Abstract
The ciliate Balantioides coli is a human enteric parasite that can cause life-threatening infections. It is a food- and waterborne parasite, with cysts being the infective stage. Despite its importance as a potential pathogen, few reports have investigated its presence in environmental samples, and some issues need attention including i) The accuracy of B. coli identification. In most cases, the protozoa is identified only by its morphological traits, which can be identical to those from other parasitic ciliates of animals. Genetic analysis of cysts recovered from environmental samples is necessary for species confirmation. In addition, genetic methods used with faecal samples need to be adequately validated with environmental matrices. ii) The methodology for searching this parasite in environmental samples. The protocols include an initial phase to isolate the cysts from the matrix followed by a second phase in which concentration procedures are usually applied. The methods may be valid but are not standardised and differences between studies could affect the results obtained. iii) The areas that needs further research. The development of genetic identification methods and standardised analytical protocols in environmental samples are required, as well as the assessment of viability and infectivity of B. coli cysts. The development of axenic culture systems will boost research on this parasite.
Keywords: Balantioides coli, Cyst identification, Environmental matrices, Future research needs
Highligths
-
•
Balantioides coli is mainly considered a foodborne parasite for humans.
-
•
Detection methods in environmental samples are not standardised.
-
•
Correct identification should be made by genetic analysis.
-
•
Methods for B. coli cyst viability and infectivity assessment are to be developed.
1. Introduction
The ciliate Balantioides coli (syn. Balantidium coli) is the largest human parasitic protozoa, and can infect a wide range of hosts, with swine being the main reservoir. The parasite has a direct life cycle, with a vegetative form, the trophozoite, inhabiting the host intestine, and a resting cyst that is released into the environment in faeces. Transmission is via the faecal-oral route through contaminated food and water. Only cysts are considered capable of infecting new hosts, although it has been suggested that trophozoites might also be infective (Pomajbíková et al., 2010).
Infected hosts may be asymptomatic or develop intestinal disorders and even life-threatening colitis (Ponce-Gordo and García-Rodríguez, 2021). People living in contact with pigs, or in areas with inadequate sanitary infrastructures, are at higher risk of infection (Ponce-Gordo and García-Rodríguez, 2021; Ponce-Gordo and Jirků-Pomajbíková, 2017). Balantioides coli is currently considered as an emerging pathogen and is included among the foodborne parasites that should be prioritised for the development of general control guidelines (Bouwknegt et al., 2018; Food and Agriculture Organization of the United Nations and World Health Organization, 2014). However, outbreaks caused by this parasite are rare, as only three have been documented in the last 100 years (Ponce-Gordo and García-Rodríguez, 2021) and balantidiosis is regarded as a zoonosis of limited public health importance in developed countries. Probably because of this, there is a lack of research aimed at investigating the potential role of B. coli as water- or foodborne pathogen (Robertson, 2014), with other diarrhoea-causing protozoa such as such as Cryptosporidium spp. or Giardia duodenalis attracting more attention from a publich health perspective. Routine monitoring for the identification of B. coli in water samples has been considered superfluous in developed countries (Ericksen and Dufour, 1986); however, its importance should not be disregarded, as fatal cases in humans and animals due to this parasite have been reported (Gomez Hinojosa et al., 2019; Viezzer Bianchi et al., 2019).
The development of methods for the detection and determination of the viability of infective stages of pathogens in the environment provides important tools for the identification and understanding of transmission routes, for determining the importance of matrices (soil, water, food) involved in transmission, and for assessing the risk of infection (Smith, 1998). This is especially important for foodborne parasites, as international commerce has now resulted in a global market in which contaminated food from developing countries can be traded in developed ones (Cork and Checkley, 2011). In the case of B. coli, data on its presence and viability in the environment are scarce, and the methodology for detecting and assessing the risk of infection in these samples is not standardised. These issues are discussed in this review.
2. Diagnosis
Trophozoites and cysts of B. coli are large and easily seen under optical microscopy at 10-40× magnification. Vegetative cells usually move slowly in a helical fashion; they are ovoid or pear-shaped, up to 150 μm in length, with cilia covering the entire cell surface. A mouth-like opening, the cytostome, is located in a short depression, or vestibule, situated subapically. Cysts are spherical or slightly ovoid, about 50 μm in diameter and contain a single cell. In both trophozoites and cysts there is an elongated or bean-shape macronucleus; a small micronucleus usually overlaps the macronucleus and, in most cases, is not observable. Detailed morphological descriptions can be found in McDonald (1922) and da Silva Barbosa et al. (2018b).
Microscopy-based studies have identified B. coli in humans, several non-human primates, swine, bovines, camels, equids, elephants, rodents and birds (ostriches and rheas) (Ponce-Gordo and García-Rodríguez, 2021; Ponce-Gordo and Jirků-Pomajbíková, 2017). However, diagnosis based on microscopy examination of the cyst morphology alone could be erroneous in some cases, as morphologically indistinguishable cysts of other ciliate species have been identified in non-human primates and bovids. In samples in which compatible cysts/trophozoites were found, B. coli has been confirmed by genetic analysis in humans, chimpanzee, bonobo, gorilla, rhesus macaques, hamadryas baboon, pigs and wild boars, sheep, elephants, guinea pigs, ostriches and rheas (Ponce-Gordo and García-Rodríguez, 2021). In rhesus macaques, hamadryas baboon, agile mangabey and collared mangabey, an unnamed Buxtonella-like species has been identified (Pomajbíková et al., 2013; Yan et al., 2018). In cattle and buffalo, there are numerous reports of B. coli (based on the finding of cysts), but to date, only Buxtonella sulcata have been identified by genetic analysis (Adhikari et al., 2013; Grim et al., 2015; Pomajbíková et al., 2013). The trophozoites of the latter species can be easily differentiated from those of B. coli due to the existence of a long vestibule extending the full length of the cell (Grim et al., 2015).
These results indicate that morphological identification of cysts in a faecal sample as of B. coli might be erroneous, at least in some hosts. The problem is compounded when considering identification of the parasite in an environmental sample, as trophozoites and resting cysts of other ciliates could be concomitantly present. Despite the increasing number of publications on the morphology and physiology of ciliate cysts (Benčaťová and Tirjaková, 2017; Gutiérrez et al., 2001; Kaur et al., 2019; Verni and Rosati, 2011), the available data are comparatively insignificant respect to the estimated number of ciliate species (Chao et al., 2006) and the cyst morphology of most of them remains unknown (Kaur et al., 2019). The resting cysts of some free-living ciliates might resemble those of B. coli/Buxtonella sp., and the protocols used in some studies for sample analysis (see section 3) could induce alterations in the cysts that may led to misdiagnosis. The presence of other parasitic structures (e.g., helminth eggs, protozoan oocysts) would support the consideration of the observed ciliate cysts as being of parasitic species; however, in the absence of molecular analysis, it would not be possible at first sight to identify whether faecal contamination and parasitic structures are of human or animal origin (Smith, 1998). For example, Entamoeba coli cysts from human and non-human primates are morphologically identical to those of Entamoeba muris from rodents; tetranucleated cysts of Entamoeba histolytica and free-living/parasitic Entamoeba moshkowskii are identical; Giardia duodenalis and Cryptosporidium parvum are zoonotic; eggs of all Taenia/Echinococcus species are identical; and eggs of Ascaris lumbricoides and Trichuris trichiura infecting humans are identical to those of Ascaris suum and Trichuris suis infecting pigs, respectively. Consequently, all options (B. coli, Buxtonella sp., and even free-living ciliates) would be feasible. Perhaps this could explain the findings by Amin (1988), who detected cysts identified as B. coli in large numbers (66–528/mL) in sewage sludge in Bahrein, but the prevalence in the human population was very low (0.01%) and non-human primates and pigs were absent in the region. In several studies on parasites contaminating soil, water and food, B. coli trophozoites were reported (Ferreira Martins et al., 2017; Karaman et al., 2017; Morais da Silva et al., 2014; Mukhtar et al., 2016; Shikara et al., 2019) but the images provided in some of the publications do not allow a clear recognition of the organism, and the identifications might correspond to free-living ciliates.
Unequivocal identifications can be made after morphological analysis of the vegetative forms. In the case of free-living ciliates, they can be obtained by inducing the excystment of the cysts; however, in the case of parasitic ciliates, excystment and maintenance of trophozoites in culture is complicated and often unfeasible. Protocols are available for in vitro culture of B. coli (da Silva Barbosa et al., 2018a; Yan et al., 2021), but they have some drawbacks (equipment requirements, culture conditions, low success rate) and cannot be used for routine diagnosis of the human ciliate. For a correct human illness source attribution (Pires et al., 2009), molecular tools are the best option for a correct and accurate diagnosis of B. coli in both faecal and environmental samples.
To date, genetic analyses for the identification of B. coli have only been performed on faecal samples; their applicability to environmental samples remains untested. In these samples, no genetic analysis for the identification of B. coli has been performed and the parasite has been identified based on the general appearance of the trophozoites/cysts without sufficiently detailed morphological analysis. It is not our intention to question the correctness of the diagnoses made in the reports cited in this review, but believe that, given the circumstances stated above, it would be more appropriate to refer to the ciliate findings as B. coli-like (e.g., Alemu et al., 2019) until confirmation by genetic analysis. From a health point of view, it may be advisable to identify compatible cysts as B. coli to adopt adequate measures (“better safe than sorry”). However, the data could be incorrect and lead to an overestimation of the real importance of this pathogen in a region or host population, and to misunderstanding of its epidemiology, transmission routes and critical control points.
