Abstract
Multiple retrospective studies have demonstrated an association between cytomegalovirus (CMV) reactivation and reduced risk of AML relapse. However, the potential mechanism explaining this association remains elusive. We investigated a homogeneous cohort of 288 adult patients with AML in remission who received allogeneic hematopoietic stem cell transplantation (HCT) from matched sibling/unrelated donors between 1995 and 2011. The 5-year cumulative incidence of relapse was greater in patients without CMV reactivation compared with those with reactivation (30.2% vs. 12.1%, p=0.001) in a landmark analysis. In multivariate analyses CMV reactivation was independently associated with reduced relapse risk (HR: 0.49 [0.25–0.95], p=0.036) and increased non-relapse mortality (26.5% vs. 13.1%, p=0.002) resulting in similar 5-year overall survival (64.5% vs. 59.1%, p=0.8). In further subgroup analyses the protective effect of CMV reactivation was significant in patients who received HCT from donors with KIR Bx compared to KIR AA (11.7% vs. 29.5%, p=0.01). Likewise, the protective effect of CMV reactivation was more significant when the donors had 2DS1 activating KIR (11.5% vs. 30.7%, p=0.05) compared with those without 2DS1 (14.3% vs. 27.5%, p=0.12). Our data independently confirm the association between CMV reactivation and AML relapse, and suggest the involvement of donor KIR genotypes and NK cell-mediated graft-versus-leukemia effect.
Keywords: CMV reactivation, AML relapse, Donor KIR genotype, NK cell mediated graft-versus-leukemia effect
1. Introduction
The effect of cytomegalovirus (CMV) infection on acute myeloid leukemia (AML) relapse was initially reported by Elmaagacli et al. who observed early CMV infection after allogeneic hematopoietic cell transplantation (alloHCT) is associated with reduced risk of relapse in a relatively homogeneous population of patients who underwent HCT with fully myeloablative conditioning (MAC).1 Similar protective effect was reported in patients with chronic myelogenous leukemia (CML) receiving T cell-depleted grafts.2 In another large single-institution study, Green et al. confirmed the association between CMV reactivation and decreased risk of relapse in AML patients, but not in patients with acute lymphoid leukemia (ALL), lymphoma, CML, or myelodysplastic syndrome (MDS).3 Further examination by Manjappa et al. suggested this protective effect might be subjective to the conditioning regimen, as CMV reactivation resulted in lower relapse only in AML patients receiving MAC but not reduced-intensity conditioning (RIC).4 More recently, a large registry study by the Japan Society for HCT, confirmed the beneficial effect of CMV reactivation on subsequent risk of relapse in AML patients but not in those with other hematological malignancies.5 However, studies from the Center for International Blood and Marrow Transplant Research (CIBMTR) showed no association between CMV reactivation and decreased relapse rate for patients with AML post-HCT.6
Although the protective effect of CMV reactivation on relapse appears robust, the biological mechanism behind this effect remains highly elusive. It is possible that CMV reactivation might not have any causative relationship with relapse prevention, but it might be a trigger for other unknown clinical/biological factor(s) leading to relapse-protection. Plausible hypotheses include upregulation and maturation of specific natural killer cell (NK) populations appearing early after CMV infection, including NKG2C+ cells (memory NK).7,8 While there is no direct evidence that these specific NK subsets are responsible for GVL effects, it is likely that CMV infection induces phenotypic and functional changes in NKs via interactions with T cells and/or stimulatory cytokines that may result in a desirable NK effector function for GVL.
Activating and inhibitory killer immunoglobulin-like receptors (aKIR, iKIR), expressed on the plasma membrane of NKs, regulate the killing function of these cells in response to viral or other intracellular pathogen infections, tumor transformed host cells, and transplanted allogeneic cells.9–11 In a study by Cook and colleagues, authors reported that in matched sibling donor HCT, presence of donor KIR haplotype B (in donors with more than one aKIR gene) was associated with a 65% reduction in CMV reactivation.12 Inhibiting effect of aKIRs was further confirmed in another study by Zaia et al, demonstrating donor aKIR profile containing aKIR2DS2 and aKIR2DS4 were predictive for low risk of CMV reactivation.13
The association between KIR genotypes and risk of AML relapse remains controversial. While multiple studies have demonstrated a beneficial role for donor NK alloreactivity in mismatched-HCT,14–16 other studies examining the KIR ligand incompatibility concept have provided conflicting results.17–20 In one study, Hsu et al, demonstrated that lack of HLA ligand for donor-iKIR (missing KIR ligand) resulted in lower relapse incidence in AML and MDS patients.21 In another study, Venstrom et al, reported that AML patients who received allografts from KIR2DS1+ donors had lower relapse rate than those with allografts from KIR2DS1 negative donors.22
These apparent associations between AML relapse, CMV reactivation, and various models for activating/inhibitory KIR-ligand interactions are the basis for the current study. We first sought to determine the impact of CMV reactivation on risk of AML relapse in a relatively homogeneous large group of adult patients in our institution with more than half receiving RIC-HCT. Then we examined the activating/inhibitory KIR-ligand interactions, which may explain the underlying immune-biology mechanism involved in CMV reactivation-induced protection against AML relapse.
2. Subjects and Methods
2.1. Patients and donors
In this retrospective study approved by the City of Hope Institutional Review Board, we identified 288 consecutive AML patients in either first or second complete remission (CR) who underwent alloHCT between 1995 and 2011 from a matched-related (MRD) or unrelated donor (MUD). Patients who were not in CR, did not received ex-vivo or in-vivo T cell depletion with anti-thymocyte globulin (ATG) or received HCT from <8/8 matched donor were excluded. From these 288 patients, 12 who experienced relapse (n=2) or death (n=10) within 55 days post-HCT were excluded to perform a landmark analysis. The remaining 276 patients and their clinical/transplant characteristics are detailed in Table 1. Briefly, majority of patients (83%) were in CR1. HCT was from a MRD in 190 and a MUD 86 patients. RIC was used in 125 patients (45%). Graft source was peripheral blood stem cells (n=240) or bone marrow cells (n=36). Sixty-nine patients (25%) had high-risk cytogenetic abnormalities according to the Southwest Oncology Group (SWOG) prognostic classification.23
Table 1a.
