Abstract
There has been limited data presented to characterize and quantify breakthrough SARS-CoV-2 infections, hospitalizations, and mortality in vaccinated patients with hematologic malignancies (HM). We performed a retrospective cohort study of patient electronic health records of 514,413 fully vaccinated patients from 63 healthcare organizations in the US, including 5956 with HM and 508,457 without malignancies during the period from December 2020 to October 2021. The breakthrough SARS-CoV-2 infections in patients with HM steadily increased and reached 67.7 cases per 1000 persons in October 2021. The cumulative risk of breakthrough infections during the period in patients with HM was 13.4%, ranging from 11.0% for acute lymphocytic leukemia to 17.2% and 17.4% for multiple myeloma and chronic myeloid leukemia respectively, all higher than the risk of 4.5% in patients without malignancies (p < 0.001). No significant racial disparities in breakthrough infections were observed. The overall hospitalization risk was 37.8% for patients with HM who had breakthrough infections, significantly higher than 2.2% for those who had no breakthrough infections (hazard ratio or HR: 34.49, 95% CI: 25.93–45.87). The overall mortality risk was 5.7% for patients with HM who had breakthrough infections, significantly higher than the 0.8% for those who had no breakthrough infections (HR: 10.25, 95% CI: 5.94–17.69). In summary, this study shows that among the fully vaccinated population, patients with HM had significantly higher risk for breakthrough infections compared to patients without cancer and that breakthrough infections in patients with HM were associated with significant clinical outcomes including hospitalizations and mortality.
Keywords: COVID-19, SARS-CoV-2, Hematologic malignancies, Vaccine, Breakthrough infections, Health outcomes
1. Introduction
Data from early in the pandemic, when vaccines were not available, showed that patients with hematologic malignancies (HM) were at increased risk for COVID-19 infection and severe outcomes [1,2]. In the United States, three COVID-19 vaccines have been authorized since December 2020 [[3], [4], [5]]. Vaccines are effective; however, breakthrough infections have been recorded [[6], [7], [8], [9], [10]]. Patients with HM have been shown to have impaired antibody response to COVID-19 vaccines [[11], [12], [13], [14], [15]]. While neutralizing antibody levels potentially correlate with protection against SARS-CoV-2 infections [16], it remains unknown how and in what degree low rate of seroconversion in patients with HM allows significant breakthrough infections and adverse outcomes. Currently, there has been little real-world data presented to systemically characterize and quantify breakthrough SARS-CoV-2 infections in fully vaccinated patients with HM and to identify which patients with HM are more vulnerable to breakthrough infections and severe outcomes. We recently reported a significantly increased breakthrough SARS-CoV-2 infection in immunized patients with multiple myeloma compared to patients without cancer [10]. We have now conducted these studies for HM, as a group, and separately for seven specific types of HM including acute myeloid leukemia (AML), chronic myeloid leukemia (CML), acute lymphocytic leukemia (ALL), chronic lymphocytic leukemia (CLL), Hodgkin lymphoma (HL), Non-Hodgkin lymphoma (NHL) and multiple myeloma (MM) through a retrospective cohort study of a large, geographically diverse real-time database of patient electronic health records (EHRs) in the US. We characterized and quantified (1) how the rates of new cases of breakthrough SARS-CoV-2 infections evolved over time from December 2020 to October 2021 in the vaccinated population with HM and the vaccinated population without cancer, (2) the cumulative risks of breakthrough infections from December 2020 to October 2021 in patients with all HM, each of the seven specific HM, and patients without cancer, stratified by race/ethnicity, (3) overall risks of hospitalization and mortality in the vaccinated HM population with and without breakthrough infections.
2. Methods
2.1. Database description
We used the TriNetX Analytics Network Platform that allows access to fully de-identified data of 84.5 million unique patients from 63 health care organizations of inpatient and outpatient settings in US [17]. TriNetX Analytics provides web-based, real-time, secure access to patient EHR data from hospitals, primary care, and specialty treatment providers, covering diverse geographic, age, race/ethnic, income and insurance groups. Though the data are de-identified, end-users can use TriNetX Analytics built-in statistical and informatics functions to work on patient-level data for cohort selection, propensity-score matching, analyzing incidence and prevalence of events in a cohort, and comparing characteristics and outcomes between matched cohorts. Because this study used only de-identified patient records and did not involve the collection, use, or transmittal of individually identifiable data, this study was exempted from Institutional Review Board approval.
2.2. Study population
The study population comprised 5956 patients with a diagnosis of HM and 508,457 fully vaccinated patients without malignancies who had fulfilled the following inclusion criteria: had documented evidence of full vaccination in the EHRs (received two doses of Pfizer-BioNTech or Moderna mRNA vaccine, or single dose of Johnson & Johnson (J&J) vaccine) between December 2020 and October 2021, and had not contracted SARS-CoV-2 infection prior to full vaccination. The latter criteria ensured that SARS-CoV-2 infections that occurred in this study population were vaccine breakthrough infections. Among 5956 fully vaccinated patients with HM, 460 patients had a diagnosis of AML, 287 of CML, 319 of ALL, 984 of CLL, 448 of HL, 2723 of NHL and 1186 of MM. Other types of HM were not examined due to their small sample sizes. Names and codes to determine the status of SARS-Cov-2 infections, vaccinations, diagnoses of HM, hospitalizations, and death from patient EHRs are described in Table 1 .
Table 1.
Status, names and codes for disease diagnosis, lab tests, and procedures.
| Status | Names and codes for disease diagnosis (ICD-10), lab tests, and procedures |
|---|---|
| SARS-CoV-2 infection | SARS coronavirus 2 and related RNA” (TNX:LAB:9088) |
| COVID-19″ (U07.1) | |
| COVID-19 vaccination | Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 30 μg/0.3 mL dosage, diluent reconstituted; second dose (0002A), by Pfizer |
| Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 100 μg/0.5 mL dosage; second dose (0012A), by Moderna | |
| Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, DNA, spike protein, adenovirus type 26 (Ad26) vector, preservative free, 5 × 10^10 viral particles/0.5 mL dosage, single dose (0031A), by Johnson & Johnson | |
| HM | Malignant neoplasms of lymphoid, hematopoietic and related tissue (ICD-10 code: C81-C96) |
| AML | Acute myeloblastic leukemia (ICD-10 code: C92.0) |
| Acute promyelocytic leukemia (ICD-10 code: C92.4) | |
| Acute myelomonocytic leukemia” (ICD-10 code: C92.5) | |
| CML | Chronic myeloid leukemia, BCR/ABL-positive (ICD-10 code: C92.1) |
| Atypical chronic myeloid leukemia, BCR/ABL-negative (ICD-10 code: C92.2). | |
| ALL | Acute lymphoblastic leukemia [ALL] (ICD-10 code: C91.0). |
| CLL | Chronic lymphocytic leukemia of B-cell type (ICD-10 code: C91.1). |
| HL | Hodgkin lymphoma (ICD-10 code: C81). |
| NHL | Follicular lymphoma (ICD-10 code: C82) |
| Non-follicular lymphoma (ICD-10 code: C83) | |
| Mature T/NK-cell lymphomas (ICD-10 code: C84) | |
| Other specified and unspecified types of non-Hodgkin lymphoma (ICD-10 code: C85) | |
| Other specified types of T/NK-cell lymphoma (ICD-10 code: C86) | |
| Cancer | Neoplasms (ICD-10 code: C00–D49) |
| Hospitalization | Hospital inpatient services (013659) |
| Death | Vital status code “deceased” that TriNetX regularly imports from the Social Security Death index. |
2.3. Statistical analysis
-
(1)
We examined how incidence proportions of breakthrough SARS-CoV-2 infections (new cases per 1000 person) evolved from December 2020 to October 2021 in vaccinated patients with all HM and patients without cancer, stratified by gender, race, and ethnicity and age groups (age ≥ 65 and < 65 years old). Time trends for each specific HM type were not separately examined due to small numbers of breakthrough infections during each time period. For security reasons, TriNetX does not report actual patient counts less than 10.
