Abstract
The ability to isolate and subsequently culture mitotically active female germ cells from adult ovaries, referred to as either oogonial stem cells (OSCs) or adult female germline stem cells (aFGSCs), has provided a robust system to study female germ cell development under multiple experimental conditions, and in many species. Flow cytometry or fluorescence-activated cell sorting (FACS) is an integral part of many isolation and characterization protocols. Here, we provide methodological details for antibody-based flow cytometric isolation of OSCs using antibodies specific for external epitopes of the proteins Ddx4 or Ifitm3, alone or in combination with the use of fluorescent reporter mice. Beginning with sample preparation, we provide point-by-point instructions to guide researchers on how to isolate OSCs using flow cytometry.
Keywords: Female germline stem cell, Oogonial stem cell, Ovary, Flow cytometry
1. Introduction
The recent identification of a relatively rare population of mitotically active germline cells that have the ability to generate oocytes and viable offspring in adult mammalian females has led to multiple published reports from different laboratories detailing isolation strategies [1–10]. Of the described methods, two approaches using antibodies targeting the germline-expressed proteins, DEAD-box polypeptide 4 (Ddx4, also referred to as Vasa or mouse Vasa homolog) and interferon-induced transmembrane protein 3, Ifitm3—also referred to as Fragilis), have been demonstrated to successfully yield a live cell population that can be expanded ex vivo, spontaneously differentiates in vitro into immature oocytes, and generates fertilization competent eggs that give rise to live offspring following intraovarian transplantation [1, 4, 11–13]. Although both FACS and magnetic-assisted cell sorting (MACS) have been used for the isolation of OSCs [1, 4, 5, 10, 12–15], only flow cytometry offers the ability to perform characterizations based on cell size, viability [4, 5] and, if desired, multiple fluorescent parameters [2, 6].
OSCs represent a relatively rare population of ovarian cells, with estimates approximating 0.014 ± 0.002 % of the total ovarian cell population in mice [4]. Although OSCs can be expanded to large numbers in vitro, a small starting number of cells obtained after isolation is to be expected. The relative rarity and small size (between 5 and 10 μm, depending on species; [4, 5, 16]) can make OSCs difficult to sort, and so additional considerations such as setting a size gate, inclusion of a viability marker, and proper secondary antibody and IgG controls are essential for successful isolation. Additionally, tissue dissociation, sample preparation, and primary antibody selection are important factors that, when not performed properly, can lead to atypical results.
Regarding antibody selection, two proteins have been reported to have cell-surface epitopes suitable for antibody-based live cell isolation: Ddx4 and Ifitm3 [1, 3–5, 15–17]. While the initial report describing Ddx4 in ovarian tissues designated expression as cytosolic [18], experimental evidence has since verified specific localization of the C-terminus on the surface of OSCs using multiple approaches [1, 4]. Alternatively, Ifitm3 is expressed in OSCs [3], contains a well-characterized extracellular N-terminus [19], and has been used to isolate functional OSCs in mice and rats [3, 11–13]. However, two caveats