Abstract
Microcephaly or reduced head circumference results from a multitude of abnormal developmental processes affecting brain growth and/or leading to brain atrophy. Autosomal recessive primary microcephaly (MCPH) is the prototype of isolated primary (congenital) microcephaly, affecting predominantly the cerebral cortex. For MCPH, an accelerating number of mutated genes emerge annually, and they are involved in crucial steps of neurogenesis. In this review article, we provide a deeper look into the microcephalic MCPH brain. We explore cytoarchitecture focusing on the cerebral cortex and discuss diverse processes occurring at the level of neural progenitors, early generated and mature neurons, and glial cells. We aim to thereby give an overview of current knowledge in MCPH phenotype and normal brain growth.
Keywords: MCPH genes, microcephaly, brain, intellectual disability, neuronal differentiation, animal models, brain malformation
Introduction
Microcephaly is clinically defined by a significant reduction of the occipito-frontal head circumference (OFC) of more than two (microcephaly) or three (severe microcephaly) SDs below the mean for a given sex, age, and ethnicity (von der Hagen et al., 2014). The prevalence of microcephaly ranges between 1.5 and 8.7 per 10,000 births in Europe and the United States, respectively (Cragan et al., 2016; Morris et al., 2016). However, 15%–20% of children with developmental delay have microcephaly (Sassaman and Zartler, 1982; Watemberg et al., 2002; Aggarwal et al., 2013). Depending on the time of appearance, microcephaly can be classified as primary/congenital or secondary/postnatal (Passemard et al., 2013; Woods and Parker, 2013; Zaqout et al., 2017). It has been suggested that the primary causes of microcephaly lead to a reduction in the number of generated neurons, while the secondary causes mainly affect the dendritic complexity and synaptic formations (Woods, 2004). Primary microcephaly is by definition present at birth, and it can be caused by environmental and/or genetic factors (Zaqout et al., 2017; Alcantara and O'Driscoll, 2014; Kaindl et al., 2010). Various environmental factors such as infections, toxins, radiation, or alcohol result in primary microcephaly. The recent identification of epidemic infections with the Zika virus as a cause for primary microcephaly has highlighted this rare condition as a key topic in neuroscience to understand normal brain development (Kleber de Oliveira et al., 2016; Subramanian et al., 2019). This condition is an addition to the genetic prototype of isolated primary microcephaly, autosomal recessive primary microcephaly (microcephaly primary hereditary (MCPH)).
MCPH is a group of rare heterogeneous neurodevelopmental disorders characterized by intellectual disability and a significant reduction in the brain volume reflected by a reduction in the head circumference already at birth (Kaindl et al., 2010; Zaqout et al., 2017; Slezak et al., 2021). The reduction in brain volume in MCPH cases affects disproportionately the neocortex, though without obvious changes in the cortical organization (Kaindl et al., 2010; Kraemer et al., 2011; Jayaraman et al., 2018). The increasing use of whole-exome sequencing (WES) has uncovered a growing number of novel and disease-causing MCPH variants (Boycott et al., 2013). Simultaneously, further radiological and postmortem studies expand the spectrum of brain malformations reported in individuals with MCPH. The prevalence of MCPH differs from 1:10,000 in populations with a high rate of consanguineous marriage to 1:250,000 in the general population (Van Den Bosch, 1959; Cox et al., 2006). In consanguineous families, most MCPH diagnosed cases reveal homozygous variants in the disease-causing gene. However, compound heterozygous variants are increasingly discovered in MCPH patients, raising the importance of using advanced and accurate diagnosis methods for such cases (Jean et al., 2020).
Currently (December 2021), twenty-eight MCPH-related genes have been identified and tagged sequentially as MCPH1–MCPH28 (MCPH; OMIM phenotypic series: PS251200; Siskos et al., 2021) (Table 1). Still, more genetic loci are expected to exist given the fact that approximately 62% of western Europeans/North Americans and 25% of Indians/Pakistani families diagnosed with MCPH fail to show linkage to any of the MCPH loci (Verloes et al., 1993; Kaindl et al., 2010; Sajid Hussain et al., 2013). Most MCPH gene variants are nonsense, frameshift, or splice site-affecting variants leading to a production of non-functional, truncated proteins (Kaindl et al., 2010; Barbelanne and Tsang, 2014; Jean et al., 2020). Most of the MCPH genes encode centrosomal and/or pericentriolar matrix (PCM) proteins that are, in turn, ubiquitously expressed (Kaindl et al., 2010; Hussain et al., 2013; Barbelanne and Tsang, 2014). It is therefore not surprising to find that many MCPH proteins are involved in centriole biogenesis including organization, maturation, and distribution (Subramanian et al., 2019; Jean et al., 2020). Furthermore, MCPH proteins play crucial roles in microtubule dynamics, mitotic spindle formation, DNA damage responses, Wnt signaling, transcriptional regulation, and cell cycle checkpoint control (Kraemer et al., 2011; Mahmood et al., 2011; Jayaraman et al., 2018; Jean et al., 2020). Disruption of one or more of these functions during cortical neurogenesis adversely affects neuronal progenitor proliferation, differentiation, and survival leading to a severe reduction in the total number of generated neurons reflected by the microcephaly phenotype. Being highly conserved among species, ongoing research on MCPH animal models deems to be an important key for understanding the pathomechanisms behind microcephaly as well as the role of MCPH proteins during normal brain development (Gilbert et al., 2005; Woods et al., 2005; Zaqout et al., 2017). Although microcephaly found in MCPH patients simulates an evolutionary retrogression of the brain size (McHenry, 1994), human brain evolution cannot be attributed solely to the protein-coding sequences of MCPH genes (Pervaiz et al., 2021). Therefore, it has been hypothesized that complex conditional effects of human-specific coding and non-coding regulatory changes in MCPH only assist this evolution process (Pervaiz et al., 2021).
TABLE 1.
List of microcephaly primary hereditary (MCPH) genes and related animal/organism models.
| Locus | Protein | Gene | Location | OMIM | Model organisms | Generation method | Key findings | Ref |
|---|---|---|---|---|---|---|---|---|
| MCPH1 | Microcephalin 1 | MCPH1 | 8p23.1 | 607117 | Xenopus | 1. Drosophila in vitro expression cloning IVEC (DIVEC) | 1. dMCPH1 is a substrate of anaphase-promoting complex (APC) | Hainline et al. (2014) |
| Fly | 2. Deletion of the mcph1 gene by imprecise excision of a P-element | 2. Lethal phenotype due to mitotic arrest, uncoordinated centrosome, and nuclear cycles | Brunk et al. (2007) | |||||
| Rodent | 3. Mcph1-knockout mice (deletion of exon 4–5) | 3. Premature increase in asymmetrical neural progenitor cell (NPC) divisions, uncoupled mitosis and centrosome cycle, misoriented mitotic spindle alignment | Gruber et al. (2011) | |||||
| 4. Mcph1-knockout mice (gene trap) | 4. Shorter survival rates, defected mitotic chromosome condensation | Trimborn et al. (2010) | ||||||
| 5. Brit1-knockout mice (gene targeting) | 5. Hypersensitive to γ-irradiation, defective DNA repair, infertility, meiotic defects | Liang et al. (2010) | ||||||
| MCPH2 | WD-repeat-containing protein 62 | WDR62 | 19q13.12 | 613583 | Fish | 1. Morpholino-mediated knockdown of wdr62 | 1. Reduction in head and eye size, prometaphase delay, increased apoptosis | Novorol et al. (2013) |
| Rodent | 2. Wdr62-knockout mice (gene trap) | 2. Abnormalities in asymmetric centrosome inheritance, neuronal migration delays, altered neuronal differentiation, prometaphase delay, infertility | Sgourdou et al. (2017) | |||||
| 3. Wdr62-knockout mice (gene trap) | 3. Mitotic arrest, cell death, reduced thickness of upper cortical neuronal layers, dwarfism | Chen et al. (2014) | ||||||
| 4. ShRNA knockdown of Wdr62 in rats (in utero electroporation) | 4. Premature differentiation of NPCs, abnormal spindle formation, and mitotic division | Xu et al. (2014) | ||||||
| 5. SiRNA knockdown of Wdr62 in mice (in utero electroporation) | 5. Spindle orientation defects, delayed mitotic progression, reduced NPC proliferation, increased cell cycle exit | Bogoyevitch et al. (2012) | ||||||
| 6. Wdr62-knockout mice (Wdr62 f/f ; homologous recombination followed by germline transmission) | 6. Mild microcephaly, reduced NPC number, impaired mitosis, increased apoptosis, increased cilium length | Zhang et al. (2019a) | ||||||
| Human cerebral organoid | 7. WDR62 −/− cerebral organoids (mutant Human pluripotent stem cell (hPSC) lines; CRISPR-Cas9) | 7. Reduced organoid size, reduced outer radial glial cell (oRGC) proliferation, impaired mitosis, increased NPC vertical division, premature differentiation, increased apoptosis, increased cilium length | ||||||
| MCPH3 | Cyclin-dependent kinase 5 regulatory subunit-associated protein 2 | CDK5RAP2 | 9q33.2 | 608201 | Fly | 1. Centrosomin (cnn) knockout flies (chemical mutagenesis) | 1. Nuclear cleavage defects, microtubule organization defects, abnormal mitotic spindle formation | Megraw et al. (1999) |
| Disconnections between centrioles and PCM | Lucas and Raff, (2007) | |||||||
| Rodent | 2. Hertwig’s anemia mouse (inversion of exon 4 of Cdk5rap2) | 2a. Fewer total neurons with special reduction in upper cortical neurons, abnormal spindle formation, and mitotic division, defective mitotic spindle orientation, premature cell cycle exit, increased cell death | Lizarraga et al. (2010) | |||||
| 2b. Reduced dendritic complexity of layer 2/3 pyramidal neurons, increased spine density, shifted excitation—inhibition balance toward excitation | Zaqout et al. (2019) | |||||||
| 3. shRNA knockdown of Cdk5rap2 in mouse (in utero electroporation) | 3. Premature differentiation of NPCs, reduced proliferation, increased cell cycle exit | Buchman et al. (2010) | ||||||
| Human cerebral organoid | 4. RNAi knockdown of CDK5RAP2 (co-electroporating green fluorescent protein (GFP) with shRNAs) and patient-derived cerebral organoids | 4. Premature neural differentiation, increased NPC oblique, and vertical divisions | Lancaster et al. (2013) | |||||
| MCPH4 | Kinetochore scaffold 1 | KNL1 | 15q15.1 | 609173 | Rodent | 1. Conditional Knl1 knockout in mouse brain | 1. Impaired NPC proliferation, missegregated chromosomes, DNA damage and p53 activation, rapid and robust apoptosis | Shi et al. (2019) |
| MCPH5 | Abnormal spindle-like, microcephaly associated protein | ASPM | 1q31.3 | 605481 | Fish | 2. Morpholino-mediated knockdown of aspm | 2a. Reduction in head and eye size, prometaphase delay, increased apoptosis | Novorol et al. (2013) |
| 2b. Reduction in head and eye size, mitotic arrest, increased apoptosis | Kim et al. (2011a) | |||||||
| Fly | 3. Mutagenesis (x-irradiation) | 3. High mitotic index, metaphase arrest, mitotic and meiotic non-disjunction, hemi-spindles formation | Gonzalez et al. (1990) | |||||
| 4. Mutagenesis (recombinant chromosomes) | 4a. Arrested mitotic cycle at metaphase, high frequency of polyploid cells, defected sex chromosome disjunction | Ripoll et al. (1985) | ||||||
| 4b. Disrupted microtubule-organizing centers, failure of cytokinesis | Riparbelli et al. (2002) | |||||||
| Rodent | 5. esiRNA knockdown of Aspm in Tis21–GFP knockin mice (in utero electroporation) | 5. Centrosome detachment, altered cleavage plane orientation, increased non-NE fate, increased neuron-like fate | Fish et al. (2006) | |||||
| 6. Aspm-knockout mice (gene trap) | 6. Mild microcephaly, midbody localization defects, Major germline defects | Pulvers et al. (2010) | ||||||
| 7. Aspm-knockout mice (removal of exons 2 and 3) | 7. Much thicker layer I and thinner layer VI cortical neurons, aberrant expression of Tbr1 and Satb2 in the subplate | Fujimori et al. (2014) | ||||||
| Ferret | 8. Aspm germline knockout ferret | 8. Severe microcephaly, displaced and altered NPC proportions, increased number of IPCs, increased apoptosis | Johnson et al. (2018) | |||||
| Human cerebral organoid | 9. RNAi knockdown of ASPM (co-electroporating GFP with shRNAs) and patient-derived cerebral organoids | 9. Reduced organoid size, proliferation defect, reduced number of RGs and oRGs | Li et al. (2017) | |||||
| MCPH6 | Centromeric protein J | CENPJ | 13q12.2 | 609279 | Fly | 1. Mutations in the DSas-4 gene (P-element insertion) | 1. Morphologically normal, no detectable centrioles or centrosomes, lack of cilia, early postnatal lethality | Basto et al. (2006) |
| 2. Point mutations | 2. Centriole loss, reduced binding affinity of the DSas-4 and Ana2 interaction | Cottee et al. (2013) | ||||||
| Rodent | 3. Conditional Cenpj knockout in mouse brain | 3. Long cilia and abnormal cilia disassembly, uncompleted cell division, reduced cell proliferation, increased apoptosis | Ding et al. (2019) | |||||
| 4. Cenpj-knockout mice (cassette insertion between exons 4 and 5) | 4. Microcephaly, dwarfism, skeletal abnormalities, increased levels of DNA damage, and apoptosis | McIntyre et al. (2012) | ||||||
| MCPH7 | SCL/TAL1-interrupting locus protein | STIL | 1p33 | 181590 | Fish | 1. Morpholino-mediated knockdown of wdr62 | 1. Reduction in head and eye size, prometaphase delay, increased apoptosis | Novorol et al. (2013) |
| 2. Cassiopeia (csp) mutant zebrafish | 2. Embryonic lethality, high mitotic index, highly disorganized mitotic spindles, lack of centrosomes, increased apoptosis | Pfaff et al. (2007) | ||||||
| Rodent | 3. Stil-knockout mice (removal of exons 3–5) | 3a. Embryonic lethality, defected neural folding, randomization of left-right asymmetry, impaired response to Sonic 3b. Hedgehog (SHH) signaling | Izraeli et al. (1999) | |||||
| Lack of centrioles and primary cilia | David et al. (2014) | |||||||
| MCPH8 | Centrosomal protein 135 kD | CEP135 | 4q12 | 611423 | Alga | 1. bld10 flagella-less mutants (insertional mutagenesis) | 1. Lack of basal bodies, disorganized mitotic spindles and cytoplasmic microtubules, abnormal cell division, and slow growth | Matsuura et al. (2004) |
| 2. bld10 null mutants (series of truncations) | 2. Basal-body defects | Hiraki et al. (2007) | ||||||
| Protozoa | 3. SiRNA knockdown of bld10 in Paramecium | 3. Abnormal basal body assembly | Jerka-Dziadosz et al. (2010) | |||||
| Fly | 4. bld10-knockout flies (transposon insertion) | 4a. Disrupted localization of the inner and outer centriole components | Roque et al. (2012) | |||||
| 4b. Short centrioles and basal bodies, immotile sperm, infertility | Mottier-Pavie and Megraw, (2009) | |||||||
| 4c. Lack of singlet microtubules and disassembly of central microtubule pair | Carvalho-Santos et al. (2012) | |||||||
| 5. plp RNAi knockdown in bld10 mutant flies | 5. Spindle alignment and centrosome segregation defects, perturbed centrosome asymmetry, mispositioned microtubule-organizing centers (MTOCs) | Singh et al. (2014) | ||||||
| MCPH9 | Centrosomal protein 152 kD | CEP152 | 15q21.1 | 613529 | Fly | 1. asterless (asl 1 , asl 2 , asl 3 ) mutant flies (P-element-mediated transformation) | 1. Defect in PCM stabilization and centrosome segregation, reduced microtubule nucleation, severe defects in meiotic spindle assembly | Varmark et al. (2007) |
| 2. asterless (asl mecD ) mutant flies (P-element-mediated transformation) | 2. Lack of centrioles, basal bodies, and cilia | Blachon et al. (2008) | ||||||
| Fish | 3. Morpholino-mediated knockdown of cep152 | 3. Curly tail (ciliary defects) | ||||||
| MCPH10 | Zinc finger protein 335 | ZNF335 | 20q13.12 | 610827 | Rodent | 1. Znf335-knockout mice (gene trap) | 1. Early embryonic lethality | Yang et al. (2012) |
| 2. Conditional Znf335 knockout in mouse brain (flanked promoter and exon1/2) | 2. Lack all cortical structure and cortical neurons, enlarged ventricles | |||||||
| 3. shRNA knockdown of Znf335 in mice (in utero electroporation) | 3. Disrupted NPC proliferation, premature differentiation, abnormal cell RGs orientation, disorganized dendritic outgrowth, lack of apical dendritic process | |||||||
| MCPH11 | Polyhomeotic-like 1 protein | PHC1 | 12p13.31 | 602978 | N/A | |||
| MCPH12 | Cyclin-dependent kinase 6 | CDK6 | 7q21.2 | 603368 | Rodent | 1. Cdk6 knockout mice (removal of 1st coding exon) | 1. Develop normally, slight hematopoiesis deficit | Malumbres et al. (2004) |
| MCPH13 | Centromeric protein E | CENPE | 4q24 | 117143 | Fly | 1. Mutations in cenp-meta gene (P-element-mediated disruption) | 1. Embryonic lethality, defects in metaphase chromosome alignment | Yucel et al. (2000) |
| Rodent | 2. Conditional and complete Cenp-e gene disruptions in mouse | 2. Early developmental arrest, mitotic chromosome misalignment | Putkey et al. (2002) | |||||
| MCPH14 | SAS-6 centriolar assembly protein | SASS6 | 1p21.2 | 609321 | Worm | 1. RNAi knockdown of sas-6 in Caenorhabditis elegans | 1. Abnormal centrosome duplication cycle | Leidel et al. (2005) |
| Fish | 2. cellular atoll (cea) mutant zebrafish | 2. Defects in nuclear division, mitotic spindle formation, and centrosome duplication | Yabe et al. (2007) | |||||
| Fly | 3. sas-6-knockout flies | 3. Lack of cohesion between centrioles | Rodrigues-Martins et al. (2007) | |||||
| MCPH15 | Major facilitator superfamily domain-containing protein 2A | MFSD2A | 1p34.2 | 614397 | Fish | 1. Morpholino-mediated knockdown of mfsd2a | 1. Embryonic lethality before neural maturation, disrupted blood–brain barrier (BBB) integrity | Guemez-Gamboa et al. (2015) |
| Rodent | 2. Mfsd2a-knockout mice (gene targeting) | 2a. Increased plasma lysophosphatidylcholine (LPC) | ||||||
| 2b. Reduced body weight and length, increased energy expenditure, increased BAT β-oxidation, increased ataxic movement | Berger et al. (2012) | |||||||
| 2c. Reduced levels of DHA in the brain, microcephaly, neuronal cell loss in hippocampus and cerebellum, cognitive deficits, and severe anxiety | Nguyen et al. (2014) | |||||||
