Abstract
Per- and polyfluoroalkyl substances (PFAS) accumulation and elimination in both wildlife and humans is largely attributed to PFAS interactions with proteins, including but not limited to organic anion transporters (OATs), fatty acid binding proteins (FABPs), and serum proteins such as albumin. In wildlife, changes in the biotic and abiotic environment (e.g. salinity, temperature, reproductive stage, and health status) often lead to dynamic and responsive physiological changes that alter the prevalence and location of many proteins, including PFAS-related proteins. Therefore, we hypothesize that if key PFAS-related proteins are impacted as a result of environmentally induced as well as biologically programmed physiological changes (e.g. reproduction), then PFAS that associate with those proteins will also be impacted. Changes in tissue distribution across tissues of PFAS due to these dynamics may have implications for wildlife studies where these chemicals are measured in biological matrices (e.g., serum, feathers, eggs). For example, failure to account for factors contributing to PFAS variability in a tissue may result in exposure misclassification as measured concentrations may not reflect average exposure levels. The goal of this review is to share general information with the PFAS research community on what biotic and abiotic changes might be important to consider when designing and interpreting a biomonitoring or an ecotoxicity based wildlife study. This review will also draw on parallels from the epidemiological discipline to improve study design in wildlife research. Overall, understanding these connections between biotic and abiotic environments, dynamic protein levels, PFAS levels measured in wildlife, and epidemiology serves to strengthen study design and study interpretation and thus strengthen conclusions derived from wildlife studies for years to come.
Keywords: Wildlife, PFAS, proteins, biotic, abiotic
1). INTRODUCTION
Per- and polyfluoroalkyl substances (PFAS), a class of man-made environmental contaminants, represent a group of aliphatic substances or organic chains (branched and linear) containing the moiety -CnF2n+1 within their structure [1]. Due to production for use in numerous industrial and consumer products [2], PFAS make their way into the environment through both point and non-point sources [3–7]. Unfortunately, the same stability that makes PFAS desirable for commercial and industrial uses also prevents degradation of many PFAS in the environment. Furthermore, many perfluoroalkyl precursors transform in the environment and biota into highly stable end products [8, 9]. This ultimate persistence of PFAS in the environment has led to the constant exposure short chain and long chain PFAS and the bioaccumulation of long chain PFAS in both wildlife and humans across the globe [10–14].
PFAS bioaccumulation within wildlife and humans is largely attributed to PFAS interactions with proteins. Specifically, in in vitro studies of proteins derived from model species, binding affinities have been documented for several PFAS, including the extensively studied perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA). [15]. PFAS are often acids with low pKa that are charged at environmentally relevant pH values, and therefore do not readily cross cell membranes by passive diffusion. Evidence suggests PFAS are substrates of a number transport proteins [16] that facilitate their entry into cells and their transport to and within cells. Proteins important to PFAS distribution include albumin [17], fatty acid binding proteins (FABP) [18], and organic anion transporters (OAT). Longer chained PFAS with larger hydrophobic regions can associate with phosphoproteins and lipoproteins [19] in biological systems. We will refer to these relevant proteins collectively as ‘PFAS-related proteins’ as we further discuss their tissue distribution as well as phospholipid bilayers [20] and how they influence the tissue distribution of PFAS within an organism [21]. However, despite the growing knowledge about PFAS and protein binding, limited information is known for protein binding affinities in wildlife species. Therefore, this review will discuss current knowledge and data on PFAS-protein interactions that may be relevant to wildlife, how the environment may impact wildlife proteins, and important knowledge gaps that may limit our ability to directly extrapolate from mammalian proteins studied under laboratory conditions to the wide variety of proteins and conditions relevant to wildlife species. We will also discuss strategies to address potential species differences in a high-throughput fashion with the use of in silico techniques such as molecular modeling.
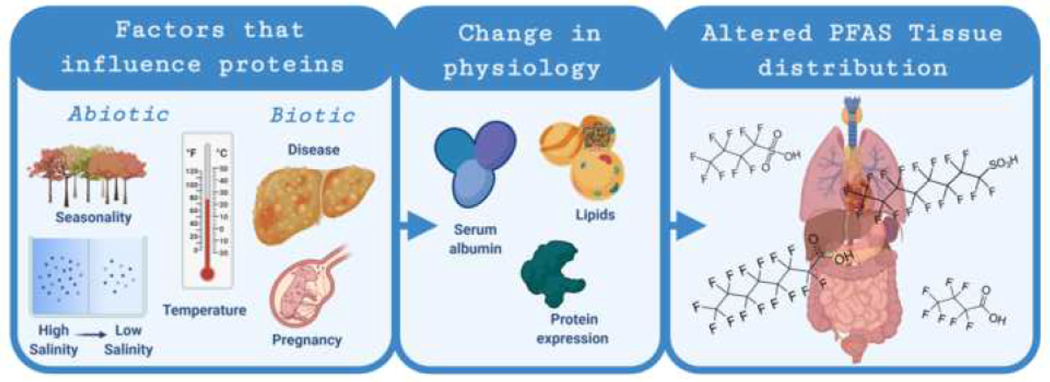
In wildlife, changes in the biotic and abiotic environment (e.g. changes in salinity, temperature, reproductive stage, and health status) often lead to dynamic and responsive physiological changes that alter the expression of many proteins including PFAS-related proteins [22, 23]. These environmentally induced changes in PFAS-related proteins then have the potential to impact PFAS tissue distribution across tissues within wildlife species (Figure 1). Specifically, we are talking about changes in proteins impacting PFAS presence or bioaccumulation rather than PFAS impacting protein function, although depending on the study and study design, the directionality of the relationship can be difficult to ascertain. We argue that considering this oftenneglected facet of PFAS-protein relationships can support wildlife study design and subsequent interpretation of results derived from wildlife biomonitoring and ecotoxicity studies. A goal of an ecotoxicity study could be to deduce causation; however, wildlife studies are often limited to crosssectional study designs which support correlations but not necessarily causation. Correlations can be the beginning step to understanding causation, but may be misinterpreted due to reverse causation [24] and can result in overinterpretation of results or, worst case, drawing erroneous conclusions. In addition, wildlife studies have high variability related to heterogeneity of sampled specimens [25], which may contribute to exposure misclassification or confounding (both of which are key epidemiological concepts). Understanding what environmentally relevant abiotic and biotic factors influence proteins and measured PFAS could go a long way towards mitigating potential sources of bias and improving study design and interpretation. Knowledge of dynamic protein changes and their impact on PFAS distribution could help researchers gather a more complete picture on the interrelated nature of PFAS, proteins, and health.
Figure 1.
The review will focus on the links between abiotic and biotic factors, how they influence changes in physiology in wildlife, and how that may potentially alter PFAS measured in wildlife tissues (Figure created with BioRender.com).
In this review, authors will (1) briefly review how protein binding has important influences on PFAS distribution, accumulation, and elimination, and (2) investigate wildlife studies for evidence of how protein changes may impact PFAS distribution in tissues. Authors will then (3) draw upon several epidemiological concepts to help inform wildlife study design, and finally (4) challenge the research community with recommendations to address current limitations of PFAS wildlife studies. Focus will largely revolve around perfluoroalkyl acids (PFAAs) including PFOS and PFOA due to the existing wealth of information in the literature but will also include existing information on emerging PFAS where possible.
2). THE IMPORTANCE OF PROTEIN BINDING TO PFAS DISTRIBUTION, ACCUMULATION, AND ELIMINATION
Serum Proteins
Within the literature, the proteins that have been most investigated for their ability to bind various PFAS are serum proteins, such as the blood carrier protein albumin. Albumin or albuminlike proteins have been observed in nearly all vertebrate species [26] and are carriers of a wide array of substances including amino acids, fatty acids, drugs and hormones [27, 28]. Even so, in vitro studies investigating direct binding of PFAS to albumin have mainly been limited to human serum albumin (HSA) [17] and bovine serum albumin (BSA) [29, 30]. These studies observe differential binding of PFAS to albumin based on chemical properties including chain length [29] and isomeric structure [31]. In wildlife it appears that other blood proteins can play a critical role. For example, one lab-based study investigated PFOS binding in tiger pufferfish (Takifugu rubripes) plasma by both in vivo and in vitro methods and observed that apolipoprotein A–I (a major component of high-density lipoprotein (HDL)), instead of albumin, was a key protein for PFOS binding and transport in the plasma of tiger pufferfish [32]. On the other hand, De Smet and colleagues reported that albumin like protein in brown trout Salmo trutta plays the same role as albumin in mammals. This suggests that PFAS binding to albumin-like proteins in plasma for wildlife is species dependent [33]. The implications for both field and laboratory studies are that blood proteins are a key medium for investigating PFAS accumulation in wildlife, but the measurement of protein-specific interactions, such as binding affinities, requires species-specific knowledge on appropriate protein targets. Further, observations of binding affinities are not necessarily transferrable across species, even within taxonomic groups [34]. For example, measured binding affinities for PFAS can differ substantially across human and bovine serum albumins, despite a greater than 75% sequence homology [35, 36]. Apolipoproteins, while having many of the same functional roles as albumin in fish species that lack albumin (i.e. lipid transport), have quite different three-dimensional structures from serum albumins [37].
Generally, the binding affinity of proteins for PFAS relies on two key features: hydrophobic interactions and polar/electrostatic interactions[38, 39]. The affinity of a particular PFAS for a particular protein will be affected, on the part of the protein, by the size of available hydrophobic grooves or binding pockets (and enhanced by greater contact, e.g. with longer-chain PFAS in a sufficiently large binding pocket) and by amino acids near the binding site that can engage in electrostatic or polar interactions with the acid head group of the PFAS. Thus, structural differences across proteins or protein isoforms can lead to differences in binding affinity for the same PFAS due to changes in the location of key amino acid residues, or to secondary structures or folding that affect the size and location of binding pockets with respect to key residues[40].
