Abstract
Introduction:
Combustible tobacco smoking and cannabis use frequently occur together, and the use of both substances is associated with overall greater severity of tobacco and cannabis related problems. Observational work has found that cannabis use is associated with tobacco cessation failure, but research directly testing the longitudinal associations of cannabis use on tobacco cessation during smoking cessation treatment is lacking. The current study examined the impact of current cannabis use on combustible tobacco cessation outcomes.
Methods:
207 daily combustible tobacco smokers (Mage = 38.24 years, SD = 14.84, 48.1% male) were enrolled in a randomized controlled smoking cessation trial. Survival analyses and multi-level modeling were used to assess lapse and relapse behavior through 12-week follow up. The current study is a secondary data analysis.
Results:
Results of the current study suggest that cannabis use is associated with faster time to lapse (OR = .644, se = .188, p = .019), but not relapse (OR = −.218, se = .403, p = .525), compared to combustible tobacco-only smokers. Additionally, cannabis use was associated with lower likelihood of achieving any 7-day point prevalence abstinence during the 12 week follow up (b = 0.93, se =0 .24, p = 0.0001).
Conclusions:
The current study provides novel evidence that cannabis use may be related to combustible tobacco use in terms of faster time to lapse and lower likelihood of any 7-day point prevalence abstinence following smoking cessation treatment. Developing integrated cannabis-tobacco cessation treatments is an important next step in research focused on tobacco-cannabis use.
Keywords: Tobacco, Cannabis, Smoking Cessation, Combustible Cigarettes
1.0. INTRODUCTION
Cigarette use and cannabis use frequently co-occur (Schauer et al., 2015), with estimates suggesting that 5.2% of adults co-use cigarettes and cannabis, 69.% of cannabis users reported tobacco use, and 17.8% of cigarette users reported cannabis use. Additionally, daily cannabis smokers are more likely to be combustible tobacco users than non-daily cannabis smokers(Goodwin et al., 2018b). Additionally, compared to the general population, cannabis use is significantly more common among combustible tobacco smokers (Goodwin et al., 2018a), and a large percentage of cannabis use occurs among combustible tobacco smokers compared to non-combustible smokers (Pacek et al., in press). Further, cannabis use and cannabis use disorder are increasing among combustible tobacco smokers (Weinberger et al., 2018).
Individuals who use both tobacco and cannabis report more severe tobacco and cannabis related problems, including withdrawal and craving (Agrawal et al., 2009; Budney et al., 2008; Peters et al., 2012). Further, research across distinct research methodologies and populations has documented a reciprocal relation between combustible tobacco and cannabis use, such that they frequently co-occur and influence one another (Badiani et al., 2015; Kristman-Valente et al., 2017). For example, combustible tobacco use has been implicated in the onset and maintenance of cannabis use and cannabis use problems (Agrawal and Lynskey, 2009). Conversely, cannabis use has been related to the initiation and severity of combustible tobacco use (Patton et al., 2005). Additionally, compared to those that only smoke cannabis, those that smoke cannabis and tobacco are more likely to meet criteria for a cannabis use disorder and have more cannabis-related problems (Peters et al., 2012; Ream et al., 2008). Longitudinal epidemiological research indicates that combustible tobacco use is associated with increased odds of cannabis use and cannabis use being associated with increased odds of combustible tobacco smoking initiation (Badiani et al., 2015; Kristman-Valente et al., 2017).
Concurrent cannabis and tobacco use is related is associated with greater tobacco and cannabis withdrawal symptoms compared to using one substance (Vandrey et al., 2008). Moreover, observational studies have found that cannabis use is related to a decreased likelihood of a combustible tobacco use cessation attempt and an increased propensity for relapse during a quit attempt over time (Weinberger et al., 2018). Additionally, among combustible tobacco smokers, cannabis use compared to non-cannabis use was associated with greater likelihood of continued tobacco use (Ford et al., 2002).
Surprisingly, little research has examined the role of cannabis use during a combustible smoking cessation treatment, and existing research is inconclusive. For example, one study reported that among heavy alcohol drinkers enrolled in a smoking cessation study, cannabis use was not related to tobacco smoking lapse (Metrik et al., 2011). However, because the sample was comprised of individuals with alcohol use disorder, it is not clear if these findings generalize to non-alcohol use disorder samples of combustible smokers. This limitation is unfortunate, as understanding if cannabis use is related to combustible tobacco cessation outcomes during smoking cessation treatment would guide assessment and intervention programming for this vulnerable group (Lee et al., 2019). Theoretically, cannabis use may impact combustible tobacco cessation outcomes by a number of mechanisms, including negative reinforcement following mitigation of tobacco withdrawal symptoms (Baker et al., 2004), enhancement motives (e.g., tobacco smoking amplifying the effects of cannabis use)(Penetar et al., 2005), or drug substitution (e.g., individuals are substituting cannabis for tobacco, and ultimately returning to tobacco) (Fairbank et al., 1993; McClure et al., 2018b).