| 2d. Specific reduction in the retinal outer rod segment length, disorganized outer rod segment discs, reduction and mislocalization of rhodopsin, activated microglia | Wong et al. (2016) | |||||||
| 3. Mfsd2a-knockout mice (gene trap) | 3. Leaky BBB, dramatic increase in central nervous system (CNS) endothelial cell vesicular transcytosis | Ben-Zvi et al. (2014) | ||||||
| 4. Mfsd2a-endothelial-specific knockout mice | 4. Reduced neuronal arborization and decreased dendrite length | Chan et al. (2018) | ||||||
| MCPH16 | Ankyrin repeat- and lem domain-containing protein 2 | ANKLE2 | 12q24.33 | 616062 | Worm | 1. ax475 mutant worms (missense mutation in the lem-4L open reading frame (ORF)) and RNAi knockdown of lem-4L in C. elegans embryos | 1. Abnormal nuclear morphology | Asencio et al. (2012) |
| Fly | 2. Ankle2 A knockout (ethyl methanesulfonate (EMS) chemical mutagenesis) | 2. Loss of thoracic bristles, severe reduction in neuroblast, impaired cell proliferation, increased apoptosis | Yamamoto et al. (2014) | |||||
| 3. Ankle2 A knockout (EMS chemical mutagenesis) and Ankle2CRIMIC knockout (CRISPR-Cas9) | 3. Disrupted endoplasmic reticulum and nuclear envelope morphology, spindle alignment defects, disrupted asymmetric cell division pathway | Link et al. (2019) | ||||||
| MCPH17 | Citron rho-interacting serine/threonine kinase | CIT | 12q24.23 | 605629 | Fly | 1. dck 2 knockout (EMS chemical mutagenesis) | 1. Defective in both neuroblast and spermatocyte cytokinesis, abnormal F actin and anillin rings | Naim et al. (2004) |
| Rodent | 2. Flathead (fh) mutant rats (spontaneous mutation, deletion within exon 1 of Citron-K) | 2a. Reduced brain size, dysgenesis of neocortex, hippocampus, cerebellum, and retina, increased apoptosis, seizures, tremor, impaired coordination, and premature death | Roberts et al. (2000) | |||||
| 2b. Reduced brain size, cytokinesis failure, binucleated cells | Sarkisian et al. (2002) | |||||||
| 2c. Decrease in the number of interneurons, hypertrophied soma and dendritic arbors of interneurons, increased apoptosis, cytokinesis failure, binucleated cells | Sarkisian et al. (2001) | |||||||
| 3. Citron-K-knockout mice (gene targeting) | 3. Depletion of microneurons in the olfactory bulb, hippocampus, and cerebellum, increased apoptosis, abnormal cytokinesis, tremor and severe ataxia, reduced life span due to lethal epilepsy | Di Cunto et al. (2000) | ||||||
| MCPH18 | WD repeat and FYVE domain-containing 3 | WDFY3 | 4q21.23 | 617485 | Fly | 1. Blue cheese (bchs) knockout flies (P-element-mediated disruption) | 1a. Extensive neurodegeneration, premature adult death, formation of protein aggregates, neuronal apoptosis | Finley et al. (2003) |
| 1b. Morphological abnormalities in motor neurons, increased apoptosis, reduced endolysosomal vesicles mobility | Lim and Kraut, (2009) | |||||||
| 2. hALFY mutant flies (single point mutation) | 2. Reduced brain volume, very fragile and malformed brain, clusters of disorganized neurons, severe rough eye phenotype | Kadir et al. (2016) | ||||||
| Rodent | 3. Disconnected mutant mice (Wdfy3 disc/disc ; spontaneous nonsense mutation in exon 59 of 67 of Wdfy3) | 3. Perinatal lethality, enlarged frontal cortical aspects, tangential expansion but lateral thinning of the neocortical neuroepithelium, focal cortical dysplasia, abnormal ganglionic eminences, enlarged ventricles, reduction in the size of the olfactory bulbs | Orosco et al. (2014) | |||||
| 4. Wdfy3-knockout mice (Wdfy3 lacZ/lacZ ; gene targeting) | 4. Perinatal lethality, more drastic thinning and lengthening of the neocortex, focal cortical dysplasias | |||||||
| 5. Wdfy3-haploinsufficiency mice (Wdfy3 +/lacZ ; gene targeting) | 5a. Deficiencies in mitochondrial function, defective mitophagy, accumulation of defective mitochondria, compromised fatty acid β-oxidation | Napoli et al. (2018) | ||||||
| 5b. Decreased mitochondrial localization at synaptic terminals, decreased synaptic density, defective brain glycophagy, cerebellar hypoplasia | Napoli et al. (2021) | |||||||
| 5c. Macrocephaly, deficits in motor coordination and associative learning, downregulation of the Wnt signaling pathway | Le Duc et al. (2019) | |||||||
| MCPH19 | Coatomer protein complex, subunit beta 2 (beta prime) | COPB2 | 3q23 | 606990 | Rodent | 1. Copb2-knockout mice Copb2 Zfn/Zfn ; Zinc-Finger nuclease mediated deletion within exon 12) | 1. Early embryonic lethality before organogenesis | DiStasio et al. (2017) |
| Copb2-knockout mice (Copb2 null/null ; CRISPR-Cas9) | ||||||||
| 2. Mice homozygous for the patient mutation (Copb2 R254C/R254C ; CRISPR-Cas9) | 2. Viable and do not have cortical malformations | |||||||
| 3. Mice heterozygous for the patient mutation and a null allele (Copb2 R254C/Zfn ; CRISPR-Cas9) | 3. Perinatal lethality, reduced brain size, reduction in layer V cortical neurons, increased apoptosis | |||||||
| MCPH20 | Kinesin family member 14 | KIF14 | 1q32.1 | 611279 | Fish | 1. kif14 mutant zebrafish (sa24165 mutant line Kettleborough et al. (2013)) | 1. Microcephaly, eye defects, body curvature, cardiac edema, glomerular cysts, high mitotic index, ciliopathy-like phenotypes | Reilly et al. (2019) |
| Fly | 2. Mutations in the Klp38B gene (P-element insertion) | 2. Semi-lethality, abnormal cell cycle progression, failure of cytokinesis, rough eyes, missing bristles, abnormal abdominal cuticles | Ohkura et al. (1997) | |||||
| Rodent | 3. Laggard (lag) mutant mice (spontaneous mutation, disruption within exon 5 of Kif14) and Kif14 knockout mice (gene targeting) | 3. Small head, tremor, ataxic gait, severe hypomyelination in the CNS, disrupted cytoarchitecture in the neocortex, hippocampus, and cerebellar cortex, increased apoptosis during late neurogenesis | Fujikura et al. (2013) | |||||
| MCPH21 | Non-SMC condensin I complex, subunit D2 | NCAPD2 | 12p13.31 | 615638 | Rodent | 1. Ncaph2 condensin II mutant mice (Ncaph2 I15N/I15N ; ENU chemical mutagenesis) | 1. Isolated T-lymphocyte developmental defect, reduced brain size, increased anaphase DNA bridge formation in apical NPCs, impaired chromosome segregation | Gosling et al. (2007), Martin et al. (2016) |
| MCPH22 | Non-SMC condensin II complex subunit D3 | NCAPD3 | 11q25 | 609276 | ||||
| MCPH23 | Non-SMC condensin I complex subunit H | NCAPH | 2q11.2 | 602332 | ||||
| MCPH24 | Nucleoporin 37 | NUP37 | 12q23.2 | 609264 | Xenopus | 1. Morpholino-mediated knockdown of nup107, nup85, or nup133 | 1. Disrupted glomerulogenesis | Braun et al. (2018) |
| Fish | 2. nup107 or nup85 knockout in zebrafish (CRISPR-Cas9) | 2. Developmental anomalies, early lethality | ||||||
| MCPH25 | Trafficking protein particle complex subunit 14 | TRAPPC14 | 7q22.1 | 618350 | Fish | 1. map11 knockout in zebrafish (CRISPR-Cas9) | 1. Microcephaly, decreased neuronal proliferation | Perez et al. (2019) |
| 2. Morpholino-mediated knockdown of c7orf43 | 2. Curved bodies, small eyes, ciliogenesis defects | Cuenca et al. (2019) | ||||||
| MCPH26 | Lamin B1 | LMNB1 | 5q23.2 | 150340 | Rodent | 1. Lmnb1-knockout mice (Lmnb1 ∆/∆ ; gene trap) | 1a. Perinatal lethality, abnormal lung development and bone ossification, abnormal skeleton and skull shape | Vergnes et al. (2004) |
| 1b. Perinatal lethality, absence of the cortical layering with reduced number of neurons, absence of lamination in the hippocampus, absence of cerebellar foliation, impaired neuronal migration, reduced NPC proliferation, solitary nuclear bleb in cortical neurons | Coffinier et al. (2011) | |||||||
| 2. Forebrain-specific Lmnb1-knockout mice (Emx1-Cre Lmnb1 fl/fl ) | 2. Very small cortex, low neuronal density, lack of upper cortical layers, nuclear blebs in embryonic neurons, nuclear membrane ruptures, increased apoptosis, asymmetric distribution of Lmnb2 | Coffinier et al. (2011), Chen et al. (2019) | ||||||
| 3. Lmnb1/Lmnb2-knockout mice (Lmnb1 −/− Lmnb2 −/− ; gene targeting) | 3. Defects in lungs, diaphragms, and brains, thinner cerebral cortex, disorganized cortical layers, impaired neuronal migration, altered cleavage plane orientation, increased cell cycle exit | Kim et al. (2011b) | ||||||
| MCPH27 | Lamin B2 | LMNB2 | 19p13.3 | 150341 | Rodent | |||
| 4. Lmnb2-knockout mice (Lmnb2 −/− ; gene targeting) | 4. Perinatal lethality, impaired neuronal migration, layering defects in the cerebral cortex and hippocampus, absence of cerebellar foliation, absence of a discrete Purkinje cell layer, elongated nuclei in cortical neurons | Coffinier et al. (2010), Coffinier et al. (2011) | ||||||
| 5. Forebrain-specific Lmnb2-knockout mice (Emx1-Cre Lmnb2 fl/fl ) | 5. Small cortex, cortical defect more pronounced after birth, abnormal layering of cortical neurons, elongated nuclei in embryonic neurons, normal distribution of Lmnb1 at the nuclear rim | Coffinier et al. (2011) | ||||||
| MCPH28 | Ribosomal RNA processing 7 homolog A | RRP7A | 22q13.2 | 619449 | Fish | 1. rrp7a mutant zebrafish (sa11429 mutant line (Kettleborough et al., 2013)) | 1. Premature lethality, reduced brain size, reduced eye size, increased apoptosis | Farooq et al. (2020) |
Classically, radiological investigations of patients with MCPH fail to show severe brain malformation except for simplified neocortical gyration. However, the increasing number of reported MCPH-linked mutations reveals that further deformities in brain architecture might occur (Table 2). The overall aim of this review is to explore the various effects of MCPH disease-causing genes on the cytoarchitecture of the cerebral cortex.
TABLE 2.
Brain malformations associated with microcephaly primary hereditary (MCPH) additional to microcephaly.
| Locus | Gene | Brain malformations | Key findings | Ref |
|---|---|---|---|---|
| MCPH1 | MCPH1 | Corpus callosum abnormalities | Mild hypoplasia | Hosseini et al. (2012) |
| Agenesis of the genu | Neitzel et al. (2002) | |||
| Pachygyria | + | Neitzel et al. (2002), Trimborn et al. (2004) | ||
| Thickening of fronto-parietal and temporal gyri | Hosseini et al. (2012) | |||
| Heterotopia | Nodular neuronal heterotopia (ventricular, infratentorial, and subependymal) | Neitzel et al. (2002), Trimborn et al. (2004) | ||
| Periventricular neuronal heterotopias | Trimborn et al. (2004) | |||
| Frontal lobe hypoplasia | + | Neitzel et al. (2002) | ||
| Ventricular system abnormalities | Dilatation of lateral ventricles (dorsal and temporal), dilated external liquor space | |||
| Myelination/white matter abnormalities | Slight retardation of myelination of cerebral medullary layer | |||
| MCPH2 | WDR62 | Corpus callosum abnormalities | Hypoplasia | Bilgüvar et al. (2010), Yu et al. (2010), Farag et al. (2013), Memon et al. (2013), Sgourdou et al. (2017), Zombor et al. (2019), Slezak et al. (2021) |
| Dysplasia | Poulton et al. (2014) | |||
| Abnormally shaped corpus callosum, agenesis of the rostral part | Bilgüvar et al. (2010) | |||
| Dysmorphic with a thick body and a small genu | Yu et al. (2010) | |||
| Incomplete genu and small splenium | ||||
| Thinning of the corpus callosum with absence of the splenium | Banerjee et al. (2016) | |||
| Pachygyria | + | Bilgüvar et al. (2010), Bhat et al. (2011), Bacino et al. (2012), Bastaki et al. (2016), Slezak et al. (2021) | ||
| Diffuse pachygyria | Sgourdou et al. (2017), Zombor et al. (2019) | |||
| Severe pachygyria | Poulton et al. (2014) | |||
| Lissencephaly/agyria | Microlissencephaly | Bilgüvar et al. (2010), Bhat et al. (2011), Bastaki et al. (2016), Slezak et al. (2021) | ||
| Polymicrogyria | + | Bilgüvar et al. (2010), Poulton et al. (2014) | ||
| Widespread polymicrogyria | Yu et al. (2010) | |||
| Polymicrogyria in the right hemisphere | Slezak et al. (2021) | |||
| Bilateral polymicrogyria | Bhat et al. (2011) | |||
| Bilateral parietal polymicrogyria | Murdock et al. (2011) | |||
| Extensive polymicrogyria in the left cerebral hemisphere | ||||
| Extensive areas of polymicrogyria in the right frontal lobe | Nardello et al. (2018) | |||
| Abnormal Sylvian fissure | Under‐opercularization | Bilgüvar et al. (2010), Poulton et al. (2014) | ||
| Widened Sylvian fissure | Farag et al. (2013) | |||
| Open Sylvian fissures | Bacino et al. (2012) | |||
| Schizencephaly | + | Memon et al. (2013) | ||
| Open-lip schizencephaly | Bilgüvar et al. (2010) | |||
| Narrow right temporoparietal open lip schizencephaly, right temporoparietal open lip schizencephaly | Yu et al. (2010) | |||
| Suspected schizencephaly in the right parietal lobe | Banerjee et al. (2016) | |||
| Closed schizencephaly in the right cerebral hemisphere | Slezak et al. (2021) | |||
| Heterotopia | Subcortical heterotopia, bilateral band heterotopia in the posterior frontal and parietal lobes | Yu et al. (2010) | ||
| Band heterotopias | Bhat et al. (2011) | |||
| A focus of gray matter heterotopia in the right parietal region | Murdock et al. (2011) | |||
| Hemispherical asymmetry | Asymmetric microcephalic hemispheres | Bilgüvar et al. (2010), Zombor et al. (2019) | ||
| Volume loss worse on the left than the right cerebral hemisphere | Yu et al. (2010), Murdock et al. (2011) | |||
| Volume loss worse on the right than the left cerebral hemisphere | Kousar et al. (2011), Rupp et al. (2014), Slezak et al. (2021) | |||
| Frontal lobe hypoplasia | + | Farag et al. (2013) | ||
| Hippocampal abnormalities | Dysmorphic | Bilgüvar et al. (2010) | ||
| Simplified hippocampal gyration | Farag et al. (2013) | |||
| Dysplasia of the temporal lobe with small hippocampus | Banerjee et al. (2016) | |||
| Infratentorial abnormalities | Unilateral cerebellar hypoplasia, unilateral brainstem atrophy | Bilgüvar et al. (2010) | ||
| Cerebellar hypoplasia | Farag et al. (2013) | |||
| Slight atrophy of the brain stem and cerebellum | Banerjee et al. (2016) | |||
| Ventricular system abnormalities | Dilated ventricles | Yu et al. (2010), Memon et al. (2013) | ||
| Prominent extra-axial cerebrospinal | Kousar et al. (2011) | |||
| Slight expansion of bilateral brain ventricles, obvious expansion of the fourth ventricle | Banerjee et al. (2016) | |||
| Asymmetrical enlargement of the ventricles, dilated Virchow–Robin spaces | Zombor et al. (2019) | |||
| Posterior horn-dominant enlargement of the lateral ventricles | Slezak et al. (2021) | |||
| Thickened gray matter | + | Poulton et al. (2014), Zombor et al. (2019), Slezak et al. (2021) | ||
| Diffusely thickened cortex | Bilgüvar et al. (2010) | |||
| Mildly thickened cortex (∼5 mm) | Nicholas et al. (2010) | |||
| Blurred gray-white matter junction | Loss of gray–white junction | Bilgüvar et al. (2010) | ||
| Ill-defined gyral and nuclei pattern | Kousar et al. (2011) | |||
| Indistinct gray–white matter border in certain areas | Zombor et al. (2019) | |||
| Gray–white matter blurring involving the left parietooccipital cortex | Nardello et al. (2018) | |||
| Myelination/white matter abnormalities | Leukodystrophy, dysplasia of the white matter | Banerjee et al. (2016) | ||
| Thin white matter | Zombor et al. (2019) | |||
| Reduced white matter volume | Slezak et al. (2021) | |||
| MCPH3 | CDK5RAP2 | Corpus callosum abnormalities | Hypoplasia | Alfares et al. (2018) |
| Agenesis/hypogenesis | Issa et al. (2013) | |||
| Hypothalamic abnormalities | Interhypothalamic adhesion | Nasser et al. (2020) | ||
| Thickened gray matter | + | Alfares et al. (2018) | ||
| Myelination/white matter abnormalities | Bilateral enhancement in the white matter (white matter disorder) | |||
| MCPH4 | KNL1 | Infratentorial abnormalities | Cerebellar vermis hypoplasia | Saadi et al. (2016) |
| Ventricular system abnormalities | Wide cyst in the posterior fossa communicating with an expanded fourth ventricle | |||
| MCPH5 | ASPM | Corpus callosum abnormalities | Thick corpus callosum | Passemard et al. (2009), Saadi et al. (2009), Létard et al. (2018) |
| Agenesis of splenium | Passemard et al. (2009) | |||
| Agenesis of rostrum | ||||
| Partial agenesis | Abdel-Hamid et al. (2016) | |||
| Hypoplasia | Abdel-Hamid et al. (2016), Létard et al. (2018) | |||
| Pachygyria | + | Létard et al. (2018), Shaheen et al. (2019) | ||
| Temporal pachygyria | Ariani et al. (2013) | |||
| Polymicrogyria | Extensive unilateral perisylvian polymicrogyria from the frontal pole to the occipital pole | Passemard et al. (2009) | ||
| Extensive bilateral posterior polymicrogyria | Létard et al. (2018) | |||
| Polymicrogyria in frontoinsular region | ||||
| Frontal lobe hypoplasia | Severe hypoplasia of the frontal lobes | Saadi et al. (2009) | ||
| Frontal lobes are short and hypoplastic | Desir et al. (2008) | |||
| Infratentorial abnormalities | Mild asymmetric cerebellar hypoplasia | Passemard et al. (2009) | ||
| Ipsilateral pons hypoplasia | ||||
| Cerebellar vermis and/or hemispheres hypoplasia | Abdel-Hamid et al. (2016), Létard et al. (2018) | |||
| Elongated superior cerebellar peduncles | Létard et al. (2018) | |||
| Relatively small pons | Abdel-Hamid et al. (2016) | |||
| Thin brain stem | Ariani et al. (2013), Létard et al. (2018) | |||
| Ventricular system abnormalities | Occipital horns of the lateral ventricles enlarged | Passemard et al. (2009) | ||
| Dysmorphic frontal ventricles | Passemard et al. (2009) | |||
| Enlarged lateral ventricles and colpocephaly | Abdel-Hamid et al. (2016) | |||
| Large porencephalic cyst communicating with lateral ventricle | ||||
| Small midline cyst | ||||
| Ventricular enlargement | Létard et al. (2018) | |||
| Arachnoid cyst in the posterior fossa | ||||
| Enlarged Virchow–Robin spaces | ||||
| Enlarged subarachnoid spaces, mega cisterna magna | ||||
| Myelination/white matter abnormalities | Reduced white matter | Abdel-Hamid et al. (2016) | ||
| Myelination delay | Létard et al. (2018) | |||
| MCPH6 | CENPJ | N/A | ||
| MCPH7 | STIL | Corpus callosum abnormalities | Partial agenesis of the corpus callosum | Mouden et al. (2015), Shaheen et al. (2019) |
| Short dysmorphic corpus callosum | Kakar et al. (2015) | |||
| Holoprosencephaly | Lobar holoprosencephaly | Kakar et al. (2015), Mouden et al. (2015) | ||
| Frontal lobe hypoplasia | Disproportionately short frontal lobes, continuity of the right and left frontal lobes at the level of the basal ganglia and lateral ventricles | Kakar et al. (2015) | ||
| Straight and atrophic frontal lobe | Cheng et al. (2020) | |||
