Abstract
Objective
Preeclampsia is a major risk factor for maternal and foetal mortality and morbidity. There have been tremendous efforts to identify serum biomarkers which can reliably predict the occurrence of preeclampsia. The study aims to assess the biomarkers that have the greatest utility in the diagnosis of preeclampsia.
Methods
A systematic search was performed on the PubMed literature database, and chain references were retrieved. Original research articles composed of case controls, cohorts, randomised control trials, and cross-sectional studies were included. The recorded variables included each study's design, type, year, and location; the value (mean ± standard deviation) of the markers in the patients and the pregnant controls; and the p-value, unit of measurement, and the sample size of each study. The results were interpreted based on the standardised mean difference (SMD) values.
Results
A total of 398 studies were retrieved from the PubMed database. After further analysis, 89 studies were selected for this review. An additional 47 studies were included based on chain referencing. Later, 136 full-text articles were reviewed in detail and their data were entered. Finally, 25 studies, in which 13 serum biomarkers were assessed, were selected for this meta-analysis. The levels of the angiogenic markers fms-like tyrosine kinase (sFlt), sFlt/placental growth factor (PlGF), and endoglin were significantly higher in patients with preeclampsia than in the pregnant controls. The levels of PlGF and the lipid biomarkers high density lipoprotein (HDL) and adiponectin were significantly lower, while the levels of triglycerides, apolipoprotein B (APO-B), and leptin were elevated in the preeclamptic patients compared to the pregnant controls (p < 0.05).
Conclusion
In our study, the values of the serum biomarkers sFlt, PlGF, sFlt/PlGF, HDL, adiponectin, leptin, triglycerides, and APO-B differed significantly between preeclampsia patients and the pregnant controls. These findings demand advanced evaluation of biomarkers to enhance diagnostic screening for preeclampsia.
Keywords: Biomarkers, Diagnosis, Meta-analysis, Pre-eclampsia, Pregnancy
الملخص
أهداف البحث
تسمم الحمل هو عامل خطر رئيس لوفيات ومراضة الأمهات والجنين. كانت هناك جهود هائلة لتحديد المؤشرات الحيوية في المصل، والتي يمكن أن تتنبأ بشكل موثوق بتسمم الحمل. تهدف الدراسة إلى تقييم المؤشرات الحيوية التي لها أفضل فائدة في تشخيص تسمم الحمل.
طرق البحث
تم إجراء بحث منهجي في قاعدة بيانات "بب ميد" وتم استرداد المراجع المتسلسلة. تم تضمين المقالات البحثية الأصلية التي تضم ضوابط الحالة، والأتراب، وتجارب التحكم العشوائية، والدراسات المقطعية. تضمنت المتغيرات المسجلة تصميم الدراسة ونوعها وسنة ومكان الدراسة وقيمة المتوسط ± الانحراف المعياري للعلامات في المرضى والضوابط والوحدة وحجم عينة مجتمع الدراسة. تم تفسير النتائج بناء على قيم فرق المتوسط المعيارية.
النتائج
تم استرجاع ما مجموعه ٣٨٩ دراسة من قاعدة بيانات "بب ميد". وأدى التحليل الإضافي ٨٩ دراسة لهذه المراجعة. تم تضمين ٤٧ دراسة إضافية من سلسلة المراجع. في وقت لاحق، تمت مراجعة ١٣٦ مقالة ذات نص كامل بالتفصيل وتم إدخال البيانات. أخيرا، تم اختيار ٢٥ دراسة لهذا التحليل التلوي، وقيمت ١٣ واصما حيويا في مصل الدم. كانت الواسمات المولدة للأوعية والتيروزين كيناز، وعامل النمو المشيمي، والإندوغلين مرتفعة بشكل ملحوظ في المرضى الذين يعانون من تسمم الحمل مقارنة بالضوابط العادية للحوامل. وكان عامل النمو المشيمي والعلامات الحيوية الدهنية عالية الكثافة والأديبونكتين منخفضة بشكل ملحوظ. في حين أن الدهون الثلاثية، وصميم البروتين ب، واللبتين كانت مرتفعة في مرضى مقدمات الارتعاج مقارنة بالضوابط العادية للحوامل.
الاستنتاجات
في دراستنا، أظهرت قيم المؤشرات الحيوية في المصل؛ الواسمات المولدة للأوعية وعامل النمو المشيمي والدهون عالية الكثافة والأديبونكتين واللبتين والدهون الثلاثية وصميم البروتين ب، اختلافات كبيرة بين المرضى والضوابط. تتطلب هذه النتائج تقييما متقدما للواسمات الحيوية من أجل الفحص التشخيصي لمقدمات الارتعاج.
الكلمات المفتاحية: المؤشرات الحيوية, التشخيص, التحليل التلوي, تسمم الحمل, الحمل
Introduction
Preeclampsia is a pregnancy disorder characterised by the new onset of hypertension accompanied by apparent proteinuria after twenty weeks of pregnancy. In the absence of proteinuria, preeclampsia presents as hypertension and any one of the following features: renal insufficiency, impaired liver function, thrombocytopenia, pulmonary oedema, or the new onset of headache (American College of Obstetrics and Gynecology, ACOG, 2019).1
The worldwide prevalence of preeclampsia is 3.8% in pregnant women, and it is responsible for more than 70,000 maternal and 500,000 foetal deaths each year.2,3 The World Health Organization (WHO) has set Sustainable Development Goals to encourage nations to reduce the maternal mortality rate to fewer than 70/100,000 births by 2030.4 The primary burden of reducing maternal mortality falls upon Sub-Saharan Africa and South Asia.5 The reported mortality rate in Pakistan in 2019 was 178/100,000 births, which trailed far behind the target rate.4 According to a WHO report, hypertensive disorders were second to haemorrhage as the leading cause of maternal mortality worldwide, accounting for 14% of deaths, and third in South Asia, following haemorrhage and sepsis.6 Preeclampsia, once diagnosed, requires specialised monitoring of both the mother and foetus.7 The complications for mothers include renal failure, hepatic failure, pulmonary oedema, placental abruption, cerebral haemorrhage, and eclampsia.7 The foetal complications include preterm birth, foetal growth restriction, stillbirth, and the necessity for neonatal intensive care; one-quarter of the neonatal deaths in developing countries are due to preeclampsia.8 Various maternal characteristics, such as hypertension, diabetes, obesity, autoimmune disease, advanced age, and nulliparity, increase the risk of preeclampsia; however, screening for maternal characteristics only results in the detection of 40–54% of preeclampsia cases.9
Preeclampsia is a heterogeneous disorder, and the use of several biomarkers for early diagnosis and screening has been investigated. The markers include ones representing angiogenic and antiangiogenic factors, inflammation, oxidative stress, endothelial damage, endocrine hormones, lipid metabolism, homeostasis, and foetal distress.10 Despite the exploration of many biomarkers, only tests for placental growth factor (PlGF) have been approved by the National Institute of Health and Care Excellence (NICE) (UK) for use alongside the standard clinical assessment, and they are only available in a handful of developed countries, which include the UK and Germany.11 However, a prospective multicentre study conducted by the WHO across eight countries on high-risk pregnant women showed that screening for the currently available biomarkers, in addition to using the standard clinical tests, did not significantly improve the ability to predict preeclampsia at <20 weeks of pregnancy.12 Therefore, additional markers and combinations that can be used to accurately predict and diagnose preeclampsia must be explored so that early antenatal surveillance and preventive treatments can be utilised to prevent maternal and foetal morbidity and mortality.
The rationale of this study: Biochemical changes in the serum of pregnant women precede the clinical signs and symptoms of preeclampsia, and these biomarkers may be utilised for the early detection of it.13 Several markers have been investigated as possible indicators for the diagnosis of, and screening for, preeclampsia; however, because inconsistent and poorly reproducible results prevail, combinations of two or three biomarkers are being investigated.10 This systemic review and meta-analysis were conducted to determine the diagnostic utility of serum biomarkers in preeclampsia through analysis of the standardised mean difference.
The review question: What is the diagnostic utility of the biomarkers associated with preeclampsia in terms of the standardised mean difference?
Materials and Methods
The systematic review and meta-analysis were conducted following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA).14 As this review involved only data from previous studies, the approval of an institutional review board was not required.