to the potential use of Ifitm3 as a cell-surface target for purification of OSCs lead us to recommend anti-Ddx4 as the antibody of choice for OSC isolation. First, Ifitm3 protein expression is not restricted to germline cells (http://www.protein-atlas.org/), and thus its utility for isolation of only OSCs is questionable. Second, while the C-terminus of Ddx4 is highly conserved among species (Fig. 1), and therefore a single antibody will work for multiple animal models, the Ifitm3 amino acid sequence that encodes for the extracellular N-terminus is poorly conserved (Fig. 1). This then requires sequence analysis and species-specific antibodies, increasing time and cost. Nonetheless, when applied to dispersed ovarian tissue, antibodies targeting Ifitm3 will successfully yield OSCs following FACS (although we caution that purity may be compromised), and so we have elected to include instruction and examples using both antibody-based FACS strategies here.
Fig. 1.
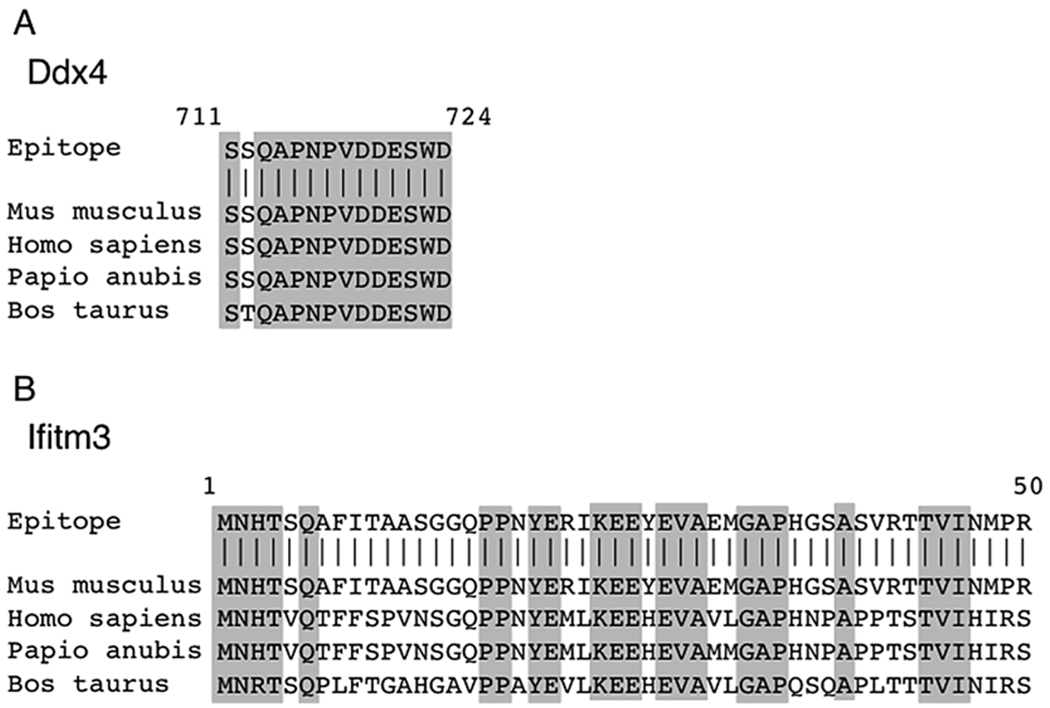
Protein alignments demonstrate conserved peptides in Ddx4 or Ifitm3 extracellular domains recognized by anti-Ddx4 (Abcam #ab13840) or Ifitm3 (Abcam #ab15592) antibodies. (a) Protein alignment of the C-terminus of Ddx4 between mouse (mus musculus), human (homo sapiens), baboon (papio anubis), and bovine (bos taurus). (b) Protein alignment of the N-terminus of Ifitm3 between mouse (mus musculus), human (homo sapiens), baboon (papio anubis), and bovine (bos taurus). Gray boxes, conserved residues across species
Another important factor to consider when attempting isolation of OSCs is the proper preparation of ovarian tissue into a single-cell suspension prior to antibody labeling. Collagenase type IV, which contains no tryptic activity, is utilized to effectively release cells from tissue [5]. Use of trypsin is not recommended, as multiple trypsin digest sites exist within the extracellular C-terminus of Ddx4 and N-terminus of Ifitm3 (Fig. 2). Accordingly, inclusion of trypsin leads to a high probability of cleaving the antigen required for antibody-based FACS isolation. Additionally, overdigestion or incomplete neutralization of collagenase type IV results in loss of viability, and as a result can lead to promiscuous (nonspecific) antibody binding.
Fig. 2.
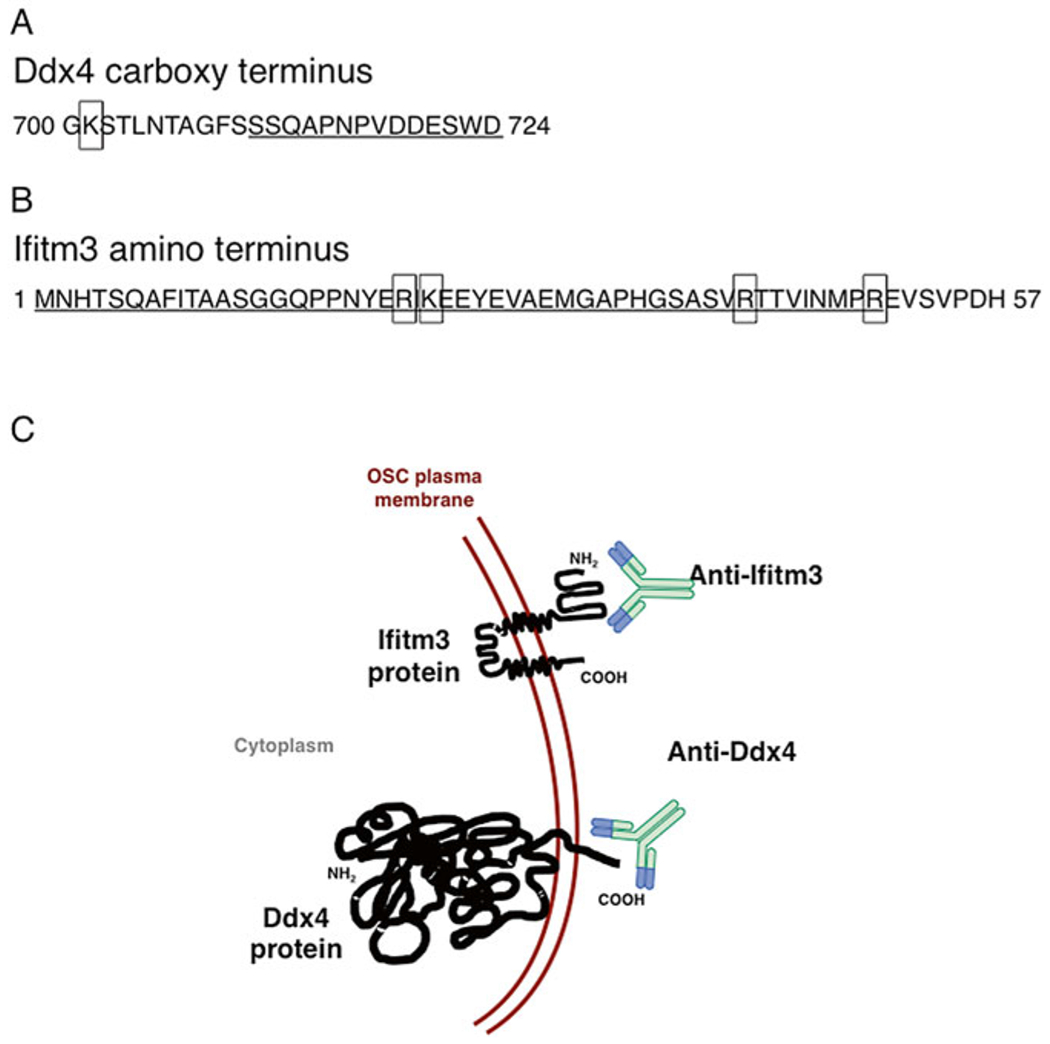
Trypsin digest sites within the extracellular mouse Ddx4 ((a), boxes) and Ifitm3 ((b), boxes) antibody recognition sites (underlined). (c) Schematic of anti-Ddx4 and anti-Ifitm3 recognition sites localized on the membranes of OSCs
Finally, FACS-based OSC isolation protocols allow for detection and analysis of multiple fluorescent parameters in a single sample, as we have previously demonstrated using Ddx4-Cre;Rosa26tdTm/+ mouse ovaries. These mice express restricted tomato red (tdTm) gene expression in cells in which the Ddx4 gene promoter has been activated. We have previously demonstrated by flow cytometry that dispersed ovaries from these animals yield a large population of non-germline tdTm-positive cells [6], indicative of promoter “leakiness” in the Ddx4-Cre mouse line. Nonetheless, a small subset of these tdTm-positive cells exhibit externalized expression of Ddx4 protein, consistent with their identification as OSCs [6]. Accordingly, genetic reporter mouse lines coupled with antibody-based FACS strategies can be useful tools for downstream studies and applications focussed on OSC biology and function.