| Infratentorial abnormalities | Atrophy of the vermis | Mouden et al. (2015) | ||
| Cerebellar hypovermis dysplasia | Cheng et al. (2020) | |||
| Ventricular system abnormalities | Absence of ventricular frontal horns | Mouden et al. (2015) | ||
| Absence of occipital lobe and a large unilateral temporal and occipital fluid cavity communicating | ||||
| Small third ventricle, enlarged lateral ventricles posteriorly | Kakar et al. (2015) | |||
| Large porencephalic cyst replacing most of the posterior right hemisphere | ||||
| Dilatation of the fourth ventricle | Cheng et al. (2020) | |||
| Blurred gray-white matter junction | + | Cheng et al. (2020) | ||
| Myelination/white matter abnormalities | Diffuse severe reduction of the white matter volume | Kakar et al. (2015) | ||
| MCPH8 | CEP135 | Heterotopia | Bilateral nodular heterotopia in the peritrigonal regions | Bamborschke et al. (2020) |
| MCPH9 | CEP152 | Corpus callosum abnormalities | Severe hypogenesis | Shaheen et al. (2019) |
| Polymicrogyria | + | |||
| Ventricular system abnormalities | Inter-hemispheric cyst at left aspect of the falx continuous with the third ventricle | |||
| MCPH10 | ZNF335 | Corpus callosum abnormalities | Thin corpus callosum | Stouffs et al. (2018) |
| Lissencephaly/agyria | Anterior agyria and a posterior simplified gyral pattern | |||
| Basal ganglia abnormalities | Absent basal ganglia | Stouffs et al. (2018) | ||
| Invisible basal ganglia | Sato et al. (2016) | |||
| Volume loss in basal ganglia (putamen atrophy) | Caglayan et al. (2021) | |||
| Patchy areas of the altered signal in the left thalamoganglionic region | Rana et al. (2019) | |||
| Infratentorial abnormalities | Hypoplasia of brainstem and cerebellum | Sato et al. (2016), Stouffs et al. (2018) | ||
| Cerebellar atrophy | Rana et al., (2019), Caglayan et al. (2021) | |||
| Ventricular system abnormalities | Enlarged ventricles | Stouffs et al. (2018) | ||
| Myelination/white matter abnormalities | Hypomyelination | Sato et al. (2016), Stouffs et al. (2018), Caglayan et al. (2021) | ||
| MCPH11 | PHC1 | N/A | ||
| MCPH12 | CDK6 | |||
| MCPH13 | CENPE | Corpus callosum abnormalities | Partial agenesis of the corpus callosum | Mirzaa et al. (2014) |
| Frontal lobe hypoplasia | Low forehead | |||
| Infratentorial abnormalities | Cerebellar hypoplasia | |||
| Lissencephaly/agyria | Diffuse severely simplified gyral pattern with virtually no gyri over the frontal lobe | |||
| Myelination/white matter abnormalities | Immature white matter | |||
| MCPH14 | SASS6 | Lissencephaly/agyria | No gyral or sulcal development | Zhang et al. (2019b) |
| Basal ganglia abnormalities | Poorly confined basal ganglia and missing delineation of the internal capsule | Khan et al. (2014) | ||
| Infratentorial abnormalities | Dysmorphic infratentorial region with hypoplasia of the vermis cerebella | |||
| Ventricular system abnormalities | No bilateral frontal horns or cavum septi pellucidi present | Zhang et al. (2019b) | ||
| Abnormal formation of the lateral ventricles | Khan et al. (2014) | |||
| MCPH15 | MFSD2A | Corpus callosum abnormalities | Hypoplasia | Guemez-Gamboa et al. (2015), Scala et al. (2020) |
| Infratentorial abnormalities | Inferior vermian hypoplasia | Scala et al. (2020) | ||
| Pontine hypoplasia | ||||
| Hypoplasia of brain stem and cerebellum | Guemez-Gamboa et al. (2015) | |||
| Ventricular system abnormalities | Enlarged ventricles | Guemez-Gamboa et al. (2015), Harel et al. (2018), Scala et al. (2020) | ||
| Hydrocephaly | Shaheen et al. (2019) | |||
| Myelination/white matter abnormalities | White matter reduction | Alakbarzade et al. (2015), Harel et al. (2018), Scala et al. (2020) | ||
| MCPH16 | ANKLE2 | Corpus callosum abnormalities | Agenesis | Yamamoto et al. (2014) |
| Partial agenesis | Shaheen et al. (2019) | |||
| Pachygyria | Coarsening of the gyral sulcal pattern and some thickening consistent with pachygyria | |||
| Polymicrogyria | Polymicrogyria-like cortical brain malformations | Yamamoto et al. (2014) | ||
| Infratentorial abnormalities | Hypoplastic cerebellum | Shaheen et al. (2019) | ||
| Ventricular system abnormalities | Small frontal horns of the lateral ventricles with mildly enlarged posterior horns | Yamamoto et al. (2014) | ||
| Thickened gray matter | Mildly thickened cortex | |||
| MCPH17 | CIT | Corpus callosum abnormalities | Hypogenesis | Li et al. (2016a) |
| Agenesis | Harding et al. (2016), Shaheen et al. (2016) | |||
| Lissencephaly/agyria | Lissencephaly | Harding et al. (2016), Shaheen et al. (2016) | ||
| Infratentorial abnormalities | Cerebellar and brainstem hypoplasia | Harding et al. (2016) | ||
| Ventricular system abnormalities | Enlarged ventricles | Harding et al. (2016), Shaheen et al. (2016) | ||
| Myelination/white matter abnormalities | Diminished white matter | Shaheen et al. (2016) | ||
| MCPH18 | WDFY3 | N/A | ||
| MCPH19 | COPB2 | Corpus callosum abnormalities | Thin corpus callosum | DiStasio et al. (2017) |
| Ventricular system abnormalities | Slight dilation of the lateral, third and fourth ventricles | |||
| Myelination/white matter abnormalities | Delayed myelination | |||
| MCPH20 | KIF14 | Corpus callosum abnormalities | Agenesis | Moawia et al. (2017), Reilly et al. (2019) |
| Partial agenesis | Makrythanasis et al. (2018) | |||
| Lissencephaly/agyria | Microlissencephaly | Makrythanasis et al. (2018) | ||
| + | Moawia et al. (2017) | |||
| Infratentorial abnormalities | Cerebellar hypoplasia | Moawia et al. (2017), Reilly et al. (2019) | ||
| Ventricular system abnormalities | Large basal cisterns | Makrythanasis et al. (2018) | ||
| Interhemispheric cyst | Moawia et al. (2017) | |||
| Thickened gray matter | Slightly thickened cortex | Moawia et al. (2017) | ||
| MCPH21 | NCAPD2 | N/A | ||
| MCPH22 | NCAPD3 | |||
| MCPH23 | NCAPH | |||
| MCPH24 | NUP37 | Infratentorial abnormalities | Cerebellar vermis hypoplasia | Braun et al. (2018) |
| MCPH25 | TRAPPC14 | Corpus callosum abnormalities | Hypoplasia | Perez et al. (2019) |
| Myelination/white matter abnormalities | Diminished white matter | |||
| MCPH26 | LMNB1 | Corpus callosum abnormalities | Thin corpus callosum | Cristofoli et al. (2020) |
| Dysgenesis | ||||
| Pachygyria | + | |||
| Lissencephaly/agyria | Lissencephaly | |||
| Ventricular system abnormalities | Enlarged ventricles | |||
| MCPH27 | LMNB2 | Ventricular system abnormalities | Enlarged ventricles | Parry et al. (2021) |
| Myelination/white matter abnormalities | Diminished white matter | |||
| MCPH28 | RRP7A | Corpus callosum abnormalities | Volume loss especially in the anterior half | Farooq et al. (2020) |
Normal Corticogenesis
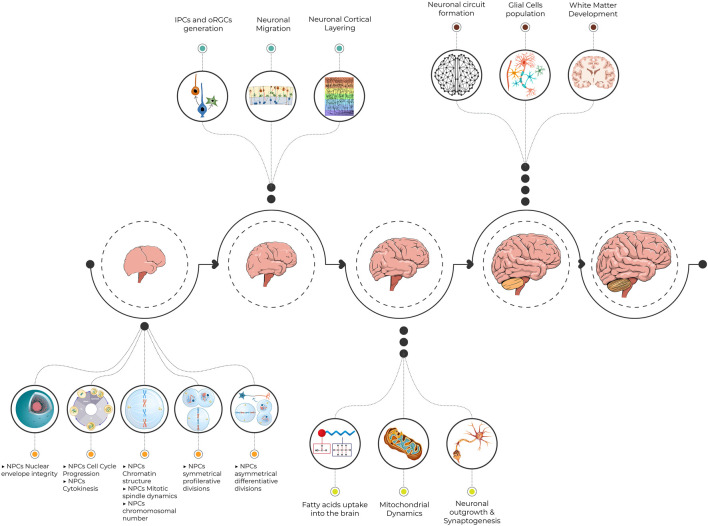
MCPH arises principally from a decreased production of neurons due to defects in progenitor proliferation, differentiation, and/or apoptosis during critical stages of brain development. Hence, it is important to briefly review the normal process of cortical neurogenesis before discussing the multiple facets of MCPH protein functions in maintaining a smooth running of this process.
Before the neurogenesis journey begins, the neural stem cells represented by neuroepithelial progenitors (NE) at the ventricular zone (VZ) undergo initial expansion in number through symmetrical cell divisions (Homem et al., 2015). Once the antiproliferative gene Tis21 starts to be expressed, NE cells begin to switch from proliferative division to neuronic division (Götz and Huttner, 2005). Simultaneously, NE cells transform gradually into more fate-restricted progenitors known as radial glial cells (RGCs) as an indication for their glial gene expressions (Götz and Huttner, 2005; Mori et al., 2005; Subramanian et al., 2019). RGCs possess apical processes attaching to the ventricular surface and basal processes reaching the basement membrane (future pial surface) (Homem et al., 2015). RGCs expand their number and exhibit a much higher number of asymmetrical cell divisions as compared with NE cells (Subramanian et al., 2019). During cell expansion, RGC nuclei show a characteristic interkinetic nuclear migration (INM) synchronized with the cell cycle phases during proliferation (Kosodo et al., 2011). The RGC nuclei migrate toward the basal side of the developing cortex during G1 phase and remain there during S phase before they migrate apically during G2 phase and proceed with M-phase once they reach the ventricular surface (Kosodo et al., 2011; Miyata et al., 2014). This pattern of migration during early neurogenesis requires functional microtubules and actin filaments (Götz and Huttner, 2005). It has been proposed that INM allows RGC rapid proliferation while maintaining their dense packing and determines cell fate through signaling gradients along their migration pathway (Götz and Huttner, 2005; Baye and Link, 2007; Del Bene et al., 2008). It is therefore very likely to find defects in neurogenesis involving RGC expansion and neuronal cell fate decisions when INM is disrupted (Latasa et al., 2009). Intriguingly, INM shows differences between species and might affect the total number of the generated neurons and thence the brain size (Okamoto et al., 2014).
Asymmetrical division of an RGC at the ventricular surface generates a self-renewing RG daughter cell and either a postmitotic neuron or a basal progenitor (intermediate progenitor cell (IPC)) (Homem et al., 2015; Subramanian et al., 2019). It has been earlier believed that the asymmetrical division of RGCs is principally driven by a change in mitotic spindle positions leading to a shift of the cleavage plane orientation from perpendicular (vertical) to parallel (horizontal) relative to the ventricular surface (Chenn and McConnell, 1995; Zhong et al., 1996; Kosodo et al., 2004; Fietz and Huttner, 2011; Homem et al., 2015). However, further investigations revealed that the rate of asymmetrical divisions in RGCs is not necessarily altered by the orientation of the cleavage plane (Morin et al., 2007; Konno et al., 2008; Postiglione et al., 2011). The asymmetrical RGC fate might be affected by inheriting centrioles with different maturity and primary cilium (Wang et al., 2009; Goetz and Anderson, 2010; Paridaen et al., 2013). It has been also shown that alterations in RGC cycle length control the shift from self-renewing divisions to neurogenic divisions (Calegari and Huttner, 2003; Calegari et al., 2005; Pilaz et al., 2009; Arai et al., 2011). Furthermore, it has been proposed that Notch signaling triggers neurogenic cell fate either by its distinct apicobasal gradient during INM or through asymmetric inheritance of endosomes positive for Sara (Smad anchor for receptor activation) (Del Bene et al., 2008; Nerli et al., 2020). This latter is achieved by targeting signaling endosomes to the central spindle by the action of plus-end kinesin motor (Klp98A) (Derivery et al., 2015).
Unlike RGCs, IPCs lack the connection with the ventricular surface and settle mainly in the subventricular zone (SVZ) basal to the VZ (Rakic, 2009; Homem et al., 2015; Subramanian et al., 2019; Heide and Huttner, 2021). IPCs undergo some proliferative divisions and terminate by generating two cortical neurons (Noctor et al., 2004; Pontious et al., 2008; Rakic, 2009). Asymmetrical divisions of RGCs in many mammals, especially in primates, yield an additional generation of a special type of basal progenitors known as basal RGCs (outer RGCs (oRGCs)) (Homem et al., 2015). Compared with IPCs, the oRGCs show a much higher proliferative capacity, which amplifies the total number of generated neurons and contribute to the characteristic folded cerebral cortex observed in primates, especially in humans (Reillo et al., 2011; Fietz et al., 2010; Hansen et al., 2010; Betizeau et al., 2013; Heide and Huttner, 2021). The newly established 3D in vitro human brain organoid model exhibits a considerable number of oRGCs (Lancaster et al., 2013; Zhang et al., 2019a).
Postmitotic cortical neurons are generated from both VZ and SVZ neural progenitors in an inside-out manner by which later-born neurons (superficial layers IV–II) bypass earlier-born neurons (deep layers VI–V) (Molyneaux et al., 2007; Leone et al., 2008). This pattern of neural generation is under spatiotemporal, cell cycle, and competency precise controls of neural progenitor fates (Kohwi and Doe, 2013). Eventually, six neural layers are created in the developing cerebral cortex, and the neurons start the formation of their distinctive dendrites, axons, and functional synapses. The fully developed cortical network contains 80% glutamatergic excitatory neurons produced by VZ and SVZ neural progenitors located in the dorsal telencephalon and 20% GABAergic inhibitory neurons that originated from the medial and caudal ganglionic eminence (Marín, 2013; Costa and Müller, 2014). The terminal number of generated cortical neurons is affected not only by the original number of neural progenitors but also by their starting and ending proliferation points and their cell lineage (Homem et al., 2015).
At the final stage of neurogenesis, RGCs lose their neuronal lineage and the connection with the apical surface switching to glial cell generators (Malatesta et al., 2000; Qian et al., 2000). Cortical astrocytes are firstly detected followed by oligodendrocytes, and the number of both glial cells is hugely expanded postnatally (Qian et al., 2000). Glial cells induce the development of white matter and axonal outgrowth by producing myelin and forming astrocytic branches (Subramanian et al., 2019). Taken together, forming a normal cerebral cortex requires highly organized spatiotemporal control for the neural progenitor populations to generate different neuronal and glial subtypes. Any defect during this process can lead to a major impact on brain development.
Brain Phenotype in Individuals With Microcephaly Primary Hereditary
The morphological changes in the brain structure of MCPH individuals have been mainly identified by radiological studies. Most MCPH cases show a reduction in brain volume associated with a simplified neocortical gyration pattern. However, the increased number of reported mutations and the ongoing neuroimaging of MCPH individuals reveal further brain malformations (Table 2). Some of these structural changes point toward the causative MCPH gene (e.g., the association between malformations of basal ganglia and mutations in gene encoding zinc finger-335 protein (ZNF335; MCPH10) (Sato et al., 2016; Stouffs et al., 2018; Rana et al., 2019; Caglayan et al., 2021)). The increasing number of reported brain malformations in MCPH individuals widens its pathogenesis spectrum. This indicates that the disruption of MCPH proteins not only is affecting the generation of neurons but could additionally affect neuronal differentiation, migration, dendritic and axonal outgrowth, and synaptogenesis. This is understandable given the fact that MCPH proteins are highly expressed in various neuroprogenitor organelles, especially the centrosome. In the following sections, we will discuss the consequences of MCPH mutations on brain development.
Accidents During the Brain Development Journey in Microcephaly Primary Hereditary
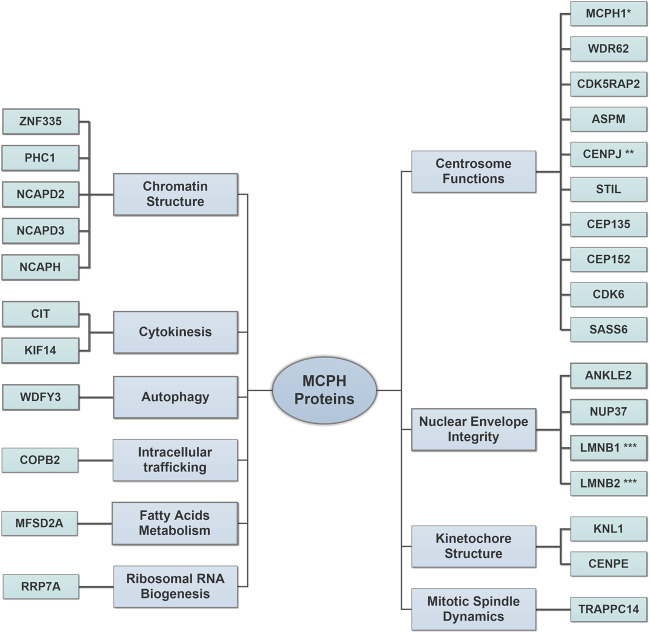
Studying the molecular mechanisms behind the pathogenesis of MCPH is very limited in humans. In fact, MCPH genes are highly conserved among different species (Woods et al., 2005; Gilbert et al., 2005), and this led to the discovery of several MCPH animal models mimicking the human phenotype (Table 1). Therefore, most of our current knowledge on the role of MCPH proteins in brain development is enriched through extensive studies on MCPH animal models. However, the pronounced difference of the human brain compared with most of the studied MCPH animal models establishes a new research direction toward 3D in vitro human brain organoid systems in studying the pathogenesis of microcephaly (Muzio and Consalez, 2013; Gabriel et al., 2020). The remarkable presence of oRGCs in this model opens the door for deeper insights into their role during the course of this disease in humans (Lancaster et al., 2013; Zhang et al., 2019a). As many MCPH proteins share overlapped functions, we saw to categorize them according to their major role(s) rather than discussing each one individually (Figure 1).
FIGURE 1.
Major roles of microcephaly primary hereditary (MCPH) proteins in brain development. The increased number of discovered MCPH proteins expands the pathomechanism spectrum to include several cellular components. Centrosome Functions: the proteins of this group regulate proper centrosomal functions to balance the transition between neural progenitor cell (NPC) proliferation and differentiation by controlling cell cycle progression and cell cycle exit fraction. Nuclear Envelope Integrity: the proteins of this group affect the proper spindle alignment and cell fate determinants during NPC proliferation and protect radial glial cell (RGC) nuclei from mechanical stress injury during INM. Kinetochore Structure: the proteins of this group assure the correct alignment of chromosomes during mitosis. Mitotic Spindle Dynamics: the proteins of this group regulate the spindle dynamics and cell division. Chromatin Structure: the proteins of this group regulate gene expression during neurogenesis and assure proper DNA damage repair. Cytokinesis: the proteins of this group regulate the terminal step in the cell cycle, which leads to a physical separation between the daughter cells. Autophagy: the proteins of this group facilitate the removal of cytosolic protein aggregates and maintain mitochondrial homeostasis. Intracellular trafficking: the proteins of this group control the cellular retrograde trafficking from the Golgi to the endoplasmic reticulum. Fatty Acid Metabolisms: the proteins of this group affect the postnatal neuronal morphogenesis, which requires a normal lipogenesis process. Ribosomal RNA Biogenesis: the proteins of this group regulate ribosomal RNA processing and affect primary cilia resorption. Please refer to (Table 1) for full protein names. *MCPH1 is also involved in chromatin structure. **CENPJ is also involved in kinetochore structure. ***LMNB1 and LMNB2 are also involved in mitotic spindle dynamics.
