Abstract
Vaccines against SARS-CoV-2 have been rapidly approved. Although pivotal studies were conducted in healthy volunteers, little information is available on the safety and efficacy of mRNA vaccines in immunocompromised patients, including recipients of allogeneic hematopoietic cell transplantation (allo-HCT). Here we used a novel assay to analyze patient- and transplantation-related factors and their influence on immune responses to SARS-CoV-2 vaccination over an extended period (up to 6 months) in a large and homogenous group of allo-HCT recipients at a single center in Switzerland. We examined longitudinal antibody responses to SARS-CoV-2 vaccination with BNT162b2 (BioNTech/Pfizer) and mRNA-1273 (Moderna) in 110 allo-HCT recipients and 86 healthy controls. Seroprofiling recording IgG, IgA, and IgM reactivity against SARS-CoV-2 antigens (receptor-binding domain, spike glycoprotein subunits S1 and S2, and nucleocapsid protein) was performed before vaccination, before the second dose, and at 1, 3, and 6 months after the second dose. Patients were stratified to 3 groups: 3 to 6 months post-allo-HCT, 6 to 12 months post-allo-HCT, and >12 months post-allo-HCT. Patients in the 3 to 6 months and 6 to 12 months post-allo-HCT groups developed significantly lower antibody titers after vaccination compared with patients in the >12 months post-allo-HCT group and healthy controls (P < .001). Within the cohort of allo-HCT recipients, patients age >65 years (P = .030), those receiving immunosuppression for prevention or treatment of graft-versus-host disease (GVHD) (P = .033), and patients with relapsed disease (P = .014) displayed low humoral immune responses to the vaccine. In contrast, the intensity of the conditioning regimen, underlying disease (myeloid/lymphoid/other), and presence of chronic GVHD had no impact on antibody levels. Antibody titers achieved the highest levels at 1 month after the second dose of the vaccine but waned substantially in all transplantation groups and healthy controls over time. This analysis of long-term vaccine antibody response is of critical importance to allo-HCT recipients and transplant physicians to guide treatment decisions regarding revaccination and social behavior during the SARS-CoV-2 pandemic.
Key Words: SARS-CoV-2, Allogeneic hematopoietic cell transplantation, Vaccination
Graphical Abstract
INTRODUCTION
Since the emergence of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in December 2019, more than 250 million people have been infected, and more than 5 million have died [1]SARS-CoV-2 vaccines have been developed and approved at unprecedented speed. In December 2020 and January 2021, Swiss authorities licensed 2 vaccines, BNT162b by Pfizer/BioNTech and mRNA-1273 by Moderna, respectively [2].
Patients with hematologic malignancies are at increased risk of SARS-CoV-2 infection [3,4]. However, individuals with immunosuppressive therapy (IST) or immunocompromising medical conditions were excluded from the pivotal phase 3 trial for BNT162b, [5], and, similarly, patients receiving systemic IST for >14 days within the previous 6 months and those receiving immunoglobulins or blood products within the previous 3 months were excluded from the phase 3 trial for mRNA-1273 [6]. Despite the presumption that immune responses to the SARS-CoV-2 vaccines may be heterogeneous in patients following allogeneic hematopoietic cell transplantation (allo-HCT), the European Society of Blood and Marrow Transplantation and the American Society of Hematology recommend administration of the vaccine as early as 3 months after allo-HCT, including in patients with controlled graft-versus-host disease (GVHD) [7].
It remains unclear whether allo-HCT recipients are able to develop a substantial immune response after mRNA vaccination. Moreover, the influences of simultaneous IST, presence of GVHD, and/or other patient- or transplantation-related parameters on the level and temporal dynamics of immune protection against SARS-CoV-2 have not yet been elucidated. Here we report the results of a prospective observational study on the efficacy of SARS-CoV-2 vaccination among 110 allo-HCT recipients and 86 healthy controls.
METHODS
Study Design and Patient Population
This study is a prospective single-center observational study with a healthy control group recruited from hospital and university staff. SARS-CoV-2-specific antibody response was measured longitudinally at the following timepoints: T0, baseline (before the first vaccination); T1, before the second dose; T2, 2 to 6 weeks after the second vaccination; T3, 3 months after the second vaccination; and T4, 6 months after the second vaccination. The primary endpoint of the study was the quantification and characterization of antibody responses in patients following allo-HCT. Additional details on study design and patient characteristics are provided in Supplementary Data, Methods. The study was conducted according to the Declaration of Helsinki and was approved by the Cantonal Ethics Committee of Zurich, Switzerland (BASEC no. 2021-00261) [8].
Serologic Assessment by Multiplex Bead-Based ABCORA Immunoassay
Longitudinal humoral response to SARS-CoV-2 was measured using the ABCORA immunoassay [9]. The assay measures IgG, IgA, and IgM reactivity to 4 SARS-CoV-2 antigens, the receptor-binding domain (RBD), spike glycoprotein subunits S1 and S2, and nucleocapsid protein (N), characterizing a total of 12 SARS-CoV-2 parameters. Details are provided in the Supplementary Data.
Elecsys Anti-SARS-CoV-2 S Assay
Samples were also evaluated with the Elecsys Anti-SARS-CoV-2 S assay (Roche Diagnostics, Risch-Rotkreuz, Switzerland). This electrochemiluminescence immunoassay was used according to the manufacturer's instructions.
SARS-CoV-2 Pseudo-Neutralization Assay
SARS-CoV-2 plasma neutralization activity was recorded using an HIV-based pseudovirus system as described previously [9,10]. Details are provided in the Supplementary Data.
Neutralization Prediction
The sum of S1 signal over cutoff (SOC) values (sum S1), defined as the sum of IgG, IgA, and IgM S1 SOC values, can be used to predict the neutralization status of a patient using a logistic regression previously developed in a cohort of 467 infected individuals [9]. A sum S1 value >17 is predictive of a 50% neutralization titer (NT50)>250, with a specificity of 94% and a sensitivity of 67% [9].
Statistical Analysis
Statistical analyses were performed in R version 4.0.5 (R Foundation for Statistical Computing, Vienna, Austria). Figures were created using the ggplot2 package. Details are provided in Supplementary Data.
RESULTS
Patient Characteristics
Allo-HCT recipients
A total of 110 allo-HCT recipients with a median age of 57 years (interquartile range [IQR], 46 to 65 years) were enrolled (Table 1 ). Most patients (n = 67; 60.9%) were at >12 months post-allo-HCT, 28 patients (25.5%) were at 3 to 6 months post-allo-HCT, and 15 patients (13.6%) were at 6 to 12 months post-allo-HCT. The study cohort comprised more male patients than female patients (62.7% versus 37.3%). Seventy percent of the patients had an underlying myeloid disease, and 25.5% underwent allo-HCT for lymphoproliferative malignancies. More patients were prepared with reduced-intensity conditioning than with myeloablative conditioning (67.3% versus 32.7%).
Table 1.