Very few data are available on the genetics of B. coli¸ and only the small subunit rRNA and the internal transcribed spacers (ITS) region have been sequenced (Ponce-Gordo et al., 2011, Ponce-Gordo et al., 2008). Two different ITS sequence variants have been identified, with the possibility of being simultaneously co-expressed by each individual cell (Ponce-Gordo et al., 2011); this is a problem when sequencing from PCR products rather than cloning, as mixed sequences are likely to occur. Sequence polymorphisms have also been detected in Buxtonella isolates (Grim et al., 2015; Pomajbíková et al., 2013), but the differences between B. coli and Buxtonella from monkeys and bovids are clear (Grim et al., 2015; Pomajbíková et al., 2013) and this marker can be used for barcoding of B. coli. There are minor variations in the ITS region between B. coli isolates of different host origin, mainly consisting of single or a few base differences scattered in the sequences, some of which are probably the consequence of PCR errors made visible by the cloning analysis (García-Rodríguez et al., 2020).
3. Parasite detection in environmental samples
There are discrepancies about how long B. coli trophozoites can survive outside the host; the available data do not come from controlled studies, but from observations of presence/absence of trophozoites in faecal material at different times, ranging from a few hours to several days at room temperature (Little, 1931; Rees, 1927). Bareja et al. (2015) maintained B. coli in culture in distilled water for up to 7 months; these results contradict the findings from previous in vitro and in vivo studies, which indicated that at least a carbohydrate source was necessary for parasite survival and growth (Ponce-Gordo and García-Rodríguez, 2021).
Data from in vitro cultures indicated that low pH values caused trophozoites to die (da Silva Barbosa et al., 2015; Yan et al., 2021), so they would be unable to survive the low pH of the stomach (Chalmers, 2014; Schuster and Visvesvara, 2004). Although Pomajbíková et al. (2010) suggested that trophozoites might be infective, it is generally accepted that cysts are the infective stage of the parasite. In the following sections, we will refer to the detection of cysts in different environmental (soil, water and food) matrices. Trophozoites, if present, would not survive most of the protocols used for sample processing and would disappear from the processed sample.
The protocols used for the diagnosis of parasites in environmental samples have an initial phase to extract parasitic structures from the matrix and reduce the sample volume to be processed, and a second phase in which concentration procedures are performed using similar or even the same methodologies as those applied to faecal samples. The initial phase is critical for the success of the analysis, as the number of parasitic structures in the soil/water/food under study may be very low, and part of them may not be recovered and therefore lost for the rest of the process. This is especially important in soil samples, as parasitic structures would tend to adhere to soil particles (Collender et al., 2015). However, there is significant lack of uniformity between the studies, and differences in the sample volume/quantity analysed in each study could have affected the results. In the soil samples, between 2 and 500 g of soil, from the surface to 50 cm depth, were taken for analysis (Table 1). For water samples, the volume considered was usually between 1 and 2 L, although it varied from 250 mL to 20 L (Table 2). In the analysis of vegetables and fruits, the amount of sample varied from 25 g to the whole vegetable piece (Table 3). In addition, as pointed out by Traub and Cuttell (2015), differences in the initial washing step (extraction solution with/without detergent and duration of agitation) can also affect the sensitivity of the analysis.
Table 1.
Methodology used in the detection of B. coli in soil matrices.
| Authors | Country | Sample type | Sample quantity | Methodology | B. coli positive samples |
|---|---|---|---|---|---|
| Charitha et al. (2015) | India | soils rich in organic matter were excluded; up to 3–5 cm depth | 200 g | Sieve filtration to remove coarse particles. Mechanical shaking in 25–30 mL of 1% Tween 80 for 2 min. Sieve filtration and centrifugation at 1500 rpm, 5 min. Sediment washed twice in distilled water by centrifugation at 1500 rpm, 5 min. Sediment resuspended for NaNO3 flotation. | 1.18% |
| Etewa et al. (2016) | Egypt | clay, sandy and silt soils, at 50 cm depth | 500 g | Sieve filtration (data on dilution liquid not provided); filtrated was used for observation in unstained wet mounts, zinc sulphate flotation, Baermann technique and charcoal coprocultures. | 8% |
| Shikara et al. (2019) | Iraq | clay in lake shore, at 1 cm depth | 200-250 g | Sieve filtration and stored in 2.5% formalin at 4 °C. Filtrated was used for formaline-ether concentration (centrifugation at 2000 rpm, 5 min). Sediment observation in unstained/iodine-stained wet mounts. |
14% |
| Ferreira Martins et al. (2017) | Brazil | not indicated | Not given | Spontaneous sedimentation (data on dilution liquid not provided) for 24 h. Sediment observation in unstained/iodine-stained wet mounts. | parasite found but no data provided |
Table 2.
Methodology used in the detection of B. coli in water samples.
| Authors | Country | Sample type | Sample quantity | Methodology | B. coli positive samples |
|---|---|---|---|---|---|
| Ajeagah and Moussima Yaka (2014) | Cameroon | river sediment | 1 L | Spontaneous sedimentation for 24–48 h. The pellet was used for different methods: 1- resuspended in formaline and centrifuged at 500 rpm, 5 min; 2- used for for zinc sulphate flotation; 3- used for Ritchie and Telemann concentration. Observations in iodine-stained wet mounts. | up to 613 cysts/L |
| Al-Tufaili et al. (2014) | Iraq | river stream at surface and at 2 m depth | 1 L | Sieve filtration and concentration (method not indicated). Sediment observation in unstained wet mounts. | parasite found but no data provided |
| Ayaz et al. (2011) | Pakistan | tap, pond and drain water | Not given | Filtration through Filtra-Max filters; filter elution and centrifugation. Sediment observation in unstained wet mounts. | 5.78% |
| Daminabo and Damen (2020) | Nigeria | abbatoir effluents | Not given | Direct observation in unstained/iodine-stained wet mounts. | 11.1% |
| Ferreira Martins et al. (2017) | Brazil | not indicated | Not given | Direct observation after collection and after spontaneous sedimentation for 24 h, in unstained/iodine-stained wet mounts. | parasite found but no data provided |
| Karaman et al. (2017) | Turkey | river stream at 30 cm depth | 5 L | Spontaneous sedimentation for 1 h. Sediment resuspended and centrifuged at 1000 rpm, 10 min. Sediment observation in unstained/iodine-stained wet mounts. | 16.77% |
| Khanum et al. (2012) | Bangladesh | wastewater | 2 L | Samples processed by formol-ether concentration. Observation in unstained wet mounts. | 4.16% |
| Nsoh et al. (2016) | Cameroon | taps, wells, boreholes and springs | Not given | Sample centrifugation at 2000 rpm, 5 min. Sediment observation in unstained/iodine-stained wet mounts. | 3.9% |
| Oyibo et al. (2016) | Nigeria | borehole and river stream | Not given | Sieve filtration (0.5 μm mesh size); residue rinsed with 50 mL distilled water and centrifuged at 500 rpm, 2 min. Sediment resuspended in saline and centrifuged. Sediment resuspended in concentrated by the formaline-ethyl acetate method. Sediment observation in unstained/iodine-stained wet mounts. |
5.3% |
| Poma et al. (2012) | Argentina | river stream | 20 L | Sieve filtration to remove solids. Filtration through ultrafiltration units. Elution with 0.05 M glycine/NaOH and 0.1% Tween 80. Filtration through gauze and further concentration by sucrose flotation and by centrifugation at 1000 rpm, 5 min. Sediment preserved in 10% formol, SAF and MIF. Observation in unstained/iodine-stained wet mounts. | parasite found but no data provided |
| Shah et al. (2016) | Pakistan | tube-wells, ponds and drain water | Not given | Filtration through Filtra-Max filters; filter elution and centrifugation. Sediment observation in unstained wet mounts. |
5.78% |
Table 3.