-
(2)
The accumulated risks of breakthrough SARS-CoV-2 infections during the period of December 2020 to October 2021 were examined in vaccinated population for all HM, seven specific HM types, and non-cancer. Risks for breakthrough SARS-CoV-2 infections were also examined and compared between White and Black patients, and between Hispanic and non-Hispanic patients.
-
(3)
The overall risks for hospitalization and death in patients with HM who had breakthrough infections were examined and compared with those in patients with HM who had no breakthrough infections. Hospitalization and death were followed starting on the day of breakthrough infections for patients with breakthroughs or 14 days after full vaccination for patients without breakthroughs up to October 23, 2021. Kaplan-Meier analysis was performed to estimate the probability of outcomes. Comparison of outcomes between patients with vs without breakthroughs were made using Cox's proportional hazards model. The proportional hazard assumption was tested using the generalized Schoenfeld approach. The hazard ratios (HRs) and 95% confidence intervals were calculated.
-
(4)
Characteristics of patients with vs without breakthroughs were compared for all HM, multiple myeloma, and non-Hodgkin lymphoma. The following characteristics were examined: demographics (age, gender, race/ethnicity); adverse socioeconomic determinants of health including problems related to employment, housing and economic circumstance that are known to be associated with COVID-19 incidence and related outcomes [18,19]; COVID-19-related comorbidities [[20], [21], [22], [23], [24], [25]] and behavior factors (tobacco smoking, alcohol use) [26]; vaccine types (Pfizer-BioNTech, Moderna, J & J) [[27], [28], [29]]; cancer treatment types (Stem cell transplant, chemotherapy, targeted therapy, radiation therapy) [30,31].
-
(5)
Characteristics of patients with HM who had breakthrough infections and were subsequently hospitalized or died were compared with those who had breakthrough infections but were not subsequently hospitalized nor died. The following patient characteristics were compared: demographics; adverse socioeconomic determinants of health, comorbidities, behavior factors, vaccine types; and cancer treatments.
All statistical analyses were performed on the TriNetX Analytics Platform. Patient characteristics were compared with chi-square tests for categorical variables and independent-sample t-tests for continuous variables, at significance set at p-value <0.05 (two-sided).
3. Results
3.1. Patient characteristics
The characteristics of fully vaccinated patients with all HM and each of seven specific HM types are shown in Table 2 . Specific HM differed in ages, race/ethnicity, adverse social determinants of health, comorbidities, and cancer treatments.
Table 2.
Characteristics of fully vaccinated population with hematological malignancy.
| HM | AML | CML | ALL | CLL | HL | NHL | MM | |
|---|---|---|---|---|---|---|---|---|
| Total no. of patients | 5956 | 460 | 287 | 319 | 984 | 448 | 2723 | 1186 |
| Age (Mean ± SD) | 65.4 ± 15.8 | 59.3 ± 17 | 63.6 ± 15.8 | 45.9 ± 21.5 | 71.3 ± 11.3 | 54.7 ± 18.5 | 66.1 ± 15.2 | 68.1 ± 11.6 |
| Gender (% Female) | 49.1 | 53.9 | 43.2 | 46.1 | 43.6 | 48.9 | 49.4 | 48.6 |
| Ethnicity (%) | ||||||||
| Hispanic/Latino | 5.5 | 8.4 | 4.2 | 12.6 | 2.9 | 8.1 | 5.4 | 5.7 |
| Not Hispanic/Latino | 81.1 | 78.9 | 86.8 | 78.8 | 84.1 | 78.5 | 80.1 | 82.8 |
| Unknown Ethnicity | 13.3 | 12.7 | 9.0 | 13.2 | 8.5 | 13.3 | 14.5 | 11.4 |
| Race (%) | ||||||||
| Caucasian | 71.2 | 69.6 | 72.5 | 64.9 | 81.4 | 70.1 | 73.0 | 61.5 |
| African American | 16.0 | 12.8 | 12.9 | 14.4 | 9.7 | 16.5 | 13.8 | 27.4 |
| Asian | 4.0 | 6.3 | 5.9 | 6.6 | 1.5 | 4.0 | 4.1 | 3.9 |
| Unknown | 8.5 | 10.4 | 8.4 | 13.8 | 7.2 | 8.0 | 8.9 | 6.8 |
| Adverse social determinants of health (%) | 3.3 | 4.3 | 3.5 | 6.9 | 2.4 | 3.8 | 3.0 | 2.8 |
| Health conditions or behavioral factors (%) | ||||||||
| Hypertension | 52.6 | 54.8 | 53.0 | 45.8 | 57.1 | 42.9 | 52.2 | 60.2 |
| Heart diseases | 18.2 | 15.2 | 20.9 | 12.2 | 19.3 | 19.9 | 19.4 | 22.4 |
| Cerebrovascular diseases | 11.6 | 12.8 | 11.5 | 15.0 | 10.0 | 10.9 | 11.2 | 13.7 |
| Obesity | 19.0 | 24.1 | 20.6 | 21.0 | 18.5 | 19.0 | 19.2 | 19.1 |
| Type 2 diabetes | 20.2 | 27.6 | 26.1 | 21.6 | 18.1 | 18.3 | 20.8 | 22.1 |
| Chronic respiratory diseases | 22.1 | 28.3 | 25.4 | 26.0 | 21.7 | 23.2 | 22.9 | 21.6 |
| Chronic kidney diseases | 17.0 | 17.6 | 23.0 | 16.6 | 17.3 | 14.5 | 15.8 | 26.2 |
| Liver diseases | 16.0 | 26.7 | 16.4 | 22.9 | 12.9 | 21.9 | 18.1 | 16.0 |
| HIV infection | 2.0 | 2.2 | 3.5 | 3.1 | 1.0 | 4.7 | 3.1 | 1.3 |
| Dementia | 1.3 | 2.2 | 3.5 | 3.1 | 1.0 | 2.2 | 1.3 | 1.2 |
| Substance use disorders | 12.1 | 13.0 | 11.5 | 12.2 | 11.5 | 15.8 | 12.8 | 12.6 |
| Depression | 18.0 | 22.2 | 23.3 | 22.3 | 17.5 | 21.7 | 18.4 | 19.4 |
| Anxiety | 24.3 | 29.8 | 24.4 | 35.1 | 23.2 | 33.7 | 25.2 | 23.3 |
| Alcohol use | 3.0 | 4.6 | 3.5 | 3.1 | 3.0 | 4.7 | 3.2 | 2.2 |
| Smoking | 3.3 | 3.0 | 3.5 | 3.1 | 3.0 | 3.8 | 3.6 | 3.2 |
| Cancer treatment (%) | ||||||||
| Stem cell transplant | 11.6 | 38.0 | 15.6 | 31.0 | 3.5 | 15.0 | 8.1 | 26.6 |
| Chemotherapy | 52.6 | 66.7 | 77.3 | 79.3 | 43.7 | 63.8 | 54.6 | 59.9 |
| Targeted therapy | 37.2 | 40.4 | 64.8 | 44.5 | 32.5 | 37.1 | 39.3 | 50.2 |
| Radiation | 12.4 | 17.6 | 10.5 | 19.7 | 5.5 | 17.9 | 15.9 | 12.1 |
| Vaccine types (%) | ||||||||
| Pfizer-BioNTech | 78.2 | 75.0 | 76.7 | 81.1 | 75.7 | 81.7 | 78.8 | 77.1 |
| Moderna | 20.8 | 24.1 | 22.3 | 18.2 | 23.6 | 17.0 | 20.1 | 22.0 |
| Johnson & Johnson | 1.0 | 0.9 | 1.0 | 0.7 | 0.7 | 2.2 | 1.1 | 0.9 |
HM – hematological malignancy, AML - acute myeloid leukemia, CML – chronic myeloid leukemia, All - acute lymphocytic leukemia, CLL - chronic lymphocytic leukemia, HL - Hodgkin lymphoma, NHL - Non-Hodgkin lymphoma, MM - multiple myeloma.