Patient Characteristics
| Characteristic | Allo-HCT Recipients | Healthy Controls (N = 86) | Overall (N = 196) | |||
|---|---|---|---|---|---|---|
| 3-6 mo (N = 28) | 6-12 mo (N = 15) | >12 mo (N = 67) | All (N = 110) | |||
| Age group, n (%) | ||||||
| <45 yr | 7 (25) | 5 (33.3) | 14 (20.9) | 26 (23.6) | 67 (77.9) | 93 (47.4) |
| 45-65 yr | 16 (57.1) | 7 (46.7) | 38 (56.7) | 61 (55.5) | 19 (22.1) | 80 (40.8) |
| >65 yr | 5 (17.9) | 3 (20) | 15 (22.4) | 23 (20.9) | 0 (0) | 23 (11.7) |
| Sex, n (%) | ||||||
| Male | 19 (67.9) | 9 (60) | 41 (61.2) | 69 (62.7) | 20 (23.3) | 89 (45.4) |
| Female | 9 (32.1) | 6 (40) | 26 (38.8) | 41 (37.3) | 66 (76.7) | 107 (54.6) |
| Vaccine, n (%) | ||||||
| mRNA-1273 | 7 (25) | 0 (0) | 9 (13.4) | 16 (14.5) | 11 (12.8) | 27 (13.8) |
| BNT162b | 21 (75) | 15 (100) | 58 (86.6) | 94 (85.5) | 75 (87.2) | 169 (86.2) |
| Conditioning regimen, n (%) | ||||||
| MAC | 5 (17.9) | 4 (26.7) | 27 (40.3) | 36 (32.7) | ||
| RIC | 23 (82.1) | 11 (73.3) | 40 (59.7) | 74 (67.3) | ||
| IST, n (%) | ||||||
| No | 3 (10.7) | 6 (40) | 50 (74.6) | 59 (53.6) | ||
| Prophylactic | 14 (50) | 2 (13.3) | 0 (0) | 16 (14.5) | ||
| Therapeutic | 11 (39.3) | 7 (46.7) | 17 (25.4) | 35 (31.8) | ||
| History of acute GVHD, n (%) | ||||||
| None/mild | 20 (71.4) | 10 (66.7) | 50 (74.6) | 80 (72.7) | ||
| Moderate/severe | 8 (28.6) | 5 (33.3) | 17 (25.4) | 30 (27.3) | ||
| Chronic GVHD, n (%) | ||||||
| None/mild | 24 (85.7) | 12 (80) | 48 (71.6) | 84 (76.4) | ||
| Moderate/severe | 4 (14.3) | 3 (20) | 19 (28.4) | 26 (23.6) | ||
| Relapse, n (%) | ||||||
| No | 25 (89.3) | 11 (73.3) | 50 (74.6) | 86 (78.2) | ||
| Yes | 3 (10.7) | 4 (26.7) | 17 (25.4) | 24 (21.8) | ||
| Underlying diagnosis, n (%) | ||||||
| Myeloid | 20 (71.4) | 11 (73.3) | 46 (68.7) | 77 (70) | ||
| Lymphatic | 8 (28.6) | 4 (26.7) | 16 (23.9) | 28 (25.5) | ||
| Other | 0 (0) | 0 (0) | 5 (7.5) | 5 (4.5) | ||
| Donor type, n (%) | ||||||
| MSD | 6 (21.4) | 3 (20) | 23 (34.3) | 32 (29.1) | ||
| MUD | 17 (60.7) | 8 (53.3) | 32 (47.8) | 57 (51.8) | ||
| Haploidentical | 5 (17.9) | 4 (26.7) | 12 (17.9) | 21 (19.1) | ||
| Mismatched | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Graft type, n (%) | ||||||
| PBSCs | 28 (100) | 14 (93.3) | 60 (89.6) | 102 (92.7) | ||
| BM | 0 (0) | 1 (6.7) | 7 (10.4) | 8 (7.3) | ||
MAC, myeloablative conditioning; RIC, reduced-intensity conditioning; MSD, matched sibling donor; MUD, matched unrelated donor; PBSCs, peripheral blood stem cells; BM, bone marrow.
Patients received their first SARS-CoV-2 vaccine dose at a median of 20 months post-HCT (range, 3 months to 35 years). Most HCT recipients (85%) received BNT162b, and only 15% received mRNA-1273. At the time of the first SARS-CoV-2 vaccination, most patients were in remission (78.2%), and 21.8% had relapsed disease. More than one-half of the patients (53.6%) were off IST, 14.5% were on prophylactic IST, and 31.8% received therapeutic IST for treatment of acute or chronic GVHD. A history of acute GVHD grade ≥II was reported in 27.3% of all patients. The presence of moderate or severe chronic GVHD at the time of the first vaccine dose was recorded in 23.6% of patients. Four allo-HCT recipients were excluded from the antibody analysis (Figure 1 ). Antibody measurements were censored by several events: third dose injection (n = 43 between August 3 and November 9, according to national guidelines of Switzerland; n = 1 outside of the guidelines), SARS-CoV-2 infection (n = 2, assessed by a positive SARS-CoV-2 PCR test), and death (n = 9, including 1 SARS-CoV-2-infected patient, 6 patients who relapsed, 1 patient with sepsis while under intensive IST for chronic GVHD, and 1 patient in whom the cause was unknown).
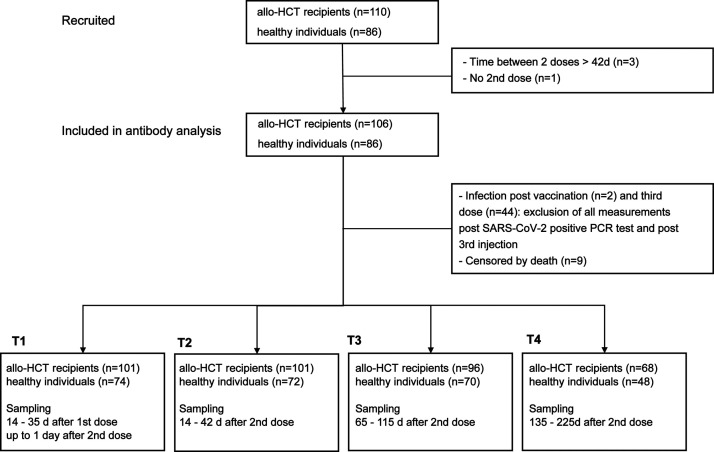
Figure 1.
Flow chart of the study. A total of 110 allo-HCT recipients and 86 healthy controls were enrolled. Results are available for 101 patients and 74 healthy controls at T1 (14 to 35 days post-first dose), for 101 patients and 72 controls at T2 (14 to 42 days post-second dose), for 96 patients and 70 controls at T3 (65 to 115 days post-second dose), and for 68 patients and 48 controls at T4 (135 to 225 days post-second dose).
Healthy controls
A total of 86 healthy controls with a median age of 35.5 years (range, 23 to 64 years) were enrolled. Compared with the patient cohort, the healthy cohort included younger and more female participants (76.7%). The majority of healthy controls (87%) received BNT162b, whereas only 11 healthy controls received mRNA-1273.
Preinfection
In our cohort, 5 of the 86 healthy controls had already seroconverted asymptomatically before vaccination, and 10 of the 106 patients had experienced previous SARS-COV-2 infection (7 of whom were seropositive at baseline). The 2-dose vaccination regimen was maintained for these patients, and they were included in the analysis.
Tolerability of SARS-CoV-2 Vaccination in Allo-HCT Recipients
The allo-HCT recipients tolerated the SARS-CoV-2 vaccines well, and no severe complications were observed. Local pain at the injection site was most commonly reported, followed by myalgia and headache. Forty percent of the patients had no side effects at all (Supplementary Table S1). Of note, 2 patients developed chronic GVHD of the lung during follow-up after vaccination.
