Abstract
Alternatively activated macrophages (M2 polarization) play an important role in asthma. Short-chain fatty acids (SCFAs) possessed immune-regulatory functions, but their effects on M2 polarization of alveolar macrophages and its underlying mechanisms are still unclear. In our study, murine alveolar macrophage MH-S cell line and human monocyte-derived macrophages were used to polarize to M2 subset with interleukin-4 (IL-4) treatment. The underlying mechanisms involved were investigated using molecule inhibitors/agonists. In vivo, female C57BL/6 mice were divided into five groups: CON group, ovalbumin (OVA) asthma group, OVA+Acetate group, OVA+Butyrate group, and OVA+Propionate group. Mice were fed with or without SCFAs (Acetate, Butyrate, Propionate) in drinking water for 20 days before developing OVA-induced asthma model. In MH-S, SCFAs inhibited IL-4-incuced protein or mRNA expressions of M2-associated genes in a dose-dependent manner. G-protein-coupled receptor 43 (GPR43) agonist 4-CMTB and histone deacetylase (HDAC) inhibitor (trichostatin A, TSA), but not GPR41 agonist AR420626 could inhibit the protein or mRNA expressions M2-associated genes. 4-CMTB, but not TSA, had no synergistic role in the inhibitory effect of SCFAs on M2 polarization. In vivo study indicated Butyrate and Propionate, but not Acetate, attenuated OVA-induced M2 polarization in the lung and airway inflammation. We also found the inhibitory effect of SCFAs on M2 polarization in human-derived macrophages. Therefore, SCFAs inhibited M2 polarization in MH-S likely through GPR43 activation and/or HDAC inhibition. Butyrate and Propionate but not Acetate could inhibit M2 polarization and airway inflammation in asthma model. SCFAs also abrogated M2 polarization in human-derived macrophages.
Keywords: asthma, M2 polarization, acetate, butyrate, propionate
SCFAs inhibited M2 polarization in MH-S likely through GPR43 activation and HDAC inhibition. Butyrate and Propionate but not Acetate could inhibit M2 polarization and airway inflammation in asthma model. SCFAs also abrogated M2 polarization in human-derived macrophages.
Graphical Abstract
Graphical Abstract.
Introduction
Asthma is characterized by airway hyper-responsiveness (AHR) and chronic airway inflammation, the immunologic dysfunction in asthma can be attributed to destruction of lung homeostasis in network of various immune cells, including eosinophil, mast cells, Th1, Th17, innate lymphoid type 2 (ILC2), regulatory T (Treg) cells and macrophages, and so on [1, 2]. As the most abundant immune cell in the lung, macrophages are thought to be sentinels of pulmonary immune responses and play an essential role in asthma through being triggered to release a series of inflammatory cytokines upon stimulus [3, 4]. The plasticity of macrophages enabled it to exhibit different phenotypes when encountering allergens or threats. Macrophages could be classically activated and alternatively activated to polarize into M1 and M2 cells, respectively. The M1 macrophages participated in the removal of pathogens, mediated reactive oxygen species (ROS)-induced tissue damage, and impaired tissue regeneration. M2 macrophages promoted tissue repair and wound healing, cleared debris and apoptotic cells, and possessed potent phagocytosis capacity and pro-angiogenic properties [5]. M2 macrophages also played an important role in triggering allergic airway inflammation [6]. Unlike M1, which was frequently triggered by Th1 cytokines such as tumor necrosis factor α (TNFα), lipopolysaccharide (LPS), and interferon γ (IFN γ), M2 differentiation was associated with IL-4 or IL-13 exposure which can bind to IL-4R or IL-4R/IL-13R, activating phosphorylation of signal transducer and activator of transcription 6 (p-Stat6) and triggering gene transcriptions related to M2 [7, 8]. Mannose receptor (MRC1) was highly expressed both in human and mouse M2, while arginase 1 (Arg1), chitinase-3-like protein-3 (Chi3l3/YM1), resistin-like molecule-α/found in inflammatory zone 1 (FIZZ1/Retnla) were only highly expressed in mouse [9–11]. Activity of Stat-1 was essential for M1 macrophage polarization, while, several genes associated with M2 macrophage profile, such as Arg1 and MRC1 were regulated by p-Stat6 activity in the presence of IL-4/IL-13 [12]. Increased p-Stat6 could promote M2 differentiation, while downregulation of p-Stat6 was associated with inhibition of M2-associated genes [13, 14]. Previous studies demonstrated the imbalance of M1/M2 in immunopathogenesis of asthma and M2 dichotomous classification was indicated in lung tissues or peripheral blood of asthma patients [15–17]. This was also supported in animal models by Moreira et al. who confirmed aggravated allergic airway inflammation in mice received adoptive transfer of M2 compared with their counterparts received M0 [6]. The expression of M2-associated genes, such as YM1, FIZZ1, and human YKL-40, can also act as chemokine of eosinophils, enhance pulmonary vascular smooth muscle cell proliferation and correlated with airway remodeling, respectively [18–20].
Short-chain fatty acids (SCFAs) were produced by gut bacteria metabolizing indigestible carbohydrates or fiber-rich diet. SCFAs gain increasing focus because of their beneficial roles in different organ systems and their crucial role in bridging intestinal microbiota and the host. Of particular interest are Acetate, Butyrate, and Propionate, which are the most abundant SCFAs in the gut. They have been shown to relieve intestinal inflammation, inhibit proliferations of cancer cells and eosinophils, enhance insulin sensitivity of islet β cells, restrain inflammatory cell infiltration through activating G-protein-coupled cell surface receptors (GPRs) (GPR41, GPR43, and GPR109a), or suppress histone deacetylases (HDAC) activity, therefore they exhibited regulatory role in glucose homeostasis [21, 22](p3) and appetite regulation [23], alleviating inflammatory bowel disease [24] and liver injury [25]. Emerging evidence turn SCFAs into the limelight due to their profound functions in regulating immune responses and inflammation process. For instance, Butyrate promoted differentiation of Treg cells while inhibited differentiation of IFNγ-producing cells [26]. Propionate impaired the capability of dendritic cells (DCs) to trigger Th2 immune response [27]. In recent studies, SCFAs were found to ameliorate allergic airway inflammation, AHR, serum IgE, eosinophils in bronchoalveolar lavage fluid (BALF) total cells and in OVA-induced asthma animal model [28] (p2). However, the role of SCFAs in M2 polarization of human-derived macrophages and murine alveolar macrophages, along with the underlying mechanisms are still not fully understood, moreover, whether SCFAs could regulate the M2 polarization in vivo remains to be elucidated.
Herein, we investigated potential role of SCFAs in M2 polarization of human macrophages and murine alveolar macrophages (MH-S) in vitro, and the effect of SCFAs on M2 polarization in the context of allergic airway inflammation.
Materials and methods
Cell culture
Murine alveolar macrophages (MH-S) and human monocytic THP1 cells were obtained from ATCC. Monocyte-derived macrophages obtained and induced from peripheral blood mononuclear cells (PBMCs) in asthma patients. These cells were cultured in 1640 RPMI supplemented with 10% fetal bovine serum (FBS) in a humidified 5% CO2 atmosphere at 37°C.
Subjects
Eight asthma patients were recruited for the study. Asthma was defined based on physician diagnosis and by the 12% or 200 ml decrease of FEV1 after bronchial dilation test using β2 agonist. Sputum was produced after induction by hypertonic saline nebulization as previously described [29], cell differential counts were performed. The demographic data, including age, sex, body mass index, history of rhinosinusitis, ICS dose, percentage of eosinophils in induced sputum (EOS%), and Asthma Control Questionnaire score (ACQ) score, FEV1 percent predicted (FEV1% pre), FEV1/forced vital capacity percentage (FEV1/FVC), and blood IgE levels were also recorded.
The study was approved by the Ruijin Hospital Ethics Committee, Shanghai Jiao Tong University School of Medicine. The study abides by principles in declaration of Helsinki for the use of human samples. Signed informed consent was obtained from all patients.
PBMC isolation and monocyte-derived macrophages
Fifteen milliliters of peripheral blood were collected and diluted with equal volumes of sterile PBS. PBMCs were isolated by Ficoll-Hypaque density and gradient centrifugation at 3000 rpm for 20 min at 20°C, then the cells were obtained for monocytes selection using Human CD14 Selection Kit (Biolegend, USA). CD14+ monocytes were cultured for 7 days in 1640 RPMI supplemented with 10% FBS and Macrophage-colony stimulating factor (M-CSF, 50 ng/ml, PeproTech, USA).
