Abstract
Background
The impact of the “test and treat” program for human immunodeficiency virus (HIV) treatment in rural areas of Uganda on cryptococcal antigen (CrAg) screening or cryptococcal meningitis (CM) is poorly understood.
Methods
We retrospectively evaluated clinical factors in 212 HIV-infected patients diagnosed with CM from February of 2017 to November of 2019 at Lira Regional Referral Hospital in northern Uganda.
Results
Among 212 patients diagnosed with CM, 58.5% were male. Median age was 35 years; CD4 count and HIV viral load (VL) were 86 cells/μL and 9463 copies/mL, respectively. Only 10% of patients had a previous history of CM. We found that 190 of 209 (90.9%) patients were ART experienced and 19 (9.1%) were ART naive. Overall, 90 of 212 (42.5%) patients died while hospitalized (median time to death, 14 days). Increased risk of death was associated with altered mental status (hazard ratio [HR], 6.6 [95% confidence interval {CI}, 2.411–18.219]; P ≤ .0001) and seizures (HR, 5.23 [95% CI, 1.245–21.991]; P = .024).
Conclusions
Current guidelines recommend CrAg screening based on low CD4 counts for ART-naive patients and VL or clinical failure for ART-experienced patients. Using current guidelines for CrAg screening, some ART-experienced patients miss CrAg screening in resource-limited settings, when CD4 or VL tests are unavailable. We found that the majority of HIV-infected patients with CM were ART experienced (90.9%) at presentation. The high burden of CM in ART-experienced patients supports a need for improved CrAg screening of ART-exposed patients.
Keywords: antiretroviral therapy, cryptococcal meningitis outcomes, screening
Mortality from meningitis in sub-Saharan Africa (SSA) remains high at 19%–68%, and the yeast Cryptococcus neoformans is the most common cause of death among people living with human immunodeficiency virus (HIV) [1, 2]. Annual global deaths from cryptococcal meningitis (CM) are estimated at 181 100, with 135 900 deaths in SSA, and CM is responsible for 15% of all AIDS-related deaths [2]. CM comprises 60% of all meningitis cases in Uganda and is associated with high morbidity and mortality [3]. Despite the increased use of antiretroviral therapy (ART) in Africa, the burden of opportunistic infections (OIs) such as CM remains unacceptably high [4, 5]. In Uganda, the median survival for CM was 26 days in the pre-ART era [6]. In the pre-ART era, the 10-week survival was 84% in cryptococcal antigen (CrAg)–positive patients and 57% in patients with CM. Fourteen-day survival was only 47% in the earlier studies, but survival has improved with appropriate antifungal therapy and ART; however, 5-year survival is only approximately 42% [7, 8].
The introduction of widespread ART use in SSA has created new challenges for detecting and treating OIs in HIV-infected patients after ART initiation. Many countries implemented a “test and treat” (TAT) program in which all persons found to be HIV-infected are immediately initiated on ART [9]. Although this approach has led to improved overall outcomes for HIV-infected persons, the problem of OIs such as CM remains. The World Health Organization (WHO) recommends CrAg screening among ART-naive patients entering into care with CD4 counts of <100 cells/µL, and CrAg screening should be considered among those with CD4 counts of <200 cells/µL [10]. In the current era of TAT, patients are often initiated on ART without first undergoing CD4 count testing and other risk assessments [11]; therefore, CrAg screening is often not routinely performed. In our experience in rural northern Uganda, the TAT program has often led to immediate initiation of ART without CD4 testing or CrAg screening. In other studies, almost half of HIV-infected persons found to have CrAg in their serum were already receiving ART [4, 5]. Thus, implementing TAT programs without CD4 testing and CrAg screening of ART-experienced patients creates new challenges, as more HIV patients on ART are presenting with CM and other OIs [6–8, 12].
Early studies that significantly influenced current WHO guidelines for CrAg screening included mostly ART-naive, HIV-infected patients. These guidelines recommend reflex CrAg screening based on CD4 and HIV viral load (VL) [10]. Little is known about cryptococcal antigenemia and the burden of CM in the current era of TAT in rural areas where CD4 and VL are not widely available [10, 13]. In Uganda, the national guidelines recommend reflex CrAg screening based on CD4 and VL, in line with WHO guidelines. However, implementation of these guidelines is challenging in rural SSA settings, such as northern Uganda, where CD4 and VL testing are not widely available [14, 15].
Here, we report the clinical presentation, risk, and outcomes among patients presenting with CM in a rural hospital in northern Uganda where a TAT program has been implemented. Almost all previous studies regarding CM outcomes in SSA have been performed in major research centers in capital cities, and little is known about the situation regarding CM in rural areas where resources are even more limited and CD4 and VL testing may not be available. Our study was conducted in rural, northern Uganda, in a real-world setting that is representative of many other rural African settings. We aimed to determine the burden of CM among ART-experienced patients, outcomes, and risk factors on hospital admission during the current TAT era. Our results provide evidence that the CM burden is high among ART-experienced patients in our rural setting.