Search method and data sources
The literature search engine PubMed was searched independently by the first two authors, RS and FB, on 5 January 2018. The terms used for the search were ‘Preeclampsia, not eclampsia’, ‘biomarkers or marker’, and ‘blood or serum or plasma’, and the dates of the search spanned from 1980/01/01 to 2017/12/31. The relevant papers cited in the articles were also retrieved.
Selection of studies
The studies that were selected met the following criteria: 1. They reported on markers in the blood of pregnant pre-eclamptic women. 2. They gave a complete description of their cases and controls. 3. They defined preeclampsia according to the ISSHP, ACOG, or comparable guidelines.1,3,11 Original research papers composed of case controls, cohorts, randomised control trials, and cross-sectional studies were included, and any papers whose full text was not in the English language and those which were reviews, abstracts of posters, and animal studies were excluded by the first two authors. The selected full texts of all the original research articles were read by the first author, and the data were compiled in Microsoft Excel datasheets.
Data extraction
The recorded variables were the study design; the type, location, and year of each study; the values of the biomarkers in the participants’ blood; the serum or plasma of preeclamptic patients and the controls, p-values, units of measurement, and techniques used; the sample size of each study; the results; and the gestational age at the time of sample collection. The type of study and year and location of each study were also recorded. The recorded statistical measures were the mean with standard deviation (mean ± SD). All studies reporting medians, interquartile ranges, and multiples of the median were excluded. The standard errors of the means were converted to standard deviations. When the units of measurement used in the studies differed, they were converted to similar ones for comparative analysis. Any studies reporting biomarkers in urine and in the cord or foetal blood were also excluded. Most of the articles reported a single value for a biomarker, which was entered as it appeared in the study. In a few of the research articles, multiple values were reported for a single biomarker; in such cases, the highest mean value was taken. Trough values were taken for a few biomarkers, such as PAPPA and PlGF, which are known to be present in low levels in patients with preeclampsia. The biomarkers that were reported in at least two studies were selected for the meta-analysis.
Statistical analysis
All the analyses were performed using Stata v.12.0 using the ‘metan’ command. I2 was used to determine the degree of heterogeneity between the markers. If the I2 value was under 50%, the fixed-effects model was used; otherwise, the random-effects model was used.15 A p-value of under 0.05 was considered significant. The results were interpreted based on the standardised mean difference (SMD) values.
Results
Studies included for meta-analysis
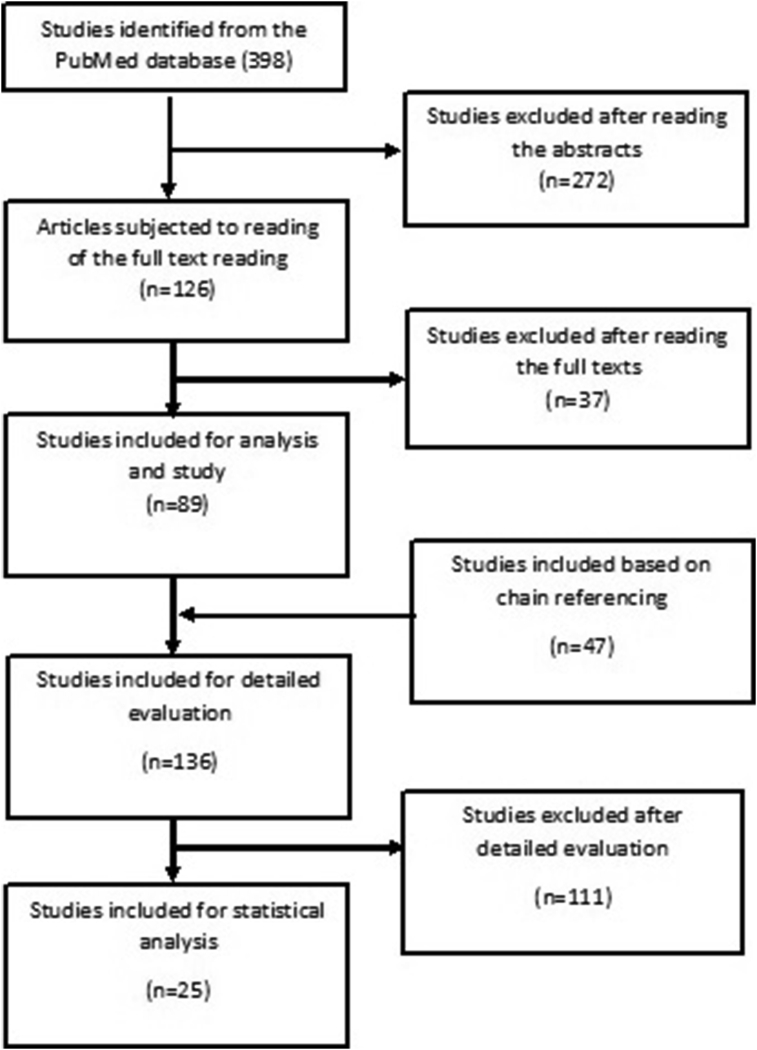
The number of articles retrieved from PubMed was 398 (Figure 1). After reading the abstracts of all the articles, we excluded 272 papers; of these, 54 were review articles, six lacked the full text in English, 11 were animal studies, 19 were abstracts from posters, and 182 were not relevant to the topic. The full texts of 126 articles were read and a further 37 articles were excluded. Thus, 89 studies were identified after reading their full texts. An additional 47 studies were identified based on chain referencing, and their full texts were read. The data of a total of 136 studies were entered in Excel datasheets and evaluated. Finally, 25 studies reporting on 13 biomarkers were selected for the meta-analysis (Figure 1).
Figure 1.
Biomarkers in patients with preeclampsia, a meta-analysis.
A brief overview of the data of these studies is given in the table (Table 1).
Table 1.
Serum biomarkers evaluated in the meta-analysis for the prediction and diagnosis of preeclampsia.
| Markers/Author of the study | Year of study | Location of study | Sample size, controls | Sample size, cases | Mean value of marker±SD in controls | Mean value of marker±SD in patients with preeclampsia |
|---|---|---|---|---|---|---|
| Marker: PlGF (pg/ml) | ||||||
| Brett Young16 | 2010 | USA | 1564 | 11 | 484 ± 412 | 117.4 ± 63.9 |
| Alexander M. Holston17 | 2009 | USA | 254 | 40 | 460 ± 796.9 | 275 ± 1106.8 |
| Richard J. Levine18 | 2004 | USA | 70 | 14 | 740 ± 1255 | 150 ± 374.2 |
| Theresa Engels19 | 2013 | Germany | 184 | 64 | 496.27 ± 44.96 | 67.74 ± 5.53 |
| Kaori Koga20 | 2010 | Japan | 21 | 8 | 16.27 ± 8.97 | 16.9 ± 9.96 |
| Markus Schmidt21 | 2009 | Germany | 44 | 7 | 80.58 ± 35.18 | 42.7 ± 23.21 |
| Yan-Qiong Ouyang22 | 2009 | China | 50 | 83 | 245.9 ± 36.1 | 173.8 ± 19 |
| Marker: sFlt1 (pg/ml) | ||||||
| Zeynep B. Güngör23 | 2017 | Turkey | 27 | 52 | 10,120 ± 5030 | 9990 ± 1970 |
| Brett Young16 | 2010 | USA | 1564 | 11 | 7300 ± 3800 | 23,500 ± 4300 |
| Alexander M. Holston17 | 2009 | USA | 254 | 40 | 9160 ± 15937.7 | 15,390 ± 15,811 |
| Kaori Koga20 | 2010 | Japan | 21 | 8 | 514.5 ± 216 | 458 ± 127 |
| Richard J. Levine18 | 2004 | USA | 70 | 14 | 1634 ± 836.6 | 4382 ± 4490 |
| Theresa Engels19 | 2013 | Germany | 184 | 64 | 2684.8 ± 138.41 | 10887.85 ± 878.37 |