M2 polarization
Primary monocyte-derived macrophages were cultured with human IL-4 (20 ng/ml) on day 8 for 24 h for M2 differentiation, then total RNA was harvested. THP-1 cells were cultured in 1640 RPMI supplemented with 10% FBS and phorbol myristate acetate (PMA, 50 ng/ml, Sigma Aldrich, USA) for 24 h, then human IL-4 (20 ng/ml, PeproTech, USA) were added for 24 h to induce M2 differentiation before harvesting RNA. MH-S was exposed to mouse IL-4 (20 ng/ml, PeproTech, USA) for 48 h to induce M2 polarization.
Immunoblotting
Equal amounts of proteins (30 μg) were separated on SDS-PAGE gel electrophoresis and transferred onto polyvinylidene fluoride membranes, then the membranes were blocked using 5% non-fat milk for 1 h at room temperature. All the primary antibodies (Arg1, p-Stat6, β-tubulin, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), total-Stat6, Cell Signaling Technology, USA; Acetyl-Histone K3, Beyotime Biotechnology, Shanghai; pan Acetyl H3, Merk, Austria) at 1:1000 dilution were incubated over night at 4°C. After washing three times, the membranes were incubated with horseradish peroxidase (HRP)-conjugated anti-mouse or anti-rabbit secondary antibodies (1:2000, Cell Signaling Technology, USA) and detected with an enhanced chemiluminescence kit (Millipore). Β-tubulin or GAPDH was used for normalization.
Quantitative real-time polymerase chain reaction (qRT-PCR) analysis
Total RNA was extracted using TRIzol reagent and reverse transcribed into cDNA using PrimeScript RT Master Mix (Takara). Quantitative PCR was performed using SYBR green (Takara) on an ABI 7500 real-time PCR system, reaction conditions were: 95°C for 30 s, 40 cycles (95°C for 5 s, 60°C for 34 s). β-actin was used for normalization. The primer sets are listed as follows: mouse Arg1, 5ʹ-GCCAGGGACTGACTACCTTAA-3ʹ and 5ʹ-AGTTCTGTCTGCTTTGCTGTG-3ʹ; mouse MRC1, 5ʹ-AGGGAAGAGAAGAAGATCCAG-3ʹ and 5ʹ-TGGGAGAAGATGAAGTCAAAC-3ʹ; mouse FIZZ1, 5ʹ-TGCCAACTGTCCTAAGAATGA-3ʹ and 5ʹ-GCACATGAGTCAGATTTCCAA-3ʹ; mouse YM1, 5ʹ-TGAGGAAGAATCTGTGGAGAA-3ʹ and 5ʹ-TGAGACAGTTCAGGGATCTTG-3ʹ; mouse β-actin, 5ʹ-CCTCTATGCCAACACAGT-3ʹ and 5ʹ-AGCCACCAATCCACACAG-3ʹ; human MRC1, 5ʹ-GCAGTCCTTTCCGATATTTGA-3ʹ and 5ʹ-CCCAGTTTCTGAACACATTCC-3ʹ; human Clec10a, 5ʹ-AGAATAAGGTGAAAGTCCAGGGG-3ʹ and 5ʹ-GCTAAAATCTGTTCTCAGGGTCAC-3ʹ; human CCL2, 5ʹ-TAGAAGAATCACCAGCAGCAAG-3ʹ and 5ʹ-CAAGTCTTCGGAGTTTGGGTTT-3ʹ; human CCL17, 5ʹ-CCTTAGAAAGCTGAAGACGTGGTA-3ʹ and 5’-TCTTCACTCTCTTGTTGTTGGGG-3ʹ; human CCL22, 5ʹ-CGTGGTGAAACACTTCTACTGGAC-3ʹ and 5ʹ-ATCATCTTCACCCAGGGCACT-3ʹ.
Flow cytometry
For MH-S, cells were harvested and washed with PBS and stained with zombie viability dye (Biolegend, USA) for 15 min at room temperature before washing and fixation with 4% paraformaldehyde for 15 min at room temperature. After washing three times, cells were permeated with intracellular staining permeabilization wash buffer (Biolegend, USA), followed by three washes and incubation with anti-mouse MRC1 antibody (Biolegend, USA) for 30 min at 4°C. The cells were acquired using Beckman cell coulter (BD, USA), analysis of the flow cytometry data was performed using Flowjo software.
For pulmonary cells, lung tissues were minced, digested with DNAse I and collagenase A in Hanks buffer for 1 h at 37°C and then filtered with 70 μm nylon strainer. Cells were centrifugated and suspended with red blood cell lysis buffer for 10 min at room temperature, followed by centrifugation at 2000 rpm for5 min at 4°C. After washing with PBS, cells were incubated with zombie, fixed with 4% paraformaldehyde, and incubated with surface-associated genes (CD45, F4/80, CD11c, Biolegend, USA) for 30 min at 4°C. All the cells were subsequentially permeated with intracellular staining permeabilization wash buffer and incubated with BV421-labeled anti-mouse MRC1 (Biolegend, USA) antibody for 30 min at 4°C. The cells were acquired using Beckman cell coulter (BD, USA), analysis of the flow cytometry data was performed using Flowjo software.
Immunofluorescence staining
MH-S were fixed with 4% paraformaldehyde (PFA) for 20 min, permeated by 0.3% TritonX-100 for 10 min and blocked with 5% BSA for 40 min at room temperature. Then, cells were incubated with anti-Arg1 (1:200) overnight at 4°C, after three washes, the cells were exposed to Alexa Fluor 594-conjugated goat anti-rabbit IgG (1:150) for 1 h at room temperature in the dark. 2-(4-Amidinophenyl)-6-indolecarbamidine dihydrochloride (DAPI) was used to stain the nuclei, and images were captured using a fluorescence microscope (CarlZeiss, Inc).
Animal models
Female C57BL/6 mice (6–8 weeks) were purchased from Shanghai SLAC laboratory (Shanghai, China) and housed in the specific pathogen free facility. All of the experiments followed protocols approved by the Institutional Animal Care and Use Committee in Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. All mice were randomly divided into five groups: CON group, OVA asthma group, OVA+Acetate group, OVA+Butyrate group, and OVA+Propinoate group. Mice in OVA+Acetate group, OVA+Butyrate group, and OVA+Propionate groups were administered with Acetate (50 mM), Butyrate (50 mM), and Propionate (80 mM) in drinking water for 20 days before developing asthma animal model.
To develop asthma animal model, mice were intraperitoneally injected with 100 µl 1 mg/ml ovalbumin (OVA, Sigma Aldrich, USA) in PBS mixed with alum or PBS on day 0 and day 7. Seven days later, the mice were challenged with aerosolized 1% OVA in PBS for 50 min for 3 days (day 14, day 15, and day 16). Twenty-four hours later, bronchoalveolar lavage was performed; bronchoalveolar lavage fluid (BALF), lung tissue, and blood were harvested for differential cell count, analysis of percentages of MRC1+ cells, measurement of cytokines IL-4, IL-5, IL-13, and IgE (ELISA). Lung tissues were also fixed in paraformaldehyde 4% overnight at 4°C, 5 μm paraffin-embedded sections were prepared and stained with periodic acid–Schiff (PAS) and hematoxylin (HE) for analysis of mucus production and cellular inflammation.
ELISA
Lungs and 0.6 ml blood were harvested 24 h after the last OVA challenge. Concentrations of IL-4, IL-5, and IL-13 per gram lung tissue were measured by ELISA kits (Raybiotech, USA and Anogen, Canada). The level of each cytokine in the supernatant of the lung homogenate was standardized with the protein concentration. Serum IgE was also measured by ELISA (Biolegend, USA).
Statistical analysis
Statistical analyses were performed using GraphPad Prism. All data represent means ± SD. P values were calculated with one-way analysis of variance, and between conditions comparisons were made by Tukey test. A P value less than 0.05 was considered as statistical significance.