METHODS
Study Design
We conducted a retrospective chart review of a cohort of patients to identify 212 patients with confirmed (n = 184) and presumed (n = 28) CM, who were admitted to the medical ward at Lira Regional Referral Hospital (LRRH), Lira, Uganda, between February 2017 and November 2019. This 350-bed capacity hospital, serving a population of 2 million, is located 340 km north of Uganda’s capital, Kampala, and serves as a referral center for other health units in the subregion and neighboring districts in rural northern Uganda. The study went through institutional approvals.
Information was collected from hospital files, clinical record forms (CRFs), and follow-up medical records for patients aged ≥13 years. During this period, data regarding clinical presentation, drug history, ART use, procedures, laboratory findings, and management of the patients were collected onto CRFs as part of an ongoing CM diagnosis and treatment program. Confirmed CM was diagnosed using the lateral flow assay–CrAg test (IMMY, Norman, Oklahoma) on cerebrospinal fluid after a lumbar puncture. Presumed CM was diagnosed and treated based on clinical judgment by the treating physicians, but without a confirmatory test performed. Patients with CM received standard of care using amphotericin B deoxycholate (0.7–1.0 mg/kg/day) for 7–10 days (or 14 days if there was a poor response to treatment) and fluconazole at 800–1200 mg/day at induction phase, 400 mg/day at consolidation phase, and 200 mg/day at maintenance phase. Patients received 1.5 L of normal saline before and after each amphotericin B deoxycholate dose. Electrolyte supplementation with magnesium and potassium chloride was routinely provided as part of the standard CM treatment protocol. Creatinine, electrolytes, and hemoglobin were evaluated before and during therapy for CM. Detailed HIV and ART status at diagnosis of CM were documented in CRFs, including CD4 count, VL, ART use and adherence, and previous CM history. ART adherence was recorded based on the information from the patient using a standard ART adherence assessment tool, which utilizes the pill-count method. Duration and the type of ART were obtained from ART cards given to patients by their HIV clinics.
Statistical Analysis
Statistical analysis was performed using Stata version 16.14 software (StataCorp, College Station, Texas). Categorical variables were presented as proportions and continuous variables as mean (standard deviation) or median (interquartile range [IQR]). Patient characteristics and outcomes were compared across different variables using a log-rank χ2 test, and a cutoff P value of .3 was used at bivariate analysis, to select variables for multivariate analysis. Adjusted and unadjusted Cox regression (Cox proportional hazard model) was used to determine variables that were significant for predicting mortality among patients with CM on admission. P values < .05 were considered significant at multivariate analysis with a 95% confidence interval (CI).
Patient Consent Statement
The study protocol was approved by the Gulu University Research Ethics Committee (GUREC-066-19), the Uganda National Council of Science and Technology (HS 2675), and the University of Minnesota Institutional Review Board (STUDY00011386). The study met the standards of international human subject protection. We used aggregated data from the hospital, where we were granted institutional permission to conduct the study. We did not seek informed consent because there was no information we used that could identify patients, and we received waiver of consent from GUREC.
RESULTS
Patient Characteristics
Records of 212 patients diagnosed with CM (184 confirmed CM and 28 presumed CM) were evaluated. Most patients were male (124 [58.5%]), and the median age was 35 (IQR, 29–45) years. The median weight was 52 (IQR, 45.2–58.7) kg, and wasting was present in 34 (26.4%) patients. Patients were considered to have wasting based on clinicians’ assessment in the hospital records at the time of admission. CD4 cell counts were available in the records for only 60 patients and VL was available for only 38 patients. The median CD4 count for those patients with records was 86 (IQR, 17.5–257) cells/μL, and 33 (55%) had a CD4 count ≤100 cells/μL. The median HIV VL for those patients with records was 9463 (IQR, 1– 48 158) copies/mL, and only 11 of 38 (34.4%) patients had HIV VL <200 copies/mL (Table 1). Most patients (170 [80%]) presented with an index episode of CM, 19 (9%) presented with CM relapse, and 21 (10%) had suspected treatment failure and/or immune reconstitution inflammatory syndrome. History of tuberculosis was present in 43 of 202 (21.3%) patients for whom this information was available. Epidemiological and laboratory characteristics are shown in Table 1 and clinical characteristics are shown in Table 2.
Table 1.
