Abstract
Background:
Cabozantinib is active in advanced prostate cancer with improvement on bone scans in men on phase II trials. This trial evaluated the efficacy and changes in bone lesions in men with metastatic castration–resistant prostate cancer (mCRPC) treated with cabozantinib.
Methods:
Eligible patients with mCRPC involving bone underwent biopsy of a bone lesion followed by cabozantinib starting at 60 milligrams daily and continuing until progression or intolerable toxicity. The primary study end point was progression free survival (PFS) at 12 weeks. The bone lesion was rebiopsied at 6 weeks. Expression of CMET, phospho-CMET, and VEGFR2 was assayed by immunohistochemistry. Serum was obtained at baseline, and at 3, 6, and 12 weeks and assayed for bone remodeling markers.
Results:
25 patients were enrolled: 22 were evaluable, and 3 were excluded before receiving cabozantinib. At 12 weeks 17/22 (77%) had stable disease or better. Median time on treatment was 24 weeks (range 3–112). Overall median PFS was 43.7 weeks (95% CI 23.7–97.0). 8 of 22 (36%) had markedly reduced uptake on bone scan. Patients with significant response on bone scan had higher BMP–2 levels at baseline, stable NTx levels, increased VEGFR2 expression, and a trend towards increased phosphor-CMET while on cabozantinib compared to patients with stable disease.
Conclusions:
Cabozantinib is active in men with mCRPC, inducing significant changes on bone scan in one third of patients with changes in markers of bone formation and the tumor microenvironment.
Introduction
Bone metastases are a significant cause of morbidity and mortality in men with mCRPC.(1–3) Multiple signaling pathways have been implicated in osseous metastases and evidence suggests that the receptor tyrosine kinase MET and its ligand hepatocyte growth factor (HGF) play key roles.(4) Over-expression of MET independently predicts invasion and metastasis in prostate cancer and expression is more common in bone metastases.(4, 5) Moreover, increased serum levels of HGF are an independent prognostic marker in patients with advanced disease.(6, 7) Up-regulation of HGF and MET are associated with the transition to androgen-independent growth of prostate cancer.(8, 9) Androgen suppression activates MET in human prostate cancer cell lines transitioning them from paracrine to autocrine signaling, resulting in androgen-independent growth. MET expression is inversely correlated with androgen receptor expression in these cell lines. (9)
Cabozantinib is a small molecule which inhibits multiple receptor tyrosine kinases including MET, VEGFR2 and RET which play critical roles in angiogenesis and tumor cell proliferation, invasion, and metastasis.(10, 11) In a xenograft model of castration-resistant prostate cancer (CRPC), cabozantinib blocks the progression of osteolytic and osteoblastic lesions.(12, 13) A phase 2 randomized discontinuation trial with a large expansion cohort demonstrated that cabozantinib has significant activity with stabilization of disease in 75% of 171 of the patients enrolled.(14) In the population with bone metastases, 68% had improvement on bone scans including complete resolution of all uptake in 12%. A confirmatory phase 2 trial in men with mCRPC post-docetaxel demonstrated a similar rate of bone scan response (63%) with an additional 19% of patients having stable disease.(15)
Despite the activity on bone scan, there was no survival benefit in a phase 3 trial in men with heavily pretreated disease.(16) While men with advanced prostate cancer appear to have some clinical benefit from cabozantinib, it is possible that the bone scan changes do not reflect any anti-neoplastic effect. To explore this question, this phase 2 trial of cabozantinib used serum markers and bone biopsy to longitudinally investigate the effects of cabozantinib on bone metastases in men with mCRPC who had not yet received chemotherapy, abiraterone, or enzalutamide.
Patients and Methods
Standard eligibility and exclusion criteria for phase 2 trials in mCRPC were used with the modification that patients were required to have one bone lesion amenable to biopsy and no prior chemotherapy. The study protocol and informed consent document were reviewed and approved by the University of Michigan Institutional Review Board (IRBMed). Informed consent was obtained from all patients before any study-specified procedures were performed. The study was conducted under an investigator-IND held by the principal investigator (DCS) granted by the US Food and Drug Administration. The study was registered at ClinicalTrials.gov (NCT01428219).
Study Design
The primary study objective was to assess the efficacy of cabozantinib in patients with CRPC metastatic to bone. Secondary objectives included assessment of the toxicity, progression free survival, response proportion in soft tissue and bone disease, duration of response, PSA response, and time to progression. Correlative studies assessed the effect of cabozantinib on bone lesions measured as changes in markers of bone metabolism, changes in MET, AKT and VEGFR2 expression and phosphorylation, and changes in perfusion and diffusion magnetic resonance (MR) and computed tomography (CT) images in bone lesions during therapy. (Results of the MR studies are reported elsewhere).(17)
Baseline evaluation consisted of CT of the chest, abdomen, pelvis, bone scan, serum markers of bone metabolism, and diffusion/perfusion MRI with biopsy of a metastatic lesion. The initial 7 patients received doxycycline 100 mg daily 12–14 days and again 2–4 days prior to bone biopsy to assess bone histomorphometry. Patients then received cabozantinib 60 mg as a daily oral dose. At 6 weeks MRI and biopsy of the metastatic bone lesion were repeated. CT and bone scan were performed at 6 and 12 weeks and then every 12 weeks. Standard laboratories and physical examination were performed every 21 days during the initial 6 weeks and every 28 days thereafter. Sera for markers were obtained at 3, 6, and 12 weeks and then every 12 weeks while on study.
