Abstract
Background
COVID-19 has recently been associated with the development of Takotsubo cardiomyopathy (TCM). This scoping review aims to summarize the existing evidence regarding TCM in COVID-19 and offer future direction for study.
Methods
Following the PRISMA Extension for Scoping Reviews, MEDLINE and EMBASE were searched for all peer-reviewed articles with relevant keywords including “Takotsubo”, “Stress-induced cardiomyopathy” and “COVID-19” from their inception to September 25, 2021.
Results
A total of 40 articles with 52 cases were included. Patients with TCM and COVID-19 showed only slight female predominance (59.6%), median age of 68.5 years, and were mostly of the apical subtype (88.6%). All-cause mortality was 36.5%. The median LVEF was 30%. Compared to those without TCM, those with TCM in COVID-19 had more critical illness, higher mortality, lower LVEF, and higher cardiac and inflammatory biomarkers. Notably, the diagnostic criteria of TCM were considerably different between case reports and observational studies.
Conclusion
This scoping review identifies that TCM in COVID-19 may have distinct features that distinguish this condition from TCM without COVID-19. Future studies are warranted to help describe risk factors, determine the utility of inflammatory biomarkers and serum catecholamine levels, and establish disease-specific diagnostic criteria.
Keywords: Takotsubo cardiomyopathy, COVID-19, Scoping review, Systematic review
1. Introduction
The spread of SARS-CoV-2 has resulted in a global pandemic with over 200 million cumulative cases and 5 million deaths as of November 2021 per the World Health Organization [1]. Coronavirus disease 2019 (COVID-19) is known for its severe respiratory illness however the spectrum of the clinical syndrome is yet to be fully comprehended [2]. This clinical syndrome is not isolated to the respiratory system, and viral particles have been confirmed in cardiac tissue via autopsy [3]. A recent meta-analysis by Santoso et al. found an association between cardiac injury in SARS-CoV2 patients, and increased mortality and need for intensive care [4]. Some examples of cardiac complications in COVID-19 include myocarditis, pericarditis, stress-induced cardiomyopathy (Takotsubo cardiomyopathy: TCM), arrhythmias, and acute coronary syndrome [5].
TCM is a heart failure syndrome with similar early and late mortality as ST-elevation and non-ST-elevation myocardial infarction [6]. The Revised Mayo Clinic Criteria is widely used as diagnostic criteria for TCM and is defined as follows: 1) Transient dyskinesis of the left ventricular mid-segments, with or without apical involvement; the regional wall-motion abnormalities extend beyond a single epicardial vascular distribution, and a stressful trigger is often, but not always, present; 2) Absence of obstructive coronary disease or absence of angiographic evidence of acute plaque rupture; 3) New electrocardiogram abnormalities (ST-segment elevation and/or T-wave inversion) or modest elevation in the cardiac troponin level; 4) Absence of pheochromocytoma and myocarditis [7]. There are also additional diagnostic guidelines per the Heart Failure Association-European Society of Cardiology Criteria and the International Takotsubo Diagnostic Criteria and the newer 2018 International Takotsubo Diagnostic Criteria (InterTAK Diagnostic Criteria) (Table 1). Clinically, the diagnosis of Takotsubo cardiomyopathy is often made when cardiac catheterization of a patient with suspected acute myocardial infarction reveals no significant blockage and the presence of anteroapical dyskinesis. Recently, the Heart Failure Association Takotsubo Syndrome Study Group and Myocardial Function Working Group of European Society of Cardiology published a joint position paper to describe pathophysiology of TCM [8]. The role of central and peripheral nervous system, autonomic-limbic integration in particular, gene profiling with microRNAs to pinpoint the genetic predisposition, as well as chronic cardiovascular abnormalities post-TCM periods, are gaining more attention in the area. Since early 2020, a number of case reports have been published reporting TCM in COVID-19 patients.
Table 1.
Summary of current diagnostic criteria for Takotsubo cardiomyopathy.