The following protocol describes in detail a procedure to successfully identify and isolate OSCs by antibody-based FACS, using adult mouse ovarian tissue as an example. All steps are included for sample preparation and enzymatic tissue dissociation, antibody-labeling, fluorescent controls, and gating strategies for flow cytometry.
2. Equipment
Laminar flow hood.
Analytical balance.
Heated orbital shaker.
CO2 asphyxiation chamber.
Dissecting microscope.
Swinging bucket centrifuge.
Fixed angle bench-top centrifuge.
37°C bead or water bath.
Assorted calibrated pipettes.
Pipette aid.
Laboratory timer.
Fluorescence-activated cell sorter with 100 μm or 70 μm nozzle and FACS software (FACS Diva 8.0.1).
GentleMACS tissue dissociator (Miltenyi Biotech #130-093-235).
3. Materials
5 C57Bl/6 mice, 6 weeks of age (Charles River Laboratories/Jackson Laboratory).
Surgical scissors.
Surgical forceps.
#9 single edge utility 2” razor blades.
No. 10 sterile scalpel.
60 mm sterile tissue culture-treated dishes.
BD Luer-Lok 10 ml syringe.
0.2 μm PES syringe filter.
Pyrex 7740 glass petri dish (Corning #3160101).
50 ml polypropylene conical centrifuge tubes.
5 ml borosilicate glass sterile disposable serological pipette.
Cell strainer, 70 μm nylon mesh (BD Falcon #352350).
Kimwipes.
15 ml polypropylene conical centrifuge tubes.
12 × 75 mm 5 ml round bottom polypropylene tubes with 35 μm cell strainer cap (BD Falcon #352235).
Steriflip 50 ml sterile disposable vacuum 0.22 μm filter unit.
Nalgene 500 ml sterile disposable filter unit with 0.2 μm PES membrane.
24-well sterile tissue culture-treated plate.
Laboratory tape.
GentleMACS C-tube (Miltenyi Biotech #130-093-237).
3.1. Chemicals/Reagents
Flow Cytometry Size Calibration Kit (Life Technologies #F13838).
0.15 M sodium chloride.
70% ethanol.
Hanks Balanced Salt Solution (HBSS) with MgCl2 and CaCl2.
HBSS without MgCl2 and CaCl2.
Collagenase type IV (Worthington #CLS-4).
DNase I (Sigma #D4263).
IgG and protease-free bovine serum albumin (BSA).
Normal goat serum (NGS).
Viability dye (SYTOX blue, Life Technologies #S34857; 7-Amino-actinomycin D (7AAD), BD #555816; 4′,6-Diamidino-2-phenylindole dihydrochloride (DAPI), Sigma D9542; Propidium Iodide (PI), Life Technologies #P3566).
Rabbit IgG (Life Technologies #10500C).
Rabbit anti-DDX4 Antibody (Abcam #ab13840) or rabbit anti-Ifitm3 Antibody (Abcam #ab15592).
Fluorophore microscale protein labeling kit of choice; recommend Alexa Fluor 488 microscale protein labeling kit (Life Technologies #A30006).
1 M HEPES buffer for cell culture.
Phosphate buffered saline, no calcium, no magnesium (PBS).
10 mM Tris pH 7.6.
MEMαGlutaMax (Life Technologies #32561-102).
Qualified fetal bovine serum (FBS), one shot (Life Technologies).
Sodium pyruvate.
MEM Non-Essential Amino Acids Solution (NEAA).
2-Mercaptoethanol (Sigma #M3148).
Leukemia inhibitory factor (LIF, ESGRO©, Milipore #ESG1106).
N-2 MAX Media Supplement (R&D Systems #AR009).
Recombinant human epidermal growth factor (EGF, Life Technologies #PHG0311).
Recombinant human glial cell line-derived neurotrophic factor (GDNF, R&D Systems #212GD050).
Recombinant human basic fibroblast growth factor (bFGF, Life Technologies #13256-029).
Penicillin-streptomycin-glutamine (PSG).
3.2. Reagent Preparation
Using the microscale protein labeling kit, conjugate primary antibody to selected fluorophore according to the manufacturer’s protocol.
DNase I: 6 U/μl (see Note 1). Store at −20°C for up to 3 months.