Results
Acetate, Butyrate and Propionate inhibit M2 polarization in AMs
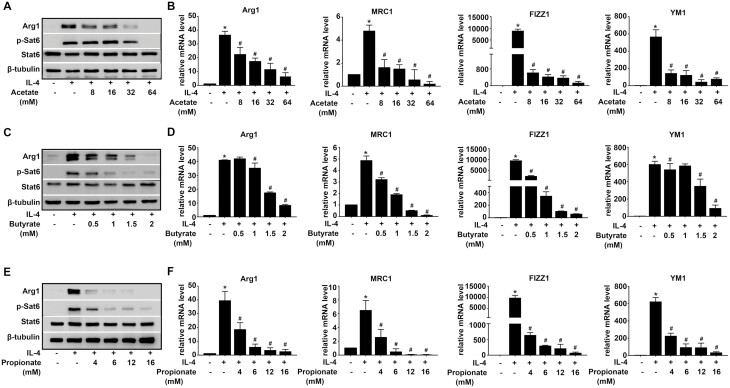
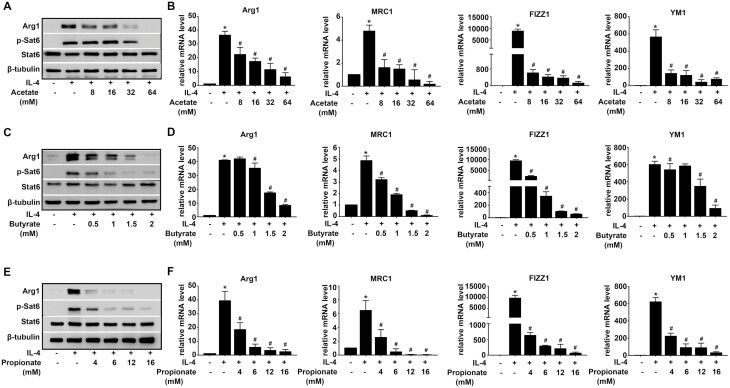
To investigate the effect of SCFAs on M2 polarization, alveolar macrophages (MH-S) were pretreated with various concentrations of Acetate (8, 16, 32, and 64mM), Butyrate (0.5, 1, 1.5, and 2mM), and Propionate (4, 6, 12, and 16 mM) for 45 min, and then stimulated with IL-4 for 48 h, mRNA or protein expressions of M2-associated genes, Arg1, MRC1, Fizz1, and Ym1 were evaluated. IL-4 could induce M2 polarization as evidenced by increased gene expressions of Arg1, MRC1, Fizz1, and Ym1, and protein expressions of Arg1 and p-Stat6, compared with CON group. Acetate clearly decreased the protein expression of Arg1 with concentration increasing, while the p-Stat6 protein expression was significantly inhibited by 32 mM and 64 mM Acetate. Acetate remarkably inhibited the gene expression of M2-associated genes (Arg1, MRC1, Fizz1, and Ym1) with the presence of IL-4 (Fig. 1A and B). Similarly, MH-S pretreated with increasing concentrations of Butyrate (0.5, 1, 1.5, and 2 mM) showed a significant decline of gene or protein expression of Arg1, p-Stat6, MRC1, Fizz1, and Ym1 (Fig. 1C and D). Meanwhile, Propionate (4, 6, 12, and 16 mM) showed an enhanced inhibition of M2-associated genes induced by IL-4, as demonstrated by rapidly descent in levels of Arg1, MRC1, Fizz1, and Ym1 gene expression, Arg1 and p-Stat6 protein expression (Fig. 1E and F). Based on these experiments, we determined 16 mM Acetate, 1.5 mM Butyrate, and 6 mM Propionate in some of the subsequent experiments.
Fig. 1.
Acetate, Butyrate, and Propionate inhibit M2 polarization in AMs. MH-S were pretreated with Acetate, Butyrate, and Propionate 30 min at the indicated concentrations before IL-4 (20 ng/ml) exposure for 48 h. (A, C, E). Protein expressions of Arg1 and p-Stat6 were evaluated. Β-tubulin was used for normalization. (B, D, F) mRNA expressions of M2-associated genes, Arg1, MRC1, Fizz1, and Ym1 were evaluated. Β-actin was used for normalization. Data are shown as means ± SD from three independent experiments. ∗P < 0.05 vs. CON group. #P < 0.05 vs. IL-4 group.
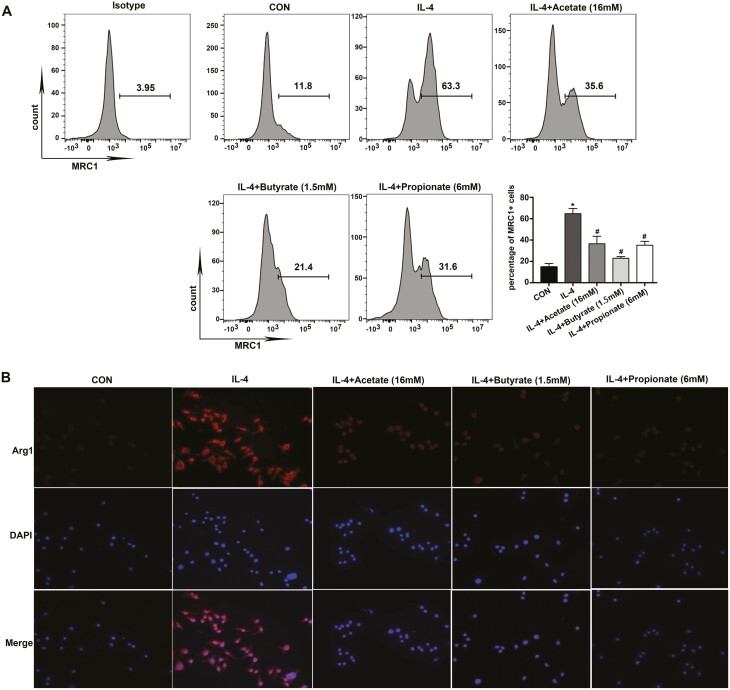
We also detected MRC1 and Arg1 protein expressions by flow cytometry and immunofluorescence, respectively. The results showed that IL-4-induced increased MRC1 expression was abrogated by Acetate (Fig. 2A). Moreover, results from immunofluorescence labeling for Arg1 performed in parallel with previous results, which revealed that the expression of Arg1 were significantly reduced in cells pretreated with Acetate, Butyrate, or Propionate compared to those without pretreatment, namely IL-4 treated only (Fig. 2B).
Fig. 2.
Acetate, Butyrate, and Propionate inhibit MRC1 and Arg1 protein expression in AMs. MH-S were pretreated with Acetate, Butyrate, and Propionate 30 min at the indicated concentrations before IL-4 (20 ng/ml) exposure for 48 h. (A) Percentage of MRC1+ cells were determined by flow cytometry. (B) Immunofluorescence staining of the expression of Arg1. Data are shown as means ± SD from three independent experiments. ∗P < 0.05 vs. CON group. #P < 0.05 vs. IL-4 group.
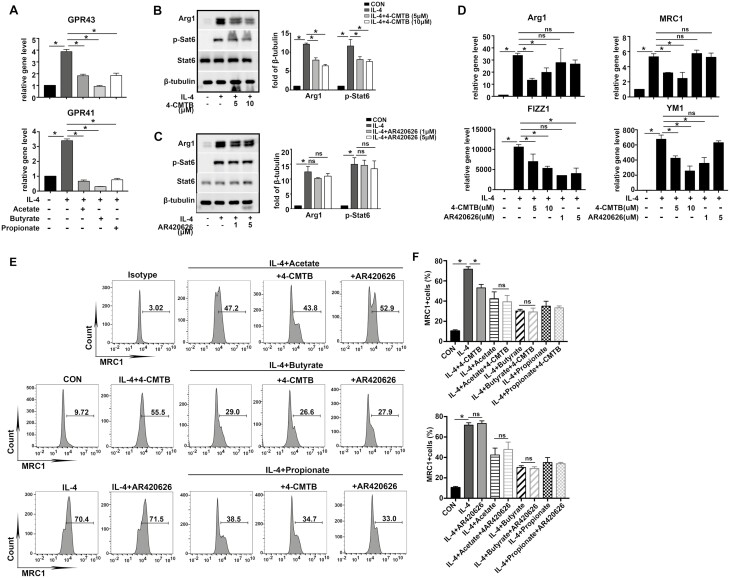
GPR43, but not GPR41 activation may be responsible for the inhibitory effects of Acetate, Butyrate, and Propionate in AMs
GPR41 and GPR43 activation have been demonstrated to be one of the main mechanisms underlying the profound functions of SCFAs in regulating immune system. We found the mRNA levels of GPR41 and GPR43 were upregulated with the treatment of IL-4 compared with CON group, however, both receptors’ expression levels were abrogated by coincubation with Acetate or Butyrate or Propionate (Fig. 3A).