| Heart Failure Association of the European Society of Cardiology | 1. Transient regional wall motion abnormalities of LV or RV myocardium which are frequently, but not always, preceded by a stressful trigger (emotional or physical). 2. The regional wall motion abnormalities usually extend beyond a single epicardial vascular distribution, and often result in circumferential dysfunction of the ventricular segments involved. 3. The absence of culprit atherosclerotic coronary artery disease including acute plaque rupture, thrombus formation, and coronary dissection or other pathological conditions to explain the pattern of temporary LV dysfunction observed (e.g. hypertrophic cardiomyopathy, viral myocarditis). 4. New and ECG abnormalities (ST-segment elevation, ST depression, LBBB, T-wave inversion, and/or QTc prolongation) during the acute phase (3 months). 5. Significantly elevated serum natriuretic peptide (BNP or NT-proBNP) during the acute phase. 6. Positive but relatively small elevation in cardiac troponin measured with a conventional assay (i.e. disparity between the troponin level and the amount of dysfunctional myocardium present). 7. Recovery of ventricular systolic function on cardiac imaging at follow-up (3–6 months). |
| International Takotsubo Diagnostic Criteria and the newer 2018 International Takotsubo Diagnostic Criteria (InterTAK Diagnostic Criteria) | 1. Patients show transienta left ventricular dysfunction (hypokinesia, akinesia, or dyskinesia) presenting as apical ballooning or midventricular, basal, or focal wall motion abnormalities. Right ventricular involvement can be present. Besides these regional wall motion patterns, transitions between all types can exist. The regional wall motion abnormality usually extends beyond a single epicardial vascular distribution; however, rare cases can exist where the regional wall motion abnormality is present in the subtended myocardial territory of a single coronary artery (focal Takotsubo). 2. An emotional, physical, or combined trigger can precede the Takotsubo syndrome event, but this is not obligatory. 3. Neurologic disorders (e.g. SAH, TIA/stroke, or seizures) as well as pheochromocytoma may serve as triggers for Takotsubo syndrome. 4. New ECG abnormalities are present (ST-segment elevation, ST-segment depression, T-wave inversion, and QTc prolongation); however, rare cases exist without any ECG changes. 5. Levels of cardiac biomarkers (troponin and CK) are moderately elevated in most cases; significant elevation of brain natriuretic peptide is common. 6. Significant coronary artery disease is not a contradiction in Takotsubo syndrome. 7. Patients have no evidence of infectious myocarditis.b 8. Postmenopausal women are predominantly affected. a. Wall motion abnormalities may remain for a prolonged period of time or documentation of recovery may not be possible. For example, death before evidence of recovery is captured. b. Cardiac magnetic resonance imaging is recommended to exclude infectious myocarditis and diagnosis confirmation of Takotsubo syndrome. |
Abbreviations: BNP, B-type natriuretic peptide; CK, creatine kinase; ECG, electrocardiography; LBBB, left bundle branch block; LV, left ventricle; NT-proBNP, N-terminal-pro-B-type natriuretic peptide; RV, right ventricle; SAH, subarachnoid hemorrhage; TIA, transient ischemic attack.
To date, however, no systematic scoping reviews are available to analyze the current evidence to identify the trends of TCM in COVID-19. In this study, we summarized the up-to-date evidence available regarding TCM in COVID-19, and identify gaps for future studies.
2. Materials and methods
2.1. Study design
This is a systematic scoping review conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension for scoping reviews (PRISMA-ScR) [9], [10]. See Appendix 1 for PRISMA-ScR Checklist of the present study.
2.2. Search strategy
We searched MEDLINE and EMBASE for all peer-reviewed articles from inception to September 25th 2021. No filters for study design and language were used. A manual screening for additional pertinent articles was done using the reference lists of all articles that met the eligibility criteria. The search strategy involved relevant keywords, including “Takotsubo”, “Stress-induced cardiomyopathy” and “COVID-19.” The search was conducted by two authors (WT and YN) independently. See Appendix 2 for detailed search terms.
2.3. Eligibility criteria
The criteria for the inclusion of articles are the following:
-
(1)
Peer-reviewed articles evaluating the relationship between COVID-19 and TCM, or reporting cases of TCM in patients with laboratory-confirmed COVID-19.
-
(2)
Randomized controlled trials (RCTs), case-control studies, cohort studies (prospective or retrospective), cross-sectional studies, and case series in adult patients
The exclusion criteria included the following:
-
(1)
Qualitative studies, review articles, and commentaries.
-
(2)
Conference abstracts.
-
(3)
Studies involving pediatric patients.
-
(4)
Diagnosis of COVID-19 made without confirmatory polymerase chain reaction (PCR) testing.
-
(5)
Concurrent myocarditis, pericarditis, or significant coronary artery disease
2.4. Study selection
Articles selected for full-text assessment were assessed independently by WT and YN using EndNote 20 reference management software. Articles considered eligible were then evaluated in full length with the inclusion and exclusion criteria.
2.5. Data extraction and definition
A standardized data collection form that followed the PRISMA and Cochrane Collaboration guidelines for systematic reviews was used to obtain the following information from each study: title, name of authors, year of publication, country of origin, study characteristics, target outcome, aims, study and comparative groups, key findings, and limitations. We also statistically analyzed data from existing case reports and case series to identify clinical characteristics of TCM in COVID-19. For the severity of COVID-19, we employed the definition proposed by the United States National Institute of Health. In brief, patients are categorized as moderate illness if there is evidence of lower respiratory disease with oxygen saturation (SpO2) ≥ 94% on room air. Severe illness is defined as a condition with SpO2 < 94% on room air, a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen < 300 mm Hg, respiratory rate > 30/min, or lung infiltrates > 50%. Patients are categorized as having critical illness when they have respiratory failure, septic shock, or multiple organ dysfunction [11].
2.6. Statistical analysis
We analyzed the data using JMP version 15.1.0 (SAS Institute Inc., Cary, North Carolina) to calculate the median and the interquartile ranges to analyze data from existing cases.