3.2. Increasing breakthrough SARS-CoV-2 infections in vaccinated populations with hematological malignancy from December 2020 to October 2021
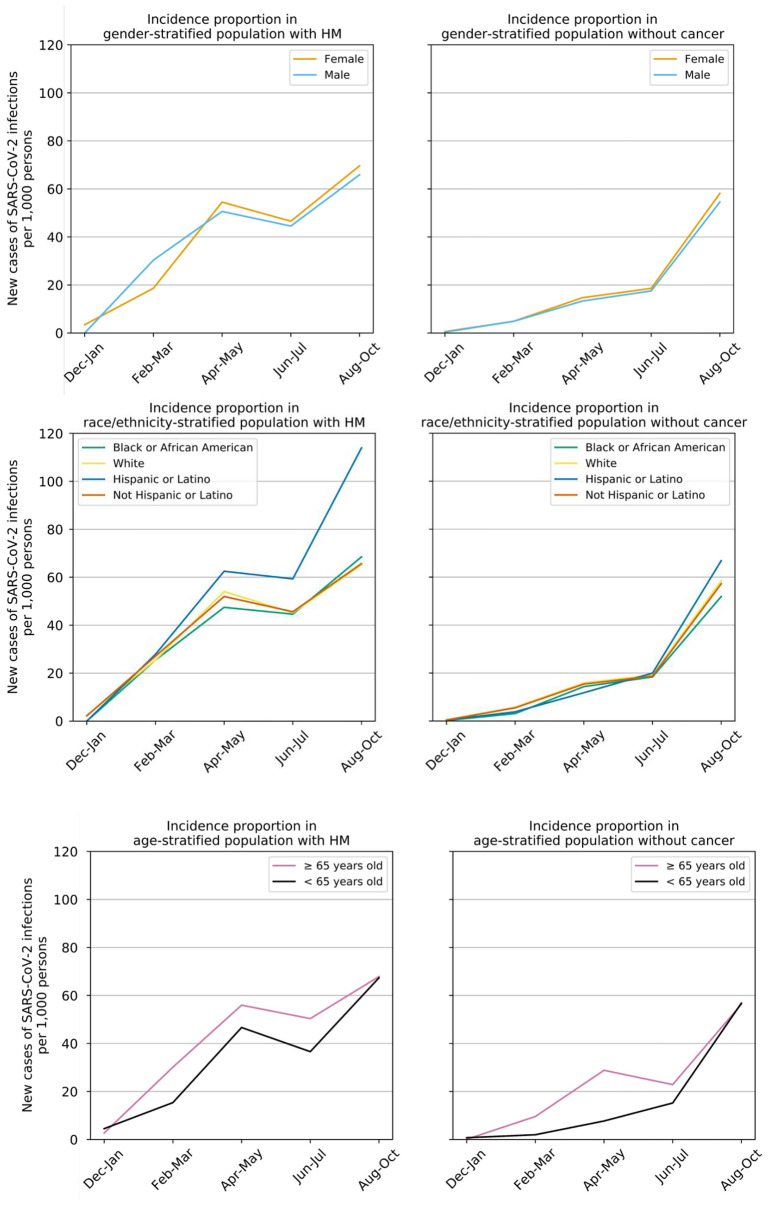
The incidence proportions of breakthrough SARS-CoV-2 infections in both patients with HM and patients without cancer steadily increased from December 2020 to October 2021 (Fig. 1 ). The rates were higher in patients with HM than those in non-cancer patient over the time period: 2.46% vs 0.49% (p < 0.001) during February–March, 5.25% vs 1.41% (p < 0.001) during April–May, 4.55% vs 1.81% (p < 0.001) during June–July, 6.78% vs 5.67% (p = 0.008) during August–October. No significant differences were observed in populations stratified by gender and race/ethnicity. Older patients (age ≥ 65 years) had consistently higher breakthrough infections than younger patients. (See Fig.1)
Fig. 1.
Time trends of incidence proportions of breakthrough SARS-CoV-2 infections in vaccinated population with hematological malifully vaccinated populations with HM and in fully vaccinated populations without cancer between December 2020 and October 2022, stratified by gender (top panel), race and ethnicity (middle panel) and age (bottom panel).
3.3. Risks of SARS-CoV-2 breakthrough infections in fully vaccinated patients with hematological malignancies
The overall risk of SARS-CoV-2 breakthrough infections in vaccinated patients with hematological malignancies was 13.4%. Among the 7 HM types, patients with CML had highest risk for breakthrough infections (17.4%), followed by MM (17.2%) and CLL (15.2%) and patients with ALL had the lowest risk (11.05). However, all of them were significantly higher than the 4.5% in fully vaccinated patients without cancer. The risks for breakthrough infection among patients with HM did not differ based on race or ethnicity, indicating that vaccines are effective against SARS-CoV-2 infections regardless of race or ethnicity. For example, among 1186 vaccinated patients with MM, the overall risk of breakthrough infections was 15.8% for White patients with MM and 18.5% for Black patients with MM and the difference was not significant (Table 3 ).
Table 3.