Correlative Studies
Bone Biopsy Immunohistochemistry:
Bone biopsy cores were immediately placed in buffered 10% formalin (Fisher Scientific, Waltham, MA) overnight at 4°C, rinsed with PBS then transferred to Immunocal (Decal Chemical Corporation, Tallman, NY) to decalcify for 1 to 2 hours. Samples were paraffin embedded, sectioned and stained with hematoxylin and eosin for immunohistochemistry. Antibodies used were: anti-cytokeratin CAM5.2 (BD349205; Becton-Dickinson. Franklin Lakes, NJ); anti-CMET (Cat# 51067; Abcam, Cambridge, MA), anti-pCMET (Cat# 44–888G; Invitrogen, Carlsbad, CA) and anti-VEGFR2/Flk-1 (SC-504; Santa Cruz Biotechnologies, Santa Cruz, CA) at 1:20, 1:200, 1:50, and 1:150 respectively. Antigen retrieval was performed with Diva Decloaker (DV2004, Biocare Medical, Concord, CA) for CMET and pCMET, and with Reveal (RV1000, Biocare Medical) for VEGFR2. Antibodies were optimized by titration using prostate cancer tissues. Results are presented as the expression index (EI) which is the product of the staining intensity x the percent positive cells.
ELISA for Bone Metabolic Markers:
Serum samples were collected and stored at −80°C. ELISA assays were performed as recommended by the manufacturer for: Bone Alkaline Phosphatase (BAP) (Quidel, San Diego, CA), bone morphogenic protein–2 (BMP-2) (DBP200, R&D Systems, Minneapolis, MN), tartrate–resistant acid phosphatase type 5b (TRAcP) (SB-TR201R, IDS, Tyne & Wear, United Kingdom), type I C-terminal collagen propeptide (CICP) (Quidel), N-telopeptides (NTx) (Osteomark serum kit X9021, Alere, Waltham, MA ), Intact Osteocalcin (Quidel), and sclerostin (SOST)(R&D). All assays were performed at room temperature and results were read using a fluorescent microplate reader.
Statistical Considerations
This study was designed as a two-stage Simon’s Mini-max trial permitting the early termination of patient entry after the first 27 evaluable patients if results were unfavorable, with a total accrual of 46 evaluable patients planned. This treatment would not be considered of interest if the progression-free proportion at 12 weeks was < 0.45, and it would be of definite clinical interest if this proportion was > 0.65. The 2-stage design had a 5% type I error and 85% power at these levels of interest. Progression was defined using a composite end point of PSA along with evidence of progression on bone scan and/or CT scans of soft tissue lesions consistent with the Prostate Cancer Working Group 2 criteria.(18) In the case of progression on bone scan or CT scan the date of progression was defined as the date of the first scan that showed progression. Although PSA was assessed, it was not sufficient to determine progression in the absence of bone scan, CT or other clinical criteria.
The count and proportion of patients who were progression-free at 12 weeks was reported. Progression-free survival and time to PSA progression were reported with Kaplan-Meier estimates. Serum markers and IHC markers were summarized at each time point of collection with means and standard deviations. Difference in markers between treatment response groups were tested using t-tests or signed rank tests if the distribution warranted. Multiple comparisons were not used in these hypotheses-generating correlative analyses.
Results:
Patients
Twenty-five patients with mCRPC were enrolled. Two were excluded for rapid disease progression prior to start of therapy, one had a comorbidity arise between enrollment and the start of treatment rendering him ineligible, leaving 22 evaluable men who took at least one dose of cabozantinib. Baseline characteristics are listed in Supplemental Table 1. All patients had bone involvement with the majority having diffuse skeletal disease (13/22 with >10 lesions). Seven patients had measureable soft tissue disease; 4 liver, 2 pelvic soft tissue masses, and one nodal disease.