Dysfunctional Centrosome
Almost one-third of MCPH mutations occur in centrosomal or mitotic spindle proteins. Defective centrosomes can affect cell cycle progression and cell division, leading to abnormal chromosomal numbers, cell cycle arrest, and apoptosis (Barbelanne and Tsang, 2014). It has been proposed that alterations of the cleavage plane orientation during NE proliferation increase asymmetric cell divisions (Buchman and Tsai, 2007; Knoblich, 2008; Zhong and Chia, 2008). This, in turn, leads to an early consumption of progenitor cells at the expense of a premature generation of neurons with ultimately reduced number, thence a smaller brain (Fish et al., 2006; Buchman et al., 2010; Kaindl et al., 2010; Lizarraga et al., 2010). In this notion, several MCPH mouse models show a shift in the cleavage plan orientation of NE cells favoring neurogenic cell fate (Fish et al., 2006; Lizarraga et al., 2010; Pulvers et al., 2010; Gruber et al., 2011; Xu et al., 2014). This evidence has been also supported by human brain organoid models (Lancaster et al., 2013; Zhang et al., 2019a). Intriguingly, most of the MCPH fly models with defected centrosomal proteins exhibit normal brain size (Basto et al., 2006; Lucas and Raff, 2007; Roque et al., 2012), indicating that changes in the cleavage plane orientation might only have a minor impact on brain growth. Alternatively, flies could have compensatory mechanisms bypassing the effect of the misoriented cleavage plane (Nigg and Raff, 2009). On the other hand, further studies discovered MCPH mouse models with the microcephaly phenotype, though unaffected cleavage plane (Pulvers et al., 2010; Marjanović et al., 2015). Furthermore, depletion of some other MCPH centrosomal proteins in mice does not affect brain growth at all (Malumbres et al., 2004).
The neural progenitor cell (NPC) symmetrical proliferation speed is frequently reduced in mutated MCPH genes, which encode centrosomal proteins (Lizarraga et al., 2010; Gruber et al., 2011; Sgourdou et al., 2017; Ding et al., 2019). This is much obvious toward the end of neurogenesis (Lizarraga et al., 2010; Gruber et al., 2011; Sgourdou et al., 2017) when the later-born neurons (superficial layers II–IV) start to be generated. Together with the premature generation of neurons, this explains why superficial cortical neurons are the most affected in most MCPH models (Lizarraga et al., 2010; Chen et al., 2014). This is in line with postmortem histological analysis described in a case of WD-repeat-containing protein 62 gene (WDR62; MCPH2) mutation (Yu et al., 2010). In addition, MCPH proteins are important for the normal distribution of cells between cortical zones. Knockout of abnormal spindle-like, microcephaly-associated gene (Aspm) in ferret increases the number of generated oRGCs, affecting the RGC overall proliferative capacity (Johnson et al., 2018). Likewise, knockdown of the cyclin-dependent kinase five regulatory subunit-associated protein two gene Cdk5rap2 in a mouse model alters the distribution of progenitor pool leading to more generation of basal progenitors (Buchman et al., 2010). By contrast, somatosensory cortical layer VI has been reported to be thinner in an Aspm knockout mouse model (Fujimori et al., 2014). Indeed, several in vivo and in vitro studies including human brain organoid models revealed that MCPH centrosomal genes balance the transition between NPC proliferation and differentiation by controlling cell cycle progression and cell cycle exit fraction (Buchman et al., 2010; Lizarraga et al., 2010; Bogoyevitch et al., 2012; Lancaster et al., 2013; Hainline et al., 2014; Zhang et al., 2019a). This explains, respectively, the reduced proliferation and premature neuronal differentiation detected in the respective MCPH animal models. Furthermore, using the conditional knockout mouse model, it has been shown that centromeric protein J (Cenpj) regulates NPC cell cycle progression by regulating cilium disassembly during neurogenesis (Ding et al., 2019). Similarly, depletion of WDR62 and centrosomal-P4.1-associated protein (CPAP) in human cerebral organoids impairs the cilium disassembly and cell cycle progression (Gabriel et al., 2016; Zhang et al., 2019a).
It has been reported that mutations in genes encoding MCPH centrosomal proteins alter the maturation and cellular number of centrosomes (Pfaff et al., 2007; Rodrigues-Martins et al., 2007; Yabe et al., 2007; Megraw et al., 2011; Hussain et al., 2012; McIntyre et al., 2012; Hussain et al., 2013; Arquint and Nigg, 2014). This, in turn, might affect the proper distribution of chromosomes to daughter cells leading to spindle instability and mitotic delay or arrest at metaphase checkpoint (Ripoll et al., 1985; Gonzalez et al., 1990; Megraw et al., 1999; Riparbelli et al., 2002; Matsuura et al., 2004; Pfaff et al., 2007; Lizarraga et al., 2010; Kim et al., 2011a; Vitale et al., 2011; Novorol et al., 2013; Chen et al., 2014). In most of these cases, such defect triggers the apoptotic cascade leading to cellular loss (Pfaff et al., 2007; Lizarraga et al., 2010; Vitale et al., 2011; McIntyre et al., 2012; Novorol et al., 2013; Chen et al., 2014). Remarkably, increased apoptosis of NPCs—associated with or without proliferation/differentiation defects—contributes to the microcephaly phenotype by depleting the neural stem cell pool (Izraeli et al., 1999; Pfaff et al., 2007; Lizarraga et al., 2010; Gruber et al., 2011; McIntyre et al., 2012; Sgourdou et al., 2017; Zhang et al., 2019a; Ding et al., 2019). Intriguingly, neuronal populations also seem to be vulnerable to apoptosis during later stages of development (Lizarraga et al., 2010).
Apparently, the impact of mutated MCPH centrosomal genes in brain development not only is restricted to NPC proliferation and differentiation phase but also exceeds it to affect neuronal migration, dendritic and axonal outgrowth, and synaptogenesis. The presence of gray matter heterotopia, polymicrogyria, lissencephaly, and pachygyria in several MCPH conditions points toward impaired neuronal migration (Table 2) (Leventer et al., 2010; Yu et al., 2010). The signs of this impairment have been reported in postmortem histopathological MCPH samples and various MCPH animal models (Yu et al., 2010; Buchman et al., 2011; Xu et al., 2014). Besides that, an interesting role of Cdk5rap2 in regulating dendritic development and synaptogenesis in superficial cortical layer II/III has been reported (Zaqout et al., 2019). The contribution of dendritic complexity deficits in the microcephaly phenotype points toward a progressive nature during the MCPH course (van Dyck and Morrow, 2017).
Defective Chromatin Structure
Chromatin structure is a golden stone for gene expression regulation during neurogenesis. Mutations in genes encoding chromatin-linked proteins expand the pathomechanism spectrum of the MCPH. Microcephalin (BRCT/BRIT1) mutated cells taken from MCPH1 individuals and mouse models displayed premature chromosome condensation (PCC) associated with a high frequency of prophase-like cells and defective DNA damage repair (Jackson et al., 2002; Neitzel et al., 2002; Liang et al., 2010; Trimborn et al., 2010). This feature has been considered as a diagnostic marker for individuals with MCPH1 gene mutations (Jackson et al., 2002). In flies, mcph1 mutants display embryonic lethality due to mitotic arrest and uncoordinated centrosome/nuclear cycles in early syncytial cell cycles (Brunk et al., 2007). Likewise, mutations in gene encoding polyhomeotic-like one protein (PHC1; MCPH11) are associated with aberrant DNA damage repair (Awad et al., 2013). In addition, PHC1 mutations disturb the expression of Nanog and the ubiquitination of histone H2A, in which the former maintains pluripotency and the latter affects the cell cycle progression by the accumulation of Geminin (Awad et al., 2013; Chen et al., 2021).
Complete ablation of Znf335 gene in mice is embryonically lethal, but conditional knockout leads to a reduction in the cortical size affecting the forebrain much severely (Yang et al., 2012). This has been attributed to disruptions in NPC proliferation, cell fate, and neuronal differentiation (Yang et al., 2012). Consistently, postmortem histopathological studies on brain samples taken from ZNF335 patients reveal a severe reduction in the neuronal number associated with abnormalities in neuronal morphogenesis, migration, and polarity (Yang et al., 2012).
Mutations in genes encoding condensin complex proteins NCAPD2, NCAPD3, and NCAPH have been linked to MCPH21, 22, and 23, respectively (Martin et al., 2016). One of the hallmarks of hypomorphic Ncaph2 mice is the formation of a chromatin bridge in apical NPCs (Martin et al., 2016). These bridges result from failed sister chromatid disentanglement leading to chromosome segregation errors and aneuploidy (Martin et al., 2016). Subsequently, NPCs undergo a reduced cell proliferation and an increased apoptosis without obvious alterations in spindle orientations or cell fat (Martin et al., 2016).
Deformed Kinetochore Proteins
Mutations in genes encoding kinetochore scaffold one protein (KNL1, previously known as CASC5) and centromere-associated protein E (CENPE) have been linked to MCPH4 (Jamieson et al., 1999; Genin et al., 2012; Mirzaa et al., 2014; Saadi et al., 2016; Szczepanski et al., 2016; Zarate et al., 2016). Proper function of proteins associated with centromeric kinetochore assures the correct alignment of chromosomes during mitosis; else, spindle assembly checkpoint (SAC) is activated and suspends the mitotic progression (Cleveland et al., 2003; Musacchio and Salmon, 2007; Santaguida and Musacchio, 2009; Hori and Fukagawa, 2012). Conditional knockout of Knl1 in mouse cortical NPCs results in DNA damage due to chromosomal segregation errors (Shi et al., 2019). This triggers a p53-dependent apoptotic cascade and leads to massive loss of NPCs and microcephaly (Shi et al., 2019). Similar to MCPH centrosomal proteins, the progressive loss of NPCs in Knl1 conditional knockout mice affects mainly the later-born neurons (superficial layers II–IV) (Shi et al., 2019). CENPE facilitates the transition from metaphase to anaphase during the cell cycle (Yen et al., 1991). Disruption of Cenpe function in mice and Drosophila leads to early embryonic lethality due to chromosomal instability (Yucel et al., 2000; Putkey et al., 2002).
Interruption of Fatty Acids Uptake Into the Brain
Proper brain development and function require essential omega-3 fatty acids, which need to be obtained from the circulation via specific transporters (Alakbarzade et al., 2015). Sodium-dependent lysophosphatidylcholine (LPC) transporter (MFSD2A) is exclusively expressed in the endothelium of the blood–brain barrier (BBB) and a major transport facilitator for docosahexaenoic acid (DHA) (Ben-Zvi et al., 2014; Nguyen et al., 2014; Alakbarzade et al., 2015; Guemez-Gamboa et al., 2015). Depending on the transporter residual activity, mutations in MFSD2A (MCPH15) gene is associated with either a progressive microcephaly syndrome or a much lethal phenotype (Berger et al., 2012; Alakbarzade et al., 2015; Guemez-Gamboa et al., 2015; Harel et al., 2018; Scala et al., 2020). The progressive feature associated with the milder form of this disease raises the possibility that LPC transportation is continuously required for membrane biogenesis in the brain (Alakbarzade et al., 2015; Scala et al., 2020). Endothelial-specific deletion of Mfsd2a in mice leads to a microcephaly phenotype accompanied by a reduction in neuronal arborization and dendritic length (Chan et al., 2018). Interestingly, neuronal loss detected in Mfsd2a knockout mice was restricted to cerebellar Purkinje cells and hippocampal CA1 and CA3 regions (Nguyen et al., 2014). Taken together, these data demonstrate that, unlike other MCPHs, Mfsd2a deficiency affects the postnatal neuronal morphogenesis, which requires a normal lipogenesis process (van Deijk et al., 2017; Ziegler et al., 2017; Chan et al., 2018). Notably, variable degrees of white matter reduction have been also reported in MCPH15 individuals (Alakbarzade et al., 2015; Harel et al., 2018; Scala et al., 2020). Further studies are required to assess to which extent do white matter deficits contribute to the microcephaly phenotype (Huang and Li, 2021).
Altered Nuclear Envelope
Mutations in several genes encoding nuclear envelop components have been recently linked to MCPH conditions. Ankyrin repeat- and lem domain-containing protein 2 (Ankle2) is localized to the endoplasmic reticulum and nuclear envelope (Link et al., 2019). Drosophila dAnkle2 mutant larvae show a reduction in the brain size due to impaired nuclear envelope integrity, which eventually affects proper spindle alignment and cell fate determinants during NPC proliferation (Link et al., 2019). Another study, however, suggests that the reduction in Drosophila dAnkle2 mutant NPCs cells is due to defects in proliferation and massive apoptosis rather than an alteration in asymmetrical cell division (Yamamoto et al., 2014). In this line, Caenorhabditis elegans ANKLE2 ortholog protein (LEM-4L) plays a critical role in mitosis by facilitating nuclear envelope reassembly during mitotic exit (Asencio et al., 2012).
Individuals with mutations in genes encoding various nuclear pore complex proteins (NPC) are diagnosed with severe forms of nephrotic syndrome (Braun et al., 2018). However, mutations in NPC subunit component nucleoporin 37 (NUP37) also exhibit intellectual disability and MCPH (Braun et al., 2018). More recently and yet to be linked to a specific OMIM MCPH number, mutations in NUP85 subunit are associated with a reduction in brain volume, delayed myelination, agenesis of the corpus callosum, gray matter heterotopia, and frontal lobe cortical malformation (Ravindran et al., 2021). Fibroblasts derived from NUP85 individuals are characterized by reduced cell viability, proliferation rate, abnormal mitotic spindle apparatus, and altered cytoskeletal protein expressions (Ravindran et al., 2021). As most of the studies performed in viable animal models with NPC defects focused on the nephrotic phenotype, further investigations to understand their effects on brain growth are still warranted.
B-type lamins 1 and 2 (LMNB1/2) are intermediate filament proteins involved in nuclear envelope reassembly, in which the deficiency leads to fragile nuclei more susceptible to nuclear membrane (NM) rupture (Coffinier et al., 2011; Chen et al., 2019). In humans, mutations in LMNB1 and LMNB2 have been linked to MCPH26 and MCPH27, respectively (Cristofoli et al., 2020; Parry et al., 2021). During early neurogenesis, RGC nuclei undergo INM, which represents mechanical stress that threatens RGCs with weakened nuclear lamina (Chen et al., 2019). Therefore, lack of murine Lmnb1/2 during this critical step triggers NPC apoptosis and leads to abnormal neuronal migration reflected by disorganized cortical layering (Kim et al., 2011b; Coffinier et al., 2011; Young et al., 2012; Chen et al., 2019). This migration defect not only is confined to the cerebral cortex but also affects the hippocampal and cerebellar layering (Coffinier et al., 2010; Coffinier et al., 2011). In addition, it has been proposed that Lmnb1/2 is localized at the mitotic spindle and plays a role in INM and neuronal migration via interaction with dynein in organizing NPC spindle orientation (Tsai et al., 2006; Kim et al., 2011b). However, no abnormal metaphase spindle formation has been noticed in lymphoblastoid cells (LCLs) derived from LMNB1 individuals (Cristofoli et al., 2020).
Defective Cytokinesis
Cytokinesis is the terminal step in the cell cycle, which leads to a physical separation between the daughter cells. Defects in this process frequently result in the formation of binucleated cells, aneuploidy, chromosomal instability, cell cycle arrest, and apoptosis (Li et al., 2016a). Notably, the elevated number of binucleated cells—including pyramidal and Purkinje cells—is considered as a key feature for cytokinesis failures (Di Cunto et al., 2000; Roberts et al., 2000; Reilly et al., 2019). Citron rho-interacting kinase (CIT) midbody protein has important roles in cytokinesis, and its defect leads to MCPH17 in humans (Li et al., 2016a; Basit et al., 2016; Harding et al., 2016; Shaheen et al., 2016). Postmortem histopathological analysis of brain samples taken from MCPH17 individuals reveals a thickened neocortex with disorganized layers and unmyelinated white matter with scattered neurons (Harding et al., 2016). In addition, the cerebellar cortex and hippocampus show dysplastic and hypoplastic features, and Purkinje cells exhibit a simplified dendritic tree where many of them are multinucleated (Harding et al., 2016). Studies conducted in Cit knockout rodent models reveal that NPCs undergo massive apoptosis due to interrupted cytokinesis (Di Cunto et al., 2000; Roberts et al., 2000). In these models, binucleated neurons have been detected in several brain and spinal cord regions; however, apoptosis seems to be more pronounced in the cerebral cortex, granular layers of cerebellum, hippocampus, and olfactory bulb (Di Cunto et al., 2000; Sarkisian et al., 2002). In addition, the high rate of cell death reported at the ganglionic eminence reduces the total number of generated interneurons (Sarkisian et al., 2001; Di Cunto et al., 2000). Consistent with human brain findings, cerebellar Purkinje cells are disorganized and show underdeveloped dendritic complexity (Di Cunto et al., 2000). The presence of disorganized cortical layering and scattered neurons in the white matter should raise the possibility of an abnormal migration process even though it is yet to be confirmed by further studies.
The role of CIT in cytokinesis requires a proper function of kinesin family 14 (KIF14) microtubule motor protein (Makrythanasis et al., 2018). It is then unsurprising to realize that mutations in KIF14 also lead to MCPH by a common mechanism as CIT mutations (Moawia et al., 2017; Makrythanasis et al., 2018; Reilly et al., 2019). This is supported by several studies conducted in various animal models with depleted KIF14 homologs (Ohkura et al., 1997; Fujikura et al., 2013; Reilly et al., 2019). Mutations of Drosophila KIF14 homolog, also known as kinesin-like protein at 38B (KLP38B), affect the cell cycle progression due to cytokinesis failure (Ohkura et al., 1997). In the same notion, Laggard mice (lag), an animal model for KIF14, are characterized by microcephaly, cortical dysgenesis, and severe hypomyelination as a consequence of massive apoptosis during late neurogenesis (Fujikura et al., 2013). Consequently, Cux1-positive upper cortical neurons are much reduced in number, and some of them are displaced (Fujikura et al., 2013). Similar to Cit knockout models, lag mice show scattered cerebellar Purkinje cells with simplified dendritic trees pointing toward abnormalities in neuronal migration and neurite formation (Fujikura et al., 2013).
Disturbed Autophagy and Mitochondrial Dynamics
MCPH18 is caused by mutations in gene encoding WD repeat and FYVE domain-containing 3 (WDFY3), also known as Autophagy-Linked FYVE (ALFY) (Kadir et al., 2016). Normally, this scaffolding protein facilitates the removal of cytosolic protein aggregates, which, in turn, maintains mitochondrial homeostasis (Kadir et al., 2016; Napoli et al., 2018; Napoli et al., 2021). Wdfy3 is highly expressed in RGCs, and its loss of function prevents the transition from symmetrical proliferative divisions to asymmetrical differentiative divisions by altering the Wnt signaling cascade (Tacchelly-Benites et al., 2013; Orosco et al., 2014; Kadir et al., 2016). The imbalance in NPCs mode of cell division leads to regional differences in neocortical thickness and opposing phenotypes of micro- and macrocephaly (Orosco et al., 2014; Le Duc et al., 2019). On the other hand, the disruption of mitochondrial dynamics in Wdfy3 mutant mice decreases the synaptic density, alters the synaptic plasticity, and probably affects dendritic development (Napoli et al., 2018; Napoli et al., 2021). Remarkably, proteins involved in GABAergic neurotransmission are downregulated in Wdfy3 mutant mice (Napoli et al., 2021). Furthermore, Wdfy3 plays a role in neural migration during early neurogenesis (Orosco et al., 2014).
Interrupted Intracellular Trafficking
Coatomer Protein Complex Subunit Beta 2 (COPB2) controls the cellular retrograde trafficking from the Golgi to the endoplasmic reticulum (Orci et al., 1993; Letourneur et al., 1994). Interestingly, mutations in COPB2 interrupt brain growth and lead to MCPH19 (DiStasio et al., 2017). While the complete loss of Copb2 is incompatible with life, partial loss of Copb2 in mice interferes with the growth of the brain (DiStasio et al., 2017). This has been associated with increased cell death and a high number of proliferative cells positive for phosphorylated histone H3 (pH3) (DiStasio et al., 2017). Still, further studies are necessitated to dissect the exact role of Copb2 in controlling brain size.
Disturbed Mitotic Spindle Dynamics
Trafficking protein particle complex subunit 14 (TRAPPC14)—also known as microtubule-associated protein 11 (MAP11)—is localized to mitotic spindles and interacts with α-tubulin regulates the spindle dynamics and cell division (Perez et al., 2019). Recently, TRAPPC14 mutations have been linked to MCPH25 in human and microcephaly phenotypes in the zebrafish model (Perez et al., 2019). This has been mainly attributed to a decreased brain cell proliferation due to altered spindle dynamics affecting the mitotic progression and probably the cytokinesis, however, without increased apoptosis (Perez et al., 2019). In addition, TRAPPC14 has been implicated in ciliogenesis and cilia stability, which, in turn, could affect brain growth (Cuenca et al., 2019).