3. Results
3.1. Search results and study selection
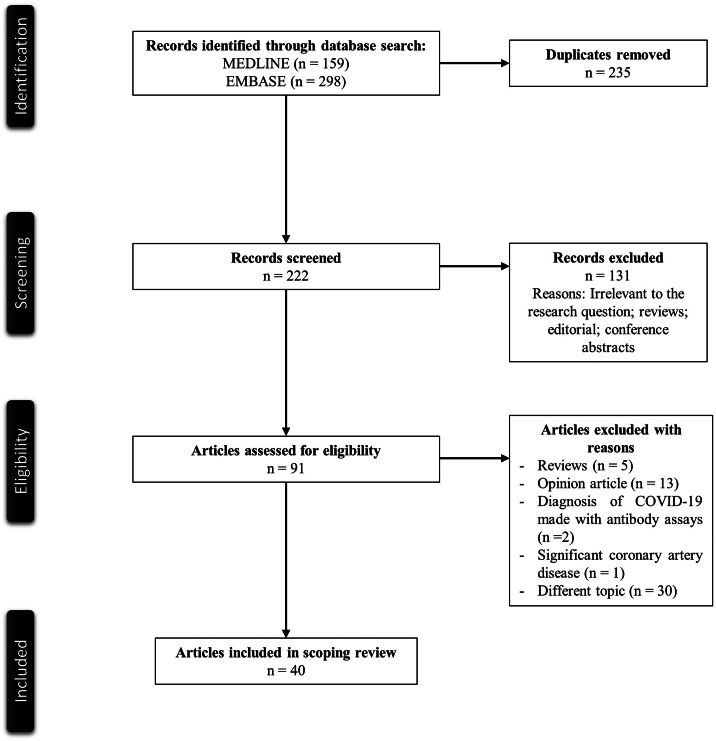
Fig. 1 illustrates a PRISMA flow diagram that depicts the process of identification, screening, eligibility, and inclusion or exclusion of the studies. The initial search of MEDLINE and EMBASE databases yielded 159 and 298 articles respectively. 255 duplicate studies were removed. 222 articles were screened based on their relevance and type of article. 131 articles that were either review articles, editorials, or conference abstracts, were excluded from the study. 91 articles were then evaluated for full text review for study inclusion per our eligibility criteria. Reviews, opinion articles, studies that diagnosed COVID-19 without confirmatory PCR testing, a case with significant coronary artery disease, and irrelevant topics were excluded. 40 articles with 52 cases were included in our review [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49].
Fig. 1.
PRISMA flowchart of the search strategy.
3.2. Description of included studies
Table 2 describes the main characteristics of two case control studies from the scoping review [50], [51]. A USA study by Giustino et al. evaluated the clinical characteristics and outcomes of patients with TCM and COVID-19 in comparison with COVID-19 patients without myocardial injury and those with myocardial injury of other causes. Outcomes included all-cause mortality, intensive care unit (ICU) admission, and development of acute respiratory distress syndrome or acute kidney injury (AKI). In their cohort, patients with TCM had significantly higher peak troponin-I levels than other groups (median: 11.40, interquartile range [IQR]: 0.55–12.55) ng/mL vs. 0.67 (0.09–2.33) ng/mL in those with other myocardial injury vs. 0.01 (0.00–0.02) ng/mL in those without myocardial injury, (p < 0.0001). Also, LVEF was significantly lower in those with TCM than comparative groups 36% (35–37) vs. 55% (49–71) in those with other myocardial injury vs. 57% (39–65) in those without myocardial injury, (p = 0.001). Those with TCM had higher mortality (40.0%) than those with other myocardial injury (30.0%) and those without myocardial injury (2.3%). Of note, peak serum D-dimer levels, interleukin-6 (IL-6), C-reactive protein (CRP), and brain natriuretic peptide (BNP), and prevalence of right ventricular (RV) dysfunction were higher in those with other myocardial injury than those with TCM. Interestingly, all of the patients with TCM were male. Four out of five had typical TCM (circumferential hypokinesis or akinesis of the apical and mid-wall segments) while the remaining patient had reverse TCM (hypokinesis of basal walls). Another study by Templin et al. from Switzerland aimed to elucidate features of COVID-19 patients who developed TCM to infer underlying pathology. They included 11 patients with COVID-19 and TCM as well as COVID-19 patients without TCM (n = 97) and TCM patients without COVID-19 (n = 3215). Those with COVID-19 and TCM were mostly female (81.8%) and elderly (mean age 72.4). The study did not provide quantitative data including serum troponin levels, BNP, and LVEF. Instead, it was noted that approximately 70% of the patients with COVID-19 and TCM had either mechanical ventilation or in-hospital death, compared to those with TCM without COVID-19 (18.6%). Interestingly, the authors described the results of autopsy of four patients who died of TCM with COVID-19. All patients had contraction band necrosis with mononuclear infiltration, suggesting considerable sympathetic nerve activation.
Table 2.