Patient and Transplant Characteristics
| Variable | Median (Range) or N (%) |
|---|---|
| Patient Age at HCT | 49 (18 – 70) |
| Patient Gender | |
| Female | 140 (51) |
| Male | 136 (49) |
| Year of HCT | |
| Before 2005 | 102 (37) |
| After 2005 | 174 (63) |
| Donor Type | |
| Sibling | 190 (69) |
| Unrelated | 86 (31) |
| Donor/Patient Gender | |
| Female Donor to Male Patient | 48 (17) |
| Other | 228 (83) |
| Cytogenetic Risk | |
| Favorable | 13 (5) |
| Intermediate | 165 (60) |
| Unfavorable | 69 (25) |
| Unknown/NA | 29 (10) |
| Stem Cell Sources | |
| BM | 36 (13) |
| PBSC | 240 (87) |
| Disease Status at HCT | |
| CR1 | 229 (83) |
| CR2 | 47 (17) |
| Donor/Patient CMV Status | |
| Negative/Negative | 34 (12) |
| Negative/Positive | 59 (21) |
| Positive/Negative | 29 (11) |
| Positive/Positive | 154 (56) |
| GVHD Prophylaxis | |
| Cyclosporine + 1 Drug | 75 (27) |
| Cyclosporine + 2/3 Drugs | 27 (10) |
| Tacro + 1 Drug | 154 (56) |
| Tacro + 2/3 Drugs | 20 (7) |
| Tacro/Siro Usage | |
| No | 114 (41) |
| Yes | 162 (59) |
| Conditioning Regimens | |
| Flu/Mel | 125 (45) |
| Other | 151 (55) |
| ABO compatibility | |
| Match | 165 (60) |
| Not-matched | 111 (40) |
2.2. Transplant regimens
Transplant conditioning included MAC: fractionated total body irradiation (FTBI) at 13.2 Gy in combination with either etoposide (120 mg/kg) or cyclophosphamide (120 mg/kg); or RIC: fludarabine 125 mg/m2 plus melphalan 140 mg/m2. Graft-versus-host-disease (GVHD) prophylaxis consisted of calcineurin inhibitor (tacrolimus or cyclosporine) plus a short course of methotrexate or tacrolimus and sirolimus with/without methotrexate. Other supportive care including infectious disease prophylaxis was provided according to the institutional standard of care procedures.
2.3. CMV monitoring and preemptive therapy
No patients in this cohort received prophylactic anti-CMV therapy. CMV was monitored by shell vial culture (until ~2005) or PCR (started ~2005) once or twice a week from day +21 through day +100. Preemptive therapy with ganciclovir or foscarnet was started when CMV reactivation was detected by a positive culture or PCR (>1500 gc/ml or 500–1500 gc/ml when considered high risk).
2.4. KIR Genotyping
DNA extraction from donor and recipient blood samples was performed using Qiamp DNA Blood Mini Kit (Qiagen, Valencia, CA) following manufacturer’s recommendations. A multiplex PCR-sequence-specific (PCR-SSP) was performed on DNA samples as previously described,24 using 28 primers in 4 multiplex PCR reactions for detection of 14 functional KIR genes. The assays were run with following primer combinations: A) iKIR2DL1, iKIR2DL2, iKIR2DL4, and aKIR2DS3; B) iKIR2DL3, aKIR2DS2, iKIR3DL1 and aKIR2DS4; C) iKIR2DL5, aKIR2DS1, and iKIR3DL2; and D) aKIR2DS4n, aKIR2DS4d, aKIR2DS5 and 3DL3. This method identifies all 14 functional KIR genes including the deletion mutant 2DS4d, but not the pseudogenes. The amplified samples were run on a 3% agarose gel for visualization and analysis. KIR2DL4, 3DL2, and 3DL3 were not included in the analyses, since they were detected in 100% of donors and recipients.
2.5. Statistical analyses
Cumulative incidence of CMV reactivation, morphologic relapse, and non-relapse mortality (NRM) were estimated, treating death (CMV reactivation and morphologic relapse) and relapse as competing risk events. Cox proportional hazards models were used to evaluate risk factors associated with morphologic relapse; the proportional hazard assumption was examined (upheld). A stepwise selection model was used and interaction between the main effect and significant covariates were examined. Clinical and transplant factors included for analysis were patients’ age (below/above median: 49 years), donor type (MRD vs. MUD), conditioning regimen (MAC vs. RIC), transplant era (1995–2005 vs. 2006–2011), CMV reactivation, disease status at HCT (CR1 vs. CR2), cytogenetic risk (low, intermediate, high), donor/recipient CMV serostatus, and acute GVHD (none or grade I vs. grade II-IV). CMV reactivation and acute GVHD were analyzed as time-dependent variables. Statistical analyses were performed using Statistical Analysis System (SAS) version 9.4. A landmark analysis was performed at day +55 to compare the cumulative incidence of relapse (CIR) by CMV reactivation status (75% of the patients who reactivated -did so by day +55). Secondary eligibility was alive and disease free at day +55. Ten patients who expired and two others who relapsed prior to day +55 were not included in the analysis.
3. Results
3.1. Overall HCT and CMV reactivation outcomes
All but 4 patients engrafted with the median of 15 days post-HCT. After the median follow-up duration of 63.1 months (range 6–211 months), 166 patients (60%) stayed alive. HCT outcomes including causes of death are summarized in Table 2. The 5-year probabilities of overall survival (OS), relapse-free survival (RFS) were 60.9% (95% CI: 54.4–66.8), 58.5% (95% CI: 52.0–64.4), respectively. At 5 years, relapse incidence and NRM were 24.5% (95% CI: 19.8–30.3), and 17.0% (95% CI: 12.8–22.5), respectively. CMV reactivation was observed in 85 patients (31%) before day +100 (median onset: 44 days) (Table 2). The cumulative incidence of CMV reactivation in patients at risk (donor or recipient seropositive) was 33.9% (95% CI: 28.4–40.4) and in seropositive patients was 38.0% (95% CI: 32.0–45.1).
