Abstract
LITMUS was a single-centre, Phase 2a study designed to investigate whether the gene biomarker FGL2/IFNG previously reported for the identification of tolerance in murine models could identify operationally tolerant liver transplant recipients. Multiplex RT-PCR was used to amplify eight immunoregulatory genes in peripheral blood mononuclear cells (PBMC) from 69 adult liver transplant recipients. Patients with PBMC FGL2/IFNG ≥ 1 and a normal liver biopsy underwent immunosuppression (IS) withdrawal. The primary end point was the development of operational tolerance. Secondary end points included correlation of tolerance with allograft gene expression and immune cell markers. Twenty-eight of 69 patients (38%) were positive for the PBMC tolerance biomarker and 23 proceeded to IS withdrawal. Nine of the 23 patients had abnormal baseline liver biopsies and were excluded. Of the 14 patients with normal biopsies, eight (57%) have achieved operational tolerance and are off IS (range 12–57 months). Additional studies revealed that all of the tolerant patients and only one non-tolerant patient had a liver gene ratio of FOXP3/IFNG ≥ 1 prior to IS withdrawal. Increased CD4+ T regulatory T cells were detected both in PBMC and livers of tolerant patients following IS withdrawal. Higher expression of SELE (gene for E-selectin) and lower expression of genes associated with inflammatory responses (GZMB, CIITA, UBD, LSP1, and CXCL9) were observed in the pre-withdrawal liver biopsies of tolerant patients by RNA sequencing. These results suggest that measurement of PBMC FGL2/IFNG may enrich for the identification of operationally tolerant liver transplant patients, especially when combined with intragraft measurement of FOXP3/IFNG. Clinical Trial Registration: ClinicalTrials.gov (LITMUS: NCT02541916).
Keywords: biomarker, gene expression, liver transplantation, tolerance, immunosuppression withdrawal
LITMUS was a single-centre, Phase 2 study designed to investigate whether the gene biomarker FGL2/IFNGpreviously reported for the identification of tolerance in murine models could identify operationally tolerant liver transplant recipients. The results suggest that measurement of PBMC FGL2/IFNGmay enrich for the identification of operationally tolerant liver transplant patients, especially when combined with intragraft measurement of FOXP3/IFNG.
Graphical Abstract
Graphical Abstract.
Introduction
The outcomes of liver transplantation have improved over the past three decades, mainly as a result of advances in surgical techniques, management of post-transplant complications, and introduction of newer immunosuppressive agents [1]. However, the need for long-term immunosuppression (IS) is associated with serious transplant-related complications reducing long-term survival [2]. Strategies to reduce or stop IS remains an important goal to prevent long-term side effects.
It has been reported that IS can be discontinued in liver transplant patients without the development of graft rejection—a phenomenon known as spontaneous operational tolerance [3]. As these patients are no longer susceptible to IS-related toxicity, investigators have sought to determine the frequency and clinical predictors of operational tolerance in liver transplantation. Over the past 25 years, a number of clinical trials have focused on operational tolerance in adult liver transplantation [4–12]. In these studies, the overall frequency of operational tolerance was shown to vary from 5.6 to 62.5% with the best results in small trials using highly selected patients. The combined success of IS withdrawal in these studies was 30.8% (140/455) in agreement with a review on operational tolerance which concluded that 20–40% of liver transplant recipients may be operationally tolerant [13]. Clinical predictors of operational tolerance included greater time post-liver transplantation, older age, and male sex [10]. Greater time post-liver transplantation was also determined to be a predictor of tolerance in paediatric liver transplantation [14].
A variety of different cellular and transcriptional markers in the peripheral blood have been identified that discriminate between tolerant and non-tolerant transplant recipients, although some studies had a retrospective cross-sectional design, in which tolerant recipients weaned off IS were compared with immunosuppressed controls, leading to a potential bias in the analysis of immunological parameters [3, 15, 16]. A more recent study suggested that the gene expression profiling of the liver biopsy may be more accurate than blood-related biomarkers in predicting the outcome of IS withdrawal [17]. This gene biomarker, which includes genes involved in the regulation of iron homeostasis, is currently undergoing validation in a multi-centre trial in Europe (LIFT trial: NCT02498977).
We previously described a gene biomarker panel that had the ability to distinguish between tolerance and rejection in preclinical murine models of heart and liver transplantation [18, 19]. The biomarker panel included 22 immunoregulatory genes, which are known to be associated with alloantigen recognition, signal transduction, T-cell activation and differentiation, adhesion/migration, and the response of immune effectors. Tolerance was characterized by high expression of immunoregulatory genes and low expression of pro-inflammatory genes. A smaller common set of genes associated with tolerance was also identified [18]. Included in this set are anti-inflammatory genes associated with regulatory T cells (Tregs), which are known to facilitate transplantation tolerance [20]. The results generated in the preclinical studies suggested that the designed novel biomarker gene set may have sufficient sensitivity and specificity to distinguish between allograft rejection and tolerance. Furthermore, the data suggested that tolerance induction was an active process in which early upregulation of a combination of a specific set of tolerogenic genes such as fibrinogen-like protein 2 (FGL2) counteracts the increased expression of an inflammatory set of genes including interferon-γ (IFNG) and granzyme B (GZMB). Based on these preclinical studies, we hypothesized that the preclinical gene biomarker panel in PBMC and a high FGL2/IFNG gene ratio could identify operationally tolerant human liver transplant recipients who could be safely weaned off IS.
Materials and methods
Study design
The Liver Immune Tolerance bioMarker Utilization Study (LITMUS, ClinicalTrials.gov NCT02541916) was a prospective observational single-centre, single-arm study conducted at the Toronto General Hospital, University Health Network (Toronto, Canada). LITMUS was approved by the Research Ethics Board of the University Health Network (14-8691). An independent data safety monitoring board monitored the trial. All patients provided written, informed consent. The inclusion and exclusion criteria for entry into the study are provided in Table 1. Twenty-three healthy living donors from the liver transplant program at the University Health Network who had normal liver biopsies were enrolled in the study and served as a control group for the gene expression analysis. Figure 1 shows the design of the trial including timing of blood and liver biopsies for GeXP analysis. In Phase 1 of LITMUS, blood samples were collected from eligible patients for PBMC gene expression analysis. Patients who had the predefined gene expression ratio (FGL2/IFNG ≥ 1) and a normal baseline liver biopsy were eligible to enter the IS withdrawal phase of the study (Phase 2). All cases eligible for Phase 2 were reviewed by an independent selection committee to confirm that there were no contraindications to IS withdrawal. In those patients who entered Phase 2 of the study, IS was withdrawn over 3 to 4 months in a stepwise fashion (25% reduction each month). Liver function tests were performed every 2 weeks during the withdrawal period, weekly for 1 month after cessation of IS, and then monthly for the next 11 months. Percutaneous liver biopsies and liver chemistry were performed at baseline, 6 months, and 1 year post-IS withdrawal. Although not formally part of the study, it was recommended patients undergo liver biopsy yearly for up to 5 years post-IS withdrawal.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria: |
| 1) Be between 18 and 70 years of age |
| 2) Be recipients of a hepatic allograft |
| 3) Be a minimum of 3 months post-transplant |
| 4) Have normal liver function tests for the 3 months prior to entry including AST, ALT, ALP, bilirubin, and prothrombin time (INR) |
| 5) Be free of rejection in the previous 3 months prior to enrolment |
| Patient exclusion criteria: |
| 1) Patients under the age of 18 and over the age of 70 |
| 2) Patients who are positive for HIV |
| 3) Patients who have detectable levels of HCV RNA or HBV DNA at the time of enrolment |
| 4) Patients who have a combined transplant and/or have been re-transplanted |
| 5) Patients taking IS for other diseases besides their liver transplant |
| 6) Patients unable to give written informed consent in accordance with research ethics board guidelines |
HBV, hepatitis B virus; HCV, hepatitis C virus; INR, international normalized ratio.
Fig. 1.
Trial design. In Phase 1, eligible patients had blood collected for PBMC gene expression profiling. In Phase 2, patients with a positive tolerance biomarker underwent IS withdrawal, following a baseline liver biopsy. Liver biopsies were performed, and blood was collected at various months post-IS withdrawal as indicated. M, months.