| Clarissa M. Tobinaga24 | 2014 | Brazil | 54 | 54 | 2885 ± 1958 | 21,807 ± 17,252 |
| Yan-Qiong Ouyang22 | 2009 | China | 50 | 83 | 1579 ± 327 | 3455.3 ± 342.6 |
| Tinnakorn Chaiworapongsa25 | 2005 | USA | 42 | 42 | 1820 + 1249 | 6568 + 5380 |
| Marker: sFlt1/PlGF | ||||||
| Brett Young16 | 2010 | USA | 1564 | 11 | 28 ± 32 | 286 ± 200 |
| Theresa Engels19 | 2013 | Germany | 184 | 64 | 18.03 ± 2.82 | 276.97 ± 38.86 |
| Yan-Qiong Ouyang22 | 2009 | China | 50 | 83 | 6.6 ± 1.8 | 20.1 ± 2.7 |
| Richard J. Levine26 | 2006 | USA | 40 | 40 | 5 ± 03 | 80 ± 30 |
| Marker: endoglin (ng/ml) | ||||||
| Zeynep B. Güngör23 | 2017 | Turkey | 27 | 52 | 8.78 ± 1.23 | 10.22 ± 1.13 |
| Pooneh Nikuei27 | 2017 | Iran | 20 | 15 | 13.58 ± 5.8 | 26.34 ± 3.37 |
| Brett Young16 | 2010 | USA | 1564 | 11 | 8.7 ± 3.6 | 43.6 ± 27.7 |
| Alexander M. Holston17 | 2009 | USA | 254 | 40 | 11.3 ± 31.87 | 18 ± 25.3 |
| Clarissa M. Tobinaga24 | 2014 | Brazil | 54 | 54 | 7.9 ± 3.8 | 46.9 ± 38.3 |
| Zhongguo Wei Zhong28 | 2010 | China | 30 | 26 | 7.49 ± 2.73 | 10.96 ± 3.21 |
| Richard J. Levine26 | 2006 | USA | 40 | 40 | 9.8 ± 1.58 | 46.6 ± 7.63 |
| Marker: HDL (mg/dl) | ||||||
| G. León-Reyes29 | 2017 | Mexico | 30 | 30 | 51.31 ± 18.16 | 49.07 ± 16.82 |
| Nansi S. Boghossian30 | 2017 | Texas, USA | 136 | 177 | 63.42 ± 13.15 | 61.48 ± 14.70 |
| Carlos Antonio Negrato31 | 2009 | Brazil | 180 | 19 | 63.15 ± 17.26 | 60.98 ± 14.56 |
| Zeynep B. Güngör32 | 2017 | Turkey | 49 | 57 | 69 ± 19 | 63 ± 18 |
| Huseyin Altug Cakmak33 | 2017 | Turkey | 40 | 100 | 47.8 ± 7.2 | 47.2 ± 8.4 |
| S. Kharb34 | 2017 | India | 25 | 25 | 52.42 ± 9.98 | 46.06 ± 11.4 |
| Pauline Mendola35 | 2017 | USA | 117 | 145 | 63.4 ± 13.1 | 61.5 ± 14.8 |
| Hakan Timur36 | 2016 | Turkey | 48 | 48 | 58.19 ± 8.09 | 46.74 ± 9.22 |
| Marker: LDL (mg/dl) | ||||||
| G. León-Reyes29 | 2017 | Mexico | 30 | 30 | 108.6 ± 38.66 | 164.6 ± 83.02 |
| Nansi S. Boghossian30 | 2017 | Texas, USA | 136 | 177 | 161.25 ± 50.27 | 148.1 ± 57.62 |
| S. Kharb34 | 2017 | India | 25 | 25 | 121.72 ± 9.48 | 139.98 ± 38.9 |
| Pauline Mendola35 | 2017 | USA | 100 | 104 | 160.3 ± 45.3 | 146 ± 62.4 |
| Hakan Timur36 | 2016 | Turkey | 48 | 48 | 131.74 ± 16.05 | 157.9 ± 32.99 |
| Marker: triglycerides (mg/dl) | ||||||
| G. León-Reyes29 | 2017 | Mexico | 30 | 30 | 236.8 ± 76.35 | 280.5 ± 115.1 |
| Nansi S. Boghossian30 | 2017 | Texas, USA | 136 | 177 | 244.46 ± 83.26 | 259.5 ± 108.95 |
| Carlos Antonio Negrato31 | 2009 | Brazil | 180 | 19 | 199.58 ± 89.81 | 223.58 ± 120.94 |
| Zeynep B. Güngör32 | 2017 | Turkey | 49 | 57 | 224 ± 94 | 205 ± 90 |
| S. Kharb34 | 2017 | India | 25 | 25 | 171.14 ± 49.43 | 269.98 ± 91.85 |
| Pauline Mendola35 | 2017 | USA | 100 | 104 | 243.9 ± 84.3 | 253.9 ± 97.6 |
| Marker: total cholesterol (mg/dl) | ||||||
| G. León-Reyes29 | 2017 | Mexico | 30 | 30 | 192.9 ± 62.43 | 213.5 ± 58.33 |
| Nansi S. Boghossian30 | 2017 | Texas, USA | 136 | 177 | 256.77 ± 48.34 | 248.65 ± 65.74 |
| Zeynep B. Güngör32 | 2017 | Turkey | 49 | 92 | 233 ± 49 | 241 ± 50 |
| S. Kharb34 | 2017 | India | 25 | 25 | 220.62 ± 51.33 | 244.62 ± 69.2 |
| Marker: APO-A (mg/dl) | ||||||
| G. León-Reyes29 | 2017 | Mexico | 30 | 30 | 178.5 ± 49.51 | 186.1 ± 46.53 |
| S. Kharb34 | 2017 | India | 25 | 25 | 157.12 ± 34.17 | 144.86 ± 41.6 |
| Pauline Mendola35 | 2017 | USA | 100 | 104 | 43.7 ± 51.5 | 46.9 ± 44.3 |
| Hakan Timur36 | 2016 | Turkey | 48 | 48 | 244.37 ± 20.84 | 167.07 ± 14.61 |
| Marker: APO-B (mg/dl) | ||||||
| G. León-Reyes29 | 2017 | Mexico | 30 | 30 | 111.2 ± 37.32 | 125.8 ± 43.94 |
| S. Kharb34 | 2017 | India | 25 | 25 | 109.46 ± 17.95 | 137.84 ± 34.82 |
| Hakan Timur36 | 2016 | Turkey | 48 | 48 | 102.39 ± 8.08 | 104.84 ± 7.05 |
| Marker: leptin (ng/ml) | ||||||
| Zeynep B. Güngör32 | 2017 | Turkey | 27 | 52 | 75.38 ± 28.76 | 126.71 ± 41.67 |
| Ali Khosrowbeygi37 | 2011 | Iran | 30 | 30 | 19.69 ± 1.5336 | 20.75 ± 2.629 |
| S. Kharb34 | 2017 | India | 25 | 25 | 21.77 ± 6.3 | 57.48 ± 18.67 |
| Yan-Qiong Ouyang22 | 2009 | China | 50 | 83 | 12.94 ± 1.36 | 20.04 ± 3.01 |
| Florian Herse38 | 2009 | China | 25 | 32 | 0.58 ± 0.14 | 0.87 ± 0.23 |
| Marker: adiponectin (ng/ml) | ||||||
| Zeynep B. Güngör32 | 2017 | Turkey | 27 | 52 | 0.01793 ± 0.00767 | 0.01733 ± 0.01075 |
| Ali Khosrowbeygi37 | 2017 | Iran | 30 | 30 | 0.0014 ± 0.00022 | 0.01294 ± 0.00034 |
| Clarissa M Tobinaga24 | 2014 | Brazil | 54 | 54 | 2.978 ± 1.245 | 3.060 ± 1.747 |
| Yan-Qiong Ouyang22 | 2009 | China | 50 | 83 | 11.97 ± 1.16 | 6.95 ± 2.88 |
| Florian Herse38 | 2009 | China | 30 | 32 | 38.03 ± 0.92 | 33.10 ± 1.55 |
| Marker: TNFα (pg/ml) | ||||||
| R. Daniela Dávila39 | 2012 | Bolivia | 15 | 20 | 1.74 ± 0.35 | 1.13 ± 0.37 |
| J Tavakkol Afshari40 | 2005 | Iran | 18 | 24 | 51.9 ± 33.8 | 53.8 ± 30 |
| Muzaffer Cakmak41 | 2015 | Turkey | 30 | 99 | 14.62 ± 5.61 | 26.49 ± 12.14 |
The markers can be divided into the following categories based on their functions:
Angiogenic markers
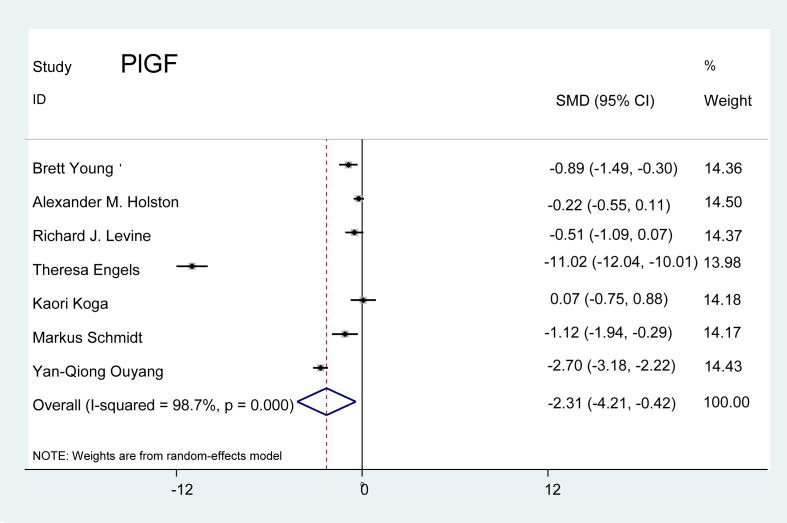
Placental growth factor (PlGF): Seven studies were extracted from the literature, and we observed that PlGF levels were markedly lower in the preeclamptic patients than in the normal pregnant controls. The value of the marker was, however, variable between the studies. As displayed in Figure 2, the combined effect demonstrated that the level of PlGF was 2.31 times lower in preeclamptic patients than in the controls (P = 0.017).