Methodology used in the detection of B. coli in vegetables.
| Authors | Country | Sample type | Sample quantity | Methodology | B. coli positive samples |
|---|---|---|---|---|---|
| Agobian et al. (2013) | Venezuela | L | 200 g | Soft washing in 2 L of filtered water and spontaneous sedimentation for 24 h. Sediment resuspended and centrifuged at 3000 rpm, 10 min. Observation in unstained/iodine-stained wet mounts. |
15% |
| Ajitha et al. (2020) | India | F, L, R, S | 200 g | Mechanical vigorous shaking for 15 min in saline; spontaneous sedimentation overnight. Sieve filtration and centrifugation at 3000 rpm, 5 min. Sediment observation in unstained/iodine-stained wet mounts. | 2.7% |
| Akoachere et al. (2018) | Cameroon | L, R | 25 g | Vigorous shaking for 15 min in 225 mL sterile distilled water; gauze filtration and spontaneous sedimentation for 10 h. Sediment resuspended and centrifuged at 1207 g, 5 min. Observation in unstained/iodine-stained wet mounts. | 32.4% |
| Al-Sanabani et al. (2016) | Yemen | L | 100 g | Sample washing in saline (time not given); spontaneous sedimentation for 6 h. Sediment resuspended and filtered, then centrifuged at 3000 rpm, 3 min. Observation in unstained wet mounts. | 3.8% |
| Alade et al. (2013) | Nigeria | L, S | 50 g | Sample washing in distilled water; filtration and centrifugation at 5000 rpm, 5 min. Sediment used for magnesium sulphate flotation. Observation in unstained wet mounts. |
8.19% |
| Alemu et al. (2019) | Ethiopia | L, R, S | 100 g | Mechanical vigorous shaking in 500 mL saline. Spontaneous sedimentation overnight. Observation in unstained/iodine-stained wet mounts. | 4.3% |
| Amaechi et al. (2016) | Nigeria | L, R, S | Not given | Sample soaking in saline for 30 min; sieve filtration and spontaneous sedimentation for 10 h. Sediment resuspended and centrifuged at 5000 rpm, 5 min. Observation in iodine-stained wet mounts. | 8.3% |
| Etewa et al. (2017) | Egypt | F, L, R | 200 g | Soaking in 1 L saline overnight; sieve filtration and centrifugation at 2000 rpm, 20 min. Supernatant used for zinc sulphate flotation. Observation in unstained/iodine-stained wet mounts. |
5.7% |
| dos Carolino Fiorido and Andrade de Souza (2020) | Brazil | F | Not given | First washing consisting in soaking in 200 mL distilled water and manual shaking for 30 s; second washing by brushing in 200 mL distilled water. In both cases, sieve filtration and spontaneous sedimentation for 24 h. Sediment resuspended and centrifuged at 4500 rpm, 1 min. Observation in iodine-stained wet mounts. | 28.57% |
| Kudah et al. (2018) | Ghana | L, R, S | 150–200 g | Sample washing in distilled water or saline (0.45–1.5% NaCl); spontaneous sedimentation for 12 h. Sediment resuspended and filtered, then centrifuged at 1500 rpm for 5 min. Observation in unstained/iodine-stained wet mounts. | 13.6% |
| Mukhtar et al. (2016) | Sudan | L, R, S | Not given | Sample washing in 30 mL 10% formalin in saline. Spontaneous sedimentation for 24 h. Sediment resuspended and centrifuged at 3000 rpm, 5 min. Observation in wet mounts. | 1.3% |
| Nazemi et al. (2012) | Iran | L, R | 200 g | Sample washing in 1 L of detergent solution (1% SDS, 0.1% Tween 80) for 10 min. Centrifugation at 3000 rpm, 10 min. Sediment fixed with 4% formaline for 10 min. Observation in unstained/iodine-stained wet mounts. | 2% |
| Ogunremi et al. (2017) | Nigeria | F, L, R, S | 200–250 g | Soaking in distiller water for 10–20 min. Sieve filtration and centrifugation at 3000 rpm for 15 min. Sediment resuspended for zinc sulphate flotation. Observation in wet mounts. | 8.29% |
| Ordoñez et al. (2018) | Philippines | F, L, R, S | 250 g | Following Nazemi et al., 2012 | 8.29% |
| Sena Barnabé et al. (2010) | Brazil | L | Not given | Sample soaking in 1 L distilled water and spontaneous sedimentation for 24 h. Sediment resuspended and centrifuged at 2500 rpm, 1 min, then resuspended for zinc sulphate flotation. Observation in iodine-stained wet mounts. | 20% |
(*) F – fruits (i.e., apple, orange, grapes, strawberries, etc.); L – leaf vegetables (i.e., lettuce, cabbage, spinach, parsley, etc.); R – root vegetables (i.e., potatoes, carrots); S – smooth surface vegetables (i.e., tomatoes, green pepper). The vegetables of each type under analysis varied among studies.
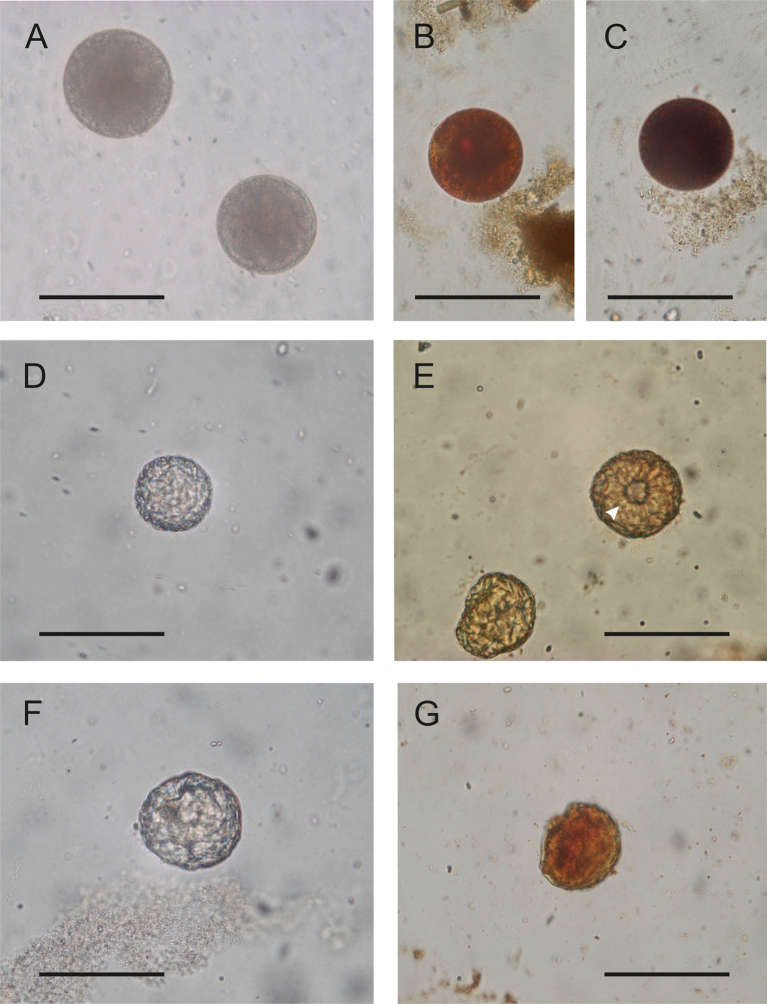
At the concentration phase, there are two sources of variation between studies: the different methods used in each case and, for a given protocol, the apparently minor differences in the methodology used (e.g., pore size of filters, dilution solutions, centrifugation speeds and times). These differences have been shown to affect parasite recovery in faecal samples (Ballweber et al., 2014; Manser et al., 2016). Comparisons between methods have been performed on faecal samples to determine which one produces fewer false-negative results (Soares et al., 2020), but there are very few studies on their actual detection limits (i.e. number of cysts/oocysts/eggs per amount or volume of original sample); and there are no studies on environmental samples. Since the minimum number of infective (oo)cysts/eggs required for infection to occur is not known for most parasites, but it is generally considered to be low (Chalmers et al., 2020), it is very important to ensure the accuracy of a negative diagnosis. The common approach is to examine several slides from each final concentrate. For this purpose, usually fresh or Lugol's iodine stained slides are prepared and observed under optical microscopy at low to medium power magnification (10-40× objectives). This is sufficient to find B. coli cysts, as their large size makes them easily discoverable and their morphology is not significantly altered by the concentration procedure (i.e. spontaneous vs forced sedimentation, or variations in centrifugation time and speed); the exception would be if flotation methods are used. The osmotic pressure of the saturated solutions does not affect oocysts or Entamoeba/Giardia cysts, or thick-shelled eggs, but can cause deformation of operculate and thin-shelled eggs and ciliate cysts (Ballweber et al., 2014; da Silva Barbosa et al., 2016), making their identification difficult (Fig. 1). Formalin does not affect the morphology of B. coli cysts and is a valid fixative for morphological analysis; however, as mentioned in section 2, it would be better to avoid its use as molecular tools would be necessary to confirm the diagnosis and thus avoid misidentifications.
Fig. 1.
Variation in the Balantioides coli cyst morphology after concentration protocols. A–C, cysts obtained after SAF-ethyl acetate sedimentation concentration. D–E, cysts obtained after sucrose flotation concentration. F–G, cysts obtained after SO4Zn flotation concentration. In A–C,; the encysted cell fills the cyst. In D–G, the osmotic pressure has caused the encysted cell to collapse and cannot be seen, and the cyst wall is deformed and even broken (E, arrowhead). The same sample (fresh faeces from a naturally infected orang-utan maintained in the Zoo-Aquarium de Madrid, Spain) was used in all procedures. A, D and F, unstained wet mounts; B, C, E and G, wet mounts stained with iodine. The cysts in B and C were observed in the same slide, one became darker than the other with iodine. All images are reproduced at the same magnification. Scale bar: 50 μm.
3.1. Cyst detection in soil matrices
Some soil-transmitted parasites can enter the host though the skin (e.g. Strongyloides, hookworms) while others enter via the oral route; in this case, apart from geophagy, the most common way of infection is through contaminated food or water. As B. coli is mainly considered as a foodborne parasite (World Health Organization, 2017), the importance of detecting it in soil samples is related to the possibility that soil would be the source of food contamination (Ajitha et al., 2020; Al-Sanabani et al., 2016; Alemu et al., 2019; Matini et al., 2017; Ordoñez et al., 2018). In general, the analysis of soil contamination with parasites is mainly focused on the detection of helminth eggs and larvae rather than enteric protozoa. The methodology used for the diagnosis of helminths in soil samples (Amoah et al., 2017; Collender et al., 2015) can be used for the search and identification of enteric protozoa, including B. coli, in soil samples. This parasite has been recorded by some authors at low rates in soil samples (Table 1), However, the images provided in some studies are unclear and could correspond indeed to free-living ciliates.