Early Vaccination Post-Allo-HCT Elicits Weak Serologic SARS-CoV-2 Responses
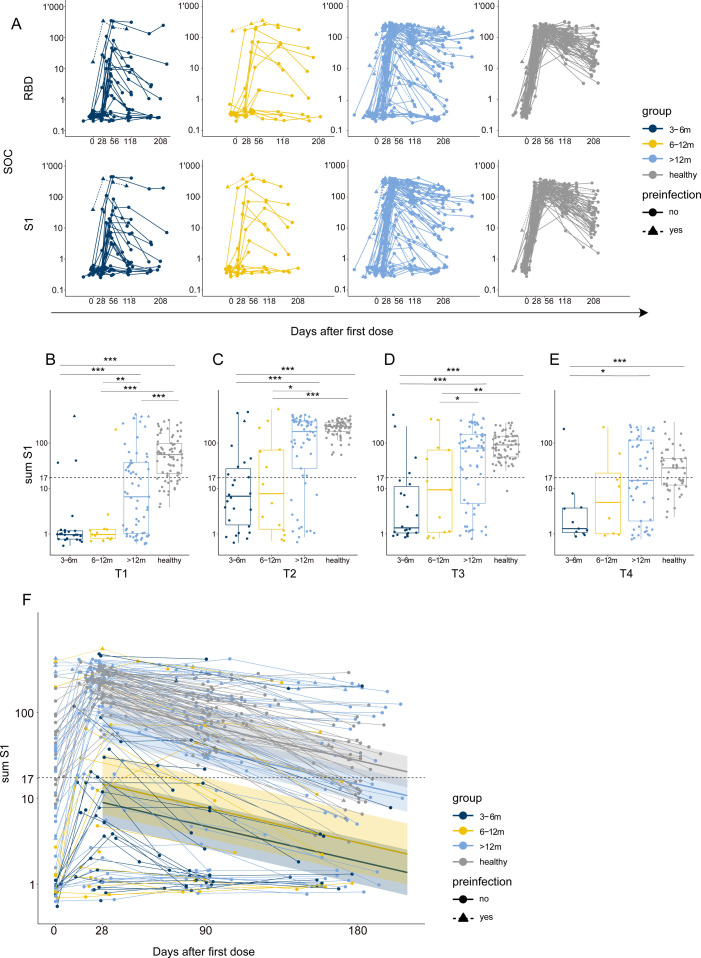
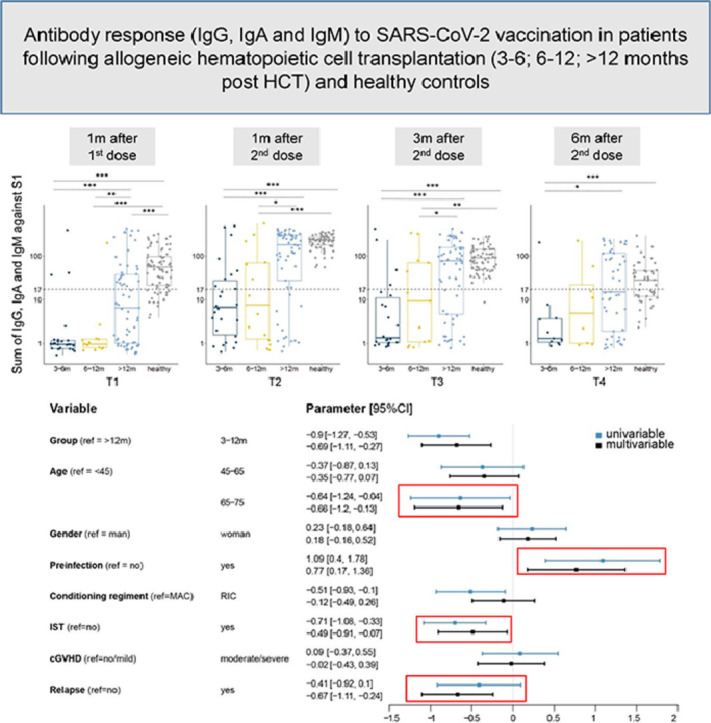
We longitudinally assessed the humoral immune responses against 4 antigens of SARS-CoV-2 —RBD, S1, S2, and N— up to 6 months after the second vaccine injection (Figure 2 A and Supplementary Figures S1 and S2). The response was homogeneous in the healthy controls group; as expected [11], we observed the highest antibody responses at 1 month after the second vaccination and declining antibody titers thereafter. Among the allo-HCT recipients, antibody responses were more heterogeneous, as some (especially those in the 3 to 6 months and 6 to 12 months post-allo-HCT groups) did not mount a response even after full 2-dose vaccination.
Figure 2.
Differences in antibody responses between the allo-HCT recipients and healthy controls. (A) Dynamics of binding IgG response against the SARS-CoV-2 antigens RBD and S1, represented as SOC values, in allo-HCT recipients stratified by time between transplantation and vaccination (dark blue, 3 to 6 months; yellow, 6 to 12 months; light blue, >12 months) and healthy controls (gray). Preinfected individuals are represented by triangles and dashed lines. (B-E) Boxplots showing sum S1 reactivity in allo-HCT recipients stratified by time between transplantation and vaccination (dark blue, 3 to 6 months; yellow, 6 to 12 months; light blue, >12 months) and healthy controls (gray) at different time points: (B) T1, 1 month post-first dose; (C) T2, 1 month post-second dose; (D) T3, 3 months post-second dose; and (E) T4, 6 months post-second dose. The dashed line corresponds to a sum S1 of 17. Preinfected individuals are represented by triangles. Results from the Wilcoxon test used to compare each group to the other groups are shown. *P < .05; **P < .01; ***P < .001. (F) Longitudinal sum S1 response since the second dose and decline prediction obtained from a single exponential decline model. Each line corresponds to 1 patient, color-coded by group (dark blue, 3 to 6 months; yellow, 6 to 12 months; light blue, >12 months; gray, healthy controls). Preinfected individuals are represented by triangles and dashed lines. The solid line corresponds to the estimated marginal mean of the non-preinfected vaccinated individuals in each group, and the shaded area corresponds to the 95% CI of the prediction.
Determining the neutralization activity of the measured antibody binding response is decisive for ascertaining protective immunity after vaccination. Assessment with ABCORA allows for predicting whether infected individuals develop high (NT50>250) or no/low neutralization titers (NT50<250) by the sum of S1 SOC values for IgG, IgA, and IgM (sum S1) [9]. To corroborate the neutralization prediction model after vaccination, we measured neutralization activity in the allo-HCT recipients and healthy controls in a pseudovirus neutralization assay. The healthy controls displayed significantly higher titers than the patients (P < .001 for the 3 to 6 months, 6 to 12 months, and >12 months post-allo-HCT groups) (Supplementary Figure S3A). In addition, we confirmed reliable neutralization prediction after vaccination (area under the curve = 0.99; Supplementary Figure S3B) and thus used the same sum S1 threshold of 17 to predict neutralization in our cohort (Supplementary Figure S3C). At T1, the majority of patients early post-allo-HCT (the 3 to 6 months and 6 to 12 months groups) showed significantly lower sum S1 responses compared with >12 months post-allo-HCT group (P = .001 for the 3 to 6 months group and P = .006 for the 6 to 12 months group versus the >12 months group) (Figure 2B). Moreover, 70% of the allo-HCT recipients (71 of 101) and 22% of the healthy controls (16 of 74) did not reach the neutralization threshold (sum S1 = 17), highlighting the importance of 2 vaccine doses.