Definition of rejection
Suspected rejection was diagnosed by disturbances in liver biochemistry including aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and bilirubin but all rejection episodes were confirmed by liver biopsy findings according to the Banff criteria [21]. Patients who had documented rejection were treated with a short course of oral steroids and reinstitution of IS.
Definition of operational tolerance
Patients were classified as operationally tolerant as long as complete cessation of IS was maintained for a minimum of 12 months and no histologic evidence of rejection was observed.
Study end points
The primary aim of the study was the development of operational tolerance in patients who had the PMBC gene ratio (FGL2/IFNG ≥ 1). Secondary end points were mortality, graft loss, changes in adverse effects associated with cessation of IS and assessment of immune markers/gene expression in peripheral blood and liver allografts including RNA sequencing described below.
Liver biopsy specimens
Percutaneous liver biopsies were performed under local anaesthesia using an 18-gauge Jamshidi needle. A 0.5-cm portion of the biopsy to be used for gene expression was stored in RNALater (Qiagen, Germantown, MA, USA) for 24 h at 4°C and then transferred to −80°C. The remainder of the biopsy was used for histologic examination and was formalin-fixed and paraffin-embedded.
Histologic examination of liver biopsies
Haematoxylin-eosin- and Masson trichrome-stained sections were examined by two local pathologists who were blinded to all clinical and biological data. Biopsies were evaluated using the Banff criteria [21, 22]. For entry into Phase 2 of the study, patients were required to have a normal liver biopsy, defined as the absence of cellular, ductopenic, antibody-mediated, or other form of rejection; absence of active interface or lobular inflammation; absence of other active parenchymal or biliary injury; and fibrosis not more than Laennec stage 2.
Isolation of peripheral blood mononuclear cells
Peripheral blood mononuclear cells (PBMC) were isolated from whole blood using Cell Preparation Tubes with sodium heparin (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) following the manufacturer’s instructions. PBMC were resuspended in RNAprotect cell reagent (Qiagen, Germantown, MA, USA) and stored at −80°C for Multiplex RT-PCR and qPCR studies. PBMC were also cryopreserved in freezing media (10% DMSO in fetal calf serum) for immunophenotyping studies.
RNA extraction from PBMC and liver biopsies
Total RNA was isolated from PBMC preserved in RNAprotect (Qiagen) using the PureLink RNA Mini Kit (Ambion, Austin, TX, USA) following the manufacturer’s instructions. The final RNA precipitate was dissolved RNA Storage Solution (Ambion). RNA aliquots were stored at −80°C for future use in GeXP multiplex reverse transcriptase polymerase chain reaction (RT-PCR), quantitative PCR (qPCR), and RNA sequencing (RNA-seq) studies.
Total RNA was extracted from liver biopsies preserved in RNALater. Specimens were thawed and transferred to a clean, RNase-free microcentrifuge tube. Tissue samples were incubated in lysis buffer (Ambion) with 2-mercaptoethanol for 10 min. Tissue samples were then homogenized using a nuclease-free disposable pellet pestle (Kimble Kontes, Vineland, NJ, USA) and processed using the PureLink RNA Mini Kit (Ambion).
GeXP multiplex RT-PCR
Thirteen genes were analysed in a single PCR using the GenomeLab GeXP Genetic Analysis System (SCIEX, Brea, CA, USA). Eight target genes [FGL2, forkhead box P3 (FOXP3), transforming growth factor-β1 (TGFB1), lymphocyte activating-3 (LAG3), T-cell immunoreceptor with Ig and ITIM domains (TIGIT), interleukin-10 (IL10), IFNG, and GZMB] were chosen for multiplexing based on previous studies [18, 19], and five housekeeping genes [hypoxanthine-guanine phosphoribosyltransferase (HPRT), TATA box binding protein (TBP), beta-2 microglobulin (B2M), cancer susceptibility candidate-3 (CASC3), and ezrin (EZR)] were used to normalize the data. Primers were designed to ensure that five nucleotides or more separated the length of each of the 13 amplicons in the gene panel, allowing for each gene amplicon to be characterized by a unique distinct peak on the electropherogram.
Total RNA from PBMC and liver biopsies were processed using the GenomeLab GeXP Start Kit (SCIEX) following the manufacturer’s protocols. RNA was first reverse-transcribed using the primer mixture and the reverse transcription reaction mixture from the GenomeLab GeXP Start Kit (SCIEX) and kanamycin resistance gene (kanR) RNA, which served as an internal control. A PCR mixture was then assembled using cDNA product from the reverse transcription step, forward primer mixture, and Thermo-Start DNA Polymerase. The forward primer mixture consisted of custom-designed gene-specific primers along with a fluorescent dye-labelled universal primer. PCR products were then separated by capillary electrophoresis using the GenomeLab GeXP Genetic Analysis System (SCIEX). Using the kanR signal as the reaction control for each well, the GeXP Quant Tool program normalized the fragment data to the kanR signals by dividing the peak area of each gene peak by the peak area of kanR. Gene expression values were then normalized to the house keeping genes and subsequently expressed as a ratio compared with gene expression in PBMC or liver tissue obtained during surgery from live liver donors who served as healthy controls.
Quantitative PCR
To validate the GeXP results, qPCR was performed. Gene expression of eight genes of interest (FGL2, FOXP3, TGFB1, LAG3, TIGIT, IL10, IFNG, and GZMB) and two housekeeping genes (HPRT and TBP) was measured by real-time qPCR (qPCR) using the LightCycler 480 SYBR Green I Master (Roche Applied Science, Indianapolis, IN, USA) and LightCycler 480 Real-Time PCR Machine (Roche Diagnostics, Rotkreuz, Switzerland). The primers for qPCR amplification of the target and housekeeping genes are shown in Supplementary Table 1. Total RNA was reverse-transcribed into complementary DNA (cDNA) using the SuperScript IV First Strand Synthesis System (Invitrogen, Waltham, MA, USA). Real-time qPCR was then performed in a 10 μl volume of 2× Master Mix (5 μl), 5 μM primer mixture (1 μl), PCR-grade water (2 μl), and cDNA diluted 1:7 (2 μl). Samples were run in triplicate and all results were normalized to HPRT. The 2−∆∆Ct method was used to calculate gene expression of target genes relative to housekeeping gene HPRT [23].
Mass cytometry
Peripheral blood immune cell subsets were characterized with a 36-parameter mass cytometry panel (Supplementary Table 2) based on the immune monitoring flow cytometry markers used in the ONE Study of immunoregulatory cell therapy in renal transplantation [24]. Cryopreserved PBMC were recovered and stained with cis-platinum and a DNA intercalator to distinguish live cells from debris. After staining with antibodies and washing, data were acquired on a CyTOF 2 mass cytometer (Fluidigm, South San Francisco, CA, USA) and analysed with conventional gating using Cytobank software (Cytobank, Santa Clara, CA, USA).
Liver immunofluorescence
Intrahepatic immunophenotyping was performed as previously described [17, 25, 26]. Briefly, the portal infiltrate size was determined by encircling portal infiltrates along the limiting plate and excluding the lumen of veins, arteries, and bile ducts. The intrahepatic infiltration of CD4+CD8−FOXP3− (CD4+), CD8+CD4−FOXP3− (CD8+), CD4+CD8−FOXP3+ (CD4+FOXP3+ Tregs), and CD8+CD4−FOXP3+ (CD8+FOXP3+) cells was quantified. In the current study 95% of portal FOXP3+ cells were CD4+, and only 5% were potentially activated CD8+FOXP3+ T effector cells excluding a significant contamination of activated T effector cells in the pool of CD4+FOXP3+ Tregs. The immunohistological Treg detection in human FFPE tissue sections was recently validated by flow cytometric and epigenetic analysis [17, 25].