Table 2.
Summary of Outcomes
| Variable | Median (Range) or N (%) |
|---|---|
| Acute GVHD Grade | |
| Yes | 171 (62) |
| Grade I | 46 (17) |
| Grade II | 76 (27) |
| Grade III | 39 (14) |
| Grade IV | 10 (4) |
| No | 105 (38) |
| Chronic GVHD | |
| Yes | 185 (67) |
| Limited | 29 (10) |
| Extensive | 156 (57) |
| No | 79 (28) |
| Died too early | 11 (4) |
| Not Available | 1 (1) |
| Time to engraftment (Days) | 15 (10 – 50) |
| No | 191 (69) |
| Yes | 85 (31) |
|
Days from HCT to CMV Reactivation Onset, n=85 (first 100 days post-trans) |
44 (14 – 100) |
| Relapse/Progression post HCT | |
| No | 209 (76) |
| Yes | 67 (24) |
| Median Follow Up (Months) | 38.0 (2.1 – 210.7) |
| Surviving Patients | 63.1 (5.7 – 210.7) |
| Deceased Patients | 13.3 (2.1 – 192.6) |
| Number of Death Events post HCT | |
| Alive | 166 (60) |
| Dead | 110 (40) |
| Cause of Death – See table below | |
| Disease Progression | 44 |
| Infection | 31 |
| GVHD | 12 |
| Other | 13 |
| Information Not Available | 10 |
Patient developed cGVHD with 100 days post-trans. * Follow up cut-off date: 03/05/2013
3.2. Incidence of AML relapse and CMV reactivation
Consistent with the earlier reports,1–5,25 the 5-year CIR was greater in patients without CMV reactivation 30.2% (95% CI: 24.1–37.7) compared with those with CMV reactivation 12.1% (95% CI: 6.7–21.6, p=0.001) when examined as a land-mark analysis for those without relapse through day 55 post-HCT (Figure 1a). However, CMV reactivation was associated with increased NRM (26.5% vs. 13.1%, p=0.002, Figure 1b) resulting in a similar 5-year RFS in both groups (61.5% vs. 56.7%, p=0.7, Figure 1c) and OS (64.5% vs. 59.1%, p=0.8, Figure 1d). By landmark analysis (Table 3), unfavorable cytogenetics (hazard ratio [HR] = 1.86, 95% CI: 1.15–3.03; p=0.01) was significantly associated with AML relapse while CMV reactivation (HR= 0.37, 95% CI: 0.20–0.70; p=0.002) was protective. There was a trend towards reduced relapse with RIC compared with MAC-HCT (HR= 0.66, 95% CI: 0.41–1.07; p=0.1). Transplant era (2005–2011) showed a trend towards increased relapse (HR= 1.65, 95% CI: 0.97–2.79; p=0.07). In multivariate analysis, only two variables, CMV reactivation (HR= 0.49, CI: 0.25–0.95; p=0.036) and cytogenetic risk (HR= 1.8, CI: 1.1–2.9; p<0.02) remained significantly associated with relapse risk (Table 3). The association between CMV reactivation and reduced AML relapse was independent of serostatus of donor/recipient.
Figure 1: 5-year cumulative incidence of relapse (CIR), cumulative incidence of non-relapse mortality (NRM), relapse free survival (RFS), and overall survival (OS) in patients with (blue line) and without (green line) CMV reactivation.
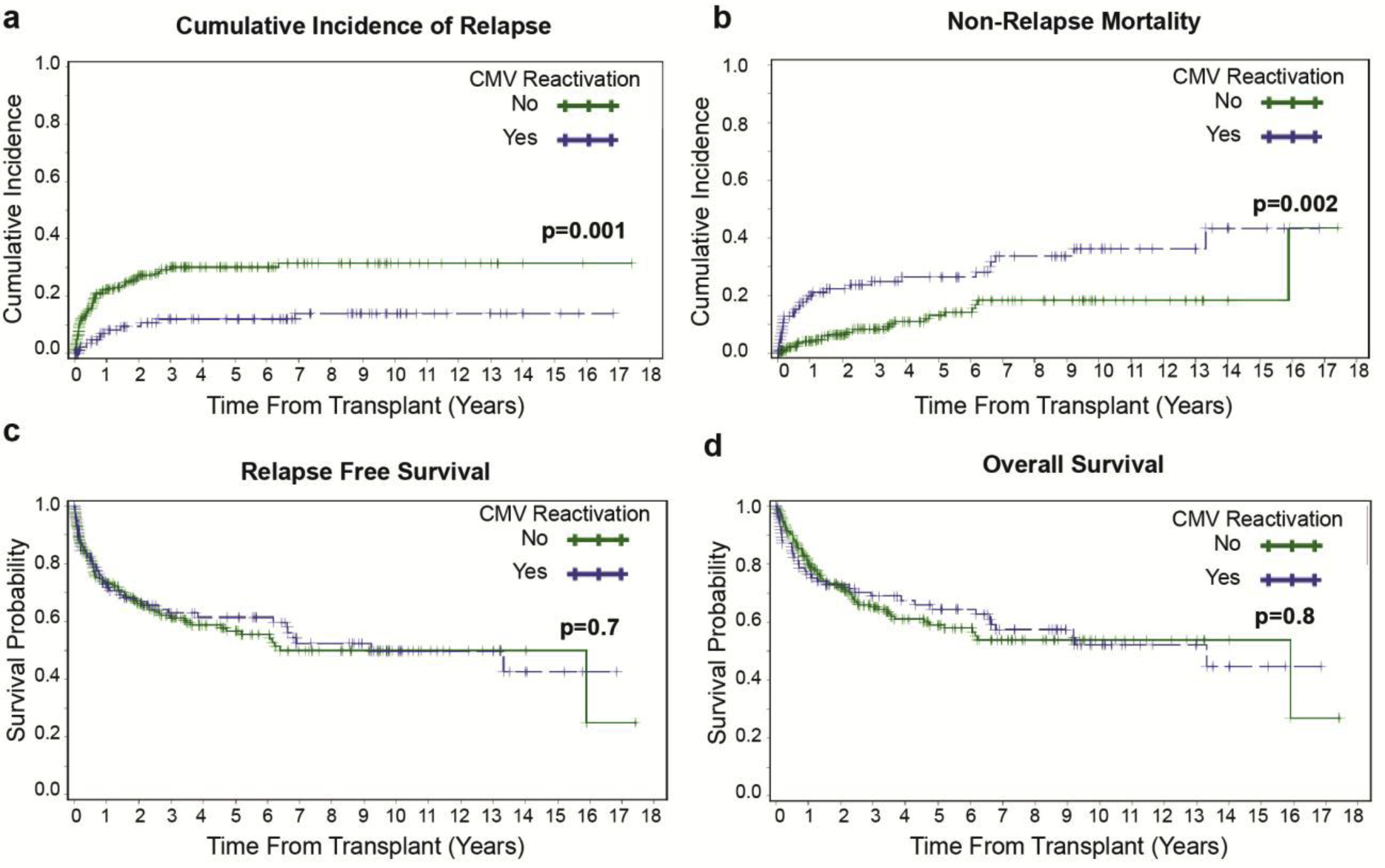
(a) CIR by CMV reactivation when examined as a landmark analysis for those without relapse by day 55 post-HCT (b) Cumulative incidence of NRM by CMV reactivation status (c) RFS by CMV reactivation status (d) OS by CMV reactivation status.
