Abstract
Voltage-sensitive fluorescent reporters can reveal fast changes in the membrane potential in neurons and cardiomyocytes. However, in many cases, illumination in the presence of the fluorescent reporters results in disruptions to the action potential shape that limits the length of recording sessions. We show here that a molecular prosthetic approach, previously limited to fluorophores, rather than indicators, can be used to substantially prolong imaging in neurons and cardiomyocytes.
Short abstract
Triplet state quenchers tethered to voltage-sensitive fluorophores substantially reduce phototoxicity associated with prolonged imaging, enabling sustained recordings in neurons and cardiomyocytes.
Introduction
Fluorophores are indispensable in the investigation of living systems. The problem is they bleach and can perturb the very system they are meant to observe. Bleaching makes it difficult to make sustained measurements, and phototoxicity introduces artifacts by disrupting the underlying physiology of the biological system. Exogenous photoprotectant cocktails, often containing antioxidants or triplet-state quenchers (TSQs), are often added to imaging media to prolong imaging duration by either reducing photobleaching or phototoxicity. However, addition of exogenous photoprotectants often requires millimolar concentrations of hydrophobic compounds,1 which can modify lipid bilayer properties.2 Pioneering work showed that intramolecular tethering of a TSQ, like the vitamin E derivative Trolox or cyclooctatetraene (COT), improves photostability and reduces the phototoxicity of common fluorophores in single molecule and cellular imaging,4,5 without the requirement for millimolar concentrations of lipophilic additives.3,4,6 In some live-cell contexts, cellular viability is improved without altering the rate of photobleaching.5
However, this self-healing fluorophore strategy has never been applied in the context of fluorescent reporters, dyes which change their optical properties in response to biological cues, such as pH, Ca2+, or membrane potential. Additionally, because the molecular mechanisms behind self-healing fluorophores rely on tuning of electron and energy transfer rates between the fluorophore and TSQ,6−8 it was not clear whether the presence of a TSQ would interfere with the sensing mechanism of fluorescent reporters that utilize photoinduced electron transfer (PeT).
Voltage-sensitive dyes have been limited by toxicity induced by the presence of the dye and the intense illumination required for fast voltage imaging. We have been exploring new molecular wire scaffolds for fluorescent voltage indicators which utilize PeT as a voltage-sensitive trigger.9,10 These molecular wire-based voltage-sensitive fluorophores (VF dyes) show decreased phototoxicity, and we found that imaging of evoked neuronal action potentials under elevated illumination intensity (21 mW/mm2) altered the observed neuronal physiology, likely because plasma membranes are especially sensitive to dye-sensitized photodamage.11 The changed neuronal physiology manifested as nonevoked spikes, after-depolarizations, shifts in the baseline, and decreases in ΔF/F (Figure S1). We wondered whether the addition of exogenous photoprotectants, like Trolox or COT, might decrease the apparent disruptions to underlying neuronal physiology.
Results
To test this, we added Trolox or COT (1 mM) to see if this reduced the number of artifacts when imaging with mVF-sarcosine12 (Figure S1) or with VF2.1.Cl (Figure S6).13 Addition of Trolox has only a modest effect on the proportion of neurons that fire normally during field stimulation (27% ± 17%, 95% C.I.) compared to mVF-sarcosine alone (12% ± 11%), while COT significantly improves the fraction of normally firing neurons to 89% (±10%) (Figure S1). However, application of millimolar concentrations of lipophilic photoprotectants is often not practical, and we wondered whether a covalently tethered photoprotectant would reduce the impact on cellular physiology while retaining the voltage sensitivity of the native VF dye.