RNA-seq and analysis
RNA previously isolated from baseline liver biopsies for GeXP studies was used for RNA-seq, which was performed at The Centre for Applied Genomics (Toronto, Canada). Briefly, RNA quality was evaluated using a Bioanalyzer 2100 (Agilent, Santa Clara, CA, USA) and samples with an RNA integrity number (RIN) > 7 were submitted for sequencing. RNA library preparation was performed with the NEBNext Ultra II Directional RNA Library Prep kit (New England Biolabs, Ipswich, MA, USA). Libraries were sequenced on a High Throughput Run Mode flowcell with V4 sequencing chemistry on a HiSeq 2500 (Illumina, San Diego, CA, USA) platform following the manufacturer’s recommended protocol. Generated sequence fragments were aligned to the reference genome (GRCh38, Gencode annotations, Release 356) using the STAR aligner, v.2.6.0c. The filtered STAR alignments were processed to extract raw read counts for genes using Htseq-count v.0.6.1p2. Only uniquely mapping reads are counted. Two-condition differential gene expression analysis was performed with DESeq2 v.1.26.0s, using R v.3.6.1. Initial minimal filtering of 10 read counts per gene for at least three samples was applied to the data set. A cut-off of P adjusted (Padj) < 0.05 by the Benjamini–Hochberg method was used to identify genes with differential expression between tolerant and non-tolerant groups.
Statistics
Continuous variables were analysed with either a t-test for normally distributed data or a Mann–Whitney U test for non-normally distributed data. Categorical variables were analysed with a Fisher’s exact test. Analysis of gene expression and liver immunohistochemistry was performed with Kruskal–Wallis test followed by Dunn’s multiple comparisons post hoc test. Statistical analyses were performed using the Graphpad Prism version 8 software package (Graphpad Software, La Jolla, CA, USA). P-values of ≤ 0.05 were considered statistically significant.
Results
Development of a human GeXP gene expression assay
A human GeXP assay was developed to quantify expression of eight target genes (FGL2, FOXP3, TGFB1, LAG3, TIGIT, IL10, IFNG, and GZMB) previously identified to be predictive of tolerance in preclinical studies [18, 19]. Comparison of gene expression using this GeXP assay with real-time qPCR showed a high degree of correlation with R-square values > 0.72 for six of the genes (FGL2, FOXP3, IFNG, IL10, LAG3, TIGIT) (Supplementary Figure 1).
Patients and clinical outcomes
Sixty-nine liver transplant recipients who were a minimum of 6 months post-liver transplant and had no documented rejection episodes in the previous 3 months were enrolled over a 54-month period from May 2015 to November 2019. Patients with autoimmune liver disease and non-active (no viral replication) viral hepatitis B and C were eligible for inclusion in the trial. After patients entered the study, their PBMC gene expression was determined using the custom GeXP assay. Similar to the murine preclinical studies of tolerance, we expressed the eight genes in the assay as increased or decreased in relation to normal healthy controls and as the ratio of anti-inflammatory to pro-inflammatory genes (e.g. FGL2/IFNG, IL10/IFNG, TIGIT/IFNG, and TGFB1/IFNG). An elevated PBMC ratio of FGL2/IFNG, which was predictive of tolerance in preclinical models of tolerance, was used to as a putative tolerance biomarker in LITMUS. Overall, 28 of the 69 patients were positive for the tolerance biomarker (FGL2/IFNG ≥ 1). Interestingly, the order of 69 patients remained relatively unchanged when patients were sorted by the ratio of FGL2/IFNG, FOXP3/IFNG, or TGFB1/IFNG, suggesting that there may be coordinated expression of other immunoregulatory genes (Supplementary Figure 2). Table 2 shows the clinical characteristics of the entire cohort and patients who were positive and negative for the tolerance biomarker. Compared with patients in the negative tolerance biomarker group, patients in the positive biomarker group had a higher percentage mycophenolate mofetil (MMF) use and a lower percentage of hepatitis B virus (HBV) as an indication for transplantation (Table 2).
Table 2.
Clinical characteristics of the entire trial cohort, patient who were positive for the tolerance biomarker, and patients who were negative for the tolerance biomarker
| Total cohort (n = 69) | Tolerance biomarker positive (n = 28) | Tolerance biomarker negative (n = 41) | P-value | |
|---|---|---|---|---|
| Age (years) | 56 ± 15 | 55 ± 16 | 57 ± 14 | 0.51 |
| Sex (% male) | 61 | 57 | 63 | 0.62 |
| Diagnosis (number of patients) | ||||
| HCV cirrhosis | 18 | 9 | 9 | 0.41 |
| HBV cirrhosis | 10 | 1 | 9 | 0.04 |
| Alcoholic cirrhosis | 12 | 3 | 9 | 0.34 |
| Autoimmune | 5 | 2 | 3 | 0.99 |
| FHF | 5 | 4 | 1 | 0.15 |
| NASH | 6 | 4 | 2 | 0.21 |
| Other | 13 | 5 | 8 | 0.99 |
| Time from transplant to trial enrolment (months) | 111 ± 74 | 110 ± 69 | 111 ± 76 | 0.98 |
| No history of rejection post-liver transplant (%) | 70 | 71 | 68 | 0.78 |
| IS regimen (%) | ||||
| Tacrolimus | 68 | 68 | 68 | 0.99 |
| Cyclosporine | 32 | 32 | 32 | 0.99 |
| MMF | 9 | 18 | 2 | 0.04 |
| Donor age (years) | 45 ± 18 | 44 ± 15 | 45 ± 18 | 0.83 |
| Type of transplantation (%) | 0.99 | |||
| Deceased donor | 83 | 82 | 83 | |
| Living donor | 17 | 18 | 17 | |
| Laboratory values at enrolment | ||||
| Creatinine | 99 ± 34 | 99 ± 25 | 99 ± 40 | 0.96 |
| AST | 25 ± 8 | 26 ± 8 | 24 ± 8 | 0.33 |
| ALT | 24 ± 13 | 27 ± 14 | 22 ± 12 | 0.16 |
| ALP | 104 ± 40 | 108 ± 45 | 102 ± 37 | 0.58 |
| Bilirubin | 14 ± 11 | 11.9 ± 5.6 | 14.8 ± 12.9 | 0.27 |
| INR | 1.1 ± 0.4 | 1.2 ± 0.6 | 1.1 ± 0.3 | 0.67 |
Data are expressed at mean ± SD. FHF, fulminant hepatic failure; HBV, hepatitis B virus; HCV, hepatitis C virus; INR, international normalized ratio; MMF, mycophenylate mofetil; NASH, non-alcoholic steatohepatitis.
Of the 28 eligible patients, 23 entered the IS withdrawal phase of LITMUS (Phase 2), three patients refused entry, and two patients were deemed medically ineligible due to pre-existing medical conditions (one patient had polycythaemia vera and preleukemia, and another patient had a history of hepatic artery and portal vein thromboses and was on systemic anticoagulation). Of the 23 patients, nine had evidence of recurrent or de novo autoimmune liver disease or subclinical cell-mediated rejection on their liver biopsies (Supplementary Figure 3) and thus were excluded from the withdrawal of IS phase of the study. Fourteen patients had normal liver biopsies and entered the IS withdrawal phase of the study. No patients with a diagnosis of autoimmune liver disease (autoimmune hepatitis, primary biliary cholangitis, or primary sclerosing cholangitis) had the tolerant profile and a normal liver biopsy. Of the patients who entered the IS withdrawal phase of the study (Phase 2), eight patients have been successfully weaned off IS and are operationally tolerant (IS-free for a range of 12–57 months). Six patients developed rejection which occurred either during IS weaning (n = 3) or post-IS withdrawal (n = 3) (Figs 2 and 3). All of the rejection episodes were mild (Banff score less than 5) and were easily reversed with a short course of steroids and reinstitution of IS. No patients suffered graft loss or any long-term side effects and their liver chemistry returned to normal following treatment.
Fig. 2.
Flowchart of patient enrolment.
Fig. 3.
Probability of patients being free of rejection from the initiation of IS withdrawal. Tick marks indicate patients who are operationally tolerant.
Table 3 shows baseline characteristics of the operationally tolerant patients versus non-tolerant patients who either developed rejection or had abnormal liver biopsies. Although there was no difference in age between the two groups, two of the patients who developed tolerance were less than 30 years of age. Compared with non-tolerant patients, operationally tolerant patients had a longer time from transplant to enrolment and a lower baseline ALT. Choice and drug levels of calcineurin inhibitor at entry into the study (pre-IS withdrawal) were not statistically different between tolerant and non-tolerant patients. Although MMF usage was increased in the high biomarker ratio patients, its use was not different between tolerant and non-tolerant patients (Table 3). The ability of other gene ratios (FOXP3/IFNG and TGFB/IFNG) to identify tolerant patients was also examined. Using these gene ratios, less patients would have been identified as having a positive ratio (ratio value ≥ 1) and less tolerant patients would have been identified than with FGL2/IFNG ratio (Supplementary Table 3).