Efficacy
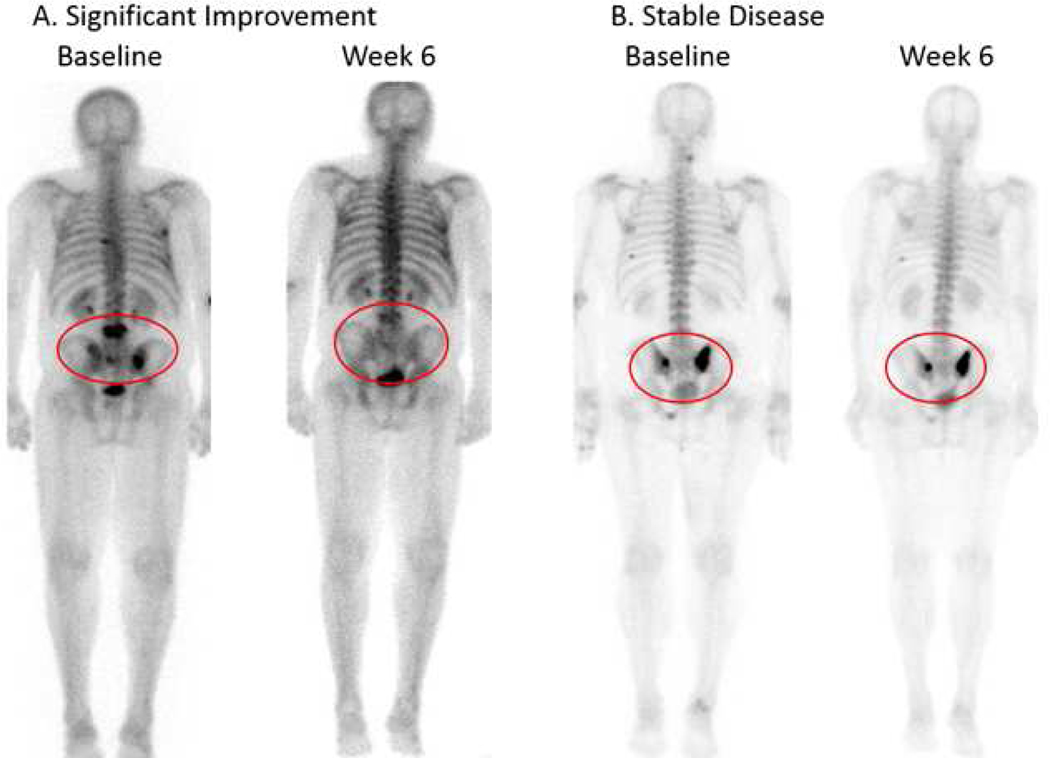
The primary efficacy endpoint was progression–free survival (PFS) at 12 weeks; 17/22 (77%, 95% confidence interval (CI) 54–92%) remained on study with stable disease or better at 12 weeks. The Kaplan-Meier estimate of PFS at 12 weeks (patients censored at time of drug discontinuation if stopped because of toxicity) was 90% (95% CI 64% – 97%) (Supplemental Figure 1), with a median overall PFS of 43.7 weeks (95% CI 23.7–97.0). One patient stopped therapy at week 3 due to a grade 4 event (trans-ischemic attack) and 4 stopped at 9 weeks (1 with progression and 3 with adverse events (AE)). Of the 3 who stopped at week 9 for AE, 2 had stable disease at 12 weeks imaging and one was unevaluable. None had evidence of progression on bone scan at 12 weeks and 8/22 (36%) had significant improvement with markedly decreased uptake, all on their first follow-up scan at 6 weeks. (Figure 1) The PSA progression-free rate at 12 weeks was 36% with a partial response rate of 18%. Four patients had a decline in PSA ≥50%, but most remained stable or had rising PSA while on cabozantinib. Therapy was discontinued due to progression (41%), adverse events (50%) or patient/physician discretion (9%). The median time on treatment was 24 weeks (range 3–112 weeks). At the time of analysis, all patients had completed therapy.
Figure 1:
Bone scan response. Signficant improvement vs stable disease
Safety
All 22 patients who received cabozantinib were evaluable for toxicity. All patients had at least one treatment-associated AE. Treatment-related AEs of any grade in over 10% of patients are summarized in Table 1. The most common all-grade AE were anorexia, diarrhea, liver function test abnormalities, dysgeusia, fatigue, and weight loss. The most common grade 3 AE were thromboembolic events, diarrhea or lipase elevation. No grade 4/5 toxicities were observed. These AE typically resolved rapidly with dose reduction or discontinuation. Only 50% (11/22) at 6 weeks and 4/22 patients (18%) at 12 weeks remained on the 60 mg daily dose. There were no significant toxicities associated with doxycycline used for bone labelling.