Figure 2.
Effect of PlGF on preeclampsia.
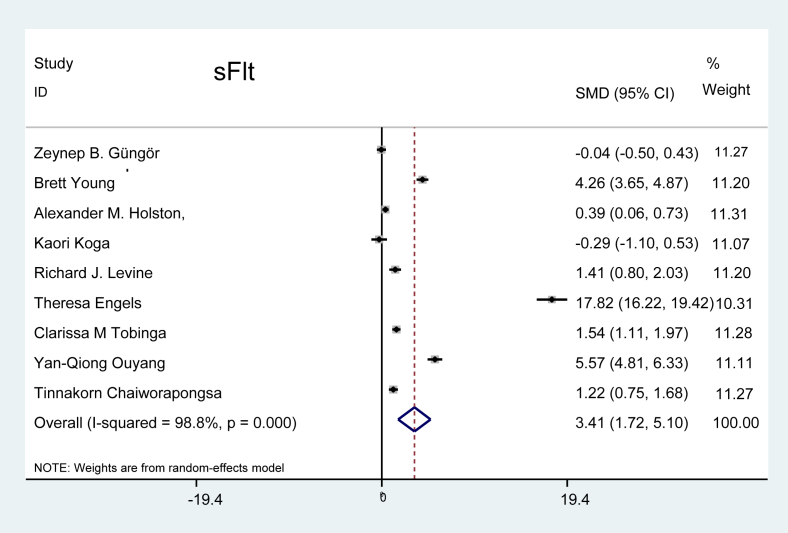
Soluble fms-like tyrosine kinase-1 (sFlt): Nine studies reported the presence of the serum marker sFlt in patients with preeclampsia, two studies showed a negative effect of sFlt on preeclampsia, and the rest of the studies showed a positive effect. The combined effect demonstrated that the level of sFlt was 3.41 times higher in patients with preeclampsia, as depicted in Figure 3 (P < 0.001).
Figure 3.
Effect of sFlt on preeclampsia.
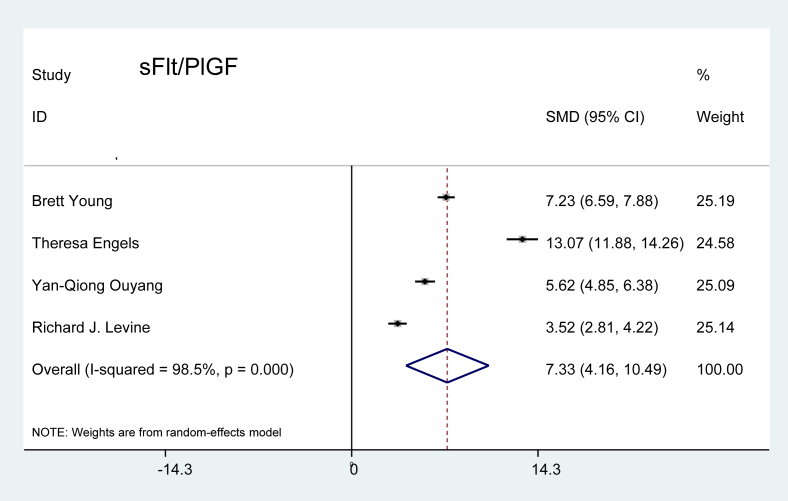
SFlt/PlGF ratio: The SMD ratio of sFlt and PlGF in preeclamptic patients compared to the controls is shown in Figure 4. Four studies related to this marker fulfilled the criteria for inclusion. The combined-effects model revealed a value 7.33 times higher in patients with preeclampsia than in the pregnant controls, which was statistically significant (P < 0.001).
Figure 4.
Effect of sFlt/PlGF on preeclampsia.
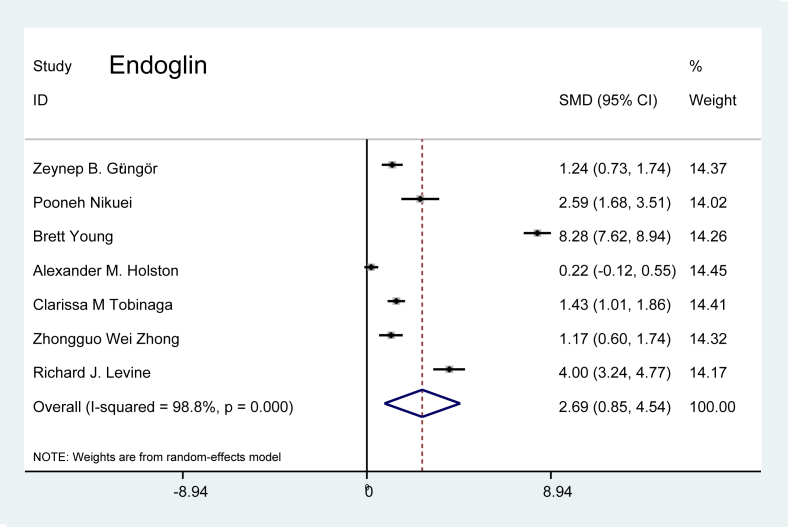
Soluble endoglin (sEng): Seven studies discussing sEng levels were evaluated. The combined-effects model showed that the level of sEng was 2.69 times higher in patients with preeclampsia than in the pregnant controls (p = 0.004), as presented in Figure 5.
Figure 5.
Effect of endoglin on preeclampsia.
Lipid markers
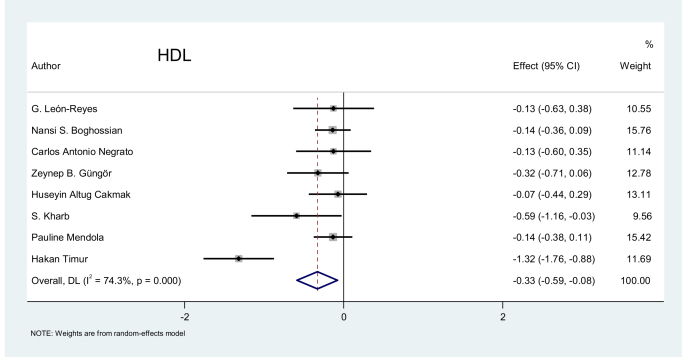
High-density lipoprotein (HDL): Eight studies related to this marker were evaluated. The pooled effect of HDL was −0.33, demonstrating the existence of a negative effect of HDL on preeclampsia. Figure 6 depicts the relatively low values for HDL in patients with preeclampsia using a random-effects model compared to the control group (p = 0.011).
Figure 6.
Effect of HDL on preeclampsia.