Bivariate Analyses Evaluating Death Based on Patients’ Demographic Characteristics, Antiretroviral Therapy Status, and Laboratory Parameters
| Characteristic | No. (%) of Patientsa | Deaths Within Hospital | χ2 | Log-Rank P Valueb |
|---|---|---|---|---|
| Cryptococcal meningitis | N = 212 | 90 (42.5) | ||
| Demographic characteristics | ||||
| Sex | N = 212 | 0.03 | .8658 | |
| Female | 88 (41.5) | 32 (31.3) | ||
| Male | 124 (58.5) | 49 (49.7) | ||
| Age | N = 212 | 0.85 | .8377 | |
| Median, y | 35 | … | ||
| Mean (SD), y | 36.8 (12.3) | … | ||
| Clinical characteristics | ||||
| ART status at presentation | n = 209 | 90 (43.1) | 4.07 | .253 |
| No ART | 19 (9.1) | 9 (4.3) | .315 | |
| On ART: adherent/uninterrupted | 156 (74.6) | 74 (35.4) | ||
| Stopped ART completely | 14 (6.7) | 4 (2.0) | ||
| Stopped but restarted | 20 (9.6) | 3 (1.4) | ||
| ART data missing | 3 | … | ||
| Weight | n = 120 | |||
| Mean (SD), kg | 51.7 (10.9) | … | ||
| Median (IQR), kg | 52 (45.2–58.7) | … | ||
| Upper arm circumference | n = 106 | |||
| Mean (SD), cm | 22.1 (2.77) | … | ||
| Wasting | n = 129 | n = 48 | 0.30 | .5818 |
| Yes | 34 (26.4) | 12 (25.0) | ||
| No | 95 (73.6) | 36 (75.0) | ||
| CM treatment history | n = 210 | n = 81 | 5.06 | .0796 |
| New CM cases | 170 (81) | 72 (88.9) | ||
| Relapse | 19 (9) | 5 (6.2) | ||
| Treatment (failure/IRIS) | 21 (10) | 4 (4.9) | ||
| History of previous TB treatment | n = 202 | n = 86 | 0.876 | .3490 |
| Yes | 43 (21.3) | 21 (24.4) | ||
| No | 159 (78.7) | 65 (75.6) | ||
| Laboratory characteristics | ||||
| CD4 count | n = 60 | n = 41 | 1.10 | .2935 |
| Mean, cells/L | 168 | … | ||
| Median (IQR), cells/µL | 86 (17.5–257) | … | ||
| ≤100 cells/µL | 33 (55) | 16 (39.0) | ||
| >100 cells/µL | 27 (45) | 25 (61.0) | ||
| HIV VL | n = 32 | n = 16 | 0.30 | .5818 |
| Mean (SD), copies/mL | 402 647 (1 774 108) | … | ||
| Median (IQR), copies/mL | 9463 (1–48 158) | … | ||
| <200 copies/mL | 11 (34.4) | 5 (31.3) | ||
| 200–1000 copies/mL | 0 | … | ||
| >1000 copies/mL | 21 (65.6) | 11 (68.7) | ||
Data are presented as No. (%) unless otherwise indicated. New CM case: not previously diagnosed and treated for CM. CM relapses: once diagnosed, treated, and CM diagnostic parameters turned negative.
Abbreviations: ART, antiretroviral therapy; CM, cryptococcal meningitis; HIV, human immunodeficiency virus; IQR, interquartile range; IRIS, immune reconstitution inflammatory syndrome; SD, standard deviation; TB, tuberculosis; VL, viral load.
The number of patients shown in each category is variable because of missing data.
Level of significance: P < .05.
Table 2.
Bivariate Analyses Evaluating Death Based on Patients’ Clinical Presentations of Cryptococcal Meningitis
| Variable | No. of Patients (N = 212) | Presence of Characteristic | Deaths Within Hospital | χ2 | Log-Rank P Value |
|---|---|---|---|---|---|
| Clinical characteristics | |||||
| Fever | 171 | 144 (84.2) | 59/67 (88.1) | 1.21 | .272 |
| Headache | 203 | 193 (95.1) | 70/76 (92.1) | 0.57 | .4513 |
| Visual changes | 138 | 24 (17.4) | 8/50 (16.0) | 3.83 | .0502 a |
| Altered mentation | 156 | 65 (41.7) | 31/59 (52.6) | 3.12 | .0771 |
| Vomiting | 155 | 82 (52.9) | 37/62 (59.7) | 1.30 | .2537 |
| Convulsions/seizures | 132 | 16 (12.1) | 8/50 (16.0) | 1.22 | .2687 |
| Nuchal rigidity | 181 | 140 (77.4) | 56/66 (84.9) | 3.95 | .0468 a |
| Kernig sign | 162 | 112 (69.1) | 47/60 (78.3) | 5.12 | .0236 a |
| Cranial nerve palsy | 126 | 12 (9.5) | 6/46 (13.1) | 0.28 | .5938 |
| Abnormal X-ray | 124 | 31 (25) | 17/45 (37.8) | 8.49 | .0036 a |
| Temperature | 144 | 52 (36.1) | 3.67 | .1595 | |
| Low (<36.5°C) | 17 (11.8) | 3 (2.1) | |||
| Normal (36.5°C–37.2°C) | 19 (13.2) | 6 (4.2) | |||
| High (>37.2°C) | 108 (75) | 43 (29.8) | |||
| Oxygen saturation | 93 | 35 (37.7) | 1.25 | .2634 | |
| Low (<90%) | 4 (4.3) | 2 (5.7) | |||
| Normal (≥90%) | 89 (95.7) | 33 (94.3) | |||
| Pulse rate, mean (SD) | 169 | 93.9 (23.2) | |||
| Glasgow Coma Scale | 164 | 164 | 62 | 9.12 | .0105 a |
| 3–7 | 9 (5.5) | 4 (6.5) | |||
| 8–11 | 21 (12.8) | 13 (20.9) | |||
| 12–15 | 134 (81.7) | 45 (72.6) | |||
Data are presented as No. (%) unless otherwise indicated.