Synthesis
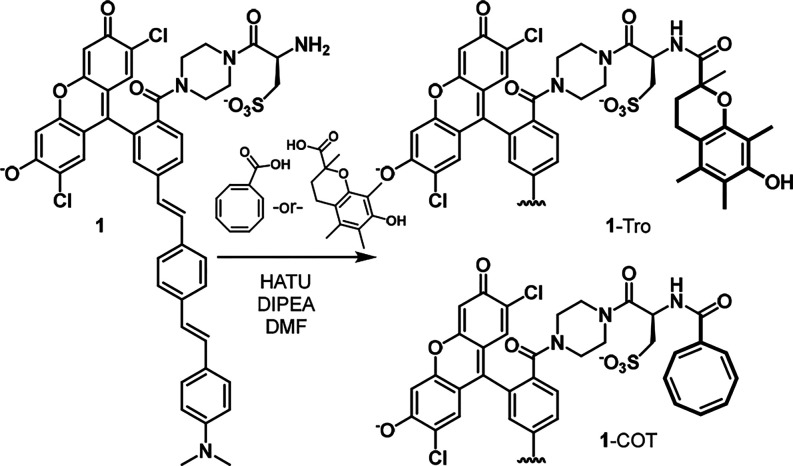
To test this hypothesis, we first synthesized a new VF dye, 1, with a cysteic acid residue that possesses a sulfonic acid group for water solubility and retention in cell membranes and a free amine for ready functionalization with Trolox or carboxy-COT (Scheme 1 and Scheme S1). From intermediate 1, we synthesized a VF dye with covalently tethered Trolox (1-Tro) or COT (1-COT) (Scheme 1).
Scheme 1. Synthesis of Triple-State Quencher VF Dyes.
In Vitro and Cellular Characterization
The new VF-conjugates show absorption and emission spectra nearly identical to the parent compound, with an absorbance maximum at 525 nm and an emission maximum near 540 nm (Figure 1a, Figure S2). In HEK293T cells, 1-COT is voltage sensitive, with a 17.9% ΔF/F per 100 mV (Figure 1b, Figure S3). Both the parent 1 and conjugates, 1-Tro and 1-COT, localize to cell membranes in HEK293T cells, neurons, and human induced pluripotent stem cell derived cardiomyocytes (hiPSC-CMs) (Figure 1c–e, Figure S4).
Figure 1.
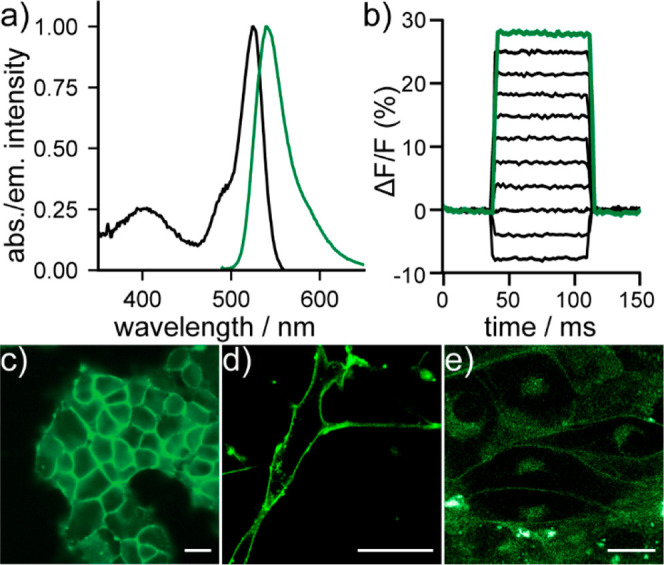
Characterization of TSQ-stabilized indicators. (a) Plot of normalized absorbance and emission intensity for 1-COT (200 nM) in phosphate-buffered saline (PBS, pH 7.2) with 0.1% Triton X-100. (b) Plot of relative change in fluorescence of 1-COT (ΔF/F) vs time in a patch-clamped HEK293T cell under whole-cell voltage-clamp conditions. (c) Widefield, epifluorescence images of 1-COT in HEK293T cells (1 μM). (d) Confocal images of 1-COT (0.5 μM) in (d) cultured rat hippocampal neurons or (e) hiPSC-derived cardiomyocytes. All scale bars are 20 μm.
Imaging in Neurons
We treated neurons with 1-Tro or 1-COT and imaged optical action potentials driven by extracellular stimulation. We compared the results to imaging with 1 alone or 1 plus the addition of exogenous photoprotective reagents Trolox or COT (Figure 2a,b). Similar to VF-sarcosine, imaging neuronal activity with 1 results in neurons without an artifact only 19% of the time (±10%, 95% confidence interval). Neurons treated with Trolox (1 mM) showed no improvement, but imaging with Trolox covalently conjugated to 1 (500 nM, 1-Tro) dramatically increased the proportion of neurons with artifact-free firing to 57% (±16%). COT had an even more dramatic effect. When 1 mM exogenous COT was added to neurons loaded with 1, the proportion of neurons without artifacts substantially increased, to 93% (±8%). However, millimolar concentrations of COT, a 2000× excess over 1, were required to observe this protective effect; at lower concentrations of 1 and 10 μM, still in stoichiometric excess over 1, a high percentage of cells still displayed artifacts (Figure S5). When COT is covalently attached to 1, 96% of neurons fire normally (±7%, Figure 2b).