Table 3.
Univariateand Multivariate Analysis for Variables Associated with AML relapse
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variable | N | Events | Hazard Ratio (95% CI) | p | Hazard Ratio (95% CI) | P |
| Age at HCT | 0.93 | N/A | ||||
| < 49 | 137 | 33 | Baseline | Baseline | ||
| > 49 | 139 | 34 | 1.02 (0.63, 1.65) | - | ||
| CMV Serostatus | 0.25 | N/A | ||||
| D-/R- | 34 | 11 | Baseline | Baseline | ||
| Other | 242 | 56 | 0.68 (0.35, 1.31) | - | ||
| CMV Serostatus | 0.13 | N/A | ||||
| R+ | 213 | 47 | Baseline | Baseline | ||
| other | 63 | 20 | 1.50 (0.89, 2.54) | - | ||
| Disease Status at HCT | 0.54 | N/A | ||||
| CR1 | 229 | 57 | Baseline | Baseline | ||
| CR2 | 47 | 10 | 0.81 (0.42, 1.57) | - | ||
| Donor Type | 0.43 | N/A | ||||
| Related | 190 | 44 | Baseline | Baseline | ||
| Unrelated | 86 | 23 | 1.22 (0.74, 2.01) | - | ||
| Transplant Era | 0.07 | N/A | ||||
| Before 2005 | 102 | 20 | Baseline | Baseline | ||
| After 2005 | 174 | 47 | 1.65 (0.97, 2.79) | - | ||
| Conditioning Regimens | 0.10 | N/A | ||||
| Fully ablative | 151 | 31 | Baseline | Baseline | ||
| RIC (Flu/Mel) | 125 | 36 | 0.66 (0.41, 1.07) | - | ||
| Cytogenetic Risk | ||||||
| Favorable | 13 | 2 | Baseline | Baseline | ||
| Intermediate | 165 | 32 | 1.34 (0.31, 5.85) | - | ||
| Unfavorable | 69 | 25 | 2.60 (0.59, 11.4) | - | ||
| Unknown | 29 | 8 | 1.89 (0.39, 9.19) | - | ||
| 0.01 | - | 0.02 | ||||
| Favorable/Intermediate/Unknown | 207 | 42 | Baseline | |||
| Unfavorable | 69 | 25 | 1.86 (1.15, 3.03) | Baseline | ||
| 0.03 | 1.86 (1.15, 3.03) | 0.04 | ||||
| CMV Reactivation before Day 55 | ||||||
| Yes | 214 | 58 | Baseline | Baseline | ||
| No | 62 | 9 | 2.15 (1.08, 4.26) | 2.1 (1.0, 4.1) | ||
| aGVHD Grade | 0.73 | N/A | ||||
| None-I | 151 | 38 | Baseline | Baseline | ||
| II-IV | 125 | 29 | 0.92 (0.57, 1.49) | - | ||
Landmark Analysis on Cumulative Incidence of Relapse: Event = Relapse, Completing risk = Death without relapse
Evaluation of CMV reactivation impact on AML relapse in patients receiving MAC and RIC, in contrast to an earlier report by Manjappa et al,4 showed a significant association between CMV reactivation and relapse in RIC-HCT patients (8.9% vs. 35.4%, p=0.007), whereas this significance was absent in MAC-HCT patients (13.5% vs. 24.7%, p=0.12, Figure 2a and 2b).
Figure 2: Impact of CMV reactivation on AML relapse in myeloablative conditioning (MAC) subgroups and reduced intensity conditioning (RIC).