The purpose of the present investigation was therefore to examine the effect of current cannabis use on time to smoking lapse and relapse, as well as 7-day point-prevalent abstinence during 12 week follow up during a combustible tobacco cessation treatment among primary combustible tobacco users. It was hypothesized that current cannabis users would evince a faster time to lapse and to relapse to combustible tobacco use. Additionally, it was hypothesized that current cannabis use would be related to poorer combustible tobacco 7-day point-prevalence abstinence throughout the follow up period.
2.0. METHOD
2.1. Measures
Marijuana Smoking History Questionnaire (MSHQ)(Bonn-Miller and Zvolensky, 2009).
The MSHQ is a 21-item self-report measure of cannabis smoking history, including patterns of use (e.g. cannabis smoking rate, years of being a regular cannabis smoker). The MSHQ has been used successfully in prior work (Manning et al., 2018) and in the current study, the item, “Please rate your cannabis use in the past 30 days” was used to identify current cannabis users given the preliminary nature of this investigation. Specifically, those that indicated smoking any cannabis in the past 30 days at baseline were coded as 1 (current user), and those that reported not smoking cannabis in the past 30 days were coded as 0 (not current user).
Smoking History Questionnaire (SHQ)(Brown et al., 2002).
The SHQ is a self-report questionnaire used to assess smoking history and patterns of smoking (e.g. smoking rate, age of onset of initiation). It has been successfully used in previous studies as a measure of smoking history (Zvolensky et al., 2004). The present study used the following variables from the SHQ to characterize the sample: average number of combustible cigarettes smoked per day, age of onset of first cigarette, and number of years as a regular, daily combustible cigarette smoker.
Fagerström Test for Cigarette Dependence (FTCD; Fagerström, 2012).
The FTCD is a well-established 6-item scale designed to assess gradations in tobacco dependence. The measure exhibits a high degree of test-retest reliability (Pomerleau et al., 1994), and positive relations with key smoking variables (Heatherton et al., 1991; Payne et al., 1994). The FTCD was administered at baseline and was used as a covariate to account for variations in cigarette dependence.
Abstinence.
The Timeline Follow-Back (TLFB; Brown et al., 1998; Sobell and Sobell, 1992) procedure was used at all assessments to assess cigarette consumption at each day since the previous assessment. The assessment has demonstrated good reliability and validity with biochemical indices of smoking (Sobell and Sobell, 1992). Biochemical verification of smoking status at baseline was completed by carbon monoxide (CO) analysis of breath samples using a CMD/CO Carbon Monoxide Monitor (Model 3110; Spirometrics, Inc.). However, previous research suggests that concurrent cannabis use may increase expired CO (Hecht and Vogt, 1985), and thus, cigarette smoking lapse and relapse during the follow-up period were determined based on self-reported TLFB. Based on past research, it was assumed that the presence of a missing smoking status data indicated that a participant had smoked a cigarette when the closest available data point indicated that they had smoked; all others were coded as not smoking (Farris et al., 2016). To model lapse behavior, a dichotomous variable was created to indicate any smoking lapse that occurred during the first 28 days post quit day. Additionally, a variable was created to indicate the number of days that elapsed from quit day to 1) the first smoking lapse (slip), defined as smoking any amount following quit day (Piper et al., 2019; Shiffman et al., 1996), and 2) relapse, defined as a return to regular smoking following a period of abstinence, as defined in the current investigation as at least 5 cigarettes per day on three consecutive days (Piper et al., 2019; Shiffman et al., 1996). A dichotomous relapse variable was also created to model relapse behavior. Further, 7-day point prevalent abstinence was defined as complete abstinence (not even a puff) for 7-days prior to each assessment point (Piper et al., 2019).
2.2. Procedure
Data for the present study are a secondary data analysis from a multi-site randomized controlled clinical trial examining the efficacy of two smoking cessation interventions (Schmidt et al., 2016). Interested persons responding to community-based advertisements (e.g., flyers, newspaper ads, radio announcements) contacted the research team and were provided with a detailed description of the study via phone. Participants were then screened for initial eligibility, and if eligible, scheduled for a baseline appointment, where they provided written informed consent, completed a diagnostic interview, a computerized self-report assessment battery as well as biochemical CO verification of smoking status to evaluate eligibility criteria (CO > 8).
Inclusion criteria for the trial included: (1) 18–65 years of age; (2) being a daily smoker for at least 1 year; (3) currently smoking a minimum of 8 cigarettes per day; and (4) self-reported motivation to quit smoking (e.g., at least 5 on a 10-point scale). Exclusion criteria for the trial included: (1) current use of pharmacotherapy for smoking cessation (except the nicotine patch, which was provided by the study); (2) limited mental competency and inability to provide informed, voluntary, written consent; (3) endorsement of current or past psychotic-spectrum symptoms; (4) current suicidality or homicidal ideation; (5) history of significant medical condition; and (6) planning to relocate within the next 6 months.