At T2, 100% (72 of 72) of the healthy controls and 62% (63 of 101) of the allo-HCT recipients attained a neutralizing antibody response. Among the allo-HCT recipients, 35% (9 of 26) of the 3 to 6 months post-allo-HCT group, 43% (6 of 14) of the 6 to 12 months group, and 79% (48 of 61) of the >12 months group reached the neutralization threshold (Figure 2C). Predicted neutralization levels were substantially lower in the 3 to 6 months and 6 to 12 months post-allo-HCT groups compared with the >12 months post-allo-HCT group and healthy subjects (P < .001 for the 3 to 6 months group and P = .012 for the 6 to 12 months group versus the >12 months group; P = .12 for the >12 months group versus the healthy controls) (Figure 2C). As antibody titers steadily decreased at T3 and continued waning at T4 (Figure 2A and D-F; Supplementary Figures S1 and S2), only 56% (27 of 48) of healthy controls and 40% (27 of 68) of allo-HCT recipients still displayed a neutralizing antibody response at T4 (9% [1 of 11] of the 3 to 6 months, 25% (2 of 8) the 6 to 12 months, and 49% (24 of 49) of the >12 months post-allo-HCT recipients) (Figure 2E).
We assessed the antibody decline after T2 using a single exponential decline model (Figure 2F) and estimated the sum S1 half-life (67 days; 95% confidence interval [CI], 62 to 74 days). This value, although lower than the estimated half-life of antibodies after infection, is in line with recent observations of an increased likelihood of breakthrough infection with longer time since mRNA vaccination 12, 13, 14.
We also performed the Elecsys S test on a subset of samples [9]. The results in both assay systems correlated well at all 3 time points (T1 to T3), with correlation coefficients between 0.93 and 0.95 (Supplementary Figure S4A). Similar patterns were observed but with a significantly lower anti-RBD antibody response in the 3 to 6 months and 6 to 12 months post-allo-HCT groups compared with the >12 months post-allo-HCT group, and the highest response in healthy controls (Supplementary Figure S4B).
Humoral Immunity to the SARS-CoV-2 Vaccine Improves Beyond 12 Months Post-Allo-HCT
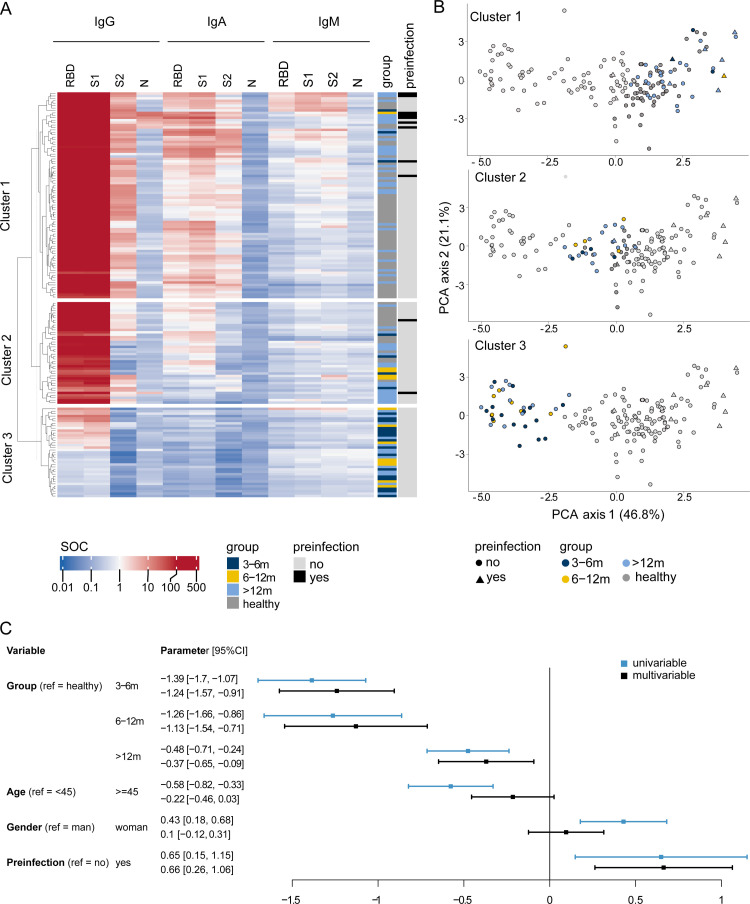
We further differentiated the influence of time since allo-HCT on antibody binding features at T2 (2 to 6 weeks after the second vaccinations) and were able to identify 3 distinct groups of patients segregating based mainly on IgG and IgA spike response in an unsupervised clustering algorithm (Figure 3 A). The most distinctive group (cluster 1) of patients was characterized by the simultaneous presence of high RBD, S1, and S2 IgG binding and some IgA and IgM binding and contained mostly >12 months post-allo-HCT recipients and healthy controls. Cluster 2 was more heterogeneous, with individuals from all groups (transplantation recipients and healthy controls), showing a robust RBD and S1 IgG response but low to no IgA, IgM, and IgG S2 responses. Cluster 3 contained only allo-HCT recipients with low antibody response (almost all patients from the 3 to 6 months and 6 to 12 months groups and some patients from the >12 months group), including low IgG RBD and S1 titers. We confirmed that patients in the >12 months post-allo-HCT group clustered mostly with healthy controls based on their humoral antibody response to the SARS-CoV-2 vaccine by a principal component analysis (Figure 3B).
Figure 3.
Humoral immunity to the SARS-CoV-2 vaccine improves over time after allo-HCT. (A) Heatmap of SARS-CoV-2 seroprofiles (IgG, IgA, and IgM SOC values against 4 SARS-CoV-2 antigens: RBD, S1, S2, and N) at T2 in allo-HCT recipients (dark blue, 3 to 6 months; yellow, 6 to 12 months; light blue, >12 months) and healthy controls (gray), and their preinfection status (black, yes; light gray, no). Patients are grouped in rows in 3 different clusters, and antibody responses are displayed in columns. (B) Principal component analysis (PCA) of SARS-CoV-2 seroprofiles (IgG, IgA, and IgM SOC values against 4 SARS-CoV-2 antigens: RBD, S1, S2, and N) at T2 of allo-HCT recipients (dark blue, 3 to 6 months; yellow, 6 to 12 months; light blue, >12 months) and healthy controls (gray), and their preinfection status (black, yes; light gray, no). Each subfigure highlights one of the clusters previously defined in the heatmap—cluster 1 (top), cluster 2 (middle) and cluster 3 (bottom)—and patients not belonging to the cluster of interest are shown in light gray. (C) Results from univariable (light blue) and multivariable (black) linear regression predicting log10 of the sum S1 value at T2 in allo-HCT recipients and healthy controls.
We used a multivariate linear regression predicting the sum S1 value at T2, adjusted for age, sex, and preinfection status, to evaluate factors affecting vaccine efficacy and further confirmed allo-HCT as a risk factor for impaired humoral immune response to the SARS-CoV-2 vaccine (Figure 3C). The allo-HCT recipients had a significantly lower antibody response than the healthy controls, with greater differences in the 3 to 6 months and 6 to 12 months post-allo-HCT groups (coefficient = -1.24 [95% CI, -1.57 to -0.91; P < .001] and -1.13 [95% CI, -1.54 to -0,71; P < .001], respectively) than in the >12 months group (coefficient = -0.37; 95% CI, -0.65 to -0.09; P = .010). In addition, preinfection led to a more potent antibody response at T2 (coefficient = 0.66; 95% CI, 0.26 to 1.06; P = .001). Age and sex were significant in the univariate analyses but not in the multivariate analysis, owing to the imbalanced distributions of age and sex between the patients and the healthy controls (Figure 3C, Table 1).