Mouse dissociation buffer: 800 U/ml collagenase type IV, 6 U/ml DNase I in HBSS with MgCl2 and CaCl2 (see Note 2).
Blocking buffer: 2% BSA, 2% NGS HBSS without MgCl2 and CaCl2 (see Note 3).
FACS buffer: 25 mM HEPES, 2% BSA in HBSS without MgCl2 and CaCl2 (see Note 4).
Recombinant human EGF: 0.1 mg/ml (see Note 5). Store at −20°C.
Recombinant human GDNF: 0.1 mg/ml (see Note 6). Store at −20°C.
Recombinant human bFGF: 0.1 mg/ml (see Note 7). Store at −20°C.
OSC culture medium: 10% FBS, 1× PSG, 1 mM sodium pyruvate, 0.1 mM NEAA, 1× N-2 Max, 0.1 mM 2-mercaptoethanol, 103 Units LIF, 0.01 μg/ml EGF, 0.04 μg/ml GDNF, 0.001 μg/ml bFGF in MEMαGlutaMax (see Note 8).
Dissociation buffer: 400 U/ml collagenase type IV, 6 U/ml DNase I in HBSS with MgCl2 and CaCl2 (see Note 9).
4. Methods
4.1. Mouse Dissection Procedure
Carbon dioxide asphyxiate and cervically dislocate five mice.
Spray mice with 70% ethanol to minimize hair sticking to surgical instruments.
With the mouse supine, hold the lower abdominal skin with surgical forceps and make a small incision using surgical scissors into the lower abdominal region, through the peritoneal cavity.
Enlarge the incision medial and lateral to expose the abdominal region. To expose the uterus flip the intestines proximal and the fat pad distal so that each is out of the field of view.
Moving proximal up the uterus, the oviduct attached to the ovary and ovarian fat pad will be visible.
Remove the ovary and place in a 60 mm tissue culture dish containing HBSS with MgCl2 and CaCl2.
Using forceps and a scalpel remove any fat and oviduct from the ovary. Combine and place all cleaned ovaries (10 total) in a new 60 mm tissue culture dish containing HBSS with MgCl2 and CaCl2.
4.2. Mouse Ovary Dissociation Procedure
Transfer ovaries to a glass petri dish with 50 μl of prewarmed dissociation buffer (800 U/ml collagenase type IV with 6 U/ml DNase I in HBSS with MgCl2 and CaCl2).
Mince ovaries in a scissoring motion between two razor blades for 2–7 min until a slurry has formed.
Transfer ovarian slurry to a 50 ml conical tube containing dissociation buffer, rinsing petri dish as needed with dissociation buffer to collect maximum amount of ovary sample.
Affix tube horizontally to the heated orbital shaker surface with laboratory tape and shake tube at 225 rpm at 37°C for 15 min.
Pipette solution ten times with 5 ml glass serological pipette (Note: do not use plastic serological pipettes, as these can damage cells).
Shake tube at 225 rpm at 37°C for 15 min.
Pipette solution ten times with 5 ml glass serological pipette.
Shake tube at 225 rpm at 37°C for 15 min.
Pipette solution ten times with 5 ml glass serological pipette.
Filter solution through a 70 μm cell strainer into a fresh 50 ml conical tube.
Rinse the original 50 ml tube with 5 ml HBSS without MgCl2 and CaCl2 and filter through same 70 μm nylon mesh cell strainer into same new 50 ml tube as sample.
Centrifuge the cell suspension at 600 ×g for 5 min, maximum acceleration, minimum brake in a swinging bucket centrifuge.
Decant and discard the supernatant, blot the top of the tube with a Kimwipe.
4.3. Antibody Labeling
Resuspend the cell pellet (see section 4.2, step 13) with 0.5 ml of blocking buffer (2% BSA, 2% NGS in HBSS without MgCl2 and CaCl2).
Incubate cells in blocking buffer at room temperature for 20 min.
Set up the following controls and samples in separate 15 ml conical tubes:
Incubate controls and samples (see step 3) in 15 ml conical tubes for 20 min at room temperature.
Add 5 ml of HBSS without MgCl2 and CaCl2 to each 15 ml conical tube to wash.
Centrifuge at 600 × g for 5 min at room temperature with maximum acceleration and brake in a swinging bucket centrifuge.
Decant supernatants and resuspend samples/controls in 0.3–0.5 ml FACS buffer (25 mM HEPES pH 7.0 and 2 % BSA in HBSS without MgCl2 and CaCl2) and strain into 5 ml round bottom tubes with 35 μm cell strainer caps).
Set up flow cytometer, perform any necessary calibration, and determine the drop delay for sorting collection.
-
Set the forward- (FSC) and side- (SSC) scatter parameters in linear scale.
FSC: Size; larger cells have greater FSC.
SSC: complexity/granularity; “smoother” or less granular cells will have a lower SSC.
Prepare the flow cytometry size calibration kit (see Note 10). Run the microspheres and set the gate on the FSC/SSC dot plot to include all objects in the 2–15 μm range of the agranular objects (Fig. 3). OSC diameter varies across species, murine OSCs are ≤10 μm.
Run unstained control and adjust voltages as needed.
Depending on the fluorophores selected, multicolor experiments require spillover correction between fluorochromes. Set up compensation as needed.
Use a viability dye to distinguish live/dead events. Depending on the fluorophore of your secondary antibody utilize SYTOX Blue at a final concentration of 1 μM, 7AAD at 10 μl per sample, propidium iodide at a final concentration of 1 μg/ml, or DAPI as described in [5]. Dead cells can bind antibodies non-specifically leading to atypical results. It is important to minimize the dead cells in the analysis. Add viability dye to the viability dye-only tube, IgG control tube, and stained sample tube. Run the viability dye control and confirm the voltages from step 31.