Fatty Acid Binding Proteins
Fatty acid binding proteins (FABPs) are a family of small transport proteins (14–16 kDa) that facilitate the transfer of fatty acids and other lipophilic substances between extra- and intracellular membranes. Studies have observed the binding affinities of several PFAS to human [41, 42] and rat-derived liver FABP isoforms [18]. As with albumin, properties of PFAS, such as chain length, impact liver FABP binding affinity, with a general agreement across observations that binding affinity increases with chain length, though a leveling off or decrease has also been observed for perfluorinated carboxylic acids with chain lengths longer than 10 perfluorinated carbons [41]. FABPs have demonstrated strong evolutionary conservation across a variety of investigated species [43]; however, similar to serum albumins, there are limited in vitro studies investigating PFAS binding to wildlife-derived FABPs. Model-based screening of wildlife proteins for binding to PFAS could help identify tissues likely to be sites of accumulation or toxic impact across different species. This was recently illustrated in a modeling study of PFAS binding to liver FABP across seven species, which predicted that FABP derived from Japanese medaka and fathead minnow would have significantly weaker binding to several perfluoroalkyl acids, whereas FABP derived from rat, chicken, rainbow trout, and human were all predicted to have similar binding affinities [34]. Of note, one of the issues around including PFAS binding to FABPs as a sink or accumulator (as well as other proteins discussed) is complicated by the fact that exposure to PFAS impacts FABP expression levels and thus may contribute to a feedback effect [44]. In depth discussion on this feedback effect is not within the scope of this review.
Organic Anion Transporters
Organic anion transporters (OATs) are members of the solute carrier (SLC) superfamily of transmembrane transporter proteins that regulate anion balance in the body and control the excretion of drugs, toxins, nutrients, and metabolites [45, 46]. Many PFAS are known substrates of OATs and have been observed to be transported across barriers such as the kidney and placenta [15, 47]. OAT isoform expression in the kidneys is well studied in humans [48] and model species [15, 49] due to their key role in excretion and reuptake of PFAS from the body. The differences in sex- and species-specific PFAS half-life observed in the literature is often attributed to hormoneregulation of isoform specific expression of OATs in the kidney [50, 51]. Han et al. 2012 provides a particularly useful review of kidney transporters and their documented interactions with PFAS [52]. Similar to FABP and albumin, the literature suggests that the ability of OATs to transport PFAS is chain length dependent [53], with maximum affinity for C7-C9 perfluorinated carboxylic acids (PFCAs) for excretion of PFAS by OAT isoforms and for C9 and C10 PFCAs for reabsorption of PFAS by OAT isoforms [52]. Current observations have been limited to the interaction of PFAS with human and rodent proteins, and despite sex differences being observed in the PFAS burden in several wildlife species, little additional information is known about OAT isoform expression in the kidneys of wildlife species [54–56]. Finally, in vivo studies in rainbow trout have reported rapid renal clearance of PFOA as a dominant elimination mechanism that could suggest facilitation by renal transporter proteins, rather than the reabsorption observed in mammalian systems [57].
ATP-binding Cassette Transporters
The ATP-binding cassette (ABC) transporters are another superfamily of proteins consisting of numerous transmembrane transporters. This superfamily of transporters plays numerous roles in living organisms including transporting drug and xenobiotics across biological membranes [58]. While limited data is available, early studies have observed transport of PFAS by select members of the ABC family. For example, the breast cancer resistance protein (BCRP) is an efflux transporter (i.e., moves molecules out of cells) that is highly expressed at biological barriers which functions to protect the brain, retina, testes, and the placenta from toxins and xenobiotics [59–61]. BCRP has been observed to transport PFOA across biological barriers in both in vitro and ex vivo studies [16, 62]. While these early studies suggest BCRP may play a role in PFAS crossing biological barriers, no other studies have investigated BCRP as a potential transporter of PFAS, and to our knowledge no information is known about how and where PFAS bind to BCRP transporters. Inter-species differences in the expression levels of BCRP between rodents and humans have been observed in a number of studies [63], and may therefore play a role in the species-specific impacts of BCRP transport of PFOA and potentially other PFAS. More studies are warranted to understand how large of a role BCRP and other ABC transporters might play in PFAS transport across biological barriers and, ultimately, their impact on tissue distribution in both model species and wildlife species.
Phospho- and lipoproteins
It is well known that a large route of maternal off-loading of PFAS for many wildlife species is through egg laying [64], with the C7 and C8 legacy PFAS dominating the yolk (i.e., PFOS, PFOA, and perfluorohexanesulfonic acid (PFHxS)) [65]; however, there are a limited number of studies that have investigated PFAS binding to proteins associated with egg laying to date. Species-specific PFAS partitioning into the fractions of eggs that contain lipoprotein and phosphoproteins (vitellin and phosvitin) has been noted [66]. To our knowledge, only one study has investigated the distribution of 17 PFAS within the yolk and albumen components of chicken eggs and followed up with in silico docking analysis to confirm binding affinities for low-density lipoprotein, high density lipoprotein, and vitellin in yolk [19]. The study observed that yolk contained a higher proportion of the detected PFAS overall as well as a higher proportion of linear isomers (n-PFOS, n-PFOA, and n-PFHxS) compared to albumen. Unfortunately, paired maternal serum was not investigated as a part of the study. Overall, more studies investigating various PFAS binding affinities to proteins associated with egg laying within the egg are warranted to better provide an improved understanding of PFAS distribution in egg laying wildlife species. Additionally, pairing these data with PFAS concentrations in maternal bird tissues would provide important insight into tissue distribution and maternal transfer in wildlife species, including discrimination in off-loading by chain length, branching, or other PFAS structural features.
Phospholipids
In addition to protein interactions, research suggests that PFAS phospholipid interactions in an organism significantly contribute to tissue-specific sorption capacity for PFAS [20]. Molecular dynamics simulations and in vitro studies show PFAS passively penetrate phospholipid bilayers, and the extent of the PFAS interaction with the phospholipid bilayer is functional group and chain length dependent [67–69]. In general, phospholipid partitioning is expected to increase with perfluoroalkyl chain length likely due to increased hydrophobicity of longer fluorinated chain lengths [70]. Longer chain PFAS partitioning to phospholipid rich tissues, such as the brain, has been observed in a variety of wildlife including seabirds [71, 72], polar bears [73], pilot whales [74], and Crucian carp [75].
In viviporous wildlife species that lactate to feed their offspring, milk production is a wellcited route of maternal off-loading [76–78]. While the rate of PFAS transfer through milk has been observed to be much lower than the rate of PFAS transfer to offspring during gestation [76, 79], the mechanism by which PFAS is transferred into maternal milk is currently unknown. One possible hypothesis is PFAS associating, even if only marginally, with lipids like phospholipids in maternal milk. In fact, PFAS have been observed to be correlated with phospholipids in one human breast milk study [80]. While early in vivo and in vitro studies suggest a significant role of phospholipids on long chain PFAS tissue partitioning, much more research is needed to fully understand (1) how physicochemical PFAS properties impact their distribution in phospholipid bilayers, (2) how the prevalence of phospholipid classes across organs might impact PFAS distribution, (3) what this means for PFAS distribution in wildlife species, and (4) differential toxicities. Of note, PFAS interaction with phospholipids is complicated by the fact that exposure to PFAS may impact energy pathways that dictate phospholipid metabolism and thus may also contribute to a feedback effect [81–84].
Additional Factors
Several additional factors related to protein interactions have arisen in the literature that may play some role in PFAS distribution in wildlife tissues including saturation of proteins in a tissue and the presence or absence of additional PFAS. For example, the renal reabsorption capabilities of OATs can be saturated at sufficiently high PFAS exposure leading to an increase in the elimination rate of PFAS in a tissue of the highly exposed organism [85, 86]. In short, this saturation effect can cause studies to underestimate the half-life of a PFAS. Evidence of this saturation effect is observed in zebrafish and chicken models [87–89]. To make things more complex, the presence of long chain PFAS has been observed to reduce bioaccumulation and tissue distribution of short-chain PFAS in a lab-based zebrafish study [90]. The authors of that study hypothesized competition for transporters and binding sites on proteins play a role in the impact of long-chain PFAS co-exposure on short-chain PFAS bioaccumulation.
Toxicokinetic and binding affinity models
As shown above, there are many different protein interactions that influence PFAS binding, serum half-lives, and subsequent tissue accumulation of specific PFAS. In silico modeling offers a powerful solution to increasing our understanding of PFAS-protein interactions for structurally diverse PFAS, for proteins across different tissue types and functions, and for evaluating protein structure and function differences across species. Multiple areas of research for in silico analysis of PFAS binding include but are not limited to individual protein binding, onecompartment models, and physiologically-based toxicokinetic (PBTK) models. Of importance, in silico modeling has revealed that inclusion of physiological parameters such as potential elimination routes, binding affinities of PFAS to specific proteins, and transport interactions with membrane proteins are needed to understand the complexity of PFAS accumulation and are key to accurately predicting tissue-specific PFAS distribution and bioaccumulation potential [21, 91, 92]. In silico whole body modeling in rainbow trout and common carp suggests that the types of proteins involved in PFAS accumulation and tissue distribution may differ between species, and that lack of available data for other tissue compartments (besides blood and liver) leads to less robust models [21]. Molecular docking is particularly attractive because it is relatively rapid and requires as input only the three-dimensional structures of the protein and chemical (PFAS) of interest. Docking to specific proteins has been used as a screening method to identify PFAS with bioaccumulation potential in various species, and has shown remarkable parallels between fish and mammals [93] as well as key differences. For example, L-FABP is an important protein for PFAS binding strength and potential half-life for rainbow trout, while in humans, albumin is most important. One limitation of molecular docking is that it does not utilize other potential factors (e.g., temperature, salinity, etc.), but it quickly screens PFAS for relative binding potential of proteins across multiple species. However, there are only a limited number of high-quality 3dimensional structures available for proteins even in well-studied mammals. For proteins without a crystal structure, tools are being developed that can predict key elements of the three-dimensional structure, but still require careful evaluation [94, 95]. Moreover, docking is rapid at the cost of accurately estimating binding energies; because of this, it is best used as a screen for relative binding affinity rather than as a method to directly predict the binding affinity. Taken together, these studies suggest that consideration of protein interactions, aided by in silico screening, could help guide sampling and monitoring of individual wildlife species.
By understanding protein interactions, we can better predict bioaccumulation and biomagnification across food webs [96]. For example, a recent food web study in the Yadkin-Pee Dee River Basin in North Carolina demonstrated that PFAS concentrations for both long and short chain PFAS (PFBS, PFOS, PFOA, and PFDA) were highest in lower trophic organisms (e.g., insects and smaller fish) compared to higher trophic organisms [97]. Understanding what proteins or lipids might be driving the relationships observed in the species in the North Carolina food web, can help understand similar food web interactions in other environments. Another potential screening tool to understand PFAS accumulation across the food web is the US EPA Sequence Alignment to Predict Across Species Susceptibility (SeqAPASS; https://seqapass.epa.gov/seqapass/) [34, 98]. This web-based application uses National Center for Biotechnology Information (NCBI) protein database to identify similarities in protein sequences across multiple species that may be a target for the chemical of interest. Given that emerging PFAS without wildlife toxicological data (e.g. Nafion byproduct 2, Cl-PFECA) are found in the environment and biota [99, 100], these types of in silico modeling and inclusion of high-throughput methods (molecular docking, SeqAPASS) will be valuable to quickly screen PFAS binding and potential half-lives in various organisms.