Risk Factors Associated with Impaired Humoral Immune Responses to the SARS-CoV-2 Vaccine in Allo-HCT Recipients
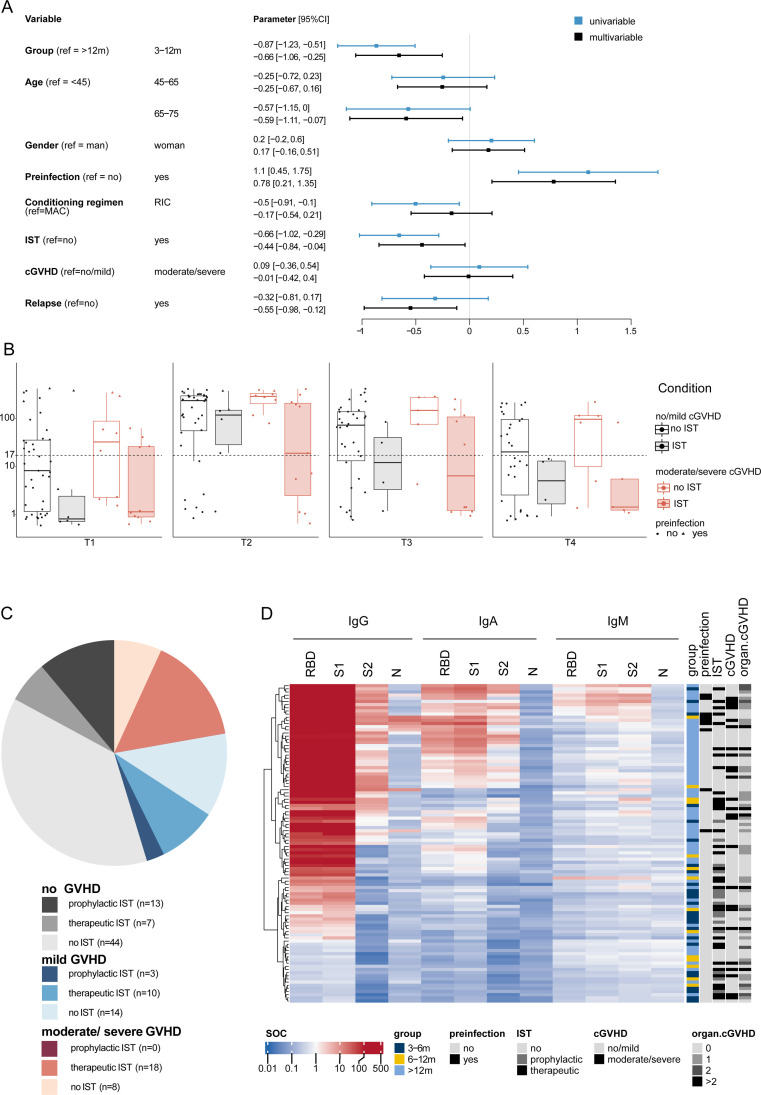
Within the allo-HCT cohort, we further evaluated the associations of age, sex, preinfection, conditioning regimen intensity (reduced intensity versus myeloablative), chronic GVHD, IST, and relapse to identify distinct risk factors for impaired humoral immunity after vaccination. In the applied multivariate regression model, we grouped patients at 3 to 6 months and 6 to 12 months post-allo-HCT together, because they showed similarly low sum S1 levels in the previous analysis (Figure 3C). Reduced predicted neutralizing titers (sum S1) at T2 were significantly associated with (1) whether patients had been vaccinated at 3 to 12 months post-allo-HCT (coefficient = -0.66; 95% CI, -1.06 to -0.25; P = .002); (2) age >65 years (coefficient = -0.59; 95% CI, -1.11 to -0.07; P = .030); (3) IST (coefficient = -0.44; 95% CI, -0.84 to -0.04; P = .033); and (4) whether patients had relapse of the underlying disease (coefficient = -0.55; 95% CI, -0.98 to -0.12; P = .014) (Figure 4 A). Again, preinfection resulted in significantly higher predicted neutralization at T2 (coefficient = 0.78; 95% CI, 0.21 to 1.35; P = .009). Sex, conditioning regimen intensity, and, unexpectedly, the presence of chronic GVHD had no influence on humoral immune responses to the vaccine.
Figure 4.
Risk factors associated with impaired immune response to the SARS-CoV-2 vaccine. (A) Results from univariable (light blue) and multivariable (dark blue) linear regression predicting log10 of the sum S1 value at T2 in allo-HCT recipients. (B) Boxplots showing sum S1 reactivity in allo-HCT recipients from the >12 months group at T1, T2, T3, and T4 stratified by immunosuppressive treatment (no, clear; yes, shaded) and chronic GVHD (none/mild, black; moderate/severe, red). (C) Repartitioning of patients receiving prophylactic, therapeutic, or no immunosuppressive treatment with no (gray shaded), mild (blue shaded), or moderate/severe (red shaded) chronic GVHD. (D) Heatmap of SARS-CoV-2 seroprofiles (IgG, IgA, and IgM SOC values against 4 SARS-CoV-2 antigens: RBD, S1, S2, and N) at T2 in allo-HCT recipients (dark blue, 3 to 6 months; yellow, 6 to 12 months; light blue, >12 months). Also shown is their preinfection status (light gray, no; black, yes), IST (light gray, no; dark gray, prophylactic; black, therapeutic), chronic GVHD (black, none/mild; light gray, moderate/severe), and the number of organs affected by chronic GVHD (shades of light gray to black: 0, 1, 2, and >2). Patients are grouped in rows, and antibody responses are displayed in columns.
Intrigued by the indistinct association of chronic GVHD with humoral vaccine response, we further explored the influence of chronic GVHD and IST on antibody response. Regardless of whether patients had no/mild or moderate/severe chronic GVHD, it was systemic IST that had a long-term impact on the humoral immune response (Figure 4B). This lack of a statistically significant impact of chronic GVHD on humoral immune reactivity may be explained by the heterogeneity within this small subgroup with moderate/severe chronic GVHD (n = 26) and the clinical condition itself, as well as its systemic and/or topical treatments (Figure 4C and D, Supplementary Table S2). Systemic treatments varied substantially, with 15 patients receiving systemic steroids, 34 receiving calcineurin inhibitors, and 20 receiving ruxolitinib. Sixteen patients received combination IST, and 35 received only single-agent IST.