Fig. 3.
GPR43, but not GPR41 activation may be responsible for the inhibitory effects of Acetate, Butyrate, and Propionate in AMs. MH-S were cultured with IL-4 (20 ng/ml) in the presence or absence of SCFAs (Acetate, Butyrate, and Propionate), GPR41 agonist (AR420626), and GPR43 agonists (4-CMTB) alone or in combination, at the indicated concentrations. (A) mRNA expressions of GPR43 and GPR41were evaluated. Β-actin was used for normalization. (B, C). Protein expressions of Arg1 and p-Stat6 were evaluated. Β-tubulin was used for normalization. (D) mRNA expressions of M2-associated genes, Arg1, MRC1, Fizz1, and Ym1 were evaluated. Β-actin was used for normalization. (E, F). Percentage of MRC1+ cells were determined by flow cytometry. Data are shown as means ± SD from three independent experiments. ∗P < 0.05.
To further investigate the role of GPR41 and GPR43 involvement in IL-4-induced macrophage polarization, GPR41 and GPR43 agonists (AR420626 and 4-CMTB) were used. MH-S cells were pretreated with AR420626 or 4-CMTB before stimulation with SCFAs and IL-4 alone or in combination.
The results showed 4-CMTB (5 µM, 10 µM) decreased the protein expressions of Arg1 and p-Stat6, and mRNA levels of M2-associated genes, including Arg1, MRC1, FIZZ1, and YM1 (Fig. 3B and D). 4-CMTB (5 µM) also significantly reduced MRC1 expression, although, a less extent than all SCFAs (Fig. 3E and F). To ascertain that 4-CMTB and SCFAs act as inhibitors M2 polarization through GPR43 activation and not through distinct independent mechanisms, we treated MH-S with the combination of SCFAs and 4-CMTB in presence of IL-4. Interestingly, SCFAs cotreatment with 4-CMTB is ineffective to affect suppression of MRC1 expression by SCFAs. Compared with IL-4+Acetate, IL-4+Butyrate or IL-4+Propionate group, there were no significant differences in MRC1 expressions in IL-4+Acetate+4-CMTB, IL-4+Butyrate+4-CMTB, and IL-4+Propionate+4-CMTB groups (Fig. 3E and F), respectively, suggesting GPR43 activation activity of SCFAs contributed to inhibition of M2 polarization induced by IL-4.
Then, we aim to figure out the effect of GPR41 activation on IL-4-induced M2 polarization. GPR41 agonist AR420626 (1 µM, 5 µM) did not affect Arg1 and p-Stat6 protein expressions, the gene expressions of Arg1, MRC1, FIZZ1, and YM1 (Fig. 3C and D). Similarly, AR420626 (5 µM) could not attenuate IL-4-induced MRC1 expression (Fig. 3E and F). To delineate probable involvement of GPR41 activation in SCFAs’ inhibitory effect, MH-S were treated with the combination of SCFAs and AR420626 in presence of IL-4. Compared with IL-4+Acetate, IL-4+Butyrate, IL-4+Propionate groups, co-incubation with AR420626 (5 µM), there were no significant differences in MRC1 expressions in IL-4+Acetate+AR420626, IL-4+Butyrate+AR420626 and IL-4+Propionate+AR420626 groups (Fig. 3E and F). These results indicated suppressive effect of SCFAs on M2 polarization is independent of GPR41 involvement.
Butyrate, and Propionate inhibit M2 polarization in AMs also partly through HDAC inhibition
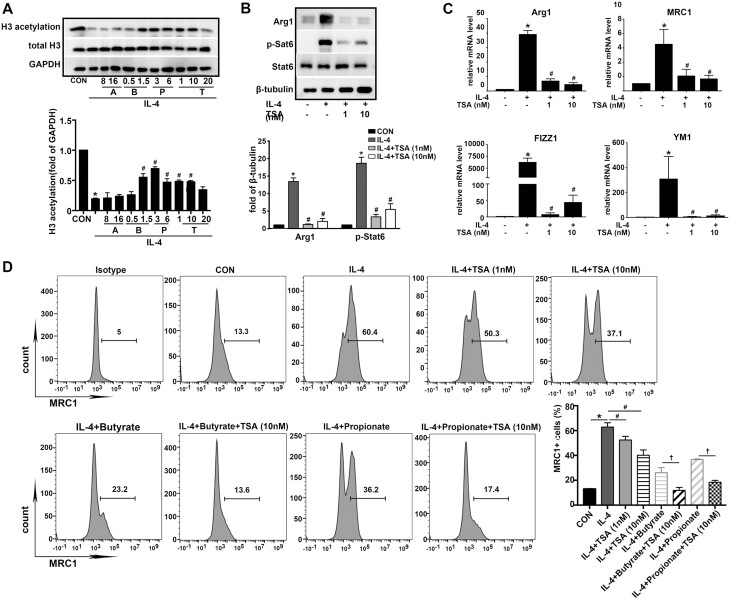
Another possibility for the mechanism through which SCFAs play an important role in regulating immune systems can also be related to HDAC inhibition. Trichostatin A (TSA) was adopted to ascertain whether TSA and SCFAs could inhibit HDAC and induce histone acetylation in alveolar macrophages. MH-S cells were pretreated with TSA or SCFAs before IL-4 stimulation. The results showed an inhibition of H3 acetylation level in cells treated with IL-4, while Butyrate (1.5 mM), Propionate (3 and 6 mM), and TSA (1, 10, and 20 nM) significantly induced an increment of H3 acetylation levels relative to IL-4-treated cells; Acetate treatment had no effect on H3 acetylation level (Fig. 4A).
Fig. 4.
Butyrate, and Propionate inhibit M2 polarization partly through HDAC inhibition in AMs. MH-S were cultured with IL-4 (20 ng/ml) in the presence or absence of SCFAs (Acetate, Butyrate, and Propionate), HDAC inhibitor (TSA) alone or in combination at the indicated concentrations. (A) Representative western blot image and relative expression of H3 acetylation level after 8 h of treatment. Data were normalized against GAPDH. (B) Protein expressions of Arg1 and p-Stat6 were evaluated. Β-tubulin was used for normalization. (C) mRNA expressions of M2-associated genes, Arg1, MRC1, Fizz1, and Ym1 were evaluated. Β-actin was used for normalization. (D) Percentage of MRC1+ cells were determined by flow cytometry. Data are shown as means ± SD from three independent experiments. ∗P < 0.05 vs. CON group. #P < 0.05 vs. IL-4 group. †P < 0.05 IL-4+Butyrate/Propionate vs. IL-4+Butyrate/Propionate +TSA.
Then, we tested the role of HDAC suppression in M2 polarization. TSA (1 and 10 nM) impaired IL-4-induced M2 polarization, as evidenced by decreased protein expression of Arg1 and p-Stat6 (Fig. 4B), the mRNA levels of Arg1, MRC1, FIZZ1, and YM1 were also inhibited (Fig. 4C). Moreover, TSA attenuated MRC1 expression in a concentration-dependent manner. We also reported a synergistic effect on the inhibitory M2 polarization of Butyrate and Propionate. Compared with IL-4+Butyrate or IL-4+Propionate group, there are significant reductions of MRC1 expressions in IL-4+Butyrate+TSA and IL-4+Propionate+TSA groups (Fig. 4D), suggesting that Butyrate and Propionate partly go through the HDAC inhibition-mediated pathway.
Butyrate and Propionate, but not Acetate ameliorate M2 polarization and allergic airway inflammation model.