Run IgG control and prepare a gating strategy to visualize a minimum of three plots: FSC/SSC (Fig. 4a, e), SSC/viability (Fig. 4b, f), and viable SSC/antigen (Fig. 4c, g or d, h).
Run the samples and include agranular objects 2–15 μm in size within the gating strategy.
Downstream of the nozzle, droplets detach from the flow cytometer stream. The drop delay is the time between a particle being interrogated by the laser and reaching the droplet break-off point. To ensure the drop collected contains the particle of interest, the drop delay must be set on the instrument.
With the cytometer sample chiller off, run stained sample using the gating strategy in step 34 (Fig. 4). Collect events of interest in microcentrifuge tube(s) containing 0.5 ml of prewarmed OSC culture medium.
Following event collection, centrifuge the microcentrifuge tubes at 800 × g for 5 min in a fixed angle centrifuge at room temperature. Carefully remove the supernatant and proceed to downstream application. If subculture and expansion of cell numbers is desired, resuspend the pellet, which may not be visible to the eye due to the small diameter and small number of cells collected, in 0.5 ml fresh prewarmed OSC culture medium and seed into one well of a 24-well tissue culture-treated plate. Culture cells in a humidified incubator at 37°C with 5% CO2 as described [5].
Fig. 3.
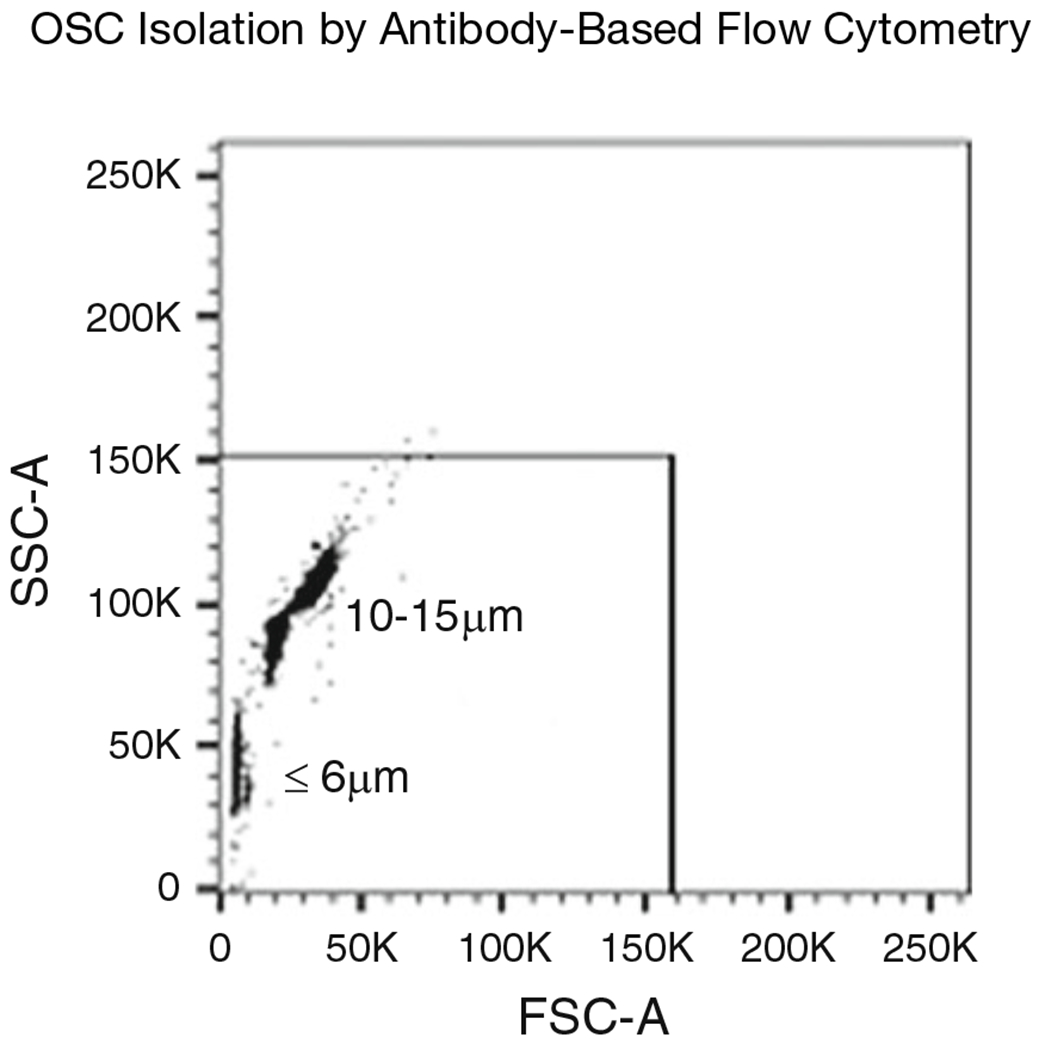
Size gating strategy using calibrated beads 2–15 μm in diameter. Size gate using forward scatter (FSC-A) and side scatter (SSC-A) parameters is set to include all events in the size range
Fig. 4.