Table 1:
Treatment Related Adverse Events Occurring in > 10% of Patients
| Adverse Event | All grades: N (%) | Grade 3: N (%) |
|---|---|---|
| Diarrhea | 16 (73) | 3 (14) |
| Increased alanine aminotransferase (ALT) | 15 (68) | 0 |
| Increased aspartate aminotransferase (AST) | 15 (68) | 0 |
| Weight loss | 11 (50) | 1 (5) |
| Palmar plantar erythrodysesthesia | 10 (45) | 1 (5) |
| Nausea | 10 (45) | 0 |
| Anemia | 10 (45) | 0 |
| Proteinuria | 9 (40) | 0 |
| Thrombocytopenia | 7 (32) | 0 |
| Vomiting | 7 (32) | 0 |
| Constipation | 6 (27) | 0 |
| Mouth pain/mucositis | 6 (27) | 0 |
| Decreased white blood cells | 5 (23) | 0 |
| Hypertension | 5 (23) | 0 |
| Venous thromboembolism | 5 (23) | 5 (23) |
| Gastroesophageal reflux | 4 (18) | 0 |
| Dry mouth | 4 (18) | 0 |
| Fatigue | 4 (18) | 0 |
| Increased alkaline phosphatase | 4 (18) | 0 |
| Muscle weakness | 4 (18) | 0 |
| Hematuria | 4 (18) | 0 |
| Hypothyroidism | 3 (14) | 0 |
| Abdominal pain | 3 (14) | 0 |
| Increased amylase | 3 (14) | 1 (5) |
| Post nasal drip | 3 (14) | 0 |
| Sore throat | 3 (14) | 0 |
Correlative Studies
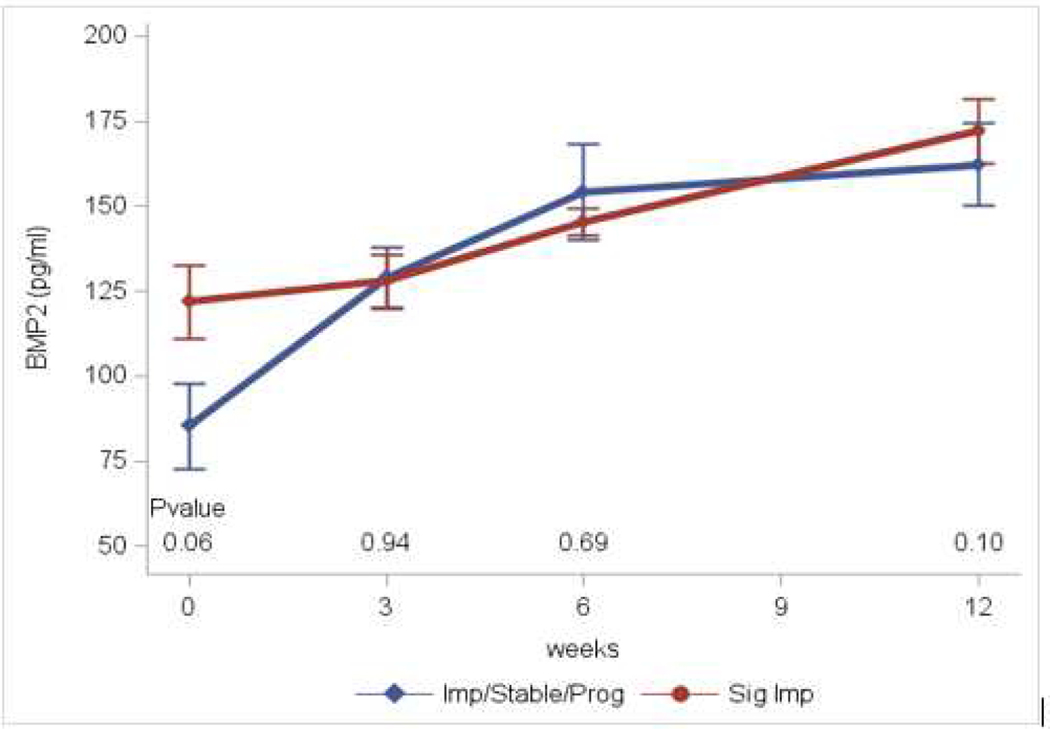
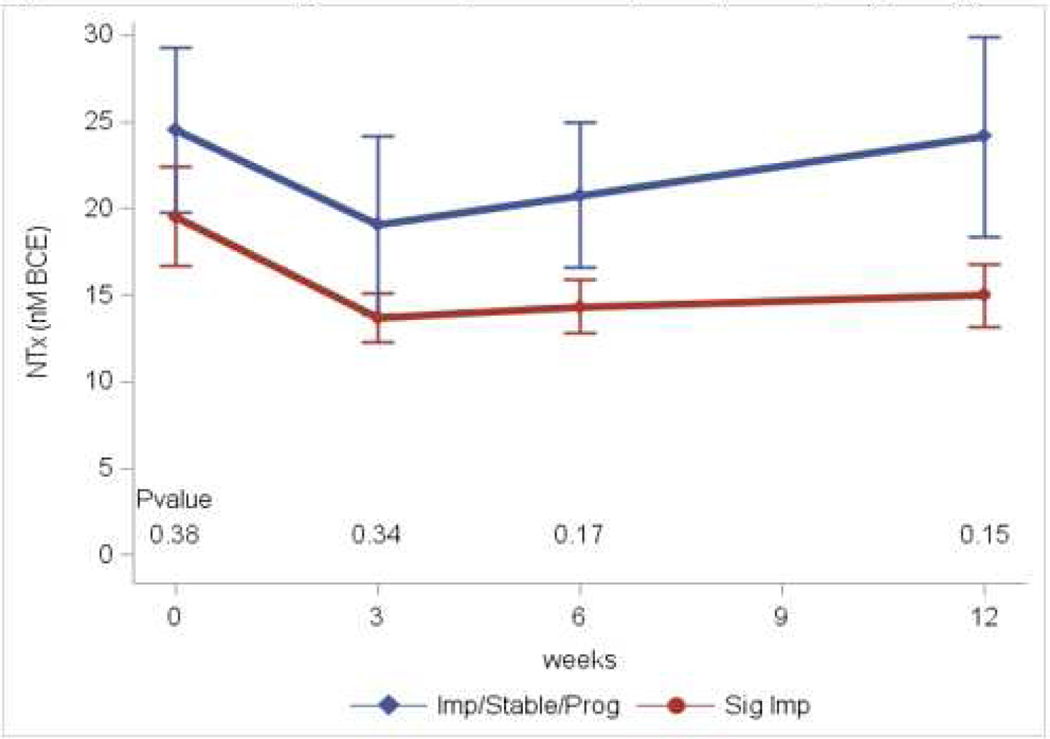
Results of serum markers of bone metabolism obtained at baseline, and 3, 6, and 12 weeks are in Table 2. These assays included markers of bone formation (osteocalcin, CICP, BAP), bone resorption (NTx, TRAcP), osteoblastogenesis (BMP-2) and inhibition of osteoblastogenesis (SOST). Across the entire population, the markers indicate a decrease in both bone formation and resorption. Two markers of bone formation (osteocalcin and CICP) were initially significantly decreased when compared to baseline. BAP initially rose then fell back to baseline, while SOST levels displayed an inverse pattern. NTx levels were significantly decreased indicating inhibition of bone resorption. TRAcP levels also decreased although this was not significant. BMP-2 levels were significantly increased at all three time points suggesting an increase in osteoblastogenesis. In all markers, the initial effects decreased over time corresponding to the decreasing dose of cabozantinib as the patients progressed on study. Comparing patients who had significant response on bone scan to those with minimal improvement/stable disease, two markers showed appreciable differences. Baseline BMP-2 was higher in those who had a significant bone scan response, while the patients with minimal response/stable disease had greater increases in this marker over time (Figure 2A). NTx levels were decreased and remained suppressed in those with a significant response, but decreased transiently then returned to baseline in those with minimal response/stable disease (Figure 2B).