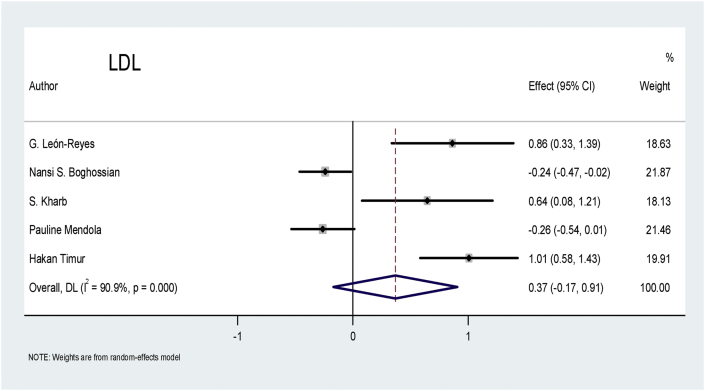
Low-density lipoprotein (LDL): Five studies related to the LDL marker fulfilled the criteria for inclusion. Two studies showed a negative effect, two showed a positive effect, and one study showed an insignificant effect of LDL on patients with preeclampsia. Figure 7 shows that the pooled effect of LDL, at 0.37, did not have a significantly greater effect on patients with preeclampsia than on the controls (p = 0.178).
Figure 7.
Effect of LDL on preeclampsia.
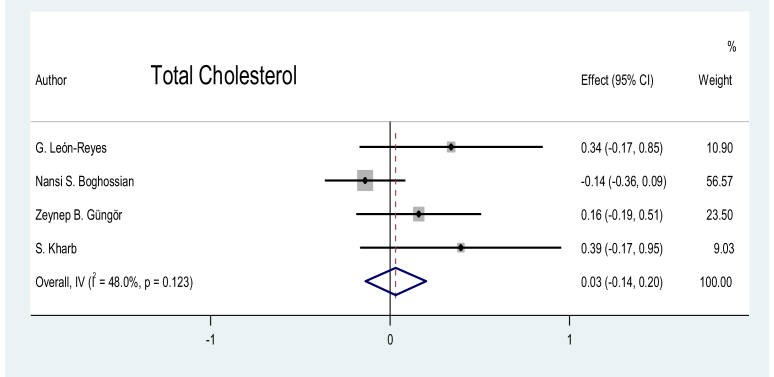
Total cholesterol: Four studies were assessed, and no significant difference in the marker levels was noted between the preeclampsia cases and the pregnant controls (p = 0.397), as shown in Figure 8.
Figure 8.
Effect of total cholesterol on preeclampsia.
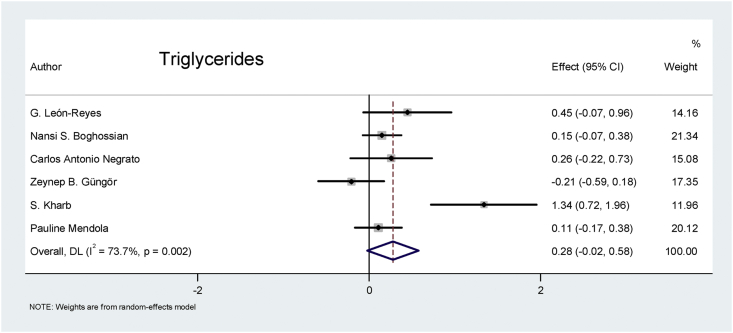
Triglycerides: The combined random-effects model for the triglyceride analysis of the six eligible studies revealed that the biomarker value was 0.28 times higher in patients with preeclampsia than in the pregnant controls, and the difference was nearly significant (p = 0.065), as shown in Figure 9.
Figure 9.
Effect of triglycerides on preeclampsia.
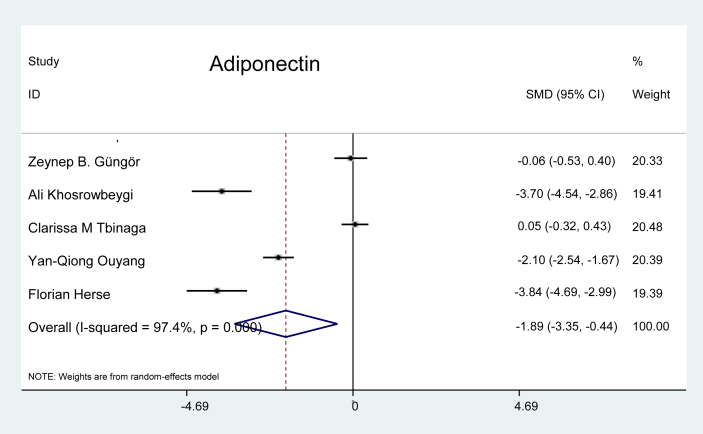
Adiponectin: Five studies were evaluated for the marker Adiponectin, (Figure 10), and the data revealed that the values for this marker were 1.89 times lower in patients with preeclampsia than in the pregnant controls (p = 0.011).
Figure 10.
Effect of adiponectin on preeclampsia.
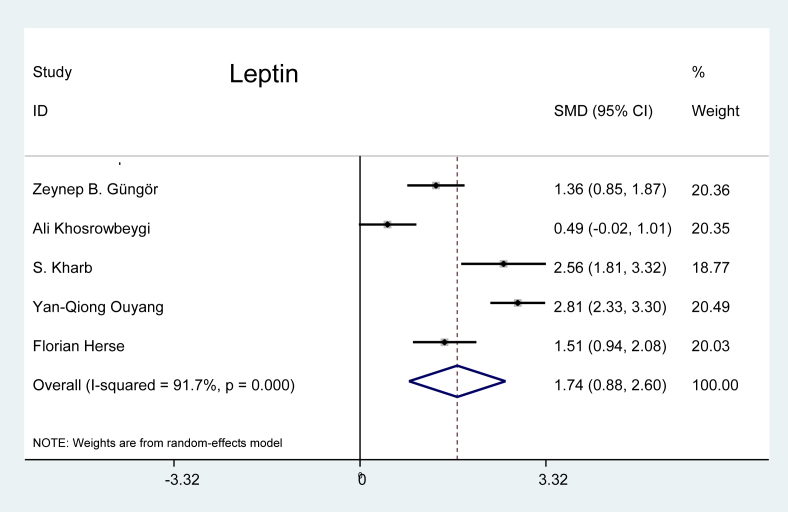
Leptin: Five studies were selected from the literature for leptin, and the random-effects model showed that the effect of this marker was 1.74 times greater on patients with preeclampsia than on the pregnant controls (p < 0.001), as shown in Figure 11.
Figure 11.
Effect of leptin on preeclampsia.
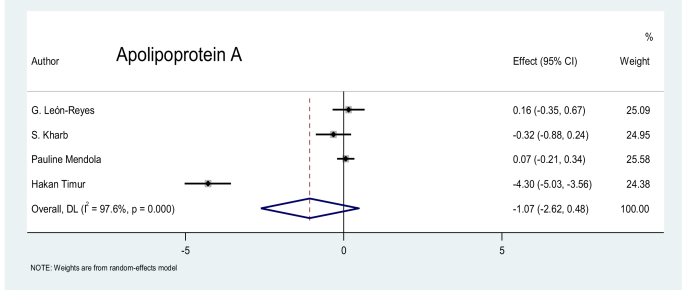
Apolipoprotein A (APO-A): No significant difference in the levels of APO-A was found between the patients with preeclampsia and the pregnant controls, as shown in the four studies in the literature (p = 0.175), the data of which are shown in Figure 12.
Figure 12.
Effect of APO-A on preeclampsia.
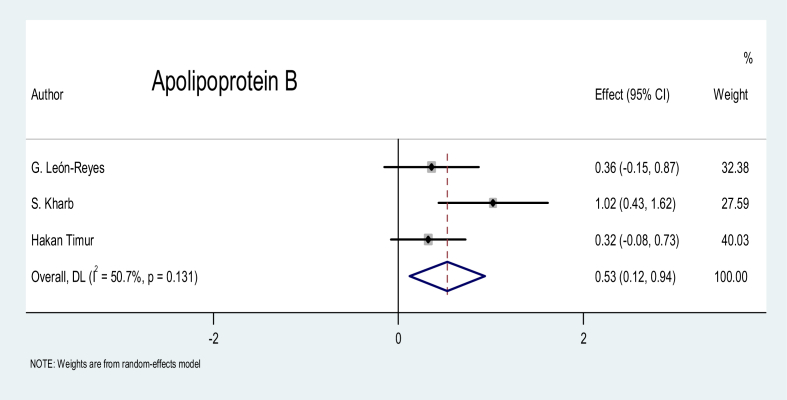
Apolipoprotein B (APO-B): A statistically significant difference was identified between patients with preeclampsia and the pregnant controls for this marker, with an SMD of 0.53 (p = 0.011), as shown in Figure 13.