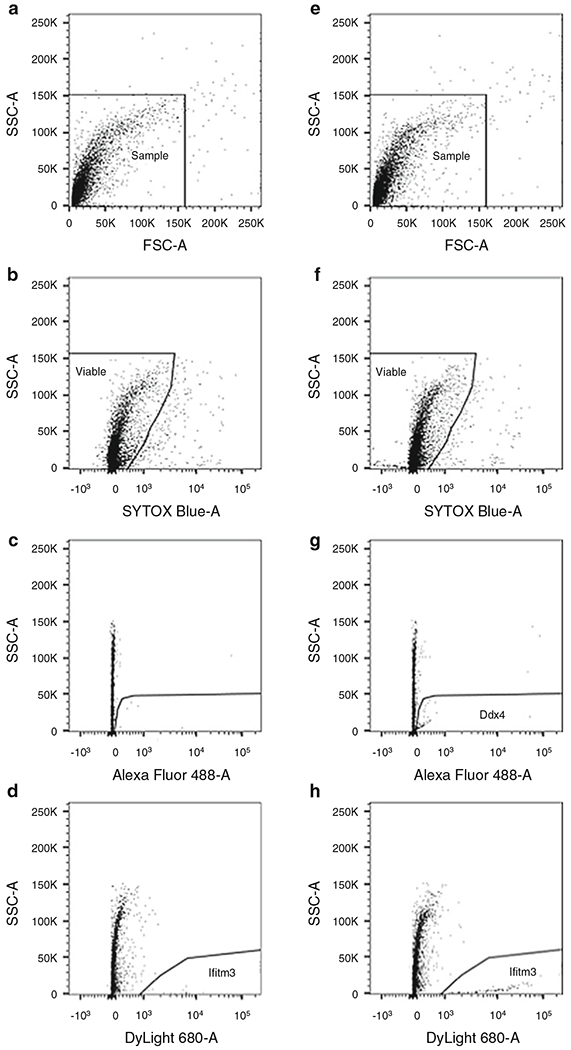
Gating strategy for sorting murine OSCs by FACS. Ovarian dispersates were prepared from 6-week-old mouse ovaries and labeled with Ddx4-AlexaFluor-488 and Ifitm3-DyLight-680. Rabbit IgG controls (fluorescent-labeled IgG; (a–d)) and antibody-labeled samples (e–h) are shown. (a)/(e). Dispersed mouse ovary is visualized by dot plot showing FSC vs. SSC signals, which are related to the size and complexity/granularity of the cell, respectively. Based on size calibration shown in Fig. 3, agranular, small events ranging from 1 to 15 μm are included in gate. (b)/(f). Events from gated region in (a)/(e) are further analyzed for SYTOX Blue to exclude nonviable events. Events from gated region in (b)/(f) are subsequently distinguished based on IgG binding (c)/(d), Ddx4 expression (g) or Ifitm3 expression (h). Samples were run using a 100 μm nozzle at 20 psi
| Control/sample | Cells (see step 2) | IgG or primary antibody |
|---|---|---|
| Unstained | 50 μl | |
| Viability dye | 50 μl | |
| Rabbit IgG control | 100 μl | 3.3 μg fluorophore-conjugated rabbit IgG |
| Stained sample | 300 μl | 10 μg fluorophore-conjugated rabbit anti-Ddx4 or rabbit anti-Ifitm3 |
4.4. Dissociation Procedure:Other Mammalian Species
For isolation of OSCs from species with larger ovaries (e.g., human, cow, non-human primate), we recommend using a dissociation instrument, such as the GentleMACS tissue dissociator. Harvest only the cortex of the ovary and proceed stepwise through the following protocol:
Prepare dissociation buffer (400 U/ml collagenase type IV with 6 U/ml DNase I in HBSS with MgCl2 and CaCl2; see Note 9) and prewarm the solution to 37 °C.
Using a scalpel, cut the ovarian cortex into ~2 × 2 mm pieces. Do not cut the pieces smaller than 2 × 2 mm as they will be too small for the GentleMACS. Do not cut the pieces larger than 2 × 2 mm as they will be too large and can jam the GentleMACS.
Transfer pieces of ovarian cortex to a GentleMACS C-tube with 5 ml of prewarmed dissociation buffer.
Load the GentleMACS C-tube into the GentleMACS tissue dissociator.
Run GentleMACS pre-programmed protocol for Human Tumor 1. If any pieces of tissue become trapped in the C-tube mechanism use forceps to return tissue to the dissociation buffer.
Affix tube horizontally to the heated orbital shaker surface with laboratory tape and shake tube at 225 rpm at 37°C for 15 min.
Pipette solution ten times with 5 ml glass serological pipette.
Run GentleMACS pre-programmed protocol for Human Tumor 2. If any pieces of tissue have become trapped in the C-tube mechanism use forceps to return tissue to the dissociation buffer.
Shake tube at 225 rpm at 37 °C for 15 min.
Pipette solution ten times with 5 ml glass serological pipette.
Run GentleMACS pre-programmed protocol for Human Tumor 3. If any pieces of tissue have become trapped in the C-tube mechanism use forceps to return tissue to the dissociation buffer.
Shake tube at 225 rpm at 37°C for 15 min.
Pipette solution ten times with 5 ml glass serological pipette.
Continue at step 10 from mouse ovary protocol (see section 4.2).
4.5. Antibody Labeling with Ddx4-Cre;Rosa26tdTm/+ Mice
The reporter mouse model Ddx4-Cre;Rosa26tdTm/+, which can exhibit leaky expression of the promoter in nongermline cells [6], can be utilized in combination with antibody-based FACS to successfully isolate OSCs.
Harvest, prepare, and stain mouse ovarian dispersates as described in mouse protocol above (mouse dissection steps 1–7, mouse ovary dissociation steps 8–20, antibody labeling steps 21–29). We recommend allophycocyanin (APC) for the Ddx4 secondary fluorophore (antibody labeling step 23).