Risks of breakthrough SARS-CoV-2 infections and 95% confidence interval in patients with hematological malignancy.
| Cohort | Patients | Overall risk (%) | Risk in White patients (%) | Risk in Black patients (%) | Risk in Not Hispanic patients (%) | Risk in Hispanic patients (%) |
|---|---|---|---|---|---|---|
| HM | 5956 | 13.4 [12.5–14.3] | 13.4 [12.4–14.5] | 14.6 [12.5–17.0] | 12.5 [11.6–13.4] | 14.7 [11.3–18.9] |
| MM | 1186 | 17.2 [15.1–19.2] | 15.8 [13.3–18.7] | 18.5 [14.5–23.2] | 16.2 [14.1–18.6] | 20.5 [12.3–31.9] |
| AML | 460 | 14.3 [11.3–17.9] | 14.1 [10.6–18.5] | NA | 15.0 [11.7–19.0] | NA |
| CML | 287 | 17.4 [13.3–22.4] | 17.3 [12.6–23.3] | NA | 14.9 [10.8–19.3] | NA |
| ALL | 319 | 11.0 [7.9–15.1] | 10.6 [6.9–15.8] | NA | 10.1 [6.9–14.5] | NA |
| CLL | 984 | 15.2 [13.0–17.6] | 15.6 [9.2–24.8] | 14.7 [12.4–17.4] | 14.7 [12.6–17.1] | NA |
| HL | 448 | 12.7 [9.8–16.2] | 14.3 [10.7–18.8] | NA | 11.9 [9.0–15.6] | NA |
| NHL | 2723 | 13.1 [11.9–14.4] | 13.3 [11.9–14.9] | 13.1 [9.9–17.0] | 12.2 [11.0–13.6] | 15.6[9.7–21.3] |
| Non-cancer | 508,457 | 4.5 [4.4–4.6] | 5.1 [5.0–5.2] | 4.7 [4.6–4.9]*** | 4.4 [4.3–4.5] | 3.6 [3.5–3.7] *** |
NA - not available. TriNetX does not report actual patient counts less than 10 for security reasons.
We then compared patients with breakthroughs to those without breakthroughs for all HM, MM, and NHL (Table 4 ). Other types of HMs were not examined due to their small sample sizes. Comparing patients with vs without breakthrough infections among the population with all HM, patients with breakthrough infections were older (68.6 ± 13.5 vs 65 ± 16 years old), had more comorbidities (e.g., 66.1% vs 50.8% for hypertension, 29.3% vs 18.9% for type 2 diabetes, 25.8% vs 18.1% for obesity) and received more chemotherapies (59.5% vs 51.9%) and targeted cancer therapies (43.3% vs 36.3). No significant differences were observed for gender, race or ethnicity, and adverse social determinants of health diagnoses. No significant differences were observed for types of vaccine take-ups between patients with breakthroughs (Pfizer-BioNTech: 78.5%, Moderna: 21.1%) and those without breakthroughs (Pfizer-BioNTech: 78.2%, Moderna: 20.8%). Similar findings were observed for patients with NHL. Among the vaccinated population with MM, patients with and without breakthrough infections did not differ in age, gender, race or ethnicity, cancer treatments, and vaccine types. However, MM patients with breakthrough infections had more comorbidities than those without breakthroughs (e.g., 69.6% vs 58.6% for hypertension, 31.9% vs 20.6% for type 2 diabetes, 28.4% vs 17.4% for obesity).
Table 4.
Characteristics of vaccinated patients with HM who had breakthrough SARS-CoV-2 infections vs those who had no breakthrough SARS-CoV-2 infections.
| Breakthrough SARS-CoV-2 infection | HM |
MM |
NHL |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes | No | P Value | Yes | No | P Value | Yes | No | P Value | |
| Total Number of Patients | 800 | 5156 | 204 | 982 | 357 | 2366 | |||
| Age (Years, Mean ± SD) | 68.6 ± 13.5 | 65 ± 16 | <0.001 | 69 ± 11.1 | 67.9 ± 11.7 | 0.21 | 69.4 ± 13.2 | 65.6 ± 15.4 | <0.001 |
| Gender (% Female) | 49.1 | 49.1 | 0.98 | 55.4 | 47.1 | 0.03 | 46.5 | 49.9 | 0.24 |
| Ethnicity (%) | |||||||||
| Hispanic/Latino | 6.6 | 6.0 | 0.47 | 7.4 | 5.9 | 0.43 | 6.7 | 5.7 | 0.47 |
| Not Hispanic/Latino | 81.8 | 81.0 | 0.58 | 84.4 | 82.5 | 0.52 | 81.2 | 80.0 | 0.57 |
| Race (%) | |||||||||
| White | 71.0 | 71.2 | 0.92 | 56.4 | 62.5 | 0.10 | 74.2 | 72.8 | 0.57 |
| Black | 17.4 | 15.8 | 0.25 | 29.4 | 27.0 | 0.48 | 13.7 | 13.8 | 0.98 |
| Asian | 4.0 | 4.0 | 0.97 | 5.3 | 3.6 | 0.22 | 4.5 | 4.1 | 0.71 |
| Adverse Social Determinants of Health (%) | 4.3 | 3.2 | 0.12 | 4.9 | 2.7 | 0.11 | 3.1 | 3.0 | 0.90 |
| Comorbidities (%) | |||||||||
| Hypertension | 66.1 | 50.8 | <0.001 | 69.6 | 58.6 | 0.003 | 67.8 | 50.1 | <0.001 |
| Heart diseases | 30.3 | 16.6 | <0.001 | 30.4 | 21.1 | 0.004 | 33.3 | 17.5 | <0.001 |
| Cerebrovascular diseases | 18.6 | 10.7 | <0.001 | 18.6 | 12.0 | 0.03 | 18.8 | 10.4 | <0.001 |
| Obesity | 25.8 | 18.1 | <0.001 | 28.4 | 17.4 | <0.001 | 26.6 | 18.2 | <0.001 |
| Type 2 diabetes | 29.3 | 18.9 | <0.001 | 31.9 | 20.6 | <0.001 | 31.9 | 19.3 | <0.001 |
| Chronic respiratory diseases | 29.4 | 21.2 | <0.001 | 25.5 | 20.9 | 0.15 | 32.5 | 21.6 | <0.001 |
| Chronic kidney diseases | 26.6 | 15.7 | <0.001 | 32.4 | 25.3 | 0.04 | 26.1 | 14.5 | <0.001 |
| Liver diseases | 20.8 | 15.4 | <0.001 | 21.1 | 15.2 | 0.04 | 22.4 | 17.6 | 0.03 |
| Substance use disorders | 15.6 | 11.7 | 0.001 | 17.6 | 11.8 | 0.02 | 17.6 | 12.2 | 0.004 |
| Anxiety | 29.2 | 23.8 | <0.001 | 27.8 | 22.9 | 0.13 | 28.7 | 24.3 | 0.07 |
| Depression | 22.3 | 17.6 | 0.001 | 23.5 | 18.5 | 0.10 | 21.0 | 18.1 | 0.19 |
| Alcohol use | 4.3 | 2.9 | 0.04 | 4.9 | 2.2 | 0.03 | 5.9 | 2.8 | 0.002 |
| Smoking | 3.1 | 3.3 | 0.78 | 4.9 | 3.4 | 0.28 | 3.6 | 3.6 | 1.00 |
| Cancer treatment (%) | |||||||||
| Stem cell transplant | 13.5 | 11.5 | 0.10 | 26.5 | 27.0 | 0.88 | 9.0 | 8.1 | 0.57 |
| Chemotherapy | 59.5 | 51.9 | <0.001 | 62.3 | 59.7 | 0.49 | 64.4 | 53.6 | <0.001 |
| Targeted therapy | 43.3 | 36.6 | <0.001 | 52.9 | 50.1 | 0.46 | 47.6 | 38.4 | <0.001 |
| Radiation | 13.5 | 12.4 | 0.37 | 11.3 | 12.4 | 0.65 | 18.5 | 15.6 | 0.17 |
| Vaccine types (%) | |||||||||
| Pfizer-BioNTech | 78.3 | 78.2 | 0.98 | 79.9 | 76.5 | 0.29 | 79.6 | 78.7 | 0.71 |
| Moderna | 21.1 | 20.8 | 0.83 | 19.1 | 22.6 | 0.27 | 19.9 | 20.2 | 0.91 |
| Johnson & Johnson | 1.3 | 1.0 | 0.53 | NA | NA | NA | NA | 1.1 | NA |
NA - not available. For security reasons, TriNetX does not report actual patient counts less than 10.