Figure 2.
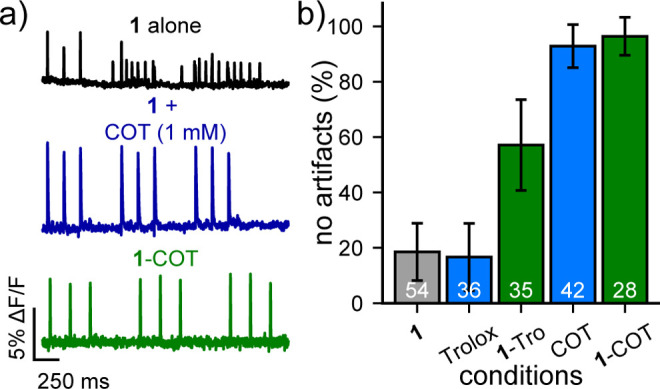
TSQ-stabilized indicators prevent unwanted physiological disruption in mammalian neurons. (a) Plots of relative fluorescence (ΔF/F) vs time for neurons loaded with 500 nM of the indicated dye (1, black; 1 + 1 mM COT, blue; or 1-COT, green) and stimulated with an extracellular electrode to evoke firing. Traces are single trials and are bleach corrected using a linear fit. (b) Plot of the fraction of neurons that exhibit no experimental artifacts during the neuronal simulation protocol, including shifts in baseline, after depolarizations, or nonevoked spikes. Data are the proportion of cells without artifacts. Error bars are the 95% confidence interval. White values indicate the number of analyzed neurons per condition.
Imaging in Cardiomyocytes
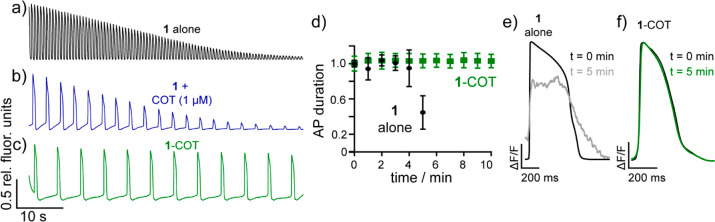
Because of the excellent performance of 1-COT in neurons, we investigated whether this protective effect could be observed in a distinct model system of electrically excitable cells. We loaded human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) with 1 alone (1 μM), 1 plus an equimolar amount of COT (1 μM), or the new 1-COT conjugate (1 μM) (Figure 3a–c). The amplitude of cardiac action potentials (AP) drops dramatically during imaging with 1 alone (Figure 3a, black). Addition of equimolar COT helps maintain AP height; however, the signal-to-noise (SNR) is substantially degraded at the end of a 60 s imaging session (Figure 3b, blue). In contrast, AP height remains nearly constant throughout the 60 s imaging bout with 1-COT (Figure 3c, green). In these experiments, the hiPSC-CMs are unpaced, which means that the beat rate, or number of APs, can vary from trial to trial.
Figure 3.
TSQ-stabilized indicators prevent unwanted physiological disruption in human induced pluripotent stem-cell derived cardiomyocytes (hiPSC-CMs). Plot of relative fluorescence vs time for unpaced hiPSC-CMs loaded with 1 μM of either (a) 1 alone (black), (b) 1 + COT (exogenously added, 1 μM), or (c) 1-COT. Recordings are single trials and are bleach corrected using an asymmetric least-squares fit. (d) Plot of normalized action potential duration (APD) vs time in spontaneously beating hiPSC-CMs loaded with 1 alone (black) or 1-COT (green) and illuminated continuously for the indicated time. At 1 min intervals, a 20 s movie was acquired, and the action potential duration was assessed. Data are mean ± SEM for n = 3 separate experiments. Example optically recorded action potentials for (e) 1 alone (black/gray) or (f) 1-COT (black/green) at t = 0 min (black) and at t = 5 min (gray or green) of continuous illumination. AP traces are the average of 21 and 24 aligned APs for 1 alone (black/gray) and 1-COT (black/green), respectively.