Prepare the flow cytometry size calibration kit (see Note 10). Run the microspheres and set the gate on the FSC/SSC dot plot to include all objects in the 2–15 μm range of the agranular objects (as demonstrated in Fig. 3). OSC diameter varies across species, murine OSCs are ≤10 μm.
Run unstained control and adjust voltages as needed.
Dead cells bind antibodies nonspecifically, and each antibody can bind differently. Therefore, every attempt must be made to minimize unintended detection of dead cells in the analysis. Use a viability dye to distinguish live/dead cell events. For this experiment, tomato red expression will be visualized with a phycoerythrin (PE) filter and Ddx4 with an APC filter. Therefore, the recommended viability dyes are SYTOX Blue at a final concentration of 1 μM or DAPI as described in [5]. Add viability dye to viability dye-only sample, control sample, and stained sample. Run viability dye control and confirm voltages from step 3.
Run negative control and prepare a gating strategy to visualize a minimum of four plots: FSC/SSC (Fig. 5a/f), SSC/viability (Fig. 5b/g), viable SSC/Tomato (PE, Fig. 5c/h), and viable Tomato + (PE+) SSC/Ddx4 (APC) (Fig. 5d/i or e/j).
Run the samples and include agranular objects 2–15 μm in size within the gating strategy.
Downstream of the nozzle, droplets detach from the flow cytometer stream. The drop delay is the time between a particle being interrogated by the laser and reaching the droplet break-off point. To ensure the drop collected contains the particle of interest, the drop delay on the instrument must be set.
With the cytometer sample chiller off, run Ddx4 stained sample using the gating strategy set up in step 5 (Fig. 5) and collect events of interest in microfuge tubes containing 0.5 ml of prewarmed OSC culture medium.
Fig. 5.
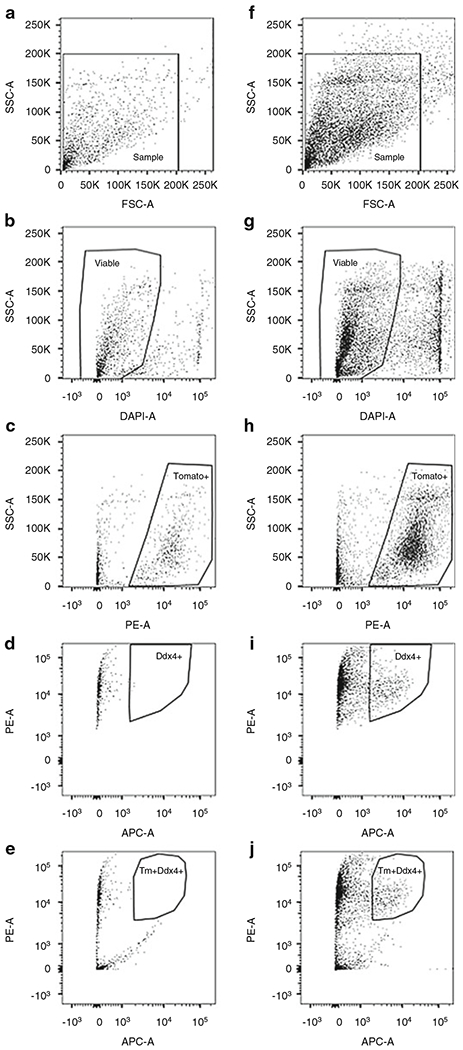
Gating strategy for sorting murine OSCs by FACS from Ddx4-Cre;Rosa26tdTm/+ mice. OSCs were isolated from 8-week-old Ddx4-Cre;Rosa26tdTm/+ mouse ovaries and stained with Ddx4-APC. Non-antibody (IgG) control (a–e) and labeled sample (f–j) are shown. (a)/(f). Dispersed mouse ovary is visualized by dot plot showing FSC vs. SSC signals, which are related to the size and complexity/granularity of the cells, respectively. Agranular, small events ranging from 2 to 15 μm are included in gate. (b)/(g). Events from gated region in (a)/(f) are further analyzed for DAPI to exclude nonviable cells. Events from gated region in (b)/(g) is subsequently distinguished based on tomato red (Tm) expression into Tomato− and Tomato+ events (c)/(h). Tomato + events are further analyzed for non-specific secondary antibody binding (d) and Ddx4 expression (i). Alternatively, events from the gated region in (b)/(g) can be distinguished based on Tomato+/nonspecific antibody binding (e) and Tomato+/Ddx4+ expression (j). Samples were run using a 70 μm nozzle at 70 psi
Following event collection, centrifuge the microfuge tubes at 800 ×g for 5 min in a fixed angle centrifuge at room temperature. Carefully remove the supernatant. Resuspend the pellet, which may not be visible due to the small number and diameter of the cells collected, in 0.5 ml fresh prewarmed OSC culture medium and seed into one well of a 24-well tissue culture treated plate. Culture cells in a humidified incubator at 37 °C with 5 % CO2 as described [5].
5 Notes
Reconstitute one vial of 2000 U DNase I with 333.3 μl of sterile 0.15 M sodium chloride to achieve a 6 U/μl solution of DNase I. Aliquot and store at −20 °C for up to 3 months.
Dissolve collagenase type IV in 10 ml of HBSS with MgCl2 and CaCl2 to a concentration of 800 U/ml. Syringe filter into a sterile 50 ml polypropylene conical tube. Add 10 μl of 6 U/μl DNase I to bring the solution to 6 U/ml DNase I. Place in 37 °C water or bead bath to warm prior to use on tissue.