Main characteristics of the included observational studies in the scoping reviews.
| Author Year Country |
Study type | Aim | Outcome | Population | Comparative groups | LVEF – median (%, IQR) | Key findings | Limitations |
|---|---|---|---|---|---|---|---|---|
| Giustino et al. 2020 USA | CC | To evaluate the clinical characteristics and outcomes of patients with TCM with COVID-19 | All-cause death Intensive care unit admission ARDS AKI |
TCM with COVID-19 (n = 5) |
Other myocardial injury (n = 69) and no myocardial injury (n = 43) with COVID-19 | TCM with COVID-19 36 (35–37) Other myocardial injury 55 (41–65) No myocardial injury 60 (57–65) |
Troponin I was significantly higher in TCM with COVID-19 than others (median 11.40 ng/mL, p < 0.0001) Significantly higher mortality in TCM with COVID-19 (40%, p = 0.001) Patients with TCM with COVID-19 were all male 4 with typical TCM (apical akinesis); 1 with reverse TCM (basal akinesis) |
Myocardial injury was defined as troponin I level ≧ 0.04 ng/mL None of the patients with TCM with COVID-19 underwent cardiac catheterization Small sample size |
| Templin et al. 2021 Switzerland |
CC | To elucidate features of COVID-19 patients who develop TCM | Mechanical ventilation or death | TCM with COVID-19 (n = 11) |
COVID-19 without TCM (n = 97) and TCM without COVID-19 (n = 3215) | Not available (mentioned as “LVEF was most significantly reduced in TCM with COVID-19” without specific data) | Significantly higher mortality in TCM with COVID-19 (70%) vs. TCM without COVID-19 (18.6%) | Heterogeneity of COVID-19 without TCM group |
Abbreviations: AKI, acute kidney injury; ARDS, acute respiratory distress syndrome; CC, case control; COVID-19, coronavirus disease 2019; LVEF, left ventricular ejection fraction; TCM, Takotsubo cardiomyopathy.
Table 3 presents baseline demographics, laboratory findings, and chief features of patients of TCM with COVID-19 from the existing cases (n = 52). TCM predominated in female COVID-19 patients (n = 31) and involved elderly patients (median 68.5 years old, IQR 58.0–78.0). The majority of these TCM patients were classified as having “critical illness” COVID-19 severity (78.8%). All-cause mortality occurred in 36.5% of the population. Most of the population had the apical TCM subtype (88.2%). Of those with reported LVEF, the median LVEF was 30% (IQR 25,0–40.0). In addition to elevated serum white blood cells, D-dimer, ferritin, lactate dehydrogenase, CRP, procalcitonin, and IL-6, which are seen in severe or critical COVID-19 patients, considerably high cardiac markers including CK-MB (median 44 IU/L, IQR 16.8–74.5), troponin I (median 0.324 ng/mL, IQR 0.134–2.81), troponin T (median 610 ng/L, IQR 423–775), BNP (median 507 pg/mL, IQR 253–1743), N-terminal-pro-BNP (NT-proBNP) (median 3787 pg/mL, IQR 1291–13,784) were noted.
Table 3.
Baseline demographics, laboratory findings, and chief features of the 52 patients from case reports and case series.
| Prevalence (%)a | Median (IQR) | |
|---|---|---|
| Age (years) | 68.5 (58.0–78.0) | |
| Sex | ||
| Male | 21/52 (40.4) | |
| Female | 31/52 (59.6) | |
| COVID-19 severity | ||
| Moderate | 2/52 (3.8) | |
| Severe | 9/52 (17.3) | |
| Critical illness | 41/52 (78.8) | |
| Death | 19/52 (36.5) | |
| Type of TCM | ||
| Takotsubo (apical) | 42/52 (80.8) | |
| Reverse | 6/52 (11.5) | |
| Midventricular | 2/52 (3.8) | |
| Biventricular | 2/52 (3.8) | |
| LVEF (%) | 37/52 (71.2) | 30.0 (25.0–40.0) |
| Laboratory findings | ||
| WBC (103/μL) | 20/52 (38.5) | 13.2 (9.5–20.1) |
| BNP (pg/mL) | 8/52 (15.4) | 507 (253–1743) |
| NT-proBNP (pg/mL) | 22/52 (42.3) | 3787 (1291–13,784) |
| Troponin T (ng/L) | 7/52 (13.5) | 610 (423–775) |
| Troponin I (ng/mL) | 30/52 (57.7) | 0.324 (0.134–2.81) |
| CK-MB (IU/L) | 6/52 (11.5) | 35.4 (10.5–64.8) |
| D-dimer (ng/mL) | 31/52 (59.6) | 1681 (910–3340) |
| Ferritin (ng/mL) | 26/52 (50.0) | 1050 (531–2615) |
| LDH (U/L) | 16/52 (30.8) | 399 (332–919) |
| CRP (mg/L) | 35/52 (67.3) | 168.8 (46.0–267) |
| Procalcitonin (ng/mL) | 8/52 (15.4) | 2.09 (0.155–5.96) |
| IL-6 (pg/mL) | 10/52 (19.2) | 460 (363–981) |
Abbreviations: BNP, B-type natriuretic peptide; NT-proBNP, N-terminal-pro-B-type natriuretic peptide; CK-MB, creatine kinase-MB isozyme; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; IL-6, interleukin 6; IQR, interquartile range; LDH, lactate dehydrogenase; LVEF, left ventricular ejection fraction; TCM, Takotsubo cardiomyopathy; WBC, white blood cell.
Prevalence here is defined as the number of cases reported the variable divided by the number of the total cases.