3.4. Overall risks of hospitalization and mortality among patients with vs. without breakthrough SARS-CoV-2 infection
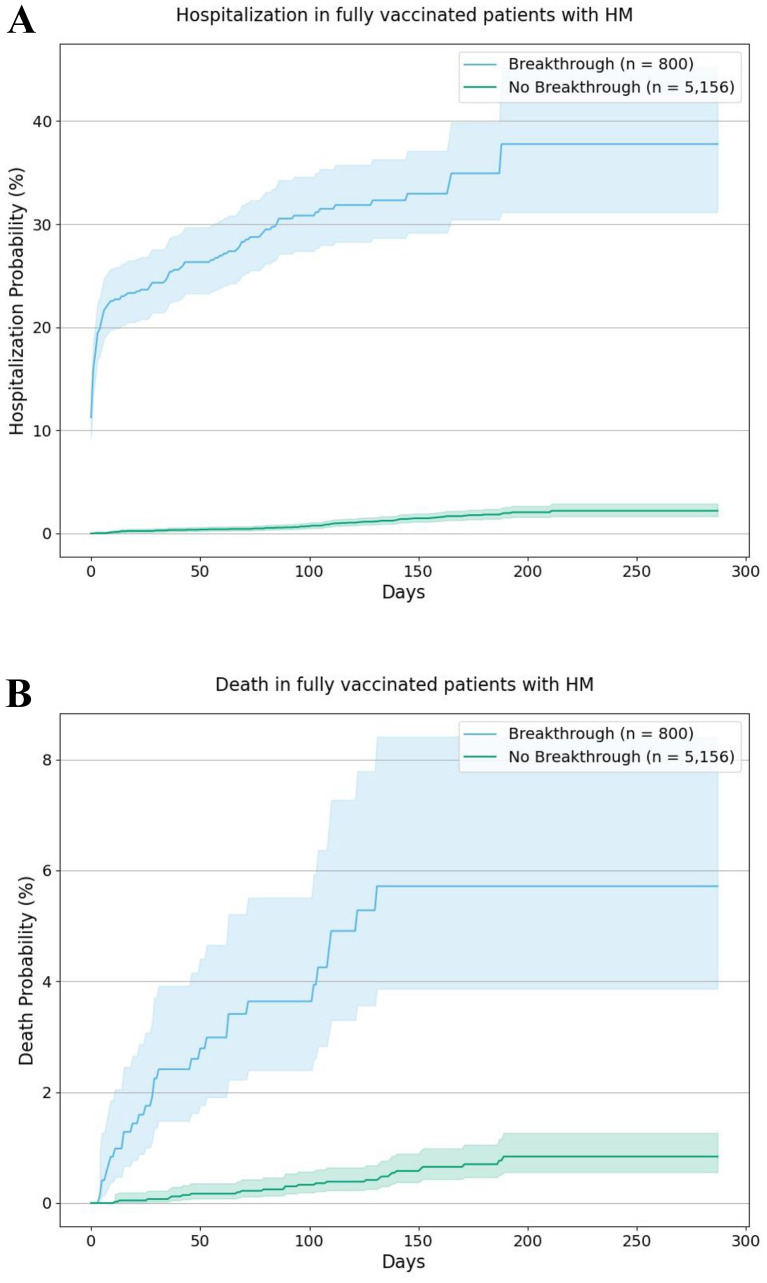
Among the population with HM, the overall hospitalization risk was 37.8% for patients with breakthrough infections, significantly higher than the 2.2% for those without breakthrough infections (HR: 34.49, 95% CI: 25.93–45.87). The overall mortality risk was 5.7% for patients with breakthrough infections, significantly higher than the 0.8% for those without breakthrough infections (HR: 10.25, 95% CI: 5.94–17.69) (Fig.2 ).
Fig. 2.
Kaplan-Meier curves for hospitalizations (2A) and death (2B) in patients with breakthroughs vs patients without breakthrough among the population with HM. For breakthrough patients, hospitalizations and death were followed starting on the day of SARS-CoV-2 infections until the end of time window (October 23, 2021). For non-breakthrough cohorts, hospitalizations and death were followed starting on 14 days after full vaccination until the end of time window (October 23, 2021). Shaded areas represent 95% CIs.
For vaccinated population with HM who had breakthrough infections, we then compared patients who were subsequently hospitalized or died to patients who were not subsequently hospitalized nor died (Table 5 ). The goal was to identify which specific subsets of patients with HM were more vulnerable to severe outcomes of breakthrough infections. Compared to patients who were not hospitalized, hospitalized patients were older, comprised of more non-Hispanic and White patients, and received more targeted therapy. No significant differences were found for other demographics, adverse social determinants of health, comorbidities, and other cancer treatments. Compared to patients who did not die, those who died after breakthrough infections were significantly older. Other factors were either not significant or the numbers were too small. Comparisons were not done for specific HM due to limited sample sizes.
Table 5.
Characteristics of vaccinated patients with HM who had breakthrough SARS-CoV-2 infections and subsequently suffered from severe outcomes (hospitalization or death) as compared with those who had breakthrough SARS-CoV-2 infections but did not subsequently suffer from severe outcomes.