Table 3.
Clinical characteristics of operationally tolerant patients and non-tolerant patients
| Operationally tolerant (n = 8) | Non-toleranta (n = 15) | P-value | |
|---|---|---|---|
| Age (years) | 56 ± 19 | 55 ± 16 | 0.93 |
| Sex (% males) | 63 | 60 | 0.99 |
| Diagnosis (number of patients) | |||
| HCV cirrhosis | 3 | 5 | 0.99 |
| Alcoholic cirrhosis | 0 | 2 | 0.53 |
| Autoimmune | 0 | 2 | 0.53 |
| FHF | 1 | 1 | 0.99 |
| NASH | 1 | 3 | 0.99 |
| Other | 3 | 2 | 0.29 |
| Time from transplant to trial enrolment (months) | 158 ± 83 | 91 ± 51 | 0.03 |
| Free of rejection prior to enrolment (%) | 75 | 68 | 0.99 |
| IS regimen prior to withdrawal (%) | |||
| Tacrolimus | 50 | 73 | 0.37 |
| Cyclosporine | 50 | 27 | 0.37 |
| MMF | 13 | 20 | 0.99 |
| CNI drug levels prior to IS withdrawal (ng/ml) | |||
| Tacrolimus (trough level) | 5.8 ± 0.5 | 5.9 ± 1.5 | 0.68 |
| Cyclosporine (C2 level) | 354 ± 245 | 278 ± 133 | 0.89 |
| Donor age (years) | 48 ± 7 | 46 ± 9 | 0.82 |
| Type of transplantation (%) | 0.99 | ||
| Deceased donor | 88 | 80 | |
| Living donor | 12 | 20 | |
| Laboratory values at enrolment | |||
| Creatinine | 107 ± 36 | 94 ± 18 | 0.27 |
| AST | 22 ± 4 | 29 ± 10 | 0.07 |
| ALT | 19 ± 6 | 33 ± 17 | 0.05 |
| ALP | 99 ± 33 | 110 ± 53 | 0.61 |
| Bilirubin | 12.6 ± 5 | 10.6 ± 10.6 | 0.33 |
| INR | 1 ± 0 | 1 ± 0.1 | 0.43 |
Data are expressed at mean ± SD. CNI, calcineurin inhibitor; FHF, fulminant hepatic failure; HCV, hepatitis C virus; INR, international normalized ratio; NASH, non-alcoholic steatohepatitis.
Patients with either unsuccessful IS withdrawal (n = 6) or abnormal baseline liver biopsy (n = 9).
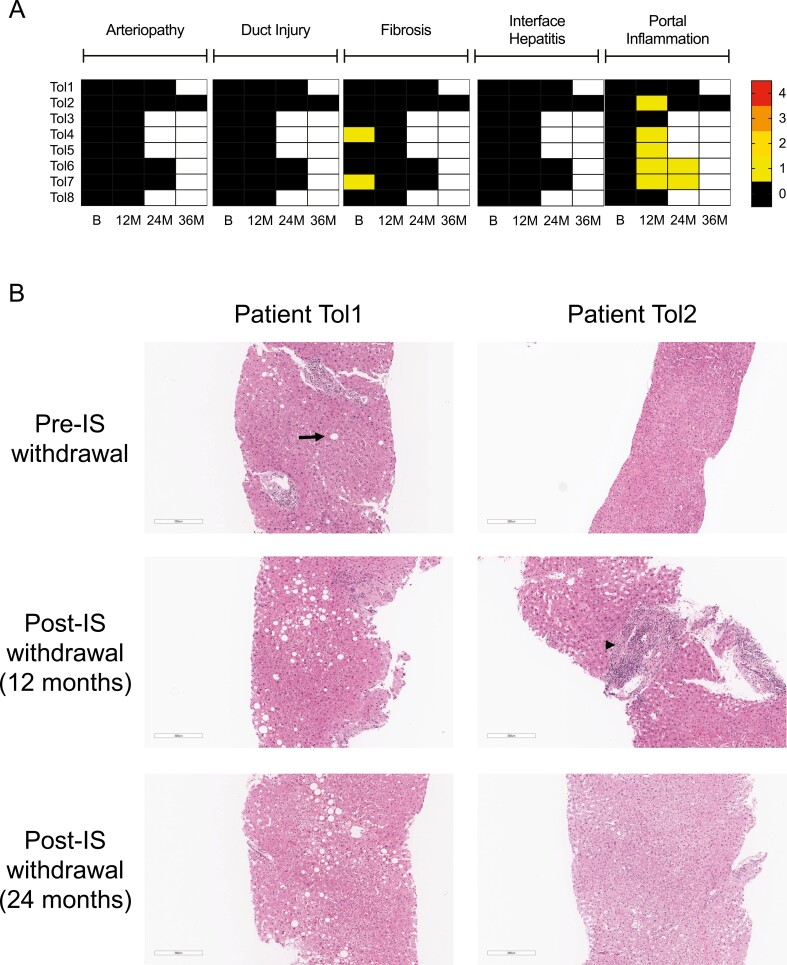
Protocol liver biopsies were performed pre- and post-IS withdrawal. Biopsies were examined especially for the presence of interface hepatitis, arteriopathy, bile duct loss, and fibrosis which have been reported to be associated with inability to wean off IS [22]. Results from a detailed analysis of biopsies from tolerant patients are shown in Fig. 4A. Pre-IS withdrawal, two of eight of the tolerant patients had mild fibrosis (Grade 1) without additional abnormalities, and six patients had no significant abnormalities. Post-IS withdrawal, there was no evidence of arteriopathy, fibrosis progression, interface hepatitis, or bile duct injury/loss in any of the biopsies from tolerant patients. However, five of the tolerant patients developed mild portal inflammation. Figure 4B shows pre- and post-IS withdrawal biopsies from a patient who had no significant change in liver pathology post-IS withdrawal (Patient Tol1) and biopsies from another patient who developed mild portal infiltrates, which resolved on a subsequent biopsy (Patient Tol2). At the time of the diagnosis of rejection, liver biopsies from the six non-tolerant patients had evidence of rejection with mild to moderate portal and/or interface hepatitis (n = 5), arteriopathy (n = 1), bile duct injury (n = 4), and increased fibrosis (n = 4) (Supplementary Figure 4).
Fig. 4.
Liver biopsy histopathology in tolerant patients. (A) Evaluation of liver biopsies for arteriopathy, duct injury, fibrosis, interface hepatitis, and portal inflammation. The absolute score for each of these parameters is represented in the heatmap as shown in the scale. Arteriopathy, duct loss, and portal inflammation were scored from 0 to 3, whereas fibrosis was scored from 0 to 4. Scoring was performed using Banff criteria [21, 22]. All eight tolerant patients had biopsies at baseline (B) and end of trial at 12 months (12M) post-IS withdrawal. Some of the tolerant patients also had biopsies available for evaluation at 24 months (24M) and 36 months (36M) post-IS withdrawal. White spaces in the heatmap represent biopsies that were not available for evaluation. (B) Liver biopsies in two patients (Patient Tol1 and Patient Tol2) pre-IS withdrawal, 12 months post-IS withdrawal, and 24 months post-IS withdrawal. Patient Tol1 was noted to have mild macrovesicular steatosis at baseline (arrow). On post-IS withdrawal biopsies, there was persistent macrovesicular steatosis and no evidence of cellular infiltrates. Patient Tol2 developed a focal portal mononuclear cell infiltrate at 1 year post-IS withdrawal (arrowhead). At 2 years post-IS withdrawal, the infiltrate had largely resolved. Liver biopsies were stained with haematoxylin and eosin (H&E). Scale bars: 300 µm.
To determine if withdrawal of IS had clinical benefit, patients were monitored for effect on renal function, hypertension, diabetes, cancer, and death at 1 year post-IS withdrawal. Patients who were maintained on IS (PBMC tolerance biomarker negative) served as a control group. Patients successfully weaned off IS had numerical improvements in the incidence and severity of renal dysfunction and no new hypertension, diabetes, cancer, or death, but none of these reached statistical significance (Table 4).