The protective effects of covalently tethered COT are even more profound after periods of extended imaging. Even after 10 min of continuous illumination, the AP duration (APD) and beat rate of hiPSC-CMs treated with 1-COT remains unchanged (Figure 3d, green; Figure S9). hi-PSC-CMs imaged with 1 alone show dramatic changes in APD even after 1 min of illumination, significant changes to APD, beat rate, and shape after 5 min, and stop beating shortly thereafter as evidenced by an arrest of beating and lack of APs after minute 6 (Figure 3d, black; Figure S9; Supplemental Videos 1, 2, 3, and 4). Examination of AP shape during the initial 10 s of imaging (Figure 3e, t = 0) and after 5 min of continuous illumination reveal profound changes to the shape of APs in hiPSC-CMs imaged with 1, while APs recorded after 5 min with 1-COT overlay closely with the initial APs (Figure 3f). Under maximum illumination, higher imaging speeds can be achieved using 1-COT, enabling high temporal resolution recording of AP rise times and upstroke velocity (Figure S7).14 Similar to the previously reported use of COT-conjugated fluorophores in live cells,51-COT has a similar bleach rate to 1 (Figure S8) but substantially decreases phototoxicity.
Safety Statement
No unexpected or unusually high safety hazards were encountered.
Conclusion
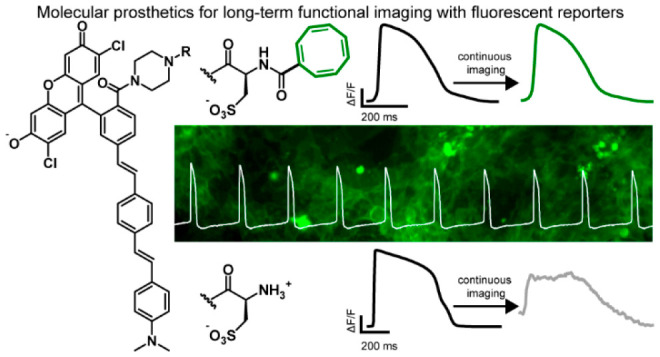
In summary, we present a generalizable molecular prosthetic that can be used to stabilize recordings made with fluorescent reporters. Previous studies elegantly showed that covalent attachment of TSQs could prolong the duty cycle of fluorophores, but to date, no study has highlighted a similar strategy for fluorescent molecules that report dynamically on their environment. Here, we show specifically that covalent attachment of TSQs like Trolox or COT allow VF dyes to retain their performance in neurons or cardiomyocytes while avoiding disruptions to underlying cellular physiology. In contrast to previous studies in our lab, which altered the molecular wire of the voltage-sensitive fluorophore,15,16 this present strategy has the potential to be more universal and could likely be applied to any VF dye, such as the silicon-rhodamine fluorophore BeRST 1 or other indicators regardless of the molecular wire or fluorescent reporter.4,17
Acknowledgments
E.W.M. thanks the NIH (Grant R35GM119855) and the Camille Dreyfus Teacher-Scholar Program for support of this research. K.N.M., B.R.B., D.M.G.-A., B.K.R., S.C.B., J.C.M., and A.M.M.G. were supported, in part by the NIH (Grant T32GM066698). V.G. was supported, in part, by a graduate fellowship from NSERC. Confocal imaging was conducted at the CRL Molecular Imaging Center RRID:SCR_017852, supported by NIH Grant S10OD025063. We thank Holly Aaron and Feather Ives for instrumental training, support, and advice.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscentsci.1c01153.