Participants deemed eligible for the larger trial were randomly assigned to active or control treatment. The two treatment conditions included: a 4-session cognitive-behavioral smoking cessation program with an added anxiety sensitivity reduction component (active; i.e., Panic-Smoking Program), or a standard cognitive-behavioral smoking cessation program (control). Both treatments took place over four, 90-minute sessions occurring once per week. Both treatment groups received nicotine replacement therapy via the transdermal nicotine patch that was initiated at treatment Session 4 (quit day). Nicotine patch dosage followed guidelines established by the Food and Drug Administration (Zvolensky et al., 2017). Participants were offered the nicotine replacement therapy for up to 12 weeks post-quit. The study protocol was approved by the Institutional Review Boards at the University of Vermont and Florida State University (clinicaltrials.gov # NCT01753141).
2.3. Data Analytic Plan
Data were analyzed using SAS version 9.4. First, descriptive statistics and bi-variate relations were examined among variables. Additionally, group differences between cannabis users and non-cannabis users were examined for demographic and smoking variables. The present study examined the effect of current cannabis use (at baseline) on time to smoking lapse and relapse during the first 28 days post-quit, and the effect of current cannabis use on any 7-day point-prevalent abstinence throughout 12 weeks. These timelines were selected to increase the odds of individuals remaining abstinent from tobacco (Farris et al., 2016; Piper et al., 2009). For all models, covariates included age, gender, baseline FTCD, and treatment condition.
To predict lapse and relapse, two multivariate Cox proportional hazards regression analyses were used to examine the predictive value of current cannabis use on time to lapse and relapse. All models included two steps, with covariates entered into the first step and current cannabis use entered into the second step.
Seven-day point-prevalent abstinence through the 12 week follow up was modeled using multi-level modeling (MLM), in accordance with guidelines put forth by the Society for Nicotine and Tobacco Research (Hall et al., 2001), which allows for all subjects to be included, regardless of missing data. First, an unconditional model, without covariates was run, followed by a conditional model including theoretically relevant covariates. Assessments from quit week, week one, week two, week four, and week twelve were included as outcome variables. To model point-prevalence abstinence throughout the 12 week follow up, multivariate MLM (MMLM) was used to avoid Type 1 error inflation and can include any assessment from which any outcome data was obtained. To account for the dichotomous structure of the outcome variable, we used generalized MMLM with a logistic linking function and robust standard errors. Random intercepts were modeled.
3.0. RESULTS
3.1. Participants
Participants (N=207, Mage = 38.24 years, SD = 14.84, 48.1% male) were treatment-seeking daily combustible tobacco smokers enrolled in a clinical trial evaluating the efficacy of a smoking cessation treatment targeting anxiety sensitivity compared to a standard smoking cessation treatment (Schmidt et al., 2016; Zvolensky et al., 2018).
In terms of demographic characteristics, 85.6% identified as White, 7.4% as Black non-Hispanic, 0.3% as Black Hispanic, 3.7% as White Hispanic, 0.7% as Asian, and 2.3% as other. In terms of education, 2.4% reported not having graduated from high school, 16.4% reported graduating from high school, 35.3% attended some college, and the remaining participants (45.9%) graduated from college (2-year, 4 year, or graduate/professional school). Additionally, 37.7% reported being married or living with a partner, 2.4% reported being widowed, 18.9% reported being separated or divorced, and 41.1% reported being never married. Participants who used cannabis reported first smoking cannabis at 16.26 (SD = 3.42) years old, reported daily cannabis use at age 16.90 (SD = 7.46), and reported being a daily cannabis user for 6.42 (SD = 8.54) years. See table 1 for demographic and smoking characteristics by group.
Table 1.
Sample Descriptive Information
| Tobacco Only (n=125) | Tobacco-Cannabis Co-Use (n=82) | |
|---|---|---|
| Age | 34.41 (SD = 8.94) years | 25.86 (SD = 8.73) years |
| Gender | 50.0% female | 50.8% female |
| Age of first cigarette | 14.39 (3.45) | 14.93 (3.05) |
| Daily smoking age | 17.52 (3.74) | 17.36 (2.71) |
| Years as a daily smoker | 16.20 (8.80) | 8.15 (8.31) |
| Average CPD | 14.66 (7.26) | 13.20 (6.94) |
| FTCD Total | 4.86 (2.20) | 4.12 (2.14) |
Note: Demographic and smoking characteristics between tobacco-only users and tobacco-cannabis co-users.
3.2. Attrition Analyses
A total of 603 people signed the consent form an entered the study. Compared to those who remained in the study and completed treatment and follow up sessions, participants who dropped out of the study were significantly younger in age (t(623) = −2.55, p = 0.01), and were significantly younger when they started regular daily smoking (t(608) = −2.16, p = 0.03). There were no significant differences on other cigarette and cannabis variables, including average cigarettes per day, number of years as a daily smoker, age of first using cannabis and age of daily cannabis use (p’s > 0.05).
3.3. Bi-Variate Correlations and Group Comparisons
Current cannabis use was significantly negatively correlated with age, years as a daily smoker, and cigarette dependence, indicating that current cannabis use was associated with lower levels of these variables (see Table 2)
Table 2.