Figure 13.
Effect of APO-B on preeclampsia.
Inflammatory marker
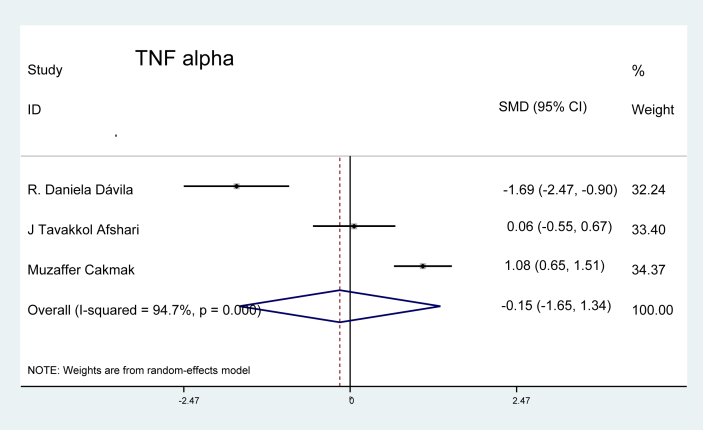
Tumour necrotic factor (TNF): This was the only inflammatory marker that met the criteria for evaluation in three studies in our analysis; however, no significant difference was identified between patients with preeclampsia and the pregnant controls (p = 0.84), as shown in Figure 14.
Figure 14.
Effect of TNF alpha on preeclampsia.
Discussion
The results of our meta-analysis show that four angiogenic markers, PlGF, sFlt, their ratio, and endoglin, presented a significant difference in their mean values between the preeclamptic patients and the normal pregnant controls. The lipid markers HDL, triglycerides, leptin, adiponectin, and APO-B were also significantly different between the two groups. The ratio of PlGF to sFlt displayed the highest significant value, 7.33, in the preeclamptic group in our meta-analysis, suggesting that it is the most useful combination of markers when screening for preeclampsia (p < 0.001).
Experimentation to uncover biomarkers in patients with preeclampsia began early in the millennium. In 2003, Maynard et al. demonstrated, through their experiments, that elevated levels of the antiangiogenic factor sFlt were produced by the placenta. SFlt acted by binding to the angiogenic factors PlGF and vascular endothelial growth factor (VEGF) and by inhibiting their interaction with endothelial cells, thus causing endothelial dysfunction, hypertension, and glomerular endotheliosis.42 Levine et al. verified that high levels of sFlt and low levels of PlGF were associated with the occurrence of preeclampsia.18 They also confirmed that soluble endoglin is produced in excess by the placenta in patients with preeclampsia and that it has antiangiogenic and hypertensive properties.26 It has also been found that maternal hyperlipidaemia, increased basal metabolic index, insulin resistance, and an unbalanced expression of lipids, apolipoproteins, and adipokines also cause endothelial dysfunction in patients with preeclampsia.32
Comparison with other meta-analyses
Angiogenic markers
Several meta-analyses have been conducted to assess the sensitivity and specificity of tests to predict preeclampsia. A meta-analysis in 2009 showed that traditional tests including ones measuring mean arterial pressure and basal metabolic rate, urinary tests, and Doppler studies, as well as tests for some serum biomarkers like Activin, Inhibin, fibrin, AFP, foetal DNA, HCG, Oestriol, and PAPPA, had lower sensitivity than specificity.43 In a meta-analysis published in 2012, the authors evaluated the SMD for sFlt, PlGF, sEng, and VEGF in pregnant women before 30 weeks of gestation and reported elevated values for sFlt and sEng and a modest negative effect of PlGF and VEGF on preeclampsia; however, the test's accuracy level was too low to accurately predict the occurrence of preeclampsia in clinical practice.44 Similarly, another meta-analysis conducted in 2015 involved an examination of PlGF, PAPPA, ADAM, INHIBIN, and PP 13 and showed a low pooled sensitivity for all the biomarkers; PlGF was the single best marker, demonstrating the highest level of sensitivity in predicting the occurrence of early-onset preeclampsia.45 PlGF has also been evaluated as a predictor of adverse outcomes in patients with preeclampsia, and a meta-analysis in 2017 reported a moderate-to-high risk of preterm birth and adverse perinatal outcomes, such as stillbirth and neonatal death; however, PlGF was unreliable in predicting maternal outcomes.46 A meta-analysis which was conducted to evaluate 20 studies on the diagnostic accuracy of the sFlt/PlGF ratio demonstrated a moderate level of accuracy for preeclampsia screening and high predictive value in relation to early-onset preeclampsia. The researchers reported a sensitivity of 0.78 and specificity of 0.84, suggesting that using the ratio was better than using a single marker in the prediction of early-onset preeclampsia.47 The SAPPHIRE study, conducted in 2018, demonstrated in the meta-analysis that the ratio of sFlt/PlGF had a pooled sensitivity of 80% and specificity of 90% in predicting preeclampsia, suggesting that it can be a powerful screening tool in the clinical assessment of pregnant patients.48
Lipid markers
Our results are comparable to those of a meta-analysis performed in 2014 that reported that triglycerides, total cholesterol, and non-HDL levels were significantly elevated in all trimesters of pregnancy in preeclamptic patients.49 A marginal increase in LDL levels and elevated levels of HDL in the third trimester were found.49 A systemic review was based on an analysis of studies in which researchers utilised proteomics and mass spectrometry and identified the pathways of the immune system, haemostasis, and lipid metabolism in patients with preeclampsia; it showed significant downregulation of APO-A in preeclamptic patients compared to normal pregnant women.50 No meta-analysis reporting on APO-B was found in the literature. Leptin is a pro-inflammatory and proangiogenic adipokine, whereas adiponectin has beneficial effects on the body and the ratio of leptin and adiponectin has been proposed as a marker for metabolic syndrome, insulin resistance, and systemic inflammation.51 High levels of leptin and low levels of adiponectin have been reported in patients with preeclampsia; however, we were unable to find any meta-analysis discussing these markers.52,53
Inflammatory marker
TNF alpha was the only marker evaluated in this meta-analysis that showed no significant association with preeclampsia. Significantly elevated levels of TNF, IL 6, and IL 10 were reported to be associated with preeclampsia in few meta-analyses.54,55
Strengths and limitations
The strength of this meta-analysis is that it was based on an assessment of a wide range of serum biomarkers that have been evaluated in the literature. To the best of our knowledge, there is no meta-analysis reporting the effect of adipokines, i.e. leptin and adiponectin, on preeclampsia. The studies in the current meta-analysis showed a high degree of heterogeneity as indicated by the I2 values. We propose several reasons for this phenomenon. First, the number of studies evaluated for each marker was small, and the results of many notable randomised trials were reported in terms of the median and multiples of the median; thus, they could not be evaluated for the SMD. Second, there was great diversity in the clinical and methodological approaches of the studies, which included cohort, case–control, and nested case–control studies. Third, the reported studies were heterogeneous regarding the inclusion of patients (low-risk versus high-risk, early versus late onset of preeclampsia), the endpoint of the disease, control selection, the timing of the index test in relation to the gestational stage, and the rationale of the test (either diagnostic or predictive). Finally, statistical variations may also be one of the causes of the heterogeneity that was observed.
Conclusion
The results of this meta-analysis demonstrate that the ratio of sFlt to PlGF accounted for the most significant difference between patients with preeclampsia and those with normal pregnancies, suggesting that this combination of antiangiogenic and angiogenic biomarkers may be the most useful tool for diagnostic tool for preeclampsia.
Recommendations
We recommend that the ratio of sFlt to PlGF be used clinically for the diagnosis of preeclampsia.
Source of funding
This research did not receive any specific grant from any funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
The authors confirm that this meta-analysis had been prepared in accordance with COPE roles and regulations. Given the nature of the meta-analysis, the IRB review was not required.
Authors' contributions
RS and FB conducted the online searches and prepared the datasheets. MH performed the statistical analysis and interpretation of data and prepared the results and figures. The manuscript was composed by RS and all authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
Footnotes
Peer review under responsibility of Taibah University.