Defective Ribosome Biogenesis
The most recently diagnosed MCPH28 cases have been linked to a mutation in Ribosomal RNA Processing seven Homolog A (RRP7A) (Farooq et al., 2020). It is known that mutations in genes involved in ribosome biogenesis are associated with neurodevelopmental defects together with other abnormalities (Hetman and Slomnicki, 2019). The encoded RRP7A protein shows high expression in RGCs and cellular localizations at the centrosome, primary cilium, and nucleolus (Farooq et al., 2020). Depletion of RRP7A alters ribosomal RNA processing and affects primary cilia resorption, causing a delay in S-phase entry and progression (Farooq et al., 2020). Mutated rrp7a zebrafish embryos display a reduction in the expression pattern of some proliferation and neural differentiation markers, while TUNEL assay analysis indicates increased apoptosis (Farooq et al., 2020). These findings might result from defective rrp7a functions at the level of centrosome and/or primary cilia. MicroRNA processing has been identified as a contributing factor in temporal fate specification (Kohwi and Doe, 2013). Thus, dysregulated ribosomal RNA processing with subsequent nucleolar stress establishes a new insight into MCPH pathomechanisms.
Microcephaly Primary Hereditary Versus Infection-Induced Microcephaly
After describing the genetic component of microcephaly, we here shed some light on some infectious agents associated with microcephaly. Particularly Toxoplasma gondii, cytomegalovirus, rubella virus, and syphilis, but also the herpes simplex virus, HIV, and Zika virus, have been reported in children born with microcephaly. The severity of microcephaly depends not only on the type of the infectious agent but much importantly on the gestational age when an infection occurs (Devakumar et al., 2018). It has been shown that neural progenitors are targeted by these pathogens; however, the mechanisms by which most of these infections lead to microcephaly are not fully understood (Devakumar et al., 2018). Nevertheless, the epidemic infections with the Zika virus and its association with congenital microcephaly triggered extensive research in this field. Several studies based on various in vitro and in vivo models point toward NPC cell cycle arrest or an increase in cell death upon the infection with the Zika virus (Adams Waldorf et al., 2016; Li et al., 2016b; Cugola et al., 2016; Garcez et al., 2016; Tang et al., 2016; Wu et al., 2016). Intriguingly, several MCPH genes including Mcph1, Aspm, Cdk5rap2, Stil, and Cep135 are downregulated in brain tissues extracted from Zika-infected mice (Li et al., 2016b; Wu et al., 2016). This raises the possibility that infection-induced microcephaly might alter brain growth via altering the expression of various MCPH genes. However, the direct impact of infectious agents on the pathogenesis of microcephaly cannot be ruled out.
Conclusion
The journey during brain growth and development is impeded at specific points with crucial steps (Figure 2). Minor defects at any of these developmental points result in various neurodevelopmental disorders including MCPH. The earlier the insult during the journey, the greater the impact on brain growth. The major obstacle is faced during the rapid NPC proliferation just before the commencing generation of neurons. Either decreased proliferation or increased NPCs apoptosis depletes the neuronal stem cell pool and ultimately leads to a smaller number of generated neurons. Obviously, most MCPHs are caused by mutations in centrosomal proteins. Hence, dysfunctional centrosome alters NPC proliferation, cell cycle progression, and cell fate determination. In addition, the resulting aneuploidy and increased DNA damage response associated with some mutated MCPH genes trigger apoptosis. The accelerated number of discovered MCPH genes expands the pathomechanism spectrum of this disease beyond the centrosomal component. Similarly, the simultaneous generation of animal models mimicking the human MCPH phenotype provides a strong platform for future studies to dissect further molecular mechanisms behind the microcephaly phenotype. This will also expand our knowledge of normal brain growth and evolution.
FIGURE 2.
An illustrative figure demonstrating the pathway toward normal brain development. Minor defects at crucial steps in neurogenesis result in various neurodevelopmental disorders including MCPH. NPCs, neural progenitor cells; IPCs, intermediate progenitor cells; oRGCs, outer radial glial cells; MCPH, microcephaly primary hereditary.
Acknowledgments
We acknowledge support from the Open Access Publication Fund of Charité—Universitätsmedizin Berlin. We also thank Mr. Ahmed Hany Amarah for his help in designing Figure 2.
Author Contributions
SZ wrote the initial manuscript draft and generated the figures and tables. AK revised the manuscript. Both authors approved the final article.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Abdel-Hamid M. S., Ismail M. F., Darwish H. A., Effat L. K., Zaki M. S., Abdel-Salam G. M. H. (2016). Molecular and Phenotypic Spectrum ofASPM-Related Primary Microcephaly: Identification of Eight Novel Mutations. Am. J. Med. Genet. 170, 2133–2140. 10.1002/ajmg.a.37724 [DOI] [PubMed] [Google Scholar]
- Adams Waldorf K. M., Stencel-Baerenwald J. E., Kapur R. P., Studholme C., Boldenow E., Vornhagen J., et al. (2016). Fetal Brain Lesions after Subcutaneous Inoculation of Zika Virus in a Pregnant Nonhuman Primate. Nat. Med. 22, 1256–1259. 10.1038/nm.4193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal A., Mittal H., Patil R., Debnath S., Rai A. (2013). Clinical Profile of Children with Developmental Delay and Microcephaly. J. neurosciences Rural Pract. 04, 288–291. 10.4103/0976-3147.118781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alakbarzade V., Hameed A., Quek D. Q. Y., Chioza B. A., Baple E. L., Cazenave-Gassiot A., et al. (2015). A Partially Inactivating Mutation in the Sodium-dependent Lysophosphatidylcholine Transporter MFSD2A Causes a Non-lethal Microcephaly Syndrome. Nat. Genet. 47, 814–817. 10.1038/ng.3313 [DOI] [PubMed] [Google Scholar]
- Alcantara D., O'Driscoll M. (2014). Congenital Microcephaly. Am. J. Med. Genet. 166, 124–139. 10.1002/ajmg.c.31397 [DOI] [PubMed] [Google Scholar]
- Alfares A., Alhufayti I., Alsubaie L., Alowain M., Almass R., Alfadhel M., et al. (2018). A New Association between CDK5RAP2 Microcephaly and Congenital Cataracts. Ann. Hum. Genet. 82, 165–170. 10.1111/ahg.12232 [DOI] [PubMed] [Google Scholar]
- Arai Y., Pulvers J. N., Haffner C., Schilling B., Nüsslein I., Calegari F., et al. (2011). Neural Stem and Progenitor Cells Shorten S-phase on Commitment to Neuron Production. Nat. Commun. 2, 154. 10.1038/ncomms1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariani F., Mari F., Amitrano S., Di Marco C., Artuso R., Scala E., et al. (2013). Exome Sequencing Overrides Formal Genetics: ASPM Mutations in a Case Study of Apparent X‐linked Microcephalic Intellectual Deficit. Clin. Genet. 83, 288–290. 10.1111/j.1399-0004.2012.01901.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arquint C., Nigg E. A. (2014). STIL Microcephaly Mutations Interfere with APC/C-mediated Degradation and Cause Centriole Amplification. Curr. Biol. 24, 351–360. 10.1016/j.cub.2013.12.016 [DOI] [PubMed] [Google Scholar]
- Asencio C., Davidson I. F., Santarella-Mellwig R., Ly-Hartig T. B. N., Mall M., Wallenfang M. R., et al. (2012). Coordination of Kinase and Phosphatase Activities by Lem4 Enables Nuclear Envelope Reassembly during Mitosis. Cell 150, 122–135. 10.1016/j.cell.2012.04.043 [DOI] [PubMed] [Google Scholar]
- Awad S., Al-Dosari M. S., Al-Yacoub N., Colak D., Salih M. A., Alkuraya F. S., et al. (2013). Mutation in PHC1 Implicates Chromatin Remodeling in Primary Microcephaly Pathogenesis. Hum. Mol. Genet. 22, 2200–2213. 10.1093/hmg/ddt072 [DOI] [PubMed] [Google Scholar]
- Bacino C. A., Arriola L. A., Wiszniewska J., Bonnen P. E. (2012). WDR62 Missense Mutation in a Consanguineous Family with Primary Microcephaly. Am. J. Med. Genet. 158a, 622–625. 10.1002/ajmg.a.34417 [DOI] [PubMed] [Google Scholar]
- Bamborschke D., Daimagüler H. S., Hahn A., Hussain M. S., Nürnberg P., Cirak S. (2020). Mutation in CEP135 Causing Primary Microcephaly and Subcortical Heterotopia. Am. J. Med. Genet. 182, 2450–2453. 10.1002/ajmg.a.61762 [DOI] [PubMed] [Google Scholar]
- Banerjee S., Chen H., Huang H., Wu J., Yang Z., Deng W., et al. (2016). Novel Mutations c.28G>T (p.Ala10Ser) and c.189G>T (p.Glu63Asp) in WDR62 Associated with Early Onset Acanthosis and Hyperkeratosis in a Patient with Autosomal Recessive Microcephaly Type 2. Oncotarget 7, 78363–78371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbelanne M., Tsang W. Y. (2014). Molecular and Cellular Basis of Autosomal Recessive Primary Microcephaly. Biomed. Res. Int. 2014, 547986. 10.1155/2014/547986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basit S., Al-Harbi K. M., Alhijji S. A. M., Albalawi A. M., Alharby E., Eldardear A., et al. (2016). CIT, a Gene Involved in Neurogenic Cytokinesis, Is Mutated in Human Primary Microcephaly. Hum. Genet. 135, 1199–1207. 10.1007/s00439-016-1724-0 [DOI] [PubMed] [Google Scholar]
- Bastaki F., Mohamed M., Nair P., Saif F., Tawfiq N., Aithala G., et al. (2016). Novel Splice-Site Mutation inWDR62revealed by Whole-Exome Sequencing in a Sudanese Family with Primary Microcephaly. Congenit. Anom. 56, 135–137. 10.1111/cga.12144 [DOI] [PubMed] [Google Scholar]
- Basto R., Lau J., Vinogradova T., Gardiol A., Woods C. G., Khodjakov A., et al. (2006). Flies without Centrioles. Cell 125, 1375–1386. 10.1016/j.cell.2006.05.025 [DOI] [PubMed] [Google Scholar]
- Baye L. M., Link B. A. (2007). Interkinetic Nuclear Migration and the Selection of Neurogenic Cell Divisions during Vertebrate Retinogenesis. J. Neurosci. 27, 10143–10152. 10.1523/jneurosci.2754-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Zvi A., Lacoste B., Kur E., Andreone B. J., Mayshar Y., Yan H., et al. (2014). Mfsd2a Is Critical for the Formation and Function of the Blood-Brain Barrier. Nature 509, 507–511. 10.1038/nature13324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger J. H., Charron M. J., Silver D. L. (2012). Major Facilitator Superfamily Domain-Containing Protein 2a (MFSD2A) Has Roles in Body Growth, Motor Function, and Lipid Metabolism. PLoS One 7, e50629. 10.1371/journal.pone.0050629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betizeau M., Cortay V., Patti D., Pfister S., Gautier E., Bellemin-Ménard A., et al. (2013). Precursor Diversity and Complexity of Lineage Relationships in the Outer Subventricular Zone of the Primate. Neuron 80, 442–457. 10.1016/j.neuron.2013.09.032 [DOI] [PubMed] [Google Scholar]
- Bhat V., Girimaji S., Mohan G., Arvinda H., Singhmar P., Duvvari M., et al. (2011). Mutations in WDR62, Encoding a Centrosomal and Nuclear Protein, in Indian Primary Microcephaly Families with Cortical Malformations. Clin. Genet. 80, 532–540. 10.1111/j.1399-0004.2011.01686.x [DOI] [PubMed] [Google Scholar]
- Bilgüvar K., Öztürk A. K., Louvi A., Kwan K. Y., Choi M., Tatlı B., et al. (2010). Whole-exome Sequencing Identifies Recessive WDR62 Mutations in Severe Brain Malformations. Nature 467, 207–210. 10.1038/nature09327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blachon S., Gopalakrishnan J., Omori Y., Polyanovsky A., Church A., Nicastro D., et al. (2008). Drosophila Asterless and Vertebrate Cep152 Are Orthologs Essential for Centriole Duplication. Genetics 180, 2081–2094. 10.1534/genetics.108.095141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogoyevitch M. A., Yeap Y. Y., Qu Z., Ngoei K. R., Yip Y. Y., Zhao T. T., et al. (2012). WD40-repeat Protein 62 Is a JNK-Phosphorylated Spindle Pole Protein Required for Spindle Maintenance and Timely Mitotic Progression. J. Cel Sci 125, 5096–5109. 10.1242/jcs.107326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boycott K. M., Vanstone M. R., Bulman D. E., MacKenzie A. E. (2013). Rare-disease Genetics in the Era of Next-Generation Sequencing: Discovery to Translation. Nat. Rev. Genet. 14, 681–691. 10.1038/nrg3555 [DOI] [PubMed] [Google Scholar]
- Braun D. A., Lovric S., Schapiro D., Schneider R., Marquez J., Asif M., et al. (2018). Mutations in Multiple Components of the Nuclear Pore Complex Cause Nephrotic Syndrome. J. Clin. Invest. 128, 4313–4328. 10.1172/jci98688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunk K., Vernay B., Griffith E., Reynolds N. L., Strutt D., Ingham P. W., et al. (2007). Microcephalin Coordinates Mitosis in the syncytialDrosophilaembryo. J. Cel. Sci. 120, 3578–3588. 10.1242/jcs.014290 [DOI] [PubMed] [Google Scholar]
- Buchman J. J., Durak O., Tsai L.-H. (2011). ASPM Regulates Wnt Signaling Pathway Activity in the Developing Brain. Genes Dev. 25, 1909–1914. 10.1101/gad.16830211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman J. J., Tsai L.-H. (2007). Spindle Regulation in Neural Precursors of Flies and Mammals. Nat. Rev. Neurosci. 8, 89–100. 10.1038/nrn2058 [DOI] [PubMed] [Google Scholar]
- Buchman J. J., Tseng H.-C., Zhou Y., Frank C. L., Xie Z., Tsai L.-H. (2010). Cdk5rap2 Interacts with Pericentrin to Maintain the Neural Progenitor Pool in the Developing Neocortex. Neuron 66, 386–402. 10.1016/j.neuron.2010.03.036 [DOI] [PubMed] [Google Scholar]
- Caglayan A. O., Yaghouti K., Kockaya T., Kemer D., Cankaya T., Ameziane N., et al. (2021). Biallelic ZNF335 Mutations Cause Basal Ganglia Abnormality with Progressive Cerebral/cerebellar Atrophy. J. Neurogenet. 35, 23–28. 10.1080/01677063.2020.1833006 [DOI] [PubMed] [Google Scholar]
- Calegari F., Haubensak W., Haffner C., Huttner W. B. (2005). Selective Lengthening of the Cell Cycle in the Neurogenic Subpopulation of Neural Progenitor Cells during Mouse Brain Development. J. Neurosci. 25, 6533–6538. 10.1523/jneurosci.0778-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calegari F., Huttner W. B. (2003). An Inhibition of Cyclin-dependent Kinases that Lengthens, but Does Not Arrest, Neuroepithelial Cell Cycle Induces Premature Neurogenesis. J. Cel. Sci. 116, 4947–4955. 10.1242/jcs.00825 [DOI] [PubMed] [Google Scholar]
- Carvalho-Santos Z., Machado P., Alvarez-Martins I., Gouveia S. M., Jana S. C., Duarte P., et al. (2012). BLD10/CEP135 Is a Microtubule-Associated Protein that Controls the Formation of the Flagellum central Microtubule Pair. Developmental Cel 23, 412–424. 10.1016/j.devcel.2012.06.001 [DOI] [PubMed] [Google Scholar]
- Chan J. P., Wong B. H., Chin C. F., Galam D. L. A., Foo J. C., Wong L. C., et al. (2018). The Lysolipid Transporter Mfsd2a Regulates Lipogenesis in the Developing Brain. Plos Biol. 16, e2006443. 10.1371/journal.pbio.2006443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.-F., Zhang Y., Wilde J., Hansen K. C., Lai F., Niswander L. (2014). Microcephaly Disease Gene Wdr62 Regulates Mitotic Progression of Embryonic Neural Stem Cells and Brain Size. Nat. Commun. 5, 3885. 10.1038/ncomms4885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Tong Q., Chen X., Jiang P., Yu H., Zhao Q., et al. (2021). PHC1 Maintains Pluripotency by Organizing Genome-wide Chromatin Interactions of the Nanog Locus. Nat. Commun. 12, 2829. 10.1038/s41467-021-22871-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N. Y., Yang Y., Weston T. A., Belling J. N., Heizer P., Tu Y., et al. (2019). An Absence of Lamin B1 in Migrating Neurons Causes Nuclear Membrane Ruptures and Cell Death. Proc. Natl. Acad. Sci. USA 116, 25870–25879. 10.1073/pnas.1917225116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C., Yang Y., Zhu X., Yu X., Zhang T., Yang F., et al. (2020). Novel Compound Heterozygous Variants in the STIL Gene Identified in a Chinese Family with Presentation of Foetal Microcephaly. Eur. J. Med. Genet. 63, 104091. 10.1016/j.ejmg.2020.104091 [DOI] [PubMed] [Google Scholar]
- Chenn A., McConnell S. K. (1995). Cleavage Orientation and the Asymmetric Inheritance of Notchl Immunoreactivity in Mammalian Neurogenesis. Cell 82, 631–641. 10.1016/0092-8674(95)90035-7 [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Mao Y., Sullivan K. F. (2003). Centromeres and Kinetochores. Cell 112, 407–421. 10.1016/s0092-8674(03)00115-6 [DOI] [PubMed] [Google Scholar]
- Coffinier C., Chang S. Y., Nobumori C., Tu Y., Farber E. A., Toth J. I., et al. (2010). Abnormal Development of the Cerebral Cortex and Cerebellum in the Setting of Lamin B2 Deficiency. Proc. Natl. Acad. Sci. 107, 5076–5081. 10.1073/pnas.0908790107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffinier C., Jung H.-J., Nobumori C., Chang S., Tu Y., Barnes R. H., 2nd, et al. (2011). Deficiencies in Lamin B1 and Lamin B2 Cause Neurodevelopmental Defects and Distinct Nuclear Shape Abnormalities in Neurons. MBoC 22, 4683–4693. 10.1091/mbc.e11-06-0504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M. R., Müller U. (2014). Specification of Excitatory Neurons in the Developing Cerebral Cortex: Progenitor Diversity and Environmental Influences. Front Cel Neurosci 8, 449. 10.3389/fncel.2014.00449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottee M. A., Muschalik N., Wong Y. L., Johnson C. M., Johnson S., Andreeva A., et al. (2013). Crystal Structures of the CPAP/STIL Complex Reveal its Role in Centriole Assembly and Human Microcephaly. eLife 2, e01071. 10.7554/eLife.01071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J., Jackson A. P., Bond J., Woods C. G. (2006). What Primary Microcephaly Can Tell Us about Brain Growth. Trends Mol. Med. 12, 358–366. 10.1016/j.molmed.2006.06.006 [DOI] [PubMed] [Google Scholar]