Bi-variate correlations among variable
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | |
|---|---|---|---|---|---|---|---|---|
| 1. Age | 1 | |||||||
| 2. Gender | 0.054 | 1 | ||||||
| 3. Age of first cigarette | −0.017 | −.168* | 1 | |||||
| 4. Age of regular daily smoking | 0.126 | −0.056 | .443** | 1 | ||||
| 5. Years as regular daily smoker | .916** | 0.082 | −.159* | −.200** | 1 | |||
| 6. Average cigarettes (as regular daily smoker) | .455** | −0.024 | 0.020 | −0.068 | .468** | 1 | ||
| 7. Average cigarettes (last week) | .339** | −0.111 | −0.017 | −0.052 | .338** | .677** | 1 | |
| 8. Cigarette Dependence | .419** | 0.010 | −0.076 | −0.074 | .448** | .635** | .627** | 1 |
| 9. Current cannabis use | −.382** | −0.107 | 0.086 | −0.047 | −.345** | −0.094 | −0.061 | −.131* |
p<0.05;
p<0.01.
Bi-variate relations among variables used in the current study.
3.4. Survival Analyses
Time to lapse.
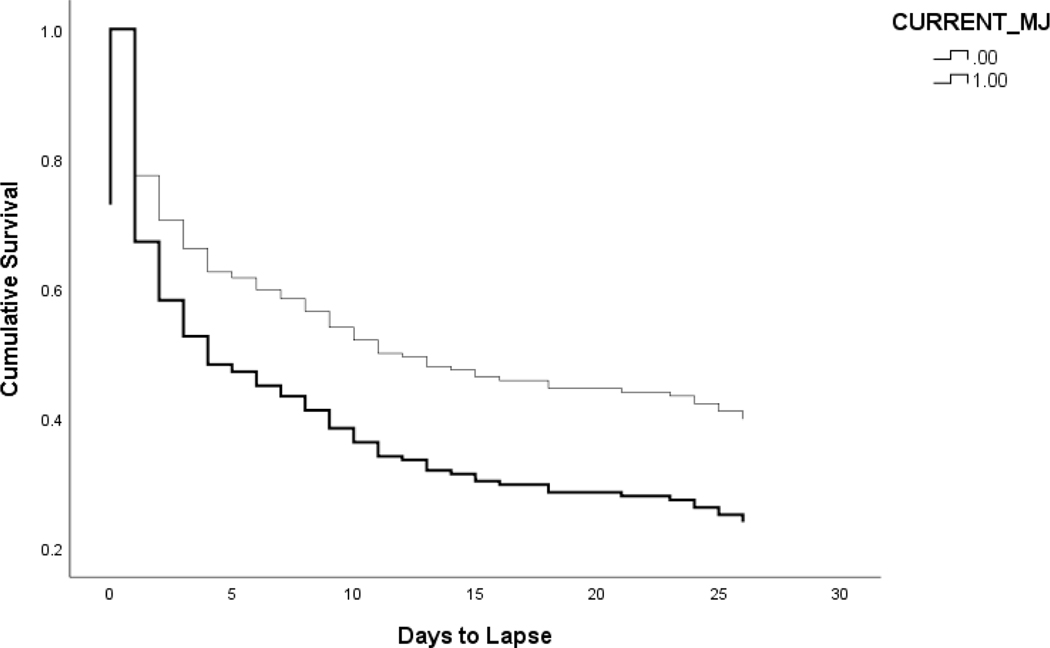
Examining step 1 of the model indicated that there was no significant improvement from the baseline model (χ2(4) = 4.90, p = .30). Age was a significant predictor of smoking lapse (OR = .989, se = .007, p = .046), such that decreased age was associated with quicker smoking lapse. For step 2 of the model, there was a significant increase in model fit (Δ χ2(1) = 5.43, p = .02). Current cannabis use was significantly associated with faster smoking lapse (OR = .644, se = .188, p = .019; see Figure 1).
Figure 1. Surival Function for Smoking Lapse.
N=207, Mage = 38.24 years, SD = 14.84, 48.1% male. The effect of current cannabis use on days to smoking lapse. Thin line is those that do not use cannabis, and thick line is those that use both cannabis and combustible tobacco.
Time to relapse.
There were no significant effects at step 1 or step 2 (OR = −.218, se = .403, p = .525).
3.5. 7-day Point Prevalence Abstinence
Unconditional Model.
Random intercept covariance was significant indicating significant variability in baseline (intercept) levels of cannabis use influencing the level of the outcome, and variability between participants in the intercept (b = 1.51, se = 0.35, p = <.001). Examining fixed effect parameters indicated that current cannabis use was significantly associated with point-prevalent abstinence (b = 0.91, se =0 .21, p < 0.0001), such that current cannabis users were less likely to be abstinent from combustible tobacco throughout the follow up.
Conditional Model.
Examining random intercept covariance indicated significant intercept covariance (b = 1.64, se = .38, p = <.001). Examining the fixed effect parameter estimates suggested that baseline cigarette dependence (b = −0.14, se =0 .05, p = 0.001) as well as current cannabis use was significantly associated with 7-day point-prevalent abstinence (b = 0.93, se =0 .24, p = 0.0001); specially current cannabis users were significantly more likely to be non-abstinent from cigarettes through week 12.