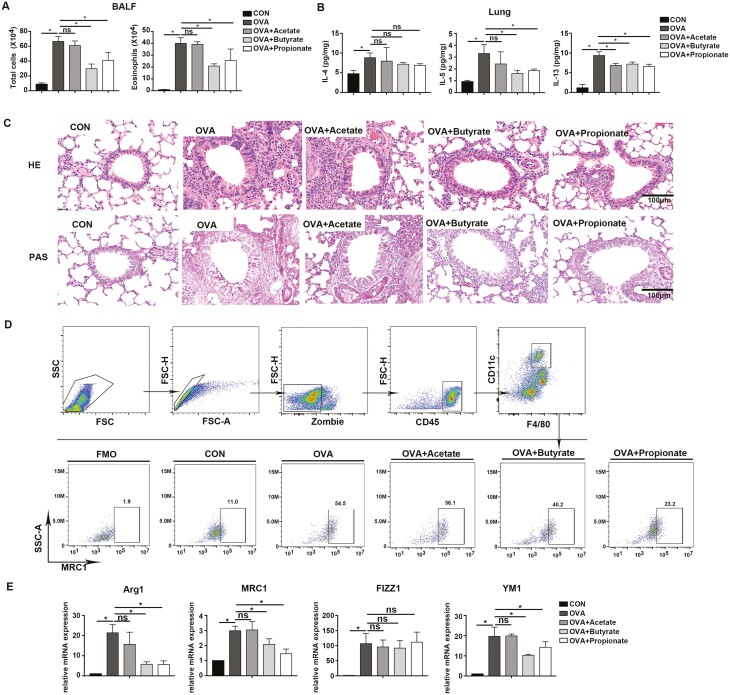
To figure out the effect of SCFAs in regulating M2 polarization in OVA asthma model, every SCFA (Acetate, Butyrate, and Propionate) was administered in drinking water for 20 days before OVA sensitization and challenge. In OVA asthma model, the mice showed augmented total cells and eosinophils in BALF, robust Th2 cytokines levels (IL-4, IL-5, and IL-13) in lung, as well as bronchial thickening and inflammation. Butyrate and Propionate, but not Acetate blunted the total cells and eosinophils in BALF, IL-5, and IL-13 levels in the lung, and airway inflammation (Fig. 5A–C). We further evaluated the percentage of M2 cells and mRNA expressions of M2-associated genes in the lung. In flow cytometry analysis, the gating strategy was performed according to previous studies [30–33], we demonstrated that the percentage of MRC1+ cells in the lung challenged by OVA was higher than mice in CON group, while this figure was statistically reduced in mice exposed to Butyrate and Propionate treatment in advance (Fig. 5D). Butyrate and propionate also suppressed mRNA expressions of Arg1, MRC1, and YM1 in the lung compared with OVA exposed mice (Fig. 5E). However, neither the percentage of MRC1+ cells nor mRNA expressions of M2-associated genes could be attenuated by Acetate (Fig. 5D and E). Our in vivo results indicated Butyrate and Propionate, but not Acetate relieved M2 polarization and allergic airway inflammation model.
Fig. 5.
Butyrate and Propionate, but not Acetate ameliorate M2 polarization and allergic airway inflammation model. Mice were treated with Acetate, Butyrate, and Propionate in drinking water for 20 days before developing OVA-induced animal model. (A) Number of total cells and eosinophils in BALF. (B) IL-4, IL-5, and IL-13 levels in lung homogenates of mice. (C) Representative images of HE- and PAS-stained histologic sections of the lungs. Scale bars = 100μm. (D) Upper: gating strategy. Lower: Percentage of M2 population (MRC1+ cells) in the lung gated from viable CD45+F4/80+CD11c+ cells. (E) RT-qPCR was performed to evaluate mRNA levels of Arg1, MRC1, Fizz1, and Ym1 in the lung. Β-actin was used for normalization. Data are shown as means ± SD from three independent experiments. ∗P < 0.05.
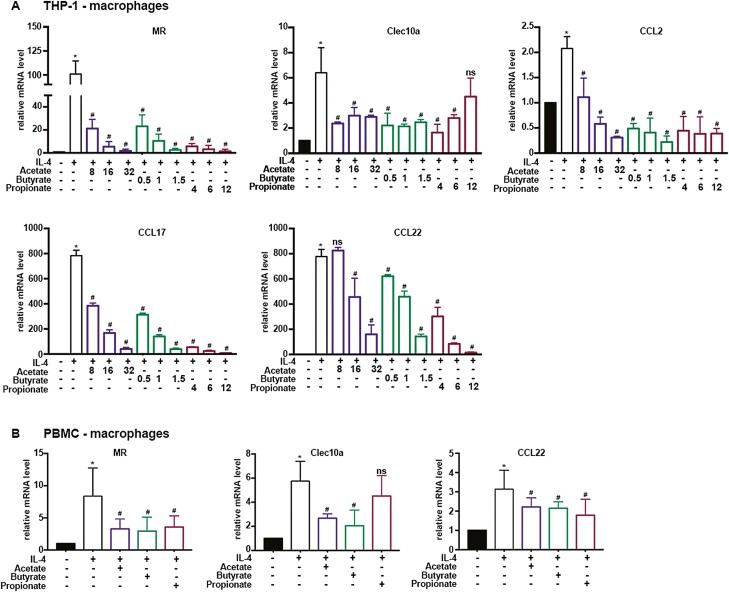
Acetate, Butyrate, and Propionate inhibit M2 polarization in human macrophages
To evaluate the effects of SCFAs on human macrophages, THP-1 and monocyte-derived macrophages from asthma patients were adopted, basic clinical information of asthma patients were given in Table 1. THP-1 and monocyte-derived macrophages were stimulated with PMA for 24 h to induce macrophages, then the cells were preincubated with Acetate, Butyrate, and Propionate at indicated concentrations followed by human IL-4 (20 ng/ml) exposure for another 24 h. Previous study indicated that human monocytes derived- and murine macrophages showed different response to IL-4 and IL-13, hence, M2-associated genes in mice (Arg1, Ym1, etc.) is not applicable for human, moreover, MRC1, C-type lectin domain family 10 member A (Clec10a), Monocyte chemoattractant protein-1 (MCP-1/CCL2), CCL17, and CCL22 may be used as indicators of M2 in human macrophages [10, 34](p1), [35–40]. As shown in Fig. 6, mRNA expressions of MRC1, Clec10a, CCL2, CCL17, and CCL22 were significantly higher than that of the untreated group, whereas these Acetate, Butyrate, and Propionate markedly reduced expressions of those genes (Fig. 6A). Simultaneously, we also found that Acetate, Butyrate, and Propionate significantly down-regulated mRNA expressions of MRC1 and CCL22 induced by IL-4 in monocyte-derived macrophages. Acetate and Butyrate also hindered Clec10a mRNA expression, however, Propionate caused an inhibitory action without statistical significance (Fig. 6B). Acetate, Butyrate, and Propionate has no effect on mRNA expressions of CCL2 and CCL17 in monocyte-derived macrophages treated with IL-4 (data not shown). Combined, these results may also suggest SCFAs as an impediment of M2 polarization in human macrophages.
Table 1.
Demographic and clinical characteristics of study subjects
| Variables | Asthma patients (n = 8) |
|---|---|
| Age, years, mean ± SD | 49.12 ± 16.63 |
| Male, no. (%) | 3 (37.5) |
| BMI, kg/m2, mean ± SD | 21.92 ± 1.87 |
| Rhinosinusitis, no. (%) | 5 (62.5) |
| ICS dosea, μg.day-1, mean ± SD | 407.5 ± 239.56 |
| FEV1 (% predicted), mean ± SD | 87.81 ± 15.39 |
| FEV1/FVC (%), mean ± SD | 73.66 ± 10.93 |
| ACQ7 score, mean ± SD | 0.625 ± 0.42 |
| Percentage of eosinophils, mean ± SD | 3.18 ± 1.57 |
| Blood IgE (IU/ml) | 166.56 ± 114.80 |
| Duration of asthma, years, mean ± SD | 9.82 ± 19.57 |
| Atopic, no. (%) | 4 (50) |
ICS dose was expressed as beclomethasone propionate equivalent dose.
Fig. 6.
Acetate, Butyrate, and Propionate inhibit M2 polarization in human macrophages. THP-1-derived macrophages were pretreated with Acetate (8, 16, and 32 mM), Butyrate (0.5, 1, and 1.5 mM), and Propionate (4, 6, 12 mM) for 30 min before IL-4 (20 ng/ml) treatment for 24 h. RT-qPCR was performed to evaluate mRNA levels of MRC1, Clec10a, CCL2, CCL17, CCL22 in THP-1. Data are shown as means ± SD from three independent experiments. (B) Monocyte-derived macrophages from eight asthma patients were pretreated with 16 mM Acetate, 1.5 mM Butyrate, and 6 mM Propionate for 30 min before IL-4 (20 ng/ml) treatment for 24 h. RT-qPCR was performed to evaluate mRNA levels of MRC1, Clec10a, and CCL22 in monocyte-derived macrophages P. ∗ < 0.05, vs. CON. #P < 0.05, vs. IL-4 group. β-actin was used for normalization.