Table 2:
Serum Markers of Bone Metabolism
| Marker | Week 0 (N=22) | Week 3 (N=22) | Week 6 (N=21) | Week 12 (N=20) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (Std) | Mean (Std) | Difference from Baseline | Mean (Std) | Difference from Baseline | Mean (Std) | Difference from Baseline | ||||
| Mean (Std) | p-value | Mean (Std) | p-value | Mean (Std) | p-value | |||||
| Osteocalcin | 9.2 (6.7) | 5.9 (3.5) | −3.3 (4.6) | <0.0001^ | 5.5 (2.9) | −3.8 (5.6) | 0.0002^ | 5.5 (2.6) | −3.3 (5.8) | 0.002^ |
| NTx | 22.3 (14.1) | 16.7 (14.6) | −5.4 (5.5) | <0.0001^ | 18.3 (12.5) | −4.3 (8.8) | 0.006^ | 20.5 (16.3) | 0.5 (15.4) | 0.11^ |
| TRAcP | 4.8 (3.3) | 3.9 (2.3) | −0.9 (1.9) | 0.04 | 4 (2.5) | −0.9 (2.2) | 0.09 | 4.4 (2.7) | −0.1 (2.4) | 0.86 |
| BMP2 | 101.2 (43.5) | 129.2 (28.3) | 28.0 (36.5) | 0.0017 | 150.7 (40.2) | 51.5 (46.4) | <0.0001 | 166.2 (36) | 65.5 (49.1) | <0.0001 |
| SOST | 324 (108.3) | 275 (76.2) | −49.1 (85.1) | 0.01 | 362.8 (162.5) | 34.2 (111.1) | 0.17 | 291.7 (104.6) | −29.8 (94.5) | 0.17 |
| BAP | 58.8 (65.2) | 68.9 (60.0) | 10.1 (29.7) | 0.13 | 61.5 (54.3) | 0.6 (32.6) | 0.94 | 50.9 (47.4) | −1.36 (56.6) | 0.92 |
| CICP | 149 (72.3) | 126.8 (61.3) | −22.1 (54.7) | 0.07 | 116.9 (54.9) | −32.9 (53.8) | 0.01 | 146 (113.8) | 4.8 (80.6) | 0.80 |
Figure 2.
A: BMP-2 levels. Significant responders vs Improved/Stable/Progressing
B: NTx levels. Significant responders vs Improved/Stable/Progressing
Bone biopsies were obtained at baseline (N=22) and after 6 weeks of treatment (N=20). In the first 7 patients, these were processed for histomorphometry, but crush artifact prohibited assessment and no further attempts for this analysis were made. After processing, prostate cancer was identified in 31 of 42 (74%) biopsy specimens. Of these, 30 had sufficient tumor for IHC with 24 paired specimens for comparison of pre- and post-treatment biopsies in 12 patients. Results are shown in Table 3 and Supplemental Figure 2. There was no significant change in mean levels of CMET, pCMET, or VEGFR2 after 6 weeks of therapy, although individual patients did have substantial changes. Comparing significant responders on bone scan to minimal and nonresponders there are statistically significant higher VEGFR2 levels (p=0.009) and a trend toward increased levels of pCMET in the responders while on cabozantinib in this small sample.