- Cragan J. D., Isenburg J. L., Parker S. E., Alverson C. J., Meyer R. E., Stallings E. B., et al. (2016). Population-based Microcephaly Surveillance in the United States, 2009 to 2013: An Analysis of Potential Sources of Variation. Birth Defects Res. A: Clin. Mol. Teratology 106, 972–982. 10.1002/bdra.23587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristofoli F., Moss T., Moore H. W., Devriendt K., Flanagan-Steet H., May M., et al. (2020). De Novo Variants in LMNB1 Cause Pronounced Syndromic Microcephaly and Disruption of Nuclear Envelope Integrity. Am. J. Hum. Genet. 107, 753–762. 10.1016/j.ajhg.2020.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenca A., Insinna C., Zhao H., John P., Weiss M. A., Lu Q., et al. (2019). The C7orf43/TRAPPC14 Component Links the TRAPPII Complex to Rabin8 for Preciliary Vesicle Tethering at the Mother Centriole during Ciliogenesis. J. Biol. Chem. 294, 15418–15434. 10.1074/jbc.ra119.008615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cugola F. R., Fernandes I. R., Russo F. B., Freitas B. C., Dias J. L. M., Guimarães K. P., et al. (2016). The Brazilian Zika Virus Strain Causes Birth Defects in Experimental Models. Nature 534, 267–271. 10.1038/nature18296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David A., Liu F., Tibelius A., Vulprecht J., Wald D., Rothermel U., et al. (2014). Lack of Centrioles and Primary Cilia inSTIL−/−mouse Embryos. Cell Cycle 13, 2859–2868. 10.4161/15384101.2014.946830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bene F., Wehman A. M., Link B. A., Baier H. (2008). Regulation of Neurogenesis by Interkinetic Nuclear Migration through an Apical-Basal Notch Gradient. Cell 134, 1055–1065. 10.1016/j.cell.2008.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derivery E., Seum C., Daeden A., Loubéry S., Holtzer L., Jülicher F., et al. (2015). Polarized Endosome Dynamics by Spindle Asymmetry during Asymmetric Cell Division. Nature 528, 280–285. 10.1038/nature16443 [DOI] [PubMed] [Google Scholar]
- Desir J., Cassart M., David P., Van Bogaert P., Abramowicz M. (2008). Primary Microcephaly with ASPM Mutation Shows Simplified Cortical Gyration with Antero-Posterior Gradient Pre- and post-natally. Am. J. Med. Genet. 146A, 1439–1443. 10.1002/ajmg.a.32312 [DOI] [PubMed] [Google Scholar]
- Devakumar D., Bamford A., Ferreira M. U., Broad J., Rosch R. E., Groce N., et al. (2018). Infectious Causes of Microcephaly: Epidemiology, Pathogenesis, Diagnosis, and Management. Lancet Infect. Dis. 18, e1–e13. 10.1016/s1473-3099(17)30398-5 [DOI] [PubMed] [Google Scholar]
- Di Cunto F., Imarisio S., Hirsch E., Broccoli V., Bulfone A., Migheli A., et al. (2000). Defective Neurogenesis in Citron Kinase Knockout Mice by Altered Cytokinesis and Massive Apoptosis. Neuron 28, 115–127. 10.1016/s0896-6273(00)00090-8 [DOI] [PubMed] [Google Scholar]
- Ding W., Wu Q., Sun L., Pan N. C., Wang X. (2019). Cenpj Regulates Cilia Disassembly and Neurogenesis in the Developing Mouse Cortex. J. Neurosci. 39, 1994–2010. 10.1523/jneurosci.1849-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiStasio A., Driver A., Sund K., Donlin M., Muraleedharan R. M., Pooya S., et al. (2017). Copb2 Is Essential for Embryogenesis and Hypomorphic Mutations Cause Human Microcephaly. Hum. Mol. Genet. 26, 4836–4848. 10.1093/hmg/ddx362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farag H. G., Froehler S., Oexle K., Ravindran E., Schindler D., Staab T., et al. (2013). Abnormal Centrosome and Spindle Morphology in a Patient with Autosomal Recessive Primary Microcephaly Type 2 Due to Compound Heterozygous WDR62 Gene Mutation. Orphanet J. Rare Dis. 8, 178. 10.1186/1750-1172-8-178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq M., Lindbæk L., Krogh N., Doganli C., Keller C., Mönnich M., et al. (2020). RRP7A Links Primary Microcephaly to Dysfunction of Ribosome Biogenesis, Resorption of Primary Cilia, and Neurogenesis. Nat. Commun. 11, 5816. 10.1038/s41467-020-19658-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fietz S. A., Huttner W. B. (2011). Cortical Progenitor Expansion, Self-Renewal and Neurogenesis-A Polarized Perspective. Curr. Opin. Neurobiol. 21, 23–35. 10.1016/j.conb.2010.10.002 [DOI] [PubMed] [Google Scholar]
- Fietz S. A., Kelava I., Vogt J., Wilsch-Bräuninger M., Stenzel D., Fish J. L., et al. (2010). OSVZ Progenitors of Human and Ferret Neocortex Are Epithelial-like and Expand by Integrin Signaling. Nat. Neurosci. 13, 690–699. 10.1038/nn.2553 [DOI] [PubMed] [Google Scholar]
- Finley K. D., Edeen P. T., Cumming R. C., Mardahl-Dumesnil M. D., Taylor B. J., Rodriguez M. H., et al. (2003). Blue cheeseMutations Define a Novel, Conserved Gene Involved in Progressive Neural Degeneration. J. Neurosci. 23, 1254–1264. 10.1523/jneurosci.23-04-01254.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish J. L., Kosodo Y., Enard W., Paabo S., Huttner W. B. (2006). Aspm Specifically Maintains Symmetric Proliferative Divisions of Neuroepithelial Cells. Proc. Natl. Acad. Sci. 103, 10438–10443. 10.1073/pnas.0604066103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikura K., Setsu T., Tanigaki K., Abe T., Kiyonari H., Terashima T., et al. (2013). Kif14 Mutation Causes Severe Brain Malformation and Hypomyelination. PLoS One 8, e53490. 10.1371/journal.pone.0053490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimori A., Itoh K., Goto S., Hirakawa H., Wang B., Kokubo T., et al. (2014). Disruption of Aspm Causes Microcephaly with Abnormal Neuronal Differentiation. Brain Development 36, 661–669. 10.1016/j.braindev.2013.10.006 [DOI] [PubMed] [Google Scholar]
- Gabriel E., Ramani A., Altinisik N., Gopalakrishnan J. (2020). Human Brain Organoids to Decode Mechanisms of Microcephaly. Front. Cell. Neurosci. 14, 115. 10.3389/fncel.2020.00115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel E., Wason A., Ramani A., Gooi L. M., Keller P., Pozniakovsky A., et al. (2016). CPAP Promotes Timely Cilium Disassembly to Maintain Neural Progenitor Pool. Embo J. 35, 803–819. 10.15252/embj.201593679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcez P. P., Loiola E. C., Madeiro da Costa R., Higa L. M., Trindade P., Delvecchio R., et al. (2016). Zika Virus Impairs Growth in Human Neurospheres and Brain Organoids. Science 352, 816–818. 10.1126/science.aaf6116 [DOI] [PubMed] [Google Scholar]
- Genin A., Desir J., Lambert N., Biervliet M., Van Der Aa N., Pierquin G., et al. (2012). Kinetochore KMN Network Gene CASC5 Mutated in Primary Microcephaly. Hum. Mol. Genet. 21, 5306–5317. 10.1093/hmg/dds386 [DOI] [PubMed] [Google Scholar]
- Gilbert S. L., Dobyns W. B., Lahn B. T. (2005). Genetic Links between Brain Development and Brain Evolution. Nat. Rev. Genet. 6, 581–590. 10.1038/nrg1634 [DOI] [PubMed] [Google Scholar]
- Goetz S. C., Anderson K. V. (2010). The Primary Cilium: a Signalling centre during Vertebrate Development. Nat. Rev. Genet. 11, 331–344. 10.1038/nrg2774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C., Saunders R. D., Casal J., Molina I., Carmena M., Ripoll P., et al. (1990). Mutations at the Asp Locus of Drosophila lead to Multiple Free Centrosomes in Syncytial Embryos, but Restrict Centrosome Duplication in Larval Neuroblasts. J. Cel Sci 96 (Pt 4), 605–616. 10.1242/jcs.96.4.605 [DOI] [PubMed] [Google Scholar]
- Gosling K. M., Makaroff L. E., Theodoratos A., Kim Y.-H., Whittle B., Rui L., et al. (2007). A Mutation in a Chromosome Condensin II Subunit, Kleisin Beta, Specifically Disrupts T Cell Development. Proc. Natl. Acad. Sci. 104, 12445–12450. 10.1073/pnas.0704870104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz M., Huttner W. B. (2005). The Cell Biology of Neurogenesis. Nat. Rev. Mol. Cel. Biol. 6, 777–788. [DOI] [PubMed] [Google Scholar]
- Gruber R., Zhou Z., Sukchev M., Joerss T., Frappart P.-O., Wang Z.-Q. (2011). MCPH1 Regulates the Neuroprogenitor Division Mode by Coupling the Centrosomal Cycle with Mitotic Entry through the Chk1-Cdc25 Pathway. Nat. Cel Biol 13, 1325–1334. 10.1038/ncb2342 [DOI] [PubMed] [Google Scholar]
- Guemez-Gamboa A., Nguyen L. N., Yang H., Zaki M. S., Kara M., Ben-Omran T., et al. (2015). Inactivating Mutations in MFSD2A, Required for omega-3 Fatty Acid Transport in Brain, Cause a Lethal Microcephaly Syndrome. Nat. Genet. 47, 809–813. 10.1038/ng.3311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainline S. G., Rickmyre J. L., Neitzel L. R., Lee L. A., Lee E. (2014). The Drosophila MCPH1-B Isoform Is a Substrate of the APCCdh1 E3 Ubiquitin Ligase Complex. Biol. open 3, 669–676. 10.1242/bio.20148318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen D. V., Lui J. H., Parker P. R. L., Kriegstein A. R. (2010). Neurogenic Radial Glia in the Outer Subventricular Zone of Human Neocortex. Nature 464, 554–561. 10.1038/nature08845 [DOI] [PubMed] [Google Scholar]
- Harding B. N., Moccia A., Drunat S., Soukarieh O., Tubeuf H., Chitty L. S., et al. (2016). Mutations in Citron Kinase Cause Recessive Microlissencephaly with Multinucleated Neurons. Am. J. Hum. Genet. 99, 511–520. 10.1016/j.ajhg.2016.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel T., Quek D. Q. Y., Wong B. H., Cazenave-Gassiot A., Wenk M. R., Fan H., et al. (2018). Homozygous Mutation in MFSD2A, Encoding a Lysolipid Transporter for Docosahexanoic Acid, Is Associated with Microcephaly and Hypomyelination. Neurogenetics 19, 227–235. 10.1007/s10048-018-0556-6 [DOI] [PubMed] [Google Scholar]
- Heide M., Huttner W. B. (2021). Human-Specific Genes, Cortical Progenitor Cells, and Microcephaly. Cells 10, 1209. 10.3390/cells10051209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetman M., Slomnicki L. P. (2019). Ribosomal Biogenesis as an Emerging Target of Neurodevelopmental Pathologies. J. Neurochem. 148, 325–347. 10.1111/jnc.14576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraki M., Nakazawa Y., Kamiya R., Hirono M. (2007). Bld10p Constitutes the Cartwheel-Spoke Tip and Stabilizes the 9-fold Symmetry of the Centriole. Curr. Biol. 17, 1778–1783. 10.1016/j.cub.2007.09.021 [DOI] [PubMed] [Google Scholar]
- Homem C. C. F., Repic M., Knoblich J. A. (2015). Proliferation Control in Neural Stem and Progenitor Cells. Nat. Rev. Neurosci. 16, 647–659. 10.1038/nrn4021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori T., Fukagawa T. (2012). Establishment of the Vertebrate Kinetochores. Chromosome Res. 20, 547–561. 10.1007/s10577-012-9289-9 [DOI] [PubMed] [Google Scholar]
- Hosseini M. M., Tonekaboni S. H., Papari E., Bahman I., Behjati F., Kahrizi K., et al. (2012). A Novel Mutation in MCPH1 Gene in an Iranian Family with Primary Microcephaly. J. Pak Med. Assoc. 62, 1244–1247. [PubMed] [Google Scholar]
- Huang B., Li X. (2021). The Role of Mfsd2a in Nervous System Diseases. Front. Neurosci. 15, 730534. 10.3389/fnins.2021.730534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain M. S., Baig S. M., Neumann S., Nürnberg G., Farooq M., Ahmad I., et al. (2012). A Truncating Mutation of CEP135 Causes Primary Microcephaly and Disturbed Centrosomal Function. Am. J. Hum. Genet. 90, 871–878. 10.1016/j.ajhg.2012.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain M. S., Baig S. M., Neumann S., Peche V. S., Szczepanski S., Nürnberg G., et al. (2013). CDK6 Associates with the Centrosome during Mitosis and Is Mutated in a Large Pakistani Family with Primary Microcephaly. Hum. Mol. Genet. 22, 5199–5214. 10.1093/hmg/ddt374 [DOI] [PubMed] [Google Scholar]
- Issa L., Mueller K., Seufert K., Kraemer N., Rosenkotter H., Ninnemann O., et al. (2013). Clinical and Cellular Features in Patients with Primary Autosomal Recessive Microcephaly and a Novel CDK5RAP2 Mutation. Orphanet J. Rare Dis. 8, 59–73. 10.1186/1750-1172-8-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izraeli S., Lowe L. A., Bertness V. L., Good D. J., Dorward D. W., Kirsch I. R., et al. (1999). The SIL Gene Is Required for Mouse Embryonic Axial Development and Left-Right Specification. Nature 399, 691–694. 10.1038/21429 [DOI] [PubMed] [Google Scholar]
- Jackson A. P., Eastwood H., Bell S. M., Adu J., Toomes C., Carr I. M., et al. (2002). Identification of Microcephalin, a Protein Implicated in Determining the Size of the Human Brain. Am. J. Hum. Genet. 71, 136–142. 10.1086/341283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson C. R., Govaerts C., Abramowicz M. J. (1999). Primary Autosomal Recessive Microcephaly: Homozygosity Mapping of MCPH4 to Chromosome 15. Am. J. Hum. Genet. 65, 1465–1469. 10.1086/302640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman D., Bae B.-I., Walsh C. A. (2018). The Genetics of Primary Microcephaly. Annu. Rev. Genom. Hum. Genet. 19, 177–200. 10.1146/annurev-genom-083117-021441 [DOI] [PubMed] [Google Scholar]
- Jean F., Stuart A., Tarailo-Graovac M. (2020). Dissecting the Genetic and Etiological Causes of Primary Microcephaly. Front. Neurol. 11, 570830. 10.3389/fneur.2020.570830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerka-Dziadosz M., Gogendeau D., Klotz C., Cohen J., Beisson J., Koll F. (2010). Basal Body Duplication in Paramecium: the Key Role of Bld10 in Assembly and Stability of the Cartwheel. Cytoskeleton (Hoboken) 67, 161–171. 10.1002/cm.20433 [DOI] [PubMed] [Google Scholar]
- Johnson M. B., Sun X., Kodani A., Borges-Monroy R., Girskis K. M., Ryu S. C., et al. (2018). Aspm Knockout Ferret Reveals an Evolutionary Mechanism Governing Cerebral Cortical Size. Nature 556, 370–375. 10.1038/s41586-018-0035-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadir R., Harel T., Markus B., Perez Y., Bakhrat A., Cohen I., et al. (2016). ALFY-controlled DVL3 Autophagy Regulates Wnt Signaling, Determining Human Brain Size. Plos Genet. 12, e1005919. 10.1371/journal.pgen.1005919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaindl A. M., Passemard S., Kumar P., Kraemer N., Issa L., Zwirner A., et al. (2010). Many Roads lead to Primary Autosomal Recessive Microcephaly. Prog. Neurobiol. 90, 363–383. 10.1016/j.pneurobio.2009.11.002 [DOI] [PubMed] [Google Scholar]
- Kakar N., Ahmad J., Morris-Rosendahl D. J., Altmüller J., Friedrich K., Barbi G., et al. (2015). STIL Mutation Causes Autosomal Recessive Microcephalic Lobar Holoprosencephaly. Hum. Genet. 134, 45–51. 10.1007/s00439-014-1487-4 [DOI] [PubMed] [Google Scholar]
- Kettleborough R. N. W., Busch-Nentwich E. M., Harvey S. A., Dooley C. M., de Bruijn E., van Eeden F., et al. (2013). A Systematic Genome-wide Analysis of Zebrafish Protein-Coding Gene Function. Nature 496, 494–497. 10.1038/nature11992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M. A., Rupp V. M., Orpinell M., Hussain M. S., Altmüller J., Steinmetz M. O., et al. (2014). A Missense Mutation in the PISA Domain of HsSAS-6 Causes Autosomal Recessive Primary Microcephaly in a Large Consanguineous Pakistani Family. Hum. Mol. Genet. 23, 5940–5949. 10.1093/hmg/ddu318 [DOI] [PubMed] [Google Scholar]
- Kim H.-T., Lee M.-S., Choi J.-H., Jung J.-Y., Ahn D.-G., Yeo S.-Y., et al. (2011). The Microcephaly Gene Aspm Is Involved in Brain Development in Zebrafish. Biochem. Biophysical Res. Commun. 409, 640–644. 10.1016/j.bbrc.2011.05.056 [DOI] [PubMed] [Google Scholar]
- Kim Y., Sharov A. A., McDole K., Cheng M., Hao H., Fan C.-M., et al. (2011). Mouse B-type Lamins Are Required for Proper Organogenesis but Not by Embryonic Stem Cells. Science 334, 1706–1710. 10.1126/science.1211222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleber de Oliveira W., Cortez-Escalante J., De Oliveira W. T. G. H., do Carmo G. M. I., Henriques C. M. P., Coelho G. E., et al. (2016). Increase in Reported Prevalence of Microcephaly in Infants Born to Women Living in Areas with Confirmed Zika Virus Transmission during the First Trimester of Pregnancy - Brazil, 2015. MMWR Morb. Mortal. Wkly. Rep. 65, 242–247. 10.15585/mmwr.mm6509e2 [DOI] [PubMed] [Google Scholar]
- Knoblich J. A. (2008). Mechanisms of Asymmetric Stem Cell Division. Cell 132, 583–597. 10.1016/j.cell.2008.02.007 [DOI] [PubMed] [Google Scholar]
- Kohwi M., Doe C. Q. (2013). Temporal Fate Specification and Neural Progenitor Competence during Development. Nat. Rev. Neurosci. 14, 823–838. 10.1038/nrn3618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konno D., Shioi G., Shitamukai A., Mori A., Kiyonari H., Miyata T., et al. (2008). Neuroepithelial Progenitors Undergo LGN-dependent Planar Divisions to Maintain Self-Renewability during Mammalian Neurogenesis. Nat. Cel Biol 10, 93–101. 10.1038/ncb1673 [DOI] [PubMed] [Google Scholar]
- Kosodo Y., Röper K., Haubensak W., Marzesco A.-M., Corbeil D., Huttner W. B. (2004). Asymmetric Distribution of the Apical Plasma Membrane during Neurogenic Divisions of Mammalian Neuroepithelial Cells. Embo J. 23, 2314–2324. 10.1038/sj.emboj.7600223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosodo Y., Suetsugu T., Suda M., Mimori-Kiyosue Y., Toida K., Baba S. A., et al. (2011). Regulation of Interkinetic Nuclear Migration by Cell Cycle-Coupled Active and Passive Mechanisms in the Developing Brain. EMBO J. 30, 1690–1704. 10.1038/emboj.2011.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kousar R., Hassan M. J., Khan B., Basit S., Mahmood S., Mir A., et al. (2011). Mutations in WDR62 Gene in Pakistani Families with Autosomal Recessive Primary Microcephaly. BMC Neurol. 11, 119. 10.1186/1471-2377-11-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer N., Issa L., Hauck S. C. R., Mani S., Ninnemann O., Kaindl A. M. (2011). What's the Hype about CDK5RAP2? Cell. Mol. Life Sci. 68, 1719–1736. 10.1007/s00018-011-0635-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster M. A., Renner M., Martin C.-A., Wenzel D., Bicknell L. S., Hurles M. E., et al. (2013). Cerebral Organoids Model Human Brain Development and Microcephaly. Nature 501, 373–379. 10.1038/nature12517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latasa M. J., Cisneros E., Frade J. M. (2009). Cell Cycle Control of Notch Signaling and the Functional Regionalization of the Neuroepithelium during Vertebrate Neurogenesis. Int. J. Dev. Biol. 53, 895–908. 10.1387/ijdb.082721ml [DOI] [PubMed] [Google Scholar]