Discussion
Recent work has brought to the forefront the pivotal role of gut microbial metabolites, SCFAs, in abrogating the cardinal features of asthma, such as ameliorate airway inflammation and AHR. However, the role of SCFAs in the regulation of alternatively activated alveolar macrophages (M2) has yet to be elucidated. We showed that SCFAs downregulated M2 polarization of human-derived and murine alveolar macrophages in vitro and they possibly act through activating GPR43, but not GPR41. Butyrate and Propionate, but not Acetate, increased H3 acetylation, and they exhibited the inhibitory effect on M2 polarization partly through HDAC inhibition. Furthermore, in vivo findings showed systemic application of Butyrate and Propionate decreased allergic airway inflammation in OVA-challenged mice, as well as the M2 polarization of alveolar macrophages.
Asthma is one of the most common chronic airway diseases mostly driven by dysfunction of Th2 immune response. Macrophages were once called ‘forgotten cell in asthma’ and now catch more and more attention given the plasticity of macrophages [41]. Alternative activation of macrophage (M2) can promote production of Th2 cytokines, accumulation of inflammatory cells, mucus secretion and AHR. It has been addressed that adoptive transfer of M2 enhanced airway inflammation and airway remodeling in Aspergillus fumigatus induced asthma animal model, reciprocally, inhibition of M2 subtype exert a protective effect against the development of airway inflammation and AHR, key players in the pathogenesis of allergic asthma [6].
The beneficial effects of SCFAs in modulating inflammatory bowel disease, liver injury, tumor growth, appetite can be attributed to regulation of innate and adaptive immune systems. Previous study provided insights into the development of IL-17 or IFN-γ producing T cells and IL-10+ producing Treg cells induced by SCFAs depending on cytokine milieu and immunological context [42]. Butyrate promoted the differentiation of colonic regulatory T cells partly through enhancing histone H3 acetylation in the promoter and conserved non-coding sequence (CNS) regions of the forkhead box P3 (Foxp3) locus [43]. In asthma patients, SCFAs have a direct effect on human eosinophils in terms of adhesion to endothelial cells, trafficking and survival [44]. Recent evidence also suggested that both intranasal administration of Butyrate and circulating Butyrate inhibited IL-13 and IL-5 production by murine ILC2s and ameliorated both A. alternata-induced AHR and airway inflammation [28]. Propionate treatment impaired the ability of seeding DCs to promote Th2 cell effector function in the lung [27]. However, conflicting data were obtained about role of SCFAs in macrophage polarization. Jiang et al. found that Butyrate ameliorated cardiac function and ventricular arrhythmia (VA) following myocardial infarction partly through facilitating M2 macrophage polarization to inhibit inflammatory responses and sympathetic neural remodeling [45]. In alcoholic liver disease (ALD) animal model, increased propionic acid and butyric acid after insulin treatment were negatively correlated with M1 and positively correlated with M2 [46]. In addition, the therapeutic function of microbial metabolite Butyrate in colitis was reported by facilitating M2 polarization to suppress inflammation [47]. However, in the present study, SCFAs, including Acetate, Butyrate, and Propionate, exhibited profound role in suppressing M2 polarization in murine alveolar macrophages, likewise, IL-4 induced alternatively activated human macrophages were also attenuated by SCFAs, moreover, Butyrate and Propionate reduced M2 polarization in the lung in OVA-induced asthma animal models. The inconsistency between previous studies and our research may be explained by different cell types and varied main driver in different diseases. For instance, classical (M1) Kupffer cell polarization is a key driver initiating liver injury in ALD, polarized M2 with proapoptotic and anti-inflammatory function could counterbalance M1-driven tissue injury [48]. However, increased M2 polarization in Th2 environment in asthma patients and animal models suggested M2 as a motivator in allergic airway inflammation. Despite the discrepancy about the function of SCFAs on macrophage polarization, these results invited the speculation that SCFAs mostly regulate the immune system to decrease inflammation and tissue injury through modulating the main triggers of different diseases, it is also worthy of future research to elucidate.
SCFAs act through binding to endogenous receptors GPR41 and GPR43, which have been reported to be expressed in the gut epithelium [49, 50], adipose tissues [51](p43),[52], immune cells [53], and nervous system [54, 55]. For instance, Maslowski et al. reported that Acetate-induced suppression of colonic inflammation was reversed in GPR43-/- animals compared with wild-type animals, as demonstrated by higher neutrophil infiltration following dextran sulfate sodium (DSS) treatment [56]. Recently, it has been found that high levels of Butyrate and Propionate in early life are associated with protection against atopy [57]. Some studies implied the protective effect of SCFAs in allergic inflammation in the lung, high-fiber diet with increasing SCFAs levels shaped the immunological environment in the lung and elicited the severity of lung inflammation [27]. Zaiss et al. [58] showed the direct link between intestinal helminth-induced increases in SCFAs and the ability to attenuate allergic airway inflammation, and this was dependent on GPR41. In line with that, the essential role of GPR41 in asthma was supported by Trompette et al. who demonstrated that Propionate lightened HDM-induced allergic airway inflammation in wild type and GPR43-/- mice, but not GPR41-/- mice [27]. We noted that these results were based on the whole animal level. GPR41 and GPR43 mRNA can be expressed by peripheral blood mononuclear cells, blood vessel endothelial cells, ILC2, eosinophils and neutrophils, dendritic cells, and macrophages [28, 56, 59–61]. Some studies indicated neither GPR41 nor GPR43 were involved in SCFAs-induced inhibitory effect on eosinophils migration and adhesion, IL-13- and IL-5-producing ILC2. In our hand, we demonstrated GPR43, but not GPR41 was activated by SCFAs, and the inhibitory role was possibly GPR43-dependent.
It is also known HDAC inhibition serves as the mechanism underlying immune-regulatory roles of SCFAs, of which Butyrate and Propionate are said to be solid HDAC inhibitor, while Acetate is a weak HDAC inhibitor or lack of this potency [62, 63]. Histone acetylation has been proposed to enzymatic activity of histone deacetylase and promote gene transcription possibly by unwinding DNA and increasing the access for binding of transcription factors [64]. Butyrate and Propionate inhibited HDAC activity to boost histone H3 acetylation at enhancer elements and promoter region of the Foxp3 gene locus, which subsequently augmented Foxp3 expression and remarkably potentiated peripheral Treg cells generation [43, 63]. Thio et al. demonstrated that Butyrate prohibited production of IL-13 and IL-5 from ILC2s and alleviated ILC2-induced airway inflammation, moreover, it functioned through downregulating HDAC activity. This is also delineated by in vitro and in vivo findings of Theiler et al. in the same manner. They highlighted Butyrate as a potential therapy in allergic inflammatory diseases through attenuating eosinophils function depending on inhibition of HDAC [44]. In the present study, Butyrate and Propionate, but not Acetate, reversed the decreased H3 acetylation induced by IL-4. However, the inhibitory activity is not existed in Acetate, this is in accordance with its ineffective role in dendritic cells and ILC2 cells [28](p2),[63]. TSA also exhibited a mock role of Butyrate and Propionate in abating M2 polarization, this is contrast to previous study which demonstrated Trichostatin A (TSA), a histone deacetylase (HDAC) inhibitor promoted peritoneal macrophage M2 phenotype to counteract excessive inflammation in a cecal ligation and puncture–induced sepsis mouse model [65]. The reason of this inconsistency may likely lie in the fact that the targets involved in the therapeutic effect of SCFAs were the main drivers which varied according to different pathological processes.
Conclusion
We demonstrated that Acetate, Butyrate, and Propionate inhibited M2 polarization in human-derived macrophages and murine alveolar macrophages. Butyrate and Propionate inhibited M2 polarization in asthma animal model and attenuated allergic airway inflammation. Mechanistically, we proved that Acetate, Butyrate, and Propionate acted partly through GPR43 activation and/or HDAC inhibition in MH-S.