4. Discussion
This is the first systematic scoping review and analysis of existing case reports of TCM in COVID-19 patients. Although there has been limited literature with low quality of evidence regarding TCM in COVID-19, several interesting findings were noted as follows. Compared to TCM without COVID-19, where more than 90% of patients are female, the present results show only 59.6% of TCM with COVID-19 are female, suggesting that TCM in COVID-19 might have different pathogenesis.
Catecholamine surge is known to play a significant role in the pathogenesis of TCM [52], [53], [54]. One of the hallmarks of COVID-19 is cytokine storm, which causes a vicious cycle of subsequent catecholamine surges [55]. Since cytokine storm in COVID-19 may worsen in proportion to its disease severity, part of the causes of death in severe COVID-19 might include undiagnosed TCM associated with catecholamine surge. However, it has been unclear whether the extent of serum catecholamine levels is associated with TCM pathogenesis and its prognosis. Future studies may target the comparison of serum catecholamine levels of the following four groups; TCM with or without COVID-19, COVID-19 with or without TCM; to evaluate the utility of serum catecholamine levels in the diagnosis and prognostication of TCM.
Regarding sex differences, about 90% of TCM patients were postmenopausal women in non-COVID-19 cases [56]. Interestingly, the present results suggest that only 59.6% of TCM with COVID-19 were female. There are several possible explanations for the difference. First, the male gender may be associated with a higher prevalence of physical stressors and thus more vulnerable to triggers such as COVID-19. A previous multi-center registry data suggested that 50% of those with TCM with male gender had physical stress defined as acute respiratory failure, infection, or other insults [57]. In comparison, only 31.3% of those with female gender had such preceding stress. Second, there may be a protective role of estrogen against TCM and severe COVID-19. Emerging evidence suggests the usefulness of exogenous estrogen against COVID-19, which may be due to its immunomodulatory effects and endothelial stabilization effects [58], [59], [60], [61]. Unfortunately, none of the existing literature has pointed out the role of estrogen in TCM with COVID-19, although the importance of estrogen has been extensively described [8]. The role of estrogen in TCM with COVID-19 and its protective effect against physical stress and the extent of pro-inflammatory cytokines needs to receive more attention in the future.
Regarding prognosis, 36.5% of the patients included in our study expired. The mortality rate is considerably higher than previously reported in those with TCM without COVID-19, ranging 0.95–2.3% in those without cardiogenic shock and 13.6–23.5% in those with cardiogenic shock [62], [63]. However, the mortality rates of patients with severe or critical COVID-19 in the ICU have been reported as around 50% [64]. Thus, whether the high mortality in patients with TCM and COVID-19 is attributable to TCM or COVID-19 itself remains to be established with a prospective study comparing the mortality of COVID-19 patients with and without TCM, preferably using propensity-matched cohorts.
This study has several limitations. First, due to the urgent need for evidence on this topic and limited time, we did not contact the authors to clarify the details of the data described in the literature. Second, since we analyzed the data of published reports, there might be publication bias related to overestimating the prevalence of clinical symptoms and laboratory data as challenging cases are more likely to be reported and published. Third, the small number of observational studies with low-quality evidence, cases, and missing data in some of the included reports might lessen the precision of our analysis. Fourth, diagnostic criteria of TCM used differed considerably between each case report and observational study, which rendered heterogeneity in the present results. Finally, some of the patients considered to have TCM by the authors in the literature review may have other conditions due to the challenges of diagnosing TCM in COVID-19.
5. Conclusions
In conclusion, limited literature with low quality of evidence is available regarding TCM in COVID-19. However, our results suggest that TCM in COVID-19 might have different clinical features from those with TCM without COVID-19, characterized by almost evenly distributed genders and higher mortality. Future research areas related to TCM in COVID-19 may include the utility of serum catecholamine levels and inflammatory markers in its diagnosis, analysis of risk factors to develop TCM in COVID-19, including gender and the role of estrogen, and establishing disease-specific diagnostic criteria of TCM in COVID-19.
Funding
None.
CRediT authorship contribution statement
WT conceived the study, searched the literature, and drafted the manuscript. YN searched the literature, assessed the quality of the studies, revised the manuscript, and supervised the study. TN, GH, RH, PS, JY, and CK revised the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
None
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ahjo.2022.100092.