Table 4.
Development of transplant complications in operationally tolerant patients and tolerance biomarker negative liver transplant recipients
| Operationally tolerant (n = 8) | Tolerance biomarker negative (n = 41) | P-value | |
|---|---|---|---|
| Renal function (change from baseline;µmol/L) | −10.1 ± 15.1 | +0.6 ± 15.3 | 0.075 |
| Hypertension (new diagnosis) | 0 (0%) | 3 (7.3%) | 0.9 |
| Diabetes (new diagnosis) | 0 (0%) | 1 (2.4%) | 0.9 |
| Malignancy (new diagnosis) | 0 (0%) | 1 (24%) | 0.9 |
| Death | 0 (0%) | 2 (4.9%) | 0.9 |
Data are presented as mean ± SD or number (percent). Change in clinical parameters since time of enrolment into trial (Phase 1).
Operationally tolerant patients have an increase in peripheral blood Treg post-IS withdrawal
Further analysis of PBMC GeXP gene expression was performed to gain insights into mechanisms of tolerance. At baseline (prior to IS withdrawal), there were no differences in the gene panel and the FGL2/IFNG gene ratio between operationally tolerant patients and non-tolerant patients (Supplementary Figure 5). In operationally tolerant patients, serial PBMC profiling revealed an early (3 months) increase in FOXP3 gene expression, consistent with an expansion of Tregs in the peripheral blood (Fig. 5A). There was also a significant increase in TIGIT (9 months) and decrease in TGFB1 (9 and 12 months) post-IS withdrawal (Fig. 5A).
Fig. 5.
PBMC gene expression and Treg in the peripheral blood. (A) Change in PBMC target gene expression at 3, 6, 9, and 12 months post-IS withdrawal in operationally tolerant recipients. For each recipient, gene expression was normalized to pre-withdrawal gene expression. Symbols represent median, and bars represent IQR. Significance was determined with the Kruskal–Wallis test followed by Dunn’s multiple comparisons post hoc test. (B) Quantification of Tregs with mass cytometry. Tregs in peripheral blood were identified based on expression of CD3+CD4+CD25+CD127low markers and are shown as a percentage of CD4+ T cells. PBMC were studied pre-IS withdrawal and 3–6 months post-IS withdrawal in operationally tolerant recipients (n = 6). Lines represent individual patients. Significance was determined used a paired t-test. ∗P < 0.05; ∗∗P < 0.01. IQR, interquartile range; M, months.
In order to confirm GeXP gene changes, mass cytometry was performed on PBMC from operationally tolerant patients. These studies demonstrated a greater than 2.5-fold expansion of Tregs as a percentage of CD4+ cells post-IS withdrawal (1.78% vs. 4.72%, P < 0.01) (Fig. 5B).
Operationally tolerant patients have an elevated baseline liver FOXP3/IFNG gene ratio and an accumulation of portal Tregs post-IS withdrawal
Gene expression from liver biopsies were also profiled with the GeXP assay to identify intrahepatic genes associated with tolerance. In contrast to baseline PBMC gene expression, there was a significant difference between baseline liver gene expression between operationally tolerant patients and patients who developed rejection (non-tolerant) with a higher intrahepatic FOXP3/IFNG gene ratio in the tolerant patients (Fig. 6A). Furthermore, at a FOXP3/IFNG ratio cut-off of 1, only one patient (17%) in the non-tolerant group had an elevated ratio, whereas all eight patients (100%) in the tolerant group had an elevated ratio (Fig. 6B). This result suggests that a higher ratio of intrahepatic Tregs to inflammatory cells at baseline may serve to identify tolerant liver transplant recipients. In the eight patients who achieved tolerance, profiling of liver gene expression over time revealed a decrease in this FOXP3/IFNG ratio, consistent with inflammatory changes in the liver post-IS withdrawal (Fig. 6C). Over time there was also an increase in TGFB1 and a decrease in FGL2 intrahepatic gene expression in operationally tolerant patients (Fig. 6C).
Fig. 6.
Baseline and longitudinal liver allograft gene expression. (A) Baseline FOXP3/IFNG liver gene ratio in operationally tolerant patients (n = 8) versus unsuccessful (n = 6) IS withdrawal. Symbols represent median, and bars represent IQR. Significance was determined by a Mann–Whitney U test. (B) Baseline FOXP3/IFNG liver gene ratio separated by a FOXP3/IFNG < 1 or FOXP3/IFNG ≥ 1. (C) Change in liver target gene expression at 6 and 12 months post-IS withdrawal in operationally tolerant recipients. For each recipient, gene expression was normalized to pre-withdrawal gene expression. Symbols represent median, and bars represent IQR. Significance was determined with the Kruskal–Wallis test followed by Dunn’s multiple comparisons post hoc test. ∗P < 0.05; ∗∗P < 0.01. IQR, interquartile range; M, months.
Immunofluorescence was performed on liver biopsies to delineate CD4+ T cells, CD8+ T cells, and FOXP3+ Tregs in operationally tolerant patients (Fig. 7A). After the complete withdrawal of IS, the size of portal tracts exhibited a non-significant trend to an approximately 50% expansion (Fig. 7B and G). T cells as well as Tregs accumulated in the portal tracts after the weaning (Fig. 7C–E). The lobular T-cell infiltration was persistently low and too variable for a systematic analysis. While both CD4+ and CD8+ T cells increased over time, Tregs accumulated disproportionately in the portal tracts compared to total T-cell numbers, as exemplified by an increasing ratio of Tregs to total CD4+ and CD8+ T cells (Fig. 7F and G). The increase in portal Treg seen at 1 year persisted out to 2 years post-IS withdrawal (Fig. 7E and G).
Fig. 7.
Immunophenotyping of the T-cell compartment in liver allograft biopsies at pre-withdrawal and 12 and 24 months post-IS withdrawal. (A) Left panel: representative histology from a tolerant patient after successful IS withdrawal. Co-staining for CD4 (red), CD8 (green), and FOXP3 (blue) of intrahepatic T-cell infiltration was performed in liver biopsy sections. Liver sinusoidal epithelial cells weakly express CD4 and can be distinguished from T cells by strength of CD4 expression, shape, and localization of cells. The white line surrounds areas of portal infiltrations and excludes lumen of veins, arteries, and bile ducts. Right panel: higher magnification of portal infiltrates with clear nuclear localization of the FOXP3 in CD4+ Tregs (white arrows). (B) Size of portal infiltrates in liver biopsies before after complete and successful IS weaning. (C) Portal infiltration density of CD4+ T cells. (D) Portal infiltration density of CD8+ T cells. (E) Portal infiltration density of CD4+FOXP3+ Tregs. (F) Portal Tregs/(CD4+ + CD8+) ratio. Symbols represent median, and bars represent IQR for patients at 12M (n = 6) and patients at 24M (n = 4). Significance was determined with the Kruskal–Wallis test followed by Dunn’s multiple comparisons post hoc test. ∗P < 0.05. (G) Mean relative changes of histological parameters during operational tolerance compared to pre-withdrawal biopsies. IQR, interquartile range; M, months.
Operationally tolerant patients have higher baseline liver expression of SELE and lower expression of genes associated with inflammatory responses
To gain further insights into mechanisms of tolerance induction, pre-IS withdrawal liver biopsies from patients who achieved operational tolerance and who were non-tolerant (abnormal biopsies at baseline or developed rejection during IS withdrawal) were analysed with RNA-seq. Overall, there were 16 genes that were differentially expressed between the two groups with five genes upregulated and 11 genes downregulated (Supplementary Table 4). Figure 8A shows a volcano plot and Fig. 8B shows a heatmap of these differentially expressed genes. The gene for E-selectin (SELE) was the most significantly upregulated gene in the tolerant group versus the non-tolerant group (Padj = 0.00026; log2 fold change = 1.67). Additional upregulated genes included tetraspanin 11 (TSPAN11), inositol hexakisphosphate kinase 6 (IP6K3), thiosulfate sulfurtransferase (TST), AC162151.2 (pseudogene), and AC136475.3 (long non-coding RNA). Genes that were reduced in the tolerant versus the non-tolerant group were associated with inflammatory responses including ubiquitin D (UBD), lymphocyte-specific protein-1 (LSP1), class II major histocompatibility complex transactivator (CIITA), C-X-C motif chemokine ligand 9 (CXCL9), and GZMB. The most significantly downregulated gene was X-inactive specific transcript (XIST). The significance of this is presently unknown but may be due to a mismatch in the sex of the liver donors between the two groups (two female liver donors in the tolerant group and five female donors in the non-tolerant group).