Characteristics of Low Responders to the SARS-CoV-2 Vaccine
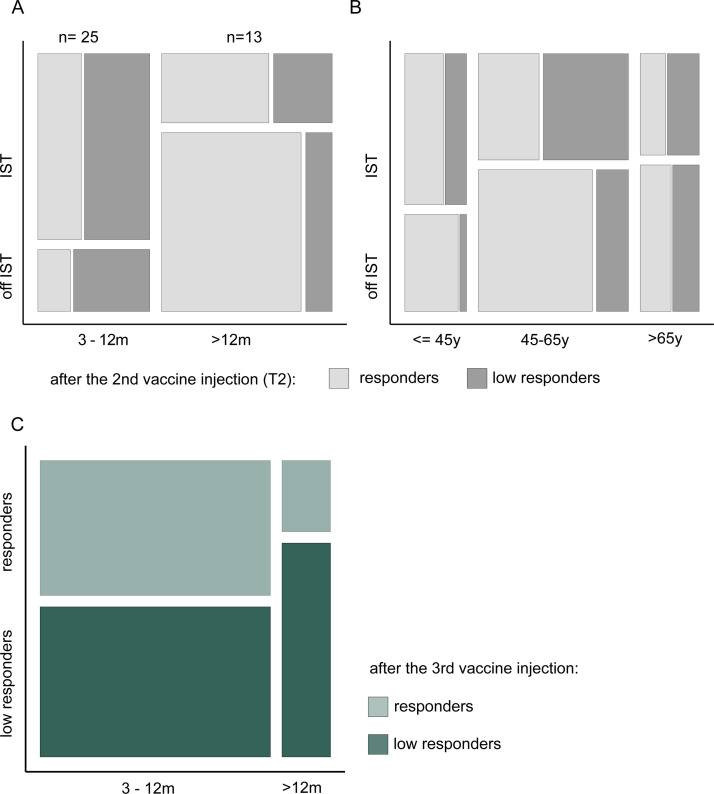
Characterizing low responders who do not reach the neutralization threshold of 17 after vaccination is of utmost clinical importance. We identified a total of 38 low responders in our cohort, including 17 patients in the 3 to 6 months post-allo-HCT group, 8 patients in the 6 to 12 months group, and 13 patients in the >12 months group. Overall, a high proportion of patients in the 3 to 12 months post-allo-HCT group were on IST (75%), and the proportion of low responders in this group was consistently high, whether patients were on IST or not (70% off IST, 60% on IST). In contrast, only 28% of the patients in the >12 months post-allo-HCR were on IST, and the low responders constituted a higher proportion of those on IST (35%) versus those not on IST (16%) (Figure 5 A). Adding an interaction term between the variables “group” and “IST” did not improve our multivariate linear regression and was not significant (P = .42). By assessing age categories, we observed consistent patterns: the proportion of low responders was higher in patients under IST regardless of their age and also was generally higher in older patients (Figure 5B). We vaccinated low responders with a third dose and evaluated the response at approximately 1 month after this third dose (median, 36 days; IQR, 31 to 37 days) in 23 patients, including 7 who died, 1 who was lost to follow-up, 1 who was excluded owing to receipt of a booster vaccination 3 months after the second vaccination, 1 who refused to receive a booster injection, and 5 for whom booster titers were not yet available. For these 23 patients, the third dose was given approximately 6 months after the second dose (median, 197 days; IQR, 138 to 221 days). We documented an increased humoral response in a fraction of patients; 47% (9 of 19) of the 3 to 12 months post-allo-HCT recipients and 25% (1 of 4) of the >12 months post-allo-HCT recipients developed a humoral immune response and became responders (sum S1>17) after the third dose (Figure 5C). Of those patients who still did not respond to the third dose, 46% (6 of 13) were on IST at the time of the injection, compared with only 20% (2 of 10) of the third dose responders.
Figure 5.
Characteristics of low vaccine responders. (A) Mosaic plot displaying the proportion of responders (NT 50>250, light gray) and low responders (NT 50<250, dark gray) at T2 in the 3 to 12 months and >12 months post-allo-HCT groups (x-axis), with or without IST (y-axis). (B) Mosaic plot displaying the proportion of responders (NT 50>250, light gray) and low responders (NT 50<250, dark gray) at T2 in the subgroups of patients age <45 years, 45 to 65 years, and >65 years (x-axis) with or without IST (y-axis). (C) Mosaic plot displaying the proportion of responders (NT 50>250, light green) and low responders (NT 50<250, dark green) (y-axis) after the third vaccine dose in the 3 to 12 months and >12 months post-allo-HCT groups (x-axis).
Plausible reasons for the poor humoral immune responses were observed in the majority of the 38 low responders. Ten low responders were vaccinated early after allo-HCT, most of them while still receiving prophylactic IST. Fifteen patients were on therapeutic IST, including 10 for treatment of moderate/severe chronic GVHD, 8 of whom were receiving ruxolitinib. Eight low responders were under treatment for relapse of their underlying disease (Supplementary Table S3).
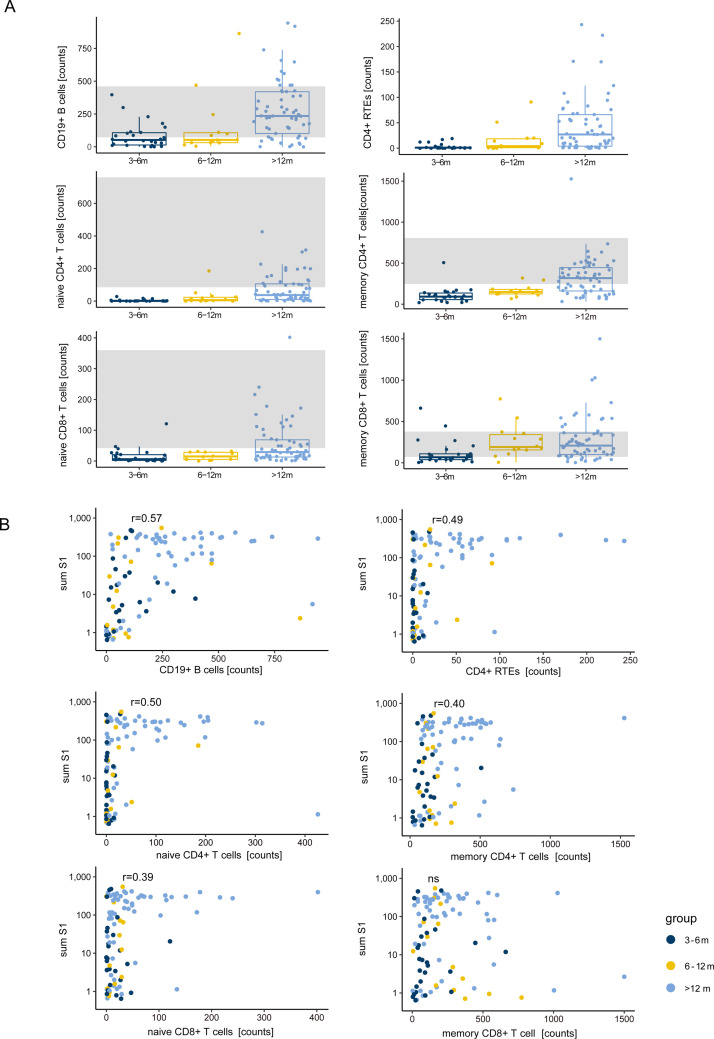
Immune Reconstitution Patterns after Allo-HCT Correlate with Serologic Profiling
Regeneration of the adaptive immune system takes weeks to months following allo-HCT. Initially, adoptively cotransferred mature lymphocytes expand, but over time (weeks to months) nascent donor hematopoietic stem cell (HSC)-derived T and B cells develop Figure 6.A displays the dynamics of the recovery of total B cells, recent thymic emigrants, and naïve and memory CD4+ and CD8+ T cells in the blood, demonstrating that reconstitution of the adaptive immune system after allo-HCT requires years rather than months, with the majority of patients achieving normal levels of B and T cells only beyond 12 months post-HCT. Antibody response to the SARS-CoV-2 vaccine was significantly correlated with higher B cell counts in the blood (Figure 6B, upper left panel), as well as with higher levels of recent thymic emigrants, naïve CD4+ and naïve CD8+ cells, and memory CD4+ cells.
Figure 6.
Immune reconstitution patterns correlate with vaccine antibody response. (A) Boxplots showing the level of immune reconstitution in allo-HCT recipients stratified by the time between transplantation and vaccination (dark blue, 3 to 6 months; yellow, 6 to 12 months; light blue, >12 months). The gray shaded areas correspond to normal ranges for each cell subpopulation. (B) Correlation of immune reconstitution and sum S1 values at T2 in allo-HCT recipients stratified by the time between transplantation and vaccination (dark blue, 3 to 6 months; yellow, 6 to 12 months; light blue, >12 months).