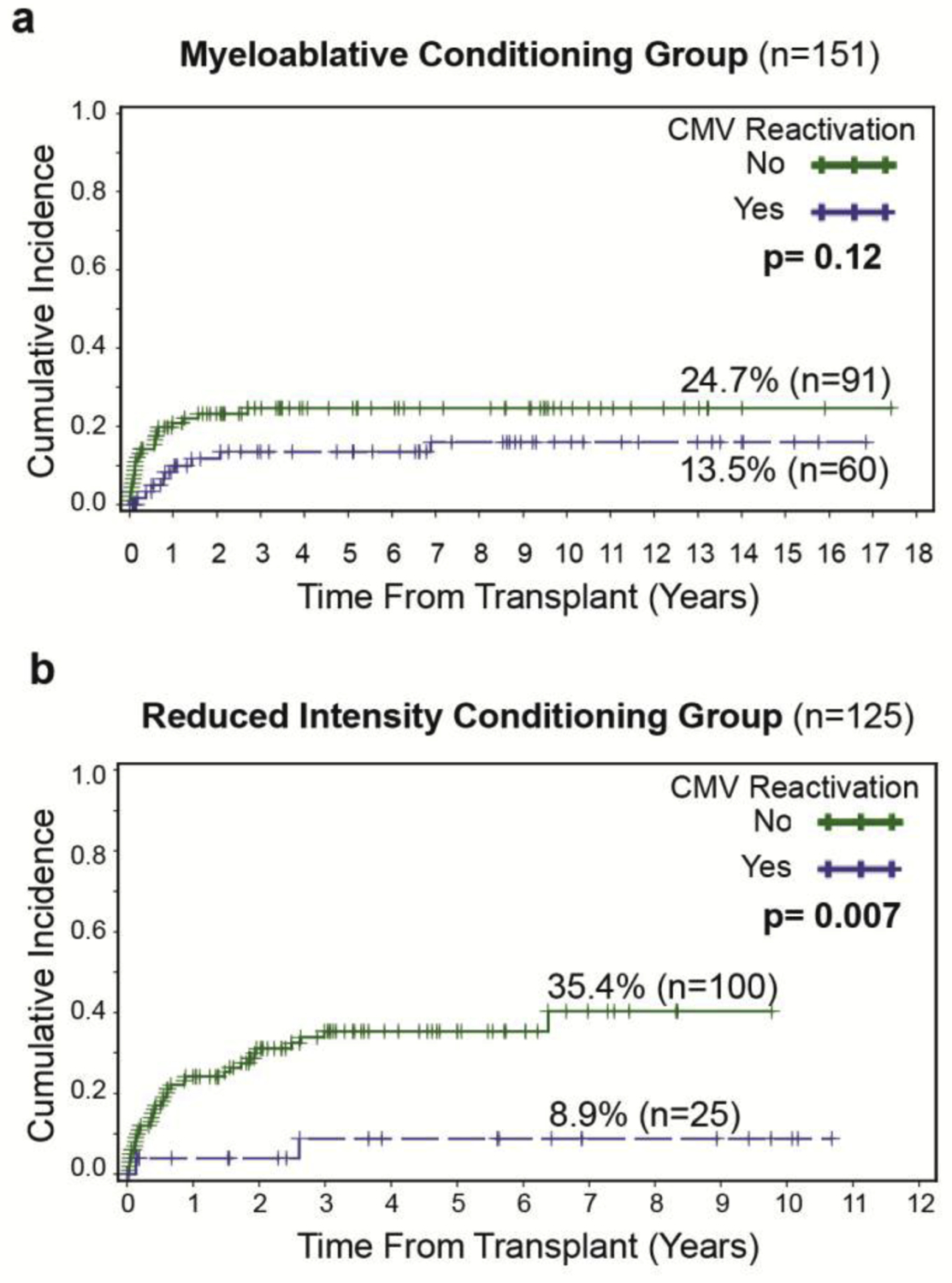
(a) Non-significant association between CMV reactivation and CIR in patients receiving MAC (b) Significant association between CMV reactivation and CIR in patients receiving RIC.
3.3. Subgroup analysis based on donor KIR genotypes
We first sought to evaluate if particular KIR genotypes were influencing the association between CMV reactivation and AML relapse. Thus, we performed subset analyses focused on the donor KIR Bx (vs. AA) and aKIR2DS1+ (vs. no KIR2DS1), which have been shown to be associated with reduced risk of relapse,22 and observed that the protective effect of CMV reactivation was maintained in patients who received HCT from donors with KIR Bx (11.7% vs. 29.5%, p=0.01). This significance was however lost in the subgroup of patients receiving HCT from KIR A/A donors (16.5% vs. 27.5%, p=0.29, Figure 3a and 3b). Likewise, the protective effect of CMV reactivation was more pronounced and significant when donors were KIR2DS1+ (11.5% vs. 30.7%, p=0.05) compared with donors without KIR2DS1 (14.3% vs. 27.5%, p=0.12, Figure 3c and 3d).
Figure 3: Cumulative incidence of relapse by CMV reactivation status based on donor KIR genotype.
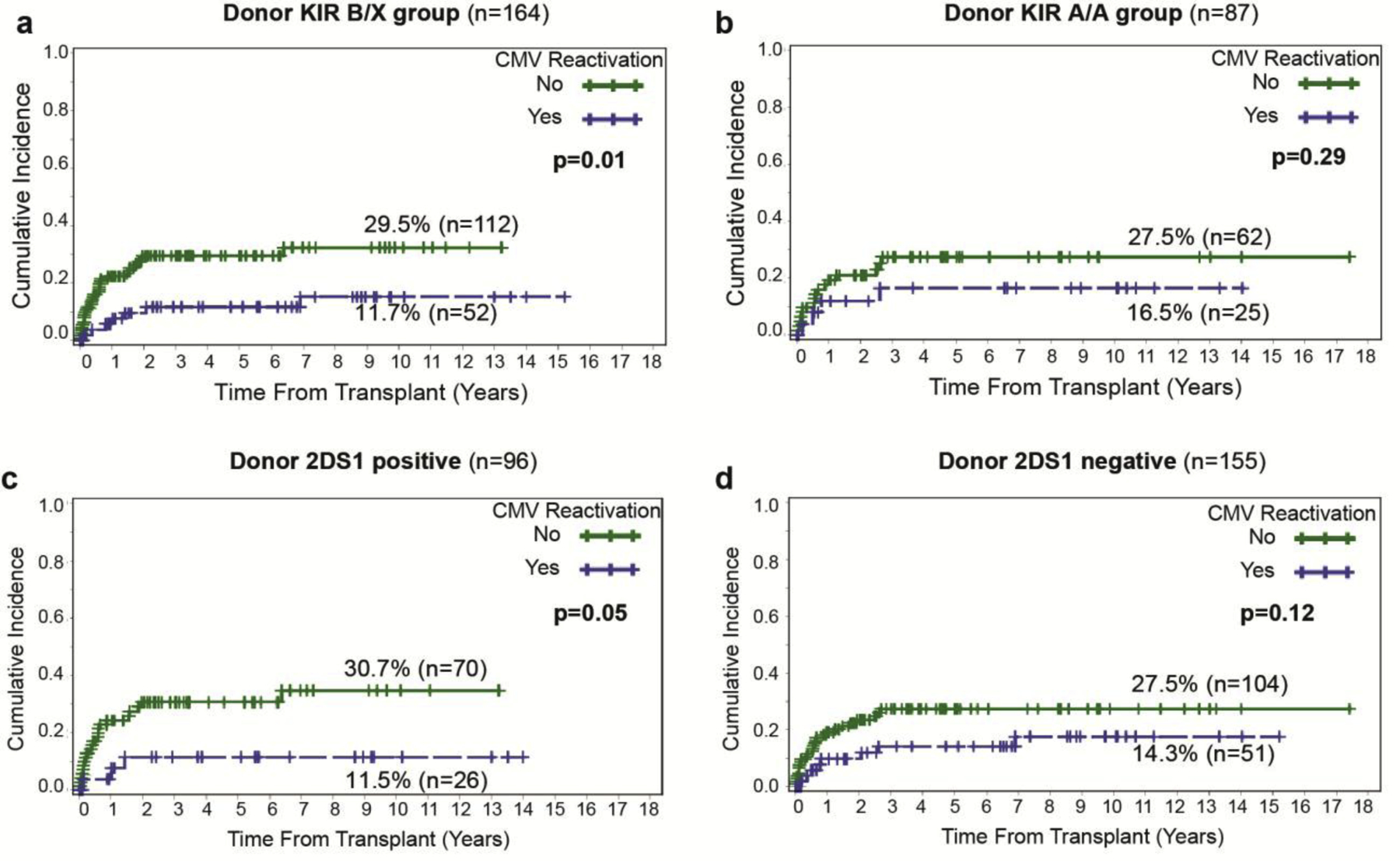
(a) Significant association between CMV reactivation and CIR in patients receiving HCT from KIR Bx donors versus (b) No significant outcomes when the donor is KIR A/A positive. (c) Protective effect of CMV reactivation when donor is 2DS1 positive and (d) Non-significant association between CIR and CMV reactivation when donor is 2DS1 negative.
3.4. Impact of KIR genotypes on AML relapse and CMV reactivation
We next examined multiple genotypes and missing-self recognition algorithms to evaluate their impact on AML relapse (supplementary table 1) and CMV reactivations (supplementary table 2). In our cohort, there were no genotypes or donor KIR-patient HLA combinations, which were significantly associated with AML relapse or CMV reactivations (Supplementary tables 1 and 2). However, when we examined the interaction between KIR and CMV in association with relapse risk, there was a trend towards reduced relapse when donor had 2DL2/3 genotype (all donors in our cohort had at least a copy of 2DL2 or 2DL3) and recipient was missing the corresponding C1 (p=0.06) (supplementary table 3).
Forcing the combination of donor KIR2DL2/3 with recipient C1(2DL2/3-C2C2) into the multivariate model showed no significant improvement on the model fit compared to using variables ≤ p=0.1 in univariate. Forcing the KIR genotype “BX vs. AA” or “2DS1+ vs. 2DS1-”, did not improve the model. We also systemically examined the interaction between KIR genotypes and CMV reactivation on the reduction of relapse risk (Supplementary Tables 3 and 4), but did not observe the KIR modifying the impact of CMV reactivation on relapse in our dataset.
4. Discussion
In this study, we independently confirmed the previous observation that CMV reactivation is associated with reduced risk of AML relapse in a relatively homogenous cohort (fully matched donors, CR1 or CR2 only). In multivariate analysis, only cytogenetic risk (high vs. others) and CMV reactivations were significant factors predicting AML relapse. Our data, in accordance with Green et al,3 indicated an association between CMV reactivation and AML relapse-protection. However, because of increased risk of NRM in the control group, the overall/disease-free survival rates between the two groups stayed similar.