Glossary
Abbreviations
- SCFAs
Short chain fatty acids
- IL-4
interleukin-4
- OVA
ovalbumin
- GPR43
G-protein-coupled receptor 43
- GPR41
G-protein-coupled receptor 43
- TSA
trichostatin A
- HDAC
histone deacetylase
- AHR
airway hyper-responsiveness
- ILC2
innate lymphoid type 2
- Treg
regulatory T
- ROS
reactive oxygen species
- LPS
lipopolysaccharide
- IFNγ
interferon γ
- p-Stat6
phosphorylation of signal transducer and activator of transcription 6
- MRC1
Mannose receptor
- Arg1
arginase 1
- Chi3l3/YM1
chitinase-3-like protein-3
- FIZZ1/Retnla
resistin-like molecule-α/found in inflammatory zone 1
- DCs
dendritic cells
- BALF
bronchoalveolar lavage fluid
- MH-S
murine alveolar macrophages
- FBS
fetal bovine serum
- PBMC
peripheral blood mononuclear cells
- BMI
body mass index
- EOS%
percentage of eosinophils in induced sputum
- ACQ
Asthma Control Questionnaire score
- FEV1% pre
FEV1 percent predicted
- EV1/FVC
FEV1/forced vital capacity percentage
- M-CSF
Macrophage-colony stimulating factor
- PMA
phorbol myristate acetate
- PVDF
polyvinylidene fluoride
- qRT-PCR
Quantitative Real Time Polymerase Chain Reaction
- PFA
paraformaldehyde
- OVA
ovalbumin
- HE
hematoxylin
- PAS
periodic acid–Schiff
- DSS
dextran sulfate sodium
- CLP
cecal ligation and puncture
- Clec10a
C-type lectin domain family 10 member A
- MCP-1/CCL2
Monocyte chemoattractant protein-1
- CNS
conserved non-coding sequence
- FOXP3
forkhead box P3
- DSS
dextran sulfate sodium
Funding
This study was supported by Grant 81770025, 81970020 from National Natural Science Foundation of China, Grant 2019SY006 from Shanghai Municipal Health Commission, Grant 20dz2261100 from Shanghai Key Laboratory of Emergency Prevention, Diagnosis and Treatment of Respiratory Infectious Diseases, Grant shslczdzk02202 from Shanghai Municipal Key Clinical Specialty, Grant 20dz2210500 from Cultivation Project of Shanghai Major Infectious Disease Research Base, Grant ZH2018QNA48 from Cross research funds of Translational Medicine and Grant 2017ZZ02014 from Shanghai key discipline for respiratory diseases.
Conflict of interest
The authors declare that they have no competing interests.
Author contributions
Conception and design of the work: G.S. In vitro study: W.D., C.H. In vivo study: C.H. Data analysis: C.H., W.D. Data interpretation: Y.N., G.L. Drafting the work or revising it critically for important intellectual content: C.H., G.S.
Ethical approval and informed consent
The study was approved by the Ruijin Hospital Ethics Committee, Shanghai Jiao Tong University School of Medicine. The study abides by principles in declaration of Helsinki for the use of human samples. Signed informed consent was obtained from all patients.
Data availability
Not applicable.
References
- 1. Holgate ST, Polosa R.. Treatment strategies for allergy and asthma. Nat Rev Immunol 2008, 8, 218–30. [DOI] [PubMed] [Google Scholar]
- 2. Holtzman MJ. Asthma as a chronic disease of the innate and adaptive immune systems responding to viruses and allergens. J Clin Invest 2012, 122, 2741–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hussell T, Bell TJ.. Alveolar macrophages: plasticity in a tissue-specific context. Nat Rev Immunol 2014, 14, 81–93. [DOI] [PubMed] [Google Scholar]
- 4. Saradna A, Do DC, Kumar S, Fu QL, Gao P.. Macrophage polarization and allergic asthma. Transl Res 2018, 191, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shapouri-Moghaddam A, Mohammadian S, Vazini H, et al.. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol 2018, 233, 6425–40. [DOI] [PubMed] [Google Scholar]
- 6. Moreira AP, Cavassani KA, Hullinger R, et al.. Serum amyloid P attenuates M2 macrophage activation and protects against fungal spore-induced allergic airway disease. J Allergy Clin Immunol 2010, 126, 712–21.e7. [DOI] [PubMed] [Google Scholar]
- 7. Leonard WJ. The molecular basis of X-linked severe combined immunodeficiency: defective cytokine receptor signaling. Annu Rev Med 1996, 47, 229–39. [DOI] [PubMed] [Google Scholar]
- 8. Heller NM, Qi X, Junttila IS, et al.. Type I IL-4Rs selectively activate IRS-2 to induce target gene expression in macrophages. Sci Signal 2008, 1, ra17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Martinez FO, Helming L, Milde R, et al.. Genetic programs expressed in resting and IL-4 alternatively activated mouse and human macrophages: similarities and differences. Blood 2013, 121, e57–69. [DOI] [PubMed] [Google Scholar]
- 10. Loke P, Nair MG, Parkinson J, Guiliano D, Blaxter M, Allen JE.. IL-4 dependent alternatively-activated macrophages have a distinctive in vivo gene expression phenotype. BMC Immunol 2002, 3, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nair MG, Cochrane DW, Allen JE.. Macrophages in chronic type 2 inflammation have a novel phenotype characterized by the abundant expression of Ym1 and Fizz1 that can be partly replicated in vitro. Immunol Lett 2003, 85, 173–80. [DOI] [PubMed] [Google Scholar]
- 12. Martinez FO, Helming L, Gordon S.. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol 2009, 27, 451–83. [DOI] [PubMed] [Google Scholar]
- 13. Kang K, Reilly SM, Karabacak V, et al.. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell Metab 2008, 7, 485–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shirakawa T, Kawazoe Y, Tsujikawa T, Jung D, Sato S, Uesugi M.. Deactivation of STAT6 through serine 707 phosphorylation by JNK. J Biol Chem 2011, 286, 4003–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Melgert BN, ten Hacken NH, Rutgers B, Timens W, Postma DS, Hylkema MN.. More alternative activation of macrophages in lungs of asthmatic patients. J Allergy Clin Immunol 2011, 127, 831–3. [DOI] [PubMed] [Google Scholar]
- 16. Chupp GL, Lee CG, Jarjour N, et al.. A chitinase-like protein in the lung and circulation of patients with severe asthma. N Engl J Med 2007, 357, 2016–27. [DOI] [PubMed] [Google Scholar]
- 17. Subrata LS, Bizzintino J, Mamessier E, et al.. Interactions between innate antiviral and atopic immunoinflammatory pathways precipitate and sustain asthma exacerbations in children. J Immunol 2009, 183, 2793–800. [DOI] [PubMed] [Google Scholar]
- 18. Teng X, Li D, Champion HC, Johns RA.. FIZZ1/RELMalpha, a novel hypoxia-induced mitogenic factor in lung with vasoconstrictive and angiogenic properties. Circ Res 2003, 92, 1065–7. [DOI] [PubMed] [Google Scholar]
- 19. Cai Y, Kumar RK, Zhou J, Foster PS, Webb DC.. Ym1/2 promotes Th2 cytokine expression by inhibiting 12/15(S)-lipoxygenase: identification of a novel pathway for regulating allergic inflammation. J Immunol 2009, 182, 5393–9. [DOI] [PubMed] [Google Scholar]
- 20. Lee CG, Da Silva CA, Dela Cruz CS, et al.. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu Rev Physiol 2011, 73, 479–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. den Besten G, Bleeker A, Gerding A, et al.. Short-chain fatty acids protect against high-fat diet-induced obesity via a PPARγ-dependent switch from lipogenesis to fat oxidation. Diabetes 2015, 64, 2398–408. [DOI] [PubMed] [Google Scholar]
- 22. Tang C, Ahmed K, Gille A, et al.. Loss of FFA2 and FFA3 increases insulin secretion and improves glucose tolerance in type 2 diabetes. Nat Med 2015, 21, 173–7. [DOI] [PubMed] [Google Scholar]
- 23. Chambers ES, Viardot A, Psichas A, et al.. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 2015, 64, 1744–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Singh N, Gurav A, Sivaprakasam S, et al.. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 2014, 40, 128–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu B, Qian J, Wang Q, Wang F, Ma Z, Qiao Y.. Butyrate protects rat liver against total hepatic ischemia reperfusion injury with bowel congestion. PLoS One 2014, 9, e106184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gurav A, Sivaprakasam S, Bhutia YD, Boettger T, Singh N, Ganapathy V.. Slc5a8, a Na+-coupled high-affinity transporter for short-chain fatty acids, is a conditional tumour suppressor in colon that protects against colitis and colon cancer under low-fibre dietary conditions. Biochem J 2015, 469, 267–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Trompette A, Gollwitzer ES, Yadava K, et al.. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 2014, 20, 159–66. [DOI] [PubMed] [Google Scholar]