3). BIOTIC AND ABIOTIC CHANGES AND THE IMPACT ON PFAS IN WILDLIFE STUDIES
Now that we have reviewed how protein binding and phospholipid association have important influences on PFAS distribution, accumulation, and elimination, the goal of this section is to begin to understand how dynamic fluctuations in the abundance of those proteins or phospholipids as a result of physiological changes could impact PFAS distribution in wildlife. Changes in abiotic and biotic factors such as salinity, temperature, and reproductive status are commonplace in a natural environment. Wildlife respond to these types of dynamic changes in their environment through adapting physiologically by altering protein abundance, lipid mobilization, or starting the process of reproduction [101–104]. Therefore, we hypothesize that if key PFAS related proteins are impacted as a result of changes in the environment, then PFAS that associate with those proteins will also be impacted. This applies to both PFAS that are currently contributing to the body burden of an organism and to fresh PFAS exposures. In brief, alterations in protein abundance drives (re)equilibration of PFAS with a larger or smaller pool of proteins, thus changing the concentration of PFAS in free circulation, as well as potentially affecting the uptake and elimination of PFAS if the expression or function of key membrane transporters are impacted. If this is the case, then knowing what environmental factors might impact proteins and thus PFAS distribution can be key to improving wildlife study design. By incorporating these kinds of abiotic and biotic factors into data collection and study design, wildlife studies may be able to better account for variability in measured PFAS concentrations and address potential misclassification or confounding that may result from the difficulties of studying animals in their natural environments.
In this section we will detail some of the ways in which wildlife studies have begun to observe associations between abiotic and biotic factors and PFAS in both terrestrial and marine wildlife tissues. This section will also describe matching protein changes where available. For the investigated abiotic factors (salinity and temperature) large aquarium-based studies are available for review. For the biotic factors (reproduction, and health status) all studies reviewed are cross sectional in design.
Salinity
Salinity is a key component of abiotic aquatic environments including marine, estuarine, and increasingly freshwater environments[105]. For successful habitation of aquatic environments, wildlife must properly osmoregulate to maintain homeostasis. Salinity in coastal and estuarine environments fluctuates daily, seasonally, and throughout a marine animal’s life course. Marine wildlife adapts to changing salinity through a variety of mechanisms to maintain osmoregulation. For example, in teleosts, a diverse group of ray-finned fishes, organs such as the kidney, gill, brain, liver, and muscle experience changes in expressed proteins, ion pumps, and percent muscle water [101]. With multiorgan osmoregulation in marine wildlife, it is not surprising that aquarium-based studies have observed associations between PFAS distribution and salinity in several species including Marine medaka [106], Blackrock fish [107], and Pacific oysters [108]. More details of these associations can be found in Table 1. Of importance, the study on Marine medaka also investigated protein expression of PFAS-related proteins and observed increased binding of OAT1 and FABP binding to PFAS in the gills with increasing salinity. It is important to note that water salinity impacts not only physiology in aquatic species but also impacts PFAS distribution within an aquatic environment [109, 110], and therefore, salinity likely impacts PFAS in wildlife tissues through a variety of complex coexisting factors.
Table 1.
Selected wildlife aquarium-based studies investigating the impact of biotic factors on PFAS levels in biological tissues. All findings listed under observations are reported as significant in the referenced publication.
| Abiotic Factor | Species | n (treatment group; time point) | Total n | Species age attreatment | Duration of study | PFAS Investigated | PFAS Treatment Concentrations | Observations | Publication |
|---|---|---|---|---|---|---|---|---|---|
| Salinity | Marine Medaka (Oryzias melastigma) | 6 | 300 | 3 months | 2 wks & 4 wks | PFOS, PFOA, PFBS, PFDoDA | 100 μ/L | Increase in salinity (0, 15, and 35 PSU) led to increased PFOS and PFOA in whole body concentrations Associated with increased gene and protein expression of FABP and OAT1 in the gill0 | Avellán – Llagun o et al. 2020 |
| Blackrock fish (Sebastes schlegeli) | 3 | 180* | 60 days | PFOS, PFOA, PFDA, PFUnDA | 10 μg/L | Reductions in salinity (34, 25, 17.5, 10 PSU) resulted in decrease d uptake and eliminatio n rate constants of PFDA, PFUnDA, PFOS but not PFOA in the serum. Serum and liver concentrations of individual PFCAs did not change over the 60-day period of investigation | Jeon et al. 2010 [90] | ||
| Pacific oysters (Crassostrea gigas) | 4 | 180* | 56 days | PFOS, PFOA, PFDA, PFUnDA | 10 μg/L | The distribution coefficients of PFOA, PFOS, PFDA, and PFUnDA in the whole oyster increased by 2.1- 2.7-fold as salinity increased from 10 to 34 PSU | Jeon al. 2010 [91] | ||
| Temperature | Rainbow trout (Oncorhy nchus mykiss) | 5 | 200 | 15 months old | 56 days | PFOS and PFHxS | 500 μg/kg per fish per day | Increased temperatures (from 7 to 19 °C) led to increased PFOS and PFHxS in liver but decreased concentrations in muscle | Vidal et al. 2019 |
estimated total n based on study design for publications where total n was not explicitly stated.
Temperature
Like salinity, temperature is a key abiotic factor in marine and freshwater environments that can change rapidly or seasonally. For the purposes of this review, temperature and seasonal changes will be discussed separately. Temperature changes will be discussed related to aquatic water temperatures that can be subject to a more rapid change, and seasonal temperature changes will represent sustained temperature differences from season to season.
Aquatic wildlife responding to rapid fluctuations in environmental temperature have been observed to adapt many physiological processes such as cardiac output, feeding, respiration, and growth [102, 103]; however, the effects of fluctuating temperature on PFAS in aquatic wildlife are not well studied at this time. To date, only one 56-day uptake and depuration study in adult rainbow trout investigated changes in PFOS and PFHxS organ distribution with fluctuations in water temperature [111]. While significant differences in PFOS and PFHxS were observed between temperature groups (see Table 1), investigations into PFAS-related protein expression or binding was not included in the study. To our knowledge, no additional studies have examined the relationship between temperature change and PFAS distribution in marine wildlife. The impact of temperature on the expression of PFAS-related proteins may or may not be important to other species of fish and mammals. Similar to salinity, changes water temperature can impact not only physiology in aquatic species but also PFAS physical properties and thus impact PFAS partitioning among substrates within an aquatic environment [112]. As a result the impact of temperature on PFAS levels in wildlife is likely a complex relationship of multiple inter-related factors.
Seasonal temperature changes/ diet changes/ body condition
For both aquatic and terrestrial wildlife, changing seasons result in numerous concurrent changes in both the biotic and abiotic environments. Not only do temperature ranges shift for several months, but often the availability of plant and prey species also shifts, resulting in large fluctuations in available diet from season to season [113]. With fluctuations in seasonal temperature and diet also come physiological changes such as the body condition of the impacted wildlife (e.g. lean versus fat). Therefore, changes in seasonal temperature, diet, and body condition are tightly coupled, and for wildlife studies, often cannot be separated from one another. While these changes often come hand in hand, understanding how such changes might impact PFAS accumulation and distribution in wildlife is key in both the design and interpretation of PFAS wildlife studies. Currently, several studies have documented associations between either diet or body condition (often related to the change in season) and PFAS within a single wildlife species including polar bears [114], artic foxes [115], tawny owls [116], and bank moles [117] (Table 2). Investigations into how PFAS-related proteins might be associated with the PFAS findings were not within the scope of these wildlife studies. Comparatively, other wildlife studies have observed no association between season and PFAS in studies investigating American alligators [118] and shad [119]. Both the alligator and shad studies observed high spatial variation within a small geographic location that could overwhelm seasonal trends in PFAS changes. This would suggest, for locations of high PFAS spatial heterogeneity such as areas with point sources of PFAS like aqueous film forming foams (AFFF) or large changes in emission sources, seasonality is of less impact on the variability of PFAS in wildlife studies [120]. Species territory range and migration patterns can also play a role in PFAS exposure and seasonality [121]. The takeaway from these studies investigating diet, body condition, and season is that each individual study (and species) is likely to have its own unique set of variables influencing PFAS levels in the tissue of interest.
Table 2.
Selected cross-sectional studies investigating the impact of biotic factors on PFAS levels in biological tissues of wildlife. All findings listed under observations are reported as significant in the referenced publication.