Abbreviation: SD, standard deviation.
Level of significance: P < .05 is indicated in bold.
ART Experience and Cryptococcal Meningitis
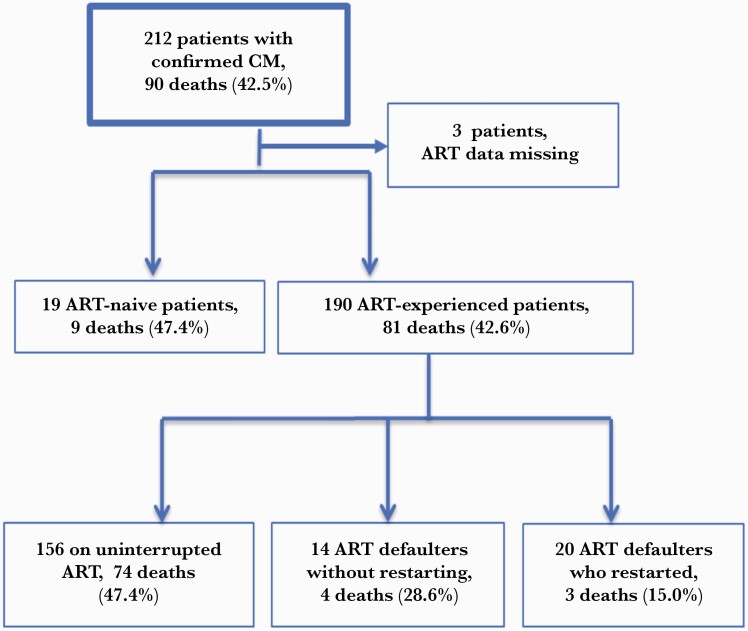
In most early studies evaluating outcomes of CM in SSA, CM occurred primarily in patients who were ART naive and in whom CM represented their first AIDS-defining illness [16–18]. Our study suggests this was not the case in our hospital since most CM occurred in patients who were ART experienced. Among the 209 CM patients with data on ART, 190 (90.9%) were ART experienced before presentation with CM, and only 19 (9.1%) were ART naive (Figure 1). These results suggest that findings from previous studies before the TAT era, which enrolled only ART-naive patients at research centers and might not apply to in rural areas such as northern Uganda where most patients with CM are ART experienced.
Figure 1.
Flow diagram for cryptococcal meningitis (CM) outcomes by antiretroviral therapy (ART) status.
We further assessed the ART history based on information extracted from patient medical records. Of the 190 patients who were ART experienced, 156 (82.1%) patients self-reported to be adherent to ART, 14 (7.4%) were ART defaulters (ART not taken for more than 3 months), and 20 (10.5%) had restarted ART after defaulting (Figure 1).
Timing of Mortality During Hospitalization
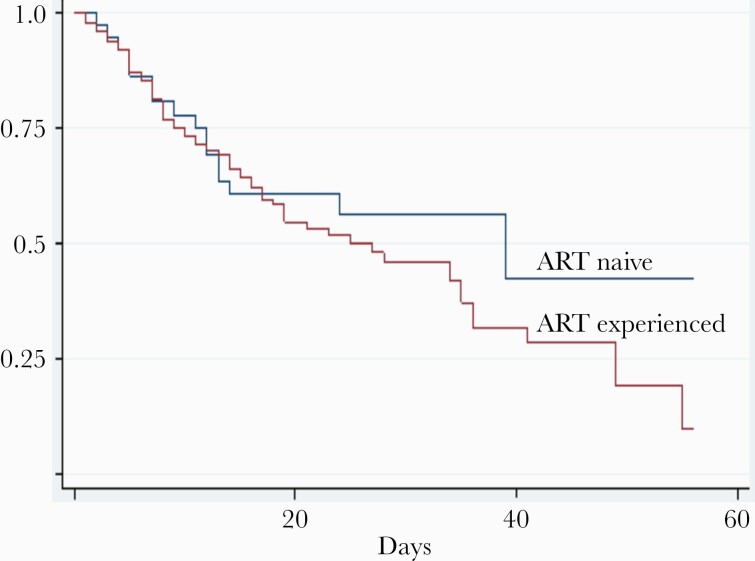
We assessed the survival of patients hospitalized with confirmed CM who were treated with an amphotericin B deoxycholate and fluconazole combination regimen. Overall, 90 (42.5%) deaths in hospital occurred among patients with confirmed CM. The median time of hospital stay was 20 (IQR, 7–40) days. The median time of death was 14 (IQR, 7–14) days. Most CM mortality occurred early during hospitalization, with 80% of all CM mortality occurring before the fifth week. At 2 weeks, 60% (54/90 patients) of all in-hospital mortality had occurred (Figure 2).