Our results are, however unique from others with regards to the impact of CMV reactivation on RIC-HCTs. The original study by Elmaagacli and colleagues included only patients who received MAC.1 In a larger retrospective study, MAC was given to 83% of their cohort (n=2138), and no detailed assessment was presented for the RIC subgroup.3 Manjappa and colleagues reported that CMV reactivation did not significantly affect the risk of relapse in their RIC cohort of 58 patients.4 However, our current data, in contrast with the study by Manjappa et al,4 suggest that the impact of CMV reactivation on the risk of relapse was greater in the RIC recipients. This observed difference might be due to the difference in the sample size of RIC cohorts (n=58 vs. 125 in our cohort), methods of CMV detection (PCR only vs. shell vial culture and PCR), use of ATG in the conditioning regimen (44 of 58 patients received ATG in Manjappa’s cohort), and differences in the exact regimens within RIC conditioning between the two cohorts. In particular, the use of ATG might have reduced the impact of CMV reactivation as observed by Busca et al.26 and Bao et al.25 Thompson et al. postulated that delayed T-cell reconstitution due to alemtuzumab-containing regimens could potentially eliminate the beneficial effects of CMV reactivation in RIC transplant patients.27 Therefore, these clinical heterogeneities in conditioning regimens, GVHD prophylaxis, and methods of CMV detection preemptive therapy are likely contributing to the conflicting results among single-center and registry studies.1–3,5
Although several studies have reported an association between CMV reactivation and decreased relapse after HCT in AML patients, the biological mechanism(s) underlying this observation remain highly elusive. The possibility of direct cytotoxic effects by ganciclovir is unlikely based on Green’s analysis in which ganciclovir-induced cytopenia (possible surrogate for cytotoxic effects) was not associated with relapse.3 It is also unlikely that CMV-specific T cells exert bystander cytotoxicity to AML blasts. At least adoptive cellular immunotherapy using CMV-specific T cells did not show a significant difference in relapse risk in a small retrospective analysis by Thompson et al, in which the 5-year CIR was 25%, vs. 25% in those not receiving cellular immunotherapy.27 Although NK cells are the predominant lymphocyte population to reconstitute early after HCT, their GVL effect is reported to be limited by delayed NK cell functional maturation throughout the first year post-HCT.28–30 Foley et al. demonstrated a robust expansion in interferon-gamma (IFN-γ)–producing NKG2C+ NK, in HCT recipients as early as 2 weeks after detection of CMV viremia.7 In a more recent study Pical-Izard et al, reported that rapidly emerging NK cells remain immature (NKG2A+ CD56dim) for more than 6 months after RIC-HCT, most probably due to GVHD prophylaxis with cyclosporine A, but the expansion of “memory-like” NK cell is faster in patients with CMV reactivation.31 Therefore, one promising hypotheses to explain the relapse protection after CMV reactivation would be the upregulation of more functional “memory NK” post-HCT.