The presence of parasitic structures in soil is due to contamination from wastewater or with polluted irrigation water (e.g. in crops), or from direct contamination with faeces from infected hosts. In the first case, their distribution in the irrigated soil would be homogeneous, while in the second case the distribution would be heterogeneous (faeces being the initial point of dispersal). Then, the first problem to solve is the spatial distribution of sampling points, and several strategies are possible (systematic aligned and non-aligned methods, walking path transect sampling, grid-based random sampling) (Collender et al., 2015). However, the sampling pattern, if followed, is usually not indicated in the reports. Once samples have been collected, the general protocol has been to mix them with a wash/extraction solution and filter them, and the obtained sediment (sometimes all of the filtrate) has been processed using concentration techniques, with the final concentrate been observed under the microscope. For helminth eggs and larvae, flotation concentration methods are commonly used, with recovery rates ranging from less than 10% to more than 100% (Collender et al., 2015). In the few studies in which B. coli was identified in soil samples, flotation and sedimentation methods were used, but published data were aggregated and differences between methods, if any, were not mentioned (Table 1).
Separation of parasitic structures potentially trapped in the particulate material of soil samples is challenging (Amoah et al., 2017; Collender et al., 2015). Detergent solutions are preferred for this washing step and Tween 40, Tween 80, Triton X, 7×, antiformin and ammonium bicarbonate have been used (Amoah et al., 2017). In the studies that have reported B. coli cysts in soil samples, one study used Tween 80 in the washing step (Charitha et al., 2015); no data were provided on the dilution fluid used in the other studies (Table 1). Comparative analysis should be conducted to determine the composition of the washing solution (with/without detergent, which detergent, concentration) that allows the best performance in terms of parasitic structures-soil particles separation.
3.2. Cyst detection in water samples
Most waterborne protozoan outbreaks reported worldwide have been caused by Giardia duodenalis and Cryptosporidium spp. (Baldursson and Karanis, 2011; Efstratiou et al., 2017; Karanis et al., 2007; Rosado-García et al., 2017), whereas only a single outbreak caused by B. coli was documented (in 1971, on Truk Island in the Pacific; Walzer et al., 1973). Although drinking water can act as a carrier of B. coli cysts, their large size allows them to be easily removed by filtration; furthermore, they do not remain in suspension as easily as smaller cysts of other waterborne protozoa (i.e., Cryptosporidium spp., Giardia duodenalis or Entamoeba spp.). Because of this, B. coli is primarily considered a foodborne parasite for humans (World Health Organization, 2017) and is not among the pathogens prioritised for regulation in drinking water (Hoffman et al., 2009). Accordingly, findings of this parasite in water samples should in most cases be considered of possible relevance to food (or soil) contamination rather than for direct infection to humans.
Standardised protocols exist for the detection of Cryptosporidium spp. and Giardia duodenalis in water samples (US Environmental Protection Agency methods 1622 and 1623, and ISO 15553:2006), which are considered the most relevant waterborne parasites due to the number of outbreaks and associated human infections caused by them. Other simpler, not standardised procedures have been proposed for the diagnosis of other protozoa (Lora-Suarez et al., 2016; Moreno et al., 2018; Tobón et al., 2018). The use of non-standardised methods makes direct comparisons between studies difficult, as there are differences in the types of samples (running or standing water sampled at the surface, at different deep or in the sediment; wastewater; sludge from treatment plants), the volume processed, and the analytical methods used.
Balantioides coli cysts have been reported in samples from sewage, drainage water, rivers, ponds and drinking water; concentration by sedimentation and filtration through Filtra-Max filters were the most commonly used methods (Table 2). Flocculation is widely used in water treatment plants for pathogen removal; however, this method is not commonly used for the isolation and diagnosis of protozoa (Bridle et al., 2014) although its possible use for the detection of Giardia duodenalis, Cryptosporidium spp. and Toxoplasma gondii in contaminated water has been suggested (Karanis and Kimura, 2002; Kourenti et al., 2003; Zhang et al., 2013). There are no reports of B. coli in the studies based on flocculation procedures; as few data are available, further research is needed to determine wether this method could be valid for B. coli.
3.3. Cyst detection in food
Transmission of B. coli through contaminated food stuff is not considered of great importance in the developed world (Dawson, 2005) probably because this parasite has rarely been implicated in foodborne outbreaks; however, it has been frequently reported in surveys of parasites in fresh produce and fruits sold in local markets in developing countries. In general, contamination of vegetables with pathogenic microorganisms, including B. coli, can occur at source (i.e., by using wastewater or night soil), or during transport, handling and sale/service to final consumers (Auad et al., 2019; Dixon, 2015; Ensink et al., 2007; Herman et al., 2015; Milinovich and Klieve, 2011; Pires et al., 2012; Rodrigues Maldonade et al., 2019). Appropriate control measures to avoid or minimise contamination has been reviewed by Dixon (2015).
Li et al. (2020) has reviewed detection methods for intestinal protozoan parasites contaminating vegetables and fruits. Standardised protocols exist for the detection of Cryptosporidium spp. and Giardia duodenalis (ISO 15553:2006 and ISO 18744:2016, respectively), and in general, the trend is to incorporate molecular tools (PCR and immunology-based methods) for parasite detection and identification (Li et al., 2020; Orlandi et al., 2002). All reports of B. coli contamination of fruits and vegetables have been made by detection of morphologically compatible cysts. The parasite is usually found in <10% of the vegetables studied, although values up to 32.4% have been reported (Table 3). The discordance between the results of the different studies has been attributed to a combination of factors, such as irrigation and fertilisation systems, lack of sanitary facilities and human behaviour in the study area (Alade et al., 2013; Amaechi et al., 2016; Hassan et al., 2012), and differences in analytical methodology (Agobian et al., 2013; Ajitha et al., 2020; Akoachere et al., 2018; Amaechi et al., 2016; Hassan et al., 2012; Kudah et al., 2018).
The most common methodological scheme for the detection of intestinal parasites in vegetables has consisted of washing the samples followed by filtration of the washing liquid and centrifugation (with or without a previous spontaneous sedimentation); in some cases flotation techniques have been used. However, differences among studies can be found in the specific protocols for detaching parasites from vegetables, washing solutions, and concentration techniques (Table 3). In addition, the same protocol is usually applied in each study to all vegetables tested despite they can have different characteristics (i.e., leafy, root or smooth surface vegetables) that could affect the performance of the method used and the results obtained (Kudah et al., 2018).
4. Viability assessment and parasite inactivation
Detection methods are in most cases not valid for determining the viability of the parasites. There are some exceptions, such as stool cultures used for hookworm diagnosis or migration methods (i.e. Baermann technique) for Strongyloides diagnosis, which are based on live eggs/larvae present in the sample. For most helminths, egg viability is determined by observing their morphology and the presence of developing and active larvae inside the eggs, as well as by vital staining (Amoah et al., 2017). In the case of enteric protozoa, the microscopic appearance and integrity of the (oo)cysts would be suggestive of viability; however, due to their very small size, morphological alterations may be overlooked. Furthermore, if they show altered morphology (lack of internal structures, retracted content, wall deformation), this may be caused by the substances used in the analytical procedure (i.e. formalin, saturated solutions, Lugol's iodine) and therefore it is not possible to determine whether the (oo)cysts were initially degenerated, or killed and altered during the analysis. Furthermore, (oo)cysts are dormant stages and no activity can be observed in them even if they are viable and infective (once oocyst sporulation has occurred, no further activity can be observed). For some protozoa, alternative methods have been developed based on dead/live selective dyes (i.e., trypan blue, ethidium bromide, propidium iodide, SYTO-dyes, DAPI), in vitro excystation, in vitro culture, or analysis of specific gene targets (Rousseau et al., 2018). In B. coli, only Etewa et al. (2017) have assessed viability by trypan blue staining. These authors considered as viable those cysts that appeared light blue and showed exclusion of the dye, while dead cysts appeared dark blue.
The results of all these viability tests may not correlate with parasite infectivity, as not all viable cysts would be infective (Erickson and Ortega, 2006; Rousseau et al., 2018). The best method to determine infectivity is by experimental infections in animal models. In the case of B. coli, there was active research on this topic in the first half of the 20th century, but the studies conducted showed variable results (Gabaldon, 1935; Schumaker, 1930; Walker, 1913; Young, 1950) and interest in this type of research on a parasite that was not (and still is not) considered a major public health threat declined. Current regulations on the use of experimental animals have discouraged research on this topic. An alternative would be the use of in vitro models for the excystation and culture of B. coli, but more research is needed. Several media have been used for the culture of the trophozoites, with variable success rates (Clark and Diamond, 2002; da Silva Barbosa et al., 2015, da Silva Barbosa et al., 2018a; Yan et al., 2021). In some cases, cultures were initiated from cysts (da Silva Barbosa et al., 2015; Yan et al., 2021). However, the specific conditions for inducing the excystation process are undefined and da Silva Barbosa et al. (2015) failed to obtain excystation in 36% of the cultures.