Appendix A. Supplementary data
Supplementary material 1 - PRISMA-ScR Checklist
Supplementary material 2 - Detailed search terms
References
- 1.World Health Organization WHO Coronavirus Dashboard. https://covid19.who.int/ Available at:
- 2.Azevedo R.B., Botelho B.G., Hollanda J.V.G., et al. Covid-19 and the cardiovascular system: a comprehensive review. J. Hum. Hypertens. 2021;35(1):4–11. doi: 10.1038/s41371-020-0387-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindner D., Fitzek A., Bräuninger H., et al. Association of Cardiac Infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020;5(11):1281–1285. doi: 10.1001/jamacardio.2020.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santoso A., Pranata R., Wibowo A., Al-Farabi M.J., Huang I., Antariksa B. Cardiac injury is associated with mortality and critically ill pneumonia in COVID-19: a meta-analysis. Am. J. Emerg. Med. 2021;44:352–357. doi: 10.1016/j.ajem.2020.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chilazi M., Duffy E.Y., Thakkar A., Michos E.D. COVID and cardiovascular disease: what we know in 2021. Curr. Atheroscler. Rep. 2021;23(7):37. doi: 10.1007/s11883-021-00935-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Redfors B., Vedad R., Angerås O., et al. Mortality in takotsubo syndrome is similar to mortality in myocardial infarction - a report from the SWEDEHEART registry. Int. J. Cardiol. 2015;185:282–289. doi: 10.1016/j.ijcard.2015.03.162. [DOI] [PubMed] [Google Scholar]
- 7.Boyd B., Solh T. Takotsubo cardiomyopathy: review of broken heart syndrome. Jaapa. 2020;33(3):24–29. doi: 10.1097/01.JAA.0000654368.35241.fc. [DOI] [PubMed] [Google Scholar]
- 8.Omerovic E., Citro R., Bossone E., et al. Pathophysiology of Takotsubo syndrome – a joint scientific statement from the Heart Failure Association Takotsubo Syndrome Study Group and Myocardial Function Working Group of the European Society of Cardiology – part 2: vascular pathophysiology, gender a. European Journal of Heart Failure. 2021 doi: 10.1002/ejhf.2368. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.McGowan J., Straus S., Moher D., et al. Reporting scoping reviews-PRISMA ScR extension. J. Clin. Epidemiol. 2020;123:177–179. doi: 10.1016/j.jclinepi.2020.03.016. [DOI] [PubMed] [Google Scholar]
- 10.Tricco A.C., Lillie E., Zarin W., et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann. Intern. Med. 2018;169(7):467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 11.National Institutes of Health Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. https://www.covid19treatmentguidelines.nih.gov/ Available at: [PubMed]
- 12.Alizadehasl A., Soleimani A., Peighambari M.M., Mostafavi A. Biventricular apical ballooning in patient with COVID-19. J. Echocardiogr. 2021:1–2. doi: 10.1007/s12574-021-00530-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alshamam M.S., Nso N., Idrees Z., Nassar M., Munira M.S. Coronavirus disease 2019 (COVID-19)-induced Takotsubo cardiomyopathy prognosis in geriatric setting. Cureus. 2021;13(7) doi: 10.7759/cureus.16211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bapat A., Maan A., Heist E.K. Stress-induced cardiomyopathy secondary to COVID-19. Case Rep. Cardiol. 2020 doi: 10.1155/2020/8842150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belli O., Ardissino M., Bottiroli M., et al. Emergency cardiac imaging for coronavirus disease 2019 (COVID-19) in practice: a case of Takotsubo stress cardiomyopathy. Cardiovasc. Ultrasound. 2021;19(1) doi: 10.1186/s12947-021-00251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernardi N., Calvi E., Cimino G., et al. COVID-19 pneumonia, takotsubo syndrome, and left ventricle thrombi. JACC: Case Rep. 2020;2(9):1359–1364. doi: 10.1016/j.jaccas.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhattacharyya P.J., Attri P.K., Farooqui W. Takotsubo cardiomyopathy in early term pregnancy: a rare cardiac complication of SARS-CoV-2 infection. BMJ Case Reports. 2020;13(9) doi: 10.1136/bcr-2020-239104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bottiroli M., De Caria D., Belli O., et al. Takotsubo syndrome as a complication in a critically ill COVID-19 patient. ESC Heart Fail. 2020;7(6):4297–4300. doi: 10.1002/ehf2.12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chao C.J., DeValeria P.A., Sen A., et al. Reversible cardiac dysfunction in severe COVID-19 infection, mechanisms and case report. Echocardiography. 2020;37(9):1465–1469. doi: 10.1111/echo.14807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chitturi K.R., Thacker S., Al-Saadi M.A., et al. Successful treatment of acute heart failure in COVID-19-induced cytokine storm with tocilizumab: a case report. Eur. Heart J. Case Rep. 2020;4(FI1):1–6. doi: 10.1093/ehjcr/ytaa188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dave S., Thibodeau J.T., Styrvoky K., Bhatt S.H. Takotsubo cardiomyopathy in a coronavirus disease-2019-positive patient: a case report. A A Pract. 