| Biotic Factor | Species | Total n | PFAS Investigated | Observations | Publication |
|---|---|---|---|---|---|
| Seasonal changes/ diet/ body condition | Polar bears (Ursus maritimus) | 77 | PFOS, PFHxS, PFOA, PFNA, PFDA, PFUnDA, PFDoDA, and PFTrDA | Fasting polar bears (stable isotopes analysis) maintained higher plasma PFOS, PFHxS, PFOA, PFNA, and PFDA than non-fasting polar bears | Tartu et al. 2017 |
| Arctic foxes (Vulpes lagopus) | 18 | PFBA, PFPeA, PFHxA, PFOA, PFNA, PFDA, PFUnDA, PFDoDA, PFTriDA, PFTeDA, PFHxS, PFHpS, PFDS and FOSA | PFDA and PFHpS concentrations in liver, kidney, and blood, and, PFNA in liver and blood, were twice as high in the lean compared to the fat foxes | Aas et al. 2014 | |
| Tawny owls (Strix aluco) | 107 | PFHxS, PFHpS, PFOS, PFDS, PFOA, PFNA, PFDA, PFUnDA, PFDoDA, PFTriDA, and PFTeDA | PFOS in eggs of tawny owls was negatively related to seasonal temperature, but the pattern was complex as there was an interaction between temperature and the feeding conditions. The PFOS accumulation was highest in years with high vole abundance and low to medium seasonal temperatures. | Bustnes et al. 2015 | |
| Bank moles (Myodes glareolus) | 28 | PFHxA, PFHpA, PFOA, PFNA, PFDA, PFUnDA, PFDoDA, PFTriDA, PFTeDA, PFPeDA, PFBS, PFHxS, PFOS, PFDS, and FOSA | Higher seasonal PFAS in adult bank mole liver in Spring compared to Autumn that was PFAS and location specific. | Ecke et al. 2020 | |
| American alligator (Alligator Mississippiensis) | 229 | PFBA, PFPeA, PFHxA, PFHpA, PFOA, PFNA, PFDA, PFUnDA, PFDoDA, PFTriDA, PFTeDA, PFBS, PFHxS, PFOS, and FOSA | No seasonal changes were observed in plasma PFAS in male or female alligators | Bangma et al. 2017 [48] | |
| Shad (Alosaagone) | 42 | PFHxA, PFHpA, PFOA, PFNA, PFDA, PFUnDA, PFDoDA, PFTriDA, PFTeDA, PFHxS, and PFOS | No seasonality was observed in PFAS in tissue compartments of sampled shad | Valsecchi et al. 2018 | |
| Reproduction | Striped mullet (Mugilcephalus) | 128 | PFBA, PFPeA, PFHxA, PFHpA, PFOA, PFNA, PFDA, PFUnDA, PFDoDA, PFTriDA, PFTeDA, PFBS, PFHxS, PFOS, and FOSA | PFOA, PFNA, and PFTriA increased in the maternal liver with sub-stage of oocyte development PFOS and its precursor PFOSA decreased in the liver with increasing stage of oocyte development |
Bangma et al. 2018 |
| Health status | Southern sea otter (Enhydra lutris nereis) | 80 | PFOS, and PFOA | Higher concentrations of PFOA and PFOS were observed in sea otters found to have died of infectious diseases compared to otters that were determined to have died of trauma | Kannan et al. 2006 |
| Mozambiqu e tilapia (Oreochromis mossambicus) | 23 | PFBA, PFPeA, PFHxA, PFHpA, PFOA, PFNA, PFDA, PFUnDA, PFDoDA, PFTriDA, PFTeDA, PFBS, PFHxS, PFOS, and FOSA | Lower PFOS and PFHxS was observed in the liver, kidney, and plasma of tilapia diagnosed with pansteatitis when compared to healthy tilapia from the same location | Bangma et al. 2017 [50] |
Reproduction
Numerous physiological changes occur for the successful reproduction of wildlife that may impact the distribution of PFAS in wildlife tissues including changes in hormone levels, key-PFAS related proteins, and reorganization of nutrients from mother to fetus/egg/newborn [122–124]. Maternal redistribution of PFAS to eggs or to newborn young through milk has been widely investigated in the literature as a source of maternal off-loading [64, 76]. While limited binding studies have been performed, PFAS transfer into eggs is hypothesized to be driven by a variety of proteins including low-density lipoprotein, high-density lipoprotein, and vitellogenin (a large phosphoprotein precursor to vitellin) [19, 125]. In addition, yolk studies have observed differences in the rate of uptake of various PFAS into developing embryos [126]. Similarly, PFAS transfer through milk is hypothesized to be through PFAS binding with milk proteins [127]. Overall, reproduction is a relatively well documented mode of maternal redistribution of PFAS to the fetus, newborn, or egg in wildlife [122, 123]. Of interest, one particular study focused on PFAS distribution in the liver of Striped mullet species as they progress through reproductive stages of oocyte development and observed interesting subtle fluctuations in several PFAS [125] (Table 2). The authors of the study hypothesized that the observed trends may be linked to a complex interplay of nutrient transport to the liver and the production and transport of vitellogenin from the liver to developing eggs; however, they were unable to measure PFAS in eggs across all the sampled individuals to confirm.
Health Status
The health status of individual wildlife can be key to physiological processes. Disease encompasses a multitude of aliments, but in very broad and general terms, disease can impact numerous physiological endpoints such as renal and hepatic function, food intake, and reproductive capacity [128, 129]. These disease driven physiological changes can be a result of concurrent changes in protein expression [130]. Authors hypothesize that depending on the circumstance, disease-associated physiological changes may lead to changes in the expression of key PFAS-related proteins, and thus alter the pharmacokinetics and distribution of PFAS in diseased wildlife. Here we survey the literature through the lens of how proteins impact PFAS rather than the impact of PFAS on proteins (as it relates to the onset, progression, or presence of disease).
Unfortunately, wildlife studies examining associations between PFAS and disease are often limited to cross-sectional study designs, which restricts the ability to determine causation and will be discussed in more depth in the next section. Cross-sectional studies have investigated PFAS in both diseased wildlife and wildlife that have died due to disease, such as studies in Mozambique tilapia [131] and sea otters [132] (Table 2). Overall, the number of studies investigating disease-associated protein changes on PFAS in wildlife is very limited. More research is needed to begin to understand how diseases impact key PFAS-related proteins and thus PFAS distribution in disease-affected wildlife.
4). WHAT CAN BE LEARNED FROM EPIDEMIOLOGICAL STUDIES ON PFAS
Designing wildlife and ecotoxicology studies that consider the breadth of factors that may influence PFAS-related protein dynamics is complex. However, there are some key parallels to consider from the epidemiologic literature on PFAS which underscore the issues presented throughout this review.
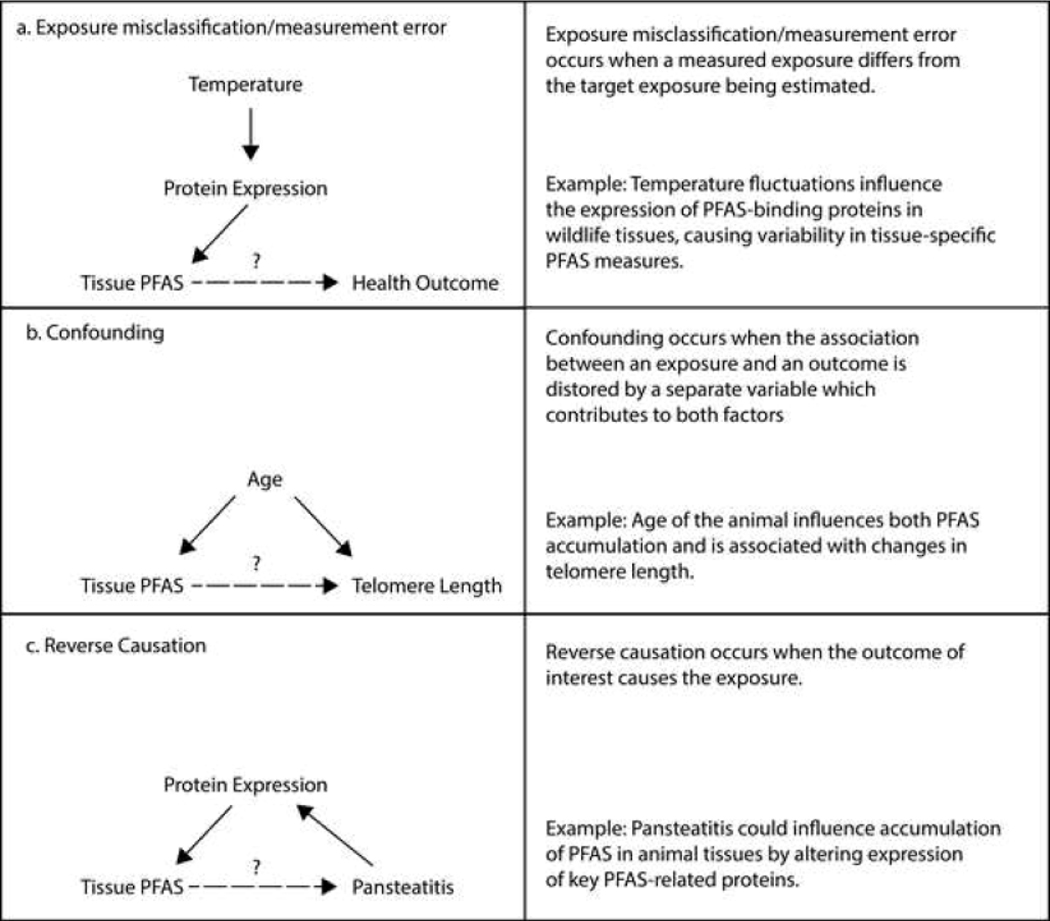
Quantifying PFAS in biological matrices in wildlife studies provides an estimate of internal exposure that integrates across exposure sources and can take into account behavioral or genetic differences that may alter the uptake or metabolism of chemicals from the environment in heterogenous populations [133]. However, under such circumstances, variability in the populations’ measured exposure may be high, making it difficult to interpret or accurately use the measure to assess associations with outcomes of interest. With relevance to the PFAS literature, we have highlighted several additional abiotic and biotic factors that may influence measured PFAS concentrations in wildlife tissues (e.g., season, temperature, salinity), but these issues are also well-documented in environmental epidemiology [133]. Overall, failure to account for factors contributing to PFAS variability may result in exposure misclassification as measured concentrations may not reflect average exposure levels (Figure 2) [134].
Figure 2.
Directed acyclic graphs (DAGs) demonstrating hypothetical associations between tissue PFAS and health outcomes. The three scenarios demonstrate potential (a) misclassification/measurement error, (b) confounding, and (c) reverse causation driven by the influence of temperature changes on the expression of PFAS-related proteins.
For example, acute temperature changes have been shown to influence tissue concentrations of PFAS in rainbow trout, as previously reported in [111], possibly via temperaturerelated changes in the expression and/or distribution of PFAS-binding proteins. Thus, temperature may be an important determinant of tissue-specific PFAS measures in some fish species. Not accounting for temperature at the time(s) of sampling may therefore result in unexplained variability in the distribution of PFAS measures and, ultimately, misclassification of the exposure. If unrelated to the outcome of interest, as depicted in the causal diagram shown in Figure 2a, the effect of this misclassification is expected to bias results towards the null (e.g., no association between exposure and outcome), although this is not always the case [135]. Nevertheless, it remains critical to account for these types of factors in study design or data collection in order to accurately describe exposure characteristics in both human and wildlife populations.
In some circumstances, the factors that influence measured PFAS concentrations may be causally related to the outcome of interest, thus serving as potential confounders (Figure 2b). For example, in one longitudinal study investigating the impact of PFAS on telomere length in adult Artic seabirds, Black-legged kittiwakes (Rissa tridactyla), the authors discussed a potential confounding effect of bird age on findings due to (1) the effect of age of PFAS accumulation, and (2) the relationship between age and telomere length [136]. Several epidemiologic studies in humans also highlight this issue. As discussed throughout this review, albumin and other PFASbinding proteins are key factors to consider in PFAS studies. In humans, kidney function (i.e., glomerular filtration rate) and albumin levels change as the result of hemodynamic changes throughout the course of pregnancy [137]. Not only are these changes related to concentrations of PFAS measured in maternal plasma, they are also suspected to relate to birth outcomes [138]. Thus, studies that measure PFAS during pregnancy without accounting for potential hemodynamic changes may report spurious associations between PFAS concentrations and birth outcomes. This has been extensively debated with respect to the association between PFAS and birth weight [139–143]. Moreover, PBTK models have demonstrated the potential for these factors to confound research findings [138]. This highlights the potential for the dynamic changes discussed throughout this paper to contribute to confounding by physiology in wildlife studies as well, although more research is warranted.