Without a suitable method to assess B. coli cyst viability, studies evaluating the efficacy of different systems for cyst inactivation are lacking. Etewa et al. (2017) tested the use of 5% acetic acid and postassium permanganate (24 mg/L) for 15 and 30 min as disinfectant solutions against helminth eggs and protozoan (oo)cysts (including B. coli) on fresh produce from rural markets in Egypt. They found a slightly higher effect with acetic acid, but in all cases, the viability loss (assessed by trypan blue staining) was less than 30%. Chlorination, UV light and ozone disinfection have been investigated against enteric protozoa in wastewater, but no single common method was found to be valid: chlorine is effective against Giardia cysts but not against Cryptosporidium oocysts, UV at reasonable doses produces little inactivation, and ozone is more effective against Giardia than against Cryptosporidium (Schaefer et al., 2004). In fresh vegetables, chlorine and hydrogen peroxide, to be effective, would need a longer contact time than that used in the processed vegetable industries; chlorine dioxide is effective against Cryptosporidium but not against Cyclospora; and ozone has not been tested against parasites contaminating fresh produce (Gérard et al., 2019). Freezing, heating and drying can be effective but may not be adequate if the organoleptic characteristics of the food are altered. This variability in the efficacy of treatments against different parasites does not allow extrapolating a valid disinfectant for B. coli, and specific studies on this parasite would be necessary.
5. Future studies and research
Balantioides coli is the only ciliate known to date to parasitize humans; however, Buxtonella-like ciliates have been found in some non-human primates, raising the question of wether humans are also suitable hosts to them. So far, very few ciliate isolates of human origin have been investigated using molecular tools and all have been confirmed as B. coli (López Arias et al., 2017; Ponce-Gordo et al., 2011); however, genetic analysis should be conducted on future isolates to rule out infections by ciliates other than B. coli. The same type of analyses should be performed on animal isolates, as Buxtonella sp. infect bovines and non-human primates. Moreover, these techniques should be mandatory in ciliate-positive environmental (food/water/soil) samples to confirm the species, as identifications as B. coli has so far been done on the basis of morphological similarity of cysts. Further research is needed to adapt existing protocols with faecal samples or to develop new ones, for molecular identification of this parasite in environmental samples. In the meantime, it would be advisable to identify compatible findings as B. coli-like until genetic analyses confirm the species.
Except for Cryptosporidium spp. and Giardia duodenalis, there are not standardised methods for the detection of parasites in environmental samples (Chalmers et al., 2020). Due to the large size of B. coli cysts, no specific protocols are needed and those applicable for the detection of helminth eggs could also be valid for the tentative identification of this parasite. However, flotation methods, which are commonly used for the diagnosis of helminth eggs and some protozoa such as Cryptosporidium spp. and Giardia duodenalis, would not be recommended for the detection of B. coli as they can produce morphological alterations in the cysts, making their identification difficult. It should also be noted that the differences between protocols could affect cyst recovery rates and limit the performance of other techniques that can be used to analyse the sample (e.g. if formalin is used, genetic analysis cannot be performed). If a common standardised procedure were used in epidemiological surveys, the data would be easely comparable and risk assessment and evaluation of critical control points would be done with a higher degree of confidence.
Another important point in parasitological surveys involving environmental samples is to determine whether the parasites are alive and infective; most detection methods do not provide this information. As for B. coli, this is an unexplored field of research. Investigation of vitality markers and defined conditions for in vitro excystment is needed. In vivo models, which could be considered the “gold standard” in infectivity experiments, pose major ethical concerns and show a lack of reproducibility in B. coli. In vitro models are the best alternative, but they have also shown variable reproducibility in B. coli and need to be improved. The development of a reliable culture system, focusing on axenic media, will boost research on this parasite.
Funding sources
This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Adhikari B.B., Rana H.B., Sultan K.M., Devkotal B., Nakao T., Kobayashi K., Sato H., Dhakal I.P. Prevalence of Buxtonella sulcata in water buffaloes and cows in Chitwan Valley, southern Nepal. Japanese J. Vet. Parasitol. 2013;11:55–60. [Google Scholar]
- Agobian G., Quiñones O., Rodríguez J., Sorondo O., Subiela J., Tamayo D., Taylor L., Tolosa L., Venegas J., Cárdenas E., Traviezo Valles L.E. Contaminación por enteroparásitos en repollos comercializados en los estados Lara, Yaracuy y Portuguesa. Rev. Venez. Salud Pública. 2013;1:7–14. [Google Scholar]
- Ajeagah G.A., Moussima Yaka D.A. Study of the influence of environmental factors on the occurrence of Balantidium coli cysts in an urban aquatic system in Cameroon. J. Ecol. Nat. Environ. 2014;6:190–199. doi: 10.5897/jene2014.0451. [DOI] [Google Scholar]
- Ajitha S., Vazhavandal G., Uma A., Prabhusaran N. Study on parasitic contamination of common edible fruits and vegetables sold in local markets of Tiruchirappalli, South India. Indian J. Microbiol. Res. 2020;7:362–368. doi: 10.18231/j.ijmr.2020.065. [DOI] [Google Scholar]
- Akoachere J.F.T.K., Tatsinkou B.F., Nkengfack J.M. Bacterial and parasitic contaminants of salad vegetables sold in markets in Fako division, Cameroon and evaluation of hygiene and handling practices of vendors. BMC Res. Notes. 2018;11:100. doi: 10.1186/s13104-018-3175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alade G.O., Alade T.O., Adewuyi I.K. Prevalence of intestinal parasites in vegetables sold in Ilorin, Nigeria. J. Agric. Environ. Sci. 2013;13:1275–1282. doi: 10.5829/idosi.aejaes.2013.13.09.11040. [DOI] [Google Scholar]
- Alemu G., Mama M., Misker D., Haftu D. Parasitic contamination of vegetables marketed in Arba Minch town, southern Ethiopia. BMC Infect. Dis. 2019;19:1–7. doi: 10.1186/s12879-019-4020-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sanabani A.-W., Abd Algalil F.M., Radman B.A., Al-Manusori R.T. Prevalence of intestinal parasites in fresh leafy vegetables in some farms at Dhamar city, Yemen. Int. J. Med. Res. 2016;1:7–13. [Google Scholar]
- Al-Tufaili R.A.N., Khayoon S.Q., Rashid A.A. Investigation of parasitic contamination in Kufa river water-Al-Najaf province. Al-Kufa Univ. J. Biol. 2014;6:11. [Google Scholar]
- Amaechi E., Ohaeri C., Ukpai O., Adegbite R.A. Prevalence of parasitic contamination of salad vegetables in Ilorin, North Central, Nigeria. Momona Ethiop. J. Sci. 2016;8:136–145. doi: 10.4314/mejs.v8i2.3. [DOI] [Google Scholar]
- Amin O.M. Pathogenic Micro-organisms and Helminths in sewage products, Arabian gulf, country of Bahrain. Am. J. Public Health. 1988;78:314–315. doi: 10.2105/AJPH.78.3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoah I.D., Singh G., Stenström T.A., Reddy P. Detection and quantification of soil-transmitted helminths in environmental samples: a review of current state-of-the-art and future perspectives. Acta Trop. 2017;169:187–201. doi: 10.1016/j.actatropica.2017.02.014. [DOI] [PubMed] [Google Scholar]
- Auad L.I., Ginani V.C., Dos Santos Leandro E., Stedefeldt E., Habu S., Nakano E.Y., Nunes A.C.S., Zandonadi R.P. Food trucks: assessment of an evaluation instrument designed for the prevention of foodborne diseases. Nutrients. 2019;11:1–12. doi: 10.3390/nu11020430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayaz S., Khan S., Khan S.N., Bibi F., Shamas S., Akhtar M. Prevalence of zoonotic parasites in drinking water of three districts of Khyber Pakhtunkhwa Province, Pakistan. Pakistan J. Life Soc. Sci. 2011;9:67–69. [Google Scholar]