4.0. DISCUSSION
The current study examined current cannabis use on a combustible tobacco cessation attempt (time to lapse, relapse, and point prevalence abstinence) among treatment seeking combustible tobacco smokers enrolled in a clinical trial for smoking cessation. Results from the current study rejected the null hypotheses, such that current cannabis use was associated with faster time to lapse and supported the null hypothesis that cannabis use was not associated with time to relapse. Current cannabis use also was inversely related to point prevalence abstinence at 12 weeks post quit attempt. Importantly, these effects were evident over the variance accounted for by age, gender, treatment condition, and baseline severity of cigarette dependence. Further, these results sit in the larger context that the cannabis users in the present sample demonstrated fewer years of being a regular combustible cigarette smoker and less cigarette dependence (FTCD total score). Thus, despite the cannabis-tobacco sample being ‘less severe’ on these tobacco-related variables, they demonstrated less success in terms of lapse and point prevalence abstinence. These findings are in line with previous research suggesting that cannabis use may impact combustible tobacco cessation over time in a longitudinal observation study (Weinberger et al., 2018), and extends past work to implicate cannabis use in smoking cessation failure among individuals making an active quit attempt during treatment.
Current cannabis use was not associated with smoking relapse following quit day. Although this finding was unexpected, there are several plausible hypotheses that may help explain these results. For instance, extant work suggests that early smoking lapse is often considered to be the most clinically significant predictor of returning to pre-quit levels of smoking (Bolman et al., 2018; Brandon et al., 1990; Brown et al., 2005; Roche et al., 2014). However, it is possible that a full relapse was not detected in the time period assessed, as has been found in past research (Zvolensky et al., 2009). Additionally, given the fact that all participants received treatment for smoking cessation, it is possible that following a smoking lapse, they were able to implement coping strategies taught during treatment strategies to prevent a relapse.
The results from the current study may have important clinical implications. Given that current cannabis use is related to several combustible smoking cessation variables, it may be important for providers to assess and target cannabis use prior to initiating a combustible tobacco cessation attempt. Yet, given the fact that tobacco use may be contributing to and driving difficulties quitting cannabis (McClure et al., 2018a), there may be more clinical utility in targeting both substances at the same time. Research examining tobacco-alcohol relations found clinical utility in concurrently targeting both substances (Prochaska et al., 2004), and thus, using this type of model for cannabis-tobacco relations may be warranted. Some previous work among individuals attempting to quit cannabis found that targeting both cannabis and tobacco use improved cannabis use outcomes, and to a lesser extent, tobacco use outcomes (Lee et al., 2014). These treatments integrate evidenced based motivational enhancement therapy, cognitive behavior therapy, and contingency management. Therefore, there may be merit in developing integrated cessation interventions that target both cannabis and combustible tobacco use. Yet, because the results from the current study suggests that cannabis use was associated with only faster time to lapse, there may need to be psychoeducation about the complexities in involved. Namely, cannabis may be associated with lapse but not relapse. To more firmly established clinical implications of cannabis-tobacco relations for intervention programming, replication and extension to other samples is first warranted.
The current study has several limitations. First, current cannabis use was coded dichotomously to determine the broadband effect of cannabis use on combustible cigarette cessation outcomes. It is possible that different levels of cannabis use may impact lapse and point-prevalence abstinence. Therefore, future work should extend these findings by examining frequency and quantity of cannabis use, as well as the perceived intoxication and/or chemical potency of cannabis, to better understand its relation to cessation outcomes for users of both cannabis and tobacco. Second, the sample was predominately White and generally well-educated. Future work may benefit from replicating these findings among a more racially and ethnically diverse sample. Third, we did not model the use of electronic cigarette use in the current sample. There is therefore a need to document the prevalence and impact of electronic cigarette use among users of both combustible cigarettes and cannabis. Fourth, information regarding past illicit substance use, including opioids, benzodiazepines, and stimulants was not collected, and it is possible that a history of substance use may impact lapse and relapse behavior. Finally, given the observed association between cannabis use and smoking cessation outcomes, there is a need for future research to explicate the mechanisms underlying the observed associations. There may be utility in exploring intra-individual difference factors, including self-efficacy for remaining abstinent (Stephens et al., 1995), false safety behavior(Buckner et al., 2019), emotion dysregulation (Rogers et al., 2019), among others, in future research on tobacco-cannabis use.
Overall, the present investigation provides novel empirical evidence that current cannabis users compared to non-cannabis users may experience poorer combustible cigarette cessation outcomes, including time to lapse and lower likelihood of any 7-day point-prevalence abstinence during 12-weeks post quit attempt during smoking cessation treatment. Future work should seek to better understand factors that play a role in greater lapse rates among cannabis users to determine whether smoking lapse occurs as a result of cannabis intoxication or whether other biopsychosocial factors.
Highlights.