2020;14(11) doi: 10.1213/XAA.0000000000001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demertzis Z.D., Dagher C., Malette K.M., et al. Cardiac sequelae of novel coronavirus disease 2019 (COVID-19): a clinical case series. Eur. Heart J. Case Rep. 2020;4(Fi1):1–6. doi: 10.1093/ehjcr/ytaa179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faqihi F., Alharthy A., Alshaya R., et al. Reverse takotsubo cardiomyopathy in fulminant COVID-19 associated with cytokine release syndrome and resolution following therapeutic plasma exchange: a case-report. BMC Cardiovasc. Disord. 2020;20(1) doi: 10.1186/s12872-020-01665-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujisaki T., Kassim F., Kassim G., Bandyopadhyay D., Singh V., Kim B. Biventricular takotsubo syndrome with COVID-19 in an Asian male. J. Cardiol. Cases. 2021;24(1):6–9. doi: 10.1016/j.jccase.2020.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gomez J.M.D., Nair G., Nanavaty P., Rao A., Marinescu K., Suboc T. COVID-19-associated Takotsubo cardiomyopathy. BMJ Case Rep. 2020;13(12) doi: 10.1136/bcr-2020-236811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hegde S., Khan R., Zordok M., Maysky M. Characteristics and outcome of patients with COVID-19 complicated by Takotsubo cardiomyopathy: case series with literature review. OpenHeart. 2020;7(2) doi: 10.1136/openhrt-2020-001360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoepler W., Traugott M.T., Christ G., et al. Clinical and angiographic features in three COVID-19 patients with takotsubo cardiomyopathy. Case report. SN Compr. Clin. Med. 2021;3(1):263–268. doi: 10.1007/s42399-020-00683-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kariyanna P.T., Chandrakumar H.P., Jayarangaiah A., et al. Apical takotsubo cardiomyopathy in a COVID-19 patient presenting with stroke: a case report and pathophysiologic insights. Am. J. Med. Case Rep. 2020;8(10):350–357. [Google Scholar]
- 29.Kong N., Singh N., Mazzone S., Burkhardt R., Anchan R., Blair J. Takotsubo’s syndrome presenting as cardiogenic shock in patients with COVID-19: a case-series and review of current literature. Cardiovasc. Revasc. Med. 2021;28S:50–53. doi: 10.1016/j.carrev.2021.01.017. [DOI] [PubMed] [Google Scholar]
- 30.Manzur-Sandoval D., Carmona-Levario P., García-Cruz E. Giant inverted T waves in a patient with COVID-19 infection. Ann. Emerg. Med. 2021;77(2):264–267. doi: 10.1016/j.annemergmed.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minhas A.S., Scheel P., Garibaldi B., et al. Takotsubo syndrome in the setting of COVID-19. JACC: Case Rep. 2020;2(9):1321–1325. doi: 10.1016/j.jaccas.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mishra A.K., Dai Q., Sahu K.K., ElMeligy A. Atypical takotsubo cardiomyopathy in COVID-19. Am. J. Med. Sci. 2021;362(5):e41–e42. doi: 10.1016/j.amjms.2021.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moderato L., Monello A., Lazzeroni D., et al. Takotsubo syndrome during SARS-CoV-2 pneumonia: a possible cardiovascular complication. 2006;21(6):417–420. doi: 10.1714/3359.33323. 2020. [DOI] [PubMed] [Google Scholar]
- 34.Ortuno S., Jozwiak M., Mira J.P., Nguyen L.S. Case report: takotsubo syndrome associated with novel coronavirus disease 2019. Front Cardiovasc. Med. 2021;8 doi: 10.3389/fcvm.2021.614562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oyarzabal L., Gómez-Hospital J.A., Comin-Colet J. Tako-tsubo syndrome associated with COVID-19. Rev. Esp. Cardiol. (Engl. Ed.) 2020;73(10):846. doi: 10.1016/j.rec.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Panchal A., Kyvernitakis A., Biederman R. An interesting case of COVID-19 induced reversed Takotsubo cardiomyopathy and insight on cardiac biomarkers. Cureus. 2020;12(11) doi: 10.7759/cureus.11296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park J.H., Moon J.Y., Sohn K.M., Kim Y.S. Two fatal cases of stress-induced cardiomyopathy in COVID-19 patients. J. Cardiovasc. Imaging. 2020;28(4):300–303. doi: 10.4250/jcvi.2020.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pasqualetto M.C., Secco E., Nizzetto M., et al. Stress cardiomyopathy in COVID-19 disease. Eur. J. Case Rep. Intern. Med. 2020;7(6) doi: 10.12890/2020_001718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roca E., Lombardi C., Campana M., et al. Takotsubo syndrome associated with COVID-19. European journal of case reportsIntern. Med. 2020;7(5) doi: 10.12890/2020_001665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sang C.J., Heindl B., Von Mering G., et al. Stress-induced cardiomyopathy precipitated by COVID-19 and influenza a coinfection. JACC: Case Rep. 2020;2(9):1356–1358. doi: 10.1016/j.jaccas.2020.