| Hospitalization |
Death |
|||||
|---|---|---|---|---|---|---|
| Yes | No | P Value | Yes | No | P Value | |
| Total Number of Patients | 228 | 572 | 28 | 772 | ||
| Age (Years, Mean ± Sd) | 71 ± 12.4 | 67.7 ± 13.8 | 0.002 | 74.8 ± 14.4 | 68.4 ± 13.4 | 0.014 |
| Gender (% Female) | 45.2 | 50.7 | 0.16 | 46.4 | 49.2 | 0.77 |
| Ethnicity (%) | ||||||
| Hispanic/Latino | 6.1 | 6.8 | 0.73 | NA | 6.6 | NA |
| Not Hispanic/Latino | 88.2 | 79.5 | <0.001 | 92.8 | 81.8 | 0.05 |
| Race (%) | ||||||
| White | 76.3 | 68.9 | 0.04 | 82.1 | 70.6 | 0.19 |
| Black | 17.1 | 17.5 | 0.90 | NA | 17.6 | NA |
| Adverse Social Determinants Of Health* (%) | 4.8 | 4.2 | 0.70 | NA | 4.0 | NA |
| Comorbidities (%) | ||||||
| Hypertension | 68.9 | 65.9 | 0.42 | 71.4 | 66.2 | 0.57 |
| Heart Diseases | 31.6 | 29.7 | 0.61 | NA | 30.2 | NA |
| Cerebrovascular Diseases | 21.5 | 18.2 | 0.28 | NA | 18.4 | NA |
| Obesity | 22.8 | 26.7 | 0.25 | NA | 25.6 | NA |
| Type 2 Diabetes | 32.5 | 28.1 | 0.23 | NA | 29.3 | NA |
| Chronic Respiratory Diseases | 29.8 | 29.7 | 0.98 | NA | 29.4 | NA |
| Chronic Kidney Diseases | 29.4 | 25.9 | 0.31 | NA | 26.7 | NA |
| Liver Diseases | 18.4 | 21.9 | 0.28 | NA | 20.7 | NA |
| Substance Use Disorders | 13.2 | 16.8 | 0.20 | NA | 15.8 | NA |
| Anxiety | 26.3 | 30.6 | 0.23 | NA | 22.4 | NA |
| Depression | 16.2 | 25 | 0.007 | NA | 22.4 | NA |
| Smoking | 4.4 | 3.5 | 0.55 | NA | 3.1 | NA |
| Alcohol Use | 4.4 | 4.2 | 0.90 | NA | 4.1 | NA |
| Cancer Treatment (%) | ||||||
| Stem Cell Transplant | 11.8 | 14.5 | 0.32 | NA | 13.6 | NA |
| Chemotherapy | 64.5 | 58.7 | 0.14 | 75.0 | 59.3 | 0.10 |
| Targeted Therapy | 50.0 | 40.9 | 0.02 | 60.7 | 42.9 | 0.06 |
| Radiation | 11.4 | 14.3 | 0.27 | NA | 13.3 | NA |
| Vaccine Types (%) | ||||||
| Pfizer-BioNTech | 77.2 | 78.7 | 0.65 | 67.9 | 78.6 | 0.18 |
| Moderna | 21.9 | 20.8 | 0.73 | NA | 21.0 | NA |
NA - not available. For security reasons, TriNetX does not report actual patient counts less than 10.
4. Summary and future directions
This study shows that the incidence proportions of breakthrough SARS-CoV-2 infections in vaccinated patients with HM steadily increased from December 2020 to October 2021, higher than those in patients without cancer, indicating general waning immunity of vaccine, especially in patients with HM. The cumulative risk of breakthrough infections in patients with HM was 13.4%, higher than 4.5% in patients without cancer, indicating that HM is a risk for breakthrough infections in fully vaccinated patients. These findings are consistent with previous reports of low seroconversion in patients with HM [[11], [12], [13], [14], [15]] and provide robust real-world evidence that impaired seroconversion could have resulted in significant breakthrough infections in patients with HM, even when they are fully vaccinated. Our previous studies, in the early pandemic when vaccines were not available, showed disproportionate COVID-19 infections between Black and White patients with HM [1]. In contrast, this study shows no signs of racial or ethnic disparities for breakthrough infections in any of the seven HMs once patients were fully vaccinated. Findings in this study show that risks for hospitalization and mortality in patients with HM who had breakthrough infections were not only significantly higher than in those who had no breakthrough infections, but also substantial, with an overall risk of 37.8% for hospitalization, and 5.7% for mortality. While our finding shows that HM itself is a risk factor for breakthrough infections in fully vaccinated patients, we identified subsets of patients with HM who were more vulnerable to breakthrough infections: older patients and patients with significant comorbidities (e.g., hypertension, heart diseases, cerebrovascular diseases, obesity, type 2 diabetes, chronic respiratory diseases, chronic kidney diseases, liver diseases, substance use disorders, depression, and anxiety) and patients who received chemotherapies or targeted therapies. In addition, we show that among vaccinated patients with HM who had breakthrough infections, age was an additional significant risk factor for both hospitalization and death.
4.1. Future considerations
Findings from this study clearly indicate increased breakthrough SARS-CoV-2 infection rates for fully vaccinated patients with HM compared to fully vaccinated patients without malignancy. Moreover, breakthrough infections in HM patients are of significant severity as indicated by substantial hospitalization and mortality. The failure to develop protective antibody response to COVID-19 vaccination in HM patients indicates the need to maintain virus mitigation strategies in patients having HM, even though they may be fully vaccinated. Our recent study comparing Moderna and Pfizer-BioNTech mRNA vaccines in general population showed that recipients of Moderna mRNA vaccine had fewer breakthrough infections and severe outcomes compared to recipients of Pfizer vaccine [29]. Whether it is true in patients with HM needs to be validated. Our results also indicate the need for careful surveillance and early diagnosis of breakthrough infections, since disease may be more severe in this group and patients may benefit from the early administration of monoclonal antibodies [32,33] and/or anti-viral therapy with drugs such as molnupiravir [34], nirmatrelvir-ritonavir [35] and/or selective serotonin reuptake inhibitors (SSRIs) [36]. The defective protective antibody response in patients with HM further calls for research to examine, in these patients, the possibility of enhanced strategies for immunization adjuvants to boost antibody response as well consideration of passive immune protection, using either convalescent serum, monoclonal antibodies [37] or prophylactic use of intravenous immunoglobulin (IVIG), which has recently been demonstrated to contain COVID-19 protective antibodies [38] and recently suggested as a protective strategy for immunosuppressed patients receiving CAR-T Therapy [39]. SARS-CoV-2 infections and hospitalizations are rising in the US after the emergence of the Omicron variant in December 2021 [40]. The Omicron variant is more transmissible than earlier variants. Reports showed lower rates of severe clinical outcomes following Omicron infection compared with Delta variant infection [[41], [42], [43], [44], [45]]. Currently it remains unknown how the Omicron variant impacts patients with HM and how well vaccination and boosters will protect against infections and severe clinical outcomes associated with Omicron.
4.2. Limitations
This study has several limitations. First, the observational, retrospective nature of this study of patient EHR data could introduce selection, information, testing and follow up biases. Second, time-series antibody level data in vaccinated patients are not available in patient EHRs. This information is important to correlate antibody data with breakthrough infections, hospitalizations, and death to advance our understanding of the immune correlates of protection in patients with HM. Third, though the TriNetX EHR Database collected de-identified data of more than 84 million unique patients across the US, patients int the EHR database represent people who had medical encounters with healthcare systems and does not necessarily represent the entire US population. While patients with HM likely have medical encounters, the generalizability of the results from the TriNetX platform remains unknown and needs to be validated in other populations. Fourth, vaccination that was done outside of healthcare organizations were not necessarily captured in patient EHRs. Consequently, patients without documented vaccination in their EHRs may have been vaccinated. This limitation may not have impacted our findings as we focused on fully vaccinated patients who had documented vaccination data in their EHRs, and we made no comparisons to unvaccinated persons. However, findings from this study need to be validated in other vaccinated populations. Fifth, differences between patient population in geographic distribution, virus circulation and types of healthcare organizations could confound the results. Finally, how timing of cancer treatments and disease characteristics (e.g., diagnosis time, disease state, stages) further affected the risks of breakthrough infections and outcomes in vaccinated patients with HM were not examined in this study due to limited sample sizes. Future studies are warranted to identify risks factors for breakthrough infections and severe outcomes in patients with HM.
Practice points
-
•
Vaccinated patients with hematologic malignancies (HM) should continue to practice COVID-19 mitigation strategies, including physical and social distancing, hand washing, respiratory etiquette, mask wearing in public and ventilation of indoor spaces.