- Le Duc D., Giulivi C., Hiatt S. M., Napoli E., Panoutsopoulos A., Harlan De Crescenzo A., et al. (2019). Pathogenic WDFY3 Variants Cause Neurodevelopmental Disorders and Opposing Effects on Brain Size. Brain. 142, 2617–2630. 10.1093/brain/awz198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidel S., Delattre M., Cerutti L., Baumer K., Gönczy P. (2005). SAS-6 Defines a Protein Family Required for Centrosome Duplication in C. elegans and in Human Cells. Nat. Cel Biol 7, 115–125. 10.1038/ncb1220 [DOI] [PubMed] [Google Scholar]
- Leone D. P., Srinivasan K., Chen B., Alcamo E., McConnell S. K. (2008). The Determination of Projection Neuron Identity in the Developing Cerebral Cortex. Curr. Opin. Neurobiol. 18, 28–35. 10.1016/j.conb.2008.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Létard P., Drunat S., Vial Y., Duerinckx S., Ernault A., Amram D., et al. (2018). Autosomal Recessive Primary Microcephaly Due to ASPM Mutations: An Update. Hum. Mutat. 39, 319–332. [DOI] [PubMed] [Google Scholar]
- Letourneur F., Gaynor E. C., Hennecke S., Démollière C., Duden R., Emr S. D., et al. (1994). Coatomer Is Essential for Retrieval of Dilysine-Tagged Proteins to the Endoplasmic Reticulum. Cell 79, 1199–1207. 10.1016/0092-8674(94)90011-6 [DOI] [PubMed] [Google Scholar]
- Leventer R. J., Jansen A., Pilz D. T., Stoodley N., Marini C., Dubeau F., et al. (2010). Clinical and Imaging Heterogeneity of Polymicrogyria: a Study of 328 Patients. Brain. 133, 1415–1427. 10.1093/brain/awq078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Xu D., Ye Q., Hong S., Jiang Y., Liu X., et al. (2016). Zika Virus Disrupts Neural Progenitor Development and Leads to Microcephaly in Mice. Cell Stem Cell 19, 120–126. 10.1016/j.stem.2016.04.017 [DOI] [PubMed] [Google Scholar]
- Li H., Bielas S. L., Zaki M. S., Ismail S., Farfara D., Um K., et al. (2016). Biallelic Mutations in Citron Kinase Link Mitotic Cytokinesis to Human Primary Microcephaly. Am. J. Hum. Genet. 99, 501–510. 10.1016/j.ajhg.2016.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Sun L., Fang A., Li P., Wu Q., Wang X. (2017). Recapitulating Cortical Development with Organoid Culture In Vitro and Modeling Abnormal Spindle-like (ASPM Related Primary) Microcephaly Disease. Protein Cell 8, 823–833. 10.1007/s13238-017-0479-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y., Gao H., Lin S.-Y., Peng G., Huang X., Zhang P., et al. (2010). BRIT1/MCPH1 Is Essential for Mitotic and Meiotic Recombination DNA Repair and Maintaining Genomic Stability in Mice. Plos Genet. 6, e1000826. 10.1371/journal.pgen.1000826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim A., Kraut R. (2009). The Drosophila BEACH Family Protein, Blue Cheese, Links Lysosomal Axon Transport with Motor Neuron Degeneration. J. Neurosci. 29, 951–963. 10.1523/jneurosci.2582-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link N., Chung H., Jolly A., Withers M., Tepe B., Arenkiel B. R., et al. (2019). Mutations in ANKLE2, a ZIKA Virus Target, Disrupt an Asymmetric Cell Division Pathway in Drosophila Neuroblasts to Cause Microcephaly. Developmental Cel 51, 713–729. e716. 10.1016/j.devcel.2019.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizarraga S. B., Margossian S. P., Harris M. H., Campagna D. R., Han A.-P., Blevins S., et al. (2010). Cdk5rap2 Regulates Centrosome Function and Chromosome Segregation in Neuronal Progenitors. Development 137, 1907–1917. 10.1242/dev.040410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas E. P., Raff J. W. (2007). Maintaining the Proper Connection between the Centrioles and the Pericentriolar Matrix Requires Drosophila Centrosomin. J. Cel Biol 178, 725–732. 10.1083/jcb.200704081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood S., Ahmad W., Hassan M. J. (2011). Autosomal Recessive Primary Microcephaly (MCPH): Clinical Manifestations, Genetic Heterogeneity and Mutation Continuum. Orphanet J. Rare Dis. 6, 39–54. 10.1186/1750-1172-6-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makrythanasis P., Maroofian R., Stray-Pedersen A., Musaev D., Zaki M. S., Mahmoud I. G., et al. (2018). Biallelic Variants in KIF14 Cause Intellectual Disability with Microcephaly. Eur. J. Hum. Genet. 26, 330–339. 10.1038/s41431-017-0088-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malatesta P., Hartfuss E., Gotz M. (2000). Isolation of Radial Glial Cells by Fluorescent-Activated Cell Sorting Reveals a Neuronal Lineage. Development 127, 5253–5263. 10.1242/dev.127.24.5253 [DOI] [PubMed] [Google Scholar]
- Malumbres M., Sotillo R., Santamaría D., Galán J., Cerezo A., Ortega S., et al. (2004). Mammalian Cells Cycle without the D-type Cyclin-dependent Kinases Cdk4 and Cdk6. Cell 118, 493–504. 10.1016/j.cell.2004.08.002 [DOI] [PubMed] [Google Scholar]
- Marín O. (2013). Cellular and Molecular Mechanisms Controlling the Migration of Neocortical Interneurons. Eur. J. Neurosci. 38, 2019–2029. [DOI] [PubMed] [Google Scholar]
- Marjanović M., Sánchez-Huertas C., Terré B., Gómez R., Scheel J. F., Pacheco S., et al. (2015). CEP63 Deficiency Promotes P53-dependent Microcephaly and Reveals a Role for the Centrosome in Meiotic Recombination. Nat. Commun. 6, 7676. 10.1038/ncomms8676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C.-A., Murray J. E., Carroll P., Leitch A., Mackenzie K. J., Halachev M., et al. (2016). Mutations in Genes Encoding Condensin Complex Proteins Cause Microcephaly through Decatenation Failure at Mitosis. Genes Dev. 30, 2158–2172. 10.1101/gad.286351.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura K., Lefebvre P. A., Kamiya R., Hirono M. (2004). Bld10p, a Novel Protein Essential for Basal Body Assembly in Chlamydomonas. J. Cel Biol 165, 663–671. 10.1083/jcb.200402022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHenry H. M. (1994). Tempo and Mode in Human Evolution. Proc. Natl. Acad. Sci. 91, 6780–6786. 10.1073/pnas.91.15.6780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre R. E., Lakshminarasimhan Chavali P., Ismail O., Carragher D. M., Sanchez-Andrade G., Forment J. V., et al. (2012). Disruption of Mouse Cenpj, a Regulator of Centriole Biogenesis, Phenocopies Seckel Syndrome. Plos Genet. 8, e1003022. 10.1371/journal.pgen.1003022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megraw T. L., Li K., Kao L. R., Kaufman T. C. (1999). The Centrosomin Protein Is Required for Centrosome Assembly and Function during Cleavage in Drosophila. Development 126, 2829–2839. 10.1242/dev.126.13.2829 [DOI] [PubMed] [Google Scholar]
- Megraw T. L., Sharkey J. T., Nowakowski R. S. (2011). Cdk5rap2 Exposes the Centrosomal Root of Microcephaly Syndromes. Trends Cel Biol. 21, 470–480. 10.1016/j.tcb.2011.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memon M. M., Raza S. I., Basit S., Kousar R., Ahmad W., Ansar M. (2013). A Novel WDR62 Mutation Causes Primary Microcephaly in a Pakistani Family. Mol. Biol. Rep. 40, 591–595. 10.1007/s11033-012-2097-7 [DOI] [PubMed] [Google Scholar]
- Mirzaa G. M., Vitre B., Carpenter G., Abramowicz I., Gleeson J. G., Paciorkowski A. R., et al. (2014). Mutations in CENPE Define a Novel Kinetochore-Centromeric Mechanism for Microcephalic Primordial Dwarfism. Hum. Genet. 133, 1023–1039. 10.1007/s00439-014-1443-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata T., Okamoto M., Shinoda T., Kawaguchi A. (2014). Interkinetic Nuclear Migration Generates and Opposes Ventricular-Zone Crowding: Insight into Tissue Mechanics. Front. Cel Neurosci 8, 473. 10.3389/fncel.2014.00473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moawia A., Shaheen R., Rasool S., Waseem S. S., Ewida N., Budde B., et al. (2017). Mutations of KIF14 Cause Primary Microcephaly by Impairing Cytokinesis. Ann. Neurol. 82, 562–577. 10.1002/ana.25044 [DOI] [PubMed] [Google Scholar]
- Molyneaux B. J., Arlotta P., Menezes J. R. L., Macklis J. D. (2007). Neuronal Subtype Specification in the Cerebral Cortex. Nat. Rev. Neurosci. 8, 427–437. 10.1038/nrn2151 [DOI] [PubMed] [Google Scholar]
- Mori T., Buffo A., Götz M. (2005). The Novel Roles of Glial Cells Revisited: the Contribution of Radial Glia and Astrocytes to Neurogenesis. Curr. Top. Dev. Biol. 69, 67–99. 10.1016/s0070-2153(05)69004-7 [DOI] [PubMed] [Google Scholar]
- Morin X., Jaouen F., Durbec P. (2007). Control of Planar Divisions by the G-Protein Regulator LGN Maintains Progenitors in the Chick Neuroepithelium. Nat. Neurosci. 10, 1440–1448. 10.1038/nn1984 [DOI] [PubMed] [Google Scholar]
- Morris J. K., Rankin J., Garne E., Loane M., Greenlees R., Addor M.-C., et al. (2016). Prevalence of Microcephaly in Europe: Population Based Study. Bmj 354, i4721. 10.1136/bmj.i4721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottier-Pavie V., Megraw T. L. (2009). DrosophilaBld10 Is a Centriolar Protein that Regulates Centriole, Basal Body, and Motile Cilium Assembly. MBoC 20, 2605–2614. 10.1091/mbc.e08-11-1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouden C., de Tayrac M., Dubourg C., Rose S., Carré W., Hamdi-Rozé H., et al. (2015). Homozygous STIL Mutation Causes Holoprosencephaly and Microcephaly in Two Siblings. PLoS One 10, e0117418. 10.1371/journal.pone.0117418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdock D. R., Clark G. D., Bainbridge M. N., Newsham I., Wu Y.-Q., Muzny D. M., et al. (2011). Whole-exome Sequencing Identifies Compound Heterozygous Mutations in WDR62 in Siblings with Recurrent Polymicrogyria. Am. J. Med. Genet. 155, 2071–2077. 10.1002/ajmg.a.34165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchio A., Salmon E. D. (2007). The Spindle-Assembly Checkpoint in Space and Time. Nat. Rev. Mol. Cel Biol 8, 379–393. 10.1038/nrm2163 [DOI] [PubMed] [Google Scholar]
- Muzio L., Consalez G. G. (2013). Modeling Human Brain Development with Cerebral Organoids. Stem Cel Res Ther 4, 154. 10.1186/scrt384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naim V., Imarisio S., Di Cunto F., Gatti M., Bonaccorsi S. (2004). DrosophilaCitron Kinase Is Required for the Final Steps of Cytokinesis. MBoC 15, 5053–5063. 10.1091/mbc.e04-06-0536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli E., Panoutsopoulos A. A., Kysar P., Satriya N., Sterling K., Shibata B., et al. (2021). Wdfy3 Regulates Glycophagy, Mitophagy, and Synaptic Plasticity. J. Cereb. Blood Flow Metab., 0271678X2110273. 10.1177/0271678x211027384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli E., Song G., Panoutsopoulos A., Riyadh M. A., Kaushik G., Halmai J., et al. (2018). Beyond Autophagy: a Novel Role for Autism-Linked Wdfy3 in Brain Mitophagy. Sci. Rep. 8, 11348. 10.1038/s41598-018-29421-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardello R., Fontana A., Antona V., Beninati A., Mangano G. D., Stallone M. C., et al. (2018). A Novel Mutation of WDR62 Gene Associated with Severe Phenotype Including Infantile Spasm, Microcephaly, and Intellectual Disability. Brain Development 40, 58–64. 10.1016/j.braindev.2017.07.003 [DOI] [PubMed] [Google Scholar]
- Nasser H., Vera L., Elmaleh-Bergès M., Steindl K., Letard P., Teissier N., et al. (2020). CDK5RAP2 Primary Microcephaly Is Associated with Hypothalamic, Retinal and Cochlear Developmental Defects. J. Med. Genet. 57, 389–399. 10.1136/jmedgenet-2019-106474 [DOI] [PubMed] [Google Scholar]
- Neitzel H., Neumann L. M., Schindler D., Wirges A., Tönnies H., Trimborn M., et al. (2002). Premature Chromosome Condensation in Humans Associated with Microcephaly and Mental Retardation: a Novel Autosomal Recessive Condition. Am. J. Hum. Genet. 70, 1015–1022. 10.1086/339518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerli E., Rocha-Martins M., Norden C. (2020). Asymmetric Neurogenic Commitment of Retinal Progenitors Involves Notch through the Endocytic Pathway. eLife 9. 10.7554/eLife.60462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L. N., Ma D., Shui G., Wong P., Cazenave-Gassiot A., Zhang X., et al. (2014). Mfsd2a Is a Transporter for the Essential omega-3 Fatty Acid Docosahexaenoic Acid. Nature 509, 503–506. 10.1038/nature13241 [DOI] [PubMed] [Google Scholar]
- Nicholas A. K., Khurshid M., Désir J., Carvalho O. P., Cox J. J., Thornton G., et al. (2010). WDR62 Is Associated with the Spindle Pole and Is Mutated in Human Microcephaly. Nat. Genet. 42, 1010–1014. 10.1038/ng.682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg E. A., Raff J. W. (2009). Centrioles, Centrosomes, and Cilia in Health and Disease. Cell 139, 663–678. 10.1016/j.cell.2009.10.036 [DOI] [PubMed] [Google Scholar]
- Noctor S. C., Martínez-Cerdeño V., Ivic L., Kriegstein A. R. (2004). Cortical Neurons Arise in Symmetric and Asymmetric Division Zones and Migrate through Specific Phases. Nat. Neurosci. 7, 136–144. 10.1038/nn1172 [DOI] [PubMed] [Google Scholar]
- Novorol C., Burkhardt J., Wood K. J., Iqbal A., Roque C., Coutts N., et al. (2013). Microcephaly Models in the Developing Zebrafish Retinal Neuroepithelium point to an Underlying Defect in Metaphase Progression. Open Biol. 3, 130065. 10.1098/rsob.130065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkura H., Török T., Tick G., Hoheisel J., Kiss I., Glover D. M. (1997). Mutation of a Gene for a Drosophila Kinesin-like Protein, Klp38B, Leads to Failure of Cytokinesis. J. Cel Sci 110 ( Pt 8) (Pt 8), 945–954. 10.1242/jcs.110.8.945 [DOI] [PubMed] [Google Scholar]
- Okamoto M., Shinoda T., Kawaue T., Nagasaka A., Miyata T. (2014). Ferret-mouse Differences in Interkinetic Nuclear Migration and Cellular Densification in the Neocortical Ventricular Zone. Neurosci. Res. 86, 88–95. 10.1016/j.neures.2014.10.006 [DOI] [PubMed] [Google Scholar]
- Orci L., Palmer D. J., Ravazzola M., Perrelet A., Amherdt M., Rothman J. E. (1993). Budding from Golgi Membranes Requires the Coatomer Complex of Non-clathrin Coat Proteins. Nature 362, 648–652. 10.1038/362648a0 [DOI] [PubMed] [Google Scholar]
- Orosco L. A., Ross A. P., Cates S. L., Scott S. E., Wu D., Sohn J., et al. (2014). Loss of Wdfy3 in Mice Alters Cerebral Cortical Neurogenesis Reflecting Aspects of the Autism Pathology. Nat. Commun. 5, 4692. 10.1038/ncomms5692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paridaen J. T. M. L., Wilsch-Bräuninger M., Huttner W. B. (2013). Asymmetric Inheritance of Centrosome-Associated Primary Cilium Membrane Directs Ciliogenesis after Cell Division. Cell 155, 333–344. 10.1016/j.cell.2013.08.060 [DOI] [PubMed] [Google Scholar]
- Parry D. A., Martin C.-A., Greene P., Marsh J. A., Ambrose J. C., Arumugam P., et al. (2021). Heterozygous Lamin B1 and Lamin B2 Variants Cause Primary Microcephaly and Define a Novel Laminopathy. Genet. Med. 23, 408–414. 10.1038/s41436-020-00980-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passemard S., Kaindl A. M., Verloes A. (2013). “Chapter 13 - Microcephaly,” in Handbook of Clinical Neurology. Editors Dulac O., Lassonde M., Sarnat H. B. (Elsevier; ), 129–141. [DOI] [PubMed] [Google Scholar]
- Passemard S., Titomanlio L., Elmaleh M., Afenjar A., Alessandri J.-L., Andria G., et al. (2009). Expanding the Clinical and Neuroradiologic Phenotype of Primary Microcephaly Due to ASPM Mutations. Neurology 73, 962–969. 10.1212/wnl.0b013e3181b8799a [DOI] [PubMed] [Google Scholar]
- Perez Y., Bar-Yaacov R., Kadir R., Wormser O., Shelef I., Birk O. S., et al. (2019). Mutations in the Microtubule-Associated Protein MAP11 (C7orf43) Cause Microcephaly in Humans and Zebrafish. Brain. 142, 574–585. 10.1093/brain/awz004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pervaiz N., Kang H., Bao Y., Abbasi A. A. (2021). Molecular Evolutionary Analysis of Human Primary Microcephaly Genes. BMC Ecol. Evo 21, 76. 10.1186/s12862-021-01801-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff K. L., Straub C. T., Chiang K., Bear D. M., Zhou Y., Zon L. I. (2007). The Zebra Fish Cassiopeia Mutant Reveals that SIL Is Required for Mitotic Spindle Organization. Mol. Cel Biol 27, 5887–5897. 10.1128/mcb.00175-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilaz L.-J., Patti D., Marcy G., Ollier E., Pfister S., Douglas R. J., et al. (2009). Forced G1-phase Reduction Alters Mode of Division, Neuron Number, and Laminar Phenotype in the Cerebral Cortex. Proc. Natl. Acad. Sci. 106, 21924–21929. 10.1073/pnas.0909894106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontious A., Kowalczyk T., Englund C., Hevner R. F. (2008). Role of Intermediate Progenitor Cells in Cerebral Cortex Development. Dev. Neurosci. 30, 24–32. 10.1159/000109848 [DOI] [PubMed] [Google Scholar]
- Postiglione M. P., Jüschke C., Xie Y., Haas G. A., Charalambous C., Knoblich J. A. (2011). Mouse Inscuteable Induces Apical-Basal Spindle Orientation to Facilitate Intermediate Progenitor Generation in the Developing Neocortex. Neuron 72, 269–284. 10.1016/j.neuron.2011.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulton C. J., Schot R., Seufert K., Lequin M. H., Accogli A., Annunzio G. D., et al. (2014). Severe Presentation ofWDR62mutation: Is There a Role for Modifying Genetic Factors? Am. J. Med. Genet. 164, 2161–2171. 10.1002/ajmg.a.36611 [DOI] [PubMed] [Google Scholar]
- Pulvers J. N., Bryk J., Fish J. L., Wilsch-Brauninger M., Arai Y., Schreier D., et al. (2010). Mutations in Mouse Aspm (Abnormal Spindle-like Microcephaly Associated) Cause Not Only Microcephaly but Also Major Defects in the Germline. Proc. Natl. Acad. Sci. 107, 16595–16600. 10.1073/pnas.1010494107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putkey F. R., Cramer T., Morphew M. K., Silk A. D., Johnson R. S., McIntosh J. R., et al. (2002). Unstable Kinetochore-Microtubule Capture and Chromosomal Instability Following Deletion of CENP-E. Developmental Cel 3, 351–365. 10.1016/s1534-5807(02)00255-1 [DOI] [PubMed] [Google Scholar]