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sattar Y., Connerney M., Ullah W., et al. COVID-19 presenting as takotsubo cardiomyopathy complicated with atrial fibrillation. Int. J. Cardiol. Heart Vasc. 2020;29 doi: 10.1016/j.ijcha.2020.100580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Solano-López J., Sánchez-Recalde A., Zamorano J.L. SARS-CoV-2, a novel virus with an unusual cardiac feature: inverted Takotsubo syndrome. Eur. Heart J. 2020;41(32):3106. doi: 10.1093/eurheartj/ehaa390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tarun T., Kumar S., Johnson J., Chockalingam A. A case report on transient cardiomyopathy with cytokine storm in SARS-CoV-2. Eur. Heart J. Case Rep. 2021;5(2) doi: 10.1093/ehjcr/ytaa519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taza F., Zulty M., Kanwal A., Grove D. Takotsubo cardiomyopathy triggered by SARS-CoV-2 infection in a critically ill patient. BMJ Case Rep. 2020;13(6) doi: 10.1136/bcr-2020-236561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Titi L., Magnanimi E., Mancone M., et al. Fatal Takotsubo syndrome in critical COVID-19 related pneumonia. Cardiovasc. Pathol. 2021;51 doi: 10.1016/j.carpath.2020.107314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Torabi A.J., Villegas-Galaviz J., Guglin M., Frick K., Rao R. Cardiogenic shock following cardiac tamponade and Takotsubo in COVID-19. Futur. Cardiol. 2021;17(4):631–635. doi: 10.2217/fca-2020-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsao C.W., Strom J.B., Chang J.D., Manning W.J. COVID-19-associated stress (Takotsubo) cardiomyopathy. Circ.Cardiovasc. Imaging. 2020;13(7) doi: 10.1161/CIRCIMAGING.120.011222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tutor A., Unis G., Ruiz B., Bolaji O.A., Bob-Manuel T. Spectrum of suspected cardiomyopathy due to COVID-19: a case series. Curr. Probl. Cardiol. 2021;46(10) doi: 10.1016/j.cpcardiol.2021.100926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Osch D., Asselbergs F.W., Teske A.J., et al. Takotsubo cardiomyopathy in COVID-19: a case report. Haemodynamic and therapeutic considerations. Eur. Heart J. Case Rep. 2020;4(FI1):1–6. doi: 10.1093/ehjcr/ytaa271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giustino G., Croft L.B., Oates C.P., et al. Takotsubo cardiomyopathy in COVID-19. J. Am. Coll. Cardiol. 2020;76(5):628–629. doi: 10.1016/j.jacc.2020.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Templin C., Manka R., Cammann V.L., et al. Takotsubo syndrome in coronavirus disease 2019. Am. J. Cardiol. 2021;138:118–120. doi: 10.1016/j.amjcard.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Akashi Y.J., Nakazawa K., Sakakibara M., Miyake F., Musha H., Sasaka K. 123I-MIBG myocardial scintigraphy in patients with "takotsubo" cardiomyopathy. J. Nucl. Med. 2004;45(7):1121–1127. [PubMed] [Google Scholar]
- 53.Kume T., Akasaka T., Kawamoto T., et al. Assessment of coronary microcirculation in patients with Takotsubo-like left ventricular dysfunction. Circ. J. 2005;69(8):934–939. doi: 10.1253/circj.69.934. [DOI] [PubMed] [Google Scholar]
- 54.Wittstein I.S., Thiemann D.R., Lima J.A., et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N. Engl. J. Med. 2005;352(6):539–548. doi: 10.1056/NEJMoa043046. [DOI] [PubMed] [Google Scholar]
- 55.Fajgenbaum D.C., June C.H. Cytokine storm. N. Engl. J. Med. 2020;383(23):2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Templin C., Ghadri J.R., Diekmann J., et al. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N. Engl. J. Med. 2015;373(10):929–938. doi: 10.1056/NEJMoa1406761. [DOI] [PubMed] [Google Scholar]
- 57.Murakami T., Yoshikawa T., Maekawa Y., et al. Gender differences in patients with takotsubo cardiomyopathy: multi-center registry from Tokyo CCU network. PLoS One. 2015;10(8) doi: 10.1371/journal.pone.0136655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vitale C., Mendelsohn M.E., Rosano G.M. Gender differences in the cardiovascular effect of sex hormones. Nat. Rev. Cardiol. 2009;6(8):532–542. doi: 10.1038/nrcardio.2009.105. [DOI] [PubMed] [Google Scholar]
- 59.Al-kuraishy H.M., Al-Gareeb A.I., Faidah H., Al-Maiahy T.J., Cruz-Martins N., Batiha G.E.-S. The looming effects of estrogen in Covid-19: a rocky rollout. FrontiersNutrition. 2021;8(82) doi: 10.3389/fnut.2021.649128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ortona E., Buonsenso D., Carfi A., et al. Long COVID: an estrogen-associated autoimmune disease? Cell Death Discov. 2021;7(1):77. doi: 10.1038/s41420-021-00464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suba Z. Prevention and therapy of COVID-19 via exogenous estrogen treatment for both male and female patients. J. Pharm. Pharm. Sci. 2020;23(1):75–85. doi: 10.18433/jpps31069. [DOI] [PubMed] [Google Scholar]
- 62.Di Vece D., Citro R., Cammann V.L., et al. Outcomes associated with cardiogenic shock in Takotsubo syndrome. Circulation. 2019;139(3):413–415. doi: 10.1161/CIRCULATIONAHA.118.036164. [DOI] [PubMed] [Google Scholar]
- 63.Almendro-Delia M., Núñez-Gil I.J., Lobo M., et al. Short- and long-term prognostic relevance of cardiogenic shock in Takotsubo syndrome: results from the RETAKO registry. JACC Heart Fail. 2018;6(11):928–936. doi: 10.1016/j.jchf.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 64.Oliveira E., Parikh A., Lopez-Ruiz A., et al. ICU outcomes and survival in patients with severe COVID-19 in the largest health care system in Central Florida. PLoS ONE. 2021;16(3) doi: 10.1371/journal.pone.0249038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 - PRISMA-ScR Checklist
Supplementary material 2 - Detailed search terms