-
•
Patients with HM, whether vaccinated or not, who developed signs of COVID-19 infection, should receive prompt medical attention, since even vaccinated patients with COVID-19 infections still have substantial severe outcomes.
-
•
Vaccinated patients with HM who have certain comorbidities including hypertension, heart diseases, cerebrovascular diseases, obesity, type 2 diabetes, chronic respiratory diseases, chronic kidney diseases, liver diseases, substance use disorders depression, and anxiety should take extra cautions as these factors may confer additional risk for COVID-19 infection.
Research agenda
-
•
Prospective studies are needed to document and compare timing, incidence, severity, and efficacy of available COVID-19 vaccines to prevent breakthrough infections and protection against severe outcomes associated with different virus variants including Omicron in patients with HM to characterize and explain disease specific differences.
-
•
Prospective studies are needed to determine timing and efficacy of vaccine boosters in producing antiviral immune reactivity in patients with HM who have already completed full recommended primary vaccine schedule.
-
•
Timing and severity of COVID-19 breakthrough infections need to be prospectively characterized in patients with HM following vaccine booster administration.
-
•
Evaluate the utility, tolerance, and toxicity of regularly repeating vaccine boosters to prevent COVID-19 breakthrough infections in patients with HM.
-
•
Study immune adjuvant strategies to enhance development of COVID-19 antiviral neutralizing efficacy and breakthrough infections in patients with HM.
-
•
Evaluate regular use of passive antiviral neutralizing strategies, such as monoclonal antibodies, hyper immune serum, IVIG, to prevent breakthrough COVID-19 infections in patients with HM, especially during periods of intense immunosuppression.
-
•
Examine how patient-specific characteristics including demographics, disease characteristics, treatment timing and types, comorbidities and behaviors may predispose patients with HM to COVID-19 infection.
-
•
Examine how different types of vaccine (e.g., mRNA-1273 and BNT162b2) and boosters protect patients with HM from COVID infections
-
•
Examine the risks, clinical outcomes, and racial and ethnic disparities of COVID infections with the Omicron variant in patients with HM and understand how vaccines and pre-existing immunity from previous infections protect patients with HM against infections with Omicron and associated clinical outcomes.
Acknowledgments
Acknowledgments
This work was supported by NIH National Cancer Institute R25CA221718, American Cancer Society Research Scholar Grant RSG-16-049-01 – MPC, NIH National Institute on Aging AG057557, AG061388, AG062272, National Institute on Alcohol Abuse and Alcoholism (grant no. R01AA029831), The Clinical and Translational Science Collaborative (CTSC) of Cleveland 1UL1TR002548-01, Case Comprehensive Cancer Center P30 CA043703, Case Comprehensive Cancer Center Cancer Health Disparities SPORE Planning Grant P20 CA2332216.
Authors' contributions
RX and NAB conceived, designed, and supervised the study. LW conducted experiments, performed data analysis, and prepared all the data, tables, and figures in the study. RX and NAB wrote the manuscript. DCK critically contributed to all aspects of this study. We confirm the originality of content.
Role of the funding source
The funding source had no role in the design of this study and will not have any role during its execution, analyses, interpretation of the data, or decision to submit results.
Declaration of Competing Interest
LW, DCK, RX and NAB have no financial interests to disclose.
References
- 1.Wang Q., Berger N.A., Xu R. When hematologic malignancies meet COVID-19 in the United States: Infections, death and disparities. Blood Rev. 2020:100775. doi: 10.1016/j.blre.2020.100775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Q., Berger N.A., Xu R. Analyses of risk, racial disparity, and outcomes among US patients with cancer and COVID-19 infection. JAMA Oncol. 2021;7(2):220–227. doi: 10.1001/jamaoncol.2020.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. 2020 Dec 31. [Epub 2020 Dec 10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadoff J., Gray G., Vandebosch A., Cárdenas V., Shukarev G., Grinsztejn B., et al. Safety and efficacy of single-dose Ad26. COV2. S vaccine against Covid-19. N Engl J Med. 2021;384(23):2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergwerk M., Gonen T., Lustig Y., Amit S., Lipsitch M., Cohen C., et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385(16):1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown C.M., Vostok J., Johnson H., Burns M., Gharpure R., Sami S., et al. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings—Barnstable County, Massachusetts, July 2021. Morb Mortal Wkly Rep. 2021;70(31):1059. doi: 10.15585/mmwr.mm7031e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CDC COVID-19 Vaccine Breakthrough Case Investigations Team COVID-19 vaccine breakthrough infections reported to CDC - United States, January 1–April 30, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(21):792–793. doi: 10.15585/mmwr.mm7021e3. May 28. [PMID: 34043615; PMCID: PMC8158893] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L., Wang Q., Davis P.B., Volkow N.D., Xu R. Increased risk for COVID-19 breakthrough infection in fully vaccinated patients with substance use disorders in the United States between December 2020 and August 2021. World Psychiatry. 2021 Oct 5 doi: 10.1002/wps.20921. [Epub ahead of print. PMID: 34612005] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L., Berger A.N., Xu R. Risks of SARS-CoV-2 breakthrough infection and hospitalization in fully vaccinated patients with multiple myeloma. JAMA Netw Open. 2021;4(11) doi: 10.1001/jamanetworkopen.2021.37575. e2137575. Nov 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ollila T.A., Lu S., Masel R., Zayac A., Paiva K., Rogers R.D., et al. Antibody response to COVID-19 vaccination in adults with hematologic malignant disease. JAMA Oncol. 2021;7(11):1714–1716. doi: 10.1001/jamaoncol.2021.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herzog Tzarfati K., Gutwein O., Apel A., Rahimi-Levene N., Sadovnik M., Harel L., et al. BNT162b2 COVID-19 vaccine is significantly less effective in patients with hematologic malignancies. Am J Hematol. 2021;96(10):1195–1203. doi: 10.1002/ajh.26284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenberger L.M., Saltzman L.A., Senefeld J.W., Johnson P.W., DeGennaro L.J., Nichols G.L. Antibody response to SARS-CoV-2 vaccines in patients with hematologic malignancies. Cancer Cell. 2021;39(8):1031–1033. doi: 10.1016/j.ccell.2021.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Oekelen O., Gleason C.R., Agte S., Srivastava K., Beach K.F., Aleman A., et al. Highly variable SARS-CoV-2 spike antibody responses to two doses of COVID-19 RNA vaccination in patients with multiple myeloma. Cancer Cell. 2021;39(8):1028–1030. doi: 10.1016/j.ccell.2021.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Re D., Barrière J., Chamorey E., Delforge M., Gastaud L., Petit E., et al. Low rate of seroconversion after mRNA anti-SARS-CoV-2 vaccination in patients with hematological malignancies. Leuk Lymphoma. 2021:1–3. doi: 10.1080/10428194.2021.1957877. [DOI] [PubMed] [Google Scholar]
- 16.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021:1–7. doi: 10.1038/s41591-021-01377-8. May 17. [DOI] [PubMed] [Google Scholar]