Cannabis smoking at baseline is prospectively related to cigarette smoking outcomes
Cannabis use at baseline is associated with quicker time to smoking lapse
Cannabis use at baseline is associated with lower likelihood of point-prevalent smoking abstinence
Acknowledgments
Author Disclosures:
Role of funding source:
This study was funded by a National Institute of Mental Health (R01-MH076629) grant to MJZ and NBS. The funding source had no involvement in the conduct of the research and/or preparation of the article.
Footnotes
Declaration of Interests
The authors declare there are no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agrawal A, Lynskey MT, 2009. Tobacco and cannabis co-occurrence: Does route of administration matter? Drug Alcohol Depend. 99, 240–247. 10.1016/j.drugalcdep.2008.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Lynskey MT, Madden PAF, Pergadia ML, Bucholz KK, Heath AC, 2009. Simultaneous cannabis and tobacco use and cannabis-related outcomes in young women. Drug Alcohol Depend. 101, 8–12. 10.1016/j.drugalcdep.2008.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badiani A, Boden JM, De Pirro S, Fergusson DM, Horwood LJ, Harold GT, 2015. Tobacco smoking and cannabis use in a longitudinal birth cohort: Evidence of reciprocal causal relationships. Drug Alcohol Depend. 150, 69–76. 10.1016/j.drugalcdep.2015.02.015 [DOI] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC, 2004. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol. Rev 111, 33–51. 10.1037/0033-295X.111.1.33 [DOI] [PubMed] [Google Scholar]
- Bolman C, Verboon P, Thewissen V, Boonen V, Soons K, Jacobs N, 2018. Predicting Smoking Lapses in the First Week of Quitting: An Ecological Momentary Assessment Study. J. Addict. Med 12, 65–71. 10.1097/ADM.0000000000000365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonn-Miller MO, Zvolensky MJ, 2009. An Evaluation of the Nature of Marijuana Use and Its Motives among Young Adult Active Users. Am. J. Addict 18, 409–416. 10.3109/10550490903077705 [DOI] [PubMed] [Google Scholar]
- Brandon TH, Tiffany ST, Obremski KM, Baker TB, 1990. Postcessation cigarette use: The process of relapse. Addict. Behav 15, 105–114. 10.1016/0306-4603(90)90013-N [DOI] [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Kahler CW, Strong DR, 2002. Distress tolerance and duration of past smoking cessation attempts. J. Abnorm. Psychol 111, 180–185. [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Kahler CW, Strong DR, Zvolensky MJ, 2005. Distress tolerance and early smoking lapse. Clin. Psychol. Rev 25, 713–733. 10.1016/j.cpr.2005.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TA, Chorpita BF, Barlow DH, 1998. Structural relationships among dimensions of the DSM-IV anxiety and mood disorders and dimensions of negative affect, positive affect, and autonomic arousal. J. Abnorm. Psychol 107, 179–192. [DOI] [PubMed] [Google Scholar]
- Buckner JD, Zvolensky MJ, Ecker AH, Schmidt NB, Lewis EM, Paulus DJ, Lopez-Gamundi P, Crapanzano KA, Bakhshaie J, 2019. Integrated cognitive behavioral therapy for comorbid cannabis use and anxiety disorders: A pilot randomized controlled trial. Behav. Res. Ther 115, 38–45. 10.1016/j.brat.2018.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Vandrey RG, Hughes JR, Thostenson JD, Bursac Z, 2008. Comparison of cannabis and tobacco withdrawal: Severity and contribution to relapse. J. Subst. Abuse Treat 35, 362–368. 10.1016/j.jsat.2008.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerström K, 2012. Determinants of tobacco use and renaming the FTND to the Fagerstrom Test for Cigarette Dependence. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob 14, 75–78. 10.1093/ntr/ntr137 [DOI] [PubMed] [Google Scholar]
- Fairbank JA, Dunteman GH, Condelli WS, 1993. Do Methadone Patients Substitute Other Drugs for Heroin? Predicting Substance Use at 1-Year Follow-up. Am. J. Drug Alcohol Abuse 19, 465–474. 10.3109/00952999309001635 [DOI] [PubMed] [Google Scholar]