Multiple groups have previously reported that the effect of CMV reactivation is most significant for reducing relapse in AML patients.3,5 Interestingly, GVL effects of NK cells are shown to be more prominent for AML compared to ALL or lymphomas that are intrinsically resistant to NK recognition.21,22 In accordance with other studies, Cichocki and colleagues recently reported that patients with reactivated CMV had lower leukemia relapse and superior DFS one year after RIC-HCT compared with CMV seronegative recipients.30 Further analysis of the reconstituting NK cells in these patients demonstrated that CMV reactivation is associated with both higher frequencies and greater absolute numbers of CD56dim CD57+ NKG2C+ NKs, particularly after RIC-HCT.30 Furthermore, expansion of the adaptive NK at 6 months post-transplant independently trended toward a lower 2-year relapse risk, suggesting that these cells might play a role in the observed protective effect of CMV reactivation.
In fact a number of studies demonstrated various associations between specific donor KIR haplotypes, genotypes, and combinations between donor KIR and recipient KIR ligands with AML relapse or survival after allo-HCT. In particular, donor aKIR haplotype (Bx) has been shown to be protective against AML relapse compared with donor KIR A/A haplotype.17–21 Moreover, KIR2DS1 status of HCT donors has shown to also been linked with an improved transplant outcome with reduced AML relapse.22 Based on this knowledge, we examined subgroups of patients in our cohort depending on these two KIR-type groups. Interestingly, the protective effects of CMV reactivation were more significant when donors had KIR Bx haplotype or activating 2DS1, suggesting that CMV infection might trigger and enhance a response by NK expressing aKIRs, leading to exert GVL effects, more so than CMV infection in less active NK cell populations. While additional examination of the interaction terms between KIR genotypes and CMV reactivation in preventing AML relapse showed no clear interaction between the two variables, further mechanistic studies are warranted to better define the NK cell response to CMV infection and its impact on GVL effects.
In summary we confirmed the previously described association between CMV and AML relapse in an independent cohort with a relatively large number of RIC-HCT patients. The decreased relapse incidence was offset by increased NRM with no survival benefit in CMV reactivation. The protective effect of CMV reactivation against AML relapse seems augmented in donors with activating KIRs; supporting the hypothesis that NK and KIR-ligand interactions are at least partially involved in the mechanism of CMV-associated protection from AML relapse, and justify further correlative/mechanistic studies.
Supplementary Material
Table 1b.
Donor KIR Classification
| Variable | Median (Range) or N (%) |
|---|---|
| Donor KIR Genotype | |
| AA | 87 (32) |
| BX | 164 (59) |
| Not Available | 25 (9) |
| Donor Centromeric | |
| A/A | 136 (49) |
| A/B | 96 (35) |
| B/B | 19 (7) |
| Not Available | 25 (9) |
| Donor Telomeric | |
| A/A | 150 (54) |
| A/B | 90 (33) |
| B/B | 11 (4) |
| Not Available | 25 (9) |
| Donor (2DL2/2DL3) | |
| 2DL2 homozygote | 20 (7) |
| 2DL3 homozygote | 140 (51) |
| 2DL2, 2DL3 | 91 (33) |
| Not Available | 25 (9) |
| KIR HLA | |
| C1C1 | 41 (15) |
| C1C2 or C2C2 | 210 (76) |
| Not Available | 25 (9) |
HIGHLIGHTS.
The incidence of AML relapse is reduced with CMV reactivation.
NK cells are involved in CMV-associated protection from AML relapse.
CMV infection trigger/enhance a response by NK expressing activating KIRs
Acknowledgements
The authors thank Dr. Sandra Thomas for her critical review of the manuscript. We also thank City of Hope staff and nurses, as well as the patients and their families, without whom this work would not be possible. This study was partially supported by NIH P30 CA033572 (Biostatistics Core).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: Authors declare no relevant COI.