Lastly, a key issue in using wildlife studies to determine causation is ensuring that temporality can be established within a given study design. In other words, exposure should precede the disease to support a causal relationship and to rule out the possibility of reverse causation (Figure 2c). This is impossible to determine in studies where the exposure and outcome are measured at the same time (e.g., cross-sectional studies). For example, in wild-caught Mozambique tilapia (Oreochromis mossambicus) impacted with a rare inflammatory condition of the adipose called pansteatitis, significantly lower PFOS and PFHxS was observed in the liver, kidney, and plasma of disease-impacted individuals [131]. Although it is relatively well understood that PFAS is not the cause of pansteatitis, in this unique case authors were not able to unequivocally determine if the disease influenced the measured PFAS concentrations or if PFAS contributed to disease progression. This study can serve as an example for wildlife researchers to approach cross-sectional study designs in diseased wildlife with caution. Again, additional examples supporting this idea can be further found in the epidemiological literature. For instance, cross-sectional analyses in the C8 Health Study have suggested that PFAS exposure is associated with higher risk of earlier-onset menopause in adult populations [144]. Notably, further research within this study population has indicated that the original findings were likely influenced by reverse causation and, instead, the onset of menopause caused an increase in serum PFAS concentrations [145]. The proper interpretation of these findings required an understanding of how the change in physiological status influenced the excretion of PFAS. These studies also underscore the importance of utilizing longitudinal study designs (e.g., prospective cohorts) to corroborate findings from cross-sectional studies before determining causation. However, it should be noted that one drawback of using tissue-specific measures of PFAS in wildlife studies is that this may necessitate the use of cross-sectional study designs as it often requires sacrificing the animals under investigation. Thus, designing longitudinal studies may require the use of proxy measures of PFAS exposure (i.e., environmental media or non-invasive biomarkers) and may suffer from different sources of bias or uncertainty.
5). CONCLUSIONS AND RECOMMENDED NEXT STEPS
The degree to which protein expression changes in response to dynamic physiological changes can vary from species to species, but overall, the current literature indicates that protein levels change in wildlife as a response to variation in both biotic and abiotic conditions. These changes may be associated with alterations in PFAS accumulation and distribution in biological matrices, which must be considered to correctly interpret observations about PFAS. This review has focused on the few observations currently available in the literature that point to a general gap in knowledge on the subject and is ultimately leading us to call for concrete steps to strengthen study designs for wildlife biomonitoring. Here we suggest some concrete take-home points and suggested next steps for wildlife researchers interested in incorporating the influence of dynamic protein fluctuations in their research which include:
Understand your species of interest in relation to natural history to understand the most important biotic and abiotic variables (salinity, temperature, reproductive status, seasonal changes/body conditions, known health problems). Assess potential variables that may vary between sites or collection times to determine what variables have the greatest potential to induce physiological changes in the species in question and ensure these variables will be monitored during periods of data collection. Researchers should draw on existing bodies of work by state, nonprofit organizations, existing literature, and national wildlife agencies. Reach out to local wildlife experts (for instance, U.S. Fish and Wildlife Service and state agencies) for information regarding the study species.
Control for variables by appropriate study design, which can include sampling consistency (e.g. all fish are collected at the same time of year from regions with similar salinities) or accounting for recorded variables in final study analysis (e.g. include as covariates in statistical analyses). Authors of this review have created a general checklist of suggested items to help with study design, analysis, and interpretation (Supplemental file S1).
Researchers working with wildlife should utilize previously developed guidelines for reporting animal research (ARRIVE checklist; Supplemental file S2). ARRIVE guidelines maximize the quality and reliability of published research and enable others to better scrutinize, evaluate and reproduce it.
If strong associations are observed in a cross-sectional ecotoxicity-focused study between PFAS and an outcome of interest that is hypothesized to be causally linked and not a result of environmentally induced physiological changes, authors suggest following up with longitudinal based study if possible (controlled lab/aquarium based where applicable). If the target species is not amenable to lab/aquarium-based study design, an evaluation should be undertaken to determine if a comparable lab species will respond to environmental perturbations in the same way as the target species.
Integration of in vitro and computational approaches could help inform, interpret, and guide field and in vivo studies. In vitro studies can help determine binding affinity for different proteins highly expressed in tissues of interest and evaluate saturable uptake and elimination processes where membrane transporters play a role. These data can then be used to parameterize toxicokinetic models for sensitivity analysis or scenario testing of how changes in protein expression (e.g. the pool of available protein for binding) may affect bioaccumulation potential and tissue distribution. Given the abundance of different proteins and their varied roles in organisms, it is simply not possible to include all proteins in experimental research. By testing through the framework of a toxicokinetic model, specific protein types and expression levels can be tested for their influence on outcomes of interest (tissue concentrations, uptake and elimination kinetics). In turn, toxicokinetic models run at a given concentration of proteins of interest can be used to tune PFAS exposure concentrations to elicit changes in kinetics and accumulation, which can then be tested in vivo. Models can also be used to help interpret changes observed across individuals by using individual protein expression levels and PFAS exposure concentrations or body burdens as model inputs.
From the perspective of health outcomes, further research is needed to develop adverse outcome pathways for PFAS that will allow for testing the influence of changes in proteins on particular molecular events.
Authors are aware we have only begun to scratch the surface of the complex and interrelated nature of environmental conditions, protein dynamics, and their influence on PFAS distribution in wildlife tissues. This niche of PFAS research is understudied and would benefit greatly from additional research efforts in the years to come. This review integrated evidence about the interplay of dynamic physiological changes, protein expression, and PFAS in wildlife to demonstrate the complexities of studying the effects of PFAS on animals in their natural environments. Ultimately, authors seek to improve the field of PFAS-related endpoints in wildlife species by encouraging investigators to consider the influence of protein dynamics on PFAS concentrations measured in animals. In light of these findings, we have made several recommendations about improving study design and data collection and to encourage transparency in the reporting of wildlife studies.
Supplementary Material
Highlights.
Biotic and abiotic changes impact many proteins in wildlife, including PFAS-related proteins
Changes in PFAS-related proteins may have implications for PFAS measured in biological matrices
Parallels from the epidemiological discipline can help guide study design in wildlife research
Acknowledgments
DISCLAIMER
This research was supported, in part, by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences. The views, interpretations, and conclusions expressed in the article are solely those of the authors and do not necessarily reflect or represent NIST, NIEHS, or EPA’s views or policies.
Footnotes
Competing interest: Authors have no competing interests to declare
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Buck RC, et al. , Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr Enviro Assess Manage, 2011. 7(4): p. 513–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glüge J, et al. , An overview of the uses of per-and polyfluoroalkyl substances (PFAS). 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun H, et al. , Long-chain perfluorinated chemicals in digested sewage sludges in Switzerland. Environmental pollution, 2011. 159(2): p. 654–662. [DOI] [PubMed] [Google Scholar]
- 4.Sun M, et al. , Legacy and emerging perfluoroalkyl substances are important drinking water contaminants in the Cape Fear River Watershed of North Carolina. Environmental Science & Technology Letters, 2016. 3(12): p. 415–419. [Google Scholar]
- 5.de Solla SR, De Silva AO, and Letcher RJ, Highly elevated levels of perfluorooctane sulfonate and other perfluorinated acids found in biota and surface water downstream of an international airport, Hamilton, Ontario, Canada. Environment International, 2012. 39(1): p. 19–26. [DOI] [PubMed] [Google Scholar]
- 6.Furl CV, et al. , Relative importance of wastewater treatment plants and non-point sources of perfluorinated compounds to Washington State rivers. Science of the Total Environment, 2011. 409(15): p. 2902–2907. [DOI] [PubMed] [Google Scholar]
- 7.Kim S-K and Kannan K, Perfluorinated acids in air, rain, snow, surface runoff, and lakes: relative importance of pathways to contamination of urban lakes. Environmental science & technology, 2007. 41(24): p. 8328–8334. [DOI] [PubMed] [Google Scholar]