- Qian X., Shen Q., Goderie S. K., He W., Capela A., Davis A. A., et al. (2000). Timing of CNS Cell Generation. Neuron 28, 69–80. 10.1016/s0896-6273(00)00086-6 [DOI] [PubMed] [Google Scholar]
- Rakic P. (2009). Evolution of the Neocortex: a Perspective from Developmental Biology. Nat. Rev. Neurosci. 10, 724–735. 10.1038/nrn2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana R., Gwasikoti N., Vaswani N. D., Kaushik J. S. (2019). Phenomenology of Epilepsy in a Child with a ZNF335 Encephalopathy. Indian J. Pediatr. 86, 967–968. 10.1007/s12098-019-02991-8 [DOI] [PubMed] [Google Scholar]
- Ravindran E., Jühlen R., Vieira-Vieira C. H., Ha T., Salzberg Y., Fichtman B., et al. (2021). Expanding the Phenotype of NUP85 Mutations beyond Nephrotic Syndrome to Primary Autosomal Recessive Microcephaly and Seckel Syndrome Spectrum Disorders. Hum. Mol. Genet. 30, 2068–2081. 10.1093/hmg/ddab160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reillo I., de Juan Romero C., García-Cabezas M. Á., Borrell V. (2011). A Role for Intermediate Radial Glia in the Tangential Expansion of the Mammalian Cerebral Cortex. Cereb. Cortex 21, 1674–1694. 10.1093/cercor/bhq238 [DOI] [PubMed] [Google Scholar]
- Reilly M. L., Stokman M. F., Magry V., Jeanpierre C., Alves M., Paydar M., et al. (2019). Loss-of-function Mutations inKIF14cause Severe Microcephaly and Kidney Development Defects in Humans and Zebrafish. Hum. Mol. Genet. 28, 778–795. 10.1093/hmg/ddy381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riparbelli M. G., Callaini G., Glover D. M., Avides M. d. C. (2002). A Requirement for the Abnormal Spindle Protein to Organise Microtubules of the central Spindle for Cytokinesis inDrosophila. J. Cel. Sci. 115, 913–922. 10.1242/jcs.115.5.913 [DOI] [PubMed] [Google Scholar]
- Ripoll P., Pimpinelli S., Valdivia M. M., Avila J. (1985). A Cell Division Mutant of Drosophila with a Functionally Abnormal Spindle. Cell 41, 907–912. 10.1016/s0092-8674(85)80071-4 [DOI] [PubMed] [Google Scholar]
- Roberts M. R., Bittman K., Li W.-W., French R., Mitchell B., LoTurco J. J., et al. (2000). TheFlatheadMutation Causes CNS-specific Developmental Abnormalities and Apoptosis. J. Neurosci. 20, 2295–2306. 10.1523/jneurosci.20-06-02295.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues-Martins A., Bettencourt-Dias M., Riparbelli M., Ferreira C., Ferreira I., Callaini G., et al. (2007). DSAS-6 Organizes a Tube-like Centriole Precursor, and its Absence Suggests Modularity in Centriole Assembly. Curr. Biol. 17, 1465–1472. 10.1016/j.cub.2007.07.034 [DOI] [PubMed] [Google Scholar]
- Roque H., Wainman A., Richens J., Kozyrska K., Franz A., Raff J. W. (2012). Drosophila Cep135/Bld10 Maintains Proper Centriole Structure but Is Dispensable for Cartwheel Formation. J. Cel. Sci. 125, 5881–5886. 10.1242/jcs.113506 [DOI] [PubMed] [Google Scholar]
- Rupp V., Rauf S., Naveed I., Windpassinger C., Mir A. (2014). A Novel Single Base Pair Duplication in WDR62 Causes Primary Microcephaly. BMC Med. Genet. 15, 107. 10.1186/s12881-014-0107-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadi A., Borck G., Boddaert N., Chekkour M. C., Imessaoudene B., Munnich A., et al. (2009). Compound Heterozygous ASPM Mutations Associated with Microcephaly and Simplified Cortical Gyration in a Consanguineous Algerian Family. Eur. J. Med. Genet. 52, 180–184. 10.1016/j.ejmg.2009.03.013 [DOI] [PubMed] [Google Scholar]
- Saadi A., Verny F., Siquier-Pernet K., Bole-Feysot C., Nitschke P., Munnich A., et al. (2016). Refining the Phenotype Associated with CASC5 Mutation. Neurogenetics 17, 71–78. 10.1007/s10048-015-0468-7 [DOI] [PubMed] [Google Scholar]
- Sajid Hussain M., Marriam Bakhtiar S., Farooq M., Anjum I., Janzen E., Reza Toliat M., et al. (2013). Genetic Heterogeneity in Pakistani Microcephaly Families. Clin. Genet. 83, 446–451. 10.1111/j.1399-0004.2012.01932.x [DOI] [PubMed] [Google Scholar]
- Santaguida S., Musacchio A. (2009). The Life and Miracles of Kinetochores. Embo J. 28, 2511–2531. 10.1038/emboj.2009.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkisian M. R., Frenkel M., Li W., Oborski J. A., LoTurco J. J. (2001). Altered Interneuron Development in the Cerebral Cortex of the Flathead Mutant. Cereb. Cortex 11, 734–743. 10.1093/cercor/11.8.734 [DOI] [PubMed] [Google Scholar]
- Sarkisian M. R., Li W., Di Cunto F., D'Mello S. R., LoTurco J. J. (2002). Citron-kinase, a Protein Essential to Cytokinesis in Neuronal Progenitors, Is Deleted in the Flathead Mutant Rat. J. Neurosci. 22, Rc217. 10.1523/JNEUROSCI.22-08-j0001.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassaman E. A., Zartler A. S. (1982). Mental Retardation and Head Growth Abnormalities. J. Pediatr. Psychol. 7, 149–156. 10.1093/jpepsy/7.2.149 [DOI] [PubMed] [Google Scholar]
- Sato R., Takanashi J., Tsuyusaki Y., Kato M., Saitsu H., Matsumoto N., et al. (2016). Association between Invisible Basal Ganglia and ZNF335 Mutations: A Case Report. Pediatrics 138, 138. 10.1542/peds.2016-0897 [DOI] [PubMed] [Google Scholar]
- Scala M., Chua G. L., Chin C. F., Alsaif H. S., Borovikov A., Riazuddin S., et al. (2020). Biallelic MFSD2A Variants Associated with Congenital Microcephaly, Developmental Delay, and Recognizable Neuroimaging Features. Eur. J. Hum. Genet. 28, 1509–1519. 10.1038/s41431-020-0669-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgourdou P., Mishra-Gorur K., Saotome I., Henagariu O., Tuysuz B., Campos C., et al. (2017). Disruptions in Asymmetric Centrosome Inheritance and WDR62-Aurora Kinase B Interactions in Primary Microcephaly. Sci. Rep. 7, 43708. 10.1038/srep43708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen R., Hashem A., Abdel-Salam G. M. H., Al-Fadhli F., Ewida N., Alkuraya F. S. (2016). Mutations in CIT, Encoding Citron Rho-Interacting Serine/threonine Kinase, Cause Severe Primary Microcephaly in Humans. Hum. Genet. 135, 1191–1197. 10.1007/s00439-016-1722-2 [DOI] [PubMed] [Google Scholar]
- Shaheen R., Maddirevula S., Ewida N., Alsahli S., Abdel-Salam G. M. H., Zaki M. S., et al. (2019). Genomic and Phenotypic Delineation of Congenital Microcephaly. Genet. Med. 21, 545–552. 10.1038/s41436-018-0140-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L., Qalieh A., Lam M. M., Keil J. M., Kwan K. Y. (2019). Robust Elimination of Genome-Damaged Cells Safeguards against Brain Somatic Aneuploidy Following Knl1 Deletion. Nat. Commun. 10, 2588. 10.1038/s41467-019-10411-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P., Ramdas Nair A., Cabernard C. (2014). The Centriolar Protein Bld10/Cep135 Is Required to Establish Centrosome Asymmetry in Drosophila Neuroblasts. Curr. Biol. 24, 1548–1555. 10.1016/j.cub.2014.05.050 [DOI] [PubMed] [Google Scholar]
- Siskos N., Stylianopoulou E., Skavdis G., Grigoriou M. E. (2021). Molecular Genetics of Microcephaly Primary Hereditary: An Overview. Brain Sci. 11. 10.3390/brainsci11050581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slezak R., Smigiel R., Obersztyn E., Pollak A., Dawidziuk M., Wiszniewski W., et al. (2021). Further Delineation of Phenotype and Genotype of Primary Microcephaly Syndrome with Cortical Malformations Associated with Mutations in the WDR62 Gene. Genes 12, 594. 10.3390/genes12040594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouffs K., Stergachis A. B., Vanderhasselt T., Dica A., Janssens S., Vandervore L., et al. (2018). Expanding the Clinical Spectrum of Biallelic ZNF335 Variants. Clin. Genet. 94, 246–251. 10.1111/cge.13260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian L., Calcagnotto M. E., Paredes M. F. (2019). Cortical Malformations: Lessons in Human Brain Development. Front. Cel Neurosci 13, 576. 10.3389/fncel.2019.00576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepanski S., Hussain M. S., Sur I., Altmüller J., Thiele H., Abdullah U., et al. (2016). A Novel Homozygous Splicing Mutation of CASC5 Causes Primary Microcephaly in a Large Pakistani Family. Hum. Genet. 135, 157–170. 10.1007/s00439-015-1619-5 [DOI] [PubMed] [Google Scholar]
- Tacchelly-Benites O., Wang Z., Yang E., Lee E., Ahmed Y. (2013). Toggling a Conformational Switch in Wnt/β-Catenin Signaling: Regulation of Axin Phosphorylation. BioEssays 35, 1063–1070. 10.1002/bies.201300101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H., Hammack C., Ogden S. C., Wen Z., Qian X., Li Y., et al. (2016). Zika Virus Infects Human Cortical Neural Progenitors and Attenuates Their Growth. Cell Stem Cell 18, 587–590. 10.1016/j.stem.2016.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimborn M., Bell S. M., Felix C., Rashid Y., Jafri H., Griffiths P. D., et al. (2004). Mutations in Microcephalin Cause Aberrant Regulation of Chromosome Condensation. Am. J. Hum. Genet. 75, 261–266. 10.1086/422855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimborn M., Ghani M., Walther D. J., Dopatka M., Dutrannoy V., Busche A., et al. (2010). Establishment of a Mouse Model with Misregulated Chromosome Condensation Due to Defective Mcph1 Function. PLoS One 5, e9242. 10.1371/journal.pone.0009242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai M.-Y., Wang S., Heidinger J. M., Shumaker D. K., Adam S. A., Goldman R. D., et al. (2006). A Mitotic Lamin B Matrix Induced by RanGTP Required for Spindle Assembly. Science 311, 1887–1893. 10.1126/science.1122771 [DOI] [PubMed] [Google Scholar]
- van Deijk A.-L. F., Camargo N., Timmerman J., Heistek T., Brouwers J. F., Mogavero F., et al. (2017). Astrocyte Lipid Metabolism Is Critical for Synapse Development and Function In Vivo . Glia 65, 670–682. 10.1002/glia.23120 [DOI] [PubMed] [Google Scholar]
- Van Den Bosch J. (1959). Microcephaly in the Netherlands: a Clinical and Genetical Study. Ann. Hum. Genet. 23, 91–116. 10.1111/j.1469-1809.1958.tb01455.x [DOI] [PubMed] [Google Scholar]
- van Dyck L. I., Morrow E. M. (2017). Genetic Control of Postnatal Human Brain Growth. Curr. Opin. Neurol. 30, 114–124. 10.1097/wco.0000000000000405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmark H., Llamazares S., Rebollo E., Lange B., Reina J., Schwarz H., et al. (2007). Asterless Is a Centriolar Protein Required for Centrosome Function and Embryo Development in Drosophila. Curr. Biol. 17, 1735–1745. 10.1016/j.cub.2007.09.031 [DOI] [PubMed] [Google Scholar]
- Vergnes L., Peterfy M., Bergo M. O., Young S. G., Reue K. (2004). Lamin B1 Is Required for Mouse Development and Nuclear Integrity. Proc. Natl. Acad. Sci. 101, 10428–10433. 10.1073/pnas.0401424101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verloes A., Drunat S., Gressens P., Passemard S. (1993). “Primary Autosomal Recessive Microcephalies and Seckel Syndrome Spectrum Disorders,” in GeneReviews(R). Editors Pagon R. A., Adam M. P., Ardinger H. H., Wallace S. E., Amemiya A., Bean L. J. H., et al. (Seattle (WA). [PubMed] [Google Scholar]
- Vitale I., Galluzzi L., Castedo M., Kroemer G. (2011). Mitotic Catastrophe: a Mechanism for Avoiding Genomic Instability. Nat. Rev. Mol. Cel Biol 12, 385–392. 10.1038/nrm3115 [DOI] [PubMed] [Google Scholar]
- von der Hagen M., Pivarcsi M., Liebe J., von Bernuth H., Didonato N., Hennermann J. B., et al. (2014). Diagnostic Approach to Microcephaly in Childhood: a Two-center Study and Review of the Literature. Dev. Med. Child. Neurol. 56, 732–741. 10.1111/dmcn.12425 [DOI] [PubMed] [Google Scholar]
- Wang X., Tsai J.-W., Imai J. H., Lian W.-N., Vallee R. B., Shi S.-H. (2009). Asymmetric Centrosome Inheritance Maintains Neural Progenitors in the Neocortex. Nature 461, 947–955. 10.1038/nature08435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watemberg N., Silver S., Harel S., Lerman-Sagie T. (2002). Significance of Microcephaly Among Children with Developmental Disabilities. J. Child. Neurol. 17, 117–122. 10.1177/088307380201700205 [DOI] [PubMed] [Google Scholar]
- Wong B. H., Chan J. P., Cazenave-Gassiot A., Poh R. W., Foo J. C., Galam D. L. A., et al. (2016). Mfsd2a Is a Transporter for the Essential ω-3 Fatty Acid Docosahexaenoic Acid (DHA) in Eye and Is Important for Photoreceptor Cell Development. J. Biol. Chem. 291, 10501–10514. 10.1074/jbc.m116.721340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods C. G., Bond J., Enard W. (2005). Autosomal Recessive Primary Microcephaly (MCPH): A Review of Clinical, Molecular, and Evolutionary Findings. Am. J. Hum. Genet. 76, 717–728. 10.1086/429930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods C. G. (2004). Human Microcephaly. Curr. Opin. Neurobiol. 14, 112–117. 10.1016/j.conb.2004.01.003 [DOI] [PubMed] [Google Scholar]
- Woods C. G., Parker A. (2013). Investigating Microcephaly. Arch. Dis. Child. 98, 707–713. 10.1136/archdischild-2012-302882 [DOI] [PubMed] [Google Scholar]
- Wu K.-Y., Zuo G.-L., Li X.-F., Ye Q., Deng Y.-Q., Huang X.-Y., et al. (2016). Vertical Transmission of Zika Virus Targeting the Radial Glial Cells Affects Cortex Development of Offspring Mice. Cell Res 26, 645–654. 10.1038/cr.2016.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D., Zhang F., Wang Y., Sun Y., Xu Z. (2014). Microcephaly-associated Protein WDR62 Regulates Neurogenesis through JNK1 in the Developing Neocortex. Cel Rep. 6, 104–116. 10.1016/j.celrep.2013.12.016 [DOI] [PubMed] [Google Scholar]
- Yabe T., Ge X., Pelegri F. (2007). The Zebrafish Maternal-Effect Gene Cellular Atoll Encodes the Centriolar Component Sas-6 and Defects in its Paternal Function Promote Whole Genome Duplication. Developmental Biol. 312, 44–60. 10.1016/j.ydbio.2007.08.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S., Jaiswal M., Charng W.-L., Gambin T., Karaca E., Mirzaa G., et al. (2014). A drosophila Genetic Resource of Mutants to Study Mechanisms Underlying Human Genetic Diseases. Cell 159, 200–214. 10.1016/j.cell.2014.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. J., Baltus A. E., Mathew R. S., Murphy E. A., Evrony G. D., Gonzalez D. M., et al. (2012). Microcephaly Gene Links Trithorax and REST/NRSF to Control Neural Stem Cell Proliferation and Differentiation. Cell 151, 1097–1112. 10.1016/j.cell.2012.10.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen T. J., Compton D. A., Wise D., Zinkowski R. P., Brinkley B. R., Earnshaw W. C., et al. (1991). CENP-E, a Novel Human Centromere-Associated Protein Required for Progression from Metaphase to Anaphase. EMBO J. 10, 1245–1254. 10.1002/j.1460-2075.1991.tb08066.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. G., Jung H.-J., Coffinier C., Fong L. G. (2012). Understanding the Roles of Nuclear A- and B-type Lamins in Brain Development. J. Biol. Chem. 287, 16103–16110. 10.1074/jbc.r112.354407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T. W., Mochida G. H., Tischfield D. J., Sgaier S. K., Flores-Sarnat L., Sergi C. M., et al. (2010). Mutations in WDR62, Encoding a Centrosome-Associated Protein, Cause Microcephaly with Simplified Gyri and Abnormal Cortical Architecture. Nat. Genet. 42, 1015–1020. 10.1038/ng.683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel J. K., Marszalek J. D., McIntosh J. R., Goldstein L. S. B., Cleveland D. W., Philp A. V. (2000). CENP-meta, an Essential Kinetochore Kinesin Required for the Maintenance of Metaphase Chromosome Alignment in Drosophila. J. Cel Biol 150, 1–12. 10.1083/jcb.150.1.1a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaqout S., Morris-Rosendahl D., Kaindl A. M. (2017). Autosomal Recessive Primary Microcephaly (MCPH): An Update. Neuropediatrics 48, 135–142. 10.1055/s-0037-1601448 [DOI] [PubMed] [Google Scholar]
- Zaqout S., Blaesius K., Wu Y.-J., Ott S., Kraemer N., Becker L.-L., et al. (2019). Altered Inhibition and Excitation in Neocortical Circuits in Congenital Microcephaly. Neurobiol. Dis. 129, 130–143. 10.1016/j.nbd.2019.05.008 [DOI] [PubMed] [Google Scholar]
- Zarate Y. A., Kaylor J. A., Bosanko K., Lau S., Vargas J., Gao H. (2016). First Clinical Report of an Infant with Microcephaly andCASC5mutations. Am. J. Med. Genet. 170, 2215–2218. 10.1002/ajmg.a.37726 [DOI] [PubMed] [Google Scholar]
- Zhang W., Yang S.-L., Yang M., Herrlinger S., Shao Q., Collar J. L., et al. (2019). Modeling Microcephaly with Cerebral Organoids Reveals a WDR62-Cep170-KIF2A Pathway Promoting Cilium Disassembly in Neural Progenitors. Nat. Commun. 10, 2612. 10.1038/s41467-019-10497-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Li H., Pang J., Peng Y., Shu L., Wang H. (2019). Novel SASS6 Compound Heterozygous Mutations in a Chinese Family with Primary Autosomal Recessive Microcephaly. Clinica Chim. Acta 491, 15–18. 10.1016/j.cca.2019.01.007 [DOI] [PubMed] [Google Scholar]
- Zhong W., Chia W. (2008). Neurogenesis and Asymmetric Cell Division. Curr. Opin. Neurobiol. 18, 4–11. 10.1016/j.conb.2008.05.002 [DOI] [PubMed] [Google Scholar]
- Zhong W., Feder J. N., Jiang M.-M., Jan L. Y., Jan Y. N. (1996). Asymmetric Localization of a Mammalian Numb Homolog during Mouse Cortical Neurogenesis. Neuron 17, 43–53. 10.1016/s0896-6273(00)80279-2 [DOI] [PubMed] [Google Scholar]
- Ziegler A. B., Thiele C., Tenedini F., Richard M., Leyendecker P., Hoermann A., et al. (2017). Cell-Autonomous Control of Neuronal Dendrite Expansion via the Fatty Acid Synthesis Regulator SREBP. Cel Rep. 21, 3346–3353. 10.1016/j.celrep.2017.11.069 [DOI] [PubMed] [Google Scholar]
- Zombor M., Kalmár T., Nagy N., Berényi M., Telcs B., Maróti Z., et al. (2019). A Novel WDR62 Missense Mutation in Microcephaly with Abnormal Cortical Architecture and Review of the Literature. J. Appl. Genet. 60, 151–162. 10.1007/s13353-019-00486-y [DOI] [PubMed] [Google Scholar]