- Farris SG, Zvolensky MJ, Schmidt NB, 2016. Difficulties with Emotion Regulation and Psychopathology Interact to Predict Early Smoking Cessation Lapse. Cogn. Ther. Res 40, 357–367. 10.1007/s10608-015-9705-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford DE, Vu HT, Anthony JC, 2002. Marijuana use and cessation of tobacco smoking in adults from a community sample. Drug Alcohol Depend. 67, 243–248. 10.1016/S0376-8716(02)00066-2 [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Cheslack-Postava K, Santoscoy S, Bakoyiannis N, Hasin DS, Collins BN, Lepore SJ, Wall MM, 2018a. Trends in Cannabis and Cigarette Use Among Parents With Children at Home: 2002 to 2015. Pediatrics 141, e20173506. 10.1542/peds.2017-3506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin RD, Pacek LR, Copeland J, Moeller SJ, Dierker L, Weinberger A, Gbedemah M, Zvolensky MJ, Wall MM, Hasin DS, 2018b. Trends in Daily Cannabis Use Among Cigarette Smokers: United States, 2002–2014. Am. J. Public Health 108, 137–142. 10.2105/AJPH.2017.304050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SM, Delucchi KL, Velicer WF, Kahler CW, Ranger-Moore J, Hedeker D, Tsoh JY, Niaura R, 2001. Statistical analysis of randomized trials in tobacco treatment: longitudinal designs with dichotomous outcome. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob 3, 193–202. 10.1080/14622200110050411 [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO, 1991. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br. J. Addict 86, 1119–1127. [DOI] [PubMed] [Google Scholar]
- Hecht E, Vogt TM, 1985. Marijuana smoking: effect on expired air carbon monoxide levels. Int. J. Addict 20, 353–361. 10.3109/10826088509044917 [DOI] [PubMed] [Google Scholar]
- Kristman-Valente AN, Hill KG, Epstein M, Kosterman R, Bailey JA, Steeger CM, Jones TM, Abbott RD, Johnson RM, Walker D, Hawkins JD, 2017. The Relationship Between Marijuana and Conventional Cigarette Smoking Behavior From Early Adolescence to Adulthood. Prev. Sci. Off. J. Soc. Prev. Res 18, 428–438. 10.1007/s11121-017-0774-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DC, Budney AJ, Brunette MF, Hughes JR, Etter J-F, Stanger C, 2014. Treatment models for targeting tobacco use during treatment for cannabis use disorder: Case series. Addict. Behav 39, 1224–1230. 10.1016/j.addbeh.2014.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DC, Walker DD, Hughes JR, Brunette MF, Scherer E, Stanger C, Etter J-F, Auty S, Budney AJ, 2019. Sequential and simultaneous treatment approaches to cannabis use disorder and tobacco use. J. Subst. Abuse Treat 98, 39–46. 10.1016/j.jsat.2018.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning K, Rogers AH, Bakhshaie J, Hogan JBD, Buckner JD, Ditre JW, Zvolensky MJ, 2018. The association between perceived distress tolerance and cannabis use problems, cannabis withdrawal symptoms, and self-efficacy for quitting cannabis: The explanatory role of pain-related affective distress. Addict. Behav 85, 1–7. 10.1016/j.addbeh.2018.05.009 [DOI] [PubMed] [Google Scholar]
- McClure EA, Baker NL, Sonne SC, Ghitza UE, Tomko RL, Montgomery L, Babalonis S, Terry GE, Gray KM, 2018a. Tobacco use during cannabis cessation: Use patterns and impact on abstinence in a National Drug Abuse Treatment Clinical Trials Network study. Drug Alcohol Depend. 192, 59–66. 10.1016/j.drugalcdep.2018.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure EA, Tomko RL, Salazar CA, Akbar SA, Squeglia LM, Herrmann E, Carpenter MJ, Peters EN, 2018b. Tobacco and cannabis co-use: Drug substitution, quit interest, and cessation preferences. Exp. Clin. Psychopharmacol 10.1037/pha0000244 [DOI] [PMC free article] [PubMed]
- Metrik J, Spillane NS, Leventhal AM, Kahler CW, 2011. Marijuana use and tobacco smoking cessation among heavy alcohol drinkers. Drug Alcohol Depend. 119, 194–200. 10.1016/j.drugalcdep.2011.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacek LR, Copeland J, Moeller SJ, in press. Trends in daily cannabis use among cigarette smokers in the United States, 2002–2014. Am. J. Public Health [DOI] [PMC free article] [PubMed]
- Patton GC, Coffey C, Carlin JB, Sawyer SM, Lynskey M, 2005. Reverse gateways? Frequent cannabis use as a predictor of tobacco initiation and nicotine dependence. Addiction 100, 1518–1525. 10.1111/j.1360-0443.2005.01220.x [DOI] [PubMed] [Google Scholar]
- Payne TJ, Smith PO, McCracken LM, McSherry WC, Antony MM, 1994. Assessing nicotine dependence: a comparison of the Fagerström Tolerance Questionnaire (FTQ) with the Fagerström Test for Nicotine Dependence (FTND) in a clinical sample. Addict. Behav 19, 307–317. [DOI] [PubMed] [Google Scholar]
- Penetar DM, Kouri EM, Gross MM, McCarthy EM, Rhee CK, Peters EN, Lukas SE, 2005. Transdermal nicotine alters some of marihuana’s effects in male and female volunteers. Drug Alcohol Depend. 79, 211–223. 10.1016/j.drugalcdep.2005.01.008 [DOI] [PubMed] [Google Scholar]
- Peters EN, Budney AJ, Carroll KM, 2012. Clinical correlates of co-occurring cannabis and tobacco use: a systematic review. Addiction 107, 1404–1417. 10.1111/j.13600443.2012.03843.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Bullen C, Krishnan-Sarin S, Rigotti NA, Steinberg ML, Streck JM, Joseph AM, 2019. Defining and measuring abstinence in clinical trials of smoking cessation interventions: An updated review. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob 10.1093/ntr/ntz110 [DOI] [PMC free article] [PubMed]