- 8.Letcher RJ, et al. , Comparative hepatic in vitro depletion and metabolite formation of major perfluorooctane sulfonate precursors in Arctic polar bear, beluga whale, and ringed seal. Chemosphere, 2014. 112: p. 225–31. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z, et al. , A Never-Ending Story of Per- and Polyfluoroalkyl Substances (PFASs)? Environmental Science & Technology, 2017. [DOI] [PubMed] [Google Scholar]
- 10.Reiner JL and Place BJ, Perfluorinated Alkyl Acids in Wildlife, in Toxicological Effects of Perfluoroalkyl and Polyfluoroalkyl Substances. 2015, Springer. p. 127–150. [Google Scholar]
- 11.Houde M, et al. , Monitoring of Perfluorinated Compounds in Aquatic Biota: An Updated Review. Environ Sci Technol, 2011. 45(19): p. 7962–7973. [DOI] [PubMed] [Google Scholar]
- 12.Houde M, et al. , Biological Monitoring of Polyfluoroalkyl Substances: A Review. Environmental Science & Technology, 2006. 40(11): p. 3463–3473. [DOI] [PubMed] [Google Scholar]
- 13.Jian J-M, et al. , A short review on human exposure to and tissue distribution of per- and polyfluoroalkyl substances (PFASs). Science of The Total Environment, 2018. 636: p. 1058–1069. [DOI] [PubMed] [Google Scholar]
- 14.Wang Q, et al. , Target, Nontarget, and Suspect Screening and Temporal Trends of Per- and Polyfluoroalkyl Substances in Marine Mammals from the South China Sea. Environmental Science & Technology, 2021. 55(2): p. 1045–1056. [DOI] [PubMed] [Google Scholar]
- 15.Nakagawa H, et al. , Roles of organic anion transporters in the renal excretion of perfluorooctanoic acid. Basic Clin Pharmacol Toxicol, 2008. 103(1): p. 1–8. [DOI] [PubMed] [Google Scholar]
- 16.Dankers AC, et al. , Endocrine disruptors differentially target ATP-binding cassette transporters in the blood-testis barrier and affect Leydig cell testosterone secretion in vitro. Toxicol Sci, 2013. 136(2): p. 382–91. [DOI] [PubMed] [Google Scholar]
- 17.D’eon JC, et al. , Determining the molecular interactions of perfluorinated carboxylic acids with human sera and isolated human serum albumin using nuclear magnetic resonance spectroscopy. Environmental Toxicology and Chemistry, 2010. 29(8): p. 1678–1688. [DOI] [PubMed] [Google Scholar]
- 18.Luebker DJ, et al. , Interactions of fluorochemicals with rat liver fatty acid-binding protein. Toxicology, 2002. 176: p. 175–185. [DOI] [PubMed] [Google Scholar]
- 19.Wang F, et al. , Protein-specific distribution patterns of perfluoroalkyl acids in egg yolk and albumen samples around a fluorochemical facility. Science of The Total Environment, 2019. 650: p. 2697–2704. [DOI] [PubMed] [Google Scholar]
- 20.Armitage JM, Arnot JA, and Wania F, Potential role of phospholipids in determining the internal tissue distribution of perfluoroalkyl acids in biota. Environ Sci Technol, 2012. 46(22): p. 12285–6. [DOI] [PubMed] [Google Scholar]
- 21.Ng CA and Hungerbühler K, Bioconcentration of Perfluorinated Alkyl Acids: How Important Is Specific Binding? Environ Sci Technol, 2013. 47(13): p. 7214–7223. [DOI] [PubMed] [Google Scholar]
- 22.Eryasa B, et al. , Physico-chemical properties and gestational diabetes predict transplacental transfer and partitioning of perfluoroalkyl substances. Environment International, 2019. 130: p. 104874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeung LWY, et al. , Profiles of perfluoroalkyl substances in the liver and serum of patients with liver cancer and cirrhosis in Australia. Ecotoxicology and Environmental Safety, 2013. 96: p. 139146. [DOI] [PubMed] [Google Scholar]
- 24.Rothman KJ, Greenland S, and Lash TL, Modern epidemiology. 2008: Lippincott Williams & Wilkins. 28–31. [Google Scholar]
- 25.Rhodes OE and Smith MH, Genetic Perspectives in Wildlife Management: The Case Of Large Herbivores, in Wildlife 2001: Populations, McCullough DR and Barrett RH, Editors. 1992, Springer Netherlands: Dordrecht. p. 985–996. [Google Scholar]
- 26.Li S, Cao Y, and Geng F, Genome-Wide Identification and Comparative Analysis of Albumin Family in Vertebrates. Evolutionary bioinformatics online, 2017. 13: p. 11769343177160891176934317716089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothschild MA, Oratz M, and Schreiber SS, Regulation of albumin metabolism. Annu Rev Med, 1975. 26: p. 91–104. [DOI] [PubMed] [Google Scholar]
- 28.Fasano M, et al. , The extraordinary ligand binding properties of human serum albumin. IUBMB Life, 2005. 57(12): p. 787–96. [DOI] [PubMed] [Google Scholar]
- 29.Bischel HN, et al. , Strong associations of short-chain perfluoroalkyl acids with serum albumin and investigation of binding mechanisms. Environmental Toxicology and Chemistry, 2011. 30(11): p. 2423–2430. [DOI] [PubMed] [Google Scholar]
- 30.Chen H, et al. , Systematic investigation of the toxic mechanism of PFOA and PFOS on bovine serum albumin by spectroscopic and molecular modeling. Chemosphere, 2015. 129: p. 217–224. [DOI] [PubMed] [Google Scholar]
- 31.Beesoon S and Martin JW, Isomer-specific binding affinity of perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) to serum proteins. Environmental science & technology, 2015. 49(9): p. 5722–5731. [DOI] [PubMed] [Google Scholar]
- 32.Honda M, et al. , Identification of perfluorooctane sulfonate binding protein in the plasma of tiger pufferfish Takifugu rubripes. Ecotoxicol Environ Saf, 2014. 104: p. 409–13. [DOI] [PubMed] [Google Scholar]
- 33.De Smet H, Blust R, and Moens L, Absence of albumin in the plasma of the common carp Cyprinus carpio: binding of fatty acids to high density lipoprotein. Fish Physiology and Biochemistry, 1998. 19(1): p. 71–81. [Google Scholar]
- 34.Cheng W, et al. , Integrative Computational Approaches to Inform Relative Bioaccumulation Potential of per- and Polyfluoroalkyl Substances (PFAS) across Species. Toxicol Sci, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang BX, Kim HY, and Dass C, Probing three-dimensional structure of bovine serum albumin by chemical cross-linking and mass spectrometry. J Am Soc Mass Spectrom, 2004. 15(8): p. 123747. [DOI] [PubMed] [Google Scholar]
- 36.Bischel HN, MacManus-Spencer LA, and Luthy RG, Noncovalent Interactions of Long-Chain Perfluoroalkyl Acids with Serum Albumin. Environmental Science & Technology, 2010. 44(13): p. 5263–5269. [DOI] [PubMed] [Google Scholar]
- 37.Andreeva AM, Structure of fish serum albumins. Journal of Evolutionary Biochemistry and Physiology, 2010. 46(2): p. 135–144. [PubMed] [Google Scholar]
- 38.Weiss JM, et al. , Competitive Binding of Poly- and Perfluorinated Compounds to the Thyroid Hormone Transport Protein Transthyretin. Toxicological Sciences, 2009. [DOI] [PubMed] [Google Scholar]
- 39.Sheng N, et al. , Interaction of perfluoroalkyl acids with human liver fatty acid-binding protein. Arch Toxicol, 2016. 90(1): p. 217–27. [DOI] [PubMed] [Google Scholar]
- 40.Stank A, et al. , Protein Binding Pocket Dynamics. Accounts of Chemical Research, 2016. 49(5): p. 809–815. [DOI] [PubMed] [Google Scholar]
- 41.Zhang L, Ren X-M, and Guo L-H, Structure-Based Investigation on the Interaction of Perfluorinated Compounds with Human Liver Fatty Acid Binding Protein. Environ Sci Technol, 2013. 47(19): p. 11293–11301. [DOI] [PubMed] [Google Scholar]
- 42.Yang D, et al. , Nontarget Screening of Per- and Polyfluoroalkyl Substances Binding to Human Liver Fatty Acid Binding Protein. Environmental Science & Technology, 2020. 54(9): p. 56765686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smathers RL and Petersen DR, The human fatty acid-binding protein family: Evolutionary divergences and functions. Human Genomics, 2011. 5(3): p. 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pfohl M, et al. , An ‘Omics Approach to Unraveling the Paradoxical Effect of Diet on Perfluorooctanesulfonic Acid (PFOS) and Perfluorononanoic Acid (PFNA)-Induced Hepatic Steatosis. Toxicol Sci, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nigam SK, et al. , The organic anion transporter (OAT) family: a systems biology perspective. Physiological reviews, 2015. 95(1): p. 83–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kell DB, et al. , The promiscuous binding of pharmaceutical drugs and their transportermediated uptake into cells: what we (need to) know and how we can do so. Drug Discovery Today, 2013. 18(5): p. 218–239. [DOI] [PubMed] [Google Scholar]
- 47.Kummu M, et al. , Organic anion transporter 4 (OAT 4) modifies placental transfer of perfluorinated alkyl acids PFOS and PFOA in human placental ex vivo perfusion system. Placenta, 2015. 36(10): p. 1185–1191. [DOI] [PubMed] [Google Scholar]
- 48.Yang C-H, Glover KP, and Han X, Characterization of Cellular Uptake of Perfluorooctanoate via Organic Anion-Transporting Polypeptide 1A2, Organic Anion Transporter 4, and Urate Transporter 1 for Their Potential Roles in Mediating Human Renal Reabsorption of Perfluorocarboxylates. Toxicological Sciences, 2010. 117(2): p. 294–302. [DOI] [PubMed] [Google Scholar]
- 49.Yang CH, Glover KP, and Han X, Organic anion transporting polypeptide (Oatp) 1a1-mediated perfluorooctanoate transport and evidence for a renal reabsorption mechanism of Oatp1a1 in renal elimination of perflurocarboxylates in rats. Toxicol. Lett, 2009. 190: p. 163. [DOI] [PubMed] [Google Scholar]
- 50.Lee JJ and Schultz IR, Sex differences in the uptake and disposition of perfluorooctanoic acid in fathead minnows after oral dosing. Environ. Sci. Technol, 2010. 44: p. 491. [DOI] [PubMed] [Google Scholar]
- 51.Kudo N, et al. , Sex hormone-regulated renal transport of perfluorooctanoic acid. Chemicobiological interactions, 2002. 139(3): p. 301–316. [DOI] [PubMed] [Google Scholar]
- 52.Han X, et al. , Renal Elimination of Perfluorocarboxylates (PFCAs). Chem Res Toxicol, 2012. 25(1): p. 35–46. [DOI] [PubMed] [Google Scholar]
- 53.Weaver YM, et al. , Roles of rat renal organic anion transporters in transporting perfluorinated carboxylates with different chain lengths. Toxicological sciences, 2009. 113(2): p. 305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bangma JT, et al. , Perfluorinated Alkyl Acids in plasma of American alligators (Alligator mississippiensis) from Florida and South Carolina. Environmental Toxicology and Chemistry, 2017. 36(4): p. 917–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fair PA, et al. , Associations between perfluoroalkyl compounds and immune and clinical chemistry parameters in highly exposed bottlenose dolphins (Tursiops truncatus). Environ Toxicol Chem, 2013. 32(4): p. 736–46. [DOI] [PubMed] [Google Scholar]