Table 3:
Immunohistochemistry Markers
| IHC Marker | Time point | Sig Improvement On Bone Scan Mean (Std) | Imp/Stable/Prog On Bone Scan Mean (Std) | p-value |
|---|---|---|---|---|
| cMet | Baseline | 132.0 (113.9) N=5 | 99.3 (98.9) N=7 | 0.61 |
| Week 6 | 110.0 (104.3) N=6 | 91.3 (89.2) N=8 | 0.71 | |
| Difference | −2.0 (114.5) N=5 | 5 (98.0) N=7 | 0.91 | |
| pcMet | Baseline | 125.0 (95.7) N=5 | 156.7 (102.9) N=6 | 0.29 |
| Week 6 | 156.7 (102.9) N=6 | 136.3 (68.2) N=8 | 0.66 | |
| Difference | 31.0 (109.3) N=5 | −35.7 (85.0) N=7 | 0.26 | |
| pcMet/cMet | Baseline | 5.3 (8.9) N=5 | 42.3 (88.9) N=7 | 0.32 |
| Week 6 | 4.7 (6.1) N=6 | 13.1 (24.0) N=8 | 0.37 | |
| Difference | −2.9 (6.1) N=5 | −37.4 (82.0) N=7 | 0.31 | |
| VEGFR2 | Baseline | 104.0 (65.4) N=5 | 86.7 (65.3) N=6 | 0.67 |
| Week 6 | 146.7 (56.1) N=6 | 51.3 (56.7) N=8 | 0.0009 | |
| Difference | 32.0 (95.5) N=5 | −30.0 (80.0) N=6 | 0.27 |
N=number of patients with tumor in specimen at given timepoint with staining by IHC
Discussion
As in prior phase II trials, this trial demonstrated that cabozantinib has a high level of activity, with three quarters of the men having stable disease or better at 12 weeks and 8 of the 22 evaluable patients had evidence of significant improvement in bone scan. The median progression free survival was 43.7 weeks. This compares favorably with the prior studies reflecting the population enrolled which had not received prior docetaxel or newer agents targeting the androgen signaling pathway. Also similar to prior studies, toxicities limited drug dosing and resulted in discontinuation of therapy in 50% of men.
In vitro and in vivo studies of cabozantinib show inhibition of prostate cancer growth in bone and changes in bone remodeling although the mechanism is not clear.(12, 13, 19) Alterations in the tumor microenvironment result in a reduction in osteoclasts and increase in osteoblasts mediated, in part, through a reduction in rank ligand (RANKL) expression. (19–21) A patient–derived xenograft (PDX) model showed consistent inhibition of bone formation with cabozantinib associated with inhibition of osteoblast proliferation and induction of differentiation.(22) A conflicting study suggested that cabozantinib induced osteoblast differentiation and secretion of proteins promoting prostate cancer survival and migration in bone.(23)
Clinical assays for markers of bone metabolism have shown mixed results. One study showed decreased alkaline phosphatase and urinary NTx, while a second showed an increase in BAP with a decrease in TRAcP.(22, 24) BAP was variable in our patients with an initial rise followed by a fall back to baseline, possibly due to the decreasing doses of cabozantanib while on treatment. Overall, the serum markers suggest that cabozantinib inhibits both bone resorption and bone formation while promoting osteoblastogenesis. Subjects with lower levels of BMP–2 appeared to have less response to cabozantinib suggesting that ongoing bone remodeling may be a prerequisite to response. Alternatively, in the context of bone metastasis, BMP–2 may be a more general indicator of disease activity as lower BMP–2 mRNA expression is associated with worse overall survival after radical nephrectomy for renal cell carcinoma.(25)
We attempted to assay ratios of CMET, pCMET, and VEGFR2 in sequential bone biopsies using immunohistochemistry to assess changes in tumor microenvironment. Overall, we were successful in obtaining sequential biopsies although the amount of viable tumor and variability of protein assays impacted the results. No clear signal emerged suggesting correlation with the target inhibition and response, although bone responders did have significantly increased levels of VEGFR2 expression. The explanation for this is not clear as it is inconsistent with plasma assays showing inhibition of VEGFR2 levels(24) and the PDX model which showed a marked drop in VEGFR2 immunofluorescence with exposure to cabozantinib.(22)
Despite the substantial activity demonstrated in men with prostate cancer cabozantinib failed to show a survival advantage in a phase 3 trial.(16) The study population (advanced disease post–chemotherapy), dose, and schedule may have played a role. While lower doses have shown activity there was increased activity with higher doses in the phase 2 nonrandomized extension study. (15, 26) Despite increased toxicity at the higher dose, virtually every measure of efficacy favored 100 mg compared to 40 mg daily. At the 60 mg daily dose the majority of men (88%) on the phase 3 trial required dose reduction or interruption,(16) and few men enrolled on this trial were able to maintain the 60 mg daily dose for the initial evaluation period due to toxicity, despite the fact that they were presumably healthier than the phase 3 population. Although the data from the PDX model suggests that continuous dosing is required for maximal tumor control,(22) a potential clinical strategy to match efficacy and tolerance would be to provide high doses on an intermittent schedule as is done with sunitinib in kidney cancer where there is no difference in efficacy with continuous versus intermittent dosing on a variety of schedules with reduced toxicity with intermittent dosing.(27–29)
This single-institution study has a number of limitations including small sample size, nonrandomized population, moderate success with tumor retrieval on bone biopsies, inter-assay variability on immunohistochemistry, and possible batch effects on serum assays. The challenge of sequential bone biopsies and the suspension of the cabozantinib development program limited accrual. Nevertheless, the results from these assays serve to generate hypotheses which can be tested in additional studies and may be generalizable to bone lesions in other bone-tropic malignancies (kidney and thyroid cancer) for which cabozantinib is approved.