DISCUSSION
Infections are a major cause of death in allo-HCT recipients, and several studies have confirmed an increased risk of morbidity and mortality for COVID-19 in these patients [3,15, 16, 17, 18]. Accordingly, this vulnerable population has been granted prioritized access to SARS-CoV-2 vaccines after their approval. In this population of allo-HCT recipients the efficacy of vaccinations in general is often insufficient, inconsistent, and unpredictable, however [19,20]. In addition to immunosuppressive agents, GVHD can cause severe immune dysfunction, in particular B lymphopenia with hypogammaglobulinemia that can persist for years [21].
Our study adds valuable information to recently published reports. We analyzed longitudinal antibody responses after SARS-CoV-2 vaccination in a relatively large allo-HCT cohort (n = 110), revealing both antibody peaks and their significant temporal decline over 6 months postvaccination. We examined and adjusted for various transplantation-related factors, including conditioning regimen intensity, chronic GVHD, IST, and relapse.
Patients age >65 years and those early post-transplantation were the subgroups at the greatest risk of failing to respond sufficiently to 2 vaccine doses, but IST also had an impact on immune function. The lower rate of seroconversion to SARS-CoV-2 and other vaccines in elderly patients is consistent with previous reports [22]. Likewise, detrimental effects of IST on immune responses to the SARS-CoV-2 vaccine have been described in solid organ transplant recipients [8]. The patients in our study were given IST either because they were early post-HCT (prophylactic IST) or because they were suffering from GVHD (therapeutic IST). Whether a third booster dose of the vaccine can overcome low responses remains to be elucidated in the coming months, but early experiences (including our own observations) indicate that low responders can benefit from a third dose and subsequently become good responders.
Unexpectedly, chronic GVHD per se was not associated with lower antibody levels in our patient cohort. Chronic GVHD is a very heterogeneous condition affecting only a single organ or several organs. Depending on the number and type of involved organs, treatment can be topical or may require single-agent or combined systemic IST. Moreover, “subclinical” GVHD of the bone marrow and lymphoid tissues can result in severe lymphopenia and immune dysfunction [21,23,24]. In this present study, the cohort subgroups of chronic GVHD were too small to allow for statistical analyses; however, we observed a substantial proportion of low responders to the vaccine were patients with chronic GVHD on systemic IST, including calcineurin inhibitors, steroids, and novel agents, such as ruxolitinib. Recent phase 3 studies demonstrated the efficacy of the STAT1/2 inhibitor for chronic GVHD [25]. Impaired responses to BTN162b2 under treatment with ruxolitinib also have been observed in patients who did not undergo allo-HCT, confirming the highly immunosuppressive effect of this agent [26].
Finally, in this study, patients treated for relapse of their underlying disease had poor humoral immune responses. This finding is again consistent with observations by others, in which patients with hematologic disorders receiving systemic cytotoxic or immune modulatory treatment developed only low to no serologic responses to the vaccine [26].
Our findings are in line with low anti-SARS-CoV-2 antibody response observed in a recently published study on patients with cancer, including hematologic malignancies [27]. In this report, positive anti-S IgG titers were measurable in 94% of healthy controls but in only 18% of patients with hematologic cancer after a single vaccine inoculum. Similarly, Herishanu et al [28]. reported an antibody response rate of 39.5% after 2 doses of BNT162b in patients with chronic lymphocytic leukemia and significant decreases in antibody titers by 6 months postvaccination in patients and healthy controls [28]. The first experiences with BNT162b in allo-HCT recipients from a single center were published only recently; 47 of 57 (75%) evaluated recipients displayed a positive serology at 7 to 14 days after the second dose [29]. In this study, longer time since allo-HCT, female sex, and a higher number of CD19+ cells were associated with positive humoral responses, whereas age, GVHD, and intensity of IST did not correlate with humoral immune responses [29]. Of note, in this study, exacerbations of GVHD were observed in 3 of 66 allo-HCT recipients following vaccination.
In a large cohort of patients (n = 885) with hematologic malignancies in Lithuania that included 122 allo-HCT recipients, allo-HCT recipients were among those with the highest levels of antibody titers against SARS-CoV-2 following BNT162b vaccination, compared with patients treated with Bruton kinase inhibitors, ruxolitinib, venetoclax, or anti-CD20 antibody therapy [26]. Consistent with our observations, the Lithuanian study found low antibody responses in the few patients evaluated early post-allo-HCT (<6 months, n = 5; 6 to 12 months, n = 13).
Our study is limited by its single-center character and its control group of healthy individuals that differed significantly in age and sex from our patient cohort, which might have hindered detection of the recently described influence of sex on vaccine efficacy [30]. Furthermore, owing to the lack of an established assay to measure T cell responses to the SARS-CoV-2 vaccine at our center, we were not able to analyze cellular immunity. Emerging data on quantification of SARS-CoV-2-specific T cells and corresponding cytokine production on exposure to specific peptides in vitro stress the importance and role of T cell-mediated immunity against SARS-CoV-2, but also reveal a wide heterogeneity in the magnitude of such T cell responses. Hence, ultimately both humoral and cellular immunity should be integrated into assessments that define the correlates of protection necessary to evaluate current vaccine strategies 31, 32, 33, 34.
In conclusion, in the light of breakthrough infections in vaccinated individuals, systematic analyses of immune responses to the SARS-CoV-2 vaccines and their temporal dynamics are urgently needed to guide treatment decisions during this pandemic. Our results highlight the importance of identifying those at highest risk with longitudinal antibody assessment and also elucidate the rapid decline of vaccine efficacy not only in high risk patients, but also in healthy individuals.
ACKNOWLEDGMENTS
The authors thank the staff of the Division of Infectious Diseases for their endless efforts in vaccinating health care workers and patients and for coordination of the sample collection. Special thanks to the study nurses of the outpatient clinics of the Department of Medical Oncology and Hematology; the staff of the Institute of Medical Virology diagnostics unit, sample triage, and administration; and the staff of the participating clinics for their support.
Financial disclosure: I.A.A. is supported by a research grant from the Promedica Foundation. Parts of this study were funded by the pandemic fund, University Hospital Zurich Foundation (to A.T.), and University Hospital Zurich.
Conflict of interest statement: There are no conflicts of interest to report.
Authorship statement: C.S.-C., A.H., C.P., I.A.A., and A.M.S.M. conceived and designed the study and analyzed data. S.E., A.A., and I.A.A. designed and performed binding antibody experiments. S.E. conducted neutralization experiments, and I.A.A. analyzed data. C.P. performed data analyses. C.S.-C., A.H., C.P., I.A.A., and A.M.S.M. were involved in patient recruitment, provided samples from study and diagnostic repositories, and analyzed patient data. A.M.S.M., I.A.A., C.S.-C., A.H, and C.P. wrote the manuscript, which all coauthors commented on. A.H., C.C.-S., and C.P. contributed equally as first authors. I.A.A. and A.M.S.M. made equal contributions as last authors.
Data sharing: Data collected for the study, including deidentified participant data, and data dictionary or other related documents (eg, informed consent form) will be made available upon request. These data will be shared with researchers who provide a methodologically sound proposal to achieve aims in the approved proposal. The data will be available starting at 3 months and ending at 36 months following publication. Proposals should be directed to irene.abela@usz.ch; data requestors will need to sign a data access agreement.
Footnotes
Financial disclosure: See Acknowledgments on page 214.e11.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jtct.2022.01.019.