- 56.Bangma JT, et al. , Perfluoroalkyl substances in diamondback terrapins (Malaclemys terrapin) in coastal South Carolina. Chemosphere, 2019. 215: p. 305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Consoer DM, et al. , Toxicokinetics of perfluorooctanoate (PFOA) in rainbow trout (Oncorhynchus mykiss). Aquat Toxicol, 2014. 156: p. 65–73. [DOI] [PubMed] [Google Scholar]
- 58.Gedeon C, et al. , Transport of Glyburide by Placental ABC Transporters: Implications in Fetal Drug Exposure. Placenta, 2006. 27(11): p. 1096–1102. [DOI] [PubMed] [Google Scholar]
- 59.Han LW, Gao C, and Mao Q, An update on expression and function of P-gp/ABCB1 and BCRP/ABCG2 in the placenta and fetus. Expert Opin Drug Metab Toxicol, 2018. 14(8): p. 817–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maliepaard M, et al. , Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res, 2001. 61(8): p. 3458–64. [PubMed] [Google Scholar]
- 61.Ildikó N, et al. , Membrane Transporters in Physiological Barriers of Pharmacological Importance. Current Pharmaceutical Design, 2016. 22(35): p. 5347–5372. [DOI] [PubMed] [Google Scholar]
- 62.Szilagyi JT, et al. , Anandamide down-regulates placental transporter expression through CB2 receptor-mediated inhibition of cAMP synthesis. Pharmacological Research, 2019. 141: p. 331342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tanaka Y, et al. , Tissue distribution and hormonal regulation of the breast cancer resistance protein (Bcrp/Abcg2) in rats and mice. Biochem Biophys Res Commun, 2005. 326(1): p. 181–7. [DOI] [PubMed] [Google Scholar]
- 64.Holmstrom KE and Berger U, Tissue distribution of perfluorinated surfactants in common guillemot (Uria aalge) from the Baltic Sea. Environ Sci Technol, 2008. 42(16): p. 5879–84. [DOI] [PubMed] [Google Scholar]
- 65.Göckener B, et al. , Transfer of Per- and Polyfluoroalkyl Substances (PFAS) from Feed into the Eggs of Laying Hens. Part 1: Analytical Results Including a Modified Total Oxidizable Precursor Assay. J Agric Food Chem, 2020. [DOI] [PubMed] [Google Scholar]
- 66.Newsted JL, et al. , Effects of perfluorooctane sulfonate on mallard and northern bobwhite quail exposed chronically via the diet. Environmental Toxicology and Pharmacology, 2007. 23(1): p. 19. [DOI] [PubMed] [Google Scholar]
- 67.Shen Z, et al. , Cholesterol-like Condensing Effect of Perfluoroalkyl Substances on a Phospholipid Bilayer. J Phys Chem B, 2020. 124(26): p. 5415–5425. [DOI] [PubMed] [Google Scholar]
- 68.Nouhi S, et al. , Interactions of perfluoroalkyl substances with a phospholipid bilayer studied by neutron reflectometry. J Colloid Interface Sci, 2018. 511: p. 474–481. [DOI] [PubMed] [Google Scholar]
- 69.Fitzgerald NJM, et al. , Partitioning and Accumulation of Perfluoroalkyl Substances in Model Lipid Bilayers and Bacteria. Environ Sci Technol, 2018. 52(18): p. 10433–10440. [DOI] [PubMed] [Google Scholar]
- 70.Armitage JM, et al. , Development and evaluation of a mechanistic bioconcentration model for ionogenic organic chemicals in fish. Environ Toxicol Chem, 2013. 32(1): p. 115–28. [DOI] [PubMed] [Google Scholar]
- 71.Gebbink WA and Letcher RJ, Comparative tissue and body compartment accumulation and maternal transfer to eggs of perfluoroalkyl sulfonates and carboxylates in Great Lakes herring gulls. Environ Pollut, 2012. 162: p. 40–7. [DOI] [PubMed] [Google Scholar]
- 72.Robuck A, et al. , Legacy and Novel Per- and Polyfluoroalkyl Substances (PFAS) in Juvenile Seabirds from the US Atlantic Coast. Environmental Science & Technology, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Greaves AK, Tissue and intra-brain distribution of perfluoroalkyl carboxylates and sulfonates, and select precursors, in East Greenland Polar Bears (Ursus maritimus). 2012, Carleton University. [Google Scholar]
- 74.Dassuncao C, et al. , Phospholipid Levels Predict the Tissue Distribution of Poly- and Perfluoroalkyl Substances in a Marine Mammal. Environmental Science & Technology Letters, 2019. 6(3): p. 119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shi Y, et al. , Probing the Differential Tissue Distribution and Bioaccumulation Behavior of Per- and Polyfluoroalkyl Substances of Varying Chain-Lengths, Isomeric Structures and Functional Groups in Crucian Carp. Environmental Science & Technology, 2018. 52(8): p. 4592–4600. [DOI] [PubMed] [Google Scholar]
- 76.Grønnestad R, et al. , Maternal transfer of perfluoroalkyl substances in hooded seals. Environ Toxicol Chem, 2017. 36(3): p. 763–770. [DOI] [PubMed] [Google Scholar]
- 77.Desportes G and Mouritsen R, Preliminary results on the diet of long-finned pilot whales off the Faroe Islands. Report of the International Whaling Commission, 1993. 14(Special Issue): p. 305324. [Google Scholar]
- 78.Huang S.l., Chou LS, and Ni IH, Comparable length at weaning in cetaceans. Marine Mammal Science, 2009. 25(4): p. 875–887. [Google Scholar]
- 79.Kim S-K, et al. , Distribution of perfluorochemicals between sera and milk from the same mothers and implications for prenatal and postnatal exposures. Environmental Pollution, 2011. 159(1): p. 169–174. [DOI] [PubMed] [Google Scholar]
- 80.Lamichhane S, et al. , Exposure to per- and polyfluoroalkyl substances associates with an altered lipid composition of breast milk. Environment International, 2021. 157: p. 106855. [DOI] [PubMed] [Google Scholar]
- 81.Geng D, et al. , Effect of perfluorooctanesulfonic acid (PFOS) on the liver lipid metabolism of the developing chicken embryo. Ecotoxicology and Environmental Safety, 2019. 170: p. 691–698. [DOI] [PubMed] [Google Scholar]
- 82.Jacobsen AV, et al. , Effects of perfluorooctane sulfonate on genes controlling hepatic fatty acid metabolism in livers of chicken embryos. Environmental Science and Pollution Research, 2018. 25(23): p. 23074–23081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yeung LW, et al. , Differential expression of chicken hepatic genes responsive to PFOA and PFOS. Toxicology, 2007. 237(1–3): p. 111–25. [DOI] [PubMed] [Google Scholar]
- 84.Yoo H, et al. , Depuration kinetics and tissue disposition of PFOA and PFOS in white leghorn chickens (Gallus gallus) administered by subcutaneous implantation. Ecotoxicology and environmental safety, 2009. 72(1): p. 26–36. [DOI] [PubMed] [Google Scholar]
- 85.Loccisano AE, et al. , Evaluation and prediction of pharmacokinetics of PFOA and PFOS in the monkey and human using a PBPK model. Regulatory Toxicology and Pharmacology, 2011. 59(1): p. 157–175. [DOI] [PubMed] [Google Scholar]
- 86.Andersen ME, et al. , Pharmacokinetic modeling of saturable, renal resoption of perfluoroalkylacids in monkeys-Probing the determinants of long plasma half-lives. Toxicology, 2006. 227: p. 156. [DOI] [PubMed] [Google Scholar]
- 87.Tarazona JV, et al. , Toxicokinetics of perfluorooctane sulfonate in birds under environmentally realistic exposure conditions and development of a kinetic predictive model. Toxicol Lett, 2015. 232(2): p. 363–8. [DOI] [PubMed] [Google Scholar]
- 88.Vogs C, et al. , Toxicokinetics of Perfluorinated Alkyl Acids Influences Their Toxic Potency in the Zebrafish Embryo (Danio rerio). Environmental Science & Technology, 2019. 53(7): p. 3898–3907. [DOI] [PubMed] [Google Scholar]
- 89.Gaballah S, et al. , Evaluation of Developmental Toxicity, Developmental Neurotoxicity, and Tissue Dose in Zebrafish Exposed to GenX and Other PFAS. Environ Health Perspect, 2020. 128(4): p. 47005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wen W, et al. , Long-Chain Perfluoroalkyl acids (PFAAs) Affect the Bioconcentration and Tissue Distribution of Short-Chain PFAAs in Zebrafish (Danio rerio). Environ Sci Technol, 2017. 51(21): p. 12358–12368. [DOI] [PubMed] [Google Scholar]
- 91.Cheng W and Ng CA, A Permeability-Limited Physiologically Based Pharmacokinetic (PBPK) Model for Perfluorooctanoic acid (PFOA) in Male Rats. Environmental Science & Technology, 2017. 51(17): p. 9930–9939. [DOI] [PubMed] [Google Scholar]
- 92.Vidal A, et al. , Elucidating the fate of perfluorooctanoate sulfonate using a rainbow trout (Oncorhynchus mykiss) physiologically-based toxicokinetic model. Sci Total Environ, 2019. 691: p. 1297–1309. [DOI] [PubMed] [Google Scholar]
- 93.Ng CA and Hungerbuehler K, Exploring the Use of Molecular Docking to Identify Bioaccumulative Perfluorinated Alkyl Acids (PFAAs). Environmental Science & Technology, 2015. 49(20): p. 12306–12314. [DOI] [PubMed] [Google Scholar]
- 94.Jumper J, et al. , Highly accurate protein structure prediction with AlphaFold. Nature, 2021. 596(7873): p. 583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang J and Zhang Y, Protein Structure and Function Prediction Using I-TASSER. Current protocols in bioinformatics, 2015. 52: p. 5.8.1–5.8.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Custer CM, et al. , Perfluoroalkyl Contaminant Exposure and Effects in Tree Swallows Nesting at Clarks Marsh, Oscoda, Michigan, USA. Archives of environmental contamination and toxicology, 2019. 77(1): p. 1–13. [DOI] [PubMed] [Google Scholar]
- 97.Penland TN, et al. , Trophodynamics of Per- and Polyfluoroalkyl Substances in the Food Web of a Large Atlantic Slope River. Environ Sci Technol, 2020. 54(11): p. 6800–6811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.LaLone CA, et al. , Editor’s highlight: sequence alignment to predict across species susceptibility (SeqAPASS): a web-based tool for addressing the challenges of cross-species extrapolation of chemical toxicity. Toxicological Sciences, 2016. 153(2): p. 228–245. [DOI] [PubMed] [Google Scholar]
- 99.Washington JW, et al. , Nontargeted mass-spectral detection of chloroperfluoropolyether carboxylates in New Jersey soils. Science, 2020. 368(6495): p. 1103–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McCord J and Strynar M, Identification of Per- and Polyfluoroalkyl Substances in the Cape Fear River by High Resolution Mass Spectrometry and Nontargeted Screening. Environ Sci Technol, 2019. 53(9): p. 4717–4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kammerer BD, et al. , Physiological effects of salinity on Delta Smelt, Hypomesus transpacificus. Fish Physiol Biochem, 2016. 42(1): p. 219–32. [DOI] [PubMed] [Google Scholar]