- Baldursson S., Karanis P. Waterborne transmission of protozoan parasites: review of worldwide outbreaks - an update 2004-2010. Water Res. 2011;45:6603–6614. doi: 10.1016/j.watres.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Ballweber L.R., Beugnet F., Marchiondo A.A., Payne P.A. American association of veterinary parasitologists’ review of veterinary fecal flotation methods and factors influencing their accuracy and use - is there really one best technique? Vet. Parasitol. 2014;204:73–80. doi: 10.1016/j.vetpar.2014.05.009. [DOI] [PubMed] [Google Scholar]
- Bareja R., Pottathil S., Grover P., Singh H. The simplest technique for cultivation and maintenance of Balantidium coli. Int. J. Heal. Allied Sci. 2015;4:218. doi: 10.4103/2278-344x.167650. [DOI] [Google Scholar]
- Benčaťová S., Tirjaková E. A study on resting cysts of an oxytrichid soil ciliate, Rigidohymena quadrinucleata (Dragesco and Njine, 1971) Berger, 2011 (Ciliophora, Hypotrichia), including notes on its encystation and excystation process. Acta Protozool. 2017;56:77–91. doi: 10.4467/16890027AP.17.007.7482. [DOI] [Google Scholar]
- Bouwknegt M., Devleesschauwer B., Graham H., Robertson L.J., van der Giessen J.W.B., Participants, the E.-F. workshop Prioritisation of food-borne parasites in Europe, 2016. Eurosurveillance. 2018;23:1–11. doi: 10.2807/1560-7917.ES.2018.23.9.17-00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridle H., Jacobsson K., Schultz A. In: Waterborne Pathogens. Bridle H., editor. Detection Methods and Applications. Elsevier; Amsterdam: 2014. Sample processing; pp. 1–24. [DOI] [Google Scholar]
- Chalmers R.M. Microbiology of Waterborne Diseases: Microbiological Aspects and Risks. Second edition. Elsevier; Amsterdam: 2014. Balantidium coli; pp. 277–286. [DOI] [Google Scholar]
- Chalmers R.M., Robertson L.J., Dorny P., Jordan S., Kärssin A., Katzer F., La Carbona S., Lalle M., Lassen B., Mladineo I., Rozycki M., Bilska-Zajac E., Schares G., Mayer-Scholl A., Trevisan C., Tysnes K., Vasilev S., Klotz C. Parasite detection in food: current status and future needs for validation. Trends Food Sci. Technol. 2020;99:337–350. doi: 10.1016/j.tifs.2020.03.011. [DOI] [Google Scholar]
- Chao A., Li P.C., Agatha S., Foissner W. A statistical approach to estimate soil ciliate diversity and distribution based on data from five continents. Oikos. 2006;114:479–493. doi: 10.1111/j.2006.0030-1299.14814.x. [DOI] [Google Scholar]
- Charitha V.G., Rayulu V.C., Kondaiah P.M. Contamination of soil with parasitic forms in urban and rural areas of Andhra Pradesh, India. J. Parasitol. Phot. 2015;105:227–231. [Google Scholar]
- Clark C.G., Diamond L.S. Methods for cultivation of luminal parasitic protists of clinical importance. Clin. Microbiol. Rev. 2002 doi: 10.1128/CMR.15.3.329-341.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collender P.A., Kirby A.E., Addiss D.G., Freeman M.C., Remais V. Methods for quantification of soil-transmitted helminths in environmental media: current techniques and recent advances. Trends Parasitol. 2015;31:625–639. doi: 10.1016/j.pt.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cork S.C., Checkley S. In: Zoonotic Pathogens in the Food Chain. Krause D.O., Hendrick S., editors. CAB International; Wallingford, U.K.: 2011. Globalization of the food supply and the spread of disease; pp. 1–20. [Google Scholar]
- da Silva Barbosa A., Pereira Bastos O.M., Uchôa C.M.A., Pissinatti A., Filho P.R.F., Dib L.V., Azevedo E.P., de Siqueira M.P., Cardozo M.L., Amendoeira M.R.R. Isolation and maintenance of Balantidium coli (Malmsteim, 1857) cultured from fecal samples of pigs and non-human primates. Vet. Parasitol. 2015;210:240–245. doi: 10.1016/j.vetpar.2015.03.030. [DOI] [PubMed] [Google Scholar]
- da Silva Barbosa A., Pereira Bastos O.M., Antunes Uchôa C.M., Pissinatti A., Pereira Bastos A.C.M., Vieira de Souza I., Verdan Dib L., Peixoto Azevedo E., Perlingeiro de Siqueira M., Lessa Cardozo M., Reis Amendoeira M.R. Comparison of five parasitological techniques for laboratory diagnosis of Balantidium coli cysts. Rev. Bras. Parasitol. Vet. 2016;25:286–292. doi: 10.1590/s1984-29612016044. [DOI] [PubMed] [Google Scholar]
- da Silva Barbosa A., Lessa Cardozo M., Verdan Dib L., Monteiro Fonseca A.B., Anutes Uchôa C.M., Pereira Bastos O.M., Reis Amendoeira M.R. Comparative study of three xenic media culture for cultivation of Balantidium coli strains. Brazil. J. Vet. Parasitol. 2018;27:19–25. doi: 10.1590/S1984-29612017075. [DOI] [PubMed] [Google Scholar]
- da Silva Barbosa A., Santos Barbosa H., de Oliveira Souza S.M., Verdan Dib L., Antunes Uchôa C.M., Pereira Bastos O.M.H., Reis Amendoeira M.R. Balantioides coli: morphological and ultrastructural characteristics of pig and non-human primate isolates. Acta Parasitol. 2018;63:287–298. doi: 10.1515/ap-2018-0033. [DOI] [PubMed] [Google Scholar]
- Daminabo V., Damen J. Prevalence of intestinal parasites from abattoir effluents in Jos Metropolis, Nigeria. Int. J. Photochem. Photobiol. 2020;4:1–10. doi: 10.11648/j.ijpp.20200401.11. [DOI] [Google Scholar]
- Dawson D. Foodborne protozoan parasites. Int. J. Food Microbiol. 2005;103:207–227. doi: 10.1016/j.ijfoodmicro.2004.12.032. [DOI] [PubMed] [Google Scholar]
- Dixon B.R. In: Foodborne Parasites in the Food Supply Web: Occurrence and Control. Gajadhar A.A., editor. Elsevier Ltd.; Amsterdam: 2015. Transmission dynamics of foodborne parasites on fresh produce; pp. 317–353. [DOI] [Google Scholar]
- dos Carolino Fiorido S.K.S., Andrade de Souza M.A. Análise parasitológica de frutas consumidas com casca, comercializadas em supermercados de uma cidade do sudeste do Brasil. Heal. Biosci. 2020;1:63–76. [Google Scholar]
- Efstratiou A., Ongerth J.E., Karanis P. Waterborne transmission of protozoan parasites: review of worldwide outbreaks - an update 2011–2016. Water Res. 2017;114:14–22. doi: 10.1016/j.watres.2017.01.036. [DOI] [PubMed] [Google Scholar]
- Ensink J.H.J., Mahmood T., Dalsgaard A. Wastewater-irrigated vegetables: market handling versus irrigation water quality. Tropical Med. Int. Health. 2007;12:2–7. doi: 10.1111/j.1365-3156.2007.01935.x. [DOI] [PubMed] [Google Scholar]
- Ericksen T.H., Dufour A.P. In: Waterborne Diseases in the United States. Craun G.F., editor. CRC Press; Boca Raton: 1986. Methods to identify waterborne pathogens and indicator organisms; pp. 195–214. [Google Scholar]
- Erickson M.C., Ortega Y.R. Inactivation of protozoan parasites in food, water, and environmental systems. J. Food Prot. 2006;69:2786–2808. doi: 10.4315/0362-028X-69.11.2786. [DOI] [PubMed] [Google Scholar]
- Etewa S.E., Abdel-Rahman S.A., Abd El-Aal N.F., Fathy G.M., El-Shafey M.A., Ewis A.M.G. Geohelminths distribution as affected by soil properties, physicochemical factors and climate in Sharkyia governorate Egypt. J. Parasit. Dis. 2016;40:496–504. doi: 10.1007/s12639-014-0532-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etewa S., Abdel-Rahman S., Fathy G., Abo El-Maaty D., Sarhan M. Parasitic contamination of commonly consumed fresh vegetables and fruits in some rural areas of Sharkyia governorate, Egypt. Afro-Egypt. J. Infect. Endem. Dis. 2017;7:192–202. doi: 10.21608/aeji.2017.17804. [DOI] [Google Scholar]
- Ferreira Martins N.P., Sidneu de Souza W., de Almeida Mota M.S. Métodos parasitológicos aplicados em espécimes de solo e água em setores do município de Guaraí/TO. Scire Salut. 2017;7:32–41. doi: 10.6008/spc2236-9600.2017.002.0004. [DOI] [Google Scholar]
- Food and Agriculture Organization of the United Nations, World Health Organization . 2014. Multicriteria-Based Ranking for Risk Management of Food-Borne Parasites. Microbiological Risk Assessment Series (MRA) p. 23. [Google Scholar]
- Gabaldon A. Balantidium coli : quantitative studies in experimental infections and variations in infectiousness for rats. J. Parasitol. 1935;21:386–392. [Google Scholar]