Figure 2.
Kaplan-Meier survival curves for patients with cryptococcal meningitis, by antiretroviral therapy (ART) status. Cumulative survival curves expressed as a ratio from day 0 (y-axis) are shown over time in days (x-axis) for patients who were ART experienced (red line) or ART naive (blue line).
High Mortality in ART-Experienced Patients With CM
Mortality was 9 of 19 (47.4%) for ART-naive patients and 81 of 190 (42.6%) in those who were ART experienced (P = .315) (Table 1). We stratified mortality by ART exposure as follows: 156 (47.4%) patients were ART adherent with 74 deaths, 14 (28.6%) were ART defaulters with 4 deaths, and 20 (15%) restarted ART after defaulting with 3 deaths (Figure 1).
Clinical Predictors of Mortality Among HIV Patients With CM
We initially evaluated the clinical factors using bivariate analysis using log-rank by category (Table 2). The presence of visual deficits, nuchal rigidity, positive Kernig sign, or low Glasgow Coma Scale value was significantly associated with CM mortality. In a multivariate analysis, significant predictors of mortality included altered mentation and seizures (Table 3). Patients with altered mentation were 6 times more likely to die at presentation than those without altered mentation (hazard ratio [HR], 6.627 [95% CI, 2.411–18.219]; standard error [SE], 3.420; P < .0001), and those with seizures at presentation had a 5-fold higher risk of death (5.23 times) than those without seizures (HR, 5.232 [95% CI, 1.245–21.991]; SE, 3.833; P = .024) (Table 3).
Table 3.
Multivariate Analyses for Clinical Predictors of Death Among Patients With Cryptococcal Meningitis
| Variables | Hazard Ratio | Standard Error | T Value | P Value | 95% CI of the Hazard Ratio |
|---|---|---|---|---|---|
| ART status | |||||
| Not on ART (n = 19) | 1.000 | … | … | … | |
| On ART (n = 156) | 0.197 | 0.167 | –1.92 | .055 | .037–1.035 |
| Stopped completely (n = 14) | 0.107 | 0.108 | –2.21 | .027a | .015–.777 |
| Restarted (n = 20) | 0.000 | … | … | … | |
| Fever | |||||
| No (n = 27) | 1.000 | … | … | … | |
| Yes (n = 144) | 3.024 | 2.020 | 1.66 | .098 | .817–11.196 |
| Altered mentation | |||||
| No (n = 91) | 1.000 | … | … | … | |
| Yes (n = 65) | 6.627 | 3.420 | 3.67 | .0001a | 2.411–18.219 |
| Vomiting | |||||
| No (n = 73) | 1.000 | … | … | … | |
| Yes (n = 82) | 2.176 | 0.984 | 1.72 | .085 | .897–5.277 |
| Seizure | |||||
| No (n = 116) | 1.000 | … | … | … | |
| Yes (n = 16) | 5.232 | 3.833 | 2.26 | .024a | 1.245–21.991 |
| Abnormal X-ray | |||||
| No (n = 93) | 1.000 | … | … | … | |
| Yes (n = 31) | 1.452 | 0.659 | 0.82 | .411 | .597–3.533 |
| Oxygen saturation | |||||
| >90% (n = 89) | 1.000 | … | … | … | |
| ≤90% (n = 4) | 1.016 | 0.022 | –3.07 | .002a | .001–.227 |
| Mean dependent variable | 18.333 | ||||
| Pseudo r2 | 0.215 | ||||
| χ2 | 39.938 | ||||
| AIC | 161.903 | ||||
| SD dependent variable | 13.726 | ||||
| No. of observations | 54.000 | ||||
| Probability > χ2 | 0.000 | ||||
| BIC | 177.814 | ||||
Cox proportional hazard regression analysis; results adjusted for confounders.
Abbreviations: AIC, Akaike information criterion; ART, antiretroviral therapy; BIC, Bayesian information criterion; SD, standard deviation.
Level of significance: P < .05.