References
- 1.Gestational hypertension and preeclampsia. Obstet Gynecol. June 2020;135(6):e237–e260. doi: 10.1097/AOG.0000000000003891. [DOI] [PubMed] [Google Scholar]
- 2.Ananth C.V., Keyes K.M., Wapner R.J. Pre-eclampsia rates in the United States, 1980-2010: age-period-cohort analysis. BMJ. 2013;347:f6564. doi: 10.1136/bmj.f6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown M.A., Magee L.A., Kenny L.C., Karumanchi S.A., McCarthy F.P., Saito S., et al. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. 2018;72(1):24–43. doi: 10.1161/HYPERTENSIONAHA.117.10803. [DOI] [PubMed] [Google Scholar]
- 4.Nugent R., Bertram M.Y., Jan S., Niessen L.W., Sassi F., Jamison D.T., et al. Investing in non-communicable disease prevention and management to advance the Sustainable Development Goals. Lancet. 2018;391(10134):2029–2035. doi: 10.1016/S0140-6736(18)30667-6. [DOI] [PubMed] [Google Scholar]
- 5.Alkema L., Chou D., Hogan D., Zhang S., Moller A.-B., Gemmill A., et al. Global, regional, and national levels and trends in maternal mortality between 1990 and 2015, with scenario-based projections to 2030: a systematic analysis by the UN Maternal Mortality Estimation Inter-Agency Group. Lancet. 2016;387(10017):462–474. doi: 10.1016/S0140-6736(15)00838-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Say L., Chou D., Gemmill A., Tuncalp O., Moller A.-B., Daniels J., et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2(6):e323–e333. doi: 10.1016/S2214-109X(14)70227-X. [DOI] [PubMed] [Google Scholar]
- 7.Sachan R., Patel M.L., Dhiman S., Gupta P., Sachan P., Shyam R. Diagnostic and prognostic significance of serum soluble endoglin levels in preeclampsia and eclampsia. Adv Biomed Res. 2016;5:119. doi: 10.4103/2277-9175.186993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeyabalan A. Epidemiology of preeclampsia: impact of obesity. Nutr Rev. 2013;71(suppl_1):S18–S25. doi: 10.1111/nure.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akolekar R., Syngelaki A., Poon L., Wright D., Nicolaides K.H. Competing risks model in early screening for preeclampsia by biophysical and biochemical markers. Fetal Diagn Ther. 2013;33(1):8–15. doi: 10.1159/000341264. [DOI] [PubMed] [Google Scholar]
- 10.De Kat A.C., Hirst J., Woodward M., Kennedy S., Peters S.A. Prediction models for preeclampsia: a systematic review. Pregnancy Hypertens. 2019;16:48–66. doi: 10.1016/j.preghy.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Hypertension in pregnancy pathway NICE guidelines. 2021. https://pathways.nice.org.uk/pathways/hypertension-in-pregnancy Accessed 5th March 2021. Available from: [Google Scholar]
- 12.Widmer M., Cuesta C., Khan K.S., Conde-Agudelo A., Carroli G., Fusey S., et al. Accuracy of angiogenic biomarkers at < 20 weeks' gestation in predicting the risk of pre-eclampsia: a WHO multicentre study. Pregnancy Hypertens. 2015;5(4):330–338. doi: 10.1016/j.preghy.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Portelli M., Baron B. Clinical presentation of preeclampsia and the diagnostic value of proteins and their methylation products as biomarkers in pregnant women with preeclampsia and their newborns. J Pregnancy. 2018;2018:2632637. doi: 10.1155/2018/2632637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D., Liberati A., Tetzlaff J., Altman D.G., Prisma Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Young Brett L.R.J.S.S., Qian Cong, Kee-Hak Lim, Ananth Karumanchi S., Rana Sarosh. The use of angiogenic biomarkers to differentiate non-HELLP related thrombocytopenia from HELLP syndrome. J Matern Fetal Neonatal Med. 2010;23(5):366–370. doi: 10.1080/14767050903184207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holston A.M., Qian C., Kai F.Y., Epstein F.H., Karumanchi S.A., Levine R.J. Circulating angiogenic factors in gestational proteinuria without hypertension. Am J Obstet Gynecol. 2009;200(4):392. doi: 10.1016/j.ajog.2008.10.033. e1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levine R.J., Maynard S.E., Qian C., Lim K.H., England L.J., Yu K.F., et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350(7):672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 19.Engels T., Pape J., Schoofs K., Henrich W., Verlohren S. Automated measurement of sFlt1, PlGF and sFlt1/PlGF ratio in differential diagnosis of hypertensive pregnancy disorders. Hypertens Pregnancy. 2013;32(4):459–473. doi: 10.3109/10641955.2013.827205. [DOI] [PubMed] [Google Scholar]
- 20.Koga K., Osuga Y., Tajima T., Hirota Y., Igarashi T., Fujii T., et al. Elevated serum soluble fms-like tyrosine kinase 1 (sFlt1) level in women with hydatidiform mole. Fertil Steril. 2010;94(1):305–308. doi: 10.1016/j.fertnstert.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt M., Dogan C., Birdir C., Kuhn U., Gellhaus A., Kimmig R., et al. Placental growth factor: a predictive marker for preeclampsia? Gynäkol-Geburtshilfliche Rundsch. 2009;49(2):94–99. doi: 10.1159/000197908. [DOI] [PubMed] [Google Scholar]
- 22.Ouyang Y.-Q., Li S.-J., Zhang Q., Xiang W.-P., Shen H.-L., Chen H.-P., et al. Plasma sFlt-1-to-PlGF ratio is correlated with inflammatory but not with oxidative stress in Chinese preeclamptic women. Arch Gynecol Obstet. 2009;280(1):91–97. doi: 10.1007/s00404-008-0874-2. [DOI] [PubMed] [Google Scholar]
- 23.Güngör Z.B., Ekmekçi H., Tüten A., Toprak S., Ayaz G., Çalışkan O., et al. Is there any relationship between adipocytokines and angiogenesis factors to address endothelial dysfunction and platelet aggregation in untreated patients with preeclampsia? Arch Gynecol Obstet. 2017;296(3):495–502. doi: 10.1007/s00404-017-4461-2. [DOI] [PubMed] [Google Scholar]
- 24.Tobinaga C.M., Torloni M.R., Gueuvoghlanian-Silva B.Y., Pendeloski K.P.T., Akita P.A., Sass N., et al. Angiogenic factors and uterine Doppler velocimetry in early- and late-onset preeclampsia. Acta Obstet Gynecol Scand. 2014;93(5):469–476. doi: 10.1111/aogs.12366. [DOI] [PubMed] [Google Scholar]
- 25.Chaiworapongsa T., Romero R., Kim Y.M., Kim G.J., Kim M.R., Espinoza J., et al. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern Fetal Neonatal Med. 2005;17(1):3–18. doi: 10.1080/14767050400028816. [DOI] [PubMed] [Google Scholar]
- 26.Levine R.J., Lam C., Qian C., Yu K.F., Maynard S.E., Sachs B.P., et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355(10):992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 27.Nikuei P., Rajaei M., Malekzadeh K., Nejatizadeh A., Mohseni F., AtashAbParvar A. Accuracy of soluble endoglin for diagnosis of preeclampsia and its severity. Iran Biomed J. 2017;21(5):312–320. doi: 10.18869/acadpub.ibj.21.5.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perucci L.O., Gomes K.B., Freitas L.G., Godoi L.C., Alpoim P.N., Pinheiro M.B., et al. Soluble endoglin, transforming growth factor-Beta 1 and soluble tumor necrosis factor alpha receptors in different clinical manifestations of preeclampsia. PloS One. 2014;9(5) doi: 10.1371/journal.pone.0097632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.León-Reyes G., Maida-Claros R.F., Urrutia-Medina A.X., Jorge-Galarza E., Guzmán-Grenfell A.M., Fuentes-García S., et al. Oxidative profiles of LDL and HDL isolated from women with preeclampsia. Lipids Health Dis. 2017;16(1):90. doi: 10.1186/s12944-017-0480-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boghossian N.S., Mendola P., Liu A., Robledo C., Yeung E.H. Maternal serum markers of lipid metabolism in relation to neonatal anthropometry. J Perinatol. 2017;37(6):629–635. doi: 10.1038/jp.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Negrato C.A., Jovanovic L., Tambascia M.A., Geloneze B., Dias A., Calderon I.D., et al. Association between insulin resistance, glucose intolerance, and hypertension in pregnancy. Metab Syndr Relat Disord. 2009;7(1):53–59. doi: 10.1089/met.2008.0043. [DOI] [PubMed] [Google Scholar]