- Piper ME, Smith SS, Schlam TR, Fiore MC, Jorenby DE, Fraser D, Baker TB, 2009. A Randomized Placebo-Controlled Clinical Trial of 5 Smoking Cessation Pharmacotherapies. Arch. Gen. Psychiatry 66, 1253–1262. 10.1001/archgenpsychiatry.2009.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerleau CS, Carton SM, Lutzke ML, Flessland KA, Pomerleau OF, 1994. Reliability of the Fagerstrom Tolerance Questionnaire and the Fagerstrom Test for Nicotine Dependence. Addict. Behav 19, 33–39. [DOI] [PubMed] [Google Scholar]
- Prochaska JJ, Delucchi K, Hall SM, 2004. A Meta-Analysis of Smoking Cessation Interventions With Individuals in Substance Abuse Treatment or Recovery. J. Consult. Clin. Psychol 72, 1144–1156. 10.1037/0022-006X.72.6.1144 [DOI] [PubMed] [Google Scholar]
- Ream GL, Benoit E, Johnson BD, Dunlap E, 2008. Smoking tobacco along with marijuana increases symptoms of cannabis dependence. Drug Alcohol Depend. 95, 199–208. 10.1016/j.drugalcdep.2008.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche DJO, Bujarski S, Moallem NR, Guzman I, Shapiro JR, Ray LA, 2014. Predictors of smoking lapse in a human laboratory paradigm. Psychopharmacology (Berl.) 231, 2889–2897. 10.1007/s00213-014-3465-x [DOI] [PubMed] [Google Scholar]
- Rogers AH, Bakhshaie J, Garey L, Piasecki TM, Gallagher MW, Schmidt NB, Zvolensky MJ, 2019. Individual differences in emotion dysregulation and trajectory of withdrawal symptoms during a quit attempt among treatment-seeking smokers. Behav. Res. Ther 115, 4–11. 10.1016/j.brat.2018.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer GL, Berg CJ, Kegler MC, Donovan DM, Windle M, 2015. Assessing the overlap between tobacco and marijuana: Trends in patterns of co-use of tobacco and marijuana in adults from 2003–2012. Addict. Behav 49, 26–32. 10.1016/j.addbeh.2015.05.012 [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Raines AM, Allan NP, Zvolensky MJ, 2016. Anxiety sensitivity risk reduction in smokers: A randomized control trial examining effects on panic. Behav. Res. Ther 77, 138–146. 10.1016/j.brat.2015.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman SM, Paty JA, Gnys M, Kassel JA, Hickcox M, 1996. First lapses to smoking: withinsubjects analysis of real-time reports. J. Consult. Clin. Psychol 64, 366–379. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, 1992. Timeline Follow-Back, in: Litten RZ, Allen JP (Eds.), Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Humana Press, Totowa, NJ, pp. 41–72. 10.1007/978-1-4612-0357-5_3 [DOI] [Google Scholar]
- Stephens RS, Wertz JS, Roffman RA, 1995. Self-efficacy and marijuana cessation: a construct validity analysis. J. Consult. Clin. Psychol 63, 1022–1031. [DOI] [PubMed] [Google Scholar]
- Vandrey RG, Budney AJ, Hughes JR, Liguori A, 2008. A within-subject comparison of withdrawal symptoms during abstinence from cannabis, tobacco, and both substances. Drug Alcohol Depend. 92, 48–54. 10.1016/j.drugalcdep.2007.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger AH, Pacek LR, Wall MM, Zvolensky MJ, Copeland J, Galea S, Nahvi S, Moeller SJ, Hasin DS, Goodwin RD, 2018. Trends in cannabis use disorder by cigarette smoking status in the United States, 2002–2016. Drug Alcohol Depend. 191, 45–51. 10.1016/j.drugalcdep.2018.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Garey L, Allan NP, Farris SG, Raines AM, Smits JAJ, Kauffman BY, Manning K, Schmidt NB, 2018. Effects of anxiety sensitivity reduction on smoking abstinence: An analysis from a panic prevention program. J. Consult. Clin. Psychol 86, 474–485. 10.1037/ccp0000288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Lejuez CW, Kahler CW, Brown RA, 2004. Nonclinical panic attack history and smoking cessation: an initial examination. Addict. Behav 29, 825–830. 10.1016/j.addbeh.2004.02.017 [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Paulus DJ, Garey L, Raines AM, Businelle M, Shankman S, 2017. Anxiety sensitivity and nicotine replacement therapy side effects: Examining the role of emotion dysregulation among treatment-seeking smokers. J. Stud. Alcohol Drugs 78, 877–883. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Stewart SH, Vujanovic AA, Gavric D, Steeves D, 2009. Anxiety sensitivity and anxiety and depressive symptoms in the prediction of early smoking lapse and relapse during smoking cessation treatment. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob 11, 323–331. 10.1093/ntr/ntn037 [DOI] [PMC free article] [PubMed] [Google Scholar]