermatol 2008, 17, 366–71. [DOI] [PubMed] [Google Scholar]
- 220. Liu L, Li C, Gao J, et al.. Promoter variant in the catalase gene is associated with vitiligo in Chinese people. J Invest Dermatol 2010, 130, 2647–53. [DOI] [PubMed] [Google Scholar]
- 221. Mansuri MS, Jadeja SD, Singh M, Laddha NC, Dwivedi M, Begum R.. The catalase gene promoter and 5’-untranslated region variants lead to altered gene expression and enzyme activity in vitiligo. Br J Dermatol 2017, 177, 1590–600. [DOI] [PubMed] [Google Scholar]
- 222. Kim HJ, Choi CP, Uhm YK, et al.. The association between endothelin-1 gene polymorphisms and susceptibility to vitiligo in a Korean population. Exp Dermatol 2007, 16, 561–6. [DOI] [PubMed] [Google Scholar]
- 223. Gokhale BB, Mehta LN.. Histopathology of vitiliginous skin. Int J Dermatol 1983, 22, 477–80. [DOI] [PubMed] [Google Scholar]
- 224. Nordlands JJ. Vitiligo. In: Thiers BH, Dobson RL (eds), In: Pathogenesis of Skin Diseases. New York: Churchill Livingstone, 1986, 99–128. [Google Scholar]
- 225. Al’Abadie MS, Warren MA, Bleehen SS, Gawkrodger DJ.. Morphologic observations on the dermal nerves in vitiligo: an ultrastructural study. Int J Dermatol 1995, 34, 837–40. [DOI] [PubMed] [Google Scholar]
- 226. Al’Abadie MS, Senior HJ, Bleehen SS, Gawkrodger DJ.. Neuropeptide and neuronal marker studies in vitiligo. Br J Dermatol 1994, 131, 160–5. [DOI] [PubMed] [Google Scholar]
- 227. Dimitrijević M, Stanojević S.. The intriguing mission of neuropeptide Y in the immune system. Amino Acids 2013, 45, 41–53. [DOI] [PubMed] [Google Scholar]
- 228. Ganea D, Hooper KM, Kong W.. The neuropeptide vasoactive intestinal peptide: direct effects on immune cells and involvement in inflammatory and autoimmune diseases. Acta Physiol (Oxf) 2015, 213, 442–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Taams LS. Neuroimmune interactions: how the nervous and immune systems influence each other. Clin Exp Immunol 2019, 197, 276–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Kundu RV, Mhlaba JM, Rangel SM, Le Poole IC.. The convergence theory for vitiligo: A reappraisal. Exp Dermatol 2019, 28, 647–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Cucchi ML, Frattini P, Santagostino G, Orecchia G.. Higher plasma catecholamine and metabolite levels in the early phase of nonsegmental vitiligo. Pigment Cell Res 2000, 13, 28–32. [DOI] [PubMed] [Google Scholar]
- 232. Gauthier Y, Cario-Andre M, Lepreux S, Pain C, Taïeb A.. Melanocyte detachment after skin friction in non lesional skin of patients with generalized vitiligo. Br J Dermatol 2003, 148, 95–101. [DOI] [PubMed] [Google Scholar]
- 233. Pichler R, Sfetsos K, Badics B, Gutenbrunner S, Auböck J.. Vitiligo patients present lower plasma levels of alpha-melanotropin immunoreactivities. Neuropeptides 2006, 40, 177–83. [DOI] [PubMed] [Google Scholar]
- 234. Harris JE. Chemical-Induced Vitiligo. Dermatol Clin 2017, 35, 151–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Westerhof W, d’Ischia M.. Vitiligo puzzle: the pieces fall in place. Pigment Cell Res 2007, 20, 345–59. [DOI] [PubMed] [Google Scholar]
- 236. Namazi MR. Neurogenic dysregulation, oxidative stress, autoimmunity, and melanocytorrhagy in vitiligo: can they be interconnected? Pigment Cell Res 2007, 20, 360–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable to this paper.