- 32.Güngör Z.B., Tüten A., Ekmekçi H., Ekmekçi O.B., Kucur M., Öncül M., et al. Possible effects of lipoprotein-associated phospholipase A2 single nucleotide polymorphisms on cardiovascular risk in patients with preeclampsia. J Matern Fetal Neonatal Med. 2018;31(23):3119–3127. doi: 10.1080/14767058.2017.1365125. [DOI] [PubMed] [Google Scholar]
- 33.Cakmak H.A., Dincgez Cakmak B., Abide Yayla C., Inci Coskun E., Erturk M., Keles I. Assessment of relationships between novel inflammatory markers and presence and severity of preeclampsia: epicardial fat thickness, pentraxin-3, and neutrophil-to-lymphocyte ratio. Hypertens Pregnancy. 2017;36(3):233–239. doi: 10.1080/10641955.2017.1321016. [DOI] [PubMed] [Google Scholar]
- 34.Kharb S., Bala J., Nanda S. Markers of obesity and growth in preeclamptic and normotensive pregnant women. J Obstet Gynaecol. 2017;37(5):610–615. doi: 10.1080/01443615.2017.1286463. [DOI] [PubMed] [Google Scholar]
- 35.Mendola P., Ghassabian A., Mills J.L., Zhang C., Tsai M.Y., Liu A., et al. Retinol-binding protein 4 and lipids prospectively measured during early to mid-pregnancy in relation to preeclampsia and preterm birth risk. Am J Hypertens. 2017;30(6):569–576. doi: 10.1093/ajh/hpx020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Timur H., Daglar H.K., Kara O., Kirbas A., Inal H.A., Turkmen G.G., et al. A study of serum Apo A-1 and Apo B-100 levels in women with preeclampsia. Pregnancy Hypertens. 2016;6(2):121–125. doi: 10.1016/j.preghy.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Khosrowbeygi A., Lorzadeh N., Ahmadvand H. Lipid peroxidation is not associated with adipocytokines in preeclamptic women. Iran J Reproductive Med. 2011;9(2):113–118. [PMC free article] [PubMed] [Google Scholar]
- 38.Herse F., Bai Y., Staff A.C., Yong-Meid J., Dechend R., Zhou R. Circulating and uteroplacental adipocytokine concentrations in preeclampsia. Reprod Sci. 2009;16(6):584–590. doi: 10.1177/1933719109332828. [DOI] [PubMed] [Google Scholar]
- 39.Dávila D.R., Julian C.G., Browne V.A., Toledo-Jaldin L., Wilson M.J., Rodriguez A., et al. Role of cytokines in altitude-associated preeclampsia. Pregnancy Hypertens. 2012;2(1):65–70. doi: 10.1016/j.preghy.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Afshari J.T., Ghomian N., Shameli A., Shakeri M.T., Fahmidehkar M.A., Mahajer E., et al. Determination of interleukin-6 and tumor necrosis factor-alpha concentrations in Iranian-khorasanian patients with preeclampsia. BMC Pregnancy Childbirth. 2005;5(1):14. doi: 10.1186/1471-2393-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cakmak M., Yilmaz H., Bağlar E., Darcin T., Inan O., Aktas A., Celik H.T., et al. Serum levels of endocan correlate with the presence and severity of pre-eclampsia. Clin Exp Hypertens. 2016;38(2):137–142. doi: 10.3109/10641963.2015.1060993. [DOI] [PubMed] [Google Scholar]
- 42.Maynard S.E., Min J.-Y., Merchan J., Lim K.-H., Li J., Mondal S., et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111(5):649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cnossen J.S., Riet G.T., Mol B.W., Van Der Post J.A., Leeflang M.M., Meads C.A., et al. Are tests for predicting pre-eclampsia good enough to make screening viable? A review of reviews and critical appraisal. Acta Obstet Gynecol Scand. 2009;88(7):758–765. doi: 10.1080/00016340903008953. [DOI] [PubMed] [Google Scholar]
- 44.Kleinrouweler C.E., Wiegerinck M.M.J., Ris-Stalpers C., Bossuyt P.M.M., van der Post J.A., von Dadelszen P., et al. Accuracy of circulating placental growth factor, vascular endothelial growth factor, soluble fms-like tyrosine kinase 1 and soluble endoglin in the prediction of pre-eclampsia: a systematic review and meta-analysis. BJOG. 2012;119(7):778–787. doi: 10.1111/j.1471-0528.2012.03311.x. [DOI] [PubMed] [Google Scholar]
- 45.Wu P., Van den Berg C., Alfirevic Z., O'Brien S., Röthlisberger M., Baker P.N., et al. Early pregnancy biomarkers in pre-eclampsia: a systematic review and meta-analysis. Int J Mol Sci. 2015;16(9):23035–23056. doi: 10.3390/ijms160923035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ukah U.V., Hutcheon J.A., Payne B., Haslam M.D., Vatish M., Ansermino J.M., et al. Placental growth factor as a prognostic tool in women with hypertensive disorders of pregnancy: a systematic review. Hypertension. 2017;70(6):1228–1237. doi: 10.1161/HYPERTENSIONAHA.117.10150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Y., Zhao Y., Yu A., Zhao B., Gao Y., Niu H. Diagnostic accuracy of the soluble Fms-like tyrosine kinase-1/placental growth factor ratio for preeclampsia: a meta-analysis based on 20 studies. Arch Gynecol Obstet. 2015;292(3):507–518. doi: 10.1007/s00404-015-3671-8. [DOI] [PubMed] [Google Scholar]
- 48.Swati A., Ana Sofia C., Christopher R., Manu V. Meta-analysis and systematic review to assess the role of soluble FMS-like tyrosine kinase-1 and placenta growth factor ratio in prediction of preeclampsia: the SaPPPhirE study. Hypertension. 2017;71(2):306–316. doi: 10.1161/HYPERTENSIONAHA.117.10182. [DOI] [PubMed] [Google Scholar]
- 49.Spracklen C.N., Smith C.J., Saftlas A.F., Robinson J.G., Ryckman K.K. Maternal hyperlipidemia and the risk of preeclampsia: a meta-analysis. Am J Epidemiol. 2014;180(4):346–358. doi: 10.1093/aje/kwu145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wong F., Cox B. Proteomics analysis of preeclampsia, a systematic review of maternal and fetal compartments. J Proteonomics Bioinf. 2014;S10 [Google Scholar]
- 51.Frühbeck G., Catalán V., Rodríguez A., Gómez-Ambrosi J. Adiponectin-leptin ratio: a promising index to estimate adipose tissue dysfunction. Relation with obesity-associated cardiometabolic risk. Adipocyte. 2018;7(1):57–62. doi: 10.1080/21623945.2017.1402151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song Y., Gao J., Qu Y., Wang S., Wang X., Liu J. Serum levels of leptin, adiponectin and resistin in relation to clinical characteristics in normal pregnancy and preeclampsia. Clin Chim Acta. 2016;458:133–137. doi: 10.1016/j.cca.2016.04.036. [DOI] [PubMed] [Google Scholar]
- 53.Thagaard I.N., Hedley P.L., Holm J.-C., Lange T., Larsen T., Krebs L., et al. Leptin and Adiponectin as markers for preeclampsia in obese pregnant women, a cohort study. Pregnancy Hypertens. 2019;15:78–83. doi: 10.1016/j.preghy.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 54.Xie C., Yao M.Z., Liu J.B., Xiong L.K. A meta-analysis of tumor necrosis factor-alpha, interleukin-6, and interleukin-10 in preeclampsia. Cytokine. 2011;56(3):550–559. doi: 10.1016/j.cyto.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 55.Lau S.Y., Guild S.-J., Barrett C.J., Chen Q., McCowan L., Jordan V., et al. Tumor necrosis factor-alpha, interleukin-6, and interleukin-10 levels are altered in preeclampsia: a systematic review and meta-analysis. Am J Reprod Immunol. 2013;70(5):412–427. doi: 10.1111/aji.12138. [DOI] [PubMed] [Google Scholar]