DISCUSSION
We report our experience on ART use and mortality among hospitalized CM patients at LRRH in rural, northern Uganda. We found that the majority (90.9%) of HIV-infected patients with CM were ART experienced at presentation. This suggests that OIs such as CM have remained a major problem in northern Uganda despite increased use of ART through the TAT program [15, 19]. These findings mirror other studies, where the majority of CM patients were ART experienced at the time of CM diagnosis [18, 20]. Similar studies in the Americas and Nigeria reported that 45% and 90%, respectively, of CM patients had prior exposure to ART [21, 22]. However, there has been a shift from almost exclusively ART-naive patients in earlier years to more and more ART-experienced patients presenting with CM, although early studies typically excluded ART-experienced populations [23, 24]. The trend of more patients being diagnosed with CM after exposure to ART may be due to the implementation of the TAT policy when ART initiation often occurs without CD4 testing or CrAg screening due to limited available resources [9].
Furthermore, the high burden of CM among ART-experienced patients may be due to poor adherence to ART, defaulting, or presence of ART drug resistance, which could lead to lack of virological suppression and susceptibility to OIs such as CM [10]. This underpins the need for continuous adherence counseling of patients initiated on ART to ensure compliance. Patients in this study who were found to be nonadherent were restarted on ART based on clinical judgment.
Additionally, virologic failure for any reason may explain an increased burden of CM among ART-experienced individuals; therefore, reflex CrAg screening of all HIV patients on ART with nonvirologic suppression is recommended [25–27]. Nonetheless, universal implementation of virological monitoring in Uganda remains a challenge [14]. Both WHO and Ugandan guidelines are silent on recommendations surrounding CrAg screening of HIV-infected patients who are ART experienced and living in areas without access to CD4 and VL monitoring [10, 15]. Many HIV-infected patients in rural areas of SSA, where most people in SSA live, appear to lack adequate testing to implement WHO CrAg screening guidelines.
Moreover, continued use of ART among CM patients may have contributed to mortality if the patient was on a failing ART regimen. It is unclear whether ART should be interrupted after CM diagnosis or be continued since the presence of virologic failure and the emergence of resistant mutations is possible. Recently, expert opinion by the Ambisome Therapy Induction Optimization-CM (AMBITION-CM) study group has suggested that ART be abrogated, because ART continuation in the context of established drug resistance could be futile and potentially lead to further resistance [28–30]. Based on the Cryptococcal Optimal ART Timing (COAT) trial among ART-naive patients, detrimental outcomes may occur if ART was started early among patients after CM diagnosis [31], and it is possible that restarting ART in patients with CM who interrupted therapy may also be detrimental. For this reason, in patients who stopped ART before a diagnosis of CM, ART should not be restarted until after approximately 4–6 weeks of antifungal therapy [31], although these recommendations for restarting ART were not conclusive in a systematic review [24]. However, an expert opinion recommends that patients who have defaulted their ART or are not adherent, regardless of prior duration of ART use, should be approached as ART naive with ART reinitiated after 4–6 weeks of antifungal treatment [30].
Mortality among CM patients who were on ART has remained significantly high despite the scale-up and use of ART during the TAT era. In this study, where we evaluated ART use and CM treatment outcomes, overall mortality was high at 42.5%, even though 90.9% of patients had prior ART exposure at CM diagnosis. Significant clinical predictors of mortality after CM diagnosis that we identified included altered mentation/low Glasgow Coma Scale, seizures, visual changes, nuchal rigidity, and positive Kernig sign. These results are consistent with results from other studies [32]. We found that mortality at 2 weeks was 60%, and the median time to death in our study was 14 days. These results demonstrate that mortality among CM patients occurred early on during hospital admission, a finding in agreement with other studies [32, 33].
There is a high burden of CM among ART-experienced HIV patients, which has direct implications for CrAg screening to prevent CM. CrAg screening among ART-experienced patients could prevent the development of overt CM. However, routine CrAg screening is often not done at enrollment of HIV patients on ART in the current era of TAT because CD4 testing and CrAg testing are not widely available in many rural areas. The WHO recommends CrAg screening among ART-naive patients entering into care with CD4 counts of <100 cells/µL, but many ART-experienced patients at risk for CM do not undergo CrAg screening as recommended based on current guidelines [10, 11]. Furthermore, only patients on ART who are known to be failing ART are screened, and it can be challenging to identify ART failure if VL testing is not widely available, as clinical failure is often a very late manifestation of advanced disease.
Previous studies in Uganda among ART-experienced patients demonstrated CrAg positivity between 3% and 10.5% [13, 14]; however, the lack of universal availability of VL testing and the lack of a standard threshold for HIV VL cutoff to trigger CrAg screening are challenges in rural settings. No cost-effectiveness studies are available for more widespread CrAg screening among ART-experienced patients; therefore, reflex screening approaches in patients with known ART failure are suboptimal, especially in rural areas where CD4 testing is not performed.