References
- 1.Elmaagacli AH, Steckel NK, Koldehoff M, et al. Early human cytomegalovirus replication after transplantation is associated with a decreased relapse risk: evidence for a putative virus-versus-leukemia effect in acute myeloid leukemia patients. Blood 2011;118(5):1402–1412. [DOI] [PubMed] [Google Scholar]
- 2.Ito S, Pophali P, Co W, et al. CMV reactivation is associated with a lower incidence of relapse after allo-SCT for CML. Bone Marrow Transplant 2013;48(10):1313–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Green ML, Leisenring WM, Xie H, et al. CMV reactivation after allogeneic HCT and relapse risk: evidence for early protection in acute myeloid leukemia. Blood 2013;122(7):1316–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manjappa S, Bhamidipati PK, Stokerl-Goldstein KE, et al. Protective effect of cytomegalovirus reactivation on relapse after allogeneic hematopoietic cell transplantation in acute myeloid leukemia patients is influenced by conditioning regimen. Biol Blood Marrow Transplant 2014;20(1):46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takenaka K, Nishida T, Asano-Mori Y, et al. Cytomegalovirus Reactivation after Allogeneic Hematopoietic Stem Cell Transplantation is Associated with a Reduced Risk of Relapse in Patients with Acute Myeloid Leukemia Who Survived to Day 100 after Transplantation: The Japan Society for Hematopoietic Cell Transplantation Transplantation-related Complication Working Group. Biol Blood Marrow Transplant 2015;21(11):2008–2016. [DOI] [PubMed] [Google Scholar]
- 6.Teira P, Battiwalla M, Ramanathan M, et al. Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: a CIBMTR analysis. Blood 2016;127(20):2427–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foley B, Cooley S, Verneris MR, et al. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood 2012;119(11):2665–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Della Chiesa M, Falco M, Podesta M, et al. Phenotypic and functional heterogeneity of human NK cells developing after umbilical cord blood transplantation: a role for human cytomegalovirus? Blood 2012;119(2):399–410. [DOI] [PubMed] [Google Scholar]
- 9.Vilches C, Parham P. KIR: diverse, rapidly evolving receptors of innate and adaptive immunity. Annu Rev Immunol 2002;20:217–251. [DOI] [PubMed] [Google Scholar]
- 10.Carrington M, Martin MP. The impact of variation at the KIR gene cluster on human disease. Curr Top Microbiol Immunol 2006;298:225–257. [DOI] [PubMed] [Google Scholar]
- 11.Lanier LL. Evolutionary struggles between NK cells and viruses. Nat Rev Immunol 2008;8(4):259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cook M, Briggs D, Craddock C, et al. Donor KIR genotype has a major influence on the rate of cytomegalovirus reactivation following T-cell replete stem cell transplantation. Blood 2006;107(3):1230–1232. [DOI] [PubMed] [Google Scholar]
- 13.Zaia JA, Sun JY, Gallez-Hawkins GM, et al. The effect of single and combined activating killer immunoglobulin-like receptor genotypes on cytomegalovirus infection and immunity after hematopoietic cell transplantation. Biol Blood Marrow Transplant 2009;15(3):315–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruggeri L, Capanni M, Casucci M, et al. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood 1999;94(1):333–339. [PubMed] [Google Scholar]
- 15.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002;295(5562):2097–2100. [DOI] [PubMed] [Google Scholar]
- 16.Giebel S, Locatelli F, Lamparelli T, et al. Survival advantage with KIR ligand incompatibility in hematopoietic stem cell transplantation from unrelated donors. Blood 2003;102(3):814–819. [DOI] [PubMed] [Google Scholar]
- 17.Bornhauser M, Schwerdtfeger R, Martin H, Frank KH, Theuser C, Ehninger G. Role of KIR ligand incompatibility in hematopoietic stem cell transplantation using unrelated donors. Blood 2004;103(7):2860–2861; author reply 2862. [DOI] [PubMed] [Google Scholar]
- 18.Davies SM, Ruggieri L, DeFor T, et al. Evaluation of KIR ligand incompatibility in mismatched unrelated donor hematopoietic transplants. Killer immunoglobulin-like receptor. Blood 2002;100(10):3825–3827. [DOI] [PubMed] [Google Scholar]
- 19.Bishara A, De Santis D, Witt CC, et al. The beneficial role of inhibitory KIR genes of HLA class I NK epitopes in haploidentically mismatched stem cell allografts may be masked by residual donor-alloreactive T cells causing GVHD. Tissue Antigens 2004;63(3):204–211. [DOI] [PubMed] [Google Scholar]
- 20.Beelen DW, Ottinger HD, Ferencik S, et al. Genotypic inhibitory killer immunoglobulin-like receptor ligand incompatibility enhances the long-term antileukemic effect of unmodified allogeneic hematopoietic stem cell transplantation in patients with myeloid leukemias. Blood 2005;105(6):2594–2600. [DOI] [PubMed] [Google Scholar]
- 21.Hsu KC, Keever-Taylor CA, Wilton A, et al. Improved outcome in HLA-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by KIR and HLA genotypes. Blood 2005;105(12):4878–4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venstrom JM, Pittari G, Gooley TA, et al. HLA-C-dependent prevention of leukemia relapse by donor activating KIR2DS1. N Engl J Med 2012;367(9):805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slovak ML, Kopecky KJ, Cassileth PA, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood 2000;96(13):4075–4083. [PubMed] [Google Scholar]
- 24.Sun JY, Gaidulis L, Miller MM, et al. Development of a multiplex PCR-SSP method for Killer-cell immunoglobulin-like receptor genotyping. Tissue Antigens 2004;64(4):462–468. [DOI] [PubMed] [Google Scholar]
- 25.Bao X, Zhu Q, Xue S, et al. Cytomegalovirus induces strong antileukemic effect in acute myeloid leukemia patients following sibling HSCT without ATG-containing regimen. Am J Transl Res 2016;8(2):653–661. [PMC free article] [PubMed] [Google Scholar]
- 26.Busca A, Passera R, Pini M, et al. The use of ATG abrogates the antileukemic effect of cytomegalovirus reactivation in patients with acute myeloid leukemia receiving grafts from unrelated donors. Am J Hematol 2015;90(6):E117–121. [DOI] [PubMed] [Google Scholar]
- 27.Thomson KJ, Mackinnon S, Peggs KS. CMV-specific cellular therapy for acute myeloid leukemia? Blood 2012;119(4):1088–1090; author reply 1090–1081. [DOI] [PubMed] [Google Scholar]
- 28.Jacobs R, Stoll M, Stratmann G, Leo R, Link H, Schmidt RE. CD16- CD56+ natural killer cells after bone marrow transplantation. Blood 1992;79(12):3239–3244. [PubMed] [Google Scholar]
- 29.Nguyen S, Dhedin N, Vernant JP, et al. NK-cell reconstitution after haploidentical hematopoietic stem-cell transplantations: immaturity of NK cells and inhibitory effect of NKG2A override GvL effect. Blood 2005;105(10):4135–4142. [DOI] [PubMed] [Google Scholar]
- 30.Cichocki F, Cooley S, Davis Z, et al. CD56dimCD57+NKG2C+ NK cell expansion is associated with reduced leukemia relapse after reduced intensity HCT. Leukemia 2016;30(2):456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pical-Izard C, Crocchiolo R, Granjeaud S, et al. Reconstitution of natural killer cells in HLA-matched HSCT after reduced-intensity conditioning: impact on clinical outcome. Biol Blood Marrow Transplant 2015;21(3):429–439. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