- García-Rodríguez J.J., Martínez-Díaz R.A., Martella M., Navarro J.L., Ponce-Gordo F. Genetic identification of the ciliates from greater rheas (Rhea americana) and lesser rheas (Rhea pennata) as Balantioides coli. Parasitol. Res. 2020;119:755–758. doi: 10.1007/s00436-019-06559-5. [DOI] [PubMed] [Google Scholar]
- Gérard C., Franssen F., La Carbona S., Monteiro S., Cozma-Petruţ A., Utaaker K.S., Režek Jambrak A., Rowan N., Rodríguez-Lazaro D., Nasser A., Tysnes K., Robertson L.J. Inactivation of parasite transmission stages: efficacy of treatments on foods of non-animal origin. Trends Food Sci. Technol. 2019;91:12–23. doi: 10.1016/j.tifs.2019.06.015. [DOI] [Google Scholar]
- Gomez Hinojosa P., Espinoza-Ríos J., Carlin Ronquillo A., Pinto Valdivia J.L., Salas Dueñas Y., Zare Morales W. Balantidiasis colónica: reporte de un caso fatal y revisión de la literatura. Rev. Gastroenterol. Peru. 2019;39:284–287. [PubMed] [Google Scholar]
- Grim J.N., Jirků-Pomajbíková K., Ponce-Gordo F. Light microscopic morphometrics, ultrastructure, and molecular phylogeny of the putative pycnotrichid ciliate, Buxtonella sulcata. Eur. J. Protistol. 2015;51:425–436. doi: 10.1016/j.ejop.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Gutiérrez J.C., Callejas S., Benïtez S.B.L., Martin-Gonzalez Ana, Martin-Gonzalez A. Ciliate cryptobiosis: a microbial strategy against environmental starvation. Int. Microbiol. 2001;4:151–157. doi: 10.1007/s10123-001-0030-3. [DOI] [PubMed] [Google Scholar]
- Hassan A., Farouk H., Abdul-Ghani R. Parasitological contamination of freshly eaten vegetables collected from local markets in Alexandria, Egypt: a preliminary study. Food Control. 2012;26:500–503. doi: 10.1016/j.foodcont.2012.01.033. [DOI] [Google Scholar]
- Herman K.M., Hall A.J., Gould L.H. Outbreaks attributed to fresh leafy vegetables, United States, 1973-2012. Epidemiol. Infect. 2015;143:3011–3021. doi: 10.1017/S0950268815000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman R., Marshall M.M., Gibson M.C., Rochelle P.A. Prioritizing pathogens for potential future regulation in drinking water. Environ. Sci. Technol. 2009;43:5165–5170. doi: 10.1021/es803532k. [DOI] [PubMed] [Google Scholar]
- Karaman U., Koloren Z., Ayaz E., Demirel E., Seferoglu O. The Protozoa and helminths in the water of Terme and Kocaman Boroughs of Samsun Province. J. Turgut Ozal Med. Cent. 2017;24:472–476. doi: 10.5455/jtomc.2017.09.124. [DOI] [Google Scholar]
- Karanis P., Kimura A. Evaluation of three flocculation methods for the purification of Cryptosporidium parvum oocysts from water samples. Lett. Appl. Microbiol. 2002;34:444–449. doi: 10.1046/j.1472-765X.2002.01121.x. [DOI] [PubMed] [Google Scholar]
- Karanis P., Kourenti C., Smith H. Waterborne transmission of protozoan parasites: a worldwide review of outbreaks and lessons learnt. J. Water Health. 2007;5:1–38. doi: 10.2166/wh.2006.002. [DOI] [PubMed] [Google Scholar]
- Kaur H., Iqbal S., Inga E., Yawe D. Encystment and excystment in ciliated protists: multidimensional approach. Curr. Sci. 2019;117:198–203. doi: 10.18520/cs/v117/i2/198-203. [DOI] [Google Scholar]
- Khanum H., Khanam S.S., Sultana M., Uddin M.H., Dhar R.C., Islam M.S. Protozoan parasites in a wastewater treatment plant of Bangladesh. Univ. J. Zool. Rajshahi Univ. 2012;31:5–8. doi: 10.3329/ujzru.v31i0.15372. [DOI] [Google Scholar]
- Kourenti C., Heckeroth A., Tenter A., Karanis P. Development and application of different methods for the detection of toxoplasma gondii in water. Appl. Environ. Microbiol. 2003;69:102–106. doi: 10.1128/AEM.69.1.102-106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudah C., Sovoe S., Baiden F. Parasitic contamination of commonly consumed vegetables in two markets in Ghana. Ghana Med. J. 2018;52:88–93. doi: 10.4314/gmj.v52i2.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Wang Z., Karim M.R., Zhang L. Detection of human intestinal protozoan parasites in vegetables and fruits: a review. Parasit. Vectors. 2020;13:380. doi: 10.1186/s13071-020-04255-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little C.J.H. A note on Balantidium coli. J. R. Army Med. Corps. 1931;56:298–299. [Google Scholar]
- López Arias L., Guillemi E., Bordoni N., Farber M., Garbossa G. Development of a PCR assay for identification of Neobalantidium coli (Pomajbíková et al., 2013) in Argentina. Vet. Parasitol. Reg. Stud. Rep. 2017;10:114–118. doi: 10.1016/j.vprsr.2017.08.010. [DOI] [PubMed] [Google Scholar]
- Lora-Suarez F., Rivera R., Triviño-Valencia J., Gomez-Marin J.E. Detection of protozoa in water samples by formalin/ether concentration method. Water Res. 2016;100:377–381. doi: 10.1016/j.watres.2016.05.038. [DOI] [PubMed] [Google Scholar]
- Manser M.M., Saez A.C.S., Chiodini P.L. Faecal parasitology: concentration methodology needs to be better standardised. PLoS Negl. Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0004579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matini M., Shamsi-Ehsan T., Maghsood A.H. The parasitic contamination of farm vegetables in Asadabad City, West of Iran, in 2014. Avicenna J. Clin. Microbiol. Infect. 2017;4 doi: 10.17795/ajcmi-32474. [DOI] [Google Scholar]
- McDonald J.D. On Balantidium coli (Malmsten) and Balantidium suis (sp. nov.), with an account of their neuro-motor apparatus. Univ. Calif. Publ. Zool. 1922;20:243–300. [Google Scholar]
- Milinovich G.J., Klieve A.V. In: Zoonotic Pathogens in the Food Chain. Krause D.O., Hendrick S., editors. CAB International; Wallingford, U.K.: 2011. Manure as a source of zoonotic pathogens; pp. 59–83. [Google Scholar]
- Morais da Silva S.R., Maldonade I.R., Ginani V.C., Lima S.A., Mendes V.S., Ximenes Azevedo M.L., Gurgel-Gonçalves R., Rodrigues Machado E. Detection of intestinal parasites on field-grown strawberries in the Federal District of Brazil. Rev. Soc. Bras. Med. Trop. 2014;47:801–805. doi: 10.1590/0037-8682-0044-2014. [DOI] [PubMed] [Google Scholar]
- Moreno Y., Moreno-Mesonero L., Amorós I., Pérez R., Morillo J.A., Alonso J.L. Multiple identification of most important waterborne protozoa in surface water used for irrigation purposes by 18S rRNA amplicon-based metagenomics. Int. J. Hyg. Environ. Health. 2018;221:102–111. doi: 10.1016/j.ijheh.2017.10.008. [DOI] [PubMed] [Google Scholar]
- Mukhtar I.M.I., Elfaki T.E.M., Alla A.B.A., Saad M.B.E.A. Role of vegetables in the transmission of intestinal parasites in Khartoum central market, Khartoum State, Sudan. Eur. Acad. Res. 2016;3:11825–11837. [Google Scholar]
- Nazemi S., Raei M., Amiri M., Chaman R. Parasitic contamination of raw vegetables in Shahroud, Semnan. Zahedan J. Res. Med. Sci. 2012;14:84–86. [Google Scholar]
- Nsoh F.A., Wung B.A., Atashili J., Benjamin P.T., Marvlyn E., Ivo K.K., Nguedia A.J.C. Prevalence, characteristics and correlates of enteric pathogenic protozoa in drinking water sources in Molyko and Bomaka, Cameroon: a cross-sectional study. BMC Microbiol. 2016;16:268. doi: 10.1186/s12866-016-0890-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogunremi T.T., Ogunniyi T.A.B., Olajide J.S. Human enteric parasitic pathogens in fresh fruits and vegetables consumed in Ile-Ife. Osun State. Food Sci. Qual. Manag. 2017;66:34–41. [Google Scholar]
- Ordoñez K.N., Lim Y.A.L., Goh X.T., Paller V.G.V. Parasite contamination of freshly harvested vegetables from selected organic and conventional farms in the Philippines. Pertanika J. Trop. Agric. Sci. 2018;41:1741–1756. [Google Scholar]
- Orlandi P.A., Chu D.M.T., Bier J.W., Jackson G.J. Parasites and the food supply. Food Technol. 2002;56:72–81. [Google Scholar]
- Oyibo I.F., Ahmadu L., Stephen O.O., Ameh Y.C. Evaluation of parasites of medical importance in drinking water sources in Okura District, Dekina Local Gobernment, Kogi State, Nigeria. Int. J. Dev. Res. 2016;6:7290–7294. [Google Scholar]
- Pires S.M., Evers E.G., Van Pelt W., Ayers T., Scallan E., Angulo F.J., Havelaar A., Hald T., Schroeter A., Brisabois A., Thebault A., Käsbohrer A., Schroeder C., Frank C., Guo C., Wong D.L.F., Döpfer D., Snary E., Nichols G., Spitznagel H., Wahlström H., David J., Pancer K., Stark K., Forshell L.P., Nally P., Sanders P., Hiller P. Attributing the human disease burden of foodborne infections to specific sources. Foodborne Pathog. Dis. 2009;6:417–424. doi: 10.1089/fpd.2008.0208. [DOI] [PubMed] [Google Scholar]