This study confirms the clinical activity and substantial toxicities of cabozantinib in men with mCRPC, demonstrating that bone remodeling is altered, and suggests that responders may be identified by elevated baseline levels of BMP–2 and with a subsequent increase in VEGR2 expression in tumor specimens. Although further development of cabozantinib in prostate cancer is currently on hold, this level of activity warrants further evaluation to determine optimal doses and schedules with exploration of predictive biomarkers.
Supplementary Material
Ackowledgements
Supported in part by grants from the National Institutes of Health (NIHP50-CA186786, NIH P01-CA1093900, and NIH P30-CA046592) and Exelixis.
Dr. Grivas has served as a consultant for Exelixis. Dr. Smith and Dr. Keller report that research at their institution (UM) has been partially funded by Exelixis. Dr. Smith and Dr. Hussain are listed as holders but receive no royalties for patents 11764665.4-1464 and 11764656.2-1464 held by Exelixis.
Footnotes
ClinicalTrials.gov Identifier: NCT01428219
Conflict of Interest
No other potential conflicts of interest were disclosed by the other authors
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Norgaard M, Jensen AO, Jacobsen JB, Cetin K, Fryzek JP, Sorensen HT. Skeletal related events, bone metastasis and survival of prostate cancer: a population based cohort study in Denmark (1999 to 2007). J Urol. 2010;184(1):162–7. [DOI] [PubMed] [Google Scholar]
- 2.Costa L, Badia X, Chow E, Lipton A, Wardley A. Impact of skeletal complications on patients’ quality of life, mobility, and functional independence. Support Care Cancer. 2008;16(8):879–89. [DOI] [PubMed] [Google Scholar]
- 3.Bubendorf L, Schopfer A, Wagner U, Sauter G, Moch H, Willi N, et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31(5):578–83. [DOI] [PubMed] [Google Scholar]
- 4.Humphrey PA, Zhu X, Zarnegar R, Swanson PE, Ratliff TL, Vollmer RT, et al. Hepatocyte growth factor and its receptor (c-MET) in prostatic carcinoma. Am J Pathol. 1995;147(2):386–96. [PMC free article] [PubMed] [Google Scholar]
- 5.Knudsen BS, Edlund M. Prostate cancer and the met hepatocyte growth factor receptor. Adv Cancer Res. 2004;91:31–67. [DOI] [PubMed] [Google Scholar]
- 6.Gupta A, Karakiewicz PI, Roehrborn CG, Lotan Y, Zlotta AR, Shariat SF. Predictive value of plasma hepatocyte growth factor/scatter factor levels in patients with clinically localized prostate cancer. Clin Cancer Res. 2008;14(22):7385–90. [DOI] [PubMed] [Google Scholar]
- 7.Yasuda K, Nagakawa O, Akashi T, Fujiuchi Y, Koizumi K, Komiya A, et al. Serum active hepatocyte growth factor (AHGF) in benign prostatic disease and prostate cancer. Prostate. 2009;69(4):346–51. [DOI] [PubMed] [Google Scholar]
- 8.Maeda A, Nakashiro K, Hara S, Sasaki T, Miwa Y, Tanji N, et al. Inactivation of AR activates HGF/c-Met system in human prostatic carcinoma cells. Biochem Biophys Res Commun. 2006;347(4):1158–65. [DOI] [PubMed] [Google Scholar]
- 9.Verras M, Lee J, Xue H, Li TH, Wang Y, Sun Z. The androgen receptor negatively regulates the expression of c-Met: implications for a novel mechanism of prostate cancer progression. Cancer Res. 2007;67(3):967–75. [DOI] [PubMed] [Google Scholar]
- 10.Yakes FM, Chen J, Tan J, Yamaguchi K, Shi Y, Yu P, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther. 2011;10(12):2298–308. [DOI] [PubMed] [Google Scholar]
- 11.You WK, Sennino B, Williamson CW, Falcon B, Hashizume H, Yao LC, et al. VEGF and c-Met blockade amplify angiogenesis inhibition in pancreatic islet cancer. Cancer Res. 2011;71(14):4758–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai J, Zhang H, Karatsinides A, Keller JM, Kozloff KM, Aftab DT, et al. Cabozantinib inhibits prostate cancer growth and prevents tumor-induced bone lesions. Clin Cancer Res. 2014;20(3):617–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen HM, Ruppender N, Zhang XT, Brown LG, Gross TS, Morrissey C, et al. Cabozantinib Inhibits Growth of Androgen-Sensitive and Castration-Resistant Prostate Cancer and Affects Bone Remodeling. Plos One. 2013;8(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith DC, Smith MR, Sweeney C, Elfiky AA, Logothetis C, Corn PG, et al. Cabozantinib in patients with advanced prostate cancer: results of a phase II randomized discontinuation trial. J Clin Oncol. 