Study Limitations
Details of ART use and history were missing for patients who were on ART prior to their referral to LRRH from other centers. Several files had missing data for VL results, CD4 count, date of ART initiation, and other pieces of preadmission data before hospitalization, possibly because these tests were not performed or recorded correctly. In this study, 74.6% of the patients self-reported being ≥95% adherent to ART, but there is an inherent bias in self-reporting and actual adherence was likely lower.
CONCLUSIONS
In rural northern Uganda, the majority of persons with HIV with CM were ART experienced (90.9%) at presentation. Current guidelines recommend CrAg screening of patients initiating or restarting ART based on CD4 count or VL. Thus, many HIV-infected patients miss screening in resource-limited settings where CD4 is not performed or VL is not widely available, and they are started on ART based on the TAT program without adequate testing. The high burden of CM in ART-experienced patients supports a need for expanding the current guidelines on CrAg screening to include ART-exposed patients, especially in resource-limited areas where CD4 counts or VL testing are not available.
Routine scheduled CrAg screening among HIV patients on ART may be needed because of the high burden and mortality of CM among these patients. Further studies should be done to address CrAg screening of ART-experienced patients. More studies are required to elucidate when to restart ART after default in patients with CM as well as the role of ART resistance among CM patients. Special attention during CM treatment must be given to CM patients who present with altered mentation or seizures, because of their high risk of mortality.
Notes
Author contributions. M. O., P. R. B., D. M., J. R., and A. L. contributed to study design, development, data collection monitoring, data analysis, and writing of the manuscript. J. O., and B. N. participated in data collection and data entry. Data analysis was done by M. O. and A. L. All authors contributed to manuscript editing and approval of the final version.
Acknowledgments. We acknowledge support from Lira Regional Referral Hospital regarding the clinical components of the study and the Infectious Diseases Institute at Makerere University for administrative support.
Financial support. P. R. B. was supported through the University of Minnesota Medical School and the Fulbright US Scholars Program. A. L. was supported through the Robert Wood Johnson Foundation, Future of Nursing Scholars; GO Health Travel Fellowship, University of Washington (UW) Department of Global Health; Boeing International Fellowship, UW Graduate School; and Hester McClaws Dissertation Fellowship, UW School of Nursing.
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Veltman JA, Bristow CC, Klausner JD.. Meningitis in HIV-positive patients in sub-Saharan Africa: a review. J Int AIDS Soc 2014; 17:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rajasingham R, Smith RM, Park BJ, et al. . Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 2017; 17:873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rajasingham R, Rhein J, Klammer K, et al. . Epidemiology of meningitis in an HIV-infected Ugandan cohort. Am J Trop Med Hyg 2015; 92:274–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jarvis JN, Boulle A, Loyse A, et al. . High ongoing burden of cryptococcal disease in Africa despite antiretroviral roll out. AIDS 2009; 23:1182–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beyene T, Woldeamanuel Y, Asrat D, Ayana G, Boulware DR.. Comparison of cryptococcal antigenemia between antiretroviral naive and antiretroviral experienced HIV positive patients at two hospitals in Ethiopia. PLoS One 2013; 8:e75585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. French N, Gray K, Watera C, et al. . Cryptococcal infection in a cohort of HIV-1-infected Ugandan adults. AIDS 2002; 16:1031–8. [DOI] [PubMed] [Google Scholar]
- 7. Kambugu A, Meya DB, Rhein J, et al. . Outcomes of cryptococcal meningitis in Uganda before and after the availability of HAART. Clin Infect Dis 2008; 46:1694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Butler EK, Boulware DR, Bohjanen PR, Meya DB.. Long term 5-year survival of persons with cryptococcal meningitis or asymptomatic subclinical antigenemia in Uganda. PLoS One 2012; 7:e51291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization. Progress report 2016: prevent HIV, test and treat all: WHO support for country impact. Geneva, Switzerland: WHO; 2016. [Google Scholar]
- 10. World Health Organization. Diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children. Geneva, Switzerland: WHO; 2018. [Google Scholar]
- 11. Gardner EM, McLees MP, Steiner JF, del Rio C, Burman WJ.. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis 2011; 52:793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lortholary O, Poizat G, Zeller V, et al. . Long-term outcome of AIDS-associated cryptococcosis in the era of combination antiretroviral therapy. AIDS 2006; 20:2183–91. [DOI] [PubMed] [Google Scholar]
- 13. Baluku JB, Mugabe P, Mwebaza S, et al. . Cryptococcal antigen screening among antiretroviral therapy–experienced people with HIV with viral load nonsuppression in rural Uganda. Open Forum Infect Dis 2021; 8:ofab010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mpoza E, Rajasingham R, Tugume L, et al. . Cryptococcal antigenemia in human immunodeficiency virus antiretroviral therapy–experienced Ugandans with virologic failure. Clin Infect Dis 2020; 71:1726–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Uganda Ministry of Health. Consolidated guidelines for prevention and treatment of HIV in Uganda. 2018. http://library.health.go.ug/publications/hivaids/consolidated-guidelines-prevention-and-treatment-hiv-uganda. Accessed 31 August 2021.