- 28. Thio CL, Chi PY, Lai AC, Chang YJ.. Regulation of type 2 innate lymphoid cell-dependent airway hyperreactivity by butyrate. J Allergy Clin Immunol 2018, 142, 1867–83.e12. [DOI] [PubMed] [Google Scholar]
- 29. Sohn SW, Lee HS, Park HW, et al.. Evaluation of cytokine mRNA in induced sputum from patients with allergic rhinitis: relationship to airway hyperresponsiveness. Allergy 2008, 63, 268–73. [DOI] [PubMed] [Google Scholar]
- 30. Li R, Shang Y, Hu X, et al.. ATP/P2X7r axis mediates the pathological process of allergic asthma by inducing M2 polarization of alveolar macrophages. Exp Cell Res 2020, 386, 111708. [DOI] [PubMed] [Google Scholar]
- 31. Mathie SA, Dixon KL, Walker SA, et al.. Alveolar macrophages are sentinels of murine pulmonary homeostasis following inhaled antigen challenge. Allergy 2015, 70, 80–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bedoret D, Wallemacq H, Marichal T, et al.. Lung interstitial macrophages alter dendritic cell functions to prevent airway allergy in mice. J Clin Invest 2009, 119, 3723–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Song YD, Li XZ, Wu YX, et al.. Emodin alleviates alternatively activated macrophage and asthmatic airway inflammation in a murine asthma model. Acta Pharmacol Sin 2018, 39, 1317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Raes G, Van den Bergh R, De Baetselier P, et al.. Arginase-1 and Ym1 are markers for murine, but not human, alternatively activated myeloid cells. J Immunol 2005, 174, 6561; author reply 6561–2. [DOI] [PubMed] [Google Scholar]
- 35. Scotton CJ, Martinez FO, Smelt MJ, et al.. Transcriptional profiling reveals complex regulation of the monocyte IL-1 beta system by IL-13. J Immunol 2005, 174, 834–45. [DOI] [PubMed] [Google Scholar]
- 36. Welch JS, Escoubet-Lozach L, Sykes DB, Liddiard K, Greaves DR, Glass CK.. TH2 cytokines and allergic challenge induce Ym1 expression in macrophages by a STAT6-dependent mechanism. J Biol Chem 2002, 277, 42821–9. [DOI] [PubMed] [Google Scholar]
- 37. Raes G, Brys L, Dahal BK, et al.. Macrophage galactose-type C-type lectins as novel markers for alternatively activated macrophages elicited by parasitic infections and allergic airway inflammation. J Leukoc Biol 2005, 77, 321–7. [DOI] [PubMed] [Google Scholar]
- 38. Munder M, Mollinedo F, Calafat J, et al.. Arginase I is constitutively expressed in human granulocytes and participates in fungicidal activity. Blood 2005, 105, 2549–56. [DOI] [PubMed] [Google Scholar]
- 39. Munder M, Eichmann K, Modolell M.. Alternative metabolic states in murine macrophages reflected by the nitric oxide synthase/arginase balance: competitive regulation by CD4+ T cells correlates with Th1/Th2 phenotype. J Immunol 1998, 160, 5347–54. [PubMed] [Google Scholar]
- 40. Hart PH, Bonder CS, Balogh J, Dickensheets HL, Donnelly RP, Finlay-Jones JJ.. Differential responses of human monocytes and macrophages to IL-4 and IL-13. J Leukoc Biol 1999, 66, 575–8. [PubMed] [Google Scholar]
- 41. Peters-Golden M. The alveolar macrophage: the forgotten cell in asthma. Am J Respir Cell Mol Biol 2004, 31, 3–7. [DOI] [PubMed] [Google Scholar]
- 42. Park J, Kim M, Kang SG, et al.. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol 2015, 8, 80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Furusawa Y, Obata Y, Fukuda S, et al.. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–50. [DOI] [PubMed] [Google Scholar]
- 44. Theiler A, Bärnthaler T, Platzer W, et al.. Butyrate ameliorates allergic airway inflammation by limiting eosinophil trafficking and survival. J Allergy Clin Immunol 2019, 144, 764–76. [DOI] [PubMed] [Google Scholar]
- 45. Jiang X, Huang X, Tong Y, Gao H.. Butyrate improves cardiac function and sympathetic neural remodeling following myocardial infarction in rats. Can J Physiol Pharmacol 2020, 98, 391–9. [DOI] [PubMed] [Google Scholar]
- 46. Wang Z, Zhang X, Zhu L, et al.. Inulin alleviates inflammation of alcoholic liver disease via SCFAs-inducing suppression of M1 and facilitation of M2 macrophages in mice. Int Immunopharmacol 2020, 78, 106062. [DOI] [PubMed] [Google Scholar]
- 47. Ji J, Shu D, Zheng M, et al.. Microbial metabolite butyrate facilitates M2 macrophage polarization and function. Sci Rep 2016, 6, 24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wan J, Benkdane M, Teixeira-Clerc F, et al.. M2 Kupffer cells promote M1 Kupffer cell apoptosis: a protective mechanism against alcoholic and nonalcoholic fatty liver disease. Hepatology 2014, 59, 130–42. [DOI] [PubMed] [Google Scholar]
- 49. Karaki S, Tazoe H, Hayashi H, et al.. Expression of the short-chain fatty acid receptor, GPR43, in the human colon. J Mol Histol 2008, 39, 135–42. [DOI] [PubMed] [Google Scholar]
- 50. Tazoe H, Otomo Y, Karaki S, et al.. Expression of short-chain fatty acid receptor GPR41 in the human colon. Biomed Res 2009, 30, 149–56. [DOI] [PubMed] [Google Scholar]
- 51. Zaibi MS, Stocker CJ, O’Dowd J, et al.. Roles of GPR41 and GPR43 in leptin secretory responses of murine adipocytes to short chain fatty acids. FEBS Lett 2010, 584, 2381–6. [DOI] [PubMed] [Google Scholar]
- 52. Kimura I, Ozawa K, Inoue D, et al.. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun 2013, 4, 1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Brown AJ, Goldsworthy SM, Barnes AA, et al.. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem 2003, 278, 11312–9. [DOI] [PubMed] [Google Scholar]
- 54. Kimura I, Inoue D, Maeda T, et al.. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc Natl Acad Sci USA 2011, 108, 8030–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. De Vadder F, Kovatcheva-Datchary P, Goncalves D, et al.. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014, 156, 84–96. [DOI] [PubMed] [Google Scholar]
- 56. Maslowski KM, Vieira AT, Ng A, et al.. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Roduit C, Frei R, Ferstl R, et al.; PASTURE/EFRAIM study group . High levels of butyrate and propionate in early life are associated with protection against atopy. Allergy 2019, 74, 799–809. [DOI] [PubMed] [Google Scholar]
- 58. Zaiss MM, Rapin A, Lebon L, et al.. The intestinal microbiota contributes to the ability of helminths to modulate allergic inflammation. Immunity 2015, 43, 998–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Li L, Ma L, Fu P.. Gut microbiota-derived short-chain fatty acids and kidney diseases. Drug Des Devel Ther 2017, 11, 3531–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ulven T. Short-chain free fatty acid receptors FFA2/GPR43 and FFA3/GPR41 as new potential therapeutic targets. Front Endocrinol (Lausanne) 2012, 3, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wilkerson EM, Johansson MW, Hebert AS, et al.. The peripheral blood eosinophil proteome. J Proteome Res 2016, 15, 1524–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Davie JR. Inhibition of histone deacetylase activity by butyrate. J Nutr 2003, 133, 2485–93S. [DOI] [PubMed] [Google Scholar]
- 63. Arpaia N, Campbell C, Fan X, et al.. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lee DY, Hayes JJ, Pruss D, Wolffe AP.. A positive role for histone acetylation in transcription factor access to nucleosomal DNA. Cell 1993, 72, 73–84. [DOI] [PubMed] [Google Scholar]
- 65. Cui SN, Chen ZY, Yang XB, et al.. Trichostatin A modulates the macrophage phenotype by enhancing autophagy to reduce inflammation during polymicrobial sepsis. Int Immunopharmacol 2019, 77, 105973. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.