2013;31(4):412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith MR, Sweeney CJ, Corn PG, Rathkopf DE, Smith DC, Hussain M, et al. Cabozantinib in chemotherapy-pretreated metastatic castration-resistant prostate cancer: results of a phase II nonrandomized expansion study. J Clin Oncol. 2014;32(30):3391–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith M, De Bono J, Sternberg C, Le Moulec S, Oudard S, De Giorgi U, et al. Phase III Study of Cabozantinib in Previously Treated Metastatic Castration-Resistant Prostate Cancer: COMET-1. J Clin Oncol. 2016;34(25):3005–13. [DOI] [PubMed] [Google Scholar]
- 17.Hoff BA, Brisset JC, Galban S, Van Dort M, Smith DC, Reichert ZR, et al. Multimodal imaging provides insight into targeted therapy response in metastatic prostate cancer to the bone. Am J Nucl Med Mol Imaging. 2018;8(3):189–99. [PMC free article] [PubMed] [Google Scholar]
- 18.Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26(7):1148–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee C, Whang YM, Campbell P, Mulcrone PL, Elefteriou F, Cho SW, et al. Dual targeting c-met and VEGFR2 in osteoblasts suppresses growth and osteolysis of prostate cancer bone metastasis. Cancer Lett. 2018;414:205–13. [DOI] [PubMed] [Google Scholar]
- 20.Stern PH, Alvares K. Antitumor agent cabozantinib decreases RANKL expression in osteoblastic cells and inhibits osteoclastogenesis and PTHrP-stimulated bone resorption. J Cell Biochem. 2014;115(11):2033–8. [DOI] [PubMed] [Google Scholar]
- 21.Haider MT, Hunter KD, Robinson SP, Graham TJ, Corey E, Dear TN, et al. Rapid modification of the bone microenvironment following short-term treatment with Cabozantinib in vivo. Bone. 2015;81:581–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varkaris A, Corn PG, Parikh NU, Efstathiou E, Song JH, Lee YC, et al. Integrating Murine and Clinical Trials with Cabozantinib to Understand Roles of MET and VEGFR2 as Targets for Growth Inhibition of Prostate Cancer. Clin Cancer Res. 2016;22(1):107–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu KJ, Li JK, Lee YC, Yu G, Lin SC, Pan T, et al. Cabozantinib-induced osteoblast secretome promotes survival and migration of metastatic prostate cancer cells in bone. Oncotarget. 2017;8(43):74987–5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leibowitz-Amit R, Pintilie M, Khoja L, Azad AA, Berger R, Laird AD, et al. Changes in plasma biomarkers following treatment with cabozantinib in metastatic castration-resistant prostate cancer: a post hoc analysis of an extension cohort of a phase II trial. Journal of Translational Medicine. 2016;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitsui Y, Hirata H, Arichi N, Hiraki M, Yasumoto H, Chang I, et al. Inactivation of bone morphogenetic protein 2 may predict clinical outcome and poor overall survival for renal cell carcinoma through epigenetic pathways. Oncotarget. 2015;6(11):9577–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee RJ, Saylor PJ, Michaelson MD, Rothenberg SM, Smas ME, Miyamoto DT, et al. A dose-ranging study of cabozantinib in men with castration-resistant prostate cancer and bone metastases. Clin Cancer Res. 2013;19(11):3088–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jonasch E, Slack RS, Geynisman DM, Hasanov E, Milowsky MI, Rathmell WK, et al. Phase II Study of Two Weeks on, One Week off Sunitinib Scheduling in Patients With Metastatic Renal Cell Carcinoma. J Clin Oncol. 2018:JCO2017771485. [DOI] [PMC free article] [PubMed]
- 28.Motzer RJ, Hutson TE, Olsen MR, Hudes GR, Burke JM, Edenfield WJ, et al. Randomized phase II trial of sunitinib on an intermittent versus continuous dosing schedule as first-line therapy for advanced renal cell carcinoma. J Clin Oncol. 2012;30(12):1371–7. [DOI] [PubMed] [Google Scholar]
- 29.Motzer RJ, Michaelson MD, Redman BG, Hudes GR, Wilding G, Figlin RA, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24(1):16–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.