- Pires S.M., Vieira A.R., Perez E., Wong D.L.F., Hald T. Attributing human foodborne illness to food sources and water in Latin America and the Caribbean using data from outbreak investigations. Int. J. Food Microbiol. 2012;152:129–138. doi: 10.1016/j.ijfoodmicro.2011.04.018. [DOI] [PubMed] [Google Scholar]
- Poma H.R., Gutiérrez Cacciabue D., Garcé B., Gonzo E.E., Rajal V.B. Towards a rational strategy for monitoring of microbiological quality of ambient waters. Sci. Total Environ. 2012;433:98–109. doi: 10.1016/j.scitotenv.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomajbíková K., Petrželková K.J., Profousová I., Petrášová J., Modrý D. Discrepancies in the occurrence of Balantidium coli between wild and captive African great apes. J. Parasitol. 2010;96:1139–1144. doi: 10.1645/ge-2433.1. [DOI] [PubMed] [Google Scholar]
- Pomajbíková K., Oborník M., Horák A., Petrželková K.J., Grim J.N., Levecke B., Todd A., Mulama M., Kiyang J., Modrý D. Novel insights into the genetic diversity of Balantidium and Balantidium-like cyst-forming ciliates. PLoS Negl. Trop. Dis. 2013;7 doi: 10.1371/journal.pntd.0002140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce-Gordo F., García-Rodríguez J.J. Balantioides coli. Res. Vet. Sci. 2021;135:424–431. doi: 10.1016/j.rvsc.2020.10.028. [DOI] [PubMed] [Google Scholar]
- Ponce-Gordo F., Jirků-Pomajbíková K. In: Global Water Pathogens Project. Rose J.B., Jiménez-Cisneros B., editors. UNESCO; 2017. Balantidium coli; p. 14. [DOI] [Google Scholar]
- Ponce-Gordo F., Jimenez-Ruiz E., Martínez-Díaz R.A. Tentative identification of the species of Balantidium from ostriches (Struthio camelus) as Balantidium coli-like by analysis of polymorphic DNA. Vet. Parasitol. 2008;157:41–49. doi: 10.1016/j.vetpar.2008.06.024. [DOI] [PubMed] [Google Scholar]
- Ponce-Gordo F., Fonseca-Salamanca F., Martínez-Díaz R.A. Genetic heterogeneity in internal transcribed spacer genes of Balantidium coli (Litostomatea, Ciliophora) Protist. 2011;162:774–794. doi: 10.1016/j.protis.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Rees C.W. Balantidia from pigs and guinea-pigs: their viability, cyst production and cultivation. Science (80-. ) 1927;66:89–91. doi: 10.1126/science.66.1699.89. [DOI] [PubMed] [Google Scholar]
- Robertson L.J. In: Food Associated Pathogens. Tham W., Danielsson-Tham M.-L., editors. CRC Press; Boca Raton: 2014. Protozoan parasites: A plethora of potentially foodborne pathogens; pp. 169–216. [Google Scholar]
- Rodrigues Maldonade I., Cortez Ginani V., Resende Riquette R.F., Gurgel-Gonçalves R., Silveira Mendes V., Rodrigues Machado E. Good manufacturing practices of minimally processed vegetables reduce contamination with pathogenic microorganisms. Rev. Inst. Med. Trop. Sao Paulo. 2019;61 doi: 10.1590/S1678-9946201961014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosado-García F.M., Guerrero-Flórez M., Karanis G., Hinojosa M.D.C., Karanis P. Water-borne protozoa parasites: the Latin American perspective. Int. J. Hyg. Environ. Health. 2017;220:783–798. doi: 10.1016/j.ijheh.2017.03.008. [DOI] [PubMed] [Google Scholar]
- Rousseau A., La Carbona S., Dumètre A., Robertson L.J., Gargala G., Escotte-Binet S., Favennec L., Villena I., Gérard C., Aubert D. Assessing viability and infectivity of foodborne and waterborne stages (cysts/oocysts) of Giardia duodenalis, Cryptosporidium spp., and Toxoplasma gondii: a review of methods. Parasite. 2018:25. doi: 10.1051/parasite/2018009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer F.W., Marshall M.M., Clancy J.L. In: World Class Parasites: Volume 8. The Pathogenic Enteric Protozoa: Giardia, Entamoeba, Cryptosporidium and Cyclospora. Sterling C.R., Adam R.D., editors. Kluwer Academic Publishers; New York: 2004. Inactivation and removal of enteric protozoa in water; pp. 117–127. [DOI] [Google Scholar]
- Schumaker E. Experimental infection of rats with the Balantidium from the pig. Science (80-. ) 1930;70:384. doi: 10.1126/science.70.1816.384. [DOI] [PubMed] [Google Scholar]
- Schuster F.L., Visvesvara G.S. Amebae and ciliated protozoa as causal agents of waterborne zoonotic disease. Vet. Parasitol. 2004;126:91–120. doi: 10.1016/j.vetpar.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Sena Barnabé A., Ribeiro R., Ferraz N., De Carvalho Pincinato E., Clayton R., Gomes F., Gabriela T., Galleguillos B., Cerqueira M.Z., Gean E., Soares L., Lage P.S., Araújo C.X., Szamszoryk M., Cristiano Y., Massara L. Análisis comparativo de los métodos para la detección de parásitos en las hortalizas para el consumo humano. Rev. Cuba. Med. Trop. Trop. 2010;62:21–27. [PubMed] [Google Scholar]
- Shah A.A., Khan M.A., Kanwal N., Bernstein R. Assessment of safety of drinking water in tank district: an empirical study of water-borne diseases in rural Khyber Pakhtunkhwa, Pakistan. Int. J. Environ. Sci. 2016;6:418–428. [Google Scholar]
- Shikara M., Dhia H., Abdul Hameed A.R. Evaluation of some Physico-chemical and detection of parasites in sediment of Hamrin Lake. Iraqi J. Sci. Technol. 2019;10:96–103. [Google Scholar]
- Smith H.V. Detection of parasites in the environment. Parasitology. 1998;117:S113–S141. doi: 10.1017/S0031182099004898. [DOI] [PubMed] [Google Scholar]
- Soares F.A., Benitez A.D.N., Dos Santos B.M., Loiola S.H.N., Rosa S.L., Nagata W.B., Inácio S.V., Suzuki C.T.N., Bresciani K.D.S., Falcão A.X., Gomes J.F. A historical review of the techniques of recovery of parasites for their detection in human stools. Rev. Soc. Bras. Med. Trop. 2020;53 doi: 10.1590/0037-8682-0535-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobón S.R., López Jiménez J., Campo Polanco L.F., Agudelo Cadavid R.M., Gutiérrez Builes L.A. Procedure optimization for concentration and detection of protozoa and helminths in great volumes of water. Rev. Cubana Med. Trop. 2018;70:71–77. [Google Scholar]
- Traub R.J., Cuttell L. In: Foodborne Parasites in the Food Supply Web: Occurrence and Control. Gajadhar A.A., editor. Elsevier Ltd.; Amsterdam: 2015. Laboratory diagnostic methods; pp. 403–428. [DOI] [Google Scholar]
- Verni F., Rosati G. Resting cysts: a survival strategy in protozoa ciliophora. Ital. J. Zool. 2011;78:134–145. doi: 10.1080/11250003.2011.560579. [DOI] [Google Scholar]
- Viezzer Bianchi M., Santos de Mello L., Wentz M.F., Panziera W., Soares J.F., Sonne L., Driemeier D., Petinatti Pavarini S. Fatal parasite-induced enteritis and typhlocolitis in horses in southern Brazil. Rev. Bras. Parasitol. Vet. 2019;28:443–450. doi: 10.1590/s1984-29612019056. [DOI] [PubMed] [Google Scholar]
- Walker E.L. Experimental balantidiasis. Philipp. J. Sci. Sect. B. Trop. Med. 1913;8:333–349. [Google Scholar]
- Walzer P.D., Judson F.N., Murphy K.B., Healy G.R., English D.K., Schultz M.G. Balantidiasis outbreak in Truk. Am. J. Trop. Med. Hyg. 1973;22:33–41. doi: 10.4269/ajtmh.1973.22.33. [DOI] [PubMed] [Google Scholar]
- World Health Organization . 4th. Ed. World Health Organization; Geneva: 2017. Guidelines for Drinking-Water Quality. incorporating the first addendum. [Google Scholar]
- Yan W., He K., Qian W., Wang T., Zong Y., Zhang M., Wei Z., Han L. First molecular identification of Buxtonella ciliates from captive-bred mangabeys (Cercocebus torquatus) from China. Parasitol. Res. 2018;117:3753–3759. doi: 10.1007/s00436-018-6075-4. [DOI] [PubMed] [Google Scholar]
- Yan W., Wang T., Zhao L., Sun C. Modified DMEM xenic culture medium for propagation, isolation and maintenance of Balantioides coli. Acta Trop. 2021;214 doi: 10.1016/j.actatropica.2020.105762. [DOI] [PubMed] [Google Scholar]
- Young M.D. Attempts to transmit human Balantidium coli. Am. J. Trop. Med. 1950;s1-30:71–72. doi: 10.4269/ajtmh.1950.s1-30.71. [DOI] [PubMed] [Google Scholar]
- Zhang H., Zhang X., Zhang S., Wei B., Jiang Q., Yu X. Detecting Cryptosporidium parvum and Giardia lamblia by coagulation concentration and real-time PCR quantification. Front. Environ. Sci. Eng. 2013;7:49–54. doi: 10.1007/s11783-012-0455-2. [DOI] [Google Scholar]