- 17.TriNetX Analytics Network. https://trinetx.com/
- 18.Mena G.E., Martinez P.P., Mahmud A.S., Marquet P.A., Buckee C.O., Santillana M. Socioeconomic status determines COVID-19 incidence and related mortality in Santiago, Chile. Science. 2021;372(6545) doi: 10.1126/science.abg5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Little C., Alsen M., Barlow J., Naymagon L., Tremblay D., Genden E., et al. The impact of socioeconomic status on the clinical outcomes of COVID-19; a retrospective cohort study. J Community Health. 2021:1–9. doi: 10.1007/s10900-020-00944-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The Center for Disease Control and Prevention (CDC) COVID-19 and people at with certain medical conditions. 2021. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html
- 21.The Center for Disease Control and Prevention (CDC) COVID-19 and people at increased risk. 2021. https://www.cdc.gov/drugoverdose/resources/covid-drugs-QA.html
- 22.Wang Q.Q., Kaelber D.C., Xu R., Volkow N.D. COVID-19 risk and outcomes in patients with substance use disorders: analyses from electronic health records in the United States. Mol Psychiatry. 2021;26(1):30–39. doi: 10.1038/s41380-020-00880-7. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Q., Xu R., Volkow N.D. Increased risk of COVID-19 infection and mortality in people with mental disorders: analysis from electronic health records in the United States. World Psychiatry. 2021;20(1):124–130. doi: 10.1002/wps.20806. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Q., Davis P.B., Xu R. COVID-19 risk, disparities and outcomes in patients with chronic liver disease in the United States. EClinicalMedicine. 2021;(31) doi: 10.1016/j.eclinm.2020.100688. Jan 1. 100688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Q., Davis P.B., Gurney M.E., Xu R. COVID-19 and dementia: analyses of risk, disparity, and outcomes from electronic health records in the US. Alzheimers Dement. 2021;17(8):1297–1306. doi: 10.1002/alz.12296. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patanavanich R., Glantz S.A. Smoking is associated with COVID-19 progression: a meta-analysis. Nicotine Tob Res. 2020;22(9):1653–1656. doi: 10.1093/ntr/ntaa082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collier A.Y., Yu J., McMahan K., et al. Differential kinetics of immune responses elicited by COVID-19 vaccines. N Engl J Med. 2021 doi: 10.1056/NEJMc2115596. Oct 15. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steensels D., Pierlet N., Penders J., Mesotten D., Heylen L. Comparison of SARS-CoV-2 antibody response following vaccination with BNT162b2 and mRNA-1273. Jama. 2021;326(15):1533–1535. doi: 10.1001/jama.2021.15125. Oct 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang L., Davis P.B., Kaelber D.C., Volkow N.D., Xu R. Comparison of mRNA-1273 and BNT162b2 vaccines on breakthrough SARS-CoV-2 infections, hospitalizations, and death during the Delta-predominant period. JAMA. 2022 doi: 10.1001/jama.2022.0210. Published online January 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ligumsky H., Safadi E., Etan T., Vaknin N., Waller M., Croll A., et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine among actively treated cancer patients. JNCI: Journal of the National Cancer Institute. 2021;djab174 doi: 10.1093/jnci/djab174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young J.A.H. Beginning to understand clinical events and immune responses of hematopoietic cell transplant recipients receiving SARS-CoV-2 vaccination. Transpl Cell Therapy. 2021;27(9):700. doi: 10.1016/j.jtct.2021.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michot J.M., Albiges L., Chaput N., Saada V., Pommeret F., Griscelli F., et al. Tocilizumab, an anti-IL-6 receptor antibody, to treat COVID-19-related respiratory failure: a case report. Ann Oncol. 2020;31(7):961. doi: 10.1016/j.annonc.2020.03.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shanmugaraj B., Siriwattananon K., Wangkanont K., Phoolcharoen W. Perspectives on monoclonal antibody therapy as potential therapeutic intervention for coronavirus disease-19 (COVID-19) Asian Pac J Allergy Immunol. 2020;38(1):10–18. doi: 10.12932/AP-200220-0773. [DOI] [PubMed] [Google Scholar]
- 34.Mahase E. Covid-19: Molnupiravir reduces risk of hospital admission or death by 50% in patients at risk, MSD reports. BMJ. 2021;(375) doi: 10.1136/bmj.n2422. n2422. Oct 4. [DOI] [PubMed] [Google Scholar]
- 35.Couzin-Frankel J. Antiviral pills could change pandemic’s course. Science. 2021;374(6569):799–800. doi: 10.1126/science.acx9605. Nov 12. [Epub 2021 Nov 11] [DOI] [PubMed] [Google Scholar]
- 36.Reis G., Silva E., Silva D., Thabane L., Milagres A., Ferreira T., et al. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalization among patients with COVID-19: the TOGETHER randomized platform clinical trial. Lancet. 2021 doi: 10.1016/S2214-109X(21)00448-4. Published:October 27, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubin R. Testing an old therapy against a new disease: convalescent plasma for COVID-19. Jama. 2020;323(21):2114–2117. doi: 10.1001/jama.2020.7456. [DOI] [PubMed] [Google Scholar]
- 38.Jawhara S. Could intravenous immunoglobulin collected from recovered coronavirus patients protect against COVID-19 and strengthen the immune system of new patients? Int J Mol Sci. 2020;21(7):2272. doi: 10.3390/ijms21072272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hill J.A., Giralt S., Torgerson T.R., Lazarus H.M. CAR-T–and a side order of IgG, to go?–Immunoglobulin replacement in patients receiving CAR-T cell therapy. Blood Rev. 2019;38 doi: 10.1016/j.blre.2019.100596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cloete J., Kruger A., Masha M., et al. Rapid rise in paediatric COVID-19 hospitalisations during the early stages of the omicron wave, Tshwane District, South Africa. bioRxiv. 2021 doi: 10.1101/2021.12.21.21268108. Published online December 21. [DOI] [Google Scholar]
- 41.Wang L., Berger N.A., Kaelber D.C., Davis P.B., Volkow N.D., Xu R. Comparison of outcomes from COVID infection in pediatric and adult patients before and after the emergence of omicron. medRxiv [Preprint] 2022;Jan 2:2021.12.30.21268495 doi: 10.1101/2021.12.30.21268495. [DOI] [Google Scholar]
- 42.Wang L., Berger N.A., Kaelber D.C., Davis P.B., Volkow N.D., Xu R. COVID infection severity in children under 5 years old before and after omicron emergence in the US. medRxiv [Preprint] 2022;Jan 13:2022.01.12.22269179 doi: 10.1101/2022.01.12.22269179. [DOI] [Google Scholar]
- 43.Wolter N., Jassat W., Walaza S., et al. Early assessment of the clinical severity of the SARS-CoV-2 Omicron variant in South Africa. bioRxiv. 2021 doi: 10.1101/2021.12.21.21268116. Published online December 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sheikh A., Kerr S., Woolhouse M., McMenamin J., Robertson C. Severity of omicron variant of concern and vaccine effectiveness against symptomatic disease: national cohort with nested test negative design study in Scotland. 2021. https://www.pure.ed.ac.uk/ws/files/245818096/Severity_of_Omicron_variant_of_concern_and_vaccine_effectiveness_against_symptomatic_disease.pdf Published online December 22. [DOI] [PMC free article] [PubMed]
- 45.Report 50 - Hospitalisation risk for Omicron cases in England. https://www.imperial.ac.uk/mrc-global-infectious-disease-analysis/covid-19/report-50-severity-omicron/ Accessed December 23, 2021.