Author Contributions
∥ V.G., K.N.M., B.R.B., and D.M.G.-A. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- Dave R.; Terry D. S.; Munro J. B.; Blanchard S. C. Mitigating unwanted photophysical processes for improved single-molecule fluorescence imaging. Biophysical journal 2009, 96, 2371–2381. 10.1016/j.bpj.2008.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alejo J. L.; Blanchard S. C.; Andersen O. S. Small-molecule photostabilizing agents are modifiers of lipid bilayer properties. Biophysical journal 2013, 104, 2410–2418. 10.1016/j.bpj.2013.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman R. B.; Terry D. S.; Zhou Z.; Zheng Q.; Geggier P.; Kolster R. A.; Zhao Y.; Javitch J. A.; Warren J. D.; Blanchard S. C. Cyanine fluorophore derivatives with enhanced photostability. Nat. Methods 2012, 9, 68–71. 10.1038/nmeth.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q.; Jockusch S.; Zhou Z.; Altman R. B.; Zhao H.; Asher W.; Holsey M.; Mathiasen S.; Geggier P.; Javitch J. A.; et al. Electronic tuning of self-healing fluorophores for live-cell and single-molecule imaging. Chemical Science 2017, 8, 755–762. 10.1039/C6SC02976K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z.; Li L.; Ling J.; Liu T.; Huang X.; Ying Y.; Zhao Y.; Zhao Y.; Lei K.; Chen L.; et al. Cyclooctatetraene-conjugated cyanine mitochondrial probes minimize phototoxicity in fluorescence and nanoscopic imaging. Chemical Science 2020, 11, 8506–8516. 10.1039/D0SC02837A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pati A. K.; El Bakouri O.; Jockusch S.; Zhou Z.; Altman R. B.; Fitzgerald G. A.; Asher W. B.; Terry D. S.; Borgia A.; Holsey M. D.; et al. Tuning the baird aromatic triplet-state energy of cyclooctatetraene to maximize the self-healing mechanism in organic fluorophores. Proc. Natl. Acad. Sci. U. S. A. 2020, 117, 24305–24315. 10.1073/pnas.2006517117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q.; Juette M. F.; Jockusch S.; Wasserman M. R.; Zhou Z.; Altman R. B.; Blanchard S. C. Ultra-stable organic fluorophores for single-molecule research. Chem. Soc. Rev. 2014, 43, 1044–1056. 10.1039/C3CS60237K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q.; Jockusch S.; Rodríguez-Calero G. G.; Zhou Z.; Zhao H.; Altman R. B.; Abruña H. D.; Blanchard S. C. Intra-molecular triplet energy transfer is a general approach to improve organic fluorophore photostability. Photochemical & Photobiological Sciences 2016, 15, 196–203. 10.1039/C5PP00400D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. W.; Lin J. Y.; Frady E. P.; Steinbach P. A.; Kristan W. B. Jr.; Tsien R. Y. Optically monitoring voltage in neurons by photo-induced electron transfer through molecular wires. Proc. Natl. Acad. Sci. U.S.A. 2012, 109, 2114–2119. 10.1073/pnas.1120694109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P.; Miller E. W. Electrophysiology, unplugged: Imaging membrane potential with fluorescent indicators. Accounts of chemical research 2020, 53, 11–19. 10.1021/acs.accounts.9b00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz M. P.; Koppel D. E. Membrane damage caused by irradiation of fluorescent concanavalin a. Proc. Natl. Acad. Sci. U. S. A. 1979, 76, 3314–3317. 10.1073/pnas.76.7.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier V.; Daws B. R.; Liu P.; Miller E. W. Spying on neuronal membrane potential with genetically targetable voltage indicators. J. Am. Chem. Soc. 2019, 141, 1349–1358. 10.1021/jacs.8b11997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. W.; Lin J. Y.; Frady E. P.; Steinbach P. A.; Kristan W. B.; Tsien R. Y. Optically monitoring voltage in neurons by photo-induced electron transfer through molecular wires. Proc. Natl. Acad. Sci. U. S. A. 2012, 109, 2114–2119. 10.1073/pnas.1120694109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra M.; Mummery C.; Wilde A.; Bezzina C.; Verkerk A. Induced pluripotent stem cell derived cardiomyocytes as models for cardiac arrhythmias. Frontiers in Physiology 2012, 3, 346. 10.3389/fphys.2012.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggess S. C.; Gandhi S. S.; Siemons B. A.; Huebsch N.; Healy K. E.; Miller E. W. New molecular scaffolds for fluorescent voltage indicators. ACS Chem. Biol. 2019, 14, 390–396. 10.1021/acschembio.8b00978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggess S. C.; Gandhi S. S.; Benlian B. R.; Miller E. W. Vinyl-fluorene molecular wires for voltage imaging with enhanced sensitivity and reduced phototoxicity. J. Am. Chem. Soc. 2021, 143, 11903. 10.1021/jacs.1c04543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. L.; Walker A. S.; Miller E. W. A photostable silicon rhodamine platform for optical voltage sensing. J. Am. Chem. Soc. 2015, 137, 10767–10776. 10.1021/jacs.5b06644. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.