Appendix. Supplementary materials
REFERENCES
- 1.World Health Organization. WHO coronavirus (COVID-19) dashboard. Available at: https://covid19.who.int/. Accessed November 8th 2021.
- 2.Swissmedic. Swissmedic grants authorisation for the COVID-19 vaccine from Moderna. Available at: https://www.swissmedic.ch/swissmedic/en/home/humanarzneimittel/authorisations/new-medicines.html. Accessed August 1st 2021.
- 3.Sharma A, Bhatt NS, St Martin A, et al. Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: an observational cohort study. Lancet Haematol. 2021;8:e185–e193. doi: 10.1016/S2352-3026(20)30429-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Passamonti F, Cattaneo C, Arcaini L, et al. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol. 2020;7:e737–e745. doi: 10.1016/S2352-3026(20)30251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med.2020;383:2603-2615. [DOI] [PMC free article] [PubMed]
- 6.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med.2021;384:403-416. [DOI] [PMC free article] [PubMed]
- 7.Ljungman P, Cesaro S, Cordonnier C, Mikulska M, Styczynski J, De la Camara R. COVID-19 vaccines. Version. 2021;7 https://www.ebmt.org/sites/default/files/2021-10/COVID%20vaccines%20version%207.22%20-%202021-10-03.pdf Available at. Accessed October 3rd 2021. [Google Scholar]
- 8.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abela IA, Pasin C, Schwarzmüller M, et al. Multifactorial seroprofiling dissects the contribution of pre-existing human coronaviruses responses to SARS-CoV-2 immunity. Nat Commun. 2021;12:6703. doi: 10.1038/s41467-021-27040-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt F, Weisblum Y, Muecksch F, et al. Measuring SARS-CoV-2 neutralizing antibody activity using pseudotyped and chimeric viruses. J Exp Med. 2021;217(11) doi: 10.1084/jem.20201181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shrotri M, Navaratnam AMD, Nguyen V, et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet. 2021;398:385–387. doi: 10.1016/S0140-6736(21)01642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamayoshi S, Yasuhara A, Ito M, et al. Antibody titers against SARS-CoV-2 decline, but do not disappear for several months. EClinicalMedicine. 2021;32 doi: 10.1016/j.eclinm.2021.100734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen KW, Linderman SL, Moodie Z, et al. Longitudinal analysis shows durable and broad immune memory after SARS-CoV-2 infection with persisting antibody responses and memory B and T cells. Cell Rep Med. 2021;2 doi: 10.1016/j.xcrm.2021.100354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Israel A, Merzon E, Schäffer AA, et al. Elapsed time since BNT162b2 vaccine and risk of SARS-CoV-2 infection in a large cohort. medRxiv. 2021 doi: 10.1101/2021.08.03.21261496. [preprint] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kontoyiannis DP, Lewis RE, Marr K. The burden of bacterial and viral infections in hematopoietic stem cell transplant. Biol Blood Marrow Transplant. 2009;15(1 suppl):128–133. doi: 10.1016/j.bbmt.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Storek J, Dawson MA, Storer B, et al. Immune reconstitution after allogeneic marrow transplantation compared with blood stem cell transplantation. Blood. 2001;97:3380–3389. doi: 10.1182/blood.v97.11.3380. [DOI] [PubMed] [Google Scholar]
- 17.Malard F, Genthon A, Brissot E, et al. COVID-19 outcomes in patients with hematologic disease. Bone Marrow Transplant. 2020;55:2180–2184. doi: 10.1038/s41409-020-0931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varma A, Kosuri S, Ustun C, et al. COVID-19 infection in hematopoietic cell transplantation: age, time from transplant and steroids matter. Leukemia. 2020;34:2809–2812. doi: 10.1038/s41375-020-01019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carpenter PA, Englund JA. How I vaccinate blood and marrow transplant recipients. Blood. 2016;127:2824–2832. doi: 10.1182/blood-2015-12-550475. [DOI] [PubMed] [Google Scholar]
- 20.Janssen MJM, Bruns AHW, Verduyn Lunel FM, et al. Predictive factors for vaccine failure to guide vaccination in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2021;56:2922–2928. doi: 10.1038/s41409-021-01437-0. [DOI] [PubMed] [Google Scholar]
- 21.Storek J, Geddes M, Khan F, et al. Reconstitution of the immune system after hematopoietic stem cell transplantation in humans. Semin Immunopathol. 2008;30:425–437. doi: 10.1007/s00281-008-0132-5. [DOI] [PubMed] [Google Scholar]
- 22.Haynes L, Swain SL. Why aging T cells fail: implications for vaccination. Immunity. 2006;24:663–666. doi: 10.1016/j.immuni.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Müller AMS, Linderman JA, Florek M, Miklos D, Shizuru JA. Allogeneic T cells impair engraftment and hematopoiesis after stem cell transplantation. Proc Natl Acad Sci U S A. 2010;107:14721–14726. doi: 10.1073/pnas.1009220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Müller AMS, Shashidhar S, Küpper NJ, et al. Co-transplantation of pure blood stem cells with antigen-specific but not bulk T cells augments functional immunity. Proc Natl Acad Sci U S A. 2012;109:5820–5825. doi: 10.1073/pnas.1120237109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeiser R, Polverelli N, Ram R, et al. Ruxolitinib for glucocorticoid-refractory chronic graft-versus-host disease. N Engl J Med. 2021;385:228–238. doi: 10.1056/NEJMoa2033122. [DOI] [PubMed] [Google Scholar]
- 26.Maneikis K, Šablauskas K, Ringelevičiūtė U, et al. Immunogenicity of the BNT162b2 COVID-19 mRNA vaccine and early clinical outcomes in patients with haematological malignancies in Lithuania: a national prospective cohort study. Lancet Haematol. 2021;8:e583–e592. doi: 10.1016/S2352-3026(21)00169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monin L, Laing AG, Muñoz-Ruiz M, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22:765–778. doi: 10.1016/S1470-2045(21)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herishanu Y, Avivi I, Levi S, et al. Six month antibody persistence after BNT162b2 mRNA COVID-19 vaccination in patients with chronic lymphocytic leukemia. Blood Adv. 2022;6:148–151. doi: 10.1182/bloodadvances.2021005998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ram R, Hagin D, Kikozashvilli N, et al. Safety and immunogenicity of the BNT162b2 mRNA COVID-19 vaccine in patients after allogeneic HCT or CD19-based CART therapy—a single-center prospective cohort study. Transplant Cell Ther. 2021;27:788–794. doi: 10.1016/j.jtct.2021.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ballin M Nordström P, Nordström A Effectiveness of Covid-19 vaccination against risk of symptomatic infection, hospitalization, and death up to 9 months: a Swedish total-population cohort study. Lancet. 2021 doi: 10.2139/ssrn.3949410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan AT, Lim JM, Le Bert N, et al. Rapid measurement of SARS-CoV-2 spike T cells in whole blood from vaccinated and naturally infected individuals. J Clin Invest. 2021;131 doi: 10.1172/JCI152379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalimuddin S, Tham CYL, Qui M, et al. Early T cell and binding antibody responses are associated with COVID-19 RNA vaccine efficacy onset. Med (N Y) 2021;2:682–688. doi: 10.1016/j.medj.2021.04.003. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sahin U, Muik A, Derhovanessian E, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586:594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 34.Harrington P, Doores KJ, Saha C, et al. Repeated vaccination against SARS-CoV-2 elicits robust polyfunctional T cell response in allogeneic stem cell transplantation recipients. Cancer Cell. 2021;39:1448–1449. doi: 10.1016/j.ccell.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.