- 102.James MO and Kleinow KM, Seasonal influences on PCB retention and biotransformation in fish. Environmental Science and Pollution Research, 2014. 21(10): p. 6324–6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Barron MG, Tarr BD, and Hayton WL, Temperature-dependence of cardiac output and regional blood flow in rainbow trout, Salmo gairdneri Richardson. Journal of Fish Biology, 1987. 31(6): p. 735–744. [Google Scholar]
- 104.Sabbir W, et al. , First report on reproductive features of the Hooghly croaker Panna heterolepis Trewavas, 1977 from the Bay of Bengal in relation to environmental factors. Environ Sci Pollut Res Int, 2021. [DOI] [PubMed] [Google Scholar]
- 105.Cañedo-Argüelles M, et al. , Saving freshwater from salts. Science, 2016. 351(6276): p. 914. [DOI] [PubMed] [Google Scholar]
- 106.Avellán-Llaguno RD, et al. , Elevated bioaccumulation of PFAAs in Oryzias melastigma following the increase of salinity is associated with the up-regulated expression of PFAA-binding proteins. Science of The Total Environment, 2020. 725: p. 138336. [DOI] [PubMed] [Google Scholar]
- 107.Jeon J, et al. , Bioconcentration of perfluorinated compounds in blackrock fish, Sebastes schlegeli, at different salinity levels. Environ Toxicol Chem, 2010. 29(11): p. 2529–35. [DOI] [PubMed] [Google Scholar]
- 108.Jeon J, et al. , Bioaccumulation of perfluorochemicals in pacific oyster under different salinity gradients. Environ. Sci. Technol, 2010. 44: p. 2695. [DOI] [PubMed] [Google Scholar]
- 109.Jeon J, et al. , Effects of salinity and organic matter on the partitioning of perfluoroalkyl acid (PFAs) to clay particles. Journal of Environmental Monitoring, 2011. 13(6): p. 1803–1810. [DOI] [PubMed] [Google Scholar]
- 110.Munoz G, Budzinski H, and Labadie P, Influence of environmental factors on the fate of legacy and emerging per-and polyfluoroalkyl substances along the salinity/turbidity gradient of a macrotidal estuary. Environmental science & technology, 2017. 51(21): p. 12347–12357. [DOI] [PubMed] [Google Scholar]
- 111.Vidal A, et al. , Does water temperature influence the distribution and elimination of perfluorinated substances in rainbow trout (Oncorhynchus mykiss)? Environmental Science and Pollution Research, 2019. 26(16): p. 16355–16365. [DOI] [PubMed] [Google Scholar]
- 112.Li H, Helm PA, and Metcalfe CD, Sampling in the Great Lakes for pharmaceuticals, personal care products, and endocrine-disrupting substances using the passive polar organic chemical integrative sampler. Environmental Toxicology and Chemistry, 2010. 29(4): p. 751–762. [DOI] [PubMed] [Google Scholar]
- 113.Ronconi R, et al. , Inter-annual variability in diet of non-breeding pelagic seabirds Puffinus spp. at migratory staging areas: evidence from stable isotopes and fatty acids. Marine Ecology Progress Series, 2010. 419: p. 267–282. [Google Scholar]
- 114.Tartu S, et al. , Diet and metabolic state are the main factors determining concentrations of perfluoroalkyl substances in female polar bears from Svalbard. Environmental Pollution, 2017. 229: p. 146–158. [DOI] [PubMed] [Google Scholar]
- 115.Aas CB, et al. , Effect of body condition on tissue distribution of perfluoroalkyl substances (PFASs) in Arctic fox (Vulpes lagopus). Environ Sci Technol, 2014. 48(19): p. 11654–11661. [DOI] [PubMed] [Google Scholar]
- 116.Bustnes JO, et al. , Perfluoroalkyl substance concentrations in a terrestrial raptor: relationships to environmental conditions and individual traits. Environmental Toxicology and Chemistry, 2015. 34(1): p. 184–91. [DOI] [PubMed] [Google Scholar]
- 117.Ecke F, et al. , Spatio-temporal variation of metals and organic contaminants in bank voles (Myodes glareolus). Sci Total Environ, 2020. 713: p. 136353. [DOI] [PubMed] [Google Scholar]
- 118.Bangma JT, et al. , Variation in perfluoroalkyl acids in the American alligator (Alligator mississippiensis) at Merritt Island National Wildlife Refuge. Chemosphere, 2017. 166: p. 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Valsecchi S, et al. , Perfluoroalkyl Substances (PFAS) in Fish from European Lakes: Current Contamination Status, Sources, and Perspectives for Monitoring. Environmental Toxicology and Chemistry, 2018: p. 1552–8618. [DOI] [PubMed] [Google Scholar]
- 120.Routti H, et al. , Emission Changes Dwarf the Influence of Feeding Habits on Temporal Trends of Per- and Polyfluoroalkyl Substances in Two Arctic Top Predators. Environ Sci Technol, 2017. 51(20): p. 11996–12006. [DOI] [PubMed] [Google Scholar]
- 121.Ortiz-Santaliestra ME, et al. , Accumulation of pollutants in nestlings of an endangered avian scavenger related to territory urbanization and physiological biomarkers. Environmental Pollution, 2019. 252: p. 1801–1809. [DOI] [PubMed] [Google Scholar]
- 122.Peng H, et al. , Tissue Distribution and Maternal Transfer of Poly- and Perfluorinated Compounds in Chinese Sturgeon (Acipenser sinensis): Implications for Reproductive Risk. Environmental Science & Technology, 2010. 44(5): p. 1868–1874. [DOI] [PubMed] [Google Scholar]
- 123.Houde M, et al. , Perfluoroalkyl compounds in relation to life-history and reproductive parameters in bottlenose dolphins (Tursiops truncatus) from Sarasota Bay, Florida, USA. Environ Toxicol Chem, 2006. 25(9): p. 2405–2412. [DOI] [PubMed] [Google Scholar]
- 124.Gebbink WA, et al. , Observation of emerging per- and polyfluoroalkyl substances (PFASs) in Greenland marine mammals. Chemosphere, 2016. 144: p. 2384–91. [DOI] [PubMed] [Google Scholar]
- 125.Bangma JT, et al. , Perfluorinated alkyl acids and fecundity assessment in striped mullet (Mugil cephalus) at Merritt Island national wildlife refuge. Science of The Total Environment, 2018. 619: p. 740–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Morganti M, et al. , Exposure assessment of PFAS-contaminated sites using avian eggs as a biomonitoring tool: a frame of reference and a case study in the Po River valley (Northern Italy). Integr Environ Assess Manag, 2021. [DOI] [PubMed] [Google Scholar]
- 127.Fromme H, et al. , Pre-and postnatal exposure to perfluorinated compounds (PFCs). Environmental science & technology, 2010. 44(18): p. 7123–7129. [DOI] [PubMed] [Google Scholar]
- 128.Lockley EC, et al. , Long-term survey of sea turtles (Caretta caretta) reveals correlations between parasite infection, feeding ecology, reproductive success and population dynamics. Sci Rep, 2020. 10(1): p. 18569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Palikova M, et al. , Proliferative kidney disease in rainbow trout (Oncorhynchus mykiss) under intensive breeding conditions: Pathogenesis and haematological and immune parameters. Vet Parasitol, 2017. 238: p. 5–16. [DOI] [PubMed] [Google Scholar]
- 130.Orth SR and Ritz E, The nephrotic syndrome. N Engl J Med, 1998. 338(17): p. 1202–11. [DOI] [PubMed] [Google Scholar]
- 131.Bangma JT, et al. , Tissue distribution of perfluoroalkyl acids and health status in wild Mozambique tilapia (Oreochromis mossambicus) from Loskop Dam, Mpumalanga, South Africa. Journal of Environmental Sciences, 2017. 61: p. 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kannan K, Perrotta E, and Thomas NJ, Association between Perfluorinated Compounds and Pathological Conditions in Southern Sea Otters. Environ Sci Technol, 2006. 40(16): p. 4943–4948. [DOI] [PubMed] [Google Scholar]
- 133.Aylward LL, et al. , Sources of Variability in Biomarker Concentrations. Journal of Toxicology and Environmental Health, Part B, 2014. 17(1): p. 45–61. [DOI] [PubMed] [Google Scholar]
- 134.Rothman KJ, Epidemiology: an introduction. 2012: Oxford university press. [Google Scholar]
- 135.Weisskopf MG and Webster TF, Trade-offs of Personal Versus More Proxy Exposure Measures in Environmental Epidemiology. Epidemiology, 2017. 28(5): p. 635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Blévin P, et al. , Perfluorinated substances and telomeres in an Arctic seabird: Cross-sectional and longitudinal approaches. Environmental Pollution, 2017. 230: p. 360–367. [DOI] [PubMed] [Google Scholar]
- 137.Sagiv SK, et al. , Early-Pregnancy Plasma Concentrations of Perfluoroalkyl Substances and Birth Outcomes in Project Viva: Confounded by Pregnancy Hemodynamics? American Journal of Epidemiology, 2017. 187(4): p. 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Verner MA, et al. , Associations of Perfluoroalkyl Substances (PFAS) with Lower Birth Weight: An Evaluation of Potential Confounding by Glomerular Filtration Rate Using a Physiologically Based Pharmacokinetic Model (PBPK). Environ Health Perspect, 2015. 123(12): p. 1317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sagiv SK, et al. , Early-Pregnancy Plasma Concentrations of Perfluoroalkyl Substances and Birth Outcomes in Project Viva: Confounded by Pregnancy Hemodynamics? American Journal of Epidemiology, 2018. 187(4): p. 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Savitz DA, Guest editorial: biomarkers of perfluorinated chemicals and birth weight. Environ Health Perspect, 2007. 115(11): p. A528–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Savitz DA and Wellenius GA, Invited Commentary: Exposure Biomarkers Indicate More Than Just Exposure. American Journal of Epidemiology, 2017. 187(4): p. 803–805. [DOI] [PubMed] [Google Scholar]
- 142.Braun JM, RE: “INVITED COMMENTARY: EXPOSURE BIOMARKERS INDICATE MORE THAN JUST EXPOSURE”. Am J Epidemiol, 2018. 187(4): p. 894–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Savitz DA and Wellenius GA, THE AUTHORS REPLY. American Journal of Epidemiology, 2018. 187(4): p. 896–896. [DOI] [PubMed] [Google Scholar]
- 144.Knox SS, et al. , Implications of Early Menopause in Women Exposed to Perfluorocarbons. The Journal of Clinical Endocrinology & Metabolism, 2011. 96(6): p. 1747–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dhingra R, et al. , A Study of Reverse Causation: Examining the Associations of Perfluorooctanoic Acid Serum Levels with Two Outcomes. Environ Health Perspect, 2017. 125(3): p. 416–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.