Fig. 8.
Results of RNA-seq on pre-withdrawal liver biopsies. (A) Volcano plot of genes upregulated and downregulated in tolerant patients (n = 8) versus non-tolerant patients with unsuccessful withdrawal/abnormal biopsy (n = 6 unsuccessful, n = 3 abnormal). (B) Heatmap display of the 16 genes with significant (Padj < 0.05) differences. Rows represent genes, and columns represent different patient samples. Hierarchical clustering was used to sort both rows and columns. A relative colour scheme was used to convert the minimum and maximum values in each row to colours. Higher gene expression is indicated by red, whereas lower gene expression is indicated by blue.
Discussion
Liver transplantation is now recognized as a highly successful therapy for patients with end-stage liver disease. Yet despite this, the need for long-term IS leads to significant morbidity and mortality [2]. The ability to safely stop IS would presumably improve the long-term success of liver transplantation especially if it could be done early post-transplantation prior to the development of long-term complications such as renal dysfunction [2]. Although it is known that selected liver transplant recipients may be operationally tolerant and can safely be weaned off IS, there is presently no reliable biomarker to identify these patients. The LITMUS study was a Phase 2a pilot study to examine whether a gene biomarker panel that was predictive of tolerance in preclinical heart and liver transplant models could identify operationally tolerant recipients. We identified 28/69 (41%) liver transplant recipients who had the putative PBMC tolerance biomarker and thus were candidates for IS withdrawal. Of these 28 patients, 23 had evaluable outcomes including eight patients who are operationally tolerant. Further analysis showed that patients who achieved tolerance had high baseline FOXP3/IFNG allograft gene expression and high mRNA levels of E-selectin as detected by RNA-seq.
Compared to studies that do not rely on biomarkers to guide IS withdrawal, this PBMC biomarker approach appears to enrich for recipients who can successfully be weaned off IS. Here we found that 8/14 (57%) of patients with the positive tolerance biomarker (FGL2/IFNG ≥ 1) and a normal liver biopsy were operationally tolerant. This is in contrast to non-biomarker-guided studies where a combined 140/455 (30.8%) of adult liver transplant recipients were operationally tolerant [4–12]. Unlike LITMUS, many of these non-biomarker-guided studies used highly selected patients (non-autoimmune, non-viral replicative liver transplant recipients), which can improve success rates of IS withdrawal. Furthermore, patients had stopped IS at a time well after complications had developed. We also examined the utility of other PBMC biomarkers identified in our preclinical studies by GeXP including FOXP3/IFNG and TGFB/IFNG. Although there was significant overlap in patients among these biomarkers (Supplementary Figure 2), the ratio of FGL2/IFNG ≥ 1 identified more patients who were operationally tolerant than either of the other two biomarkers. These results support the use of FGL2/IFNG in future larger clinical studies.
The biomarker represents the ratio between an anti-inflammatory gene (FGL2) and the pro-inflammatory gene (IFNG). In preclinical models of heart transplantation, we demonstrated that both Tregs and FGL2 are necessary for tolerance induction as treatment of tolerant animals with either an anti-CD25 (PC61) or an anti-FGL2 antibody led to rejection of allografts [18]. FGL2 is known to be primarily secreted by Tregs and inhibits dendritic cell maturation following binding to its cognate receptor, FcγRIIb [27]. FGL2 has also been shown to inhibit a subset of effector CD8+ T cells that express FcγRIIb [28]. Recently, FGL2 has been shown to be an effector molecule of T follicular regulatory cells, which are known to limit antibody responses in germinal centres [29]. Upregulation of FGL2 gene expression may therefore serve to inhibit cellular and humoral allo- and autoimmune responses. IFN-γ is a pro-inflammatory cytokine with important roles in T-cell activation and allograft rejection [30]. A high FGL2/IFNG ratio therefore selects for low IFNG gene expression and lower levels of T-cell activation. Transplant recipients who can be successfully weaned off IS presumably have less T-cell activation while they are immunosuppressed compared with non-tolerant patients and therefore have a higher FGL2/IFNG gene ratio. The choice and level of calcineurin inhibitor and use of MMF was not different between tolerant and non-tolerant patients. However, there was an increased proportion of patients on MMF in the high FGL2/IFNG biomarker group, which may reflect an inhibition of IFNG by MMF as has been described previously [31]. In favour of using ratios of anti-inflammatory to pro-inflammatory factors to predict patient outcomes is a recent study that showed a high cytokine IL-10 to Tumour necrosis factor-α (TNF-α) ratio in transitional B cells was associated with freedom of rejection in kidney transplant recipients [32].
In LITMUS, we also showed that monitoring of allograft gene expression may be critical to identifying operationally tolerant patients as the baseline liver allograft FOXP3/IFNG gene ratio was higher in tolerant versus non-tolerant patients. This is consistent with previous studies showing that a higher intragraft Foxp3/Ifng ratio correlated with tolerance versus rejection in preclinical transplant models [18, 19]. The intragraft and not the PBMC FOXP3/IFNG ratio was higher in tolerant versus non-tolerant patients, suggesting that local (intragraft) cell populations mediate tolerance. Importantly, FOXP3 gene expression by itself was not predictive of tolerance, consistent with prior studies showing no significant difference in graft infiltrating Tregs in baseline liver biopsies of tolerant and non-tolerant patients [17].
One of the strengths of the LITMUS study is that we provide data from multiple time points post-IS withdrawal. The data are supportive that operational tolerance is an active process involving peripheral regulation. We observed an increase in both FOXP3 gene expression and numbers of Tregs by mass cytometry in the peripheral blood of operationally tolerant recipients. This is similar to a previous report of increased numbers of CD4+CD25+ T cells and FOXP3 mRNA expression in the peripheral blood in liver transplant recipients who successfully discontinued immunosuppressive therapy [33]. Within the liver allograft, we observed an increase in numbers of T cells in portal tracts and a proportionally larger increase in FOXP3+ Tregs, similar to what has been observed in previous IS weaning trials [10, 26]. Although there was not an increase in intrahepatic FOXP3 gene expression during the development of operational tolerance, there was increase in gene expression for TGFB1, a known Treg effector molecule. These findings of the intrahepatic T-cell compartment point to active immune regulation involving immunoregulation by Tregs in the graft itself rather than a deletion of T-cell alloreactivity. However, the expansion of intragraft Treg may at least in part be related to withdrawal of calcineurin inhibitors, which are known to suppress Treg proliferation [34]. At this point, we cannot distinguish between Treg expanding to control alloimmune responses versus expanding as a result of calcineurin withdrawal. In support of Treg playing an active role in transplantation tolerance are spontaneous models of liver transplant tolerance, which are characterized by an inflammatory infiltrate in the liver and an accompanying expansion of Tregs [19]. By post-operative day 100, there was near-complete resolution of the inflammatory infiltrate liver in this model (similar to liver biopsies of Patient Tol2), while operationally tolerant human liver grafts usually exhibit a long persistence of mild portal infiltrates [26]. That Tregs are necessary for tolerance in preclinical models has been confirmed as depletion of Tregs with an anti-CD25 antibody leads to rejection [35].