Dissolve 0.5 g IgG and protease-free BSA in 24.5 ml HBSS without MgCl2 and CaCl2, add 0.5 ml NGS and filter-sterilize using a 0.2 μm filter.
Dissolve 0.5 g IgG and protease-free BSA in 24.375 ml HBSS without MgCl2 and CaCl2, add 625 μl of 1 M HEPES and filter-sterilize using a 0.2 μm filter.
Dissolve 100 μg EGF in 1 ml sterile PBS for a concentration of 0.1 mg/ml. Aliquot and store at −20 °C.
Dissolve 50 μg GDNF in 0.5 ml sterile PBS with 0.1 % IgG and protease-free BSA for a final concentration of 0.1 mg/ml. Aliquot and store at −20 °C.
Dissolve 10 μg bFGF in 0.1 ml sterile-filtered 10 mM Tris pH 7.6 for a final concentration of 0.1 mg/ml. Aliquot and store at −20 °C.
Prepare OSC culture medium using the following recipe: 430 ml MEMαGlutaMax, 50 ml FBS, 5 ml of 100× PSG, 5 ml of 100 mM sodium pyruvate, 5 ml of 10 mM NEAA, 5 ml of 100× N-2 Max, 3.5 μl of 14.3 M2-mercaptoethanol, 0.5 ml of 106 Units LIF, 50 μl of 0.1 mg/ml EGF, 200 μl of 0.1 mg/ml GDNF, 5 μl of 0.1 mg/ml bFGF. Filter-sterilize through 0.22 μm PES filter. Store at 4 °C for 2 weeks or −80 °C for 3 months.
Dissolve collagenase type IV in 10 ml of HBSS with MgCl2 and CaCl2 to a concentration of 400 U/ml. Syringe filter into a sterile 50 ml polypropylene conical tube. Add 10 μl of 6 U/μl DNase I to bring the solution to 6 U/ml DNase I. Place in 37 °C water or bead bath to warm prior to use on tissue.
Mix the flow cytometry size calibration kit microsphere suspensions and add 20 μl of each size suspension to 0.5 ml flow cytometer sheath fluid and gently vortex.
References
- 1.Zou K et al. (2009) Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat Cell Biol 11:631–636 [DOI] [PubMed] [Google Scholar]
- 2.Pacchiarotti J et al. (2010) Differentiation potential of germ line stem cells derived from the postnatal mouse ovary. Differentiation 79:159–170 [DOI] [PubMed] [Google Scholar]
- 3.Zou K et al. (2011) Improved efficiency of female germline stem cell purification using Fragilis-based magnetic bead sorting. Stem Cells Dev 20:2197–2204 [DOI] [PubMed] [Google Scholar]
- 4.White YAR et al. (2012) Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat Med 18:413–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woods DC, Tilly JL (2013) Isolation, characterization and propagation of mitotically active germ cells from adult mouse and human ovaries. Nat Protoc 8:966–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park ES, Tilly JL (2015) Use of DEAD-box polypeptide 4 (Ddx4) gene promoter-driven fluorescent reporter mice to identify mitotically active germ cells in postnatal mouse ovaries. Mol Hum Reprod 21:58–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu Z et al. (2016) Improvement in isolation and identification of mouse oogonial stem cells. Stem Cells Int 2016:2749461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bai Y et al. (2013) Localization and characterization of female germ line stem cells (FGSCs) in juvenile porcine ovary. Cell Prolif 46: 516–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo K et al. (2016) Germ stem cells are active in postnatal mouse ovary under physiological conditions. Mol Hum Reprod. doi: 10.1093/molehr/gaw015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fakih MH et al. (2015) The AUGMENT treatment: physician reported outcomes of the initial global patient experience. JFIV Reprod Med Genet 3:154. [Google Scholar]
- 11.Zhang Y et al. (2011) Production of transgenic mice by random recombination of targeted genes in female germline stem cells. J Mol Cell Biol 3:132–141 [DOI] [PubMed] [Google Scholar]
- 12.Zhou L et al. (2014) Production of fat-1 transgenic rats using a post-natal female germline stem cell line. Mol Hum Reprod 20:271–281 [DOI] [PubMed] [Google Scholar]
- 13.Xiong J et al. (2015) Intraovarian transplantation of female germline stem cells rescue ovarian function in chemotherapy-injured ovaries. PLoS One 10:e0139824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie W, Wang H, Wu J (2014) Similar morphological and molecular signatures shared by female and male germline stem cells. Sci Rep 4:5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khosravi-Farsani S et al. (2015) Isolation and enrichment of mouse female germ line stem cells. Cell J 16:406–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woods DC, Tilly JL (2015) Reply to adult human and mouse ovaries lack DDX4-expressing functional oogonial stem cells. Nat Med 21:1118–1121 [DOI] [PubMed] [Google Scholar]
- 17.Terraciano P et al. (2014) Cell therapy for chemically induced ovarian failure in mice. Stem Cells Int 2014:720753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noce T, Okamoto-Ito S, Tsunekawa N (2001) Vasa homolog genes in mammalian germ cell development. Cell Struct Funct 26:131–136 [DOI] [PubMed] [Google Scholar]
- 19.Yount JS et al. (2012) Palmitoylome profiling reveals S-palmitoylation-dependent antiviral activity of IFITM3. Nat Chem Biol 6:610–614 [DOI] [PMC free article] [PubMed] [Google Scholar]