- 16. Park BJ, Wannemuehler KA, Marston BJ, Govender N, et al. . Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 2009; 23:525–30. [DOI] [PubMed] [Google Scholar]
- 17. Bhagwan S, Naidoo K.. Aetiology, clinical presentation, and outcome of meningitis in patients coinfected with human immunodeficiency virus and tuberculosis. AIDS Res Treat 2011; 2011:180352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rhein J, Hullsiek KH, Evans EE, et al. . Detrimental outcomes of unmasking cryptococcal meningitis with recent ART initiation. Open Forum Infect Dis 2018; 5:ofy122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McCreesh N, Andrianakis I, Nsubuga RN, et al. . Universal test, treat, and keep: improving ART retention is key in cost-effective HIV control in Uganda. BMC Infect Dis 2017; 17:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Flynn AG, Meya DB, Hullsiek KH, et al. . Evolving failures in the delivery of human immunodeficiency virus care: lessons from a Ugandan meningitis cohort 2006-2016. Open Forum Infect Dis 2017; 4:ofx077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Crabtree Ramírez B, Caro Vega Y, Shepherd BE, et al. . Outcomes of HIV-positive patients with cryptococcal meningitis in the Americas. Int J Infect Dis 2017; 63:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Oladele RO, Akanmu AS, Nwosu AO, Ogunsola FT, Richardson MD, Denning DW.. Cryptococcal antigenemia in Nigerian patients with advanced human immunodeficiency virus: influence of antiretroviral therapy adherence. Open Forum Infect Dis 2016; 3:ofw055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hakyemez IN, Erdem H, Beraud G, et al. . Prediction of unfavorable outcomes in cryptococcal meningitis: results of the multicenter Infectious Diseases International Research Initiative (ID-IRI) cryptococcal meningitis study. Eur J Clin Microbiol Infect Dis 2018; 37:1231–40. [DOI] [PubMed] [Google Scholar]
- 24. Eshun-Wilson I, Okwen MP, Richardson M, Bicanic T.. Early versus delayed antiretroviral treatment in HIV-positive people with cryptococcal meningitis. Cochrane Database Syst Rev 2018; 7:CD009012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Meya DB, Manabe YC, Castelnuovo B, et al. . Serum cryptococcal antigen (CRAG) screening is a cost-effective method to prevent death in HIV-infected persons with CD4 ≤100/μL starting HIV therapy in resource-limited settings. Clin Infect Dis 2010; 51:448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rajasingham R, Meya DB, Greene GS, et al. . Evaluation of a national cryptococcal antigen screening program for HIV-infected patients in Uganda: a cost-effectiveness modeling analysis. PLoS One 2019; 14:e0210105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Baluku JB, Mugabe P, Mwebaza S, et al. . Cryptococcal antigen screening among antiretroviral therapy–experienced people with HIV with viral load nonsuppression in rural Uganda. Open Forum Infect Dis 2021; 8:ofab010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shroufi A, Van Cutsem G, Cambiano V, et al. . Simplifying switch to second line ART: predicted effect of defining failure of first-line efavirenz-based regimens in sub-Saharan Africa by a single viral load more than 1000 copies/ml. AIDS 2019; 33:1635–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gupta RK, Gregson J, Parkin N, et al. . HIV-1 drug resistance before initiation or re-initiation of first-line antiretroviral therapy in low-income and middle-income countries: a systematic review and meta-regression analysis. Lancet Infect Dis 2018; 18:346–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Alufandika M, Lawrence DS, Boyer-Chammard T, et al. . A pragmatic approach to managing antiretroviral therapy-experienced patients diagnosed with HIV-associated cryptococcal meningitis: impact of antiretroviral therapy adherence and duration. AIDS 2020; 34:1425–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boulware DR, Meya DB, Muzoora C, et al. . Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N Engl J Med 2014; 370:2487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jarvis JN, Bicanic T, Loyse A, et al. . Determinants of mortality in a combined cohort of 501 patients with HIV-associated cryptococcal meningitis: implications for improving outcomes. Clin Infect Dis 2014; 58:736–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bisson GP, Nthobatsong R, Thakur R, et al. . The use of HAART is associated with decreased risk of death during initial treatment of cryptococcal meningitis in adults in Botswana. J Acquir Immune Defic Syndr 2008; 49:227–9. [DOI] [PMC free article] [PubMed] [Google Scholar]