As part of the study design, liver biopsy samples were analysed with RNA-seq technology to identify additional genes associated with tolerance. Liver allografts from tolerant patients compared with non-tolerant patients expressed higher mRNA levels of SELE (gene for E-selectin) at baseline prior to IS withdrawal. E-selectin, which is an inducible adhesion molecule expressed by endothelial cells, plays an important role in recruitment of lymphocytes and neutrophils to sites of inflammation [36]. However, in the tolerant livers expression of E-selectin was increased in the presence of lower inflammatory gene expression. Interestingly, Tregs are reported to express ligands for E-selectin and thus expression of E-selectin may also be important for recruitment of Tregs [37, 38]. Furthermore, upregulation of E-selectin may be involved in the skewing the FOXP3/IFNG ratio to tolerance through increased Treg recruitment. The gene for a tetraspanin protein (TSPAN11) was also upregulated in tolerant livers. Tetraspanins play an important role in cell adhesion and signalling, although little is known of the role of tetraspanin 11 in either the liver or immune function [39]. Additionally, we found decreased inflammatory gene expression (UBD, LSP1, CIITA, CXCL9, and GZMB) in patients with successful IS withdrawal. Although IFNG was not significantly decreased, we did observe a decrease in IFN-γ inducible genes including UBD and CIITA in patients undergoing successful withdrawal. Thus, the data lend support to using a ratio of gene expression (anti-inflammatory to pro-inflammatory) to identify liver transplant candidates for IS withdrawal. Based on results of the RNA-seq data, we plan to add SELE and TSPAN11 to the GeXP gene expression assay to determine if these additional genes will help in the identification of patients who can be weaned off IS.
In agreement with other studies, no patients with a history of autoimmune liver disease achieved operational tolerance [40]. Two patients with autoimmune liver disease did have the PBMC gene profile for tolerance but were found on liver biopsy to have histologic evidence of recurrent disease and were excluded from withdrawal of IS as per protocol. Therefore, the use of the biomarker even in these patients proved valuable in that histologic evidence of recurrent disease was detected despite normal liver biochemistry. Both of these patients were successfully treated with increased IS with resolution of liver inflammation.
Another important finding was that time after transplantation may be an important predictor of ability to wean IS, which is consistent with a larger study of operational tolerance in liver transplantation [10] and suggests host-graft adaptation over time [41]. Although age was not significantly higher in the group with successful wean, six of eight patients in this group were greater than 60 years old. In other studies, greater age is known to correlate with increased frequency of operational tolerance in liver transplantation, an observation which is likely related to diminished immune responses (immunosenescence) during the ageing process [42]. Of interest, in the present study, two patients who developed operational tolerance were young (less than 30 years of age). The PBMC biomarker may thus help in identifying young patients who are candidates for IS withdrawal. That young patients may develop tolerance is also supported by a previous study of IS withdrawal in paediatric liver transplant recipients [14].
Our study has several limitations. First, it was conducted at a single site, which may introduce a bias, and only 69 patients were enrolled. Although none of the tolerant patients developed rejection after 1 year of IS withdrawal, we do not know long-term consequences of IS withdrawal and we plan to continue to follow the operationally tolerant patients with blood test monitoring and yearly liver biopsies. Additionally, as we did not attempt IS withdrawal in patients who did not have the PBMC biomarker profile (FGL2/IFNG ≥ 1), it is not possible to firmly conclude that patients with low FGL2/IFNG ratio cannot be weaned off IS. In this pilot trial, the research ethics board of our hospital did not allow us to withdraw IS in patients with a low ratio in PBMC due to the risk of developing rejection and graft loss. In a future validation study, we plan to include a biomarker negative group so that numbers of operationally tolerant patients can be directly compared in between biomarker positive and negative groups. Finally, we had hoped to show that cessation of IS would lead to a clinical benefit such as improvement in renal function. Although patients who achieved operational tolerance did have improvements in renal function, this did not reach statistical significance due to the small numbers of patients and will have to be addressed in future studies.
In conclusion, we have developed a human GeXP gene expression assay to monitor anti-inflammatory and pro-inflammatory genes associated with transplant tolerance. The results of the study suggest that the PBMC biomarker (FGL2/IFNG ≥ 1) enriches the patient pool for liver transplant recipients who have developed operational tolerance and can be successfully weaned off IS. The data derived from LITMUS also suggest that the combined use of the PBMC and a liver gene biomarker (FOXP3/IFNG ≥ 1) may increase the precision of the biomarker approach to identify tolerant patients. Furthermore, the immunological studies and gene expression studies over time point to active immune regulation involving Tregs as the mechanism underlying spontaneous operational tolerance.
Supplementary Material
Acknowledgements
The authors would like to thank the LITMUS coordinators and support staff (Meaghan Macarthur, Melissa Ruttan, and Nellie Kamkar) for making this study possible. We also thank biostatistical staff (Dr Bhooma Thiruv and Dr Roumiana Alexandrova) at The Centre for Applied Genomics.
Glossary
Abbreviations
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- B2M
beta-2 microglobulin
- CASC3
cancer susceptibility candidate-3
- CIITA
class II major histocompatibility complex transactivator
- CXCL9
C-X-C motif chemokine ligand 9
- EZR
ezrin
- FGL2
fibrinogen-like protein 2
- FHF
fulminant hepatic failure
- FOXP3
forkhead box P3
- GZMB
granzyme B
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HPRT
hypoxanthine-guanine phosphoribosyltransferase
- IL10
interleukin-10
- IFNG
interferon-γ
- INR
international normalized ratio
- IP6K3
inositol hexakisphosphate kinase 6
- IS
immunosuppression
- KANR
kanamycin resistance gene
- LAG3
lymphocyte activating-3
- LSP1
lymphocyte-specific protein-1
- MMF
mycophenolate mofetil
- NASH
non-alcoholic steatohepatitis
- PBMC
peripheral blood mononuclear cells
- qPCR
quantitative PCR
- RNA-seq
RNA sequencing
- RT-PCR
reverse transcriptase polymerase chain reaction
- SELE
E-selectin
- TBP
TATA box binding protein
- TGFB1
transforming growth factor-β1
- TIGIT
T cell immunoreceptor with Ig and ITIM domains
- Tregs
regulatory T cells
- TSPAN11
tetraspanin 11
- TST
thiosulfate sulfurtransferase
- UBD
ubiquitin D
- XIST
X-inactive specific transcript
Funding
LITMUS was supported by a grant from the Heart and Stroke Foundation of Canada (G-17-0018658) and a generous donation from Dr. Ellen Bialystok and Dr. Franklin Bialystok.
Conflict of interest
G.A.L. is the CEO of Veritas Therapeutics Inc. H.Y. and J.L. are employees of SCIEX. SCIEX provided GEXP reagents to the investigators at no cost and in return requested the opportunity to review the manuscript prior to submission for publication.
Author contributions
E.R., D.G., N.S., and G.A.L. designed the study. Data were collected and analysed by A.C., V.R.-L., S.M., A.I., J.L., H.Y., R.S., J.Z., M.E., C.M., A.K., F.H.V., R.T., E.J., S.J., N.S., and G.A.L. Clinical evaluations were performed by L.L., E.R., D.G., A.H., S.J., N.S., and G.A.L. Pathology was interpreted by O.A. and S.F. First draft of the manuscript was written by A.C., S.J., and G.A.L. All authors edited the manuscript.
Ethical approval
LITMUS was approved by the Research Ethics Board of the University Health Network (14-8691) and was performed according to the Declaration of Helsinki. An independent data safety monitoring board monitored the trial.
Patient consent
All patients provided written, informed consent.
Data availability
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. RNA sequencing data will be made available at the GEO repository database (GSE175397).
References
- 1. Jain A, Reyes J, Kashyap R, et al. Long-term survival after liver transplantation in 4,000 consecutive patients at a single center. Ann Surg 2000, 232, 490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lechler RI, Sykes M, Thomson AW, Turka LA.. Organ transplantation—how much of the promise has been realized? Nat Med 2005, 11, 605–13. [DOI] [PubMed] [Google Scholar]
- 3. Thomson AW, Vionnet J, Sanchez-Fueyo A.. Understanding, predicting and achieving liver transplant tolerance: from bench to bedside. Nat Rev Gastroenterol Hepatol 2020, 17, 719–39. [DOI] [PubMed] [Google Scholar]
- 4. Mazariegos GV, Reyes J, Marino IR, et al. Weaning of immunosuppression in liver transplant recipients. Transplantation 1997, 63, 243–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Devlin J, Doherty D, Thomson L, et al. Defining the outcome of immunosuppression withdrawal after liver transplantation. Hepatology 1998, 27, 926–33. [DOI] [PubMed] [Google Scholar]
- 6. Eason JD, Cohen AJ, Nair S, Alcantera T, Loss GE.. Tolerance: is it worth the risk? Transplantation 2005, 79, 1157–9. [DOI] [PubMed] [Google Scholar]
- 7. Tryphonopoulos P, Tzakis AG, Weppler D, et al. The role of donor bone marrow infusions in withdrawal of immunosuppression in adult liver allotransplantation. Am J Transplant 2005, 5, 608–13. [DOI] [PubMed] [Google Scholar]
- 8. Tisone G, Orlando G, Cardillo A, et al. Complete weaning off immunosuppression in HCV liver transplant recipients is feasible and favourably impacts on the progression of disease recurrence. J Hepatol 2006, 44, 702–9. [DOI] [PubMed] [Google Scholar]
- 9. Pons JA, Ramírez P, Revilla-Nuin B, et al. Immunosuppression withdrawal improves long-term metabolic parameters, cardiovascular risk factors and renal function in liver transplant patients. Clin Transplant 2009, 23, 329–36. [DOI] [PubMed] [Google Scholar]
- 10. Benítez C, Londoño MC, Miquel R, et al. Prospective multicenter clinical trial of immunosuppressive drug withdrawal in stable adult liver transplant recipients. Hepatology 2013, 58, 1824–35. [DOI] [PubMed] [Google Scholar]
- 11. de la Garza RG, Sarobe P, Merino J, et al. Trial of complete weaning from immunosuppression for liver transplant recipients: factors predictive of tolerance. Liver Transplant 2013, 19, 937–44. [DOI] [PubMed] [Google Scholar]
- 12. Takatsuki M, Uemoto S, Inomata Y, et al. Weaning of immunosuppression in living donor liver transplant recipients. Transplantation 2001, 72, 449–54. [DOI] [PubMed] [Google Scholar]
- 13. Appenzeller-Herzog C, Hartleif S, Vionnet J.. Clinical parameters and biomarkers predicting spontaneous operational tolerance after liver transplantation: a scoping review. Am J Transplant 2021, 21, 3312–23. [DOI] [PubMed] [Google Scholar]
- 14. Feng S, Ekong UD, Lobritto SJ, et al. Complete immunosuppression withdrawal and subsequent allograft function among pediatric recipients of parental living donor liver transplants. JAMA 2012, 307, 283–93. [DOI] [PubMed] [Google Scholar]
- 15. Martínez-Llordella M, Puig-Pey I, Orlando G, et al. Multiparameter immune profiling of operational tolerance in liver transplantation. Am J Transplant 2007, 7, 309–19. [DOI] [PubMed] [Google Scholar]
- 16. Li L, Wozniak LJ, Rodder S, et al. A common peripheral blood gene set for diagnosis of operational tolerance in pediatric and adult liver transplantation. Am J Transplant 2012, 12, 1218–28. [DOI] [PubMed] [Google Scholar]
- 17. Bohne F, Martínez-Llordella M, Lozano JJ, et al. Intra-graft expression of genes involved in iron homeostasis predicts the development of operational tolerance in human liver transplantation. J Clin Invest 2012, 122, 368–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Urbanellis P, Shyu W, Khattar R, et al. The regulatory T cell effector molecule fibrinogen-like protein 2 is necessary for the development of rapamycin-induced tolerance to fully MHC-mismatched murine cardiac allografts. Immunology 2015, 144, 91–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xie L, Ichimaru N, Morita M, et al. Identification of a novel biomarker gene set with sensitivity and specificity for distinguishing between allograft rejection and tolerance. Liver Transpl 2012, 18, 444–54. [DOI] [PubMed] [Google Scholar]
- 20. Wood KJ. Regulatory T cells in transplantation. Transplant Proc 2011, 43, 2135–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Demetris AJ, Bellamy C, Hübscher SG, et al. 2016 Comprehensive update of the Banff working group on liver allograft pathology: introduction of antibody-mediated rejection. Am J Transplant 2016, 16, 2816–35. [DOI] [PubMed] [Google Scholar]
- 22. Banff Working Group on Liver Allograft P. Importance of liver biopsy findings in immunosuppression management: biopsy monitoring and working criteria for patients with operational tolerance. Liver Transplant 2012, 18, 1154–70. [DOI] [PubMed] [Google Scholar]
- 23. Livak KJ, Schmittgen TD.. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 2001, 25, 402–8. [DOI] [PubMed] [Google Scholar]
- 24. Streitz M, Miloud T, Kapinsky M, et al. Standardization of whole blood immune phenotype monitoring for clinical trials: panels and methods from the ONE study. Transplant Res 2013, 2, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Taubert R, Hardtke-Wolenski M, Noyan F, et al. Intrahepatic regulatory T cells in autoimmune hepatitis are associated with treatment response and depleted with current therapies. J Hepatol 2014, 61, 1106–14. [DOI] [PubMed] [Google Scholar]
- 26. Taubert R, Danger R, Londoño MC, et al. Hepatic infiltrates in operational tolerant patients after liver transplantation show enrichment of regulatory T cells before proinflammatory genes are downregulated. Am J Transplant 2016, 16, 1285–93. [DOI] [PubMed] [Google Scholar]
- 27. Chruscinski A, Sadozai H, Rojas-Luengas V, et al. Role of regulatory T cells (Treg) and the Treg effector molecule fibrinogen-like protein 2 in alloimmunity and autoimmunity. Rambam Maimonides Med J 2015, 6, e0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Morris AB, Farley CR, Pinelli DF, et al. Signaling through the inhibitory Fc receptor FcγRIIB induces CD8+ T cell apoptosis to limit T cell immunity. Immunity 2020, 52, 136–50.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sungnak W, Wagner A, Kowalczyk MS, et al. T follicular regulatory cell-derived fibrinogen-like protein 2 regulates production of autoantibodies and induction of systemic autoimmunity. J Immunol 2020, 205, 3247–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hidalgo LG, Halloran PF.. Role of IFN-gamma in allograft rejection. Crit Rev Immunol 2002, 22, 317–49. [PubMed] [Google Scholar]
- 31. Lui SL, Ramassar V, Urmson J, Halloran PF.. Mycophenolate mofetil reduces production of interferon-dependent major histocompatibility complex induction during allograft rejection, probably by limiting clonal expansion. Transpl Immunol 1998, 6, 23–32. [DOI] [PubMed] [Google Scholar]
- 32. Cherukuri A, Salama AD, Mehta R, et al. Transitional B cell cytokines predict renal allograft outcomes. Sci Transl Med 2021, 13, eabe4929. [DOI] [PubMed] [Google Scholar]
- 33. Pons JA, Revilla-Nuin B, Baroja-Mazo A, et al. FoxP3 in peripheral blood is associated with operational tolerance in liver transplant patients during immunosuppression withdrawal. Transplantation 2008, 86, 1370–8. [DOI] [PubMed] [Google Scholar]
- 34. Furukawa A, Wisel SA, Tang Q.. Impact of immune-modulatory drugs on regulatory T cell. Transplantation 2016, 100, 2288–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li W, Carper K, Liang Y, et al. Anti-CD25 mAb administration prevents spontaneous liver transplant tolerance. Transplant Proc 2006, 38, 3207–8. [DOI] [PubMed] [Google Scholar]
- 36. McEver RP. Selectins: initiators of leucocyte adhesion and signalling at the vascular wall. Cardiovasc Res 2015, 107, 331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Abeynaike LD, Deane JA, Westhorpe CL, et al. Regulatory T cells dynamically regulate selectin ligand function during multiple challenge contact hypersensitivity. J Immunol 2014, 193, 4934–44. [DOI] [PubMed] [Google Scholar]
- 38. Zhang N, Schröppel B, Lal G, et al. Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity 2009, 30, 458–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Charrin S, Jouannet S, Boucheix C, Rubinstein E.. Tetraspanins at a glance. J Cell Sci 2014, 127, 3641–8. [DOI] [PubMed] [Google Scholar]
- 40. Levitsky J. Operational tolerance: past lessons and future prospects. Liver Transpl 2011, 17, 222–32. [DOI] [PubMed] [Google Scholar]
- 41. Dai H, Zheng Y, Thomson AW, Rogers NM.. Transplant tolerance induction: insights from the liver. Front Immunol 2020, 11, 1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Goronzy JJ, Weyand CM.. Mechanisms underlying T cell ageing. Nat Rev Immunol 2019, 19, 573–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. RNA sequencing data will be made available at the GEO repository database (GSE175397).